Abstract
Background
Metabolic acidosis is a feature of chronic kidney disease (CKD) due to the reduced capacity of the kidney to synthesise ammonia and excrete hydrogen ions. It has adverse consequences on protein and muscle metabolism, bone turnover and the development of renal osteodystrophy. Metabolic acidosis may be corrected by oral bicarbonate supplementation or in dialysis patients by increasing the bicarbonate concentration in dialysate fluid.
Objectives
To examine the benefits and harms of treating metabolic acidosis in patients with CKD, both prior to reaching end‐stage renal disease (ESRD) or whilst on renal replacement therapy (RRT), with sodium bicarbonate or increasing the bicarbonate concentration of dialysate.
Search methods
We searched the Cochrane Renal Group's Specialised Register [up to 4 March 2013] through contact with the Trials' Search Co‐ordinator using search terms relevant to this review.
Selection criteria
Randomised controlled trials (RCTs), crossover RCTs and quasi‐RCTs investigating the correction of chronic metabolic acidosis in adults or children with CKD.
Data collection and analysis
Outcomes were analysed using risk ratio (RR) and mean difference (MD) for continuous measures.
Main results
We identified three trials in adult dialysis patients (n = 117). There were insufficient data for most outcomes for meta‐analysis. In all three trials acidosis improved in the intervention group though there was variation in achieved bicarbonate level. There was no evidence of effect on blood pressure or sodium levels. Some measures of nutritional status/protein metabolism (e.g. SGA, NP NA) were significantly improved by correction in the one trial that looked in these in detail. There was heterogeneity of the effect on serum albumin in two trials. Serum PTH fell significantly in the two trials that estimated this, with no significant effect on calcium or phosphate though both fell after correction. Complex bone markers were assessed in one study, with some evidence for a reduction in bone turnover in those with initial high bone turnover and an increase in low turnover patients. The studies were underpowered to assess clinical outcomes, in the one study that did there was some evidence for a reduction in hospitalisation after correction.
Authors' conclusions
The evidence for the benefits and risks of correcting metabolic acidosis is very limited with no RCTs in pre‐ESRD patients, none in children, and only three small trials in dialysis patients. These trials suggest there may be some beneficial effects on both protein and bone metabolism but the trials were underpowered to provide robust evidence.
Plain language summary
There is limited evidence from three small trials suggesting that the correction of metabolic acidosis trials may have some beneficial effects on both protein and bone metabolism
In health, protein and amino acids remain in equilibrium however in CKD this balance is disturbed. Metabolic acidosis has been shown to have deleterious effects on protein balance, leading to a negative nitrogen balance, increased protein degradation, increased essential amino acid oxidation, reduced albumin synthesis and a lack of adaption to a low protein diet, and hence is associated with protein energy malnutrition, loss of lean body mass and muscle weakness. Metabolic acidosis is also a factor in the development of renal bone disease, as bone acts as a buffer for excess acid, with loss of mineral resulting from the increase in acid. This review found three small trials in adult haemodialysis patients (n = 117). The evidence for the benefits and risks of correcting metabolic acidosis is very limited with no RCTs in pre‐ESRD patients and none in children. These trials suggest there may be some beneficial effects on both protein and bone metabolism but the trials were underpowered to provide strong evidence.
Background
Metabolic acidosis is a feature of chronic kidney disease (CKD) due to the reduced capacity of the kidney to synthesise ammonia and excrete hydrogen ions. It has consequences on protein and muscle metabolism, bone turnover and the development of renal osteodystrophy (Kalandar‐Zadeh 2004; Kopple 2005).
In health, protein and amino acids remain in equilibrium however in CKD this balance is disturbed (Mitch 1993). Metabolic acidosis has been shown to have deleterious effects on protein balance, leading to a negative nitrogen balance, increased protein degradation, increased essential amino acid oxidation, reduced albumin synthesis and a lack of adaption to a low protein diet, and hence is associated with protein energy malnutrition, loss of lean body mass and muscle weakness (Kalandar‐Zadeh 2004). Metabolic acidosis is also associated with inflammatory mediators, reduced leptin, insulin resistance, increased corticosteroid production and PTH production (Kalandar‐Zadeh 2004; Mehrotra 2003). The mechanism for reducing protein may include effects on ATP‐dependent ubiquitin proteosome and increased activity of branched chain keto acid dehydrogenases. In the NHANES III prevalence study hypoalbuminaemia (a marker of protein energy malnutrition and a powerful predictive marker of mortality in renal failure and the general population) was independently associated with low bicarbonate as well as the inflammatory marker CRP (Eustace 2004).
Metabolic acidosis is a factor in the development of renal osteodystrophy, as bone acts as a buffer for excess acid, with loss of mineral resulting (Kalandar‐Zadeh 2004; Kraut 2000). Acidosis may interfere with vitamin D metabolism, and patients who are persistently more acidotic are more likely to have osteomalacia or low‐turnover bone disease (Bushinsky 1995).
Most observational studies of nutrition and metabolic acidosis in dialysis patients have found an inverse relationship thought to be due to reverse epidemiology with higher protein intake (i.e. good appetite) associated with a degree of acidosis (Kalandar‐Zadeh 2004; Lin 2002). In the DOPPS study of haemodialysis (HD) patients, severe acidosis (< 16 mEq/L) was associated with higher risk of mortality and hospitalisation though patients with mild acidosis had better outcomes than patients with normal range bicarbonate (Bommer 2004). This J‐shaped curve has also been found by Lowrie 1990 in analysis of dialysis patients.
Metabolic acidosis may be corrected in dialysis by increasing the bicarbonate in dialysate fluid or by giving oral sodium bicarbonate. In pre end‐stage renal disease (ESRD) some correction of acidosis is observed when alkaline calcium salts are used as phosphate binders, but the main method is the administration of oral sodium bicarbonate. Patients prescribed bicarbonate have to take between 6 to 12 tablets/day. The switch to a non‐calcium containing binder such as sevelamer hydrochloride may lead to more acidosis. One potential undesired effect of sodium bicarbonate is the exacerbation of salt and water retention resulting in a worsening of hypertension or oedema or both. Limited evidence in humans suggests that this is more of a problem when sodium chloride is used (Husted 1975).
There have been several short‐term non‐randomised intervention studies showing various effects of correction of acidosis on markers of protein balance (Kooman 1997; Movilli 1998; Reaich 1993; Verove 2002) and to lesser extent on markers of bone turnover and calcium phosphate balance (Lu 1994).
The absolute benefits to patients of acidosis correction are not clearly identified. It is important therefore to systematically review the randomised evidence on the correction of metabolic acidosis. This review focuses on the treatment of metabolic acidosis in patients with CKD both prior to reaching end stage renal failure (ESRF) and whilst on renal replacement therapy (RRT). The prevention of acidosis by different dialysis buffers (acetate‐free haemodialysis (HD) and lactate buffering in peritoneal dialysis (PD)) were not reviewed here.
Objectives
Does correction of metabolic acidosis improve the nutritional state of CKD patients?
Does the correction of metabolic acidosis alter bone turnover and so reduce the development of renal osteodystrophy in CKD patients?
Is the use of oral bicarbonate to correct metabolic acidosis safe in relation to hypertension and fluid overload?
Does correction of metabolic acidosis improve patients' quality of life, reduce hospitalisation or reduce mortality?
Methods
Criteria for considering studies for this review
Types of studies
Inclusion criteria
Randomised controlled trials (RCTs), crossover RCTs and quasi‐RCTs (defined by randomisation methods that do not ensure concealed allocation ‐ e.g. alternation, date of birth, days of week, hospital number, open lists) investigating the correction of chronic metabolic acidosis in patients with CKD were considered.
Exclusion criteria
Uncontrolled trials or observational studies.
