Abstract
Background
Methotrexate (MTX) is a disease modifying antirheumatic drug (DMARD) used as a first line agent for treating rheumatoid arthritis (RA). Pharmacologically, it is classified as an antimetabolite due to its antagonistic effect on folic acid metabolism. Many patients treated with MTX experience mucosal, gastrointestinal, hepatic or haematologic side effects. Supplementation with folic or folinic acid during treatment with MTX may ameliorate these side effects.
Objectives
To identify trials of supplementation with folic acid or folinic acid during MTX therapy for rheumatoid arthritis and to assess the benefits and harms of folic acid and folinic acid (a) in reducing the mucosal, gastrointestinal (GI), hepatic and haematologic side effects of MTX, and (b) whether or not folic or folinic acid supplementation has any effect on MTX benefit.
Search methods
We originally performed MEDLINE searches, from January 1966 to June 1999. During the update of this review, we searched additional databases and used a sensitive search strategy designed to retrieve all trials on folic acid or folinic acid for rheumatoid arthritis from 1999 up to 2 March 2012.
Selection criteria
We selected all double‐blind, randomised, placebo‐controlled clinical trials (RCTs) in which adult patients with rheumatoid arthritis were treated with MTX (at a dose equal to or less than 25 mg/week) concurrently with folate supplementation. In this update of the review we only included trials using 'low dose' folic or folinic acid (a starting dose of ≤ 7 mg weekly).
Data collection and analysis
Data were extracted from the trials, and the trials were independently assessed for risk of bias using a predetermined set of criteria.
Main results
Six trials with 624 patients were eligible for inclusion. Most studies had low or unclear risk of bias for key domains. The quality of the evidence was rated as 'moderate' for each outcome as assessed by GRADE, with the exception of haematologic side effects which were rated as 'low'. There was no significant heterogeneity between trials, including where folic acid and folinic acid studies were pooled.
For patients supplemented with any form of exogenous folate (either folic or folinic acid) whilst on MTX therapy for rheumatoid arthritis, a 26% relative (9% absolute) risk reduction was seen for the incidence of GI side effects such as nausea, vomiting or abdominal pain (RR 0.74, 95% CI 0.59 to 0.92; P = 0.008). Folic and folinic acid also appear to be protective against abnormal serum transaminase elevation caused by MTX, with a 76.9% relative (16% absolute) risk reduction (RR 0.23, 95% CI 0.15 to 0.34; P < 0.00001), as well as reducing patient withdrawal from MTX for any reason (60.8% relative (15.2% absolute) risk reduction, RR 0.39, 95% CI 0.28 to 0.53; P < 0.00001).
We analysed the effect of folic or folinic acid on the incidence of stomatitis / mouth sores, and whilst showing a trend towards reduction in risk, the results were not statistically significant (RR 0.72, 95% CI 0.49 to 1.06)
It was not possible to draw meaningful conclusions on the effect of folic or folinic acid on haematologic side effects of methotrexate due to small numbers of events and poor reporting of this outcome in included trials.
It does not appear that supplementation with either folic or folinic acid has a statistically significant effect on the efficacy of MTX in treating RA (as measured by RA disease activity parameters such as tender and swollen joint counts, or physician's global assessment scores).
Authors' conclusions
The results support a protective effect of supplementation with either folic or folinic acid for patients with rheumatoid arthritis during treatment with MTX.
There was a clincally important significant reduction shown in the incidence of GI side effects, hepatic dysfunction (asmeasured by elevated serum transaminase levels) as well as a clincally important significant reduction in discontinuation of MTX treatment for any reason. A trend towards a reduction in stomatitis was demonstrated however this did not reach statistical significance.
This updated review with its focus on lower doses of folic acid and folinic acid and updated assessment of risk of bias aimed to give a more precise and more clinically relevant estimate of the benefit of folate supplementation for patients with rheumatoid arthritis receiving methotrexate.
Plain language summary
Folic acid or folinic acid for reducing side effects of methotrexate for people with rheumatoid arthritis
Researchers in The Cochrane Collaboration conducted a review of the effect of folic acid or folinic acid for people taking methotrexate for rheumatoid arthritis. After searching for all relevant studies, six studies with up to 624 people were included in the review. Their findings are summarized below.
In people with rheumatoid arthritis who take methotrexate (MTX):
‐ Taking either folic or folinic acid probably improves some side effects of MTX such as nausea and abdominal pain
‐ Taking either folic or folinic acid probably reduces the chance of developing abnormal liver blood tests
‐ Taking either folic or folinic acid probably helps people continue on their MTX treatment
‐ Taking either folic or folinic acid may improve some side effects of MTX such as mouth sores
‐ We are unable to ascertain whether or not taking folic or folinic acid with MTX prevents neutropenia (problems with producing white blood cells)
‐ Taking folic or folinic acid with MTX probably has no effect on how well MTX is able to treat rheumatoid arthritis.
What are folic acid and folinic acids and why do people take them with MTX?
Folic acid and folinic acid are forms of vitamin B9. The human body needs folate to perform many functions, including cell division, growth, and the production of new red blood cells. Folinic acid is chemically different to folic acid but both work in a similar way. If a person does not have enough folic acid or folinic acid, it is called a folate deficiency. MTX (a medication that is commonly prescribed to treat rheumatoid arthritis) works by blocking some of the effects of folic acid. A folate deficiency may cause side effects such as mouth sores, stomach problems such as nausea or abdominal pain, liver problems or problems with producing blood cells. These side effects are sometimes bad enough that they cause people to stop taking MTX (discontinue treatment).
Best estimate of what happens to people who take folic acid or folinic acid while on MTX
Stomach problems such as nausea, vomiting or abdominal pain:
‐ 9 fewer people out of 100 experienced stomach problems such as nausea up to 6 to 12 months after starting folic acid or folinic acid with their MTX (9.0% absolute improvement);
‐ 35 people out of 100 experienced stomach problems such as nausea when they took MTX alone for their rheumatoid arthritis;
‐ 26 people out of 100 experienced stomach problems such as nausea when they took folic acid or folinic acid with their MTX.
Liver problems (as measured by abnormal liver blood tests):
‐ 16 fewer people out of 100 had liver problems up to 6 to 12 months after they starting folic acid or folinic acid with their MTX (16.0% absolute improvement);
‐ 21 people out of 100 experienced abnormal liver blood tests when they took MTX alone for their rheumatoid arthritis;
‐ 5 people out of 100 experienced abnormal liver blood tests when they took folic acid or folinic acid with their MTX.
Ability to continue on MTX treatment:
‐ 15 fewer people out of 100 who took folic acid or folinic acid dropped out of the studies for any reason (15.2% absolute improvement);
‐ 25 people out of 100 who took a placebo (fake folic acid or folinic acid) with their MTX dropped out of the studies for any reason;
‐ 10 people out of 100 who took folic acid or folinic acid with their MTX dropped out of the studies for any reason.
Mouth sores or ulcers:
‐ 6 fewer people out of 100 who took folic acid or folinic acid with their MTX developed mouth sores (6.2% absolute improvement);
‐ 22 people out of 100 who took a placebo (fake folic acid) with their MTX developed mouth sores or ulcers;
‐ 16 people out of 100 who took folic acid or folinic acid with their MTX developed mouth sores or ulcers.
Summary of findings
Background
Description of the condition
Rheumatoid arthritis is a chronic, systemic inflammatory disorder that can affect many tissues and organs, but principally attacks synovial joints. Rheumatoid arthritis can produce diffuse inflammation in the lungs, pericardium, pleura and sclera, and also nodular lesions, which are most common in subcutaneous tissue. Although the cause of rheumatoid arthritis is unknown, autoimmunity plays a pivotal role in both its chronicity and progression, and as such it is considered a systemic autoimmune disease. About 1% of the world's population is afflicted by rheumatoid arthritis, women three times more often than men. Onset is most frequent between the ages of 40 and 50 years, but people of any age can be affected. It can be a disabling and painful condition, which can lead to substantial loss of functioning and mobility if not adequately treated (Majithia V 2007).
Methotrexate (MTX) is classified pharmacologically as an antimetabolite due to its antagonistic effect on folic acid metabolism. Although its exact mechanism of action in rheumatoid arthritis is uncertain, it has become many rheumatologists' first line drug of choice (Kremer 1995). The evidence for its efficacy is well documented at least over the short term. MTX has been shown to be efficacious in observational studies, showing short term clinical improvement in 48% to 90% of patients (Tugwell 1987; Tugwell 1989), and in 10% to 70% of participants in placebo‐controlled studies (Anderson 1985; Thompson 1984; Weinblatt 1985; Williams 1985). Three meta‐analyses (Felson 1990; Suarez‐Almazor 1998; Tugwell 1987) estimate that a third of the patients show a major improvement. Methotrexate is often used in combination with other disease modifying drugs and biologics (Katchamart 2010; Maxwell 2009; Navarro Sarabia 2005; Singh 2009). Felson also compared the benefit‐toxicity trade offs of various second line drugs and ranked MTX ahead of azathioprine, sulfasalazine, gold salts and penicillamine (Felson 1992). However, toxicity does prevent many patients from obtaining benefit from the drug. It has been reported that mild toxicity occurs in about 60% of patients, and roughly 7% to 30% of patients discontinue MTX therapy within the first year of treatment due to toxicity (Kremer 1995; Schnabel 1994).
Description of the intervention
Folic acid (also known as vitamin B9) has demonstrated health benefits in a variety of areas. Folinic acid (5‐formyl tetrahydrofolate) is one active form in the group of vitamins known as folates. In contrast to folic acid (which is a synthetic form of folate) folinic acid is one of the forms of folate found naturally in foods. In the body folinic acid can be converted into any of the other active forms of folate.
How the intervention might work
Both MTX dose and length of exposure influence toxicity relating to MTX treatment (Wallace 1995). Other predisposing factors include existing folate deficiency, advanced age, cumulative dose, renal insufficiency and concomitant use of other antifolates (Jackson 1984). Depleted intracellular folate levels have been documented in hepatocytes and peripheral blood lymphocytes of MTX treated patients (Kremer 1986; Leeb 1995; Morgan 1987; Morgan 1991; Stenger 1992; Stewart 1991). Folate deficiency occurs frequently in patients with rheumatoid arthritis, and folate stores are further decreased in patients with rheumatoid arthritis who receive MTX (Leeb 1995). Gastrointestinal and haematologic side effects have been related to folate deficiency. Therefore, it could be expected that folate supplementation may reduce side effects associated with MTX therapy.
There has been concern that folate supplementation may reduce the efficacy of, and therefore benefit seen with, MTX if the antirheumatic effects are also mediated through folate antagonism. It has been suggested that since the mechanism of action of MTX in rheumatoid arthritis is not fully known, the beneficial effect of folate supplementation could be the result of a relative reduction of the dose of MTX (Stenger 1992). Several open prospective studies and randomised controlled trials (RCTs) have been conducted to determine if folate supplementation, with folic acid or folinic acid, has a beneficial effect in reducing the frequency and severity of MTX side effects, and to investigate potential changes to the therapeutic benefit. It is thought folate supplementation could therefore help 'rescue' or reverse the toxic effects of MTX.
Why it is important to do this review
These studies have produced varying estimates of the reduction in adverse effects, ranging from 0% to 50%. Clinicians and patients need to have a 'best estimate' to make informed decisions on whether to use folate supplementation; therefore, a meta‐analysis was undertaken.
Objectives
To examine the effect of low doses of folic acid and folinic acid in reducing gastrointestinal, hepatic (liver toxicity) and haematologic side effects of low dose MTX in patients with rheumatoid arthritis.
To determine if folate supplementation with folic acid or folinic acid reduces the arthritis benefit of MTX therapy.
Methods
Criteria for considering studies for this review
Types of studies
All double‐blind, randomised, placebo‐controlled clinical trials.
Types of participants
Patients older than 18 years who fulfilled the American Rheumatism Association's criteria for rheumatoid arthritis (Arnett 1988).
Types of interventions
Treatment with low doses of MTX (equal or less than 25 mg/week) concurrently with low dose folate supplementation (either folic or folinic acid), with a starting dose equal to or less than 7 mg/week.
Types of outcome measures
Major outcomes
Results relating to at least one of the following: gastrointestinal symptoms (such as nausea, vomiting or abdominal pain), mouth ulcers (stomatitis), liver toxicity (as measured by raised serum transaminases), haematologic side effects (anaemia or cytopenia), or withdrawal or discontinuation of MTX therapy.
Minor outcomes
Alteration of the beneficial effect of MTX (loss of efficacy) as measured by any of the following.
Swollen joint count.
Tender joint count.
Pain.
Disability score.
Grip strength.
Patient's global assessment.
Physician's global assessment.
Search methods for identification of studies
In the first version of the review, to identify trials of supplementation with folic acid or folinic acid during MTX therapy for rheumatoid arthritis we performed MEDLINE searches from January 1966 to June 1999. We also searched the Controlled Clinical Trials Register (CCTR) Issue 2, 1999 and the Cochrane Musculoskeletal Group's specialized register. We used the strategy developed by The Cochrane Collaboration as well as the search adopted and modified for the Cochrane Musculoskeletal Group (Dickersin 1994).
