Abstract
Background
Ursodeoxycholic acid is administered to patients with primary biliary cirrhosis, a chronic progressive inflammatory autoimmune‐mediated liver disease with unknown aetiology. Despite its controversial effects, the U.S. Food and Drug Administration has approved its usage for primary biliary cirrhosis.
Objectives
To assess the beneficial and harmful effects of ursodeoxycholic acid in patients with primary biliary cirrhosis.
Search methods
We searched for eligible randomised trials in The Cochrane Hepato‐Biliary Group Controlled Trials Register, The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded, LILACS, Clinicaltrials.gov, and the WHO International Clinical Trials Registry Platform. The literature search was performed until January 2012.
Selection criteria
Randomised clinical trials assessing the beneficial and harmful effects of ursodeoxycholic acid versus placebo or 'no intervention' in patients with primary biliary cirrhosis.
Data collection and analysis
Two authors independently extracted data. Continuous data were analysed using mean difference (MD) and standardised mean difference (SMD). Dichotomous data were analysed using risk ratio (RR). Meta‐analyses were conducted using both a random‐effects model and a fixed‐effect model, with 95% confidence intervals (CI). Random‐effects model meta‐regression was used to assess the effects of covariates across the trials. Trial sequential analysis was used to assess risk of random errors (play of chance). Risks of bias (systematic error) in the included trials were assessed according to Cochrane methodology bias domains.
Main results
Sixteen randomised clinical trials with 1447 patients with primary biliary cirrhosis were included. One trial had low risk of bias, and the remaining fifteen had high risk of bias. Fourteen trials compared ursodeoxycholic acid with placebo and two trials compared ursodeoxycholic acid with 'no intervention'. The percentage of patients with advanced primary biliary cirrhosis at baseline varied from 15% to 83%, with a median of 51%. The duration of the trials varied from 3 to 92 months, with a median of 24 months. The results showed no significant difference in effect between ursodeoxycholic acid and placebo or 'no intervention' on all‐cause mortality (45/699 (6.4%) versus 46/692 (6.6%); RR 0.97, 95% CI 0.67 to 1.42, I² = 0%; 14 trials); on all‐cause mortality or liver transplantation (86/713 (12.1%) versus 89/706 (12.6%); RR 0.96, 95% CI 0.74 to 1.25, I² = 15%; 15 trials); on serious adverse events (94/695 (13.5%) versus 107/687 (15.6%); RR 0.87, 95% CI 0.68 to 1.12, I² = 23%; 14 trials); or on non‐serious adverse events (27/643 (4.2%) versus 18/634 (2.8%); RR 1.46, 95% CI 0.83 to 2.56, I² = 0%; 12 trials). The random‐effects model meta‐regression showed that the risk of bias of the trials, disease severity of patients at entry, ursodeoxycholic acid dosage, and trial duration were not significantly associated with the intervention effects on all‐cause mortality, or on all‐cause mortality or liver transplantation. Ursodeoxycholic acid did not influence the number of patients with pruritus (168/321 (52.3%) versus 166/309 (53.7%); RR 0.96, 95% CI 0.84 to 1.09, I² = 0%; 6 trials) or with fatigue (170/252 (64.9%) versus 174/244 (71.3%); RR 0.90, 95% CI 0.81 to 1.00, I² = 62%; 4 trials). Two trials reported the number of patients with jaundice and showed a significant effect of ursodeoxycholic acid versus placebo or no intervention in a fixed‐effect meta‐analysis (5/99 (5.1%) versus 15/99 (15.2%); RR 0.35, 95% CI 0.14 to 0.90, I² = 51%; 2 trials). The result was not supported by the random‐effects meta‐analysis (RR 0.56, 95% CI 0.06 to 4.95). Portal pressure, varices, bleeding varices, ascites, and hepatic encephalopathy were not significantly affected by ursodeoxycholic acid. Ursodeoxycholic acid significantly decreased serum bilirubin concentration (MD ‐8.69 µmol/l, 95% CI ‐13.90 to ‐3.48, I² = 0%; 881 patients; 9 trials) and activity of serum alkaline phosphatases (MD ‐257.09 U/L, 95% CI ‐306.25 to ‐207.92, I² = 0%; 754 patients, 9 trials) compared with placebo or no intervention. These results were supported by trial sequential analysis. Ursodeoxycholic acid also seemed to improve serum levels of gamma‐glutamyltransferase, aminotransferases, total cholesterol, and plasma immunoglobulin M concentration. Ursodeoxycholic acid seemed to have a beneficial effect on worsening of histological stage (random; 66/281 (23.5%) versus 103/270 (38.2%); RR 0.62, 95% CI 0.44 to 0.88, I² = 35%; 7 trials).
Authors' conclusions
This systematic review did not demonstrate any significant benefits of ursodeoxycholic acid on all‐cause mortality, all‐cause mortality or liver transplantation, pruritus, or fatigue in patients with primary biliary cirrhosis. Ursodeoxycholic acid seemed to have a beneficial effect on liver biochemistry measures and on histological progression compared with the control group. All but one of the included trials had high risk of bias, and there are risks of outcome reporting bias and risks of random errors as well. Randomised trials with low risk of bias and low risks of random errors examining the effects of ursodeoxycholic acid for primary biliary cirrhosis are needed.
Plain language summary
Ursodeoxycholic acid for primary biliary cirrhosis
Primary biliary cirrhosis is an uncommon and slowly progressive autoimmune disease of the liver that primarily affects middle‐aged women. The cause of the disease is unknown. Over the last 30 years, the prevalence of primary biliary cirrhosis has increased substantially. Primary biliary cirrhosis is now a frequent cause of liver morbidity, and the patients are significant users of health resources, including liver transplantation.
Ursodeoxycholic acid is the only drug approved by the U.S. Food and Drug Administration for primary biliary cirrhosis, but the effects of ursodeoxycholic acid remain controversial. This review contains updated evidence and re‐evaluates beneficial and harmful effects of ursodeoxycholic acid on patients with primary biliary cirrhosis. The review includes 16 randomised clinical trials with a total of only 1447 patients. The primary outcomes were all‐cause mortality, all‐cause mortality or liver transplantation, adverse events, and quality of life. Although ursodeoxycholic acid indicated a reduction in liver biochemistry, jaundice, and histological progression, this review did not demonstrate any benefits of ursodeoxycholic acid on all‐cause mortality, all‐cause mortality or liver transplantation, or symptoms (pruritus and fatigue). The use of ursodeoxycholic acid is associated with costs and may cause adverse events. All but one of the trials had high risk of bias and the trials seem to have selective reporting of outcomes.
Summary of findings
Summary of findings for the main comparison. Ursodeoxycholic acid compared with placebo or no intervention for primary biliary cirrhosis.
| Ursodeoxycholic acid compared with placebo or no intervention for primary biliary cirrhosis | ||||||
| Patient or population: patients with primary biliary cirrhosis. Settings: out‐patients. Intervention: ursodeoxycholic acid. Comparison: placebo or no intervention. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | UDCA versus placebo or no intervention | |||||
| All‐cause mortality | Study population | RR 0.97 (0.67 to 1.42) | 1391 (14 trials) | ⊕⊝⊝⊝ very low1 | ||
| 66 per 1000 | 64 per 1000 (45 to 94) | |||||
| All‐cause mortality or liver transplantation | Study population | RR 0.96 (0.74 to 1.25) | 1419 (15 trials) | ⊕⊝⊝⊝ very low1,2 | ||
| 126 per 1000 | 121 per 1000 (93 to 158) | |||||
| Serious adverse events | Study population | RR 0.87 (0.68 to 1.12) | 1382 (14 trials) | ⊕⊝⊝⊝ very low1,2 | ||
| 156 per 1000 | 136 per 1000 (106 to 174) | |||||
| Non‐serious adverse events | Study population | RR 1.46 (0.83 to 2.56) | 1277 (12 trials) | ⊕⊝⊝⊝ very low1,2 | ||
| 28 per 1000 | 41 per 1000 (24 to 73) | |||||
| Pruritus | Study population | RR 0.96 (0.84 to 1.09) | 630 (6 trials) | ⊕⊕⊝⊝ low1,3 | ||
| 537 per 1000 | 516 per 1000 (451 to 586) | |||||
| Serum bilirubin (µmol/l) | The mean serum bilirubin concentration in the intervention groups was 8.69 lower (13.9 to 3.48 lower) | 881 (9 trials) | ⊕⊕⊝⊝ low1,4,5 | |||
|
Liver biopsy findings (worsening of histological stage) |
Study population | RR 0.62 (0.44 to 0.88) | 551 (7 trials) | ⊕⊝⊝⊝ very low1,2,6 | ||
| 381 per 1000 | 237 per 1000 (168 to 336) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Plausible bias that raises some doubt about the results. Potential limitations are likely to lower confidence in the estimate of effect. 2 Total sample size is lower than the calculated optimal information size (OIS) and total number of events is less than 300 (a threshold rule‐of‐thumb value). So, we have little knowledge about the intervention effect and further information is needed. 3 Total sample size is lower than the calculated optimal information size (OIS) but the total number of events is not less than 300 (a threshold rule‐of‐thumb value). Anyway, we have little knowledge about the intervention effect and further information is needed. 4 Serum bilirubin concentration is a surrogate endpoint instead of patient‐important outcome. 5 Total sample size is lower than the calculated optimal information size (OIS). On the other hand, according to the results of trial sequential analysis there is a evidence for a beneficial effect of ursodeoxycholic acid versus placebo or no intervention on serum bilirubin concentration when the cumulative meta‐analysis is adjusted for sparse data and multiple testing on accumulating data. Therefore, there is no risk for random errors. 6 Statistical heterogeneity I2 = 35%.
Background
Description of the condition
Primary biliary cirrhosis is a chronic autoimmune‐mediated liver disease characterised by progressive destruction of intrahepatic bile ducts, resulting in chronic cholestasis, portal inflammation, and fibrosis that can lead to cirrhosis and, ultimately, liver failure. The disease was first comprehensively described around 1950 (MacMahon 1949; Ahrens 1950). The disease primarily affects middle‐aged women with a sex ratio of 10:1. Data about the incidence and prevalence of primary biliary cirrhosis have generally been obtained passively and might not indicate the true rates of the disease in the general population. Reported annual incidence of primary biliary cirrhosis ranges from 1 to 49 persons per million, and the prevalence has been estimated between 7 to 402 persons per million (Prince 2003; Poupon 2010). Primary biliary cirrhosis is now a frequent cause of liver morbidity, and the patients are significant users of health resources, including liver transplantation (Prince 2003).
The aetiology of primary biliary cirrhosis is still unclear, but it is thought to involve multiple genetic factors and environmental triggers leading to an intense autoimmune response against the biliary epithelial cells. Histological staging is based on Ludwig’s (Ludvig 1978) and Scheuer’s classifications (Scheuer 1967), ranging from portal tract inflammation with predominantly lymphoplasmacytoid infiltrates and septal and interlobular bile‐duct loss (stage I) to frank cirrhosis (stage IV). The most common symptoms and findings are fatigue, pruritus, hyperlipidaemia, hypothyroidism, osteoporosis, and coexisting autoimmune diseases (Kaplan 2005). Diagnosis is made upon the elevated biochemical markers of cholestasis (particularly alkaline phosphatases) for more than six months in the presence of detectable serum antimitochondrial antibodies, and on exclusion of other possible aetiologies of liver damage (Heathcote 2000; EASL 2009). Characteristic liver histological changes confirm the diagnosis and are used for staging and assessing disease activity before therapeutic intervention. However, according to the latest clinical guidelines (EASL 2009), a liver biopsy shall not necessarily be used for diagnosis of primary biliary cirrhosis.
The clinical features and natural history of primary biliary cirrhosis vary greatly between patients. It may manifest as asymptomatic, slowly progressive, symptomatic, or rapidly evolving. Asymptomatic patients have about equivalent short‐term survival compared to an age‐matched and sex‐matched healthy population (Lee 2005). Most asymptomatic people with primary biliary cirrhosis will develop symptoms within five years after the diagnosis has been made. The progress to cirrhosis and end stage liver disease may necessitate liver transplantation as the only treatment option (Prince 2004). On the other hand, the overall median survival for symptomatic patients is between 10 and 15 years. Serum bilirubin level is an independent predictor of survival and is used for prognosis in patients with primary biliary cirrhosis (Shapiro 1979).
Description of the intervention
Ursodeoxycholic acid is a secondary bile acid, which is a metabolic byproduct of intestinal bacteria. After oral ingestion and intestinal absorption, ursodeoxycholic acid enters the portal circulation and is taken up by the hepatocytes where ursodeoxycholic acid is conjugated to glycine or taurine and is subsequently transported into the bile ducts (Kullak‐Ublick 2000). Ursodeoxycholic acid undergoes extensive enterohepatic recycling along with the other bile acids (Hofmann1994). Because of its high first‐pass metabolism (70%), the blood level of ursodeoxycholic acid in the systemic circulation is low (Saksena 1997). In the colon, the unabsorbed ursodeoxycholic acid is transformed to lithocholic acid by colonic microbial flora and is excreted via the faeces (Kullak‐Ublick 2000). The half life of ursodeoxycholic acid is about 100 hours (Setchell 1996).
Ursodeoxycholic acid is theoretically a safe and well tolerated drug but can induce modest weight gain (2 to 3 kg) during the first year of treatment (Siegel 2003).
Ursodeoxycholic acid acts through several pathways, such as alteration of the bile‐acid pool, choleresis (the flow of bile from the liver), immune‐modulation effects, and cytoprotective mechanisms. One of the main mechanisms of ursodeoxycholic acid is displacement of endogenous hepatotoxic bile by expansion of the hydrophilic bile acid pool which may correlate with competitive displacement of endogenous bile acids, either at the level of ileal absorption or at the hepatocyte (Stiehl 1999).
Ursodeoxycholic acid is the only drug approved for primary biliary cirrhosis by the U.S. Food and Drug Administration. Doses of 13 to 15 mg/kg/day seem to cause significant improvements in liver tests and immunoglobulin levels and reduce titers of antimitochondrial antibodies (Heathcote 1994; Pares 2000). The dose of ursodeoxycholic acid appears to be important. A study comparing three different doses showed that a dose of 13 to 15 mg/kg of body weight per day appeared to be optimal, as compared with a dose of either 5 to 7 mg or 23 to 25 mg (Angulo 1999a).
How the intervention might work
Bile duct destruction leads to the retention of hydrophobic bile acids within the liver cell. This most likely contributes to the gradual deterioration of liver function and liver histology observed in patients with primary biliary cirrhosis. Ursodeoxycholic acid increases the transportation of intracellular bile acids across the liver cell and into the canaliculus in patients with primary biliary cirrhosis (Jazrawi 1994). Mechanisms of action of ursodeoxycholic acid in primary biliary cirrhosis remain unclear, yet the hydrophilic nature of this agent could lead to a reduction in amounts of primary bile acids, and the substance might also regulate cellular signalling and protect against apoptosis (Crosignani 1991; Paumgartner 2002).
Ursodeoxycholic acid treatment in patients with primary biliary cirrhosis might reduce the serum level of IgM class antimitochondrial antibodies and IgG antibodies to pyruvate dehydrogenase. Ursodeoxycholic acid might also reduce the T‐cell‐mediated hepatocellular damage by decreasing hepatocellular and biliary expression of major histocompatibility complex (MHC) class I and MHC class II molecules (Lazaridis 2001).
Why it is important to do this review
The effect of ursodeoxycholic acid on mortality and histological progression remains still controversial (Goulis 1999; Gluud 2001b; Gong 2008; EASL 2009; AASLD 2010). Our previously updated Cochrane systematic review did not provide sufficient information on benefits and harms of ursodeoxycholic acid in patients with primary biliary cirrhosis to recommend or reject the drug for this indication (Gong 2008). The present update aimed at gathering all additional information published after 2007, and through updated methodology and checking results with trial sequential analysis aimed at conducting reassessment of the evidence.
Objectives
To assess the beneficial and harmful effects of ursodeoxycholic acid in patients with primary biliary cirrhosis.
Methods
Criteria for considering studies for this review
Types of studies
We considered for inclusion randomised clinical trials assessing ursodeoxycholic acid in patients with primary biliary cirrhosis, irrespective of blinding, language, publication year, or publication status. For cross‐over trials, we only used data from the first intervention period. For assessment of harms, we also considered quasi‐randomised studies and observational studies, but we did not perform specific electronic searches for these studies.
Types of participants
Patients with primary biliary cirrhosis, i.e., patients having at least two of the following: elevated serum activity of alkaline phosphatases, a positive antimitochondrial antibody, and liver biopsy compatible with primary biliary cirrhosis (EASL 2009; AASLD 2010).
Types of interventions
Ursodeoxycholic acid administered perorally at any dose versus placebo or 'no intervention'. Any concomitant intervention was allowed if it was delivered equally to all treatment groups in the trial.
Types of outcome measures
Primary outcomes
All‐cause mortality.
All‐cause mortality or liver transplantation.
Adverse events. Serious adverse events were defined as any untoward medical occurrence that was life threatening, resulted in death, or was persistent or led to significant disability; or any medical event, which had jeopardised the patient or required intervention to prevent it (ICH‐GCP 1997). All other adverse events (that is, any medical occurrence not necessarily having a causal relationship with the treatment) were considered as non‐serious.
Quality of life.
Secondary outcomes
Liver transplantation.
Pruritus: number of patients with pruritus or pruritus score.
Fatigue: number of patients with fatigue.
Liver‐related morbidity (number of patients who developed jaundice, portal hypertension, oesophageal varices, gastric varices, upper gastrointestinal haemorrhage, ascites, hepatic encephalopathy, hepato‐renal syndrome).
Biochemical markers: serum bilirubin, serum alkaline phosphatases, serum gamma‐glutamyltransferase, serum aspartate aminotransferase, serum alanine aminotransferase, serum albumin, total cholesterol, plasma immunoglobulins, prothrombin index.
Liver biopsy findings: worsening of liver histological stage or score.
Cost‐effectiveness: the estimated costs connected with the interventions were weighed against any possible health gains.
Search methods for identification of studies
Electronic searches
Relevant randomised clinical trials were identified by electronic searching of The Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2011), The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded, and LILACS until January 2012 (Royle 2003). The search strategies and the time spans of the searches are presented in Appendix 1.
Searching other resources
The reference lists of relevant articles were scanned for additional trials. In order to obtain unpublished trials, the principal authors of the identified clinical trials and pharmaceutical companies involved in the production of ursodeoxycholic acid were inquired about additional trials they might know of. We searched Clinicaltrials.gov (http://clinicaltrials.gov/) and the WHO International Clinical Trials Registry Platform (http://www.who.int/ictrp/en/) for ongoing trials.
Data collection and analysis
Selection of studies
We listed the identified studies, and two of the authors (JR and GP) independently assessed their fulfilment of the inclusion and exclusion criteria. Disagreements were resolved by discussion and arbitrated by CG.
Data extraction and management
JR and GP extracted data independently using data extraction forms that were developed for the purpose. If relevant information was not available in the published trial, in order to obtain missing data and assess the trials correctly, we contacted authors of the trial publications. We added information obtained through correspondence with these authors to the data extraction form. We provided the date when the information was requested and received in the 'Notes' section of the respective trial ('Characteristics of included studies'). Disagreements were resolved by discussion among the review authors.
From each trial we extracted the following information: first author; country of origin; trial design (parallel or cross‐over); inclusion and exclusion criteria; number of patients randomised; characteristics of patients: age range (mean or median) and sex ratio; dose of ursodeoxycholic acid, duration, frequency of administration; type of intervention in the control group; type and dose of additional interventions, and outcomes.
Assessment of risk of bias in included studies
The confidence that the design and the report of the randomised clinical trial would restrict bias in the comparison of the intervention defines methodological quality, and hence risk of bias, which we assessed using the following domains (Schulz 1995; Moher 1998; Kjaergard 2001; Gluud 2006; Higgins 2008; Wood 2008; Savovic 2012).
Allocation sequence generation ‐ Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice are adequate if performed by an independent adjudicator. ‐ Uncertain risk of bias: the trial is described as randomised, but the method of sequence generation was not specified. ‐ High risk of bias: the sequence generation method is not, or may not be, random. Quasi‐randomised studies, those using dates, names, or admittance numbers in order to allocate patients are inadequate for assessment of benefits and were excluded for the assessment of benefits but not for harms.
Allocation concealment ‐ Low risk of bias: allocation was controlled by a central and independent randomisation unit, sequentially numbered, opaque and sealed envelopes or similar, so that intervention allocations could not have been foreseen in advance of, or during, enrolment. ‐ Uncertain risk of bias: the trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. ‐ High risk of bias: if the allocation sequence was known to the investigators who assigned patients or if the study was quasi‐randomised. Quasi‐randomised studies were excluded for the assessment of benefits but not for harms.
Blinding ‐ Low risk of bias: the trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. ‐ Uncertain risk of bias: the trial was described as blind, but the method of blinding was not described, so that knowledge of allocation was possible during the trial. ‐ High risk of bias, the trial was not blinded, so that the allocation was known during the trial.
Incomplete outcome data ‐ Low risk of bias: the numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals. ‐ Uncertain risk of bias: the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated. ‐ High risk of bias: the number or reasons for dropouts and withdrawals were not described.
Selective outcome reporting ‐ Low risk of bias: pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. ‐ Uncertain risk of bias: not all pre‐defined, or clinically relevant and reasonably expected outcomes are reported on or are not reported fully, or it is unclear whether data on these outcomes were recorded or not. ‐ High risk of bias: one or more clinically relevant and reasonably expected outcomes were not reported on; data on these outcomes were likely to have been recorded.
Other bias ‐ Low risk of bias: the trial appears to be free of other information that could put it at risk of bias. ‐ Uncertain risk of bias: the trial may or may not be free of other information that could put it at risk of bias. ‐ High risk of bias: there are other factors in the trial that could put it at risk of bias, eg, for‐profit involvement, authors have conducted trials on the same topic etc.
Trials assessed as having 'low risk of bias' in all of the specified individual domains were considered trials with low risk of bias. Trials assessed as having 'uncertain risk of bias' or 'high risk of bias' in one or more of the specified individual domains were considered trials with high risk of bias (Higgins 2008; Gluud 2011).
Measures of treatment effect
Dichotomous data were expressed as relative risk (RR) with 95% confidence intervals (CI). When continuous scales of measurement were used to assess the treatment effects, we used the mean difference (MD) (Thompson 2002). Mean differences based on changes from baseline can usually be assumed to be addressing exactly the same underlying intervention effects as analyses based on final measurements (Higgins 2011). Therefore, we combined data reported as change from baseline values with final measurement values in meta‐analysis when using the mean difference method in RevMan (RevMan 2011). We did not use standardised mean differences (SMD) when we combined change scores and final measurements. For trials addressing the same outcome but using different scales of measuring, SMD were used.
Dealing with missing data
For the previous up‐date of this review, review authors sought missing data by contacting trial authors. This is why we have not tried to do this again.
We performed analyses according to the intention‐to‐treat method for dichotomous outcomes, except for worsening of liver histology, including all patients irrespective of compliance or follow‐up. However, for continuous outcomes we performed available patient analysis and included data only on those whose results were known.
Regarding the primary outcome measures, we included patients with incomplete or missing data in sensitivity analyses, by imputing the missing data following the scenarios below (Hollis 1999; Gluud 2011).
‐ Available patient analysis which simply excludes all patients with the missing outcome from the analysis. ‐ Extreme‐case analysis favouring the experimental intervention ('best‐worse' case scenario): none of the dropouts/patients lost from the experimental arm but all of the dropouts/patients lost from the control group experienced the outcome, including all randomised patients in the denominator. ‐ Extreme‐case analysis favouring the control ('worst‐best' case scenario): all dropouts/patients lost from the experimental arm but none from the control arm experienced the outcome, including all randomised patients in the denominator.
Assessment of heterogeneity
We explored the presence of statistical heterogeneity by the chi‐squared test with significance less than or equal to P = 0.10. We measured the quantity of heterogeneity by I². Values of I² between 0% to 40% were graded as: heterogeneity might not be important; I² statistic between 30% to 60% was graded as representing moderate heterogeneity; I² between 50% to 90% was graded as substantial heterogeneity; and I² between 75% to 100% was graded as considerable heterogeneity (Higgins 2008).
Between‐trial heterogeneity was explored by meta‐regression, depending on the available data. We performed a meta‐regression analysis with STATA 8.2 (STATA Corp, College Station, Tex). We used the STATA meta reg command for the random‐effects meta‐regression to assess which covariates influenced the intervention effect across trials. The covariates were: risk of bias of the trials, disease severity of patients at entry, ursodeoxycholic acid dosage, and trial duration (treatment and follow‐up). Univariate and multivariate analyses including all covariates were performed. The results are presented with regression coefficients and 95% CI.
Assessment of reporting biases
Funnel plots were drawn to provide visual assessment as to whether treatment effects were associated with trial size (Higgins 2008). We explored publication bias and other bias according to Begg's and Egger's methods (Begg 1994; Egger 1997) with STATA®.
Data synthesis
We performed this review according to the recommendations of The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (Gluud 2011). For the statistical analyses, we used Review Manager 5.1 (RevMan 2011). We meta‐analysed the data with both a random‐effects model (DerSimonian 1986) and a fixed‐effect model (DeMets 1987) to ensure robustness of the results. In case of differences of the results that the two models produced, we presented the result from both methods and discussed discrepancies. We presented the results with the fixed‐effect model if the results of the two models did not differ (Higgins 2002).
We summarised the evidence in the summary of findings table using GRADEpro (http://ims.cochrane.org/revman/other‐resources/gradepro).
Complimentary analyses
Trial sequential analysis
In order to assess the risks of random errors due to sparse data and multiplicity, we performed trial sequential analysis (Brok 2008; Wetterslev 2008; Thorlund 2009). We calculated the required information size (i.e., the number of patients needed in a meta‐analysis to detect or reject a certain intervention effect) (Wetterslev 2008). The required information size calculation should also account for the diversity present in the meta‐analysis (Wetterslev 2009). In our meta‐analysis, the required information size was based on the proportion of patients with the outcome in the control group, assumption of a plausible RR reduction of 20%, a type I error of 5%, and a type II error of 20% (power 80%) (Wetterslev 2008). We adjusted the required information size for diversity unless otherwise stated (Wetterslev 2009).
The underlying assumption of trial sequential analysis is that testing for significance may be performed each time a new trial is added to the meta‐analysis. We added the trials according to the year of publication, and if more than one trial was published in a year, trials were added alphabetically according to the last name of the first author (Wetterslev 2008).
On the basis of the required information size, trial sequential alpha‐spending and beta‐spending monitoring boundaries were constructed (Wetterslev 2008). These boundaries determine the statistical inference one may draw regarding the cumulative meta‐analysis that has not reached the required information size. If the cumulative Z‐curve crosses the trial sequential alpha‐spending or beta‐spending monitoring boundary, a sufficient level of evidence may have been reached and no further trials are needed. If the Z‐curve does not cross the trial sequential alpha‐spending or beta‐spending monitoring boundary, then there is insufficient evidence to reach a conclusion.
We applied trial sequential analysis with the Trial Sequential Analysis (TSA) program (TSA manual 2011; TSA program 2011) since it allows us to assess the risk of type I error due to sparse data and multiple updating in a cumulative meta‐analysis, and it provides us with important information in order to estimate the level of evidence of the experimental intervention. Additionally, trial sequential analysis provides us with important information regarding the need for additional trials and the required information size of such trials.
Subgroup analysis and investigation of heterogeneity
The following subgroup analyses were planned: ‐ To assess if the effects of ursodeoxycholic acid differ between trials with low risk of bias compared to trials with high risk of bias. ‐ To assess if the effects of ursodeoxycholic acid differ between different durations of administration of ursodeoxycholic acid. ‐ To assess if the effects of ursodeoxycholic acid differ between different doses of ursodeoxycholic acid.
Sensitivity analysis
We conducted sensitivity analyses to investigate the robustness of our main analyses. These sensitivity analyses were only performed on the primary outcomes, i.e., all‐cause mortality and all‐cause mortality or liver transplantation. The missing data could be due to patient dropouts or lost to follow‐up.
