Abstract
Background:
An outbreak of Nipah virus infection was confirmed in Kerala, India in May 2018. Five out of 23 cases including the first laboratory-confirmed case were treated at Baby Memorial Hospital (BMH), Kozhikode. The study describes the clinical characteristics and epidemiology of the Nipah virus outbreak at Kozhikode during May 2018.
Objective:
To study the clinical and epidemiological profile of Nipah virus epidemic that occurred in Kerala in May 2018.
Methods:
A collaborative team of physicians and epidemiologists from BMH, Medical College Hospital (MCH) Kozhikode and from the Indian Medical Association (IMA) conducted this study. The clinical and exposure history and the data on outbreak response were gathered from hospital medical records and through interviewing patient relatives and health workers using questionnaires.
Results:
It was identified that out of the 23 patients with Nipah virus infection, 21 (91.3%) expired. Out of the 21 patients, 18 tested positive for Nipah virus by Real Time polymerase chain reaction (RT-PCR). It has been found that only the index case was infected in the community from fruit bats. Rest of the cases were due to transmission of the virus at three public hospitals. Median age was 45 years. 65% of them were males. Median incubation period was 9.5 days. Fever (100%), altered sensorium (84.2%), tachycardia (63.1%), hypertension (36.8%), segmental myoclonus (15.7%), segmental sweating (15.7%) and shortness of breath (73.6%) were common features. Mean duration of illness was 6.4 days.
Conclusion:
The rapid spread of infection uncovered the miserable state of health care system in implementing infection control measures. The case fatality and the socio-economic burden warrant developing appropriate treatments, vaccines and diagnostics.
Keywords: Acute respiratory distress syndrome, encephalitis, henipavirus, Nipah virus, Pteropus bats
INTRODUCTION
Nipah virus (NiV) was first reported among pig farmers of Malaysia between September 1998 and April 1999.[1] The virus was isolated for the first time from a patient who lived in Sungai Nipah village of peninsular Malaysia and hence the name.[2] Bangladesh experienced its first NiV epidemic in 2001 and has been a regular major public health problem since then. Two outbreaks were reported in India during 2001 and 2007, both from the state of West Bengal that borders Bangladesh.[3] Fruit bats (Pteropus species) are the natural hosts, and pigs are the intermediate hosts of NiV.[4] Transmission of NiV occurs through close contact with infected pigs or infected bats or other infected humans. Unprotected exposure carries a greater risk of transmission.[3] Human-to-human transmission was first noticed in the Bangladesh epidemic of 2004.[4,5]
Background
Kerala, the southern state of India, is geographically set apart from the regions which experience NiV outbreaks usually. An outbreak of NiV infection was confirmed for the first time in Kerala in May 2018. NiV infection was unprecedented in this part of the country that posed an initial challenge in diagnosing the cases. The diagnosis was made from the sixth case within a span of 14 days. Five out of 23 cases including the first laboratory-confirmed case were treated at Baby Memorial Hospital (BMH), Kozhikode.
Objective
The objective of the study was to describe the clinical characteristics and epidemiology of the outbreak of NiV infection in Kerala in May 2018.
METHODS
The retrospective study was conducted by a collaborative team of physicians and epidemiologists from the Research Cell of Indian Medical Association (IMA) and the BMH and the Medical College Hospital (MCH) Kozhikode, the two hospitals where most of the cases were being admitted. The clinical and exposure history and the data on outbreak response were gathered from hospital medical records and by interviewing patient relatives and health-care workers using questionnaires. We visited residential places and health-care institutions where the spread occurred. Thirty respondents were interviewed and two target group discussions involving health-care workers and patient relatives were held. The study period was from May 20 to June 5.
Case definition
We followed the guidelines of the National Centre for Disease Control for case definitions.[6] A probable case is defined as a suspect case who resided in the same village as the confirmed case or who came in direct contact with a confirmed case in a hospital setting during the outbreak period and developed symptoms of respiratory distress and/or encephalitis and died before diagnostic specimen could be collected. A confirmed case is defined as a suspect case whose serum or urine or respiratory secretions or cerebrospinal fluid (CSF) tested positive for NiV RNA by Real Time polymerase chain reaction (RT-PCR) or by isolation of NiV from any of these fluids.
