Abstract
Background/Aim:
To study the impact of computer-aided detection (CADe) system on the detection rate of polyps and adenomas in colonoscopy.
Materials and Methods:
A total of 1026 patients were prospectively randomly scheduled for colonoscopy with (the CADe group, CADe) or without (the control group, CON) the aid of the CADe system, together with visual notification and voice alarm, so as to compare the detection rate of polyp.
Results:
Compared with group CON, the detection rate of adenomas increased in group CADe, the average number of adenomas increased, the number of small adenomas increased, the number of proliferative polyps increased, and the differences were statistically significant (P < 0.001), but the comparison for the number of larger adenomas showed no significant difference between the groups (P > 0.05).
Conclusions:
The CADe system is feasible for increasing the detection of polyps and adenomas in colonoscopy.
Keywords: Adenoma, artificial intelligence, colonoscopy, detection rate, polyp
INTRODUCTION
In recent years, the incidence of colorectal cancer (CRC) in China has increased and brought the country to the third place in the world, in terms of incidence of the disease.[1,2] At present, colonoscopy is considered to be the gold standard for reducing the morbidity and mortality of CRC.[3,4] It is generally believed that 80%–95% of CRC evolves from colorectal polyps step by step, especially adenomatous polyps. Timely removal of polyps can effectively prevent the occurrence of CRC. Therefore, colonoscopy reduces the incidence of CRC by detecting and excising adenomatous polyps. Nowadays, the workload of colonoscopy in third-class level A hospitals is heavy. Doctors' fatigue state, as well as doctors' lack of experience, may easily lead to mis- and missed diagnosis of diseases, which then directly affects the detection rate and diagnostic accuracy of colonoscopy.[5] The factors affecting the detection rate of intestinal polyps include blind areas and human errors. Blind areas can be solved by wide-angle range or remote connection, and artificial intelligence (AI) can solve human errors and is attracting wide attention.[6,7,8,9,10,11] To solve human errors, we obtained a large number of colonoscopic videos and used an automatic image feature extraction and machine-learning-based AI system algorithm to evaluate the performance of computer-aided detection (CADe) system, so that the detection rate of polyps and adenomas can be increased through the CADe system.
MATERIALS AND METHODS
CADe system of polyp
The CADe system of polyps (Henan Xuanweitang Medical Information Technology Co., LTD., Zhengzhou City, Henan Province, China) (Former name: Henan Tongyu Medical Technology Co., Ltd) was developed on the basis of in-depth learning architecture with the help of endoscopists and modelers. In our study, one endoscopy specialist of Endoscopy Center (had performed >20,000 cases of colonoscopy) retrospectively commented on the presence of polyps in each frame of each video, which was used as the gold standard for the detection of polyps. To use these videos for machine learning and evaluation, we divided these full-length videos into short videos according to the method described in the document and produced 151 polyp-positive videos and 384 polyp-negative videos. These 535 videos were randomly divided into two groups: the study samples (101 polyp-positive cases and 300 polyp-negative cases) and the test samples (46 polyp-positive cases and 88 polyp-negative cases). The study samples were used for machine learning, and the test samples were used to evaluate the performance of our system. Therefore, the test sample set was a completely independent data set that had never been used during machine learning. From the colonoscopy system, the video stream was captured by a high-definition frame capture device. The aperture describing image information in each successively captured frame can be clipped to ignore all unnecessary image data, such as black frames or screen displays. The clipped images were then color transformed, and the information from three color channels was weighted according to their participation degree in the information. Next, the weighted combination of color, structure, texture, and motion information was used to detect the image area where the polyp may be located. Once the image content of the detected area remained constant for at least three consecutive frames, an enhancement mask was provided, which was repeated for each frame of the captured image stream. Therefore, endoscopists were encouraged to pay special attention to each area and check whether such markers matched polyps. The system was designed to highlight potential polypoid structures during real-time colonoscopy.
System algorithms
In this study, we developed a new original algorithm based on convolutional three-dimensional (3D) neural network, which was a kind of deep learning. Deep learning is the latest machine learning method using deep neural network. If a large number of learning samples are available, it can automatically extract specific features from data without human resources. The convolutional 3D network is designed for spatiotemporal data. Therefore, compared with previous deep learning methods, it is more suitable for video data sets.