Short duration of correction of less than four weeks.
Types of participants
Adults and children with CKD, whether or not they were receiving RRT.
Presence of metabolic acidosis at entry to trial (initial venous bicarbonate must be stated).
Types of interventions
Oral sodium bicarbonate supplements versus placebo or no treatment.
Increase or variation in HD or PD dialysate bicarbonate concentration.
Types of outcome measures
Where available the following outcome measures were collected.
Nutritional state and protein turnover
Body weight
Body mass measures (lean)
Serum albumin
Nitrogen balance studies
Isotope protein turnover studies
Skinfold thickness (e.g. triceps)
Subjective measures of nutrition status (e.g. Subjective Global Assessment (SGA))
Markers of bone metabolism and renal bone disease
Bone biopsy, histomorphometry (e.g. osteoid volume, osteoclasts/mm²)
X‐ray changes
Symptoms (e.g. bone pain, fractures)
Parathyroid hormone level
Calcium and phosphate levels, alkaline phosphatase
Osteocalcin
Isotopic bone turnover studies
Adverse reactions
Fluid overload
Systolic and diastolic blood pressure
General clinical outcomes
Hospitalisation (hospitalised or not, days in hospital)
All‐cause mortality
Quality of life
Generic
Disease specific
Search methods for identification of studies
We searched the Cochrane Renal Group's Specialised Register [up to 4 March 2013] through contact with the Trials' Search Co‐ordinator using search terms relevant to this review.
The Cochrane Renal Group’s Specialised Register contains studies identified from:
Quarterly searches of the Cochrane Central Register of Controlled Trials CENTRAL;
Weekly searches of MEDLINE OVID SP;
Handsearching of renal‐related journals & the proceedings of major renal conferences;
Searching of the current year of EMBASE OVID SP;
Weekly current awareness alerts for selected renal‐journals;
Searches of the International Clinical Trials Register (ICTRP) Search Portal & ClinicalTrials.gov
Studies contained in the Specialised register are identified through search strategies for CENTRAL, MEDLINE, EMBASE based on the scope of the Cochrane Renal Group. Details of these strategies as well as a list of handsearched journals, conference proceedings and current awareness alerts are available in the 'Specialised Register' section of information about the Cochrane Renal Group
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of nephrology textbooks, e.g. Oxford Textbook of Nephrology, and reference lists from studies were scanned, as well as review articles
Letters seeking information about unpublished or incomplete trials to investigators known to be involved in previous studies
Data collection and analysis
The review was largely undertaken by two authors (PR, NW). The search strategy described was used to obtain titles and abstracts of studies that might have been relevant to the review. The titles and abstracts were screened independently and discarded if not applicable, however studies and reviews that might include relevant data or information on trials were retained initially. The retrieved abstracts were screened independently and, if necessary the full text of these studies, to determine which studies satisfied the inclusion criteria. Data extraction was carried out independently by the same authors using standard data extraction forms. There were no studies reported in non‐English language journals. Where more than one publication of one trial existed, only the publication with the most complete data was included.
Study quality
Two authors independently performed assessment of study quality (allocation concealment, blinding, intention‐to‐treat analysis, completeness of follow‐up), without blinding to authorship or journal, using the checklist below.
Quality checklist
Allocation concealment
Adequate (A): Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study.
Unclear (B): Randomisation stated but no information on method used is available.
Inadequate (C): Method of randomisation used such as alternate medical record numbers or unsealed envelopes; any information in the study that indicated that investigators or participants could influence intervention group.
Blinding
Blinding of investigators: Yes/No/not stated
Blinding of participants: Yes/No/not stated
Blinding of outcome assessor: Yes/No/not stated
Blinding of data analysis: Yes/No/not stated
In trials where no placebo is used, the participants was considered non‐blinded.
Intention‐to‐treat analysis
Yes: Specifically reported by authors that intention‐to‐treat analysis (ITT) was undertaken and this was confirmed on study assessment, or not stated but evident from study assessment that ITT was undertaken.
Unclear. Reported but unable to confirm on study assessment, or not reported and unable to confirm by study assessment.
No: Lack of intention‐to‐treat analysis confirmed on study assessment (Patients who were randomised were not included in the analysis because they did not receive the study intervention, they withdrew from the study or were not included because of protocol violation) regardless of whether ITT reported or not.
Completeness of follow‐up
The percentage of participants for whom data were complete at defined study end‐point.
Data extraction
Two authors extracted data independently. Discrepancies were resolved by discussion. Data were double entered into RevMan.
Statistical analysis
For dichotomous data, risk ratios (RR) was used with 95% confidence intervals (CI) and for continuous data, mean difference (MD). Data was pooled using the random‐effects model but the fixed‐effect model was also to be analysed to ensure robustness of the model chosen and susceptibility to outliers. Heterogeneity was analysed using the Chi‐squared and I² tests with a P of 0.05 used for statistical significance (Higgins 2003).
Sensitivity analysis was to be performed on aspects of quality where possible:
Concealed allocation: two group/crossover/quasi‐randomised methods
Blinded assessment
Loss to follow‐up
Dose of bicarbonate
Duration of bicarbonate
Degree of acidosis at start
Severity of renal failure at start of intervention (pre‐ESRD versus RRT)
Mode of RRT
If sufficient RCTs were identified, an attempt was to be made to examine for publication bias using a funnel plot (Egger 1997).
Results
Description of studies
1708 titles and abstracts were retrieved of which 87 were potentially relevant. We excluded 84 ‐ 19 reviews, 23 observational studies, 17 non‐randomised interventions, 16 RCTs undertaken in all patients irrespective of acidosis (10 of differing PD fluid pH, 5 of differing HD fluid and 1 in pre‐ESRD patients) and 9 duplicates.
No eligible trials were identified in pre‐ESRD patients or in children. Three trials were found in dialysis patients, one in PD (Szeto 2003) and two in HD (Brady 1998; Lefebrve 1989) with a total of 117 patients being randomised. Two trials recruited patients on the basis of definite acidosis, though with different levels (<16 mEq/L (Brady 1998), <25 mEq/L (Szeto 2003) and in the third acidosis was presumed though baseline bicarbonate was 16 mEq/L (Lefebrve 1989). The intervention was oral bicarbonate in one, increase in dialysis fluid bicarbonate in one and a combination in the two in the third trial. The duration of treatment varied from 16 weeks (Brady 1998) to 18 months (Lefebrve 1989).
Risk of bias in included studies
Allocation concealment was clear in one (Szeto 2003), unclear in the other two trials.
Blinding of patients and clinicians was only used in one trial (Szeto 2003).
Loss to follow‐up was under 15% in all trials.
Intention to treat was used though the outcome data presented are largely taken from patients completing follow‐up.
Effects of interventions
A diverse and varied set of measures was assessed in each of the trials making formal meta‐analysis not possible for most outcomes.
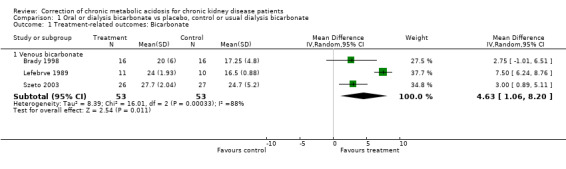
In all three trials acidosis improved in the intervention group though there was variation in achieved bicarbonate level and heterogeneity in the difference.
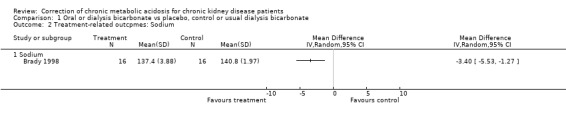
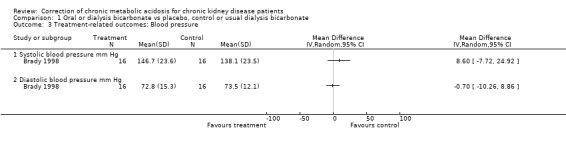
There was no evidence for an effect on blood pressure or sodium levels.
