Abstract
Background
Randomised clinical trials have compared portosystemic shunting procedures with endoscopic therapy for variceal haemorrhage, but there is no consensus as to which approach is preferable.
Objectives
To compare the effects of shunts (total surgical shunt (TS); distal spleno‐renal shunts (DSRS) or transjugular intrahepatic porto‐systemic shunts (TIPS) with endoscopic therapy (ET, sclerotherapy and/or banding) for prevention of variceal rebleeding in patients with cirrhosis.
Search methods
The Cochrane Hepato‐Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded, conference proceedings, and the references of identified trials were searched (last search September 2006). Researchers in the field and in industry were contacted.
Selection criteria
Randomised clinical trials comparing TS, DSRS or TIPS with ET in patients who had recovered from a variceal haemorrhage and were known to be cirrhotic.
Data collection and analysis
Data were collected to allow intention‐to‐treat analysis where possible. For each outcome, a pooled estimate of treatment effect (log hazard ratio for time to outcome, Peto odds ratio for binary outcomes, and differences in means for continuous outcomes) across trials was calculated.
Main results
Twenty‐two trials evaluating 1409 patients were included. All trials had problems of method. Shunt therapy compared with ET demonstrated significantly less rebleeding (OR 0.24, 95% CI 0.18 to 0.30) at the cost of significantly increased acute hepatic encephalopathy (OR 2.07, 95% CI 1.59 to 2.69) and chronic encephalopathy (OR 2.09, 95% CI 1.20 to 3.62). There were no significant differences regarding mortality (hazard ratio 1.00, 95% CI 0.82 to 1.21) and duration of in‐patient stay (weighed mean difference 0.78 day, 95% CI ‐1.48 to 3.05). The proportion of patients with shunt occlusion or dysfunction was 3.1% (95% CI 0.4 to 10.7%) following TS (two trials), 7.8% (95% CI 3.8 to 13.9%) following DSRS (four trials), and 59% (range 18% to 72%) following TIPS (14 trials).
Authors' conclusions
All shunts resulted in a significantly lower rebleeding rate at the expense of a higher incidence of encephalopathy. TIPS was complicated by a high incidence of shunt dysfunction. No survival advantage was demonstrated with any shunt.
Plain language summary
Portosystemic shunts compared with sclerotherapy/banding lowers variceal rebleeding, but increases hepatic encephalopathy
A third of deaths from cirrhosis are due to variceal bleeding. Randomised clinical trials have compared three types of portosystemic shunting separately against endoscopic therapy. The shunts included in these trials have been total portocaval shunts, distal splenorenal shunts, and transjugular intrahepatic portocaval shunts. The authors found that when compared to endoscopic therapy all three types of shunt lowered the rate of rebleeding at the cost of a higher incidence of hepatic encephalopathy, without any statistically significant difference in survival.
Background
Variceal haemorrhage is a formidable clinical challenge. Bleeding from gastro‐oesophageal varices is a significant cause of early mortality, at 30% to 50% for a first bleed (Graham 1981; Bornman 1994). Those who survive a first bleed are more likely than not to have further bleeding episodes in the first few days of the initial bleed (Smith 1982). There is general agreement that early and vigorous resuscitation should be undertaken, preferably in specialist units (Khan 1997). Early endoscopy has emerged to be mandatory not only to ascertain the cause of such bleeding but also to achieve haemostasis (Grace 1997).
Once preliminary control of bleeding has been achieved, regular endoscopic measures are used to prevent rebleeding in most centres. This requires multiple visits of the patient to the hospital and sometimes is associated with recurrent bleeding episodes (Terblanche 1983; McIntyre 1996). However, rebleeding is significantly less frequent during and after endoscopic variceal obliteration, compared to controls (Pagliaro 1989). Adjunct measures include long‐term beta‐blocker treatment to reduce portal hypertension (Bernard 1997). More recently, variceal banding has appeared as a more effective measure than sclerotherapy to prevent rebleeding (Laine 1995).
One way of decompressing the varices and hence preventing rebleeding is to create a portosystemic shunt. Portosystemic shunts have been classified according to their haemodynamic consequences into total surgical shunts (TS), which have no prograde hepatopetal flow through the portal vein, and selective or partial shunts, which preserve pre‐existing hepatopetal portal vein flow (Sutton 1994). The distal spleno‐renal shunt (DSRS) is a selective shunt that has been associated with improved preservation of liver function and hence lower morbidity as compared to TS, although a lower mortality has not been conclusively demonstrated (D'Amico 1995). The selectivity of DSRS can further be improved if the venous collaterals between the splenic vein and pancreas are disconnected, an additional procedure that is particularly important in alcoholics (Warren 1986).
Since the early 1990s the radiologically placed transjugular intrahepatic portosystemic shunt (TIPS) has been popularised (LaBerge 1993). It is inserted radiologically by minimal access and can usually be placed more quickly than a surgical shunt (Brown 1997). In essence it is a side‐to‐side portosystemic shunt. TIPS can, however, have serious acute and chronic complications and has a small but significant mortality rate (Casado 1998). Stenosis and occlusion rates have been reported to exceed 75% at two years in randomised trials using TIPS (Papatheodoridis 1999).
Since shunts are usually a once only treatment and have been associated with low rebleeding rates, can a case be made for wider use of shunting as a more effective and safer alternative to long‐term endoscopic measures? No previous Cochrane review has addressed this question, although several meta‐analyses have been published in the past two decades. Surgical shunts have been compared to medical therapy in two previous meta‐analyses (Pagliaro 1989; Spina 1992a), but TS have not been compared to ET. Pagliaro and co‐workers reported results comparing prophylactic TS in patients who had no history of previous variceal bleeding, and the medical arm did not include ET (Pagliaro 1989). The second meta‐analysis compared DSRS versus ET (Spina 1992a), whilst two published reports assess TIPS versus ET (Luca 1999; Papatheodoridis 1999, which has recently been up‐dated by Burroughs et al Burroughs 2002). To comprehensively address the question we have conducted a systematic review to compare the outcome of shunts (TS, DSRS, and TIPS) versus endoscopic therapy (ET, sclerotherapy and/or banding) in the long‐term management of variceal bleeding, by assessment of the rate of rebleeding, as well as the incidence of encephalopathy, complications, in‐patient stay (cost) and survival following either form of treatment. We have combined comparison of various types of shunt with ET in a single review to obtain a more complete picture of alternatives, while guarding against over‐simplification of the comparisons. While drawing issues related to each shunt together, this review also provides improvements in methods of data analysis (Parmar 1998; Williamson 2002), inclusion of up‐dated studies (for example, Rikkers 1993), and application of standardised methods in evaluating the different types of shunt. In addition, possible improvements in the future design and conduct of randomised trials in this important area have been addressed.
Objectives
To compare shunts (TS, DSRS, or TIPS) with ET in the long‐term management of variceal bleeding, by assessment of the rate of rebleeding, the incidence of encephalopathy, complications, the length of in‐patient stay (and costs where available), and survival following each treatment.
Methods
Criteria for considering studies for this review
Types of studies
We endeavoured to identify all possible randomised trials (published and unpublished) where shunts have been compared with ET. Studies employing pseudo‐/quasi‐randomisation methods (for example, alternate allocation) were evaluated for inclusion as well.
Types of participants
Patients known to have cirrhosis (preferably proven by biopsy) and who had bled from oesophago‐gastric varices but had subsequently stabilised (prior to randomisation), either spontaneously or by the use of non‐surgical options, including vasoactive drugs and/or balloon tamponade and/or endoscopic measures.
Types of interventions
Surgical shunts (TS or DSRS or TIPS versus ET, with or without concomitant long‐term drug therapy (for example, beta‐blockers).
Types of outcome measures
(1) Rebleeding (time to rebleeding). Incidence of endoscopically proven, clinically significant rebleeding (that is, requiring transfusion) from oesophagogastric varices or portal hypertensive gastropathy. (2) Time to development of acute and chronic encephalopathy (defined by recurrent episodes of acute encephalopathy or inability of the patient to attain their previous level of function because of post‐treatment encephalopathy), as defined by: (a) Classical signs detected on physical examination. (b) Signs unequivocally described by patient's relatives. (c) Psychometric testing. (d) Electroencephalogram (EEG). (3) Procedure‐related complications. (4) In‐patient stay. Total days spent in hospital (or actual cost) due to complications of cirrhotic portal hypertension or procedure‐related complications during the follow‐up period. (5) Mortality (time to death).
Search methods for identification of studies
We searched The Cochrane Heptao‐Biliary Group Controlled Trials Register (September 2006), the Cochrane Central Register of Controlled Trials in The Cochrane Library (Issue 3, 2006), MEDLINE (1950 to September 2006), EMBASE (1980 to September 2006), and Science Citation Index Expanded (1945 to September 2006) (Royle 2003). An all language search was carried out and only human studies were evaluated. The search strategies are given in Appendix 1.
The reference lists of identified trials were investigated for relevant trials. Conference proceedings/abstracts for the European Association for the Study of the Liver, American Association for the Study of Liver Disease, British Society of Gastroenterology, and Digestive Diseases Week were full text searched for three years (2001, 2002, and 2003). All authors of studies identified to be pertinent were asked to review the list of identified trials and add any unidentified trials. Manufacturers (TIPS, pharmacological firms) were contacted as well.
Data collection and analysis
SK and RS independently applied the inclusion criteria to all identified studies and independently extracted data from reports. Unpublished data were sought by writing to the authors. Data were sought so as to allow an intention‐to‐treat analysis. Differences were resolved by consensus or by consulting an arbiter (PW or RS).
Methodological quality assessment The quality of the included trials was assessed in a standard way using four components, adopted from The Cochrane Reviewers' Handbook (Clarke 2003), as well as Schulz 1995; Moher 1998; and Kjaergard 2001:
Generation of the allocation sequence
Adequate, if the allocation sequence was generated by a computer or random number table. Drawing of lots, tossing of a coin, shuffling of cards, or throwing dice were considered as adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure;
Unclear, if the trial was described as randomised, but the method used for the allocation sequence generation was not described;
Inadequate, if a system involving dates, names, or admittance numbers was used.
Allocation concealment
Adequate, if the allocation of patients was undertaken by a central independent unit, or made use of an on‐site locked computer, or identically appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed envelopes;
Unclear, if the trial was described as randomised, but the method used to conceal the allocation was not described;
Inadequate, if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised.
Blinding (or masking)
Adequate, if the trial was described as double blind and the method of blinding included sham operation. Anticipating that no trials would include sham operation, trials that used blinded outcome assessors were considered adequate;
Unclear, if the trial was described as double blind, but the method of blinding was not described;
Not performed, if the trial was not double blind.
Follow‐up (inclusion of all randomised participants at evaluation)
Adequate, if the numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals;
Unclear, if the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated;
Inadequate, if the number or reasons for dropouts and withdrawals were not described.
Data extraction Standardised forms were used to extract the following data: patient characteristics, trial design, pattern of patient recruitment, exclusions, losses to follow‐up, and cross‐over patients. Data extracted in relation to the shunt groups included suitability for shunt, whether the shunt was emergency or elective, and in patients undergoing DSRS, whether spleno‐pancreatic disconnection was undertaken. Data were also extracted on the timing and method of assessing shunt patency.
The following data were sought for each treatment group after randomisation: estimates of log hazard ratio and its variance for time to rebleeding, time to the development of encephalopathy and time to death; incidence of rebleeding, encephalopathy and procedure‐related complications; surrogate measures of cost such as total length of hospital stay during the follow‐up period. When a trial had more than two arms, data were extracted only from the arms which corresponded to the treatment options compared in this review.
Data analysis Analyses were performed according to the intention‐to‐treat method, whenever possible, ie, with all randomised patients included in the analysis within the group into which they were randomised. In some trials, however, this approach was not clearly stated or insufficient information was given (Cello 1987; Rikkers 1987; GDEAIH 1995; Sauer 1998; Garcia‐V 1999), so authors were contacted to retrieve pertinent data. Further information was not given, however, so the information reported was used. For each outcome in each comparison (TS versus ET, DSRS versus ET, TIPS versus ET) a pooled estimate of treatment effect was calculated as a hazard ratio (HR) for time to event outcomes, odds ratio (OR) for binary outcomes, and differences in means (weighted mean difference (WMD)) for continuous outcomes across the trials. If estimates of log HR and its variance were not quoted directly in trial reports, alternative aggregate data (eg, log rank test P‐value) were extracted, and methods proposed by Parmar 1998 and Williamson 2002 were used to estimate log HR and its variance. In the output of the RevMan analyses 'odds ratio' actually means HR when observed‐expected number of events and the variance of each trial has been entered into RevMan analyses as individual patient data (IPD). Where information was insufficient for estimation of HR, binary data were used to calculate the OR (eg, rebleeding and encephalopathy data). Further details and discussion of the reliability of results is given in Tudur 2001.
Data were analysed with both the fixed‐effect model and the random‐effects model meta‐analyses, but only the fixed effect results were reported unless the analyses produced contradictory results for significance, in which case the most conservative estimate was reported.
Results
Description of studies
After removing duplications, a total of 66 trial reports or abstracts were identified by the search strategies and assessed for inclusion in the review. Twenty‐seven trials did not meet the inclusion criteria. Thirteen trial reports were excluded, the reasons for which are given in the table entitled 'Characteristics of excluded studies'. Twenty‐six trial reports or abstracts met the inclusion criteria. Due to duplicate publications of the same trial, these 26 reports refer to 22 randomised clinical trials. For the four trials with duplicate or up‐dated publications, the most recent report was used for data retrieval from three trials (Cello 1987; Rikkers 1993; P‐Layrargues 2001) and the report that included the most relevant information was used for data retrieval from the fourth trial (Teres 1987). One included trial (Korula 1987) comparing TS versus ET is awaiting analysis by the trialists, therefore data are not yet available for inclusion in meta‐analysis here.
Of the 22 included trials, six trials were identified from bibliographic lists.
The trials were carried out in nine countries including the United States (n = 7), Germany (n = 5), Spain (n = 3), Italy (n = 2), Sweden (n = 1), United Kingdom (n = 1), France (n = 1), Japan (n = 1), and Canada (n = 1). Results from three trials were only reported in abstracts (Korula 1987; GDEAIH 1995; Sauer 1998). The results of all trials were available in English.
A total of four trials compared TS versus ET (228 patients), four trials compared DSRS versus ET (284 patients), and 14 trials compared TIPS versus ET (1034 patients).
Only four trials (Jalan 1997; Sauer 1998; P‐Layrargues 2001; Sauer 2002) employed banding in the ET arm. Propranolol was used in addition to sclerotherapy in three trials, but only in the ET arm (GDEAIH 1995; Rossle 1997; Sauer 1997) and in addition to banding in one trial, again only in the ET arm (Sauer 2002). In all other trials, sclerotherapy was used alone in the ET arm.
Risk of bias in included studies
Shunt therapy versus ET Twenty‐two trials evaluating 1409 patients were included. All trials had at least one problem with their methods, which could account for systematic error/s (bias).
TS versus ET Four trials (Korula 1987; Cello 1987; Planas 1991; Isaksson 1996) were eligible for inclusion. One trial, however, which was published as an abstract, is awaiting analysis by the authors (Korula 1987, personal communication); therefore data are not yet available. The method of generation of the allocation sequence was unclear in all trials (Cello 1987; Planas 1991; Isaksson 1996). Planas 1991 did not describe the method used to conceal the allocation, but Cello 1987 and Isaksson 1996 used adequate methods. None of the trials employed blinded assessment of outcomes. Both Cello 1982 and Planas 1991 reported adequate follow‐up, and Planas 1991 reported both intention‐to‐treat and treatment‐received analyses. Isaksson 1996 did not mention the number of patients excluded or ineligible for the trial, and the analyses reported were based on treatment received.