We conducted sensitivity analyses to exclude a large intervention effect of a 40%, 30%, and 20% relative risk reduction (RRR) of mortality using Trial Sequential Analysis (TSA) (Wetterslev 2008; TSA manual 2011; TSA program 2011).
In the original protocol for this systematic review (Gluud 1999a), we only intended to extract data from the time when patients were on ursodeoxycholic acid versus placebo/no intervention in order to secure data from the most unbiased comparisons. We also extracted data on mortality and/or liver transplantation at the maximal follow‐up of each trial, including data from patients switched from blinded ursodeoxycholic acid onto open label ursodeoxycholic acid (ursodeoxycholic acid→ursodeoxycholic acid) versus patients switched from placebo onto open label ursodeoxycholic acid (placebo→ursodeoxycholic acid). The interpretation of these latter data, however, should be performed with caution (see Discussion).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies
Results of the search
Our search strategy identified 1365 publications, out of which 637 were duplicates. Of the remaining 728 publications, 623 were excluded because they were reviews, because they did not relate to primary biliary cirrhosis, or because they did not describe a randomised clinical trial investigating the effect of ursodeoxycholic acid in patients with primary biliary cirrhosis. The remaining 105 publications referred to 16 randomised clinical trials (Figure 1).
1.
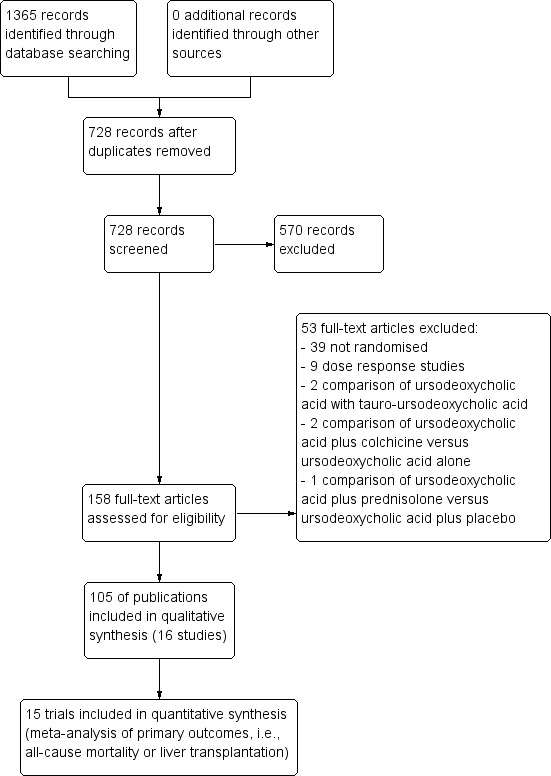
Flow diagram.
Fourteen of the included trials consisted of more than one publication. Two out of the 16 randomised clinical trials were published as abstracts only (De la Mora 1994; Goddard 1994), and the De la Mora 1994 trial provided no extractable data on the trial's characteristics and outcomes.
Most of the primary authors and manufacturers of the ursodeoxycholic acid were contacted for further information and data relating to the trials while conducting the previous up‐date of this review. Dr. Albert Pares kindly provided data on the method of sequence generation.
Through a search for ongoing trials in Clinicaltrials.gov (http://clinicaltrials.gov/) and WHO International Clinical Trials Registry Platform (http://www.who.int/ictrp/en/) we have not identified any registered ongoing or planned trials.
Included studies
A total of 1476 patients with primary biliary cirrhosis were randomised in the 16 randomised clinical trials. Ursodeoxycholic acid dose varied from 7.7 to 15.0 mg/kg/day with a median of 10 mg/kg/day. The duration of the trials varied from 3 to 92 months with a median of 24 months. The percentage of symptomatic patients and patients with advanced primary biliary cirrhosis at baseline varied from 15% to 83% with a median of 51%. The details are displayed in Table 5. From the publications which reported sex of the patients, more than 89.5% were females. Three trials were conducted in United States (Senior 1991; Lindor 1994; Combes 1995) and two trials were conducted in United Kingdom (Goddard 1994; Turner 1994). Other trials were conducted each in different countries: Italy, Mexico, Sweden, Canada, China, Germany, Japan, Greece, Spain, France, and Finland (see Characteristics of included studies). Fiftheen trials had the parallel group design and one trial had the cross‐over group design (Hwang 1993).
1. Summary of characteristics of the included trials.
| Trial | Risk of bias | Ursodeoxycholic acid dose* | Trial duration (months) | Severity of PBC#¤ |
| Papatheodoridis 2002 | High | 13.5 | 92.4 | 0.6400 |
| Pares 2000 | Low | 15.0 | 40.8 | 0.2708 |
| Combes 1995 | High | 11.0 | 24.0 | 0.6689 |
| Leuschner 1989 | High | 10.0 | 9.0 | 0.1500 |
| Eriksson 1997 | High | 7.7 | 24.0 | 0.3350 |
| Vuoristo 1995 | High | 13.5 | 24.0 | 0.3333 |
| Goddard 1994 | High | 10.0 | 15.0 | 0.3200 |
| Lindor 1994 | Low | 14.0 | 48.0 | 0.6833 |
| Battezzati 1993 | Low | 8.7 | 12.0 | 0.4950 |
| Senior 1991 | High | 10.0 | 6.0 | 0.6666 |
| Turner 1994 | Low | 10.0 | 24.0 | 0.8261 |
| Hwang 1993 | High | 9.2 | 3.0 | 0.5833 |
| Oka 1990 | High | 9.2 | 6.0 | 0.3795 |
| Heathcote 1994 | Low | 14.0 | 24.0 | 0.5270 |
| Poupon 1991 | High | 14.0 | 24.0 | 0.4658 |
* ursodeoxycholic acid dose in mg/kg/day. # PBC= primary biliary cirrhosis. ¤ proportion of patients with stage III or IV at entry; or proportion of symptomatic patients at entry.
Following the stipulated follow‐up in the ursodeoxycholic acid‐group and the placebo‐group, six trials (Poupon 1991; Battezzati 1993; Heathcote 1994; Lindor 1994; Combes 1995; Eriksson 1997) continued ursodeoxycholic acid treated patients on open label ursodeoxycholic acid (ursodeoxycholic acid→ursodeoxycholic acid) and offered open label ursodeoxycholic acid to the patients originally given placebo (placebo→ursodeoxycholic acid). The Papatheodoridis 2002 trial continued to administer ursodeoxycholic acid to all patients randomised to the ursodeoxycholic acid arm and switched 14/43 'no intervention' patients to ursodeoxycholic acid after they had been followed for a mean duration of 3.5 years. It was not possible to separate the data of the original period (ursodeoxycholic acid versus no intervention) from the total period (ursodeoxycholic acid→ursodeoxycholic acid versus no intervention→ursodeoxycholic acid), as only data from the total period were given.
Excluded studies
The excluded studies are listed under 'Characteristics of excluded studies' and the reasons for exclusion are given there.
Risk of bias in included studies
Risk of bias was assessed according to six domains: allocation sequence generation; allocation concealment; blinding; handling of incomplete outcome data; selective outcome reporting; and other potential sources of bias. One out of 16 trials was considered as having low risk of bias (Lindor 1994). Our statistical analyses are, therefore, based mainly on trials with high risk of bias. For details of the judgements made for the individual trials, please see Figure 2 and Figure 3.
2.
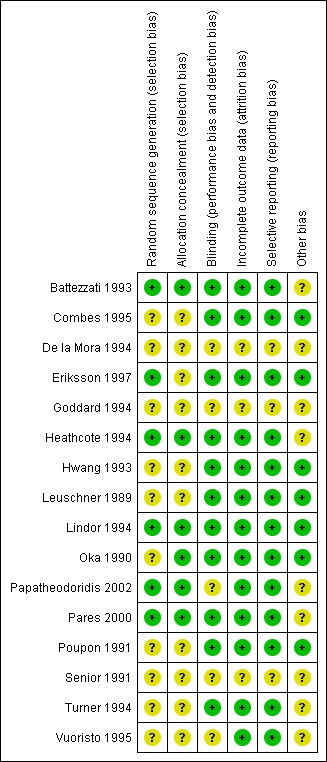
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
3.
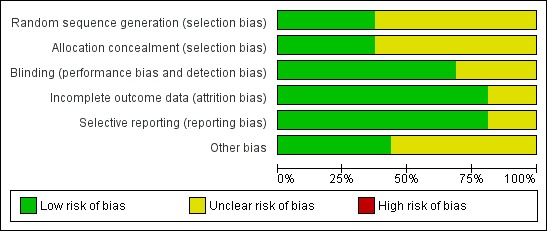
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
The generation of the allocation sequence was adequately described in six trials (Battezzati 1993; Heathcote 1994; Lindor 1994; Eriksson 1997; Pares 2000; Papatheodoridis 2002). The remaining ten trials were described as randomised, but the method for sequence generation was not described (Leuschner 1989; Oka 1990; Poupon 1991; Senior 1991; Hwang 1993; De la Mora 1994; Goddard 1994; Turner 1994; Combes 1995; Vuoristo 1995).
The method used to conceal allocation was adequately described in six trials (Oka 1990; Battezzati 1993; Heathcote 1994; Lindor 1994; Pares 2000; Papatheodoridis 2002). The method for allocation concealment was judged as unclear in 10 trials (Leuschner 1989; Oka 1990; Poupon 1991; Heathcote 1994; Lindor 1994; Turner 1994; Vuoristo 1995; Eriksson 1997; Pares 2000; Papatheodoridis 2002).
Blinding
The method of blinding was adequately described in 11 trials (Leuschner 1989; Oka 1990; Poupon 1991; Battezzati 1993; Hwang 1993; Heathcote 1994; Lindor 1994; Turner 1994; Combes 1995; Eriksson 1997; Pares 2000). The method of blinding was unclear or not used in five trials (Senior 1991; De la Mora 1994; Goddard 1994; Vuoristo 1995; Papatheodoridis 2002).
Incomplete outcome data
Incomplete data were addressed adequately in the included trials except for three trials (Senior 1991; De la Mora 1994; Goddard 1994).
Selective reporting
Predefined primary and secondary outcomes were adequately assessed in all included trials except three (Senior 1991; De la Mora 1994; Goddard 1994). Whenever less than 16 trials reported on an outcome, there was risk of outcome reporting bias as we had no access to any of the trial protocols.
Other potential sources of bias
Following the information provided in the trial publication, one trial may be free of other causes of bias (Lindor 1994).
Effects of interventions
See: Table 1
Primary outcomes
All‐cause mortality
Fourteen trials provided information on all‐cause mortality and could be included in the analyses. The included trials reported a total of 91 (6.5%) deaths in 1391 patients (Analysis 1.1). In the ursodeoxycholic acid group, 45 (6.4%) out of 699 patients died versus 46 (6.6%) out of 692 patients in the control group. Meta‐analyses with both the fixed‐effect model and random‐effects model showed that ursodeoxycholic acid had no effect on all‐cause mortality (RR 0.97; 95% CI 0.67 to 1.42, I² = 0%) (Analysis 1.1).
1.1. Analysis.
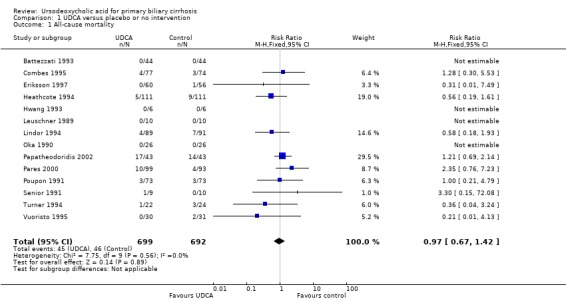
Comparison 1 UDCA versus placebo or no intervention, Outcome 1 All‐cause mortality.
Inspection of the funnel plot did not indicate bias (Figure 4).
4.
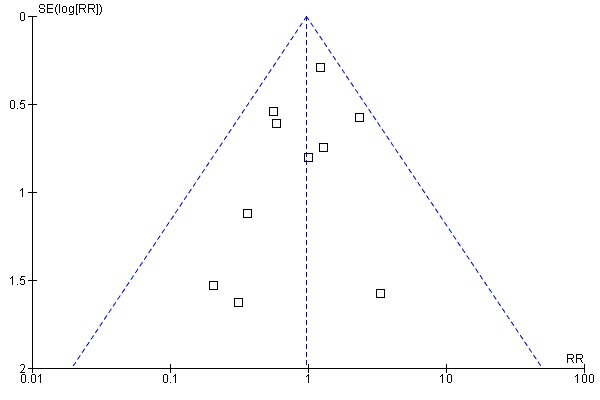
Funnel plot of comparison: 1 Ursodeoxycholic acid versus placebo or no intervention, outcome: 1.1 All‐cause mortality.
The subgroup analyses stratifying the trials according to risk of bias, risk of bias including industry involvement, trial duration, and dose of ursodeoxycholic acid did not reveal any differences in effect on all‐cause mortality (Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5). Heterogeneity was absent (I² = 0%, P = 0.56).
1.2. Analysis.
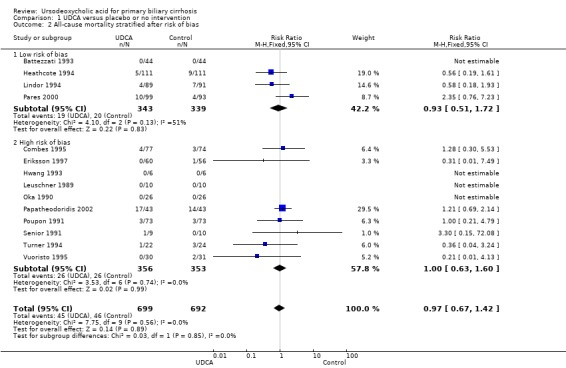
Comparison 1 UDCA versus placebo or no intervention, Outcome 2 All‐cause mortality stratified after risk of bias.
1.3. Analysis.
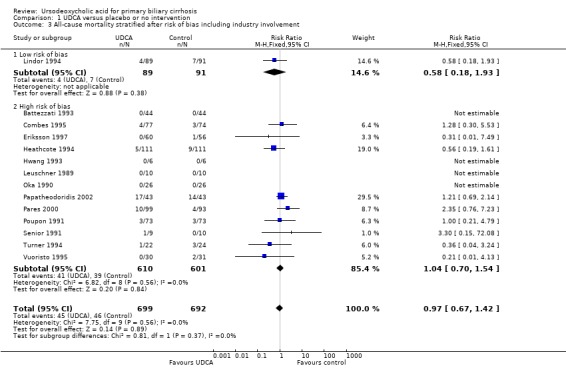
Comparison 1 UDCA versus placebo or no intervention, Outcome 3 All‐cause mortality stratified after risk of bias including industry involvement.
1.4. Analysis.
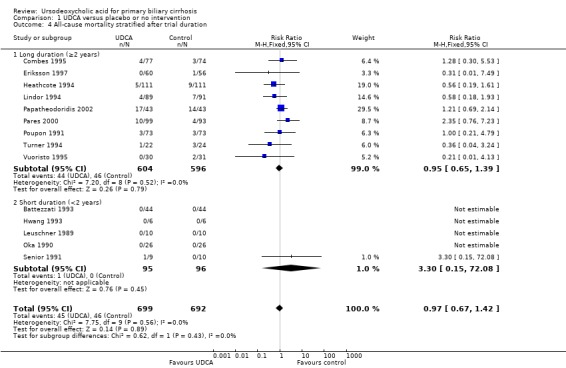
Comparison 1 UDCA versus placebo or no intervention, Outcome 4 All‐cause mortality stratified after trial duration.
1.5. Analysis.
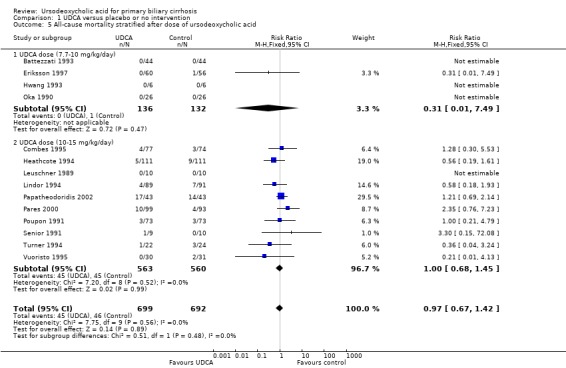
Comparison 1 UDCA versus placebo or no intervention, Outcome 5 All‐cause mortality stratified after dose of ursodeoxycholic acid.
Trial sequential analysis with data from all included trials showed that only 1382 patients of the diversity‐adjusted required information size of 8539 were accrued (16%) and no firm evidence for benefit or harm was reached (Figure 5). The cumulative Z‐curve did not cross the red trial sequential alpha‐spending monitoring boundaries for benefit or harm. Therefore, there is no evidence to support or reject that ursodeoxycholic acid influences mortality.
5.
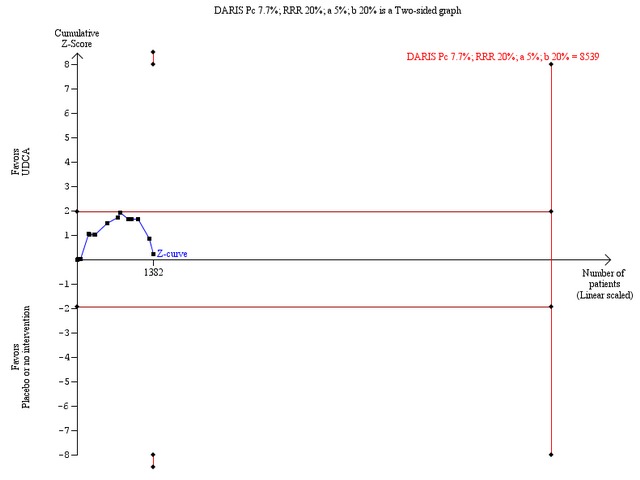
Trial sequential analysis of the random‐effects meta‐analysis of the effect of ursodeoxycholic acid versus placebo or no intervention on all‐cause mortality. The trial sequential analysis is performed with an assumed control proportion of death of 7.7%, an anticipated relative risk reduction (RRR) of 20%, a type 1 error risk of 5% (two‐sided) (a), and a power of 80% (a type II error risk of 20%) (b). The diversity‐adjusted required information size (DARIS) to detect or reject a RRR of 20% with a between trial heterogeneity of 0% is estimated to 8539 patients. The actually accrued number of patients is 1382, which is only 16% of the required information size. The blue cumulative Z‐curve does not cross the red trial sequential alpha‐spending monitoring boundaries for benefit or harm. Therefore, there is no evidence to support or refute that ursodeoxycholic acid influences mortality with a 20% RRR of mortality. The cumulative Z curve does not reach the futility area delineated by the trial sequential beta‐spending monitoring boundaries (which are not even drawn by the program), demonstrating that further randomised trials are needed.
Sensitivity analyses to assess intervention effects of 40% or 30% relative risk reduction of mortality showed that we could exclude a very large intervention effect of 40% relative risk reduction of deaths (Figure 6). However, we were unable to prove or disprove a relative risk reduction of 30% (Figure 7), and below (data not shown). For such smaller intervention effects, the number of trial patients has to be increased substantially.
6.
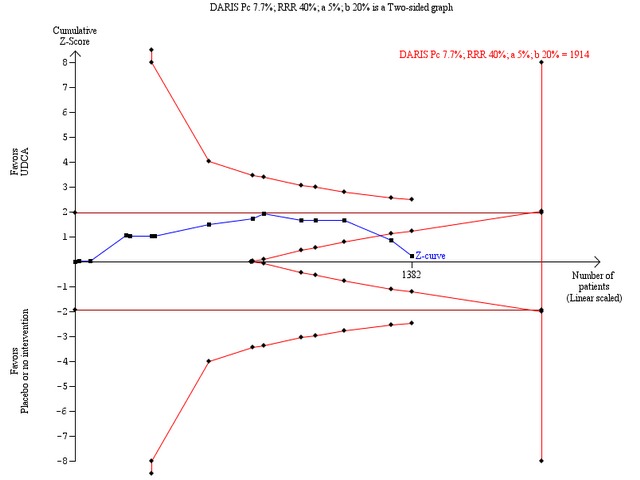
Trial sequential analysis of the random‐effects meta‐analysis of the effect of ursodeoxycholic acid versus placebo or no intervention on all‐cause mortality. The trial sequential analysis is performed with an assumed control proportion of death of 7.7%, an anticipated relative risk reduction (RRR) of 40%, a type 1 error risk of 5% (two‐sided) (a), and a power of 80% (type 2 error risk of 20%) (b). The diversity‐adjusted required information size to detect or reject a RRR of 40% with a between trial heterogeneity of 0% is estimated to 1914 patients. The actually accrued number of patients is 1382, which is 72% of the required information size. The blue cumulative Z‐curve does not cross the red trial sequential alpha‐spending monitoring boundaries for benefit or harm. However, the boundaries for futility (the red inner wedge boundaries showing the trial sequential beta‐spending monitoring boundaries) are crossed. The red conventional boundaries (horizontal line at Z = 1.96 and Z = ‐1.96) for harm or benefit are not crossed. Therefore, there is no evidence to support ursodeoxycholic acid and we can refute that ursodeoxycholic acid influences mortality by a 40% RRR of mortality with the chosen error risks.
7.
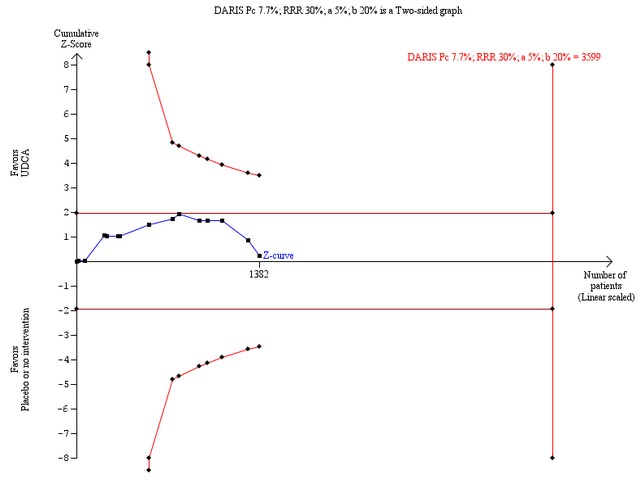
Trial sequential analysis of the random‐effects meta‐analysis of the effect of ursodeoxycholic acid versus placebo or no intervention on all‐cause mortality. The trial sequential analysis is performed with an assumed control proportion of death of 7.7%, an anticipated relative risk reduction (RRR) of 30%, a type 1 error risk of 5% (two‐sided) (a), and a power of 80% (a type 2 error risk of 20%) (b). The diversity‐adjusted required information size (DARIS) to detect or reject a RRR of 30% with a between trial heterogeneity of 0% is estimated to 3599 patients. The actually accrued number of patients is 1382, which is only 38% of the required information size. The blue cumulative Z‐curve does not cross the red trial sequential monitoring boundaries for benefit or harm. Therefore, there is no evidence to support that ursodeoxycholic acid influences mortality. The cumulative Z‐curve does not reach the futility area delineated by the trial sequential beta‐spending monitoring boundaries (which are not even drawn by the program), demonstrating that further randomised trials are needed.
Available patient analysis did not result in any changes of effect estimates (RR 0.98; 95% CI 0.67 to 1.43; I² = 0%; 1247 patients, 14 trials) (Analysis 2.1). Analysing the missing data in the best‐case scenario (assuming that patients with unknown vital status receiving ursodeoxycholic acid were alive and that all patients from the control group with unknown vital status were dead) or in the worst‐case scenario (assuming that patients with unknown vital status receiving ursodeoxycholic acid were dead and all patients with unknown vital status from the control group were alive) showed statistical significant effects of ursodeoxycholic acid ranging from a beneficial effect (best‐case scenario: RR 0.35; 95% CI 0.26 to 0.48; 1 391 patients, 14 trials) to a harmful effect (worst‐case scenario: RR 2.16, 95% CI 1.57 to 2.97; 1391 patients, 14 trials) (Analysis 2.1).
2.1. Analysis.
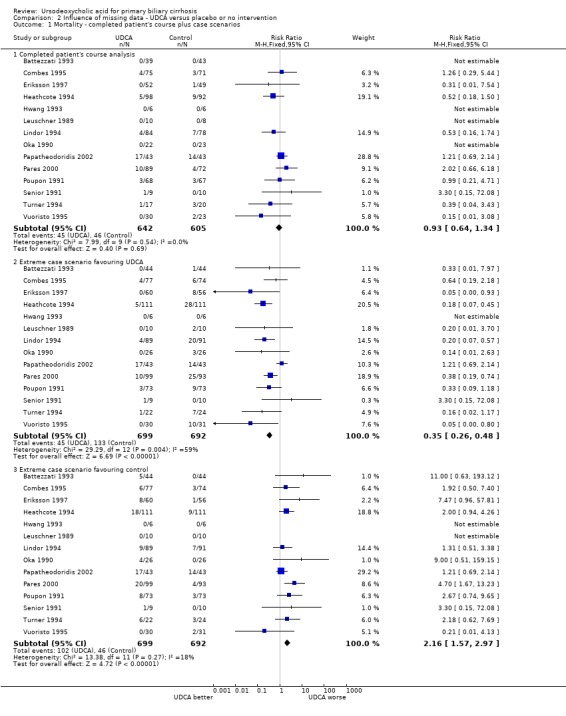
Comparison 2 Influence of missing data ‐ UDCA versus placebo or no intervention, Outcome 1 Mortality ‐ completed patient's course plus case scenarios.
Univariate meta‐regression analyses revealed that none of examined covariates (risk of bias of the trials, disease severity of patients at entry, ursodeoxycholic acid dosage, and trial duration) were significantly associated with the estimated intervention effect on mortality. In multivariate meta‐regression analysis including all covariates, none were significantly associated with the estimated intervention effect on mortality. See Table 6.
2. UDCA* effects on mortality adjusted for trial‐level covariates.
| Covariates | Coefficient | 95% CI | P‐value |
| Risk of bias (low versus high) | 0.225 | ‐1.153 to 1.630 | 0.749 |
| UDCA* dose (mg/kg/day) | ‐0.284 | ‐1.004 to 0.437 | 0.440 |
| Trial duration (year) | 0.014 | ‐0.012 to 0.040 | 0.296 |
| Severity of PBC# | ‐4.938 | ‐10.459 to 0.582 | 0.080 |
* UDCA= ursodeoxycholic acid. # PBC= primary biliary cirrhosis.
Analysis of data from the extended follow‐up for ursodeoxycholic acid→ursodeoxycholic acid versus placebo→ursodeoxycholic acid into the analyses demonstrated a RR of 0.97 with 95% CI 0.73 to 1.30 (Analysis 3.1). It compared 76 (10.9%) deaths in 699 patients originally randomised to ursodeoxycholic acid with 78 (11.2%) deaths in 692 patients originally randomised to placebo or no intervention.
3.1. Analysis.
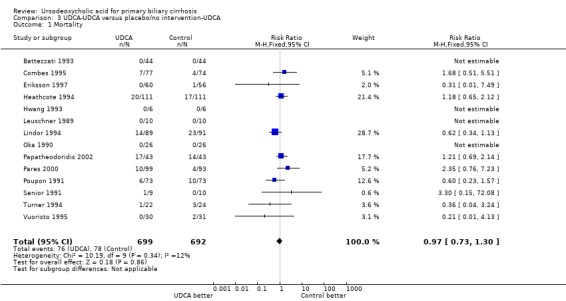
Comparison 3 UDCA‐UDCA versus placebo/no intervention‐UDCA, Outcome 1 Mortality.
All‐cause mortality or liver transplantation
Fifthteen trials provided information on all‐cause mortality or liver transplantation and could be included in the analyses. The included trials reported a total of 175 (12.3%) deaths or transplants in 1419 patients (Analysis 1.6). In the ursodeoxycholic acid group, 86 (12.0%) out of 713 patients died or were transplanted versus 89 (12.6%) out of 706 patients in the control group. Meta‐analyses with both the fixed‐effect model and random‐effects model showed no significant difference in effect between the compared interventions (RR 0.96; 95% CI 0.74 to 1.25, I² = 15%) (Analysis 1.6).
