Abstract
Context
Longstanding type 1 diabetes (T1D) may lead to alterations in hippocampal neurochemical profile. Upregulation of hippocampal glucose transport as a result of recurrent exposure to hypoglycemia may preserve cognitive function during future hypoglycemia in subjects with T1D and impaired awareness of hypoglycemia (IAH). The effect of T1D on hippocampal neurochemical profile and glucose transport is unknown.
Objective
To test the hypothesis that hippocampal neurochemical composition is altered in T1D and glucose transport is upregulated in T1D with IAH.
Design and participants
Hippocampal neurochemical profile was measured with single-voxel magnetic resonance spectroscopy at 3T during euglycemia in 18 healthy controls (HC), 10 T1D with IAH, and 12 T1D with normal awareness to hypoglycemia (NAH). Additionally, 12 HC, 8 T1D-IAH, and 6 T1D-NAH were scanned during hyperglycemia to assess hippocampal glucose transport with metabolic modeling.
Setting
University medical center.
Main Outcome Measures
Concentrations of hippocampal neurochemicals measured during euglycemia and ratios of maximal transport rate to cerebral metabolic rate of glucose (Tmax/CMRGlc), derived from magnetic resonance spectroscopy–measured hippocampal glucose as a function of plasma glucose.
Results
Comparison of hippocampal neurochemical profile revealed no group differences (HC, T1D, T1D-IAH, and T1D-NAH). The ratio Tmax/CMRGlc was not significantly different between the groups, T1D-IAH (1.58 ± 0.09) and HC (1.65 ± 0.07, P = 0.54), between T1D-NAH (1.50 ± 0.09) and HC (P = 0.19), and between T1D-IAH and T1D-NAH (P = 0.53).
Conclusions
Subjects with T1D with sufficient exposure to recurrent hypoglycemia to create IAH did not have alteration of Tmax/CMRglc or neurochemical profile compared with participants with T1D-NAH or HC.
The hippocampus plays an essential role in learning and memory processing and is thought to be particularly vulnerable to severe changes in glycemia (1–4). Several studies have shown evidence of hippocampal atrophy and reduced performance on neurocognitive testing in subjects with type 2 diabetes (T2D) (5, 6). Less is known about the effects of diabetes on the hippocampus in subjects with type 1 diabetes (T1D). In children with T1D, exposure to recurrent severe hypoglycemia particularly before age 5 years negatively affects spatial long-term memory performance, which is a hippocampally mediated task (7). Greater exposure to severe hypoglycemia has also been associated with enlargement of hippocampal gray matter volume in children with T1D (8). Adults with longstanding T1D, as with children with the disease, are exposed to recurrent hypoglycemia. Similar to T2D, they are also exposed to chronic hyperglycemia, often without exposure to the other metabolic risk factors seen in T2D. However, little is known about the impact of glycemic variations on hippocampal function in adults with T1D.
Recurrent episodes of hypoglycemia in subjects with diabetes can lead to the development of impaired awareness of hypoglycemia (IAH) (9), a clinical syndrome in which the first sign of low blood sugar could be the loss of consciousness. Interestingly, healthy subjects exposed to antecedent hypoglycemia and adults with T1D and IAH show less impairment of cognitive performance during a subsequent episode of hypoglycemia (10–12), an observation that suggests antecedent hypoglycemia may precondition hippocampal metabolism to support regional activity during subsequent hypoglycemia. Increased glucose uptake has been proposed as a potential cerebral adaptation to recurrent hypoglycemia, which allows neurons to maintain metabolism during the stress of hypoglycemia (13). In animal experiments, antecedent hypoglycemia has been shown to increase blood–brain barrier glucose transport and glucose transporter expression (14, 15). Using magnetic resonance spectroscopy (MRS), a previous study demonstrated that steady-state glucose concentrations in the occipital cortex are higher in subjects with T1D and IAH compared with healthy controls (16), suggesting that glucose transport may be upregulated after exposure to recurrent hypoglycemia. Hippocampal glucose transport has not been studied previously in humans.
The aim of this study was to examine effects of longstanding T1D on the neurochemical composition of the hippocampus and to identify if T1D, along with sufficient exposure to recurrent hypoglycemia to cause IAH, alters hippocampal glucose transport kinetics. We hypothesized that subjects with T1D and IAH will have upregulation of hippocampal glucose transport as compared to healthy controls and subjects with T1D who do not have IAH.
Material and Methods
Participants
Eighteen healthy controls (HC) and 22 subjects with T1D were enrolled after giving informed consent using procedures approved by the University of Minnesota Institutional Review Board: Human Subjects Committee. Volunteers were recruited with the goal to frequency-match age and sex between groups. Magnetic resonance (MR) data were obtained at 3T from all participants to compare neurochemical profiles in the hippocampus during euglycemia. A subset of volunteers (13 HC and 17 T1D patients) underwent a hyperglycemic clamp study, and MR data were continuously collected during hyperglycemia to assess hippocampal glucose transport kinetics. Participants with T1D were categorized as either having IAH or normal awareness of hypoglycemia (NAH) based on scoring with a standardized questionnaire (17).
Exclusion criteria included history of central nervous system or cardiac disease, use of drugs that can alter glucose metabolism (other than insulin for the patients with diabetes), alcohol abuse, history of renal insufficiency with serum creatinine levels above 1.5 mg/dL, pregnancy, and breastfeeding. Subjects also had to meet requirements for a study in the MR scanner, which include weight less than 300 lb, and the absence of metallic substances in their body.
