Abstract
Background
Adherence to prescribed regimens is required to derive maximal benefit from many highly active antiretroviral therapy (HAART) regimens in people living with HIV/AIDS.
Objectives
To conduct a systematic review of the research literature on the effectiveness of patient support and education to improve adherence to HAART.
Search methods
A systematic search of electronic databases was performed from January 1996 to May 2005.
Selection criteria
Randomized controlled trials examining the effectiveness of patient support and education to improve adherence to HAART were considered for inclusion. Only those studies that measured adherence at a minimum of six weeks were included.
Data collection and analysis
Study selection, quality assessments and data abstraction were performed independently by two reviewers.
Main results
Nineteen studies involving a total of 2,159 participants met criteria for inclusion. It was not possible to conduct a meta‐analysis due to study heterogeneity with respect to populations, interventions, comparison groups, outcomes, and length of follow‐up. Sample sizes ranged from 22 to 367. The populations studied ranged from general HIV‐positive populations to studies focusing exclusively on children, women, Latinos, or adults with a history of alcohol dependence, to studies focusing almost exclusively on men. Study interventions included cognitive behavioral therapy, motivational interviewing, medication management strategies, and interventions indirectly targeting adherence, such as programs directed to reduce risky sexual behaviours. Ten studies demonstrated a beneficial effect of the intervention on adherence. We found that interventions targeting practical medication management skills, those administered to individuals vs groups, and those interventions delivered over 12 weeks or more were associated with improved adherence outcomes. We also found that interventions targeting marginalized populations such as women, Latinos, or patients with a past history of alcoholism were not successful at improving adherence. We were unable to determine whether effective adherence interventions were associated with improved virological or immunological outcomes. Most studies had several methodological shortcomings leaving them vulnerable to potential biases.
Authors' conclusions
We found evidence to support the effectiveness of patient support and education interventions intended to improve adherence to antiretroviral therapy. Interventions targeting practical medication management skills, those interventions administered to individuals vs groups, and those interventions delivered over 12 weeks or more were associated with improved adherence outcomes. There is a need for standardization and increased methodological rigour in the conduct of adherence trials.
Plain language summary
Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS
People living with HIV/AIDS are required to achieve high levels of adherence to benefit from many antiretroviral regimens. This review identified 19 studies involving a total of 2,159 participants that evaluated an intervention intended to improve adherence. Ten of these studies demonstrated a beneficial effect of the intervention. We found that interventions targeting practical medication management skills, those administered to individuals vs groups, and those interventions delivered over 12 weeks or more were associated with improved adherence to antiretroviral therapy. We also found that interventions targeting marginalized populations such as women, Latinos, or patients with a past history of alcoholism were not successful at improving adherence. We did not find studies that evaluated the quality of the patient‐provider relationship or the clinical setting. Most studies had several methodological shortcomings.
Background
High adherence to antiretroviral therapy is a key determinant of the degree and durability of viral suppression (Paterson 2000, Yeni 2002, Yeni 2004, Gross 2001, Wood 2003) and has been associated with lower rates of disease progression, hospitalization, and mortality (Press 2002). In contrast, poor adherence correlates with treatment failure (Yeni 2002, Nieuwkerk 2001, Haubrich 1999, Knobel 2001) and can limit options for future antiretroviral therapies due to cross‐resistance between HIV drugs. Thus, even brief periods of non‐adherence to certain antiretrovirals can have lifelong implications (Perno 2002, Wainberg 1998). Complicated HIV regimens, medication side effects and toxicities, high pill burdens, and psychosocial factors all contribute to non‐adherence for persons on HAART (Singh 1999, Ferguson 2002, Carpenter 2000, Ickovics 2002, Catz 2000, Ammassari 2002, Golin 2002). Patient characteristics and the nature of health‐care delivery are additional contributors (Ickovics 2002a, Ickovics 1997).
Previous studies have demonstrated that the rates of non‐adherence for medications within the general population can range from 15% to 93%, with the average rate being 50% non‐adherence for chronic health conditions (Haynes 1979, McDonald 2002). In a study of HIV treatment naïve patients, 95% adherence was associated with maximal therapeutic effects of antiretroviral therapy; however, only 30% of patients in the study were able to attain this level (Paterson 2000). While the optimal level of adherence may vary across regimens, strategies to improve adherence remain particularly important for persons taking antiretroviral medications.
Few systematic reviews of interventions to improve adherence to antiretroviral therapy have been published. Early reviews yielded a limited number of underpowered or poor quality studies (Haddad 2000, Fogarty 2002) and were inconclusive (Ickovics 2002a). More recent reviews have reported preliminary success of several interventions while reiterating the need for more rigour in the conduct of intervention trials (Cote 2005, Simoni 2003, Simoni 2006) . The most recent systematic review, which described the literature to December 2004 and performed a meta‐analysis of studies using between‐ and within‐group designs, found that the average intervention had a small and variable effect on adherence and that stronger effects were demonstrated in populations with poor baseline adherence (Amico 2006). However, this review included uncontrolled studies and excluded controlled studies that did not provide enough information to calculate an effect size.
Objectives
To conduct a systematic review of the research literature on the effectiveness of patient support strategies and education for improving adherence to highly active antiretroviral therapy (HAART) in people living with HIV/AIDS.
Methods
Criteria for considering studies for this review
Types of studies
We only considered randomized controlled trials (RCTs) for inclusion in this review because they are considered the gold standard to assess the effects of interventions. Carefully planned and properly conducted RCTs are thought to provide the highest strength of evidence to establish causality, considering their ability to minimize potential biases by controlling for both known and unknown confounding factors.
Types of participants
Studies of people infected with HIV and receiving highly active antiretroviral therapy (HAART) were included. HAART was defined as at least three antiretroviral drugs consisting of: at least one protease inhibitor; at least one non‐nucleoside reverse transcriptase inhibitor; or three or more nucleoside reverse transcriptase inhibitors, of which one was abacavir. We included studies of adults and children in which at least 80% of the study population had been prescribed HAART.
Types of interventions
We considered support‐ and education‐based interventions intended to improve adherence to HAART. The interventions, which could be directed to individuals or groups, included all types of patient education, counseling, support, health promotion, reminders, provision of resources, supervision, consultation, and telephone hotlines. We imposed no criteria concerning the "dose" or intensity of the intervention, but the control arm had to receive usual or standard adherence support or an alternate intervention of any type.
Types of outcome measures
We included studies that measured and reported at least one measure of adherence to HAART for both the intervention and control arms at least 6 weeks after study initiation. Possible measures included electronic monitoring, pill counts, medication diaries, patient self‐report, provider report, clinic and pharmacy records. Dropouts or withdrawals from drug trials were not considered measures of adherence. We recorded immunologic and virologic outcomes as secondary endpoints when available, but these were not a requirement for study selection.
Search methods for identification of studies
Electronic Databases The search strategy covered the literature from January 1996 (following the introduction of the first protease inhibitor) to May 2005. We made minor modifications to the search strategy over time to update the list of antiretroviral medications. We searched the following databases: Medline, CINAHL, HEALTHSTAR, Cochrane CENTRAL, PsycInfo, EMBASE, International Pharmaceutical Abstracts, Sociological Abstracts and Science Citation Index.
Conference Proceedings We also searched the following conference databases in the same time frame: Conference on Retroviruses and Opportunistic Infections (CROI), International AIDS Conference, International AIDS Society (IAS), Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), and the Canadian Association of HIV/AIDS Research (CAHR).
We employed the following librarian‐developed search strategy to identify studies relevant to the review. The strategy was modified as required for various databases.
1 exp human immunodeficiency virus/ (25780) 2 exp Human Immunodeficiency Virus Infection/ (55232) 3 exp Anti Human Immunodeficiency Virus Agent/ (1163) 4 exp Proteinase Inhibitor/ (44472) 5 exp Rna Directed DNA Polymerase Inhibitor/ (16016) 6 Protease inhibitors/ (8939) 7 NEVIRAPINE/ (3068) 8 DELAVIRDINE/ (1197) 9 INDINAVIR/ (4956) 10 NELFINAVIR/ (3157) 11 SAQUINAVIR/ (3651) 12 RITONAVIR/ (4212) 13 hiv.tw. (51525) 14 human immunodeficiency.tw. (19547) 15 acquired immunodeficiency syndrome.tw. (2837) 16 acquired immune deficiency syndrome.tw. (756) 17 aids.tw. (22140) 18 (Fosamprenavir or Atazanavir or Indinavir or Nelfinavir or Saquinavir or Ritonavir or Amprenavir or Lopinavir or ritonavir or kaletra or Tripanavir or tipranavir).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name] (7561) 19 (Nevirapine or Delavirdine or Efavirenz).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name] (4061) 20 Tenofovir.mp. (436) 21 (Abacavir or Stavudine or Didanosine or Lamivudine or Zidovudine or Zalcitabine or Combivir or "AZT + 3TC" or Trizivir or "AZT + 3TC + abacavir").mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name] (13735) 22 or/1‐21 (116766) 23 Patient Compliance/ (16643) 24 exp Patient Education/ (11307) 25 complian:.tw. (18802) 26 comply.tw. (1480) 27 complied.tw. (555) 28 noncomplian:.tw. (1510) 29 non‐complian:.tw. (1023) 30 adher:.tw. (19834) 31 non‐adher:.tw. (522) 32 nonadher:.tw. (836) 33 or/23‐32 (57959) 34 randomization/ (6224) 35 random:.tw. (136023) 36 Double Blind Procedure/ (30795) 37 Single Blind Procedure/ (3517) 38 Clinical Trial/ (210296) 39 Placebo/ (27809) 40 Methodology/ (26448) 41 exp comparative study/ (73529) 42 exp Evaluation/ (19562) 43 exp Follow Up/ (88126) 44 exp Prospective Study/ (25781) 45 Crossover Procedure/ (10286) 46 exp types of study/ or exp controlled study/ (2917667) 47 Meta Analysis/ (13759) 48 meta‐analy:.tw. (6674) 49 exp POSTMARKETING SURVEILLANCE/ (3124) 50 exp Case Control Study/ (6621) 51 exp Cohort Analysis/ (14622) 52 clinic: trial:.tw. (41235) 53 ((singl: or doubl: or tripl: or trebl:) adj (mask: or blind:)).tw. (29579) 54 placebo:.tw. (36863) 55 latin square:.tw. (343) 56 control:.tw. (492259) 57 prospectiv:.tw. (93042) 58 volunteer:.tw. (32818) 59 metaanaly:.tw. (465) 60 ((systematic: or quantitativ:) adj (review: or overview)).tw. (4319) 61 (medline or embase or cinahl or psycinfo or psychinfo or psyclit or psychlit or scisearch).tw. and review.pt. (3763) 62 data synthes:.tw. (1734) 63 usual care.tw. (820) 64 intervention.tw. (58279) 65 strateg$.tw. (97031) 66 enhance.tw. (34962) 67 improve.tw. (72635) 68 Major Clinical Study/ (398303) 69 treatment outcome/ (159281) 70 controlled study/ (1090196) 71 or/34‐70 (2983259) 72 22 and 33 and 71 (3199) 73 limit 72 to human (3004) 74 ((2002: or 2003:) not (200201: or 200202: or 200203: or 200204:)).em. (916265) 75 73 and 74 (1082) 76 from 75 keep 1‐500 (500) 77 from 75 keep 501‐1000 (500) 78 from 75 keep 1001‐1082 (82) 79 from 75 keep 1 (1)
Data collection and analysis
Study Selection Two reviewers independently assessed the studies for potential relevance by reading the abstract and title to eliminate obvious exclusions. If the abstract was unavailable, the full text paper was retrieved. We used a customized form reflecting the selection criteria to facilitate the selection process. Studies published in all languages were considered for inclusion.
Full‐text versions of the papers identified from the abstract screening process were subsequently retrieved and assessed independently by two reviewers for inclusion in the review. Full‐text papers were not examined in cases where both reviewers agreed that the study did not meet inclusion criteria. In the case of uncertainty regarding the relevance of a study, the authors of the study were contacted to clarify the necessary aspects of the study design. When disagreements about the relevance of a study emerged, the reviewers met to discuss their disagreement until consensus was reached. If consensus could not be reached, a third reviewer was available to arbitrate the decision. References from retrieved studies were also reviewed in an attempt to locate additional relevant studies.
Data Abstraction Data abstraction was performed independently by two reviewers. We attempted to contact study authors to obtain data identified as missing during data abstraction. Disagreements were discussed between the reviewers until consensus was reached. If consensus could not be reached, a third reviewer was invited to settle the disagreement.