RESULTS
We identified 23 patients including 5 probable cases 21 of whom (91.3%) died. Eighteen were confirmed cases and tested positive for NiV by RT-PCR. It was found that all cases, except one, contracted the virus in three public hospitals. The index case was assumed to be infected from fruit bats. Out of 22 cases (excluding index case), 19 had exposure to the index case at various locations. Three cases contracted the infection from some of the primary cases. The median age was 45 years. Sixty-five percent of them were males.
Clinical features
The most common symptom was fever (100%) followed by altered sensorium (84.2%). Tachycardia, hypertension, segmental myoclonus and segmental sweating were seen in 63.1%, 36.8%, 15.7% and 15.7% respectively. Shortness of breath was seen in 14 out of 19 patients (73.6%). The median incubation period was 9.5 days (range, 4–14 days), and the mean duration of illness was 6.4 days. A comparative account of clinical features of patients admitted to the two hospitals, i.e., BMH and MCH are shown in Table 1.
Table 1.
Comparative account of clinical features of patients admitted to two major hospitals
| Clinical feature | BMH | MCH | Cumulative data (%) |
|---|---|---|---|
| Number of patients (n) | 5 | 14 | 19 |
| Median age (years) | 52 | 29.5 | 36 |
| Sex | 4:1 | 8:6 | 12:7 |
| Median incubation period (days) | 10.8 | 10.2 | 10.5 |
| Fever | 5 | 14 | 19/19 (100) |
| Sore throat | 1 | 0 | 1/19 (5.2) |
| Myalgia | 3 | 9 | 12/19 (63.1) |
| Headache | 2 | 5 | 7/19 (36.8) |
| Vomiting | 3 | 5 | 8/19 (42.1) |
| Altered sensorium | 5 | 11 | 16/19 (84.2) |
| Seizures | 1 | 1 | 2/19 (10.5) |
| Segmental myoclonus | 3 | 0 | 3/19 (15.7) |
| Segmental sweating | 2 | 1 | 3/19 (15.7) |
| Hypertension | 5 | 2 | 7/19 (36.8) |
| Tachycardia | 5 | 7 | 12/19 (63.1) |
| Ataxia | 1 | 0 | 1/19 (5.2) |
| Cranial nerve palsy | 2 | 1 | 3/19 (15.7) |
| Limb weakness | 2 | 0 | 2/19 (10.5) |
| Hypotonia | 5 | 0 | 5/19 (26.3) |
| Meningism | 0 | 1 | 1/19 (5.2) |
| Shortness of breath | 5 | 9 | 14/19 (73.6) |
| Loose stools | 0 | 4 | 4/19 (21) |
It includes the index as well as the serologically confirmed cases. Data not available for probable cases. BMH: Baby Memorial Hospital, MCH: Medical College Hospital
First laboratory-confirmed case
A 28-year-old male with fever, vomiting, and altered sensorium was referred to BMH from Sub-district hospital (SDH), Perambra, in the early hours of May 17, 2018. He was diaphoretic, restless and confused on arrival at the emergency room (ER). He had hypertension (180/100 mmHg), tachycardia (180/min), and tachypnea (36/min). Nervous system examination revealed hypotonia of all four limbs, diminished deep tendon reflexes and limb myoclonus. Chest examination showed bilateral diffuse fine crackles. He was admitted to the multidisciplinary intensive care unit. Blood tests like complete blood counts, hepatic, renal, and coagulation parameters were normal, except mild thrombocytopenia (140,000 cells/mm3). Arterial blood gases (ABGs) was normal. Computed tomography (CT) brain revealed cerebral edema. He was electively intubated, and intermittent positive pressure ventilation was instituted. His initial bedside echocardiogram (ECHO) was normal. Intravenous (IV) mannitol was administered, and lumbar puncture was done. A CSF study showed elevated protein and sugar levels. Cell count was normal. He was started on empirical treatment with IV acyclovir and ceftriaxone for probable central nervous system infection. His father and aunt who had similar signs and symptoms were brought to BMH on the same morning. As per the relatives' account, his brother had died following a similar illness 12 days earlier. The family cluster of similar clinical conditions prompted the treating team to test his blood for NiV along with other viruses that cause encephalitis and toxins. A detailed microbiological and virological analysis was run by Manipal Centre for Virus Research (MCVR). The results proved NiV infection by late evening of May 18, 2018. The test results were reconfirmed by the National Institute of Virology (NIV), Pune on May 20, 2018.
