Abstract
This summary article reviews the literature on neural correlates of age-related changes in temporal processing in the auditory brainstem. Two types of temporal processing dimensions are considered, (i) static, which can be measured using a gap detection or forward masking paradigms, and (ii) dynamic, which can be measured using amplitude and frequency modulation. Corresponding data from physiological studies comparing neural responses from young and old animals using acoustic stimuli as silent gaps-in-noise, amplitude modulation, and frequency modulation are considered in relation to speech perception. Evidence from numerous investigations indicates an age-related decline in encoding of temporal sound features which may be a contributing factor to the deficits observed in speech recognition in many elderly listeners.
Keywords: Aging, Temporal resolution, Neural correlates, Animal models
1. Introduction
The ability to precisely encode complex temporal features inherent in species-specific vocalizations and environmental sounds is critical for survival in mammals. This capacity requires high resolution temporal processing, often on the order of fractions of a millisecond. The fine-tuned temporal processing demands of the auditory system are met through morphological and physiological adaptations, which serve to counteract the temporal variability inherent in chemical neurotransmission by neurons across multiple synapses (Trussell, 2002). Age-related changes in this complex neuromachinery can result in altered neural timing which may be partly responsible for deficits in speech recognition observed in aged listeners (Gordon-Salant and Fitzgibbons, 1993).
Speech comprehension difficulties observed in aged human listeners has motivated research efforts to identify age-related changes in more central processes affecting speech comprehension. In contrast to age-related changes in audibility, which are the direct consequence of sensory receptor pathology, age-related deficits in speech perception are thought to be related to alteration in coding of sounds in both the peripheral and central auditory system. Central effects have been attributed most often when listeners are processing signals in difficult listening situations (Gordon-Salant and Fitzgibbons, 1995; Snell and Frisina, 2000). These findings have led clinicians to infer a central aging component, which has been substantiated by recent psychoacoustic investigations in which poor speech recognition performance in old listeners is degraded, as compared to young listeners matched for peripheral sensitivity (Fitzgibbons and Gordon-Salant, 1996; Fozard and Gordon-Salant, 2001; Frisina et al., 2001; Dubno et al., 2003). Similarly, impaired temporal acuity in elderly subjects with and without hearing loss has been reported using several different temporal resolution paradigms, such as gap detection or sinusoidal amplitude modulation (Glasberg et al., 1987; Moore and Glasberg, 1988; Moore et al., 1992; Schneider et al., 1994; Snell and Frisina, 2000). In aged listeners, including those with normal hearing, degraded temporal processing has been directly linked to speech identification difficulties (Fitzgibbons and Gordon-Salant, 1996; Snell, 1997; Frisina et al., 2001; Snell and Frisina, 2000). The relative importance of temporal cues is supported by the demonstration of good speech recognition in the absence of spectral cues (Rosen, 1992; Shannon et al., 1995) as well as by the capability of single channel cochlear implants to restore spoken language comprehension (Green et al., 2004).
Typically, two types of stimuli have been used to assess age-related changes in neural correlates of temporal acuity. Static temporal acuity can be measured using the gap detection task and dynamic temporal acuity by measuring the response to amplitude modulation. The gap detection paradigm is probably the most common method used to measure static temporal resolution in animal models (Salvi and Arehole, 1985; Klump and Gleich, 1991; Walton et al., 1997). It consists of placing a silent gap in between two carriers and models similar types of silent intervals found in the speech signal, for example in the perception of voice onset time. After a series of various gap durations are presented, the shortest detectable gap, or gap threshold, is obtained. Also, inherent in most species-specific vocalizations are intensity fluctuations in the temporal envelope, commonly referred to as amplitude modulation (AM). AM features in speech are known to provide important cues for discrimination of specific phonemic features, such as voicing or vowel perception. In addition, certain parameters of amplitude-modulated speech have been implicated in auditory scene analysis, or a listener’s ability to segregate one speaker from many (Grimault et al., 2002). Typically, a range of amplitude-modulated signals is presented using tone or noise carriers whose range of modulation frequencies extends over several octaves, e.g., from 10 to 1000 Hz.
The neural response to sinusoidal AM (SAM) is typically measured in three ways: (i) by measuring the magnitude of the driven rate; (ii) by measuring the degree of synchronization, or phase-locking, of the neural response to each cycle of the envelope and (iii) by quantifying response gain. Rate measures are simply a metric of the number of spikes elicited by the AM signal. Phase-locking can be described using the synchronization index or vector strength which can vary from 0.0 to 1.0, with a value of 1.0 indicating perfect synchrony. Response gain (in dB) is another measure of synchronization to AM, and is calculated by dividing the percent modulation in the response by the percent modulation in the stimulus (Frisina et al., 1990). These measures of AM encoding are used to derive two types of modulation transfer functions (MTFs), the rate MTF (rMTF) which plots driven rate as a function of modulation frequency and the temporal MTF (tMTF) which plots either vector strength or dB gain by modulation frequency. The MTF characterizes the temporal filter properties of the auditory neuron under study.
The neural encoding of AM signals in the auditory nerve and caudal brainstem suggest that the temporal envelope is represented in the phase-locked response of auditory neurons, rather than changes in driven rate (Frisina et al., 1990). However, as one ascends the auditory system the temporal code for AM becomes degraded and appears to be replaced by a rate code (Langner and Schreiner, 1988; Joris et al., 2004). Auditory midbrain neurons show a wide variety of temporal filter properties which can be represented as changes in response rate or phase-locking depending on the modulation frequency. Many neurons display tuning, or have a best modulation frequency (BMF) where a peak is present in both the rMTF and tMTF. These neurons are referred to as band-pass, where other units, showing no such preference for a given modulation frequency are referred to as all-pass. Band-pass type units show tuning for both narrow (tones) and broadband (broadband noise) carrier signals. It is also the case that a unit’s BMF for rate is also near the BMF for phase-locking (Langner and Schreiner, 1988; Schreiner and Langner, 1988; Krishna and Semple, 2000). Age-related changes in neural coding of AM in the auditory brainstem have been reported in only the cochlear nucleus and inferior colliculus (IC).
