Abstract
Context:
India has adopted active case finding (ACF) as an additional strategy to find its missing tuberculosis (TB) cases since 2017. Treatment outcomes of patients identified through ACF may be similar or different from those detected through routine passive case finding (PCF); currently, there are limited studies on this in India.
Aim:
The aim of this study was to assess differences in treatment outcomes of patients detected through ACF and PCF under the national TB program.
Study Design:
A study was conducted in six TB units of Haridwar district where ACF campaigns were conducted in 2017–2018.
Methods:
Data from patients detected by ACF (n = 72) and PCF (n = 184) were extracted from program records.
Results:
Of 72 patients detected by ACF, only 54 (75%) were initiated on treatment. A high proportion of initial loss to follow-up (25% vs. 0%) and delay in treatment initiation (4 days vs. 0 days) was observed in ACF patients as compared to PCF. The proportion of unsuccessful treatment outcome was 33% (n = 18) among ACF patients compared to 14% (n = 25) among PCF patients (adjusted relative risk: 2.6, 95% confidence interval: 1.7–4.0).
Conclusion:
High initial loss to follow-up, delay in treatment initiation, and poor treatment outcome among ACF patients are a major concern. The study results call for active follow-up after diagnosis and close monitoring during treatment for patients detected by ACF.
Keywords: Case finding, India, loss to follow-up, operational research, stigma, treatment outcome, tuberculosis
INTRODUCTION
Tuberculosis (TB) remains a global public health crisis with an estimated 10.8 million new cases in 2017, of which only 6.4 million (64%) were diagnosed and notified to national programs.[1] The World Health Organization's (WHO) goal to end the TB epidemic by 2035 will not be realized without finding these 3.6 million “missing” people.[1] The WHO End TB Strategy highlights the importance of “active case finding (ACF)” in finding the missing cases. ACF is defined as “the systematic identification and screening of people with presumptive TB, in high-risk groups, using tests, examinations or other procedures that can be applied rapidly.”[2] This includes interventions such as contact screening of index TB patients or mass community screening of asymptomatic individuals and other high-risk groups.
India continues to have the highest number of TB cases in the world with nearly 2.11 million cases in 2017 and 1.3 million missing cases.[3] We need to identify them to achieve the ambitious targets set by the Government of India for ending TB by 2025.[4] In line with this, the Revised National Tuberculosis Control Program (RNTCP) has decided to shift from the passive case strategy (PCF) to PCF + ACF strategy for TB detection in 300 high-risk districts.[5] In Uttarakhand state, Haridwar district was selected for ACF campaign. The first and second phases of ACF campaign were conducted in January 2017 and February 2018. Since most of the patients detected through ACF would be asymptomatic or comparatively healthier than those detected by PCF, the treatment outcomes of ACF patients may or may not be similar to PCF patients. However, currently, there are limited studies on this in India.[6]
Thus, the present study was designed to describe the diagnostic and treatment cascade of ACF patients and report their treatment outcomes versus those detected by PCF.
METHODS
Study design
It was a retrospective cohort study involving review of existing program records.
Study setting
Uttarakhand is a hilly state in the northern part of India which is administratively divided into 13 districts. Haridwar is the second most populated district in the state with nearly 1.9 million inhabitants. Due to its holy importance, Haridwar has a large migrant population, mostly pilgrims. Haridwar district was selected for ACF campaign due to large migrant population. Haridwar leads the state in terms of TB case detection (4255 cases) in the year 2017.[7] RNTCP was launched in the district in 2004. There are six tuberculosis units (TUs) and 16 designated microscopy centers in the district. Till now, two phases of ACF campaign under RNTCP have been completed in Haridwar district: the first phase in January 2017 and the second phase in February 2018. The first phase was conducted in all six TUs (Haridwar, Roorkee, Laksar Narsan, Bahadrabad, and Bhagwanpur) and the second phase involved three TUs (Roorkee, Bahadrabad, and Narsan). The present study included records of both the campaigns.