Quality Assessment The methodological quality was appraised for the included studies. The purpose of this appraisal was not to exclude studies based on a pre‐determined cut‐off, but to discuss the overall quality of the studies available for inclusion. We created a list of quality criteria, based on Sackett (Sackett 1979) and the Consort Statement (Moher 2001, Altman 2001), outlining the following important design issues specific to adherence enhancing intervention studies:
Randomization Method: The method used to generate the random allocation sequence was described and appropriate. We were unable to judge whether or not the randomization method was appropriate when the authors used the following terms without elaboration: random, randomization, or random allocation. Following the Consort Statement (item 8a) (Moher 2001, Altman 2001), several sequence generation methods were considered appropriate, including the use of a random‐number table or a computerized random‐number generator.
Allocation Concealment: The method used to implement the random allocation sequence needed to be described and appropriate. This criterion refers to the requirement that the investigators or clinicians should not have prior information of the treatment group to which the patient would be randomized. Following the Consort Statement (Moher 2001, Altman 2001) (item 9), the authors needed to clarify whether or not the allocation sequence was concealed until the groups were assigned. The best methods to allocation concealment include the involvement of external sources, such as a central telephone randomization system. We also considered the use of opaque, sealed envelopes to be an appropriate concealment mechanism.
Objective Measure of Adherence: Subjective measures of adherence, such as patient self‐report, are commonly used but tend to yield overestimates (Sackett 1979). Objective measures of adherence include electronic monitoring, pill counts, pharmacy or clinic medication data, and direct observation.
Attention Control: Control patients should receive similar contact time with the adherence support provider as patients receiving the intervention (Sackett 1979). Otherwise, the observed effects may be due to either the intervention or the non‐specific effects of the increased attention paid to the intervention group (McDonald 2002).
Participants Lost to Follow‐up: Details regarding participants lost to follow‐up should be provided for both the intervention and control arms. We considered follow‐up to be successful if 80% or more of both the intervention and the control groups were available at the end of the study. Following the Consort Statement (Moher 2001, Altman 2001) (item 13a), attrition as a result of loss to follow up should be distinguished from exclusion, withdrawal from the study, and poor adherence to the protocol.
Length of Follow‐up: We considered at least 6 months from study initiation to be an adequate length of follow‐up because HAART regimens are life‐long and adherence interventions need to show persistent effects to be beneficial. In addition, we considered (as a separate criterion) whether follow‐up measurements were available for 80% or more of the participants in both the intervention and control groups at six months since study initiation.
Analysis of All Randomized Participants: This criterion refers to whether or not the investigators included in the analysis all the participants who underwent randomization and were analyzed in the groups to which they were randomized.
Results
Description of studies
We identified 7,512 citations from the searches conducted of the electronic databases from January 1996 to May 2005. Nineteen studies involving a total of 2,159 participants met criteria for inclusion (Berrien 2004, DiIorio 2003, Fairley 2003, Goujard 2003, Jones 2003, Knobel 1999, Levy 2004, Murphy 2002, Pradier 2003, Rathbun 2005, Rawlings 2003, Safren 2001, Safren 2003, Samet 2005, Smith 2003, Tuldra 2000, van Servellen 2003, Weber 2004, Wyatt 2004). The most common reasons for exclusion were the absence of a comparison group and the failure to report a measure of adherence for both the intervention and control groups at 6 weeks from study initiation. Studies were heterogeneous with respect to populations, interventions, comparison groups, outcomes, and lengths of follow‐up. Accordingly, we did not attempt to pool results using meta‐analysis.
All studies were randomized controlled trials and were published in English with the exception of one paper that was published in Spanish (Knobel 1999). Twelve studies were conducted in the US, two in Spain, two in France, two in Australia, and one in Switzerland. Sample sizes ranged from 22 to 367. Length of follow‐up ranged from 6 weeks to 15 months. While some studies focused exclusively on children (Berrien 2004), women (Jones 2003, Wyatt 2004), Latinos (van Servellen 2003), or adults with a history of alcohol dependence (Samet 2005), others included all patients with HIV, some of which had a large proportion of men in the study population (>80%). Six studies specifically targeted patients who reported difficulties with adherence or who presented with poor adherence at study baseline (Safren 2001, Murphy 2002, Jones 2003, van Servellen 2003, Safren 2003; Weber 2004). The duration of the interventions ranged from a single session to multiple sessions delivered over one year. Five studies included participants who were either antiretroviral naïve or switching to a new antiretroviral regimen (Knobel 1999, Tuldra 2000, Smith 2003, Rawlings 2003, Rathbun 2005); the remainder included treatment‐experienced participants who were taking antiretroviral medications at baseline.
The types of interventions varied from cognitive behavioral therapy, motivational interviewing, or medication management strategies, to interventions indirectly targeting adherence such as programs directed to reduce risky sexual behaviours. All interventions were directed at patients, individually or in groups, rather than at providers or health care systems. The interventions were delivered by lay individuals, health advocates, social workers, psychologists, nurses, pharmacists, and physicians. Adherence outcomes were calculated as dichotomous and/or continuous variables, ranging from the proportion of participants reaching a pre‐determined threshold of adherence (e.g., ³95% adherence) to the mean difference in adherence levels from the end of the study to baseline. In general, the overall level of adherence at baseline in most studies ranged between 55% and 95%, which is consistent with reported estimates from other studies (Paterson 2000, Bangsberg 2002, Bangsberg 2000, Bangsberg 2001). Between 68% and 82% of study populations reported 95% or greater adherence at baseline, which is also consistent with the wide range seen in past studies (Paterson 2000, Hugen 2002, Howard 2002). Virological or immunological outcomes were reported in 12 studies and ranged from the number of participants with a viral load above a pre‐determined level to a mean difference in viral load from the end of the study to baseline. Some statistical analyses controlled for baseline values, others used the differences between follow‐up and baseline values, and others used only follow‐up and neglected baseline values.
Risk of bias in included studies
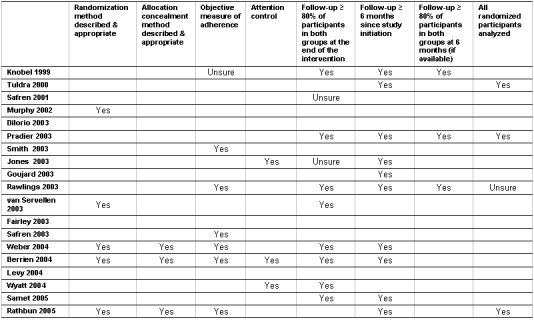
The quality assessment showed that the examined studies had several methodological shortcomings (Figure 1). In general, the quality of the studies was weak leaving most of the studies vulnerable to several potential biases. Because there were so few studies of good quality, we were unable to assess the effect of quality on study outcomes.
1.
Quality assessment.
The randomization method was described and was considered appropriate in only 5 studies (Murphy 2002, van Servellen 2003, Weber 2004, Berrien 2004, Rathbun 2005). All the included studies implemented the traditional two‐group, parallel, randomized controlled trial design with the exception of three studies. Two studies from the same research group (Fairley 2003, Levy 2004) used an unconventional randomized design (a "Stepped‐Wedge" design) while another study (Wyatt 2004) used a time‐lagged wait‐list control.
We identified four design features that may have resulted in an overestimation of the treatment effects:
(1) Allocation concealment: Only 3 studies described the method of allocation concealment (Weber 2004, Berrien 2004, Rathbun 2005). It has been shown that studies that failed to report allocation concealment may overestimate the intervention effect by 41% and studies that incompletely report allocation concealment may overestimate the intervention effect by 30% (Schulz 1995).
(2) Objective measure of adherence: Only 6 studies used an objective measure of adherence (Smith 2003, Rawlings 2003, Safren 2003, Weber 2004, Berrien 2004, Rathbun 2005) (an additional study used an objective measure of adherence to corroborate the validity of the self‐reports (Knobel 1999)). A major limitation of using subjective reports is low sensitivity (i.e., many patients with low adherence would not be identified by self‐report). In several studies where self‐reported adherence methods were compared to Medication Event Monitoring System (MEMS) methods, self‐reported methods have consistently overestimated adherence by 5‐20% (Liu 2001, Miller 2000, Gallant 1998).
(3) Analysis of all randomized participants: Only 3 studies analyzed all randomized participants (Tuldra 2000, Pradier 2003, Rathbun 2005). The conclusions derived from studies that fail to analyze all randomized participants can be misleading, particularly when there are imbalances between the intervention and control groups. Only two studies reported a method to handle missing data. One study imputed adherence rates by regression and the other study imputed missing data as last observation carried forward.
(4) Attention control: Only 3 studies reported that participants in both the intervention and control groups received a similar amount of contact time (Jones 2003, Berrien 2004, Wyatt 2004). This factor is particularly important when the intervention involves a considerable amount of contact time with the participants. If the contact time variable is not accounted for, the treatment effects may be the result of the non‐specific effects of the increased attention paid to the intervention group as opposed to the intervention itself (McDonald 2002).
Regarding loss of participants to follow‐up, less than half the studies (8 studies) reported following up successfully 80% or more of the participants in both the intervention and control groups at the end of the intervention (Knobel 1999, Pradier 2003, Rawlings 2003, van Servellen 2003, Weber 2004, Berrien 2004, Wyatt 2004, Samet 2005) (this was unclear for two additional studies (Safren 2001, Jones 2003)). A high level of attrition is a particular concern for adherence trials given the observation that adherence trials may already be intrinsically biased since they focus on participants who are already willing to take part on studies (McDonald 2002).
In addition, regarding the length of the follow‐up, just over half the studies (10 studies) reported follow‐up times of 6 months or more since study initiation (Knobel 1999, Tuldra 2000, Pradier 2003, Jones 2003, Goujard 2003, Rawlings 2003, Weber 2004, Berrien 2004, Samet 2005, Rathbun 2005). This is particularly important for interventions designed to improve adherence to long‐term treatments, such as HAART. Of these, however, only 3 studies reported 80% or more participants in both the intervention and control groups at six months (Knobel 1999, Pradier 2003, Rawlings 2003).
Effects of interventions
Effects on Adherence Outcomes Ten studies demonstrated a beneficial effect of the intervention on antiretroviral adherence (Knobel 1999, Tuldra 2000, Pradier 2003, Smith 2003, Goujard 2003, Fairley 2003, Safren 2003, Weber 2004, Berrien 2004, Levy 2004), eight studies failed to demonstrate an improved level of adherence resulting from the intervention (Safren 2001, Murphy 2002, DiIorio 2003, Rawlings 2003, van Servellen 2003, Wyatt 2004, Samet 2005, Rathbun 2005), and we were unable to determine a clear presence or absence of a benefit for one study because it failed to report a between‐group comparison (Jones 2003).
We examined a number of intervention features to identify any recurring characteristics that may be linked to successful adherence outcomes. This semi‐qualitative approach has been used by others to help identify the relationship between intervention characteristics and study outcomes (Kawamoto 2005). Features that tended to be associated with success included those interventions provided to individuals vs groups, those provided over longer periods of time (³ 12 weeks), and those interventions targeting practical medication management skills vs cognitive behavioral or motivational approaches.
When interventions were provided at the individual level, 67% (10/15) were successful (Knobel 1999, Tuldra 2000, Pradier 2003, Smith 2003, Goujard 2003, Fairley 2003, Safren 2003, Weber 2004, Berrien 2004, Levy 2004) compared to a 0% (0/4) success rate observed with interventions provided in a group setting. In addition, 86% (6/7) of the interventions provided over 12 weeks or more successfully improved adherence (Knobel 1999, Pradier 2003, Smith 2003, Goujard 2003, Safren 2003, Berrien 2004) compared to those interventions delivered under 12 weeks (0/8 or 0% success rate). Interventions that targeted the improvement of patient's medication management skills were fairly successful (6/8 or 75% success rate) (Tuldra 2000, Smith 2003, Goujard 2003, Fairley 2003, Safren 2003, Levy 2004) when compared to interventions designed around cognitive behavioral therapy and motivational interviewing approaches (1/7 or 14% success rate) (Weber 2004). The interventions that focused on medication management skills consistently used reminders or memory aids, such as beepers, alarms, medication boxes, planning cards, paging systems, text messaging, or visual aids (i.e., a study had to provide these reminders exclusively to the intervention group for that study to be labeled as having this feature; we excluded from this category those studies that also provided these reminders to the control group). In general, these interventions were designed to improve the levels of adherence by improving the participant's medication management skills (e.g., medication information, tailored drug schedules, medication dossettes, side‐effect management, and reminder devices) and by helping patients identify and address barriers to adherence (e.g., problems patients might face in real‐life situations or strategies to manage side‐effects).
Several of the studies that looked at marginalized populations such as women (Wyatt 2004), Latinos (van Servellen 2003), or patients with a past history of alcoholism (Samet 2005) were not successful at improving adherence. This may suggest that a different approach needs to be developed in order to design effective interventions for these populations.
