Abstract
For better understanding of brain functions, optogenetic neural modulation has been widely employed in neural science research. For deep tissue in vivo applications, large-scale two-photon based near simultaneous 3D laser excitation is needed. Although 3D holographic laser excitation is nowadays common practice, the inherent short coherence length of the commonly used femtosecond pulses fundamentally restricts the achievable field-of-view. Here we report a technique for near simultaneous large-scale femtosecond holographic 3D excitation. Specifically, we achieved two-photon excitation over 1.3 mm field-of-view within 1.3 milliseconds, which is sufficiently fast even for spike timing recording. The method is scalable and compatible with the commonly used two-photon sources and imaging systems in neuroscience research.
1. Introduction
Among the over 400 neurological disorders, the majority have no cure, largely due to the very limited understanding of brain function [1,2]. With the large number of interlinked neurons and the supporting glial cells, we need precise tools for quantitatively investigating cell functions [3–5]. Thanks to genetic function indicators and modulators [6–8], we can now image and modulate neurons with cellular specificity and sub-cellular spatial resolution. In particular, optogenetics have emerged over the past decades as a widely employed tool to excite or suppress neurons [9–22].
For deep tissue spatially confined neural modulation, two-photon excitation has been employed [23–26]. The near infrared excitation inherently has longer scattering path length and offers greater penetration depth. Moreover, the nonlinear excitation can effectively achieve 3D confined excitation volume [20]. To cover multiple cells of interest, laser scanning and holography have been applied [14,19,20,22,27–34]. Holographic excitation allows truly simultaneous excitation [35,36]. However, as the laser pulse energy is split between multiple spatial foci, holographic excitation in general benefits from laser sources of greater pulse energy. Laser scanning has the advantage of great power efficiency for nonlinear excitation. However, for millisecond (ms) scale neural modulation, the number of spots that can be visited by scanning is very limited. For example, fast galvo scanner can typically offer 125 µs small step response time. Thus, one can only visit ∼8 closely spaced targets within 1 ms. In comparison, holography allows the simultaneous access of one to two orders magnitude more targets limited by the pulse energy. However, the achievable field-of-view (FOV) of femtosecond holography is very limited, which is typically less than 200 µm in the majority of applications. As most of the brain functions involve neurons across multiple function areas and millimeter scale imaging [37–39] is nowadays routine practice, the FOV of femtosecond holography needs to be greatly increased.
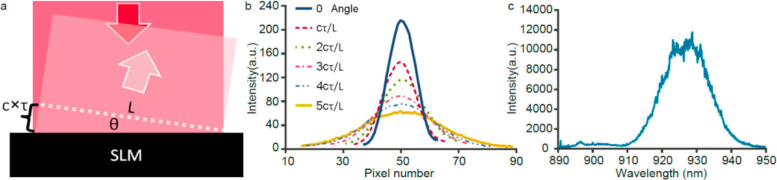
The FOV of femtosecond holography is often limited by the inherent short coherence length of the laser pulse (Fig. 1) rather than limited by the pixel numbers on the spatial light modulator (SLM). Currently available SLM (e.g. GAEA-2 from Holoeye and JD7714 from Jasper) can provide ∼4000 pixels per line. At the limit of using 4 pixels for one λ, the SLM can support 1000 λ in wavefront tilting. The transverse FOV at the focal plane of the objective lens is 1000 λ/NA, where NA is the numerical aperture of the excitation. With a NA of 0.5 and λ of 0.93 µm, the FOV can reach 1.84 mm, which is larger than the FOV of most two-photon microscopes. However, the coherence length of 100-200 femtosecond pulses of the commonly used femtosecond oscillator is typically 30-60 µm, which is only 32-64 λ. At greater wavefront tilting, the femtosecond pulse front tilt occurs. Another way to look at this problem is from the spectral domain. The femtosecond pulse spectrum is broad and the SLM is like a grating. A higher grating spatial frequency (steeper tilting) will cause more severe angular dispersion (pulse front tilt or spatial chirp). With the maximum tilting limited by the coherence length c×τ, where c is the speed of light and τ is the pulse duration, the FOV will be reduced to c×τ/NA (e.g. 120 µm for NA 0.5 and 200 fs pulse duration). To illustrate this effect, we focused the output beam of the SLM with a telecentric lens onto a camera and quantified the focus profile as a function of beam tilting angle (Fig. 1b, 1c). As expected, the focus was stretched along the diffraction direction and the focus peak intensity dropped accordingly.
