Abstract
Background:
Pediatric Acute Respiratory Distress Syndrome (PARDS) occurs frequently in children, but until recently there were no pediatric specific diagnostic criteria. The Pediatric Acute Lung Injury Consensus Conference (PALICC) definition was developed to overcome limitations of the Berlin definition in children, but the incidence and outcomes of children who meet this definition are unclear.
Methods:
Prospective, observational, cross-sectional study amongst invasively (IMV) or non-invasively (NIV) ventilated children with PARDS in a participating pediatric intensive care unit (PICU) during 10 study weeks.
Findings:
From 145 international PICUs, new PARDS occurred in 744 children (3.2% of PICU patients). 708 had complete data for analysis, with 17.1% mortality. Only 32% met Berlin criteria at PARDS diagnosis, and had higher severity of illness, different PARDS triggers and co-morbidities, and more cardiac dysfunction than the larger PARDS cohort. Based on hypoxemia severity at PARDS onset, mortality was similar amongst NIV, mild, or moderate PARDS (10–15%), but higher for severe PARDS (33%). Half of NIV PARDS patients were subsequently intubated, with 25% mortality. PARDS severity 6 hours after initial diagnosis discriminates PICU mortality (AUC 0.689 (95%CI 0.624, 0.754)) better than at PARDS diagnosis, and outperforms Berlin hypoxemia categories (0.639 (0.575, 0.703), p=0.01). Adjusted mortality is nearly 3 times higher for those with severe PARDS or those from a low/middle-income country.
Interpretation:
PARDS affects 3% of PICU patients internationally. The PALICC definition identifies many more children with ARDS than the Berlin definition, and PARDS severity groupings stratify mortality risk, particularly when applied 6 hours after PARDS diagnosis.
Introduction
Acute Respiratory Distress Syndrome (ARDS) continues to be a major source of morbidity and mortality in critically ill patients.1 Although early descriptions of ARDS included children,2 pediatric considerations were not addressed in either American European Consensus Conference (AECC) or Berlin definitions of ARDS.3,4 A specific definition5 for Pediatric ARDS (PARDS)6 has recently been created, through the Pediatric Acute Lung Injury Consensus Conference (PALICC). Differences in practice patterns and etiologies of ARDS in children and adults suggest a substantial number of children with high morbidity and mortality do not meet the Berlin definition, but have ARDS.7,8–10
The PALICC definition was modeled after the Berlin definition when the pathobiology and practice patterns of adult ARDS were thought to be similar to PARDS.11,12 However, unlike the Berlin Definition,4,13 PALICC uses: (1) pulse oximetry when PaO2 is unavailable; (2) oxygenation index (OI) or oxygenation saturation index (OSI) to stratify severity instead of PaO2/FiO2 (PF) ratio; and (3) evidence of pulmonary parenchymal disease rather than bilateral infiltrates on chest imaging. Diagnostic criteria for PALICC and Berlin are provided in the electronic supplement. Single center or regional studies have evaluated the PALICC definition, but its performance in a large international sample is unknown.8,9,14–16
We sought to understand the implications of the PALICC definition on the incidence and outcomes of children with PARDS through a prospective, international, multi-center, cross-sectional observational study, the Pediatric ARDS Incidence and Epidemiology (PARDIE) study. The main research goals were to (1) determine the incidence and outcomes of children diagnosed with PARDS by PALICC criteria; (2) compare performance of PALICC with the Berlin definition; and (3) determine how the timing of hypoxemia metrics within the first three days of PARDS onset discriminates PICU mortality. We hypothesized that PALICC would identify more children with PARDS than the Berlin definition, that PALICC PARDS severity groups would have prognostic relevance, and that hypoxemia severity gauged after the initial PARDS diagnosis but within the first 24 hours would retain the strongest independent association with mortality.
Methods
Study Design and Patient Selection
PARDIE involved 5 continuous days of screening all PICU patients, repeated over 10 study weeks from May 9, 2016-June 16, 2017 in multiple international pediatric intensive care units (PICUs). Included patients had a new diagnosis of PARDS during one of the study periods. Data collection concentrated on the first 3 days of PARDS diagnosis and followed mortality to hospital discharge or 90 days, whichever came first.
An international executive committee oversaw the study. PICUs were recruited through a variety of international organizations. Children’s Hospital Los Angeles (CHLA) was the clinical and data coordinating center, and the protocol was first approved by the CHLA Institutional Review Board (IRB). PARDIE sites either obtained local IRB approval, or used the central CHLA IRB. In all but one site, waiver of informed consent was granted. Institutions had to submit at least 3 weeks of valid screening statistics or complete data on at least 1 PARDS patient to meet minimum requirements for participation.
Inclusion Criteria: (1) hypoxemia within 7 days of a clinical insult; (2) respiratory failure not fully explained by cardiac failure or fluid overload; (3) chest imaging with new infiltrate(s) consistent with pulmonary parenchymal disease; (4) PF ratio ≤ 300 or SpO2/FiO2 (SF) ratio ≤ 264 if on Non Invasive Ventilation (NIV, oro-nasal mask CPAP ≥ 5 cmH2O or BiPAP) or OI ≥ 4 or OSI ≥ 5 if on Invasive Mechanical Ventilation (IMV). Children with chronic home mechanical ventilation must have also had an acute deterioration in oxygenation from their baseline (ESM for full definition).
Exclusion Criteria: (1) preparation for or recovering from cardiac intervention; (2) cyanotic heart disease; (3) active perinatal lung disease; (4) within 7 days of cardiopulmonary bypass; (5) previously met PARDS criteria during the illness > 24 hours prior to screening.
Paper case report forms were available in English and Spanish, along with the detailed study protocol and a data dictionary. All data were entered electronically through a secure, password enabled website developed by the “Laboratoire de Télématique Biomedicale,” Sherbrooke, Quebec, Canada in collaboration with the investigators. All protected health information remained at the local site. Dates and times were converted to time from PICU admission. Data queries surrounding completeness and validity were sent routinely.
Data elements included characteristics of the PICUs, demographics, elements for PARDS, co-morbidities, chest imaging, arterial blood gas, and pulse oximetry results. PICU mortality was the primary outcome. Secondary outcomes included 90 day mortality (censored at hospital discharge), length of IMV and NIV, and cause of death (as gauged by the site investigator). Sites could participate in additional studies surrounding ancillary therapies (V.1), ventilator management (V.2), and chest imaging (V.3) (data to be presented in other manuscripts). Vasoactive medication data from V.1 and blinded, standardized interpretation of chest imaging by radiologists and pediatric intensivists from V.3 are presented in this manuscript. The protocol, case report forms, and methods regarding site training and data management are provided in the Electronic Supplementary Material (ESM).
Analysis
Based on preliminary data5,9,14 we anticipated a roughly equal distribution of patients in NIV, mild, moderate, and severe PARDS, and expected severe PARDS mortality to be ≥ 22% with NIV/mild/moderate PARDS morality to be around 13%. Hence, we targeted a sample size of 800 patients (200 with severe PARDS) to determine whether severe PARDS patients had higher mortality than the other groups (alpha =0.05, Power =0.84). Although we recruited approximately 100 fewer patients than targeted, higher mortality rates were in the severe group yielded a post-hoc power of 0.99. Hypoxemia for IMV was based on PALICC PARDS OI/OSI severity groups. For NIV, PF ratio (or SF ratio when PF was not available) cut points from the Berlin Definition were used.4,17 28 Day Ventilator Free Days (VFD) were calculated for IMV only and for a combination of IMV_NIV (ESM). Countries were grouped by geographic region and economic status using 2016 World Bank classifications18,19 for high, middle-high, and middle-low income countries. There was 1 middle-low income country, therefore, middle-low and middle-high were combined and treated as middle-income (Table S1).
Continuous variables are described as median (interquartile range) and analyzed with a Mann Whitney U test or Kruskal-Wallis ANOVA. Categorical data are reported as number (percent) and analyzed with Chi-squared or Fisher’s exact tests. Area Under the Curve (AUC) of the Receiver Operating Characteristic Plots were calculated with respective 95% Confidence intervals against PICU mortality and compared with each other using Chi-squared tests. 90-day survival curves (with the assumption that patients discharged prior to 90 days were alive at 90 days) were stratified by PARDS severity. Inter-observer reliability regarding bilateral infiltrates on chest imaging was computed with a kappa statistic. Hierarchical multivariable logistic regression models (using the ICU as the random effect)20,21 were created to determine whether the PARDS severity group retained an independent association with PICU mortality. All variables with a univariate association (p<0.1) with mortality were considered, and those which remained associated with mortality in multivariable modeling (p<0.05) or were confounders (> 20% change in relationship between hypoxemia severity and mortality regardless of p-value) were retained in the final model. After each step of the multivariable model, if the addition of the new variable had large effect on the point estimate for another parameter in the model, we evaluated for co-linearity, starting first with a correlation matrix. When variables were highly correlated, we retained the variable with the highest univariate association with the outcome in the multi-variable model. Pseudo-likelihood methods evaluating the ratio of the generalized chi-square statistic and its degrees of freedom (with a goal close to 1) were used to indicate that variability in these data has been properly modeled, with no residual over dispersion. Hierarchical multivariate survival analysis is presented in the supplement. Analyses were performed in SAS version 9.4, and STATA Version 15. All analyses were verified by two statisticians. Data is been presented in line with the STROBE checklist for cross-sectional studies.
