Abstract
The cattle fever tick, Rhipicephalus annulatus (Say), is an economically destructive arthropod because of its ability to vector bovine babesiosis. Cattle fever ticks can spend more than 90% of their life cycle as questing larvae, but the effect of climatic factors on their off-host behavior and survival is unclear. The goal of this study was to measure the effects of specific ecological factors on off-host larvae in nature. The study was conducted in a south Texas pasture over a 20-mo period, during which time larval populations were surveyed and ambient weather variables - relative humidity and temperatures – were recorded. Oviposition success and larval survival varied between cattle fever tick cohorts and was affected by relative humidity and canopied (with tree cover) versus exposed habitat. The results show that relative humidity and the interaction of relative humidity and inhabiting canopied habitats play a key role in oviposition success. Additionally, canopied habitats have a positive influence on off-host larval survival in the spring and summer.
Keywords: cattle tick, questing, habitat, climatic factors
Graphical Abstract
Introduction
Rhipicephalus (Boophilus) annulatus (Say), the cattle fever tick, is a vector of hemoparasites that cause babesiosis (Awad et al., 2011). Babesiosis took a toll on the American cattle industry and economy in the nineteenth century which eventually led to the implementation of an eradication program in 1906. The cattle fever tick, R. annulatus, as well as the related Rhipicephalus (Boophilus) microplus, were almost entirely eradicated from the United States by 1946 with the exception of a buffer (quarantine) zone along the Rio Grande in south Texas (Graham and Hourrigan, 1977). While measures are being taken to prevent the reintroduction of these cattle fever ticks (disinfestation of imported cattle and surveillance and interception of stray cattle), there are periodically recurring infestations along the south Texas-Mexico border (Davey, 1986; Lohmeyer et al., 2011). Difficulty in controlling free-roaming ungulate hosts, such as nilgai and white-tailed deer, along with the fact that most of the cattle fever tick life cycle is off-host complicates eradication efforts (Nuñez et al., 1985). Thus, adding better off-host management protocols to the existing host prevention methods would strengthen eradication efforts and reduce the risk of re-infestation.
Rhipicephalus ticks are one-host ticks, with a mean incubation period of approximately three weeks (Davey et al., 1982). The off-host portion of the larval stage typically consists of greater than 90% of the life cycle. Questing larval ticks seek hosts by climbing up vegetation and forming clusters with other larvae often clinging to one another. This clustering helps the larvae maintain moisture (Davey et al., 1991; Yoder and Knapp, 2009). While questing is a vital part of the tick’s life cycle, it can also result in high mortality because the larvae lose water and energy while having no source of nutrition. Under optimal lab conditions Rhipicephalus annulatus larvae have been shown to survive up to 8 mo before succumbing to starvation (Hooker et al., 1912). A clear understanding of the ecological conditions that influence cattle fever tick survival could be implemented to help predict future outbreaks and develop off-host control strategies.
Ecological factors such as seasonality, ambient and soil temperatures, humidity, sun exposure, vegetation, and precipitation all can affect tick survival. To date, what we have learned about the effect of climate and ecological factors on the questing behavior of Rhipicephalus tick larvae is from Rhipicephalus (Boophilus) australis. Complicating matters, these studies were conducted in Australia, a climate and ecosystem different from that of the quarantine zone in south Texas, USA. While morphologically similar, R. australis, R. annulatus, and R. microplus have been proven to be different species through cross mating and genetic studies (Ali et al., 2016; Burger et al., 2014; Estrada Peña et al., 2012; Low et al., 2015), signifying a need for field ecological studies specific to R. microplus and R. annulatus in south Texas.
Rhipicephalus microplus and R. annulatus have a parapatric distribution in Texas along the north side of the Rio Grande Valley (Lohmeyer et al., 2011). As a temperate zone species, R. annulatus is found in Webby County, Texas extending west into north central Mexico (Estrada Peña and Venzal, 2006). While an ecological study has been done on R. microplus larval survivability in south Texas (Leal et al., 2018), there is a paucity of literature on the ecological effects on R. annulatus questing behavior and survival in nature. The population dynamics model developed by Teel, 1991 aimed to predict the influence of season and habitat on tick populations in Texas, requires validation. The goal of this study is to strengthen the understanding of the effects of a south Texas climate on the development and survival of off-host R. annulatus larvae, to create a clearer foundation for future ecological studies and help enhance off-host eradication efforts in south Texas.
Materials and Methods:
Study Site
This study was conducted in a pasture at Moore Air Base located near Edinburg, TX, USA (26.3871◦ N, 98.3376◦ W; elevation 66 m) at the United States Department of Agriculture (USDA)-Agricultural Research Service, Cattle Fever Tick Research Laboratory. The lower Rio Grande Valley is a semi-arid, subtropical region with ambient temperatures averaging between lows of 8°C in the winter and highs of 36°C in the summer. Annual rainfall ranges between 380–750 mm and is highly erratic both seasonally and annually. The experimental pasture contains vegetation characterized as Tamaulipan scrub brushland (Correll and Johnston, 1970). The soil is a shallow calcareous clay with caliche near the surface. Vegetative cover within the pasture is around 90%, with a canopy cover of around 20%. The dominant tree species is honey mesquite, Prosopis glandulosa (Torr.), with shrubby acacias, Vachellia farnesiana (L.) Willd., Vachellia rigidula (Benth.), and spiny hackberry, Celtis ehrenbergiana (Klotzsch). Typical of pastureland of south Texas, the dominant understory plant is buffelgrass, Pennisetum ciliare (L.), with the common forbs silverleaf nightshade, Solanum elaeginifolium (Cav.) and cowpen daisy, Verbesiana encelioides (Cav.). Plant names follow the USDA Plants Database. (United States Department of Agriculture NRCS, 2006)
Rearing of Ticks
Ticks were reared as described previously (Leal et al., 2017). Briefly, larval ticks were placed on stanchioned cattle and allowed to develop until females were engorged and dropped from the host. These females were held in petri dishes (at 27±1◦C, 80±5% relative humidity (RH)) for oviposition. Experimental colonies of R. annulatus were maintained under optimal conditions in a climate-controlled room (Davey, 1986; Davey et al., 1984). The strain designated as “Klein Grass” was used to infest study arenas as described below.
