Abstract
Background/Aims:
The development of infection in pancreatitis increases the mortality rate up to 32%. Therefore, it is important to identify patients who are at high risk of developing infection, at an early stage. The objectives of the study were (a) to analyze the quantitative parameters of diffusion-weighted magnetic resonance imaging (DW-MRI) and apparent diffusion coefficient (ADC) in infected as well as sterile pancreatic collections (b) to establish “cut-off” values for ADC that can identify infected pancreatic collections.
Materials and Methods:
Prospective observational study of pancreatitis cases who underwent DW-MRI from August 2018 to July 2019 were enrolled in the study. The collections were analyzed for diffusion restriction. The average of the three ADC values from the wall and center of collection was noted.
Results:
Infected collections were seen in 7 and sterile collections observed in 11 cases. The optimal cut-off ADC value to differentiate sterile and infected collection in our study was 1.651 × 10-3 mm2/s (sensitivity of 81.8%; specificity of 100.0%). ROC curve for mean ADC from the wall showed a significant diagnostic accuracy with AUC: 0.91; 95% CI: 0.77-1.0 (P = 0.004).
Conclusion:
DW-MRI is a reliable noninvasive technique to differentiate sterile and infected pancreatic collections. ADC values from the periphery of the collection can predict infected pancreatic collections at an early stage. DW-MRI should not be considered as a substitute for aspiration cytology in patients with septic symptoms and absent diffusion restriction on MRI.
Keywords: Apparent diffusion coefficient, diffusion MRI, infected, pancreatic collections, sterile
INTRODUCTION
The incidence of acute pancreatitis (AP) is increasing worldwide, implying a significant burden on healthcare systems. In 2012, two new classification systems for AP were introduced, namely, revised Atlanta classification (RAC) and the determinant-based classification (DBC).[1,2] The RAC provides a three-category severity classification based on organ failure, local, and systemic complications. The DBC is a four-category severity classification that includes critical pancreatitis based on the presence of infection in pancreatic collections.[2]
The incidence of infection in AP ranges from 7 to 12% whereas it increases dramatically from 30 to 71% in necrotizing pancreatitis.[3] The development of infection in pancreatitis increases mortality to 32%.[4] Therefore, it is important to identify patients who are at high risk of developing the infection, at an early stage. Early differentiation from inflammation versus infection in pancreatitis on cross-sectional imaging remains a challenge. However, in the last decade various studies have been published considering various clinical and biochemical parameters (procalcitonin and artificial neural networks which include 16 blood parameters) to predict early infection in AP.[5,6] The cost, availability, and reliability of these biochemical tests still remains an issue and precludes their use in clinical practice.
The objectives of the study were:
To study the quantitative parameters of diffusion-weighted magnetic resonance imaging (DW-MRI) and apparent diffusion coefficient (ADC) in infected and sterile pancreatic collections
To establish “cut-off” values for ADC that can identify infected pancreatic collections at an early stage.
MATERIALS AND METHODS
Patients
This prospective observational study was approved by the Institutional Review Board. From August 2018 to July 2019, all patients suspected of AP who underwent MRI including DW-imaging were enrolled in the study. The exclusion criteria were previous drainage, surgery, and general contraindications to MRI. A total of 18 patients were enrolled in the study. The sampling of collections by fine-needle aspiration (FNA) was done in patients who were clinically suspected for infection using the diagnostic criteria of systemic inflammatory response syndrome (SIRS). Of the 18 patients recruited in the study, 11 patients had symptoms of sepsis and 7 patients did not have septic symptoms. FNA from the collection was done in 8 cases. Around 4 cases turned out to be infective and 4 cases were sterile on FNA. However, we could not sample 3 patients, though these patients had symptoms of sepsis. Hence, we included these 3 cases in the infective group. Thus, the total number of infected collections was 7 and 11 cases had sterile collections [Figure 1].
Figure 1.
