Abstract
Objective:
Quantifying risk for cardiovascular disease (CVD) events among adolescents is difficult owing to the long latent period between risk factor development and disease outcomes. This study examined the 30-year CVD event risk among adolescents with severe obesity treated with and without metabolic and bariatric surgery (MBS), compared to youth with moderate obesity, overweight, or normal-weight.
Methods:
Cross-sectional and longitudinal comparisons of five frequency-matched (age and diabetes status) groups were performed: Normal-weight (n=247), overweight (n=54), obesity (n=131), severe obesity without MBS (n=302), and severe obesity undergoing MBS (n=215). A 30-year CVD event score developed by the Framingham Heart Study was the primary outcome. Data are mean(SD) with differences between time-points for MBS examined using linear mixed-models.
Results:
Preoperatively, the likelihood of CVD events was higher among adolescents undergoing MBS (7.9[6.7]%) compared to adolescents with severe obesity not referred for MBS (5.5[4.0]%), obesity (3.9[3.0]%), overweight (3.1[2.4]%), and normal-weight (1.8[0.8]%; p<0.001 all). One-year post-MBS, event risk was significantly reduced (7.9[6.7]% to 4.0[3.4]%, p<0.0001), and was sustained up to 5 years post-MBS (p<0.0001 all years versus baseline).
Conclusion:
Adolescents with severe obesity are at elevated risk for future cardiovascular events. Following MBS, predicted risk of CV events was substantially and sustainably reduced.
Keywords: Adolescent, MBS, Cardiovascular, Obesity, Cost-effectiveness
Table of Contents Summary:
The cardiovascular event risk of pediatric severe obesity is significant in comparison to peers with normal-weight, overweight and moderate obesity. Metabolic and bariatric surgery substantially and sustainably reduces the risk for cardiovascular events.
Introduction
The long latent period between risk factor development and disease onset presents a challenge for evaluating cardiovascular disease (CVD) outcomes in pediatric cohorts. Evidence from several established longitudinal studies clearly demonstrates that obesity in childhood and adolescence is an independent risk factor for early CVD morbidity and mortality.(1, 2, 3, 4, 5, 6, 7) However, this risk may be underestimated because the cohorts from which this evidence was derived were established prior to or early in the onset of the current obesity epidemic. Furthermore, the continued rise in obesity prevalence and the increased proportion of youth with severe obesity (i.e. BMI ≥ 120% of the 95th percentile or BMI ≥ 35kg/m2),(8, 9) suggests that a current assessment of long-term risks of CVD in youth with obesity is needed. Moreover, from a healthcare policy and economics standpoint, it would be very helpful to understand how effective obesity treatment impacts the long-term CVD risk.
Measuring the actual impact of severe obesity and its treatment on CVD events is difficult until sufficient time has elapsed, usually requiring several decades for a critical mass of events to occur. However, the impact can be estimated using CVD prediction algorithms using relatively simple clinical data such as serum lipids, systolic blood pressure, diabetes status, and anthropometric measures. The Framingham Heart Study has developed a 30-year CVD event risk estimate (validated in 20 to 59 year olds) which offers the opportunity to prospectively evaluate CVD event risk long-term.(10) Given the preponderance of evidence suggesting childhood obesity is a risk factor for early CVD morbidity and mortality,(3, 4, 6, 7, 11) coupled with the elevated CVD risk profile exhibited among youth with severe obesity,(12, 13, 14, 15, 16, 17, 18) it is probable that the risk of CVD events later in life is high among youth with severe obesity.
Currently, the only interventions with high rates of clinically meaningful and sustained weight loss among youth with severe obesity is MBS.(12, 13, 14, 16, 17, 18, 19, 20) Data in adults undergoing MBS have demonstrated a significant reduction in CVD events and mortality compared with non-surgical controls.(21, 22, 23) Whether these findings translate to pediatric populations cannot be determined without long-term follow-up. However, the impact of MBS on long term CVD event risk can be modeled using the Framingham methodology. Therefore, the aims of this analysis were to: 1) Examine the risk of developing a CVD event within 30-years among adolescents with severe obesity compared with contemporaries from differing weight strata; 2) Determine the effect of MBS on 30-year CVD event risk among adolescents with severe obesity; 3) Evaluate the cost-effectiveness of MBS for CVD event reduction.
Patients and Methods
Surgical Study Cohort and Measurement Time-Points
Data from the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study were utilized for this analysis.(24) Teen-LABS is an ongoing National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) - funded, prospective, longitudinal, multicenter observational study that enrolled consecutive adolescents (< 20 years of age) undergoing MBS at five clinical centers. Parental permission and participant assent (<18 years old), and consent from older adolescents (≥18 years old) were obtained. Medical and surgical care were provided for each patient as specified by patient care pathways at each institution. No attempts were made to standardize or alter care within this observational research protocol.(25) Data collection time-points used for this analysis were baseline (pre-operative) and annually up to 5 years post-operative assessments.
Teen-LABS Risk Factor Measurements
At each assessment, height was measured on a wall-mounted stadiometer and weight on an electronic scale (Scale-Tronix 5200, Scale Tronix, White Plains, NY, USA or Tanita TBF310, Arlington Heights, Illinois, USA) and BMI was calculated. Diabetes was defined by using self-reported diagnoses, medical record review, medication use for the treatment of diabetes (excluding metformin with a concomitant diagnosis of polycystic ovary syndrome), hemoglobin A1c (HbA1c) ≥6.5%, impaired fasting glucose (≥126 mg/dL), or 2-hour oral glucose ≥200 mg/dL within 2 weeks before enrollment. Total cholesterol (TC) and high-density lipoprotein cholesterol (HDL-c) were measured from fasting blood samples at a central laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories, Seattle, WA). Average systolic blood pressure (SBP) was taken from ≥2 separate measurements obtained using a Welch Allyn Spot Vital Signs Monitor (4200B, Hillrom, Batesville, Indiana). Subject-reported use of antihypertensive medications and smoking status were documented.