DSRS versus ET Four trials were eligible for inclusion. Henderson 1990 mentioned randomisation but not the exact method, whereas two trials used random number tables (Teres 1987; Spina 1990) and one trial used Efron's biased coin design (Rikkers 1993). Two trials used envelopes for allocation concealment (Teres 1987; Henderson 1990). Two trials did not provide information on allocation concealment (Spina 1990; Rikkers 1993). None of the trials employed blinded assessment of outcomes. Teres 1987 excluded (after randomisation) 14 patients from the DSRS group and four patients from the ET group from analysis for clinical reasons. Rikkers 1993 switched one patient who had been randomised to DSRS to ET for analysis, after the patient withdrew consent for surgery. All authors were contacted to clarify these discrepancies, but either trial data were inaccessible (Teres 1987; Henderson 1990; Spina 1990) or trialists did not respond (Rikkers 1993).
TIPS versus ET Fourteen trials met the inclusion criteria. Two trials were reported as abstracts only (GDEAIH 1995; Sauer 1998) and trial quality could not be evaluated. Six trials had adequate generation of randomisation sequence: five of the trials used computer generated random numbers (Cabrera 1996; Rossle 1997; Sanyal 1997; Sauer 1997; Sauer 2002) and one trial used "random blocks of four" (Merli 1998). Three trials (Jalan 1997; Garcia‐V 1999; P‐Layrargues 2001) had unclear generation of the randomisation sequence. Four trials ( Cello 1987; Merli 1998; Nahara 2001; Gulberg 2002) used sealed envelopes to conceal allocation and thus had adequate allocation concealment. Three trials (Cabrera 1996; Sauer 1998; Garcia‐V 1999) did not describe a method to conceal allocation. None of the trials employed blinded assessment of outcomes. Intention‐to‐treat analysis was used by most trialists, although this was not mentioned by Garcia‐V 1999. Merli 1998 randomised one patient in error and excluded that patient from analyses. Similarly, Cabrera 1996 excluded one patient from each group who died after randomisation but before the allocated treatment was given. Attrition to follow‐up was less than 5% in all cases (where such data could be obtained from reports or authors).
Effects of interventions
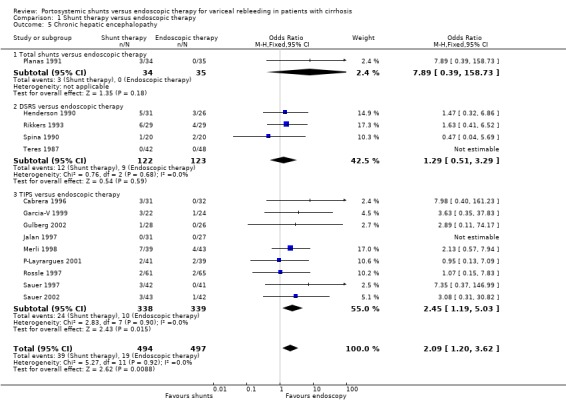
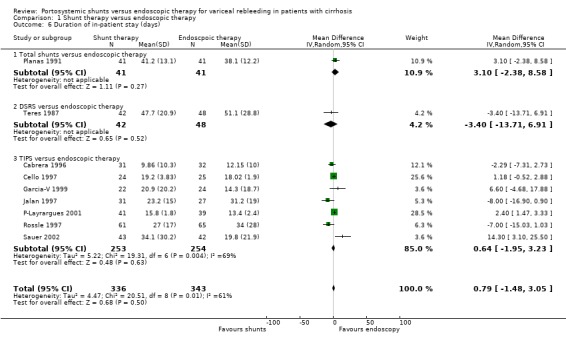
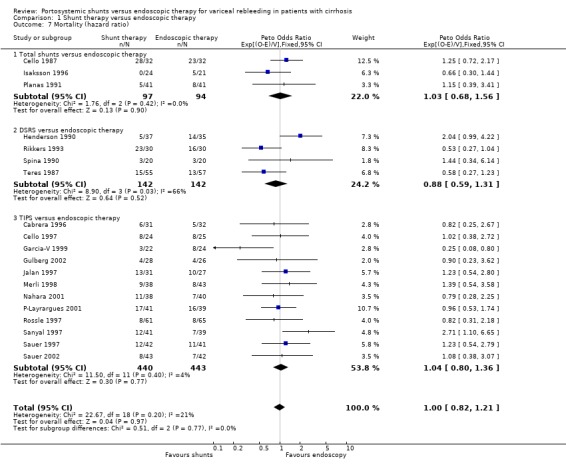
Shunt therapy versus ET Shunt therapy compared with ET demonstrated significantly less rebleeding (OR 0.24, 95% CI 0.18 to 0.30) at the cost of significantly increased acute hepatic encephalopathy (OR 2.07, 95% CI 1.59 to 2.69) and chronic encephalopathy (OR 2.09, 95% CI 1.20 to 3.62). There were no significant differences between shunts and ET in mortality (HR 1.00, 95% CI 0.82 to 1.21) and duration of in‐patient stay (WMD 0.78 day, 95% CI ‐1.48 to 3.05). The proportion of patients with shunt occlusion or dysfunction was 3.1% (95% CI 0.4 to 10.7%) following TS (two trials), 7.8% (95% CI 3.8 to 13.9%) following DSRS (four trials), and 59% (range 18% to 72%) following TIPS (14 trials). Funnel plots Figures 1 to 4 (Figure 1; Figure 2; Figure 3; Figure 4) show the funnel plots of shunts versus ET for rebleeding, acute hepatic encephalopathy, chronic encephalopathy, and mortality. There was visual indication of bias favouring shunt therapy with respect to rebleeding (Figure 1), but no indication of bias with respect to hepatic encephalopathy, chronic encephalopathy, or mortality (Figures 2 to 4).
1.
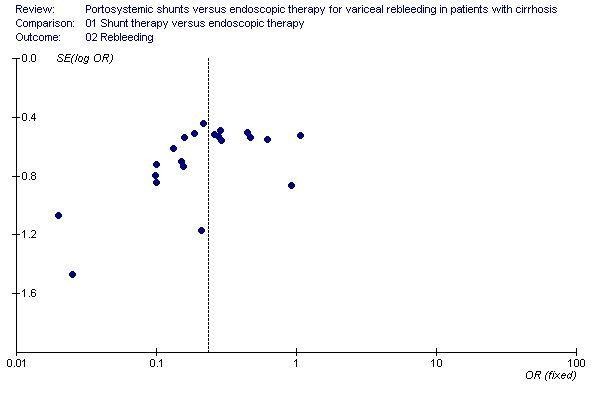
Funnel plot of shunt therapy versus endoscopic therapy, showing bias in favour of shunt therapy.
2.
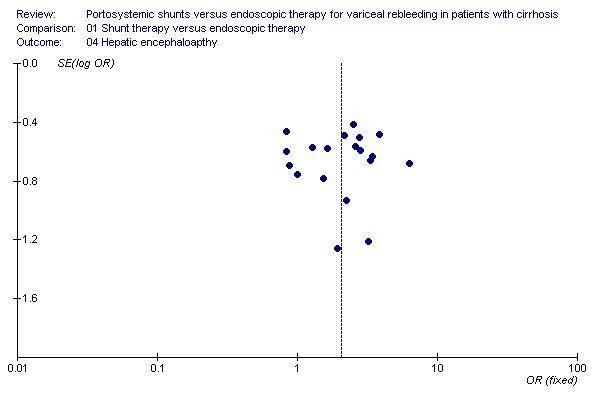
Funnel plot of shunt therapy versus endoscopic therapy, showing no bias in favour of shunt therapy on hepatic encephalopathy.
3.
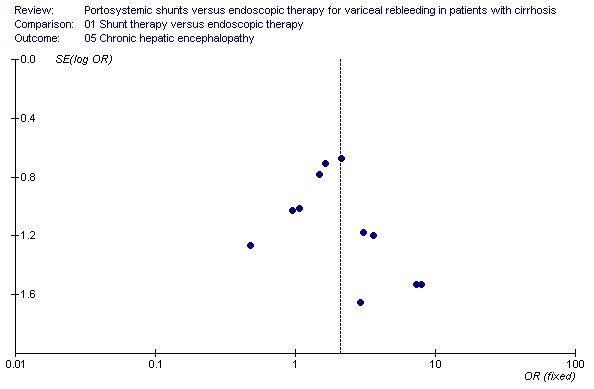
Funnel plot of shunt therapy versus endoscopic therapy, showing no bias in favour of shunt therapy on chronic hepatic encephalopathy.
4.
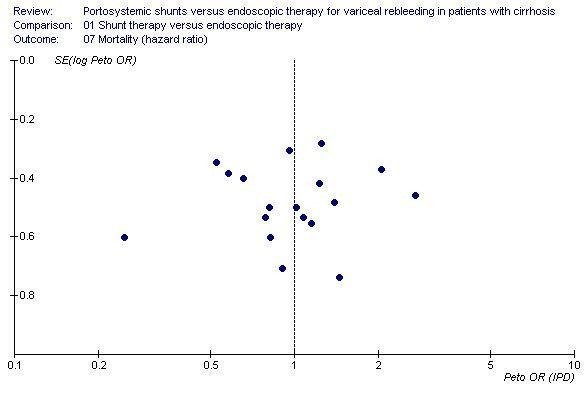
Funnel plot of shunt therapy versus endoscopic therapy, showing no bias in favour of shunt therapy on mortality.
TS versus ET
Rebleeding The number of patients rebleeding in the follow‐up period was significantly less in the TS group (OR 0.14; 95% CI 0.07 to 0.27). Data were not available to allow a time to rebleeding analysis.
Encephalopathy Data were not available to perform a time to development of a first episode of encephalopathy analysis. Patients treated with TS appeared to be more susceptible to either new episodes or worsening of their pre‐existing encephalopathy, which was seen as a trend (OR 1.52, 95% CI 0.67 to 3.44). Isaksson 1996 did not report the incidence of chronic encephalopathy whereas Planas 1991 reported three cases in the TS group with chronic disabling encephalopathy compared to none in the ET group (OR 8.09; 95% CI 0.81 to 80.51).
Complications Complications are summarised in Table 2. Shunt dysfunction was recorded in two trials (Planas 1991; Isaksson 1996) and 95% confidence intervals for the percentage with shunt dysfunction were calculated using the method proposed by Wilson 1927 giving results of 2% (0.4% to 13%) and 4% (0.7% to 20%), respectively.
1. TS versus ET, shunt surveillance and complications.
| Trial | SH surveillance | SH dysfunction | SH complications | ET complications |
| Planas 1991 | Angiography or ultrasound 3‐10 months later or at the time of rebleeding. | 1/41 [percentage and 95% CI: 2(0.4 to 13)%] | Wound abscess 2, Sepsis 1, pneumonia 2, chylous pleural effusion 1, cholestasis 1 | Ulcers 3, stenosis 1, pneumonia 1, dysphagia 4. |
| Isaksson 1996 | Angiography at 4 months then annual ultrasound. | 1/24 [percentage and 95% CI: 4(0.7 to 20)%] | Oesophagitis 8. | Oesophageal stenosis 2, oesophagitis 7. |
| Cello 1987 | Not mentioned. | Not mentioned. | Not mentioned. | Not mentioned. |
Cost/in‐patient stay Only Planas 1991 reported the mean (+/‐ standard deviation) in‐patient stay (days) (WMD between groups 3.1 days; 95% CI ‐2.3 to 8.5 days); Isaksson 1996 reported the median in‐patient stay in the TS group as 34.5 days (range 9‐122 days) and in the ET group as 33 days (range 15‐64 days).
Cello 1987 reported the sum of all costs for in‐patient hospital care (including hospital and professional charges). Planas 1991 reported data but did not provide detail on costs. Isaksson 1996 calculated the hospital cost by including the cost of the laboratory, radiology, transfusions, drugs, grafts, hotel service, endoscopy, and surgical treatment. Mean (+/‐standard deviation) or *median (range) health care cost per patient were as follows (US dollars):
TS/ET Cello 1987 $ 28,043 (+/‐ 2,920) $ 23,077 (+/‐ 3,375) Planas 1991 $ 9,761 (+/‐ 750) $ 9,047 (+/‐ 704) Isaksson 1996 *$ 12,049 (7,802‐64,853) *$ 12,027 (2,525‐171,681)
Mortality There was no significant difference in survival between the two groups (HR 1.03; 95% CI 0.68 to 1.56).
DSRS versus ET
Rebleeding Data were not available to allow an analysis of time to first rebleeding. The DSRS group had a significantly lower likelihood of rebleeding than the ET group over the follow‐up period (OR 0.17; 95% CI 0.10 to 0.29).
Encephalopathy Time to the development of encephalopathy could not be calculated. There tended to be a higher incidence of either new episodes of encephalopathy or worsening of pre‐existing encephalopathy with DSRS (OR 1.74; 95% CI 0.84 to 3.63). There was no evidence of a difference in chronic encephalopathy between the two groups (OR 1.29; 95% CI 0.51 to 3.22). Henderson 1990 did not report data on encephalopathy.
Complications Complications are summarised in Table 3. Shunt dysfunction was recorded in four trials (Teres 1987; Henderson 1990; Spina 1990; Rikkers 1993) and 95% confidence intervals were calculated using the method proposed by Wilson 1927 giving results of 3% (0.5% to 15%), 10% (4% to 26%), 0% (0% to 16%), and 14% (7% to 27%), respectively.
2. DSRS versus ET, shunt surveillance and complications.
| Trial | SH surveillance | SH dysfunction | SH complications | ET complications |
| Henderson 1990 | Not mentioned. | 1/35 [percentage and 95% CI: 3(0.5 to 15)%] | Not mentioned. | Not mentioned. |
| Rikkers 1993 | Angiography 3 months and then 1, 3, and 6 years. | 3/30 [percentage and 95% CI: 10(4 to 26)%] | Not mentioned. | Stenosis 2. |
| Spina 1990 | Angiography 10th day, 1, 3, and 6 months and then 6 monthly for 2 years. | 0/20 [percentage and 95% CI: 0(0 to 16)%] | Intestinal obstruction (one death). | Ulcers 2, stenosis 2, dysphagia 5. |
| Teres 1987 | Angiography or ultrasound or splenoportography 7 to 10 months after surgery or when rebleeding. | 6/43 [percentage and 95% CI: 14(7 to 27)%] | Not mentioned. | Ulcers 2, stenosis 3, dysphagia 15. |
Cost/in‐patient stay Teres 1987 reported the number of in‐patient days following randomisation (WMD between groups ‐3.4; 95% CI ‐13.7 to 6.9) and was the only trialist to do so.
Rikkers 1993 provided a cost analysis in the preliminary report but only included patients who were admitted to the University Hospital, and did not include the full cohort of patients. The mean (+/‐standard deviation) health care cost per patient was given as $ 34,474 (+/‐ $ 5499) for DSRS and $ 37,6488 (+/‐$ 6392) for ET (US dollars). Mortality There was no significant difference in survival between the two groups (HR 0.88; 95% CI 0.59 to 1.31). There is evidence of significant statistical heterogeneity (P = 0.031). The cause of heterogeneity appears to be divergent results from two trials (Henderson 1990; Rikkers 1993). Sensitivity analyses were not performed because of the small number of trials. A random‐effects model produced similar results to that of the fixed‐effect model (HR 0.89; 95% CI 0.61 to 1.49).
TIPS versus ET
Rebleeding Rebleeding due to portal hypertension was considered as a time to event outcome and showed a significant advantage for TIPS (eleven trials included) (HR 0.42; 95% CI 0.32 to 0.54). However, three trial reports did not provide the causes of rebleeding but implied that the rebleeding episodes were related to portal hypertension (Cabrera 1996; Sanyal 1997; Sauer 1997). A second meta‐analysis for rebleeding (all causes) was also carried out, showing a similar advantage for TIPS (OR 0.31; 95% CI 0.24 to 0.41). All eligible trials were included in the second meta‐analysis (14 trials). Sensitivity analyses were performed excluding trials employing pharmacotherapy in the ET arm, but no difference in overall results was apparent.