Some measures of nutritional status/protein metabolism such as SGA and NPNA were significantly improved by correction in the one trial that looked in these in detail (Szeto 2003). There was heterogeneity of the effect on serum albumin in two trials.
Serum PTH fell significantly in the two trials that estimated this, there was no significant effect on calcium or phosphate though both fell after correction.
Complex bone markers were assessed in one study (Lefebrve 1989), with some evidence for a reduction in bone turnover in those with initial high bone turnover and an increase in low turnover patients.
The studies were underpowered to assess clinical outcomes, in the one study that did (Szeto 2003) there was some evidence for a reduction in hospitalisation after correction.
Discussion
The evidence for the benefits and risks of correcting metabolic acidosis is very limited with no RCTs in pre‐ESRD patients, none in children, and only three small trials in dialysis patients. These trials suggest there may be some beneficial effects on both protein and bone metabolism but the trials are underpowered to provide robust evidence. One trial only partly corrected the acidosis (Brady 1998) which may have explained why there was no change in the primary marker of nutritional status albumin. Szeto 2003 suggested that correction of even mild acidosis maybe beneficial. Lefebrve 1989 suggested that the secondary hyperparathyroidism seen in patients with high bone turnover could be reversed, and bone turnover stimulated in those with low turnover.
Only Szeto 2003 had any data on clinical outcomes (hospitalisation and mortality). There were no data on patient quality of life after correction. Whilst correction of acidosis appeared well tolerated with no evident effects on weight, sodium or blood pressure it is important to note a non significant increase in admissions for heart failure in Szeto 2003. This suggests that one cannot reliably extrapolate results to pre‐ESRD where the long‐term use of oral sodium bicarbonate has not been evaluated especially in those at risk from sodium fluid loading (e.g. those with oedema, hypertension or heart failure).
In PD there have been some trials which have evaluated the effect of buffering the unphysiologic acidic PD dialysis fluid in all patients not just those with acidosis (e.g. Carrasco 2001; Cooker 2001; Jones 2002; Stein 1997). Primary outcomes have often been markers of intra‐peritoneal inflammation. However Stein 1997 was a good sized (n = 200) RCT within one year follow‐up using more lactate in the PD fluid to increase serum bicarbonate as well as using oral bicarbonate supplementation in the high lactate group. The difference in bicarbonate achieved was 27 mmol/L versus 23 mmol/L in the high versus low groups. The high group had some better nutritional markers at one year (weight gain, mid arm circumference) and reduced hospital admissions and days in hospital. There is a need to systematically review such studies.
The widespread use of bicarbonate HD will have reduced the frequency of acidosis in HD patients. Use of acetate‐free biofiltration (AFB) compared to standard bicarbonate HD in all HD patients (e.g. Verzetti 1998) may further reduce acidosis. A systematic review of such studies found that overall the trials were small, poorly designed, of insufficient duration with variable reporting of outcomes (Rabindranath 2006), No significant differences were found for dialysis related symptoms; data on bicarbonate levels, protein and bone metabolism were not reported, and there was insufficient evidence to favour any method. Gabutti 2003 showed that overcorrection can lead to metabolic alkalosis which was associated with symptomatic hypotension on HD.
Some small non‐randomised studies in HD patients have shown that correction may reduce secondary hyperparathyroidism (Movilli 2001), reduce protein degradation and increase albumin levels (Movilli 1998; Wiederkehr 2004) and increase branched chain amino acids (Kooman 1997).
There have been some non‐randomised uncontrolled studies (Jenkins 1989; Reaich 1993; Rustom 1998) and a few cross‐over studies (Passfall 1997; Roberts 1996; Roberts 2002), but most have been small (< 20 patients) and of short duration (< 8 weeks), the exception being a before and after study by Verove 2002 (six months). In these studies there was no standard definition of acidosis nor a target level to which it should be corrected. There was variation between patients' diets during the studies, in some dietary intake was controlled in others the standard diet was used. Such studies provide some supporting evidence that sodium bicarbonate can correct acidosis and that it is well tolerated in the short‐term with effects on protein metabolism as indicated by falls in urea levels (Jenkins 1989; Verove 2002), rising albumin (Verove 2002) fall in protein catabolic rate (Verove 2002) and reduction in more complex measures of protein degradation (Reaich 1993). Less data on the effect of calcium phosphate metabolism or bone changes are available (Lin 2002).
The main limitation of this review was the scarcity of robust adequately powered RCTs of long duration in either pre‐ESRD or dialysis patients.
Authors' conclusions
Implications for practice.
There is no randomised evidence to support the correction of acidosis in pre‐ESRD patients by sodium bicarbonate supplementation or in children. Evidence in dialysis patients is very limited.
Sodium bicarbonate is an inexpensive and well tolerated drug, and increasing bicarbonate concentration in dialysis fluid is relatively straightforward: both are effective in correcting acidosis. These considerations no doubt help support positive guideline recommendations for acidosis correction.
Current guidelines have to rely on extrapolation from the few dialysis trials, non randomised data and consensus. KDOQI recommends maintaining predialysis bicarbonate ≥ 22 mEq/L in all patients (KDOQI 2000), the UK Renal Association has different targets for CAPD (25‐29 mmol/L) and HD (20‐26 mmol/L) and a non specific target 'within normal range' for pre‐ESRD patients (UKRA 2002). The Australian CARI Guidelines for pre‐ESRD patients recommend correction to over 22 mol/L based on Grade III evidence (CARI 2005).
However it is not known how rigorously identification and correction of acidosis is achieved in pre‐ESRD patients. Registry data enable audit in dialysis patients. The potential adverse consequences of volume loading in patients at risk of fluid retention, or of over‐correction leading to alkalosis which may precipitate calcium phosphorus and lead to symptomatic hypotension have not been fully addressed. Whether mild acidosis is actually beneficial in dialysis patients as indicated by observational data or whether this is just reverse epidemiology reflecting higher protein intake is uncertain.
Implications for research.
RCTs are needed to determine the benefits and risks of correction of acidosis in both pre‐ESRD and dialysis patients and could consider at what bicarbonate level to initiate correction and what target level to achieve.