1.6. Analysis.
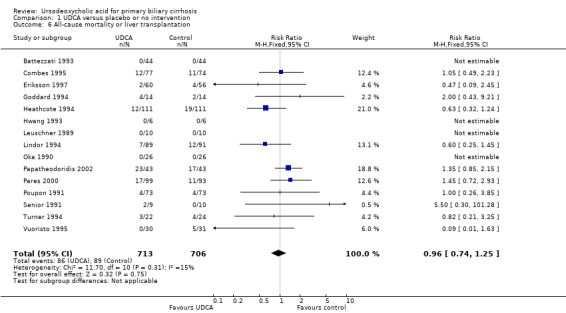
Comparison 1 UDCA versus placebo or no intervention, Outcome 6 All‐cause mortality or liver transplantation.
Inspection of the funnel plot did not indicate bias (Figure 8).
8.
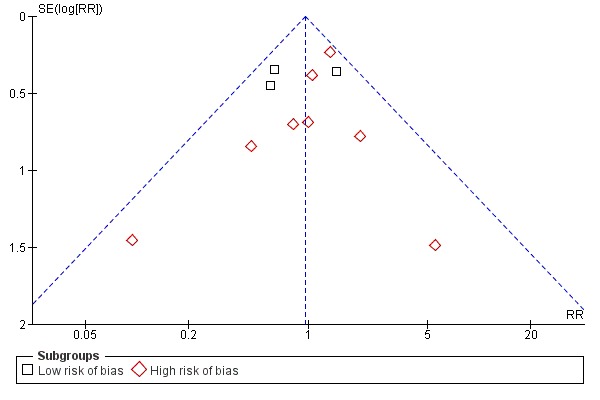
Funnel plot of comparison: 1 UDCA versus placebo or no intervention, outcome: 1.7 All‐cause mortality or liver transplantation stratified after risk of bias.
The subgroup analyses stratifying the trials according to risk of bias, risk of bias including industry involvement, trial duration, and dose of ursodeoxycholic acid did not reveal any differences in effect estimates in the risk of all‐cause mortality or liver transplantation (Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10). Heterogeneity might not be important (I² = 15%, P = 0.31).
1.7. Analysis.
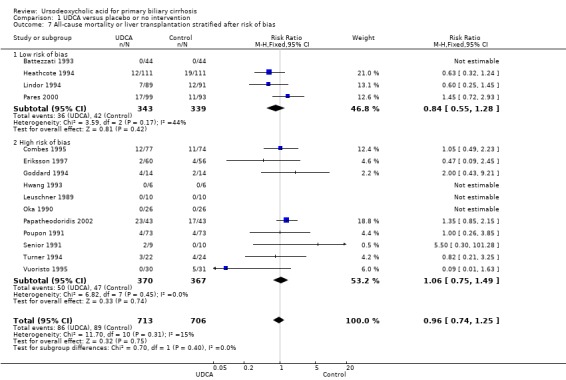
Comparison 1 UDCA versus placebo or no intervention, Outcome 7 All‐cause mortality or liver transplantation stratified after risk of bias.
1.8. Analysis.
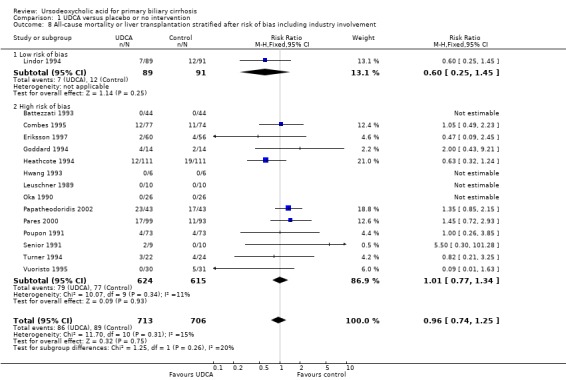
Comparison 1 UDCA versus placebo or no intervention, Outcome 8 All‐cause mortality or liver transplantation stratified after risk of bias including industry involvement.
1.9. Analysis.
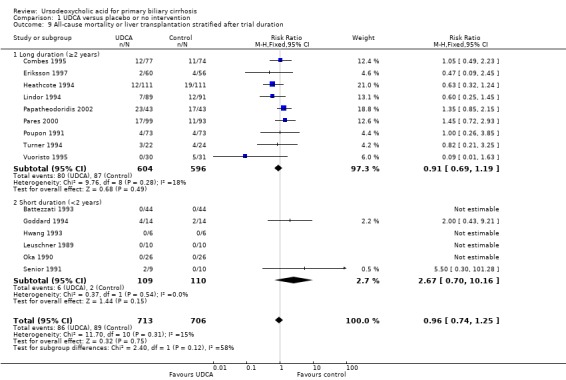
Comparison 1 UDCA versus placebo or no intervention, Outcome 9 All‐cause mortality or liver transplantation stratified after trial duration.
1.10. Analysis.
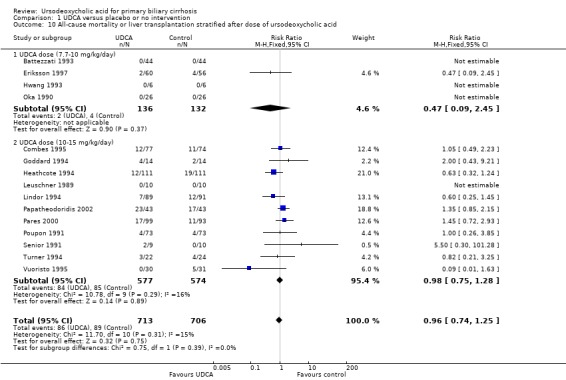
Comparison 1 UDCA versus placebo or no intervention, Outcome 10 All‐cause mortality or liver transplantation stratified after dose of ursodeoxycholic acid.
Trial sequential analysis with data from all included trials showed that only 1 410 patients of the required diversity‐adjusted information size of 4 043 were accrued (35%) and no firm evidence for benefit or harm was therefore reached (Figure 9). The cumulative Z‐curve did not cross the red trial sequential alpha‐spending monitoring boundaries for benefit or harm. Therefore, there is no evidence to support or refute that ursodeoxycholic acid influences mortality or transplantation. Sensitivity analyses showed that an intervention effect corresponding to a 30% relative risk reduction of all‐cause mortality or liver transplantation can be excluded (Figure 10).
9.
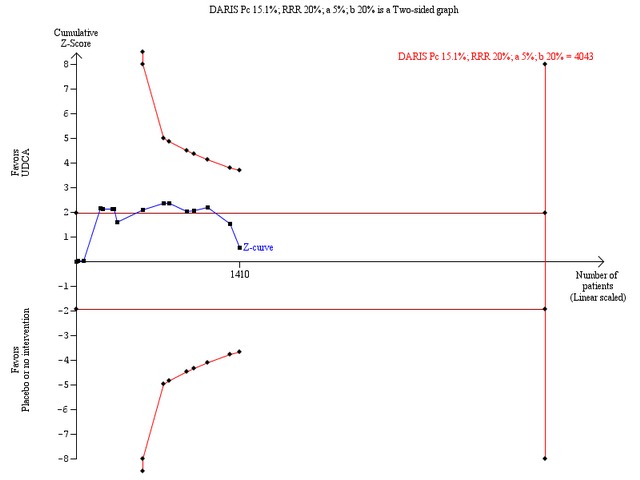
Trial sequential analysis of the random‐effects meta‐analysis of the effect of ursodeoxycholic acid versus placebo or no intervention on all‐cause mortality or liver transplantation. The trial sequential analysis is performed with an assumed control proportion of death of 15.1%, an anticipated relative risk reduction (RRR) of 20%, a type 1 error risk of 5% (two‐sided), and a power of 80% (a type 2 error risk of 20%) (b). The diversity‐adjusted required information size (DARIS) to detect or reject a RRR of 20% with a between trial heterogeneity of 37% is estimated to 4043 patients. The actually accrued number of patients is 1410, which is only 35% of the required information size. The blue cumulative Z‐curve does not cross the red trial sequential monitoring boundaries for benefit or harm. Therefore, there is no evidence to support or refute that ursodeoxycholic acid influences mortality or transplantation. The cumulative Z curve does not reach the futility area delineated by the trial sequential beta‐spending monitoring boundaries (which are not even drawn by the program), demonstrating that further randomised trials are needed.
10.
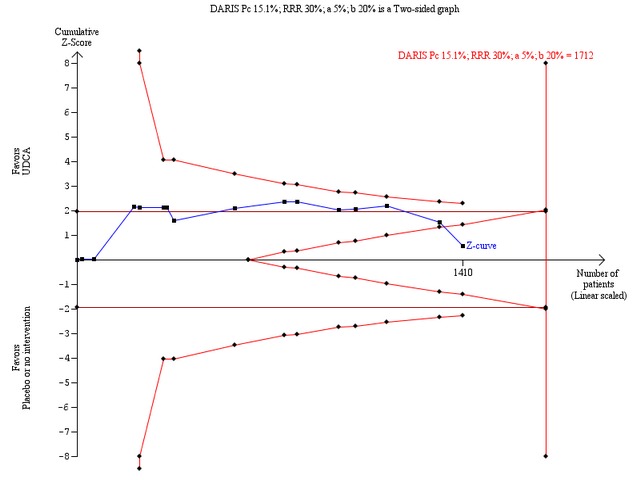
Trial sequential analysis of the random‐effects meta‐analysis of the effect of ursodeoxycholic acid versus placebo or no intervention on all‐cause mortality or liver transplantation. The trial sequential analysis is performed with an assumed control proportion of death of 15.1%, an anticipated relative risk reduction (RRR) of 30%, a type 1 error risk of 5% (two‐sided), and a power of 80% (a type 2 error risk of 20%) (b). The diversity‐adjusted required information size (DARIS) to detect or reject a RRR of 30% with a between trial heterogeneity of 37% is estimated to 1712 patients. The actually accrued number of patients is 1410, which is 82% of the required information size. The blue cumulative Z‐curve does not cross the red trial sequential alpha‐spending monitoring boundaries for benefit or harm. However, the boundaries for futility delineated by the trial sequential beta‐spending monitoring boundaries (the red inner wedge boundaries) are crossed. Accordingly, the red conventional boundaries (horizontal line at z =1.96 and z =‐1.96) for harm or benefit are not crossed. Therefore, there is no evidence to support that ursodeoxycholic acid influences mortality or transplantation. Moreover, a 30% RRR of mortality or transplantation can be rejected with the chosen error risks.
Available patient analysis did not result in any significant changes of effect estimates (RR 0.93; 95% CI 0.64 to 1.34; I² = 23%; 1 275 patients, 15 trials) (Analysis 2.2). The best‐case scenario and worst‐case scenario analyses on missing data showed statistical significant effects of ursodeoxycholic acid ranging from a beneficial effects (best‐case scenario: RR 0.49; 95% CI 0.30 to 0.80; 1419 patients, 15 trials) to a harmful effects (worst‐case scenario: RR 1.60; 95% CI 1.21 to 2.10; 1419 patients, 15 trials) (Analysis 2.2). These data show that we have too little knowledge about the true effect of ursodeoxycholic acid on all‐cause mortality or liver transplantation, also due to poor outcome reporting of the included trials on mortality and liver transplantation.
2.2. Analysis.
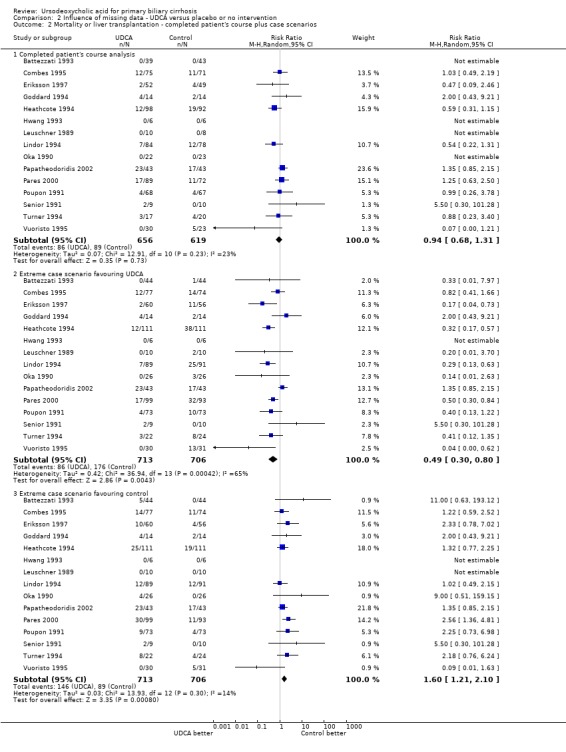
Comparison 2 Influence of missing data ‐ UDCA versus placebo or no intervention, Outcome 2 Mortality or liver transplantation ‐ completed patient's course plus case scenarios.
Univariate meta‐regression analyses revealed that none of the examined covariates (risk of bias, disease severity of patients at entry; ursodeoxycholic acid dosage, and trial duration) were significantly associated with the estimated intervention effect on mortality or liver transplantation. In multivariate meta‐regression analysis including all covariates, none were significantly associated with the estimated intervention effect on mortality or liver transplantation (Table 7).
3. UDCA* effects on mortality or transplantation adjusted for trial‐level covariates.
| Covariate | Coefficient | 95% CI | P‐value |
| Risk of bias (low vs. high) | ‐0.487 | ‐1.484 to 0.510 | 0.338 |
| UDCA* (mg/kg/day) | 0.039 | ‐0.244 to 0.322 | 0.787 |
| Trial duration (year) | 0.008 | ‐0.011 to 0.027 | 0.408 |
| Severity of PBC# | ‐1.282 | ‐3.637 to 1.073 | 0.286 |
* UDCA= ursodeoxycholic acid. # PBC= primary biliary cirrhosis.
Including data from the extended follow‐up for ursodeoxycholic acid→ursodeoxycholic acid versus placebo/no intervention→ursodeoxycholic acid demonstrated a RR of 0.88 with 95% CI from 0.73 to 1.06 (Analysis 3.2). The meta‐analysis showed 147 (20.6%) deaths or liver transplantations out of 713 patients originally randomised to ursodeoxycholic acid, and 165 (23.3%) deaths or liver transplantations out of 706 patients originally randomised to placebo or 'no intervention'.
3.2. Analysis.
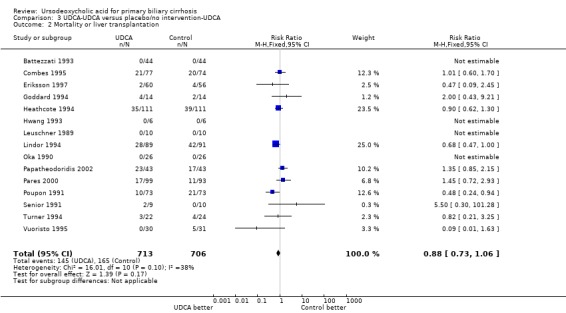
Comparison 3 UDCA‐UDCA versus placebo/no intervention‐UDCA, Outcome 2 Mortality or liver transplantation.
Adverse events
We divided the reporting of adverse events into the following types: serious adverse events and non‐serious adverse events (ICH‐GCP 1997).
There was no significant difference in the risk ratio for overall proportion of serious adverse events when comparing ursodeoxycholic acid with placebo or no intervention (RR 0.87; 95% CI 0.68 to 1.12; I² = 23%; 1382 patients, 14 trials) (Analysis 1.11). In the ursodeoxycholic group 94 serious adverse events were reported versus 107 serious adverse events in the control group of the included trials.
1.11. Analysis.
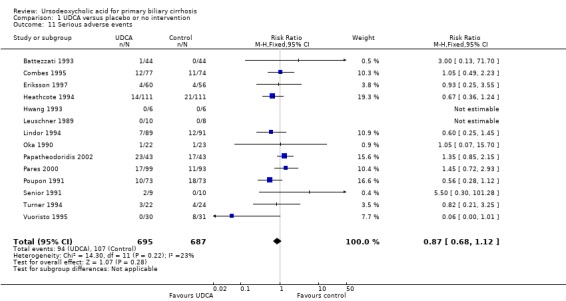
Comparison 1 UDCA versus placebo or no intervention, Outcome 11 Serious adverse events.
There was also no significant difference in the risk ratio for overall incidence of non‐serious adverse events when comparing ursodeoxycholic acid with placebo or 'no intervention' (RR 1.46; 95% CI 0.83 to 2.56; I² = 0%; 1 277 patients, 12 trials) (Analysis 1.12). In the ursodeoxycholic group 27 non‐serious adverse events were reported versus 18 non‐serious adverse events in the control group of the included trials.
1.12. Analysis.
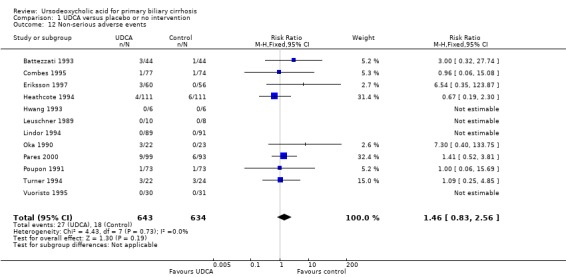
Comparison 1 UDCA versus placebo or no intervention, Outcome 12 Non‐serious adverse events.
For assessment of harm, besides the data provided by randomised clinical trials which are included in our analyses (Analysis 1.11; Analysis 1.12) we also included data from eleven non‐randomised studies which reported on harm (Podda 1989; Lotterer 1990; Kneppelhout 1992; Peridigoto 1992; Shibata 1992; Ikeda 1996; Poupon 1996; Schonfeld 1997; Van Hoogstraten 1998; Angulo 1999 a; Verma 1999). For details regarding description of these non‐randomised studies see Characteristics of excluded studies. In Lotterer 1990, there were 7 patients out of 12 who experienced adverse events. One patient died, two patients had acute upper gastrointestinal bleeding, one patient developed ascites, one patient had transient diarrhoea, and one patient had transient exacerbation of pruritus (Table 8). In Ikeda 1996, in the colchicine‐ursodeoxycholic acid group, there were 2 patients out of 10 who experienced diarrhoea versus 0 patients out of 12 in the ursodeoxycholic acid group. In Poupon 1996, in the colchicine‐ursodeoxycholic acid group, there were 4 patients out of 37 who experienced an adverse event such as death (2 patients), variceal bleeding (1 patient) and peripheral polyneuropathy (1 patient) versus 2 patients out of 37 in the ursodeoxycholic acid‐placebo group (Table 9). The two former studies may say more about adverse events associated with colchicine than with ursodeoxycholic acid. In Angulo 1999 a, 155 patients with primary biliary cirrhosis were treated with three different doses of ursodeoxycholic acid, there were 21 patients out of 155 who experienced adverse events such as hypertension (2 patients), creatinine elevation (2 patients), thrombocytopenia (3 patients), leukopenia (1 patient), nausea and vomiting (6 patients), diarrhoea (3 patients), fever (1 patient), and rash (3 patients) (Table 10). In Van Hoogstraten 1998, 61 patients with primary biliary cirrhosis were treated with two different doses of ursodeoxycholic acid, there were 2 patients out of 61 who experienced adverse events such as liver failure (1 patient) and diarrhoea (1 patient) (Table 11). In Peridigoto 1992, there were 3 patients who experienced adverse events such as variceal bleeding and ascites and more than one event occurred in some patient (Table 12). In Podda 1989, there were 2 patients out of 30 who experienced pruritus. In Kneppelhout 1992, there were 9 patients out of 17 who experienced adverse events such as liver transplantation, ascites, nausea, increased pruritus, increase in pre‐existent hyperbilirubinaemia, fever, weakness, and more than one event occurred in some patient (Table 13). In Schonfeld 1997, there was one patient out of 15 who experienced severe and progressive fatigue, weight loss, ascites, an increase in serum bilirubin concentration and was liver transplanted. In Shibata 1992, there were 3 patients out of 12 who experienced adverse events such as death, bleeding varices, hepatocellular carcinoma, diarrhoea, gallstones, and more than one event occurred in some patient (Table 14). In Verma 1999, there was one patient out of 24 who experienced severe migraine.
4. Adverse events (Lotterer 1990).
| Adverse event | UDCA* |
| Death | 1/12 |
| Transient exacerbation of pruritus | 1/12 |
| Transient diarrhoea | 2/12 |
| Ascites | 1/12 |
| Acute upper GI bleeding | 2/12 |
* UDCA = ursodeoxycholic acid.
5. Adverse events (Poupon 1996).
| Adverse event | Colchicin‐UDCA* | UDCA‐placebo |
| Variceal bleeding | 1/37 | 2/37 |
| Death | 2/37 | 0/37 |
| Peripheral polyneuropathy | 1/37 | 0/37 |
* UDCA = ursodeoxycholic acid.
6. Adverse events (Angulo 1999 a).
| Adverse event | UDCA* |
| Hypertension | 2/155 |
| Creatinine elevation | 2/155 |
| Thrombocytopenia | 3/155 |
| Leukopenia | 1/155 |
| Nausea and vomiting | 6/155 |
| Diarrhoea | 3/155 |
| Fever | 1/155 |
| Rash | 3/155 |
*UDCA = ursodeoxycholic acid.
7. Adverse events (Van Hoogstraten 1998).
| Adverse event | UDCA* |
| Liver failure | 1/61 |
| Diarrhoea | 1/61 |
*UDCA = ursodeoxycholic acid.
8. Adverse events (Peridigoto 1992).
| Adverse event | UDCA* |
| Variceal bleeding | 3/3 |
| Ascites | 2/3 |
* UDCA = ursodeoxycholic acid.
9. Adverse events (Kneppelhout 1992).
| Adverse event | UDCA* |
| Nausea | 2/17 |
| Increased pruritus | 4/17 |
| Increase in pre‐existent hyperbilirubinaemia | 3/17 |
| Ascites | 1/17 |
| Liver transplantation | 1/17 |
| Fever | 1/17 |
| Weakness | 1/17 |
* UDCA = ursodeoxycholic acid.
10. Adverse events (Shibata 1992).
| Adverse event | Colchicin‐UDCA* |
| Diarrhoea | 1/12 |
| Gallstones | 1/12 |
| Bleeding varices | 1/12 |
| Death | 1/12 |
| Hepatocellular carcinoma | 1/12 |
* UDCA = ursodeoxycholic acid.
Quality of life
None of the trials used specific quality‐of‐life scales. Two trials (Turner 1994; Eriksson 1997) evaluated symptoms using visual analogue scales. None of these showed any significant difference between the ursodeoxycholic acid group and placebo group. However, significantly (P < 0.01) more patients felt better or much better following ursodeoxycholic acid intervention than after placebo in the Eriksson 1997 trial.
Secondary outcomes
Liver transplantation
Fourteen trials provided information on liver transplantation and could be included in the analyses. The included trials reported 78 (5.6%) transplants in 1391 patients (Analysis 1.13). In the ursodeoxycholic acid group, 37 (5.3%) out of 699 patients were transplanted versus 41 (5.9%) out of 692 patients in the control group. Meta‐analyses with both the fixed‐effect model and random‐effects model showed no significant difference in effect of ursodeoxycholic acid on liver transplantation (RR 0.89; 95% CI 0.59 to 1.36, I² = 0%) (Analysis 1.13).
1.13. Analysis.
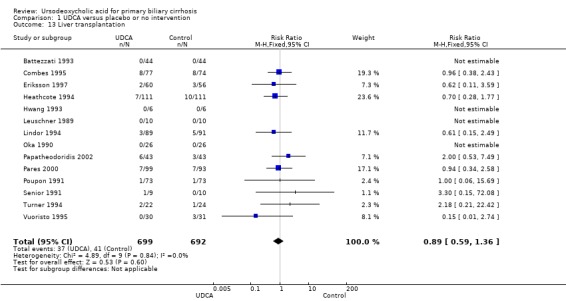
Comparison 1 UDCA versus placebo or no intervention, Outcome 13 Liver transplantation.
Including data from the extended follow‐up for ursodeoxycholic acid→ursodeoxycholic acid versus placebo/'no intervention'→ursodeoxycholic acid (now comprising 65 (9.3%) liver transplantations in 699 patients originally randomised to ursodeoxycholic acid versus 85 (12.3%) liver transplantations in 692 patients originally randomised to placebo/no intervention) demonstrated an RR of 0.76 with 95% CI from 0.57 to 1.03 (Analysis 3.3).
3.3. Analysis.
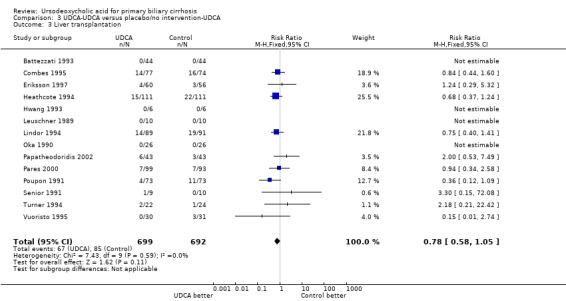
Comparison 3 UDCA‐UDCA versus placebo/no intervention‐UDCA, Outcome 3 Liver transplantation.
Pruritus and fatigue
Ursodeoxycholic acid did not significantly influence neither the number of patients with pruritus (RR 0.96; 95% CI 0.84 to 1.09; I² = 0%; 630 patients, 6 trials) (Analysis 1.14) nor the pruritus score (SMD ‐0.10; 95% CI ‐0.33 to 0.12; I² = 0%; 314 patients, 3 trials) (Analysis 1.15). Trial sequential analysis of these data supports the finding in the meta‐analysis Analysis 1.14 (Figure 11). Fatigue was not significantly improved by ursodeoxycholic acid (RR 0.90; 95% CI 0.81 to 1.00; I² = 62%; 506 patients, 4 trials) (Analysis 1.16).
1.14. Analysis.
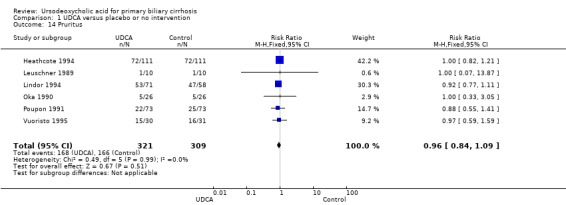
Comparison 1 UDCA versus placebo or no intervention, Outcome 14 Pruritus.
1.15. Analysis.
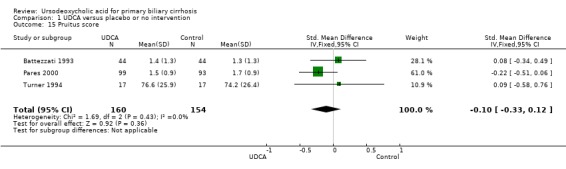
Comparison 1 UDCA versus placebo or no intervention, Outcome 15 Pruitus score.
11.
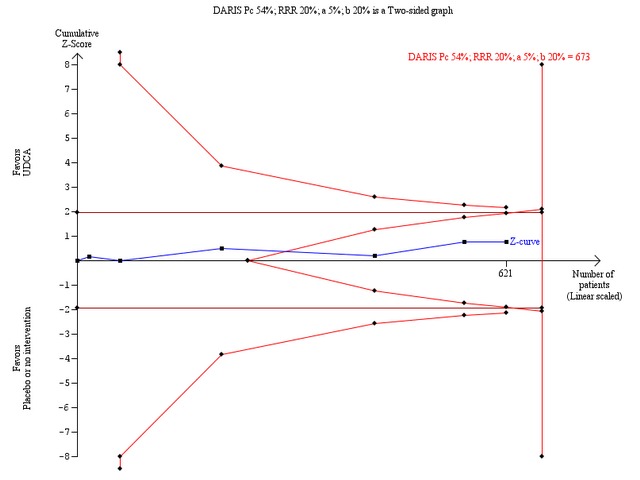
Trial sequential analysis of the random‐effects meta‐analysis of the effect of ursodeoxycholic acid versus placebo or no intervention on pruritus. The trial sequential analysis is performed with an assumed control proportion of pruritus of 54%, an anticipated relative risk reduction (RRR) of 20%, a type 1 error risk of 5% (two‐sided), and a power of 80% (a type 2 error risk of 20%) (b). The heterogeneity‐adjusted required information size (DARIS) to detect or reject a RRR of 20% with a between trial heterogeneity of 0% is estimated to 673 patients. The actually accrued number of patients is 621, which is 92% of the required information size. The blue cumulative Z‐curve does not cross the red trial sequential monitoring boundaries for benefit or harm. However, the boundaries for futility delineated by the trial sequential beta‐spending monitoring boundaries (the red inner wedge boundaries) are crossed. Therefore, there is no evidence to support that ursodeoxycholic acid influences pruritus and a 20% RRR of pruritus can be rejected with the chosen error risks.