Encephalopathy TIPS resulted in significantly more episodes of new encephalopathy or of worsening of pre‐existing encephalopathy when time to development of encephalopathy was analysed (HR 1.96; 95% CI 1.47 to 2.61). As is evident from the meta‐analysis, not all trials could be included because of the lack of available data (nine trials included). However, when development of encephalopathy was considered as a binary outcome, a further four trials could be included, again demonstrating a significantly increased risk of encephalopathy following TIPS (OR 2.17; 95% CI 1.62 to 2.90). Patients treated with TIPS also showed a significantly higher risk of developing chronic encephalopathy (OR 2.52; 95% CI 1.26 to 5.05).
Complications Complications are summarised in Table 4. Shunt dysfunction/occlusion was the commonest problem following TIPS. Shunt dysfunction was recorded in twelve trials (Cabrera 1996; Cello 1997; Jalan 1997; Rossle 1997; Sanyal 1997; Sauer 1997; Merli 1998; Sauer 1998; Garcia‐V 1999; P‐Layrargues 2001; Nahara 2001; Sauer 2002) giving a median percentage with shunt dysfunction of 59% (range 18% to 72%). The confidence intervals for individual studies, calculated using the method proposed by Wilson 1927, are wide and vary across studies.
3. TIPS versus ET, shunt surveillance and complications.
| Trial | SH surveillance | SH dysfunction | SH complications | ET complications |
| Cabrera 1996 | Angiography 6 monthly. | 15/26 [percentage and 95% CI: 58(39 to 75)%] | Portal thrombosis 2, spontaneous bacterial peritonitis 2, haemobilia 1, sepsis 1. | Ulcers 5, stenosis 4, pneumonia 2, sepsis 1, spontaneous bacterial peritonitis 2. |
| Cello 1997 | Duplex ultrasound. | 4/22 [percentage and 95% CI: 18(7 to 39)%] | Not mentioned. | Not mentioned. |
| Garcia‐V 1999 | Angiography at 1 and 6 months. | 13/18 [percentage and 95% CI: 72(49 to 88)%] | Not mentioned. | Ulcers 5, stenosis 1. |
| Jalan 1997 | Duplex ultrasound at 1 week, angiography at 1 and 6 months, and then 6 monthly. | 9/28 [percentage and 95% CI: 32(18 to 51)%] | Sepsis 3, perforation of the capscule 1 (death). | Ulcers 12, sepsis 4, pneumonia 2. |
| Merli 1998 | Duplex ultrasound 6 monthly and angiography 6 monthly. | 21/33 [percentage and 95% CI: 64(47 to 78)%] | Haemolysis 1, intra‐hepatic haematoma 1, cardiac arrest 1, pulmonary embolism 1. | Ulcers 2, stenosis 2, pneumonia 1, stroke 1. |
| Rossle 1997 | Duplex ultrasound at 1, 3, 6, 9, and 12 months and then 6 monthly. | 18/60 [percentage and 95% CI: 30(20 to 43)%] | Stent migration 1, haemobilia 3, haemoperitoneum 2, bleeding in the liver 1 and sepsis 1. | Ulcers 8, dysphagia 5, mediastinitis 1, hypopyon 1. |
| Sanyal 1997 | Duplex ultrasound at day 1, first week, 1 and 3 months and then 3 monthly. | 20/34 [percentage and 95% CI: 59(42 to 74)%] | Haemolysis 5. | Ulcers 22, stenosis 3, dysphagia 5. |
| Sauer 1997 | Duplex ultrasound every 3 months and angiography every 3 months. | 29/42 [percentage and 95% CI: 69(54 to 81)%] | Shunt dislocation 4. | Ulcers 19, haemorrhage 5. |
| G‐P Layrargues 2001 | Duplex ultrasound at 24 hours and then 3 monthly for two years. | 24/41 [percentage and 95% CI: 59(43 to 72)%] | Haemoperitoneum causing death 1, 30 episodes of shunt dysfunction in 24. patients. | Sepsis 2. |
| GDEAIH 1995 | Information not reported in abstract. | Information not reported. | Information not reported. | Information not reported. |
| Sauer 1998 | Information not reported in abstract. | Reported as 56% after one year'. | Information not reported. | Information not reported. |
| Sauer 2002 | Angiography or Duplex scanning every 3 months. | Cumulative dysfunction 89% during follow‐up, re‐intervention rate 62%. | pneumonia (4 patients), haemobilia (2 patients), stent dislocation (1 patient). | Dysphagia 3, pneumonia 3, septicaemia 2, post‐therapeutic haemorrhage 2. |
| Gulberg 2002 | Three monthly doppler sonography. | Not mentioned. | Perforation of liver capsule 1 (death). | Perforation of oesophagus 1. |
| Nahara 2001 | Three monthly duplex scanning. | Shunt dysfunction 71%. | Haemobilia 2, segmental hepatic infarction 1. | Dysphagia 2, pleural effusion 6, oesophageal stenosis requiring dilatation 1. |
Cost/in‐patient stay Seven trials reported the mean number of days (with standard deviations) spent as an in‐patient following randomisation in each treatment group due to complications of portal hypertension or due to complications of the allocated treatment. The data (where reported) were found to be skewed. A meaningful interpretation was not possible and caution is required in interpretation (significant statistical heterogeneity, P = 0.01). However, there appeared to be a difference between the two groups in favour of ET (WMD between groups 1.92 days, 95% CI 1.12 to 2.72 days).
Taking total estimated costs associated with either treatment, Cello 1997 reported TIPS to cost a mean (+/‐standard deviation, US dollars) of $ 29,790 (+/‐$ 3422) per patient whereas ET costed $ 27,540 (+/‐$ 5088) per patient. Jalan 1997 reported TIPS to cost £ 5782 whereas ET costed £ 4020 per patient (mean only, pounds sterling).
Mortality There was no significant difference in survival between the two therapies (HR 1.04; 95% CI 0.80 to 1.36). Data from the trials reported as abstracts could not be extracted for this time‐to‐death analysis (GDEAIH 1995; Sauer 1998).
Discussion
This systematic review has compared a range of portosystemic shunting procedures (TS, DSRS, and TIPS) with ET. All types of shunt were found to be significantly more effective at preventing recurrent variceal haemorrhage. However, all forms of shunting were also associated with an increased incidence of hepatic encephalopathy, a major disadvantage of shunting that was most obvious following TIPS. There was no marked difference in cost between shunting and ET, although full interpretation of the cost analysis was not possible because of divergent reporting. Considerable caution is required in the interpretation of these results. TIPS was found to be associated with a longer hospital stay than ET in fixed‐effect model analysis, but this was associated with significant clinical as well as statistical heterogeneity and the significance disappeared in a random‐effects model analysis. Where data could be collected, the TS or DSRS versus ET comparisons suggested no notable difference in treatment costs. Nor was there any evidence of a difference in survival between any type of shunt and ET. Whilst death tended to result from bleeding in patients treated with ET, death tended to result from liver failure in patients treated with TS, DSRS, or TIPS. Therefore, in the absence of surveys of patient preference, published guidelines recommend ET as the preferable first line long‐term treatment in the secondary prevention of recurrent variceal haemorrhage from cirrhotic portal hypertension (Jalan 2000). In centres where expertise for shunting exists or where ET fails, the option of shunting should be considered after an informed discussion with the patient. It is necessary to note that the treatment of these patient requires dedicated specialist team effort and the chosen treatment has to be given in an optimal manner to achieve results equivalent to those of randomised clinical trials studied and selected series (Orloff 1995). Thus patients who develop clinically significant rebleeding after ET in non‐specialist hospitals should be referred early for shunting in specialist centres.
Systematic study of three different shunts (TS, DSRS, and TIPS), which were predominant during three different eras (TS in the 1960s to 1970s, DSRS in the 1970s to 1980s, and TIPS in the 1990s to 2000s), provides both a historical perspective and insight into the multiple options in the treatment of a difficult condition. The design and conduct of randomised clinical trials have developed during the last several decades. Thus it could be misleading to combine the three groups of trials in order to provide an overall assessment of ET versus shunting. Although there was minimal heterogeneity in the three comparisons, the patients in each trial were drawn from different populations, and subjects in trials of differing design. Even though randomised trials from the 1960s to the 1990s have been combined in a single meta‐analyses (Antman 1992), we were hesitant to combine the three groups of trials. When we did so, however, we observed no introduction of major heterogeneity.
TS has not previously been compared with ET in a meta‐analysis, so it was interesting not only to study this comparison, but also to place randomised clinical trials comparing TS versus ET alongside comparisons of DSRS versus ET, as well as TIPS versus ET. It was expected that a gain from a very low rebleeding rate following TS would be lost by a high incidence of new or worsened encephalopathy, associated with total decompression and diversion of all portal blood flow. However, the results show only a trend towards a higher encephalopathy rate in the TS groups. There could be several reasons for this apparent discrepancy. Firstly, Cello 1987 recruited only patients who were Child‐Pugh grade C for both trial arms, and such patients are more prone to encephalopathy because of their poor hepatic functional reserve, compared to patients of Child‐Pugh grades A and B. Secondly, a significant proportion of patients were not evaluated for encephalopathy in the trial reported by Isaksson 1996 (seven in the TS group and five in the ET group). Thirdly, the trials of TS versus ET were undertaken in an earlier era, when the conduct and reporting of randomised clinical trials were at an earlier stage of development, and the issue of encephalopathy has since received greater attention.
DSRS resulted in a trend to more episodes of new encephalopathy or worsening of pre‐existing encephalopathy than ET, without this trend reaching statistical significance. DSRS also resulted in an almost identical incidence of chronic encephalopathy as ET. These data are in agreement with those of others, who conducted a meta‐analysis of four trials comparing DSRS versus ET (Spina 1992a). The meta‐analysis of DSRS versus ET reported here includes a further report of one of these trials (Rikkers 1993) that has not changed the overall conclusion. It is not possible to comment on the value of spleno‐pancreatic disconnection in DSRS, as there were insufficient data to allow a sub‐group analysis. However, when contrasted with data on the other forms of shunt, the apparently low frequency of encephalopathy associated with DSRS suggests that the preservation of prograde portal flow by this procedure is advantageous. Interestingly the survival analysis shows significant heterogeneity. Henderson 1990 reported a survival benefit for the ET arm whereas Rikkers 1993 produced results in the opposite direction. The likely explanation for this discrepancy may lie in the trial design of the Emory group (Henderson 1990) who employed successful shunt rescue in a significant proportion of patients within the ET group (12 patients rebled after ET with a survival rate of 58%).
TIPS resulted in a significantly higher incidence of new or worsened encephalopathy, as well as a significantly higher incidence of chronic encephalopathy. In all instances, however, this was reported to be treatable and did not lead to disabling encephalopathy. A further important complication in the TIPS group was shunt insufficiency or dysfunction as well as shunt occlusion, as a result of thrombosis. Such TIPS failure was the commonest cause of rebleeding in this group. As the follow‐up period of most trials was short (under two years), one would expect that the rate of rebleeding would increase over time in the TIPS group. Therefore, vigorous surveillance is necessary for the early recognition and treatment of TIPS dysfunction, which reached 77% at one year in a study from the Barcelona group (Casado 1998). The development of covered TIPS stents has reduced the frequency of shunt dysfunction and occlusion (Bureau 2004), but trials of covered TIPS versus ET have yet to be undertaken. The drawback of encephalopathy remains with covered TIPS, so such trials might not alter our overall conclusions about the place of shunting. Two previous meta‐analyses have compared uncovered TIPS with ET (Luca 1999; Papatheodoridis 1999, which has been up‐dated by Burroughs et al Burroughs 2002). Although both groups of authors draw similar conclusions about TIPS to ours, greater confidence can be placed in our conclusions because of more robust methods. As most of the outcome variables are time dependent, these are better studied by time‐to‐event analysis, using the novel techniques described here, rather than as binary outcomes, as in previous meta‐analyses (Buyse 1987). Furthermore, both groups of authors allude to surgical shunts as an alternative that is preferable in some patients, but present no data to support this assertion (Luca 1999; Papatheodoridis 1999).
A lower incidence of encephalopathy following DSRS than following TIPS is plausible. DSRS diverts oesophageal, gastric, and splenic blood only from the portal circulation, whereas TIPS also diverts flow from the mesenteric veins, carrying more of the products of digestion and absorption implicated in the pathogenesis of encephalopathy (Weissenborn 1992). A randomised trial comparing DSRS with TIPS (140 patients, refractory to medical treatment of banding/medication, less than 65 years, Child‐Pugh grade A or B) with follow‐up between two and eight years has been published (Henderson 2006). The results suggest DSRS and TIPS are similarly efficacious in the control of refractory variceal bleeding in Child‐Pugh class A and B patients. Reintervention is significantly greater for TIPS (82%) compared with DSRS (11%). Interpretation of data is being performed to address economic comparison (personal communication). Because both procedures have equivalent outcomes, the choice is dependent on available expertise and ability to monitor the shunt and reintervene when needed. These recommendations are in keeping with those derived from studies of an alternative shunt, the narrow diameter (8 mm) portocaval shunt, which is a short shunt (under 3 cm) usually fashioned from ringed polytetrafluoroethylene (Bismuth 1974; Sarfeh 1986). This shunt is considered a partial shunt as it maintains pre‐existing prograde portal flow, albeit at a reduced level. Randomised clinical trials have shown this shunt to result in significantly lower rates of encephalopathy than TS (Collins 1994) and to result in significantly fewer treatment failures than TIPS (Rosemurgy 1997). It has the advantage of being easier to undertake than DSRS, making it more applicable in an emergency setting. The narrow diameter portocaval shunt has not been compared to ET in any randomised clinical trial, so it was not included in this systematic review.
Meta‐analysis of randomised clinical trials comparing endoscopic injection sclerotherapy with variceal ligation (banding) indicates that banding is associated with a lower rate of rebleeding, fewer sessions to achieve obliteration, and fewer complications (Laine 1995). However, at the time of an endoscopy conducted out of hours by a less experienced clinician who is confronted by active bleeding, sclerotherapy is likely to be safer.
We have not attempted to assess the effect of pharmacotherapy in this systematic review, even though a few trials used pharmacotherapy in the ET arm. Sensitivity analyses excluding these trials did not change the overall results, especially in relation to rebleeding. The addition of pharmacotherapy is rational when no portal decompression has been undertaken, even though there is a need for further trials to test this approach. Nor has this systematic review addressed the possibility of pharmacotherapy instead of ET. However, ET is probably more effective in preventing rebleeding than propranolol (Bernard 1997), and although the complication rate is higher, it is not prohibitive, particularly with banding (Sarin 1999; Jalan 2000). Again, more randomised clinical trials are needed to compare banding with pharmacotherapy.
This systematic review has shown considerable variation in the quality of randomised trials in cirrhotic portal hypertension, the majority of which were unclear with respect to generation of allocation sequence and allocation concealment. This may raise the risk of selection bias (Schulz 1995; Kjaergard 2001). None of the trials performed blinded outcome assessment. This may raise the risk of assessment bias (Schulz 1995; Kjaergard 2001). Furthermore, a number of trials had unclear reporting of follow‐up, which may introduce attrition bias. The statistical power of trials is also a major problem in this field, where modest survival advantages are unlikely to be detected unless large‐scale, multicentre randomised trials are undertaken incorporating hundreds of patients. Further issues have been highlighted at the Baveno Consensus Conferences (Baveno I, de Franchis 1992; Baveno II, de Franchis 1996; Baveno III, de Franchis 2001), including appropriate forms of randomisation and blinding, as well as the need for accurate data on all evaluable patients, trial events, and costs. In particular the need for accurate data on the timing and assessment of all individual components of Child‐Pugh grading should be emphasised, especially for encephalopathy.