Fig. 1.
(a) Coherence length fundamentally limits the achievable FOV of femtosecond holography. (b) Experimentally measured focus profile as a function of wavefront tilting angle. : Laser coherence length; L: SLM width. (c) The spectrum of the femtosecond laser (Mira-HP, Coherent) employed for this work.
Recently, time-division multiplexing has been demonstrated for large FOV femtosecond optogenetic neural modulation [40]. The essence is to utilize high speed SLM to update the hologram and utilize galvo to deliver the different holograms to different spatial locations. Limited by the update speed of SLM, the switching time reached 7.5 ms. Tens of ms will be needed to cover multiple regions. Towards millisecond scale near simultaneous 3D excitation, we need to greatly increase the wavefront switching speed.
2. Methods
Here, we report a new method for millisecond scale near simultaneous large FOV femtosecond holography. The key point is that there are actually sufficient pixels on modern SLM to simultaneously display multiple wavefront. For 120 µm FOV (NA 0.5, λ 0.93 µm), we will need only ∼300 pixels. Thus the current 4k SLM can simultaneously display wavefront for ∼90 regions. All we need is to find a way to quickly read out the wavefront and deliver them to different locations.
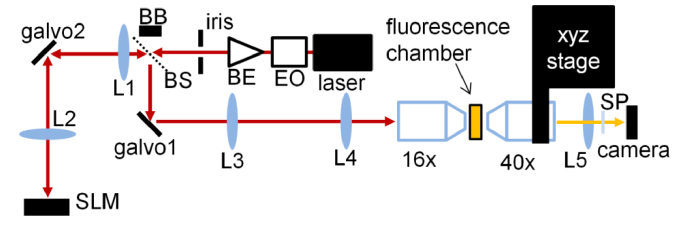
Galvo scanner can offer sub millisecond switching time. Therefore, we can utilize galvo for both wavefront switching and position switching. To evaluate the maximum speed and FOV, we implemented the large FOV femtosecond holography system (Fig. 2). Specifically, we utilized an 8 MHz EO modulator for precise timing control of the laser illumination. We used a beam expander to increase the beam size to fill the 4.9 mm aperture iris and controlled its divergence. The expanded beam was relayed by a pair of telecentric scan lenses (f = 200 mm and f = 120 mm) onto a 1920×1152 pixel SLM, such that the aperture occupied 320 pixels (1/6 of the 1920 pixels) across its diameter. We displayed 6 holograms side by side on the SLM. The laser beam position on the SLM was controlled by a galvo which is positioned at the common focal plane of the two relay lenses (L1 and L2 in Fig. 2). To avoid potential damage, we utilized the beam expander to introduce slight divergence such that the laser focus was shifted away by 4 mm from the lens focal plane. The beam reflected from the SLM was directed by a femtosecond low group delay dispersion beam splitter onto another galvo which formed mirror image with respect to the input iris. The galvo was subsequently imaged by telecentric relay lenses (f = 150 mm and f = 429 mm) onto the back focal plane of a Nikon 16x NA 0.8 water dipping objective lens. The laser beam aperture on the pupil was 14 mm, resulting in 0.56 in excitation NA. To visualize the 3D two-photon excitation profile, we filled a glass chamber with fluorescence dye solution (Qtracker 625, Invitrogen). The light path within the liquid was 200 µm. The two-photon excited fluorescence was recorded by a wide field fluorescence microscope equipped with a Nikon 40x NA 0.8 water dipping objective lens whose position was controlled by a 3-axis motorized linear stage.