This computer search was complemented by the following handsearches: (i) bibliographic references, (ii) Current Contents from June to December 1996, (iii) abstracts of rheumatology meetings from 1993 to 1996: American College of Rheumatology, International League Against Rheumatism, British College of Rheumatologists, Canadian College of Rheumatologists and European League Against Rheumatism, (iv) all issues of the four journals: Journal of Rheumatology, Arthritis and Rheumatism, Clinical and Experimental Rheumatology, and British Journal of Rheumatology from January 1992 to April 1996.
As part of the update of this review, a sensitive search strategy was designed to retrieve all trials on folic acid or folinic acid for rheumatoid arthritis from 1999 to March 2012. We did not limit the search by language. We searched the following databases:
1. The Cochrane Library 2012, Issue 2, Wiley InterScience (www.thecochranelibrary.com) including the Cochrane Central Register of Controlled Trials (CENTRAL), Database of Reviews of Effectiveness (DARE), HTA database, NHS EED, and the Methods Studies database;
2. MEDLINE (via Ovid), 1999 to February week 4, 2012;
3. MEDLINE® In‐Process & Other Non‐Indexed Citations (via Ovid), 2 March 2012;
4. EMBASE, 1999 to Week 9 ‐2012;
5. NIH clinical trials registry Clinical Trials.gov.
The search strategy was devised on Ovid MEDLINE (Appendix 1) and then adapted for the other databases (Appendix 2; Appendix 3). In all cases the databases were searched from 1999 to 2 March 2012. All references were imported into an EndNote library and tagged with the name of the database.
Data collection and analysis
Selection of studies
Following an a priori protocol, at least two review authors (BS, ZO, WK, MS, TR) independently reviewed the eligibility criteria for abstracts for inclusion in this review. We screened all titles or abstracts generated by the searches for potentially relevant studies based on the following criteria: the type of study; type of participants; type of intervention; and type of outcome measurements. We assessed the full‐length articles of the selected titles or abstracts for eligibility (for a full description see 'Criteria for considering studies for this review'). We resolved disagreements by consensus or third‐party adjudication.
Data extraction and management
Data were independently extracted by four authors (ZO, BS, WK, MS). Folate supplementation was considered as 'the administration of folic or folinic acid in any dose, and at any time with respect to MTX'. The following gastrointestinal effects were combined (where possible) because of lack of consistency in the reporting and to obtain sufficient events for analysis: abdominal pain, anorexia, dyspepsia, nausea and vomiting. The incidence of stomatitis as well as the incidence of abnormal serum liver enzymes were analysed independently. The included studies had defined 'abnormal liver function test' differently, and for data extraction purposes we defined 'abnormal' as either aspartate aminotransferase (AST), alanine aminotransferase (ALT) or alkaline phosphatase (ALP) at least equal to or greater than two times baseline values, or at least two times the upper limit of the reference range used in the study. The following haematologic side effects were included, if reported: cytopenia, macrocytosis, or pancytopenia.
To assess changes in disease activity, the following measurements were considered a priori: swollen joint count, tender joint count, pain, disability score, grip strength, patient's global assessment, and physician's global assessment. Most trials reported swollen and tender joint counts, or indices, and patient global assessments. These were the outcomes finally included for the MTX efficacy analysis. Joint counts included the number of swollen and tender joints, if reported. Alternatively, when the number of involved joints was not reported indices (for example Ritchie index) were included.
A starting dose of ≤ 7 mg/wk folic or folinic acid was used as a cut off value for studies to be included in the analysis. The aim of this was to exclude studies which gave very high doses of folic or folinic acid. It was felt by the authors that by doing so, it would allow the results of the updated review to provide a better estimate of the effects of folate supplementation at doses currently prescribed in practice. Worldwide guidelines currently support co‐administration of folic acid with MTX, and where a dose value is suggested it usually falls in the range of 0.5 to 2 mg daily (Chakravarty 2008; Dutch Society of Rheumatology 2011; GUIPCAR group 2007; Singh 2012).
Assessment of risk of bias in included studies
Assessment of risk of bias was undertaken for each included study using the Cochrane Collaboration's 'Risk of bias' tool (Higgins 2011). The following seven key domains were assessed by two review authors: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and 'other issues' (comparability of treatment and control group at entry, and appropriateness of duration of surveillance). Pairs of review authors judged the key domains as either 'high risk', 'low risk' or 'unclear' rsk of bias. In cases of disagreement between the review authors, the decision was made by consensus.
Measures of treatment effect
For each trial, risk ratio and the 95% confidence interval were calculated for dichotomous outcomes. Data analysis employed a standard meta‐analysis using methods for continuous data developed by Hedges and Olkin (Hedges 1982) and described by Petitti (Petitti 1994). Mean differences (MD) and 95% confidence intervals (CIs) were calculated for continuous outcomes (reporting mean and standard deviation or standard error of the mean). If the scale for each assessment varied among the studies and the mean and standard deviation of the change was provided for the folic and folinic acid arm and control arm, we calculated a standardised mean difference. If the standard deviations of the change scores were not available we used the standard deviation of the baseline score for each group. This provides a conservative estimate of the significance of differences between groups.
Unit of analysis issues
The level at which randomisation occurred in the included studies was reported as specified by the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
Dealing with missing data
If we noticed missing data during data extraction we attempted to contact the original investigators of the study to request the required information. It was anticipated that it may also have been necessary to conduct a sensitivity analysis if assumptions were made (Deeks 2011). The potential effect of missing data upon conclusions drawn from this review would also be described.
Assessment of heterogeneity
Heterogeneity between comparable trials was tested using a standard Chi2 test and considered statistically significant at P < 0.10 after due consideration of the value of the I2 statistic, a value greater than 50% may indicate substantial heterogeneity. If results were determined to be heterogeneous (that is I2 > 50%) a random‐effects model would have been used to further analyse the results.
Assessment of reporting biases
If there were sufficient studies it was intended to assess the possibility of publication bias with funnel plots.
Data synthesis
Where appropriate, results of comparable groups of trials were pooled using the fixed‐effect model and 95% CIs were calculated. If heterogeneity existed between studies a random‐effects model was to be used however there was no heterogeneity between included trials.
Since folic and folinic acid do not act at the same point on the folate pathway, they were analysed separately as well as together. For side effects, the overall treatment effect across trials was calculated using a fixed‐effect model (Mantel‐Haenszel).
Meta‐analysis was facilitated by RevMan 5.2 using the statistics as described below.
Continuous outcomes
The trials included in this review used different indices to evaluate changes in disease activity. For instance, different total numbers of joints were considered and global assessments were measured with various scales. To enhance comparability across trials all the disease activity outcome variables (joint counts and patient global assessments) were compared using standardised mean differences between the treatment (folic or folinic acid) and placebo groups. The standardised mean differences were based on end‐of‐trial results. When appropriate, the standardised mean differences were pooled by applying the inverse of the variance of each trial as a weight.
Mean differences (MD) were calculated using a fixed‐effect model as outcomes were measured on the same standard scales.
Dichotomous outcomes
Risk ratio (RR) (Mantel‐Haenszel) and 95% CI were calculated for dichotomous outcomes. A fixed‐effect model was selected for interpretation of the dichotomous outcome measures in this review since this is the most appropriate statistic for the interpretation of pooled data where the event is common (Deeks 1998) and where there is no significant statistical heterogeneity between trials.
Additional data were obtained from the authors for one of the studies. Another study did not report any measures of dispersion. The end‐of‐trial variance for this study was estimated using the mean coefficient of variation of the other trials weighted by the sample size of each study. The pooled estimates were calculated using RevMan 5.1. The analysis was conducted separately for folic and folinic acid and pooled.
Appropiate statistical analysis was performed using RevMan in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
Subgroup analysis and investigation of heterogeneity
In the presence of significant heterogeneity, the results from comparable groups of trials were pooled using the random‐effect model and the 95% CIs calculated.
Sensitivity analysis
We anticipated that sensitivity analyses would be undertaken, when indicated, to investigate the effects of methodological quality, for example allocation concealment and intention‐to‐treat analysis or where cluster randomised trials were combined with each other or with other studies in a meta‐analysis.
Grading of evidence and summary of findings table
Major outcomes (including benefits and adverse events) were presented in Table 1, Table 2 and Table 3, which provide information on the quality of evidence and the magnitude of the intervention effect, as well as a summary of the main outcome data (Schünemann 2011). An assessment of the overall quality of evidence per outcome (high, moderate, low and very low) using the GRADE approach was also presented as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011).
Summary of findings for the main comparison. Folic or folinic acid (any) compared to placebo for reducing side effects in patients receiving methotrexate for rheumatoid arthritis.
| Folic acid or folinic acid (any) compared to placebo for reducing side effects in patients receiving methotrexate for rheumatoid arthritis | ||||||
| Patient or population: Patients receiving methotrexate for rheumatoid arthritis Settings: International hospital and clinic settings Intervention: Supplementation with either folic or folinic acid Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Supplementation with either folic or folinic acid | |||||
|
GI side effects (nausea, vomiting, abdominal pain) Follow‐up: 24 to 52 weeks |
346 per 1000 | 256 per 1000 (204 to 318) | RR 0.74 (0.59 to 0.92) | 644 (6 studies) | ⊕⊕⊕⊝ moderate1 | Absolute risk reduction: ‐9.0% (‐14.2% to ‐2.8%) (P = 0.008) Relative risk reduction: ‐26.0% (‐41.0% to ‐8.1%) NNT: 11 (7 to 35) |
|
Stomatitis / mouth sores (incidence) Follow‐up: 24 to 52 weeks |
223 per 1000 | 161 per 1000 (109 to 236) | RR 0.72 (0.49 to 1.06) | 575 (4 studies) | ⊕⊕⊕⊝ moderate1 | Absolute risk difference: ‐6.2% (‐11.4% to +1.3%) Relative risk difference: ‐27.8% (‐51.1% to +5.8%) Not statistically significant |
|
Liver toxicity (incidence of transaminase elevation) Follow‐up: 8 to 52 weeks |
208 per 1000 | 48 per 1000 (31 to 71) | RR 0.23 (0.15 to 0.34) | 551 (4 studies) | ⊕⊕⊕⊝ moderate1 | Absolute risk reduction: ‐16.0% (‐17.7% to ‐13.7%) (P <0.00001) Relative risk reduction: ‐76.9% (‐85.1% to ‐65.9%) NNT: 6 (6 to 7) |
|
Haematological disorders (neutropenia, etc) Follow up: 24 to 52 weeks |
<10 per 10002 | See comment | RR 1.55 (0.40 to 5.91) | 443 (2 studies) | ⊕⊕⊝⊝ low1 | This is a rare event2. The studies included in this review were underpowered to detect a meaningful difference in rates of neutropenia. |
|
Total withdrawals Follow‐up: 8 to 52 weeks |
250 per 1000 | 98 per 1000 (70 to 133) | RR 0.39 (0.28 to 0.53) | 640 (6 studies) | ⊕⊕⊕⊝ moderate1 | Absolute risk reduction: ‐15.2% (‐18.0% to ‐11.7%) (P <0.00001) Relative risk reduction: ‐60.8% (‐72.0% to ‐46.8%) NNT: 7 (6 to 9) |
|
Number of swollen joints Change in number of swollen joints Follow‐up: 8 to 52 weeks |
Mean no. of swollen joints per patient in the control group is 18.24 | Mean no. of swollen joints per patient = 18.47 | See comment | 142 patients (4 studies) | ⊕⊕⊕⊝ moderate1 | Standardized Mean Difference (SMD) between groups in number of swollen joints = 0.05 (‐0.28 to 0.38) Absolute risk difference: 4.82% (‐27.01% to 36.6%) Relative risk difference: 26.42% (‐148.08% to 201.04%) Not statistically significant |
|
Number of tender joints Change in number of tender joints Follow‐up: 8 to 52 weeks |
Mean no. of tender joints per patient in the control group is 15.23 | Mean no. of tender joints per patient = 16.05 | See comment | 122 patients (3 studies) |
⊕⊕⊕⊝ moderate1 | Standardized Mean Difference (SMD) between groups in number of tender joints = 0.09 (‐0.27 to 0.45) Absolute risk difference: 4.55% (‐13.65% to 22.75%) Relative risk difference: 29.88% (‐89.63% to 149.38%) Not statistically significant |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; NNT; number needed to treat | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Number of events is less than 300.
2The incidence of clinically important cytopenia in patients treated with low‐dose MTX is estimated to be less than 1%.