1.1. Age-related temporal processing deficits in the cochlear nucleus
The diversity in anatomical and connectional architecture of the cochlear nucleus (CN) is mirrored in the diversity of different types of response properties to simple sounds found in each of the sub-nuclei. Two stimulus coding pathways are postulated to have their origin in response properties of CN neurons. The “what” pathway is comprised of the anteroventral (AVCN) and posterior-ventral (PVCN) nuclei where neurons perform simple and complex analysis of sound features, such as frequency, intensity, duration, and envelope periodicity. While the “where” pathway originates in the AVCN and dorsal nuclei (DCN) (Frisina, 2001; Sutherland et al., 1998). Spherical and globular bushy cells of the AVCN are involved in processing localization cues in the horizontal plane, while the principle neurons, or fusiform cells of the DCN have been implicated in the processing of elevation cues important for sound localization (Davis, 2002; Davis et al., 2007). Furthermore, fusiform cells (FCs) show evidence of enhancement of SAM stimuli as compared to auditory nerve fibers by restricting the modulation frequencies they respond to. In effect FCs sharpen the temporal filter for coding AM (Joris and Smith, 1998). Unfortunately, there are few neurophysiological studies which have studied the neural correlates of age-related dysfunction in the CN from aged animal models.
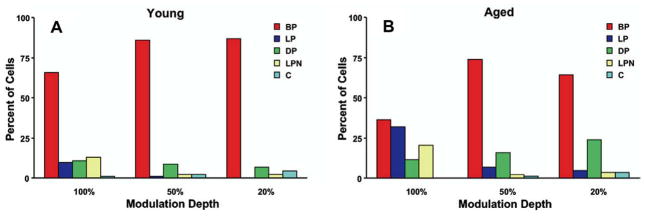
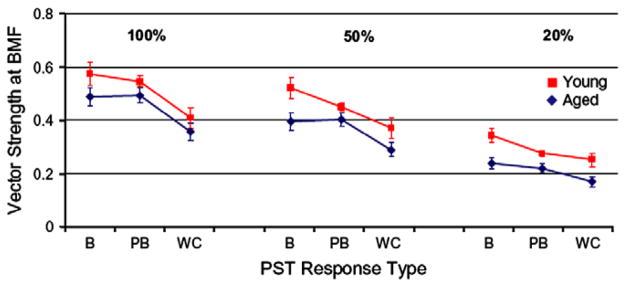
Recently, Caspary and colleagues successfully recorded responses to SAM tones from FCs in young adult and old Fisher Brown Norway rats (Schatteman et al., 2008). Neural coding of SAM tones using three modulation depths (20%, 50% and 100%) were presented 30 dB above the neuron’s best frequency (BF), and rate and synchronization MTFs were collected. The authors observed an age-related reduction in band-pass tMTF filter types from FCs recorded from old rats (Fig. 1). The most pronounced shift occurs for 100% AM where the proportion of units having bandpass filter shapes declined from approximately 60% in young rats to 30% in old rats. There was a concomitant increase in the number of low-pass and double-peaked filter types with increasing age. The authors also report a significant reduction in the strength of phase-locking with age at the BMF and this occurred for all three types of unit classifications (Fig. 2). Buildup units (B) tended to have the strongest phase-locking, followed by pauser-buildup (PB), then wideband chopper (WC), with 100% modulation depth SAM producing the greatest vector strength. Regardless of the modulation depth, FCs from old rats consistently showed poorer synchronization to SAM BF tones as compared to units from young rats. In contrast to the age-related alterations in tMTFs, filter properties for rate measures of AM coding remained stable age.
Fig. 1.
Distribution of synchronization filter properties of fusiform cells from young (A, N = 91) and old (B, N = 84) rats plotted as a function of the three modulation depths that were tested. Filter properties were classified from temporal MTFs into band-pass (BP), low-pass (LP), double-peaked (DP), low-pass notched (LPN) and complex (C). Note the significant loss of BP units in the aged rats, especially for the 100% modulation depth carrier, with a concomitant increase in low-pass units. (adapted with permission from Schatteman et al. (2008).)
Fig. 2.
Age-related decline in temporal coding of AM signals at all modulation depths plotted as a function of the unit’s PSTH type, where B = buildup, PB = pauser-buildup and WC = wideband chopper. The mean vector strength is shown for the three response types. Phase-locking declines for all unit types with decreases in modulation depth and is strongest for buildup units regardless of the depth of modulation. Responses from old rats show reduced phase-locking for all three unit types as compared to units from young rats, but the age effect is most pronounced for buildup units. (adapted with permission from Schatteman et al. (2008).)
The authors conclude that these results are consistent with an age-related change in the manner that excitatory and inhibitory circuits shape responses to AM. Evidence suggests that the inhibitory neurotransmitter glycine plays a crucial role in shaping responses to SAM tones presented at a units BF. When glycinergic receptors are blocked using strychnine, vector strength is reduced in FCs (Backoff et al., 1997). Thus, the present results suggest that an age-related alteration glycinergic neurotransmission may play a role in the etiology of age-related changes in response to SAM stimuli in the DCN (Backoff et al., 1999; Wang et al., 2009).