Prospective comparative study
The prospective study was designed as a randomized controlled trial to study the effect of endoscopist-assisted CAD system on the detection rate of polyps and adenomas. The study was approved by the local ethics committee. Colonoscopy videos in the study were selected from the participants who underwent colonoscopy in Digestive Endoscopy Center of the 988 Hospital of the Joint Logistics Support Force of the Chinese People's Liberation Army from October 2018 to March 2019. All the participants signed the informed consent, and then routine intestinal preparation was performed, followed by oral administration of 3 L of compound polyethylene glycol electrolyte powder. (Beaufour Ipsen Industrie France; Registration No: H20140560; specification: the product is a compound preparation, containing per pouch: 64.000 g of polyethylene glycol 4000, 5.700 g of anhydrous sodium fulfate, 1.460 g of NaCl, 0.750 g of KCl, and 1.680 g of NAHCO3.) Intestinal preparation referred to APP video education of digestive endoscopy. Colonoscopy was performed using one high-definition colonoscopy (Fuji EPX-4450HD) and one high-definition monitor. Exclusion criteria: any participant with inflammatory bowel disease, history of CRC surgery, history of radiotherapy and/or chemotherapy, and biopsy contraindications. Before colonoscopy, nurses recorded the basic information of each participant, including the gender, age, colonoscopy indications, body mass index, history of smoking, constipation, diabetes, stroke, family history of cancer, abdominal surgery, colonoscopy, aspirin, or other nonsteroidal anti-inflammatory drug use. Before colonoscopy, each participant was randomly divided into group CON (routine colonoscopy group) and group CADe. In group CADe, real-time polyp CADe system was used to assist colonoscopy. The CADe system was only opened when colonoscopy exited, especially started from the point the endoscopist identified the opening of the appendix. The system was connected with the endoscope generator and the video stream was collected synchronously. In addition, the system processed each frame and displayed the detected polyps. The CADe system represented the probability of polyps by percentage (0%–100%) in each frame. Figure 1 shows the probability provided by the CADe system in the upper left corner of the endoscopic image. When the probability exceeded the critical value, the CADe system warned of the possibility of polyps by changing the color of the four corners of the endoscopic image to red. When lesions appeared on the screen, a warning was issued, and when lesions disappeared from the screen, the warning would stop. Endoscopists focused mainly on the main monitor during the examination process, and a voice alarm prompted them to view the system monitor to check the location of each polyp detected by the system. The above operations were done without the assistance of nurses, trainees, or staff. In both groups, nurses needed to record the insertion time, withdrawal time, and Boston Bowel Preparation Scale (BBPS). When polyps were detected, nurses also assisted in biopsy pathological examination. Nurses also recorded the location, size, and morphological characteristics according to the Paris Classification.[12] In group CADe, missed polyps and system error alarms were also recorded. These missed polyps were defined as polyps confirmed by the colonoscopist but not detected by the system. False alarms were defined as lesions detected and continuously tracked but were not identified as polyps by the system.
Figure 1.
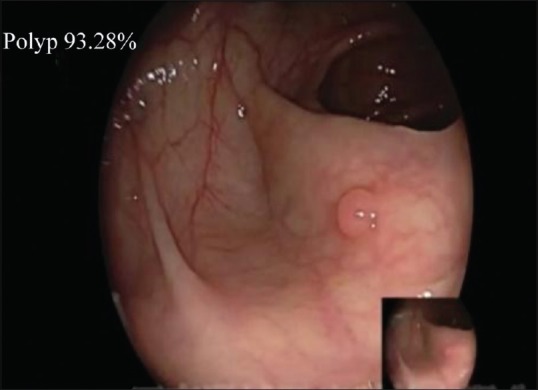
CADe system provides the probability in the upper left corner of the endoscopic image
Statistical analysis
SPSS 21.0 was used for statistical analysis. The intergroup comparison of the basal clinical and demographic characteristics was performed by the χ2 test of categorical variables and double-sample t-test of continuous variables. Logistic regression analysis was used to analyze the impact of the CADe system on the detection rate of polyps/adenomas in colonoscopy. The response variable was the binary result of whether polyps/adenomas were detected. The covariate was the group variable indicating whether patients belong to group CADe. Regarding the number of polyps and adenomas detected, Poisson's regression was used to evaluate the effectiveness of computer-aided diagnosis of colonoscopy, with P < 0.05 (bilateral) as statistically significant. If any basal clinical and demographic characteristics showed statistical differences between the two groups, an additional covariate was constructed to adjust the logistic/Poisson's regression model to address possible confusion effects by adding these significant features as covariates to the model. The main result was the detection rate of adenoma. The secondary results included were the detection rate of polyp, the average number of polyps detected by colonoscopy in each case, the average number of adenomas detected by colonoscopy in each case, and false-positive and false-negative rates.