ACF campaigns were conducted as per the RNTCP Technical and Operational Guidelines 2016, as shown in Figure 1.[5] Due to resource limitation in the first two phases of ACF campaign in Haridwar district, chest X-ray and CBNAAT could not be performed. Following the diagnosis of TB, patients were started on a 6/8-month RNTCP treatment regimen.
Figure 1.
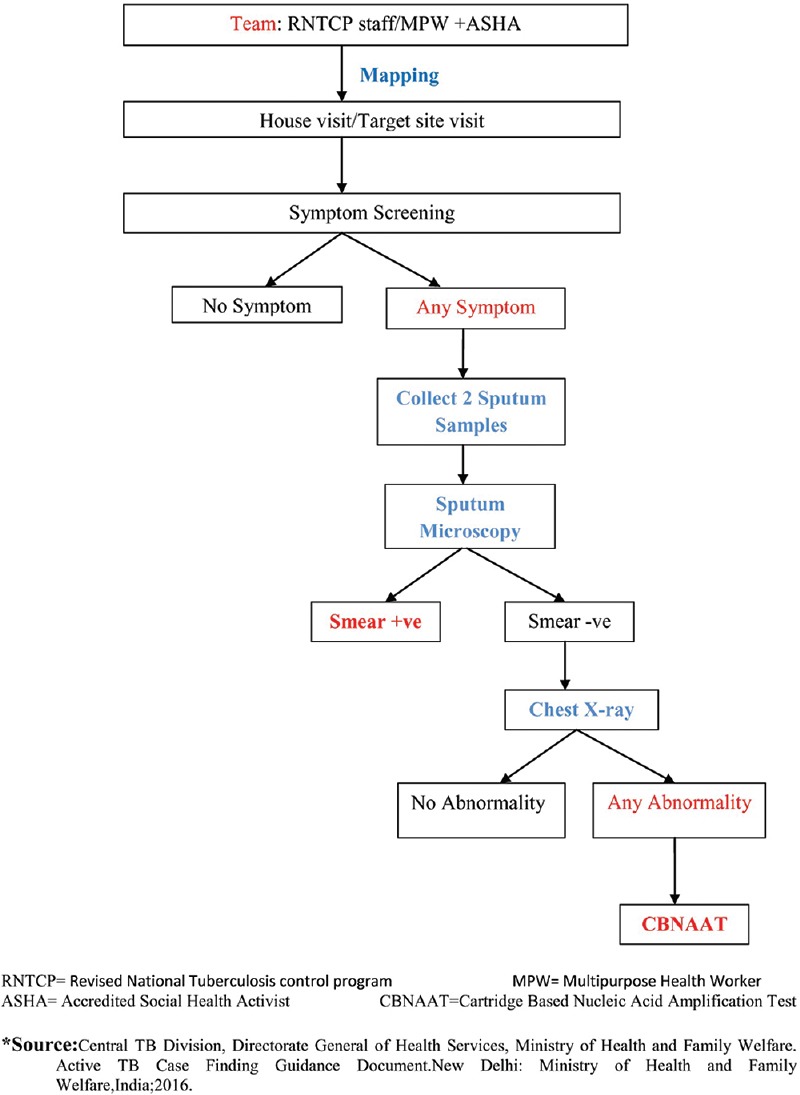
Screening workflow chart of active tuberculosis case finding campaign under the Revised National Tuberculosis Control Program
Details of the ACF screening activity in terms of aggregate figures (number screened, number of presumptive TB patients identified, number tested, and number diagnosed with TB) were recorded in an ACF register on a daily basis at the TU level. Once a patient is diagnosed with TB, he is put on treatment and his details are recorded in the Nikshay portal at the TU by the data entry operator.
Study participants
Study participants were included a ll TB patients who diagnosed by two ACF campaigns and detected by routine PCF in January 2017 and February 2018 in Haridwar district. In the first (involved six TUs) and second (involved three TUs) phases of ACF campaign, 33 and 39 TB patients were diagnosed, respectively. In the same period, 111 and 73 TB patients were detected by PCF under routine RNTCP in these TUs. All these 72 TB patients diagnosed by ACF campaign and 184 TB patients diagnosed by PCF activity were included in this study.