Index case
On retrospective investigation, it was found that a 26-year-old brother of the first confirmed case had similar symptoms since May 2, 2018, and was admitted to SDH, Perambra, on May 3, 2018. He later died at MCH on May 5, 2018. This is considered as the index case. His clinical features included fever, altered sensorium, headache, myalgia, cough, shortness of breath, and vomiting which were consistent with encephalitis, sepsis, and acute respiratory distress syndrome (ARDS). Nonspecific presentation besides limited time to investigate the case led to the ignorance of an impending epidemic.
In search of the source of outbreak, the Nipah link with Pteropus bats was confirmed and established by the NIV and Indian Council of Medical Research. About 19.2% (10/52) of the bats collected from the outbreak area tested positive for NiV by RT-PCR.[7] The timing of the outbreak coincided with the breeding season of bats, a known zoonotic reservoir. The index case, who was an animal lover, might have come in contact with NiV-infected bat pup, or might have consumed bat-bitten fruits which were explained as the likely modes of transmission. The possible date of infection to the index case could be between April 11–27, 2018, based on the documented incubation period of the disease (4–21 days).
Further transmission
Among the 23 cases identified, 19 were primary cases who contracted the infection from the index case while he was admitted to SDH, Perambra, and MCH between May 4–5, 2018. The wards, ER, and radiology room were the places of exposure. Of the 3 secondary cases, 2 contracted the disease at MCH and 1 at SDH, Balussery, where the primary cases were treated. The cases comprised of immediate family members, patients and their bystanders, and caregivers. Three patients on May 17, 2018, and one patient on May 19, 2018, had succumbed with features of encephalitis and ARDS. All these patients had come into close contact with the index case either at SDH, Perambra, or at MCH. They were identified as probable cases because NiV serology was not tested in them. Table 2 summarizes the list of patients source of infection, nature of exposure, and outcome. Figure 1 shows the incidence of NiV cases on each day from 1st May to 31st May. Peak incidence was noted between 11th to 23rd May which corresponds to the incubation period of NiV. Figure 2 shows the epidemic curve with incidence and mortality rates. Figure 3 shows number of patients exposed to index case each location.
Table 2.
Demographic details and outcome of patients
| Case | Age (years) | Sex | Location of exposure | Type of contact | Source of infection | Date of exposure | Date of onset of illness | Outcome | Date of death |
|---|---|---|---|---|---|---|---|---|---|
| 1X | 26 | Male | Not known | Consumption of bat-bitten fruit* | Bats* | Unknown | May 2, 2018 | Died | May 5, 2018 |
| 2a | 45 | Male | 1 | Bystander | Case 1 | May 4, 2018 | May 13, 2018 | Died | May 17, 2018 |
| 3a | 100 | Male | 1 | Inpatient | Case 1 | May 4, 2018 | May 15, 2018 | Died | May 17, 2018 |
| 4a | 17 | Male | 3 | Bystander | Case 1 | May 5, 2018 | May 11, 2018 | Died | May 17, 2018 |