RESULTS
Patient enrolment and basal data
A total of 1100 consecutive patients were enrolled. Of these patients, 84 cases (40 in group CON and 44 in group CADe) were excluded because they met the exclusion criteria. A total of 1026 eligible patients were analyzed. They were prospectively divided into group CON (518 cases) and group CADe (508 cases). The basal characteristics are shown in Table 1. There was no significant difference in the demographic data and risk factors for adenoma detection between the two groups. No complications were reported. The total withdrawal time of group CON and group CADe was 6.74 and 6.82 min, respectively (P < 0.001). Totally 232 biopsies were performed in group CADe. The withdrawal time of biopsy was 6.11 min in group CON and 6.16 min in group CADe (P > 0.05).
Table 1.
Comparison of basal data between the two groups
| Characteristic | CON (n=518) | CADe (n=508) | P |
|---|---|---|---|
| Age (years) | 50.13±12.68 | 51.02±12.26 | 0.21 |
| BMI (kg/m2) | 24.13±2.96 | 23.98±2.98 | 0.87 |
| Withdrawal time (min) | 6.74±1.62 | 6.82±1.78 | <0.001 |
| Full time (min) | 12.70±4.16 | 12.41±4.25 | 0.16 |
| Insertion time (min) | 5.96±4.06 | 5.68±4.09 | 0.72 |
| Time of polyp-free withdrawal (min) | 6.32±1.09 | 6.37±0.98 | 0.52 |
| Biopsy withdrawal time (min) | 6.11±1.00 | 6.16±1.26 | 0.16 |
| Display n (%) | 0.68 | ||
| Screen | 36 (6.95) | 30 (5.91) | |
| Symptom | 482 (93.05) | 478 (94.09) | |
| Sex, n (%) | 0.18 | ||
| Female | 231 (44.59) | 244 (48.03) | |
| Male | 287 (55.41) | 264 (51.97) | |
| BMI classification, n (%) | 0.61 | ||
| <25 | 383 (73.94) | 370 (72.84) | |
| 25-30 | 126 (24.32) | 133 (26.18) | |
| >30 | 9 (1.74) | 5 (0.98) | |
| Inspection time, n (%) | 0.72 | ||
| Morning | 295 (56.95) | 268 (52.76) | |
| Afternoon | 223 (43.05) | 240 (47.24) | |
| Anesthesia, n (%) | 0.88 | ||
| No | 20 (3.86) | 18 (3.54) | |
| Yes | 498 (96.14) | 490 (96.46) | |
| BBPS | 6.71±1.24 | 6.68±1.22 | 0.92 |
| BBPS rank, n (%) | 0.76 | ||
| Insufficient (total score <6, single intestinal segment <2) | 71 (13.71) | 66 (12.99) | |
| Sufficiency (total score (>6 points), single intestinal segment (>2 points) | 447 (86.29) | 442 (87.01) |
BMI: Body mass index; BBPS: Boston Bowel Preparation Scale; P: χ2 test or t-test
Polyp characteristics, average number of polyps per patient, and detection rate of polyp
A total of 734 polyps were detected, including 392 cases of adenoma (53.41%) and 31 cases of sessile serrated adenoma (4.22%). In all, 248 polyps were found in group CON (33.79%) and 486 polyps in group CADe (66.21%) [Table 2]. The average number of polyps detected by colonoscopy in group CON and group CADE was 0.57 and 0.87, respectively (P < 0.001). The average number of polyps detected in both groups increased by 1.53 times [95% confidence interval (CI) was 1.652–2.297, P < 0.001] [Table 3]. The detection rates of polyps in group CON and group CADs were 0.28 and 0.44, respectively [odds ratio (OR) =1.57, 95% CI 1.586–2.483, P < 0.001] [Table 3]. There was no significant difference in the basal clinical and demographic variables between the two groups. Therefore, the covariate adjustment model was not considered to solve the potential confusion effect.