Authors' conclusions
Implications for practice.
Endoscopic therapy should be the first‐line treatment in the prevention of variceal rebleeding but in centres with expertise and experience in shunting procedures the latter options should carefully be discussed with the patient. When recurrent rebleeding occurs after endoscopic therapy, selected shunting procedures should be offered at an early stage to those who are fit to undergo these procedures.
Implications for research.
We propose adequately powered, adequately conducted, properly reported multicentre trials in this important area. Since a survival benefit has not been shown for either therapy there is a need for larger sample sizes and longer follow‐up periods. The issue of cost has not been adequately addressed. Patient recruitment will continue to be an impediment and the only way around this is the pooling of resources across different centres with similar interests. Furthermore, trial reporting should be such that it facilitates future meta‐analyses. These recommendations are not specific to the comparisons addressed here, but have implications for randomised clinical trials in the management of portal hypertension in general.
Importantly only three trials reported as full publications employed variceal banding as a means of obliterating varices (Jalan 1997; P‐Layrargues 2001; Sauer 2002). We suggest that future trials employ banding in the endoscopic therapy arm.
Future trials should be reported following the CONSORT recommendations (www.consort‐statement.org).
What's new
| Date | Event | Description |
|---|---|---|
| 9 November 2008 | Amended | Converted to new review format. |
Notes
The protocol for this review was published under the title 'Shunts versus endoscopic therapy for long‐term management of variceal haemorrhage'. The authors find the new title 'Portosystemic shunts versus endoscopic therapy for variceal rebleeding in patients with cirrhosis' to better reflect the contents of the review.
Acknowledgements
We are deeply indebted to Christian Gluud, Dimitrinka Nikolova, and Anne Gethe Hee of The Cochrane Hepato‐Biliary Group for their patience, help, and support.
Appendices
Appendix 1. Search strategies
| Database | Timespan | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | September 2006 (26 references). | (shunt* OR dsrs OR tips) AND (sclerotherap* OR band*) AND ('portal hypertension*' OR cirrho* OR varic*) AND prevent* AND rebleed* |
| Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library | Issue 3, 2006 (15 references). | #1 MeSH descriptor Portasystemic Shunt, Surgical explode all trees #2 ((surgical or total or distal splenorenal or transjugular intrahepatic portosystemic) and shunt) or dsrs or tips #3 (#1 OR #2) #4 MeSH descriptor Sclerotherapy explode all trees #5 endoscopic and (sclerotherap* or band*) #6 (#4 OR #5) #7 MeSH descriptor Hypertension, Portal explode all trees #8 MeSH descriptor Liver Cirrhosis, Biliary explode all trees #9 MeSH descriptor Esophageal and Gastric Varices explode all trees #10 portal hypertension* or cirrho* or varic* #11 (#7 OR #8 OR #9 OR #10) #12 prevent* and rebleed* #13 (#3 AND #6 AND #11 AND #12) |
| MEDLINE (WinSPIRS 5.0) | 1950 to September 2006 (19 references). | #1 explode "Portasystemic‐Shunt‐Surgical"/ all subheadings #2 ((surgical or total or distal splenorenal or transjugular intrahepatic portosystemic) and shunt) or dsrs or tips #3 #1 or #2 #4 explode "Sclerotherapy"/ all subheadings #5 endoscopic and (sclerotherap* or band*) #6 #4 or #5 #7 explode "Hypertension‐Portal"/ all subheadings #8 explode "Liver‐Cirrhosis‐Biliary"/ all subheadings #9 explode "Esophageal‐and‐Gastric‐Varices"/ all subheadings #10 portal hypertension* or cirrho* or varic* #11 #7 or #8 or #9 or #10 #12 prevent* and rebleed* #13 #3 and #6 and #11 and #12 #14 random* or blind* or placebo* or meta‐analysis #15 #13 and #14 |
| EMBASE (WinSPIRS 5.0) | 1980 to September 2006 (36 references). | #1 explode "portosystemic‐anastomosis"/ all subheadings #2 ((surgical or total or distal splenorenal or transjugular intrahepatic portosystemic) and shunt) or dsrs or tips #3 #1 or #2 #4 explode "sclerotherapy"/ all subheadings #5 endoscopic and (sclerotherap* or band*) #6 #4 or #5 #7 explode "portal‐hypertension"/ all subheadings #8 explode "liver‐cirrhosis"/ all subheadings #9 explode "esophagus‐varices"/ all subheadings #10 portal hypertension* or cirrho* or varic* #11 #7 or #8 or #9 or #10 #12 prevent* and rebleed* #13 #3 and #6 and #11 and #12 #14 random* or blind* or placebo* or meta‐analysis #15 #13 and #14 |
| Science Citation Index Expanded (http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame) | 1945 to September 2006. | #1 TS=(((surgical or total or distal splenorenal or transjugular intrahepatic portosystemic) and shunt) or dsrs or tips) #2 TS=(endoscopic and (sclerotherap* or band*)) #3 TS=(portal hypertension* or cirrho* or varic*) #4 TS=(prevent* and rebleed*) #5 #4 AND #3 AND #2 AND #1 #6 TS=(random* or blind* or placebo* or meta‐analysis) #7 #6 AND #5 |
Data and analyses
Comparison 1. Shunt therapy versus endoscopic therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rebleeding (hazard ratio) | 11 | 835 | Peto Odds Ratio (95% CI) | 0.42 [0.32, 0.54] |
| 1.1 Total shunts versus endoscopic therapy | 0 | 0 | Peto Odds Ratio (95% CI) | 0.0 [0.0, 0.0] |
| 1.2 DSRS versus endoscopic therapy | 0 | 0 | Peto Odds Ratio (95% CI) | 0.0 [0.0, 0.0] |
| 1.3 TIPS versus endoscopic therapy | 11 | 835 | Peto Odds Ratio (95% CI) | 0.42 [0.32, 0.54] |
| 2 Rebleeding | 21 | 1487 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.18, 0.30] |
| 2.1 Total shunt versus endoscopic therapy | 3 | 191 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.03, 0.23] |
| 2.2 DSRS versus endoscopic therapy | 4 | 262 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.07, 0.26] |
| 2.3 TIPS versus endoscopic therapy | 14 | 1034 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.22, 0.39] |
| 3 Development of hepatic encephalopathy (hazard ratio) | 10 | 725 | Peto Odds Ratio (95% CI) | 1.96 [1.47, 2.61] |
| 3.1 Total shunts versus endoscopic therapy | 0 | 0 | Peto Odds Ratio (95% CI) | 0.0 [0.0, 0.0] |
| 3.2 DSRS versus endoscopic therapy | 0 | 0 | Peto Odds Ratio (95% CI) | 0.0 [0.0, 0.0] |
| 3.3 TIPS versus endoscopic therapy | 10 | 725 | Peto Odds Ratio (95% CI) | 1.96 [1.47, 2.61] |
| 4 Hepatic encephaloapthy | 19 | 1338 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [1.59, 2.69] |
| 4.1 Total shunt versus endoscopic therapy | 3 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.67, 3.54] |
| 4.2 DSRS versus endoscopic therapy | 3 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.83, 3.69] |
| 4.3 TIPS versus endoscopic therapy | 13 | 969 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.20 [1.63, 2.98] |
| 5 Chronic hepatic encephalopathy | 14 | 991 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.09 [1.20, 3.62] |
| 5.1 Total shunts versus endoscopic therapy | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.89 [0.39, 158.73] |
| 5.2 DSRS versus endoscopic therapy | 4 | 245 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.51, 3.29] |
| 5.3 TIPS versus endoscopic therapy | 9 | 677 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.45 [1.19, 5.03] |
| 6 Duration of in‐patient stay (days) | 9 | 679 | Mean Difference (IV, Random, 95% CI) | 0.79 [‐1.48, 3.05] |
| 6.1 Total shunts versus endoscopic therapy | 1 | 82 | Mean Difference (IV, Random, 95% CI) | 3.10 [‐2.38, 8.58] |
| 6.2 DSRS versus endoscopic therapy | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐13.71, 6.91] |
| 6.3 TIPS versus endoscopic therapy | 7 | 507 | Mean Difference (IV, Random, 95% CI) | 0.64 [‐1.95, 3.23] |
| 7 Mortality (hazard ratio) | 19 | 1358 | Peto Odds Ratio (95% CI) | 1.00 [0.82, 1.21] |
| 7.1 Total shunts versus endoscopic therapy | 3 | 191 | Peto Odds Ratio (95% CI) | 1.03 [0.68, 1.56] |
| 7.2 DSRS versus endoscopic therapy | 4 | 284 | Peto Odds Ratio (95% CI) | 0.88 [0.59, 1.31] |
| 7.3 TIPS versus endoscopic therapy | 12 | 883 | Peto Odds Ratio (95% CI) | 1.04 [0.80, 1.36] |
1.1. Analysis.
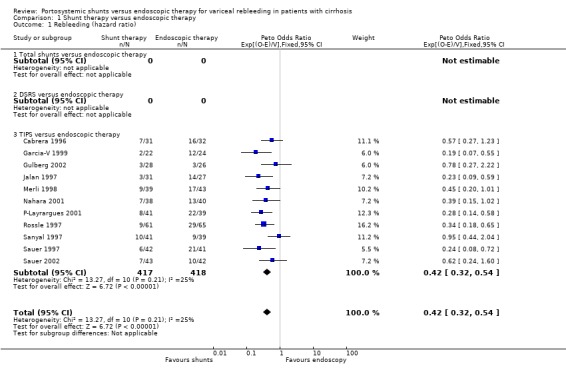
Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 1 Rebleeding (hazard ratio).
1.2. Analysis.
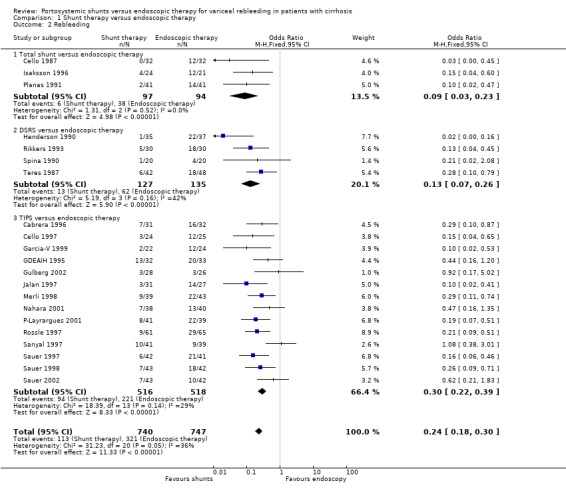
Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 2 Rebleeding.
1.3. Analysis.
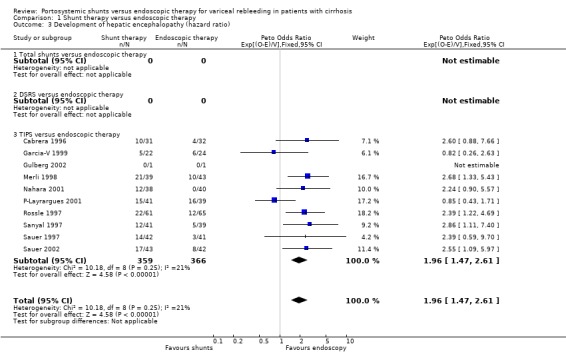
Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 3 Development of hepatic encephalopathy (hazard ratio).
1.4. Analysis.
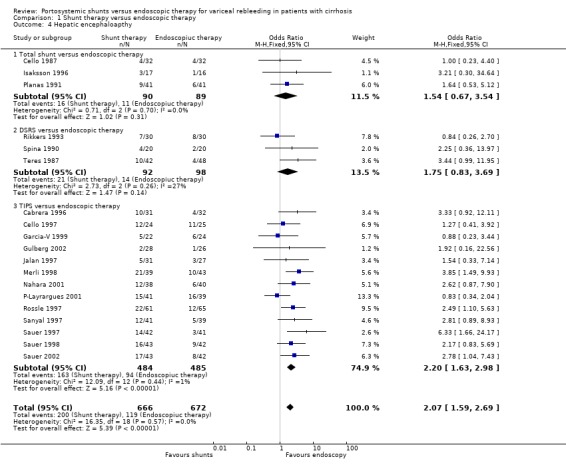
Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 4 Hepatic encephaloapthy.
1.5. Analysis.
Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 5 Chronic hepatic encephalopathy.
1.6. Analysis.
Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 6 Duration of in‐patient stay (days).
1.7. Analysis.
Comparison 1 Shunt therapy versus endoscopic therapy, Outcome 7 Mortality (hazard ratio).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cabrera 1996.
| Methods | Randomised trial.
A. Generation of allocation sequence: adequate. Computer generated random numbers.
B. Allocation concealment: unclear. No information.
C. Blinding: unclear. No information.
D. Follow‐up: adequate. Inclusion of all randomised participants at evaluation: 1 patient from each group died before treatment and not included in the analysis. Time from bleeding episode to randomisation: Three days after bleeding was controlled. Time from randomisation to treatment in days (mean, SD): TIPS ( 8.4, 3.6), ET (2.7, 3.2). Total number of patients evaluated and found eligible: 63 (90 evaluated). Randomised to TIPS: 31, randomised to ET: 32. Adequate reasons provided for those not randomised: yes. Nine patients in the ET group crossed over to TIPS during follow‐up. There were no losses to follow‐up. Intention to treat analysis. Follow‐up period (mean days, SD) TIPS (452, 298) range: 20 to 020 days. ET (455, 298) range: 70 to 951 days. Assessment of suitability for shunt carried out prior to randomisation: no. Shunt patency assessed with angiography at six months or at the time of rebleeding. Method of Child's grading: Child‐Pugh. Method of encephalopathy testing: clinical. Rebleeding episodes endoscopically verified: yes. Specified whether rebleeding episode clinically significant: yes. |
|
| Participants | Inclusion criteria: all cirrhotic patients admitted with an episode of acute oesophageal variceal bleeding. Exclusions (one or more of the following): presence of gastric varices with active bleeding, episodes of chronic encephalopathy, severe acute alcoholic hepatitis, end‐stage cirrhosis, neoplastic disease, septicaemia and portal vein thrombosis. The two groups comparable in‐terms of age, Child's status and number of alcoholic patients. |
|
| Interventions | ET:
sclerotherapy, intra‐ and para‐variceal technique, sclerosant = 1% polidocanol. Shunt: TIPS (wall and Strecker) |
|
| Outcomes | Incidence of rebleeding. Incidence of complications. Incidence of deaths. | |
| Notes | Long‐term follow‐up published as abstract in Hepato‐Gastroenterology 1998, Third International Congress of Hepato‐Pancreato‐Biliary Association. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Cello 1984.
| Methods | Duplicate publication of Cello 1987. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Cello 1987.
| Methods | Randomised trial.
A. Generation of allocation sequence: unclear. No information.
B. Allocation concealment: adequate. Serially numbered opaque envelopes.
C. Blinding: unclear. No information.
D. Follow‐up: adequate.
Inclusion of all randomised participants at evaluation: yes. Time from bleeding episode to randomisation, not mentioned. Time from randomisation to treatment: six hours for shunt and two hours for endoscopic treatment. Total number of patients evaluated: 68. Randomised to shunt: 32, randomised to ET: 32. Adequate reasons provided for those not randomised ‐ yes. Two patients in the shunt group did not receive the allocated treatment. No losses to follow‐up. Intention to treat analysis. Mean follow‐up period (range): 530 days mean, 21 to 1830 days. Assessment of suitability for shunt carried out prior to randomisation: no. Method of Child's grading: single worst Child’s criteria. Method of encephalopathy testing: clinical testing. Rebleeding episodes endoscopically verified: yes. Specified whether rebleeding episode clinically significant = yes. (Only 16 patients in the shunt group and 14 in the ET group discharged alive after the initial hospitalisation). |
|
| Participants | Inclusion criteria: Child's C patients with actively bleeding varices confirmed on endoscopy, requiring six or more units of blood transfusion. Exclusions: moribund patients. Pre‐treatment variables were comparable across the two groups other than active alcoholics which were significantly greater in the ES group. |
|
| Interventions | ET:
Sclerotherapy, intra‐variceal technique, sclerosant = sodium morrhuate. Shunt: Portocaval shunt. |
|
| Outcomes | Rebleeding. Encephalopathy. Survival. Cost. | |
| Notes | Only 16 patients in the shunt group and 14 in the ET group discharged after the index hospitalisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Cello 1997.
| Methods | Randomised trial.