What's new
| Date | Event | Description |
|---|---|---|
| 30 April 2014 | Amended | Minor edits made to study names |
| 4 May 2013 | Amended | Contact details updated. |
| 4 March 2013 | Amended | New reports of existing studies identified |
History
Protocol first published: Issue 4, 1999 Review first published: Issue 1, 2007
| Date | Event | Description |
|---|---|---|
| 12 May 2008 | Amended | Converted to new review format. |
Acknowledgements
We are very grateful to Gail Higgins from the Cochrane Renal Group for advice and assistance with literature searching.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Data and analyses
Comparison 1. Oral or dialysis bicarbonate vs placebo, control or usual dialysis bicarbonate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment‐related outcomes: Bicarbonate | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Venous bicarbonate | 3 | 106 | Mean Difference (IV, Random, 95% CI) | 4.63 [1.06, 8.20] |
| 2 Treatment‐related outcpmes: Sodium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Sodium | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Treatment‐related outcomes: Blood pressure | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Systolic blood pressure mm Hg | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Diastolic blood pressure mm Hg | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Treatment‐related outcomes: Kt/V | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
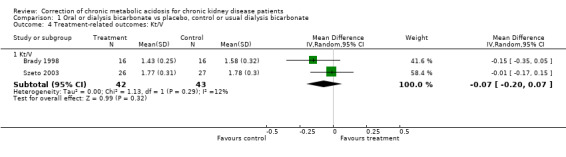
| 4.1 Kt/V | 2 | 85 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.20, 0.07] |
| 5 Nutritional outcomes | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Subjective global assessment (SGA) at 12 months | 1 | 53 | Mean Difference (IV, Random, 95% CI) | 0.61 [0.07, 1.15] |
| 5.2 Normalized protein nitrogen appearance (NPNA) at 12 months | 1 | 53 | Mean Difference (IV, Random, 95% CI) | 0.21 [0.07, 0.35] |
| 5.3 Protein catabolic rate (PCR) | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.08, 0.24] |
| 5.4 Albumin | 2 | 85 | Mean Difference (IV, Random, 95% CI) | 2.23 [‐2.71, 7.17] |
| 5.5 Oedema | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.75, 0.17] |
| 5.6 Fat‐free oedema‐free body mass | 1 | 53 | Mean Difference (IV, Random, 95% CI) | 2.80 [‐0.82, 6.42] |
| 5.7 Lean body mass | 1 | 53 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐4.20, 5.60] |
| 6 Bone metabolism outcomes: Phosphorus, osteocalcin and calcium | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Phosphorus | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.22, 0.42] |
| 6.2 Pre‐dialysis calcium (mg/dL) | 2 | 53 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.55, 0.19] |
| 6.3 Osteocalcin (ng/mL) | 2 | 23 | Mean Difference (IV, Random, 95% CI) | ‐25.0 [‐76.93, 26.93] |
| 7 Bone metabolism outcomes: PTH | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 PTH (1‐84 pg/mL) | 2 | 53 | Mean Difference (IV, Random, 95% CI) | ‐74.99 [‐207.55, 57.57] |
| 7.2 PTH (53‐84 pg/mL) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐38.0 [‐250.90, 174.90] |
| 7.3 PTH (44‐68 pg/mL) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐1872.00 [‐5486.99, 1742.99] |
| 8 Bone metabolism outcomes: Volume, surfaces, number and formation | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Bone volume (%) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Osteoid volume (%) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Osteoid surfaces (%) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Osteoblast surfaces (%) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 Osteoclast surfaces (%) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.6 Osetoclasts/sq mm | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.7 Bone formation rate (cubic um/sq um/d) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.8 Osteoid thickness (um) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Clinical outcomes: All‐cause mortality, hosptalisation | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 Hospitalisation | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 All‐cause mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Clinical outcomes: Hospital admissions/patient, length of hospital stay | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 Hospital admissions/patient | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Average length of hospital stay | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
Comparison 1 Oral or dialysis bicarbonate vs placebo, control or usual dialysis bicarbonate, Outcome 1 Treatment‐related outcomes: Bicarbonate.
1.2. Analysis.
Comparison 1 Oral or dialysis bicarbonate vs placebo, control or usual dialysis bicarbonate, Outcome 2 Treatment‐related outcpmes: Sodium.
1.3. Analysis.
Comparison 1 Oral or dialysis bicarbonate vs placebo, control or usual dialysis bicarbonate, Outcome 3 Treatment‐related outcomes: Blood pressure.
1.4. Analysis.
Comparison 1 Oral or dialysis bicarbonate vs placebo, control or usual dialysis bicarbonate, Outcome 4 Treatment‐related outcomes: Kt/V.
1.5. Analysis.
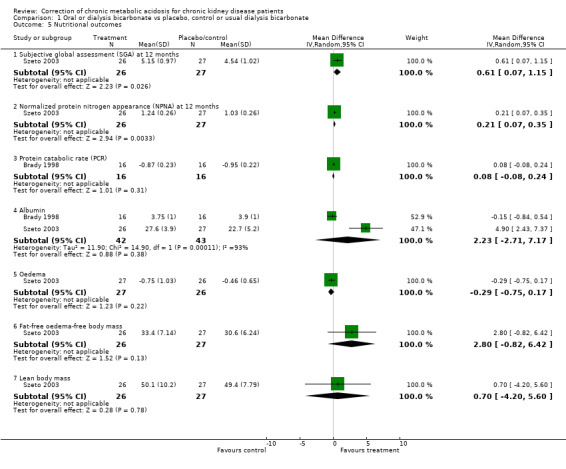
Comparison 1 Oral or dialysis bicarbonate vs placebo, control or usual dialysis bicarbonate, Outcome 5 Nutritional outcomes.
1.6. Analysis.
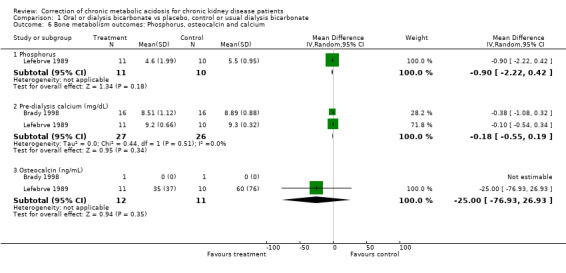
Comparison 1 Oral or dialysis bicarbonate vs placebo, control or usual dialysis bicarbonate, Outcome 6 Bone metabolism outcomes: Phosphorus, osteocalcin and calcium.
1.7. Analysis.
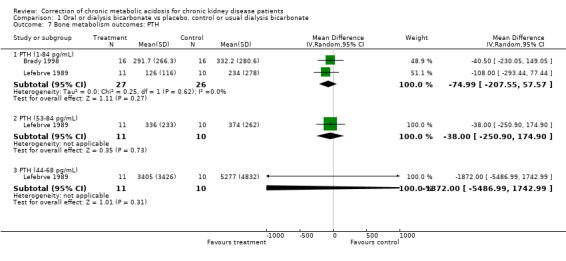
Comparison 1 Oral or dialysis bicarbonate vs placebo, control or usual dialysis bicarbonate, Outcome 7 Bone metabolism outcomes: PTH.
1.8. Analysis.
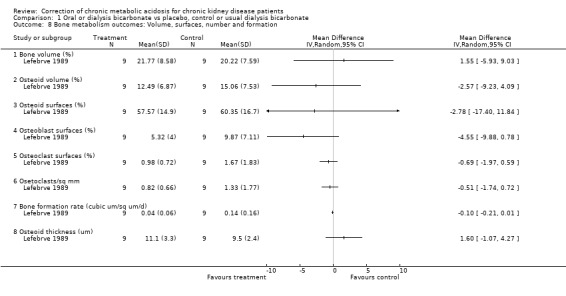
Comparison 1 Oral or dialysis bicarbonate vs placebo, control or usual dialysis bicarbonate, Outcome 8 Bone metabolism outcomes: Volume, surfaces, number and formation.
1.9. Analysis.
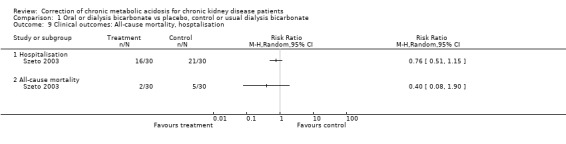
Comparison 1 Oral or dialysis bicarbonate vs placebo, control or usual dialysis bicarbonate, Outcome 9 Clinical outcomes: All‐cause mortality, hosptalisation.