Role of Funding Source
Funding was used to create the study database and support personnel at the clinical and data coordinating center. Funding agencies had no other role in the study. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
PALICC PARDS Incidence and Outcome
A total of 170 PICUs obtained approval, and 145 PICUs from 27 countries met minimum requirements for participation (ESM Collaborator List). Over the 10 study weeks, 23,280 patients were admitted to participating PICUs, and 12,242 required invasive or non-invasive mechanical ventilation (MV). There were 744 new cases of PARDS identified by the PALICC definition over the 10 study weeks, yielding an international PARDS incidence of 3.2% (95% CI 3.0, 3.4%) amongst PICU patients and 6.1% (95% CI 5.7, 6.5%) amongst those on MV. Amongst MV patients, PARDS incidence is higher in North America, in high-income countries, in non-summer months, and lower in medium volume PICUs (all p<0.01, Table S2).
Thirty six of the 744 patients with PARDS were excluded because they were either not entered into the study database or did not have complete data on mortality or PARDS severity, leaving 708 for analysis (Figure 1). Overall mortality was 17.1% (95% CI 14.4, 20.1%; 121/708), and initial PARDS severity groups were nearly equally distributed amongst NIV, mild, moderate, and severe (Figure 1). When stratifying by initial PARDS severity group, mortality was similar amongst those with NIV, mild, or moderate PARDS (10–15%), but was significantly higher for those with severe PARDS (33%; 54/165) (Table 1a, Figure 1). There were fewer VFDs and longer length of MV in survivors as PARDS severity worsened (Table 1a).
Figure 1:
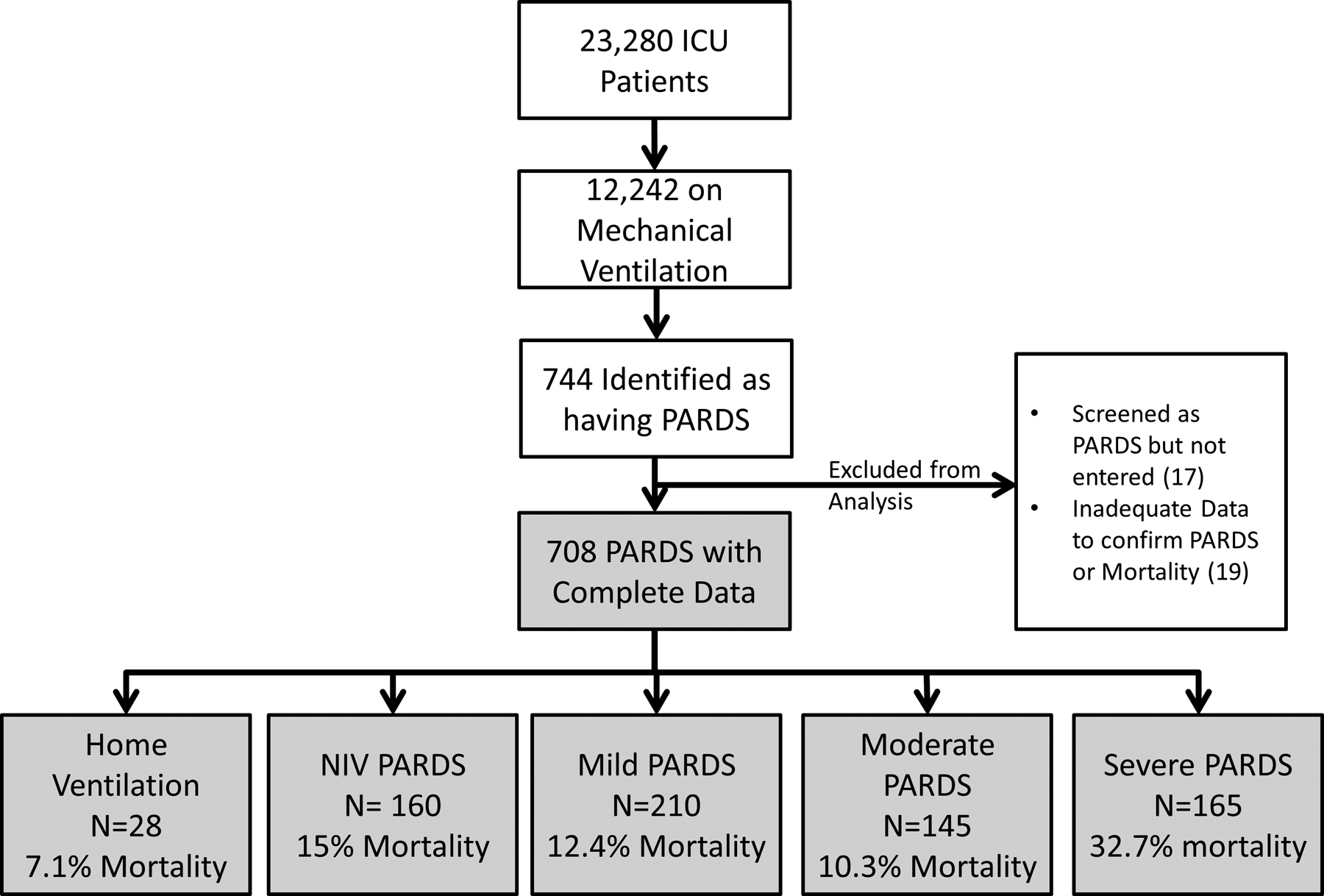
Screened and enrolled patients, stratified by initial PARDS severity. As per the PALICC definition, patients normally on home ventilation were identified as having PARDS, but not included in the severity stratification.
Table 1.
Table 1a (top) Study outcomes by initial PARDS severity amongst those on IMV; excluding those on home mechanical ventilation. Data are presented as number (percentage) or median (interquartile range). Medians were compared using Kruskal-Wallis Analysis of Variance, with multiple comparisons based on mean ranks. Table 1b (bottom) Study outcomes for initial NIV PARDS group, stratified by whether they were subsequently intubated or remained on NIV for the entire PARDS illness.
| Mild PARDS | Moderate PARDS | Severe PARDS | p value | |
|---|---|---|---|---|
| 210 | 145 | 165 | ||
| PICU Mortality | 26 (12.4%) (95% CI 8.2%, 17.6%) |
15 (10.3%) (95% CI 5.9%, 16.5%) |
54 (32.7%) (95% CI 25.6%, 40.5%) |
<0.0001 |
| 90 Day Mortality& | 30 (14.3%) (95% CI 9.9%, 19.8%) |
21 (14.5%) (95% CI 9.2%, 21.3%) |
56 (34.0%) (95% CI 26.8%, 41.7%) |
<0.0001 |
| 28 Day VFDS IMV Only | 21.1 (12.9,23.9) | 20.5 (13.2,23.9) | 13.1 (0,21.0)* # | <0.0001 |
| 28 Day VFDS IMV or NIV | 20.5 (10.6,23.6) | 18.4 (8.9,23.4) | 10.1 (0,20.4)* # | <0.0001 |
| Length IMV Survivors | 5.9 (4,10.2) | 7.0 (4.0,12.4) | 7.9 (5.0,14.7)* | 0.016 |
| Length IMV/NIMV Survivors | 6.5 (4.1,11.6) | 7.5 (4.0,14.6) | 9.3 (5.9,17.1)* | 0.0050 |
| NIV PARDS | NIV PARDS Intubated | NIV PARDS Not Intubated | ||
| 160 | 80 | 80 | ||
| PICU Mortality | 24 (15%) (95% CI 9.9%, 21.5%) |
20 (25%) (95% CI 16.0%, 35.9%) |
4 (5%) (95% CI 1.4%, 12.3%) |
0.0004 |
| 90 Day Mortality& | 27 (17.1%); n=158 (95% CI 11.4%, 23.6%) |
21 (26.6%); n=79 (95% CI 17.0%, 37.6%) |
6 (7.6%); n=79 (95% CI 2.8%, 15.6%) |
0.0015 |
| 28 Day VFDS IMV Only | 25.3 (14.2,28) | 17.5 (0,23.1) | 28 (28,28) | <0.0001 |
| 28 Day VFDS IMV or NIV | 20.4 (3.3,24.9) | 14.4 (0, 21) | 24.5 (20.1,25.9) | <0.0001 |
| Length IMV Survivors | 0 (0,6.6) | 7.6 (4.3,12.5) | NA | |
| Length IMV/NIMV Survivors | 5.9 (2.7,10.7) | 10.7 (5.7,20.5) | 3.2 (2.0,6.9) | <0.0001 |
indicates different than mild PARDS in multiple comparisons;
indicates different than moderate PARDS in multiple comparisons.