A. Generation of allocation sequence: unclear. No information.
B. Allocation concealment: adequate. Sealed, opaque envelopes.
C. Blinding: unclear. No information.
D. Follow‐up: adequate.
Inclusion of all randomised participants at evaluation: yes. Time from bleeding episode to randomisation (mean, SD) = TIPS (35.4,5.6 hours), ET (37.4, 4.7 hours). Time from randomisation to treatment (mean, SD) = TIPS ( 59.5, 6.7 hours). Total number of patients evaluated = 300. Randomised to TIPS = 24, randomised to ET = 25. Adequate reasons provided for those not randomised. Reasons mentioned but numbers not provided. One patient did not receive the allocated treatment in TIPS group and six patients in ET group were crossed over to TIPS during follow‐up. Follow‐up period in days (mean, SD) TIPS (575, 109) ET (567, 104) Assessment of suitability for shunt carried out prior to randomisation = yes. Shunt patency assessed with Duplex scanning. Method of Child's grading = Child‐Pugh, however, patients were not stratified according to the Child‐Pugh system. Method of Encephalopathy testing = clinical. Rebleeding episodes endoscopically verified = yes. Specified whether rebleeding episode clinically significant = not stated. |
|
| Participants | Inclusion criteria: all patients admitted with massive or submassive acute gastrointestinal tract haemorrhage from large oesophageal varices. Exclusions (one or more of the following): prisoners, <18 or >75 years of age, Cerebrovascular accident three months before the onset of bleeding, refusal to accept blood products, gastric variceal haemorrhage, ECG changes compatible with myocardial infarction, specified limits of PO2, creatinine, bilirubin, prothrombin time and platelet count measurements, Grade IV encephalopathy, cancer other than skin cancer, AIDS, sepsis, pneumonia, peritonitis, alcoholic hepatitis (clinical evidence only), thrombosis of portal, hepatic or inferior vena cava. Patients across the two strata were comparable in‐terms of age, child score and alcoholics. |
|
| Interventions | ET:
Sclerotherapy, para‐variceal technique, sclerosant = ethanolamine oleate. Shunt: TIPS (wall stents). |
|
| Outcomes | Rebleeding. Encephalopathy. Survival. Cost analysis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Garcia‐V 1999.
| Methods | Randomised trial.
A. Generation of allocation sequence: unclear. Randomisation mentioned, but method not specified.
B. Allocation concealment: unclear. No information.
C. Blinding: unclear.
D. Follow‐up: unclear.
Inclusion of all randomised participants at evaluation: exclusions less than 10%. Time from variceal bleeding to therapy in days, mean (SD): TIPS 5.4 (2.1), ET 5.6 (2.2). 22 randomised in TIPS group and 24 in the ET group. Follow‐up period in days mean (SD): TIPS 760 (390), ET 503 (460) Assessment of suitability for shunt carried out prior to randomisation: not specified. No cross‐overs, no information on attrition to follow‐up. Shunt patency assessed with duplex scanning and portography at one month and then every six month. Method of Child's grading: Child‐Pugh. Method of encephalopathy testing: Parson‐Smith criteria. Rebleeding episodes endoscopically verified: yes. Specified whether rebleeding episode clinically significant: not specified. |
|
| Participants | Inclusion criteria: endoscopically proven oesophageal variceal bleeding, diagnosis of cirrhosis based on clinical history and laboratory, ultrasonography, and/ or liver biopsy findings, age between 18 to 75 years and informed consent from the patient. Exclusions (one or more of the following): history of chronic encephalopathy, portal vein thrombosis, hepatocellular carcinoma and end‐stage liver disease. Comparable with respect to age, gender, etiology. Endoscopic group had a significantly greater proportion of patients with pre‐existing encephalopathy but were comparable in‐terms of Child‐Pugh class. |
|
| Interventions | ET:
Sclerotherapy, ?intra‐variceal and para‐variceal technique, sclerosant = ethanolamine oleate. Shunt: TIPS (wall stent). |
|
| Outcomes | Rebleeding. Survival. Encephalopathy. Rebleeding index. Days spent as an in‐patient. Causes of death. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
GDEAIH 1995.
| Methods | Randomised trial (Abstract). Randomised to TIPS: 32; ET: 33. | |
| Participants | Child's C cirrhotic patients presenting with variceal bleeding. Treated with sclerotherapy prior to randomisation. | |
| Interventions | ET:
Sclerotherapy plus propranolol. Shunt: TIPS (type not specified). |
|
| Outcomes | Variceal rebleeding. Survival. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gulberg 2002.
| Methods | Randomised trial.
A. Generation of allocation sequence: unclear. No information.
B. Allocation concealment: adequate. Sealed, opaque envelopes.
C. Blinding: unclear. No information.
D. Follow‐up: adequate.
Inclusion of all randomised participants at evaluation: yes. Time from bleeding episode to randomisation (mean, SD) = TIPS 13, 3(days), ET 14, 3 (days). Time from randomisation to treatment = unclear. Total number of patients evaluated = 86 Randomised to TIPS = 28, randomised to ET = 26. Adequate reasons provided for those not randomised = yes. Two patients did not receive the allocated treatment in TIPS group and six patients in ET group were crossed over to TIPS and one to a surgical shunt during follow‐up. Follow‐up period in years (mean) TIPS 1.8 ET 2 . Assessment of suitability for shunt carried out prior to randomisation = yes. Shunt patency assessed with Duplex scanning. Method of Child's grading = Child‐Pugh, patients were stratified according to the Child‐Pugh system. Method of encephalopathy testing = clinical. Rebleeding episodes endoscopically verified = yes. Specified whether rebleeding episode clinically significant = yes. |
|
| Participants | Inclusion criteria: age > 18 years, endoscopic evidence of variceal bleeding within 2 months before randomisation, stable heamodynamic condition. Exclusion criteria: isolated gastric varices, isolated bleeding from gastric varices, large or diffuse liver tumour, liver transplantation intended in six months, hepatic encephalopathy > grade 2, Child Pugh >13, extra hepatic cholestasis, heart failure, sepsis, multi‐organ failure. |
|
| Interventions | ET:
Variceal band ligation. TIPS: Expandable 8‐10 mm stents. |
|
| Outcomes | Rebleeding. Death. Treatment failure. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Henderson 1990.
| Methods | Randomised trial.
A. Generation of allocation sequence: unclear. No information.
B. Allocation concealment: adequate. Serially numbered opaque envelopes.
C. Blinding: unclear. No information.
D. Follow‐up: adequate.
Inclusion of all randomised participants at evaluation: yes. Time from bleeding episode to randomisation, not mentioned. Time from randomisation to treatment: Six hours for shunt and two hours for endoscopic treatment. Total number of patients evaluated: 68. Randomised to SHUNT: 32, randomised to ET: 32. Adequate reasons provided for those not randomised ‐ yes. Two patients in the shunt group did not receive the allocated treatment. No losses to follow‐up. Intention to treat analysis. Mean follow‐up period (range): 530 days mean, 21 to 1830 days. Assessment of suitability for shunt carried out prior to randomisation: no. Method of Child's grading: single worst Child’s criteria. Method of encephalopathy testing: clinical testing. Rebleeding episodes endoscopically verified: yes. Specified whether rebleeding episode clinically significant = yes. (Only 16 patients in the shunt group and 14 in the ET group discharged alive after the initial hospitalisation). |
|
| Participants | Inclusion criteria: biopsy proven cirrhosis, endoscopic evidence of varices and suitability for a DSRS shunt established with angiography. Exclusions (one or more of the following): living more than 200 miles from the base hospital, referred for specific therapy, previous chronic sclerotherapy, emergent or urgent surgery, noncirrhotic variceal bleed. Patients comparable in‐terms of age, Child's class and alcoholics. |
|
| Interventions | ET:
Sclerotherapy, intra‐variceal and para‐variceal technique, sclerosant: 0.75 ‐1.0% sodium tetradecyl sulfate or 1.5 ‐2.0% sodium morrhuate. Shunt: DSRS (Warren). |
|
| Outcomes | Survival. Rebleeding. Hepatic function. Hemodynamics and liver and spleen volumes. | |
| Notes | Encephalopathy not considered as an outcome. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Isaksson 1996.
| Methods | Randomised trial.
A. Generation of allocation sequence: unclear. Randomisation mentioned but method not specified.
B. Allocation concealment: adequate. Sealed opaque envelopes.
C. Blinding: unclear. No information.
D. Follow‐up: inadequate.
Inclusion of all randomised participants at evaluation: incomplete information. Time from bleeding episode to randomisation and treatment: not specified, but patients were included after their variceal bleeding was arrested with emergency endoscopic sclerosis and if they fulfilled the entry criteria. Total number of patients evaluated: 228. Randomised to shunt: 24, randomised to ET: 21. Adequate reasons provided for those not randomised: yes. No patient was crossed over. No losses to follow‐up. Intention to treat analysis. Follow‐up period in months (mean): shunt 69.5, ET 60.2. Assessment of suitability for shunt carried out prior to randomisation: not specified. Method of Child's grading: Child's (version not specified). Method of encephalopathy testing: clinical and psychometric testing. Rebleeding episodes endoscopically verified: not specified. Specified whether rebleeding episode clinically significant: yes. (Isaksson et al. were unable to assess encephalopathy in 7/24 patients in the shunt group and in 5/21 group in the ET group). |
|
| Participants | Inclusion criteria: age between 20 to 75 years, endoscopically verified varices as the source of bleeding, portal hypertension, biopsy confirmed cirrhosis. Exclusions: not specified. Patients comparable in‐terms of age and Child's status but alcoholics slightly greater in the ES group. |
|
| Interventions | ET:
Sclerotherapy, submucosal, and paravariceal technique, sclerosant = ethoxy‐sclerol. Shunt: Interpositional 14 mm mesocaval Goretex shunt. |
|
| Outcomes | Survival. Rebleeding. Encephalopathy. Complications. Cost and hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Jalan 1997.
| Methods | Randomised trial.
A. Generation of allocation sequence: unclear. Randomisation mentioned but method not specified.
B. Allocation concealment: adequate. Sealed envelopes in batches of 25.
C. Blinding: unclear. No information.
D. Follow‐up: adequate.
Inclusion of all randomised participants at evaluation: yes. Time from bleeding episode to randomisation: 24 hours after cessation of bleeding. Time from randomisation to treatment in days (mean): TIPS (2.2), VB ie, variceal banding (2.4). Total number of patients evaluated and found eligible: 61 (105 evaluated). Randomised to TIPS: 31, randomised to ET: 27. Adequate reasons provided for those not randomised: yes. Three patiens in the TIPS group did not receive the allocated treatment and six patients in the endoscopic therapy were crossed over to TIPS during follow‐up. No losses to follow‐up. Intention‐to‐treat analysis. Follow‐up period months (mean, SD) TIPS (15.7,10.2) ET (16.8,10.9). Assessment of suitability for shunt carried out prior to randomisation: yes. Shunt patency assessed with duplex scanning and portography at one week, one month and then every six months. Method of Child's grading: Child‐Pugh. Method of encephalopathy testing: Parson‐Smith criteria. Rebleeding episodes endoscopically verified: yes. Specified whether rebleeding episode clinically significant: yes. |
|
| Participants | Inclusion criteria: all cirrhotic patients between 18 and 75 yrs of age who presented with a first (index) episode of variceal bleeding. Exclusions (one or more of the following): rebleeding from varices from varices within 24 hours of initial endoscopy, bleeding from ectopic varices, previous endoscopic treatment for varices, malignancy, portal vein thrombosis. Patients characteristic similar in the two groups. |
|
| Interventions | ET:
Variceal banding ligation, single application. Shunt: TIPS (wall stent). |
|
| Outcomes | Variceal rebleeding. Survival. Encephalopathy. Complications: sepsis, shunt dysfunction. Cost analysis and amount of time spent in‐patient. | |
| Notes | Only trial to employ variceal banding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Korula 1987.
| Methods | Randomised trial.
A. Generation of allocation sequence: unclear. Randomisation by balanced design.
B. Allocation concealment: unclear. Not mentioned.
C. Blinding: unclear. No information.
D. Follow‐up: adequate.
Inclusion of all randomised participants at evaluation: yes. Time from bleeding episode to randomisation: not mentioned. Time from randomisation to treatment in days (mean): not mentioned. Total number of patients evaluated and found eligible: 37 (55 evaluated). Randomised to surgical shunt: 18, randomised to ET: 19. Adequate reasons provided for those not randomised: no information. No losses to follow‐up. Follow‐up period months (mean, SD): ET 10.5 (9.5) Shunt 13.1(8.8). Assessment of suitability for shunt carried out prior to randomisation: not mentioned Shunt patency assessed: no mention. Method of Child's grading: Child‐Pugh. Method of encephalopathy testing: not mentioned. Rebleeding episodes endoscopically verified: no information. |
|
| Participants | Inclusion criteria: all patients with cirrhotic portal hypertension (Child‐Pugh A) with minimum of two variceal bleeding episodes who received less than one session of ET. Exclusions: not mentioned. Characteristic similar in the two groups. |
|
| Interventions | ET:
Sclerotherapy. Shunt: TS (13) DSRS (3) Meso‐caval (1). |
|
| Outcomes | Variceal rebleeding. Survival. Transfusion requirement. | |
| Notes | Only trial to employ variceal banding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Merli 1998.
| Methods | Randomised trial.
A. Generation of allocation sequence: adequate. Randomisation in blocks of four, three centres had a separate list.
B. Allocation concealment: adequate. Sealed envelopes.
C. Blinding: unclear. No information.
D. Follow‐up: adequate.
Inclusion of all randomised participants at evaluation: yes. Time from bleeding episode to randomisation: Three strata of time intervals used: I = acutely bleeding patients (one to seven days), II = patients referred from other centres following a bleed but without endoscopic treatment (one to six weeks), III = patients referred following various intervals after a variceal bleed for advice and treatment (seven weeks to six months). Time from randomisation to treatment : According to strata specified but active bleeding had to have been controlled for a minimum of 24 hrs. Total number of patients evaluated and found eligible: 82 (120 evaluated). Randomised to TIPS: 39, randomised to ET: 43. Adequate reasons provided for those not randomised: yes. Two patients crossed over to ET from TIPS and six crossed over from ET to TIPS during follow‐up. Six patients in TIPS group and four patients in the ET group did not receive the allocated treatment. One patient in each group lost to follow‐up. Intention to treat analysis but one patient erroneously assigned to TIPS and excluded from the analysis. Follow‐up period in weeks (mean, SE) TIPS (77.7, 7.12) ET (73.9, 7.3) Assessment of suitability for shunt carried out prior to randomisation: yes. Shunt patency assessed with Duplex ultrasound at six months or when shunt malfunction was suspected. Method of Child's grading: Child‐Pugh. Method of encephalopathy testing: Parson‐Smith criteria. Rebleeding episodes endoscopically verified: yes. Specified whether rebleeding episode clinically significant: yes. |
|
| Participants | Inclusion criteria: patients with variceal bleeding (proven or presumed) according to pre‐specified criteria. Exclusions (one or more of the following): complete portal vein thrombosis, previous episode/s of chronic recurrent hepatic encephalopathy, advanced hepatocellular carcinoma, previous multiple sessions of sclerotherapy, ongoing pharmacological prophylaxis of rebleeding (one emergency session during the acute bleeding phase was permissible), severe cardio‐vascular contraindications; or concomitant morbid condition/s with a life expectancy of less than a year. Patients characteristic comparable other than alcoholics which were higher in the endoscopic group. |
|
| Interventions | ET:
Sclerotherapy, technique not mentioned, sclerosant = 1‐2% polidocanol. Shunt: TIPS (wall or Strecker). |
|
| Outcomes | Rebleeding. Encephalopathy. Survival. Treatment failure and complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Nahara 2001.
| Methods | Randomised trial.