Non-surgical Comparators
Cincinnati Children’s Hospital Non-Surgical Cohort
Data were obtained from a study of youth, aged 10 to 23 years who had been enrolled in a study of obesity and type 2 diabetes mellitus (T2DM) on the heart and vasculature. The detailed methodology of this study has been previously published.(26) Diagnosis of T2DM was made by the participant’s primary care provider. This study was approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center.
Following an overnight fast (minimum 10-hours), participants underwent anthropometric, SBP, and laboratory assessments. Height, weight, and waist circumference were measured in a standardized manner, as previously published.(26) Two measures of height were obtained with a calibrated stadiometer (Veeder-Rood, Elizabethtown, North Carolina) and two measures of weight with a Health-O-Meter electronic scale (Jarden Consumer Solutions, Rye, New York) were averaged. SBP was measured according to standards described in the Fourth Report.(27) The average of three SBP measurements was taken using a mercury manometer. Fasting plasma lipid profiles (TC and HDL-c) were performed with standardized methods from the National Heart Lung and Blood Institute–Centers for Disease Control and Prevention. Smoking exposure and medication use was evaluated by self-report questionnaire.
University of Minnesota Non-Surgical Cohort
The University of Minnesota cohort included data from a cross-sectional and longitudinal study examining cardiovascular risk factors in youth ranging from normal-weight to severe obesity. Height and weight were determined using a wall-mounted stadiometer and an electronic scale, respectively. Seated SBP was measured after the participant had been resting quietly (10 minutes) with 3 consecutive times with an automated BP cuff at approximately 3-minute intervals and the average used. Fasting blood samples (>10hr) were used to measure TC and HDL-c using standard methods by the Fairview Diagnostics Laboratories, Fairview-University Medical Center (Minneapolis, MN, USA) - a Center for Disease Control and Prevention certified laboratory. Smoking exposure and medication use was evaluated by self-report questionnaire. Patients with diabetes were not enrolled into this Minnesota cohort.
Main Statistical Analysis
Standard descriptive statistics summarized participant characteristics at baseline. Categorical variables were calculated as frequencies and percentages, continuous variables as means and standard deviations. The analysis used two models for estimating full CVD event risks including coronary death, myocardial infarction, and stroke (fatal and nonfatal), angina pectoris, intermittent claudication, and congestive heart failure: 1) the first model included BMI but excluded lipids (TC and HDL-c), sex, age, SBP, anti-HTN treatment, smoking status, and diabetes status; 2) the second model excluded BMI but included lipids (TC and HDL-c), sex, age, SBP, anti-HTN treatment, smoking status, and diabetes status. Age, SBP, TC and HDL-c levels were treated as continuous variables. Sex, anti-HTN treatment, smoking status, and diabetes status were treated as categorical variables. The CVD event models from the Framingham Heart Study were developed in, and modeled for, persons aged 20 to 59 years of age. Due to the model constraint and to eliminate the bias, ages less than 20 were adjusted to 20 for all groups in this study. Subjects with age > 20 in comparison groups were removed to match the age range of Teen-LABS baseline (13–<20 years old). Due to group difference in diabetes status and low prevalence, normal-weight participants with diabetes were removed from the study. Next, we matched the proportion of overweight, obese, and severely obese groups with diabetes to the proportion with diabetes in the Teen-LABS cohort. Missing data patterns and percentages were examined before calculating risk scores. We detected no evidence of informative missing data. Prior to matching, only complete data were used for calculating risk scores. Main analyses were performed using SAS software version 9.4. (SAS Institute Inc., Cary, NC). Data were presented as mean with 95% confidence intervals. Statistical significance level was set at α=0.05. Tukey’s adjustment method for multiple testing was used for pairwise post hoc comparisons among normal, overweight, obese, severely obese groups and Teen-LABS baseline cohort within each hypothesis considered in this study. Linear mixed model was used for risk score comparisons between baseline and follow-up time points.
Effectiveness and Cost-effectiveness Modeling
A state-transition model was developed to assess the long-term and cost-effectiveness of MBS for CVD event risk reduction. The analyses used Teen-LABS baseline and post-surgical results compared to modeled non-surgical comparators using Teen-LABS baseline characteristics. The analysis had a time horizon of 30 years and the model cycle length was one year with modeling beginning at five years following MBS. To minimize potential bias favoring our hypothesis, we created the statistical model under the conservative assumption that non-surgical comparators would not gain weight over time (despite the existence of data to the contrary)(14, 19) but instead would remain at their initial BMI of 53 kg/m2. In the MBS group, we modeled either receiving Roux-en-Y gastric bypass (71%) or vertical sleeve gastrectomy (29%) based upon Teen-LABS distributions. We modeled BMI data showing a decline at 1-year with a slight increase each subsequent year with plateau at 10-years based on the results of the Swedish Obese Subjects study.(21) Transitions from the event-free state to cardiovascular event states were based on the 30-year composite risk score measured in the Teen-LABS study. Event states include coronary death, myocardial infarction, and stroke (fatal and nonfatal), angina pectoralis, intermittent claudication, and congestive heart failure. The distribution of cardiovascular events for males and females was based on incidence rates from the National Heart, Lung, and Blood Institute.(28) The probability of death from cardiovascular events was estimated from the American Heart Association event statistics.(29) The model also incorporated costs of MBS and cardiovascular events. Cardiovascular event costs and utilities were estimated separately for acute and post-first-year events.(11, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39) Both cohorts had a baseline Quality Adjusted Life Years (QALY) of 0.72, and the MBS group gained 0.0056 QALY per unit of BMI lost.(28, 40) All costs from prior years were adjusted to 2015-year dollars using the Consumer Price Index. Endpoints included QALYs, total costs (US $2015), and incremental cost-effectiveness ratios (ICERs). A willingness-to-pay (WTP) threshold of $100,000/QALY was used to determine cost-effectiveness. Costs and QALYs were each discounted at 3%. All cost effectiveness analyses were conducted using the R statistical platform.(41)
Results
Descriptive and clinical characteristics of the non-surgical and MBS cohorts used for these analyses are presented in Table 1. Of note, participants were matched on the age range of Teen-LABS (13–<20 years old) and prevalence of diabetes among the differing obesity groups.