1.16. Analysis.
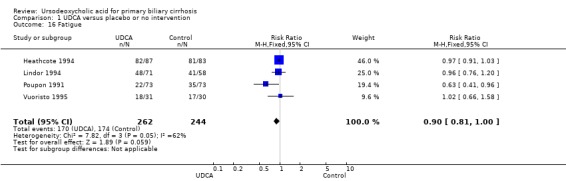
Comparison 1 UDCA versus placebo or no intervention, Outcome 16 Fatigue.
Liver‐related morbidity
In fixed‐effect meta‐analysis, two trials in which the number of patients with jaundice was reported led to a significant effect of ursodeoxycholic acid versus placebo or no intervention (RR 0.35; 95% CI 0.14 to 0.90; I² = 51%; 198 patients, 2 trials). However, in random‐effects meta‐analysis, two trials in which the number of patients with jaundice was reported showed no significant effect of ursodeoxycholic acid versus placebo or no intervention (RR 0.56; 95% CI 0.06 to 4.95; I² = 51%; 198 patients, 2 trials) (Analysis 1.17).
1.17. Analysis.
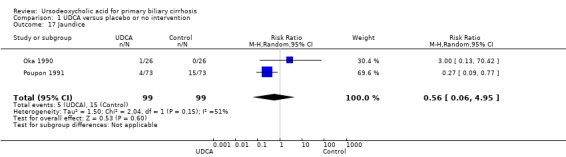
Comparison 1 UDCA versus placebo or no intervention, Outcome 17 Jaundice.
Neither portal pressure (MD 0.60 mmHg; 95% CI ‐2.78 to 3.98; 28 patients, 1 trial) (Analysis 1.18), varices (RR 1.16; 95% CI 0.64 to 2.09; I² = 0%; 341 patients, 3 trials) (Analysis 1.19), bleeding varices (RR 1.05; 95% CI 0.52 to 2.15; I² = 0%; 767 patients, 7 trials) (Analysis 1.20), ascites (RR 0.55; 95% CI 0.24 to 1.26; I² = 0%; 547 patients, 5 trials) (Analysis 1.21) nor hepatic encephalopathy (RR 0.47; 95% CI 0.04 to 5.09; 212 patients, 2 trials) (Analysis 1.22) were significantly affected by ursodeoxycholic acid treatment.
1.18. Analysis.
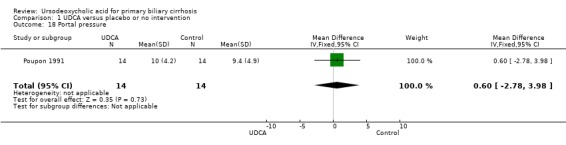
Comparison 1 UDCA versus placebo or no intervention, Outcome 18 Portal pressure.
1.19. Analysis.
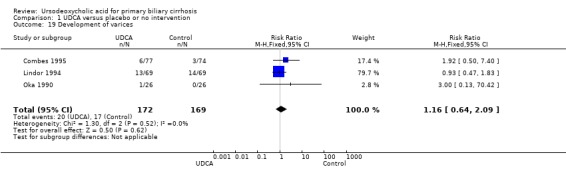
Comparison 1 UDCA versus placebo or no intervention, Outcome 19 Development of varices.
1.20. Analysis.
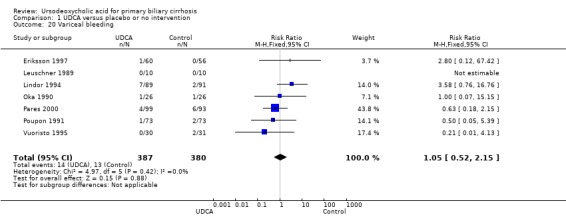
Comparison 1 UDCA versus placebo or no intervention, Outcome 20 Variceal bleeding.
1.21. Analysis.
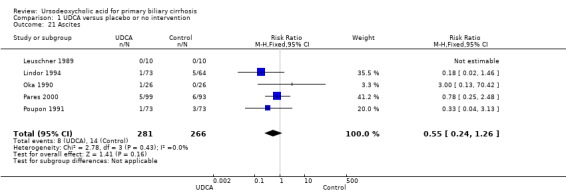
Comparison 1 UDCA versus placebo or no intervention, Outcome 21 Ascites.
1.22. Analysis.
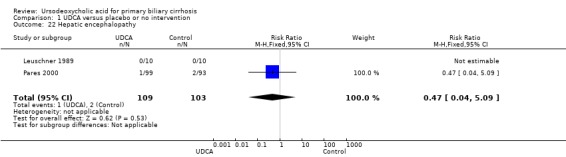
Comparison 1 UDCA versus placebo or no intervention, Outcome 22 Hepatic encephalopathy.
Biochemical markers
Ursodeoxycholic acid significantly decreased serum bilirubin concentration (MD ‐8.69 µmol/l; 95% CI ‐13.90 to ‐3.48; I² = 0%; 881 patients, 9 trials) (Analysis 1.24). Trial sequential analysis of these data supports the finding in the meta‐analysis (Figure 12).
1.24. Analysis.
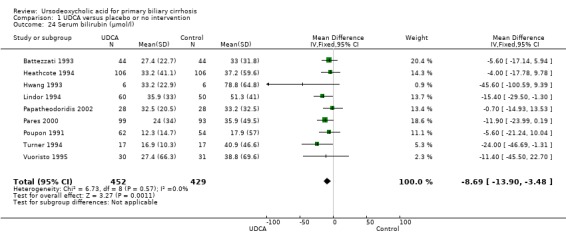
Comparison 1 UDCA versus placebo or no intervention, Outcome 24 Serum bilirubin (µmol/l).
12.
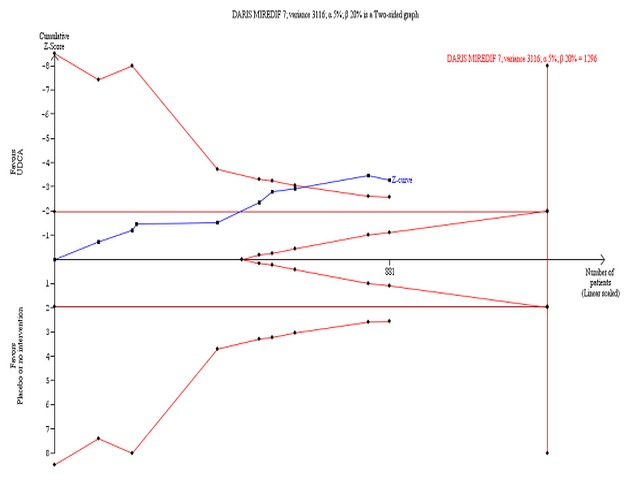
Trial sequential analysis of the cumulative meta‐analysis of the effect of ursodeoxycholic acid versus placebo or no intervention on serum bilirubin concentration in patients with primary biliary cirrhosis. The diversity‐adjusted required information size (DARIS) of 1296 patients is calculated based on a minimal relevant intervention effect (MIREDIF) of 7 µmol/l, a standard deviation of 56 µmol/l (variance 3116), a risk of type I error of 5%, a power of 80% (a type 2 error risk of 20%) (b), and a diversity of 0%. The cumulated Z‐curve (blue curve) crosses the trial sequential monitoring boundary (red curve) implying that there is evidence for a beneficial effect of 7 µmol/l decrease in the serum bilirubin concentration when the cumulative meta‐analysis is adjusted for sparse data and multiple testing on accumulating data.
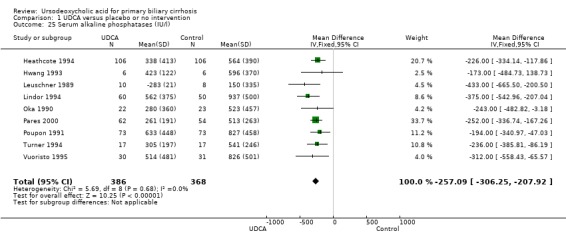
Ursodeoxycholic acid significantly decreased the activity of serum alkaline phosphatases (MD ‐257.09 U/l; 95% CI ‐306.25 to ‐207.92; I² = 0%; 754 patients, 9 trials) (Analysis 1.25). Trial sequential analysis of these data supports the finding in the meta‐analysis (Figure 13).
1.25. Analysis.
Comparison 1 UDCA versus placebo or no intervention, Outcome 25 Serum alkaline phosphatases (IU/l).
13.
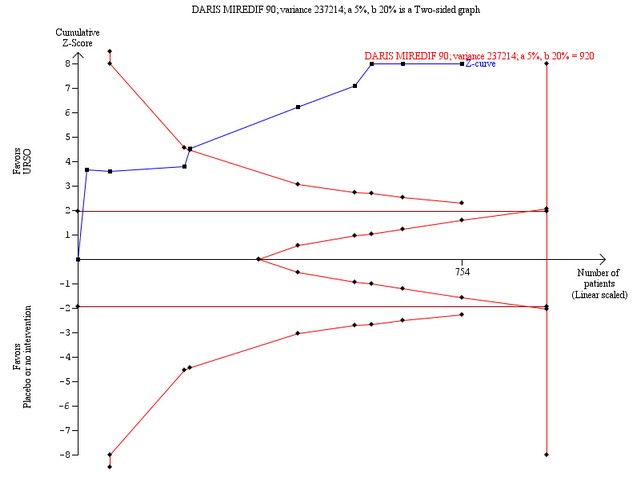
Trial sequential analysis of the cumulative meta‐analysis of the effect of ursodeoxycholic acid versus placebo or no intervention on the activity of serum alkaline phosphatases in patients with primary biliary cirrhosis. The diversity‐adjusted required information size (DARIS) of 920 patients is calculated based on a minimal relevant intervention effect (MIREDIF) of 90 IU/L, a standard deviation of 487 IU/L (variance 237214), a risk of type I error of 5%, a power of 80% (a type 2 error risk of 20%) (b), and a diversity of 0%. The cumulated Z‐curve (blue curve) crosses the trial sequential monitoring boundary (red curve) implying that there is evidence for a beneficial effect of 90 IU/L decrease in the activity of serum alkaline phosphatases when the cumulative meta‐analysis is adjusted for sparse data and multiple testing on accumulating data.
Ursodeoxycholic acid significantly decreased the activity of serum gamma‐glutamyltransferase (MD ‐277.57 U/l; 95% CI ‐337.84 to ‐217.30; I² = 52%; 426 patients, 5 trials) (Analysis 1.26), serum aspartate aminotransferase (MD ‐35.59 U/l; 95% CI ‐42.88 to ‐28.30; I² = 0%; 782 patients, 8 trials) (Analysis 1.27), serum alanine aminotransferase (MD ‐34.68 U/l; 95% CI ‐43.04 to ‐26.33; I² = 32%; 712 patients, 8 trials) (Analysis 1.28), total cholesterol (MD ‐0.78 mmol/l; 95% CI ‐1.04 to ‐0.52; I² = 19%; 712 patients, 9 trials) (Analysis 1.30), and plasma immunoglobulin M concentration (MD ‐1.33 g/l; 95% CI ‐1.81 to ‐0.86; I² = 0%; 704 patients, 7 trials) (Analysis 1.31).
1.26. Analysis.
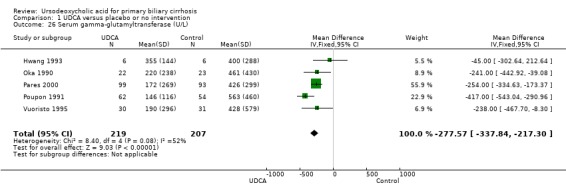
Comparison 1 UDCA versus placebo or no intervention, Outcome 26 Serum gamma‐glutamyltransferase (U/L).
1.27. Analysis.
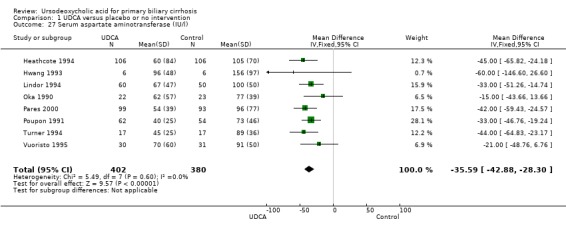
Comparison 1 UDCA versus placebo or no intervention, Outcome 27 Serum aspartate aminotransferase (IU/l).
1.28. Analysis.
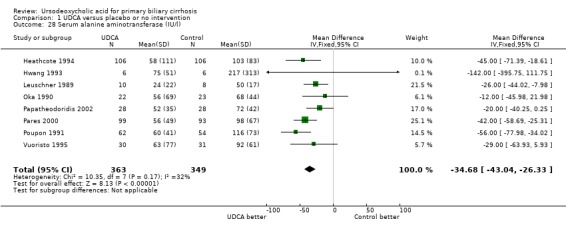
Comparison 1 UDCA versus placebo or no intervention, Outcome 28 Serum alanine aminotransferase (IU/l).
1.30. Analysis.
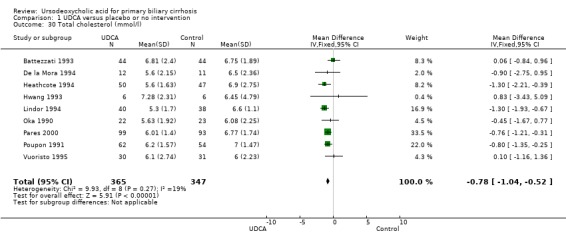
Comparison 1 UDCA versus placebo or no intervention, Outcome 30 Total cholesterol (mmol/l).
1.31. Analysis.
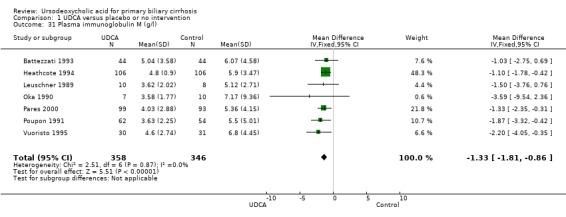
Comparison 1 UDCA versus placebo or no intervention, Outcome 31 Plasma immunoglobulin M (g/l).
Ursodeoxycholic acid had no significant effect on serum albumin concentration (MD 0.34 mmol/l; 95% CI ‐0.45 to 1.13; I² = 0%; 457 patients, 4 trials) (Analysis 1.29) and on prothrombin index (MD 2.05 %; 95% CI ‐0.62 to 4.71; I² = 0%; 308 patients, 2 trials) (Analysis 1.32).
1.29. Analysis.
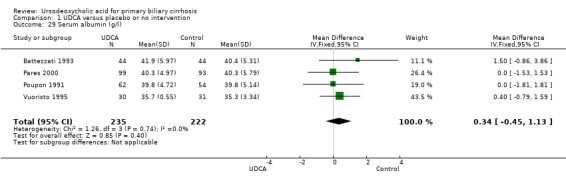
Comparison 1 UDCA versus placebo or no intervention, Outcome 29 Serum albumin (g/l).
1.32. Analysis.
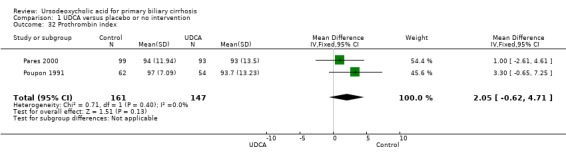
Comparison 1 UDCA versus placebo or no intervention, Outcome 32 Prothrombin index.
Liver histology
Liver biopsies at the end of treatment were performed and reported in seven (Leuschner 1989; Poupon 1991; Lindor 1994; Turner 1994; Combes 1995; Pares 2000; Papatheodoridis 2002) out of 16 trials. Ursodeoxycholic acid had statistically significant effect on histological stage (random, RR 0.62; 95% CI 0.44 to 0.88; I² = 35%; 551 patients, 7 trials) (Analysis 1.33). There was no effect of ursodeoxycholic acid on fibrosis (RR 0.88, 95% CI 0.57 to 1.38; 139 patients, 1 trial) or on florid duct lesions (RR 0.84, 95% CI 0.40 to 1.76; 115 patients, 1 trial). About half of the patients in the Pares 2000 trial observed statistically significant improvements in histological stage, portal inflammation, and piecemeal necrosis in the ursodeoxycholic acid group, but not regarding ductular proliferation or cholestasis. The placebo group had significantly fewer bile ducts per portal tract. Our analyses were based on presented available patient data at the end of treatment.
1.33. Analysis.
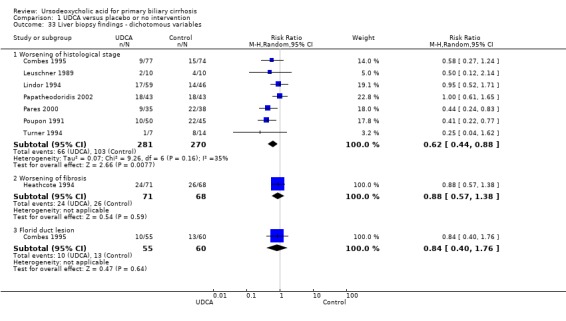
Comparison 1 UDCA versus placebo or no intervention, Outcome 33 Liver biopsy findings ‐ dichotomous variables.
Publication bias and other biases Neither the Egger's nor the Begg's graphs and their corresponding tests on mortality provided evidence for asymmetry (Egger's test, P = 0.47; Begg's test, P = 0.83).
Discussion
Summary of main results
This review included 16 randomised clinical trials assessing the effects of ursodeoxycholic acid versus placebo or 'no intervention' in patients with primary biliary cirrhosis. With the inclusion of updated data from 2007 to January 2012, the present systematic review did not demonstrate any benefit or harm of ursodeoxycholic acid on all‐cause mortality or on all‐cause mortality or liver transplantation. The results from this present review are in agreement with the main findings of Goulis et al meta‐analyses (Goulis 1999) and our previous versions of the present Cochrane review (Gluud 2001b; Gong 2008). New trial data were added to liver biochemistry and clinical symptoms since 2008, and we confirm a reduction in liver biochemistry and jaundice following ursodeoxycholic acid administration. However, these results are based mainly on trials with high risk of bias and there are risks of outcome reporting bias. Due to addition of data on ascites and histology, the result of the effect for ascites changed from significant into insignificant, and the result of the effect for histology changed from insignificant into significant. Ursodeoxycholic acid seems generally well tolerated in patients with primary biliary cirrhosis.
Results on all‐cause mortality and all‐cause mortality or liver transplantation as well as the following secondary outcomes ‐ pruritus, serum bilirubin, and serum alkaline phosphatases ‐ were analysed with trial sequential analysis. According to the results of the trial sequential analyses, there seems to be firm evidence for a beneficial effects of ursodeoxycholic acid on decreasing serum bilirubin concentration and the activity of serum alkaline phosphatases in patients with primary biliary cirrhosis compared with placebo or 'no intervention'. However, these beneficial effects may still be due to systematic errors (bias), as estimated intervention effects were calculated using data from trials assessed as having ’high risk of bias’ except one. Additionally, trial sequential analyses provide us with important information regarding the need for additional trials and the required information size.
The relationship between ursodeoxycholic acid effect and the severity of primary biliary cirrhosis was indicated in the classical meta‐regression (Sharp 1998), suggesting that ursodeoxycholic acid effect on mortality (if any) is more likely to be observed in patients with more severe primary biliary cirrhosis. However, this relationship was not supported by our univariate and multivariate meta‐regression analyses, which included 'severity' as a co‐variate. Therefore, whether the intervention effect of ursodeoxycholic acid (if any) is related to the severity of primary biliary cirrhosis should be investigated further.
We found statistically significant effect of ursodeoxycholic acid on histological stage. However, the observed effect could be due to systematic errors or random errors. There is a theoretical possibility that ursodeoxycholic acid may still delay progression from early stage disease to late stage disease and then ultimately prolong survival. However, the effects of ursodeoxycholic acid should primarily be assessed via patient relevant outcomes.
We found no difference regarding serious adverse events between the intervention groups. Ursodeoxycholic acid was generally well tolerated. We previously observed that ursodeoxycholic acid was associated with non‐serious adverse events, mostly weight gain (Gong 2008). This finding ensued from new data from the Lindor 1994 trial. However, it is presently unclear whether the weight gain should be considered a beneficial or a harmful effect, and it needs to be studied further.
Other non‐serious adverse events included mild gastrointestinal disorders like diarrhoea, nausea, vomiting, etc. However, there was no significant difference regarding non‐serious adverse events between intervention groups.
It has been claimed that ursodeoxycholic acid is a cost‐effective therapy for primary biliary cirrhosis (Pasha 1999). However, this claim rests on the extrapolation of results from two selected randomised clinical trials (Heathcote 1994; Lindor 1994). It is evident that cost‐effectiveness analyses ought to be performed on the basis of all available high‐quality evidence and not just on selected evidence. Considering the annual cost of ursodeoxycholic acid of about $2500 (Pasha 1999) and the findings of the present review, we challenge the conclusion drawn by Pasha et al that ursodeoxycholic acid is cost‐effective for primary biliary cirrhosis.
Quality of the evidence
We conducted this review according to The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (Gluud 2011). The results of our meta‐analyses, however, are only as strong as the primary trials included.
The main limitations in the design and implementation was the lack of clarity of the generation of allocation sequence, concealment of allocation, blinding, and the small number of patients enrolled in the trials. Of the sixteen included trials, six trials (37%) reported adequate allocation sequence generation and allocation concealment; eleven trials (69%) reported adequate blinding; thirteen trials (81%) adequately addressed incomplete data, and reported on clinically relevant and reasonably expected outcomes; and seven trials (44%) appeared to be free of additional risks of bias. Fifteen out of the sixteen trials were considered at high risk of bias and our evidence base is therefore severely limited even when trial sequential analyses did not show risk of random errors.
Potential biases in the review process
In this systematic review, a comprehensive and extensive literature search was performed (Appendix 1), but the searches did not retrieve any additional trials. A potential limitation of our approach may be that we have not specifically searched for trials in the grey literature (written material that is not published commercially) which may have introduced a slight risk of bias into our meta‐analysis (Egger 2003). This bias, however, is unlikely to influence our results in a beneficial way as trials found in grey literature rarely report beneficial effect (Higgins 2011).
Most of the included trials are of a relatively small size, which increases the risk of providing a more unrealistic estimate of the intervention effects due to bias (systematic errors) and chance (random errors). Risk of bias is known to impact the estimated intervention effect, with trials of a high risk of bias tending to overestimate the beneficial intervention effects (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Savovic 2012). We divided the analyses for the primary outcomes into trials with high risk of bias and trials with low risk of bias to examine any influence of bias on the effect estimates of our primary outcomes. Of the sixteen included trials, four trials had low risk of bias regarding five domains (allocation sequence generation, allocation concealment, blinding, handling of incomplete outcome data, and selective outcome reporting), and only one trial had low risk of bias regarding all six domains (including other potential sources of bias).
There was no statistical signs of publication bias and other bias. Small trials have less power, meaning that there is less chance of detecting a small but true effect as statistically significant (Kjaergard 2001). The risk of random error is higher when data come from small information sizes (or 'sample sizes' for individual trials), so information sizes need to be sufficiently large in order to reduce the risk of random error and increase the chance of observing a true intervention effect (Brok 2008; Wetterslev 2008). This review meta‐analysed data (all‐cause mortality or liver transplantation) from 15 trials involving a total of 1447 patients. The median length of trial duration was 24 months. This is not sufficiently long considering that the estimated median survival of a patient with primary biliary cirrhosis is 10 to 15 years (Prince 2002). Therefore, it is difficult to detect a significant difference on mortality based on the trials, most of which are under‐powered. To reduce the risk of random errors we have conducted trial sequential analysis on the primary outcomes and some secondary outcomes which showed significant effect estimates applying both the random‐effects and fixed‐effect models.
Heterogeneity among trials might be due to differences in dose of ursodeoxycholic acid, trial duration, and disease severity of patients at entry. To reflect our concern about heterogeneity, we conducted all analyses using both the random‐effects model and fixed‐effect model. We presented the results with the fixed‐effect model if the results of the two models did not differ. We also considered other important and pre‐defined trial‐level covariates, including trial risk of bias, severity of primary biliary cirrhosis, ursodeoxycholic acid dose, and trial duration. The random‐effects model meta‐regression showed that none of examined covariates were significantly associated with the estimated intervention effect on all‐cause mortality or all‐cause mortality or liver transplantation.
Agreements and disagreements with other studies or reviews
In consistency with previous meta‐analyses and reviews (Goulis 1999; Gluud 2001b; Gong 2008), this updated systematic review did not demonstrate any benefit of ursodeoxycholic acid on all‐cause mortality, and all‐cause mortality or liver transplantation in patients with primary biliary cirrhosis. On the other hand, ursodeoxycholic acid seemed to improve biochemical outcomes. This seems to place clinicians and researchers in a dilemma: if therapeutic decisions are based on clinical outcomes (e.g., mortality), there is insufficient evidence to support the use of ursodeoxycholic acid in primary biliary cirrhosis, but if based on non‐validated 'surrogate' outcomes (e.g., serum bilirubin level or serum alkaline phosphatases), there is evidence favouring the ursodeoxycholic acid interventions for the disease (Gluud 2007). This dilemma was reflected in a survey regarding the use of ursodeoxycholic acid for primary biliary cirrhosis among Danish doctors (Kürstein 2005), who had very different answers to the question why they prescribed ursodeoxycholic acid for primary biliary cirrhosis patients. Sixteen per cent of the doctors thought ursodeoxycholic acid reduced mortality, twenty‐seven per cent thought ursodeoxycholic acid reduced morbidity, and twenty‐three per cent thought it benefited 'surrogate' outcomes (Kürstein 2005). We believe that clinical practice should be based on results from randomised trials using clinically and patient relevant outcomes.
This systematic review did not demonstrate a benefit of ursodeoxycholic acid on our predefined primary outcomes: all‐cause mortality and all‐cause mortality or liver transplantation, neither in the period during which patients were treated with ursodeoxycholic acid or placebo/no intervention nor during the later period in which all the patients were treated with open label ursodeoxycholic acid. This observation is in contrast to some previous attempts to aggregate data from studies assessing ursodeoxycholic acid interventions for primary biliary cirrhosis (Simko 1994; Poupon 1997; Poupon 2000). However, Simko et al included non‐randomised studies in their meta‐analysis that are more liable to bias, that is systematic overestimation of benefit (Simko 1994). Poupon et al only included three and five out of the 16 randomised clinical trials in their meta‐analyses, respectively (Poupon 1997; Poupon 2000). Such meta‐analyses largely run the risk of trial selection bias (Gluud 2001a).
The previous Lancet meta‐analysis by Goulis 1999 and our first Cochrane systematic review (Gluud 2001b) were mainly criticised for including many trials of only two‐year duration and with very heterogeneous lengths of follow‐up (Talwalker 2003; Kaplan 2005). Furthermore, updated evidence from randomised clinical trials and analyses on longer follow‐up data from our previous review (Gong 2008) did not seem to support long‐term ursodeoxycholic acid treatment for primary biliary cirrhosis. The main finding in our present updated review does not seem to support long‐term ursodeoxycholic acid intervention, which was suggested in observational studies (Rust 2005; Pares 2006). Thus, the results suggest no benefit of ursodeoxycholic acid on mortality.
The Mayo Risk Score Model has identified several prognostic biomarkers for primary biliary cirrhosis, e.g., serum bilirubin. These biomarkers may respond to ursodeoxycholic acid and may be predictive of survival (Dickson 1989). But they do not necessarily predict clinical benefit of the intervention in question because 'a perfect correlation does not a surrogate make' (Baker 2003). In the absence of validated surrogate outcomes in ursodeoxycholic acid for primary biliary cirrhosis, confirmatory trials assessing the ursodeoxycholic acid effect should only be based on clinical outcomes, e.g., mortality. We believe that evaluation based on such clinical outcomes‐based evaluation will benefit patients in the long run (Gluud 2007).