| 5a | 48 | Female | 3 | Hospital staff | Case 1 | May 5, 2018 | May 12, 2018 | Died | May 19, 2018 |
| 6y | 28 | Male | 1 | Family (brother) | Case 1 | May 4, 2018 | May 13, 2018 | Died | May 18, 2018 |
| 7 | 51 | Female | 1 | Family (aunt) | Case 1 | May 5, 2018 | May 13, 2018 | Died | May 19, 2018 |
| 8b | 50 | Male | 1 | Inpatient | Case 1 | May 4, 2018 | May 17, 2018 | Died | May 20, 2018 |
| 9 | 36 | Female | 3 | Inpatient | Case 1 | May 5, 2018 | May 16, 2018 | Died | May 20, 2018 |
| 10 | 23 | Female | 3 | Inpatient | Case 1 | May 5, 2018 | May 13, 2018 | Died | May 20, 2018 |
| 11 | 48 | Male | 2 | Bystander | Case 1 | May 5, 2018 | May 16, 2018 | Died | May 20, 2018 |
| 12 | 32 | Female | 1 | Caregiver | Case 1 | May 4, 2018 | May 15, 2018 | Died | May 20, 2018 |
| 13 | 48 | Female | 1 | Bystander | Case 1 | May 4, 2018 | May 18, 2018 | Died | May 21, 2018 |
| 14 | 52 | Male | 1 | Bystander | Case 1 | May 5, 2018 | May 15, 2018 | Died | May 22, 2018 |
| 15 | 45 | Male | 1 | Bystander | Case 1 | May 4, 2018 | May 15, 2018 | Died | May 22, 2018 |
| 16 | 58 | Male | 1 | Family (father) | Case 1 | May 4, 2018 | May 15, 2018 | Died | May 24, 2018 |
| 17c | 75 | Female | 4 | Inpatient | Cases 9,10,11 | May 17, 2018 | May 23, 2018 | Died | May 26, 2018 |
| 18 | 27 | Male | 3 | Bystander | Case 1 | May 5, 2018 | May 14, 2018 | Died | May 27, 2018 |
| 19 | 54 | Male | 3 | Bystander | Case 1 | May 5, 2018 | May 17, 2018 | Died | May 30, 2018 |
| 20c | 28 | Male | 2 | Bystander | Case 2 | May 14, 2018 | May 23, 2018 | Died | May 30, 2018 |
| 21c | 26 | Male | 5 | Inpatient | Case 8b | May 19, 2018 | May 26, 2018 | Died | May 31, 2018 |
| 22 | 19 | Female | 2 | Caregiver | Case 1 | May 5, 2018 | May 13, 2018 | Survived | Not applicable |
| 23 | 27 | Male | 2 | Outpatient at MCH | Case 1 | May 5, 2018 | May 19, 2018 | Survived | Not applicable |
xIndex case, yFirst confirmed case, aProbable cases (NiV serology not done), bCase 8 was initially admitted to SDH, Perambra on May 4, 2018, where he contracted NiV from case 1. He was again, admitted to SDH, Balussery, on May 17, 2018, after the onset of fever. Case 21 was already admitted to SDH, Balussery, from May 14, 2018, where he contracted NiV from case 8, cSecondary cases without exposure to case 1, *Unconfirmed hypothesis. Location 1: SDH, Perambra, Location 2: Casualty, MCH, Kozhikode, Location 3: Radiology room, MCH, Location 4: ICU, MCH, Location 5: SDH, Balussery, NiV: Nipah virus, MCH: Medical College Hospital, SDH: Sub-district hospital
Figure 1.
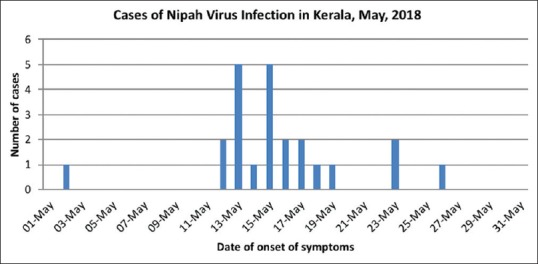
Incidence of Nipah virus infection
Figure 2.
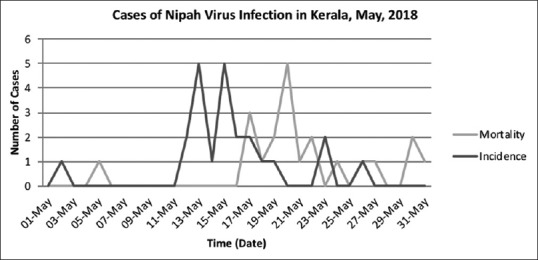
Epidemic curve
Figure 3.
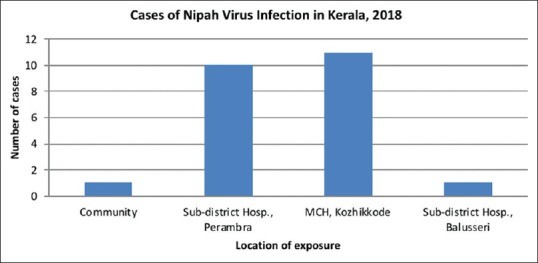
Location of exposure of Nipah virus infection
Overcrowding, inadequate ventilation, and improper infection control practices in the wards, ERs, and the corridors of some public hospitals contributed to the rapid spread of infection. The hospital staff was not using hand gloves, face mask, and other personal protective equipment, and they were not following hand hygiene precautions. This led to an easy transmission of infection to patients, bystanders, and caregivers in these hospitals.