Data variables, data source, and data collection
The variables included were as follows: age, sex, clinical profile (category of disease, type of diagnosis, site of disease, type of drug regimen, and initiation of treatment), date of diagnosis, date of treatment initiation, and treatment outcomes of patients detected by ACF and PCF. Quantitative data were collected from multiple sources (Nikshay portal, lab register, treatment register, and ACF register) and entered into a structured data collection pro forma.
Data analysis
Quantitative data were double-entered and validated using EpiData version 3.1 (EpiData Association, Odense, Denmark). Descriptive statistics (frequencies and proportions) was used to summarize the characteristics of patients stratified by the type of case finding (ACF and PCF). We compared the demographic and clinical characteristics of patients detected by ACF and PCF using the Chi-square test and t-test, wherever applicable. The main exposure variable of interest was type of case finding activity (ACF or PCF). The primary outcome of interest was unsuccessful or successful treatment outcome. Unsuccessful treatment outcome included those recorded as died, loss to follow-up, failed, and not evaluated. Successful treatment outcomes included treatment completed and cured categories. We used log-binomial regression to assess the differences in treatment outcomes between cases detected by ACF and PCF after adjusting for potential sociodemographic and clinical confounders. The strength of association was presented using adjusted relative risks (aRRs). The analysis was done using STATA software (version 13.0 College Station, TX: StataCorp, LP, USA).
Ethical issues
Ethical approval was obtained from the Institutional Ethics Committee of AIIMS, Rishikesh, India (AIIMS/IEC/18/474), and from the Ethics Advisory Group of the International Union against Tuberculosis and Lung Disease, Paris, France (The Union/EAG/87/18). Administrative approval was taken from the District and State TB Officer for conducting the study.
RESULTS
Diagnosis and treatment cascade of patients identified through active case finding campaign
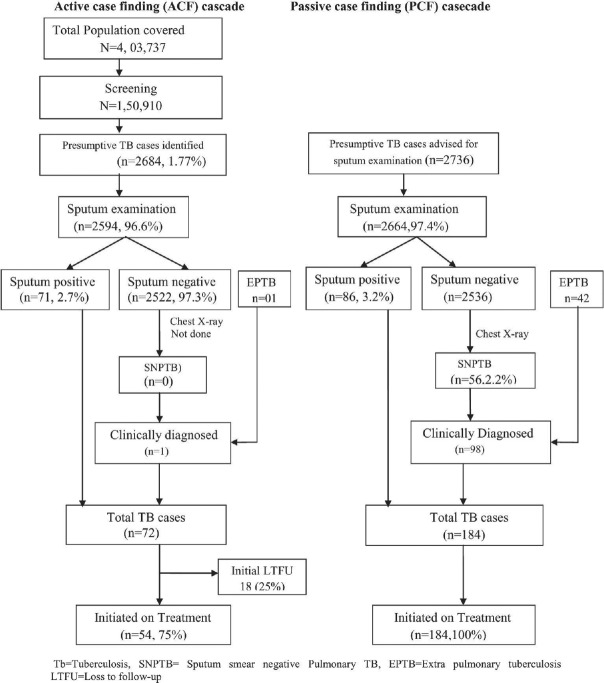
The diagnostic and treatment cascade of patients identified through ACF and PCF is detailed in Figure 2. The ACF campaigns covered a population of 403,737 and screened 150,910 individuals identifying 2684 presumptive TB cases (2.8%), of whom 2594 (96.6%) underwent sputum examination. A total of 72 cases of TB were diagnosed including 1 case of clinically diagnosed extrapulmonary TB, of whom 54 (75%) were initiated on anti-TB treatment. A total of 184 TB cases (86 bacteriologically confirmed, 42 extrapulmonary TB, and 56 sputum smear-negative TB) were identified through PCF, all of whom were initiated on treatment. The initial loss to follow-up (LTFU) was higher among patients detected through ACF as compared to patients detected through PCF (25% vs. 0%).
Figure 2.