1.10. Analysis.
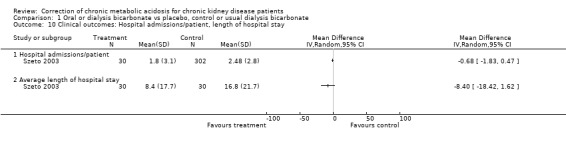
Comparison 1 Oral or dialysis bicarbonate vs placebo, control or usual dialysis bicarbonate, Outcome 10 Clinical outcomes: Hospital admissions/patient, length of hospital stay.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brady 1998.
| Methods | Country: USA Setting/Design: University Outpatient Centre, randomised trial Randomisation method: Not stated Blinding ‐ Participants: No ‐ Investigators: No ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: No Follow‐up period: 16 weeks Loss to follow‐up: 2 per group | |
| Participants | INCLUSION CRITERIA
Mean predialysis serum bicarbonate </= 18 mEq/L on monthly laboratory analyses during the previous 3 months
Medically stable, life expectancy of at least 4 months
Prescribed dialysate bicarbonate of 35 mEq/L TREATMENT GROUP Number: 18 Age: 51 ± 14.6 years Sex : 61% female Diabetes: 44% Ethnicity: 78% black Time on dialysis: 57.9 ± 57.4 months CONTROL GROUP Number: 18 Age: 52.3 ± 15.8 years Sex : 67% female Diabetes: 33% Ethnicity: 83% black Time on dialysis: 62.3 ± 61.0 months EXCLUSIONS Supplemental oral sodium bicarbonate or citrate before initiation of study Pregnant or of childbearing potential Participating in any other interventional research study during the same time period |
|
| Interventions | TREATMENT GROUP
Increase dialysis bicarbonate from 35 mEq/L to 40 mEq/L + oral sodium bicarbonate 1 mEq/kg if pre‐dialysis bicarbonate < 22 mmol/L CONTROL GROUP Usual dialysis prescription of 35 mEq/L CO‐INTERVENTIONS |
|
| Outcomes | Serum albumin Blood pressure Interdialytic weight gain Protein catabolic rate (PCR) Pre‐ and postdialysis BUN PTH Calcium Phosphorus KT/V Total lymphocyte count (TLC) | |
| Notes | Two subjects were withdrawn from each group ‐ control group: hosptialised at another facility (1); missed multiple dialyses (1) ‐ treatment group: transplant (1); declined to take prescribed sodium bicarbonate (1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Lefebrve 1989.
| Methods | Country: France Setting/Design: Hospital, randomised trial Time frame: 18 months Randomisation method: Stratification according to prestudy bone morphology Blinding ‐ Participants: No ‐ Investigators: No ‐ Outcome assessors: No ‐ Data analysis: No Intention‐to‐treat: Yes Loss to follow‐up: none | |
| Participants | INCLUSION CRITERIA
Informed consent including 2 bone biopsies TREATMENT GROUP Number: 11 Age: 51.2 ± 9.1 years Sex (M/F): 5/6 Duration of dialysis: 6.6 ± 3.3 years CONTROL GROUP Number: 10 Age: 49.9 ± 14.1 years Sex (M/F): 5/5 Duration of dialysis: 6.2 ± 3.0 years EXCLUSIONS Severe radiological osteitis fibrosa, plasma calcium > 11 mg/dL, parathyroidectomy, diabetes mellitus, treatment with corticosteroids |
|
| Interventions | TREATMENT GROUP
Bicarbonate added to dialysis fluid to achieve venous bicarbonate > 24 mEq/L CONTROL GROUP Standard bicarbonate concentration 33 mEq/L CO‐INTERVENTIONS Phosphate binder (1‐5 g aluminium gel when plasma phosphate > 6 mg/dL), calcium carbonate (up to 4 g/d to obtain plasma calcium > 9.0 mg/dL), vitamin D (up to 0.75 µg/d when 9.0 mg/dL plasma calcium level could not be achieved with 4 g calcium carbonate alone) Treatment group: 5 patients took antihypertensive drugs Control group: 6 patients took antihypertensive drugs |
|
| Outcomes | Predialysis pH PTH (1‐84, 44‐68,53‐84) Pre‐ and post dialysis creatinine, sodium, calcium, phosphate measured every 2 weeks Plasma alkaline phosphatase, aluminium, iPTH, osteocalcin measured every 3 months Bone volume, osteoid volume, osteoid surfaces, osteoclast surfaces (%), osteoclasts/mm², bone formation rate, osteoid thickness | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Szeto 2003.
| Methods | Country: Hong Kong Setting/Design: University, randomised trial Time frame: 12 months Randomisation method: not stated Blinding ‐ Participants: Yes ‐ Investigators: Yes ‐ Outcome assessors: Yes ‐ Data analysis: Yes Intention‐to‐treat: Yes Loss to follow‐up: 4/30 and 3/30 in intervention and control respectively | |
| Participants | INCLUSION CRITERIA
Plasma bicarbonate level measured twice in the 3 months prior to enrolment.
Total weekly Kt/V < 2.1
Venous bicarbonate ≤ 25 mmol/L on 2 consecutive measurements
Stable clinical condition and CAPD regimen for at least 12 months TREATMENT GROUP Number: 30 Age: 54.3 ± 12.4 years Sex (M/F): 16/14 Duration of dialysis: 39.9 ± 20.8 months Diabetes: 12 CONTROL GROUP Number: 30 Age: 56.6 ± 13.2 years Sex (M/F): 19/11 Duration of dialysis: 39.4 ± 26.0 months Diabetes: 8 EXCLUSIONS Patients unlikely to survive, planned living‐related kidney transplant, transfer to other renal centre within 6 months |
|
| Interventions | TREATMENT GROUP
Oral sodium bicarbonate 0.9 g thrice daily CONTROL GROUP Placebo (pure starch tablet) CO‐INTERVENTIONS None stated |
|
| Outcomes | Total Kt/V Residual GFR Subjective global assessment of nutrition (SGA) Normalised protein nitrogen appearance (NPNA) Anthropometric lean body mass (LBM) Anthropmetric measures (e.g. biceps skinfold thickness) Oedema, weight, hospitalisation, technique failure, mortality | |
| Notes | Appearance, packaging and labeling of tablets were identical | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
iPTH ‐ immunoactive parathormone
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cancarini 1998 | RCT of differing PD fluid pH in all patients 8 weeks duration |
| Carrasco 2001 | RCT of differing PD fluid pH in all patients |
| Chu 2001 | Non‐randomised, IV bicarconate correction pre‐ESRD |
| Cochran 1975 | Non‐randomised correction in pre‐ESRD patients |
| Coles 1997 | RCT of differing PD fluid pH in all patients |
| Cooker 2001 | RCT of differing PD fluid pH in all patients |
| Dalal 1989 | Non‐randomised HD fluid pH in all patients |
| Dratwa 2003 | Non‐randomised PD fluid pH in all patients 6 weeks duration |
| Feriani 1997 | Non‐randomised PD fluid pH in all patients |
| Feriani 2004 | Non‐randomised correction in PD patients |
| Gabutti 2003 | RCT of differing HD fluid pH in all patients |
| Harris 1995 | RCT of differing HD fluid pH in all patients |
| Jones 2002 | RCT of differing PD fluid pH in all patients |
| Lin 1994 | Non randomised in acidotic pre‐ESRD patients |
| Mak 1999 | Non‐randomised correction in acidotic HD patients 2 weeks duration |
| Marangoni 1995 | Non‐randomised fluid pH in HF all patients |
| Movilli 2001 | Non‐randomised, sodium bicarbonate in HD |
| Panichi 1994 | Non‐randomised differing HD fluid in all patients |
| Passfall 1997 | Crossover RCT sodium bicarbonate/water in pre‐ESRD all patients |
| Pickering 2002 | RCT of differing PD fluid pH in all patients 4 weeks duration |
| Plum 1997 | RCT of differing PD fluid pH in all patients 6 hour duration |
| Reaich 1993 | Non‐randomised correction in pre‐ESRD patients 4 weeks duration |
| Roberts 1996 | Non‐randomised correction in acidotic pre‐ESRD patients |
| Roberts 2002 | Non‐randomised correction in acidotic pre‐ESRD patients |
| Rustom 1998 | Non‐randomised correction in pre‐ESRD patients |
| Schrander 1998 | RCT of AFB in HD in all patients |
| Stein 1997 | RCT of differing PD fluid pH in all patients and also used oral bicarbonate supplementation in the high lactate group rather than just using differing PD fluid pH |
| Tranaeus 2000 | RCT of differing PD fluid pH in all patients |
| Verzetti 1998 | RCT of AFB in HD in all patients |
| Wiederkehr 2004 | Non‐randomised HD study 4 weeks duration |
| Yamamoto 1992 | Non‐randomised PD fluid pH study |
Contributions of authors