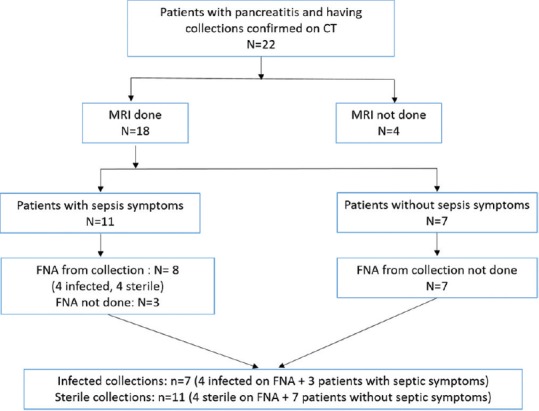
Flow of patients through the study
Imaging technique
All abdominal CT scans were performed on GE Discovery 750 HD Single-source Dual Energy CT scanner. About 100 mL of nonionic iodinated contrast material (iodine concentration, 400 mg/mL) was injected through an 18–20-gauge antecubital intravenous cannula at a rate of 4 mL/s. Scans were acquired in pancreatic parenchymal and hepatic venous phase using a smart prep protocol with an enhancement threshold set at 100 HU. Examination parameters were detector coverage 40 mm, 98.43 mm/s table speed, 0.6 s rotation time, pitch and speed of 0.984:1, 5 mm section thickness, 5-mm reconstruction interval, 100-120 kVp, and 200-360 mA. Additional images were reconstructed with 0.625 mm reconstruction intervals for detailed interpretation.
All MRI examinations were performed on a 3.0 T magnet (Discovery MR750 w, GE Healthcare, USA) using a 32-channel abdominal array coil. Our protocol included axial T2-weighted nonfat saturated fast spin echo sequence (TR, 2800 ms; TE, 90 ms; matrix, 320 × 224; slice thickness, 5 mm; gap, 1 mm; FOV, 34 × 34 mm2), coronal T2-weighted nonfat saturated fast spin echo sequence (TR, 894 ms; TE, 90 ms; matrix, 416 × 288; slice thickness, 3 mm; gap, 0.2 mm; FOV, 40 × 36 mm2), axial fast imaging employing steady-state acquisition (FIESTA) sequence (TR, 3.7 ms; TE, 1.4 ms; matrix, 256 × 320; slice thickness, 5 mm; gap, 1 mm; FOV, 34 × 34 mm2), T1-weighted three-dimensional gradient-echo sequence with fat suppression (TR, 3.5 ms; TE, 1.6 ms; matrix, 288 × 160; FOV, 40 × 28 mm2; section thickness, 5 mm), and an axial respiratory-triggered diffusion-weighted single-shot echo-planar sequence with fat suppression (TR, 4865 ms; TE, 70 ms; slice thickness, 5 mm; gap, 1 mm; FOV, 40 × 40 mm2; acquisition time, 4 min 30 s; b values, 50, 700, and 1000 s/mm2). ASSET (array coil spatial sensitivity encoding) parallel imaging method was used to reduce imaging acquisition time.
Fine-needle aspiration procedure
Diagnostic aspiration and if required therapeutic drainage was performed in patients presenting with one or more signs of septicemia. All interventions were carried out after MRI, either under ultrasound or CT guidance. In patients with more than two pockets of fluid collections, aspiration of diffusion restricting pocket was performed. Access was achieved with an 18-gauge puncture needle followed by an 8/10 F pigtail drainage if needed. Special care was taken to avoid visceral organ injury. Approximately 5 to 20 mL of fluid was collected and transferred into a sterile container. All samples were analyzed by direct microscopic examination, gram staining, and culture. The collection was considered infected when the culture results were positive.
Image analysis
Three radiologists (B.S. with over 12 years, N.S. with 9 years, and B.R. with 2 years of experience) interpreted the CT and MRI of the patients. The findings were recorded only after all the three radiologists reached a mutual consensus. CT images were analyzed for collections according to RAC. Modified CTSI scoring was given in all cases. The presence of gas bubbles, peripheral enhancement around the collection were considered to be positive for infection. For MRI interpretation, the collections were analyzed for the presence or absence of diffusion restriction on images with a b value of 1000 s/mm2. All ADC values were measured directly on the workstation console (AW server, version 3.2; GE) for quantitative analysis. ADC values from the wall and central parts of the collections were measured. The average of the three ADC values was accepted as the final ADC value separate from the wall and center of the collection.