Summary of findings 2. Folic acid compared to placebo for reducing side effects in patients receiving methotrexate for rheumatoid arthritis.
| Folic acid compared to placebo for reducing side effects in patients receiving methotrexate for rheumatoid arthritis | ||||||
| Patient or population: Patients receiving methotrexate for rheumatoid arthritis Settings: International hospital and clinic settings Intervention: Supplementation with folic acid Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Folic Acid | |||||
| GI side effects (ie, incidence of nausea, vomiting, abdominal pain) Follow‐up: 24 to 52 weeks | 346 per 1000 | 263 per 1000 (197 to 349) | RR 0.76 (0.57 to 1.01) | 355 (3 studies) | ⊕⊕⊕⊝ moderate1,2 | Absolute risk difference = ‐8.3% (‐14.9% to 0.3%) Relative risk difference = ‐24.0% (‐43.1% to 0.8%) Not statistically significant |
|
Stomatitis / mouth sores (incidence) Follow‐up: 24 to 52 weeks |
223 per 1000 |
201 per 1000 (118 to 343) |
RR 0.90 (0.53 to 1.54) | 302 (2 studies) |
⊕⊕⊕⊝ moderate1,2 | Absolute risk difference = ‐2.2% (‐10.5% to 12.0%) Relative risk difference = ‐9.9% (‐47.1% to 53.8%) Not statistically significant |
|
Liver toxicity (ie, incidence of transaminase elevation) Follow‐up: 24 to 52 weeks |
208 per 1000 | 40 per 1000 (21 to 75) | RR 0.19 (0.10 to 0.36) | 302 (2 studies) | ⊕⊕⊕⊝ moderate1,2 | Absolute risk reduction = ‐16.8% (‐18.7% to ‐13.3%) (P < 0.00001) Relative risk reduction = ‐80.8% (‐89.9% to ‐63.9%) NNT = 6 (5 to 8) |
|
Haematological disorders (incidence of neutropenia, etc) Follow up: 24 to 48 weeks |
<10 per 10003 | See comment |
RR 1.70 (0.42 to 6.96) |
443 (2 studies) |
⊕⊕⊝⊝ low1 | This is a rare event3. The studies included in this review were underpowered to detect a meaningful difference in rates of neutropenia. |
|
Total withdrawals Follow‐up: 24 to 48 weeks |
250 per 1000* | 108 per 1000 (73 to 160) | RR 0.43 (0.29 to 0.64) | 343 (3 studies) | ⊕⊕⊕⊝ moderate1,2 | Absolute risk reduction = ‐14.2% (‐17.7% to ‐9.0%) (P=0.000039) Relative risk difference = ‐56.8% (‐70.8% to ‐36.0%) NNT = 7 (6 to 11) |
|
Number of swollen joints with folic acid (≤7 mg/wk)
Change in number of swollen joints Follow‐up: 48 weeks |
Mean no. of swollen joints per patient = 16.00 | Mean no. of swollen joints per patient = 14.35 | See comment | 42 (1 study) | ⊕⊕⊕⊝ moderate1,2 | Mean difference between groups in number of swollen joints (Absolute difference)= ‐1.65 (‐7.96 to 4.66)4 Relative risk difference: ‐10.4% (‐49.8% to 29.1%) Not statistically significant |
|
Number of tender joints with folic acid (≤7 mg/wk)
Change in number of tender joints Follow‐up: 48 weeks |
Mean no. of tender joints per patient = 17.63 | Mean no. of tender joints per patient = 20.09 | See comment | 42 (1 study) | ⊕⊕⊕⊝ moderate1,2 | Mean difference between groups in number of tender joints = 2.46 (‐6.08 to 11.00)5 Absolute risk difference: Relative risk difference: 14.0% (‐34.5% to 62.4%) Not statistically significant |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; NNT: Number needed to treat | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Number of events is less than 300
2 Less than 400 participants
3The incidence of clinically important cytopenia in patients treated with low dose MTX is estimated to be less than 1%.
4 Post‐treatment number of swollen joints not reported. Change scores presented here.
5 Post‐treatment number of tender joints not reported. Change scores presented here.
Summary of findings 3. Folinic acid compared to placebo for reducing side effects in patients receiving methotrexate for rheumatoid arthritis.
| Folinic acid compared to placebo for reducing side effects in patients receiving methotrexate for rheumatoid arthritis | ||||||
| Patient or population: Patients receiving methotrexate for rheumatoid arthritis Settings: International hospital and clinic settings Intervention: Supplemtation with folinic acid Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Folinic Acid | |||||
|
GI side effects (nausea, vomiting, abdominal pain) Follow‐up: 24 to 52 weeks |
346 per 1000 | 270 per 1000 (204 to 353) | RR 0.78 (0.59 to 1.02) | 426 (4 studies) | ⊕⊕⊕⊝ moderate1,2 | Absolute risk difference: ‐7.6% (‐14.2% to 0.7%) Relative risk difference: ‐22.0% (‐41.0 to 2.0%) Not statistically significant |
|
Stomatitis / mouth sores (incidence) Follow‐up: 24 to 52 weeks |
223 per 1000 |
156 per 1000 (103 to 239) |
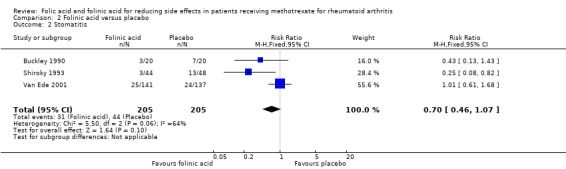
RR 0.70 (0.46 to 1.07) | 410 (3 studies) | ⊕⊕⊕⊝ moderate1,2 | Absolute risk difference: ‐6.7% (‐12.0% to 0.16%) Relative risk difference: ‐30.0% (‐53.8% to 7.2%) Not statistically significant |
|
Liver toxicity (incidence of transaminase elevation) Follow‐up: 8 to 52 weeks |
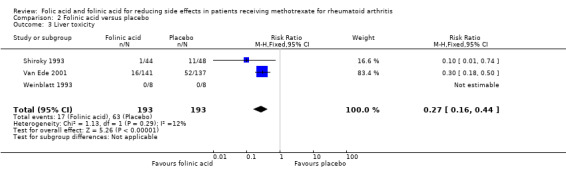
208 per 1000 | 56 per 1000 (33 to 92) | RR 0.27 (0.16 to 0.44) | 358 (3 studies) | ⊕⊕⊕⊝ moderate1,2 | Absolute risk reduction: ‐15.2% (‐17.5% to ‐11.6%) Relative risk reduction: ‐73.1% (‐84.1% to ‐55.8%) NNT: 7 (6 to 9) |
|
Haematological disorders (neutropenia, etc) Follow‐up: 52 weeks |
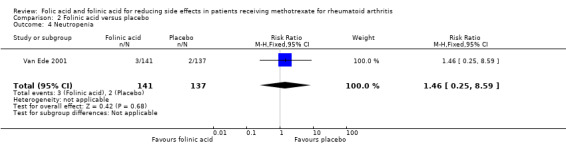
<10 per 10003 | See comment | RR 1.46 (0.25 to 8.59) | 278 (1 study) |
⊕⊕⊝⊝ low1 | This is a rare event3. The studies included in this review were underpowered to detect a meaningful difference in rates of neutropenia. |
|
Total withdrawals Follow‐up: 8 to 52 weeks |
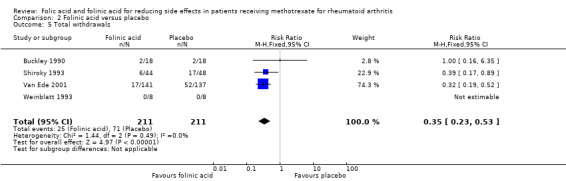
250 per 1000 | 88 per 1000 (58 to 133) | RR 0.35 (0.23 to 0.53) | 386 (3 studies) | ⊕⊕⊕⊝ moderate1,2 | Absolute risk reduction: ‐16.2% (‐19.2% to ‐11.7%) Relative risk reduction: ‐64.8% (‐76.8% to ‐46.8%) NNT: 6 (5 to 9) |
|
Number of swollen joints with folinic acid (≤7 mg/wk)
Change in number of swollen joints Follow‐up: 8 to 52 weeks |
Mean no. of swollen joints per patient = 19.13 | Mean no. of swollen joints per patient = 20.29 | See comment | 100 (3 studies) |
⊕⊕⊕⊝ moderate1,2 | Mean difference between groups in number of swollen joints (Absolute difference) = 1.72 (‐3.47 to 6.92)4 Relative risk difference: 8.1% (‐16.3% to 32.6%) Not statisticaly significant |
|
Number of tender joints with folinic acid (≤7 mg/wk)
Change in number of tender joints Follow‐up: 8 to 52 weeks |
Mean no. of tender joints per patient = 14 | Mean no. of tender joints per patient = 13.88 | See comment | 80 (2 studies) |
⊕⊕⊕⊝ moderate1,2 | Mean difference between groups in number of tender joints (Absolute difference) = 1.13 (‐4.25 to 6.51)5 Relative risk difference: 6.3% (‐23.9% to 36.6%) Not statistically significant |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; NNT; number needed to treat | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Number of events is less than 300. 2 Less than 400 participants.
3The incidence of clinically important cytopenia in patients treated with low‐dose MTX is estimated to be less than 1%. 4 Post‐treatment number of swollen joints not reported. Change scores presented here. 5 Post‐treatment number of tender joints not reported. Change scores presented here.
Results
Description of studies
Results of the search
We initially screened 133 references as well as an additional 192 during the update, from which 15 papers in total were selected for full text appraisal.
Six RCTs met the eligibility criteria. The other nine (two abstracts and seven articles) were excluded.
All of the RCTs included in the analysis assessed haematologic side effects with a complete blood count including platelet count. Some trials also included mean corpuscular volume. Three of six included trials reported the results as 'there was no statistically significant difference in the frequency of haematologic side effects between folate group and placebo group'. Hence, they did not report the number of patients with haematologic side effects, mean values or measures of variance and could not be included in the analysis. Overall, the included trials reported 624 participants of which 385 were treated with either folinic (211 participants) or folic acid (174 participants).
See the study flow diagram (Figure 1) and search summary (Table 4).
1.
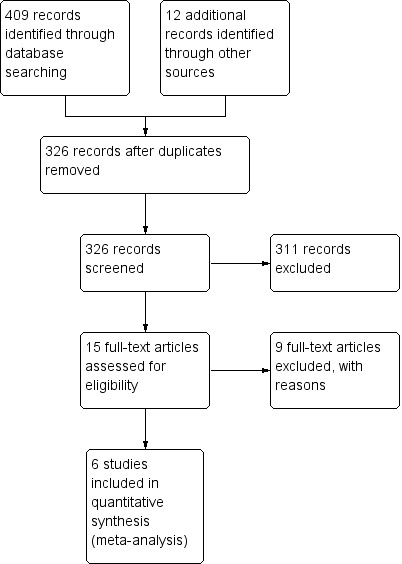
Study flow diagram.
1. Search summary.
| Database(s) | Search Date | Results (n=) | Notes |
| MEDLINE (1966‐1999), CCTR | January 1966‐June 1999 | 150 | 133 after duplicates removed |
| MEDLINE, PubMed | 1999 ‐ June 2011 | ‐ | See footnote1 |
| MEDLINE, PubMed, EMBASE, CENTRAL | 1999‐March 2012 | 259 | 192 after duplicates removed |
| Total | 409 | ||
| Total after duplicates removed | 326 |
1In 2011 this review was assessed as suitable for a 'streamlined' update search procedure. A Boolean serach of intervention and condition terms was combined with a Randomised Controlled Trial filter from 1999 until June 2011. A Pubmed 'related items' search was also carried out of the 3 largest and 3 most recent included studies from the original review.
During the 2012 review, the search was re‐run, and back‐dated to follow on from the original search (1999).
Included studies
Six RCTs met the updated eligibility criteria (Buckley 1990; Morgan 1990; Morgan 1994; Shiroky 1993; Van Ede 2001; Weinblatt 1993).
Morgan 1994 evaluated two different doses of folic acid and only the patients given a dose of folate within our criteria for acceptance were included in this meta‐analysis. In the trial by Buckley 1990 patients received folinic acid at a dose which varied according to their MTX dose. The mean MTX dose was 9.9 mg/wk (5 to 15 mg/wk). The side effects were not reported separately for patients at above and below 7 mg/wk folinic acid but we decided to include it in the analysis. The inclusion of this study did not change the overall results substantially.
In the trial by Van Ede 2001 patients received folic or folinic acid at a starting dose of 1 mg/day and 2.5 mg/week respectively. The MTX dose used was variable (7.5 mg to 25 mg/week). When the MTX dose increased to (or beyond) 15 mg/week the doses of folic and folinic acid were doubled.
For details of the content of individual interventions see Characteristics of included studies.
Excluded studies
Nine RCTs (two abstracts and seven articles) were excluded: two abstracts were published as full‐length publications, one trial was not double‐blind, one was uncontrolled, two did not include the outcomes of interest and three gave doses of folic or folinic acid outside the range of interest for this review (see Characteristics of excluded studies).
Risk of bias in included studies
The methodological quality summary for each included study is presented in Figure 2 and the review authors' judgements about each methodological quality item are presented as percentages across all included studies in Figure 3.
2.
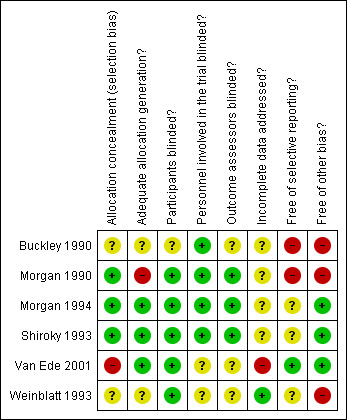
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
3.
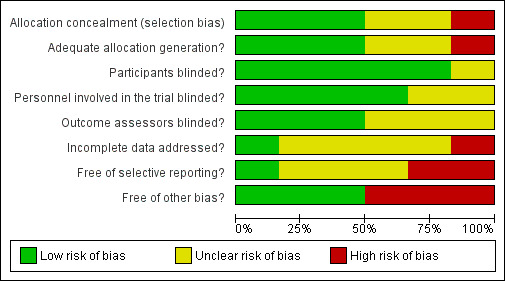
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Allocation
Three studies adequately described sequence generation (low risk of bias) (Morgan 1990; Morgan 1994; Shiroky 1993). One study reported a high risk of bias as there was nothing mentioned about concealment (Van Ede 2001) and the other studies did not clearly describe these methods (unclear risk of bias).