Outbreak response
After the confirmation of the outbreak by NIV, Pune, on May 20, 2018, it was notified to the World Health Organization (WHO) on May 23, 2018. MCH Kozhikode was made as the nodal center for admitting all suspected NiV cases, all health-care providers in the region were instructed to follow the instructed protocols and transfer the suspected cases to the nodal center. Stringent infection control measures were instituted in all hospitals of the district. Round-the-clock helplines were established, and outreach programs were organized to reduce panic and facilitate people in contacting the health authorities. Awareness campaigns were run on the media to educate the general public on identifying suspected cases and reporting to the nodal center. Contact tracing was done based on hospital admission records. The last case was reported on May 29, 2018. A total of 2649 contacts were traced and followed up through the incubation period. Samples of blood, urine, and throat swab were collected from 337 suspected cases and sent for testing at MCVR. Eighteen samples tested positive for NiV by RT-PCR and the rest 319 were negative. The WHO published the outbreak news on May 31, 2018, and the state was declared epidemic-free by July 1, 2018.
Laboratory features
Laboratory data were available for 18 patients, who tested positive for NiV RT-PCR. Serology was not done in the index case and four other epidemiologically linked cases. RT-PCR was weakly positive in the urine sample of 1 patient (case 19). tested on May 22, 2018, who had a history of contact with the index case on May 5, 2018, and was admitted to BMH on May 18, 2018. The throat and urine samples tested strongly positive, but the blood sample remained negative when the test was repeated on May 28, 2018. The patient expired on May 30, 2018. Most of the patients tested positive for NiV only in the later part of the illness.
Complete blood count showed leukopenia in 2 cases, and 2 others developed leukocytosis probably due to sepsis in the terminal stage of the illness. Total leukocyte count was normal in rest of the patients. Mild thrombocytopenia (130,000–140,000/mm3) was detected in 42% of the cases. Abnormal liver function test characterized by elevated aspartate aminotransferase in the range of 60–78 IU/L was noted in 3 patients. Urine biochemistry showed 3 + proteinuria in 2 patients. CSF specimens showed mildly elevated protein, sugar, and opening pressure (250-350 mmH2O) in three patients. One patient showed elevated protein alone. CSF study was not carried out in rest of the cases. Blood culture done in five patients did not show any growth. Serum troponin I (trop I) was high in 7 patients. ECHO showed global left ventricular hypokinesia in two patients. Myocardial function as indicated by ECHO and trop I became abnormal on the 2nd or 3rd day of admission. ECHO and trop I estimation were not done in a majority of patients. ABG showed type 1 respiratory failure in 73.6% of the cases. The investigation details of patients admitted to BMH are summarised in Table 3.
Table 3.
The investigation details of patients admitted to Baby Memorial Hospital
| Parameter | Case 6 | Case 7 | Case 14 | Case 16 | Case 19 |
|---|---|---|---|---|---|
| TC | Normal | Normal | Low | Low | Normal |
| Serum creatinine | Normal | Elevated in late stages | Elevated in late stages | Elevated in late stages | Normal |
| Transaminases | Normal | Normal | Normal | Elevated | Normal |
| Serum electrolytes | Hyponatremia | Normal | Normal | Hyponatremia | Normal |
| Urine biochemistry | Proteinuria (3+) | Normal | Normal | Proteinuria (3+) | Proteinuria (3+) |
| CSF | High protein, high sugar | High protein, high sugar | High protein, high sugar | High protein | Normal |
| CT brain | Diffuse cerebral edema with compression of ventricles | Hydrocephalus | Diffuse cerebral atrophy with chronic infarcts | Normal | Normal |
| MRI brain | Nonspecific white matter hyperintensities on T2W FLAIR sequence | Not done | Not done | Not done | Not done |
| Echocardiogram | Global LV dysfunction | Global LV dysfunction | Normal | Normal | Normal |
| Trop I | Elevated | Elevated | Normal | Normal | Normal |
| LDH | Elevated | Elevated | Elevated | Not done | Not done |
| ABG | Hypoxia | Hypoxia | Hypoxia | Hypoxia | Hypoxia |
| Date of contact | May 2, 2018 | May 2, 2018 | May 5, 2018 | May 2, 2018 | May 2, 2018 |
| Number of days of symptoms | 4 | 2 | 4 | 4 | 10 |
| Date of admission | May 17, 2018 | May 17, 2018 | May 20, 2018 | May 17, 2018 | May 18, 2018 |
| Date of death | May 18, 2018 | May 19, 2018 | May 22, 2018 | May 24, 2018 | May 30, 2018 |
| Date of NiV serology positivity | May 18, 2018 | May 18, 2018 | May 20, 2018 | May 18, 2018 | May 28, 2018 |
TC: Total leukocyte count, CSF: Cerebrospinal fluid, MRI: Magnetic resonance imaging, LDH: Lactate dehydrogenase, ABG: Arterial blood gas, NiV: Nipah virus, FLAIR: Fluid-attenuated inversion recovery, LV: Left ventricular
Radiological abnormalities
Abnormal chest X-ray was seen in 5 out of 18 patients (27.7%). Of these, 3 patients had bilateral fluffy shadows and 1 had patchy opacities in the right lower zone suggestive of ARDS [Figure 4]. CT brain was done in 5 patients, of which 2 had normal findings, 2 had diffuse brain edema and early hydrocephalus and 1 had diffused cerebral atrophy with chronic infarcts. Magnetic resonance imaging brain of 1 patient showed nonspecific white matter hyperintensities on T2-weighted fluid-attenuated inversion recovery sequence [Figure 5].