Statistical analysis
Data were analyzed using Statistical Package for Social Sciences (SPSS) version 23. Qualitative variables like gender were described using frequency and percentages and compared using the Chi-square test or Fischer's exact test as applicable. Quantitative variables like age, ADC values were described using median and interquartile range (IQR) and compared using the Mann-Whitney U test. Diagnostic accuracy was assessed using the receiver operator characteristic (ROC) curve and cut-offs were estimated using the Youden's Index. Those variables whose area under the curve (AUC) was significantly higher than 0.5 were considered as clinically useful. A P- value of less than 0.05 was considered significant.
RESULTS
Of the 18 patients recruited in the study, infected collections were present in 7, and 11 cases had sterile collections. On CT, air foci within the collection were present only in 3 of the 7 infected cases in our study. The clinical and radiological presentation, types of collections and modified CTSI scores of all the cases are illustrated in Table 1.
Table 1.
Clinical and radiological profile of the patients with pancreatitis
| Age (years)/sex | Septic symptoms (±) | Total Leucocyte count (1000/µl) | Collection | CT air (±) | Modified CTSI score | FNA from collection |
|---|---|---|---|---|---|---|
| 50/M | + | 13.5 | ANC | + | 8 | Infected |
| 18/M | + | 14.5 | ANC | - | 10 | Infected |
| 34/F | + | 10.9 | APFC | + | 6 | Infected |
| 30/M | + | 11.9 | WOPN | - | 10 | Infected |
| 78/F | + | 14 | ANC | - | 10 | Sterile |
| 61/M | + | 10.1 | APFC | - | 6 | Sterile |
| 55/M | + | 22.18 | ANC | - | 8 | Sterile |
| 52/M | + | 11.2 | APFC | - | 8 | Sterile |
| 40/M | + | 16.4 | ANC | - | 6 | Not Done |
| 38/M | + | 16.6 | ANC | - | 10 | Not Done |
| 58/F | + | 18.4 | ANC | + | 6 | Not Done |
| 35/M | - | 14 | ANC | - | 10 | Not Done |
| 45/M | - | 14.3 | APFC | - | 8 | Not Done |
| 63/M | - | 7.57 | ANC | - | 6 | Not Done |
| 31/M | - | 8.51 | ANC | - | 6 | Not Done |
| 70/F | - | 11.9 | ANC | - | 6 | Not Done |
| 45/M | - | 10.9 | APFC | - | 4 | Not Done |
| 48/M | - | 15.6 | WOPN | - | 8 | Not Done |
ANC: Acute necrotic collection; WOPN: Walled-off pancreatic necrosis; APFC: Acute pancreatic fluid collection
The ADC values from the wall of the collection were statistically significant between the sterile and the infected group [infected group - median (IQR), 1.091 (0.437) × 10-3 mm2/s; sterile group – median (IQR), 2.184 (0.633) × 10-3 mm2/s, P = 0.004]. The ADC values from the central part of the collections were not significant between the sterile and infected group [infected group - median (IQR), 1.761 (1.841) × 10-3 mm2/s; sterile group – median (IQR), 3.161 (1.472) × 10-3 mm2/s]. Quantitative analysis of the ADC values is illustrated in Table 2.
Table 2.