Study Arenas
As in the earlier study with R. microplus (Leal et al., 2018), female R. annulatus were released into study arenas. These study arenas consisted of 18 individual metal tubs (American Metalcraft, Franklin Park, IL, USA) with the dimensions measuring 60 cm in diameter and 30 cm in height. Tubs were filled 22 cm deep with soil from the surrounding pasture and a selected grown plant was transferred to each tub to establish a study arena (Fig. 1A). Each was planted with one of three common south Texas pasture plants: buffelgrass, silverleaf nightshade, or cowpen daisy. These plants were ideal for the local conditions because they thrive in semiarid pastures. Buffelgrass is an invasive dominant pasture grass in south Texas and northern Mexico, native to Africa (Arriaga et al., 2004). Silverleaf nightshade is a plant native to south Texas that contains spines with a sticky texture (Mekki, 2007). Cowpen daisy or yellow-top, is also native to south Texas. It grows throughout the year in regions with mild winters (Grichar and Sestak, 1998). If a plant died, it was replaced between tick cohort introductions. A total of 18 study arenas (14 for cohort 1) were scattered throughout the eight-hectare pasture (Fig. 2). The study arenas were arrayed so that some (n = 8) were situated under the canopy of a large mesquite tree (Fig. 1B) with others (n = 10) placed in exposed situations away from the trees. In cohort 1, the corresponding numbers were eight and six study arenas. It is important to note that we did not measure or observe a difference in biotic factors in the arenas as compared to open ground.
Figure 1. Study arena and habitat in south Texas pasture.

A.) A representative image of a study arena planted with Pennisetum ciliare. B.) A representative image of a study arena under a canopied habitat.
Figure 2.
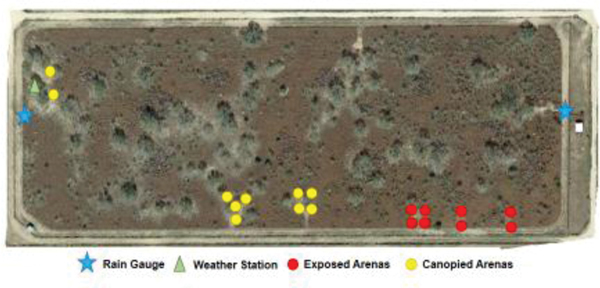
Satellite photo of the eight-hectare study pasture showing the locations of canopied and exposed study arenas.
Data collection
At the beginning of each cohort all study arenas were infested by releasing one engorged female at the center of each tub near the stem of the plant. Females would most often seek a hiding place in the root mass or less often, would dig into the soil. Consequently, they were not generally observable after the first day and in any case, once placed, these females were not disturbed during oviposition (typically a 3- to 4-wk period). No adult ticks were observed completing oviposition near the rim of the study arenas, nor were any larvae observed crawling over the rim of the study arenas. Presumably some female cattle fever ticks suffered depredation, but the cause of mortality was not directly determined. Starting at week 3, larval ticks were sampled using the standard flag method as previously described (Leal et al., 2018). A white flannel cloth (dimension, 25 × 20 cm) was placed directly over the plant then dragged in opposite directions to represent a potential passing host (collection time of approximately 40 s). Each flannel cloth was then placed in a numbered zip-lock bag corresponding to each tub. Larvae attached to the cloth were collected with clear adhesive tape then mounted directly on a data sheet following the recording methods of Wilkinson (1961). Thus, the means of determining population response to environmental variables was a destructive sampling method which provided numbers captured as a proxy for larval abundance. Twelve censuses were taken per month, with 2 to 3 d between each census. All arenas were sampled at each collection date. Sampling clock-times were varied to include all periods of day and night. Data were collected continuously over a 20-mo period. Abiotic factors were measured by a HOBO Pro model V2 micro weather station (Onset Computer Corporation, Bourne, MA, USA) to record ambient temperatures and percent relative humidity. A rain gauge was installed at each end of the experimental pasture and read after each rain event.
Cohorts
There was a total of 9 cohorts in this study. The cohorts typically extended over more than one season so for the purposes of this study cohorts were defined by the date on which engorged females were introduced into the study arenas, in accordance with the parallel study of R. microplus (Leal et al. 2018). A new cohort would begin as the previous one ended (day range is stated in the results). To ensure no larvae remained from a previous cohort, sampling continued in positive arenas approximately 2 weeks after the last larvae was collected. If a couple of arenas still had larvae, counting would continue and a new set of arenas would be filled, planted and used to start the next cohort. Each cohort consisted of a full set of 14–18 study arenas that were infested at the same time by releasing individual engorged females into each arena and the resulting populations were monitored as described above. For analyzing potential seasonal effects each cohort was assigned to the season that corresponded to the month when the cohort was initiated, i.e, winter (November-February), spring (March-May), summer (June-August), and fall (September-October). For each study arena in each cohort we measured the time interval from the introduction of females to the first positive larval sample (incubation stage), and from the first to the last positive larval sample (larval stage).