Flow diagram of active case finding and passive case finding cascade under the Revised National Tuberculosis Control Program in Haridwar district in the year 2017 and 2018
Demographic and clinical profile of patients detected by active case finding versus passive case finding
The demographic, clinical, and treatment characteristics of patients stratified by ACF and PCF are presented in Table 1. The major differences between the patients detected by ACF versus PCF were in their age group, proportion of category 2 TB disease (6% vs. 18%), and extrapulmonary TB (1% vs. 23%). The median (IQR) number of days from diagnosis to treatment initiation was 4 (10.5-2.0) to 0 (0-0) days for patients detected by ACF and PCF, respectively (P < 0.001).
Table 1.
Comparison of the sociodemographic and clinical profiles of tuberculosis patients detected by active case finding and passive case finding under the Revised National Tuberculosis Control Program in Haridwar district (2017-2018)
| Characteristics | Total | Detected by ACF | Detected by PCF | P |
|---|---|---|---|---|
| Sex | ||||
| Male | 158 (62) | 47 (65) | 111 (60) | 0.4 |
| Female | 98 (38) | 25 (35) | 73 (40) | |
| Age group in years | ||||
| 0-14 | 6 (2) | 0 (0) | 6 (3) | 0.1 |
| 15-44 | 153 (60) | 39 (54) | 114 (62) | |
| 45 and above | 97 (38) | 33 (46) | 64 (35) | |
| Category | ||||
| Category I | 218 (85) | 68 (94) | 150 (82) | 0.009 |
| Category II | 38 (15) | 4 (6) | 34 (18) | |
| Site of disease | ||||
| Pulmonary | 213 (83) | 71 (99) | 142 (77) | 0.0000 |
| Extrapulmonary | 43 (17) | 1 (1) | 42 (23) | |
| Type of diagnosis | ||||
| Microbiologically confirmed | 157 (62) | 71 (100) | 86 (47) | 0.0000 |
| Clinically confirmed | 99 (38) | 1 (0) | 98 (53) | |
| Initiation of treatment* | ||||
| Yes | 238 (93) | 54 (75) | 184 (100) | 0.0000 |
| No | 18 (7) | 18 (25) | 0 (0) | |
| Type of regimen* | ||||
| Daily | 101 (42) | 28 (52) | 73 (40) | 0.1114 |
| Intermittent | 137 (58) | 26 (48) | 111 (60) | |
| Median (IQR) time interval from diagnosis to treatment (in days)* | 0 | 4 | 0 | 0.0000 |
| Total | 256 (0.0-0.0) | 72 (10.5-2.0) | 184 (0.0-0.0) |
*Only for those who were started on treatment, 54 for ACF and 184 for PCF. RNTCP: Revised National Tuberculosis Control Program, ACF: Active case finding, PCF: Passive case finding, IQR: Interquartile range
Treatment outcome (active case finding versus passive case finding)
Of those initiated on treatment, the proportion of unsuccessful treatment outcome was 33% (n = 18) among ACF patients compared to 14% (n = 25) among PCF patients. Of those with unsuccessful outcome, majority of them were lost to follow-up (n = 19, 44%) or death (n = 14, 33%). A similar pattern was observed in both PCF and ACF [Table 2].
Table 2.
Tuberculosis treatment outcomes of patients detected by active case finding and passive case finding under the Revised National Tuberculosis Control Program in Haridwar district during 2017-2018
| Treatment outcome | Total | Detected by ACF | Detected by PCF | P |
|---|---|---|---|---|
| Successful outcome | 195 (82) | 36 (67) | 159 (86) | |
| Cured | 106 (44) | 36 (67) | 70 (38) | |
| Completed | 89 (38) | 0 (0) | 89 (48) | |
| Unsuccessful treatment outcome | 43 (18) | 18 (33)# | 25 (14)# | 0.0009* |
| Died | 14 (6) | 5 (9) | 9 (5) | |
| Failed | 2 (1) | 1 (2) | 1 (<1) | |
| Loss to follow-up | 19 (8) | 7 (13) | 12 (7) | |
| Not evaluated | 4 (1.5) | 4 (7) | 0 (0) | |
| Change in treatment regimen | 4 (1.5) | 1 (2) | 3 (2) | |
| Transferred out | 0 (0) | 0 (0) | 0 (0) | |
| Total | 238 (100) | 54 (100) | 184 (100) |
*P value represents a significant difference of unsuccessful treatment outcome between patients detected by PCF (14%) versus ACF (33%) which are marked by a #mark. RNTCP: Revised National Tuberculosis Control Program, ACF: Active case finding, PCF: Passive case finding
Adjusted comparison of treatment outcome of patients detected by active case finding versus passive case finding
After adjusting for potential confounders, the risk of unsuccessful outcome among patients detected by the ACF activities was significantly higher compared to cases detected by PCF (aRR: 2.6, 95% confidence interval [CI]: 1.7–4.0). The other factor associated with unsuccessful treatment outcome was the category of TB, i.e., Category II (aRR: 3.2, 95% CI: 2.1–4.9) [Table 3].