Study protocol
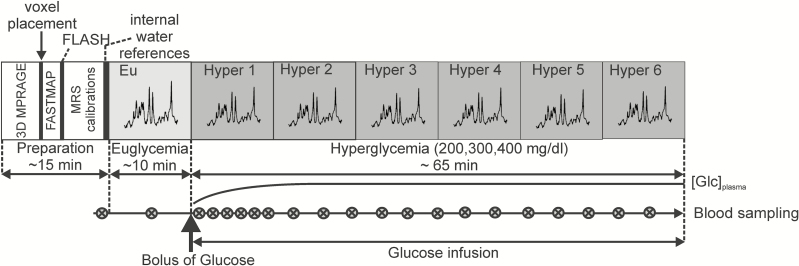
On the day of the hyperglycemic clamp study, subjects arrived at the Center for Magnetic Resonance Research in the morning after an overnight fast. The patients with diabetes were instructed to manage their diabetes in such a way as to minimize the amount of subcutaneous insulin present at the time of the study without creating hyperglycemia (ie, patients were asked to stop their pump on the morning of the clamp experiment and glargine-treated patients were asked to take last glargine dose 24 hours before study start). For the clamp study, 1 intravenous (IV) catheter was inserted into 1 vein in each antecubital fossa for the later infusion of glucose, insulin, and potassium. A third IV catheter was placed retrograde into a vein in the distal leg for later blood sampling. Subjects with diabetes were administered insulin intravenously at a rate of 1.0 mU/kg/min during the clamp study. HC did not receive insulin infusion. An IV infusion of 20% dextrose was administered as necessary to maintain target blood glucose. Blood samples were collected every 5 minutes for measurement of glucose on an Analox machine (Analox Instruments, Stourbridge, UK) to guide adjustments in the glucose infusion rate. During the baseline (euglycemia) MRS data acquisition, blood glucose was maintained at 95 mg/dL (Fig. 1). The hyperglycemic clamp protocol, which was previously successfully used by our group (18, 19), was initiated with an IV bolus injection of 20% glucose over 2 to 3 minutes using the formula of administering 2 mg glucose/kg body weight for each 1 mg/dL increase desired. Immediately after the bolus injection, a continuous infusion of 20% dextrose was started and the rate of administration was adjusted to maintain the desired glucose target. Subjects were randomly assigned to 1 of 3 target hyperglycemic levels: 200, 300, or 400 mg/dL. Insulin levels were drawn before the start of the clamp study and every 20 minutes during the hyperglycemia protocol. MRS data were collected in ~10-minute blocks with minimal interruptions, starting with ~10-minute baseline (during euglycemia) MRS acquisition followed by 6 MRS blocks for up to ~65 minutes (during hyperglycemia) postglucose bolus. The design was sufficient to reach and maintain steady-state glucose concentrations in the brain for at least 20 minutes (Fig. 1).
Figure 1.
Magnetic resonance imaging/MRS acquisition protocol. Schematic representation of the experimental protocol, highlighting MRS data acquisition during euglycemia and hyperglycemia. MRS data were collected in ~10-minute blocks, starting with ~10 minutes during euglycemia, followed by 6 MRS blocks during hyperglycemia. Abbreviations: Eu, euglycemia; FLASH, fast low angle shot; Glc, glucose; hyper, hyperglycemia; MRS, magnetic resonance spectroscopy; 3D, 3-dimensional.
Subjects with diabetes who participated in the baseline-only (euglycemia) hippocampal neurochemical measurement did not receive a continuous insulin infusion. They were instructed to adjust insulin so that blood glucose was in the range 90 to 140 mg/dL before the start of the MR imaging (MRI) study. If the blood glucose was not in the target range, insulin was given to bring glucose into the target range or the study was rescheduled.
MRI and MRS data acquisition
All data were obtained on a 3T Siemens Tim Trio scanner with a standard 32-channel receive-array coil and whole-body coil for excitation. The position of the subjects’ head was fixed with memory foam. High-resolution sagittal magnetization prepared rapid acquisition gradient echo images (repetition time [TR] = 2530 ms, echo time [TE] = 3.65 ms, flip angle = 7°, slice thickness = 1 mm, 224 slices, field-of-view = 256 × 176 mm2, matrix size = 256 × 256) were acquired. Fast low angle shot (FLASH) images were obtained as a reference for the subjects head position (70 sagittal slices of 3-mm thickness; TR/TE 212/2.42 ms; field-of-view of 256 × 256 mm with an acquisition matrix of 256 × 256 and 6/8 partial Fourier encoding with a GRAPPA factor of 3).
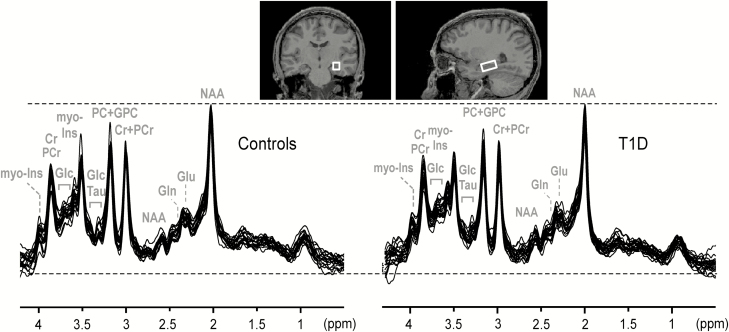
MRS data were collected with methodology recently developed and validated by our group. We demonstrated feasibility to obtain spectra of high quality and reproducibility from the human hippocampus (20), a region challenging for 1H MRS because of its small size and proximity to susceptibility gradients (21). The semi-LASER pulse sequence (TR = 5 seconds, TE = 28 ms) (22) was used in conjunction with the FASTMAP B0 shimming tool (23) to acquire spectra from a volume of interest (VOI) (13 × 26 × 12 mm3 = ~4 mL) in the left hippocampal region (Fig. 2). The shoulders of the subjects were supported by a 3-cm-thick pad, with the head being slightly hyperextended to get the long axis of the hippocampus aligned closer to the transversal plane, thus minimizing rotation of the MRS VOI. Power calibration of the localization and water suppression pulses was performed before collecting metabolite spectra. Outer volume suppression was used to suppress unwanted coherences originating from outside the VOI. The water signal was suppressed with the VAPOR technique (24). Unsuppressed water spectra were obtained as an internal reference for spectra quantification in absolute units (µmol/g) and for residual eddy current removal.