1.2. Neural correlates of age-related changes in gap encoding in the inferior colliculus
The inferior colliculus integrates auditory input from over 10 different brainstem nuclei and serves a gateway to the thalamus and cortex (Winer and Schreiner, 2005). IC neurons demonstrate a sophisticated level for processing of complex signals, including species-specific vocalizations (Aitkin et al., 1994), amplitude modulation (Langner and Schreiner, 1988; Rees and Sarbaz, 1997), frequency modulation (Pollak and Bodenhamer, 1981; Hage and Ehret, 2003), spatial localization (Semple et al., 1983; Aitkin et al., 1984, 1985), and gap detection (Walton et al., 1997). In addition, there is definitive evidence which points to an age-related decline in inhibitory neurotransmission in the IC of aged rodents (Milbrandt et al., 1994, 1996, 2000; Caspary et al., 1995; Helfert et al., 1999). For these reasons it has been postulated that age-related alterations in inhibitory neurotransmitters and synaptic neurochemistry could form the neural bases for the perceptual temporal processing deficits observed in aged listeners.
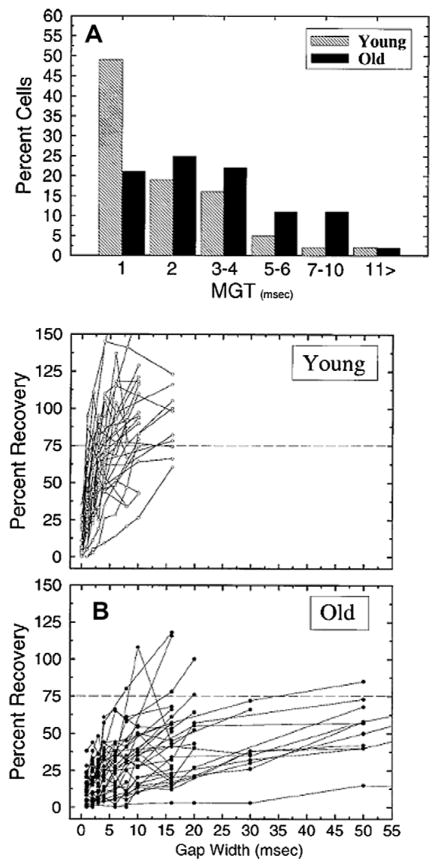
Similar to the transformation in coding of envelope periodicity, as one moves from caudal to rostral nuclei, neural mechanisms for coding silent gaps embedded in ongoing sound are also transformed. At the level of the auditory nerve the fundamental means of coding silent gaps is observed as a cessation of activity in response to the gap (Zhang et al., 1990). In a series of studies Walton and colleagues also characterized the temporal processing capabilities of IC neurons, using the gap-in-noise paradigm. Initial studies concentrated on the examination of neural correlates of the gap detection paradigm in young adult and old CBA mice. Walton et al. (1997) found two primary types of responses to gaps-in-noise, tonic units which are similar to auditory nerve fibers and phasic units which respond to the onsets of the two stimuli comprising the gap stimulus. Thus, phasic units encode the gap by discharging to the rapid onset in the second marker. Importantly, they also found that many IC neurons from young mice had minimal gap thresholds (MGTs) which were equivalent to that found behaviorally in mice using the identical stimuli (Walton et al., 1997).
In aged mice neural responses to silent gaps were altered in the following ways: (1) there was an age-related decline in the number of IC neurons that were able to encode very short gap durations, e.g., a greater proportion of neurons from old animals have elevated neural gap thresholds (Fig. 3, panel A) and (2) the rate of recovery in the nearly all phasic-type units from old mice was much slower when compared to recovery times from phasic units in young mice, panel B (Walton et al., 1998; Barsz et al., 2002). The importance of the latter may be especially significant, as recovery from prior stimulation is required for rapid processing of complex sounds, such as speech. As the speech signal is comprised of very short elements which vary in amplitude, forward masking by preceding phonemic elements is a potential problem, if recovery is not rapid. Phasic units, which are ideally suited to indicate the initiation of a signal, typically recover within 10 ms in young mice, but require more than 25–50 ms in units from old mice (Fig. 3, panel B). As the intensity of the first and second noise bursts which mark the gap were equal in these studies, one would predict that if the second marker of the gap was reduced in intensity, as occurs in many phonemes, deficits in recovery would be even more pronounced in units from aged mice.
Fig. 3.
Age-related alteration in encoding silent gaps embedded in noise carriers. Stimuli were two noise bursts presented at 65 dB SPL, of 100 ms and 50 ms duration with a silent gap varying from 1 to 96 ms placed after the first burst. (A) The distribution of minimum gap thresholds (MGTs) from units from young and old CBA mice. There is a significant shift to longer gap thresholds in units recorded from old mice. (B) The percent recovery in driven rate to the second noise burst which delineates the end of the silent gap from young (top panel) and old (bottom panel) mice. A value of 100% indicates that the driven activity to noise burst 1 equaled that to noise burst 2. The horizontal dotted line denotes the 75% recovery point. (adapted with permission from Walton et al. (1998).)
These results were replicated for narrow band stimuli by Finlayson (2002) who measured the duration of suppression of IC units to paired-tone stimuli presented at the BF in young adult and aged Fisher rats (Finlayson, 2002). Finlayson reported that units from aged rats had significantly longer recovery times, as compared to young rats and that this effect depended on a units BF, but not on threshold. Low frequency (<10 kHz) units are shown in the left panel of Fig. 4 where the duration of suppression is plotted as a function of the units threshold and the same is done for high frequency (>10 kHz) units in the right panel. The solid (old units) and dashed (young units) best fit regression show a shift of over 200 ms in post-stimulus recovery time for units from old rats. Recently, Wang and colleagues reported behavioral temporal processing deficits in aged Fisher rats, where gap thresholds in aged rats were also found to be significantly elevated (Wang et al., 2009).