We also realise that the challenge of performing a new trial on intervention for primary biliary cirrhosis is high. The estimated median survival of primary biliary cirrhosis is 10 to 15 years. To spend 15 years planning and carrying out a trial for each new potential treatment of primary biliary cirrhosis would consume many patients' lifetimes, not to mention the expense and difficulty of retaining patients in such a long trial (Mayo 2005). Nevertheless, there are at least an estimated one million patients with primary biliary cirrhosis world‐wide. Therefore, it is possible to conduct large trials with appropriate statistical power if international groups of primary biliary cirrhosis investigators collaborate. Such large trials do not need to be conducted for more than two to four years.
Authors' conclusions
Implications for practice.
This updated review confirms and extends previous observations showing no benefit of ursodeoxycholic acid on all‐cause mortality and on all‐cause mortality or liver transplantation. Although based on a small number of trials with risk of bias, ursodeoxycholic acid seems to improve liver biochemical variables, including serum bilirubin concentration, and liver histology. This review does not support or refute short‐term or long‐term use of ursodeoxycholic acid.
Implications for research.
It is less likely to find any benefit of ursodeoxycholic acid on patient's survival in a new trial with the average size of the trials included into this updated review. Integration of international groups of investigation for primary biliary cirrhosis will make large trial sizes feasible. Randomised clinical trials which assess ursodeoxycholic acid versus placebo in primary biliary cirrhosis with larger sample sizes and minimised risk of bias are needed. Trials should mainly be based on clinical outcomes, e.g., mortality. Multi‐centre trials and multinational trials would be necessary to secure patient recruitment as primary biliary cirrhosis is a relatively rare disease. Such trials ought to be reported according to the CONSORT guidelines (www.consort‐statement.org).
What's new
| Date | Event | Description |
|---|---|---|
| 10 February 2012 | New citation required but conclusions have not changed | The searches were updated. No new trials were identified. However, all relevant to the review additional information found in recent publications was added. |
| 6 February 2012 | New search has been performed | The review was thoroughly updated following the Guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Trial sequential analysis (TSA program 2011) as well as Summary of Findings tables were performed. A detailed list of what is new is under "Differences between previous published version of the review and this review". |
| 3 January 2012 | New search has been performed | The authors' team is renewed; all but one are new authors. |
Notes
This is an updated systematic review to the Gong et al (Gong 2008).
Acknowledgements
We thank the patients who entered the trials and the investigators who conducted them. We also wish to express special thanks to Dr. Albert Pares who kindly responded to our requests for further information on the trial he was involved in. We thank especially Dimitrinka Nikolova, The Cochrane Hepato‐Biliary Group, for expert assistance during the preparation of this review and excellent collaboration. We are very grateful to Sarah Louise Klingenberg, The Cochrane Hepato‐Biliary Group, for her contribution to this review. We thank Janus Jakobsen, The Cochrane Hepato‐Biliary group, for many important linguistic improvements.
Peer reviewer: Janus Jakobsen, Denmark. Contact editor: Rosa Simonetti, Italy.
Appendices
Appendix 1. Search strategies
| Database | Searching period | Search term |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | January 2012. | ('primary biliary cirrhosis' OR PBC) AND (urso* OR ursodeoxycholic acid OR actigall OR 'deoxycholic acid' OR 'cholit ursan' OR cholofalk OR delursan OR destolit OR urdox OR arsacol OR 'de ursil' OR deursil OR paptarom) |
| Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library | Issue 1, 2012. | #1 MeSH descriptor Liver Cirrhosis, Biliaryexplode all trees #2 primary biliary cirrhosis or PBC #3 (#1 OR #2) #4 MeSH descriptor Ursodeoxycholic Acidexplode all trees #5 urso* or ursodeoxycholic acid or actigall or deoxycholic acid or cholit ursan or cholofalk or delursan or destolit or urdox or arsacol or de ursil or deursil or paptarom #6 (#4 OR #5) #7 (#3 AND #6) |
| MEDLINE (Ovid SP) | January 1946 to January 2012. | 1. exp Liver Cirrhosis, Biliary/ 2. (primary biliary cirrhosis or PBC).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 3. 1 or 2 4. exp Ursodeoxycholic Acid/ 5. (urso* or ursodeoxycholic acid or actigall or deoxycholic acid or cholit ursan or cholofalk or delursan or destolit or urdox or arsacol or de ursil or deursil or paptarom).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 6. 4 or 5 7. 3 and 6 8. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 9. 7 and 8 |
| EMBASE (OvidSP) | 1974 to January 2012. | 1. exp primary biliary cirrhosis/ 2. (primary biliary cirrhosis or PBC).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 3. 1 or 2 4. exp ursodeoxycholic acid/ 5. (urso* or ursodeoxycholic acid or actigall or deoxycholic acid or cholit ursan or cholofalk or delursan or destolit or urdox or arsacol or de ursil or deursil or paptarom).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 6. 4 or 5 7. 3 and 6 8. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 9. 7 and 8 |
| LILACS | 1982 to January 2012. | ursodeoxycholic OR UDCA [Words] and primary biliary cirrhosis OR PBC [Words] |
| Science Citation Index Expanded (http://apps.webofknowledge.com) | 1927 to January 2012. | # 5 495 #4 AND #3 # 4 996,355 TS=(random* or blind* or placebo* or meta‐analysis) # 3 1,647 #2 AND #1 # 2 8,707 TS=(urso* or ursodeoxycholic acid or actigall or deoxycholic acid or cholit ursan or cholofalk or delursan or destolit or urdox or arsacol or de ursil or deursil or paptarom) # 1 11,286 TS=(primary biliary cirrhosis or PBC) |
Data and analyses
Comparison 1. UDCA versus placebo or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 14 | 1391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.67, 1.42] |
| 2 All‐cause mortality stratified after risk of bias | 14 | 1391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.67, 1.42] |
| 2.1 Low risk of bias | 4 | 682 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.51, 1.72] |
| 2.2 High risk of bias | 10 | 709 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.63, 1.60] |
| 3 All‐cause mortality stratified after risk of bias including industry involvement | 14 | 1391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.67, 1.42] |
| 3.1 Low risk of bias | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.18, 1.93] |
| 3.2 High risk of bias | 13 | 1211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.70, 1.54] |
| 4 All‐cause mortality stratified after trial duration | 14 | 1391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.67, 1.42] |
| 4.1 Long duration (≥2 years) | 9 | 1200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.65, 1.39] |
| 4.2 Short duration (<2 years) | 5 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.3 [0.15, 72.08] |
| 5 All‐cause mortality stratified after dose of ursodeoxycholic acid | 14 | 1391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.67, 1.42] |
| 5.1 UDCA dose (7.7‐10 mg/kg/day) | 4 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.49] |
| 5.2 UDCA dose (10‐15 mg/kg/day) | 10 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.68, 1.45] |
| 6 All‐cause mortality or liver transplantation | 15 | 1419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.74, 1.25] |
| 7 All‐cause mortality or liver transplantation stratified after risk of bias | 15 | 1419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.74, 1.25] |
| 7.1 Low risk of bias | 4 | 682 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.55, 1.28] |
| 7.2 High risk of bias | 11 | 737 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.75, 1.49] |
| 8 All‐cause mortality or liver transplantation stratified after risk of bias including industry involvement | 15 | 1419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.74, 1.25] |
| 8.1 Low risk of bias | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.25, 1.45] |
| 8.2 High risk of bias | 14 | 1239 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.77, 1.34] |
| 9 All‐cause mortality or liver transplantation stratified after trial duration | 15 | 1419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.74, 1.25] |
| 9.1 Long duration (≥2 years) | 9 | 1200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.69, 1.19] |
| 9.2 Short duration (<2 years) | 6 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.70, 10.16] |
| 10 All‐cause mortality or liver transplantation stratified after dose of ursodeoxycholic acid | 15 | 1419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.74, 1.25] |
| 10.1 UDCA dose (7.7‐10 mg/kg/day) | 4 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.09, 2.45] |
| 10.2 UDCA dose (10‐15 mg/kg/day) | 11 | 1151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.75, 1.28] |
| 11 Serious adverse events | 14 | 1382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.68, 1.12] |
| 12 Non‐serious adverse events | 12 | 1277 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.83, 2.56] |
| 13 Liver transplantation | 14 | 1391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.59, 1.36] |
| 14 Pruritus | 6 | 630 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.84, 1.09] |
| 15 Pruitus score | 3 | 314 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.33, 0.12] |
| 16 Fatigue | 4 | 506 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.81, 1.00] |
| 17 Jaundice | 2 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.06, 4.95] |
| 18 Portal pressure | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐2.78, 3.98] |
| 19 Development of varices | 3 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.64, 2.09] |
| 20 Variceal bleeding | 7 | 767 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.52, 2.15] |
| 21 Ascites | 5 | 547 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.24, 1.26] |
| 22 Hepatic encephalopathy | 2 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.04, 5.09] |
| 23 Variceal bleeding, ascites, and/or encephalopathy | 3 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.91, 2.01] |
| 24 Serum bilirubin (µmol/l) | 9 | 881 | Mean Difference (IV, Fixed, 95% CI) | ‐8.69 [‐13.90, ‐3.48] |
| 25 Serum alkaline phosphatases (IU/l) | 9 | 754 | Mean Difference (IV, Fixed, 95% CI) | ‐257.09 [‐306.25, ‐207.92] |
| 26 Serum gamma‐glutamyltransferase (U/L) | 5 | 426 | Mean Difference (IV, Fixed, 95% CI) | ‐277.57 [‐337.84, ‐217.30] |
| 27 Serum aspartate aminotransferase (IU/l) | 8 | 782 | Mean Difference (IV, Fixed, 95% CI) | ‐35.59 [‐42.88, ‐28.30] |
| 28 Serum alanine aminotransferase (IU/l) | 8 | 712 | Mean Difference (IV, Fixed, 95% CI) | ‐34.68 [‐43.04, ‐26.33] |
| 29 Serum albumin (g/l) | 4 | 457 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [‐0.45, 1.13] |
| 30 Total cholesterol (mmol/l) | 9 | 712 | Mean Difference (IV, Fixed, 95% CI) | ‐0.78 [‐1.04, ‐0.52] |
| 31 Plasma immunoglobulin M (g/l) | 7 | 704 | Mean Difference (IV, Fixed, 95% CI) | ‐1.33 [‐1.81, ‐0.86] |
| 32 Prothrombin index | 2 | 308 | Mean Difference (IV, Fixed, 95% CI) | 2.05 [‐0.62, 4.71] |
| 33 Liver biopsy findings ‐ dichotomous variables | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 33.1 Worsening of histological stage | 7 | 551 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.44, 0.88] |
| 33.2 Worsening of fibrosis | 1 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.57, 1.38] |
| 33.3 Florid duct lesion | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.40, 1.76] |
| 34 Liver biopsy findings ‐ continuous variables | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 34.1 Histological stage | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.91, ‐0.17] |
| 34.2 Portal inflammation | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.61, 0.15] |
| 34.3 Piecemeal necrosis | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐0.98, ‐0.14] |
| 34.4 Lobular necrosis | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.66, 0.06] |
| 34.5 Ductular proliferation | 1 | 489 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.46, ‐0.00] |
| 34.6 Cholestasis | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.28, 0.12] |
| 35 Liver biopsy findings ‐ continuous variables | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 35.1 Bile duct/portal tract | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [0.10, 0.36] |
1.23. Analysis.
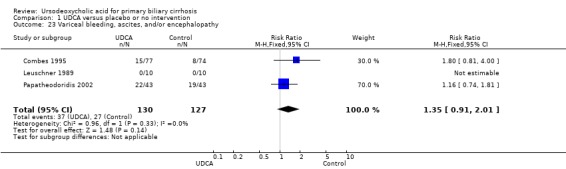
Comparison 1 UDCA versus placebo or no intervention, Outcome 23 Variceal bleeding, ascites, and/or encephalopathy.
1.34. Analysis.
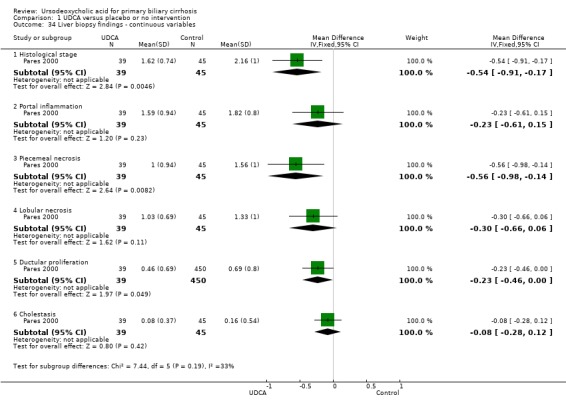
Comparison 1 UDCA versus placebo or no intervention, Outcome 34 Liver biopsy findings ‐ continuous variables.
1.35. Analysis.
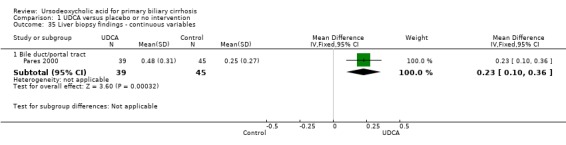
Comparison 1 UDCA versus placebo or no intervention, Outcome 35 Liver biopsy findings ‐ continuous variables.
Comparison 2. Influence of missing data ‐ UDCA versus placebo or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality ‐ completed patient's course plus case scenarios | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Completed patient's course analysis | 14 | 1247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.64, 1.34] |
| 1.2 Extreme case scenario favouring UDCA | 14 | 1391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.26, 0.48] |
| 1.3 Extreme case scenario favouring control | 14 | 1391 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [1.57, 2.97] |
| 2 Mortality or liver transplantation ‐ completed patient's course plus case scenarios | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Completed patient's course analysis | 15 | 1275 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.68, 1.31] |
| 2.2 Extreme case scenario favouring UDCA | 15 | 1419 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.30, 0.80] |
| 2.3 Extreme case scenario favouring control | 15 | 1419 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [1.21, 2.10] |
Comparison 3. UDCA‐UDCA versus placebo/no intervention‐UDCA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 14 | 1391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.73, 1.30] |
| 2 Mortality or liver transplantation | 15 | 1419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.73, 1.06] |
| 3 Liver transplantation | 14 | 1391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.58, 1.05] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Battezzati 1993.
| Methods | Multicenter double‐blind, placebo controlled randomised clinical trial with parallel group design (two interventions groups).
Trial duration 1 year (six months treatment and six months follow‐up). Follow‐up: 5 patients receiving ursodeoxycholic acid and 1 placebo dropped out. |
|
| Participants | Country: Italy.
Number of patients randomised: 88, mean age 54.5 years (88.5% females), histological stage IV 49%.
Inclusion criteria: Primary biliary cirrhosis (PBC) defined as: ‐ positive AMA ≥ 1:40 and liver biopsy compatible with PBC. If one of these were missing, patients could enter provided they had three of the following: ‐ serum alkaline phosphatase > 2.0 times upper normal limit; ‐ immunoglobulin M ≥ 280 mg/l; ‐ pruritus; ‐ serum bilirubin > 2 mg/l; ‐ a positive Schyrimer's test plus absence of extrahepatic obstruction. Exclusion criteria: ‐ serum bilirubin levels > 10 mg/dl; ‐ ascites; ‐ previous episodes of variceal bleeding or encephalopathy; ‐ evidence of malignant conditions; ‐ alcohol abuse. |
|
| Interventions | Patients were randomly assigned to receive: Intervention group 1: ursodeoxycholic acid 500 mg daily in two dived doses at mealtime ( ˜8.7 mg/kg/day; range 5.4‐11.6 mg/kg/day), n = 44; Intervention group 2: placebo, n = 44. No patient was taking any medication known to be hepatotoxic nor had been treated with corticosteroids, immunosuppressant agents, colchicine, penicillamine or ursodeoxycholic acid in the previous six months. |
|
| Outcomes | Symptoms. Liver biochemistry. Serum bile acids. Serum cholesterol. | |
| Notes | Patients switched onto ursodeoxycholic acid at the end of the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using computer random number generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central pharmacy. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It was reported that the trial was double‐blinded, that placebo was 'identical in appearance', and outcome assessment was performed centrally. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Other bias | Unclear risk | It was reported that ursodeoxycholic acid and placebo were obtained through the courtesy of ABC Farmaceutici, Torino, Italy. |
Combes 1995.
| Methods | Multicenter double‐blind, placebo controlled randomised clinical trial with parallel group design (two interventions groups).
Trial duration 2 years. Follow‐up: 2 patients from the ursodeoxycholic acid and 3 patients from the placebo groups withdrew from the trial during the placebo controlled period (0 to 2 year). |
|
| Participants | Country: USA
Number of patients randomised: 151, from six centres, mean age 49.2 years (89% females), histological stage I‐II 32.5%, III‐IV 67.5%.
Inclusion criteria: ‐ cholestatic liver disease for at least six months; ‐ serum alkaline phosphatase > 1.5 times upper normal limit; ‐ positive AMA; ‐ no biliary obstruction; ‐ liver biopsy compatible with PBC. Exclusion criteria: ‐ PBC treatment during the last three months; ‐ recurrent bleeds from varices; ‐ spontaneous encephalopathy; ‐ diuretic‐resistant ascites; ‐ serum bilirubin ≥ 20 mg/dl; ‐ pregnancy; ‐ age < 19 years; ‐ other cause of liver disease. |
|
| Interventions | Patients were randomly assigned to receive: Intervention group 1: ursodeoxycholic acid 10 to 12 mg/kg/day once at bedtime (Ciba‐Geigy Corporation), n = 77; Intervention group 2: placebo (2 years) and open‐label ursodeoxycholic acid (4 years), n = 74. |
|
| Outcomes | Mortality free of liver transplantation. Liver transplantation. Symptoms. Liver biochemistry. Liver histology. ursodeoxycholic acid enrichment in bile. | |
| Notes | Three patients randomised to receive placebo had high bile‐ursodeoxycholic acid concentrations, suggesting ursodeoxycholic acid intake. All patients were offered open label ursodeoxycholic acid following completion of the first 2‐year of the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised, but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double‐blind, placebo described as 'comparable‐appearing' and it was reported that 'coded medications were provided'. All investigators remained blinded throughout the trial to the treatment allocation for each patient. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Other bias | Low risk | The trial appears to be free of information that could put it at risk of bias. |
De la Mora 1994.
| Methods | Randomised trial. Follow‐up: information not provided. |
|
| Participants | Patients with PBC (n = 28) from one centre in Mexico. | |
| Interventions | Experimental: ursodeoxycholic acid (details were not given). Control: placebo. |
|
| Outcomes | Serum cholesterol. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised, but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | 'Placebo' employed, but it is not known if it was indeed double blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated. |
| Selective reporting (reporting bias) | Unclear risk | Not all pre‐defined, or clinically relevant and reasonably expected outcomes are reported on or are not reported fully, or it is unclear whether data on these outcomes were recorded or not. |
| Other bias | Unclear risk | The trial may or may not be free of information that could put it at risk of bias. |
Eriksson 1997.
| Methods | Multicenter double‐blind, placebo controlled randomised clinical trial with parallel group design (two interventions groups).
Trial duration 2 years. Follow‐up: 8 patients from the ursodeoxycholic acid and 7 patients from the placebo withdrew. Patients were stratified into symptomatic and asymptomatic. |
|
| Participants | Country: Sweden.
Number of patients randomised: 116, from six centres in Sweeden, mean age 57 years (85.5% females).
Inclusion criteria: PBC defined as chronic cholestatic liver disease of more than six months duration with histology typical of or compatible with PBC plus at least two of the following: ‐ positive anti‐mitochondrial antibodies; ‐ alkaline phosphatases > 1.5 times the upper reference value; ‐ IgM > 1.5 times the upper reference value during the year preceding the entry into the trial. Exclusion criteria: ‐ patients with severe end‐stage liver disease; ‐ diuretic‐resistant ascites; ‐ repeated variceal bleeding in spite of sclerosing treatment; ‐ patients waiting for liver transplantation; ‐ pregnancy; ‐ alcohol or drug abuse. |
|
| Interventions | Patients were randomly assigned to receive: Intervention group 1: 500 mg ursodeoxycholic acid (˜7.7 mg/kg/day) as two capsules in the evening, n = 60; Intervention group 2: placebo, n = 56. |
|
| Outcomes | Mortality. Liver transplantation. Symptoms ‐ pruritus, fatigue, ascites, jaundice. Liver biochemistry and bile acids. Histology ‐ portal inflammation, spill‐over, interface hepatitis, bile duct proliferation, portal fibrosis. Quality of life. | |
| Notes | At 24 months, 32 of 49 patients allocated to placebo and still remaining in the trial were switched to ursodeoxycholic acid and 42 of 52 patients allocated to ursodeoxycholic acid and still remaining in the trial continued with ursodeoxycholic acid. Anti‐hepatitis C virus tests not performed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using a randomisation list which was produced for every clinic. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as 'double‐blind', and placebo looked identical to ursodeoxycholic acid, but details on taste and smell not given. However outcome assessment was blinded and the possible non‐blinding of others unlikely to introduce bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Other bias | Low risk | Trial appears to be free of information that could put it at risk of bias. |
Goddard 1994.
| Methods | Double‐blind, placebo controlled randomised clinical trial with parallel group design (three interventions groups and one control group). Mean follow‐up: 15 months (range: 0 to 30 months). |
|
| Participants | Country: UK.
Number of patients randomised: 57, mean age and sex ratio not provided.
Inclusion criteria:patients with PBC.
Exclusion criteria: none listed. Diagnostic criteria (data being sought). |
|
| Interventions | Patients were randomly assigned to receive: Intervention group 1: ursodeoxycholic acid 10mg/kg/day. Intervention group 2: colchicine 1 mg/day. Intervention group 3: ursodeoxycholic acid plus colchicine. Control: placebo. |
|
| Outcomes | Mortality (being sought). Liver transplantation (being sought). Liver biochemistry. | |
| Notes | No exact data on number of patients randomised to each arm. Data on mortality and liver transplantation are not given separately. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised, but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | 'Placebo' employed, but it is not known if it was indeed double blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Treatment failures were reported but the exact numbers and reasons for dropouts and withdrawals were not described in all intervention groups. |
| Selective reporting (reporting bias) | Unclear risk | One or more clinically relevant and reasonably expected outcomes were not reported fully, or it is unclear whether data on these outcomes were recorded or not. |
| Other bias | Unclear risk | The trial may or may not be free of information that could put it at risk of bias. |
Heathcote 1994.
| Methods | Multicenter double‐blind, placebo controlled randomised clinical trial with parallel group design (two interventions groups).
Trial duration 2 years. Follow‐up: 13 patients receiving ursodeoxycholic acid and 19 placebo withdrew. |
|
| Participants | Country: Canada. Number of patients randomised: of 408 patients assessed, 222 patients were randomised (1:1) during a 26 months period, mean age 56.3 years (93% females), histological stage I 18.5%, II 27%, III 29%, IV 25.5%. Inclusion criteria: ‐ positive AMA; ‐ serum alkaline phosphatase > 1.0 times upper normal limit; ‐ liver biopsy compatible with PBC; ‐ age > 18 years. Exclusion criteria: ‐ patients on liver transplant list; ‐ patients needed to take enzyme‐inducing drugs; ‐ pregnancy; ‐ severe coexisting condition that was likely to affect survival within five years of trial entry. |
|
| Interventions | Patients were randomly assigned to receive: Intervention group 1: ursodeoxycholic acid 14mg/kg/day swallowed with the evening meal, n = 111; Intervention group 2: placebo, n = 111. |
|
| Outcomes | Mortality. Liver transplantation. Symptoms ‐ pruritus, fatigue. Liver biochemistry and bile acids. Histology. | |
| Notes | Patients offered ursodeoxycholic acid at the end of the trial for 6 to 24 months. Data for serum cholesterol were extracted from Heathcote 1993 (Heathcote 1994). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The method of sequence generation was generated using consecutive identification numbers. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled separately at each centre by the trial pharmacist stratified for symptomatic/asymptomatic. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double‐blind, and the placebo tablets were identical and 'equally bitter tasting', this was confirmed by the research coordinator. Also, outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Other bias | Unclear risk | It was reported that trial medications were kindly provided by Interfalk and Jouveinal Inc., Canada. |
Hwang 1993.
| Methods | Double‐blind, placebo controlled randomised clinical trial with cross‐over group design (two interventions groups).
Trial duration: 3 months. Follow‐up: no patients withdrew. |
|
| Participants | Country: China.