Table 3.
Association of the type of case finding (active versus passive) with unsuccessful tuberculosis treatment outcomes among patients registered under the Revised National Tuberculosis Control Program in Haridwar district during 2017-2018
| Characteristics | Total | Unsuccessful outcomes, n (%) | RR (95% CI) | P | aRR (95% CI) | P |
|---|---|---|---|---|---|---|
| Type of case finding | ||||||
| ACF | 54 | 18 (33) | 2.5 (1.5-4.2) | <0.001 | 2.6 (1.7-4.0) | <0.001 |
| PCF | 184 | 25 (14) | 1.0 | |||
| Sex | ||||||
| Male | 145 | 30 (21) | 1.5 (0.8-2.7) | 0.19 | 1.3 (0.7-2.2) | 0.4 |
| Female | 93 | 13 (14) | 1.0 | 1.0 | ||
| Age | ||||||
| 0-14 | 6 | 1 (17) | 1.0 | |||
| 15-44 | 149 | 22 (15) | 0.9 (0.2-5.5) | 0.9 | ||
| 45 and above | 83 | 20 (24) | 1.5 (0.3-9.0) | 0.8 | ||
| Category of patient | ||||||
| Category I | 200 | 31 (15) | 1.0 | 1.0 | ||
| Category II | 38 | 12 (32) | 2.0 (1.2-3.6) | 0.02 | 3.2 (2.1-4.9) | 0.0001 |
| Site of TB | ||||||
| Pulmonary | 195 | 42 (21) | 9.3 (1.3-35.5) | 0.003 | 5.6 (0.8-39.8) | 0.08 |
| Extrapulmonary | 43 | 1 (2) | 1.0 | 1.0 | ||
| Bacteriological status | ||||||
| Bacteriologically confirmed | 140 | 33 (24) | 2.3 (1.2-4.5) | 0.008 | ||
| Clinically diagnosed | 98 | 10 (10) | 1.0 | |||
| Type of regimen | ||||||
| Daily | 101 | 16 (16) | 1.0 | |||
| Intermittent | 137 | 27 (20) | 1.2 (0.7-2.2) | 0.4 | ||
| Treatment delay (days) | ||||||
| ≤7 | 210 | 32 (15) | 1.0 | |||
| >7 | 19 | 5 (26) | 1.7 (0.8-3.9) | 0.2 | 1.5 (0.8-2.8) | 0.2 |
RR: Relative risk, aRR: Adjusted relative risk, CI: Confidence interval, PCF: Passive case finding, ACF: Active case finding
DISCUSSION
There were two key findings in this study: (i) TB patients detected by ACF had significantly worse treatment outcomes compared to those detected by PCF and (ii) there is a high initial LTFU among patients detected through ACF. Nearly one-third of the patients detected by ACF had an unsuccessful treatment outcome which is significantly higher than those in the PCF arm. This supports the general notion that patients detected by ACF are relatively healthier and thus are more likely to drop out or be noncompliant. This is probably because ACF is a provider-driven activity with no active role of the patient in the process of diagnosis and treatment initiation. Similar to our study, other studies have also found ACF patients to perform poorly compared to those identified under the routine PCF activity.[6,8] However, it contradicts findings from recent studies in India, Myanmar, Cambodia, and South Africa, which showed that the treatment outcomes of patients detected by ACF and PCF were similar.[9,10,11,12] A systematic review in 2013 also found no difference in treatment outcomes in both the arms.[13] The differences could be due to varying sample sizes, specific strategies used for ACF, and the level of implementation, considering that our ACF strategy was a campaign approach with several data through the cascade missing.