The presence of inconsistent outcomes or intervention features that appeared in only a small number of studies limited our ability to reach conclusions about the relationship between other types of interventions and outcomes. We were unable to ascertain whether success was associated with the type of provider administering the intervention, the number of visits over which the intervention was provided, the antiretroviral experiences of patients, delivery at tertiary or academic centers, or the targeting of patients reporting poor adherence at baseline.
We were unable to determine the effect of the type of provider because many interventions were delivered by different combinations of providers (e.g., pharmacists/ nurses, physicians/nurses) and it was not feasible to disentangle the effects of a single provider or a particular combination of providers. The effect of providing the intervention over multiple visits was also difficult to interpret as six out of twelve interventions (50%) that included 3 visits or more were successful, but 3 out of 4 (75%) that included less than 3 visits were also successful. Whether the intervention was provided to patients already on established regimens or those initiating/switching to a new regimen affected the success of the interventions was also difficult to interpret. Three of the five studies (60%) that offered the intervention to patients initiating or switching to new antiretroviral regimens were positive studies, compared to 7 of 14 studies targeting patients who were already on existing regimens (50% success rate). Even though the interventions that were implemented in tertiary or academic centers had a 62% success rate (8/13), those interventions that were delivered in non‐tertiary centers had a 33% success rate (1/3). Lastly, two out of six interventions (33%) that targeted patients presenting with poor adherence or who reported difficulties with adherence at baseline were successful while 8 out of 13 studies (62%) that did not mention whether or not they targeted patients reporting poor adherence were also successful.
Effects on Virological and/or Immunological Outcomes We were unable to determine whether improved adherence extended to improved viral or immunologic outcomes. Six studies included virological outcomes only (Tuldra 2000, Pradier 2003, Smith 2003, Weber 2004, Berrien 2004, Rathbun 2005) and six studies included both virological and immunological outcomes (Knobel 1999, Goujard 2003, Rawlings 2003, Fairley 2003, Levy 2004, Samet 2005). Only 5 out of 12 studies that reported virological and/or immunological results found at least one significant effect associated with the intervention (Knobel 1999, Tuldra 2000, Pradier 2003, Samet 2005, Rathbun 2005). These studies reported conflicting statistical findings. For example, three studies reported a statistical significant result for one outcome (e.g., reduction in viral load) but not another (e.g., proportion of patients with a viral load below a pre‐determined level) (Knobel 1999, Pradier 2003, Samet 2005). Another study reported a significant finding when the analysis included only those participants who completed the study but not when the analysis included all those participants that were randomized at study entry (Tuldra 2000). Another study reported statistically significant findings for some points in time (e.g., week 4 and 28), but not for others (e.g, week 16) (Rathbun 2005).
Discussion
We found evidence to support the effectiveness of some types of patient support and education interventions in improving adherence to antiretroviral therapy. Overall, several key intervention features such as interventions delivered to individuals vs groups, those delivered over 12 weeks or more, and those interventions targeting practical medication management skills vs cognitive behavioural or motivational approaches, were present in many of the successful intervention studies. To our knowledge, our systematic review is the first to suggest that interventions directly targeting practical medication management skills tended to be effective at improving adherence, and that interventions targeting psychological constructs (e.g., self‐efficacy, attitudes, motivation, emotions, stress management, cognitive distortions and automatic thoughts) may be ineffective or may take longer to have an impact.
We also found that interventions targeting marginalized groups such as women, Latinos, or patients with a past history of alcohol dependence were not associated with improved adherence.
We were unable to determine whether the advantage associated with adherence outcomes translated to improved virological or immunological outcomes. This finding may be attributable to participant selection, since laboratory responses may be more difficult to demonstrate in patients using antiretroviral drugs at study baseline, or due to time factors, since virological and immunological responses may lag behind improved adherence. Alternatively, our results may indicate that average incremental improvements in adherence are nevertheless too small to improve clinical outcomes. Studies of adequate power and duration are required to resolve this question.
Our findings are in contrast to a recent systematic review (Amico 2006), which found that patients reporting low levels of adherence at baseline benefited the most from adherence interventions. This difference may be the result of differing inclusion criteria. For example, while we included only randomized controlled trials, Amico et al included both between‐ and within‐group designs (Amico 2006). Furthermore, we included 9 more randomized controlled trials because our search window extended until May 2005 and we included all eligible studies, even if we could not calculate effect sizes.
Inconsistencies and small number of studies limited our ability to reach conclusions about the effectiveness of the type of providers, the type of settings (e.g., tertiary vs community settings), the status of antiretroviral regimen at the time of consultation (whether patients are initiating a new regimen, switching to a new regimen, or already on an existing regimen), or the "dose" or intensity of the interventions.
There were several limitations to our systematic review. We were unable to pool the data for meta‐analysis because of significant heterogeneity in many study features. As well, we were unable to assess the clinical significance of the reported adherence outcomes. Such concerns may be particularly important for future studies as the understanding of optimal adherence evolves. In addition, some studies included unvalidated adherence measures (e.g., an ordinal score ranging from one to four (Goujard 2003) or the proportion of patients reporting being adherent on 14 consecutive days (Wyatt 2004)).
Lastly, some of the included studies were conceived as pilot studies, a development that is consistent with the increasing level of maturity of any new technology. These studies, either by definition or as a result of feasibility issues, may have lacked the power to detect meaningful differences between the intervention and control groups. McDonald et al suggested as a rule of thumb that adherence studies require the inclusion of at least 60 participants per group in order to have 80% power to detect an absolute difference of 25% in the proportion of participants judged to have adequate adherence (McDonald 2002). Only 7 studies met this rule of thumb (Knobel 1999, Pradier 2003, Jones 2003, Goujard 2003, Rawlings 2003, Wyatt 2004, Samet 2005). Of these, three reported a statistical advantage favouring the intervention (Knobel 1999, Pradier 2003, Goujard 2003) and an additional one reported an improvement in mean CD4 cell counts (Samet 2005) (this study did not report significant findings for the adherence outcome).
Authors' conclusions
Implications for practice.
This systematic review found evidence to support the effectiveness of some patient support and education interventions to improve adherence to antiretroviral therapy. We found that those interventions targeting practical medication management skills, those administered to individuals vs groups, and those delivered over 12 weeks or more could improve adherence to antiretroviral therapy. We did not find evidence to suggest that interventions targeting more complex psychological constructs were effective at improving adherence outcomes. We also failed to find evidence that interventions targeting marginalized populations such as women, Latinos, or patients with a past history of alcoholism were successful at improving adherence. Further studies should focus on medication management skills interventions to confirm the effectiveness of these approaches and the generalizability of these results to a wider range of populations.
We did not find any studies that examined the effectiveness of provider‐level interventions (e.g., those interventions that provide feedback to practitioners) and system‐level interventions (e.g., those interventions that address access and affordability to services). Future efforts may need to look at the effectiveness of these interventions as evidence suggests that the quality of the patient‐provider relationship and clinical setting are also important influences on adherence (Ickovics 2002a).
Implications for research.
There is a need for standardization and improved methodological rigour in the conduct of adherence trials. Adherence studies should follow a randomized design that implements and reports appropriate randomization and allocation concealment methods, uses an objective measure of adherence, follows up participants over at least 6 months since study initiation, and analyzes patients to the groups to which they were randomized.
The statistical analyses should also control for baseline values or use the differences between follow‐up and baseline values as the main outcome to make sure that the intervention and control groups are equivalent at study entry. In addition, adherence trials need to be powered to detect clinically significant differences using appropriate adherence outcomes that are clearly specified a priori.
What's new
| Date | Event | Description |
|---|---|---|
| 29 October 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 3, 2000
| Date | Event | Description |
|---|---|---|
| 24 May 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Ms. Angela Eady, MLS, McMaster University Library for her help in literature searching, defining terms and developing search strategies. We would also like to thank the following: Ms Sarah Lyons, Ontario Institute for Studies in Education, University of Toronto and Neurobehavioural Research Unit, St. Michael's Hospital; Ms Lorraine Lee, Queens University, for their assistance in reviewing abstracts and studies for this review.
Finally, thanks to Damian Rzeznikiewiz, Kinesiology and Health Science, York University, for his help in creating the tables and entering the references.
We gratefully acknowledge the support of the Ontario Ministry of Health and Long‐Term Care (MOHLTC). The opinions, results, and conclusions are those of the authors and no endorsement by the Ministry is intended or should be inferred.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Berrien 2004.
| Methods | RCT Setting: Tertiary outpatient clinic (Connecticut, US) Adherence measure(s): Pharmacy refill history (scored from 0‐3) (3 points: monthly; 2 points: less than monthly; 1 point: much less than monthly; 0 points: never) Self‐report (10 questions for a maximum score of 37, 37‐perfect adherence) |
|
| Participants | Children/Youth Mean age:10 (range 1.5‐20) 45‐55% Male 50‐64% Hispanic 29‐35% African‐ American | |
| Interventions | Intervention: Individual education program designed to improve knowledge of HIV & resolve barriers to adherence, delivered by a nurse in 8 home visits over 3 months (including aids). The interventions was also offered in Spanish Control: Standard medication adherence education, including visual aids, medication boxes, beepers & emotional support + 1 home visit |
|
| Outcomes | Adherence outcome(s): Mean pharmacy refill score at 3 or 8 months Follow‐up: I: 2.7 C: 1.7 p‐value between groups: .002 Difference in mean adherence scores at 3 or 8 months Baseline: I:32.2 (1.1) C:31.7(.61) Follow‐up: I:34.8 (.41) C:31.9(1.0) Change: I: 2.7 (.88) C: .2 (.96) p‐value between groups: .07 |
|
| Notes | Severity: CDC‐C: 10%‐24%, Mean CD4: 839‐861, Mean VL: 3.67‐3.92 Regimen at study baseline: Already existing regimen Duration of intervention: 3 months Length of follow‐up: Average of 8 months? |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
DiIorio 2003.
| Methods | RCT Setting: Clinic in large city (US) Adherence measure(s): Self‐report 5‐item scale recording ease and ability to be adherent in previous 30 days |
|
| Participants | Mean age: 42(SD 8) 53% Male 82% Single 88% African American | |
| Interventions | Intervention: 3 motivational interviewing sessions delivered 2 weeks apart by trained and supervised nurses + motivational materials (e.g., videotape, journal, calendar) Control: Usual adherence education |
|
| Outcomes | Adherence outcome(s): Mean adherence from a scale with a total score ranging from 5 to 30 Follow‐up: I: 26.5(4.8) C: 23.4 (5.1) p‐value between groups: .22 |
|
| Notes | Severity: Mean Yrs since HIV Dx: 11(4), CD4<200 at clinic entry Regimen at study baseline: Already existing regimen Duration of intervention: 6 weeks Length of follow‐up: 8 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Fairley 2003.
| Methods | RCT Setting: Sexual health centre (Melbourne, Australia) Adherence measure(s): Self‐report (number of missed doses in previous 4, 7 & 28 days) |
|
| Participants | Mean age: 38 95‐100% Male 90‐91% MSM 5‐14% IDU Difficulty with adherence | |
| Interventions | Intervention: Individual education program delivered by a nurse in a single session (2‐3 hours) including regimen analysis, computer‐generated medication planner, and a choice of a variety of aids (e.g., short message service and medication alarm). Nurse or pharmacist available for advice after clinic hours Control: Usual care. Delay in receiving the education program |
|
| Outcomes | Adherence outcome(s):
Mean number of missed doses Baseline: 4 days: .76 (SD 1.5) 7 days: 1.5 (SD 2.5) 28 days 2.5 (SD 4.1) Follow‐up: 4 days .38 (SD .9) 7 days .74 (SD 1.5) 28 days: 2.5 (SD 6.3) p‐value between groups: 4 days .03 7 days .008 28 days .96 |
|
| Notes | Severity: Mean VL: 3.71, Mean CD4: 513, Undetectable VL: 73% Regimen at study baseline: Already existing regimen Duration of intervention: Variable (1 visit + electronic reminder for up to 20 weeks) Length of follow‐up: Up to 20 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Goujard 2003.
| Methods | RCT Setting: Tertiary outpatient clinic (France) Adherence measure(s): Self‐report (global adherence score based on non‐specified qualitative criteria) |
|
| Participants | Mean age: 41 80% Male | |
| Interventions | Intervention: Educational program of 4 individual 1‐hour sessions, delivered over 12 months by trained professionals, including provision of pill boxes and therapeutic planning cards Control: Provision of a therapeutic planning card at enrolment |
|
| Outcomes | Adherence outcome(s): Adherence ordinal score ranging from 1 (poor) to 4 (good)
Change:
6 months:
I:+.25
C:‐.19 12 months: I:+.22 C:‐.05 p‐value between groups: 6 months: .025 12 months: .22 |
|
| Notes | Severity: AIDS: 33%, CD4 <200 : 17%, CD4 200‐500: 49%, CD4>500: 34%, VL<200: 55%, VL<5k: 23%, VL>5k: 23% Regimen at study baseline: Already existing regimen Duration of intervention: Minimum of 3 sessions during first 12 months Length of follow‐up: 6 months + 12 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Jones 2003.