Figure 2.
MR spectra acquired during euglycemia. The MRS voxel placed in the left hippocampal region is shown on MR images with coronal (left) and sagittal (right) orientation. The spectra (semi-LASER, repetition time = 5 sec, echo time = 28 ms, 128 transients) acquired from each subject were overlaid for healthy controls (N = 18) and subjects with T1D (N = 22) separately. Gaussian apodization was used for display purposes with σ = 13 sec. Spectra were scaled to the 2.01 ppm NAA peak. Abbreviations: MR, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; T1D, type 1 diabetes.
Data quality was monitored during acquisition by an experienced MR operator (P.B.). When deterioration of the spectral quality was noticed (insufficient water suppression or linewidth deterioration), acquisition was interrupted and quick sagittal FLASH images were obtained in 35 seconds. The FLASH scans allowed visual assessment of the voxel position relative to the initially acquired reference FLASH scan. If a shift in the VOI was visually detected, the VOI position was corrected and 3 steps followed: readjustment of B0 shims with FASTMAP, recalibration of water suppression pulses, and reacquisition of internal water references.
MR spectra processing and quantification
Metabolite spectra were saved as single shots, which allowed corrections for small frequency and phase drifts resulting from physiological head motion (25) and removal of single shots affected by larger head movements. After removal of the effects of residual eddy currents (26), the spectra were summed in blocks of 128 single shots (~10 minutes of acquisition). All preprocessing steps were performed with the MRspa software (https://www.cmrr.umn.edu/downloads/mrspa). Finally, the sums were quantified using LCModel (http://s-provencher.com/lcmodel.shtml), version 6.3-0G (Fig. 2) (27).
The T2 relaxation time of tissue water obtained in the hippocampus of 74 ms (20) was taken into account in LCModel fitting, assuming that the T2 of water under Carr-Purcell conditions (28) is 1.5× longer than the measured free precession T2 (25). The smaller effect of T2 relaxation of metabolite signals was neglected. Average brain tissue water content of 78% was calculated from average within-VOI fraction of gray matter (GM) and white matter (WM) (20). The cerebrospinal fluid (CSF) within-VOI fraction (CSFfr) obtained per subject (as described later) was used to correct concentrations from each session.
Signal-to-noise ratio (SNR) was measured on metabolite spectra (number of excitations = 128) in frequency domain as height of N-acetyl-aspartate (NAA) peak at 2 ppm divided by root mean square of the noise, whereas the spectral linewidth was measured on the unsuppressed water spectrum as the full-width-at-half-maximum (FWHM). Both parameters were used as descriptors of spectral quality and were included in the statistical model.
Quality control criteria of MRS data
Quality control criteria based on Cramér-Rao lower bounds (CRLB) and correlation coefficients provided by LCModel were used to select metabolites quantified with sufficient reliability, as described previously (29). Specifically, metabolites quantified with mean CRLB <30% in at least 1 of the compared groups were used for further analysis. All concentrations except those for which CRLB was 999% were used when calculating mean CRLBs for each cohort (30). Only the sums of metabolites were reported if correlation coefficients were r < -0.7 (ie, creatine + phosphocreatine = total creatine); or if 1 of the pair of moderately correlating metabolites (-0.5 > r > -0.7) was not quantifiable (CRLB = 999%) in most cases, such as NAA + N-acetyl-aspartyl-glutamate = total NAA and glycerophosphocholine + phosphocholine = total choline. Glucose (Glc) correlated moderately with taurine (Tau, r = -0.6), which translated to higher quantification accuracy and reduced between-subject variance of their sum (Glc+Tau) compared with glucose quantified alone.
Metabolic modeling
To obtain kinetic parameters of hippocampal glucose transport, we fitted (Glc+Tau) data in each group using a steady-state reversible Michaelis-Menten model (18) given by the following equation:
where CMRGlc is the cerebral metabolic rate of Glc, Tmax is the maximal transport rate, Kt is the Michaelis-Menten constant of glucose transport, and Vd is the physical distribution volume of glucose (in the brain Vd = 0.77 mL/g). The expression predicts that the Glcbrain concentration is a linear function of plasma glucose Glcplasma concentration. The slope of the linear fit depends only on the ratio Tmax/CMRGlc and on Vd (but not on Kt). The ratio Tmax/CMRGlc is readily derived from the slope of the fit: if r = Tmax/CMRGlc and s is the slope of the linear fit of Glcbrain versus Glcplasma, then from the equation is: s = Vd(r-1)/(r+1), from which we obtain: r = (Vd+s)/(Vd-s). Assuming Tau does not change during hyperglycemia, the slope of Glcbrain data is expected to be the same as the slope of (Glcbrain+Tau) data. Therefore we used (Glc+Tau) concentrations, which are determined more reliably by LCModel than Glc concentrations alone.