Statistical Analysis
Parameters measured for each cohort were as follows: total numbers of larval ticks per individual arena, total larvae per each positive arena, mean larvae per arena in all, canopied, and exposed habitats and percentage of arenas positive for larvae. A “positive” arena was one in which larvae were detected, indicating survival and reproductive success by the released gravid female. Difference between mean numbers of larvae per cohort, mean numbers of larvae per canopied and exposed habitats within and between cohorts, mean relative humidity between cohorts, mean maximum and mean minimum relative humidity within and among cohorts, mean temperature between cohorts, mean maximum and mean minimum temperature within and among cohorts, duration of the incubation stage by cohort, and duration of the larval stage by cohort were conducted by one-way analysis of variance (ANOVA) with Student-Newman Keul’s multiple comparison posttest. For analyzing potential seasonal effects, mean numbers of larvae by season and percentage of arenas positive for larvae by season, and by habitat were done by pair-wise t-test assuming unequal variance. Linear regression was used to measure correlation between mean larval numbers by cohort and corresponding weather variables. The aforementioned analyses were performed using Graphpad Instat (Graphpad Software Inc., 2009).
Overall survival, oviposition success, and post-incubation survival were analyzed using multifactorial ANOVAs with temperature, relative humidity, precipitation and habitat (input categorically) as main factors. These multifactorial ANOVAs were performed using JMP Pro 13 (SAS Institute Inc., 2016). Stepwise reduction of all full models was used, removing highest-order interactions and always the least significant effect first (Crawley, 1993). Factors were removed when p > .05. Model reduction stopped when no further interactions could be removed. No non-significant main effects were removed. Interactions or differences were considered significant when p < .05.
Results:
Mean (± SD) larval tick numbers were analyzed by cohort, by season, and by habitat, including all study arenas, and separately for positive study arenas only (Table 1 and Table 2). The mean numbers of larvae counted from arenas containing one of the three plant species included in this study were not consistent. Cowpen daisy arenas had the highest numbers (242.7 ± 367.5, n = 17); silver-leaf nightshade the lowest (71.8 ± 220.1, n =17); with buffelgrass intermediate (158.3 ± 261.4, n = 78). These differences, though large, were not statistically significant (p = 0.14) because of the high variation within and among cohorts. Similar results were reported in our parallel study of R. microplus (Leal et al. 2018). Therefore, data from arenas with different plant species were combined in the further analysis.
Table 1.
Total and mean ± SD number of Rhipicephalus annulatus larvae collected in canopied and exposed habitats. Statistical comparison of means was performed by one-way ANOVA assuming unequal variance with Student-Newman Keul’s posttest. Means followed by the same letter are not significantly different at p = 0.05. Oviposition success indicated by the percentage of study arenas (% positive) that had at least one larval tick collected. Means calculated using all studied arenas (not positive only) within the identified categories.
| Cohorts | Season Started (Duration) | N Arenas | Positive Arenas | Total Larvae | Mean ±SD Larvae | Mean ±SD Larvae Canopied | Mean ±SD Larvae Exposed | |
|---|---|---|---|---|---|---|---|---|
| N | (%) | |||||||
| 1 | Winter (Jan 18 - Mar 7) | 14 | 3 | 21.4 | 7 | 0.38 ± 1.6 b | 0 b | 1.2 ± 1.6 a |
| 2 | Winter (Feb 10 – Mar 21) | 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | Spring (Mar 3 - Jul 11) | 18 | 17 | 94.4 | 4998 | 277.7 ± 343.9 a | 403.1 ± 404.0 a | 120.9 ± 163.9 a |
| 4 | Summer (Jun 21 - Sep 6) | 18 | 13 | 72.2 | 1254 | 69.7 ± 160.5 b | 107.5 ± 206.2 b | 22.4 ± 56.9 a |
| 5 | Fall (Sept 19 - Feb 7) | 18 | 18 | 100 | 4640 | 257.8 ± 343.9 a | 346 ± 337.2 a | 147.5 ± 197.5 a |
| 6 | Winter (Jan 3 - May 8) | 18 | 18 | 100 | 2276 | 126.4 ± 221.3 b | 115.3 ± 224.5 b | 140.4 ± 231.9 a |
| 7 | Spring (April 24-Jul 17) | 18 | 18 | 100 | 6706 | 372.5 ± 415.0 a | 568.8 ± 460.1 a | 127.2 ± 149.7 a |
| 8 | Summer (Jun 11 – Jul 20) | 17 | 1 | 5.9 | 2 | 0.11 | 0.22 ± .67 | 0 |
| 9 | Summer (Jun 11 - Aug 7) | 18 | 7 | 38.9 | 237 | 13.2 ± 47.5 b | 18.6 ± 60.8 b | 4.6 ± 10.0 a |
Table 2.
Collections of larval Rhipicephalus annulatus in relation to season and habitat. Collective (over 20 mo) results comparing mean ± SD, seasonal larval numbers by habitat. Statistical comparison of means was performed by pair-wise t-test assuming unequal variance. Means followed by the same letter are not significantly different at p = 0.05.
| Habitat | Winter | Spring | Summer | Fall |
|---|---|---|---|---|
| Canopied | 63.9 ± 173.7 cd | 485.9 ± 429.8 a | 60.9 ± 151.8 cd | 346 ± 337.1ab |
| Exposed | 80.7 ± 184.5 c | 124.0 ± 151.6 bc | 14.2 ± 41.7 e | 147.5 ± 197.5 bc |
| All | 71.3 ± 175.8 c | 325.1 ± 378.7 ab | 41.1 ± 102.8 d | 257.7 ± 294.1 ab |
| Positive Arenas Only | 108.6 ± 208.8 c | 334.4 ± 380.0 ab | 67.8 ± 148.8 cd | 257.7 ± 294.1 ab |
The mean number of larvae per cohort were markedly and significantly higher in cohorts 3 (spring), 5 (fall), and 7 (spring) (Table 1). Mean larval numbers in cohorts 3, 5, and 7 were not significantly different from each other. Not surprisingly oviposition success (positive study arenas) was high in these three cohorts (94.4%, 100%, 100% respectively). In contrast, cohorts 2 (winter) and 8 (summer) essentially failed (Table 1), the lack of larvae indicating a failure of the females to lay eggs or for those eggs to hatch, and therefore these two cohorts were excluded from the further analysis. In comparison, cohort 1 (winter) and cohorts 4 and 9, both summer cohorts, had low to moderate levels (21–70%) of reproductive success, and low to moderate numbers of larvae.