Table 1.
Descriptive and clinical characteristics of the non-surgical comparators and Teen-LABS prior to surgery.
| 247 | 54 | 131 | 302 | 215 |
|---|---|---|---|---|
| 15.9 (2.5) | 16.8 (2.2) | 16.3 (2.3) | 16.4 (2.0) | 17.0 (1.5) |
| 41% | 37% | 44% | 38% | 25% |
| 56% | 56% | 47% | 55% | 72% |
| 42% | 37% | 51% | 43% | 22% |
| 2% | 7% | 2% | 2% | 6% |
| 164.7 (10.8) | 168.8 (8.9) | 167.2 (10.2) | 168.6 (9.0) | 168.2 (8.6) |
| 56.7 (11.5) | 76.8 (10.9) | 87.3 (13.5) | 117.8 (20.8) | 149.9 (30.5) |
| 20.7 (2.4) | 26.9 (2.2) | 31.1 (2.5) | 41.3 (5.8) | 52.9 (9.4) |
| 73.5 (6.9) | 92.9 (5.1) | 109.3 (5.7) | 144.8 (21.1) | 168.0 (30.6) |
| 107.5 (10.2) | 113.3 (8.9) | 116.8 (10.2) | 121.5 (11.8) | 125.4 (13.4) |
| 158(29) | 168(34) | 167 (32) | 167 (32) | 157(30) |
| 56(12) | 53 (13) | 46(10) | 44 (12) | 38 (9) |
| 1.6% | 1.9% | 0.0% | 2.7% | 1.9% |
| 0% | 13.0% | 13.7% | 13.6% | 13.5% |
| 0% | 5.6% | 3.8% | 5.3% | 22.8% |
Data are presented as mean (SD) and percentages.
Abbreviations (in order of appearance): pre-op = Before Surgery; BMI = body mass index; SBP = systolic blood pressure; TC = total cholesterol; HDL-c = high-density lipoprotein cholesterol; ant-HTN meds = prescribed anti-hypertension medications.
Baseline (pre-op) and post-surgical follow-up data are presented in Table 2 with by surgical type presented as supplements (RYGB: supplemental Table A; VSG supplemental Table B). The sample size at each time-point represents those with complete data. The nadir for post-surgical weight loss (BMI reduction and weight loss) was achieved at 1-year with small increases in 3-year, 4-year, and 5-year. SBP and TC decreased at 1-year and increased in each subsequent year. HDL-c increased at 1-year with a further increase at 2-years which was sustained out to 5-years. Diabetes prevalence decreased post-MBS (13.5% to 2.2% prevalence from baseline to 1-year) and the decrease was sustained to 5-years after surgery. A reduction in BP-medication use was also observed. Smoking prevalence increased from baseline.
Table 2.
Baseline and changes over a 5-year period in clinical characteristics among Teen-LABS participants.
| 215 | 181 | 168 | 162 | 166 | 158 |
| 17.0 (1.5) | 18.1 (1.5) | 19.3 (1.5) | 20.2 (1.5) | 21.1 (1.5) | 22.1 (1.6) |
| 25% | 26% | 23% | 24% | 24% | 25% |
| 72% | 72% | 69% | 70% | 71% | 72% |
| 22% | 23% | 25% | 24% | 23% | 22% |
| 6% | 5% | 6% | 6% | 6% | 6% |
| 168.2 (8.6) | 168.2 (9.1) | 168.8 (8.9) | 168.1 (9.5) | 168.8 (9.1) | 168.3 (10.0) |
| 149.9 (30.5) | 104.8 (27.0) | 105.5 (28.7) | 108.5 (31.7) | 111.5 (33.9) | 113.9 (32.0) |
| 52.9 (9.4) | 37.0 (8.9) | 37.0 (9.8) | 38.4 (10.9) | 39.1 (11.4) | 40.3 (10.9) |
| 125 (13) | 117(13) | 119 (13) | 118 (14) | 120(14) | 122 (14) |
| 157 (30) | 147(30) | 150(29) | 151 (29) | 156 (35) | 154(29) |
| 38 (9) | 48 (11) | 53 (12) | 53 (14) | 55 (15) | 54 (15) |
| 1.9% | 3.3% | 6.6% | 8.6% | 6.6% | 10.8% |
| 13.5% | 2.2% | 1.8% | 0.6% | 2.4% | 2.5% |
| 22.8% | 8.3% | 4.8% | 5.6% | 6.0% | 5.1% |
Data are presented as mean (SD) and percentages.