PR ‐ trial selection, quality assessment, data extraction, data analysis, writing of protocol and review NW ‐ trial selection, quality assessment, data extraction, data analysis, writing of review SB ‐ trial selection, quality assessment, data extraction, data analysis, writing of protocol and review CJ ‐ data analysis, writing of protocol and review CT ‐ analysis, writing of protocol and review
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Brady 1998 {published data only}
- Brady JP, Hasbargen JA. Correction of metabolic acidosis and its effect on albumin in chronic hemodialysis patients. American Journal of Kidney Diseases 1998;31(1):35‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brady JP, Hasbargen JA. Correction of metabolic acidosis and its effect on nutrition in hemodialysis patients [abstract no:A0788]. Journal of the American Society of Nephrology 1996;7(9):1403. [Google Scholar]
Lefebrve 1989 {published data only}
- Lefebvre A, Vernejoul MC, Gueris J, Goldfarb B, Graulet AM, Morieux C. Optimal correction of acidosis changes progression of dialysis osteodystrophy. Kidney International 1989;36(6):1112‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Szeto 2003 {published data only}
- Szeto CC, Chow KM, Kwan BCH, Chung KY, Leung CB, Li PKT. The beneficial effect of oral sodium bicarbonate in peritoneal dialysis patients ‐ How long does it last after stopping treatment?. Hong Kong Journal of Nephrology 2005;7(1):14‐21. [EMBASE: 2005204080] [Google Scholar]
- Szeto CC, Wong TY, Chow KM, Leung CB, Li PK. Oral sodium bicarbonate for the treatment of metabolic acidosis in peritoneal dialysis patients: a randomized placebo‐control trial. Journal of the American Society of Nephrology 2003;14(8):2119‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cancarini 1998 {published data only}
- Cancarini GC, Faict D, Vos C, Guiberteau R, Tranaeus A, Minetti L, et al. Clinical evaluation of a peritoneal dialysis solution with 33 mmol/L bicarbonate. Peritoneal Dialysis International 1998;18(6):576‐82. [MEDLINE: ] [PubMed] [Google Scholar]
Carrasco 2001 {published data only}
- Carrasco AM, Rubio MA, Sanchez Tommero JA, Fernandez Giron F, Gonzalez Rico M, Peso Gilsanz G, et al. Acidosis correction with a new 25 mmol/l bicarbonate/15 mmol/l lactate peritoneal dialysis solution. Peritoneal Dialysis International 2001;21(6):546‐53. [MEDLINE: ] [PubMed] [Google Scholar]
Chu 2001 {published data only}
- Chu P, Lu KC, Lin YF. Acute correction of metabolic acidosis increases serum procollagen type I carboxyterminal propeptide in patients with chronic renal failure. Journal of the Formosan Medical Association 2001;100(11):748‐52. [MEDLINE: ] [PubMed] [Google Scholar]
Cochran 1975 {published data only}
- Cochran M, Wilkinson R. Effect of correction of metabolic acidosis on bone mineralisation rates in patients with renal osteomalacia. Nephron 1975;15(2):98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coles 1997 {published data only}
- Coles GA, Gokal R, Ogg C, Jani F, O'Donoghue DT, Cancarini GC, et al. A randomized controlled trial of a bicarbonate‐ and a bicarbonate/lactate‐containing dialysis solution in CAPD. [see comments.] [erratum appears in Perit Dial Int 1997 May‐Jun;17(3):223.][ Note:Cancarinu GC[corrected to Cancarini GC]]. Peritoneal Dialysis International 1997;17(1):48‐51. [MEDLINE: ] [PubMed] [Google Scholar]
- Coles GA, O'Donoghue DJ, Pritchard N, Ogg CS, Jani FM, Gokal R, et al. A controlled trial of two bicarbonate‐containing dialysis fluids for CAPD‐‐final report. Nephrology Dialysis Transplantation 1998;13(12):3165‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mackenzie RK, Jones S, Moseley A, Holmes CJ, Argyle R, Williams JD, et al. In vivo exposure to bicarbonate/lactate‐ and bicarbonate‐buffered peritoneal dialysis fluids improves ex vivo peritoneal macrophage function. American Journal of Kidney Diseases 2000;35(1):112‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Topley N, Mackenzie R, Williams JD, Coles GA, Tranaeus A, Faict D, et al. In vivo exposure to bicarbonate/lactate‐buffered pdf (tbl) improves ex vivo pmo function, compared to bicarbonate‐(tb) or lactate‐buffered pdf (pd4) [abstract]. Nephrology 1997;3(Suppl 1):S408. [Google Scholar]
- Williams JD, Holmes CJ, Jones S, Mackenzie R, Coles GA, Faict D, et al. Long‐term in vivo exposure to bicarbonate/lactate dialysis fluid improves ex vivo peritoneal macrophage (PMO) TNFa secretion [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):292A. [Google Scholar]
Cooker 2001 {published data only}
- Cooker LA, Luneburg P, Holmes CJ, Jones S, Topley N. Bicarbonate/Lactate Study Group. Interleukin‐6 levels decrease in effluent from patients dialyzed with bicarbonate/lactate‐based peritoneal dialysis solutions. Peritoneal Dialysis International 2001;21 Suppl 3:S102‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Dalal 1989 {published data only}
- Ajam M, Gupta DK, Nawab ZM, Gandhi VC, Ing TS, Daugirdas JT. l‐lactate versus dl‐lactate as a base for hemodialysis. [abstract]. Kidney International 1989;35(1):237. [Google Scholar]
- Dalal S, Yu AW, Gupta DK, Kar PM, Ing TS, Daugirdas JT. L‐lactate high‐efficiency hemodialysis: hemodynamics, blood gas changes, potassium/phosphorus, and symptoms. Kidney International 1990;38(5):896‐903. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dalal SP, Ajam M, Gupta DK, Gupta R, Nawab Z, Manahan FJ, et al. L‐lactate for high‐efficiency hemodialysis: feasibility studies and a randomized comparison with acetate and bicarbonate. International Journal of Artificial Organs 1989;12(10):611‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Dalal SP, Gupta D, Manahan F, Ing TS, Daugirdas JT. L‐Lactate for high‐efficiency hemodialysis: a double‐blind crossover study. [abstract]. Kidney International 1990;37(1):292. [Google Scholar]
Dratwa 2003 {published data only}
- Dratwa M, Wilkie M, Ryckelynck JP, ter Wee PM, Rutherford P, Michel C, et al. Physioneal APD Research Group. Clinical experience with two physiologic bicarbonate/lactate peritoneal dialysis solutions in automated peritoneal dialysis. Kidney International ‐ Supplement 2003, (88):S105‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Feriani 1997 {published data only}
- Feriani M, Carobi C, Greca G, Buoncristiani U, Passlick‐Deetjen J. Clinical experience with a 39 mmol/L bicarbonate‐buffered peritoneal dialysis solution. Peritoneal Dialysis International 1997;17(1):17‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Feriani 2004 {published data only}
- Feriani M, Passlick‐Deetjen J, Jaeckle‐Meyer I, Greca G, Study Group. Individualized bicarbonate concentrations in the peritoneal dialysis fluid to optimize acid‐base status in CAPD patients. Nephrology Dialysis Transplantation 2004;19(1):195‐202. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gabutti 2003 {published data only}
- Gabutti L, Ferrari N, Giudici G, Mombelli G, Marone C. Unexpected haemodynamic instability associated with standard bicarbonate haemodialysis. Nephrology Dialysis Transplantation 2003;18(11):2369‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harris 1995 {published data only}
- Harris DC, Yuill E, Chesher DW. Correcting acidosis in hemodialysis: effect on phosphate clearance and calcification risk. Journal of the American Society of Nephrology 1995;6(6):1607‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jones 2002 {published data only}
- Jones S, Holmes CJ, Mackenzie RK, Stead R, Coles GA, Williams JD, et al. Continuous dialysis with bicarbonate/lactate‐buffered peritoneal dialysis fluids results in a long‐term improvement in ex vivo peritoneal macrophage function. Journal of the American Society of Nephrology 2002;13 Suppl 1:S97‐103. [MEDLINE: ] [PubMed] [Google Scholar]