Blinding
Five studies adequately blinded participants for the intervention (low risk of bias) (Morgan 1990; Morgan 1994; Shiroky 1993; Van Ede 2001; Weinblatt 1993). Four studies adequately blinded assessors to type of intervention (low risk of bias) (Buckley 1990; Morgan 1990; Morgan 1994; Shiroky 1993).
Incomplete outcome data
Only one study was judged as appropriately addressing incomplete outcome data (low risk of bias) (Weinblatt 1993).
Selective reporting
Only one was judged as appropriately addressing incomplete outcome data (low risk of bias) (Van Ede 2001).
Other potential sources of bias
Treatment and control groups were comparable at entry in three studies (low risk of bias) (Morgan 1994; Shiroky 1993; Van Ede 2001) and significant differences were present in three studies indicating a high risk of bias (Buckley 1990; Morgan 1990; Weinblatt 1993).
Effects of interventions
See: Table 1; Table 2; Table 3
Six trials with 624 participants met the inclusion criteria. There was no heterogeneity between included trials.
Folic acid versus placebo
An 81% relative (16.8% absolute) reduction in risk was observed for the incidence of abnormal serum transaminase levels (RR 0.19, 95% CI 0.10 to 0.36; P ≤ 0.00001), and there was a statistically significant decrease in the number of people who dropped out of the studies for any reason whilst taking folic acid (‐14.2% absolute difference; RR 0.43, 95% CI 0.29 to 0.64; P ≤ 0.0001).
A 24% relative (8.1% absolute) reduction was seen for the risk of developing gastrointestinal (GI) side effects such as nausea, vomiting or abdominal pain although this failed to reach statistical significance (RR 0.76, 95% CI 0.57 to 1.01; P = 0.057). A trend towards a reduction in the incidence of stomatitis (mouth sores) was seen however this also failed to reach statistical significance (RR 0.90, 95% CI 0.53 to 1.54; P = 0.71).
Folinic acid versus placebo
A 73% relative (15.2% absolute) reduction in risk was observed for the incidence of abnormal serum transaminase levels (RR 0.27, 95% CI 0.16 to 0.44; P ≤ 0.00001), and there was a statistically significant decrease in the number of people who dropped out of the studies for any reason whilst taking folinic acid (‐16.2% absolute difference; RR 0.35, 95% CI 0.23 to 0.53; P ≤ 0.00001).
A 22% relative (7.6% absolute) reduction was seen for the risk of developing GI side effects such as nausea, vomiting or abdominal pain however this failed to reach statistical significance (RR 0.78, 95% CI 0.59 to 1.02; P = 0.066). Similarly, a trend towards a reduction in the incidence of stomatitis (mouth sores) was seen. This too failed to reach statistical significance (RR 0.70, 95% CI 0.46 to 1.07; P = 0.10).
It was not possible to draw meaningful conclusions on the effect of folic or folinic acid on haematologic side effects of MTX due to the small numbers of events and poor reporting of this outcome in included trials, as described above.
Effect of folic or folinic acid on disease activity (efficacy of methotrexate)
No statistically significant difference in disease activity (ie, no statistically significant lowering of the effectiveness of the methotrexate to treat rheumatoid arthritis) was observed between placebo and folic or folinic acid at low dosages. There was a slight trend towards an increased number of tender joints in patients treated with folic acid (+2.46 swollen joints per patient [95% CI ‐6.08 to 11.00; P 0.61]), and both tender and swollen joints for folinic acid (+1.13 tender joints per patient [95% CI ‐4.25 to 6.51; P 0.68], and +1.72 swollen joints per patient [95% CI ‐3.47 to 6.92; P 0.52]), however the wide confidence intervals suggest this is likely due to chance.
The mean differences (MD) in disease activity (swollen and tender joint count, patient global assessment) between placebo or folate supplementation were analysed. There was no evidence of a reduction in the MDs in disease activity (swollen and tender joint count, patient global assessment) in the folate supplementation groups compared to placebo.
Folic acid or folinic acid versus placebo
When studies using either folic acid or folinic acid were pooled together, the results were similar to the analyses of the individual agents versus placebo.
A funnel plot to assess publication bias was not provided as there were not enough included studies to conduct this type of analysis.
Discussion
Summary of main results
The results support the protective effect of folic or folinic supplementation in patients with rheumatoid arthritis during treatment with MTX. There was a statistically significant reduction in the incidence of abnormal transaminase elevation as well as a statistically significant reduction in discontinuation of MTX treatment for any reason in the population studied. A trend towards a reduction in gastrointestinal side effects and stomatitis was demonstrated and although this did not reach statistical significance the concurrent statistically significant reduction in discontinuation of MTX treatment for any reason may indicate that the decrease in these side effects was greatest where the side effects were severe enough to result in MTX withdrawal.
Although the analysis of haematologic side effects was made difficult by small numbers of events and the outcome being poorly reported in included studies, pooled trials reported no statistically significant differences between rheumatoid arthritis patients who received folate supplementation or placebo. The incidence of clinically important cytopenia in patients treated with low dose MTX is estimated to be less than 1% (Canadian Pharmaceuticals Association 1996), and therefore the size of a trial designed to detect any differences would be enormous.
Overall completeness and applicability of evidence
Sample size could potentially be a confounding variable since only one folinic acid study entered more than 40 patients per group. Interestingly, it appears that the benefit shown was greater for trials with higher numbers of patients and overall higher quality. Several authors (Dijkmans 1995; Furst 1994) have reported that the inverse is usually true with lower quality studies showing greater benefits, suggesting biases from poor design. The finding of a trend in the opposite direction in this meta‐analysis is reassuring and indicates the likely validity of the reduction of side effects from folate co‐administration.
A concern with meta‐analysis is the potential existence of publication bias. It is possible that some trials have been completed that found no benefit of folic or folinic acid supplementation. It is difficult to be more definitive about publication bias in this review but we feel it is unlikely that we would be unaware of negative studies of sufficient size to eliminate the benefit seen in this meta‐analysis.
Three studies (excluded from our analysis) have suggested that high dose folinic acid supplementation may reduce the beneficial effects of MTX on rheumatoid arthritis (Griffith 2000; Joyce 1991; Tishler 1988). In a previous version of this review (where these trials were included) there was a difference observed for high dose folinic acid which may have suggested a decrease in benefit of the MTX on the arthritis (an isolated increase in the number of tender joints but not in other clinical variables such as patient global assessment). These results were mostly driven by the study by Joyce et al and in our view are still non‐conclusive. We analysed the effect of adding these studies back into our meta‐analysis, and even when these studies were added back the overall results did not show a statistically significant decrease in MTX efficacy.
There are no studies that suggest folic acid may alter the efficacy of MTX, despite a folic acid to MTX ratio in some trials higher than the folinic acid to MTX ratio used in the study by Joyce 1991 which suggested a decrease in MTX efficacy. We did not find any major differences in disease activity between placebo and folic acid at low dosages.
It is possible that the timing of administration of folinic acid and MTX, as well as the folinic acid to MTX ratio, may alter the efficacy of MTX and it should be noted that this question was not possible to include in the design of this review.
Our results support the protective effects of low dose folate supplementation in reducing GI and hepatic side effects of MTX in patients with rheumatoid arthritis. This is consistent with the recommendations by some authors (Drummond 1995; Morgan 1993; Shiroky 1993; Weinblatt 1993), as well as current prescribing guidelines.
Deciding which of the two forms of folate supplementation should be recommended is more difficult. Experts have differing recommendations, often acknowledging that there is insufficient evidence for advising the use of one compound over the other (Borigini 1995; Dijkmans 1995; Furst 1994; Salach 1994; Schnabel 1994; Weinblatt 1993). There is no evidence to date of a significant difference between folic or folinic acid. The results in this meta‐analysis were less impressive for folinic acid, but four of the five studies had small sample sizes and the larger study by Shiroky et al did show a benefit.
Given both the efficacy of folic acid in reducing MTX side effects and its low cost compared to folinic acid, the use of folic acid is likely to be the more cost‐effective therapy. For folinic acid to be considered cost‐effective it must be proven more effective than folic acid at reducing MTX side effects. One study (Hartman 2004) examined the economics of folate supplementation in rheumatoid arthritis patients in detail and concluded that, aside from the differences in cost between folic and folinic acid, potentially the largest impact on overall treatment costs relates to the increased drug survival seen with either agent. If folate supplements can help patients tolerate MTX for longer, it may delay or prevent a change in treatment to a far more expensive biologic agent.
It is unclear whether all patients on MTX, or only those with side effects, should receive folate supplementation. This systematic review cannot address this issue. Most guidelines and texts recommend folate be given to all patients receiving methotrexate. Yet the effects of folic or folinic acid on the development of liver disease are unknown. It has been suggested that supplementation may have a protective effect on the development of liver disease, in which case universal administration could perhaps be considered.
In summary, supplementation with folic or folinic acid in MTX treated rheumatoid arthritis patients provides a reduction in the incidence of abnormal liver function tests and a reduction in overall withdrawal from treatment. There is also a trend towards a reduction in the incidence of gastrointestinal side effects and stomatitis. The results of our review do not suggest any clear clinical advantage of one form of folate over the other.
Quality of the evidence
A major problem in synthesising evidence is the lack of uniformity in outcome measures. In 1994, during the Conference on Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT), it was determined that a core set of toxicity measures needed to be established for antirheumatic drugs. This set will allow researchers to compare data across all trials with accurate meta‐analyses of toxicity (Brooks 1995). Published guidelines also urge investigators to provide simple tabulation of the incidence of adverse events per patient even where indices are used to allow the reader to understand how the index was computed and the extent to which more patients had single or multiple adverse experiences. We also encourage investigators to describe fully the numbers and flow of patients by treatment group throughout the trial and to clearly report the reasons for dropouts for each group. This meta‐analysis was hampered by lack of uniformity in the way these items were reported.
Potential biases in the review process
The review was restricted to RCTs; we excluded clinical controlled trials (CCTs) thus limiting the potential for bias. All studies described themselves as randomised mostly without giving details of how the randomisation sequence was generated and what precautions were taken in relation to concealment of allocation.
Authors' conclusions
Implications for practice.
Our results support the protective effect of low doses of folic or folinic acid supplementation in reducing GI and liver side effects of MTX in rheumatoid arthritis patients as well as in reducing patient discontinuation of MTX therapy.
Implications for research.
A multicentre RCT comparing both folate compounds and including an economic analysis may be necessary to adequately assess potential differences between the drugs.
Feedback
Comments regarding Cochrane Review by Shea et al. 2013 (Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis), 10 May 2014
Summary
Submitted by Joan Chung Yan Ng, B.Sc.(Pharm); Karin Ng, B.Sc.(Pharm); Li‐Ching Alice Wang, B.Sc.(Pharm); Elaine Wong, B.Sc.(Pharm).
We certify that we have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
***
Dear Editors,
We read with interest the review by Shea et al. regarding folic acid and folinic acid (FA/FNA) for reducing side effects in patients receiving methotrexate for rheumatoid arthritis.(1) The authors of this review should be commended for their efforts in compiling the evidence on the effects of folic or folinic acid use in reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Withdrawals from methotrexate therapy due to toxicity are very common, so we appreciate the efforts of the authors in compiling the evidence into a review that is very relevant to practice.
After analyzing the review in depth, we identified some issues that we would like to bring to your attention. Our main concern pertains to the outcome of liver toxicity. We understand that the conclusion that FA/FNA decreases methotrexate‐induced liver side effects is based on meta‐analysis of 4 out of 6 trials included in this review using the surrogate marker of liver transaminases. However, a retrospective analysis found that alterations of liver transaminase levels were not predictive of progressive hepatic changes (confirmed with percutaneous liver biopsy) with long‐term methotrexate therapy.(2) Therefore, we do not believe that liver transaminase levels are an appropriate surrogate marker for acute or chronic liver toxicity induced by methotrexate. Given the available evidence, we feel that it cannot be concluded that “liver side effects” are decreased; we can only definitively conclude that FA/FNA reduced liver transaminase increase compared to no treatment, and it should be made clear to the reader that it is uncertain whether there is any clinical significance in liver transaminase reduction. Further, we noticed that the study Buckley 1990, which was not included in this liver toxicity analysis, initially outlined a liver transaminase toxicity outcome, but did not report their results.(3) We wonder if you were able to contact the investigators for their unpublished results to include in your meta‐analysis. Although the study was very small (n = 20) and would likely have minimal impact on the overall effect, we feel it is still important to include for completeness.