In our study, initial LTFU among TB patients detected by ACF was very high (25%) as compared to PCF (0%). Although the available evidence is limited with varying definitions used in different studies, the initial LTFU rates among ACF patients reported in other countries ranged from 26% to 32% which support the findings in this study.[6,11] Refusal to start treatment might be attributed to the lack of motivation on the part of the patient due to mild symptomatology and no felt need for receiving care.
The study showed that there were more bacteriologically positive pulmonary TB cases among cases detected by ACF when compared to PCF similar to previous assessments in the literature.[9] This is because the focus of ACF campaigns has always been on picking up smear-positive TB cases from the community in order to curb disease transmission. Another reason could be that X-ray was not used during ACF campaigns due to which clinically diagnosed smear-negative cases were missed. The major strengths of the study are: (i) this is one of the first studies in India to evaluate the pretreatment loss to follow-up and treatment outcomes of patients identified through ACF campaign and compare them with patients diagnosed routinely through PCF under programmatic settings. Although the national TB program has initiated ACF in a campaign mode since 2017, there is no systematic analysis of patient data to inform/improve the activity. Often, the aggregate TB cases identified only are reported.
(ii) This study was conducted under routine program settings, thereby reflecting the true ground picture, (iii) the chances of misclassification of patients into ACF and PCF arms are minimal because the details of patients detected through ACF were recorded in a separate ACF register and the Nikshay portal, and (iv) double data entry and validation was done which minimizes data entry errors.
There were few limitations in this study. First, there was no information on other predictors for TB treatment outcomes such as HIV, diabetes, socioeconomic status, smear grade, smoking status, and nutritional status of patients, and some of them could be associated with the exposure of interest (ACF and PCF). Hence, there could be some bias due to these unexplained confounders. The second missing data in these key predictor variables reflect poor recording and reporting system which needs urgent attention through strict supervision, feedback, and corrective action.
Despite these limitations, there are important policy implications of the results of the study. Our study highlighted high initial LTFU, higher proportion of unsuccessful outcome, and delay in treatment initiation among TB patients detected by ACF. There is a need to motivate and improve patients' perception about the disease and the need for DOTS (Directly observed treatment, short-course) and convince them about the need for initiating and completing treatment. The study also highlights the need to improve the health system's role in ensuring early initiation and completion of treatment. Close monitoring of patients diagnosed through ACF campaigns is needed to reduce initial loss to follow-up and ensure treatment adherence so as to improve the overall program indicators. We also need to understand the reasons for poor treatment outcome and high LTFU among the ACF patients which requires a systematic qualitative inquiry. This will feed into the designing of interventions to improve adherence and treatment outcomes among patients diagnosed through ACF.
CONCLUSION
ACF is an important strategy to find India's missing TB cases. However, in the current campaign strategy, the program is losing patients at various levels and these patients go back to their communities and continue to spread the infection. Measures to reduce loss to follow-up among patients identified through ACF are important in order to improve the effectiveness of ACF campaigns.
Financial support and sponsorship
The training course under which this research was conducted and open access publication charges was funded by The Global Fund to Fight AIDS, Tuberculosis and Malaria (GFATM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This research was conducted as a part of the “National Operational Research Training Course 2018–2019” organized by Project Axshya, funded by The Global Fund, and implemented by The International Union against Tuberculosis and Lung Diseases (The Union), South-East Asia Regional Office, New Delhi, India. The training course was conducted in collaboration with Revised National Tuberculosis Control Program, Ministry of Health and Family Welfare, Government of India, and National Institute of Tuberculosis and Respiratory Diseases, New Delhi, India.