Fig. 2.
Optical design for near simultaneous large FOV femtosecond holography. L1-4, telecentric relay lenses; L5, Nikon tube lens; SP, shortpass filter; EO, 8MHz electro-optic modulator; BE, beam expander; BB, beam block; BS, femtosecond beam splitter; 16x, Nikon 16x NA 0.8 objective for two-photon excitation; 40x, Nikon 40x NA 0.8 objective for detection.
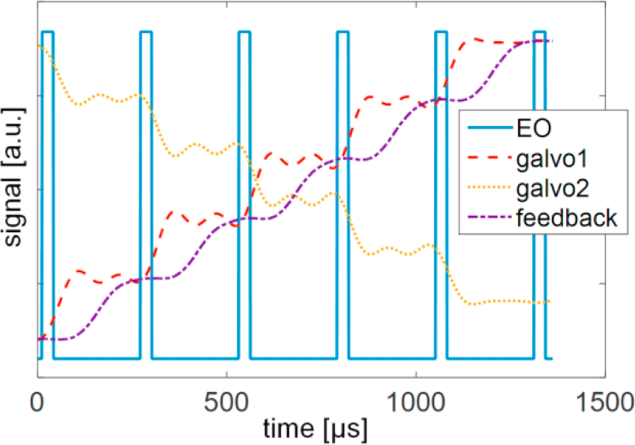
A key requirement for high-speed operation is the precise timing control of the galvo movement and the laser illumination. Although the small step response of galvo (6215 H, Cambridge technology) can reach 125 µs, the step size for large FOV holography can potentially reach ∼20% of the full travel range. Directly driving galvo with such fast stepwise signal can trigger the high current limit of the galvo, resulting in inconsistent unsustainable motion. Through experimental test, we discovered that applying low pass filter to the driving signal can avoid triggering high current limit. Experimentally, we applied 4 kHz low-pass filter to the driving signal of both galvo mirrors and achieved consistent operation (Fig. 3). With the low-pass filter, the driving signal deviated from the desired stepwise signal. However, the resulting galvo motion (see the galvo feedback signal in Fig. 3) approached the desired stepwise motion. We controlled the laser exposure using the 8 MHz EO modulator such that the two-photon excitation occurred when the galvo positon was near stationary. The illumination duty cycle was 12%. With such a configuration, we can apply 6 wavefront to 6 locations within 1.3 ms covering 1.3 mm distance, ∼30 times faster than relying on SLM for updating wavefront.
Fig. 3.
The timing control of EO modulator and galvo and the recorded galvo feedback signal.
3. Results
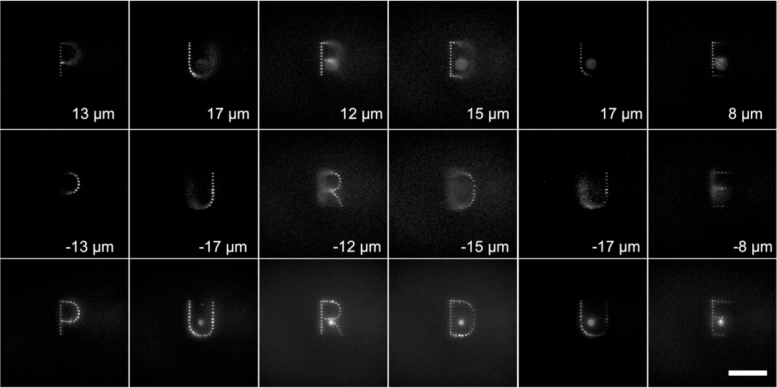
We utilized the near simultaneous large FOV femtosecond holography system to implement both 2D and 3D holographic two-photon excitation. Specifically, we display 6 letters formed by two-photon excitation distributed across 1.3 mm FOV. The holograms were computed based on 2D and 3D Gerchberg–Saxton algorithm [41,42]. The wide-field fluorescence microscope’s observation FOV is only 333 µm. Therefore, we utilize the 3-axis stage to move the observation objective lens to record 6 images to cover the 1.3 mm FOV (Fig. 4).