Quantitative analysis of ADC values of the collection
| ADC values from the content and wall of collection | Infected (n=7) × 10-3 mm2/s | Sterile (n=11) × 10-3 mm2/s | P |
|---|---|---|---|
| ADC value from the content (Minimum), Median (IQR) | 1.488 (1.862) | 2.944 (1.709) | 0.085a |
| ADC value from the content (Maximum), Median (IQR) | 2.077 (1.760) | 3.378 (1.213) | 0.069a |
| ADC value from the content (Mean), Median (IQR) | 1.767 (1.841) | 3.161 (1.472) | 0.104a |
| ADC value from the wall (Minimum), Median (IQR) | 0.898 (0.481) | 1.735 (0.678) | 0.004 a |
| ADC value from the wall (Maximum), Median (IQR) | 1.423 (0.403) | 2.541 (0.845) | 0.003a |
| ADC value from the wall (Mean), Median (IQR) | 1.091 (0.437) | 2.184 (0.633) | 0.004a |
aMann-Whitney U test; ADC: Apparent diffusion coefficient. P value of less than 0.05 is significant
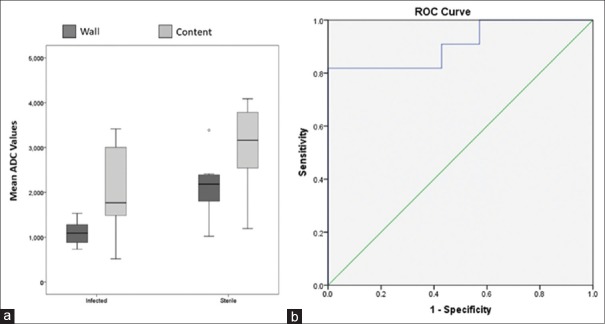
The optimal cut-off ADC value to differentiate sterile and infected collection in our study was 1.651 × 10-3 mm2/s from the collection wall by Youden's J statistic method where sensitivity is 81.8% and specificity is 100.0%. The ROC curve for mean ADC values from the wall shows a significant diagnostic accuracy (AUC: 0.91; 95% CI: 0.77-1.0, P = 0.004, Figures 2–4).
Figure 2.
(a) Box-and-whisker plots for the mean ADC values from both wall and content of sterile and infected pancreatic collections. Centre horizontal line = median, bottom and top edges of box = 25th and 75th percentiles, vertical line = range of data. (b) ROC curves showing the diagnostic performance of apparent diffusion coefficients (ADC) from the wall of infected pancreatic collection
Figure 4.
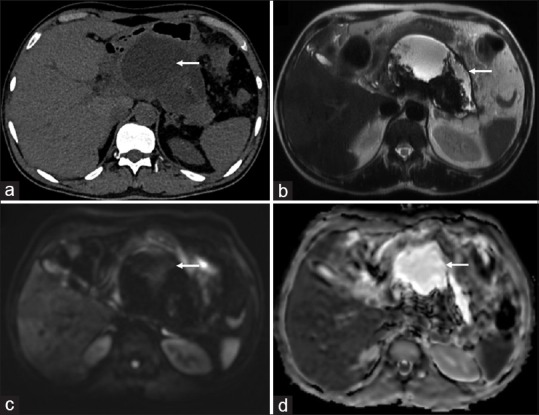
A 60-year-old male presenting with pancreatitis. Sterile collection confirmed on fine-needle aspiration. (a) Axial non-contrast CT scan showing collection (arrow) in lesser sac (b) Axial T2-weighted MRI image confirming the walled-of necrotic collection (arrow) with thick hypointense debris (c) No bright signal (arrow) is seen in the collection on diffusion-weighted images (b = 1000 s/mm2) (d) Corresponding high signal on the apparent diffusion coefficient map (arrow) is seen suggestive of no diffusion restriction
Figure 3.