Considering the results by season, the data in Table 2 shows significantly higher numbers of larval ticks in the spring and fall cohorts compared to winter and summer, with the lowest survival in summer. Overall, the summer cohorts had the lowest mean numbers, 41.1 larval ticks recovered per study arena. Among these, cohort 4 had a mean of 68.7 and cohort 9 had a mean of 13.2 larvae recovered per study arena (n.s. at p = 0.05). Notably cohort 4 had relatively strong reproductive success (72.2%) compared to cohort 9 with only 38.9% positive arenas. The numbers in the winter cohorts 1 (mean = 0.38) and 6 (mean = 126.4) were very different from one another (p < 0.01) and this was in large part due to a much greater oviposition success (100%) in cohort 6 compared to cohort 1 (only 21.4%). Overall spring and fall cohorts had significantly higher means (325.1 and 257.7 larvae recovered) than winter (mean = 71.3) and summer cohorts (mean = 41.1) (Table 2).
Multifactorial analysis confirmed that habitat significantly influenced tick survivability. Figure 3 shows mean numbers of larvae per cohort collected from canopied habitats and exposed habitats (total numbers per cohort shown in Table 1). With the exception of the winter cohorts, canopied habitats had markedly higher mean numbers of ticks per study arena compared to exposed habitats. Specifically, spring cohort 7 had fourfold higher mean numbers of larvae in canopied habitats than in its corresponding exposed habitats. In the fall cohort canopied arenas had threefold higher numbers than in the corresponding exposed arenas. Similarly, the summer cohorts had four to fivefold mean numbers of larvae in the canopied study arenas than in their respective exposed study arenas. In contrast, for the winter cohorts there was no significant difference in mean numbers between the exposed and canopied study arenas.
Figure 3.
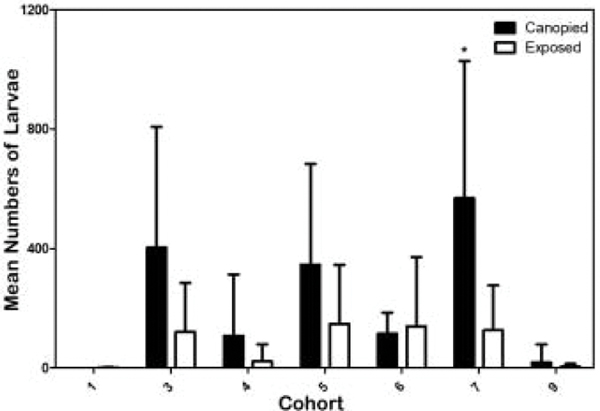
The number (mean + SD) of Rhipicephalus annulatus larvae collected from canopied (black bars) and exposed (white bars) habitats per cohort. No larvae were found in study arenas from the canopied habitat in cohort 1. * indicates a statistical difference between canopied and exposed study arenas within a cohort (p < 0.01). Number of ticks collected from each habitat per cohort presented in Table 2. Cohorts 2 and 8 excluded from analysis.
Habitat significantly affected the mean larval numbers by cohort (Table 3). In the multifactorial analysis, relative humidity and temperature showed no detectable effect on total larval survivability (Table 3). However, the multifactorial analysis did indicate that habitat and RH had an effect on oviposition success (Table 4). But during the larval phase only habitat had a detectable effect on survivability (Table 5). This was an interesting result given the variability in RH (Fig. 4) and a similar variability trend in temperature among the different cohorts (Fig. 5). Specifically, spring cohort 3 and fall cohort 5 recorded significantly higher mean RH (72.7% and 71.6% respectively) compared to the rest of the cohorts. In contrast, cohorts 1 (winter), 7 (spring) and 9 (summer) exhibited relatively similar mean RHs which were significantly (p < 0.001) lower (61.7 – 63.7%) than the other cohorts. Although winter cohort 1 did have a mean-max RH similar to the other cohorts, the mean-min RH during cohort 1 was significantly lower than all other cohorts (Fig. 4). The other winter cohort (cohort 6), had significantly higher mean RH and mean-min RH compared to cohort 1 (Fig. 4). Winter cohort 6 was among the larger larval populations compared to the other cohorts (Table 1). The mean-max RH was similar between cohorts that included winter, fall, summer and spring (cohorts 1, 3–6). However, these cohorts had significantly higher mean-max RH levels compared to spring cohort 7 and summer cohort 9.
Table 3:
Multifactor ANOVA results comparing the effects of mean temperature, mean relative humidity (RH), precipitation, and habitat on the number of Rhipicephalus annulatus larvae collected in each cohort. Habitat was input in the analysis as either canopied or exposed.
| Source | Nparm | DF | F Ratio | Prob > F |
|---|---|---|---|---|
| Average Temp | 1 | 1 | 0.0000 | 0.5526 |
| Average RH | 1 | 1 | 0.0000 | 0.4920 |
| Habitat | 1 | 1 | 8.8792 | 0.0038* |
| Precipitation | 1 | 1 | 1.2535 | 0.6967 |
= statistically significant
Table 4:
Multifactor ANOVA results comparing the effects of mean temperature, mean relative humidity (RH), and habitat on Rhipicephalus annulatus oviposition success per cohort. Habitat was input in the analysis as either canopied or exposed.
| Oviposition Success- Number of Positive Arenas | ||||
|---|---|---|---|---|
| Source | Nparm | DF | F Ratio | Prob > F |
| Mean Incubation Temp | 1 | 1 | 2.0999 | 0.1500 |
| Mean Incubation RH | 1 | 1 | 40.6334 | <0.0001* |
| Habitat (+) | 1 | 1 | 1.4206 | 0.2357 |
| Habitat * Mean Incubation RH | 1 | 1 | 4.0174 | 0.0473* |
= statistically significant
Table 5:
Multifactor ANOVA results comparing the effects of mean temperature, mean relative humidity (RH), and habitat on Rhipicephalus annulatus larvae survival post-oviposition per cohort. Habitat was input in the analysis as being canopied or exposed.