Fig. 4.

Effects of age on gap encoding to pure tones presented at the best frequency of each unit (figure adapted from Finlayson (2002)). Duration of suppression is plotted as a function of each units thresholds, with units with BFs < 10 kHz on the left and those with BFs > 10 kHz on the right. Units from young mice are denoted by the filled symbols (circles and diamonds) and those from old rats by the open symbols (triangles). Threshold does not correlate with the duration of suppression, but for units with BFs < 10 kHz there is a strong effect of age, with the duration of suppression from units of old rats nearly 400 ms longer (dotted versus solid line) than the young data.. (adapted with permission from Finlayson (2002).)
1.3. Neural correlates of age-related changes in amplitude and frequency modulation encoding in the inferior colliculus
Dynamic encoding of amplitude fluctuations has been extensively studied in both cat and rodent auditory midbrain. In several species, investigators have found most IC units had band-pass MTFs with BMFs of less than 120 Hz (Rees and Möller, 1983, 1987; Frisina et al., 1990; Krishna and Semple, 2000). Phase-locking to AM increased monotonically with increases in modulation depth, typically saturating before modulation depths reached 100%. Modulation thresholds, as small as 2%, were encountered and in many IC units BMF remained constant with large changes in modulation depth. The maximum SAM gain (response modulation divided by stimulus modulation) observed in IC was about 20–30 dB, roughly equivalent to the findings in rat and cat CN (Langner and Schreiner, 1988) and gerbil CN (Frisina et al., 1990). The ability of IC neurons to encode temporal variations in SAM stimuli was found to be intermediate between that of the auditory nerve and cortex, with BMF ranging between 3 and 1000 Hz, with the majority of cells having BMFs of less than 400 Hz (Rees and Möller, 1983).
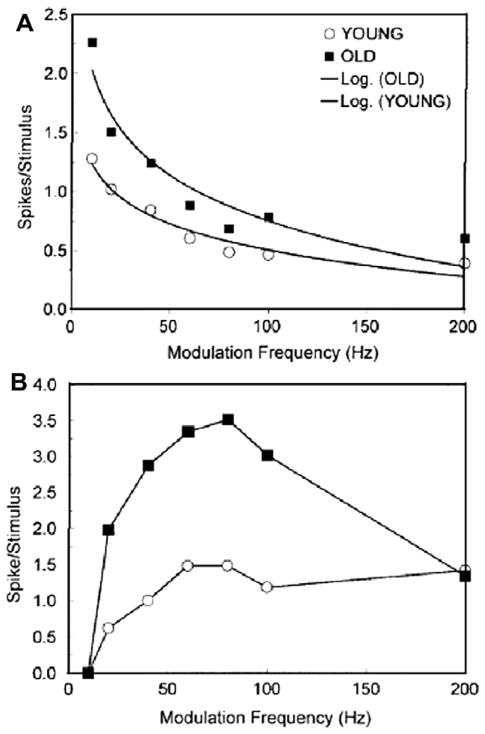
Age-related changes in both dynamic and static temporal processing have been reported to occur in responses from auditory midbrain neurons from old animals. Shaddock Palombi et al. (2001) examined modulation encoding to BF tones, using a fixed 30 dB intensity above threshold, in the IC of young adult and old rats and reported that temporal coding to AM tones, as measured by tMTFs, changed with age (Shaddock Palombi et al., 2001). Temporal MTFs obtained from aged rats displayed a greater number of low-pass type tMTFs as compared to units from younger rats, which showed a greater number of band-pass units (Fig. 5). However, the proportions of different types of rate-based MTFs were comparable across age. Walton et al. (2002), found that when wideband noise carriers were used, rate measures of periodicity coding by IC neurons were significantly affected by age (Walton et al., 2002). First, the overall rate coding of SAM noise at the best modulation frequency (BMF) in units from old mice was significantly greater, as compared to units from young mice, as shown in Fig. 6. The authors measured changes in the transient response, defined as the magnitude of the response (driven activity) to the first cycle of modulation (Fig. 6A), and periodic activity which was measured during the remaining cycles of modulation (Fig. 6B). Median spikes/stimulus are plotted as a function of modulation frequency. The transient response for units from old mice were significantly greater for modulation frequencies ranging from 20 to 100 Hz, with driven activity decreasing as modulation frequency increases for units from both young and old mice. The median driven activity for units from old mice to the periodic portion of the AM stimulus was two times greater than the response from units from young mice.
Fig. 5.