The Glcbrain concentrations deemed to be at steady state were selected independently by 3 different observers (P.G.H., G.O., A.M.) based on visual observation from the time courses of Glcbrain and Glcplasma. The ratio was obtained per group and observer and the coefficients of variation (standard deviation/mean) were calculated to assess interrater variability in the ratios.
Segmentation
Segmentation of the within-VOI brain volume was performed using SPM8 software (https://www.fil.ion.ucl.ac.uk/spm/software/spm8/) and an in-house written MATLAB script to compare the VOI composition between groups. The within-VOI CSF fraction was used to adjust metabolite concentrations per subject; the mean fractions of GM (GMfr), WM (WMfr), and CSF were critical to interpret between-group comparisons.
The hippocampal volumes were obtained using the automated Freesurfer segmentation tool (version 5.3) (31). The ratio of hippocampal volumes and the estimated intracranial volume (obtained by Freesurfer) (32) provided hippocampal volumes normalized to intracranial volume (ICV).
Statistical analysis
Statistical comparison of participants’ characteristics.
Participant characteristics such as age, sex, body mass index (BMI), duration of diabetes, and hemoglobin A1c (HbA1c) were reported for T1D (and IAH and NAH subgroups) and controls (Table 1). Numeric variables were compared between status groups using the 2-tailed independent t test and categorical variables were compared using Fisher’s exact test.
Table 1.
Demographic Data
| Group | n | Age (y) | Sex | BMI (kg/m2) | T1D Duration (y) | HbA1C (%) | HbA1C (mmol/mol) |
|---|---|---|---|---|---|---|---|
| Subjects included in neurochemical profiling at euglycemia | |||||||
| Controls | 18 | 40 ± 17 | 12M/6F | 24 ± 3 | – | – | – |
| T1D | 22 | 41 ± 14 | 11M/11F | 27 ± 4* | 26 ± 12 | 7.4 ± 0.9 | 57 ± 10 |
| T1D-IAH | 10 | 42 ± 15 | 4M/6F | 27 ± 4 | 29 ± 14 | 7.4 ± 0.8 | 57 ± 9 |
| T1D-NAH | 12 | 41 ± 14 | 7M/5F | 27 ± 4 | 24 ± 10 | 7.3 ± 1.0 | 57 ± 11 |
| Subjects included in glucose transport cohort | |||||||
| Controls | 12 | 33 ± 13 | 8M/4F | 23 ± 3 | – | – | – |
| T1D | 14 | 37 ± 12 | 6M/8F | 26 ± 4 | 22 ± 11 | 7.5 ± 0.8 | 58 ± 8 |
| T1D-IAH | 8 | 40 ± 15 | 3M/5F | 26 ± 4 | 26 ± 13 | 7.5 ± 0.5 | 58 ± 6 |
| T1D-NAH | 6 | 34 ± 7 | 3M/3F | 26 ± 5 | 18 ± 5 | 7.5 ± 1.1 | 58 ± 12 |
*P = 0.008 T1D vs. healthy controls. All participants contributed data to the measurement of hippocampal neurochemical profile at euglycemia. A subgroup of this cohort underwent a hyperglycemic clamp in the magnetic resonance scanner to determine glucose transport kinetics in the hippocampus. Data in age, body mass index, T1D duration, and HbA1C columns are mean ± standard deviation.
Abbreviations: BMI, body mass index; F, female; HbA1c, hemoglobin A1c; IAH, impaired awareness of hypoglycemia; M, male; NAH, normal awareness of hyperglycemia; T1D—type 1 diabetes,.
Cross-sectional comparison of neurochemical profiles during euglycemia and hyperglycemia.
HC were compared with the group of subjects with T1D and separately with the IAH and NAH subgroups of T1D, which were also compared with each other and outcomes of the comparison were controlled for false-positive errors with false discovery rate method. However, some factors on the subject status (HC, T1D, IAH, NAH) could potentially confound the effects of diabetes by affecting metabolite concentrations by mechanisms unrelated to diabetes such as normal physiologic processes (age, sex, BMI), variable spectral quality (SNR, FWHM), and variable voxel composition among subjects (GMfr and WMfr). Therefore, those factors were used as covariates in a multiple linear regression model where the response variable was a particular metabolite and the main coefficient of interest was the status. GMfr and WMfr as well as SNR and FWHM are by definition negatively correlated, hence we first regressed GMfr versus WMfr and SNR versus FWHM and used the residuals of these respective models (WMresid and SNRresid) in the final multiple regression models instead of GMfr and SNR. In addition, to compare neurochemical profiles during hyperglycemia, a mixed effect model was used to account for multiple observations. Specifically, metabolite concentrations obtained during several steady-state timepoints and the respective FWHM and SNR provided by LCModel were used for this analysis. Regression models were run using R (version 3.4). P values < 0.05 were considered statistically significant and false positive observations were limited to 5% with false discovery rate correction. Finally, the 2-tailed independent t test was used to compare average CRLB across groups during euglycemia and hyperglycemia.
Comparison of hippocampal volumes.
Hippocampal volumes normalized to ICV were compared between the 2 groups with the independent 2-tailed t test.
Comparison of Glc transport.
Comparison of Glc transport (ie, Tmax/CMRGlc) among groups was performed with z statistics, whereas error of measurement was expressed as standard error of the mean calculated with Monte Carlo simulations (33), because only 1 value per group was obtained by fitting of the steady state concentration (Glc+Tau) selected by single observer (P.G.H.).