The training is based on “The Union/Medécins sans Frontières (MSF)” model OR course and has been acknowledged/accredited by the Special Programme for Research and Training in Tropical Diseases at the WHO/TDR under the Structured Operational Research and Training Initiative. Mentorship and facilitation for this course was provided through The Union South-East Asia Office, New Delhi; the Centre for Operational Research, The Union, Paris, France; Baroda Medical College, Vadodara; MSF, New Delhi; ESIC Medical College and PGIMSR, Bengaluru; North Delhi Municipal Corporation Medical College, Hindu Rao Hospital, New Delhi; GMERS Medical College, Vadodara; Postgraduate Institute of Medical Education and Research, Chandigarh, India; and Yenepoya Medical College, Mangalore, India.
REFERENCES
- 1.World Health Organization. Global Tuberculosis Report 2018. Geneva: World Health Organization; 2018. [Google Scholar]
- 2.World Health Organization. Systematic Screening for Active Tuberculosis: Principles and Recommendations. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 3.Revised National TB Control Programme: Annual Status Report. New Delhi: Ministry of Health and Family Welfare, India; 2018. Mar, [Last accessed on 2019 May 08]. Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare. TB India Report 2018. Available from: https://tbcindia.gov.in/showfile.php?lid=3314 . [Google Scholar]
- 4.Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare. Revised National Tuberculosis Control Programme: National Strategic Plan for Tuberculosis Control. Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare. 2012. [Last accessed on 2019 Feb 19]. pp. 108–10. Available from: https://www.tbfacts.org/wp-content/uploads/2016/01/NSP-2012-2017.pdf .
- 5.Active TB Case Finding Guidance Document. New Delhi, India: Ministry of Health and Family Welfare; 2016. Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare. [Google Scholar]
- 6.Santha T, Renu G, Frieden TR, Subramani R, Gopi PG, Chandrasekaran V, et al. Are community surveys to detect tuberculosis in high prevalence areas useful? Results of a comparative study from Tiruvallur District, South India. Int J Tuberc Lung Dis. 2003;7:258–65. [PubMed] [Google Scholar]
- 7.Uttarakhand Sees Rise in TB Cases; Haridwar Tops List. Times of India. 2017. Dec 26, [Last accessed on 2019 May 10]. Available from: https://timesofindia.indiatimes.com/city/dehradun/uttarakhand-sees-rise-in-tb-cases-haridwar-tops-list/articleshow/62257897.cms .
- 8.Cassels A, Heineman E, LeClerq S, Gurung PK, Rahut CB. Tuberculosis case-finding in Eastern Nepal. Tubercle. 1982;63:175–85. doi: 10.1016/s0041-3879(82)80028-7. [DOI] [PubMed] [Google Scholar]
- 9.Khaing PS, Kyaw NTT, Satyanarayana S, Oo NL, Aung TH, Oo HM, et al. Treatment outcome of tuberculosis patients detected using accelerated vs. passive case finding in Myanmar. Int J Tuberc Lung Dis. 2018;22:1145–51. doi: 10.5588/ijtld.18.0038. [DOI] [PubMed] [Google Scholar]
- 10.Eang MT, Satha P, Yadav RP, Morishita F, Nishikiori N, van-Maaren P, et al. Early detection of tuberculosis through community-based active case finding in Cambodia. BMC Public Health. 2012;12:469. doi: 10.1186/1471-2458-12-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.den Boon S, Verver S, Lombard CJ, Bateman ED, Irusen EM, Enarson DA, et al. Comparison of symptoms and treatment outcomes between actively and passively detected tuberculosis cases: The additional value of active case finding. Epidemiol Infect. 2008;136:1342–9. doi: 10.1017/S0950268807000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shewade HD, Gupta V, Satyanarayana S, Kumar S, Pandey P, Bajpai UN, et al. Active versus passive case finding for tuberculosis in marginalised and vulnerable populations in India: Comparison of treatment outcomes. Glob Health Action. 2019;12:1656451. doi: 10.1080/16549716.2019.1656451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kranzer K, Afnan-Holmes H, Tomlin K, Golub JE, Shapiro AE, Schaap A, et al. The benefits to communities and individuals of screening for active tuberculosis disease: A systematic review. Int J Tuberc Lung Dis. 2013;17:432–46. doi: 10.5588/ijtld.12.0743. [DOI] [PubMed] [Google Scholar]