Number of patients randomised: 12, mean age 58 years (100% females). Inclusion criteria: ‐ elevated serum alkaline phosphatase and gamma‐glutamyl transferase with lack of large bile duct abnormalities; ‐ positive AMA with elevated immunoglobulin M, G or A; ‐ liver biopsy compatible with PBC. Exclusion criteria: ‐ previous PBC treatment. |
|
| Interventions | Patients were randomly assigned to receive: Intervention group 1: ursodeoxycholic acid 600 mg/day. Intervention group 2: placebo. |
|
| Outcomes | Mortality. Symptoms. Liver biochemistry. | |
| Notes | All patients switched to ursodeoxycholic acid on completion of the six months cross‐over trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised, but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It was reported that placebo was 'identical tablet form containing starch'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It was specified that there were no dropouts or withdrawals, and that all 12 patients completed a six month course of treatment. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes are reported. |
| Other bias | Low risk | The trial appears to be free of information that could put it at risk of bias. |
Leuschner 1989.
| Methods | Double‐blind, placebo controlled randomised clinical trial with parallel group design (two interventions groups). Trial duration: 9 months. Follow‐up: 2 patients from placebo arm left the trial. |
|
| Participants | Country: Germany. Number of patients randomised: 20, mean age not provided (90% females). Inclusion criteria: PBC defined as at least three of the following: ‐ alkaline phosphatase > 1.7 times upper normal limit; ‐ gamma‐glutamyl transferase > 5.0 times upper normal limit; ‐ immunoglobulin M > 2.0 times upper normal limit; ‐ positive AMA plus no obstruction of the extrahepatic biliary tract. Exclusion criteria: ‐ oesophageal varices; ‐ ascites; ‐ pancreatitis; ‐ cardiac failure or renal failure; ‐ pregnancy; ‐ age < 30 years; ‐ any previous PBC treatment within the four weeks; ‐ alcohol or drug abuse. |
|
| Interventions | Patients were randomly assigned to receive: Intervention group 1: ursodeoxycholic acid 10 mg/kg/day, divided into two doses, n = 10. Intervention group 2: placebo, n = 10. |
|
| Outcomes | Outcome measure(s): ‐ mortality; ‐ symptoms; ‐ liver biochemistry; ‐ liver histology. | |
| Notes | Two patients from the placebo arm left the trial for reasons unrelated to the trial and are not considered in the analysis of the results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised, but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It was reported that placebo was 'identical tablet'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes are reported. |
| Other bias | Low risk | The trial appears to be free of information that could put it at risk of bias. |
Lindor 1994.
| Methods | Multicenter double‐blind, placebo controlled randomised clinical trial with parallel group design (two interventions groups). Trial duration: 4 years. Follow‐up: five voluntary withdrawals in ursodeoxycholic acid arm and 13 voluntary withdrawals in the placebo arm. |
|
| Participants | Country: USA. Number of patients randomised: 180, enrolled from four USA centres, mean age 53 years (89% females). However, 162 patients (90%) came from one centre. Inclusion criteria: PBC defined as: ‐ chronic cholestatic liver disease for at least six months; ‐ serum alkaline phosphatase level > 1.5 times upper normal limit; ‐ antimitochondrial antibody positivity; ‐ absence of biliary obstruction; ‐ liver biopsy compatible with PBC. Exclusion criteria: ‐ previous PBC treatment in preceding 3 months; ‐ anticipated need for liver transplantation within one year; ‐ recurrent variceal haemorrhage; ‐ spontaneous encephalopathy, or diuretic resistant ascites; ‐ pregnancy; ‐ age less than 18 or more than 70 years; ‐ other co‐existent liver disease. |
|
| Interventions | Patients were randomly assigned to receive: Intervention group 1: ursodeoxycholic acid in the form of 250 mg tablets at a dose of 13 to 15mg/kg/day in four divided doses, n = 89; Intervention group 2: placebo, n = 91. |
|
| Outcomes | Outcome measure(s): ‐ mortality; ‐ liver transplantation; ‐ symptoms; ‐ autoimmune conditions; ‐ liver biochemistry; ‐ liver histology; ‐ adverse events. | |
| Notes | Patients originally receiving placebo switched to ursodeoxycholic acid after four years and were followed for an additional eight years. Data for the following outcomes were extracted from (Lindor 1994): ‐ development of varices (Angulo 1999); ‐ bleeding varices (Lindor 1997); ‐ ascites (Lindor 1997); ‐ cholesterol (Balan 1994). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed separately for each strata using 'a blocked, randomised assignment schedule'. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled so that intervention allocations could not have been foreseen in advance of, or during enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Other bias | Low risk | The trial appears to be free of other information that could put it at risk of bias. |
Oka 1990.
| Methods | Multicenter double‐blind, placebo controlled randomised clinical trial with parallel group design (two interventions groups). Trial duration: 24 weeks. Follow‐up: 4 patients receiving ursodeoxycholic acid and 3 placebo dropped out. |
|
| Participants | Country: Japan. Number of patients randomised: 52, from 13 departments in Japan, mean age 59 years (91% females). Inclusion criteria: ‐ PBC was diagnosed clinically and histologically. Exclusion criteria: ‐ patients with severe symptoms or having received other medications for their PBC within the last three months. |
|
| Interventions | Patients were randomly assigned to receive: Intervention group 1: ursodeoxycholic acid 600 mg/day in three divided doses, n = 26; Intervention group 2: placebo, n = 26. |
|
| Outcomes | Symptoms (itching). Complications (oesophageal varices). Liver biochemistry. Serum cholesterol. Serum bile acids. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised, but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a single monitor according to a randomisation scheme (1:1), so that intervention allocations could not have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Placebo tablets could not be distinguished from ursodeoxycholic acid tablets". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Other bias | Low risk | The trial appears to be free of information that could put it at risk of bias. |
Papatheodoridis 2002.
| Methods | Randomised clinical trial with parallel group design (two interventions groups). Trial duration: 92 months. Follow‐up: no patients lost to follow‐up. |
|
| Participants | Country: Greece. Number of patients randomised: 86, mean age 54 years (89% females). Inclusion criteria: ‐ liver histology compatible with PBC; ‐ positive antimitochondrial antibodies; ‐ alkaline phosphatase levels more than twice the upper limit of normal. Exclusion criteria: ‐ extrahepatic biliary obstruction or other cause of liver disease; ‐ patients aged > 70 years; ‐ patients treated with any immunosuppressive agent within the 12 months before entry; ‐ patients with decompensated cirrhosis (Child class B or C); ‐ baseline bilirubin levels ≥ 3 mg/dl. |
|
| Interventions | Patients were randomly assigned to receive: Intervention group 1: ursodeoxycholic acid 12 to 15 mg/kg/day, n = 43; Intervention group 2: no intervention, n = 43. |
|
| Outcomes | Liver decompensation. Mortality or liver transplantation. Symptoms. Liver biochemistry. Liver histology. | |
| Notes | 14/43 control patients were crossed‐over to ursodeoxycholic acid at their own request at a median of 3.5 years (range 2 to 8 years) after entry in the trial. Mean follow‐up was 7.3 ± 3.0 years in the ursodeoxycholic acid group and 8.1 ± 3.1 years in the control group. The authors did both intention‐to‐treat analysis and treatment‐as‐received analysis. Data for the following outcomes were extracted from graphs from Hadziyannis 1990 (Papatheodoridis 2002): ‐ serum bilirubin; ‐ serum alanine aminotransferase. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was achieved using random number table. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by serially numbered sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial did not address this component and it was likely unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It was specified that there were no dropouts or withdrawals. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Other bias | Unclear risk | The trial reported a grant from the pharmaceutical company Galenica Hellas. |
Pares 2000.
| Methods | Double‐blind, placebo controlled randomised clinical trial with parallel group design (two interventions groups). Trial duration: at least 2 years (median follow‐up was 3.4 years). Follow‐up: 10 ursodeoxycholic acid treated patients and 21 placebo treated patients discontinued. |
|
| Participants | Country: Spain. Number of patients randomised: 192, from 16 hospitals in Spain, mean age 54 years (93% females). Inclusion criteria: ‐ compatible liver biopsy; ‐ alkaline phosphatase > 2 upper normal limit; ‐ positive antimitochondrial antibodies; ‐ patients with negative antimitochondrial antibodies were accepted if there was no evidence of extrahepatic biliary obstruction. Exclusion criteria: ‐ age > 72 years; ‐ previous PBC treatment in the 6 months before entry; ‐ life expectancy less than 6 months; ‐ drug addiction; ‐ pregnancy; ‐ other cause of liver disease. |
|
| Interventions | Patients were randomly assigned to receive: Intervention group 1: ursodeoxycholic acid 14 to 16 mg/kg/day in three divided doses, n = 99; Intervention group 2: no intervention, n = 93. |
|
| Outcomes | Mortality.
Liver transplantation.
Symptoms.
Complications.
Liver biochemistry.
Liver histology. Adverse events. |
|
| Notes | Data for liver biopsy findings ‐ dichotomous variables outcome were extracted from Pares 2001 (Pares 2000). Additional information requested on 26th January 2012 and reply received on 31st January 2012 through personal communication with the principal author Dr. Albert Pares who provided data on the method of sequence generation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised to take ursodeoxycholic acid or placebo (ratio 1: 1), using a randomisation code generated by computer. |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by serially numbered sealed and opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described ('placebo was identical in appearance, smell, and taste'), so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Other bias | Unclear risk | It was reported that trial medications were provided by Zambon S. A., Laboratorio Farmaceutico. |
Poupon 1991.
| Methods | Multicenter double‐blind, placebo controlled randomised clinical trial with parallel group design (two interventions groups). Trial duration: 2 years. Follow‐up: 5 patients receiving ursodeoxycholic acid and 6 placebo withdrew. |
|
| Participants | Country: France and Canada. Number of patients randomised: 146, from 22 centres in France and Canada, mean age 56 years (92% females). Inclusion criteria: ‐ liver biopsy compatible with PBC; ‐ serum alkaline phosphatase > 2.0 upper normal limit; ‐ positive AMA. Exclusion criteria: ‐ PBC treatment within last six months; ‐ serum bilirubin > 150 µmol/l; ‐ serum albumin < 25 g/l; ‐ past or active bleeding oesophageal varices; ‐ presence of extrahepatic obstruction; ‐ excessive alcohol consumption; ‐ positive hepatitis B surface antigen. |
|
| Interventions | Patients were randomly assigned to receive: Intervention group 1: ursodeoxycholic acid 13 to 15 mg/kg/day, n = 73; Intervention group 2: placebo, n = 73. |
|
| Outcomes | Mortality. Liver transplantation. Symptoms. Liver biochemistry. Liver histology. | |
| Notes | All patients treated for two years with placebo were offered ursodeoxycholic acid and further followed‐up for another two years together with patients continuing on ursodeoxycholic acid. One patient, included in the publications of the study up to 1993, was excluded from the 1994 publication due to a raised serum bilirubin at entry, which violated the entry criteria. Data were extracted at the maximum follow‐up where applicable, if not the end of treatment was used for data extraction. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised, but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described ‐ placebo was 'identical capsule', so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Other bias | Low risk | The trial appears to be free of information that could put it at risk of bias. |
Senior 1991.
| Methods | Double‐blind randomised clinical trial with parallel group design (two interventions groups). Trial duration: six months. Follow‐up: no patients withdrew. |
|
| Participants | Country: USA. Number of patients randomised: 19, mean age 53 years (75% females). Inclusion criteria: ‐ PBC confirmed by liver biopsy and supporting clinical tests within six months of entry into the trial. Exclusion criteria: ‐ none listed. |
|
| Interventions | Patients were randomly assigned to receive: Intervention group 1: ursodeoxycholic acid 10 mg/kg/day, n = 9; Intervention group 2: placebo, n = 10. |
|
| Outcomes | Mortality. Symptoms. Liver biochemistry. | |
| Notes | Data for the following outcomes were extracted from O'Brian 1990 (Senior 1991): ‐ mortality; ‐ liver transplantation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised, but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was described as double‐blind, but the method of blinding was not described, so that knowledge of allocation was possible during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated. |
| Selective reporting (reporting bias) | Unclear risk | Not all pre‐defined, or clinically relevant and reasonably expected outcomes are not reported fully and properly. |
| Other bias | Unclear risk | The trial reported partial support for ursodiol supplies by Ciba‐Geigy Corporation. |
Turner 1994.
| Methods | Double‐blind, placebo controlled randomised clinical trial with parallel group design (two interventions groups). Trial duration: 2 years. Follow‐up: 5 patients receiving ursodeoxycholic acid and 4 placebo withdrew. |
|
| Participants | Country: UK. Number of patients randomised: 46, mean age 57 years (96% females). Inclusion criteria: ‐ liver biopsy compatible with PBC; ‐ positive AMA; ‐ abnormal liver function tests; ‐ no medication within six months of trial entry. Exclusion criteria: ‐ none listed. |
|
| Interventions | Patients were randomly assigned to receive: Intervention group 1: ursodeoxycholic acid 10mg/kg/day (mean actual dose (+/‐SD): 11.4+/‐0.9 mg/kg/day), n = 22; Intervention group 2: placebo, n = 24. |
|
| Outcomes | Mortality. Liver transplantation. Symptoms. Liver biochemistry. Liver histology. Quality of life. | |
| Notes | Data for the following outcomes were extracted from the preliminary report of the included trial (Myszor 1990): ‐ pruritus score; ‐ serum bilirubin; ‐ serum alkaline phosphatases; ‐ serum aspartate aminotransferase. Number of patients randomised 34, follow‐up 1 year. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised, but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as blinded, the parties that were blinded, and the method of blinding was described, so that knowledge of allocation was adequately prevented during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Other bias | Unclear risk | It was reported that trial medications were generously donated by Thames Laboratories, Wrex‐ham, Wales. |
Vuoristo 1995.
| Methods | Double‐blind, placebo controlled randomised clinical trial with parallel group design (two interventions groups and one control group). Trial duration: 2 years. Follow‐up: 0 patients receiving ursodeoxycholic acid and 8 placebo withdrew. |
|
| Participants | Country: Finland. Number of patients randomised: 90, from four centres in Finland, mean age 55 years (82% females). Inclusion criteria: ‐ elevated serum alkaline phosphatases activity; ‐ liver biopsy compatible with PBC; ‐ positive AMA. Exclusion criteria: ‐ other cause of liver disease; ‐ positive hepatitis B surface antigen and hepatitis C antibodies; ‐ end‐stage PBC; ‐ patients treated with drugs that might affect prognosis; ‐ serum bilirubin level > 150 µmol/L; ‐ serum albumin level < 25 g/L; ‐ drug‐resistant ascites; ‐ patients in whom liver transplantation was indicated; ‐ previous PBC treatment for 6 months before the trial. |
|
| Interventions | Patients were randomly assigned to receive: Intervention group 1: ursodeoxycholic acid 12 to 15 mg/kg/day in two doses, n = 30; Intervention group 2: colchicine 1 mg/day, n = 29; Control: placebo, n = 31. |
|
| Outcomes | Mortality.
Liver transplantation.
Symptoms.
Liver biochemistry.
Liver histology. Adverse events. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial is described as randomised, but the method of sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | The trial was described as randomised but the method used to conceal the allocation was not described, so that intervention allocations may have been foreseen in advance of, or during, enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was described as blind, but the method of blinding was not described fully (it was only reported that placebo was used, but no mention on appearance), so knowledge of allocation was possible during the trial. The outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for dropouts and withdrawals in all intervention groups were described. |
| Selective reporting (reporting bias) | Low risk | Pre‐defined, or clinically relevant and reasonably expected outcomes are reported on. |
| Other bias | Unclear risk | It was reported that ursodeoxycholic acid tablets were donated by Leiras Oy, Helsinki, Finland. |
PBC = primary biliary cirrhosis.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Angulo 1999 | This is not a randomised trial, but a comparison of liver histology of 16 ursodeoxycholic acid treated patients from one randomised trial to the liver histology of 51 patients from another randomised trial. |
| Angulo 1999 a | There is no placebo or no intervention group in this randomised trial, which compares low (5 to 7 mg/kg/day), standard (13 to 15 mg/kg/day), and high (23 to 25 mg/kg/day) doses of ursodeoxycholic acid in 155 patients with PBC. The improvements in alkaline phosphatase, aspartate aminotransferase, Mayo risk score, and biliary ursodeoxycholic acid enrichment were significantly greater in the standard‐ and high‐dose groups compared to the low‐dose group, but not between the standard‐ and high‐dose group. No significant effects were noted on symptoms with any dose. |
| Bateson 1998 | This is a case series of 40 PBC patients with symptomatic disease treated with ursodeoxycholic acid. The results were compared to 12 historic ursodeoxycholic acid‐untreated PBC patients. |
| Brodanova 1997 | This is a case series of 13 PBC patients treated with ursodeoxycholic acid. |
| Cauch‐Dudek 1998 | This is a case series of 88 patients with PBC evaluating fatigue. A self‐rated fatigue. Severity score did not correlate with ursodeoxycholic acid use. |
| Crippa 1995 | The trial is not randomised, but compares 18 ursodeoxycholic acid treated PBC patients to eight untreated PBC patients. |
| Crosignani 1996 | This is a dose‐response study examining the effects of three doses of tauro‐ursodeoxycholic acid in 24 patients with PBC. |
| Eisenburg 1988 | This is a case series of 21 PBC patients during ursodeoxycholic acid administration. |
| Ferri 1993 | This is a controlled comparison of ursodeoxycholic acid with tauro‐ursodeoxycholic acid for PBC. |
| Grippa 1995 | This is a non‐randomised study comparing 18 ursodeoxycholic acid treated PBC patients to eight ursodeoxycholic acid‐untreated PBC patients. |
| Ideo 1990 | Out of three PBC patients treated with ursodeoxycholic acid (600 mg/day), ursodeoxycholic acid was stopped in one of these patiens 'randomly selected'. |
| Ikeda 1996 | This is a randomised trial comparing ursodeoxycholic acid plus colchicine versus ursodeoxycholic acid alone in 22 patients with PBC. |
| Kehagioglou 1991 | The study is not described as randomised, but compares 16 PBC patients treated with ursodeoxycholic acid (14 mg/kg/day for a mean period of 22 months (range 3 months to 35 months) to a control group consisting of 10 PBC patients treated with placebo. |
| Kim 1997 | This is a case series of eight ursodeoxycholic acid‐treated PBC patiens who lacked antimitochondrial antibodies. |
| Kneppelhout 1992 | This is a case series of 19 patients with PBC during ursodeoxycholic acid administration. |
| Krzeski 1999 | This is a case series of 60 PBC patients treated with ursodeoxycholic acid. |
| Larghi 1997 | This is a randomised trial with crossover design comparing ursodeoxycholic acid versus tauro‐ursodeoxycholic acid. |
| Leuschner 1996 | This randomised trial compared ursodeoxycholic acid plus prednisolone versus ursodeoxycholic acid plus placebo for PBC. |
| LONDON 1998 | This trial compared placebo to different doses of URSO (300 mg/day, 600 mg/day, 900 mg/day and 1200 mg/day) in 23 biopsy proven early stage PBC patients. There is no mention of randomisation. Patients were followed for eight weeks with a four week washout period between doses. A significant trend toward normalising of abnormal liver function tests was observed together with a significant increase in lethargy, irrespective of ursodeoxycholic acid dose, compared to placebo. |
| Lotterer 1990 | This is a case series of twelve PBC patients during ursodeoxycholic acid administration. |
| Matsuzaka 1994 | This is a case series of three PBC patients during ursodeoxycholic acid administration. |
| Matsuzaki 1990 | This is a case series of ten PBC patients during ursodeoxycholic acid administration. |
| MAYO‐II 1997 | This trial randomised 150 PBC patients to three doses of ursodeoxycholic acid (5 to 7 mg/kg/day; 13 to 15 mg/kg/day; 22 to 25 mg/kg/day) and followed the patients for one year. No differences were observed between the medium and the high dose with respect to liver biochemistry changes, but both these dose groups had significantly greater improvement of liver biochemistry compared to the low dose group. Clinical events such as death, transplantation, or complications of liver disease were rare and were not different between the three dose groups. |
| NEWARK‐I | The study is not randomised. The study included only four patients with PBC and apparently these were treated first with placebo for three months and then with ursodeoxycholic acid (10‐15 mg/kg/day) for three‐six months. No major outcome variables are reported. |
| NEWARK‐III | This study investigated biochemical features, including biliary bile acids, in 14 patients with PBC using a paired design. First, all patients received placebo for three months. Then, the patients were treated with 900 mg ursodeoxycholic acid (10‐12 mg/kg/day) for six months (n = 11) to 12 months (n = 8). The latter patients were then treated with placebo for three months and restarted on ursodeoxycholic acid for another 12 months. Due to the paired design, the observed improvements may be due to the fluctuating course of PBC. |
| Ogino 1993 | This is a case series of 28 PBC patients treated with ursodeoxycholic acid and compared to seven PBC patiens not treated with ursodeoxycholic acid. |
| Okuyama 1988 | This is a study of a single PBC patient during ursodeoxycholic acid administration. |
| Osuga 1989 | This is a case series of eight PBC patients during ursodeoxycholic acid administration. |
| Peridigoto 1992 | This is a study of three PBC patiens during ursodeoxycholic acid administration. |
| Podda 1989 | This is a randomised trial examining three doses of ursodeoxycholic acid in PBC patients and patients with primary sclerosing cholangitis and chronic hepatitis. |
| Poupon 1987 | This is a case series of 15 PBC patients during ursodeoxycholic acid administration. |
| Poupon 1989 | This study is not randomised. |
| Poupon 1996 | This is a randomised trial comparing ursodeoxycholic acid plus colchicine versus ursodeoxycholic acid in 74 patients with PBC. |
| Schonfeld 1997 | This is a case series of 15 PBC patients during ursodeoxycholic acid administration. |
| Shibata 1992 | This is a case series of 12 PBC patients during ursodeoxycholic acid administration. |
| Stiehl 1990 | This is a case series of 29 patients with PBC during ursodeoxycholic acid administration. |
| Taha 1994 | This is a case series of patients with PBC during different drug administrations (cholestyramine, wash out, ursodeoxycholic acid, and ursodeoxycholic acid plus cholestyramine). |
| Takezaki 1991 | This is a study of a single PBC patient during ursodeoxycholic acid administration. |
| Toda 1998 | No placebo or no intervention group are included. The trial compares the efficacy of three doses of ursodeoxycholic acid (150 mg/day; 600 mg/day; 900 mg/day) in 82 PBC patients for 24 months. |
| Unoura 1990 | Not a randomised trial, but compares 16 ursodeoxycholic acid treated PBC‐patients to eight patients without ursodeoxycholic acid treatment. |
| Van de Meeberg 1996 | No placebo or no intervention group. Five patients treated 'in random order' with 10 mg ursodeoxycholic acid/kg/day in either a single or in three divided doses ‐ no difference in liver biochemistry improvement. |
| Van Hoogstraten 1998 | This RCT compares 10 versus 20 mg ursodeoxycholic acid/kg/day during six months in 61 PBC patients. Liver biochemistry improved in PBC patients receiving 20 mg/kg/day compared to a dose of 10 mg/kg/day. |
| Verma 1999 | This cross‐over RCT compares different doses of ursodeoxycholic acid in twenty‐four biopsy‐proven early‐stage PBC patients (one male, 23 female) who received five doses of ursodeoxycholic acid (0, 300, 600, 900, 1200 mg/day) each for eight weeks with four‐week washout periods between doses. Symptoms (pruritus, fatigue, diarrhoea) were assessed on a four‐point scale (none, mild, moderate, severe). Liver function tests were performed using conventional methods, and serum bile acids were measured using gas liquid chromatography. The dose of 900 mg/day produced the greatest enrichment of ursodeoxycholic acid in serum bile acids, although there was no difference in the enrichment of ursodeoxycholic acid between the different doses. There was a trend towards normalization of the abnormal LFTs in a dose‐dependent manner (for Y‐glutamyl transferase (yGT), alkaline phosphatase (ALP), alanine transaminase (ALT) and IgM). Multi‐factorial analysis showed that ursodeoxycholic acid treatment, irrespective of dose, was significantly better than placebo for all the variables. The 900 mg and 1200 mg doses were better than both 300 mg and 600 mg using gamma‐glutamyl transpeptidase and total bilirubin as variables, better than 300 mg using alkaline phosphatase and IgM as variables, and better than 600 mg using albumin as a variable. No variables showed a significant difference between 900 and 1200 mg. The study concluded that the optimum dose of ursodeoxycholic acid is 900 mg/day (equivalent to 13.5 mg/kg/day). This trial is excluded due to the cross‐over design and due to the fact that it did not provide any data on the primary outcome variables. |
| Wirth 1994 | This is a case series of 14 patients with PBC examined before and during ursodeoxycholic acid administration. |
| Wirth 1995 | This is a case series of 22 patients with PBC, who have their subtypes of antimitochondrial antibodies examined and related to response to ursodeoxycholic acid administration. |
| Wolfhagen 1994 | No randomisation, combination therapy with ursodeoxycholic acid and prednisone in seven patients. |
| Yamazaki 1992 | This is a study of a single PBC patient with eosinophilic infiltration. |
| Yamazaki 1996 | This is a case series of 38 PBC patients, of which 55 per cent exhibited eosinophilia. The eosinophilia was reduced during ursodeoxycholic acid treatment. |
| Yokomori 1996 | This is a study of a single patient with PBC and pruritus responding to treatment with ursodeoxycholic acid and cholestyramine. |
PBC = primary biliary cirrhosis.
Differences between protocol and review
Peer reviewers requested that we included data from the trials after the period in which fair comparisons could be made.
Differences between previous published version of the review and this review
The whole review protocol part was thoroughly updated following the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): text was added or rewritten for greater precision and understanding. Outcomes were rearranged so that they now are patient oriented. An attempt to collect evidence on harm was also made. The statistical approach was also revised. Based on updated domains for bias risk, we judged bias risk domains anew (Higgins 2011). In the present update, only one trial was assessed as having low risk of bias. Minor inaccuracies found in data previously extracted were now corrected. Number of patients who died, were liver transplanted, obtained hepatic encephalopathy or were diagnosed with bleeding varices are now added to the analyses on serious adverse events. New trial data were added to some of the secondary outcome measures. Due to addition of data to the outcome measures on ascites and histology, the result of the effect for ascites changed from significant into insignificant, and the result of the effect for histology changed from insignificant into significant. We performed subgroup analyses using data in the already included trials as well data found in follow‐up publications of the included trials. Results on all‐cause mortality and mortality or liver transplantation as well as the following secondary outcomes ‐ pruritus, serum bilirubin and serum alkaline phosphatases ‐ were analysed with trial sequential analysis. The random‐effects model meta‐regression showed that none of examined covariates (bias risk of the trials, disease severity of patients at entry, ursodeoxycholic acid dosage, and trial duration) were significantly associated with the estimated intervention effect on mortality or mortality or liver transplantation. Summary of Findings tables and grading of the evidence were also performed. We abandoned our Baysian analyses as they did not seem to add new information.
Contributions of authors
JR and GP screened the literature, identified trials with updated information, extracted data, and made the risk of bias judgements. JR, GB, and CG analysed and interpreted the data and results. JR drafted the manuscript and performed the meta‐analyses. GB performed the meta‐regression analyses. MK, GB, and CG were involved in critical revision of the manuscript for important intellectual content.
Sources of support
Internal sources
The Cochrane Hepato‐Biliary Group, Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Denmark.
External sources
Ministry of Science (Grant No. 41004), Serbia.
Clinic of Gastroenterology, Clinical Centre of Serbia, Belgrade, Serbia.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Battezzati 1993 {published data only}
- Battezzati PM, Podda M, Bianchi FB, Naccarato R, Orlandi F, Surrenti C, et al. Ursodeoxycholic acid for symptomatic primary biliary cirrhosis. Preliminary analysis of a double‐blind multicenter trial. Italian Multicenter Group for the Study of UDCA in PBC. Journal of Hepatology 1993;17:332‐8. [DOI] [PubMed] [Google Scholar]
- Italian Multicenter Project for UDCA Treatment in PBC. Ursodeoxycholic acid (UDCA) for symptomatic primary biliary cirrhosis (PBC): a double‐blind multicenter trial (EASL abstract). Journal of Hepatology 1989;9(Suppl 1):87. [Google Scholar]
- Podda M, Almasio P, Battezzati PM, Crosignani A, and Italian Multicenter Group for the Study of UDCA in PBC. Long‐term effect of the administration of ursodeoxycholic acid alone or with colchicine in patients with primary biliary cirrhosis: a double‐blind multicenter study. Bile Acids and the Hepatobiliary System. From Basic Science to Clinical Practice. Falk Symposium 68. 1993:310‐5.
- Podda M, Battezzati PM, Crosignani A, Bianchi FB, Fusconi M, Chiaramonte M, et al. Urodeoxycholic acid (UDCA) for symptomatic primary biliary cirrhosis (PBC): a double‐blind multicenter trial [AASLD abstract]. Hepatology 1989;10:639. [Google Scholar]
Combes 1995 {published data only}
- Carithers RL, Luketic VA, Peters M, Zetterman RK, Garcia‐Tsao G, et al. Extended follow‐up of patients in the U.S. multicenter trial of ursodeoxycholic acid for primary biliary cirrhosis (Abstract). Gastroenterology 1996;110(4):A1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes B, Carithers RL Jr, Maddrey WC, Lin D, McDonald MF, Wheeler DE, et al. A randomised, double‐blind, placebo‐controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology 1995;22:759‐66. [PubMed] [Google Scholar]
- Combes B, Carithers RL Jr, Maddrey WC, Munoz S, Garcia Tsao G, Bonner GF, et al. Biliary bile acids in primary biliary cirrhosis: effect of ursodeoxycholic acid. Hepatology 1999;29(6):1649‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes B, Carithers RL Jr, McDonald MF, Maddrey WC, Munoz SJ, Boyer JL, et al. Ursodeoxycholic acid therapy in patients with primary biliary cirrhosis [AASLD abstract]. Hepatology 1991;14:91A. [Google Scholar]
- Combes B, Carithers RL, Maddrey WC, Munoz SJ, McDonald MF, Garcia‐Tsao G, et al. A randomised, double‐blind, placebo‐controlled trial of ursodeoxycholic acid (UDCA) in primary biliary cirrhosis (AASLD Abstract). Hepatology 1993;18:175A. [PubMed] [Google Scholar]
- Combes B, Carithers RL, Maddrey WC, Munoz SJ, McDonald MF, Garcia‐Tsao G, et al. A randomised, double‐blind, placebo‐controlled trial of ursodeoxycholic acid (UDCA) in primary biliary cirrhosis (PBC). Falk Symposium No. 68. XII International Bile Acid Meeting. Bile Acids and the Hepatobiliary System. From Basic Science to Clinical Practice. Basel. 1992:43.
- Combes B, Carithers RL, Maddrey WC, Munoz SJ, McDonald MF, Garcia‐Tsao G, et al. A randomised, double‐blind, placebo‐controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Bile Acids and the Hepatobiliary System. From Basic Science to Clinical Practice. Falk Symposium 68. 1993:289‐91.
- Combes B, Carithers RL, Maddrey WC, Munoz SJ, McDonald MF, Garcia‐Tsao G, et al. The American multicenter primary biliary cirrhosis, Ursodiol versus placebo study group (PUPS) trial. Falk Symposium No. 80. XIII International Bile Acid Meeting. Bile Acids in Gastroenterology: Basic and Clinical Aspects. San Diego. 1994:67.