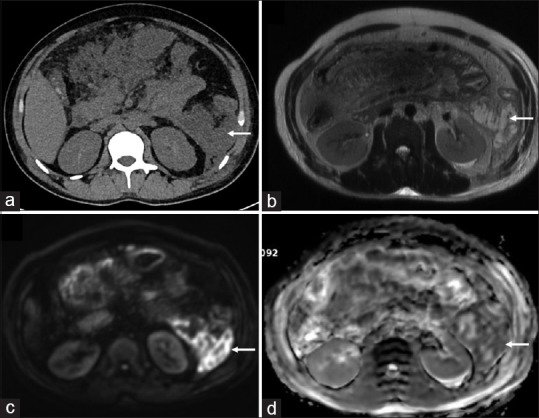
A 31-year-old male presenting with biliary pancreatitis. Infected collection confirmed on fine-needle aspiration. (a) Axial non-contrast CT scan showing collection (arrow) in left paracolic gutter (b) Axial T2-weighted MRI image confirming the collection (arrow) with wall formation (c) Bright signal (arrow) is seen in the wall of the collection on diffusion-weighted images (b = 1000 s/mm2) (d) corresponding low signal on the apparent diffusion coefficient map (arrow) suggestive of diffusion restriction
DISCUSSION
Diffusion-weighted MRI (DW-MRI) has now established a sequence for various intracranial pathologies such as stroke, subdural empyema, and brain abscesses.[7] In the last decade, the utility of the DW-MRI sequence for various abdominal oncological and nononcological pathologies has dramatically increased. The molecular diffusion of the molecules in tissue forms the basis of DW-MRI. In living tissue, this depends upon the cellular compartmentalization, cell type and number, cell membrane density, macromolecule size, type and temperature.[8] The degree of diffusion restriction is quantified by a diffusion coefficient, which reflects the average distance a particle will move in a second (measured in mm2/s). Areas of diffusion restriction will not lose the signal on high b value images (because their protons are not moving), giving dark pixels on ADC image. On the other hand, areas of fast diffusion will lose the most signal as b values increase, thus giving bright pixels on the ADC image.[9]
Early detection of intra-abdominal fluid collections and their differentiation from the sterile and infected fluid is critical in cases diagnosed with pancreatitis. The presence of gas bubbles within the collection is the only reliable marker of infection on CT but this occurs late in the disease course.[3] In our study, air foci within the infected collections on CT were present in only 3 of 7 cases. The various sources of infection in these collections are colonic necrosis/perforation, secondary to catheter drainage and sometimes the liquefied pancreatic tissue and blood products within the abdomen itself promote bacterial growth. Nuclear medicine imaging techniques are available to diagnose infection but are expensive and not easily accessible to all.[10] DW-MRI is a noninvasive diagnostic tool to detect infection in abdominal collections and is also easy to interpret for the nonexperienced radiologist.[11,12]
The optimal cut-off ADC value from the wall was 1.651 × 10-3 mm2/s to differentiate sterile and infected collection in our study. Borens et al.[11] have shown that the minimum cut-off for ADC values for the infected collection was 1.012 × 10-3 mm2/s. The ADC values of the peripheral diffusion-restricted parts of the collections ranged from 0.81 to 1.49 × 10-3 mm2/s (mean ± SD, 1.16 ± 0.14) in the study conducted by Islim et al.[12] However, Oto et al.[8] have shown an ADC value of 2.0 × 10-3 mm2/s as an optimum threshold to differentiate abscess and noninfected fluid.
Infected collection or pus is a viscous fluid that consists of bacteria, dead white cells (neutrophils), inflammatory cells, and cellular debris. Biochemically, the infected fluid contains elevated levels of protein, albumin, and lactate dehydrogenase (LDH).[13] In pancreatic collections, in addition to the above mentioned fluid characteristics, there are many pancreatic enzymes and tissue necrosis within the collection itself which may lead to unequal diffusion restrictions at the center and peripheral region of the collections. This was the rationale for calculating ADC values from the center as well as from the walls of the collection. In our study, the ADC values were lower in the wall as compared to the central part of infected collections. The reason for the different ADC values could be due to higher density of inflammatory cells, and pancreatic enzymes in the periphery as compared to the centre of the fluid collection.
Our study had a few limitations. We could not do a cytological examination of fluid in 7 cases without septic symptoms and in 3 patients with septic symptoms due to operational and ethical issues. This did not alter the management of the disease as patients with septic symptoms and diffusion-restriction on MRI were treated with antibiotics. All the patients with septic symptoms had received at least one course of antibiotics before MRI examination either before transfer from another hospital or in our institution for suspicion of infection. This could be another limitation to our findings as 4 cultures were sterile in patients with septic symptoms. Although our sample size was small, this is an important pilot noninvasive study to predict early infection in pancreatic collections. We did not perform interobserver agreement analysis in our study, and average of the three ADC measurements were taken on mutual consensus of lowest ADC region. This could limit the reproducibility of ROI. In future, studies with larger sample size and without prior antibiotic treatment should be conducted.