| Post-incubation Survival-Positive Study Arenas Only | ||||
|---|---|---|---|---|
| Source | Nparm | DF | F Ratio | Prob > F |
| Mean Larval Stage Temp | 1 | 1 | 0.4154 | 0.2954 |
| Mean Larval Stage RH | 1 | 1 | 0.7748 | 0.3751 |
| Habitat (+) | 1 | 1 | 11.2175 | 0.0021* |
| Precipitation | 1 | 1 | 1.0324 | .3123 |
= statistically significant
Figure 4.
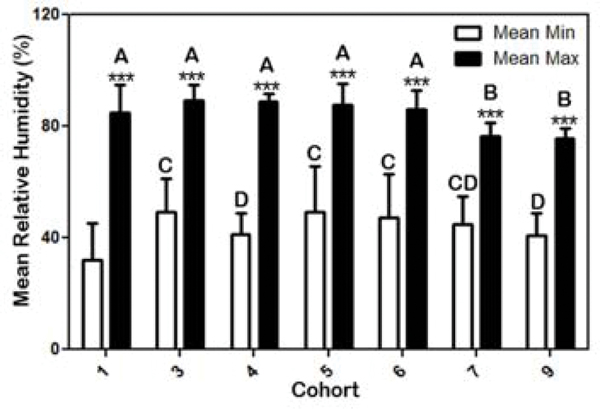
The mean minimum + SD (white bars) and maximum (black bars) percent relative humidity (%RH) in each cohort during Rhipicephalus annulatus larval collections for the duration of each cohort. Different letters indicate p < 0.001 comparing %RH between cohorts; *** = p < 0.001 comparing max and min %RH within cohorts.
Figure 5.
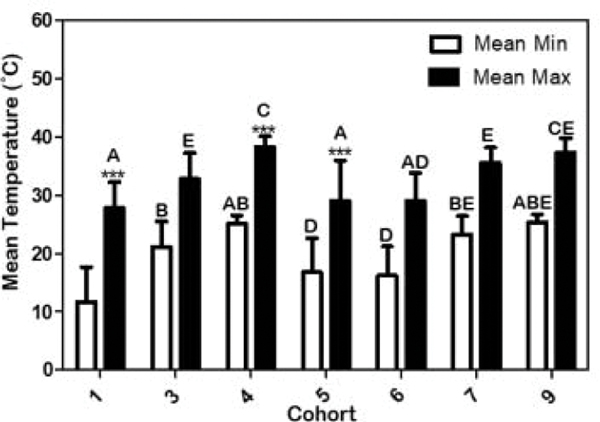
The mean minimum + SD (white bars) and mean maximum (black bars) temperature in degrees Celsius during Rhipicephalus annulatus larval collections for the duration of each cohort. Different letters indicate p < 0.05 comparing temperature between cohorts; *** = p < 0.001 comparing max and min temp within cohorts. Cohorts 2 and 8 excluded from analysis.
Ambient temperature showed a more predictable pattern than RH, with summer cohorts 4 and 9 having the highest mean temperatures (cohort 4 with a mean temperature of 30.6°C, and cohort 9 with a mean temperature of 30.5°C) whereas winter/fall cohorts had the lowest (winter cohorts with a mean temperature of 18.1°C for cohort 1 and 21.9°C for cohort 6, and fall cohort with a mean temperature of 19.4°C). Not surprisingly, cohort 1 (winter) recorded the lowest overall mean ambient temperature and mean-min temperature (Fig. 5) compared to all other cohorts. However, cohort 1 did have a mean-max temperature similar to fall cohort 5 and cohort 6 (the other winter cohort). As expected, summer cohorts 4 and 9 had significantly higher mean ambient temperatures as well as mean-min and mean-max temperatures (Fig. 5). However, mean-min temperatures in the summer cohorts were similar to those of the spring cohorts (3 and 7) and fall cohort 5 (Fig. 5). These similarities are not unexpected given the overlapping months among several of the cohorts (Table 1).
Rainfall by cohort is shown in Figure 6. Spring cohort 3 had the most precipitation. There was no measurable precipitation in winter cohort 1 and virtually no precipitation in cohorts 7 and 9. Cohorts with meaningful amounts of rainfall generally had large mean numbers of larvae compared to the dry cohorts. The clear exception was cohort 7 which had virtually no precipitation but large mean numbers of larvae. Consequently, the multifactorial analysis failed to detect an influence of precipitation (Tables 3–5). This result seems counterintuitive and even contradictory considering that there was a detectable effect from relative humidity (Table 4). A simple regression analysis of RH against precipitation gave a very significant (p=0.007, r2= 0.79) correlation (Fig. 7). Based on that result we ran a regression analysis of precipitation against larval numbers and found a correlation between rainfall and the larval phase but only in exposed habitats (Fig. 8).
Figure 6.
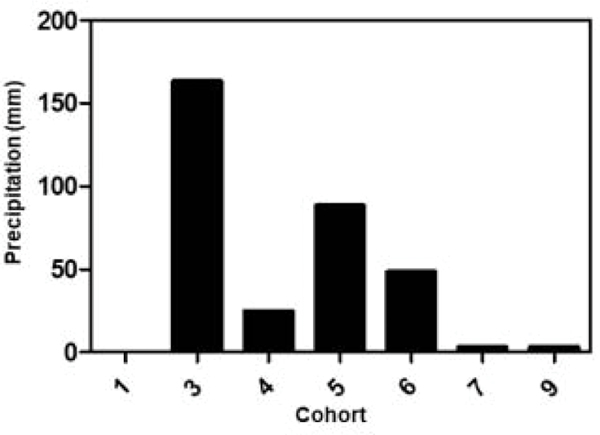
Total millimeters of precipitation measured per cohort.