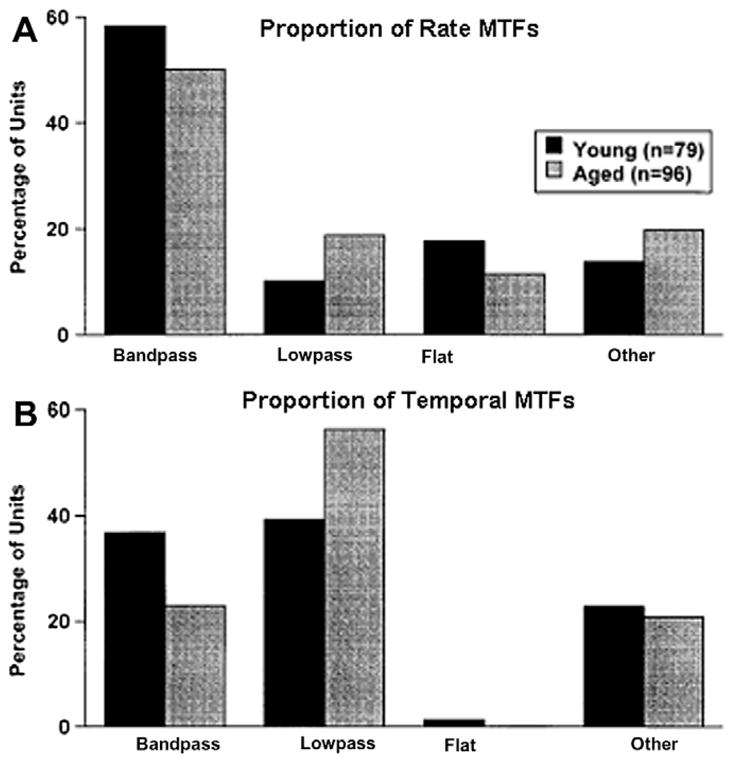
Age-related changes in SAM coding to best frequency tones in auditory midbrain neurons of young and old rats. Signals were 100% SAM tones centered at the units BF. (A) The proportion of units in the different classes of rate MTFs. Neurons from both young and old rats had similar percentages of units. (B) The same comparison for temporal MTFs. Note that neurons from young rats had a higher proportion of band-pass type tMTFs as compared to old rats. As the proportion of flat and other types of tMTFs were nearly identical the data suggests that neurons from aged rats are changing filter shapes, becoming more low-pass than band-pass. (adapted with permission from Shaddock Palombi et al. (2001).)
Fig. 6.
Age-related changes in SAM coding to broadband noise of auditory midbrain neurons in young and old CBA mice. Signals were 100% SAM wideband noise presented at 65 dB SPL. (A) The effect of MF on the transient response, or first cycle of the SAM noise, on driven activity measured in spikes/stimulus (median data plotted). Data were fit with a negative log function. Data sets from both young and old mice show a systematic decrease in median driven response to the onset of the first cycle of modulation as modulation frequency increases. (B) The same comparison for the median periodic response, or the driven response to the remaining cycles of modulation. Note that neurons from young mice tended to display a stable driven response with increases in modulation frequency. In contrast, units from old mice show a sharp increase in driven rate which then declines above 100 Hz MF. (adapted with permission from Walton et al. (2002).)
These results suggest a loss of gain control to wideband amplitude-modulated signals in the IC of old mice. If the overall gain of a system’s output is an important factor in determining, or discriminating, between different modulation frequencies, then an increase in rate might disrupt the coding of envelope periodicities. This may have a secondary influence on coding of higher modulation frequencies as they also found that the upper cutoff frequency for AM rate encoding was nearly half of that found for IC neurons from young adult mice. Although the median values were similar, the 75th percentile was 200 Hz for units from young mice and 80 Hz for units from old mice. Overall, these results could also be due to a combination of a loss of “on” CF inhibition together with alteration in side-band inhibition. The latter was recently reported to occur in middle and old aged CBA mice (Leong et al., 2009). Their results showed that IC units from middle and old mice exhibited broader excitatory receptive fields when compared to units from young mice. A plausible explanation of these results is that there is a reduction in side-band inhibition at moderate to loud sound levels, where the wideband noise AM carriers were found to generate increased excitability in units from old mice.
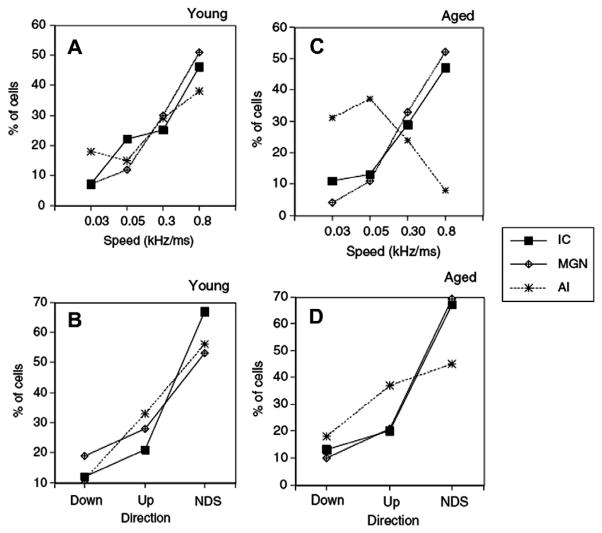
Species-specific vocalizations contain various forms of frequency modulation (FM), such as formant transitions which occur in speech, the constant frequency modulated component in bat echolocation calls, and mother–pup ultrasonic calls in rodents. Two fundamental components of sinusoidal FM are the speed in which frequency is changing over a given bandwidth and the direction of modulation, either upward or downward. Deficits in processing simple frequency modulations have been associated with poor speech recognition (Buss et al., 2004) and age-related changes in neural correlates of FM have been reported in extracellular responses from aged rats. In a series of studies, Mendelson and colleagues investigated the effects of aging on neural coding of linear upward and downward FM sweeps (0.15–45 kHz) in the IC, medial geniculate body (MGB) and primary auditory cortex (AI). They found that units from young rats, in all three areas, preferred fast FM (Fig. 7A and C) and that the proportion of IC and MGB units was similar in old rats (Lee et al., 2002; Lui and Mendelson, 2003; Mendelson and Lui, 2004). However, the proportion of cortical units which preferred fast FM dropped to less than 10% as compared to nearly 40% in young rats. The majority of units from both young and old rats did not show directional preference (NDS) to linear sweeps and this was observed for all threes structures (Fig. 7B and D). These data indicate that linear FM processing is largely unaffected by age in the auditory midbrain and thalamus. The age-related decline in the proportion of cortical units which can respond to fast FM sweeps mirrors the deficit found in gap detection, in that coding faster FM and shorter gaps is impaired in the aged animal.