90 Day mortality censored at Hospital Discharge
Pneumonia/lower respiratory tract infection (63%; 445/708) and sepsis (19%; 136/708) were the most common PARDS triggers. A pre-existing comorbidity was present in 63% (419/708) of children, the most common being chronic pulmonary disease (28%; 197/708). Unadjusted mortality was higher amongst patients with sepsis (30%; 41/136), drowning (67%; 4/6), non-septic shock (60%; 6/10), any co-morbidity (21%; 93/419), left ventricular dysfunction (38%; 15/40), cancer (51%; 30/59), immune suppression (including cancer) (46%; 44/95), those with an arterial blood gas (ABG) (24%; 75/312), and those on vasoactive medications (31.7%; 72/227) at PARDS diagnosis. Pneumonia as the PARDS trigger (12%; 52/445) or history of prematurity (11%; 14/131) was associated with lower unadjusted mortality (Table 2, Figure S1). Refractory hypoxemia was assigned as the cause of death in 34% (41/121) of patients, multi-system organ failure in 43% (52/121), and brain death or neurologic injury in 28% (35/121). Geographic region, country income, and other ICU level characteristics also had unadjusted associations with mortality (Table 3).
Table 2:
Patient characteristics stratified by PICU mortality. Data presented as number (percentage) or median (interquartile range). P values represent Chi-Squared, Fisher’s Exact or Mann-Whitney U between survivors and non-survivors.
| Survivors | Non Survivors | Total | Mortality | P Value | |
|---|---|---|---|---|---|
| 587 (82.9%) | 121 (17.1%) | 708 | 17.1% | ||
| PARDS Risk Factor | |||||
| Pneumonia | 393 (67%) | 52 (43%) | 445 (62.9%) | 11.7% | <0.0001 |
| Sepsis | 95 (16.2%) | 41 (33.9%) | 136 (19.2%) | 30.1% | <0.0001 |
| Aspiration | 47 (8%) | 13 (10.7%) | 60 (8.5%) | 13 21.7% | 0.33 |
| Trauma | 24 (4.1%) | 3 (2.5%) | 27 (3.8%) | 11.1% | 0.60 |
| Drowning | 2 (0.3%) | 4 (3.3%) | 6 (0.9%) | 66.7% | 0.0092 |
| Other Shock | 4 (0.7%) | 6 (5.0%) | 10 (1.4%) | 60.0% | 0.0026 |
| Other | 22 (3.8%) | 2 (1.7%) | 24 (3.4%) | 8.3% | 0.41 |
| Demographics | |||||
| Age (years) (n=701) | 3.2 (0.71,9.5) | 3.7 (0.9,11.1) | 3.3 (0.73,9.6) | N/A | 0.33 |
| Male (n=706) | 356 (60.8%) | 76 (63.3%) | 432 (61.2%) | 17.6% | 0.60 |
| Weight (kg) (n=704) | 14 (7.95,30.2) | 15.2 (8,41.3) | 14.5 (8,31.5) | N/A | 0.22 |
| Race (n=701) | 0.11 | ||||
| Asian/PI/AI | 37 (6.4%) | 14 (11.7%) | 51 (7.3%) | 27.5% | |
| Black | 82 (14.1%) | 21 (17.5%) | 103 (14.7%) | 20.4% | |
| White | 357 (61.5%) | 63 (52.5%) | 420 (59.9%) | 15.0% | |
| Other/Unknown Race | 105 (18.1%) | 22 (18.3%) | 127 (18.1%) | 17.3% | |
| Ethnicity (n=702) | 0.013 | ||||
| Non-Hispanic | 357 (61.3%) | 62 (51.7%) | 419 (59.7%) | 14.8% | |
| Hispanic | 130 (22.3%) | 42 (35%) | 172 (24.5%) | 24.4% | |
| Other/Unknown Ethnicity | 95 (16.3%) | 16 (13.3%) | 111 (15.8%) | 14.4% | |
| Co-morbidities | |||||
| Any Comorbidity | 352 (60%) | 93 (76.9%) | 445 (62.9%) | 20.9% | 0.0005 |
| LV Dysfunction | 25 (4.3%) | 15 (12.4%) | 40 (5.7%) | 37.5% | 0.0004 |
| Prematurity | 117 (19.9%) | 14 (11.6%) | 131 (18.5%) | 10.7% | 0.031 |
| Pre-existing Pulmonary Disease | 166 (28.3%) | 31 (25.6%) | 197 (27.8%) | 15.7% | 0.55 |
| Chronic Respiratory Support | 103 (17.6%) | 17 (14.1%) | 120 (17%) | 14.2% | 0.35 |
| Home Ventilation | 26 (4.4%) | 2 (1.7%) | 28 (4%) | 7.1% | 0.20 |
| Congenital Heart Disease | 70 (11.9%) | 8 (6.6%) | 78 (11%) | 10.3% | 0.089 |
| Acquired Heart Disease | 43 (7.3%) | 13 (10.7%) | 56 (7.9%) | 23.2% | 0.21 |
| Neuromuscular Disease | 104 (17.7%) | 18 (14.9%) | 122 (17.2%) | 14.8% | 0.45 |
| Cancer | 29 (4.9%) | 30 (24.8%) | 59 (8.3%) | 50.9% | <0.0001 |
| Immune Suppression | 51 (8.7%) | 44 (36.4%) | 95 (13.4%) | 46.3% | <0.0001 |
| ABG at PARDS | 237 (40.4%) | 75 (62%) | 312 (44.1%) | 24% | <0.0001 |
| PIM 3 Probability of Death | 3.1% (0.5,5.7) | 7.5% (1.9,31) | 3.2% (0.6,6.8) | N/A | <0.0001 |
| Vasoactive at PARDS (n=621)* | 155 (30.2%) | 72 (67.3%) | 227 (36.6%) | 31.7% | <0.0001 |
| Vasoactive 3 days of PARDS (n=624)* | 213 (41.3%) | 85 (78.7%) | 298 (47.8%) | 28.5% | <0.0001 |
From V.1 ancillary study, so not recorded in all patients.
Table 3:
Hospital/ICU based characteristics stratified by PICU mortality. Data presented as number (percentage) or median (interquartile range). P values represent Chi-Squared or Fisher’s Exact between survivors and non survivors. ECMO= Extracorporeal membrane oxygenation; HFOV= High Frequency Oscillatory Ventilation.
| Survivors | Non Survivors | Total | % Mortality | P | |
|---|---|---|---|---|---|
| Season | 587 | 121 | 708 | 0.084 | |
| Fall | 113 (19.3%) | 31 (25.6%) | 144 (20.3%) | 21.5% | |
| Winter | 240 (40.9%) | 35 (28.9%) | 275 (38.8%) | 12.7% | |
| Spring | 164 (27.9%) | 37 (30.6%) | 201 (28.4%) | 18.4% | |
| Summer | 70 (11.9%) | 18 (14.9%) | 88 (12.4%) | 20.5% | |
| Region | 0.0095 | ||||
| North America | 380 (64.7%) | 68 (56.2%) | 448 (63.3%) | 15.2% | |
| Europe | 102 (17.4%) | 17 (14.1%) | 119 (16.8%) | 14.3% | |
| Central/South America | 72 (12.3%) | 29 (24%) | 101 (14.3%) | 28.7% | |
| Middle East/Asia/Australia/NZ | 33 (5.6%) | 7 (5.8%) | 40 (5.7%) | 17.5% | |
| Country Income | <0.0001 | ||||
| High Income | 516 (87.9%) | 89 (73.6%) | 605 (85.5%) | 14.7% | |
| Middle Income | 71 (12.1%) | 32 (26.5%) | 103 (14.6%) | 31.1% | |
| PICU Beds (n=707) | 0.56 | ||||
| ≤10 | 87 (14.9%) | 19 (15.7%) | 106 (15%) | 17.9% | |
| 11 to 20 | 164(28%) | 37 (30.6%) | 201 (28.4%) | 18.4% | |
| 21 to 30 | 126 (21.5%) | 31 (25.6%) | 157 (22.2%) | 19.7% | |
| 31 to 40 | 127 (21.7%) | 19 (15.7%) | 146 (20.7%) | 13.0% | |
| > 40 | 82 (14%) | 15 (12.4%) | 97 (13.7%) | 15.5% | |
| PICU Admissions per Year (n=698) | 0.0029 | ||||
| ≤250 | 17 (2.9%) | 8 (6.8%) | 25 (3.6%) | 32.0% | |
| 251–500 | 65 (11.2%) | 13 (11%) | 78 (11.2%) | 16.7% | |
| 501–750 | 58 (10%) | 11 (9.3%) | 69 (9.9%) | 15.9% | |
| 751–1000 | 76 (13.1%) | 25 (21.2%) | 101 (14.5%) | 24.8% | |
| 1001–1250 | 32 (5.5%) | 13 (11%) | 45 (6.5%) | 28.9% | |
| >1250 | 332 (57.2%) | 48 (40.7%) | 380 (54.4%) | 12.6% | |
| PICU Characteristics* | |||||
| Cardiac Surgery Center | 479 (81.7%) | 98 (81%) | 577 (81.6%) | 17.0% | 0.85 |
| ICU Physician In House | 391 (69.6%) | 87 (71.9%) | 478 (70%) | 18.2% | 0.61 |
| ICU Fellowship Program | 514 (87.7%) | 102 (84.3%) | 616 (87.1%) | 16.6% | 0.31 |
| Trauma Center | 522 (89.1%) | 109 (90.1%) | 631 (89.2%) | 17.3% | 0.75 |
| ECMO Center | 418 (71.3%) | 79 (65.3%) | 497 (70.3%) | 15.9% | 0.19 |
| HFOV Capabilities | 579 (98.8%) | 115 (95%) | 694 (98.2%) | 16.6% | 0.014 |
| Respiratory Therapists | 470 (80.2%) | 97 (80.2%) | 567 (80.2%) | 17.1% | 0.99 |
| Burn Center | 267 (47.5%) | 61 (50.4%) | 328 (46.0%) | 18.6% | 0.56 |
| Point of Care Blood Gas | 467 (83.7%) | 103 (90.4%) | 570 (84.8%) | 18.1% | 0.071 |
Data not available from all centers: denominator was not always the same in each column but percentages correctly reflect the number of patients with data available
PALICC vs. Berlin Definition
Of the 708 patients who met PALICC PARDS criteria (17.1% mortality), 230/708 (32.5%) concurrently met all Berlin criteria for ARDS (bilateral infiltrates on chest imaging and arterial PF ratio ≤ 300) at the time of PALICC PARDS diagnosis (26.5% (61/230) mortality). An additional 183/708 (25.9%) met Berlin criteria in the subsequent 3 days after the initial PARDS diagnosis (20.8% (38/183) mortality), and 295/708 (41.7%) did not meet Berlin criteria within 3 days of PARDS diagnosis (7.5% (22/295) mortality; Figure 2). For those who met Berlin criteria after PARDS diagnosis, the median time to diagnosis was 12.8 hours (IQR 3.2, 31.6) after PALICC. In multivariable modeling, patients were more likely to meet Berlin at initial PARDS diagnosis if they were from a medium income country, had clinical evidence of left ventricular dysfunction, had higher PIM 3 score, were from an ICU with < 1250 admissions per year, were on invasive mechanical ventilation, were not on chronic respiratory support before ICU admission, or had a PARDS risk factor other than pneumonia (all p≤ 0.04) (Tables S3–S5). Patients meeting Berlin were also more likely to be on vasoactive medications (Table S3, subset of 621 children with data available).