Abbreviations (in order of appearance): pre-op = Before Surgery; BMI = body mass index; SBP = systolic blood pressure; TC = total cholesterol; HDL-c = high-density lipoprotein cholesterol; Anti-HTN Meds = prescribed anti-hypertension medications.
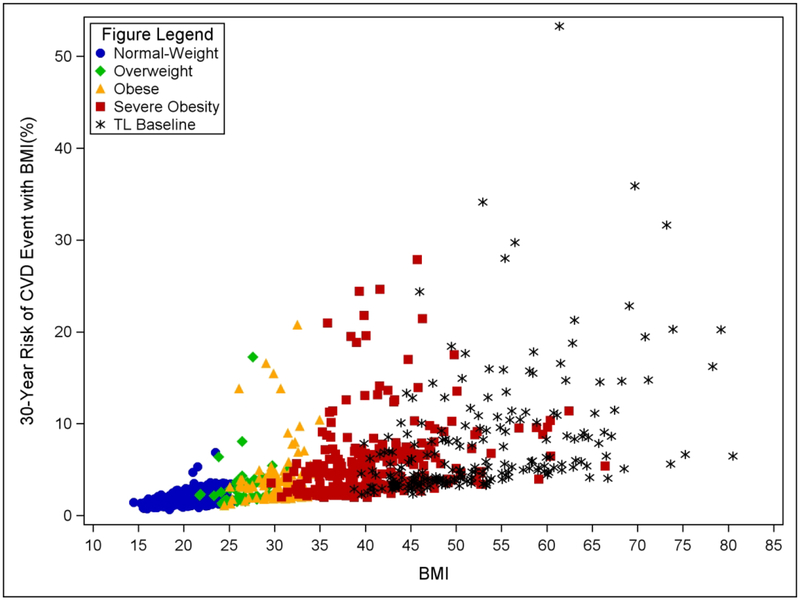
Figure 1 displays individual 30-year risk of CVD events (with BMI included in the model; Supplemental Figure A shows without BMI in the model) for each comparison group and Teen-LABS pre-op versus BMI. Individual variation was observed in each group and was most pronounced among adolescents with obesity and severe obesity (Table 3). Teen-LABS pre-op: 52.6% of the cohort presented with >5% risk of CVD events, 22.3% with >10% risk, 10.7% with >15% risk, and 5.6% with >20% risk; Severe obesity: 38.7% of the cohort presented with >5% risk of CVD events, 8.9% with >10% risk, and 3.6% with >15% risk; Obesity: 13.0% of the cohort presented with >5% risk of CVD events, 4.6% with >10% risk, and 2.3% with >15% risk; Overweight: 9.3% with overweight presented with >5% risk of CVD events and 1.9% had >10% risk of CVD events. Conversely, only 2 out of 247 (0.8%) youth with normal weight presented with >5% risk of CVD events and no youth with normal weight had >10% risk of CVD events.
Figure 1.
Individual 30-year Risk for CVD events versus BMI. Data use full CVD model with BMI included and HDL-c and TC excluded.
Table 3.
Proportion of individuals with estimated CVD event risk at different thresholds in each comparison group and Teen-LABS preoperatively.
| Risk Level | Normal-weight (n=247) | Overweight (n=54) | Obese (n=131) | Severe Obesity (n=302) | Teen-LABS Pre-op (n=215) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | n | n | n | n | ||||||
| >5% | 2 | 5 | 17 | 117 | 113 | |||||
| >10% | 0 | 1 | 6 | 27 | 48 | |||||
| >15% | 0 | 1 | 3 | 11 | 23 | |||||
| >20% | 0 | 0 | 1 | 6 | 12 | |||||
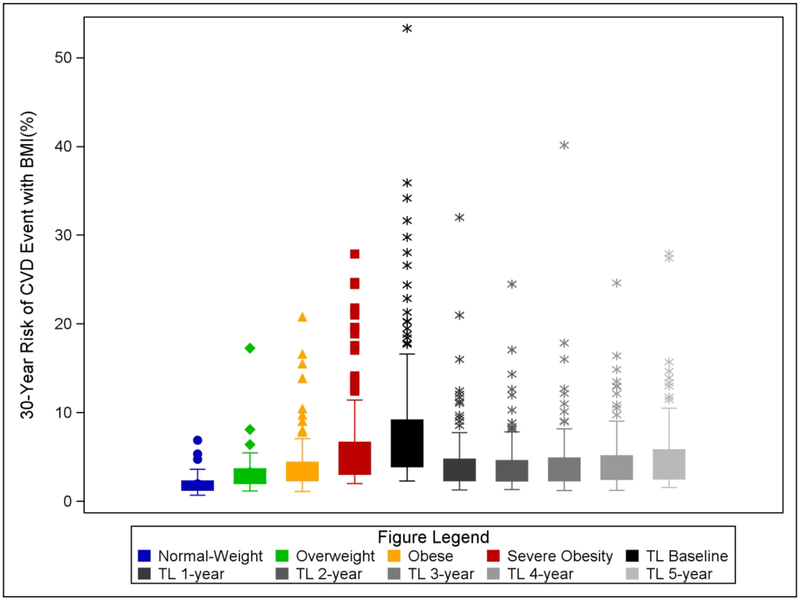
Figure 2 depicts the 30-year risk of CVD events (with BMI included in the model; Supplemental Figure B shows without BMI in the model) of each non-surgical cohort group and Teen-LABS at each time-point. Post-hoc pairwise comparisons were conducted (using Tukey method) between groups. No statistical differences in 30-year CVD event risk were observed between youth with normal-weight (1.8[0.8]% and overweight (3.1[2.4]%; p=0.24) nor overweight and obese youth (3.9[3.0]%; p=0.76). The Teen-LABS group at baseline (before operation) had a significantly higher 30-year CVD event risk (7.9[6.7]%) as compared to all comparisons groups (all p<0.0001). Youth with severe obesity had higher 30-year CVD event risk (5.5[4.0]%) than youth with normal-weight, overweight, and obesity (all p<0.01). Following MBS in Teen-LABS participants, a statistical significant reduction in 30-year CVD event risk was observed at 1-year which was sustained at each time-point up to 5 years after surgery (P<0.0001 all). The proportion of participants with elevated CVD risk scores fell [>5% (52.6% to 22.1%), >10% (22.3% to 4.4%), and >15% (10.7% to 1.7%)] at 1-year (supplemental Table C). These reductions were sustained with no statistically significant changes by years 4 or 5.