Figure 7.
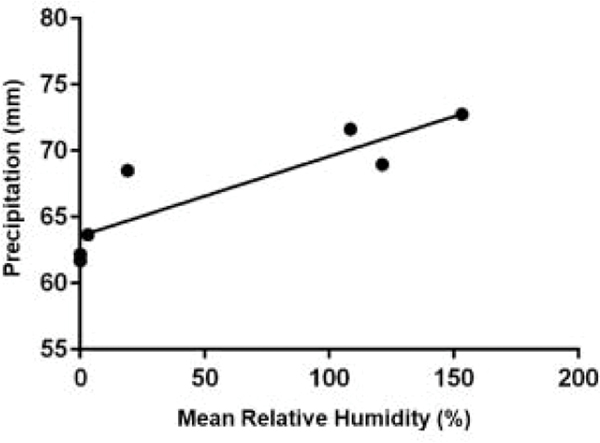
Regression graph representing a correlation between mean relative humidity and precipitation throughout the study. (p = 0.007, r2 = 0.79)
Figure 8.
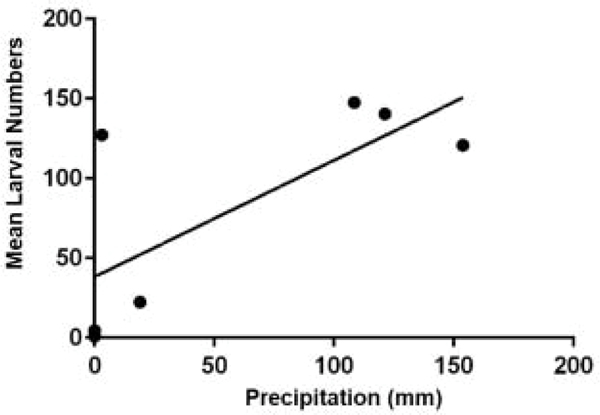
Regression graph suggesting a correlation between mean numbers of Rhipicephalus annulatus larvae collected from exposed habitats and precipitation in millimeters (p = 0.06, r2 = 0.52)
Five of the nine cohorts started in one season and finished in another (Table 1). So, we looked at the possibility that habitat, relative humidity, temperature and precipitation might have preferentially affected different stages of the tick life cycle (female incubation-oviposition success vs. = larval survival). Figure 9 shows the differences in the mean number of days cohorts spent in the two different life cycle stages. Cohort 6, one of the cohorts that started in winter and finished in spring, had a significantly (p <0.01) longer mean incubation stage indicated by the time females were placed in study arenas until the first larvae was found (Figure 9). This cohort had 100% positive study arenas and one of the highest larval populations among the cohorts (Table 1). The other winter cohort (cohort 1) also had a slightly longer incubation period compared to the other non-winter cohorts (Figure 9). However, cohort 1 had only 21.4% positive study arenas, a low larval population and was shorter in duration compared to cohort 6 (Table 1). The exception was cohort 3, which started in spring and finished in summer and had a significantly (p <0.01) longer mean larval stage (post-incubation) compared to cohorts 4, 6, 7, 9 and 1 (Figure 9). The length of the larval stage was not significantly different between the majority of cohorts (1, 4, 6–9). Yet, the mean number of larval ticks collected during this time was significantly different between some of these cohorts (Table 1). Indicating that the destructive sampling method used minimally affected tick survivability. As expected, multifactor analysis showed that mean RH significantly influenced oviposition success (Table 4), while only habitat significantly influenced larval survivability (Table 5).
Figure 9.
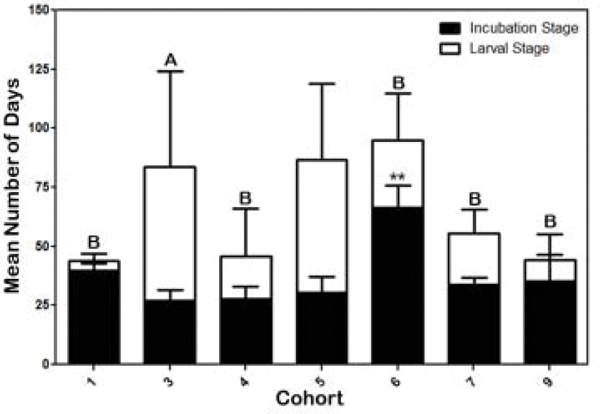
Mean (+ SD) number of days of Rhipicephalus annulatus egg incubation (black bars) and larval (white bars) stages in only positive study arenas from each cohort. The duration of the egg incubation period was defined as the day an engorged female was placed in a study arena until the day the first larvae were collected in each study arena. Larval stage was defined by the day the first larvae was collected in each study arena until the last day larvae was collected from the same study arena. ** = p <0.01 = incubation stage cohort 6 vs incubation stage cohort 3, 4, 7, 9; A vs. B = p <0.01 in larval stage cohort 3 vs. larval stage 1, 4, 6, 7, and 9. Number of ticks collected and number of positive study arenas per cohort presented in Table 1. Cohorts 2 and 8 excluded from analysis.
Discussion:
Ecological studies on tick population dynamics almost uniformly report a strong effect of humidity on off-host survival (Needham and Teel, 1991). This was true in the present study as well. The contrastingly large difference in larval numbers between the two winter cohorts was traceable to rainfall during the incubation phase of cohort 6, resulting in 100% oviposition success among study arenas vs only 21% success in cohort 1 which had no measurable precipitation. The multifactorial analysis detected a strong interaction between RH and oviposition success. The incubation mean RH for cohort 1 was 60% while the mean RH during incubation of cohort 6 was 68%, and this difference in RH was statistically significant. Not surprisingly, the regression analysis showed a significant correlation between rainfall and relative humidity. In contrast to the results of the parallel study with R. microplus in which there was an inverse relationship between larval survival and rainfall (Leal et al., 2018), the data in this study with R. annulatus showed a positive correlation. The cause of the difference in result may be the fact that there was no rain during any of the incubation phases of the cohorts in this study. Heavy rains dispersing the egg masses was suspected as the cause of the negative interaction in the R. microplus study.