| Methods | RCT Setting: Tertiary outpatient clinics & Community health centres (Miami, NY, NJ; US) Adherence measure(s): Self‐report (rate of adherence over the previous 7 days) |
|
| Participants | 100% Women Mean age: 37(+/‐10) 76% unemployed 54% African‐ American 18% Hispanic 55% History of drug dependence | |
| Interventions | Intervention: 10 weekly 2‐hr group cognitive‐behavioural stress management/expressive supportive therapy sessions delivered by trained and supervised therapists Control: 10 weekly 2‐hr individual informational and educational sessions (including entertainment tapes) |
|
| Outcomes | Adherence outcome(s):
Mean adherence at week 10‐baseline Change: I: +30.4% C:+19.6% p‐value within groups: I: <.01 C: >.05 |
|
| Notes | Severity: AIDS: 100% Regimen at study baseline: Already existing regimen Duration of intervention: 10 weeks Length of follow‐up: 15 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Knobel 1999.
| Methods | RCT Setting: Tertiary outpatient clinic (Barcelona, Spain) Adherence measure(s): Self‐report questions + Pill counts every 2 months |
|
| Participants | Age: 36(+/‐ 8) 73% Male 48‐50% current or past IDU | |
| Interventions | Intervention: Individual intervention consisting of educational and supportive counseling delivered by a pharmacist at first medication fill + telephone support + monthly visits to outpatient unit Control: Conventional dispensing of pills every two months |
|
| Outcomes | Adherence outcome(s):
Proportion of patients reaching >=90% adherence at week 24 Follow‐up: I: 77% (46/60) C:53% (58/110) RR=1.45 (1.16‐1.82) p‐value between groups: .002 |
|
| Notes | Severity: CD4: 232‐245*, VL: 5.02‐5.15†, CDC‐C: 32% Regimen at study baseline: Initial regimen Duration of intervention: 24 weeks Length of follow‐up: 24 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Levy 2004.
| Methods | RCT Setting: Tertiary outpatient clinic (Melbourne, Australia) Adherence measure(s): Self‐report |
|
| Participants | Mean age: 41‐42 81‐88% Male 38‐58% MSM 21‐25% IDU 0‐22% Higher education | |
| Interventions | Intervention: Individual education program delivered by a pharmacist and/or nurse in a single session (2 hours) including counseling, a computerized medication planner and adherence aids (i.e., dosette boxes and electronic alarms). Pharmacist available for advice after clinic hours Control: Usual care. Delay in receiving the education program |
|
| Outcomes | Adherence outcome(s):
Mean number of missed doses in previous 4, 7 & 28 days, measured on monthly basis Baseline: 4 days: 1.9 (SD 3.0) 7 days: 3.0 (SD 4.1) 28 days: 7.4 (SD 11.5) Follow‐up: 4 days: 1.0 (SD 2.6) 7 days: 1.8 (SD 3.7) 28 days: 4.2 (SD 8.3) p‐value between groups: 4 days: <.001 7 days: <.001 28 days: <.001 |
|
| Notes | Severity: Mean CD4: 382, Mean VL: 4.34 Regimen at study baseline: Already existing regimen Duration of intervention: Variable (1 visit + electronic reminder for up to 20 weeks) Length of follow‐up: Up to 20 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Murphy 2002.
| Methods | RCT Setting: Tertiary outpatient clinic (California, US) Adherence measure(s): Self‐report (percent of medications taken in previous 3 days) |
|
| Participants | Mean age: 39(SD 7) 88% Male 46% African American 18% Hispanic 73% Unemployed Poor adherence at study baseline | |
| Interventions | Intervention: 3 group and 2 individual cognitive behavioural sessions (including role‐playing and homework assignments) delivered by a psychologist and a nurse, over 7 weeks, using a manual Control: Regular care provided at the clinic. One 30 minute consultation and medication schedule |
|
| Outcomes | Adherence outcome(s):
Mean adherence (SD) at week 7‐8 & 20 Baseline: I: 69% (41%) C: 62% (46%) Follow‐up: 7‐8 wks: I: 87% (SD 30%) C: 87% (SD 28%) 20 wks: I: 86% (SD 33%) C: 83% (SD 36%) Change: 7‐8 wks: +18% (SD 35%) +25% (SD 45%) 20 wks: +18% (SD 48%) +21% (SD 41%) p‐value between groups: 7‐8 wks: .62 20 wks: .82 |
|
| Notes | Severity: Mean CD4: 340(291), VL<400: 33%, VL<10k: 30%, VL<50k: 15%, VL>50k: 9% Regimen at study baseline: Already existing regimen Duration of intervention: 7‐8 weeks Length of follow‐up: 20 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pradier 2003.
| Methods | RCT Setting: Tertiary outpatient clinic (Nice, France) Adherence measure(s): Self‐report (doses taken/ prescribed in previous 4 days) |
|
| Participants | Median age: 38‐40 87‐91% Male 64‐70% Single 30‐33% IDU transmission 24‐25% Unemployed | |
| Interventions | Intervention: 3 individual counseling and educational sessions (addressing cognitive, emotional, behavioral and social components) delivered by trained, supervised nurses using a manual (at baseline, 2 and 4 months) Control: Standard of care (usual clinical follow‐up; medical consultations every 2‐3 months) |
|
| Outcomes | Adherence outcome(s):
Proportion of patients reaching 100% adherence at month 6 Baseline: I: 58% C: 63% Follow‐up: I: 75% C: 61% Change: OR=2.5±‡§ (1.4‐4.2) p‐value within groups: I:.004 C: >.05 p‐value between groups: .001 |
|
| Notes | Severity: CDC‐C: 30‐32%, Median CD4: 340‐361, Mean VL: 2.6‐2.7, Undetectable VL: 40‐41% Regimen at study baseline: Already existing regimen Duration of intervention: 4 months Length of follow‐up: 6 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rathbun 2005.
| Methods | RCT Setting: Tertiary clinic (Oklahoma, US) Adherence measure(s): Electronic monitoring (doses consumed/ prescribed) |
|
| Participants | Adult Indigents Median age: 38 85% Male 65‐75% Caucasian 63‐76% MSM 6‐19% IDU transmission | |
| Interventions | Intervention: Education on adherence strategies and monitoring of patient progress, including visual aids and reminder devices, delivered by pharmacists in clinic visits and phone follow‐up for up to 12 weeks Control: Standard of care, delivered by patient's primary care providers (physician or nurse) |
|
| Outcomes | Adherence outcome(s):
Mean adherence rate at 4, 16 & 28 weeks (number of consumed/ prescribed doses)¦ Follow‐up: Week 4: I: 86% (SD 27%) C: 73% (SD 32%) Week 16: I: 77% (SD 28%) C: 56% (SD 39%) Week 28: I: 74% (SD 31%) C: 51% (SD 41%) p‐value between groups: Week 4: .23 Week 16: .08 Week 28: .08 |
|
| Notes | Severity: Median CD4: 103‐296, Median VL: 4.34‐ 5.25, AIDS: 79%, CDC C: 56‐59% Regimen at study baseline: Initial or switching to new regimen Duration of intervention: 2 weeks (additional visits allowed up to 12 weeks for participants needing more assistance) Length of follow‐up: 28 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Rawlings 2003.
| Methods | RCT Setting: Tertiary outpatient clinic (25 sites, US) Adherence measure(s): Electronic monitoring (doses observed/ expected in 24 weeks) |
|
| Participants | Mean age: 37(SD 9) 65% Male 71% African American 21% Hispanic 20% current IDU 43% MSM transmission 91% Treatment naïve | |
| Interventions | Intervention: Group educational program delivered in 4 sessions (one per week) to patients and caregivers and facilitated by a health care professional with skills‐building exercises, including videotapes, charts and patient logbooks + Routine counselling Control: Routine counseling alone |
|
| Outcomes | Adherence outcome(s):
Mean overall adherence in 24 weeks Follow‐up: I: 70% C: 74% p‐value between groups: >.05 |
|
| Notes | Severity: Median CD4: 379, Median log VL: 4.18, CDC Asympt: 80%, CDC AIDS: 2% Regimen at study baseline: Initial regimen Duration of intervention: 4 weeks Length of follow‐up: 24 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Safren 2001.
| Methods | RCT Setting: Community health centre (Boston, US) Adherence measure(s): Self‐report (pills taken/ prescribed in previous 2 weeks) |
|
| Participants | Mean age: 41(SD 8) 88% Male 29% African American 20% Hispanic 67% MSM Poor adherence at study baseline | |
| Interventions | Intervention: Single‐session individual intervention utilizing cognitive‐behavioral, problem‐solving, and motivational interviewing techniques, including a videotape presentation + one follow‐up telephone review (10 min) one week later Control: Self‐monitoring medication diary. One follow‐up interview |
|
| Outcomes | Adherence outcome(s):
Mean adherence at week 12 Baseline: I: 74% C: 84% Follow‐up: I: 94% C: 93% p‐value between groups: >.05‡ |
|
| Notes | Severity: None provided Regimen at study baseline: Already existing regimen Duration of intervention:One session Length of follow‐up: 12 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Safren 2003.
| Methods | RCT Setting: Community health centre (Boston, US) Adherence measure(s): Electronic monitoring (doses taken/ prescribed in previous 2 weeks) |
|
| Participants | 74% Unemployed or on disability 30% African‐American 17% Hispanic Adherence <90% | |
| Interventions | Intervention: An on‐line pager reminder system, which used a website (Medi‐Mom) to deliver alerts in the form of text messages at the designated times Control: Medication monitoring only |
|
| Outcomes | Adherence outcome(s):
Mean adherence at week 2 & 12 Baseline: I: 55% (n=30) C: 57% (n=30) Follow‐up: Week 2: I: 70% (n=30) C: 56% (n=30) Week 12: I: 64% (n=19) C: 52% (n=25) p‐value between groups: Week 2: <.004 Week 12: <.03 |
|
| Notes | Severity: CD4<200: 19%, CD4 200‐350: 23%, CD4>350: 58%, VL<400: 51%, VL<5k: 29%, VL>5k: 20% Regimen at study baseline: Already existing regimen Duration of intervention: 12 weeks Length of follow‐up: 12 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Samet 2005.
| Methods | RCT Setting: Tertiary outpatient clinic (Boston, US) Adherence measure(s): Self‐report (pills taken/ prescribed in previous 3 days and 30 days) |
|
| Participants | Adults with history of alcohol dependence Mean age: 43 78‐84% Male 42‐52% African American 20‐25% Homeless 57‐60% IDU Transmission 22‐25% MSM transmission 6‐7% IDU in past 6 months | |
| Interventions | Intervention: 4 (15‐60min) educational sessions over 3 months (including 1 home visit). Nurse trained in motivational interviewing provided individualized counseling, discussed alcohol use, and provided a timer. The intervention was also offered in Spanish Control: Standard of care (verbal or written instructions) |
|
| Outcomes | Adherence outcome(s):
Proportion of patients with 100% adherence in previous 3 days at 6 and 12‐13 months Baseline: I: 58% C: 65% Follow‐up: 1‐6 month: I: 65% C: 63% 12‐13 month: I: 71% C: 62% p‐value between groups: 1‐6 month: >.05 12‐13 month: >.05 Proportion of patients with >=95% adherence in previous 30 days at 6 months and 12‐13 months Baseline: I: 68% C: 69% Follow‐up: 1‐6 month: I: 63% C: 62% 12‐13 month: I: 67% C: 64% p‐value between groups: 1‐6 month: >.05 12‐13 month: >.05 |
|
| Notes | Severity: Mean CD4: 364‐480, Mean VL: 1.9‐2.2, Undetectable VL: 28‐30%, AIDS: 24‐31% Regimen at study baseline: Already existing regimen Duration of intervention: 3 months Length of follow‐up: 12‐13 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Smith 2003.
| Methods | RCT Setting: Tertiary outpatient clinic (North Carolina, US) Adherence measure(s): Electronic monitoring (doses taken/ prescribed in previous week) |
|
| Participants | Mean age: 37 91% Male 74% Non‐White 53% Unemployed 2‐5% IDU transmission 21‐33% MSM transmission | |
| Interventions | Intervention: Individual medication self‐management program including 3 monthly follow‐up education sessions, assistance with scheduling of doses and skills training, delivered by a pharmacist or a nurse Control: Education session + assistance with scheduling of doses + electronic monitoring |
|
| Outcomes | Adherence outcome(s):
Mean adherence at week 12 Follow‐up: I: 96% (n=8) C: 37% (n=9) p‐value between groups: <.001 |
|
| Notes | Severity: None provided Regimen at study baseline: Initial or switching to new regimen Duration of intervention: 12 weeks Length of follow‐up: 12 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tuldra 2000.