Our next issue pertains to missing data from the trials included. We noted that in the methods of your review, it was described that a sensitivity analysis would be done to deal with missing data; however, there was no further mention of it in the results or discussion sections. Upon further investigation, we discovered that the methodologies of each study were very unclear, and we are generally unsure of the risk of bias in terms of missing data as details were often not reported. For the study that carried the most weight, Van Ede 2001, it was outlined that 18 patients (equally distributed between the three groups) were excluded from analysis after it was discovered that they did not actually meet inclusion criteria. This is concerning to us as we feel that all patients randomized should be included, and it is unclear whether these excluded patients experienced events during the investigation period or how it was dealt with. Using RevMan, we considered a worst‐case scenario for the GI upset/nausea outcome in comparing FA/FNA with placebo (Analysis 3.1) by adding back in the 18 patients who were excluded to assess the impact of the missing data. The current forest plot shows a risk ratio of 0.74 (0.59 to 0.92) favouring treatment. Assuming that the 18 patients were distributed evenly between the 3 groups (FA, FNA, and placebo) as described in the report, we added 12 events /12 to the FA/FNA group and 0 events /6 to the placebo group, which changes the risk ratio to 0.81 (0.65 to 1.01). This decreases the benefit that FA/FNA appears to have in improving GI upset/nausea and shifts the overall effect to cross 1. This may be a statistical difference when compared to the initial outcome analysis, but there is likely no clinical significance as GI upset/nausea is extremely subjective, and it is difficult to come to a definitive conclusion based on studies with varying designs. The same data manipulation for liver toxicity (Analysis 3.3) and total withdrawal (Analysis 3.4), which both have clear benefit for treatment in the current Cochrane review, has close to no effect on the final overall effect. Therefore, although including this missing data appears to have little impact in the overall analysis, we feel it is important that it still be addressed, and we would encourage you to consider including your sensitivity analyses into the review.
Lastly, we would also would like to request clarification on the rationale for limiting FA/FNA to less than or equal to 7 mg per week. We are aware that guidelines typically recommend folate supplementation of 5 to 10 mg per week (or 0.5 to 2 mg per day) but we are unsure how this threshold of 7 mg was decided for this review.
Thank you again for your work, and we look forward to hearing back from you.
Sincerely,
Joan Chung Yan Ng, B.Sc.(Pharm)
Karin Ng, B.Sc.(Pharm)
Li‐Ching Alice Wang, B.Sc.(Pharm)
Elaine Wong, B.Sc.(Pharm)
References
1. Shea B, Swinden MV, Tanjong Ghogomu E, Ortiz Z, Katchamart W, Rader T, et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. 2013;5:CD000951.
2. Shergy WJ, Polisson RP, Caldwell DS, Rice JR, Pisetsky DS, Allen NB. Methotrexate‐associated hepatotoxicity: retrospective analysis of 210 patients with rheumatoid arthritis. Am J Med. 1988 Dec;85(6):771–4.
3. Buckley LM, Vacek PM, Cooper SM. Administration of folinic acid after low dose methotrexate in patients with rheumatoid arthritis. J Rheumatol. 1990 Sep;17(9):1158–61.
Reply
Dear Ms Chung Yan Ng, Ms Ng, MsAlice Wang, Ms Elaine Wong,
Thank you for your letter.
We will respond to each of the issues you raise.
1. We understand that the conclusion that FA/FNA decreases methotrexate‐induced liver side effects is based on meta‐analysis of 4 out of 6 trials included in this review using the surrogate marker of liver transaminases. However, a retrospective analysis found that alterations of liver transaminase levels were not predictive of progressive hepatic changes (confirmed with percutaneous liver biopsy) with long‐term methotrexate therapy.(2) Therefore, we do not believe that liver transaminase levels are an appropriate surrogate marker for acute or chronic liver toxicity induced by methotrexate. Given the available evidence, we feel that it cannot be concluded that “liver side effects” are decreased; we can only definitively conclude that FA/FNA reduced liver transaminase increase compared to no treatment, and it should be made clear to the reader that it is uncertain whether there is any clinical significance in liver transaminase reduction.
You are correct that the liver transaminase is a surrogate outcome. Despite some conflicting papers such as the one you mention, the assessment of the American College of Rheumatology is that transaiminases do indeed predict clinical liver damage [Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease‐modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008; 59:762.]
2] Further, we noticed that the study Buckley 1990, which was not included in this liver toxicity analysis, initially outlined a liver transaminase toxicity outcome, but did not report their results.(3) We wonder if you were able to contact the investigators for their unpublished results to include in your meta‐analysis. Although the study was very small (n = 20) and would likely have minimal impact on the overall effect, we feel it is still important to include for completeness.
We did attempt to contact the authors of this paper but received no reply. As you point out this will likely have minimal impact on the overall effect. 3.Our next issue pertains to missing data from the trials included. We noted that in the methods of your review, it was described that a sensitivity analysis would be done to deal with missing data; however, there was no further mention of it in the results or discussion sections. Upon further investigation, we discovered that the methodologies of each study were very unclear, and we are generally unsure of the risk of bias in terms of missing data as details were often not reported. For the study that carried the most weight, Van Ede 2001, it was outlined that 18 patients (equally distributed between the three groups) were excluded from analysis after it was discovered that they did not actually meet inclusion criteria. This is concerning to us as we feel that all patients randomized should be included, and it is unclear whether these excluded patients experienced events during the investigation period or how it was dealt with. Using RevMan, we considered a worst‐case scenario for the GI upset/nausea outcome in comparing FA/FNA with placebo (Analysis 3.1) by adding back in the 18 patients who were excluded to assess the impact of the missing data. The current forest plot shows a risk ratio of 0.74 (0.59 to 0.92) favouring treatment. Assuming that the 18 patients were distributed evenly between the 3 groups (FA, FNA, and placebo) as described in the report, we added 12 events /12 to the FA/FNA group and 0 events /6 to the placebo group, which changes the risk ratio to 0.81 (0.65 to 1.01). This decreases the benefit that FA/FNA appears to have in improving GI upset/nausea and shifts the overall effect to cross 1. This may be a statistical difference when compared to the initial outcome analysis, but there is likely no clinical significance as GI upset/nausea is extremely subjective, and it is difficult to come to a definitive conclusion based on studies with varying designs. The same data manipulation for liver toxicity (Analysis 3.3) and total withdrawal (Analysis 3.4), which both have clear benefit for treatment in the current Cochrane review, has close to no effect on the final overall effect. Therefore, although including this missing data appears to have little impact in the overall analysis, we feel it is important that it still be addressed, and we would encourage you to consider including your sensitivity analyses into the review.
We disagree with this approach. The patients that did not meet the inclusion criteria are by definition inappropriate patients for this study so it would be clinically misleading to include them since it is inappropriate to use data from them for any clinically relevant conclusion. Furthermore there were 6 in each group so it does not introduce an imbalance between groups. 4. Lastly, we would also would like to request clarification on the rationale for limiting FA/FNA to less than or equal to 7 mg per week. We are aware that guidelines typically recommend folate supplementation of 5 to 10 mg per week (or 0.5 to 2 mg per day) but we are unsure how this threshold of 7 mg was decided for this review.
We chose this as it reflects the most frequent clinical practice of 1 mg per day. Best wishes
Contributors
Beverley Shea, George Wells, Peter Tugwell on behalf of the authors.
What's new
| Date | Event | Description |
|---|---|---|
| 7 July 2014 | Feedback has been incorporated | Submitted by Joan Chung Yan Ng, B.Sc.(Pharm); Karin Ng, B.Sc.(Pharm); Li‐Ching Alice Wang, B.Sc.(Pharm); Elaine Wong, B.Sc.(Pharm). |
History
Review first published: Issue 1, 1998
| Date | Event | Description |
|---|---|---|
| 25 June 2013 | Amended | Minor edits |
| 17 January 2013 | New search has been performed | New search with one new study. |
| 1 June 2012 | New citation required and conclusions have changed | This review update involves new authors. The search was updated to include studies up to March 2012. The protocol has been updated to exclude high doses of folic or folinic acid > 7 mg/week in order to obtain a better estimate of effect in doses currently used in clinical practice. As a result, three previously included studies have been removed from the meta‐analysis. |
| 11 June 2011 | New citation required but conclusions have not changed | This review update involved new authors. All analyses were new but the conclusions did not change. |
| 6 September 2008 | Amended | Converted to new review format. |
| 29 August 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to acknowledge the following for their contribution to the original systematic review, Maria E Suarez‐Almazor, and David Moher. The authors would like to acknowledge Alicia White for preparing the retrospective risk of bias tables and the original summary of findings tables and Jessie McGowan and Margaret Sampson for preparing and executing the original search strategy. We would also like to acknowledge Kendra Tonkin, Lara Maxwell and the Cochrane Musculoskeletal Group for their ongoing support and their help with the preparation of this manuscript.
Appendices
Appendix 1. MEDLINE search strategy
Database: Ovid MEDLINE(R) without Revisions <1996 to February Week 4 2012>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp rheumatoid arthritis/ (38870)
2 ((rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat$ or reumat$ or revmarthrit$) adj3 (arthrit$ or artrit$ or diseas$ or condition$ or nodule$)).tw. (41694)
3 (felty$ adj2 syndrome).tw. (119)
4 (caplan$ adj2 syndrome).tw. (16)
5 (sjogren$ adj2 syndrome).tw. (5585)
6 (sicca adj2 syndrome).tw. (323)
7 still$ disease.tw. (770)
8 bechterew$ disease.tw. (57)
9 or/1‐8 (54888)
10 exp folic acid/ (14003)
11 exp folinic acid/ (4311)
12 (folic adj2 acid).tw. (6828)
13 folate.tw. (10636)
14 vitamin b9.tw. (34)
15 Leucovorin.tw. (2841)
16 (folinic adj2 acid).tw. (1240)
17 or/10‐16 (21797)
18 random$.tw. (413278)
19 factorial$.tw. (10329)
20 crossover$.tw. (22574)
21 cross over.tw. (8050)
22 cross‐over.tw. (8050)
23 placebo$.tw. (87688)
24 (doubl$ adj blind$).tw. (58798)
25 (singl$ adj blind$).tw. (6120)
26 assign$.tw. (116804)
27 allocat$.tw. (39041)
28 volunteer$.tw. (76814)
29 crossover procedure/ (0)
30 double blind procedure/ (0)
31 randomized controlled trial/ (223436)
32 single blind procedure/ (0)
33 or/18‐32 (646472)
34 9 and 17 and 33 (42)
35 limit 34 to yr="1999 ‐Current" (38)
Appendix 2. Embase search strategy
Database: Embase <1980 to 2012 Week 09>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp rheumatoid arthritis/ (119243)
2 ((rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat$ or reumat$ or revmarthrit$) adj3 (arthrit$ or artrit$ or diseas$ or condition$ or nodule$)).tw. (101138)
3 (felty$ adj2 syndrome).tw. (641)
4 (caplan$ adj2 syndrome).tw. (127)
5 (sjogren$ adj2 syndrome).tw. (11942)
6 (sicca adj2 syndrome).tw. (806)
7 still$ disease.tw. (1679)
8 bechterew$ disease.tw. (401)
9 or/1‐8 (155574)
10 exp folic acid/ (34466)
11 exp folinic acid/ (22285)
12 (folic adj2 acid).tw. (13750)
13 folate.tw. (18246)
14 vitamin b9.tw. (48)
15 Leucovorin.tw. (5463)
16 (folinic adj2 acid).tw. (2598)
17 or/10‐16 (64358)
18 random$.tw. (685835)
19 factorial$.tw. (17939)
20 crossover$.tw. (40770)
21 cross over.tw. (18017)
22 cross‐over.tw. (18017)
23 placebo$.tw. (165691)
24 (doubl$ adj blind$).tw. (121746)
25 (singl$ adj blind$).tw. (11572)
26 assign$.tw. (191900)
27 allocat$.tw. (64571)
28 volunteer$.tw. (149381)
29 crossover procedure/ (31987)
30 double blind procedure/ (103334)
31 randomized controlled trial/ (298222)
32 single blind procedure/ (14881)
33 or/18‐32 (1135921)
34 9 and 17 and 33 (169)
35 limit 34 to yr="1999 ‐ 2012" (144)
Appendix 3. The Cochrane Library search strategy
#1 MeSH descriptor Folic Acid explode all trees
#2 folic
#3 MeSH descriptor Leucovorin explode all trees
#4 folate
#5 folinic
#6 (#1 OR #2 OR #3 OR #4 OR #5)
#7 MeSH descriptor Arthritis, Rheumatoid explode all trees
#8 MeSH descriptor Rheumatoid Nodule explode all trees
#9 rheum*
#10 rheumatoid arthritis
#11 (#7 OR #8 OR #9 OR #10)
#12 (#6 AND #11)
Data and analyses
Comparison 1. Folic acid versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea / GI upset | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.57, 1.01] |
| 2 Stomatitis | 2 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.53, 1.54] |
| 3 Liver toxicity | 2 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.10, 0.36] |
| 4 Neutropenia | 2 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.42, 6.96] |
| 5 Total withdrawals | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.29, 0.64] |
| 6 Tender Joints | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 2.46 [‐6.08, 11.00] |
| 7 Swollen Joints | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐1.65 [‐7.96, 4.66] |
| 8 Patient global assessment | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.50, 0.52] |
1.1. Analysis.
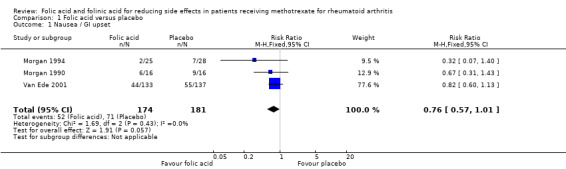
Comparison 1 Folic acid versus placebo, Outcome 1 Nausea / GI upset.
1.2. Analysis.
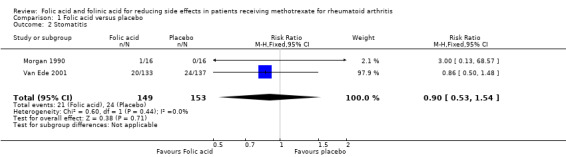
Comparison 1 Folic acid versus placebo, Outcome 2 Stomatitis.