Figure 2.
Box and whiskers plot of by each comparison group and Teen-LABS (TL) at baseline and each subsequent post-surgical time point for 30-year Risk for CVD events. Data use full CVD model with BMI included and HDL-c and TC excluded. Individual data points are shown in corresponding colors.
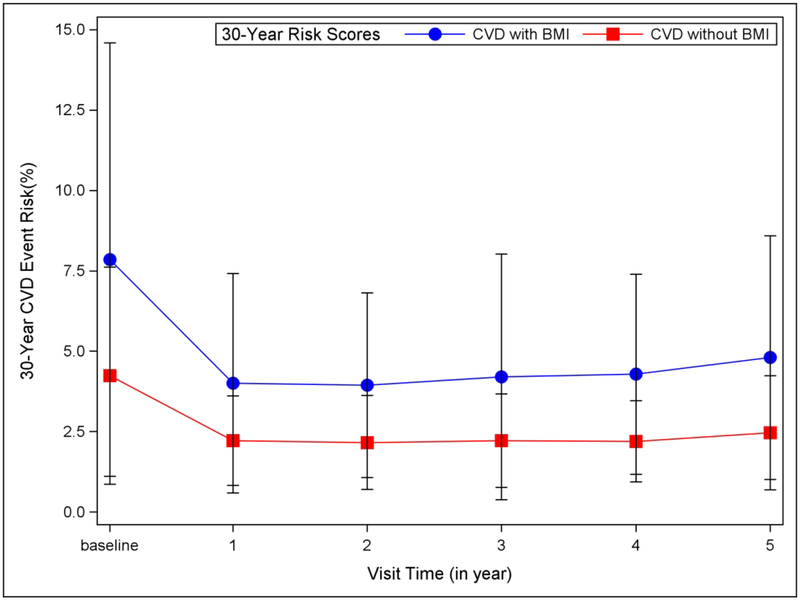
Figure 3 compares the 30-year risk of CVD events for two models (1] with BMI excluding HDL and TC; 2] without BMI but including TC and HDL) for the Teen-LABS cohort at each time-point. While CVD events were reduced in both models following MBS and sustained for 3 years, the estimates of CVD event risk were lower without the inclusion of BMI in model. Analysis by surgical type were conducted as well showing a similar patter (RYGB: Supplemental Figure C; VSG Supplemental Figure D). It should be noted that these analyses are underpowered due to sample size limitations.
Figure 3.
Mean (±1 SD) 30-year Risk for CVD event risk for the Teen-LABS cohort at each time point with 2 models: 1) Blue: Full CVD with BMI included and HDL-c and TC excluded, 2) Red: Full CVD with BMI excluded and HDL-c and TC included.
Table 4 shows effectiveness models of MBS versus nonsurgical controls for CVD events and deaths over 30-years. The number of qualifying youth with severe obesity needing to be treated with MBS to prevent one cardiovascular event was 36.5 and to prevent one cardiovascular death was 379. These analyses were expanded (supplemental table D) to examine the cost-effectiveness of MBS for preventing cardiovascular events. Despite the initial cost of MBS ($24,675.59), the MBS models indicate higher QALY (18.5 versus 16.8) than control with an ICER of $13,432.64/QALY. Using a willingness-to-pay threshold of $100,000/QALY, MBS was cost effective as it is well below the cutoff. Detailed cardiovascular event probability and costs are included in Supplemental Table E.
Table 4.
Effectiveness of Bariatric Surgery on Cardiovascular Events and Death.
| Outcome | Group | Estimated Event/Death Rate | Number Needed to Treat |
|---|---|---|---|
| Total Cardiovascular Events | Control | 7.31% | 36.5 |
| Bariatric Surgery | 4.58% | ||
| Cardiovascular Deaths | Control | 0.70% | 379 |
| Bariatric Surgery | 0.44% |
Discussion
The main results of these analyses indicate that adolescents with severe obesity, especially those qualifying for MBS, appear to be at pronounced risk for a major cardiovascular event within 30 years, and that by undergoing MBS as an adolescent, this risk of hard cardiovascular endpoints was substantially mitigated despite a post-operative plateau of BMI that did not drop below the cutoff for severe obesity for most participants. From a health care cost perspective, despite the initial cost of surgery, these data indicate that MBS in adolescents with severe obesity is cost-effective by preventing later CVD events and early CVD deaths.