- Combes B, Luketic VA, Peters MG, Zetterman RK, Garcia‐Tsao G, Munoz SJ, et al. Prolonged follow‐up of patients in the U.S. multicenter trial of ursodeoxycholic acid for primary biliary cirrhosis. The American Journal of Gastroenterology 2004;99(9):264‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes B, Markin RS, Wheeler DE, Rubin R, West AB, Mills AS, et al. The effect of ursodeoxycholic acid on the florid duct lesion of primary biliary cirrhosis. Hepatology 1999;30:602‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emond M, Carithers RL Jr, Luketic VA, Peters M, Zetterman RK, Garcia‐Tsao G, et al. Does ursodeoxycholic acid improve survival in patients with primary biliary cirrhosis? Comparison of outcome in the US multicenter trial to expected survival using the Mayo Clinic prognostic model [AASLD abstract]. Hepatology 1996;24:168A. [Google Scholar]
De la Mora 1994 {published data only}
- Mora G, Bobadilla J, Romero P, Rodríguez‐Leal G, Morán S, Kershenobich D, et al. Does treatment with ursodeoxycholic acid (UDCA) really diminish cholesterol serum levels in primary biliary cirrhosis (PBC)? [EASL abstract]. Hepatology 1994;19:57I. [Google Scholar]
Eriksson 1997 {published and unpublished data}
- Eriksson LS, Olsson R, Glauman H, Prytz H, Befrits H, Lindgren S, et al. Ursodeoxycholic acid (UDCA) in patients with primary biliary cirrhosis (PBC) Results of a two‐year randomised placebo‐controlled study (abstract). Scandinavian Journal of Gastroenterology 1995;30(Suppl 209):35. [DOI] [PubMed] [Google Scholar]
- Eriksson LS, Olsson R, Glauman H, Prytz H, Befrits R, Ryden BO, et al. Ursodeoxycholic acid treatment in patients with primary biliary cirrhosis. A Swedish multicenter, double‐blind, randomised controlled study. Scandinavian Journal of Gastroenterology 1997;32:179‐86. [DOI] [PubMed] [Google Scholar]
Goddard 1994 {published data only}
- Goddard CJR, Hunt L, Smith A, Fallowfield G, Rowan B, Warnes TW. A trial of ursodeoxycholic acid (UDCA) and colchicine in primary biliary cirrhosis (PBC) (AASLD abstract). Hepatology 1994;20:151A. [Google Scholar]
- Goddard CJR, Smith A, Hunt L, Halder T, Hillier V, Rowan B, et al. Surrogate markers of response in a trial of ursodeoxycholic acid (UDCA) and colchicine in primary biliary cirrhosis (PBC). Gut 1995;36(Suppl 1):A30. [Google Scholar]
Heathcote 1994 {published data only}
- Ghent CN, Cauch‐Dudek K, Heathcote EJ, and the Canadian PBC Trial Group. Ursodeoxycholic acid therapy effects on pruritus and fatigue in primary biliary cirrhosis. Hepatology 1997;26:438 A. [Google Scholar]
- Heathcote EJ, Cauch DK, Walker V, Bailey RJ, Blendis LM, Ghent CN, et al. The Canadian Multicenter Double‐blind Randomized Controlled Trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology 1994;19:1149‐56. [PubMed] [Google Scholar]
- Heathcote EJL, Cauch K, Walker V, Bailey RJ, Blendis LM, Ghent CN, et al. A double blind randomised controlled multi‐centre trial of ursodeoxycholic acid (UDCA) in primary biliary cirrhosis (PBC): results from a blinded interim analysis. XII International Bile Acid Meeting. Bile Acids and the Hepatobiliary System. From Basic Science to Clinical Practice. Basel. Falk Symposium No. 68. 1992:45.
- Heathcote EJL, Cauch K, Walker V, Bailey RJ, Blendis LM, Ghent CN, et al. The Canadian multi‐centre double blind randomised controlled trial of ursodeoxycholic acid in primary biliary cirrhosis [AASLD abstract]. Hepatology 1992;16:91A. [PubMed] [Google Scholar]
- Heathcote EJL, Cauch K, Walker V, Blendis LM, Ghent CN, Pappas SC, et al. A four‐year follow‐up study of ursodeoxycholic acid therapy for primary biliary cirrhosis. Gastroenterology 1993;104:A914. [Google Scholar]
- Heathcote EJL, Cauch K, Walker V, Blendis LM, Pappas SC, Wanless IR, et al. A double‐blind randomised controlled multicenter trial of ursodeoxycholic acid in primary biliary cirrhosis: results from a 1991 interim analysis. Bile Acids and the Hepatobiliary System. From Basic Science to Clinical Practice. Falk Symposium 68. 1993:294‐8.
- Kilmurry MR, Cauch‐Dudek K, O`Rourke K, Heathcote EJ, the Canadian PBC Trial Group. Is the Mayo model useful for predicting survival following the introduction of ursodeoxycholic acid treatment for primary biliary cirrhosis? [abstract]. Gastroenterology 1995;108(Suppl 4):96. [Google Scholar]
- Kilmurry MR, Heathcote EJ, Cauch DK, O'Rourke K, Bailey RJ, Blendis LM, et al. Is the Mayo model for predicting survival useful after the introduction of ursodeoxycholic acid treatment for primary biliary cirrhosis?. Hepatology 1996;23:1148‐53. [DOI] [PubMed] [Google Scholar]
- Michieletti P, Witt‐Sullivan H, Steiner G, Cauch K, Walker V, Heathcote EJL. No evidence for a more atherogenic serum lipid profile with ursodeoxycholic acid (UDCA) therapy in patients with primary biliary cirrhosis (PBC) [abstract]. Hepatology 1993;18(4):217A. [Google Scholar]
- Neuman MG, Cameron RG, Shear NH, Blendis LM. Ursodeoxycholic acid reduces fibrosis in primary biliary cirrhosis (Abstract). XV International Bile Acid Meeting. Bile Acids and Cholestasis. Titisee, Germany. Falk Symposium No 108. 1998:59.
Hwang 1993 {published and unpublished data}
- Hwang SJ, Chan CY, Lee SD, Wu JC, Tsay SH, Lo KJ. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis: a short‐term, randomised, double‐blind controlled, cross‐over study with long‐term follow up. Journal of Gastroenterology and Hepatology 1993;8:217‐23. [DOI] [PubMed] [Google Scholar]
Leuschner 1989 {published and unpublished data}
- Güldütuna S, Leuschner U, Imhof M, Zimmer G. Treatment of chronic active hepatitis and primary biliary cirrhosis with ursodeoxycholic acid. Zeitschrift fur Gastroenterologie 1992;30 Suppl 1:49‐54. [PubMed] [Google Scholar]
- Leuschner M, Güldütuna S, Imhof M, Bhati S, You T, Leuschner U. Ursodeoxycholic acid therapy in primary biliary cirrhosis. Bile acids and the hepatobiliary system. From Basic Science to Clinical Practice. Falk Symposium 68. 1993:299‐302.
- Leuschner U, Fischer H, Güldütuna S, Kurtz W, Gatzen M, Hellstern A, et al. Does ursodeoxycholic acid (UDCA) influence cell membrane architecture in patients with primary biliary cirrhosis (PBC)?. Gastroenterology 1989;96:A621. [DOI] [PubMed] [Google Scholar]
- Leuschner U, Fischer H, Hübner K. [Ursodeoxycholic acid in the treatment of primary biliary cirrhosis: results of a controlled study]. Zeitschrift fur Gastroenterologie. Verhandlungsband 1989;24:133. [PubMed] [Google Scholar]
- Leuschner U, Fischer H, Kurtz W, Güldütuna S, Hubner K, Hellstern A, et al. Ursodeoxycholic acid in primary biliary cirrhosis: results of a controlled double‐blind trial. Gastroenterology 1989;97:1268‐74. [DOI] [PubMed] [Google Scholar]
- Leuschner U, Fisher H, Hübner K, Güldütuna S, Gatzen M, Hellstern A, et al. Ursodeoxycholic acid (UDCA) treatment of primary biliary cirrhosis: clinical and histological results of a controlled study. Trends in Bile Acid Research. Falk Symposium 52. 1989:355‐8.
Lindor 1994 {published and unpublished data}
- Angulo P, Lindor KD, Therneau TM, Jorgensen RA, Malinchoc M, Dickson ER. Utilization of the Mayo risk score in patients with primary biliary cirrhosis receiving ursodeoxycholic acid. Liver 1999;19(2):115‐21. [DOI] [PubMed] [Google Scholar]
- Balan V, Dickson ER, Jorgensen RA, Lindor KD. Effect of ursodeoxycholic acid on serum lipids of patients with primary biliary cirrhosis [see comments]. Mayo Clinic Proceedings 1994;69:923‐9. [DOI] [PubMed] [Google Scholar]
- Batts KP, Jorgensen RA, Dickson ER, Hofmann AF, Rossi SS, Ludwig J, et al. The effects of ursodeoxycholic acid on hepatic inflammation and histological stage in patients with primary biliary cirrhosis (AASLD Abstract). Hepatology 1993;18:175A. [PubMed] [Google Scholar]
- Batts KP, Jorgensen RA, Dickson ER, Lindor KD. Effects of ursodeoxycholic acid on hepatic inflammation and histological stage in patients with primary biliary cirrhosis. American Journal of Gastroenterology 1996;91:2314‐7. [PubMed] [Google Scholar]
- Dickson ER, Lindor KD. Beneficial effects of ursodeoxycholic acid in an open trial of patients with primary biliary cirrhosis. Bile Acids as Therapeutic Agents. From Basic Science to Clinical Practice. Falk Symposium 58. 1991:271‐2.
- Dickson ER, Lindor KD, Baldus WP, Jorgensen RA, Ludwig J, Murtaugh PA. Ursodiol (URSO) is effective therapy for patients with primary biliary cirrhosis (PBC). Falk Symposium No. 68. XII International Bile Acid Meeting. Bile Acids and the Hepatobiliary System. From Basic Science to Clinical Practice. Basel. 1992:44.
- Dickson ER, Lindor KD, Baldus WP, Jorgensen RA, Ludwig J, Murtaugh PA. Ursodiol is effective therapy for patients with primary biliary cirrhosis. Bile Acids and the Hepatobiliary System. From Basic Science to Clinical Practice. Falk Symposium 68. 1993:292‐3.
- Jorgensen RA, Angulo P, Dickson ER, Lindor K. Results of long‐term ursodiol treatment for patients with primary biliary cirrhosis. American Journal of Gastroenterology 2002;97(10):2647‐50. [DOI] [PubMed] [Google Scholar]
- Jorgensen RA, Dickson ER, Hofmann AF, Rossi SS, Lindor KD. Characterisation of patients with a complete biochemical response to ursodeoxycholic acid. Gut 1995;36:935‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda MA, Lindor KD, Jorgensen RA, Rossi SS, Hofmann AF, Salen GR, et al. Dissimilar patterns of serum and biliary bile acids in primary biliary cirrhosis (PBC) patients treated with ursodeoxycholic acid (UDCA). Hepatology 1993;18(4):174 A. [Google Scholar]
- Laurin JM, DeSotel CK, Jorgensen RA, Dickson ER, Lindor KD. The natural history of abdominal pain associated with primary biliary cirrhosis. American Journal of Gastroenterology 1994;89:1840‐3. [PubMed] [Google Scholar]
- Lindor KD, Baldus WP, Jorgensen RA, Ludwig J, Murtaugh PA, Dickson ER. Ursodeoxycholic acid (UDCA) is beneficial therapy for patients with primary biliary cirrhosis (PBC) [AASLD abstract]. Hepatology 1992;16:91A. [Google Scholar]
- Lindor KD, Dickson ER, Baldus WP, Jorgensen RA, Ludwig J, Murtaugh PA, et al. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis [see comments]. Gastroenterology 1994;106:1284‐90. [DOI] [PubMed] [Google Scholar]
- Lindor KD, Jorgensen RA, Dickson ER. Ursodeoxycholic acid delays the onset of oesophageal varices in primary biliary cirrhosis [abstract]. Hepatology 1995;22(4):125A. [DOI] [PubMed] [Google Scholar]
- Lindor KD, Jorgensen RA, Therneau TM, Malinchoc M, Dickson ER. Ursodeoxycholic acid delays the onset of oesophageal varices in primary biliary cirrhosis. Mayo Clinic Proceedings 1997;72:1137‐40. [DOI] [PubMed] [Google Scholar]
- Lindor KD, Lacerda MA, Jorgensen RA, DeSotel CK, Batta AK, Salen G, et al. Relationship between biliary and serum bile acids and response to ursodeoxycholic acid in patients with primary biliary cirrhosis. American Journal of Gastroenterology 1998;93:1498‐504. [DOI] [PubMed] [Google Scholar]
- Lindor KD, Therneau TM, Jorgensen RA, Malichoc M, Dickson ER. Effects of ursodeoxycholic acid on survival in patients with primary biliary cirrhosis. Gastroenterology 1996;110:1515‐8. [DOI] [PubMed] [Google Scholar]
- Lindor KD, Therneau TM, Jorgensen RA, Malinchoc M, Dickson ER. Effects of ursodeoxycholic acid (UDCA) on survival in patients with primary biliary cirrhosis (PBC) [AASLD abstract]. Gastroenterology 1995;108(4):A1111. [DOI] [PubMed] [Google Scholar]
- Siegel JL, Jorgensen R, Angulo P, Lindor KD. Treatment with ursodeoxycholic acid is associated with weight gain in patients with primary biliary cirrhosis. Journal of Clinical Gastroenterology 2003;37(2):183‐5. [DOI] [PubMed] [Google Scholar]
- Zukowski TH, Jorgensen RA, Dickson ER, Lindor KD. Autoimmune conditions associated with primary biliary cirrhosis: response to ursodeoxycholic acid therapy. American Journal of Gastroenterology 1998;93:958‐61. [DOI] [PubMed] [Google Scholar]
Oka 1990 {published data only}
- Oka H, Toda G, Ikeda Y, Hashimoto N, Hasumura Y, Kamimura T, et al. A multicenter double‐blind controlled trial of ursodeoxycholic acid for primary biliary cirrhosis. Gastroenterologia Japonica 1990;25:774‐80. [DOI] [PubMed] [Google Scholar]
- Toda G, Oka H, Hasumura Y, Kamimura T, Ohat Y, Tsuji T, et al. A multicenter double‐blind controlled trial of ursodeoxycholic acid for primary biliary cirrhosis in Japan. XI International Bile Acid Meeting. Bile Acids as Therapeutic Agents ‐ From Basic Science to Clinical Practice. Freiburg. 1990:76.
Papatheodoridis 2002 {published data only}
- Hadziyannis S, Hadziyannis E. A randomised controlled trial of ursodeoxycholic acid (UDCA) in primary biliary cirrhosis (PBC) (AASLD Abstract). Hepatology 1988;8:1421. [Google Scholar]
- Hadziyannis SJ. Long‐term treatment of primary biliary cirrhosis with ursodeoxycholic acid: the third year of a controlled trial. XI International Bile Acid Meeting. Bile Acids as Therapeutic Agents ‐ From Basic Science to Clinical Practice. Freiburg. 1990:57‐8.
- Hadziyannis SJ, Hadziyannis ES, Lianidou E, Makris A. Long‐term treatment of primary biliary cirrhosis with ursodeoxycholic acid: the third year of a controlled trial. Bile Acids as Therapeutic Agents. From Basic Science to Clinical Practice. Falk Symposium 58. 1990:287‐96.
- Hadziyannis SJ, Hadziyannis ES, Makris A. A randomised controlled trial of ursodeoxycholic acid (UDCA) in primary biliary cirrhosis (PBC) [abstract]. Hepatology 1989;10:580. [Google Scholar]
- Hadziyannis SJ, Hadziyannis ES, Makris A. A randomised controlled trial of Ursodeoxyccholic acid (UDCA) in primary biliary cirrhosis (PBC). European Journal of Clinical Investigation 1989;19(Pt 2):A15. [Google Scholar]
- Papatheodoridis GV, Deutsch M, Hadziyannis E, Tzakou A, Hadziyannis SJ. Ursodeoxycholic‐acid for primary biliary cirrhosis: final results of a 12‐year prospective, randomised, controlled trial. Journal of Hepatology 2000;32(Suppl 2):40. [DOI] [PubMed] [Google Scholar]
- Papatheodoridis GV, Hadziyannis ES, Deutsch M, Hadziyannis SJ. Ursodeoxycholic acid for primary biliary cirrhosis: final results of a 12‐year, prospective, randomised, controlled trial. American Journal of Gastroenterology 2002;97:2063‐70. [DOI] [PubMed] [Google Scholar]
Pares 2000 {published and unpublished data}
- Pares A, Caballeria L, Bruguera M, Rodes J. Factors influencing histological progression of early primary biliary cirrhosis. Effect of ursodeoxycholic acid. Journal of Hepatology 2001;34(Suppl 1):189‐90. [Google Scholar]
- Pares A, Caballeria L, Rodes J. Long‐term ursodeoxycholic acid treatment delays progression of mild primary biliary cirrhosis. Journal of Hepatology 2001;34(Suppl 1):187‐8. [Google Scholar]
- Parés A, Caballeria L, Rodes J, Bruguera M, Rodrigo L, Garcia‐Plaza A, et al. Long‐term effects of ursodeoxycholic acid in primary biliary cirrhosis: results of a double‐blind controlled multicentric trial. Journal of Hepatology 2000;32:561‐6. [DOI] [PubMed] [Google Scholar]
- Parés A, for the Spanish Association for the Study of the Liver. Long‐term treatment of primary biliary cirrhosis with ursodeoxycholic acid: results of a randomised, double‐blind, placebo‐controlled trial (abstract). Journal of Hepatology 1997;26(Suppl 1):166. [Google Scholar]
Poupon 1991 {published data only}
- Calmus Y, Poupon R. Ursodeoxycholic acid (UDCA) in the treatment of chronic cholestatic diseases. Biochimie 1991;73:1335‐8. [DOI] [PubMed] [Google Scholar]
- Chazouilleres O, Legendre C, StMaur P, Ismail PR, Poupon R. Histological course of primary biliary cirrhosis (PBC) treated with ursodeoxycholic acid (UDCA) [abstract]. Hepatology 1995;2(4):125A. [Google Scholar]
- Corpechot C, Carrat F, Bonnand A‐M, Poupon RE, Poupon R. A Markov model for assessment of histological progression in PBC provides evidence that UDCA therapy is effective in early stages [abstract]. Hepatology 1999;30(4):474A. [Google Scholar]
- Corpechot C, Carrat F, Bonnand A‐M, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on liver fibrosis progression in primary biliary cirrhosis. Hepatology 2000;32:1196‐9. [DOI] [PubMed] [Google Scholar]
- Degott C, Zafrani ES, Callard P, Balkau B, Poupon RE, Poupon R. Histopathologic study of primary biliary cirrhosis and the effect of ursodeoxycholic acid treatment on histological progression. Hepatology 1999;29:1007‐12. [DOI] [PubMed] [Google Scholar]
- Huet PM, Huet J, Hotte S. Long term effect of ursodeoxycholic acid (UDCA) on hepatic function and portal hypertension in primary biliary cirrhosis (PBC) [AASLD abstract]. Hepatology 1994;20:202A. [Google Scholar]
- Huet PM, Willems B, Huet J, Poupon R. Effects of ursodeoxycholic acid (UDCA) on hepatic function and portal hypertension in primary biliary cirrhosis (PBC). XII International Bile Acid Meeting. Bile Acids and the Hepatobiliary System. From Basic Science to Clinical Practice. Basel. Falk Symposium No. 68. 1992:118.
- Huet PM, Willems B, Huet J, Poupon R. Effects of ursodeoxycholic acid (UDCA) on hepatic function and portal hypertension in primary biliary cirrhosis (PBC) [AASLD abstract]. Hepatology 1990;12:907. [Google Scholar]
- Poupon R, Chazouilleres O, Balkau B, Poupon RE. Clinical and biochemical expression of the histopathological lesions of primary biliary cirrhosis UDCA‐PBC Group. Journal of Hepatology 1999;30(3):408‐12. [DOI] [PubMed] [Google Scholar]
- Poupon R, Poupon RE, The UDCA‐PBC Group. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Strategies for the Treatment of Hepatobiliary Diseases. Falk Symposium 53. 1990:79‐81.
- Poupon R, Poupon RE, the UDCA‐PBC Group. Ursodeoxycholic acid for primary biliary cirrhosis. International Lugano Symposium on Biliary Physiology and Diseases: Strategies for the Treatment of Hepatobiliary Diseases. Lugano. Falk Symposium No. 53. 1989:22.
- Poupon R, the UDCA‐PBC Group. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. First International Symposium. Trends and Discovery in Bile Acid Research. Bora‐Bora (French Polynesia). 1990:123‐6.
- Poupon RE, Balkau B, Eschwege E, Poupon R, Kaplan MM. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. Annals of Internal Medicine 1991;115(6 Suppl 2):48. [DOI] [PubMed] [Google Scholar]
- Poupon RE, Balkau B, Eschwege E, Poupon R, The UDCA‐PBC Study Group. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. New England Journal of Medicine 1991;324:1548‐54. [DOI] [PubMed] [Google Scholar]
- Poupon RE, Balkau B, Guechot J, Heintzmann F. Predictive factors in ursodeoxycholic acid‐treated patients with primary biliary cirrhosis: role of serum markers of connective tissue. Hepatology 1994;19:635‐40. [DOI] [PubMed] [Google Scholar]
- Poupon RE, Balkau B, Poupon R, The UDCA‐PBC Group. Beneficial effect of ursodeoxycholic acid (UDCA) in primary biliary cirrhosis (PBC). Final results of the French Canadian trial [AASLD abstract]. Hepatology 1990;12:872. [Google Scholar]
- Poupon RE, Chretien Y, Poupon R, Paumgartner G. Serum bile acids in primary biliary cirrhosis: effect of ursodeoxycholic acid therapy. Hepatology 1993;17:599‐604. [DOI] [PubMed] [Google Scholar]
- Poupon RE, Chrétien Y, Balkau B, Niard AM, Poupon R, and the UDCA‐PBC Study Group. Ursodeoxycholic therapy for primary biliary cirrhosis: a four year controlled study. Hepatology 1992;16:91A. [Google Scholar]
- Poupon RE, Eschwege E, Poupon R, Attali P, Capron JP, Erlinger S, et al. Ursodeoxycholic acid for the treatment of primary biliary cirrhosis. Interim analysis of a double‐blind multicenter randomised trial. The UDCA‐PBC Study Group. Journal of Hepatology 1990;11(1):16‐21. [DOI] [PubMed] [Google Scholar]
- Poupon RE, Ouguerram K, Chretien Y, Verneau C, Eschwege E, Magot T, et al. Cholesterol‐lowering effect of ursodeoxycholic acid in patients with primary biliary cirrhosis. Hepatology 1993;17:577‐82. [DOI] [PubMed] [Google Scholar]
- Poupon RE, Poupon R, Balkau B, The UDCA‐PBC Study Group. Ursodiol for the long‐term treatment of primary biliary cirrhosis. The UDCA‐PBC Study Group [see comments]. New England Journal of Medicine 1994;330:1342‐7. [DOI] [PubMed] [Google Scholar]
- Poupon RE, Poupon R, UDCA‐PBC Group. Ursodeoxycholic acid (UDCA) for treatment of primary biliary cirrhosis (PBC). Interim analysis of a double‐blind multicenter randomised trial. Hepatology 1989;10:639. [DOI] [PubMed] [Google Scholar]
Senior 1991 {published data only}
- Arora R, Batta AK, Salen G, O'Brien C, Senior JR. Effect of ursodiol on bile acid conjugation in patients with primary biliary cirrhosis [AASLD abstract]. Hepatology 1990;12(4):994. [Google Scholar]
- Batta AK, Arora R, Salen G, O'Brian C, Senior JR. Effect of ursodiol on biliary bile acid composition and conjugation in patients with primary biliary cirrhosis. Gastroenterology 1990;98(2):A567. [Google Scholar]
- O'Brian CB, Senior JR, Sternlieb JM, Sample M, Saul SM, Arora R, et al. Ursodiol treatment of primary biliary cirrhosis. Gastroenterology 1990;98:A617. [Google Scholar]
- O'Brian CB, Senior JR, Sternlieb JM, Saul SM. Caution: not all patients with primary biliary cirrhosis may successfully be treated by ursodiol. Second International Meeting on Pathochemistry, Pathophysiology and Pathomechanisms of the Biliary System and New Strategies for the Treatment of Hepato‐Biliary Diseases. Bologna. 1990:208.
- Senior JR, O'Brian CB, Dickson ER. Effect of oral ursodiol treatment on the predicted probability of mortality in primary biliary cirrhosis. Hepatology 1990;12:438. [Google Scholar]
- Senior JR, O'Brien CB. Mortality risk indices as outcome measures of the effectiveness of ursodeoxycholic acid treatment of cholestatic liver diseases. Bile Acids as Therapeutic Agents. From Basic Science to Clinical Practice. Falk Symposium 58. 1991:273‐85.