| Methods | RCT Setting: Tertiary outpatient clinic (Baladona, Spain) Adherence measure(s): Self‐report (pills taken/ prescribed in previous month) |
|
| Participants | Mean age: 39(+/‐ 8) 75% Male 38% IDU transmission 38% MSM transmission | |
| Interventions | Intervention: Education program including patient tailored medication schedule, medication management skills, problem solving skills and telephone support + follow‐up visits Control: Standard assessment with a psychologist following a regular medical visit |
|
| Outcomes | Adherence outcome(s):
Proportion of patients reaching >= 95% adherence at week 48§ Follow‐up: 48 wks: I: 94% (32/34) C: 69% (25/36) I: 58% (32/55) C: 41% (25/61) p‐value between groups: .008 .064± |
|
| Notes | Severity: Mean CD4: 355, Median VL: 4.02, Yrs since HIV Dx: 6(4) Regimen at study baseline: Initial or switching to new regimen Duration of intervention: Unclear Length of follow‐up: 48 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
van Servellen 2003.
| Methods | RCT Setting: Community‐based not‐for profit clinics (Los Angeles, US) Adherence measure(s): Self‐report (number of missed doses in previous 4 days & 24 hours) |
|
| Participants | Spanish‐Speaking low income/ homeless adults Mean age: 40‐42 88‐93% Male 93‐98% Born outside US Poor adherence at study baseline | |
| Interventions | Intervention: 5‐week instructional support modular program conducted in Spanish by treatment advocates and a nurse practitioner in small groups of 3‐7 participants Control: Standard care only |
|
| Outcomes | Adherence outcome(s):
4 day and 24 hour total number of doses missed at 6 weeks Baseline: 4 days: I: 2.38 (SD 4.55) C: 1.82 (SD 4.86) 24 hours: I: .56 (SD 1.45) C: .29 (SD 1.21) Follow‐up: 4 days: I: 1.26 (SD 3.02) C: 2.16 (SD 3.23) 24 hours: I: .29 (SD 0.96) C: .32 (SD 0.96) p‐value between groups: 4 days: >.05 24 hours: >.05 |
|
| Notes | Severity: Mean CD4: 214‐353, Mean VL: 4.84‐4.52, Mean Yrs since HIV Dx: 5‐8 Regimen at study baseline: Already existing regimen Duration of intervention: 5 weeks Length of follow‐up: 6 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Weber 2004.
| Methods | RCT Setting: Tertiary outpatient clinic + Private practice psychotherapists (Zurich, Switzerland) Adherence measure(s): Electronic monitoring (doses observed/ expected) Electronic monitoring (doses observed/ prescribed in previous month) Self‐report (VAS on whether antiretrovirals taken as prescribed ranging from 0‐never‐ to 10‐always) |
|
| Participants | Median age: 40‐42 50‐60% MSM 13‐25% Past IDU No current IDU/methadone | |
| Interventions | Intervention: Median of 11 45‐min cognitive behavioral therapy sessions (range 2‐25) in 1 year, delivered by licensed and supervised psychotherapists + standard of care Control: Standard of care |
|
| Outcomes | Adherence outcome(s):
Mean adherence at month 12 Baseline: I: 94.3% C: 94.9% Follow‐up: I: 92.8% C: 88.9% p‐value within groups: I: .14 C: .006 p‐value between groups: .15‡ Proportion of patients reaching >= 95% adherence at month 12 Baseline: I: 82.1% C:69.6% Follow‐up: I: 70.8% C: 50% p‐value between groups: .29 at BL .014 at F/U Mean adherence at month 10‐12 Baseline: I: 9.97 C: 9.92 Follow‐up: I: 9.93 C:9.80 p‐value between groups: .4 at BL .012 at F/U |
|
| Notes | Severity: Median CD4 nadir: 120‐173, Median CD4: 380‐499, VL<50: 100%, AIDS: 33% Regimen at study baseline: Already existing regimen Duration of intervention: Variable (Range 2‐25 visits within 12 months, median of 11 visits) Length of follow‐up: 12 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Wyatt 2004.
| Methods | RCT Setting: County hospitals, community‐ based clinics, AIDS service organizations, drug rehab centers (Los Angeles, US) Adherence measure(s): Self‐report (number of days participants took their medication "exactly as prescribed" in the past 2 weeks) |
|
| Participants | 100% Women with history of child sexual abuse Mean age: 41 (SD 8.2) 51% African American 49% Latina 56% High school 93% Unemployed | |
| Interventions | Intervention: Psychoeducational group program to reduce sexual risks and increase adherence in women with a history of child sexual abuse, delivered in 11 weekly sessions (2.5 hours each) by a facilitator with a peer mentor in English or Spanish. Control: Wait‐list group with a one‐time group meeting focusing on HIV prevention and child sexual abuse information. Participants were offered the intervention at the end of the study. |
|
| Outcomes | Adherence outcome(s):
Proportion of patients reporting adherence on all 14 days Follow‐up: I: 75.6% C: 73.3% Change: OR: 1.13 p‐value between groups: .41‡ |
|
| Notes | Severity: AIDS: 13% Regimen at study baseline: Already existing regimen Duration of intervention: 11 weeks Length of follow‐up: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
* CD4 units reported as cells/mm3 or cells/ml † Viral load reported as log10 copies/ml IDU: Injection Drug Use MSM: Men who have Sex with Men ± All randomized participants analyzed ‡ Controlling for baseline measures § Participants lost to follow up = failure ¦ Missing adherence rates imputed by regression
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adamian 2004 | Not a RCT Adherence not measured at 6 weeks for both groups No control arm |
| Albert 1999 | Not a RCT Not an adherence intervention Adherence not measured at 6 weeks for both groups |
| Aloisi 2002 | Not a RCT Not an adherence intervention No control arm |
| Altice 2004 | Not a RCT Adherence not measured at 6 weeks for both groups No control arm |
| Ammassari 2002 | Not a RCT Not an adherence intervention No control arm |
| Ammassari 2002 (a) | Review |
| Bamberger 2000 | Program Description |
| Bangsberg 2002 | Description of an adherence assessment technique |
| Barreiro 2002 | Review |
| Bouhnik 2002 | Not a RCT Not an adherence intervention |
| Broadhead 2002 | Not a RCT 80% of people not on HAART No control arm |
| Cegala 2000 | 80% of people not on HAART Adherence not measured at 6 weeks for both groups |
| Chesney 1999 | Review |
| DeFino 2004 | Not a RCT Adherence not measured at 6 weeks for both groups No control arm |
| Dorz 2003 | Not a RCT Not an adherence intervention Adherence not measured at 6 weeks for both groups No control arm . |
| Dunbar 2003 | Not a RCT No control arm |
| Flandre 2002 | Not an adherence intervention No control arm |
| Fogarty 2002 | Review |
| Fourney 2003 | Not a RCT Adherence not measured at 6 weeks for both groups No control arm |
| Frick 2001 | 80% of people not on HAART Adherence not measured at 6 weeks for both groups |
| Garcia 2003 | Review |
| GarciaP 2003 | Review |
| GarciaR 2003 | Description of an adherence assessment technique |
| Gifford 1998 | Not an adherence intervention Adherence not measured at 6 weeks for both groups No control arm |
| Greenberg 1999 | Not a RCT Not an adherence intervention Adherence not measured at 6 weeks for both groups No control arm |
| Guarinieri 2002 | Review |
| Haddad 2000 | Review |
| Haubrich 1999 | Not a RCT Not an adherence intervention Adherence not measured at 6 weeks for both groups |
| Holzemer 2000 | Not a RCT Adherence not measured at 6 weeks for both groups No control arm |
| Ickovics 2002 | Review |
| Ickovics 2002a | Review article. |
| Ironson 2005 | Not an adherence intervention |
| Jane 2004 | Not a RCT No control arm |
| Kalichman 2003 | Not a RCT Not an adherence intervention Adherence not measured at 6 weeks for both groups No control arm |
| Khanlou 2003 | Not a RCT Adherence not measured at 6 weeks for both groups No control arm |
| Knobel 2001 | Not a RCT Not an adherence intervention Adherence not measured at 6 weeks for both groups No control arm |
| Knobel 2004 | Review |
| Lanzafame 2000 | Not a RCT Adherence not measured at 6 weeks for both groups |
| Lucas 2002 | Review |
| Lucas 2004 | Adherence not measured at 6 weeks for both groups |
| Lyon 2003 | Not a RCT No control arm |
| Macalino 2004 | Not a RCT No control arm |
| Malow 1998 | Not a RCT No control arm |
| Mann 2001 | Adherence not measured at 6 weeks for both groups |
| Margolin 2003 | Not an adherence intervention |
| Martin 2001 | Adherence not measured at 6 weeks for both groups No control arm |
| McCance‐Katz 2002 | Not a RCT No control arm |
| McDonald 2002 | Review |
| McPherson‐Baker 2000 | Not a RCT 80% of people not on HAART |
| Molassiotis 2003 | Not a RCT No control arm |
| Murri 2002 | Review |
| Orrell 2003 | Not a RCT Not an adherence intervention No control arm |
| Palepu 2004 | Not a RCT 80% of people not on HAART Not an adherence intervention Adherence not measured at 6 weeks for both groups No control arm |
| Park‐Wyllie 2003 | Review |
| Parsons 2005 | Not a RCT No control arm |
| Paterson 2002 | Review |
| Perno 2002 | Review |
| Poppa 2004 | Review /Guidelines |
| Press 2002 | Review |
| Reynolds 2004 | Review |
| Rigsby 2000 | 80% of people not on HAART |
| Safren 1999 | Description of intervention |
| Schroeder 2004 | Commentary |
| Selnes 2002 | Review |
| Simoni 2003 | Review |
| Sommers 2001 | Not a RCT No control arm |
| Sorensen 1998 | Not a RCT 80% of people not on HAART |
| Stenzel 2001 | Not a RCT No control arm |
| Stone 2004 | Review |
| Sullivan 2004 | Review |
| Tesoriero 2003 | Not a RCT No control arm |
| Trotta 2002 | Review |
| Tucker 2003 | Not a RCT Not an adherence intervention No control arm |
| Tuldra 2002 | Review |
| Wagner 2002 | Adherence not measured at 6 weeks for both groups |
| Wagner 2002a | Not a RCT Adherence not measured at 6 weeks for both groups No control arm |
| Wagner 2003 | Not a RCT Not an adherence intervention Adherence not measured at 6 weeks for both groups No control arm |
| Weiss 2003 | Not a RCT Not an adherence intervention Adherence not measured at 6 weeks for both groups No control arm |
| Wohl 2004 | Adherence not measured at 6 weeks for both groups No control arm |
| Wu 2002 | Review |
| Yun 2005 | Not a RCT Not an adherence intervention |
Contributions of authors
S Rueda: Designing the review, coordinating the review, screening search results, organizing retrieval of papers, screening retrieved papers against inclusion criteria, appraising quality of papers, extracting data from papers, analysis and interpretation of data, providing a methodological perspective, providing a clinical perspective, providing a policy perspective, writing the review, providing general advice on the review LY Park‐Wyllie: Designing the review, coordinating the review, screening search results, screening retrieved papers against inclusion criteria, appraising quality of papers, extracting data from papers, analysis and interpretation of data, providing a methodological perspective, providing a clinical perspective, providing a policy perspective, writing the review, performing previous work that was the foundation of the current review AM Bayoumi: Designing the review, analysis and interpretation of data, providing a methodological perspective, providing a clinical perspective, providing a policy perspective, performing previous work that was the foundation of the current review, writing the review, providing general advice on the review AM Tynan: Coordinating the review, screening search results, organizing retrieval of papers, screening retrieved papers against inclusion criteria, writing to authors of papers for additional information, entering data into RevMan TA Antoniou: Screening search results, screening retrieved papers against inclusion criteria, appraising quality of papers, extracting data from papers, providing a clinical perspective, performing previous work that was the foundation of the current review, providing general advice on the review SB Rourke: Designing the review, analysis and interpretation of data, providing a methodological perspective, providing a clinical perspective, providing a policy perspective, performing previous work that was the foundation of the current review, general advice on the review RH Glazier: Conceiving the review, analysis and interpretation of data, providing a methodological perspective, providing a clinical perspective, writing the review, performing previous work that was the foundation of the current review, providing general advice on the review
Sources of support
Internal sources
Centre for Research on Inner City Health, St Michael's Hospital, Toronto, Canada.
Department of Family and Community Medicine, St Michael's Hospital, Toronto, Canada.