Prior to undergoing MBS, adolescents with severe obesity face a greater than 4-fold higher risk of a CVD events within 30-years compared to normal-weight peers. Even when compared with age- and diabetes frequency-matched adolescents with severe obesity who did not undergo MBS, a statistically significant and clinically meaningfully elevated risk of 30-year CVD events was found prior to surgery. This finding emphasizes the truly unique nature of adolescents who qualify for and elect to undergo MBS, as they appear to harbor a very concerning CVD risk profile. However, despite having lower estimated CVD event risk than the Teen-LABS cohort, youth with severe obesity who did not undergo MBS (the comparison group) were also at pronounced risk for CVD events when compared with less obese and normal-weight peers. These results support the position that more effective treatment options are needed in this group to mitigate this risk.(20)
The findings from this study, despite differences in methodology, are similar to data in adults undergoing MBS that demonstrate significant reductions in measured CVD events and mortality.(21, 22, 42) Our study is limited by using estimated risk models, however, the models have been validated in adults using hard endpoints (e.g. stroke, heart failure). In addition, our use of contemporaneous comparison groups demonstrates striking treatment effects of surgery, irrespective of the residual high BMI values in many of the postoperative cohort members. Despite adult studies being able to measure actual events, the similarity in terms of relative treatment effects are striking. The Swedish Obese Subjects study demonstrated after a median follow-up of 14.7 years (range, 0–20 years) that MBS reduced the risk for total CVD events by 33%, while our analysis of these adolescent data estimated a 39% risk reduction at 30 years. Swedish Obese Subjects showed that the number needed to treat to prevent 1 event was 50 persons,(21) while our modeling with adolescent data estimated only 36.5 persons. In addition, another cohort composed entirely of adults undergoing Roux-en-y gastric bypass has demonstrated similar findings with a reduction in composite CVD outcomes (HR: 0.58) and reduced 10-year Framingham Risk Score at 5 years when compared to non-surgical controls.(23) Collectively, our data using risk models are consistent with these findings using hard endpoints, despite the limitations inherent in a modeling approach to prediction of long-term CVD outcomes.
Importantly, the reduced risk was a stable finding in spite of the majority of surgical participants remaining in the severe obesity category (50.8%[92/181]) following MBS. The reduced weight, combined with improvements in dyslipidemia, reductions in blood pressure, and diabetes prevalence all contributed to the risk reduction. Moreover, the sustained risk reduction was observed despite a significant increase in smoking prevalence from pre-op (1.9%) to 5 year (10.8%). These findings further highlight the multifaceted metabolic effects of MBS which are more profound than weight loss alone. Given these findings it will be important to continue to follow adolescents post-MBS long-term in order to better understand the implications, particularly considering the relatively high degree of heterogeneity that was projected. Future work could focus on better understanding the sources of variability and whether patient selection in terms of number and severity of co-morbidities may play a role. Furthermore, these data highlight the need for creation of CVD risk factor scoring longer than 30 years which encompass adolescent age groups.
This study has several strengths including a large sample size of the surgical and comparison cohorts, longitudinal follow-up for 5-years after surgery with excellent cohort retention, and the use of clinically relevant outcomes and measures. However, there are several limitations inherent in this work as well. The algorithm used to calculated risk reduction makes certain assumptions that deserve mention. The 30-year risk scores were originally developed for persons 20–<50 years old, therefore, our models used an age of 20 for calculations despite primary data from individuals with mean ages of ~17 years. While this assumption represents a constraint and a study limitation, there is little reason to believe that there are substantive differences in CVD event risk between the mean age of 17 and the 20 years of age used to conduct analysis, therefore, this necessary assumption likely does not alter the risk assessment. The racial distribution of the Framingham cohort is largely white and the diversity difference documented in our cohort may lead to bias in calculation of risk. While the risk scores were developed in the Framingham Heart study whose benefits and limitations have been well documented,(43, 44) the predicted risk of events does not always equate to actual event occurrence and may be an overestimation as presented in this study. We conducted analysis examining differences by surgical procedure, however due to sample size these data are underpowered and should be view with caution, it will be important for future studies to examine these important differences.
Conclusion
Adolescents with severe obesity are at a much higher risk of experiencing a major CVD event within 30-years in comparison to peers with normal-weight, overweight, or obesity. Following MBS, risk of CVD events was substantially and durably reduced. The estimated number of adolescents with severe obesity needed to treat in order to prevent 1 event is 36.5. MBS appears to be cost-effective due in part to CVD event prevention.
Supplementary Material
Supplemental Figure A. Individual 30-year Risk for CVD events versus BMI. Data use full CVD model with BMI excluded and HDL-c and TC included.
Supplemental Figure B. Box and whiskers plot of by each comparison group and Teen-LABS (TL) at baseline and each subsequent post-surgical time point for 30-year Risk for CVD events. Data use full CVD model with BMI excluded and HDL-c and TC included.
Individual data points are shown in corresponding colors.
Supplemental Figure C. RYGB only. Mean (±1 SD) 30-year Risk for CVD event risk for the Teen-LABS cohort at each time point with 2 models: 1) Blue: Full CVD with BMI included and HDL-c and TC excluded, 2) Red: Full CVD with BMI excluded and HDL-c and TC included.
Supplemental Figure D. VSG only. Mean (±1 SD) 30-year Risk for CVD event risk for the Teen-LABS cohort at each time point with 2 models: 1) Blue: Full CVD with BMI included and HDL-c and TC excluded, 2) Red: Full CVD with BMI excluded and HDL-c and TC included.
What is Known on This Subject
Adolescents with severe obesity often present with elevated levels of many chronic disease risk factors including cardiovascular disease.
Metabolic and bariatric surgery causes significant weight reduction which is associated with improvements in risk factors and co-morbidities for many chronic diseases.