Figure 2a (top) and b (bottom):
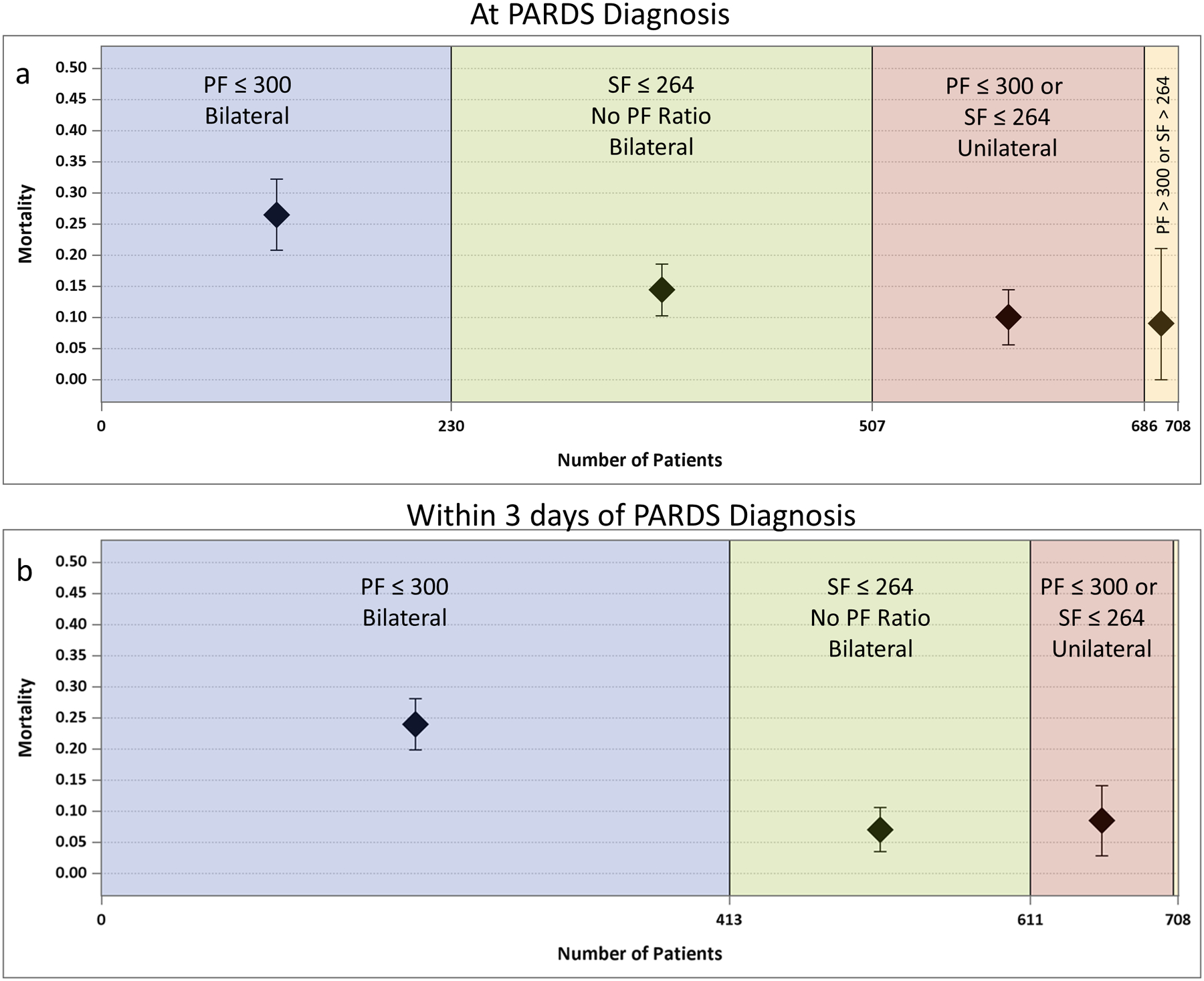
Berlin criteria at (a) PARDS diagnosis and (b) within 3 days of PARDS diagnosis. The size of the box represents the number of patients in each area and the cumulative total is represented on the x-axis. The diamond represents the point estimate for ICU mortality with the 95% Confidence Interval (whiskers). Bilateral = bilateral infiltrates on chest x-ray. Unilateral= unilateral infiltrate on chest x-ray. (a) Only 32% of children have an arterial blood gas with PF <300 and bilateral infiltrates on chest imaging at the time of PARDS diagnosis (patients who met All Berlin Criteria, blue). Allowing for the SF ratio to be substituted for the PF ratio in the Berlin Definition, then 72% met modified Berlin Criteria (blue + green). Bilateral infiltrates at PARDS diagnosis were not present in 25% of PARDS patients who would have otherwise met Berlin SF/PF criteria (pink). Only 3% of patients who met PARDS criteria did not have a PF ratio ≤ 300 or SF ratio ≤ 264 at PARDS diagnosis (tan). (b) In the first 3 days of PARDS diagnosis, 58% of patients met all Berlin criteria (blue), and 86.3% met Berlin criteria if SF ratio was included (blue + green). *Note only 3 patients did not have a PF ratio ≤ 300 or SF ratio ≤ 264 within the first 3 days of PARDS diagnosis, so they are not labeled on the graph (tan). None of these 3 patients died.
If the SF ratio could be substituted for PF ratio, then 507/708 (72%) of patients would have met Berlin criteria at PALICC PARDS diagnosis (20% (101/507) mortality), and 611/708 (86.3%) would have met it within 3 days of PARDS diagnosis (18.5% (113/611) mortality). For those who met Berlin criteria allowing for SF modification after PARDS diagnosis, the median time to diagnosis was 31 hours (IQR 19, 45.8) after PALICC. In multivariable modeling, factors which remained independently associated with meeting Berlin (with SF modification) at initial PARDS diagnosis included being from a medium income country, being in an ICU which does not routinely care for adult patients, and having severe PARDS (all p<0.02).
Radiological bilateral infiltrates were identified in 523/708 (74%) of children at PARDS diagnosis, and 613/708 (87%) within 3 days of PARDS diagnosis by site principal investigators. Bilateral infiltrates were more common for those with severe PARDS (142/172; 86%) (p<0.001) and were associated with higher mortality as compared with unilateral infiltrates both at PARDS onset (102/523 (19.5%) vs. 19/185 (10.3%), p=0.004) and within 3 days of PARDS diagnosis (113/613 (18.4%) vs. 8/95 (8.4%), p=0.02). When stratifying by hypoxemia at PARDS onset, mortality was higher with bilateral infiltrates for the NIV PARDS (p=0.03) group but not statistically different in the severe PARDS group (p=0.07) (Table S6). Notably, there was very poor agreement between pediatric intensivist and radiologist interpretations of bilateral infiltrates (kappa= 0.34) in 759 chest x-rays evaluated by both.