Fig. 4.
Two-photon excited 2D fluorescence patterns formed by six holograms covering 1.3 mm. The overall excitation time was 1.3 ms. Scale bar: 100 µm.
The measured fluorescence spot was 1.5 and 3.1 µm in full-width-half-maximum (FWHM), perpendicular and parallel to the galvo movement direction, respectively. The elongation in the galvo movement direction indicates that the galvo was not completely stationary during the exposure. Shorter exposure or slower galvo movement is expected to reduce the elongation. However, for two-photon excitation of soma, this micron level elongation is acceptable. In fact, laser scanning has been utilized to deliberately cover more area of the soma for efficient optogenetic neural modulation [27,28].
For 3D hologram, we essentially tilted each letter so that the left side and right side of the letter were at different depth (Fig. 5). For each letter, we recorded 3D image stack. We show the images at different depth and their maximum intensity projection (bottom row in Fig. 5). We note that there were out of focus fluorescence signal in the wide-field microscope images. Although the two-photon excitation is well confined in 3D, the recording system is based on wide-field microscope and therefore the fluorescence above or below the imaging plane was not rejected. The fluorescence spot in the middle of the letter was due to the residual beam power not controlled by the SLM.
Fig. 5.
3D two-photon excited fluorescence patterns formed by six holograms. The overall excitation time was 1.3 ms. The bottom row shows the maximum intensity projection of the recorded wide-field image stack. Scale bar: 100 µm.
4. Discussion
In our experiment, we utilized 1D switching and 1D positioning to switch between 6 holograms covering 1.3 mm within 1.3 ms. For even more holograms, we can utilize 2D galvo switching and positioning. As the switching time is now limited only by the galvo, the SLM wavefront update speed is no longer important. In this work, we utilized a 1920×1150 pixel SLM which can provide ∼16 holograms. Potentially, 4k SLM can simultaneously display ∼90 holograms. For even more holograms, we can combine the SLM switching [40] with the galvo switching to cover even larger FOV [37].
The switching speed is limited by the employed galvo scanner to 260 µs. For covering 6 regions with 5 transitions, we used 1.3 ms in total with 12% illumination duty cycle. Although acousto-optic deflector (AOD) and electro-optic deflector can both offer microsecond level transitions speed, their achievable spot number or optical etendue is very limited. Consequently, they cannot cover as many holograms as galvo scanners. Moreover, for large FOV operation, we will still need to rely on galvo for large-scale positioning control. Therefore, the overall speed is still fundamentally limited by the galvo scanner.
For higher laser throughput, we can replace the femtosecond beam splitter that coupled the laser beam in and out of the SLM with a pickoff mirror and titled the SLM upward so that the return beam is higher than the input beam. This will yield four times higher laser excitation power.
5. Conclusion
In summary, we present a new method to achieve near simultaneous large FOV 3D femtosecond holography for optogenetic neural modulation application. The key idea is that the achievable FOV in most cases is limited by the short laser coherence length rather than by the SLM pixel number. Modern SLM can in fact simultaneously display a huge number of holograms. Therefore, we will just need to find a fast method to switch between the displayed holograms and deliver them to the right locations. Using frequency filtered galvo control signal, we achieved sustainable high speed operation, in which we displayed 6 holograms over 1.3 mm FOV within 1.3 ms. The illumination time difference between holograms is 260 µs. Such speed is sufficient for spike timing recording. The hologram number can be further increased by using 2D galvo control and even larger format SLM. We expect that this method will help increase the throughput and temporal accuracy of optogenetic neural modulation research.