Table 2.
Characteristics of polyps and adenomas
| Characteristic | CON (n=248) | CADe (n=486) | P |
|---|---|---|---|
| Pathology | 0.210 | ||
| Cance | 0 (0.00) | 0 (0.00) | 1.000 |
| Sessile serrated adenoma/polyp | 13 (5.24) | 18 (3.70) | 0.538 |
| Adenoma | |||
| Advanced adenoma | 16 (6.45) | 14 (2.88) | 0.821 |
| Other | 128 (51.61) | 236 (48.56) | <0.001 |
| Benign lesion | |||
| Proliferative and inflammatory | 92 (37.10) | 203 (41.77) | <0.001 |
| Hamartoma | 0 (0.00) | 0 (0.00) | 1.000 |
| Normal colonic mucosa | 1 (0.40) | 1 (0.21) | 0.565 |
| Polyp position | <0.001 | ||
| Cecum | 3 (1.21) | 6 (1.23) | 0.481 |
| Ascending colon | 45 (18.15) | 100 (20.58) | <0.001 |
| Transverse colon | 48 (19.36) | 112 (23.05) | <0.001 |
| Descending colon | 23 (9.27) | 73 (15.02) | <0.001 |
| Sigmoid colon | 75 (30.24) | 133 (27.37) | <0.001 |
| Rectum | 54 (21.77) | 62 (12.75) | 0.260 |
| Polypoid shape | 0.088 | ||
| Pedicle | 36 (14.52) | 52 (10.70) | 0.211 |
| Sessile | 96 (38.71) | 180 (37.04) | <0.001 |
| Flat | 116 (46.77) | 254 (52.26) | <0.001 |
| Polyp size (mm) | 5.33±2.89 | 4.88±2.67 | 0.053 |
| Classification of polyp size (mm) | 0.098 | ||
| 0-5 | 169 (68.15) | 378 (77.77) | <0.001 |
| 6-10 | 63 (25.40) | 86 (17.70) | 0.042 |
| >10 | 16 (6.45) | 22 (4.53) | 0.211 |
| Adenoma location | 0.388 | ||
| Cecum | 3 (2.11) | 6 (2.40) | 0.322 |
| Ascending colon | 40 (28.17) | 50 (20.00) | 0.320 |
| Transverse colon | 38 (26.76) | 75 (30.00) | <0.001 |
| Descending colon | 22 (15.49) | 48 (19.20) | 0.003 |
| Sigmoid colon | 20 (14.09) | 35 (14.00) | 0.016 |
| Rectum | 19 (13.38) | 36 (14.40) | 0.188 |
| Adenoma shape | 0.267 | ||
| Pedicle | 36 (25.35) | 40 (16.00) | 0.416 |
| Sessile | 46 (32.40) | 88 (35.20) | 0.001 |
| Flat | 60 (42.25) | 122 (48.80) | <0.001 |
| Adenoma size (mm) | 5.63±3.09 | 5.38±3.01 | 0.380 |
| Classification of adenoma size (mm) | 0.172 | ||
| 0-5 | 89 (62.68) | 166 (66.40) | <0.001 |
| 6-10 | 43 (30.28) | 63 (25.20) | 0.241 |
| >10 | 10 (7.04) | 21 (8.40) | 0.096 |
P: χ2 test
Table 3.
Detection of polyps and adenomas
| CON (n=518) | CADe (n=508) | P | FC/OR | 95% CI | |
|---|---|---|---|---|---|
| PDR | 0.2781 | 0.4365 | <0.001 | 1.570 | 1.586-2.483 |
| ADR | 0.2389 | 0.3910 | <0.001 | 1.637 | 1.201-2.220 |
| Average number of polyps detected | 0.5684 | 0.8720 | <0.001 | 1.534 | 1.652-2.297 |
| Average number of adenomas detected | 0.3420 | 0.5178 | <0.001 | 1.514 | 1.423-2.016 |
FC: Fold change; OR: odds ratio; CI: confidence interval; PDR: polyp detection rate; ADR: adenoma detection rate; P: χ2 test or t-test
Adenoma characteristics, average number of adenomas per patient, and detection rate of adenomas
A total of 392 adenomas were detected [Table 2]. The average number of adenomas detected by colonoscopy in group CON and group CADe was 0.34 and 0.52, respectively (P < 0.001). The average number of adenomas detected in both groups increased by 1.51 times (95% CI 1.423–2.016, P < 0.001) [Table 3]. The detection rates of adenomas in group CON and group CADe were 0.23 and 0.39, OR = 1.64, 95% CI 1.201–2.220, respectively, P < 0.001 [Table 3]. The number of polyps detected in group CADe was significantly higher than group CON when considering sessile polyps, polyps ranging from 0 to 1 cm, and polyps in all parts of the colon. The number of adenomas detected in group CADe also increased significantly when considering sessile polyps, polyps less than 0.5 cm, and polyps in all parts of the colon except cecum and ascending colon [Table 3].