What This Study Adds
We demonstrate that adolescents with severe obesity have significant risk for having a cardiovascular event prior to the age of 50 years old.
Metabolic and bariatric surgery produces a marked and sustained reduction in cardiovascular event risk.
Acknowledgements
We would like to thank all of the adolescents and young adults who participated in these studies.
Funding Source: Funding for Teen-LABS was provided by the National Institutes of Health (NIH) (U01DK072493 / UM1 DK072493 to T.H.I.) and the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH (8UL1TR000077). Support also came from National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, (UL1TR000114). Non-surgical comparison cohorts were provided in part by funding from NIH grants F32-HL127881 (J.R.R.), R01-HL110957 (A.S.K), and R01-HL105591 (E.M.U). Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114 (J.R.R.).
Abbreviations (in order of appearance):
- CVD
cardiovascular disease
- MBS
metabolic and bariatric surgery
- BMI
body mass index
- Teen-LABS
Teen-Longitudinal Assessment of Bariatric Surgery
- TC
Total cholesterol
- HDL-c
high-density lipoprotein cholesterol
- SBP
systolic blood pressure
- T2DM
type 2 diabetes mellitus
- anti-HTN
anti-hypertension
Footnotes
Trial Registration: Teen-LABS ClinicalTrials.gov number, .
Author Disclosures: Dr. Ryder receives support from Boehringer Ingelheim Pharmaceuticals in the form of drug/placebo. Dr. Inge has served as consultant for Standard Bariatrics and Independent Medical Expert Consulting Services, both unrelated to this project. Dr. Kelly serves as a consultant for Vivus Pharmaceuticals, Novo Nordisk Pharmaceuticals, and WW but does not accept personal or professional income for his services. Dr. Kelly also receives research support from Astra Zeneca Pharmaceuticals in the form of drug/placebo. Dr. Urbina receives compensation from Astellas for participation on a DSMB for a drug study unrelated to this study.
Bibliography
- 1.Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med 2011;365: 1876–1885. [DOI] [PubMed] [Google Scholar]
- 2.Twig G, Yaniv G, Levine H, Leiba A, Goldberger N, Derazne E, et al. Body-Mass Index in 2.3 Million Adolescents and Cardiovascular Death in Adulthood. N Engl J Med 2016;374: 2430–2440. [DOI] [PubMed] [Google Scholar]
- 3.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med 2010;362: 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker JL, Olsen LW, Sørensen TIA. Childhood Body-Mass Index and the Risk of Coronary Heart Disease in Adulthood. New England Journal of Medicine 2007;357: 2329–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med 1992;327: 1350–1355. [DOI] [PubMed] [Google Scholar]
- 6.Bjorge T, Engeland A, Tverdal A, Smith GD. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol 2008;168: 30–37. [DOI] [PubMed] [Google Scholar]
- 7.DiPietro L, Mossberg HO, Stunkard AJ. A 40-year history of overweight children in Stockholm: life-time overweight, morbidity, and mortality. Int J Obes Relat Metab Disord 1994;18: 585–590. [PubMed] [Google Scholar]
- 8.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. Jama 2016;315: 2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pencina MJ, D’Agostino RB, Larson MG Sr., Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: the framingham heart study. Circulation 2009;119: 3078–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen KK, Olsen TS, Dehlendorff C, Kammersgaard LP. Hemorrhagic and ischemic strokes compared: stroke severity, mortality, and risk factors. Stroke 2009;40: 2068–2072. [DOI] [PubMed] [Google Scholar]
- 12.Beamish AJ, Olbers T, Kelly AS, Inge TH. Cardiovascular effects of bariatric surgery. Nature reviews Cardiology 2016;13: 730–743. [DOI] [PubMed] [Google Scholar]
- 13.Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML, et al. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. New England Journal of Medicine 2016;374: 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inge TH, Jenkins TM, Xanthakos SA, Dixon JB, Daniels SR, Zeller MH, et al. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): a prospective follow-up analysis. The lancet Diabetes & endocrinology 2017;5: 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation 2013;128: 1689–1712. [DOI] [PubMed] [Google Scholar]
- 16.Michalsky MP, Inge TH, Jenkins TM, Xie C, Courcoulas A, Helmrath M, et al. Cardiovascular Risk Factors After Adolescent Bariatric Surgery. Pediatrics 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryder JR, Edwards NM, Gupta R, Khoury J, Jenkins TM, Bout-Tabaku S, et al. Changes in Functional Mobility and Musculoskeletal Pain After Bariatric Surgery in Teens With Severe Obesity: Teen-Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA pediatrics 2016;170: 871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryder JR, Gross AC, Fox CK, Kaizer AM, Rudser KD, Jenkins TM, et al. Factors associated with long-term weight loss maintenance following bariatric surgery in adolescents with severe obesity. Int J Obes 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olbers T, Beamish AJ, Gronowitz E, Flodmark CE, Dahlgren J, Bruze G, et al. Laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity (AMOS): a prospective, 5-year, Swedish nationwide study. The lancet Diabetes & endocrinology 2017;5: 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.RJ R, FC K, KA S. Treatment Options for Severe Obesity in the Pediatric Population: Current Limitations and Future Opportunities. Obesity 2018;26: 951–960. [DOI] [PubMed] [Google Scholar]
- 21.Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, et al. Bariatric surgery and long-term cardiovascular events. Jama 2012;307: 56–65. [DOI] [PubMed] [Google Scholar]