Hypoxemia Severity Timing: IMV PARDS
For those on IMV, hypoxemia severity at PARDS onset using PALICC OI/OSI groups had slightly better discrimination of PICU mortality AUC 0.644 (95% CI 0.58, 0.71), as compared with Berlin PF/SF ratio groups AUC 0.605 (0.55, 0.66), although this was not statistically significant (p=0.09). (Figure 3a, Table S7, Figure S2a, S3a). PF/SF groups categorize more patients as moderate/severe and fewer as mild as compared with OI/OSI groups (Figure 3a).
Figure 3:
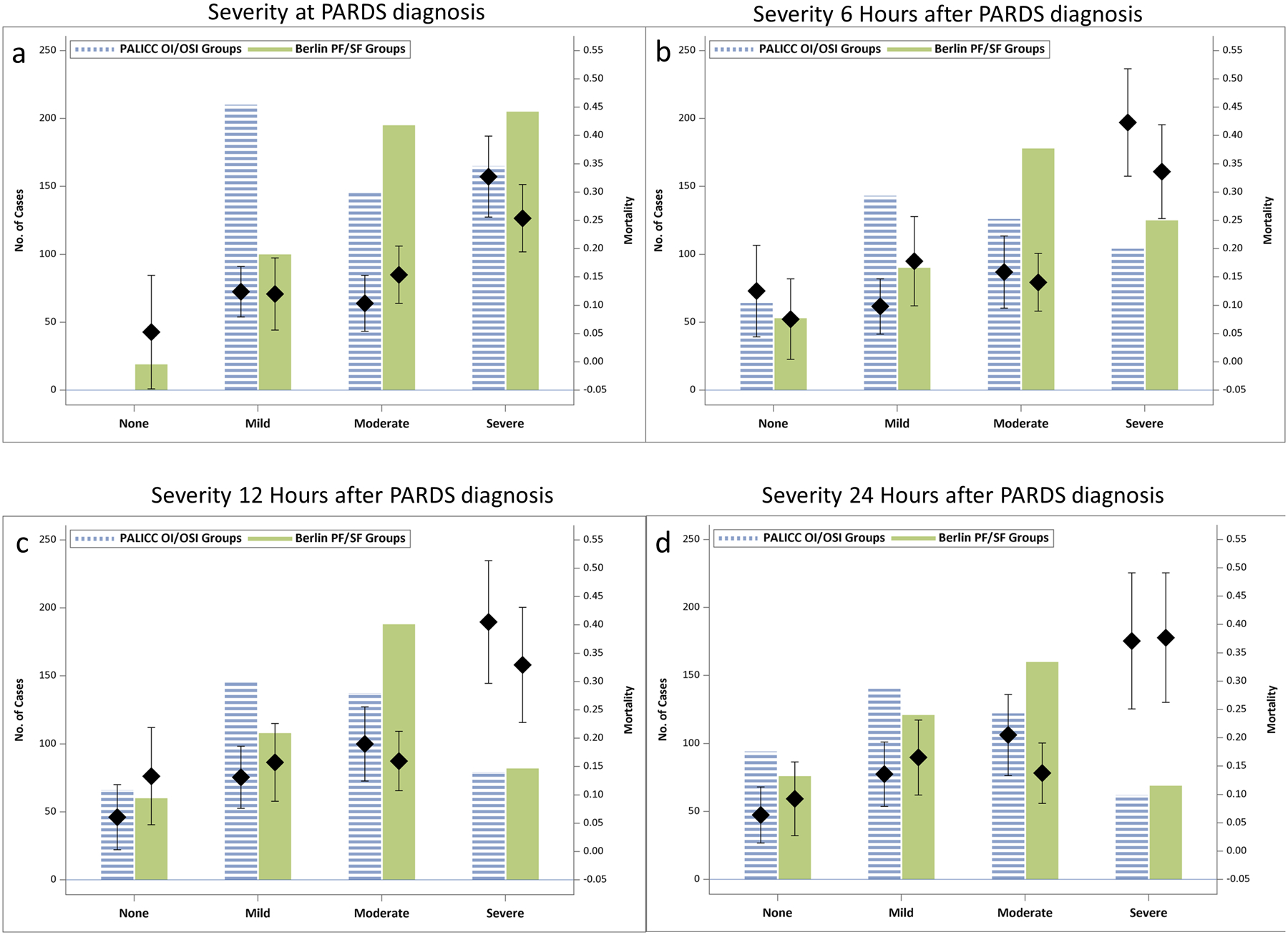
OI/OSI versus PF/SF ratio based severity stratifications for those initially on IMV (excluding home ventilation). Each graph represents the number of patients who would fall in each severity group (bars) using hypoxemia metrics at distinct time points after PARDS diagnosis: (a) PARDS diagnosis (b) 6 hours, (c) 12 hours, and (d) 24 hours after PARDS diagnosis. ICU mortality for each group is represented with the diamond, and the whiskers represent 95% Confidence Interval around the point estimate for mortality. For PALICC OI/OSI groups: Severe = OI ≥ 16 or OSI ≥ 12.3; Moderate = OI ≥ 8 and < 16 or OSI ≥ 7.5 and < 12.3; Mild = OI ≥ 4 and < 8 or OSI ≥ 5 and < 7.5; None = OI<4 or OSI <5, or no longer on mechanical ventilation. For Berlin PF/SF groups: Severe = PF ≤ 100 or SF ≤ 150; Moderate = PF ≤ 200 and > 100 or SF ≤ 221 and > 150; Mild = PF ≤ 300 and > 200 or SF ≤ 264 and SF> 221; None = PF >300 or SF > 264, or no longer on mechanical ventilation.
When examining OI at PARDS onset (OSI equivalent when OI not available22), mortality increased substantially at an OI of 15 (Figure S4), corresponding with the PALICC severe group. For those on IMV, 278/520 (53%) met criteria with an ABG (OI instead of OSI) and were nearly twice as likely to die (67/278 (24.1%) vs. 28/242 (11.6%), p=0.0002), although mortality similarly increased amongst those who met criteria with OSI at an OI equivalent of 15. The presence of an OI (vs. OSI) at PARDS diagnosis did not appear to be related to hypoxemia severity (Figure S5).
Discrimination of PICU mortality using PALICC OI/OSI groups improves over baseline 6 hours after PARDS diagnosis (AUC 0.689 (0.624, 0.754), Figure 3b–d, Figure S2b–d). These changed marginally from 6–48 hours after PARDS diagnosis. Similar patterns are seen with Berlin PF/SF ratio groups (Figure 3b–d, Figure S3b–d), but OI/OSI groups discriminate PICU mortality better than PF/SF groups 6–24 hours (p<0.05, Table S7) after PARDS diagnosis. The pattern of PF/SF groups categorizing more patients as moderate/severe and fewer as mild versus OI/OSI groups becomes less prominent (although still present) over the first 24 hours (Figure 3).
NIV PARDS
For those with NIV PARDS, only 28/160 (17.5%) met diagnostic criteria with an ABG (PF ratio). Half of the 160 patients with NIV PARDS were eventually intubated (Table 1b), with 69/80 (86%) of intubations occurring within 48 hours of PARDS diagnosis (Figure 4). PICU mortality 20/80 (25%), number of VFDs and length of MV (amongst survivors) for NIV patients who are subsequently intubated approximates those with initial moderate to severe IMV PARDS (Table 1a–b). Hypoxemia severity at PARDS diagnosis on NIV is strongly associated with risk for intubation (log-rank, p=0.002, Figure 4), and mortality (AUC 0.64 (0.52, 0.75)).
Figure 4.