Results of good bowel preparation (BBPS <7)
The adenoma detection rate in group CADe was 5.8% higher than group CON when intestinal preparation was good. However, due to the insufficient sample size of subgroup analysis, this study failed to show statistical significance. Other outcomes in group CADe, including the average number of adenomas detected, the average number of polyps detected, and the detection rate of polyps, increased significantly [Table 4].
Table 4.
Results of good bowel preparation (BBPS ≥7)
| CON (n=288) | CADe (n=280) | P | FC/OR | 95% CI | |
|---|---|---|---|---|---|
| PDR | 0.2834 | 0.4007 | 0.003 | 1.414 | 1.189-2.303 |
| ADR | 0.2159 | 0.2700 | 0.17 | 1.251 | 1.946-1.998 |
| Average number of polyps detected | 0.5048 | 0.8328 | <0.001 | 1.650 | 1.302-1.968 |
| Average number of adenomas detected | 0.2714 | 0.4309 | 0.006 | 1.588 | 1.423-2.016 |
BBPS: Boston Bowel Preparation Scale; FC: Fold change; OR: odds ratio; CI: confidence interval; PDR: polyp detection rate; ADR: adenoma detection rate; P: χ2 test or t-test
Misreporting of polyp by CADe system
There were 36 false alarms (false-positive) in group CADe, with an average of 0.071 false alarms per colonoscopy [Table 5]. Of all polyps detected in group CADe, no polyps were missed [Table 5].
Table 5.
Mis- and missed report of polyps
| CADe, n (%) | |
|---|---|
| False-positive | 36 (100.00) |
| Bubble | 12 (33.33) |
| Feces | 6 (16.67) |
| Undigested fecal residue | 5 (13.89) |
| Crumpled mucosa | 7 (19.44) |
| Local inflammation | 4 (11.11) |
| Local hemorrhage | 1 (2.78) |
| Drug capsule | 1 (2.78) |
| Other (scar/diverticulum, etc.) | 0 (0.00) |
| Missed polyp | 0 (0.00) |
DISCUSSION
CRC is the third most common malignant tumor in the world and the fourth leading cause of cancer death.[13] Colonoscopy has been demonstrated to be effective in detecting and removing tumors to prevent CRC.[1] However, the diagnostic performance of colonoscopy varies, which leads to the importance of improving quality and reducing operator dependence. Adenoma detection rate is an independent predictor of the risk of interval CRC[14,15] and even taking patient-related factors into account, this key indicator varies considerably among endoscopists in similar environments.[16,17] The application of AI in colonoscopy is attracting more and more attention because it may improve the quality of colonoscopy.[6,18] The main research focused in this field includes automatic paired polyp detection[19,20] and feature analysis,[8,10] which may help improve the detection rate of adenomas, respectively. The detection rate of adenomas can also be increased by the shortest withdrawal time, fractional-dose bowel preparation, and right colon inversion.[21,22,23,24] A preliminary study had shown that 94% of test polyps (47 out of 50) were detected by AI system, and the false-positive rate was 60% (51 out of 85).[25] Despite these advances, polyp specificity still produces many false-positives. On the contrary, inadequate sensitivity will not increase the detection rate of polyps. In addition, to improve the efficiency of real-time detection, the analysis time must be fast, and there is should be no obvious delay by endoscopists. Because of these preconditions, most of the current studies on automatic polyp detection are small-scale nonclinical studies. Nonetheless, with the rapid increase in interest in this field and the emergence of in-depth learning, great progress can be expected in the next few years.