Fig. 7.
Distribution of FM selectivity for preferred speed (top row) and direction-selectivity (bottom row) from units in three auditory locations: inferior colliculus (IC), auditory thalamus (MGN), and auditory cortex (AI), for units from young (A and B) and aged (C and D) rats. Speed of the FM shown in (A) and (C) ranged from 0.03 to 0.8 kHz/ms. Note that in units from young rats most units preferred faster speeds. In old rats, there was a significant difference in preferred speed between AI and the IC and MGN, where the majority of cells recorded from AI preferred slow speeds while cells from IC and MGN preferred fast sweeps. Similarly, most units from both young and old rats were not sensitive to the direction of FM sweeps (B and C, NDS). (adapted with permission from Mendelson and Lui (2004).)
2. Conclusions
Age-related hearing impairments affect over one-half of the adults over 75 years of age and can consist of both a loss in peripheral function and an alteration in central auditory processing. As reviewed in this summary report, there is a growing body of evidence which indicates that temporal encoding of sounds is altered in the aged auditory brainstem. The caudal auditory brainstem has several types of cells which appear to be specialized for coding rapid temporal events. For example, octopus cells in the CN can precisely encode onset to sound in the microsecond range by virtue of their intrinsic membrane properties (Oertel et al., 2000). Octopus cells appear to be specialized to detect both the end of gaps-in-noise and noise increments. That there are such onset-sensitive cells in the inferior colliculus and in auditory cortex indicates that this early stage coding of stimulus onsets is preserved in cortical processing and forms a central component of the neural bases of temporal resolution (Walton et al., 1997; Eggermont, 1999). Age-related alteration in the manner in which these specialized neurons function to preserve timing information may contribute to the perceptual deficits observed in the aged listener. Complete understanding of the neural bases of temporal coding deficits will assist in the diagnosis and rehabilitation, and most importantly be crucial to further advances in the design of hearing aids or other therapeutic intervention for older listeners.
Acknowledgments
Supported by NIH-NIA Grant P01 AG009524.
Abbreviations
- AM
amplitude modulation
- AVCN
anterior-ventral cochlear nucleus
- B
buildup
- BMF
best modulation frequency
- BF
best frequency
- CN
cochlear nucleus
- DCN
dorsal cochlear nucleus
- FC
fusiform cell
- FM
frequency modulation
- IC
inferior colliculus
- MGB
medial geniculate body
- MGT
minimal gap threshold
- MTF
modulation transfer function
- PB
pauser-buildup
- PVCN
posterior-ventral cochlear nucleus
- rMTF
rate modulation transfer function
- SAM
sinusoidal amplitude modulation
- tMTF
temporal modulation transfer function
- WC
wideband chopper
References
- Aitkin LM, Gates GR, et al. Responses of neurons in inferior colliculus to variations in sound-source azimuth. J Neurophysiol. 1984;52 (1):1–17. doi: 10.1152/jn.1984.52.1.1. [DOI] [PubMed] [Google Scholar]
- Aitkin LM, Pettigrew JD, et al. Representation of stimulus azimuth by low-frequency neurons in inferior colliculus of the cat. J Neurophysiol. 1985;53 (1):43–59. doi: 10.1152/jn.1985.53.1.43. [DOI] [PubMed] [Google Scholar]
- Aitkin L, Tran L, et al. The responses of neurons in subdivisions of the inferior colliculus of cats to tonal, noise and vocal stimuli. Exp Brain Res. 1994;98 (1):53–64. doi: 10.1007/BF00229109. [DOI] [PubMed] [Google Scholar]
- Backoff PM, Shadduck Palombi P, et al. Glycinergic and GABAergic inputs affect short-term suppression in the cochlear nucleus. Hear Res. 1997;110 (1–2):155–163. doi: 10.1016/s0378-5955(97)00081-6. [DOI] [PubMed] [Google Scholar]
- Backoff PM, Shadduck Palombi P, et al. Gamma-aminobutyric acidergic and glycinergic inputs shape coding of amplitude modulation in the chinchilla cochlear nucleus. Hear Res. 1999;134 (1–2):77–88. doi: 10.1016/s0378-5955(99)00071-4. [DOI] [PubMed] [Google Scholar]
- Barsz K, Ison JR, et al. Behavioral and neural measures of auditory temporal acuity in aging humans and mice. Neurobiol Aging. 2002;23 (4):565–578. doi: 10.1016/s0197-4580(02)00008-8. [DOI] [PubMed] [Google Scholar]
- Buss E, Hall Iii JW, et al. Temporal fine-structure cues to speech and pure tone modulation in observers with sensorineural hearing loss. Ear Hear. 2004;25 (3):242. doi: 10.1097/01.aud.0000130796.73809.09. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Milbrandt JC, et al. Central auditory aging: GABA changes in the inferior colliculus. Exp Gerontol. 1995;30 (3–4):349–360. doi: 10.1016/0531-5565(94)00052-5. [DOI] [PubMed] [Google Scholar]
- Davis KA. Evidence of a functionally segregated pathway from dorsal cochlear nucleus to inferior colliculus. J Neurophysiol. 2002;87 (4):1824–1835. doi: 10.1152/jn.00769.2001. [DOI] [PubMed] [Google Scholar]