Results
Hippocampal neurochemical profile in T1D during euglycemia
Subjects with T1D and HC who participated in the euglycemia study had similar distribution of age (P = 0.75, Table 1) and sex (P = 0.35). BMI was significantly higher in volunteers with T1D (P = 0.008, uncorrected) compared with HC. In the T1D group, diabetes was generally well controlled and of long duration, with an average HbA1c of 7.4 ± 0.9% and duration of 26 ± 12 years (Table 1). Diabetes duration was slightly longer in T1D participants with IAH than in T1D patients with NAH, but was not statistically significant (P = 0.37). The distribution of age, sex, and HbA1c values were very similar between the diabetes groups (P = 0.80, P = 0.67, and P = 0.92, respectively).
Spectral quality during euglycemia was high and consistent between the T1D and HC groups. The reproducible spectral patterns are shown in Fig. 2. Average water linewidths (all P > 0.15) and SNR (all P > 0.31, uncorrected) were similar across 4 groups (HC vs. T1D, HC vs. T1D-IAH, HC v.s T1D-NAH, and T1D-NAH vs. T1D-IAH; Table 2) and were consistent with previously published data (20). The average within-VOI GM, WM, and CSF fractions were similar among groups (all P > 0.08, uncorrected) and are presented in Table 2. Consistent fractional VOI content is demonstrated per subject in Reference (27) and further verifies reproducible voxel placement across subjects and groups. In addition, the comparison of normalized hippocampal volumes among groups did not reveal hippocampal atrophy in subjects with T1D, T1D-IAH, and T1D-NAH when compared with HC and there was no difference in hippocampal volumes between T1D-IAH and T1D-NAH (all P > 0.58, uncorrected (27)).
Table 2.
Measures of Spectral Quality and MRS-Voxel Composition
| SNR | FWHM | GM | WM | CSF | |
|---|---|---|---|---|---|
| (Hz) | (%) | (%) | (%) | ||
| Controls | 68.5 ± 9.5 | 8.8 ± 0.8 | 61.8 ± 3.6 | 32.6 ± 4.5 | 5.4 ± 3.3 |
| T1D | 66.2 ± 9.9 | 8.6 ± 0.9 | 63.0 ± 3.4 | 31.0 ± 5.0 | 5.8 ± 3.5 |
| T1D-IAH | 64.4 ± 11.0 | 8.9 ± 1.1 | 64.1 ± 2.6 | 29.6 ± 3.8 | 6.2 ± 2.9 |
| T1D-NAH | 67.6 ± 9.0 | 8.4 ± 0.5 | 62.2 ± 3.9 | 32.1 ± 5.6 | 5.5 ± 4.0 |
Numbers are means ± SD.
Abbreviations: CSF, cerebrospinal fluid; FWHM, full width half maximum; GM, gray matter; IAH, impaired awareness of hypoglycemia; NAH, normal awareness of hyperglycemia; SNR, signal-to-noise ratio; T1D, type 1 diabetes; WM, white matter.
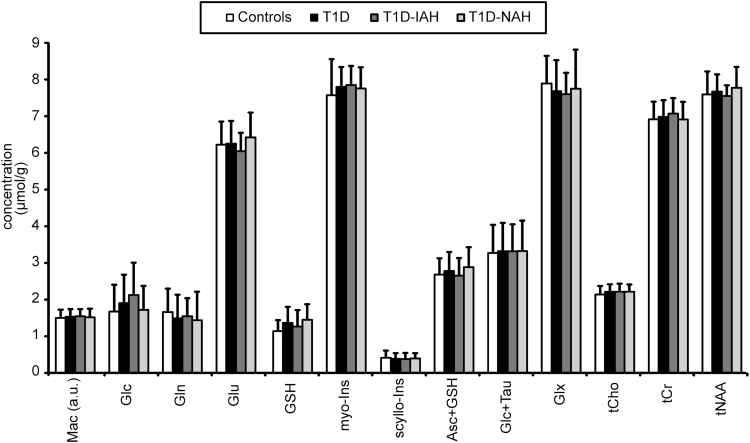
The comparison of neurochemical profiles during euglycemia across 4 groups revealed no difference for any of the 12 metabolites and macromolecules displayed in Fig. 3. The statistical comparison was limited for glucose (Glc), glutamine (Gln), and scyllo-inositol (scyllo-Ins) when quality control criteria were applied (ie, data were compared only when 1 or both of the compared groups had mean CRLB <30% [CRLB are listed in Reference (27), specifically T1D-IAH and T1D-NAH were not compared with each other for Gln and HC were not compared with other groups for scyllo-Ins and Glc.
Figure 3.
Comparison of neurochemical profiles during euglycemia. Bar plot demonstrates similarity of neurochemical levels between healthy controls, (n = 18), subjects with type 1 diabetes (T1D) (n = 22) and subgroup of volunteers with T1D and impaired awareness of hypoglycemia (T1D-IAH) (n = 10), and with T1D and normal awareness of hypoglycemia (NAH) (n = 12). Bars represent mean metabolite concentrations; error bars, standard deviations. Abbreviations: Asc, ascorbate; Cr, creatine; Glc, glucose; Gln, glutamine; Glu, glutamate; Glx = Glu+Gln; GSH, glutathione; Mac, macromolecules; myo-Ins, myo-inositol; scyllo-Ins, scyllo-inositol; tCho, total choline; tCr, total creatine; tNAA, total NAA.
When potential factors that might possibly confound the effect of interest were taken into account (ie, water linewidth, SNR, GMfr, WMfr, BMI, sex, and age) in the regression model, no neurochemical differences across groups were observed.