However, part of missed detection cases may be due to endoscopists not adequately recognizing the lesions shown on video monitors. It is well-known that the detection rate of polyps in afternoon colonoscopy is lower than that in morning colonoscopy, which may be attributed to the fatigue of endoscopists, consistent with Marcondes et al.[5] CADe polyp detection may be suitable to solve this problem. Computer monitoring of colorectal lesions may be appropriate to prevent the omission of polyps during endoscopy.
Video sequences collected during colonoscopy can be reviewed using CADe retrospectively. The CADe system used in this study has also been successfully tested on video clips recorded in the past.
One reason why colorectal polyps can be detected by colonoscopy may be that frame grabbers are used to capture image signals from the colonoscopy system and process images, which have no (short) delays between final depicting images on the second screen. However, this delay may be reduced by directly accessing the image stream of the colonoscopy system (without using a frame grabber).
In this study, the detection rates of adenoma and the average number of polyps and adenomas by colonoscopy in group CADe were significantly higher than group CON; however, the overall increase in adenoma detection was mainly due to the increase in small adenomas. Most of the small adenomas detected by CADe system were smaller, which also proves that smaller polyps are more easily ignored in the field of vision than larger and more prominent polyps. Smaller adenomas have a lower risk of malignancy than larger adenomas, but higher overall adenoma detection may ultimately help reduce the risk of CRC, consistent with the results by Klare et al.[11] Further research should address the role of CADe system in reducing polyps, which is the main objective of colonoscopy.
The results of this study show that detection of microproliferative polyps increases significantly, which may mean that additional unnecessary polypectomy will increase the workload. The CADe system is expected to be combined with the CADx system to support the detection and diagnosis of lesions, thus avoiding excessive workload.
This system failed to help endoscopists detect more adenomas in the cecum and ascending colon, which may be due to the high instability of colonoscopy in these areas, which reduces vision. In addition, there was no significant difference in the rectum, which may be due to the good visibility and stability of colonoscopy in this segment.
This study is a prospective randomized controlled trial using a deep-learning-based CADe system to help endoscopists detect colon polyps in a large number of patients. The results show that the CADe system can effectively deal with unidentified polyps. However, the remaining polyps in the blind area remains an unsolved problem.
This study has several limitations. First, in this study, we removed the biopsy time from each corresponding withdrawal time as an indirect reminder of attention [Table 1]. The withdrawal time of the two groups was similar (6.11 and 6.16 min, P = 0.16), which may represent similar observation attention. The CADe system had an average of 0.071 false alarms per colonoscopy, while having no prolonged withdrawal time.
In the future, double-blinded studies can be used to study the application of CADe system in improving the detection rate of polyps/adenomas. This study may also help determine whether polyps can be detected simultaneously by endoscopists and systems or those initially missed by endoscopists, which is a problem not designed in current studies.
The detection rate of polyps and adenomas in this study is not as high as reported in the Western countries. It may be affected by a variety of factors, including the differences in genetics, lifestyle, diet and habits, as well as the incidence of colonic polyps/adenomas, between Eastern and Western populations. Therefore, the results of this study may not be extended to the areas with higher detection rate of polyps/adenomas in the world. The application of CADe system in these fields needs further study.
Although the false-positives rate was very low, the system designer did not take some false-positive into account. These false-positive results were caused by feces, fecal residue, local bleeding, inflammation, or wrinkled mucosa, which lead to distraction during examination or operation. This can be corrected by adding enough training samples to the system. This study did not control the fatigue level of endoscopists participating in the examination, which may be an independent factor affecting adverse reactions. The application of CADe system at different fatigue levels needs to be further studied. This study was conducted using Fuji Colonoscopy equipment. Therefore, we should also discuss the application of CADe system in other colonoscope manufacturers.