We found no significant effect of temperature on larval numbers in this study. Strey et al. (1991) reported data from lab experiments that a range of temperatures from 17–36 °C are optimal for egg viability and Davey et al. (1991) found a similar range was optimal for the larval stage. During this field study the temperatures experienced were generally within this optimal range and hence no detrimental effect would be expected. During winter cohort 6 the only freeze dates during the experiment occurred (7–8 January 2017) just four days after release of the female and it is likely that eggs had not yet been laid, hence this extreme temperature had no measurable effect. The summer cohorts had significantly fewer larval numbers than the spring and fall cohorts which might have been in part due to high temperatures, but the analysis could not separate this parameter from relative humidity. Davey et al. (1991) likewise found no effect of temperature on R. annulatus larvae in the lab except when RH was below optimal.
In the parallel study arena study by Leal et al. (2018), R. microplus was found to have highest numbers in the spring and lowest in the fall. In the present study with R. annulatus the highest numbers were in spring and fall with lowest in summer and winter. In the multifactorial analysis much of this difference was explained as a habitat interaction. Survival was significantly better in spring and summer in canopied habitats, whereas there was no difference between exposed and canopied habitats in terms of survival in fall and winter. Contrastingly, with R. microplus, canopied habitats were better in summer, but exposed habitats had better survival in winter (Leal et al., 2018). These results are in accord with the general observation that R. microplus is a tropical tick, finding optimal conditions in hot, humid regions, whereas R. annulatus is a temperate zone tick adapted to cooler, drier regions (Estrada Peña and Venzal, 2006).
Conclusions:
This study supports previous literature that identify a relationship between habitat, relative humidity, and larval cattle fever tick survival. We showed that successful oviposition and egg hatching is dependent on relative humidity and habitat, while larval survival is dependent mainly on habitat. These results are particularly relevant for infestations in south Texas. Our study also suggests the environment of south Texas has become a favorable area for R. annulatus to inhabit, as suggested by the model by Teel (1991) and our weather data. With this new information on R. annulatus larvae, better off-host control can be implemented. This study can provide program managers and the scientific community with knowledge about how larval population dynamics respond to the interaction between seasonality and habitat. For example, it will inform researchers where and when the larvae will be most abundant – in canopied habitats and when relative humidity is high. This study also provides results based on natural conditions validating model predictions based on laboratory studies done previously on R. annulatus. This information, in return, can provide the foundation for future ecological studies on R. annulatus larvae.
Acknowledgements:
The illustration for the graphic abstract was designed and provided by Austin “Wolfe” Fuentes, a graduate student at the University of Texas Rio Grande Valley. We would like to give special thanks to Alexis Racelis and Christopher Vitek of the University of Texas Rio Grande Valley, Michael Moses, Jason Tidwell, Summer De Luna, Joni Ortiz, Ruby Martinez, Bethany Olivarez, James Hellums, Cesario Agádo, Homero Vazquez, Ariel Hinojosa, and Charluz Arocho Rosario of the United States Department of Agriculture for their unwavering support and technical assistance. This research was conducted in part to complete the requirements of the Master of Science Degree in the Department of Biology at the University of Texas Rio Grande Valley. Mention of trade names or commercial products in this article is for information purposes only and does not constitute endorsement by the USDA. The USDA is an equal opportunity provider and employer. In conducting the research described in this report, the Investigators adhered to the “Guide for the Care and Use of Laboratory Animals,” as promulgated by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council. The facilities are fully accredited by the American Association of Laboratory Animal Care.
Funding:
This project was funded in part by the USDA-Agricultural Research Service, National Program 104, and Project # 3094-32000-039-00D, STEP 2 USDA Research Success program (USDA grant 2015-38422-24061) and the National Institute of General Medical Sciences (1R25GM100866).