1.3. Analysis.
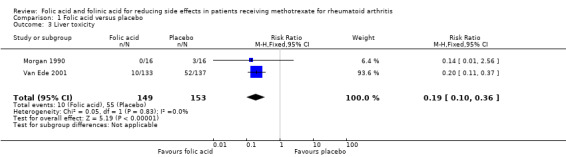
Comparison 1 Folic acid versus placebo, Outcome 3 Liver toxicity.
1.4. Analysis.
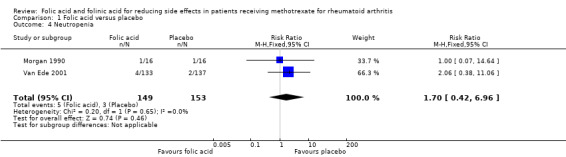
Comparison 1 Folic acid versus placebo, Outcome 4 Neutropenia.
1.5. Analysis.
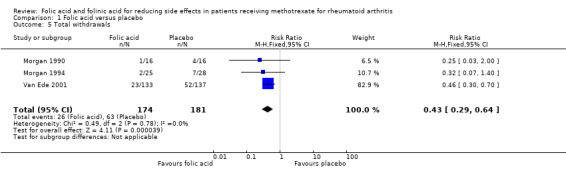
Comparison 1 Folic acid versus placebo, Outcome 5 Total withdrawals.
1.6. Analysis.
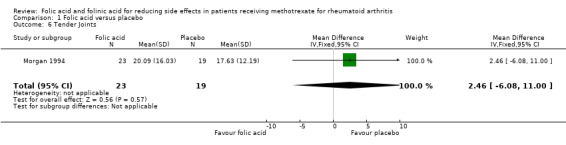
Comparison 1 Folic acid versus placebo, Outcome 6 Tender Joints.
1.7. Analysis.
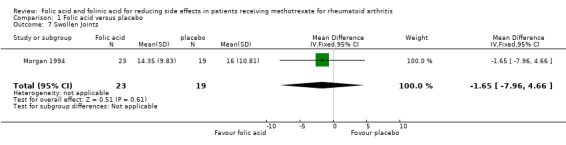
Comparison 1 Folic acid versus placebo, Outcome 7 Swollen Joints.
1.8. Analysis.
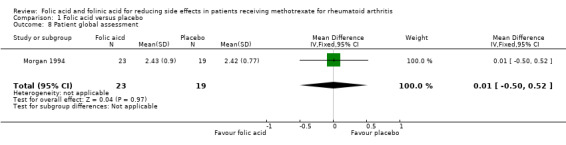
Comparison 1 Folic acid versus placebo, Outcome 8 Patient global assessment.
Comparison 2. Folinic acid versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea / GI upset | 4 | 426 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.59, 1.02] |
| 2 Stomatitis | 3 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.46, 1.07] |
| 3 Liver toxicity | 3 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.16, 0.44] |
| 4 Neutropenia | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.25, 8.59] |
| 5 Total withdrawals | 4 | 422 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.23, 0.53] |
| 6 Tender Joints | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.13 [‐4.25, 6.51] |
| 7 Swollen Joints | 3 | 100 | Mean Difference (IV, Fixed, 95% CI) | 1.72 [‐3.47, 6.92] |
| 8 Patient global assessment | 3 | 100 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.25, 0.54] |
2.1. Analysis.
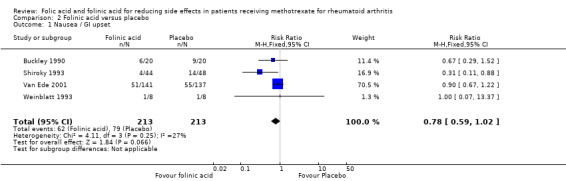
Comparison 2 Folinic acid versus placebo, Outcome 1 Nausea / GI upset.
2.2. Analysis.
Comparison 2 Folinic acid versus placebo, Outcome 2 Stomatitis.
2.3. Analysis.
Comparison 2 Folinic acid versus placebo, Outcome 3 Liver toxicity.
2.4. Analysis.
Comparison 2 Folinic acid versus placebo, Outcome 4 Neutropenia.
2.5. Analysis.
Comparison 2 Folinic acid versus placebo, Outcome 5 Total withdrawals.
2.6. Analysis.
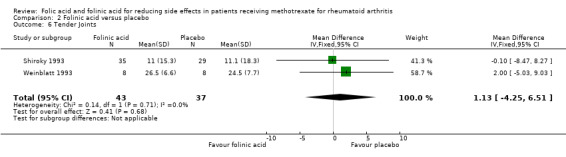
Comparison 2 Folinic acid versus placebo, Outcome 6 Tender Joints.
2.7. Analysis.
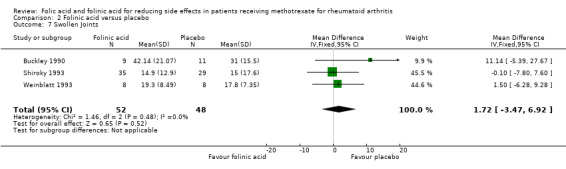
Comparison 2 Folinic acid versus placebo, Outcome 7 Swollen Joints.
2.8. Analysis.
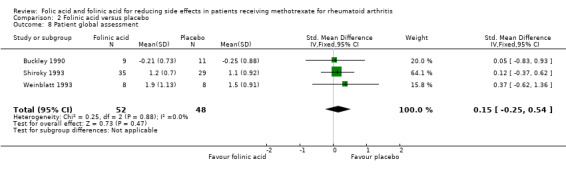
Comparison 2 Folinic acid versus placebo, Outcome 8 Patient global assessment.
Comparison 3. Folic or folinic acid versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea / GI upset | 6 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.59, 0.92] |
| 2 Stomatitis | 4 | 575 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.49, 1.06] |
| 3 Liver toxicity | 4 | 551 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.15, 0.34] |
| 4 Neutropenia | 2 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.40, 5.91] |
| 5 Total withdrawals | 6 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.28, 0.53] |
| 6 Tender Joints | 3 | 122 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.27, 0.45] |
| 7 Swollen Joints | 4 | 142 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.28, 0.38] |
| 8 Patient Global Assessment | 4 | 142 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.22, 0.44] |
3.1. Analysis.
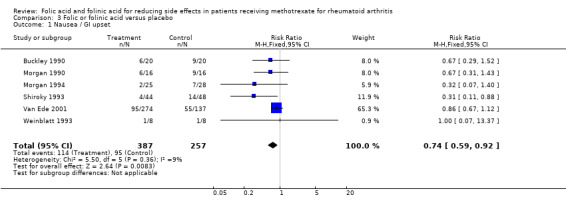
Comparison 3 Folic or folinic acid versus placebo, Outcome 1 Nausea / GI upset.
3.2. Analysis.
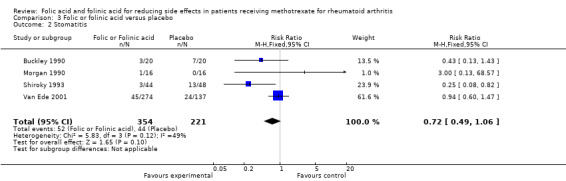
Comparison 3 Folic or folinic acid versus placebo, Outcome 2 Stomatitis.
3.3. Analysis.
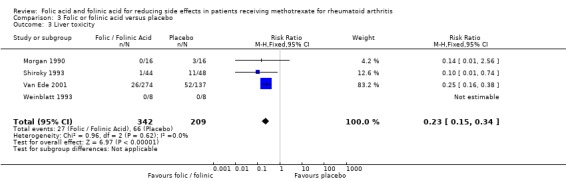
Comparison 3 Folic or folinic acid versus placebo, Outcome 3 Liver toxicity.
3.4. Analysis.
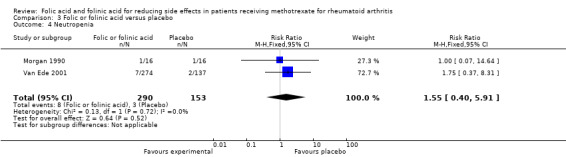
Comparison 3 Folic or folinic acid versus placebo, Outcome 4 Neutropenia.
3.5. Analysis.
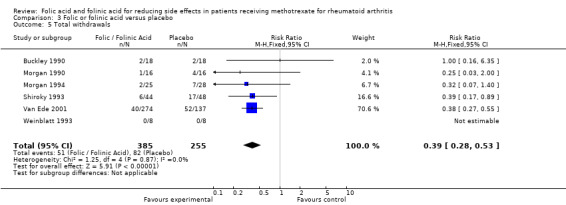
Comparison 3 Folic or folinic acid versus placebo, Outcome 5 Total withdrawals.
3.6. Analysis.
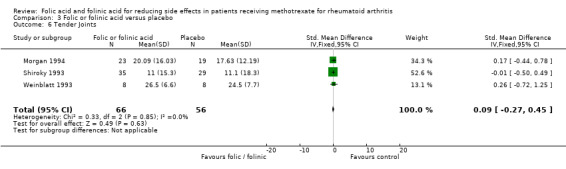
Comparison 3 Folic or folinic acid versus placebo, Outcome 6 Tender Joints.
3.7. Analysis.
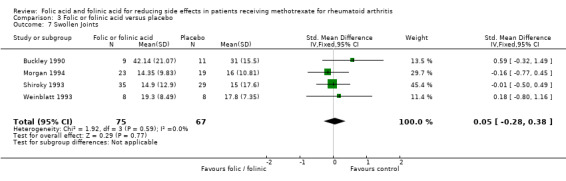
Comparison 3 Folic or folinic acid versus placebo, Outcome 7 Swollen Joints.
3.8. Analysis.
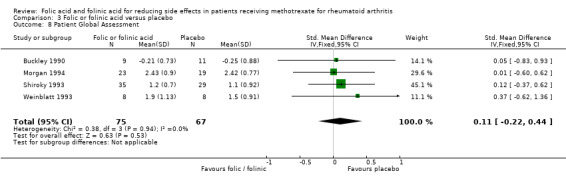
Comparison 3 Folic or folinic acid versus placebo, Outcome 8 Patient Global Assessment.
Comparison 4. Folic acid versus folinic acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 GI side effects | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.56, 6.19] |
| 2 Liver toxicity | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.28, 2.83] |
| 3 Total withdrawals | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.80, 2.56] |
4.1. Analysis.
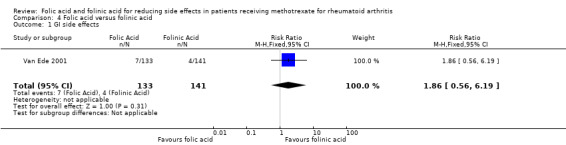
Comparison 4 Folic acid versus folinic acid, Outcome 1 GI side effects.
4.2. Analysis.
Comparison 4 Folic acid versus folinic acid, Outcome 2 Liver toxicity.
4.3. Analysis.
Comparison 4 Folic acid versus folinic acid, Outcome 3 Total withdrawals.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Buckley 1990.
| Methods | Randomized controlled, double‐blind clinical trial. A crossover study design. |
|
| Participants | Twenty patients (7 males, 13 females), with rheumatoid arthritis, older than 18 years, reated with low dose of MTX (less than 20mg /week) were included into a 48‐week trial. | |
| Interventions | Patients were randomized to receive either folinic acid or placebo weekly for 24 weeks, then they were crossed over to receive the alternate treatment. The dose of folinic acid was about equal to the dose of MTX, between 5 and 15mg per week. The mean dose of MTX was 9.9 mg /week. | |
| Outcomes | The reduction of MTX toxicity (gastrointestinal and haematologic side effects) and the reduction of MTX efficacy (joint count, 50' walk time, grip stregnth, rheumatoid factor, ESR, patient and physician assessent, a questionnaire asking the patient to self rate fatigue and pain on a scale from 0‐3, and to report the duration of morning stiffness. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Nothing mentioned about concealment |
| Adequate allocation generation? | Unclear risk | Quote: “We used block randomization (groups of 4) and patients were prospectively assigned to received either folinic acid of placebo initially” Comment: Says patients were randomized |
| Participants blinded? | Unclear risk | Quote: “double blind” Comment: The study is described as double blind in the abstract, but no further details are provided (i.e. whether the intervention and placebo were identical in appearance, taste, smell etc.) |
| Personnel involved in the trial blinded? | Low risk | Quote: “double blind” Comment: Physicians blinded to the treatment regimen. |
| Outcome assessors blinded? | Unclear risk | Quote: “physicians blinded to the treatment regimen” Comment: Not clear if double blind means that participants, study personnel and outcome assessors were blinded |
| Incomplete data addressed? | Unclear risk | Quote: All patients who dropped out did so during the initial drug treatment before crossover.” Comment: Does not use intent to treat |
| Free of selective reporting? | High risk | Quote: “hemogram, platelet count, liver transaminases, creatinine, stomatitis, GI toxicity, alopecia” Comment: Does not report all data, reported only stomatitis and GI upset |
| Free of other bias? | High risk | Quote: Group I received leucovorin first and repletion could have carried over into the placebo period Comment: The groups cannot be compared |
Morgan 1990.