External sources
Ontario HIV Treatment Network, Canada.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Berrien 2004 {published data only}
- Berrien VM, Salazar JC, Reynolds E, McKay K. Adherence to antiretroviral therapy in HIV‐infected pediatric patients improves with home‐based intensive nursing intervention. AIDS Patient Care STDS 2004;18(6):355‐363. [DOI] [PubMed] [Google Scholar]
DiIorio 2003 {published data only}
- DiIorio C, Resnicow K, McDonnell M, Soet J, McCarty F, Yeager K. Using motivational interviewing to promote adherence to antiretroviral medications: a pilot study. Journal of the Assoc of Nurses in AIDS Care 2003;14(2):52‐62. [DOI] [PubMed] [Google Scholar]
Fairley 2003 {published data only}
- Fairley CK, Levy R, Rayner CR, Allardice K, Costello K, et al. Randomized trial of an adherence proramme for clients with HIV. International Journal of STD & AIDS 2003;14:805‐809. [DOI] [PubMed] [Google Scholar]
Goujard 2003 {published data only}
- Goujard C, Bernard N, Sohier N, et al. Impact of a patient education program on adherence to HIV medication: a randomized clinical trial. J Acquir Immune Defic Syndr 2003;34:191‐194. [DOI] [PubMed] [Google Scholar]
Jones 2003 {published data only}
- Jones DL, Ishii M, LaPerriere A, Stanley H, Antoni M, Ironson G, et al. Influencing medication adherence among women with AIDS. AIDS CARE 2003;15(4):463‐474. [DOI] [PubMed] [Google Scholar]
Knobel 1999 {published data only}
- Knobel H, Carmona A, Lopez JL, Gimeno JL, Saballs P, Gonzalez A, Guelar A, Diez A. Adherence to highly active antiretroviral treatment: impact of individualized assessment [Adherencia al tratamiento antirretroviral de gran actividad: impacto de una intervencion de asesoramiento individualizado]. Enfermedades Infecciosas y Microbiologia Clinica 1999;17:78‐81. [PubMed] [Google Scholar]
Levy 2004 {published data only}
- Levy RW, Rayner CR, Fairley CK, et al. Multidisciplinary HIV adherence intervention: a randomized study. AIDS Patient Care STDS 2004;18:728‐735. [DOI] [PubMed] [Google Scholar]
Murphy 2002 {published data only}
- Murphy DA, Lu Michael C, Martin D, Hoffman D, Marelich WD. Results of a pilot intervention trial to improve antiretroviral adherence among HIV‐positive patients. Journal of the Association of Nurses in AIDS Care 2002;13(6):57‐69. [DOI] [PubMed] [Google Scholar]
Pradier 2003 {published data only}
- Pradier C, Bentz L, Spire B, Tourette‐Turgis C, Morin M, et al. Efficacy of an educational and counseling intervention on adherence to highly active antiretroviral therapy: French prospective controlled study. HIV Clin Trials 2003;4(2):121‐131. [DOI] [PubMed] [Google Scholar]
Rathbun 2005 {published data only}
- Rathbun RC, Farmer KC, Stephens JR, Lockhart SM. Impact of an adherence clinic on behavioral outcomes and virologic response in the treatment of HIV infection: a prospective, randomized, controlled pilot study. Clinical Therapeutics 2005;27(2):199‐209. [DOI] [PubMed] [Google Scholar]
Rawlings 2003 {published data only}
- Rawlings MK, Thompson MA, Farthing CF, Brown LS, Racine J, et al. Impact of an educational program on efficacy and adherence with a twice‐daily lamivudine/zidovudine/abacavir regimen in underrepresented HIV‐infected patients. J Acquir Immune Defic Syndr 2003;34(2):174‐183. [DOI] [PubMed] [Google Scholar]
Safren 2001 {published data only}
- Safren SA, et al. Two strategies to increase adherence to HIV antiretroviral medication: life‐steps and medication monitoring. Behaviour Research & Therapy 2001;39(10):1151‐62. [DOI] [PubMed] [Google Scholar]
Safren 2003 {published data only}
- Safren, SA, et al. Use of an on‐line pager system to increase adherence to antiretroviral medications. Aids Care 2003;15(6):787‐793. [DOI] [PubMed] [Google Scholar]
Samet 2005 {published data only}
- Samet JH, Horton NJ, Meli S, Dukes K, Tripp T, et al. A randomized controlled trial to enhance antiretroviral therapy adherence in patients with a history of alcohol problems. Antiviral Therapy 2005;10(1):83‐93. [DOI] [PubMed] [Google Scholar]
Smith 2003 {published data only}
- Smith SR, Rublein JC, Marcus C, Brock TP, Chesney MA. A medication self‐management program to improve adherence to HIV therapy regimens. Patient Education and Counseling 2003;50:187‐199. [DOI] [PubMed] [Google Scholar]
Tuldra 2000 {published data only}
- Tuldra A, et al. Prospective randomized two‐arm controlled study to determine the efficacy of a specific intervention to improve long‐term adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2000;25(3):221‐8. [DOI] [PubMed] [Google Scholar]
van Servellen 2003 {published data only}
- Servellen G, Carpio F, Lopez M, et al. Program to enhace health literacy and treatment adherence in low‐income HIV‐infected Latino men and women. AIDS Patient Care STDS 2003;17:581‐594. [DOI] [PubMed] [Google Scholar]
Weber 2004 {published data only}
- Weber R, Christen L, Christen S, Tschopp S, Znoj Hansjoerg, Schnedier C, et al. Effect of individual cognitive behaviour intervention on adherence to antiretroviral therapy: prospective randomized trial. Antiviral Therapy 2004;9(1):85‐95. [PubMed] [Google Scholar]
Wyatt 2004 {published data only}
- Wyatt GE, Longshore D, Chin D, et al. The efficacy of an integrated risk reduction intervention for HIV‐positive women with child sexual abuse histories. AIDS and Behavior 2004;8:453‐462. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adamian 2004 {published data only}
- Adamian MS, Golin CE, Shain LS, DeVellis B. Brief motivational interviewing to improve adherence to antiretroviral therapy: development and qualitative pilot assessment of an intervention. AIDS Patient Care and STDs 2004;18(4):229‐. [DOI] [PubMed] [Google Scholar]
Albert 1999 {published data only}
- Albert SM, Weber CM, Todak G, Polanco C, Clouse R, et al. An observed performance test of medication management ability in HIV: relation to neuropsychological status and medication adherence outcomes. AIDS and Behavior 1999;3(2):121‐128. [Google Scholar]
Aloisi 2002 {published data only}
- Aloisi MS, Arici C, Balzano R, Noto P, Piscopo R, et al. Behavioral correlates of adherence to antiretroviral therapy. JAIDS 2002;31:S145‐S148. [DOI] [PubMed] [Google Scholar]
Altice 2004 {published data only}
- Altice FL, Mezger JA, Hodges J, Bruce RD, Marinovich A, et al. Developing a directly administered antiretroviral therapy intervention for HIV‐infected drug users: implications for program replication. Clinical Infectious Diseases 2004;38:S376‐87. [DOI] [PubMed] [Google Scholar]
Ammassari 2002 {published data only}
- Ammassari A, Antinori A, Cozzi‐Lepri A, Trotta MP, Nasti G, et al. Relationship between HAART adherence and adipose tissue alterations. JAIDS 2002;31:S140‐S144. [DOI] [PubMed] [Google Scholar]
Ammassari 2002 (a) {published data only}
- Ammassari A, Trotta MP, Murri R, Castelli F, et al. Correlates and predictors of adherence to highly active antiretroviral therapy: overview of published liteature. JAIDS 2002;31:S123‐S127. [DOI] [PubMed] [Google Scholar]
Bamberger 2000 {published data only}
- Bamberger JD, Unick J, Klein P, Fraser M, Chesney M, Katz MH. Helping the urban poor stay with antiretroviral HIV drug therapy. Am J Public Health 2000;90(5):699‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bangsberg 2002 {published data only}
- Bangsberg DR, Bronstone A, Chesney MA, Hecht FM. Computer‐assisted self‐interviewing (CASI) to improve provider assessment of adherence in routine clinical practice. JAIDS 2002;31:S107‐S111. [DOI] [PubMed] [Google Scholar]
Barreiro 2002 {published data only}
- Barreiro P, Garcia‐Benayas T, Soriano V, Gallant J. Simplification of antiretroviral treatment ‐ how to sustain success, reduce toxicity and ensure adherence avoiding PI use. AIDS Review 2002;4:233‐241. [PubMed] [Google Scholar]
Bouhnik 2002 {published data only}
- Bouhnik AD, Chesney M, Carrieri P, Gallais H, Moreau J, et al. Nonadherence among HIV‐infected injecting drug users: the impact of social instability. JAIDS 2002;31:S149‐153. [DOI] [PubMed] [Google Scholar]
Broadhead 2002 {published data only}
- Broadhead RS, Heckathorn DD, Altice FL, Hulst Y, Carbone M, et al. Increasing drug users' adherence to HIV treatment: results of a peer‐driven intervention feasibility study. Social Science and Medicine 2002;55:235‐46. [DOI] [PubMed] [Google Scholar]
Cegala 2000 {published data only}
- Cegala DJ, Marinelli T, Post D. The effects of patient communication skills training on compliance. Arch Fam Med 2000;j9:57‐. [DOI] [PubMed] [Google Scholar]
Chesney 1999 {published data only}
- Chesney MA, Ickovics J, Hecht FM, Sikipa G, Rabkin J. Adherence: a necessity for successful HIV combination therapy. AIDS 1999;13(Suppl A):S271‐S278. [PubMed] [Google Scholar]
DeFino 2004 {published data only}
- DeFino M, Clark J, Mogyoros D, Shuter J. Predictors of virologic success in patients completing a structured antiretroviral adherence program. Journal of the Assoc of Nurses in AIDS Care 2004;15(5):60‐67. [DOI] [PubMed] [Google Scholar]
Dorz 2003 {published data only}
- Dorz S, Lazzarini L, Cattelan A, Meneghetti F, Novara C, et al. Evaluation of adherence to antiretroviral therapy in Italian HIV patients. AIDS Patient Care and STDs 2003;17(1):33‐41. [DOI] [PubMed] [Google Scholar]
Dunbar 2003 {published data only}
- Dunbar PJ, Madigan D, Grohskope LA, Revere D, Woodward J, et al. A two‐way messaging system to enhance antiretroviral adherence. J of the Am Medical Informatics Association 2003;10(1):11‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flandre 2002 {published data only}
- Flandre P, Peytavin G, Meiffredy V, Saidi Y, Descamps D, et al. Adherence to antiretroviral therapy and outcomes in HIV‐infected patients enrolled in an induction/maintenance randomized trial. Antiviral Therapy 2002;7(2):113‐121. [PubMed] [Google Scholar]
Fogarty 2002 {published data only}
- Fogarty L, Roter D, Larson S, Burke J, Gillespie J, Levy R. Patient adherence to HIV medication regimens: a review of published and abstract reports. Patient Education and Counselling 2002;46:93‐108. [DOI] [PubMed] [Google Scholar]
Fourney 2003 {published data only}
- Fourney AM, Williams ML. Formative evaluation of an intervention to increase compliance to HIV therapies: the ALP project. Health Promotion Practice 2003;4(2):165‐170. [DOI] [PubMed] [Google Scholar]
Frick 2001 {published data only}
- Frick PA, Lavreys L, Mandaliya K, Kreiss JK. Impact of an alarm device on medication compliance in women in Mombasa, Kenya. Intl J of STD & AIDS 2001;12:329‐333. [DOI] [PubMed] [Google Scholar]
Garcia 2003 {published data only}
- Garcia PB, Lahoz JG, Vazquez VS. Optimization of adherence to antiretroviral therapy [Optimizacion de la adherencia al tratamiento antirretrovirico]. Rev Clin Esp 2003;203(6):299‐302. [DOI] [PubMed] [Google Scholar]
GarciaP 2003 {published data only}
- Garcia PR, Cote JK. Factors affecting adherence to antiretroviral therapy in people living with HIV/AIDS. Journal of the Assocation of Nurses in AIDS Care 2003;14(4):37‐45. [DOI] [PubMed] [Google Scholar]
GarciaR 2003 {published data only}
- Garcia R, Schooley RT, Badaro R. An adherence trilogy is essential for long‐term HAART success. Brazilian Journal of Infectious Diseases 2003;7(5):307‐314. [DOI] [PubMed] [Google Scholar]
Gifford 1998 {published data only}
- Gifford AL, Laurent DD, Gonzales VM, Chesney MA, Lorig KR. Pilot randomized trial of education to improve self‐management skills of men with symptomatic HIV/AIDS. JAIDS 1998;18(2):136‐44. [DOI] [PubMed] [Google Scholar]