Footnotes
Conflicts of Interests: There is no conflict of interest with this study to any previous study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali A, Parizi LF, Ferreira BR, da Silca Vaz IJ, 2016. A revision of two distinct species of Rhipicephalus: R. microplus and R. australis. Parasitology 46, 1240–1248. 10.1590/0103-8478cr20151416 [DOI] [Google Scholar]
- Arriaga L, V AEC, Moreno E, Alarcon J, 2004. Potential ecological distribution of alien invasive species and risk assessment: A case study of buffel grass in arid regions of Mexico. Conserv. Biol. 18, 1504–1514. 10.1111/j.1523-1739.2004.00166.x [DOI] [Google Scholar]
- Awad H, Antunes S, Galindo RC, do Rosario VE, Domingos A, El Hussein AM, 2011. Prevalence and genetic diversity of Babesia and Anaplasma species in cattle in Sudan. Vet. Parasitol. 181, 146–152. [DOI] [PubMed] [Google Scholar]
- Burger TD, Shao R, Barker SC, 2014. Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species. Mol. Phylogenet. Evol. 76, 241–253. 10.1016/j.ympev.2014.03.017 [DOI] [PubMed] [Google Scholar]
- Correll DS, Johnston MC, 1970. Manual of the Vascular Plants of Texas. Texas Research Foundation, Renner, Texas. [Google Scholar]
- Crawley MJ, 1993. Methods in Ecology: GLIM for Ecologists. Oxford Blackwell Scientific Publications, London, England. [Google Scholar]
- Davey RB, 1986. Daily dynamics of egg development and fecundity and effect of age of larve on attachment rate to cattle in Boophilus annulatus. Southwest. Entomol. 11, 17–22. [Google Scholar]
- Davey RB, Cooksey LM, Despins JL, 1991. Survival of larvae of Boophilus annulatus, Boophilus microplus, and Boophilus hybrids (Acari: Ixodidae) in different temperature and humidity regimes in the laboratory. Vet. Parasitol. 40, 305–313. 10.1016/0304-4017(91)90110-H [DOI] [PubMed] [Google Scholar]
- Davey RB, Garza J, Thompson GD, 1982. Seasonal observation on the development and ovipositional capability of Boophilus annulatus and B. microplus (Acari: Ixodidae) Reared on Bovines. J. Med. Entomol. 19, 24–28. 10.1093/jmedent/19.1.24 [DOI] [PubMed] [Google Scholar]
- Davey RB, Osburn RL, Miller JA, 1984. Ovipositional and morphological comparisons of Boophilus microplus (Acari: Ixodidae) collected from different geographic areas. Ann. Entomol. Soc. Am. 1–5. 10.1093/aesa/77.1.1 [DOI] [Google Scholar]
- Estrada Peña A, Venzal JM, 2006. High-resolution predictive mapping for Boophilus annulatus and B . microplus (Acari: ixodidae) in Mexico and southern Texas. Vet. Parasitol. 142, 350–358. 10.1016/j.vetpar.2006.07.003 [DOI] [PubMed] [Google Scholar]
- Estrada Peña A, Venzal JM, Nava S, Mangold A, Guglielmone AA, Labruna MB, De La Fuente J, 2012. Reinstatement of Rhipicephalus (Boophilus) australis (Acari: Ixodidae) with redescription of the adult and larval stages. J. Med. Entomol. 49, 794–802. 10.1603/ME11223 [DOI] [PubMed] [Google Scholar]
- Graham OH, Hourrigan JL, 1977. Eradication programs for the arthropod parasites of livestock. J. Med. Entomol. 13, 629–658. 10.1093/jmedent/13.6.629 [DOI] [PubMed] [Google Scholar]
- Grichar WJ, Sestak DC, 1998. Control of golden crownbeard (Verbesina encelioides) in peanut (Arachis hypogaea) with postemergence herbicides. Peanut Sci. 25, 39–43. 10.3146/i0095-3679-25-1-10 [DOI] [Google Scholar]
- Hooker WA, Bishopp FC, Wood HP, 1912. The life history and bionomics of some North American ticks. U.S Department of Agriculture - Bureau of Entomology, Washington, D.C, USA: 10.5962/bhl.title.65064 [DOI] [Google Scholar]
- Leal B, Thomas DB, Dearth R, 2017. Cattle fever tick, Rhipicephalus (Boophilus) microplus (Acari: Ixodidae): Potential control on pastures by the application of urea fertilizer. Vet. Parasitol. 241, 39–42. 10.1016/j.vetpar.2017.05.010 [DOI] [PubMed] [Google Scholar]
- Leal B, Thomas DB, Dearth RK, 2018. Population dynamics of oOff-host Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) larvae in response to habitat and seasonality in south Texas. Vet. Sci. 5, 33 10.3390/vetsci5020000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmeyer KH, Pound JM, May MA, Kammlah DM, 2011. Distribution of Rhipicephalus (Boophilus) microplus and Rhipicephalus (Boophilus) annulatus (Acari: Ixodidae) infestations detected in the United States along the Texas / Mexico border. J. Med. Entomol. 48, 770–774. 10.1603/ME10209 [DOI] [PubMed] [Google Scholar]
- Low VL, Tay ST, Kho KL, Koh FX, Tan TK, Lim YAL, Ong BL, Panchadcharam C, Norma-Rashid Y, Sofian-Azirun M, 2015. Molecular characterisation of the tick Rhipicephalus microplus in Malaysia: New insights into the cryptic diversity and distinct genetic assemblages throughout the world. Parasit. Vectors 8 10.1186/s13071-015-0956-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekki M, 2007. Biology, distribution and impacts of silverleaf nightshade (Solanum elaeagnifolium Cav.)*. EPPO Bull. 37, 114–118. 10.1111/j.1365-2338.2007.01094.x [DOI] [Google Scholar]
- Needham GR, Teel PD, 1991. Off-host physiological ecology of Ixodid ticks. Annu. Rev. Entomol. 36, 659–681. 10.1146/annurev.en.36.010191.003303 [DOI] [PubMed] [Google Scholar]
- Nuñez JL, Muñoz-Cobeñas ME, Horacio LM, 1985. Boophilus microplus: The Common Cattle Tick. Springer-Verlag Berlin Heidelberg, Berlin, Germany. [Google Scholar]
- Strey OF, Teel PD, Ring DR, Longnecker MT, 1991. Modeling embryo development and emergence of Boophilus annulatus (Acari: Ixodidae). J. Med. Entomol. 28, 165–173. 10.1093/jmedent/28.1.165 [DOI] [PubMed] [Google Scholar]
- Teel PD, 1991. Application of modelling to the ecology of Boophilus annulatus (Say) (Acari: Ixodidae). J. Agric. Entomol. 8, 291–296. [Google Scholar]
- United States Department of Agriculture, NRCS, 2006. The PLANTS Database. https://plants.sc.egov.usda.gov/java (accessed 28 March 2019).
- Wilkinson PR, 1961. The use of sampling methods in studies of the distribution of larvae of Boophilus microplus on pastures. Aust. J. Zool. 9, 752–783. 10.1071/ZO9610752 [DOI] [Google Scholar]
- Yoder JA, Knapp DC, 2009. Cluster-promoted water conservation by larvae of the American dog tick, Dermacentor variabilis (Acari: Ixodidae). Int. J. Acarol. 25, 55–57. 10.1080/01647959908683613 [DOI] [Google Scholar]