- Davis KA, Lomakin O, et al. Response properties of single units in the dorsal nucleus of the lateral lemniscus of decerebrate cats. J Neurophysiol. 2007;98 (3):1475. doi: 10.1152/jn.00451.2007. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Horwitz AR, et al. Recovery from prior stimulation: masking of speech by interrupted noise for younger and older adults with normal hearing. J Acoust Soc Am. 2003;113:2084. doi: 10.1121/1.1555611. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Neural correlates of gap detection in three auditory cortical fields in the Cat. J Neurophysiol. 1999;81 (5):2570–2581. doi: 10.1152/jn.1999.81.5.2570. [DOI] [PubMed] [Google Scholar]
- Finlayson PG. Paired-tone stimuli reveal reductions and alterations in temporal processing in inferior colliculus neurons of aged animals. J Assoc Res Otolaryngol. 2002;3 (3):321–331. doi: 10.1007/s101620020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbons PJ, Gordon-Salant S. Auditory temporal processing in elderly listeners. J Am Acad Audiol. 1996;7 (3):183. [PubMed] [Google Scholar]
- Fozard JL, Gordon-Salant S. Changes in vision and hearing with aging. Handb Psychol Aging. 2001;241:266. [Google Scholar]
- Frisina RD. Subcortical neural coding mechanisms for auditory temporal processing. Hear Res. 2001;158 (1–2):1–27. doi: 10.1016/s0378-5955(01)00296-9. [DOI] [PubMed] [Google Scholar]
- Frisina RD, Smith RL, et al. Encoding of amplitude modulation in the gerbil cochlear nucleus: I. A hierarchy of enhancement. Hear Res. 1990;44 (2–3):99. doi: 10.1016/0378-5955(90)90074-y. [DOI] [PubMed] [Google Scholar]
- Frisina DR, Frisina RD, et al. Auditory temporal processing during aging. Funct Neurobiol Aging. 2001;565:579. [Google Scholar]
- Glasberg BR, Moore BC, et al. Gap detection and masking in hearing-impaired and normal-hearing subjects. J Acoust Soc Am. 1987;81 (5):1546–1556. doi: 10.1121/1.394507. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ. Temporal factors and speech recognition performance in young and elderly listeners. J Speech Hear Res. 1993;36 (6):1276–1285. doi: 10.1044/jshr.3606.1276. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ. Recognition of multiply degraded speech by young and elderly listeners. J Speech Hear Res. 1995;38 (5):1150–1156. doi: 10.1044/jshr.3805.1150. [DOI] [PubMed] [Google Scholar]
- Green T, Faulkner A, et al. Enhancing temporal cues to voice pitch in continuous interleaved sampling cochlear implants. J Acoust Soc Am. 2004;116:2298. doi: 10.1121/1.1785611. [DOI] [PubMed] [Google Scholar]
- Grimault N, Bacan S, Micheyl C. Auditory stream segregation on the basis of amplitude-modulation rate. J Acoust Soc Am. 2002;111:1340–1348. doi: 10.1121/1.1452740. [DOI] [PubMed] [Google Scholar]
- Hage SR, Ehret G. Mapping responses to frequency sweeps and tones in the inferior colliculus of house mice. Eur J Neurosci. 2003;18 (8):2301. doi: 10.1046/j.1460-9568.2003.02945.x. [DOI] [PubMed] [Google Scholar]
- Helfert RH, Sommer TJ, et al. Age-related synaptic changes in the central nucleus of the inferior colliculus of Fischer-344 rats. J Comp Neurol. 1999;406:3. [PubMed] [Google Scholar]
- Joris PX, Smith PH. Temporal and binaural properties in dorsal cochlear nucleus and its output tract. J Neurosci. 1998;18 (23):10157–10170. doi: 10.1523/JNEUROSCI.18-23-10157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris PX, Schreiner CE, et al. Neural processing of amplitude-modulated sounds. Physiol Rev. 2004;84 (2):541–577. doi: 10.1152/physrev.00029.2003. [DOI] [PubMed] [Google Scholar]
- Klump G, Gleich O. Gap detection in the European starling (Sturnus vulgaris): III. Processing in the peripheral auditory system. J Comp Physiol. 1991;168:469–475. [Google Scholar]
- Krishna BS, Semple MN. Auditory temporal processing: responses to sinusoidally amplitude-modulated tones in the inferior colliculus. J Neurophysiol. 2000;84 (1):255–273. doi: 10.1152/jn.2000.84.1.255. [DOI] [PubMed] [Google Scholar]
- Langner G, Schreiner CE. Periodicity coding in the inferior colliculus of the cat: I. Neuronal mechanisms. J Neurophysiol. 1988;60 (6):1799–1822. doi: 10.1152/jn.1988.60.6.1799. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Wallani T, et al. Temporal processing speed in the inferior colliculus of young and aged rats. Hear Res. 2002;174 (1–2):64–74. doi: 10.1016/s0378-5955(02)00639-1. [DOI] [PubMed] [Google Scholar]
- Leong UC, Barsz K, et al. Neural correlates of age-related declines in frequency selectivity in the auditory midbrain. Neurobiol Aging. 2009 doi: 10.1016/j.neurobioaging.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Lui B, Mendelson JR. Frequency modulated sweep responses in the medial geniculate nucleus. Exp Brain Res. 2003;153 (4):550–553. doi: 10.1007/s00221-003-1618-y. [DOI] [PubMed] [Google Scholar]
- Mendelson JR, Lui B. The effects of aging in the medial geniculate nucleus: a comparison with the inferior colliculus and auditory cortex. Hear Res. 2004;191 (1–2):21–33. doi: 10.1016/j.heares.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Albin RL, et al. Age-related decrease in GABAB receptor binding in the Fischer 344 rat inferior colliculus. Neurobiol Aging. 1994;15 (6):699–703. doi: 10.1016/0197-4580(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Albin RL, et al. GABAA receptor binding in the aging rat inferior colliculus. Neuroscience. 1996;73 (2):449–458. doi: 10.1016/0306-4522(96)00050-4. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Holder TM, et al. GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear Res. 2000;147 (1–2):251–260. doi: 10.1016/s0378-5955(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Moore BC, Glasberg BR. Gap detection with sinusoids and noise in normal, impaired, and electrically stimulated ears. J Acoust Soc Am. 1988;83 (3):1093–1101. doi: 10.1121/1.396054. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Peters RW, et al. Detection of temporal gaps in sinusoids by elderly subjects with and without hearing loss. J Acoust Soc Am. 1992;92:1923. doi: 10.1121/1.405240. [DOI] [PubMed] [Google Scholar]