| Methods | Randomized controlled, double‐blind, clinical trial. A parallel study design. | |
| Participants | Thirty‐two patients (16/16 placebo/control respectively) with rheumatoid arthritis, older than 18 years, treated with low dose of MTX (less than 20mg/week), were included into a 24‐week trial. Six males, 26 females, with a mean age of 52.0 and 50.9 years for the folic acid and placebo group respectively. | |
| Interventions | Patients were randomized to receive either FA 1mg/d or placebo for 24 weeks. The median dose of MTX was 7.5 mg/week (2.5‐15 mg/week). | |
| Outcomes | The reduction of MTX toxicity (gastrointestinal , hepatic and haematologic side effects) and the reduction of MTX efficacy (swelling joint count, tenderness joint count, joint swelling index, joint tenderness index, joint pain (VAS), patient global assessment of disease activity, the duration of morning stiffness, grip strength. The definition of elevated liver enzymes in this study was AST or ALP greater than or equal to 2x baseline values. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Quote: “Identical FA‐ and placebo containing capsules were prepared by the Investigational Drug Service of the University of Alabama Hospital” |
| Adequate allocation generation? | High risk | Quote: “The 2 groups were matched, by the study statistician, on the basis of sex, previous use of folate‐containing vitamins, rheumatoid factor (RF) serology, and age.” Comment: patients were matched, was not described as random allocation |
| Participants blinded? | Low risk | "The study was a double‐blind” |
| Personnel involved in the trial blinded? | Low risk | “Neither the investigators, the patients, nor the treating rheumatologists were aware of the placebo/folic acid capsule assignments until the study was completed.” |
| Outcome assessors blinded? | Low risk | “Neither the investigators, the patients, nor the treating rheumatologists were aware of the placebo/folic acid capsule assignments until the study was completed.” Comment: outcome assessors blinded adequately |
| Incomplete data addressed? | Unclear risk | Quote: “Seven patients could not be included in the data analysis...Five of the patients (16%) dropped out of the study before visit 3. Four of these patients were in the placebo group” Comment: Information about missing data was not mentioned |
| Free of selective reporting? | High risk | Not all measured outcomes were reported |
| Free of other bias? | High risk | Quote: “Although the placebo group had a longer mean duration of disease, the 2 study groups were comparable in all other parameters measured, suggesting that longer disease duration could not have accounted for the differences observed.” Comment: Not all the baseline characteristics were the same between two groups |
Morgan 1994.
| Methods | Randomized controlled double‐blind, clinical trial. A parallel study design. | |
| Participants | Seventy‐nine, 20 males and 59 females, with rheumatoid arthritis older than 18 years, treated with low dose MTX (less than 20 mg/week), were included into a 48 week‐trial. The mean age was 54.4 years for the FA and 52.2 for the placebo group. | |
| Interventions | Patients were randomized to receive either FA or placebo weekly for 48 weeks. The dose of FA was 5 mg/week or 27.5 mg/week. The mean dose of MTX was 9.16 mg/week. Only the patients receiving low dose (5 mg/wk) FA or placebo were included in the study [25 and 28 patients respectively]. 26 patients were excluded from the analysis. | |
| Outcomes | The reduction of MTX GI and haematological side effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A coded vial number represented the treatment assignment. Unclear if concealed from outcome assessors |
| Adequate allocation generation? | Low risk | “randomization using a computer program ... Patients were assigned to treatment groups by a sequential treatment assignment process designed to balance the sample with respect to baseline features, including age, sex, folate‐containing vitamin use, rheumatoid factor status, and prednisone use.” |
| Participants blinded? | Low risk | Quote: “double‐blind status” Comment: patients received placebo or folic acid and were blinded to vitamin capsule |
| Personnel involved in the trial blinded? | Low risk | Adequate “double blind status” |
| Outcome assessors blinded? | Low risk | Adequate “double blind status” |
| Incomplete data addressed? | Unclear risk | Quote: “Patients contributed to data analyses until therapy with methotrexate was discontinued. For patients who withdrew before receiving 12 months of therapy, we computed toxicity and efficacy data based on data collected until drug therapy was discontinued.” Comment: Did not use intent to treat |
| Free of selective reporting? | Unclear risk | Quote: “At the initial visit, complete blood cell count, Westergren erythrocyte sedimentation rate, liver enzyme (aspartate amino transferase and alkaline phosphatase), rheumatoid factor by nephelometry, and serum creatinine values were obtained.” Comment: Measures and outcomes not all reported |
| Free of other bias? | Low risk | Quote: “We found no statistical differences in mean cumulative methotrexate dose among the three groups.” Comment: similar baseline values and mean methotrexate dose were present between the groups |
Shiroky 1993.
| Methods | Randomized controlled, double‐blind, clinical trial. A parallel study design. | |
| Participants | Ninety‐two patients (48/44 placebo/FNA respectively) with rheumatoid arthritis, older than 18 years, treated with low dose of MTX (less than 20 mg/week), were included into a 52‐week trial. Thirty males, 62 females, with a mean age of 53.1 and 53.4 years for the FNA and placebo group respectively. | |
| Interventions | Patients were randomized to receive either FNA 2.5‐5mg/wk or placebo for 52 weeks. The mean dose of MTX was 13.6 mg/week (2.5‐30 mg/week). | |
| Outcomes | The reduction of MTX toxicity (gastrointestinal, hepatic and haematologic side effects) and the reduction of MTX efficacy (swelling joint count, tenderness joint count, patient global assessment, physician assessment of disease activity, the duration of morning stiffness, grip strength, 50 foot walking time, morning stiffness and HAQ). Side effects relating to toxicity were reported in terms of number of clinic visits at which the side effect occurred, not by number of patients. The number of patients suffering particular side effects were reported when severe enough to result in withdrawal from the study protocol. We used the latter data in the review. We extracted liver enzyme elevation data defined in the study as 'moderate' or 'severe' derangement, which was AST or ALT greater than or equal to 2x the upper limit of the normal range. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Quote: “all supplies of leucovorin and placebo were prepared using identical labeling and bottling.” Comment: concealed from personnel and participants |
| Adequate allocation generation? | Low risk | “Study drug assignment was based on randomization, using a table of random numbers and stratified by center.” |
| Participants blinded? | Low risk | Quote: “The placebo tablets were identical to the leucovorin tablets and both were tasteless.” Comment: double blind |
| Personnel involved in the trial blinded? | Low risk | Quote: “double‐blind” Comment: Personnel did not know whether patients were receiving placebo or leucovorin |
| Outcome assessors blinded? | Low risk | Outcome assessors were adequately blinded. They were not aware if the patients were receiving placebo or leucovorin |
| Incomplete data addressed? | Unclear risk | Quote: “Patients withdrawing early because of an unrelated medical illness were included in the baseline analysis (not the 52‐week analysis of efficacy)” Comment: did not use intent to treat |
| Free of selective reporting? | Unclear risk | Quote: “All patients had a baseline evaluation which consisted of a complete history and physical examination, complete blood cell count (CBC) with differential count, blood biochemistry profile consisting of glucose, urea, serum creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl transferase (GGT), alkaline phosphatase, total bilirubin, total protein, and serum albumin levels, urinalysis, and a 24‐hour urine collection for creatinine clearance and protein excretion analyses” Comment: Study does not show all measured outcomes |
| Free of other bias? | Low risk | Quote: “There were no significant differences noted in the 15 variables analyzed... there were no significant differences noted at baseline No differences were noted in any of the other laboratory values examined” Comment: no bias since there was no statistical differences |
Van Ede 2001.
| Methods | Randomized controlled, double‐blind, clinical trial. A parallel study. | |
| Participants | Four‐hundred and eleven (137/133/141 placebo/folic acid /folinic acid respectively) with rheumatoid arthritis. older than 18 years. | |
| Interventions | Patients were randomized to receive either FA 1 mg/d, FNA 2.5 mg/wk or placebo for 48 weeks. The mean dose of MTX was 13.6 mg/week (2.5‐20 mg/week). | |
| Outcomes | The reduction of MTX toxicity (gastrointestinal, hepatic and haematologic side effects) and the reduction of MTX efficacy (swelling joint count, tender joint count, Ritchie index, Pain score, patient global assessment, physician assessment of disease activity and ESR). Liver toxicity data extracted from this study was reported as "moderate" or "severe" and was defined as values greater than or equal to 3x the upper limit of normal. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Nothing mentioned about concealment |
| Adequate allocation generation? | Low risk | Adequate “randomly assigned” |
| Participants blinded? | Low risk | Adequate “double blind” |
| Personnel involved in the trial blinded? | Unclear risk | Quote: “double blind” Comment: Not sure if double blind includes patient and personnel or patient and outcome assessors |
| Outcome assessors blinded? | Unclear risk | Quote: “double blind” Comment: Not sure if double blind includes patient and personnel or patient and outcome assessors |
| Incomplete data addressed? | High risk | Nothing mentioned about what was done with missing data |
| Free of selective reporting? | Low risk | Everything mentioned about results and measurements was properly reported in the paper |
| Free of other bias? | Low risk | Baseline variables showed no statistically significant between group differences |
Weinblatt 1993.
| Methods | Randomized controlled, double‐blind, clinical trial. A parallel design | |
| Participants | Sixteen (8/8 placebo/control respectively) with rheumatoid arthritis, older than 18 years, treated with low dose of MTX (less than 20 mg/week), were included into a 8‐week trial. Six males, 10 females. The mean age was 55.9 years for the MTX group and 62.3 for the placebo group. | |
| Interventions | Patients were randomized to receive either FNA 1 mg/wk or placebo for 8 weeks. The mean dose of MTX was 13.6 mg/week (2.5‐20 mg/week). | |
| Outcomes | The reduction of MTX toxicity (gastrointestinal and haematologic side effects) and the reduction of MTX efficacy (swelling joint count, tenderness joint count, patient global assessment, physician assessment of disease activity, the duration of morning stiffness, grip strength, 50 foot walking time, morning stiffness and HAQ). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Nothing mentioned about concealment |
| Adequate allocation generation? | Unclear risk | Comment: randomized trial, no sequence was mentioned |
| Participants blinded? | Low risk | Quote: “Our study was an 8‐week double blind study” Comment: Participants adequately blinded |
| Personnel involved in the trial blinded? | Unclear risk | Not clear whether “double blind” refers to personnel |
| Outcome assessors blinded? | Unclear risk | Not clear whether “double blind” refers to outcome assessors |
| Incomplete data addressed? | Low risk | Quote: “All patients completed the study.” Comment: there was no incomplete data to be reported |
| Free of selective reporting? | Unclear risk | Values and outcomes mentioned, but not all reported |
| Free of other bias? | High risk | “There was no statistically significant difference (P > 0.05) in disease activity measurements or laboratory variables between study groups at baseline.” |
FA = folic acid RA = rheumatoid arthritis FNA = folinic acid HAQ = Health Assessment Questionnaire
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andersen 1997 | Does not include outcomes of interest |
| Canadian Group 1992 | Abstract published as full paper |
| Griffith 2000 | Starting dose of folic acid >7mg weekly |
| Hanrahan 1988 | Starting dose of folinic acid >7mg weekly |
| Joyce 1991 | Starting dose of folinic acid >7mg weekly |
| Morgan 1998 | Does not include outcomes of interest |
| Stewart 1991 | Uncontrolled clinical trial |
| Tishler 1988 | Unblinded clinical trial |
| Weinblatt 1992 | This study was published after its presentation on ACR '92 meeting |
Differences between protocol and review
In the update of this review, trials that gave high doses of folic or folinic acid (that had been included in previous versions of this review) were excluded from the analysis. This decision was made based on the authors' view that excluding these studies would increase the clinical relevance of the findings by more accurately estimating the effects of folic or folinic acid at doses currently prescribed in practice. Since the last published version of this review, several groups have published guidelines recommending the administration of folate supplements at dosages around 5 to 10 mg/week. Studies examining doses of folic or folinic acid that were significantly higher than this were therefore excluded.
Contributions of authors
BS ‐ designed and reviewed the protocol for the updated review, extracted data and assessed quality, conducted methodological analysis, and contributed to and reviewed the manuscript.
MS ‐ assisted in designing the updated review protocol, reviewed the protocol, screened and extracted data, and contributed to and reviewed the manuscript.
ETG ‐ contributed to and reviewed drafts of the document.
ZO ‐ designed and reviewed the initial protocol for the review, extracted data and assessed quality, conducted methodological analysis, and contributed to and reviewed the manuscript.
WK ‐ applied the inclusion and exclusion criteria for accepting studies in the review, classified the studies, helped with classifying the interventions, extracted and entered data and assessed quality, and contributed to and reviewed the manuscript.
TR ‐ wrote the search strategies and identified the literature.
CB ‐ contributed methodological expertise and reviewed drafts of the document.
GW ‐ contributed statistical methodological expertise and reviewed drafts of the document.
PT ‐ contributed methodological expertise and reviewed drafts of the document.
Sources of support
Internal sources
University of Ottawa, Clinical Epidemiology Unit, Ottawa,, Canada.
University of Ottawa, Institute of Population Health, Canada.