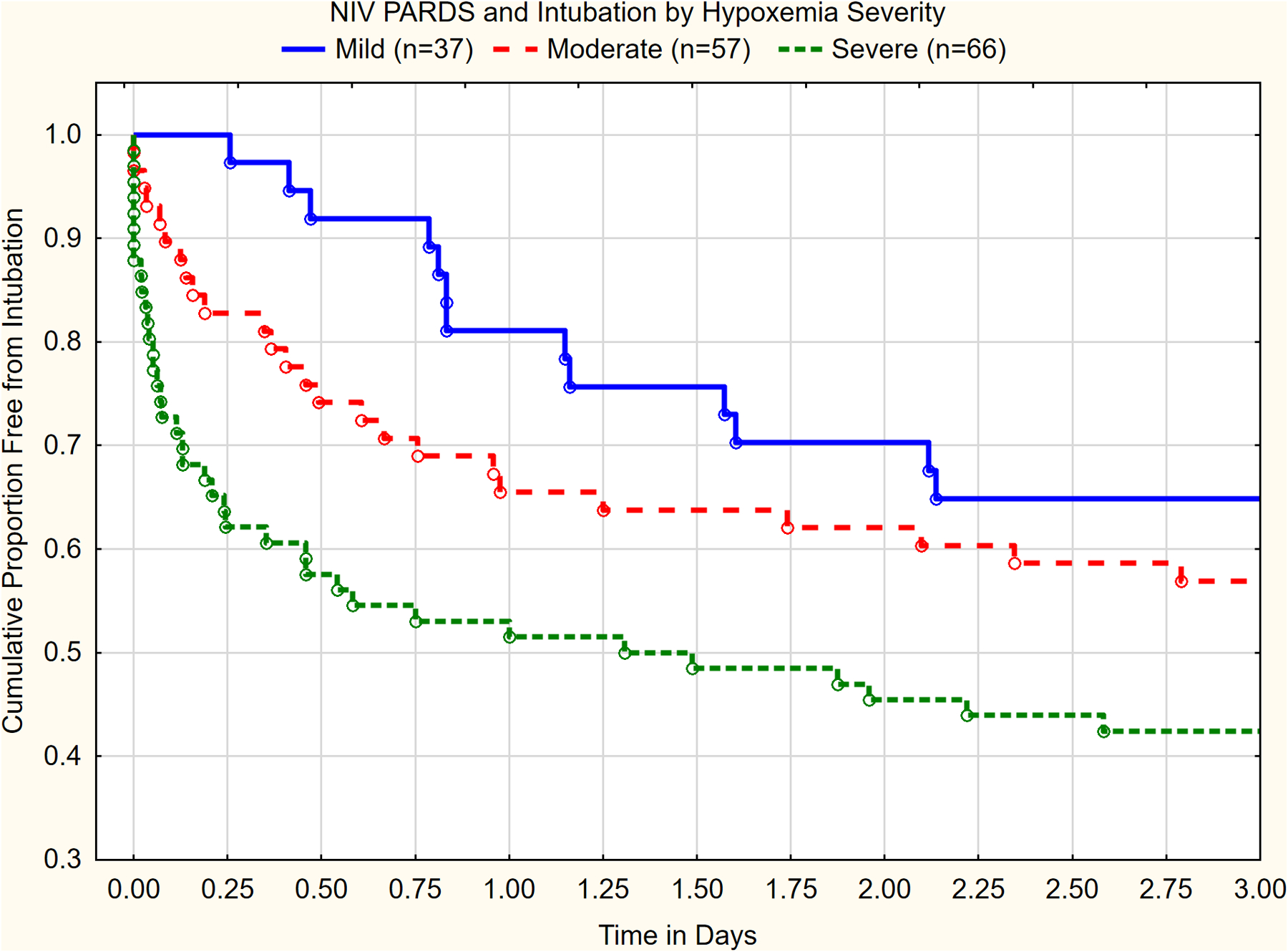
Cumulative proportions free of intubation as a function of initial hypoxemia severity for those on NIV. Very few children are intubated after 3 days (graph cut off at this point). Hypoxemia severity is highly associated with time to intubation (p<0.002, Log-rank test). Severe = PF ≤ 100 or SF ≤ 150; Moderate = PF ≤ 200 and > 100 or SF ≤ 221 and > 150; Mild = PF ≤ 300 and > 200 or SF ≤ 264 and SF> 221. No patients were censored.
Multivariable Model
Variables at PARDS onset that retained an independent association with higher mortality included: severe PARDS, being from a medium income country, PARDS risk factors of drowning and non-septic shock, and immune suppression. A PARDS risk factor of pneumonia was independently associated with lower mortality (Table 4). Bilateral infiltrates at PARDS onset (p>0.2) or presence of an ABG at PARDS onset (p=0.055) were not independently associated with mortality. Multivariable survival analysis yielded nearly identical results (Table S8).
Table 4:
Independent risk factors for mortality at PARDS onset. All variables have been adjusted for individual ICU as a random effect in the hierarchical model. Vasoactive agents were not considered for inclusion in this model, since data was only available for 621 of the 708 patients. PIM 3 score was also not included because PF ratio and immune suppression are components of the score, and the primary goal was to evaluate whether PARDS severity is independently associated with mortality (which has significant overlap with PF ratio). The ratio of Generalized Chi-Square/Degrees of Freedom = 0.85, indicating the variability in data has been properly modeled, with no residual over dispersion.
| Risk factors for Mortality | OR (95% CI) | P value |
|---|---|---|
| Severe PARDS at onset | 3.04 (1.89, 4.87) | <0.0001 |
| Medium Income Country | 2.85 (1.56, 5.22) | 0.0008 |
| PARDS Risk Factor Pneumonia | 0.48 (0.30, 0.76) | 0.0022 |
| PARDS Risk Factor Other Shock | 10.9 (2.56, 46.5) | 0.0012 |
| PARDS Risk Factor Drowning | 12.7 (2.0, 79.4) | 0.0078 |
| Immune Suppression | 6.3 (3.6, 11.2) | <0.0001 |
Discussion
This is the largest international prospective study of ARDS in children using the recently created PALICC definition. We found that PARDS affects 3% of PICU patients, and mortality exceeds 30% in those with severe hypoxemia. Factors related to health-care delivery (i.e. income of country), patient co-morbidities, and PARDS triggers are associated with mortality, but PALICC defined severe PARDS retained a strong association with mortality, reinforcing its importance in a PARDS definition. PALICC identified 40% more children as having ARDS as compared with Berlin (with arterial PF ratio), and diagnosed ARDS a median of 12–30 hours sooner. PALICC based OI/OSI severity groups appear to stratify mortality better than PF/SF groups, particularly by 6 hours after PARDS diagnosis, and are well calibrated for VFD and length of MV in survivors.
Our study highlights important disparities in PARDS outcomes internationally. Multivariable modeling suggests that mortality is higher in middle-income counties as compared to high-income countries. This income variable is likely a surrogate for factors related to health-care delivery, resources, and time to treatment (among others). Previous regional studies support that these outcome disparities exist,1,8,16,23 but our study provides the advantage of using common methodology across PICUs to identify PARDS patients and describe their outcomes. This was not an a priori hypothesis of the study, so the results should be interpreted with caution. However, we would encourage further study to explore residual confounding or reasons for these disparities, and methods to improve them.
We believe our data justifies the three major differences between PALICC and Berlin: pulse oximetry criteria when an ABG is not available, radiographic criteria, and OI instead of PF ratio on IMV.5 We found that half of PARDS patients do not have an ABG at PARDS diagnosis. Echoing previous findings,10 children who met ABG based Berlin criteria at PARDS diagnosis had: higher mortality, higher severity of illness, more left ventricular dysfunction, more vasoactive medications, more indirect causes of lung injury, less respiratory co-morbidity, and were less likely to be on NIV. Furthermore, there were ICU specific factors associated with meeting Berlin criteria, such as being from a middle income country, and a smaller ICU. These may reflect institutional differences in severity of illness or in the decision to place arterial lines. The presence of an ABG at PARDS diagnosis was no longer independently associated with mortality after controlling for other factors. Furthermore, when allowing for pulse oximetry in the Berlin definition, many of the noted differences were no longer significant. The value of pulse oximetry based criteria (including OSI) has also been demonstrated in adults with ARDS.24 Therefore, SpO2 based criteria are essential for PARDS diagnosis because requiring the subjective decision to obtain a PaO2 will underestimate the number and select for a different subset of children with PARDS.
The elimination of bilateral infiltrates in the PARDS definition was controversial. From a pathophysiologic standpoint, ARDS must involve diffuse inflammation in the lungs. While this has historically been characterized by bilateral infiltrates on chest imaging in addition to hypoxemia, PALICC argued to remove the requirement for bilateral infiltrates because of: 1) high inter-observer variability in the assessment of bilateral infiltrates25; (2) CXR findings may lag behind the degree of hypoxemia; (3) lack of independent prognostic relevance of bilateral infiltrates after controlling for hypoxemia; and (4) lack of sensitivity of the CXR to detect lung inflammation. We have confirmed the first three, but did not specifically test the fourth. We found (1) poor inter-rater reliability in the interpretation of bilateral infiltrates between intensivists and radiologists; (2) most children have bilateral infiltrates at the time of PARDS diagnosis (74%), but a substantial number develop them over the first three days of PARDS diagnosis, resulting in a median delay in ARDS diagnosis of 31 hours; and (3) bilateral infiltrates (regardless of when) are not independently associated with mortality in multivariable modeling. Therefore, we believe these data confirm the rationale for elimination of bilateral infiltrates in the PARDS definition. Nevertheless, the low mortality (8%) amongst the 13% of PARDS patients who do not develop bilateral infiltrates in the first 3 days may imply these patients are different than the rest of the cohort. This should be an ongoing area of investigation to inform future modifications to the PARDS definition. As PALICC recommends, PARDS studies should stratify patients by the presence or absence of bilateral infiltrates, but it is crucially important to focus future investigation on ways to reduce variability, such as automated computer generated interpretation, newer standardized scoring systems,26 or other imaging methods.27–29 Simply using common training films, as recommended by the Berlin Definition and some other investigators, may be inadequate.30
PALICC recommends OI for hypoxemia severity, rather than PF ratio.5 Neither definition provides clarity regarding when to measure hypoxemia severity (at PARDS onset, or at some point after PARDS onset). We found that OI/OSI groups generally outperform PF/SF ratio groups to discriminate mortality, which echoes previous findings in both adults and children with ARDS.31–34 Berlin PF/SF ratio groups have a gradual increase in mortality risk from mild to severe, but the severe ARDS group (i.e. PF ratio < 100 or SF < 150) is substantially larger, with lower mortality than the PALICC severe group. Using PALICC classifications at PARDS onset, there is little difference in mortality between mild, moderate, or NIV PARDS, but significantly higher mortality in severe PARDS (OI > 16 or OSI > 12.3). By 6 hours after PARDS diagnosis, there is improved gradation of mortality risk with OI/OSI groups. While other studies have advocated waiting 12–24 hours after ARDS diagnosis to stratify risk, our data supports that 6 hours is adequate.9,14,35–37 Future refinements to ARDS definitions should consider a delayed measure of hypoxemia (i.e. 6 hours) to confirm the diagnosis of ARDS and stratify risk. Regardless, it appears that the severe PARDS group (whenever this is classified) has substantially higher mortality.