Greenberg 1999 {published data only}
- Greenberg B, Berkman A, Thomas R, Hoos D, Finkelstein R, Astemborski J, Vlahov D. Evaluating supervised HAART in late‐stage HIV among drug users: a preliminary report. Journal of Urban Health: Bulletin of the New York Academy of Medicine 1999;76(4):468‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guarinieri 2002 {published data only}
- Guarinieri M. Highly active antiretroviral therapy adherence: the patient's point of view. JAIDS 2002;31:S167‐S169. [DOI] [PubMed] [Google Scholar]
Haddad 2000 {published data only}
- Haddad M, Inch C, Glazier RH, Wilkins AL, Urbshott GB, Bayoumi A, Rourke S. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS (Cochrane Review). Cochrane Library 2000;3:CD001442. [DOI] [PubMed] [Google Scholar]
Haubrich 1999 {published data only}
- Haubrich RH, Little SJ, Currier JS, Forthal DN, Kemper CA, et al. The value of patient‐reported adherence to antiretroviral therapy in predicting virologic and immunologic response. AIDS 1999;13(9):1099‐1107. [DOI] [PubMed] [Google Scholar]
Holzemer 2000 {published data only}
- Holzemer WL, Henry SB, Portillo CJ, Miramontes H. The client adherence profiling intervention tailoring (CAP‐IT) intervention for enhancing adherence to HIV/AIDS medications: a pilot study. J Assoc Nurses AIDS Care 2000;11(1):36‐44. [DOI] [PubMed] [Google Scholar]
Ickovics 2002 {published data only}
- Ickovics JR, Meade CS. Adherence to HAART among patients with HIV: breakthroughs and barriers. AIDS Care 2002;14(3):309‐318. [DOI] [PubMed] [Google Scholar]
Ickovics 2002a {published data only}
- Ickovics JR, Meade CS. Adherence to antiretroviral therapy among patients with HIV: a critical link between behavioral and biomedical sciences. JAIDS 2002;31:98‐102. [DOI] [PubMed] [Google Scholar]
Ironson 2005 {published data only}
- Ironson G, Weiss S, Lydston D, Ishii M, Jones D, et al. The impact of improved self‐efficacy on HIV viral load and distress in culturally diverse women living with AIDS: the SMART/EST Women's Project. AIDS Care 2005;17(2):222‐236. [DOI] [PubMed] [Google Scholar]
Jane 2004 {published data only}
- Jane CC, MT Creus, OI Barrueta, OD Sanchez, OM Echevarria, et al. Evaluation of a pharmaceutical program aimed at improving adherence to antiretroviral therapy [Evaluacion de un programa de atencion farmaceutica dirigido a mejorar la adherencia al tratamiento antiretroviral]. Farm Hosp (Madrid) 2004;28(Suppl 1):19‐26. [PubMed] [Google Scholar]
Kalichman 2003 {published data only}
- Kalichman SC, Rompa D. HIV treatment adherence and unprotected sex practices in people receiving antiretroviral therapy. Sex Transm Infect 2003;79:59‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khanlou 2003 {published data only}
- Khanlou H, Kandula VR, Yeh V, Stein TG, Sanchez S, et al. Pilot study of directly observed therapy in highly nonadherenct HIV‐infected patients in an urban community‐based institution. JAIDS 2003;33(5):651‐653. [DOI] [PubMed] [Google Scholar]
Knobel 2001 {published data only}
- Knobel H, Guelar A, Carmona A, Espona M, Gonzalez A, et al. Virologic outcome and predictors of virologic failure of highly active antiretroviral therapy containing protease inhibitors. AIDS Patient Care and STDs 2001;15(4):193‐199. [DOI] [PubMed] [Google Scholar]
Knobel 2004 {published data only}
- Knobel H, Guela A. Strategies to optimize the adherence to antiretroviral treatment. Therapeutic interventions [Estrategias para optimizar la adherencia al traitamiento antirretroviral. Intervenziones en la pauta terapeutica]. Enfermedades Infecciosas y Microbiologia Clinica 2004;22(2):106‐12. [DOI] [PubMed] [Google Scholar]
Lanzafame 2000 {published data only}
- Lanzafame M, Trevenzoli M, Cattelan AM, Rovere P, Parrinello A. Directly observed therapy in HIV therapy: a realistic perspective?. JAIDS 2000;25(2):200‐201. [DOI] [PubMed] [Google Scholar]
Lucas 2002 {published data only}
- Lucas GM, Flexner CW, Moore RD. Directly administered antiretroviral therapy in the treatment of HIV infection: benefit or burden?. AIDS Patient Care and STDs 2002;16(11):527‐535. [DOI] [PubMed] [Google Scholar]
Lucas 2004 {published data only}
- Lucas GM, Weidle PJ, Hader S, Moore RD. Directly administered antiretroviral therapy in an urban methadone maintenance clinic: a nonrandomized comparative study. Clinical Infectious Diseases 2004;38:S409‐13. [DOI] [PubMed] [Google Scholar]
Lyon 2003 {published data only}
- Lyon ME, Trexler C, Akpan‐Townsend C, Pao M, Selden K, et al. A family group approach to increasing adherence to therapy in HIV‐infected youths: results of a pilot project. AIDS Pt Care and STDs 2003;17(6):299‐308. [DOI] [PubMed] [Google Scholar]
Macalino 2004 {published data only}
- Macalino GE, Mitty JA, Bazerman LB, Singh K, McKenzie M, Flanigan T. Modified directly observec therapy for the treatment of HIV‐seropositive substance users: lessons learned from a pilot study. Clinical Infectious Diseases 2004;38:S393‐7. [DOI] [PubMed] [Google Scholar]
Malow 1998 {published data only}
- Malow RM, McPherson S, Klimas N, Antoni MH, Schneiderman N, Penedo FJ, Zaskind D, Page B, McMahon R. Adherence to complex combination antiretroviral therapies by HIV positive drug abusers. Psychiatr Serv 1998;49:1021‐1022. [DOI] [PubMed] [Google Scholar]
Mann 2001 {published data only}
- Mann T. Effects of future writing and optimism on health behaviors in HIV‐infected women. Ann Behav Med 2001;23(1):26‐33. [DOI] [PubMed] [Google Scholar]
Margolin 2003 {published data only}
- Margolin A, Avants SK, Warburton LA, Hawkins KA, Shi J. A randomized clinical trial of a manual‐guided risk reduction intervention for HIV‐positive injection drug users. Health Psychology 2003;22(2):223‐228. [PubMed] [Google Scholar]
Martin 2001 {published data only}
- Martin J, Sabugal GM, Rubio R, Sainz‐Maza M, Blanco JM, Alonso JL, Dominguez J. Outcomes of a health education intervention in a sample of patients infected by HIV, most of them injection drug users: possibilities and limitations. AIDS CARE 2001;13(4):467‐473. [DOI] [PubMed] [Google Scholar]
McCance‐Katz 2002 {published data only}
- McCance‐Katz EF, Gourevitch MN, Arnsten J, Sarlo J, Rainey P, Jatlow P. Modified directly observed therapy (MDOT) for injection drug users with HIV disease. Am J on Addictions 2002;11:271‐278. [DOI] [PubMed] [Google Scholar]
McDonald 2002 {published data only}
- McDonald HP, Garg AX, Haynes RB. Review: evidence on the effectiveness of interventions to assist patients' adherence to prescribed medications is limited. JAMA 2002;288:2868‐79. [Google Scholar]
McPherson‐Baker 2000 {published data only}
- McPherson‐Baker S, Malow RM, Penedo F, et al. Enhancing adherence to combination antiretroviral therapy in non‐adherent HIV‐positive men. AIDS Care 2000;12:399‐404. [DOI] [PubMed] [Google Scholar]
Molassiotis 2003 {published data only}
- Molassiotis A, Lopez‐Nahas V, Chung WY, Lam SW. A pilot study of the effects of a behavioural intervention on treatment adherence in HIV‐infected patients. AIDS Care 2003;15(1):125‐135. [DOI] [PubMed] [Google Scholar]
Murri 2002 {published data only}
- Murri R, Antinori A, Ammassari A, Nappa S, Orofino G, Abrescia N, Ussini. Physician estimate of adherence and the patient‐physician relationship as a setting to improve adherence to antiretroviral therapy. JAIDS 2002;31:S158‐S162. [DOI] [PubMed] [Google Scholar]
Orrell 2003 {published data only}
- Orrell C, Bangsberg DR, Badri M, Wood R. Adherence is not a barrier to successful antiretroviral therapy in South Africa. AIDS 2003;17(9):1369‐1375. [DOI] [PubMed] [Google Scholar]
Palepu 2004 {published data only}
- Palepu A, Horton NJ, Tibbetts N, Meli S, Samet JH. Uptake and adherence to highly active antiretroviral therapy among HIV‐infected people with alcohol and other substance use problems: the impact of substance abuse treatment. Addiction 2004;99:361‐368. [DOI] [PubMed] [Google Scholar]
Park‐Wyllie 2003 {published data only}
- Park‐Wyllie LY, Phillips EJ. Challenges of adherence management in human immunodeficiency virus pharmacotherapy. Canadian Journal of Clinical Pharmacology 2003;10(4):189‐195. [PubMed] [Google Scholar]
Parsons 2005 {published data only}
- Parsons JT, Rosof E, Punzalan JC, DiMaria L. Integration of motivational interviewing and cognitive behavioral therapy to improve HIV medication adherence and reduce substance abuse use among HIV‐positive men and women: results of a pilot project. AIDS Patient Care and STDs 2005;19(1):31‐39. [DOI] [PubMed] [Google Scholar]
Paterson 2002 {published data only}
- Paterson DL, Potoski B, Capitano B. Measurement of adherence to antiretroviral medications. JAIDS 2002;31:S103‐S106. [DOI] [PubMed] [Google Scholar]
Perno 2002 {published data only}
- Perno CF, Ceccherini‐Silberstein F, Luca A, Cozzi‐Lepri A, Gori C, Cingolani A, et al. Virologic correlates of adherence to antiretroviral medications and therapeutic failure. JAIDS 2002;31:S118‐S122. [DOI] [PubMed] [Google Scholar]
Poppa 2004 {published data only}
- Poppa A, Davidson O, Deutsch J, Godfrey D, Fisher M, Head S, et al. British HIV association (BHIVA)/British association for sexual health and HIV (BASHH) guidelines for provision of adherence support to individuals receiving antiretroviral therapy. BHIVA 2004;5(Suppl. 2):46‐60. [DOI] [PubMed] [Google Scholar]
Press 2002 {published data only}
- Press N, Tyndall MW, Wood E, Hogg RS, Montaner JSG. Virologic and immunologic response, clinical progression, and highly active antiretroviral therapy adherence. JAIDS 2002;31:S112‐S117. [DOI] [PubMed] [Google Scholar]
Reynolds 2004 {published data only}
- Reynolds NR. Adherence to antiretroviral therapies: state of the science. Current HIV Research 2004;2:207‐214. [DOI] [PubMed] [Google Scholar]
Rigsby 2000 {published data only}
- Rigsby MO, Rosen MI, Beauvais JE, et al. Cue‐dose training with monetary reinforcement. J Gen Intern Med 2000;15:841‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Safren 1999 {published data only}
- Safren SA, Otto MW, Worth JL. Life‐steps: applying cognitive behavioral therapy to HIV medication adherence. Cognitive and Behavioral Practice 1999;6:332‐341. [Google Scholar]
Schroeder 2004 {published data only}
- Schroeder K, Fahey T, Ebrahim S, Peters TJ. Adherence to long‐term therapies: recent WHO report provides some answers but poses even more questions. Journa of Clinical Epidemiology 2004;57:2‐3. [DOI] [PubMed] [Google Scholar]
Selnes 2002 {published data only}
- Selnes, OA. Neurocognitive aspects of medication adherence in HIV infection. JAIDS 2002;31:S132‐S135. [DOI] [PubMed] [Google Scholar]
Simoni 2003 {published data only}
- Simoni JM, Frick PA, Pantalone DW, Turner BJ. Antiretroviral adherence interventions: a review of current literature and ongoing studies. Topics in HIV Medicine 2003;11(6):185‐198. [PubMed] [Google Scholar]
Sommers 2001 {published data only}
- Sommers SD, Kent DJ, Beam B, Boles M, Antoniskis D. A team approach to address antiretroviral therapy adherence barriers in a managed care organization. J Managaed Care Pharm 2001;7(3):214‐218. [Google Scholar]
Sorensen 1998 {published data only}
- Sorensen JL, Mascovich A, Wall TL, DePhilippis D, Batki SL, Chesney M. Medication adherence strategies for drug abusers with HIV/AIDS. AIDS Care 1998;10:297‐312. [DOI] [PubMed] [Google Scholar]
Stenzel 2001 {published data only}
- Stenzel MS, McKenzie M, Adelson‐Mitty J, Flanigan TP. Enhancing adherence to HAART: a pilot program of modified directly observed therapy. The AIDS Reader 2001;11(6):317‐28. [PubMed] [Google Scholar]