External sources
No sources of support supplied
Declarations of interest
None known
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Buckley 1990 {published data only}
- Buckley L, Vacek P, Cooper S. Administration of folinic acid after low dose methotrexate in patients with rheumatoid arthritis. Journal of Rheumatology 1990;17:1158‐61. [PubMed] [Google Scholar]
Morgan 1990 {published data only}
- Morgan SL, Baggott J, Vaughn W, Young P, Austin J, Krumdieck C, Alarcon S. The effect of folic acid supplementation on the toxicity of low‐dose methotrexate in patients with rheumatoid arthritis. Arthritis and Rheumatism 1990;33(1):9‐18. [DOI] [PubMed] [Google Scholar]
Morgan 1994 {published data only}
- Morgan S, Baggot J, Vaughn W, et al. Supplementation with folic acid during methotrexate therapy for rheumatoid arthritis. A double‐blind, placebo controlled trial. Annals of Internal Medicine 1994;121(11):833‐41. [DOI] [PubMed] [Google Scholar]
Shiroky 1993 {published data only}
- Shiroky J, Neville C, Esdaile J, et al. Low dose methotrexate with leucovorin (folinic acid) in the management of rheumatoid arthritis. Results of a randomized, double‐blind, placebo controlled trial. Arthritis and Rheumatism 1993;36(3):795‐803. [DOI] [PubMed] [Google Scholar]
Van Ede 2001 {published data only}
- Ede A, Laan R, Blom H, Huizinga T, Haagsma C, Giesendorf B, et al. The C677T mutation in the methylenetetrahydrofolate reductase gene. A genetic risk factor for methotrexate‐related elevation of liver enzymes in rheumatoid arthritis patients. Arthritis and Rheumatism 2001;44(11):2525‐30. [DOI] [PubMed] [Google Scholar]
Weinblatt 1993 {published data only}
- Weinblatt M, Maier A, Coblyn J. Low dose leucovorin does not interfere with the efficacy of methotrexate in rheumatoid arthritis: an 8 weeks randomized placebo controlled trial. Journal of Rheumatology 1993;20:950‐2. [PubMed] [Google Scholar]
References to studies excluded from this review
Andersen 1997 {published data only}
- Andersen LS, Hansen EL, Knusdsen JB, Wester JU, Hansen GVO, Hansen TM. Propectively measured red cell folate levels in methotrexate treated patients with rheumatoid arthritis: Relation to withdrawal and side effects. Journal of Rheumatology 1997;24(5):830‐7. [PubMed] [Google Scholar]
Canadian Group 1992 {unpublished data only}
- The Canadian Leucovorin Study Group. Low dose methotrexate with leucovorin in the management of rheumatoid arthritis [Abstract]. Arthritis and Rheumatism 1993;36(6):795‐803. [DOI] [PubMed] [Google Scholar]
Griffith 2000 {published data only}
- Griffith S, Fisher J, Clarke S, Montgomery B, Jones P, Saklatvala J, et al. Do patients with rheumatoid arthritis established on methotrexate and folic acid 5 mg daily need to continue folic acid supplements long term?. Rheumatology 2000;39:1102‐9. [DOI] [PubMed] [Google Scholar]
Hanrahan 1988 {published data only}
- Hanrahan P, Russell A. Concurrent use of folinic acid and methotrexate in rheumatoid arthritis. Journal of Rheumatology 1988;15:1078‐80. [PubMed] [Google Scholar]
Joyce 1991 {published data only}
- Joyce D, Will D, Hoffman D, Laing B, Blackbourn S. Exacerbation of rheumatoid arthritis in patients treated with methotrexate after administration of folinic acid. Annals of the Rheumatic Diseases 1991;50:913‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 1998 {published data only}
- Morgan SL, Baggott JE, Lee JY, Alarcon GS. Folic acid supplementation prevents deficient blood folate levels and hyperhomocysteinemia during longterm, low dose methotrexate therapy for rheumatoid arthritis: Implications for cardiovascular disease prevention. Journal of Rheumatology 1998;25:441‐6. [PubMed] [Google Scholar]
Stewart 1991 {published data only}
- Stewart K, Mackenzie A, Clough J, Wilke W. Folate supplementation in methotrexate‐treated rheumatoid arthritis patients. Seminars in Arthritis and Rheumatism 1991;20(5):332‐8. [DOI] [PubMed] [Google Scholar]
Tishler 1988 {published data only}
- Tishler M, Caspi D, Fishel B, Yaron M. The effects of leucovorin (folinic acid) on methotrexate therapy in rheumatoid arthritis patients. Arthritis and Rheumatism 1988;31(7):906‐8. [DOI] [PubMed] [Google Scholar]
Weinblatt 1992 {published data only}
- Weinblatt M, Maier A, Coblyn J. Low dose leucovorin plus methotrexate (MTX) in rheumatoid arthritis (RA): an eight week placebo controlled randomized trial [Abstract]. Arthritis and Rheumatism 1992;Suppl:S340. [Google Scholar]
Additional references
Anderson 1985
- Andersen P, West S, O'Dell J, et al. Weekly pulse methotrexate in rheumatoid arthritis. Clinical and immunologic effects in a randomized, double‐blind study. Annals of Internal Medicine 1985;103:489‐96. [DOI] [PubMed] [Google Scholar]
Arnett 1988
- Arnett F, Edworthy S, Bloch D, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheumatism 1988;31:315‐24. [DOI] [PubMed] [Google Scholar]
Brooks 1995
- Brooks P, Day R. Toxicity of antirheumatic drugs. Journal of Rheumatology 1995;22(5):998‐90. [PubMed] [Google Scholar]
Chakravarty 2008
- Chakravarty K, McDonald H, Pullar T, Taggart A, Chalmers R, Oliver S, et al. BSR/BHPR guideline for disease‐modifying anti‐rheumatic drug (DMARD) therapy in consultation with the British Association of Dermatologists. Rheumatology 2008;47(6):924‐5. [PUBMED: 16940305] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). The Cochrane Collaboration, 2011. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT,Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dijkmans 1995
- Dijkmans B. Folate supplementation and methotrexate. British Journal of Rheumatology 1995;34:1172‐4. [DOI] [PubMed] [Google Scholar]
Drummond 1995
- Drummond M, Bosi Ferraz M, Mason J. Assessing the cost effectiveness of NSAID: an international perspective. Journal of Rheumatology 1995;22(7):1408‐14. [PubMed] [Google Scholar]
Dutch Society of Rheumatology 2011
- Dutch Society of Rheumatology. Guidelines for Medication: Methotrexate [Nederlandse Vereniging voor Reumatologie. Richtlijnen Medicijnen: Methotrexaat]. Available via http://www.nvr.nl/richtlijnen/Totstandkoming_richtlijnen September 2009 (Updated 2011).
Felson 1990
- Felson D, Anderson J, Meenan R. The comparative efficacy and toxicity of second‐line drugs in rheumatoid arthritis. Results of two meta‐analyses. Arthritis and Rheumatism 1990;33(10):1449‐61. [DOI] [PubMed] [Google Scholar]
Felson 1992
- Felson D, Anderson J, Meenan R. Use of short‐term efficacy/toxicity tradeoffs to select second‐line drugs in rheumatoid arthritis: A meta‐analysis of published clinical trials. Arthritis and Rheumatism 1992;35:1117‐25. [DOI] [PubMed] [Google Scholar]
Furst 1994
- Furst D, Clements P. Pharmacological approaches. SAARDs‐ II. Rheumatology 1994;13:1‐8. [Google Scholar]
GUIPCAR group 2007
- GUIPCAR Group. Clinical practice guideline for the management of rheumatoid arthritis in Spain. Spanish Society of Rheumatology, Madrid 2007:301.
Hartman 2004
- Hartman M, VanEde AE, Severens JL, Laan R, Putte L, Wilt G. Economic evaluation of folate supplementation during methotrexate treatment in rheumatoid arthritis. Journal Of Rheumatology 2004;31(5):902‐8. [PubMed] [Google Scholar]
Hedges 1982
- Hedges LV. Estimation of effect size from a series of independent experiments. Psychological Bulletin 1982;92:490‐499. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jackson 1984
- Jackson R. Biological effects of folic acid antagonists with antineoplastic activity. Pharmacology & Therapeutics 1984;25:61‐82. [DOI] [PubMed] [Google Scholar]
Katchamart 2010
- Katchamart W, Trudeau J, Phumethum V, Bombardier C. Methotrexate monotherapy versus methotrexate combination therapy with non‐biologic disease modifying anti‐rheumatic drugs for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2010;4:pp CD008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kremer 1986
- Kremer J, Galivan J, Streckfuss A, Kamen B. Methotrexate metabolism analysis in blood and liver of rheumatoid arthritis patients. Association with hepatic folate deficiency and formation of polyglutamates. Arthritis and Rheumatism 1986;29:832‐4. [DOI] [PubMed] [Google Scholar]
Kremer 1995
- Kremer J. The changing face of therapy for rheumatoid arthritis. Rheumatic Diseases Clinics of North America 1995;21(3):845‐52. [PubMed] [Google Scholar]
Leeb 1995
- Leeb BF, Witzmann G, Ogris E, Studnicka‐Benke A, Andel I, Schweitzer H, Smolen JS. Folic acid and cyanocobalamin levels in serum and erythrocytes during low‐dose methotrexate therapy of rheumatoid arthritis and psoriatic arthritis patients. Clinical and Experimental Rheumatology 1995 Jul‐Aug;13(4):459‐63. [PubMed] [Google Scholar]
Majithia V 2007
- Majithia V, Geraci SA. Rheumatoid arthritis: diagnosis and management. American Journal of Medicine 2007;120 (11):36–9. [DOI] [PubMed] [Google Scholar]
Maxwell 2009
- Maxwell L, Singh J. Abatacept for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2009;4:pp CD007277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 1987
- Morgan S, Baggot J, Altz‐Smith M. Folate status of rheumatoid arthritis patients receiving long‐term, low‐dose methotrexate therapy. Arthritis and Rheumatism 1987;30:1348‐56. [DOI] [PubMed] [Google Scholar]
Morgan 1991
- Morgan S, Baggot J, Refsum H, Ueland P. Homocysteine levels in patients with rheumatoid arthritis treated with low‐dose methotrexate. Clinical Pharmacology and Therapeutics 1991;50:547‐56. [DOI] [PubMed] [Google Scholar]
Morgan 1993
- Morgan S, Alarcón G, Krumdieck C. Folic acid supplementation during methotrexate therapy: it makes sense [Editorial]. Journal of Rheumatology 1993;20(6):929‐30. [PubMed] [Google Scholar]
Navarro Sarabia 2005
- Navarro Sabia F, Ariza Ariza R, Hernandez Cruz B, Villanueva I. Adalimumab for treating rheumatoid arthritis. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005113.pub2] [DOI] [PubMed] [Google Scholar]
Petitti 1994
- Petitti D. Meta‐analysis, decision analysis, and cost‐effectiveness analysis: methods for quantitative synthesis in medicine. First Edition. New York: Oxford University Press, 1994:90‐114. [Google Scholar]
Salach 1994
- Salach R, Cash J. Methotrexate: The emerging drug of choice for serious rheumatoid arthritis. Clinical Therapeutics 1994;16(6):912‐22. [PubMed] [Google Scholar]
Schnabel 1994
- Schnabel A, Gross Wl. Low‐dose methotrexate in rheumatic diseases: efficacy, side effects and risk factors for side effects. Seminars in Arthritis and Rheumatism 1994;23:310‐27. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Singh 2009
- Singh J, Christensen R, Wells G, Suarez Alamor M, Buchbinder R, Lopez Olivo A, Tanjong Ghogomu E, Tugwell P. Biologics for rheumatoid arthritis: an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007848.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2012
- Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, Moreland LW. 2012 Update of the 2008 American College of Rheumatology Recommendations for the Use of Disease‐Modifying Antirheumatic Drugs and Biologic Agents in the Treatment of Rheumatoid Arthritis. Arthritis Care and Research May 2012;64(5):625‐39. [PUBMED: 22473917] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stenger 1992
- Stenger A, Houtman P, Bruyn G. Does folate supplementation make sense in patients with rheumatoid arthritis treated with methotrexate?. Annals of the Rheumatic Diseases 1992;51:1019‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suarez‐Almazor 1998
- Suarez‐Almazor ME, Belseck E, Shea B, Tugwell P, Wells GA. Methotrexate for treating rheumatoid arthritis. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000957] [DOI] [Google Scholar]
Thompson 1984
- Thompson R, Watts C, Edelman J, et al. A controlled two‐centre trial of parenteral methotrexate therapy for refractory rheumatoid arthritis. Journal of Rheumatology 1984;11:760‐3. [PubMed] [Google Scholar]
Tugwell 1987
- Tugwell P, Bennett K, Gent M. Methotrexate in rheumatoid arthritis. Annals of Internal Medicine 1987;107:358‐66. [DOI] [PubMed] [Google Scholar]
Tugwell 1989
- Tugwell P, Bennett K, Gent M. Methotrexate in rheumatoid arthritis. Feedback on American College of Physicians guidelines. Annals of Internal Medicine 1989;110:581‐3. [DOI] [PubMed] [Google Scholar]
Wallace 1995
- Wallace C, Sherry D. A practical to avoidance of methotrexate toxicity [Editorial]. Journal of Rheumatology 1995;22(6):1009‐12. [PubMed] [Google Scholar]
Weinblatt 1985
- Weinblatt M, Coblyn J, Fox D, et al. Efficacy of low‐dose methotrexate in rheumatoid arthritis. New England Journal of Medicine 1985;312:818‐22. [DOI] [PubMed] [Google Scholar]
Williams 1985
- Williams H, Wilkens R, Samuelson C, et al. Comparison of low‐dose oral pulse methotrexate and placebo in the treatment of rheumatoid arthritis. A controlled clinical trial. Arthritis and Rheumatism 1985;28:721‐30. [DOI] [PubMed] [Google Scholar]