In conclusion, DW-MRI is a reliable noninvasive technique to predict early infection in pancreatic collections. ADC values less than 1.651 × 10-3 mm2/s from the wall of the collection in patients with septic symptoms is sufficient justification to start the patients on antibiotics. DW-MRI should not be considered as a substitute for aspiration cytology in patients with septic symptoms and absent diffusion restriction on MRI.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis– 2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger EP, Forsmark CE, Layer P, Lévy P, Maraví-Poma E, Petrov MS, et al. Determinant-based classification of acute pancreatitis severity: An international multidisciplinary consultation. Ann Surg. 2012;256:875–80. doi: 10.1097/SLA.0b013e318256f778. [DOI] [PubMed] [Google Scholar]
- 3.Triantopoulou C, Delis S, Dervenis C. Imaging evaluation of post-pancreatitis infection. Infect Disord Drug Targets. 2010;10:15–20. doi: 10.2174/187152610790410873. [DOI] [PubMed] [Google Scholar]
- 4.Petrov MS, Shanbhag S, Chakraborty M, Phillips ARJ, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139:813–20. doi: 10.1053/j.gastro.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Siriwardena AK, Jegatheeswaran S, Mason JM, Baltatzis M, Chan A, Sheen AJ, et al. PROCalcitonin-based algorithm for antibiotic use in Acute Pancreatitis (PROCAP): Study protocol for a randomised controlled trial. Trials. 2019;20:463. doi: 10.1186/s13063-019-3549-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu Q, Nian YJ, Tang L, Guo Y, Wen LZ, Wang B, et al. Artificial neural networks accurately predict intra-abdominal infection in moderately severe and severe acute pancreatitis. J Dig Dis. 2019;20:486–94. doi: 10.1111/1751-2980.12796. [DOI] [PubMed] [Google Scholar]
- 7.Carney O, Falzon A, MacKinnon AD. Diffusion-weighted MRI in paediatric neuroimaging. Clin Radiol. 2018;73:999–1013. doi: 10.1016/j.crad.2018.07.101. [DOI] [PubMed] [Google Scholar]
- 8.Oto A, Schmid-Tannwald C, Agrawal G, Kayhan A, Lakadamyali H, Orrin S, et al. Diffusion-weighted MR imaging of abdominopelvic abscesses. Emerg Radiol. 2011;18:515–24. doi: 10.1007/s10140-011-0976-1. [DOI] [PubMed] [Google Scholar]
- 9.MRI Physics: Diffusion-Weighted Imaging [Internet] 2019. [Last cited on 2019 Aug 11]. Available from: http://xrayphysics.com/dwi.html .
- 10.Bhattacharya A, Kochhar R, Sharma S, Ray P, Kalra N, Khandelwal N, et al. PET/CT with 18F-FDG-labeled autologous leukocytes for the diagnosis of infected fluid collections in acute pancreatitis. J Nucl Med. 2014;55:1267–72. doi: 10.2967/jnumed.114.137232. [DOI] [PubMed] [Google Scholar]
- 11.Borens B, Arvanitakis M, Absil J, El Bouchaibi S, Matos C, Eisendrath P, et al. Added value of diffusion-weighted magnetic resonance imaging for the detection of pancreatic fluid collection infection. Eur Radiol. 2017;27:1064–73. doi: 10.1007/s00330-016-4462-8. [DOI] [PubMed] [Google Scholar]
- 12.Islim F, Salik AE, Bayramoglu S, Guven K, Alis H, Turhan AN. Non-invasive detection of infection in acute pancreatic and acute necrotic collections with diffusion-weighted magnetic resonance imaging: Preliminary findings. Abdom Imaging. 2014;39:472–81. doi: 10.1007/s00261-014-0076-2. [DOI] [PubMed] [Google Scholar]
- 13.Mönkemüller KE, Harewood GC, Curioso WH, Fry LC, Wilcox CM, Morgan DE, et al. Biochemical analysis of pancreatic fluid collections predicts bacterial infection. J Gastroenterol Hepatol. 2005;20:1667–73. doi: 10.1111/j.1440-1746.2005.04067.x. [DOI] [PubMed] [Google Scholar]