Acknowledgment
S. S. acknowledges the financial support from the program of China Scholarships Council. Z.-Y.C. acknowledges the support from the Ph.D. abroad visiting scholar program of the Nanchang University. M.C. acknowledges the scientific equipment support from HHMI.
Funding
National Institute of Neurological Disorders and Stroke10.13039/100000065 (U01NS094341, U01NS107689).
Disclosures
The authors declare no conflicts of interest.
References
- 1.Price D. L., “New order from neurological disorders,” Nature 399(6738), A3–A5 (1999). 10.1038/399a003 [DOI] [PubMed] [Google Scholar]
- 2.Collins P. Y., Patel V., Joestl S. S., March D., Insel T. R., Daar A. S., Bordin I. A., Costello E. J., Durkin M., Fairburn C., Glass R. I., Hall W., Huang Y., Hyman S. E., Jamison K., Kaaya S., Kapur S., Kleinman A., Ogunniyi A., Otero-Ojeda A., Poo M.-M., Ravindranath V., Sahakian B. J., Saxena S., Singer P. A., Stein D. J., Anderson W., Dhansay M. A., Ewart W., Phillips A., Shurin S., Walport M., “Grand challenges in global mental health,” Nature 475(7354), 27–30 (2011). 10.1038/475027a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagai J., Rajbhandari A. K., Gangwani M. R., Hachisuka A., Coppola G., Masmanidis S. C., Fanselow M. S., Khakh B. S., “Hyperactivity with Disrupted Attention by Activation of an Astrocyte Synaptogenic Cue,” Cell 177(5), 1280–1292.e20 (2019). 10.1016/j.cell.2019.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkhurst C. N., Yang G., Ninan I., Savas J. N., Yates J. R., Lafaille J. J., Hempstead B. L., Littman D. R., Gan W.-B., “Microglia Promote Learning-Dependent Synapse Formation through Brain-Derived Neurotrophic Factor,” Cell 155(7), 1596–1609 (2013). 10.1016/j.cell.2013.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Bartheld C. S., Bahney J., Herculano-Houzel S., “The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting,” J. Comp. Neurol. 524(18), 3865–3895 (2016). 10.1002/cne.24040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen T.-W., Wardill T. J., Sun Y., Pulver S. R., Renninger S. L., Baohan A., Schreiter E. R., Kerr R. A., Orger M. B., Jayaraman V., Looger L. L., Svoboda K., Kim D. S., “Ultrasensitive fluorescent proteins for imaging neuronal activity,” Nature 499(7458), 295–300 (2013). 10.1038/nature12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giepmans B. N. G., Adams S. R., Ellisman M. H., Tsien R. Y., “Review - The fluorescent toolbox for assessing protein location and function,” Science 312(5771), 217–224 (2006). 10.1126/science.1124618 [DOI] [PubMed] [Google Scholar]
- 8.Tsien R. Y., “The green fluorescent protein,” Annu. Rev. Biochem. 67(1), 509–544 (1998). 10.1146/annurev.biochem.67.1.509 [DOI] [PubMed] [Google Scholar]
- 9.Lin M. Z., Schnitzer M. J., “Genetically encoded indicators of neuronal activity,” Nat. Neurosci. 19(9), 1142–1153 (2016). 10.1038/nn.4359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deisseroth K., “Optogenetics,” Nat. Methods 8(1), 26–29 (2011). 10.1038/nmeth.f.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrillo-Reid L., Han S., Yang W., Akrouh A., Yuste R., “Controlling Visually Guided Behavior by Holographic Recalling of Cortical Ensembles,” Cell 178(2), 447–457.e5 (2019). 10.1016/j.cell.2019.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yizhar O., Fenno L. E., Davidson T. J., Mogri M., Deisseroth K., “Optogenetics in Neural Systems,” Neuron 71(1), 9–34 (2011). 10.1016/j.neuron.