Reinforcing previous studies,38 we found that PARDS patients on NIV are at high risk for intubation, and NIV PARDS patients who are intubated have 25% mortality. NIV hypoxemia severity is associated with mortality and time to intubation.39 While these findings have not been adjusted for other potential confounding factors such as immunosuppressed status,40,41 we should look more closely at our practice of NIV in PARDS, particularly for those with severe hypoxemia.41–44 Identifying when and how to use NIV in PARDS should continue to be an active area of investigation.45
Both the Berlin and PALICC definitions created severity groups in part because therapeutic approaches should differ as a function of ARDS severity.4,5,13 We have found that although PALICC groups are not well calibrated for mortality at PARDS onset, they do have stepwise associations with length of MV and VFDs (albeit a large reduction in VFDs in the severe group). We found that nearly 30% of PARDS deaths are associated with neurologic injury, with only about 30% related to refractory hypoxemia. This, coupled with 17% mortality and a PARDS incidence of 3% in this study, reinforces difficulties in using mortality as a primary endpoint. Given that most PARDS therapeutic research now uses VFDs or length of MV amongst survivors, it is reassuring that the PALICC severity groups are calibrated for these outcomes, implying they can be used to stratify or target enrollment in future trials.
While this study represents the largest ever international cohort of children with PARDS, there are limitations. First, this was a voluntary effort and many sites did not have research coordinators, so not all data queries were resolved. Strict data management and cleaning strategies were applied, and out of range or potentially inaccurate values were removed. Second, while this study was an international sample from 145 PICUs, we did not have adequate representation of low or middle-low income countries. Third, there is the potential for seasonal bias. We had ten study weeks throughout 1 year, with 2 “winter” months in the Southern Hemisphere and 3 in the Northern Hemisphere. Fourth, since we did not follow patients after hospital discharge, we felt the primary analysis should use logistic regression on the outcome of ICU mortality, rather than survival analysis. However, if we assume patients who survived to ICU discharge were alive at 90 days, then survival analysis yields similar results (Table S8). The choice of survival analysis versus logistic regression in ICU studies is an area of controversy because it is unclear if shorter or longer time to death is clinically meaningful.46 Fifth, patients were included if they met PALICC PARDS criteria. We cannot draw conclusions regarding patients who met Berlin Criteria, but not PARDS criteria. Given the nature of the definitions, and previous investigations, we anticipate that fewer than 2% of patients who meet Berlin criteria do not meet PARDS criteria.9,14 Finally, the diagnosis of ARDS lacks a gold standard. While our study shows the PALICC definition identifies many more children with ARDS than the Berlin definition, we cannot determine whether ARDS is being “over-diagnosed” with PALICC.
In conclusion, PARDS occurs in approximately 6% of all MV children in PICUs internationally, and has high associated mortality for those with severe hypoxemia. The PALICC definition for PARDS can be applied internationally, identifies significantly more patients than the Berlin Definition, and appears to adequately stratify mortality and length of ventilation amongst PARDS patients when applied 6 hours after PARDS diagnosis. The PALICC definition can be used as a framework for future research in PARDS, particularly in studies focused on minimizing potential disparities in PARDS outcomes and evaluating treatment strategies which may have different risk-benefit profiles based on PARDS severity.
Supplementary Material
Research in context.
Evidence before this study:
Acute Respiratory Distress Syndrome (ARDS) has been described in children for over 50 years, but an understanding of the international epidemiology of pediatric ARDS (PARDS) has been limited. The recent Pediatric Acute Lung Injury Consensus Conference (PALICC) definition of PARDS was developed to overcome limitations of definitions of ARDS designed and validated for adults (i.e. the Berlin Definition), but the effect of this new definition on the incidence and outcomes of PARDS is unknown. We searched PubMed on July 31, 2018 for the terms “Acute Respiratory Distress Syndrome”; “Pediatric” and “Epidemiology” and identified several studies which report pediatric ARDS epidemiology using either American European Consensus (AECC) Conference or Berlin Definitions of ARDS. There were a handful of studies which report PARDS epidemiology using PALICC criteria, but they were single center or regional in nature.
Added Value of this study:
This prospective international cross sectional observational study including 145 international pediatric intensive care units and over 700 children with PARDS using the PALICC definition confirms that the Berlin Definition underestimates the burden of ARDS in children, and variably identifies only a high-risk subset of children with ARDS. PARDS occurs in approximately 3% (744/23280) of children admitted to intensive care units and is associated with approximately 17% (121/708) mortality. The PARDS severity groupings proposed by PALICC have important prognostic relevance throughout a diverse group of international pediatric intensive care units.
Implications of all the available evidence:
The PALICC definition can be used as an international framework for clinical care and PARDS research.
Acknowledgements:
The authors would like to thank Justin Hotz, RCP for all his assistance throughout the project, and Dr. Carolyn Wong, PhD for statistical consultation.
Funding:
University of Southern California Clinical Translational Science Institute
CHU- Sainte Justine, University of Montreal, Canada
Réseau en Santé Respiratoire du Fonds de Recherche Quebec-Santé (FRQS)
Children’s Hospital Los Angeles, Department of Anesthesiology and Critical Care Medicine
Footnotes
The corresponding author has had full access to all the data in the study and had final responsibility for the decision to submit for publication
Declaration of Interests
Dr. Khemani reports grants from University of Southern California CTSI, grants and non-financial support from Children’s Hospital Los Angeles, Department of Anesthesiology and Critical Care, during the conduct of the study; grants from National Institutes of Health, personal fees from OrangeMed, personal fees from Hamilton Medical, outside the submitted work; .Dr. Jouvet reports grants from CHU- Sainte Justine, University of Montreal Canada, grants from Reseau en Sante Respiratoire du Fonds de Recherche Quebec-Sante (FRQS), grants and non-financial support from Air Liquide Sante, outside the submitted work; Dr. Yehya reports grants from NIH, during the conduct of the study; Dr. Thomas reports personal fees from Therabron, personal fees from CareFusion, grants from Gene Fluidics, outside the submitted work; Dr. Newth reports personal fees from Philips Research North America, outside the submitted work; All other authors declared no conflicts of interest.