Stone 2004 {published data only}
- Stone VE, Smith KY. Improving adherence to HAART. Supplement to the Journal of the National Medical Association 2004;96(Suppl. 2):27S‐29S. [PMC free article] [PubMed] [Google Scholar]
Sullivan 2004 {published data only}
- Sullivan LE, Fiellin DA. Hepatitis C and HIV infections: implications for clinical care in injection drug users. The American Journal on Addictions 2004;13:1‐20. [DOI] [PubMed] [Google Scholar]
Tesoriero 2003 {published data only}
- Tesoriero J, French T, Weiss L, Waters M, Finkelstein R, et al. Stability of adherence to highly active antiretroviral therapy over time among clients enrolled in the treatment adherence demonstration project. JAIDS 2003;33:484‐493. [DOI] [PubMed] [Google Scholar]
Trotta 2002 {published data only}
- Trotta MP, Ammassari A, Melzi S, Zaccarelli M, Ladisa N, Sighinolfi L, Mura MS, et al. Treatment‐related factors and highly active antiretroviral therapy adherence. JAIDS 2002;31:S128‐S131. [DOI] [PubMed] [Google Scholar]
Tucker 2003 {published data only}
- Tucker JS, Burnam MA, Sherbourne CD, Kung F‐Y, Gifford AL. Substance abuse and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. The American Journal of Medicine 2003;114:573‐580. [DOI] [PubMed] [Google Scholar]
Tuldra 2002 {published data only}
- Tuldra A, Wu AW. Interventions to improve adherence to antiretroviral therapy. JAIDS 2002;31(Suppl. 3):S154‐S157. [DOI] [PubMed] [Google Scholar]
Wagner 2002 {published data only}
- Wagner GJ, Ghosh‐Dastidar B. Electronic monitoring: adherence assessment or intervention?. HIV Clin Trials 2002;3(1):45‐51. [DOI] [PubMed] [Google Scholar]
Wagner 2002a {published data only}
- Wagner G, Iguchi M, Schneider S, Scott J, Anderson D. Placebo practice trials: a tool to assess and improve adherence readiness. HIV Clin Trials 2002;3(6):475‐481. [DOI] [PubMed] [Google Scholar]
Wagner 2003 {published data only}
- Wagner GJ, Kanouse DE, Koegel P, Sullivan G. Adherence to HIV antiretrovirals among persons with serious mental illness. AIDS Patient Care and STDs 2003;17(4):179‐186. [DOI] [PubMed] [Google Scholar]
Weiss 2003 {published data only}
- Weiss L, French T, Finkelstein R, Waters M, Mukherjee R, Agins B. HIV‐related knowledge and adherence to HAART. AIDS Care 2003;15(5):673‐679. [DOI] [PubMed] [Google Scholar]
Wohl 2004 {published data only}
- Wohl AR, Garland WH, Squires K, Witt M, Larsen R, et al. The feasibility of a community‐based directly administered antiretroviral therapy program. Clinical Infectious Diseases 2004;38(Suppl. 5):S388‐92. [DOI] [PubMed] [Google Scholar]
Wu 2002 {published data only}
- Wu AW, Ammassari A, Antinori A. Adherence to antiretroviral therapy: where are we and where do we go from here?. JAIDS 2002;31:S95‐S97. [DOI] [PubMed] [Google Scholar]
Yun 2005 {published data only}
- Yun L, Maravi M, Kobayashi JS, Barton PL, Davidson AJ. Antidepressant treatment improves adherence to antiretroviral therapy among depressed HIV‐infected patients. J Acquir Immune Defic Syndr 2005;38(4):432‐. [DOI] [PubMed] [Google Scholar]
Additional references
Altman 2001
- Altman DG, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: Explanation and elaboration. Annals of Internal Medicine 2001;134:663‐694. [DOI] [PubMed] [Google Scholar]
Amico 2006
- Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions ‐ A research synthesis of trials, 1996 to 2004. J Acquir Immune Defic Syndr 2006;41:285‐297. [DOI] [PubMed] [Google Scholar]
Ammassari 2002
- Ammassari A, Trotta MP, Murri R, et al. Correlates and predictors of adherence to highly active antiretroviral therapy: overview of published literature. J Acquir Immune Defic Syndr 2002;31(3):S123‐127. [DOI] [PubMed] [Google Scholar]
Bangsberg 2000
- Bangsberg DR, Hecht FM, Charlebois ED, et al. Adherence to protease inhibitors, HIV‐1 viral load, and development of drug resistance in an indigent population. AIDS 2000;14:357‐366. [DOI] [PubMed] [Google Scholar]
Bangsberg 2001
- Bangsberg DR, Perry S, Charlebois ED, et al. Non‐adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS 2001;15:1181‐1183. [DOI] [PubMed] [Google Scholar]
Bangsberg 2002
- Bangsberg DR, Deeks SG. Is average adherence to HIV antiretroviral therapy enough?. Journal of General Internal Medicine 2002;17(10):812‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carpenter 2000
- Carpenter CC, Cooper DA, Fischl MA, et al. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society‐USA Panel. JAMA 2000;283:381‐390. [DOI] [PubMed] [Google Scholar]
Catz 2000
- Catz SL, Kelly JA, Bogart LM, et al. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychology 2000;19:124‐133. [PubMed] [Google Scholar]
Cote 2005
- Cote JK, Godin G. Efficacy of interventions in improving adherence to antiretroviral therapy ‐ Review. International Journal of STD & AIDS 2005;16:335‐343. [DOI] [PubMed] [Google Scholar]
Ferguson 2002
- Feguson TF, Stewart KE, Funkhouser E, et al. Patient‐perceived barriers to antiretroviral adherence: associations with race. AIDS Care 2002;14:607‐617. [DOI] [PubMed] [Google Scholar]
Fogarty 2002
- Fogarty L, Roter D, Larson S, et al. Patient adherence to HIV medication regimens: a review of published and abstract reports. Patient Educ Couns 2002;46:93‐108. [DOI] [PubMed] [Google Scholar]
Gallant 1998
- Gallant J, Block D. Adherence to antiretroviral regimens in HIV‐infected patients: results of a survey among physicians and patients. Journal of the International Association of Physicians in AIDS Care 1998;4:32‐35. [PubMed] [Google Scholar]
Golin 2002
- Golin CE, Liu H, Hays RD, et al. A prospective study of predictors of adherence to combination antiretroviral medication. Journal of General Internal Medicine 2002;17:756‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gross 2001
- Gross R, Bilker WB, Friedman HM, et al. Effects of adherence to newly initiated antiretroviral therapy on plasma viral load. AIDS 2001;15:2109‐2117. [DOI] [PubMed] [Google Scholar]
Haddad 2000
- Haddad M, Inch C, Glazier RH, et al. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database Syst Rev 2000;3:CD001442. [DOI] [PubMed] [Google Scholar]
Haubrich 1999
- Haubrich RH, Little SJ, Currier JS, et al. The value of patient‐reported adherence to antiretroviral therapy in predicting virologic and immunologic response. AIDS 1999;13:1099‐1107. [DOI] [PubMed] [Google Scholar]
Haynes 1979
- Haynes RB, Taylor DW, Sackett DL. Compliance in health care. Compliance in health care. Baltimore, MD: John Hopkins University Press, 1979. [Google Scholar]
Howard 2002
- Howard AA, Arnsten JH, Li YT, et al. A prospective study of adherence and viral load in a large multi‐center cohort of HIV‐infected women. AIDS 2002;16:2175‐2182. [DOI] [PubMed] [Google Scholar]
Hugen 2002
- Hugen PWH, Langebeek N, Burger DM, et al. Assessment of adherence to HIV protease inhibitors: Comparison and combination of various methods, including MEMS (electronic monitoring), patient and nurse report, and therapeutic drug monitoring.. Journal of Acquired Immune Deficiency Syndromes 2002;30:324‐334. [DOI] [PubMed] [Google Scholar]
Ickovics 1997
- Ickovics JR, Meisler AW. Adherence in AIDS clinical trials: a framework for clinical research and clinical care. Journal of Clinical Epidemiology 1997;50:385‐391. [DOI] [PubMed] [Google Scholar]
Ickovics 2002
- Ickovics JR, Cameron A, Zackin R, et al. Consequences and determinants of adherence to antiretroviral medication: results from Adult AIDS Clinical Trials Group protocol 370. Antiviral Therapy 2002;7:185‐193. [DOI] [PubMed] [Google Scholar]
Ickovics 2002a
- Ickovics JR, Meade CS. Adherence to antiretroviral therapy among patients with HIV: a critical link between behavioral and biomedical sciences. J Acquir Immune Defic Syndr 2002;31(3):S98‐102. [DOI] [PubMed] [Google Scholar]
Kawamoto 2005
- Kawamoto K, Houlihan CA, Balas EA, et al. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. British Medical Journal 2005;330:765‐768E. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knobel 2001
- Knobel H, Guelar A, Carmona A, et al. Virologic outcome and predictors of virologic failure of highly active antiretroviral therapy containing protease inhibitors. AIDS Patient Care STDS 2001;15:193‐199. [DOI] [PubMed] [Google Scholar]
Liu 2001
- Liu H, Golin C, Miller L, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med 2001;134:968‐977. [DOI] [PubMed] [Google Scholar]
McDonald 2002
- McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions ‐ Scientific review. JAMA 2002;288:2868‐2879. [DOI] [PubMed] [Google Scholar]
Miller 2000
- Miller L, Hays R. Measuring adherence to antiretroviral medications in clinical trials. HIV Clin Trials 2000;1:36‐46. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. The Lancet 2001;357:1191‐1194. [PubMed] [Google Scholar]
Nieuwkerk 2001
- Nieuwkerk PT, Sprangers MAG, Burger DM, et al. Limited patient adherence to highly active antiretroviral therapy for HIV‐1 infection in an observational cohort study. Archives of Internal Medicine 2001;161:1962‐1968. [DOI] [PubMed] [Google Scholar]
Paterson 2000
- Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000;133:21‐30. [DOI] [PubMed] [Google Scholar]
Perno 2002
- Perno CF, Ceccherini‐Silberstein F, Luca A, et al. Virologic correlates of adherence to antiretroviral medications and therapeutic failure. J Acquir Immune Defic Syndr 2002;31(3):S118‐122. [DOI] [PubMed] [Google Scholar]
Press 2002
- Press N, Tyndall MW, Wood E, et al. Virologic and immunologic response, clinical progression, and highly active antiretroviral therapy adherence. J Acquir Immune Defic Syndr 2002;31(3):S112‐117. [DOI] [PubMed] [Google Scholar]
Sackett 1979
- Sackett DL, Snow LC. The magnitude of adherence and nonadherence. In: Haynes RB, Taylor DW, Sackett DL editor(s). Compliance in Health Care. John Hopkins University Press, 1979:11‐22. [Google Scholar]
Schulz 1995
- Schulz K, Chalmers I, Hayes R, et al. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐412. [DOI] [PubMed] [Google Scholar]
Simoni 2003
- Simoni JM, Frick PA, Pantalone DW, et al. Antriretroviral adherence interventions: a review of current literature and ongoing studies. Top HIV Med 2003;11:185‐198. [PubMed] [Google Scholar]
Simoni 2006
- Simoni JM, Pantalone DW, Frick PA, et al. Enhancing antiretroviral adherence: A review of published reports of RCTs and on‐going NIH‐funded research. In: Trafton JA, Gordon WP, eds. Best Practices in the Behavioral Management of Chronic Disease. Los Altos: Institute for Disease Management, 2006. [Google Scholar]
Singh 1999
- Singh N, Berman SM, Swindells S, et al. Adherence of human immunodeficiency virus‐infected patients to antiretroviral therapy. Clinical Infectious Diseases 1999;29:824‐830. [DOI] [PubMed] [Google Scholar]
Wainberg 1998
- Wainberg MA, Friedland G. Public health implications of antiretroviral therapy and HIV drug resistance. JAMA 1998;279:1977‐1983. [DOI] [PubMed] [Google Scholar]
Wood 2003
- Wood E, Hogg RS, Yip B, et al. Is there a baseline CD4 cell count that precludes a survival response to modern antiretroviral therapy?. AIDS 2003;17:711‐720. [DOI] [PubMed] [Google Scholar]
Yeni 2002
- Yeni PG, Hammer SM, Carpenter CC, et al. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society‐USA Panel [Erratum appears in JAMA 2003 Jan‐Feb; 11(1):32]. JAMA 2002;11:222‐235. [DOI] [PubMed] [Google Scholar]
Yeni 2004
- Yeni PG, Hammer SM, Hirsch MS, et al. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society‐USA Panel. JAMA 2004;292:251‐265. [DOI] [PubMed] [Google Scholar]