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 13.Adam Y., Kim J. J., Lou S., Zhao Y., Xie M. E., Brinks D., Wu H., Mostajo-Radji M. A., Kheifets S., Parot V., “Voltage imaging and optogenetics reveal behaviour-dependent changes in hippocampal dynamics,” Nature 569(7756), 413–417 (2019). 10.1038/s41586-019-1166-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stamatakis A., Schachter M., Gulati S., Malanowski S., Tajik A., Fritz C., Trulson M., Otte S., “Simultaneous optogenetics and cellular resolution calcium imaging during active behavior using a miniaturized microscope,” Front. Neurosci. 12, 496 (2018). 10.3389/fnins.2018.00496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shemesh O. A., Tanese D., Zampini V., Linghu C., Piatkevich K., Ronzitti E., Papagiakoumou E., Boyden E. S., Emiliani V., “Temporally precise single-cell-resolution optogenetics,” Nat. Neurosci. 20(12), 1796–1806 (2017). 10.1038/s41593-017-0018-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pawela C., DeYoe E., Pashaie R., “Intracranial Injection of an Optogenetics Viral Vector Followed by Optical Cannula Implantation for Neural Stimulation in Rat Brain Cortex,” Optogenetics: Methods and Protocols(Springer, 2016), pp. 227–241. [DOI] [PubMed] [Google Scholar]
- 17.Packer A. M., Roska B., Häusser M., “Targeting neurons and photons for optogenetics,” Nat. Neurosci. 16(7), 805–815 (2013). 10.1038/nn.3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow B. Y., Boyden E. S., “Optogenetics and translational medicine,” Sci. Transl. Med. 5(177), 177ps5 (2013). 10.1126/scitranslmed.3003101 [DOI] [PubMed] [Google Scholar]
- 19.Vaziri A., Emiliani V., “Reshaping the optical dimension in optogenetics,” Curr. Opin. Neurobiol. 22(1), 128–137 (2012). 10.1016/j.conb.2011.11.011 [DOI] [PubMed] [Google Scholar]
- 20.Oron D., Papagiakoumou E., Anselmi F., Emiliani V., “Two-photon optogenetics,” in Progress in brain research (Elsevier, 2012), pp. 119–143. [DOI] [PubMed] [Google Scholar]
- 21.Boyden E. S., “A history of optogenetics: the development of tools for controlling brain circuits with light,” F1000 Biol. Rep. 3, 12 (2011). 10.3410/B3-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoham S., “Optogenetics meets optical wavefront shaping,” Nat. Methods 7(10), 798–799 (2010). 10.1038/nmeth1010-798 [DOI] [PubMed] [Google Scholar]
- 23.Zipfel W. R., Williams R. M., Webb W. W., “Nonlinear magic: multiphoton microscopy in the biosciences,” Nat. Biotechnol. 21(11), 1369–1377 (2003). 10.1038/nbt899 [DOI] [PubMed] [Google Scholar]
- 24.Hoover E. E., Squier J. A., “Advances in multiphoton microscopy technology,” Nat. Photonics 7(2), 93–101 (2013). 10.1038/nphoton.2012.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helmchen F., Denk W., “Deep tissue two-photon microscopy,” Nat. Methods 2(12), 932–940 (2005). 10.1038/nmeth818 [DOI] [PubMed] [Google Scholar]
- 26.Svoboda K., Yasuda R., “Principles of Two-Photon Excitation Microscopy and Its Applications to Neuroscience,” Neuron 50(6), 823–839 (2006). 10.1016/j.neuron.2006.05.019 [DOI] [PubMed] [Google Scholar]
- 27.Rickgauer J. P., Deisseroth K., Tank D. W., “Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields,” Nat. Neurosci. 17(12), 1816–1824 (2014). 10.1038/nn.3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rickgauer J. P., Tank D. W., “Two-photon excitation of channelrhodopsin-2 at saturation,” Proc. Natl. Acad. Sci. 106(35), 15025–15030 (2009). 10.1073/pnas.0907084106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shim E., Chen Y., Masmanidis S., Li M., “Multisite silicon neural probes with integrated silicon nitride waveguides and gratings for optogenetic applications,” Sci. Rep. 6(1), 22693 (2016). 10.1038/srep22693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reutsky-Gefen I., Golan L., Farah N., Schejter A., Tsur L., Brosh I., Shoham S., “Holographic optogenetic stimulation of patterned neuronal activity for vision restoration,” Nat. Commun. 4(1), 1509 (2013). 10.1038/ncomms2500 [DOI] [PubMed] [Google Scholar]