Hippocampal Glc transport in T1D
The demographic and clinical characteristics of the groups used for the Glc transport assessment are summarized in Table 1 (lower half). None of the characteristics (ie, age, sex, BMI, T1D duration, and HbA1c levels) were statistically different between groups (all P > 0.05, uncorrected). The clamp study under hyperglycemia was either not completed or data were discarded because of unacceptable motion in 4 participants (1 HC, 3 T1D) and thus only their euglycemic neurochemical profiles (reported in the previous section) were analyzed in these cases. Mean insulin levels during hyperglycemia were not significantly different in T1D (51.2 ± 20.4 mU/L) compared with HC (51.6 ± 30.9 mU/L) (P = 0.97). Similar to the comparison during euglycemia, we also compared hippocampal neurochemical profiles measured during hyperglycemia among groups and we observed no differences for any of the 12 metabolites and macromolecules (27).
The capability of 1H MRS to reliably quantify Glc+Tau from data acquired in individual subjects is demonstrated in Fig. 4a,b. The spectral patterns are clearly different between euglycemia and hyperglycemia in the spectral segment from 3.2 to 4.0 ppm where the Glc+Tau peaks are located (Fig. 4a) (27). In this cohort, Glc was quantified with average CRLB ~35% during euglycemia and ~9% during steady-state hyperglycemia. The average CRLBs of Glc+Tau were ~10% during euglycemia and ~6% during steady-state hyperglycemia. The average timecourse of Glc+Tau measured in the brain is shown along with the timecourse of Glc measured in plasma for 3 different plasma Glc target levels (Fig. 4b)
Figure 4.
Comparison of spectral patterns during euglycemia and hyperglycemic clamp in 2 individuals (1 healthy control and 1 subject with T1D. (a) Left: Spectra (sLASER, repetition time/echo time: 5 sec/28 ms, 128 transients) acquired during hyperglycemia (in red) are superimposed over the spectrum collected during euglycemia (in blue). Right: Timecourses of the plasma Glc and brain Glc+Tau concentrations obtained from the same subjects. (b) Timecourses of Glc+Tau in brain and Glc in plasma in all subjects. The timecourses were constructed for 3 distinct targets of plasmatic Glc concentrations using a smoothing algorithm implemented in R (statistical software package). Data from healthy controls (N = 12) and subjects with T1D (N = 14) are pooled. T1D, type 1 diabetes.
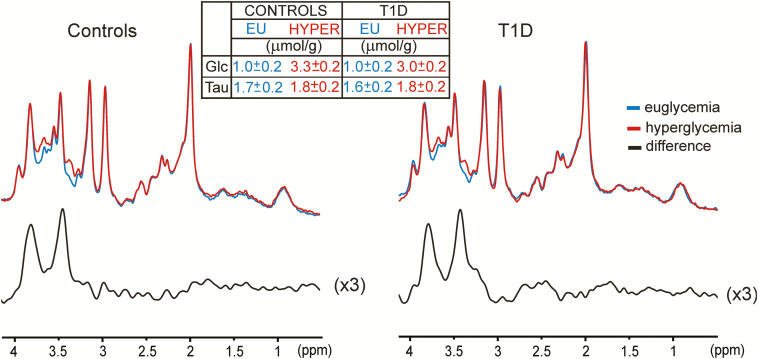
To verify the assumption that Tau does not change because of hyperglycemia and thereby justify using Glc+Tau for glucose transport modeling, we pooled spectra from all subjects and summed them per group (HC and T1D) and condition (euglycemia, hyperglycemia) to obtain 4 high SNR spectra. Those spectra were quantified with LCModel. with resulting Glc and Tau concentrations shown in Fig. 5. The difference in Tau concentration between euglycemia and hyperglycemia is within quantification error (given by CRLBs ~10% [ie, ~0.2 µmol/g]). Therefore, we opted to use the concentration of Glc+Tau, rather than brain Glc alone for metabolic modeling. Finally, we subtracted euglycemia and hyperglycemia spectra within groups (HC and T1D). Only a small linewidth adjustment was necessary to match linewidth of spectra before subtraction (0.1 Hz for T1D, 0 Hz for HC), thus documenting overall stability of the spectral quality and voxel position in the hippocampus. Only glucose peaks are visible in the difference spectrum, thereby ruling out contamination with unwanted signals and confirming good spectral quality throughout the study. Also, the difference spectrum does not suggest changes of metabolites other than Glc.
Figure 5.
The spectra obtained during euglycemia and hyperglycemia (128 transients) were pooled from all controls and T1D and summed by obtaining one pair of high SNR spectra per group. The EU and HYPER spectra were quantified with LCModel. Resulting concentrations for Glc and Tau are shown in the table. Difference spectra reflect the average increase in brain Glc content resulting from hyperglycemia. Abbreviations: EU, euglycemia; HYPER, hyperglycemia.
The ratio of Tmax/CMRGlc was calculated by fitting the model described in the methods section. Between-rater variability of Tmax/CMRGlc was assessed as coefficients of variation (standard deviation/mean), which were below 3% for all groups (0.8%, 1.4%, 0.7%, and 2.3% for HC, T1D, T1D-IAH, and T1D-NHA, respectively). Such a low variance among raters allowed us to use Glc+Tau values selected as steady state by a single observer (P.G.H.) to perform the final comparison of Glc transport kinetics among groups.