Lin 1994 {published data only}
- Lin YF, Shieh SD, Diang LK, Lin SH, Chyr SH, Li BL, et al. Influence of rapid correction of metabolic acidosis on serum osteocalcin level in chronic renal failure. ASAIO Journal 1994;40(3):M440‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mak 1999 {published data only}
- Mak RH. Effect of metabolic acidosis on branched‐chain amino acids in uremia. Pedatric Nephrology 1999;13(4):319‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marangoni 1995 {published data only}
- Marangoni R, Civardi F, Masi F, Savino R, Cimino R, Colombo R, et al. Lactate versus bicarbonate on‐line hemofiltration: a comparative study. Artificial Organs 1995;19(6):490‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Movilli 2001 {published data only}
- Movilli E, Zani R, Carli O, Sangalli L, Pola A, Camerini C, et al. Direct effect of the correction of acidosis on plasma parathyroid hormone concentrations, calcium and phosphate in hemodialysis patients: a prospective study. Nephron 2001;87(3):257‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Panichi 1994 {published data only}
- Panichi V, Parrini M, Bianchi AM, Andreini B, Cirami C, Finato V, et al. Mechanisms of acid‐base homeostasis in acetate and bicarbonate dialysis, lactate hemofiltration and hemodiafiltration. International Journal of Artificial Organs 1994;17(6):315‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Passfall 1997 {published data only}
- Passfall J, Pai J, Spies KP, Haller H, Luft FC. Effect of water and bicarbonate loading in patients with chronic renal failure. Clinical Nephrology 1997;47(2):92‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Pickering 2002 {published data only}
- Pickering WP, Price SR, Bircher G, Marinovic AC, Mitch WE, Walls J. Nutrition in CAPD: serum bicarbonate and the ubiquitin‐proteasome system in muscle. Kidney International 2002;61(4):1286‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Plum 1997 {published data only}
- Plum J, Erren C, Fieseler C, Kirchgessner J, Passlick‐Deetjen J, Grabensee B. An amino acid‐based peritoneal dialysis fluid buffered with bicarbonate versus glucose/bicarbonate and glucose/lactate solutions: an intraindividual randomized study. Peritoneal Dialysis International 1999;19(5):418‐28. [MEDLINE: ] [PubMed] [Google Scholar]
- Plum J, Fussholler A, Schoenicke G, Busch T, Erren C, Fieseler C, et al. In vivo and in vitro effects of amino‐acid‐based and bicarbonate‐buffered peritoneal dialysis solutions with regard to peritoneal transport and cytokines/prostanoids dialysate concentrations. Nephrology Dialysis Transplantation 1997;12(8):1652‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Plum J, Fussholler A, Schonicke G, Busch T, Erren C, Fieseler C, et al. Effects of alternative peritoneal dialysis solutions on peritoneal transport and cytokines/prostanoids dialysate concentrations. Nieren‐und Hochdruckkrankheiten 1997;26(Suppl 1):S80‐5. [EMBASE: 1997355309] [Google Scholar]
Reaich 1993 {published data only}
- Reaich D, Channon SM, Scrimgeour CM, Daley SE, Wilkinson R, Goodship TH. Correction of acidosis in humans with CRF decreases protein degradation and amino acid oxidation. American Journal of Physiology 1993;265(2 Pt 1):E230‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Roberts 1996 {published data only}
- Roberts RG, Gilmour ER, Goodship THJ. The correction of acidosis does not increase dietary protein intake in chronic renal failure patients. American Journal of Kidney Diseases 1996;28(35):350. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Roberts 2002 {published data only}
- Roberts RG, Redfern CP, Graham KA, Bartlett K, Wilkinson R, Goodship TH. Sodium bicarbonate treatment and ubiquitin gene expression in acidotic human subjects with chronic renal failure. European Journal of Clinical Investigation 2002;32(7):488‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rustom 1998 {published data only}
- Rustom R, Grime JS, Costigan M, Maltby P, Hughes A, Taylor W, et al. Oral sodium bicarbonate reduces proximal renal tubular peptide catabolism, ammoniogenesis, and tubular damage in renal patients. Renal Failure 1998;20(2):371‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schrander 1998 {published data only}
- Schrander‐vd Meer AM, ter Wee PM, Donker AJ, Dorp WT. Dialysis efficacy during acetate‐free biofiltration. Nephrology Dialysis Transplantation 1998;13(2):370‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stein 1997 {published data only}
- Stein A, Moorhouse J, Iles‐Smith H, Baker F, Johnstone J, James G, et al. Role of an improvement in acid‐base status and nutrition in CAPD patients. Kidney International 1997;52(4):1089‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tranaeus 2000 {published data only}
- Tranaeus A. A long‐term study of a bicarbonate/lactate‐based peritoneal dialysis solution‐‐clinical benefits. The Bicarbonate/Lactate Study Group. Peritoneal Dialysis International 2000;20(5):516‐23. [MEDLINE: ] [PubMed] [Google Scholar]
Verzetti 1998 {published data only}
- Verzetti G, Navino C, Bolzani R, Galli G, Panzetta G. Acetate‐free biofiltration versus bicarbonate haemodialysis in the treatment of patients with diabetic nephropathy: a cross‐over multicentric study. Nephrology Dialysis Transplantation 1998;13(4):955‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wiederkehr 2004 {published data only}
- Wiederkehr MR, Kalogiros J, Krapf R. Correction of metabolic acidosis improves thyroid and growth hormone axes in haemodialysis patients. Nephrology Dialysis Transplantation 2004;19(5):1190‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yamamoto 1992 {published data only}
- Yamamoto T, Sakakura T, Yamakawa M, Horiuchi N, Hirata S, Iritani Y, et al. Clinical effects of long‐term use of neutralized dialysate for continuous ambulatory peritoneal dialysis. Nephron 1992;60(3):324‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Brito‐Ashurst 2009 {published data only}
- Brito‐Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status.[see comment]. Journal of the American Society of Nephrology 2009 Sep;20(9):2075‐84. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito‐Ashurst I, Varagunam M, Yaqoob MM. A randomized controlled trial (RCT) to study the effect of bicarbonate supplementation on the rate of progression of renal failure and nutritional status in chronic kidney disease (CKD) stage 4&5 patients [abstract no: F‐FC001]. Journal of the American Society of Nephrology 2006;17(Abstracts):37A. [˜] [Google Scholar]
De Santo 2006 {published data only}
- Santo NG, Frangiosa A, Anastasio P, Marino A, Correale G, Perna A, et al. Sevelamer worsens metabolic acidosis in hemodialysis patients. Journal of Nephrology 2006;19 Suppl 9:S108‐14. [MEDLINE: ] [PubMed] [Google Scholar]
Feriani 1993 {published data only}
- Feriani M, Dissegna D, Greca G, Passlick‐Deetjen J. Short‐term clinical study with bicarbonate‐containing peritoneal dialysis solution. Peritoneal Dialysis International 1993;13(4):296‐301. [MEDLINE: ] [PubMed] [Google Scholar]
Goraya 2013 {published data only}
- Goraya N, Simoni J, Jo CH, Wesson DE. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clinical Journal of the American Society of Nephrology: CJASN 2013;8(3):371‐81. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lofberg 2006 {published data only}
- Lofberg E, Gutierrez A, Anderstam B, Wernerman J, Bergstrom J, Price SR, et al. Effect of bicarbonate on muscle protein in patients receiving hemodialysis. American Journal of Kidney Diseases 2006;48(3):419‐29. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mathur 2006 {published data only}