A. Generation of allocation sequence: unclear. No information.
B. Allocation concealment: adequate. Sealed, opaque envelopes.
C. Blinding: unclear. No information.
D. Follow‐up: adequate.
Inclusion of all randomised participants at evaluation: yes. Time from bleeding episode to randomisation (mean, S.D) = TIPS (19.1,2 days), ET (17.9,1.9). Time from randomisation to treatment (mean, SD) = Not clear. Total number of patients evaluated = 101 Randomised to TIPS = 38, randomised to ET = 40. Adequate reasons provided for those not randomised = yes. One patient in ET group crossed over to TIPS during follow‐up. Follow‐up period in days (mean, SD) TIPS (1116,92) ET (1047,102) Assessment of suitability for shunt carried out prior to randomisation = no. Shunt patency assessed with Duplex scanning. Method of Child's grading = Child‐Pugh, however, patients were not stratified according to the Child‐Pugh system. Method of Encephalopathy testing = Parson Smith criteria. Rebleeding episodes endoscopically verified = yes. Specified whether rebleeding episode clinically significant = not stated. |
|
| Participants | Inclusions: cirrhosis with recent variceal haemorrhage, clinical stability at randomisation, age between 20 to 69 years. Exclusion criteria: hepatocellular carcinoma, episodes of chronic encephalopathy, portal vein thrombosis, Child‐Pugh > 13, serum creatinine >2.5 milligram per decilitre, serum bilirubin > 5 milligram per decilitre, active infection and severe cardiopulmonary disease. |
|
| Interventions | ET: Sclerotherapy using 5% ethanolamine TIPS: Rosch‐Uchida as well as wall stents (8 to 10 mm). | |
| Outcomes | Rebleeding. Death. Hepatic encephalopathy. Complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
P‐Layrargues 1997.
| Methods | Randomised trial, reported as abstract only. Duplicate publication of P‐Layrargues 2001. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
P‐Layrargues 2001.
| Methods | Randomised trial.
A. Generation of allocation sequence: unclear. Randomisation mentioned but method not specified.
B. Allocation concealment: unclear. No information.
C. Blinding: no information.
D. Follow‐up: adequate.
Inclusion of all randomised participants at evaluation: yes. Time from bleeding to randomisation: After patients had been haemodynamically stabilised for 24 hours. TIPS (hours): 44. ET (hours): 42. Time from randomisation to Traitement for TIPS (mean): 13 hours. 158 patients evaluated, reasons provided for those excluded: yes. 39 included in the ET group and 41 in the TIPS group. Follow‐up period in days (mean). TIPS: 678. ET: 581. Assessment of suitability for shunt carried out prior to randomisation: Yes. Shunt patency assessed with duplex scanning at 24 hours and then 3 monthly. Two patients in the TIPS group and four in the ET underwent transplantation, one patient crossed over from ET to TIPS. Intention‐to‐treat analysis. Method of Child's grading: Child‐Pugh. Method of encephalopathy testing: clinical. Rebleeding episodes endoscopically verified: yes. Specified whether clinically significant : yes. |
|
| Participants | Inclusion criteria: cirrhosis, Child‐Pugh grade B and C, age between 18 to 75 years, with an episode of endoscopically verified variceal bleeding. Exclusions: portal vein thrombosis, previous endoscopic therapy within three months, previous shunt, fundal varices, hepatocellular carcinoma, cardiac, renal or respiratory failure, non‐compliance, sepsis, and uncontrolled bleeding. |
|
| Interventions | ET:
Variceal band ligation. Shunt: TIPS (type not specified). |
|
| Outcomes | Survival. Rebleeding. Encephalopathy. Shunt dysfunction. Duration of hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pera 1991.
| Methods | Duplicate publication of Teres 1987. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Planas 1991.
| Methods | Randomised trial.
A. Generation of allocation sequence: unclear. Randomisation mentioned but method not specified.
B. Allocation concealment: unclear. No information.
C. Blinding: unclear. No information.
D. Follow‐up: adequate.
Inclusion of all randomised participants at evaluation: yes. Time from bleeding episode to randomisation: Three days after stabilisation following a variceal bleed. Time from randomisation to treatment, days (mean,SD): Shunt (14.7,6.3), ET (7.2,3.4). Total number of patients evaluated: 189. Randomised to SHUNT: 41, randomised to endoscopic therapy (ET): 41. Adequate reasons provided for those not randomised: yes. Seven patients in the shunt group and six in the ET group did not receive the allocated treatment. One patient in each group was lost to follow‐up. Intention to treat analysis. Mean follow‐up period in months (SD): SHUNT 20.9 (13.9), ET 20.8 (15). Assessment of suitability for shunt carried out prior to randomisation: no. Method of Child's grading: Child‐Campbell. Method of Encephalopathy testing: clinical testing and history. Rebleeding episodes endoscopically verified: yes. Specified whether rebleeding episode clinically significant: not specified. |
|
| Participants | Inclusion criteria: Child‐Campbell A and B cirrhotic patients who were considered following endoscopically proven variceal haemorrhage. Exclusions (one or more of the following): Child‐Campbell class C, uncontrollable haemorrhage, or early rebleeding between admission and randomisation. Patient characteristics comparable, age slightly less in the ES group. |
|
| Interventions | ET:
Sclerotherapy, intra‐ and para‐variceal technique, sclerosant polidocanol. Shunt: End‐to‐side portocaval shunt. |
|
| Outcomes | Rebleeding. Encephalopathy. Mortality. Complications. Days of hospitalisation and cost. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rikkers 1987.
| Methods | Duplicate publication of Rikkers 1993. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rikkers 1993.
| Methods | Randomised trial.
A. Generation of allocation sequence: adequate. Efron's biased coin design.
B. Allocation concealment: unclear. No information.
C. Blinding: unclear. No information.
D. Follow‐up: adequate.
Inclusion of all randomised participants at evaluation: one patient switched from shunt to ET. Time from bleeding episode to randomisation: not specified. Time from randomisation to treatment: not specified. Randomised to shunt: 31 (DSRS 26, Total = 4), randomised to ET: 29. (One patient switched to ET from shunt group after he withdrew consent and he was assessed as being randomised to ET.) Adequate reasons provided for those not randomised: not specified. Two patients in each group lost to follow‐up. Per protocol analysis, non intention to treat. Mean follow‐up period in months (SE): shunt 85.5 (5), ES 92 (7). Assessment of suitability for shunt carried out prior to randomisation: yes. Suitability for shunt assessed with angiography. Method of Child's grading: modified Child's with four parameters. Method of encephalopathy testing: clinical, EEG, psychometric, number connection test. Rebleeding episodes endoscopically verified: yes. Specified whether rebleeding episode clinically significant: not specified. |
|
| Participants | Inclusion criteria: portal hypertension secondary to cirrhosis, endoscopic documentation of acute or recent oesophageal variceal haemorrhage requiring a minimum transfusion of 3 U of blood, residence within 500 miles of Salt Lake City or Omaha, non‐operative control of acute variceal haemorrhage, patency of splenic and portal veins documented by selective angiography Exclusions: not specified. Patients comparable in the two groups. |
|
| Interventions | ET:
Sclerotherapy, intra‐variceal technique, sclerosant 0.75% sodium tetradecyl sulfate and 50% dextrose or 5% sodium morrhuate. Shunts: DSRS (n = 26), side to side portocaval (n = 3), end‐to‐side portocaval (n = 1). |
|
| Outcomes | Survival. Recurrent haemorrhage. Therapy failure. Quantitative liver function and haemodynamics. Encephalopathy. Cost. | |
| Notes | Portacaval shunts were performed on 3 patients with medically intractable ascites and on 1 with massive rebleeding. SPD was not used in any of the patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rossle 1997.
| Methods | Randomised trial.
A. Generation of allocation sequence: adequate. Computer generated random numbers.
B. Allocation concealment: adequate. Study groups read by person not involved in the clinical setting. Random setting could not be previewed.
C. Blinding: unclear. No information.
D. Follow‐up: adequate.
Inclusion of all randomised participants at evaluation: yes. Time from bleeding episode to randomisation in hours (SD): TIPS 6.3 (5.5), ET 4.4 (5). Time from randomisation to treatment: 48 hrs. Total number of patients evaluated and found eligible: 190. Randomised to TIPS: 61, randomised to ET: 65. Adequate reasons provided for those not randomised: Not individually specified. Three patients lost to follow‐up in TIPS group and one in ET group. Nine patients were crossed over from ET to TIPS during follow‐up. Intention‐to‐treat analysis. Follow‐up period in months (mean, interquartile range). TIPS (14, 8‐23) ET (13, 8‐25). Assessment of suitability for shunt carried out prior to randomisation: Not mentioned. Shunt patency assessed with Duplex ultrasound at 1,3, 6 ,9, and 12 months and then every six months. Method of Child's grading: Child‐Pugh. Method of encephalopathy testing = clinical testing, trail making test, mental state examination. Rebleeding episodes endoscopically verified: yes. Specified whether rebleeding episode clinically significant: yes. |
|
| Participants | Inclusion criteria: liver cirrhosis, variceal bleeding within 2 weeks before randomisation, and age over 18 years. Exclusions (one or more of the following): hepatic encephalopathy grade 3 and 4, liver insufficiency (bilirubin more than 5mg/dl), cavernomatous portal‐vein thrombosis, advanced malignancy, contra‐indications to propranolol (obstructive lung disease, severe hypotension) and bleeding emergency Patients comparable in the two groups. |
|
| Interventions | ET:
Sclerotherapy plus propranolol, technique of sclerotherapy not mentioned, sclerosant (number of patients): polidocanol (59), bucrylate or histoacryl and lipiodol mixture (5), fibrin glue (4), polidocanol plus bucrylate (8). Shunts: TIPS (Palmaz stent (n = 39), memotherm stent (n = 16), wallstent (n = 6). |
|
| Outcomes | Rebleeding. Complications. Encephalopathy. Hospital stay and mortality. Liver function and Child‐Pugh class. | |
| Notes | Propranolol used in‐addition to sclerotherapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Sanyal 1997.
| Methods | Randomised trial.
A. Generation of allocation sequence: adequate. Computer generated numbers.
B. Allocation concealment: adequate. Sealed opaque envelopes.
C. Blinding: unclear. Not mentioned.
D. Follow‐up: adequate.
Inclusion of all randomised participants at evaluation: yes. Time from bleeding episode to randomisation: Clinical stability for at least 72 hours following a variceal bleed. Time from randomisation to treatment: 72 hours. Total number of patients evaluated and found eligible: 100. Randomised to TIPS: 41, randomised to ET: 39. Adequate reasons provided for those not randomised = yes. Two patients lost to follow‐up in TIPS group and one in ET group. Six patients in ET group crossed over to TIPS during follow‐up. Five patients in the TIPS group and three in the ET group underwent hepatic transplantation. Intention to treat analysis. Follow‐up period in days (median) TIPS (990) ET (956). Assessment of suitability for shunt carried out prior to randomisation: yes. Shunt patency assessed with Duplex ultrasound at one week, one and three months and then every three months, angiography carried out at six monthly intervals. Method of Child's grading: Child‐Pugh. Method of encephalopathy testing: not mentioned. Rebleeding episodes endoscopically verified: yes. Specified whether rebleeding episode clinically significant: yes. |
|
| Participants | Inclusion criteria: clinical stability in the absence of re bleeding 72 hours following a variceal bleed. Exclusions (one or more of the following): portal venous thrombosis, hepatoma, end‐stage cancer or systemic disease which would limit the patients life span to less than one year. Patients slightly younger in the TIPS group. |
|
| Interventions | ET:
Sclerotherapy, intra‐variceal technique, sclerosant sodium morrhuate (patients on beta‐blockade prior to randomisation were asked to stop taking it for the duration of the follow‐up period). Shunt: TIPS (wall stent). |
|
| Outcomes | Rebleeding. Survival. Complications. Rates or re‐hospitalisations. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Sauer 1997.
| Methods | Randomised trial.
A. Generation of allocation sequence: adequate. Computer generated random numbers.
B. Allocation concealment: unclear. No information.
C. Blinding: unclear. No information.
D. Follow‐up: adequate.
Inclusion of all randomised participants at evaluation: yes. Time from bleeding episode to randomisation in days (SD): 1.1 days (1.1). Time from randomisation to treatment in days (SD): 3.4 (2.8) Total number of patients evaluated and found eligible: 98. Randomised to TIPS: 42, randomised to ET: 41. Adequate reasons provided for those not randomised: yes. No losses to follow‐up. Five patients in ES group crossed over to TIPS during follow‐up. Intention‐to‐treat analysis. Assessment of suitability for shunt carried out prior to randomisation = yes. Shunt patency assessed with Duplex ultrasound or angiography at three monthly intervals. Method of Child's grading: Child‐Pugh. Method of encephalopathy testing: clinical and trail making test. Rebleeding episodes endoscopically verified: yes. Specified whether rebleeding episode clinically significant: yes. Median observation time in years TIPS (1.6) ET (1.4) Assessment of suitability for shunt carried out prior to randomisation = not mentioned. Shunt patency assessed with Duplex ultrasound and angiography at three monthly intervals. Method of Child's grading = Child‐Pugh. Method of encephalopathy testing = clinical and trail‐making tests. Rebleeding episodes endoscopically verified = yes. Specified whether rebleeding episode clinically significant = no. |
|
| Participants | Inclusion criteria: cirrhosis and acute oesophageal haemorrhage. Exclusions (one or more of the following): gastric varices, prior endoscopic or surgical treatment for varices, portal venous thrombosis, hepatoma, end‐stage cancer or systemic disease which would limit the patients life span to less than six months and uncontrolled bleeding requiring an emergency TIPS procedure. Slightly younger patients in the TIPS group. |
|
| Interventions | ET:
Sclerotherapy plus propranolol, sclerotherapy intra‐variceal and para‐variceal technique, sclerosant = 5% ethanolamine oleate. Shunt: TIPS (Palmaz stent). |
|
| Outcomes | Rebleeding. Mortality. Encephalopathy. Complications. | |
| Notes | Propranolol used in‐addition to sclerotherapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sauer 1998.
| Methods | Randomised trial (abstract). 85 patients randomised to TIPS (43) or EB 42). | |
| Participants | Patients with cirrhosis and portal hypertension. | |
| Interventions | ET:
Variceal band ligation. Shunt: TIPS (Palmaz stent ). |
|
| Outcomes | Rebleeding. Survival. Encephalopathy. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sauer 2002.
| Methods | Randomised trial.