References
- 1.Schouten LR, Veltkamp F, Bos AP, et al. Incidence and Mortality of Acute Respiratory Distress Syndrome in Children: A Systematic Review and Meta-Analysis. Critical care medicine. 2016;44(4):819–829. [DOI] [PubMed] [Google Scholar]
- 2.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–323. [DOI] [PubMed] [Google Scholar]
- 3.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American journal of respiratory and critical care medicine. 1994; 149:(3)818–24.. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Medicine. 2012;38(10):10. [DOI] [PubMed] [Google Scholar]
- 5.Khemani RG, Smith LS, Zimmerman JJ, Erickson S, PALICC Group. Pediatric Acute Respiratory Distress Syndrome: Definition, Incidence, and Epidemiology: Proceedings From the Pediatric Acute Lung Injury Consensus Conference. Pediatric Critical Care Medicine. 2015;16(5_suppl):S23–S40 [DOI] [PubMed] [Google Scholar]
- 6.PALICC Group. Pediatric Acute Respiratory Distress Syndrome. Pediatric Critical Care Medicine. 2015;16(5):428–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta S, Sankar J, Lodha R, Kabra SK. Comparison of Prevalence and Outcomes of Pediatric Acute Respiratory Distress Syndrome Using Pediatric Acute Lung Injury Consensus Conference Criteria and Berlin Definition. Frontiers in Pediatrics. 2018;(6):93.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong JJ, Phan HP, Phumeetham S, et al. Risk Stratification in Pediatric Acute Respiratory Distress Syndrome: A Multicenter Observational Study. Critical care medicine. 2017;45(11):1820–1828. [DOI] [PubMed] [Google Scholar]
- 9.Parvathaneni K, Belani S, Leung D, Newth CJ, Khemani RG. Evaluating the Performance of the Pediatric Acute Lung Injury Consensus Conference Definition of Acute Respiratory Distress Syndrome. Pediatric Critical Care Medicine. 2017;18(1):17–25. [DOI] [PubMed] [Google Scholar]
- 10.Khemani RG, Rubin S, Belani S, et al. Pulse oximetry vs. PaO2 metrics in mechanically ventilated children: Berlin definition of ARDS and mortality risk. Intensive Care Medicine. 2015;41(1):94–102. [DOI] [PubMed] [Google Scholar]
- 11.Sapru A, Flori H, Quasney MW, Dahmer MK, PALICC Group. Pathobiology of acute respiratory distress syndrome. Pediatric Critical Care Medicine.16(5 Suppl 1):S6–22. [DOI] [PubMed] [Google Scholar]
- 12.Flori H, Dahmer MK, Sapru A, Quasney MW, PALICC Group. Comorbidities and Assessment of Severity of Pediatric Acute Respiratory Distress Syndrome: Proceedings From the Pediatric Acute Lung Injury Consensus Conference. Pediatric Critical Care Medicine. 2015;16(5_suppl) Supplement(1):S41–S50. [DOI] [PubMed] [Google Scholar]
- 13.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012; 307 (23) : 2526–33. [DOI] [PubMed] [Google Scholar]
- 14.Yehya N, Servaes S, Thomas NJ. Characterizing degree of lung injury in pediatric acute respiratory distress syndrome. Critical care medicine. 2015;43(5):937–946. [DOI] [PubMed] [Google Scholar]
- 15.Rowan CM, Smith LS, Loomis A, et al. Pediatric Acute Respiratory Distress Syndrome in Pediatric Allogeneic Hematopoietic Stem Cell Transplants: A Multicenter Study. Pediatric Critical Care Medicine. 2017;18(4):304–309. [DOI] [PubMed] [Google Scholar]
- 16.Wong JJ, Jit M, Sultana R, et al. Mortality in Pediatric Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. J Intensive Care Med. 2017:885066617705109. [DOI] [PubMed] [Google Scholar]
- 17.Khemani RG, Rubin S, Belani S, et al. Pulse Oximetry vs. PaO2 metrics in mechanically ventialted children: Berlin definition of ARDS and mortality risk. Intensive Care Medicine. 2015; 2015; 41(1):94–102t. [DOI] [PubMed] [Google Scholar]
- 18.The World Bank. GNI per capita, Atlas Method. 2016; https://data.worldbank.org/indicator/NY.GNP.PCAP.CD. Last accessed July 18, 2018.
- 19.Laffey JG, Madotto F, Bellani G, et al. Geo-economic variations in epidemiology, patterns of care, and outcomes in patients with acute respiratory distress syndrome: insights from the LUNG SAFE prospective cohort study. Lancet Respir Med. 2017;5(8):627–638. [DOI] [PubMed] [Google Scholar]
- 20.Epstein D, Wong CF, Khemani RG, et al. Race/Ethnicity is not associated with mortality in the PICU. Pediatrics. 2011;127(3):e588–597. [DOI] [PubMed] [Google Scholar]
- 21.Wong GY, Mason WM. The Hierarchical Logistic Regression Model for Multilevel Analysis. Journal of the American Statistical Association. 1985;80(391):513–524. [Google Scholar]
- 22.Khemani RG, Thomas NJ, Venkatachalam V, et al. Comparison of SpO2 to PaO2 based markers of lung disease severity for children with acute lung injury. Critical care medicine. 2012;40(4):1309–1316. [DOI] [PubMed] [Google Scholar]
- 23.Erickson SE, Shlipak MG, Martin GS, et al. Racial and ethnic disparities in mortality from acute lung injury. Critical care medicine. 2009;37(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DesPrez K, McNeil JB, Wang C, Bastarache JA, Shaver CM, Ware LB. Oxygenation Saturation Index Predicts Clinical Outcomes in ARDS. 2017;1(6):1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubenfeld GD, Caldwell E, Granton J, Hudson LD, Matthay MA. Interobserver variability in applying a radiographic definition for ARDS. Chest. 1999;116(5):1347–1353. [DOI] [PubMed] [Google Scholar]
- 26.Warren MA, Zhao Z, Koyama T, et al. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax. 2018;14:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newth CJL, Khemani RG, Jouvet PA, Sward KA. Mechanical Ventilation and Decision Support in Pediatric Intensive Care. Pediatric Clinics of North America.64(5):1057–1070. [DOI] [PubMed] [Google Scholar]
- 28.Zaglam N, Jouvet P, Flechelles O, Emeriaud G, Cheriet F. Computer-aided diagnosis system for the Acute Respiratory Distress Syndrome from chest radiographs. Comput Biol Med.52:41–48. [DOI] [PubMed] [Google Scholar]
- 29.Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135(4):714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goddard SL, Rubenfeld GD, Manoharan V, et al. The Randomized Educational Acute Respiratory Distress Syndrome Diagnosis Study: A Trial to Improve the Radiographic Diagnosis of Acute Respiratory Distress Syndrome. Critical care medicine. 2018;12:12. [DOI] [PubMed] [Google Scholar]
- 31.Seeley E, McAuley DF, Eisner M, Miletin M, Matthay MA, Kallet RH. Predictors of mortality in acute lung injury during the era of lung protective ventilation. Thorax. 2008;63(11):994–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.NHLBI Acute Respiratory Distress Syndrome Clinical Trials Network, Wiedemann HP, et al. Comparison of two fluid-management strategies in acute lung injury. New England Journal of Medicine. 2006;354(24):2564–2575. [DOI] [PubMed] [Google Scholar]
- 33.Trachsel D, McCrindle BW, Nakagawa S, Bohn D. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. American Journal of Respiratory & Critical Care Medicine. 2005;172(2):206–211. [DOI] [PubMed] [Google Scholar]
- 34.Khemani RG, Conti D, Alonzo TA, Bart RD, Newth CJL. Effect of tidal volume in children with acute hypoxemic respiratory failure. Intensive Care Medicine. 2009;35(8):1428–1437. [DOI] [PubMed] [Google Scholar]
- 35.Yehya N, Thomas NJ, Khemani RG. Risk Stratification Using Oxygenation in the First 24 Hours of Pediatric Acute Respiratory Distress Syndrome. Critical care medicine. 2018; 46 (4): 619–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.López-Fernández Y, Azagra AM-d, de la Oliva P, et al. Pediatric Acute Lung Injury Epidemiology and Natural History Study: Incidence and outcome of the acute respiratory distress syndrome in children. Critical care medicine. 2012; 40(12): 3238–45. [DOI] [PubMed] [Google Scholar]
- 37.Villar J, Fernandez RL, Ambros A, et al. A clinical classification of the acute respiratory distress syndrome for predicting outcome and guiding medical therapy. Critical care medicine. 2015;43(2):346–353. [DOI] [PubMed] [Google Scholar]
- 38.Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. American Journal of Respiratory and Critical Care Medicine. 2005;171(9):995–1001. [DOI] [PubMed] [Google Scholar]
- 39.Mayordomo-Colunga J, Pons M, Lopez Y, et al. Predicting non-invasive ventilation failure in children from the SpO2/FiO2 (SF) ratio. Intensive Care Medicine. 2013;39(6):1095–1103. [DOI] [PubMed] [Google Scholar]
- 40.Zinter MS, Dvorak CC, Spicer A, Cowan MJ, Sapru A. New Insights Into Multicenter PICU Mortality Among Pediatric Hematopoietic Stem Cell Transplant Patients. Critical care medicine. 2015;43(9):1986–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piastra M, De Luca D, Pietrini D, et al. Noninvasive pressure-support ventilation in immunocompromised children with ARDS: a feasibility study. Intensive Care Medicine. 2009;35(8):1420–1427. [DOI] [PubMed] [Google Scholar]
- 42.Antonelli M, Conti G, Moro ML, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi-center study. Intensive Care Medicine. 2001;27(11):1718–1728. [DOI] [PubMed] [Google Scholar]
- 43.Essouri S, Carroll C, PALICC Group. Noninvasive support and ventilation for pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatric Critical Care Medicine. 2015;16(5 Suppl 1):S102–110. [DOI] [PubMed] [Google Scholar]
- 44.Mayordomo-Colunga J, Pons-Odena M, Medina A, et al. Non-invasive ventilation practices in children across Europe. Pediatric Pulmonology. 2018;24:24. [DOI] [PubMed] [Google Scholar]
- 45.Piastra M, De Luca D, Marzano L, et al. The number of failing organs predicts non-invasive ventilation failure in children with ALI/ARDS. Intensive Care Medicine. 2011;37(9):1510–1516. [DOI] [PubMed] [Google Scholar]
- 46.Schoenfeld D Survival methods, including those using competing risk analysis, are not appropriate for intensive care unit outcome studies. Critical Care (London, England). 2006;10(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