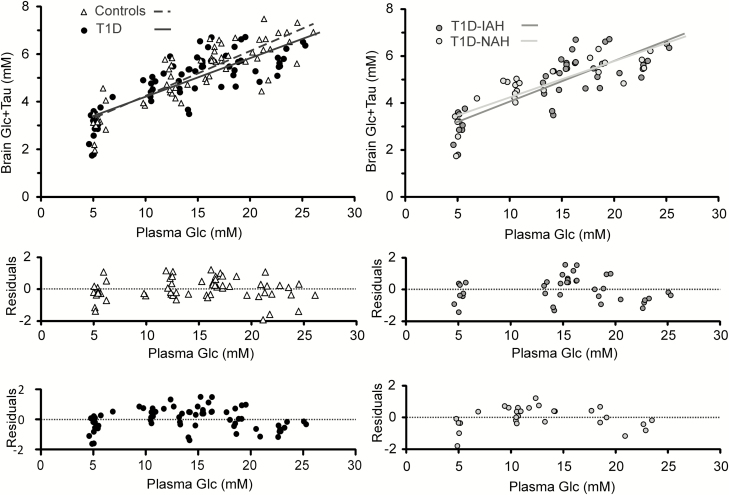
There was no significant difference in Tmax/CMRGlc when T1D (1.53 ± 0.06) was compared with HC (1.65 ± 0.07, P = 0.19, Fig. 6), between T1D-IAH (1.58 ± 0.09) and HC (P = 0.54), between T1D-NHA (1.50 ± 0.09,) and HC (P = 0.19) and between T1D-IAH and T1D-NAH (P = 0.53).
Figure 6.
Steady-state hippocampal Glc+Tau vs plasma Glc concentrations. Also shown are the best fits of the steady-state, reversible Michaelis-Menten model to the data for each of the groups (top row). There was no difference in transport parameters between the groups. Residuals of the linear fits are plotted (a separate panel per group) in the bottom part of the figure. Abbreviations: Glc, glucose; T1D, type 1 diabetes; T1D-IAH, type 1 diabetes with impaired awareness of hypoglycemia; T1D-NAH, type 1 diabetes with normal awareness of hypoglycemia.
We assumed Vd constant (Vd = 0.77 mL/g) and neglected the effect of CSF on Vd. Our data show a similar ~5% CSF content in the voxel in all groups. Correcting for this effect would lead to Vd = 0.78 mL/g (0.77 × 0.95 + 1 × 0.05) instead of 0.77 mL/g, assuming Vd = 1 in CSF. This correction had a negligible effect on our results (<1% change in the values of Tmax/CMRglc).
Discussion
In this study, we measured the neurochemical profile and Tmax/CMRglc in the hippocampus of subjects with T1D and IAH, subjects with T1D and NAH, and HC. Using state-of-the-art MRS methods, we were able to acquire data with good spectral quality, which allowed reliable quantification of 12 hippocampal neurochemicals (Fig. 3). CRLBs were not statistically different among groups for any of the reliably quantified metabolites, thus indicating the same quality of the spectra used for quantification and statistical assessment of group differences. We did not observe any difference in the level of NAA or other metabolites during euglycemia and hyperglycemia in subjects with T1D compared with healthy controls. Comparison of metabolites in the subgroups of T1D-IAH, T1D-NAH, and HC also did not show any significant differences. We also did not observe any difference in Tmax/CMRglc between subjects with T1D and IAH compared with T1D with NAH or HC. This could mean either that Tmax and CMRglc are both unaltered by the disease or that both Tmax and CMRglc are altered by a similar amount, leaving the ratio unchanged. However, a previous study showed no difference in CMRglc between subjects with T1D-IAH and HC, which would support the notion that Tmax is also not different between groups (34).
In subjects with T1D, poor glycemic control, recurrent hypoglycemia, and long duration of diabetes have been associated with atrophy of cortical and subcortical GM (35, 36). Hippocampal structural abnormalities and cognitive dysfunction have been reported in children with T1D who are exposed to recurrent hypoglycemia (7, 8). Subjects with T2D have been shown to have hippocampal atrophy and reduced performance on neurocognitive testing (37). NAA and Glu are highly abundant in the neuronal compartment, and their lower levels may therefore indicate neuronal loss or dysfunction (38). A previous study conducted at 4T found that NAA and Glu levels in the occipital cortex were lower by about 6% in subjects with T1D compared with matched HC (39). MRS data in that study were acquired during hyperglycemic conditions, whereas a recent study reported higher Glu concentration in T1D than HC in a WM region during euglycemia (40). Those results might suggest an association between Glu and Glc levels. However, our data do not show any between-group differences in Glu levels neither during euglycemia, nor during hyperglycemia and do not support association between Glc and Glu levels in the hippocampus (Fig. 3) (27). In addition, we did not observe any changes in the levels of osmolytes myo-inositol and Tau with hyperglycemia, which were shown to increase on induction of hyperglycemia in streptozotocin-induced diabetic rats (41, 42). Although Tau is of substantially lower concentration and thus more difficult to quantify in the human than rodent brain, myo-inositol was quantified with high precision (CRLB ~3%) and also did not increase with hyperglycemia in the current study (27). The discrepant observations between animal and human studies might be caused by the difference in the hyperglycemia onset and duration. Namely, hyperglycemia was induced within minutes in the current study but over days in streptozotocin-induced diabetes in rats. The findings may also reflect differences between animal and human brain physiology (41, 42) or that diabetes was well controlled in our participants (~7.5% HbA1C, see Table 1), making comparison of the animal and human data challenging. In other human studies, lower NAA levels in subjects with T1D compared with HC have been shown in visual cortex (43), pons, parietal white matter (44), basal ganglia, and frontal lobe (45, 46). In these studies, lower NAA was associated with poor glycemic control. Another study that compared neurochemical profiles in the occipital cortex did not find any differences in NAA levels between a small sample of T1D and HC (47). Thus, our results are not consistent with several previous studies in T1D that showed lower NAA levels in different brain regions. However, these studies did not examine hippocampus, which is a challenging brain region to study using 1H MRS methods (20, 39). We also examined hippocampal volumes and comparison among groups including T1D, T1D-IAH, and T1D-NAH to HC, which did not show any significant differences (27). This is in agreement with previous reports, which do not suggest hippocampal atrophy in adults with T1D (1).