Turner 1994 {published and unpublished data}
- Myszor M, Turner I, Mitshison H, Bennett M, Burt AD, James OFW. No symptomatic or histological benefit from ursodeoxycholic acid treatment in PBC after 1 year. Controlled pilot study [IASL abstract]. Hepatology 1990;12:415. [Google Scholar]
- Turner IB, Myszor M, Mitchison HC, Bennett MK, Burt AD, James OF. A two year controlled trial examining the effectiveness of ursodeoxycholic acid in primary biliary cirrhosis. Journal of Gastroenterology and Hepatology 1994;9:162‐8. [DOI] [PubMed] [Google Scholar]
Vuoristo 1995 {published and unpublished data}
- Kisand KE, Karvonen A‐L, Vuoristo M, Färkkilä M, Lehtola J, Inkovaara J, et al. Ursodeoxycholic acid treatment lowers the serum levels of antibodies against pyruvate dehydrogenase and influences their inhibitory capacity for the enzyme complex in patients with primary biliary cirrhosis. Journal of Molecular Medicine 1996;74:269‐74. [DOI] [PubMed] [Google Scholar]
- Miettinen TA, Farkkila M, Vuoristo M, Karvonen AL, Leino R, Lehtola J, et al. Serum cholestanol, cholesterol precursors, and plant sterols during placebo‐controlled treatment of primary biliary cirrhosis with ursodeoxycholic acid or colchicine. Hepatology 1995;21:1261‐8. [PubMed] [Google Scholar]
- Miettinen TA, Färkkila M, Vuoristo M, Karvonen A‐L, Leino R, Lehtola J, et al. Improvement of serum non cholesterol sterols may indicate retarded progression of primary biliary cirrhosis (PBC) in a randomised placebo controlled two‐year trial with colchicine and ursodeoxycholic acid (AASLD abstract). Gastroenterology 1993;104:A954. [Google Scholar]
- Vuoristo M, Farkkila M, Karvonen AL, Leino R, Lehtola J, Makinen J, et al. A placebo‐controlled trial of primary biliary cirrhosis treatment with colchicine and ursodeoxycholic acid [see comments]. Gastroenterology 1995;108:1470‐8. [DOI] [PubMed] [Google Scholar]
- Vuoristo M, Färkkilä M, Gylling H, Karvonen A‐L, Leino R, Lehtola J, et al. Expression and therapeutic response related to apolipoprotein E polymorphism in primary biliary cirrhosis. Journal of Hepatology 1997;27:136‐42. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Angulo 1999 {published data only}
- Angulo P, Batts KP, Therneau TM, Jorgensen RA, Dickson ER, Lindor KD. Long‐term ursodeoxycholic acid delays histological progression in primary biliary cirrhosis. Hepatology 1999;29(3):644‐7. [DOI] [PubMed] [Google Scholar]
Angulo 1999 a {published data only}
- Angulo P, Dickson ER, Therneau TM, Jorgensen RA, Smith C, DeSotel CK, et al. Comparison of three doses of ursodeoxycholic acid in the treatment of primary biliary cirrhosis: a randomised trial. Journal of Hepatology 1999;30:830‐5. [DOI] [PubMed] [Google Scholar]
Bateson 1998 {published data only}
- Bateson MC, Gedling P. Ursodeoxycholic acid therapy for primary biliary cirrhosis. A 10‐year British single‐centre population‐based audit of efficacy and survival. Postgraduate Medical Journal 1998;74(874):482‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brodanova 1997 {published data only}
- Brodanova M, Perlik F. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Casopis Lekaru Ceskych 1997;136(7):215‐20. [PubMed] [Google Scholar]
Cauch‐Dudek 1998 {published data only}
- Cauch‐Dudek K, Abbey S, StewartDE, Heathcote EJ. Fatigue in primary biliary cirrhosis. Gut 1998;43(5):705‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crippa 1995 {published data only}
- Crippa G, Cagnoni C, Castelli A, Concesi C, Girometta S, Pancotti D, et al. Prolonged treatment with ursodeoxycholic acid for primary biliary cirrhosis. Clinical Therapeutics 1995;146:367‐72. [PubMed] [Google Scholar]
Crosignani 1996 {published data only}
- Crosignani A, Battezzati PM, Setchell KDR, Invernizzi P, Covini G, Zuin M, et al. Tauroursodeoxycholic acid for treatment of primary biliary cirrhosis. A dose‐response study. Digestive Diseases and Sciences 1996;41(4):809‐15. [DOI] [PubMed] [Google Scholar]
Eisenburg 1988 {published data only}
- Eisenburg J, Eder M, Spengler U, Berg PA, Caselmann W, Mannes AG, et al. Treatment of primary biliary cirrhosis with ursodeoxycholic acid. Part 2: Prospective long‐term trial in 21 patients. Fortschritte der Medizin 1988;106(34):695‐8. [PubMed] [Google Scholar]
Ferri 1993 {published data only}
- Ferri F, Bernocchi P, Fedeli S. Tauroursodeoxycholic acid for treatment of primary biliary cirrhosis. A controlled comparison with ursodeoxycholic acid. Clinica Terapeutica 1993;143(4):321‐6. [PubMed] [Google Scholar]
Grippa 1995 {published data only}
- Crippa G, Cagnoni C, Castelli A, Concesi C, Girometta S, Pancotti D, et al. Prolonged treatment with ursodeoxycholic acid for primary biliary cirrhosis. Clinica Terapeutica 1995;146(5):367‐72. [PubMed] [Google Scholar]
Ideo 1990 {published data only}
- Idéo G, Bellati G, Pedraglio E, Bottelli R, Maggi G. Efficacy of ursodeoxycholic acid in lowering alanine aminotransferase and gamma‐glutamyl transpeptidase serum levels in patients with chronic active hepatitis and primary biliary cirrhosis. Current Therapeutic Research Clinical and Experimental 1990;47(1):62‐6. [Google Scholar]
Ikeda 1996 {published data only}
- Ikeda T, Tozuka S, Noguchi O, Kobayashi F, Sakamoto S, Marumo F, et al. Effects of additional administration of colchicine in ursodeoxycholic acid‐treated patients with primary biliary cirrhosis: A prospective randomised study. Journal of Hepatology 1996;24(1):88‐94. [DOI] [PubMed] [Google Scholar]
Kehagioglou 1991 {published data only}
- Kehagioglou K, Dritsas S, Kanatakis S, Tsatsa E, Mastora M, Chrissikos N, et al. Effect of UDCA on the natural course of PBC. Journal of Hepatology 1991;13(Suppl 2):S134. [Google Scholar]
Kim 1997 {published data only}
- Kim WR, Poterucha JJ, Jorgensen RA, Batts KP, Hombuger HA, Dickson‐ER, et al. Does antimitochondrial antibody status affect response to treatment in patients with primary biliary cirrhosis? Outcomes of ursodeoxycholic acid therapy and liver transplantation. Hepatology 1997;26(1):22‐6. [DOI] [PubMed] [Google Scholar]
Kneppelhout 1992 {published data only}
- Kneppelhout JC, Mulder CJJ, Berge Henegouwen GP, Vries RA, Brandt K‐H. Ursodeoxycholic acid treatment in primary biliary cirrhosis with the emphasis on late stage disease. Netherlands Journal of Medicine 1992;41(1):11‐6. [PubMed] [Google Scholar]
Krzeski 1999 {published data only}
- Krzeski P, Habior A, Zych W, Walewska‐Zielecka B, Butruk E. Effects of ursodeoxycholic acid treatment on bilirubin concentration and survival of patients with primary biliary cirrhosis. Gastroenterologia Polska 1999;6(3):231‐4. [Google Scholar]
Larghi 1997 {published data only}
- Larghi A, Crosignani A, Battezzati PM, De‐Valle G, Allocca M, Invernizzi P, et al. Ursodeoxycholic and tauro‐ursodeoxycholic acids for the treatment of primary biliary cirrhosis: A pilot crossover study. Alimentary Pharmacology and Therapeutics 1997;11(2):409‐14. [DOI] [PubMed] [Google Scholar]
Leuschner 1996 {published data only}
- Leuschner M, Guldutuna S, You T, Hubner K, Bhatti S, Leuschner U. Ursodeoxycholic acid and prednisolone versus ursodeoxycholic acid and placebo in the treatment of early stages of primary biliary cirrhosis. Journal of Hepatology 1996;25(1):49‐57. [DOI] [PubMed] [Google Scholar]
LONDON 1998 {published data only}
- Verma A, Ahmed HA, Jazrawi RP, Davis T, Bland M, Benson M, et al. Determining the most efficacious dose of ursodeoxycholic acid in primary biliary cirrhosis (Abstract). XV International Bile Acid Meeting. Bile Acids and Cholestasis. Falk Symposium No 108. Titisee, Germany. 1998:62.
Lotterer 1990 {published data only}
- Lotterer E, Stiehl A, Raedsch R, Foelsch UR, Bircher J. Ursodeoxycholic acid in primary biliary cirrhosis: No evidence for toxicity in the stages I to III. Journal of Hepatology 1990;10(3):284‐90. [DOI] [PubMed] [Google Scholar]
Matsuzaka 1994 {published data only}
- Matsuzaki Y, Doy M, Tanaka N, Shoda J, Osuga T, Nakano M, et al. Biochemical and histological changes after more than four years of treatment of ursodeoxycholic acid in primary biliary cirrhosis. Journal of Clinical Gastroenterology 1994;18(1):36‐41. [DOI] [PubMed] [Google Scholar]
Matsuzaki 1990 {published data only}
- Matsuzaki Y, Tanaka N, Osuga T, Aikawa T, Shoda J, Doi M, et al. Improvement of biliary enzyme levels and itching as a result of long‐term administration of ursodeoxycholic acid in primary biliary cirrhosis. American Journal of Gastroenterology 1990;85(1):15‐23. [PubMed] [Google Scholar]
MAYO‐II 1997 {published data only}
- Lindor KD, Jorgensen R, Theneau TM, Smith C, Mahoney DW, Dickson ER. Comparison of three different doses of ursodeoxycholic acid in the treatment of primary biliary cirrhosis: a randomised trial. Hepatology 1997;26:438 A. [DOI] [PubMed] [Google Scholar]
NEWARK‐I {published data only}
- Batta AK, Arora R, Salen G, Katz S. Ursodeoxycholic acid improves liver function and reduces serum and urinary endogenous bile acids in primary biliary cirrhosis. Hepatology 1988;8:1221A. [Google Scholar]
- Batta AK, Arora R, Salen G, Tint GS, Eskreis D, Katz S. Characterization of serum and urinary bile acids in patients with primary biliary cirrhosis by gas‐liquid chromatography‐mass spectrometry: effect of ursodeoxycholic acid treatment. Journal of Lipid Research 1989;30:1953‐62. [PubMed] [Google Scholar]
- Batta AK, Salen G, Arora R, Shefer S, Tint GS, Abroon J, et al. Effect of ursodeoxycholic acid on bile acid metabolism in primary biliary cirrhosis. Hepatology 1989;10:414‐9. [DOI] [PubMed] [Google Scholar]
- Eskreis D, Abroon J, Katz S, Salen G, Arora R. Ursodeoxycholic acid treatment of primary biliary cirrhosis. American Journal of Gastroenterology 1988;83:1065A. [Google Scholar]
NEWARK‐III {published data only}
- Batta AK, Salen G, Mirchandani R, Tint GS, Shefer S, Batta M, et al. Effect of long‐term treatment with ursodiol on clinical and biochemical features and biliary bile acid metabolism in patients with primary biliary cirrhosis. American Journal of Gastroenterology 1993;88:691‐700. [PubMed] [Google Scholar]
Ogino 1993 {published data only}
- Ogino H, Unoura M, Kawai H, Terasaki S, Yanagi M, Matsushita E, et al. Effect of ursodeoxycholic acid therapy on lymphocyte function of patients with primary biliary cirrhosis. Acta Hepatologica Japonica 1993;34(4):306‐12. [Google Scholar]
Okuyama 1988 {published data only}
- Okuyama S, Higuchi T, Ichimiya H, Hayashi H, Sakamoto N. A case of primary biliary cirrhosis ‐ 4 years' treatment with 300 mg/day ursodeoxycholic acid. Acta Hepatologica Japonica 1988;29(6):799‐802. [Google Scholar]
Osuga 1989 {published data only}
- Osuga T, Tanaka N, Matsuzaki Y, Aikawa T. Effect of ursodeoxycholic acid in chronic hepatitis and primary biliary cirrhosis. Digestive Diseases and Sciences 1989;34(12 Suppl):49S‐51S. [DOI] [PubMed] [Google Scholar]
Peridigoto 1992 {published data only}
- Perdigoto R, Wiesner RH. Progression of primary biliary cirrhosis with ursodeoxycholic acid therapy. Gastroenterology 1992;102(4):1389‐91. [PubMed] [Google Scholar]
Podda 1989 {published data only}
- Podda M, Ghezzi C, Battezzati PM, Bertolini E, Crosignan A, Petroni ML, et al. Ursodeoxycholic acid for chronic liver diseases. Journal of Clinical Gastroenterology 1988;10(Suppl 2):S25‐S31. [PubMed] [Google Scholar]
- Podda M, Ghezzi C, Battezzati PM, Bertolini E, Crosignani A, Petroni ML, et al. Effect of different doses of ursodeoxycholic acid in chronic liver disease. Digestive Diseases and Sciences 1989;34(12 Suppl):59S‐65S. [DOI] [PubMed] [Google Scholar]
Poupon 1987 {published data only}
- Poupon R, Chrétien Y, Poupon RE, Ballet F, Calmus Y, Darnis F. Is ursodeoxycholic acid an effective treatment for primary biliary cirrhosis?. Lancet 1987;1(8537):834‐6. [DOI] [PubMed] [Google Scholar]
Poupon 1989 {published data only}
- Poupon R, Balkau B, Legendre C, Lévy VG, Chrétien Y, Poupon RE. Ursodeoxycholic acid improves histologic features and progression of primary biliary cirrhosis. Hepatology 1989;10(4):637. [Google Scholar]
Poupon 1996 {published data only}
- Poupon RE, Huet PM, Poupon R, Bonnand A‐M, Nhieu JT, Zafrani ES, et al. A randomised trial comparing colchicine and ursodeoxycholic acid combination to ursodeoxycholic acid in primary biliary cirrhosis. Hepatology 1996;24(5):1098‐103. [DOI] [PubMed] [Google Scholar]
Schonfeld 1997 {published data only}
- Schonfeld JV, Breuer N, Zotz RB, Beste M, Goebell H. Serial quantitative liver function tests in patients with primary biliary cirrhosis: A prospective long‐term study. Digestion 1997;58(4):396‐401. [DOI] [PubMed] [Google Scholar]
Shibata 1992 {published data only}
- Shibata J, Fujiyama S, Honda Y, Sato T. Combination therapy with ursodeoxycholic acid and colchicine for primary biliary cirrhosis. Journal of Gastroenterology and Hepatology 1992;7(3):277‐82. [DOI] [PubMed] [Google Scholar]
Stiehl 1990 {published data only}
- Stiehl A, Rudolph G, Raedsch R, Moller B, Hopf U, Lotterer E, et al. Ursodeoxycholic acid‐induced changes of plasma and urinary bile acids in patients with primary biliary cirrhosis. Hepatology 1990;12(3 I):492‐7. [DOI] [PubMed] [Google Scholar]
Taha 1994 {published data only}
- Taha AS, Allison MC, Myara A, Trivin F, Duncan A, Russell RI. Does cholestyramine reduce the efficacy of ursodeoxycholic acid in primary biliary cirrhosis?. European Journal of Gastroenterology and Hepatology 1994;6(6):535‐8. [Google Scholar]
Takezaki 1991 {published data only}
- Takezaki E, Nishibayashi H, Murakami S, Kagawa K, Ohmori H, Kohda T, et al. A case of primary biliary cirrhosis with a histological improvement after long‐term therapy with ursodeoxycholic acid. IRYO Japanese Journal of National Medical Services 1991;45(4):376‐81. [Google Scholar]
Toda 1998 {published data only}
- Toda G, Tanaka N, Ikeda Y, Kobayashi K, Inoue K, Onji M, et al. Dose‐dependency of effect of ursodeoxycholic acid on primary biliary cirrhosis: a randomised, double‐blind controlled study. Kan‐Tan‐Sui (Japan) 1998;37:443‐60. [Google Scholar]
Unoura 1990 {published data only}
- Unoura M, Ogino H, Mizuno Y, Urabe T, Matsushita E, Kaneko S, et al. Effects of ursodeoxycholic acid on lymphocyte functions in primary biliary cirrhosis. XI International Bile Acid Meeting. Bile Acids as Therapeutic Agents ‐ From Basic Science to Clinical Practice. Freiburg. 1990:Abstract No. 77.
Van de Meeberg 1996 {published data only}
- Meeberg PC, Wolfhagen FH, Berge‐Henegouwen GP, Salemans JM, Tangerman A, Buuren HR, et al. Single or multiple dose ursodeoxycholic acid for cholestatic liver disease: biliary enrichment and biochemical response. Journal of Hepatology 1996;25:887‐94. [DOI] [PubMed] [Google Scholar]
Van Hoogstraten 1998 {published data only}
- Hoogstraten HJ, Smet MB, Renooij W, Breed JG, Engels LG, Ouden‐Muller JW, et al. A randomised trial in primary biliary cirrhosis comparing ursodeoxycholic acid in daily doses of either 10 mg/kg or 20 mg/kg. Dutch Multicentre PBC Study Group. Alimentary Pharmacology and Therapeutics 1998;12:965‐71. [DOI] [PubMed] [Google Scholar]
Verma 1999 {published data only}
- Verma A, Jazrawi RP, Ahmed HA, Davis T, Bland JM, Benson M, et al. Optimum dose of ursodeoxycholic acid in primary biliary cirrhosis. European Journal of Gastroenterology and Hepatology 1999;11(10):1069‐76. [DOI] [PubMed] [Google Scholar]
Wirth 1994 {published data only}
- Wirth HP, Meyenberger C, Altorfer J, Ammann R, Blum HE. Eosinophilia in primary biliary cirrhosis: Regression under therapy with ursodeoxycholic acid. Schweizerische Medizinische Wochenschrift 1994;124(19):810‐5. [PubMed] [Google Scholar]
Wirth 1995 {published data only}
- Wirth HP, Zala G, Meyenberger Ch, Ammann R. Subtype pattern of antimitochondrial antibodies in primary biliary cirrhosis and response to ursodeoxycholic acid. Schweizerische Medizinische Wochenschrift 1995;125(15):750‐4. [PubMed] [Google Scholar]
Wolfhagen 1994 {published data only}
- Wolfhagen FH, Buuren HR, Schalm SW. Combined treatment with ursodeoxycholic acid and prednisone in primary biliary cirrhosis. The Netherlands Journal of Medicine 1994;44:84‐90. [PubMed] [Google Scholar]
Yamazaki 1992 {published data only}
- Yamazaki M, Morimoto H, Wakabayashi T, Suzuki K, Kida H, Sugioka G, et al. A patient with asymptomatic primary biliary cirrhosis associated with eosinophilic infiltration and peripheral eosinophilia improved by the administration of ursodeoxycholic acid. Acta Hepatologica Japonica 1992;33(4):348‐52. [Google Scholar]
Yamazaki 1996 {published data only}
- Yamazaki K, Nakadate I, Suzuki K, Sato S, Masuda T. Eosinophilia in primary biliary cirrhosis. American Journal of Gastroenterology 1996;91(3):516‐22. [PubMed] [Google Scholar]
Yokomori 1996 {published data only}
- Yokomori H, Oda M, Kamegaya Y, Motoori T, Ohbu M, Ishii H. Rapid improvement of intractable pruritus in a case with primary biliary cirrhosis by a combined therapy of ursodeoxycholic acid (UDCA) and cholestyramine (CS) ‐ Serum bile acid analysis. Acta Hepatologica Japonica 1996;37(2):102‐8. [Google Scholar]
Additional references
AASLD 2010
- Silveira MG, Brunt EM, Heathcote J, Gores GJ, Lindor KD, Mayo MJ. American Association for the Study of Liver Diseases endpoints conference: design and endpoints for clinical trials in primary biliary cirrhosis. Hepatology 2010;52(1):349‐59. [DOI] [PubMed] [Google Scholar]
Ahrens 1950
- Ahrens EH Jr, Payne MA, Kunkel HG, Eisenmenger WJ, Blondheim SH. Primary biliary cirrhosis (classical article). Medicine‐Baltimore 1994;73(5):264‐80. [PubMed] [Google Scholar]
Angulo 1999a
- Angulo P, Dickson ER, Therneau TM, Jorgensen RA, Smith C, DeSotel CK, Lange SM, Anderson ML, Mahoney DW, Lindor KD. Comparison of three doses of ursodeoxycholic acid in the treatment of primary biliary cirrhosis: a randomised trial. Journal of Hepatology 1999;30(5):830‐5. [DOI] [PubMed] [Google Scholar]
Baker 2003
- Baker SG, Kramer BS. A perfect correlate dose not a surrogate make. BMC Medical Research Methodology 2003;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐1101. [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [DOI] [PubMed] [Google Scholar]
Crosignani 1991
- Crosignani A, Podda M, Battezzati PM, Bertolini E, Zuin M, Watson D, Setchell KD. Changes in bile acid composition in patients with primary biliary cirrhosis induced by ursodeoxycholic acid administration. Hepatology 1991;14(6):1000‐7. [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods of combining randomised clinical trials: strengths and limitations. Statistics in Medicine 1987;6:341‐8. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Dickson 1989
- Dickson ER, Grambsch PM, Fleming TR, Fisher LD, Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology 1989;10(1):1‐7. [DOI] [PubMed] [Google Scholar]
EASL 2009
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. Journal of Hepatology 2009;51(2):237‐67. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. British Medical Journal 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 2003
- Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study.. Health Technology Assessment 2003;7(1):1‐76. [PubMed] [Google Scholar]
Gluud 2001a
- Gluud C, Christensen E. Ursodeoxycholic acid for primary biliary cirrhosis ‐ lessons for the future. Journal of Hepatology 2001;34(5):787‐8. [DOI] [PubMed] [Google Scholar]
Gluud 2006
- Gluud LL. Bias in clinical intervention research. American Journal of Epidemiology 2006;163(6):493‐501. [DOI] [PubMed] [Google Scholar]
Gluud 2007
- Gluud C, Brok J, Gong Y, Koretz R. Hepatology may have problems with putative surrogate outcome measures. Journal of Hepatology 2007;46:734‐42. [DOI] [PubMed] [Google Scholar]
Gluud 2011
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2011, Issue 10. Art. No.: LIVER.
Goulis 1999
- Goulis J, Leandro G, Burroughs AK. Randomised controlled trials of ursodeoxycholic‐acid therapy for primary biliary cirrhosis: a meta‐analysis. Lancet 1999;354:1053‐60. [DOI] [PubMed] [Google Scholar]
Heathcote 2000
- Heathcote EJ. Management of primary biliary cirrhosis. The American Association for the Study of Liver Diseases practice guidelines. Hepatology 2000;31(4):1005‐13. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmann1994
- Hofmann AF. Pharmacology of ursodeoxycholic acid, an enterohepatic drug. Scandinavian Journal of Gastroenterology. Supplement 1994;204:1‐15. [DOI] [PubMed] [Google Scholar]
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. British Medical Journal (Clinical Research Ed.) 1999;319:670‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation of Technical Requiements for Registration of Pharmaceuticals for Human Use. Code of Federal Regulation & ICH Guidelines. Media: Parexel Barnett, 1997. [Google Scholar]
Jazrawi 1994
- Jazrawi RP, Caestecker JS, Goggin PM, Britten AJ, Joseph AEA, Maxwell JD, et al. Kinetics of hepatic bile acid handling in cholestatic liver disease: effect of ursodeoxycholic acid. Gastroenterogy 1994;106:134‐42. [DOI] [PubMed] [Google Scholar]
Kaplan 2005
- Kaplan MM, Gershwin ME. Primary biliary cirrhosis. New England Journal of Medicine 2005;353:1261‐73. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomised trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Kullak‐Ublick 2000
- Kullak‐Ublick GA, Stieger B, Hagenbuch B, Meier PJ. Hepatic transport of bile salts. Seminars in Liver Disease 2000;20:273‐92. [DOI] [PubMed] [Google Scholar]
Kürstein 2005
- Kürstein P, Gluud L, Willemann M, Olsen K, Kjellberg J, Sogaard J, et al. Agreement between reported use of interventions for liver diseases and research evidence in Cochrane systematic reviews. Journal of Hepatology 2005;43:984‐9. [DOI] [PubMed] [Google Scholar]
Lazaridis 2001
- Lazaridis KN, Gores GJ, Lindor KD. Ursodeoxycholic acid 'mechanisms of action and clinical use in hepatobiliary disorders. Journal of Hepatology 2001;35(1):134‐46. [DOI] [PubMed] [Google Scholar]
Lee 2005
- Lee YM, Kaplan MM. The natural history of PBC: has it changed?. Seminars in Liver Disease 2005;25(3):321‐6. [DOI] [PubMed] [Google Scholar]
Ludvig 1978
- Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Archiv. A. Pathological Anatomy and Histology 1978;379(2):103‐12. [DOI] [PubMed] [Google Scholar]
MacMahon 1949
- MacMahon HE, Thannhauser SJ. Xanthomatous biliary cirrhosis (a clinical syndrome). Annals of Internal Medicine 1949;30:121. [DOI] [PubMed] [Google Scholar]
Mayo 2005
- Mayo MJ. Patients and patience: the pitfalls of primary biliary cirrhosis trials. Nature Clinical Practise Gastroenterology & Hepatology 2005;2:552‐3. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Myszor 1990
- Myszor M, Turner I, Mitshison H, Bennett M, Burt AD, James OFW. No symptomatic or histological benefit from ursodeoxycholic acid treatment in PBC after 1 year. Controlled pilot study [IASL abstract]. Hepatology 1990;12:415. [Google Scholar]
Pares 2006
- Pares A, Caballeria L, Rodes J. Excellent long‐term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology 2006;130(3):715‐20. [DOI] [PubMed] [Google Scholar]
Pasha 1999
- Pasha T, Heathcote J, Gabriel S, Cauch‐Dudek K, Jorgensen R, Therneau T, et al. Cost‐effectiveness of ursodeoxycholic acid therapy in primary biliary cirrhosis. Hepatology 1999;29:21‐6. [DOI] [PubMed] [Google Scholar]
Paumgartner 2002
- Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology 2002;36(3):525‐31. [DOI] [PubMed] [Google Scholar]
Poupon 1997
- Poupon RE, Lindor KD, Cauch‐Dudek K, Dickson RE, Poupon R, Heathcote JE. Combined analysis of randomised controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology 1997;113:884‐90. [DOI] [PubMed] [Google Scholar]
Poupon 2000
- Poupon RE. Ursodeoxycholic acid for primary biliary cirrhosis: lessons from the past ‐ issues for the future. Journal of Hepatology 2000;32:685‐8. [DOI] [PubMed] [Google Scholar]
Poupon 2010
- Poupon R. Primary biliary cirrhosis: a 2010 update. Journal of Hepatology 2010;52(5):745‐58.. [DOI] [PubMed] [Google Scholar]
Prince 2002
- Prince M, Chetwynd A, Newman W, Metcalf JV, James OFW. Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: follow‐up for up to 28 years. Gatroenterology 2002;123:1044‐51. [DOI] [PubMed] [Google Scholar]
Prince 2003
- Prince MI, James OFW. The epidemiology of primary biliary cirrhosis. Clinics in Liver Disease 2003;7:795‐819. [DOI] [PubMed] [Google Scholar]
Prince 2004
- Prince MI, Chetwynd A, Craig WL, Metcalf JV, James OF. Asymptomatic primary biliary cirrhosis: clinical features, prognosis, and symptom progression in a large population based cohort. Gut 2004;53(6):865‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Royle 2003
- Royle P, Milne R. Literature searching for randomised controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Rust 2005
- Rust C, Beuers U. Medical treatment of primary biliary cirrhosis and primary sclerosing cholangitis. Clinical Review in Allergy & Immunology 2005;28(2):135‐45. [DOI] [PubMed] [Google Scholar]
Saksena 1997
- Saksena S, Tandon RK. Ursodeoxycholic acid in the treatment of liver diseases. Postgraduate Medical Journal 1997;73:75‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Savovic 2012
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, Als‐Nielsen B, Balk EM, Gluud C, Gluud LL, Ioannidis JP, Schulz KF, Beynon R, Welton NJ, Wood L, Moher D, Deeks JJ, Sterne JA. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Scheuer 1967
- Scheuer P. Primary biliary cirrhosis. Proceedings of the Royal Society of Medicine 1967;60(12):1257‐60. [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes, R, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Setchell 1996
- Setchell KD, Rodrigues CM, Podda M, Crosignani A. Metabolism of orally administered tauro‐ursodeoxycholic acid in patients with primary biliary cirrhosis. Gut 1996;38(3):439‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shapiro 1979
- Shapiro JM, Smith H, Schaffner F. Serum bilirubin: a prognostic factor in primary biliary. Gut 1979;20:137‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharp 1998
- Sharp SJ. Metaanalysis regression. STATA Techn Bull 1998:4216‐22. [Google Scholar]
Siegel 2003
- Siegel JL, Jorgensen R, Angulo P, Lindor KD. Treatment with ursodeoxycholic acid is associated with weight gain in patients with primary biliary cirrhosis. Journal of Clinical Gastroenterology 2003;37(2):183‐5. [DOI] [PubMed] [Google Scholar]
Simko 1994
- Simko V, Michael S, Prego V. Ursodeoxycholic therapy in chronic liver disease: a meta‐analysis in primary biliary cirrhosis and in chronic hepatitis. American Journal of Gastroenterology 1994;89:392‐8. [PubMed] [Google Scholar]
Stiehl 1999
- Stiehl A, Benz C, Sauer P. Mechanism of hepatoprotective action of bile salts in liver disease. Gastroenterology Clinics of North America 1999;28(1):195‐209, viii. [DOI] [PubMed] [Google Scholar]
Talwalker 2003
- Talwalkar JA, Lindor KD. Primary biliary cirrhosis. Lancet 2003;362:53‐61. [DOI] [PubMed] [Google Scholar]
Thompson 2002
- Thompson SG, Higgins JP. How should meta‐regression analyses be undertaken and interpreted?. Statistics in Medicine 2002;21(11):1559‐73. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
TSA manual 2011 [Computer program]
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for trial sequential analysis (TSA). Copenhagen, Denmark: Copenhagen Trial Unit, Centre for Clinical Intervention Research, 2011.
TSA program 2011 [Computer program]
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. Trial Sequential Analysis. Copenhagen, Denmark: Copenhagen Trial Unit, Centre for Clinical Intervention Research, 2011.
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. British Medical Journal (Clinical Research Ed.) 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Christensen 1997
- Christensen E, Gluud C. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database of Systematic Reviews 1997, Issue 4. [DOI] [PubMed] [Google Scholar]
Gluud 1999a
- Gluud C, Christensen E. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI] [PubMed] [Google Scholar]
Gluud 1999b
- Gluud C, Christensen E. Ursodeoxycholic acid (UDCA) in primary biliary cirrhosis (PBC) ‐ a Cochrane Hepato‐Biliary systematic review. Journal of Hepatology 1999;30(Suppl 1):83. [Google Scholar]
Gluud 2001b
- Gluud C, Christensen E. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD000551] [DOI] [PubMed] [Google Scholar]
Gong 2008
- Gong Y, Huang ZB, Christensen E, Gluud C. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database of Systematic Reviews 2008;16(3):CD000551. [DOI] [PubMed] [Google Scholar]