- 31.Packer A. M., Peterka D. S., Hirtz J. J., Prakash R., Deisseroth K., Yuste R., “Two-photon optogenetics of dendritic spines and neural circuits,” Nat. Methods 9(12), 1202–1205 (2012). 10.1038/nmeth.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quirin S., Peterka D. S., Yuste R., “Instantaneous three-dimensional sensing using spatial light modulator illumination with extended depth of field imaging,” Opt. Express 21(13), 16007–16021 (2013). 10.1364/OE.21.016007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikolenko V., Watson B. O., Araya R., Woodruff A., Peterka D. S., Yuste R., “SLM microscopy: scanless two-photon imaging and photostimulation with spatial light modulators,” Front. Neural Circuits 2, 14 (2008). 10.3389/neuro.04.005.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang W., Carrillo-Reid L., Bando Y., Peterka D. S., Yuste R., “Simultaneous two-photon imaging and two-photon optogenetics of cortical circuits in three dimensions,” eLife 7, e32671 (2018). 10.7554/eLife.32671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernandez O., Papagiakoumou E., Tanese D., Fidelin K., Wyart C., Emiliani V., “Three-dimensional spatiotemporal focusing of holographic patterns,” Nat. Commun. 7(1), 11928 (2016). 10.1038/ncomms11928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaigneau E., Ronzitti E., Gajowa M. A., Soler-Llavina G. J., Tanese D., Brureau A. Y. B., Papagiakoumou E., Zeng H., Emiliani V., “Two-Photon Holographic Stimulation of ReaChR,” Front. Cell. Neurosci. 10, 234 (2016). 10.3389/fncel.2016.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sofroniew N. J., Flickinger D., King J., Svoboda K., “A large field of view two-photon mesoscope with subcellular resolution for in vivo imaging,” eLife 5, 055947 (2016). 10.7554/eLife.14472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai P. S., Mateo C., Field J. J., Schaffer C. B., Anderson M. E., Kleinfeld D., “Ultra-large field-of-view two-photon microscopy,” Opt. Express 23(11), 13833–13847 (2015). 10.1364/OE.23.013833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stirman J. N., Smith I. T., Kudenov M. W., Smith S. L., “Wide field-of-view, multi-region, two-photon imaging of neuronal activity in the mammalian brain,” Nat. Biotechnol. 34(8), 857–862 (2016). 10.1038/nbt.3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang S. J., Allen W. E., Kauvar I., Andalman A. S., Young N. P., Kim C. K., Marshel J. H., Wetzstein G., Deisseroth K., “Extended field-of-view and increased-signal 3D holographic illumination with time-division multiplexing,” Opt. Express 23(25), 32573–32581 (2015). 10.1364/OE.23.032573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerchberg R. W., “A practical algorithm for the determination of phase from image and diffraction plane pictures,” Optik 35, 237–246 (1972). [Google Scholar]
- 42.Sinclair G., Leach J., Jordan P., Gibson G., Yao E., Laczik Z. J., Padgett M. J., Courtial J., “Interactive application in holographic optical tweezers of a multi-plane Gerchberg-Saxton algorithm for three-dimensional light shaping,” Opt. Express 12(8), 1665–1670 (2004). 10.1364/OPEX.12.001665 [DOI] [PubMed] [Google Scholar]