Figure 4.
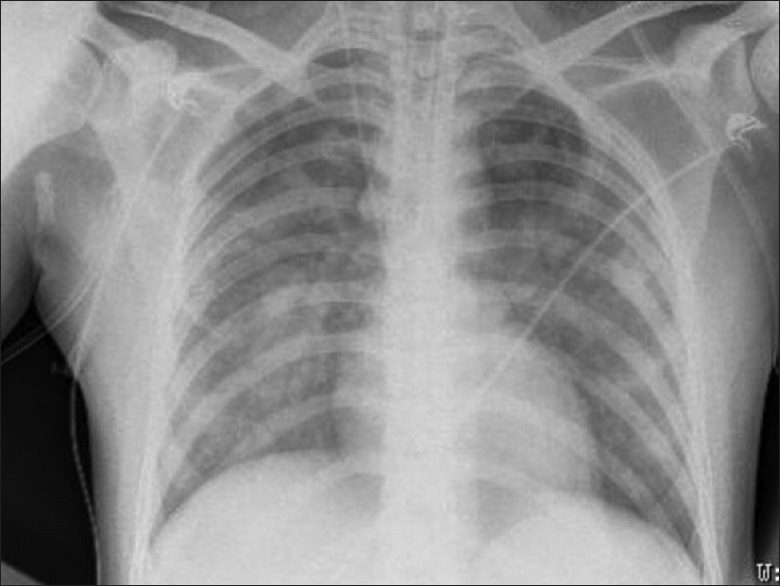
Chest X-ray (anteroposterior view) showing bilateral fluffy shadows involving middle and lower zones – suggestive of acute respiratory distress syndrome
Figure 5.
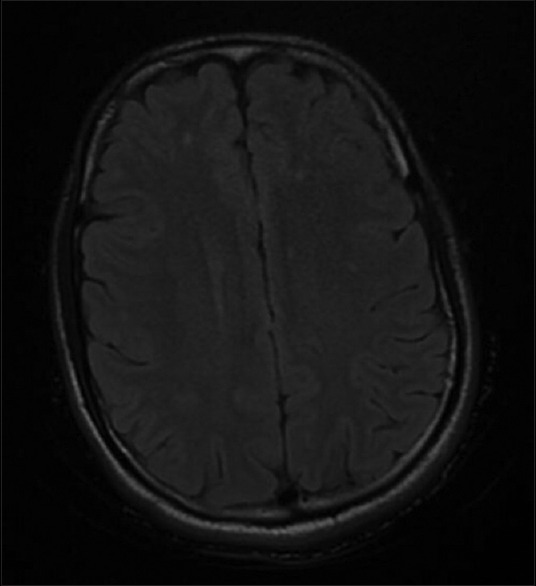
Axial section – T2-weighted fluid-attenuated inversion recovery sequence of magnetic resonance imaging brain showing discrete nonspecific white matter hyperintensities
Treatment
Patients with respiratory distress received both noninvasive ventilation and intermittent positive pressure ventilation. A majority received IV antibiotics to prevent secondary bacterial infection. Patients with seizures received antiepileptics. Raised intracranial tension and brain edema were managed with mannitol and dexamethasone. Those who developed hypertensive crisis received antihypertensives. Patients in septic shock received vasopressors and crystalloids. Out of 19 patients, 8 (42.1%) received ribavirin. IV acyclovir was initially given to 3 patients, and later, it was changed to ribavirin 1200 mg/day (in 6 divided doses). The 2 patients who survived received ribavirin for 14 days, and it was started in the early stage of illness.
DISCUSSION
This retrospective study is aimed at providing an insight into the clinical manifestations, laboratory features, and epidemiology of the first NiV outbreak experienced in Kerala. The case fatality rate (91.3%) was very high in the outbreak. It was found that only the index case was infected in the community from fruit bats. Rest of the cases were due to transmission of the virus in 3 public hospitals.