In conclusion, this study shows that the CADe system based on in-depth learning increases the detection rate of colorectal polyps and adenomas. Therefore, the CADe system is feasible for the detection of polyps and adenomas in colonoscopy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 2.Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–96. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, et al. Colorectal cancer screening: Recommendations for physicians and patients from the U.S. multi-society task force on colorectal cancer. Am J Gastroenterol. 2017;112:1016–30. doi: 10.1038/ajg.2017.174. [DOI] [PubMed] [Google Scholar]
- 5.Marcondes FO, Gourevitch RA, Schoen RE, Crockett SD, Morris M, Mehrotra A. Adenoma detection rate falls at the end of the day in a large multi-site sample. Dig Dis Sci. 2018;63:856–9. doi: 10.1007/s10620-018-4947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne MF, Shahidi N, Rex DK. Will computer-aided detection and diagnosis revolutionize colonoscopy? Gastroenterology. 2017;153:1460–4.e1. doi: 10.1053/j.gastro.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Misawa M, Kudo SE, Mori Y, Misawa M, Kudo SE, Mori Y, et al. Characterization of colorectal lesions using a computer-aided diagnostic system for narrow-band imaging endocytoscopy. Gastroenterology. 2016;150:1531–2.e3. doi: 10.1053/j.gastro.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Chen PJ, Lin MC, Lai MJ, Lin JC, Lu HH, Tseng VS. Accurate classification of diminutive colorectal polyps using computer-aided analysis. Gastroenterology. 2018;154:568–75. doi: 10.1053/j.gastro.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Mori Y, Kudo SE, Misawa M, Mori K. Simultaneous detection and characterization of diminutive polyps with the use of artificial intelligence during colonoscopy. Video GIE. 2019;4:7–10. doi: 10.1016/j.vgie.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrne MF, Chapados N, Soudan F, Oertel C, Linares Pérez M, Kelly R, et al. Real-time differentiation of adenomatous and hyperplastic diminutive colorectal polyps during analysis of unaltered videos of standard colonoscopy using a deep learning model. Gut. 2019;68:94–100. doi: 10.1136/gutjnl-2017-314547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klare P, Sander C, Prinzen M, Haller B, Nowack S, Abdelhafez M, et al. Automated polyp detection in the colorectum: A prospective study (with videos) Gastrointest Endosc. 2019;89:576–82.e1. doi: 10.1016/j.gie.2018.09.042. [DOI] [PubMed] [Google Scholar]
- 12.Endoscopic Classification Review Group. Update on the Paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570–8. doi: 10.1055/s-2005-861352. [DOI] [PubMed] [Google Scholar]
- 13.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 14.Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298–306. doi: 10.1056/NEJMoa1309086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 16.Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol. 2007;102:856–61. doi: 10.1111/j.1572-0241.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- 17.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–41. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 18.Mori Y, Kudo SE, Berzin TM, Misawa M, Takeda K. Computer-aided diagnosis for colonoscopy. Endoscopy. 2017;49:813–9. doi: 10.1055/s-0043-109430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Esparrach G, Bernal J, Lopez-Ceron M, Córdova H, Sánchez-Montes C, Rodríguez de Miguel C, et al. Exploring the clinical potential of an automatic colonic polyp detection method based on the creation of energy maps. Endoscopy. 2016;48:837–42. doi: 10.1055/s-0042-108434. [DOI] [PubMed] [Google Scholar]
- 20.Urban G, Tripathi P, Alkayali T, Mittal M, Jalali F, Karnes W, et al. Deep learning localizes and identifies polyps in real time with 96% accuracy in screening colonoscopy. Gastroenterology. 2018;155:1069–78. doi: 10.1053/j.gastro.2018.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halpern Z, Gross SA, Gralnek IM, Shpak B, Pochapin M, Hoffman A, et al. Comparison of adenoma detection and miss rates between a novel balloon colonoscope and standard colonoscopy: A randomized tandem study. Endoscopy. 2015;47:238–44. doi: 10.1055/s-0034-1391437. [DOI] [PubMed] [Google Scholar]
- 22.Chin M, Karnes W, Jamal MM, Lee JG, Lee R, Samarasena J, et al. Use of the endocuff during routine colonoscopy examination improves adenoma detection: A meta-analysis. World J Gastroenterol. 2016;22:9642–9. doi: 10.3748/wjg.v22.i43.9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–41. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 24.Horton N, garber A, Hasson H, Lopez R, Burke CA. Impact of single- vs. split-dose low-volume bowel preparations on bowel movement kinetics, patient inconvenience, and polyp detection: A prospective trial. Am J Gastroenterol. 2016;111:1330–7. doi: 10.1038/ajg.2016.273. [DOI] [PubMed] [Google Scholar]
- 25.Misawa M, Kudo SE, Mori Y, Cho T, Kataoka S, Yamauchi A, et al. Artificial intelligence-assisted polyp detection for colonoscopy: Initial experience. Gastroenterology. 2018;154:2027–9 e3. doi: 10.1053/j.gastro.2018.04.003. [DOI] [PubMed] [Google Scholar]