- 22.Romeo S, Maglio C, Burza MA, Pirazzi C, Sjöholm K, Jacobson P, et al. Cardiovascular Events After Bariatric Surgery in Obese Subjects With Type 2 Diabetes. Diabetes Care 2012;35: 2613–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benotti PN, Wood GC, Carey DJ, Mehra VC, Mirshahi T, Lent MR, et al. Gastric Bypass Surgery Produces a Durable Reduction in Cardiovascular Disease Risk Factors and Reduces the Long-Term Risks of Congestive Heart Failure. Journal of the American Heart Association 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML, et al. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. New England Journal of Medicine;0: null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inge TH, Zeller MH, Jenkins TM, Helmrath M, Brandt ML, Michalsky MP, et al. Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. JAMA pediatrics 2014;168: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urbina EM, Kimball TR, McCoy CE, Khoury PR, Daniels SR, Dolan LM. Youth with obesity and obesity-related type 2 diabetes mellitus demonstrate abnormalities in carotid structure and function. Circulation 2009;119: 2913–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114: 555–576. [PubMed] [Google Scholar]
- 28.National Heart, Lung, and Blood Institute, National Institutes of Health. Incidence and Prevalence: 2006 Chart Book on Cardiovascular and Lung Diseases. Bethesda, United States: National Institutes of Health (NIH), 2006. [Google Scholar]
- 29.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation 2015;131: e29–322. [DOI] [PubMed] [Google Scholar]
- 30.O’Sullivan AK, Rubin J, Nyambose J, Kuznik A, Cohen DJ, Thompson D. Cost estimation of cardiovascular disease events in the US. PharmacoEconomics 2011;29: 693–704. [DOI] [PubMed] [Google Scholar]
- 31.Fox KM, Wang L, Gandra SR, Quek RGW, Li L, Baser O. Clinical and economic burden associated with cardiovascular events among patients with hyperlipidemia: a retrospective cohort study. BMC cardiovascular disorders 2016;16: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thijs V, Butcher K. Challenges and misconceptions in the aetiology and management of atrial fibrillation-related strokes. European journal of internal medicine 2015;26: 461–467. [DOI] [PubMed] [Google Scholar]
- 33.Yong JH, Thavorn K, Hoch JS, Mamdani M, Thorpe KE, Dorian P, et al. Potential Cost-Effectiveness of Ambulatory Cardiac Rhythm Monitoring After Cryptogenic Stroke. Stroke 2016;47: 2380–2385. [DOI] [PubMed] [Google Scholar]
- 34.Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation 2011;123: 2562–2570. [DOI] [PubMed] [Google Scholar]
- 35.Coyle D, Coyle K, Cameron C, Lee K, Kelly S, Steiner S, et al. Cost-effectiveness of new oral anticoagulants compared with warfarin in preventing stroke and other cardiovascular events in patients with atrial fibrillation. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research 2013;16: 498–506. [DOI] [PubMed] [Google Scholar]
- 36.Chapman RH, Liu LZ, Girase PG, Straka RJ. Determining initial and follow-up costs of cardiovascular events in a US managed care population. BMC cardiovascular disorders 2011;11: 11–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matza LS, Stewart KD, Gandra SR, Delio PR, Fenster BE, Davies EW, et al. Acute and chronic impact of cardiovascular events on health state utilities. BMC health services research 2015;15: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fanari Z, Weintraub WS. Cost-effectiveness of medical, endovascular and surgical management of peripheral vascular disease. Cardiovascular revascularization medicine : including molecular interventions 2015;16: 421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merchant RM, Becker LB, Abella BS, Asch DA, Groeneveld PW. Cost-Effectiveness of Therapeutic Hypothermia After Cardiac Arrest. Circulation: Cardiovascular Quality and Outcomes 2009. [DOI] [PubMed] [Google Scholar]
- 40.Karlsson J, Taft C, Ryden A, Sjostrom L, Sullivan M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes (Lond) 2007;31: 1248–1261. [DOI] [PubMed] [Google Scholar]
- 41.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing,. 2015. [Google Scholar]
- 42.Benotti PN, Wood GC, Carey DJ, Mehra VC, Mirshahi T, Lent MR, et al. Gastric Bypass Surgery Produces a Durable Reduction in Cardiovascular Disease Risk Factors and Reduces the Long-Term Risks of Congestive Heart Failure. Journal of the American Heart Association 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlendorf KH, Nasir K, Blumenthal RS. Limitations of the Framingham risk score are now much clearer. Preventive medicine 2009;48: 115–116. [DOI] [PubMed] [Google Scholar]
- 44.Hemann BA, Bimson WF, Taylor AJ. The Framingham Risk Score: an appraisal of its benefits and limitations. The American heart hospital journal 2007;5: 91–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure A. Individual 30-year Risk for CVD events versus BMI. Data use full CVD model with BMI excluded and HDL-c and TC included.
Supplemental Figure B. Box and whiskers plot of by each comparison group and Teen-LABS (TL) at baseline and each subsequent post-surgical time point for 30-year Risk for CVD events. Data use full CVD model with BMI excluded and HDL-c and TC included.
Individual data points are shown in corresponding colors.
Supplemental Figure C. RYGB only. Mean (±1 SD) 30-year Risk for CVD event risk for the Teen-LABS cohort at each time point with 2 models: 1) Blue: Full CVD with BMI included and HDL-c and TC excluded, 2) Red: Full CVD with BMI excluded and HDL-c and TC included.
Supplemental Figure D. VSG only. Mean (±1 SD) 30-year Risk for CVD event risk for the Teen-LABS cohort at each time point with 2 models: 1) Blue: Full CVD with BMI included and HDL-c and TC excluded, 2) Red: Full CVD with BMI excluded and HDL-c and TC included.