A. Generation of allocation sequence: adequate. Computer generated random numbers.
B. Allocation concealment: unclear. No information.
C. Blinding: unclear. No information.
D. Follow‐up: adequate.
Inclusion of all randomised participants at evaluation: yes. Time from bleeding episode to randomisation in days : 1.2 ‐ 3.2. Time from randomisation to treatment in days (SD): 2.4 ‐ 3.1. Total number of patients evaluated and found eligible: 112. Randomised to TIPS: 43, randomised to ET: 42. Adequate reasons provided for those not randomised: yes. No losses to follow‐up. Three patients in ET group crossed over to TIPS during follow‐up. Intention to treat analysis. Assessment of suitability for shunt carried out prior to randomisation = yes. Shunt patency assessed with Duplex ultrasound or angiography at three monthly intervals. Method of Child's grading: Child‐Pugh. Method of encephalopathy testing: clinical. Rebleeding episodes endoscopically verified: yes. Specified whether rebleeding episode clinically significant: yes. Mean observation time in years TIPS (4.1) ET (3.6). Assessment of suitability for shunt carried out prior to randomisation = not mentioned. Shunt patency assessed with Duplex ultrasound and angiography at three monthly intervals. Method of Child's grading = Child‐Pugh Method of encephalopathy testing = clinical. Rebleeding episodes endoscopically verified = yes. Specified whether rebleeding episode clinically significant = no. |
|
| Participants | Inclusion criteria: cirrhosis and acute first oesophageal haemorrhage. Exclusions (one or more of the following): gastric varices, prior endoscopic or surgical treatment for varices, portal venous thrombosis, hepatoma, end‐stage cancer or systemic disease which would limit the patients life span to less than six months and uncontrolled bleeding. |
|
| Interventions | ET:
Variceal band ligation plus propranolol. Shunt: TIPS (Palmaz stent) or wallstents (Schneider). |
|
| Outcomes | Rebleeding. Mortality. Encephalopathy. Complications. | |
| Notes | Propranolol used in‐addition to variceal banding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Spina 1990.
| Methods | Randomised trial.
A. Generation of allocation sequence: adequate. Random number table.
B. Allocation concealment: unclear. No information.
C. Blinding: unclear. No information.
D. Follow‐up: adequate.
Inclusion of all randomised participants at evaluation: yes. Time from bleeding episode to randomisation: ? When the patient achieved haemodynamic stability. Time from randomisation to treatment: 24 hours. Total number of patients evaluated: 77. Adequate reasons provided for not randomising: yes. Randomised to SHUNT: 20, randomised to ET: 20. No losses to follow‐up. Mean follow‐up period in months: shunt 29.2, ET 23.8. Confidence interval in months: (24.2 to 34.1), (23.8 to 30.3). Assessment of suitability for shunt carried out prior to randomisation: yes. Method of Child's grading: Child‐Pugh. Method of encephalopathy testing: mental status, asterixis, trail making tests, EEG. Rebleeding episodes endoscopically verified: mes. Specified whether rebleeding episode clinically significant: mes. |
|
| Participants | Inclusion criteria: biopsy confirmed cirrhosis, endoscopically verified variceal bleed requiring at least one unit of blood transfusion, arrest of variceal haemorrhage either spontaneously or by the use drugs and or tamponade and or sclerotherapy, good liver function as reflected by Child‐Pugh class A and B, patency of the portal venous system, eligible for either shunt or sclerotherapy, absence of life threatening diseases and willingness to return for regular follow‐up. Exclusions: not specified. Patients in the ES group slightly older and with a larger number of alcoholics. |
|
| Interventions | ET:
Sclerotherapy, intra‐variceal and para‐variceal technique, sclerosant 0.5% to 1% polidocanol. Shunt: DSRS (Warren). |
|
| Outcomes | Rebleeding. Mortality. Encephalopathy. Mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Teres 1987.
| Methods | Randomised trial.
A. Generation of allocation sequence: adequate. Random number table.
B. Allocation concealment: adequate. Sealed envelopes.
C. Blinding: unclear. No information.
D. Follow‐up: adequate.
Inclusion of all randomised participants at evaluation: yes. Time from bleeding episode to randomisation: 10 to 15 days. Time from randomisation to treatment in DSRS group in days: 11‐65. Total number of patients evaluated: 189. Randomised to SHUNT: 57, randomised to ET: 55. 14 patients in the shunt group and 55 patients in the endoscopic group did not receive the allocated treatment and were excluded, reasons provided. Two patients in the endoscopic group were lost to follow‐up. Mean follow‐up period in months (SD): shunt 27.45 (15.6), ES 26.5 (16.9). Follow‐up range in months DSRS (1‐58), ET (1‐64). Assessment of suitability for shunt carried out prior to randomisation: no. Method of Child's grading: Child‐Campbell. Method of encephalopathy testing: clinical testing and history. Rebleeding episodes endoscopically verified: yes. Specified whether rebleeding episode clinically significant: not specified. |
|
| Participants | Inclusion criteria: Child‐Campbell A and B cirrhotic patients with at least one episode of variceal haemorrhage. Exclusions (one or more of the following): continual variceal bleeding despite medical treatment and early rebleeding between admission and randomisation. Child‐Campbell score greater and number of alcoholics greater in the ES group. |
|
| Interventions | ET:
Sclerotherapy, technique ?intra‐variceal, sclerosant 5% ethanolamine oleate. Shunt: DSRS (retroperitoneal approach). |
|
| Outcomes | Mortality. Rebleeding. Hepatic encephalopathy. Complications. Days of hospitalisation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
SD: standard deviation. SE: standard error of the mean. ITT: intention to treat. ET: endoscopic therapy. TS: total shunt. DSRS: distal splenorenal shunt. TIPS: transjugular intrahepatic porto‐systemic shunt. yrs: years.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cello 1982 | Randomisation was to two groups, sclerotherapy and oesophageal transection. The results were compared retrospectively to those of a separate group of patients who had received total shunts. |
| Escorsell 1997 | Randomised groups included patients who received TIPS versus medical therapy. Endoscopic therapy was used acutely in after a variceal bleed. |
| Kitano 1992 | Patients were included who had not previously bled from varices. |
| Krieger 1997 | The study end‐points are not the subject of this review. |
| Meddi 1999 | Cost‐analysis study, possible overlap of previously published results. |
| Orloff 1994 | Variceal bleeding not controlled prior to randomisation. Endoscopic therapy not used in the medically treated group of patients. |
| Paquet 1990 | Non‐randomised study. |
| Resnick 1974 | Endoscopic therapy not employed in the medically treated group. |
| Reynolds 1981 | Endoscopic therapy not employed in the medically treated group. |
| Rossi 1994 | The study outcome measures are not the subject of this review. Only seven patients randomised. |
| Sanyal 1994 | The study outcome measures not a subject of this review. |
| Teres/Baroni 1987 | Non randomised study. Variceal bleeding not controlled prior to randomisation. |
| Tripathi 2001 | Randomised groups included those who received TIPS compared to those who received TIPS and variceal banding. |
| Urbistondo 1996 | Child's C patients not randomised to the DSRS arm. Unable to extract data only for Child's A and B patients from the study. Unable to contact the authors. In addition, small study with unacceptably large attrition to follow‐up (more than 40%). |
Contributions of authors
SK designed and co‐ordinated the project, performed the literature search, wrote to authors, screened and identified relevant trials, developed the data extraction protocol, extracted data from eligible trials, performed quality assessment, entered data into Review Manager, and led the writing of the review.
CTS performed quality assessment, extracted data independently from eligible trials, provided statistical support, performed the survival analyses, and contributed to writing the review.
PW supervised the survival calculations, provided a methodological perspective, and contributed to writing the review.
RS conceived and supervised the project, secured funding, identified relevant trials, established consensus where there was initial divergence of opinion, provided an experienced clinical perspective, and finalised the writing of the review.
Sources of support
Internal sources
University Department of Surgery, University of Liverpool, UK.
External sources
European Commission (BMH4‐CT96‐0373), Belgium.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Cabrera 1996 {published data only}
- Cabrera J, Maynar M, Granados R, Gorriz E, Reyes R, Pulido‐Duque J M, et al. Transjugular intrahepatic portosystemic shunt versus sclerotherapy in the elective treatment of variceal hemorrhage. Gastroenterology 1996;110(3):832‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cello 1984 {published data only}
- Cello P, Grendell JH, Crass R A, Trunkey DD, Cobb EE, Heilbron DC. Endoscopic sclerotherapy versus portacaval shunt in patients with severe cirrhosis and variceal hemorrhage. New England Journal of Medicine 1984;311:1589‐94. [DOI] [PubMed] [Google Scholar]
Cello 1987 {published data only}
- Cello J P, Grendell JH, Crass RA, Weber TE, Trunkey DD. Endoscopic sclerotherapy versus portacaval shunt in patients with severe cirrhosis and acute variceal hemorrhage. Long‐term follow‐up. New England Journal of Medicine 1987;316:11‐5. [DOI] [PubMed] [Google Scholar]
Cello 1997 {published data only}
- Cello JP, Ring EJ, Olcott EW, Koch J, Gordon R, Sandhu J, et al. Endoscopic sclerotherapy compared with percutaneous transjugular Intrahepatic portosystemic shunt after initial sclerotherapy in patients with acute variceal haemorrhage. A randomised controlled trial. Annals of Internal Medicine 1997;126(11):858‐65. [DOI] [PubMed] [Google Scholar]
Garcia‐V 1999 {published data only}
- Garcia‐Villareal L, Martinez‐Lagares F, Sierra A, Guevara C, Marrero JM, Jimenez E, et al. Transjugular Intrahepatic portosystemic shunt versus prevention of rebleeding after recent varcieal hemorrhage. Hepatology 1999;29:27‐32. [DOI] [PubMed] [Google Scholar]
GDEAIH 1995 {published data only}
- Groupe d'Etude de Anastomoses Intra‐Hepatiques. TIPS versus sclerotherapy + propranolol in the prevention of variceal rebleeding: preliminary results of a multicenter randomised trial. Hepatology 1995;22:297A. [Google Scholar]
Gulberg 2002 {published data only}
- Gulberg N, Schepke G, Geigenberger G, Holl J, Brensing KA, Waggershauser T, et al. Transjugular intrahepatic portosystemic shunting is not superior to endoscopic variceal band ligation for prevention of variceal rebleeding in cirrhotic patients: a randomized, controlled trial. Scandinavian Journal of Gastroenterology 2002;37:338‐43. [DOI] [PubMed] [Google Scholar]
Henderson 1990 {published data only}
- Henderson JM, Kutner MH, Millikan WJ, Galambos JT, Riepe SP, Brooks S, et al. Endoscopic variceal sclerosis compared with distal splenorenal shunt to prevent recurrent variceal bleeding in cirrhosis, a prospective randomized trial. Annals of Internal Medicine 1990;112(4):262‐9. [DOI] [PubMed] [Google Scholar]
- Warren WD, Henderson JM, Millikan WJ, Galambos J T, Brooks WS, Riepe SP, et al. Distal Splenorenal shunt versus Endoscopic Sclerotherapy for long‐term management of variceal bleeding; preliminary report of a prospective, randomised trial. Annals of Surgery 1986;203(5):454‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Isaksson 1996 {published data only}
- Isaksson B, Jeppsson B, Bengtsson F, Hannesson P, Herlin P, Bengmark S. Mesocaval shunt or repeated sclerotherapy: effects on rebleeding and encephalopathy ‐ a randomized trial. Surgery 1995;117:498‐504. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jalan 1997 {published data only}
- Jalan R, Forrest EH, Stanley AJ, Redhead DN, Forbes J, Dillon JF, et al. A randomized trial comparing transjugular intrahepatic portosystemic stent‐shunt with variceal band ligation in the prevention of rebleeding from esophageal varices. Hepatology 1997;26:1115‐22. [DOI] [PubMed] [Google Scholar]
Korula 1987 {published data only}
- Korula J, Yelin A, Yamada S, Weiner J, Cohen H, Reynolds TB. A prospective randomised controlled comparison of chronic endoscopic variceal sclerotherapy and portalsystemic shunt for variceal hemorrhage in Childs Class A cirrhotics: A preliminary report. Gastroenterology 1987;92:1745. [Google Scholar]
Merli 1998 {published data only}
- Merli M, Salerno F, Riggio O, Franchis R, Fiaccadori F, and the Grupppo Italiano Studio TIPS (G.I.S.T). Transjugular intrahepatic portosystemic shunt versus endoscopic sclerotherapy for the prevention of variceal bleeding in cirrhosis: a randomized multicenter trial. Gruppo Italiano Studio TIPS (G.I.S.T.). Hepatology 1998;27(1):40‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nahara 2001 {published data only}
- Nahara Y, Kanazawa H, Kawamata H, Tada N, Saitoh H, Matsuzaka S, et al. A randomized clinical trial comparing transjugular intrahepatic portosystemic shunt with endoscopic sclerotherapy in the long‐term management of patients with cirrhosis after recent variceal hemorrhage. Hepatology Research 2001;21:189‐98. [DOI] [PubMed] [Google Scholar]
P‐Layrargues 1997 {published data only}
- Pomier‐Layrargues G, Dufresne MP, Buli B, Lambert J, Fenyves D, Willems B, et al. TIPS versus endoscopic variceal ligation in the prevention of variceal rebleeding in cirrhotic patients: a comparative randomized clinical trial (interim analysis). Hepatology 1997;26(4):137A. [Google Scholar]
P‐Layrargues 2001 {published data only}
- Layrargues GP, Villeneuve JP, Deschenes M, Bui B, Perreault P, Fenyves D, et al. Transjugular intrahepatic portosystemic shunt (TIPS) versus endsocopic variceal ligation in the prevention of variceal rebleeding in patients with cirrhosis: a randomised trial. Gut 2001;48:390‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pera 1991 {published data only}
- Pera C, Vista J, Garcia‐Valdecasas J C, Grande L, Fuster J. The modified Distal‐Splenorenal Shunt in the elective treatment of variceal hemorrhage. Hepato‐Gastroenterology 1991;38:12‐5. [PubMed] [Google Scholar]
Planas 1991 {published data only}
- Planas R, Boix J, Broggi M, Cabre E, Gomes‐Vieira MC, Morillas R, et al. Portacaval shunt versus endoscopic sclerotherapy in the elective treatment of variceal hemorrhage. Gastroenterology 1991;100(4):1078‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rikkers 1987 {published data only}
- Rikkers LF, Burnett DA, Volentine GD, Buchi KN, Cormier RA. Shunt surgery versus endoscopic sclerotherapy for long‐term treatment of variceal bleeding. Early results of a randomized trial. Annals of Surgery 1987;206(3):261‐71. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rikkers 1993 {published data only}
- Rikkers LF, Jin G, Burnett DA, Buchi KN, Cormier RA. Shunt surgery versus endoscopic sclerotherapy for variceal hemorrhage: late results of a randomized trial. American Journal of Surgery 1993;165(1):27‐32 (discussion 32‐3). [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rossle 1997 {published data only}
- Rossle M, Deibert P, Haag K, Ochs A, Olschewski M, Siegerstetter V, et al. Randomised trial of transjugular intrahepatic portosystemic shunt versus endoscopy plus propranolol for prevention of variceal rebleeding. Lancet 1997;349(9058):1043‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sanyal 1997 {published data only}