There are important differences between the clinical and epidemiological descriptions of NiV infection in Kerala and those of the Malaysian outbreak. Unlike in Malaysian outbreak where direct contact with infected pigs was identified as the mode of transmission to humans, here, fruit bats are suspected to be the source of infection, and ingestion of bat-bitten fruits is propounded to be the mode of transmission to the index case.[8] All other cases resulted from human-to-human spread by close contact. In Bangladesh epidemics between 2001 and 2007, NiV was contracted from an infected domestic cow, consumption of date palm sap contaminated by bat secretions, and human-to-human transmission.[9]
In a study by Goh et al., 91 out of 94 (97%) patients had fever as compared to Kerala where all of them presented with fever.[10] Headache was present in 36.8% (7/19), whereas it was noted in 74.9% of the patients in Malaysian outbreak.[11] In Siliguri, fever was present in all and headache and myalgia in 5%.[4] Altered sensorium was present in 16 84.2% (16/19) in comparison to 71.6% in Malaysia[11] and 97% in Siliguri outbreak.[4] Segmental myoclonus and segmental sweating were present in 15.7% cases (3/19), whereas it was noted in 49.3% and 26.2% of the patients, respectively, in Malaysia.[11] Hypertension was present in 36.8% (7/19) and tachycardia in 63.1% (12/19) towards the end of illness. Many patients developed hypertension before death in Siliguri outbreak.[4] Hypertension and tachycardia were noted in 43.3% and 42.3% of Malaysian patients, respectively.[11] These features are because of brainstem dysfunction during later stages of the illness. The incidence of seizures was 10.5% (2/19), in contrast to Malaysian outbreak where it was 23.2%.[11] In Siliguri, it was not possible to differentiate between involuntary movements and seizures.[4] Hyporeflexia was present in 26.3% (5/19) and limb weakness in 10.5% (2/19) patients. These features were also present in seriously ill patients of Malaysian and Siliguri outbreaks.[4,11] Only 5.2% (one patient) had meningism, whereas signs of meningeal irritation were not noted in Siliguri,[4] and 19.1% of the cases had meningism in Malaysia.[11] One patient had cerebellar ataxia, and this was a unique finding because it was not recorded in Malaysia and Siliguri.[4,11] Cranial nerve involvement was seen in 15.7% of the patients (2 patients had bilateral ptosis and 1 had lateral rectus palsy). In Malaysia, pinpoint pupils were noted in a majority of the patients, whereas in Siliguri, all patients had dilated pupils.[4,11] In Singapore, 2 out of 11 patients presented with pneumonia without encephalitis.[12] Kerala had both respiratory and neurological manifestations of NiV, unlike Malaysian where only neurological disease was seen.[11] About 73.6% (14/19) of the patients had ARDS (characterized by fall in oxygen saturation, bilateral crackles on respiratory examination, and diffuse pulmonary infiltrates on chest X-ray requiring ventilation support). In Siliguri outbreak, respiratory involvement was seen in 51% of the cases.[4] In Bangladesh epidemics between 2001 and 2004, respiratory distress was noted in 69% of the cases.[13] genomic similarity was observed among the NiV strains that caused Bangladesh, Siliguri, and Kerala outbreaks. Nucleotide sequences of Siliguri strain showed 99% similarity to Bangladesh strain.[4] Kerala strain was 97% identical to Bangladesh strain.[14] Variations in genomic structure could be the reason for the absence of respiratory system involvement in Malaysian outbreak, while it was a chief manifestation in Bangladesh, Siliguri, and Kerala outbreaks. High rates of human-to-human transmission in Bangladesh, Siliguri, and Kerala outbreaks might also be because of the genomic similarity. Respiratory symptoms and diarrhea could have contributed to high transmission rates among humans in Kerala.[15] Another unique feature was myocardial dysfunction in 7 patients, which was not reported in previous outbreaks. The mortality rate was 91.3% (21/23) which was much higher than the Malaysian (40%) and Siliguri (68%) outbreaks. One of the major confounding factors might be poor infection control precautions at SDH, Perambra, and MCH, Kozhikode. The two patients, who survived, received ribavirin in the early stages. This might be a coincidence or a treatment response which can be established only by further studies on ribavirin.