- Mathur RP, Dash SC, Gupta N, Prakash S, Saxena S, Bhowmik D. Effects of correction of metabolic acidosis on blood urea and bone metabolism in patients with mild to moderate chronic kidney disease: a prospective randomized single blind controlled trial. Renal Failure 2006;28(1):1‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pickering 1998 {published data only}
- Pickering WP, Russ Price S, Mitch WE, Walls J. The effects of correction of metabolic acidosis on the ubiquitin pathway in CAPD patients [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):287A. [Google Scholar]
Starke 2012 {published data only}
- Starke A, Corsenca A, Kohler T, Knubben J, Kraenzlin M, Uebelhart D, et al. Correction of metabolic acidosis with potassium citrate in renal transplant patients and its effect on bone quality. Clinical Journal of the American Society of Nephrology: CJASN 2012;7(9):1461‐72. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
UBI Study 2012 {published data only}
- Iorio B, Aucella F, Conte G, Cupisti A, Santoro D. A prospective, multicenter, randomized, controlled study: the correction of metabolic acidosis with use of bicarbonate in Chronic Renal Insufficiency (UBI) Study. Journal of Nephrology 2012;25(3):437‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Van Stone 1984 {published data only}
- Stone J, Haynie J, Carey J. A double blind study of oral base replacement in chronic hemodialysis patients [abstract]. Kidney International 1984;25(1):194. [Google Scholar]
- Stone JC. Oral base replacement in patients on hemodialysis. Annals of Internal Medicine 1984;101(2):199‐201. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ward 1987 {published data only}
- Ward RA, Wathen RL, Williams TE, Harding GB. Hemodialysate composition and intradialytic metabolic, acid‐base and potassium changes. Kidney International 1987;32(1):129‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Williams 1997 {published data only}
- Williams AJ, Dittmer ID, McArley A, Clarke J. High bicarbonate dialysate in haemodialysis patients: effects on acidosis and nutritional status. Nephrology Dialysis Transplantation 1997;12(12):2633‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Bommer 2004
- Bommer J, Locatelli F, Satayathum S, Keen ML, Goodkin DA, Saito A, et al. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS). American Journal of Kidney Diseases 2004;44(4):661‐71. [MEDLINE: ] [PubMed] [Google Scholar]
Bushinsky 1995
- Bushinsky DA. The contribution of acidosis to renal osteodystrophy. Kidney International 1995;47(6):1876‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CARI 2005
- Voss D. Acidosis in pre‐dialysis patients. www.cari.org.au/Acidosis 20in 20pre‐dialysis.pdf (accessed June 2006).
Cochrane Renal Group 2008
- Willis NS, Mitchell R, Higgins GY, Webster AC, Craig JC. Cochrane Renal Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2008, Issue 1. Art. No.: RENAL (accessed March 2008).
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309(6964):1286‐91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315(7109):629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eustace 2004
- Eustace JA, Astor B, Muntner PM, Ikizler TA, Coresh J. Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney International 2004;65(3):1031‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Husted 1975
- Husted FC, Nolph KD, Maher JF. NaHCO3 and NaCl tolerance in chronic renal failure. Journal of Clinical Investigation 1975;56(2):414‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jenkins 1989
- Jenkins D, Burton PR, Bennett SE, Baker F, Walls J. The metabolic consequences of the correction of acidosis in uraemia. Nephrology Dialysis Transplantation 1989;4(2):92‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Kalandar‐Zadeh 2004
- Kalandar‐Zadeh K, Mehotra R, Fouque D, Koople JD. Metabolic acidosis and malnutrition‐inflammation complex syndrome in chronic renal failure. Seminars in Dialysis 2004;17(6):455‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDOQI 2000
- National Kidney Foundation. Clinical practice guidelines for nutrition in chronic renal failure. http://www.kidney.org/professionals/kdoqi/guidelines/doqi_nut.html (accessed June 2006).
Kooman 1997
- Kooman JP, Deutz NEP, Zijlmans P, Wall BA, Gerlag PGG, Hooff JP, et al. The influence of bicarbonate supplementation on plasma levels of branched‐chain amino acids in haemodialysis patients with metabolic acidosis. Nephrology Dialysis Transplantation 1997;12(11):2397‐401. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kopple 2005
- Kopple JD, Kalantar‐Zadeh K, Mehotra R. Risks of chronic metabolic acidosis in patients with chronic kidney disease. Kidney International ‐ Supplement 2005, (95):21‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kraut 2000
- Kraut JA. Disturbances of acid base balance and bone disease in end‐stage renal disease. Seminars in Dialysis 2000;13(4):261‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 1996
- Lefebvre C, McDonald S. Development of a sensitive search strategy for reports of randomized controlled trials in EMBASE. Fourth International Cochrane Colloquium; 1996 Oct 20‐24; Adelaide (Australia). 1996.
Lin 2002
- Lin S, Lin Y, Chin H, Wu C. Must metabolic acidosis be associated with malnutrition in haemodialysed patients?. Nephrology Dialysis Transplantation 2002;17(11):2006‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lowrie 1990
- Lowrie E, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. American Journal of Kidney Diseases 1990;15(5):458‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lu 1994
- Lu KC, Shieh SD, Li BL, Chu P, Jan SY, Lin YF, et al. Rapid correction of metabolic acidosis in chronic renal failure: effect on parathyroid hormone activity. Nephron 1994;67(4):419‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Master List 2007
- United States Cochrane Center. Master list of journals being searched. http://apps1.jhsph.edu/cochrane/masterlist.asp (accessed May 2007).
Mehrotra 2003
- Mehrotra R, Kopple JD. Protein and energy nutrition among adult patients treated with chronic peritoneal dialysis. Advances in Renal Replacement Therapy 2003;10(3):194‐212. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mitch 1993
- Mitch WE, Jurkovitz C, England BK. Mechanisms that cause protein and amino acid catabolism in uremia. American Journal of Kidney Diseases 1993;21(1):91‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Movilli 1998
- Movilli E, Zani R, Carli O, Sangalli L, Pola A, Camerini C, et al. Correction of metabolic acidosis increases serum albumin concentrations and decreases kinetically evaluated protein intake in haemodialysis patients: A prospective study. Nephrology Dialysis Transplantation 1998;13(7):1719‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rabindranath 2006
- Rabindranath KS, Strippoli GF, Daly C, Roderick PJ, Wallace S, MacLeod AM. Haemodiafiltration, haemofiltration and haemodialysis for end‐stage kidney disease. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006258] [DOI] [PubMed] [Google Scholar]
UKRA 2002
- Renal Association. Treatment of adults and children with renal failure: standards and audit measures. 3rd Edition. London: Royal College of Physicians of London and the Renal Association. http://www.renal.org/Standards/standards.html (accessed June 2006).
Verove 2002
- Verove C, Maissoneuve N, Azouzi A, Boldron A, Azar R. Effect of the correction of metabolic acidosis on nutritional status in elderly patients with chronic renal failure. Journal of Renal Nutrition 2002;12(4):224‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]