- Sanyal AJ, Freedman AM, Luketic VA, Purdum PP 3rd, Shiffman ML, Cole PE, et al. Transjugular intrahepatic portosystemic shunts compared with endoscopic sclerotherapy for the prevention of recurrent variceal hemorrhage. A randomized, controlled trial. Annals of Internal Medicine 1997;126(11):849‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sauer 1997 {published data only}
- Sauer P, Theilmann L, Stremmel W, Benz C, Richter G M, Stiehl A. Transjugular intrahepatic portosystemic stent shunt versus sclerotherapy plus propranolol for variceal rebleeding. Gastroenterology 1997;113:1623‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sauer 1998 {published data only}
- Sauer P, Benz C, Theilmann G, Richter W, Stremmel W, Stiehl A. Tranjugular intrahepatic portosystemic stent shunt (TIPS) vs. endoscopic banding in the prevention of variceal rebleeding: final results of a randomized study. Gastroenterology 1998;114(4):A1334. [Google Scholar]
Sauer 2002 {published data only}
- Sauer P, Hansmann J, Richter GM, Stremmel W, Stiehl A. Endoscopic variceal ligation plus propranolol versus transjugular intrahepatic portosystemic stent shunt: A long‐term randomized trial. Endoscopy 2002;34:690‐7. [DOI] [PubMed] [Google Scholar]
Spina 1990 {published data only}
- Spina GP, Santambrogrio R, Opocher E, Cosentino F, Zambelli A, Passoni GR, et al. Distal splenorenal shunt versus endoscopic sclerotherapy in the prevention of variceal rebleeding. First stage of a randomized, controlled trial. Annals of Surgery 1990;211(2):178‐86. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Teres 1987 {published data only}
- Terés J, Bordas JM, Bravo D, Visa J, Grande L, Garcia‐Valdecasas JC, et al. Sclerotherapy vs. distal splenorenal shunt in the elective treatment of variceal hemorrhage: a randomized controlled trial. Hepatology 1987;7(3):430‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cello 1982 {published data only}
- Cello JP, Crass RC, Trunkey DD. Endoscopic sclerotherapy versus esophageal transection of Child's class C patients with variceal hemorrhage. Comparison with results of portacaval shunt: preliminary report. Surgery 1982;91(3):333‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Escorsell 1997 {published data only}
- Escorsell A, Bandi JC, Moitinho E, Feu F, Garcia Pagan JC, Bosch J, et al. Time profile of the haemodynamic effects of terlipressin in portal hypertension. Journal of Hepatology 1997;26(3):621‐7. [DOI] [PubMed] [Google Scholar]
Kitano 1992 {published data only}
- Kitano S, Iso Y, Hashizume M, Yamaga H, Koyanagi N, Wada H, et al. Sclerotherapy vs. esophageal transection vs. distal splenorenal shunt for the clinical management of esophageal varices in patients with child class A and B liver function: a prospective randomized trial. Hepatology 1992;15(1):63‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krieger 1997 {published data only}
- Krieger S, Jauss M, Jansen O, Stiehl A, Sauer P, Geissler M, et al. MRI findings in chronic hepatic encephalopathy depend on portosystemic shunt: results of a controlled prospective clinical investigation. Journal of Hepatology 1997;27(1):121‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meddi 1999 {published data only}
- Meddi P, Merli M, Lionetti R, Santis A, Valeriano V, Masini A, et al. Cost analysis for the prevention of variceal rebleeding: a comparison between transjugular intrahepatic portosystemic shunt and endoscopic sclerotherapy in a selected group of Italian cirrhotic patients. Hepatology 1999;29(4):1074‐7. [DOI] [PubMed] [Google Scholar]
Orloff 1994 {published data only}
- Orloff MJ, Bell RH, Orloff MS, Hardison WGM, Greenburg AG. Prospective randomized trial of emergency portacaval shunt and emergency medical therapy in unselected cirrhotic patients with bleeding varices. Hepatology 1994;20((4 Pt 1)):863‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Paquet 1990 {published data only}
- Paquet KJ, Lazar A, Gad HA. Surgical measures in recurrent hemorrhage from esophageal and gastric varices after sclerotherapy ‐ a prospective study [Chirurgische Massnahmen bei Rezidivblutungen aus Osophagus‐ und Magenvarizen nach Sklerosierungstherapie ‐ eine prospektive Studie]. Langenbecks Archiv für Chirurgie. Supplement‐II, Verhandlungen der Deutschen Gesellschaft für Chirurgie 1990;Suppl II:397‐402. [MEDLINE: ] [PubMed] [Google Scholar]
Resnick 1974 {published data only}
- Resnick RH, Iber FL, Ishihara AM, Chalmers TC, Zimmerman H. A controlled study of the therapeutic portacaval shunt. Gastroenterology 1974;6(5):843‐57. [MEDLINE: ] [PubMed] [Google Scholar]
Reynolds 1981 {published data only}
- Reynolds TB, Donovan AJ, Mikkelsen WP, Redeker AG, Turrill FL, Weiner JM. Results of a 12‐year randomized trial of portacaval shunt in patients with alcoholic liver disease and bleeding varices. Gastroenterology 1981;80(5 Pt 1):1005‐11. [PubMed] [Google Scholar]
Rossi 1994 {published data only}
- Rossi P, Maccioni F, Salvatori FM, Bezzi M, Gandini R, Broglia L, et al. Transjugular intrahepatic portosystemic shunt (TIPS): indications and results after 22 months of experience [Derivazione porto‐sistemica intraepatica transgiugulare (TIPS): indicazioni e risultati dopo 22 mesi di esperienza]. La Radiologia Medica 1994;87(5):585‐96. [MEDLINE: ] [PubMed] [Google Scholar]
Sanyal 1994 {published data only}
- Sanyal AJ, Freedman AM, Shiffman ML, Purdum PP 3rd, Luketic VA, Cheatham AK. Portosystemic encephalopathy after transjugular intrahepatic portosystemic shunt: results of a prospective controlled study. Hepatology 1994;20(1 Pt 1):46‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sauer P, Theilmann L, Stremmel W, Stiehl A. Hepatic encephalopathy after transjugular intrahepatic portosystemic stent shunt (TIPS) [Hepatische Enzephalopathie nach transjugularem intrahepatischem porto‐systemischem Stent‐Shunt (TIPS)]. Zeitschrift für Gastroenterologie 1995;33(5):277‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Teres/Baroni 1987 {published data only}
- Teres J, Baroni R, Bordas JM, Visa J, Pera C, Rodes J. Randomized trial of portacaval shunt, stapling transection and endoscopic sclerotherapy in uncontrolled variceal bleeding. Journal of Hepatology 1987;4(2):159‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tripathi 2001 {published data only}
- Tripathi D, Helmy A, Lui HF, Stanley AJ, Forrest E, Redhead DN, et al. Randomized controlled trial of transjugular intra‐hepatic portosystemic stent‐shunt (TIPSS) versus TIPSS and variceal band ligation (VBL) in the prevention of variceal re‐bleeding [abstract]. Journal of Hepatology 2001;34(1):69. [Google Scholar]
Urbistondo 1996 {published data only}
- Urbistondo M, Torres EA, Castro F, Oharriz J, Medina R, Molina A, et al. Prevention of recurrent esophageal bleeding and survival in patients with alcoholic cirrhosis: a randomized study. Puerto Rico Health Sciences Journal 1996;15(3):195‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Additional references
Antman 1992
- Antman EM, Lau J, Kupelnick B, Mosteller F, Chalmers TC. A comparison of results of meta‐analyses of randomized control trials and recommendations of clinical experts. Treatments for myocardial infarction. JAMA 1992;268(2):240‐8. [PubMed] [Google Scholar]
Bernard 1997
- Bernard B, Lebrec D, Mathurin P, Opolon P, Poynard T. Propranolol and sclerotherapy in the prevention of gastrointestinal rebleeding in patients with cirrhosis: a meta‐analysis. Journal of Hepatology 1997;26(2):312‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bismuth 1974
- Bismuth H, Franco D, Hepp J. Portal‐systemic shunt in hepatic cirrhosis: does the type of shunt decisively influence the clinical result?. Annals of Surgery 1974;179:209‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bornman 1994
- Bornman PC, Krige JEJ, Terblanche J. Management of oesophageal varices. Lancet 1994;343(8905):1079‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brown 1997
- Brown RS Jr, Lake JR. Transjugular intrahepatic portosystemic shunt as a form of treatment for portal hypertension: indications and contraindications. Advances in Internal Medicine 1997;42:485‐504. [MEDLINE: ] [PubMed] [Google Scholar]
Burroughs 2002
- Burroughs AK, Vangeli M. Transjugular Intrahepatic portosystemic shunt versus endoscopic therapy: randomized trials for secondary prophylaxis of variceal bleeding: an updated meta‐analysis. Scandinavian Journal of Gastroenterology 2002;3:248‐52. [DOI] [PubMed] [Google Scholar]
Buyse 1987
- Buyse M, Ryan LM. Issues of efficiency in combining proportions of deaths from several clinical trials. Statistics in Medicine 1987;6:565‐76. [DOI] [PubMed] [Google Scholar]
Casado 1998
- Casado M, Bosch J, Garcia‐Pagan JC, Bru C, Banares R, Bandi JC, et al. Clinical events after transjugular intrahepatic portosystemic shunt: correlation with hemodynamic findings. Gastroenterology 1998;114(6):1296‐303. [DOI] [PubMed] [Google Scholar]
Clarke 2003
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.1.5 [updated January 2003]. The Cochrane Library 2003, Issue 1. [Google Scholar]
Collins 1994
- Collins JC, Rypins EB, Sarfeh IJ. Narrow‐diameter portacaval shunts for management of variceal bleeding. World Journal of Surgery 1994;18(2):211‐5. [DOI] [PubMed] [Google Scholar]
D'Amico 1995
- D'Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta‐analytic review. Hepatology 1995;22(1):332‐54. [DOI] [PubMed] [Google Scholar]
de Franchis 1992
- Franchis R, Pascal JP, Ancona E, Burroughs AK, Henderson M, Fleig W, et al. Definitions, methodology and therapeutic strategies in portal hypertension. A Consensus Development Workshop, Baveno, Lake Maggiore, Italy, April 5 and 6, 1990. Journal of Hepatology 1992;15(1‐2):256‐61. [DOI] [PubMed] [Google Scholar]
de Franchis 1996
- Franchis R. Developing consensus in portal hypertension. Journal of Hepatology 1996;25(3):390‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Franchis 2001
- Franchis R. Portal hypertension III: Proceedings of the Third Baveno International Consensus Workshop on Definitions, Methodology and Therapeutic Strategies. Oxford: Blackwell Science Ltd, 2001. [ISBN 0‐632‐05918‐4] [Google Scholar]
Grace 1997
- Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. American Journal of Gastroenterology 1997;92:1081‐91. [PubMed] [Google Scholar]
Graham 1981
- Graham DY, Smith JL. The course of patients after variceal haemorrhage. Gastroenterology 1981;80:800‐9. [PubMed] [Google Scholar]
Henderson 2006
- Henderson JM, Boyer TD, Kutner MH, Galloway JR, Rikkers LF, Jeffers LJ, Abu‐Elmagd K, Connor J, DIVERT Study Group. Distal splenorenal shunt versus transjugular intrahepatic portal systematic shunt for variceal bleeding: a randomized trial. Gastroenterology 2006;130(6):1643‐51. [DOI] [PubMed] [Google Scholar]
Jalan 2000
- Jalan R, Hayes PC. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 2000;46(Suppl III):iii1‐iii15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 1997
- Khan S, Sutton R. Oesophageal varices and portal hypertension. Surgery 1997;15(8):175‐81. [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
LaBerge 1993
- LaBerge JM, Ring EJ, Gordon RL, Lake JR, Doherty MM, Somberg KA, et al. Creation of transjugular intrahepatic portosystemic shunts with the wall stent endoprosthesis: results in 100 patients. Radiology 1993;187:413‐20. [DOI] [PubMed] [Google Scholar]
Laine 1995
- Laine L, Cook D. Endoscopic ligation compared with sclerotherapy for treatment of esophageal variceal bleeding. A meta‐analysis. Annals of Internal Medicine 1995;123:280‐7. [DOI] [PubMed] [Google Scholar]
Luca 1999
- Luca A, D'Amica G, Galla R, Midiri M, Morabito A, Pagliaro L. TIPS for prevention of recurrent bleeding in patients with cirrhosis: meta‐analysis of randomized clinical trials. Radiology 1999;212:411‐21. [DOI] [PubMed] [Google Scholar]
McIntyre 1996
- McIntyre N, Burroughs AK. Cirrhosis and Portal Hypertension. In: Weatherall DJ, Ledingham JGG, Warrell DA editor(s). Oxford Textbook of Medicine. Churchill Livingstone, 1996:2085‐100. [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Orloff 1995
- Orloff MJ, Orloff MS, Orloff SL. Three decades of experience with emergency portacaval shunt for acutely bleeding esophageal varices in 400 unselected patients with cirrhosis of the liver. Journal of the American College of Surgeons 1995;180(3):257‐63. [PubMed] [Google Scholar]
Pagliaro 1989
- Pagliaro L, Burroughs AK, Sorensen TIA, Lebrec D, Morabito A, D'Amico G, et al. Therapeutic controversies and randomised controlled trials (RCTs); prevention of bleeding and rebleeding in cirrhosis. Gastroenterology International 1989;2(2):71‐84. [Google Scholar]
Papatheodoridis 1999
- Papatheodoridis GV, Goulis J, Leandro G, Patch D, Burroughs AK. Transjugular intrahepatic portosystemic shunt compared with endoscopic treatment for prevention of variceal rebleeding: A meta‐analysis. Hepatology 1999;30(3):612‐22. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Rosemurgy 1997
- Rosemurgy AS 2nd, Bloomston M, Zervos EE, Goode SE, Pencev D, Zweibel B, et al. Transjugular intrahepatic portosystemic shunt versus H‐graft portacaval shunt in the management of bleeding varices: a cost‐benefit analysis. Surgery 1997;122(4):794‐9; discussion 799‐800. [DOI] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Sarfeh 1986
- Sarfeh IJ, Rypins EB, Mason GR. A systematic appraisal of portacaval H‐graft diameters: clinical and haemodynamic perspectives. Annals of Surgery 1986;204:356‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarin 1999
- Sarin SK, Lamba GS, Kumar M, Misra A, Murthy NS. Comparison of endoscopic ligation and propranolol for the primary prevention of variceal bleeding. New England Journal of Medicine 1999;340:988‐93. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Smith 1982
- Smith JL, Graham DY. Variceal haemorrhage, a critical evaluation of survival analysis. Gastroenterology 1982;82:968‐72. [PubMed] [Google Scholar]
Spina 1992a
- Spina GP, Henderson JM, Rikkers LF, Teres J, Burroughs AK, Conn HO, et al. Distal spleno‐renal shunt versus endoscopic sclerotherapy in the prevention of variceal rebleeding. A meta‐analysis of 4 randomized clinical trials. Journal of Hepatology 1992;16(3):338‐45. [DOI] [PubMed] [Google Scholar]
Sutton 1994
- Sutton R, Shields R. The place of portal‐systemic shunting. In: Blumgart LH editor(s). Surgery of the liver and biliary tract. Churchill Livingstone, 1994. [Google Scholar]
Terblanche 1983
- Terblanche J, Bornman PC, Kahn D, Jonker MA, Campbell JA, Wright J, et al. Failure of repeated injection sclerotherapy to improve long‐term survival after oesophageal variceal bleeding. A five‐year prospective controlled clinical trial. Lancet 1983;2:1328‐32. [DOI] [PubMed] [Google Scholar]
Tudur 2001
- Tudur C, Williamson PR, Khan S, Best LY. The value of the aggregate data approach in meta‐analysis with time‐to‐event outcomes. Journal of the Royal Statistical Society. Series A (Statistics in Society) 2001;2(164):357‐70. [Google Scholar]
Warren 1986
- Warren WD, Millikan WJ Jr, Henderson JM, Abu‐Elmagd KM, Galloway JR, Shires GT 3rd, et al. Splenopancreatic disconnection. Improved selectivity of distal splenorenal shunt. Annals of Surgery 1986;204(4):346‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weissenborn 1992
- Weissenborn K. Recent developments in the pathophysiology and treatment of hepatic encephalopathy. Bailliere's Clinical Gastroenterology 1992;6(3):609‐30. [DOI] [PubMed] [Google Scholar]
Williamson 2002
- Williamson PR, Tudur Smith C, Hutton JL, Marson AG. Aggregate data meta‐analysis with time‐to‐event outcomes. Statistics in Medicine 2002;21(22):3337‐51. [DOI] [PubMed] [Google Scholar]
Wilson 1927
- Wilson EB. Probable inference, the law of succession, and statistical inference. Journal of the American Statistical Association 1927;22:209‐12. [Google Scholar]