CONCLUSION
Although the first confirmation of diagnosis was made in one of the primary cases and a swift response from various stakeholders curtailed the spread of the infection in less than a month, rapid initial spread of infection through public hospitals uncovered the miserable state of health-care system in implementing infection control measures. The transmission seemed to have stopped in health facilities which had stricter infection control protocols. Hence, adoption of stringent institution-based infection control practices in all hospitals is a need of the hour to avert epidemics. Considering the fact that NiV is conquering new territories each year, the role of ecological factors such as deforestation and urbanization needs to be studied. “One health” approach that includes animal surveillance enhances early detection of zoonotic infections. Taking high case fatality and socioeconomic burden caused by NiV into account, there is a need for appropriate treatment strategies, vaccines, and diagnostics for fatal diseases such as NiV infection.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient's guardians have given their consent for the patient's images and other clinical information to be reported in the journal. The patient's guardians understand that the patient's name and initial will not be published, and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Chua KB. Nipah virus outbreak in Malaysia. J Clin Virol. 2003;26:265–75. doi: 10.1016/s1386-6532(02)00268-8. [DOI] [PubMed] [Google Scholar]
- 2.Mohd Nor MN, Gan CH, Ong BL. Nipah virus infection of pigs in peninsular Malaysia. Rev Sci Tech. 2000;19:160–5. doi: 10.20506/rst.19.1.1202. [DOI] [PubMed] [Google Scholar]
- 3.Kulkarni DD, Tosh C, Venkatesh G, Senthil Kumar D. Nipah virus infection: Current scenario. Indian J Virol. 2013;24:398–408. doi: 10.1007/s13337-013-0171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chadha MS, Comer JA, Lowe L, Rota PA, Rollin PE, Bellini WJ, et al. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg Infect Dis. 2006;12:235–40. doi: 10.3201/eid1202.051247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Centre for Diarrheal Disease Research, Bangladesh. Person-to-person transmission of Nipah virus during outbreak in Faridpur district. Health Sci Bull. 2004;2:5–9. [Google Scholar]
- 6.National Centre for Disease Control. Case Definitions of NIPAH Virus Disease. [Last accessed on 2018 Nov 24]. Available from: https://ncdc.gov.in/showfile.php?lid=232 .
- 7.Sadanadan R, Arunkumar G, Laserson KF, Heretik KH, Singh S, Mourya DT, et al. Towards global health security: Response to the May 2018 Nipah virus outbreak linked to Pteropus bats in Kerala, India. BMJ Glob Health. 2018;3:e001086. doi: 10.1136/bmjgh-2018-001086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, et al. Nipah virus: A recently emergent deadly paramyxovirus. Science. 2000;288:1432–5. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- 9.Luby SP, Gurley ES, Hossain MJ. Transmission of human infection with Nipah virus. Clin Infect Dis. 2009;49:1743–8. doi: 10.1086/647951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goh KJ, Tan CT, Chew NK, Tan PS, Kamarulzaman A, Sarji SA, et al. Clinical features of Nipah virus encephalitis among pig farmers in Malaysia. N Engl J Med. 2000;342:1229–35. doi: 10.1056/NEJM200004273421701. [DOI] [PubMed] [Google Scholar]
- 11.Sherrini BA, Chong TT. Nipah encephalitis – An update. Med J Malaysia. 2014;69(Suppl A):103–11. [PubMed] [Google Scholar]
- 12.Paton NI, Leo YS, Zaki SR, Auchus AP, Lee KE, Ling AE, et al. Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet. 1999;354:1253–6. doi: 10.1016/S0140-6736(99)04379-2. [DOI] [PubMed] [Google Scholar]
- 13.Hossain MJ, Gurley ES, Montgomery JM, Bell M, Carroll DS, Hsu VP, et al. Clinical presentation of Nipah virus infection in Bangladesh. Clin Infect Dis. 2008;46:977–84. doi: 10.1086/529147. [DOI] [PubMed] [Google Scholar]
- 14.Arunkumar G, Chandni R, Mourya DT, Singh SK, Sadanandan R, Sudan P, et al. Outbreak investigation of Nipah virus disease in Kerala, India, 2018. J Infect Dis. 2019;219:1867–78. doi: 10.1093/infdis/jiy612. [DOI] [PubMed] [Google Scholar]
- 15.Thulaseedaran NK, Kumar KGS, Kumar J, Geetha P, Jayachandran NV, Kamalasanan CG, et al. A case series on the recent Nipah epidemic in Kerala. J Assoc Physicians India. 2018;66:63–7. [PubMed] [Google Scholar]