- Oertel D, Bal R, et al. Detection of synchrony in the activity of auditory nerve fibers by octopus cells of the mammalian cochlear nucleus. Natl Acad Sci. 2000;97:11773–11779. doi: 10.1073/pnas.97.22.11773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak GD, Bodenhamer RD. Specialized characteristics of single units in inferior colliculus of mustache bat: frequency representation, tuning, and discharge patterns. J Neurophysiol. 1981;46 (3):605–620. doi: 10.1152/jn.1981.46.3.605. [DOI] [PubMed] [Google Scholar]
- Rees A, Möller AR. Responses of neurons in the inferior colliculus of the rat to AM and FM tones. Hear Res. 1983;10:301–330. doi: 10.1016/0378-5955(83)90095-3. [DOI] [PubMed] [Google Scholar]
- Rees A, Möller AR. Stimulus properties influencing the responses of inferior colliculus neurons to amplitude-modulated sounds. Hear Res. 1987;27:129–143. doi: 10.1016/0378-5955(87)90014-1. [DOI] [PubMed] [Google Scholar]
- Rees A, Sarbaz A. The influence of intrinsic oscillations on the encoding of amplitude modulation by neurons in the inferior colliculus. Plenum Publishing Corporation; 1997. [Google Scholar]
- Rosen S. Temporal information in speech: acoustic, auditory and linguistic aspects. Philos Trans: Biol Sci. 1992;336:367–373. doi: 10.1098/rstb.1992.0070. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Arehole S. Gap detection in chinchillas with temporary high-frequency hearing loss. J Acoust Soc Am. 1985;77:1173–1177. doi: 10.1121/1.392181. [DOI] [PubMed] [Google Scholar]
- Schatteman TA, Hughes LF, et al. Aged-related loss of temporal processing: altered responses to amplitude modulated tones in rat dorsal cochlear nucleus. Neuroscience. 2008;154 (1):329–337. doi: 10.1016/j.neuroscience.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BA, Pichora-Fuller MK, et al. Gap detection and the precedence effect in young and old adults. J Acoust Soc Am. 1994;95 (2):980–991. doi: 10.1121/1.408403. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Langner G. Periodicity coding in the inferior colliculus of the cat: II. Topographical organization. J Neurophysiol. 1988;60 (6):1823–1840. doi: 10.1152/jn.1988.60.6.1823. [DOI] [PubMed] [Google Scholar]
- Semple MN, Aitkin LM, et al. Spatial receptive fields in the cat inferior colliculus. Hear Res. 1983;10 (2):203. doi: 10.1016/0378-5955(83)90054-0. [DOI] [PubMed] [Google Scholar]
- Shaddock Palombi P, Backoff PM, et al. Responses of young and aged rat inferior colliculus neurons to sinusoidally amplitude modulated stimuli. Hear Res. 2001;153 (1–2):174–180. doi: 10.1016/s0378-5955(00)00264-1. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, et al. Speech recognition with primarily temporal cues. Science. 1995;270 (5234):303. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Snell KB. Age-related changes in temporal gap detection. J Acoust Soc Am. 1997;107 (3):1615–1626. doi: 10.1121/1.418205. [DOI] [PubMed] [Google Scholar]
- Snell KB, Frisina DR. Relationships among age-related differences in gap detection and word recognition. J Acoust Soc Am. 2000;107 (3):1615–1626. doi: 10.1121/1.428446. [DOI] [PubMed] [Google Scholar]
- Sutherland DP, Glendenning KK, Masterton RB. Role of acoustic striae in hearing: discrimination of sound-source elevation. Hear Res. 1998;120:86–108. doi: 10.1016/s0378-5955(98)00056-2. [DOI] [PubMed] [Google Scholar]
- Trussell LO. Modulation of transmitter release at giant synapses of the auditory system. Curr Opin Neurobiol. 2002;12 (4):400–404. doi: 10.1016/s0959-4388(02)00335-5. [DOI] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, et al. Neural correlates of behavioral gap detection in the inferior colliculus of the young CBA mouse. J Comp Physiol A. 1997;181 (2):161–176. doi: 10.1007/s003590050103. [DOI] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, et al. Age-related alteration in processing of temporal sound features in the auditory midbrain of the CBA mouse. J Neurosci. 1998;18 (7):2764–2776. doi: 10.1523/JNEUROSCI.18-07-02764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP, Simon H, et al. Age-related alterations in the neural coding of envelope periodicities. J Neurophysiol. 2002;88 (2):565–578. doi: 10.1152/jn.2002.88.2.565. [DOI] [PubMed] [Google Scholar]
- Wang H, Turner JG, et al. Age-related changes in glycine receptor subunit composition and binding in dorsal cochlear nucleus. Neuroscience. 2009;160 (1):227–239. doi: 10.1016/j.neuroscience.2009.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Schreiner C. The Inferior Colliculus. Springer; 2005. [Google Scholar]
- Zhang W, Salvi RJ, et al. Neural correlates of gap detection in auditory nerve fibers of the chinchilla. Hear Res. 1990;46 (3):181–200. doi: 10.1016/0378-5955(90)90001-6. [DOI] [PubMed] [Google Scholar]