Glucose is the primary source of fuel for the brain and cognitive function can be impaired during acute hypoglycemia (48). Previous studies suggest that exposure to recurrent hypoglycemia may result in cerebral adaptation, which attenuates the cognitive impairment seen during subsequent hypoglycemia. Short-term memory performance was preserved during hypoglycemia in healthy subjects who are exposed to antecedent hypoglycemia (11). Similarly, subjects with T1D and IAH perform better on cognitive testing during hypoglycemia as compared with subjects with T1D without IAH (12). Exposure to recurrent hypoglycemia blunts counterregulatory hormonal and symptom responses to further hypoglycemia, leading to development of IAH. Previous data suggest that these adaptations may result from increased fuel availability to the brain during hypoglycemia (49). In rodents, antecedent chronic hypoglycemia has been shown to increase expression of GLUT1 transporters in the endothelial cells of blood–brain barrier and upregulate glucose transport (14, 50). Using in vivo microdialysis technique in rats, McNay et al. (51) showed that exposure to moderate recurrent hypoglycemia resulted in markedly increased glucose concentration in the hippocampal interstitial fluid following intraperitoneal glucose administration compared with controls, suggesting upregulation of glucose transport. Our group has previously demonstrated that steady-state glucose concentrations during hyperglycemia were higher in the occipital cortex in subjects with T1D and IAH compared with matched controls, suggesting that glucose transport may be upregulated in this region in response to recurrent hypoglycemia (16). In one study (52), brain glucose uptake (calculated as the arteriovenous difference in the glucose concentration across the brain multiplied by the cerebral blood flow) was reported to be maintained during hypoglycemia in subjects with T1D and tight glycemic control based on low A1C (suggesting possibly higher exposure to recent hypoglycemia). In comparison, brain glucose uptake decreased during hypoglycemia in healthy controls and subjects with less well-controlled T1D. However, other studies in animal models and humans have not shown that glucose uptake is increased following exposure to recurrent hypoglycemia (53–55). In a recent study, we examined glucose transport kinetics in hypothalamus of healthy subjects who underwent a protocol of repeated antecedent hypoglycemia to experimentally induce IAH. In this study, Tmax/CMRglc in the hypothalamus of healthy humans with experimentally induced IAH was not different from those measured in the absence of IAH (19). In other studies, global brain glucose transport parameters measured by positron emission tomography were normal in healthy subjects exposed to hypoglycemia (53) and similar between hypoglycemia aware and unaware subjects with T1D (54).
Our study had 73% power to detect a 10% change in Tmax/CMRglc between HC and T1D (2-tail z test) with P = 0.05. Therefore, significant differences lower than 10% may not have been detected. Tmax/CMRGlc values estimated for the hippocampus in this study (1.5-1.7) were similar to those estimated for hypothalamus (1.6-1.8) and lower compared with cortical regions (1.8-2.3) using similar MRS methods and reversible Michaelis-Menten modeling in previous studies (18, 19). These data suggest that the rates of brain glucose transport and/or metabolism may be different in cortex and subcortical regions. In addition, it is not clear how the brain glucose concentration may be affected by other factors such as chronic hyperglycemia, glycemic variability. and blood–brain barrier integrity, potentially disrupted by diabetes (56). In a recent paper, Hwang et al. (57) showed that during acute hyperglycemia subjects with T1D had a blunted rise in brain glucose in the occipital cortex compared with HC. In this study, change in brain glucose levels during acute hyperglycemia in subjects with T1D correlated positively with glycemic variability in the 5 days before MR scanning. Conflicting data from in vivo human studies, in regard to brain glucose transport in T1D, can be plausibly explained by differences in study participants, experimental design, and methods of data analysis.
Because of endogenous insulin production, HC participants did not receive insulin infusion during the hyperglycemic clamp. Studies have shown conflicting results on role of insulin on brain glucose uptake and metabolism (58–60). Previous studies provide data that both support or refute the hypothesis that brain glucose uptake/metabolism is insulin-independent. In 1 previous in vivo human study, insulin infusion with plasma insulin concentrations up to 100 pmol/L did not have any significant impact on brain Glc concentrations or glucose transport kinetics (59). In our current study, the mean insulin level during hyperglycemic clamp were not significantly different between T1D and HC.
In this study, we used a commonly used standardized questionnaire to assess hypoglycemia awareness status. We did not assess counterregulatory and symptom response during hypoglycemia. In our previous studies, we have reported that IAH classification by standard questionnaires may not always correlate with counterregulatory hormone and symptoms response during hypoglycemia (61, 62). Therefore, some overlap of hypoglycemia awareness status between the groups with T1D cannot be excluded. In our study, the data were acquired under euglycemic and hyperglycemic conditions; therefore, it is possible that any acute changes in glucose uptake kinetics during hypoglycemia may not be captured with this experimental design. Previous studies using MRS have shown that there is a linear relationship between plasma glucose concentrations and the measured brain glucose content and that this linear relationship extends into the hypoglycemia range (59, 63). Another limitation of this study is that we did not perform neurocognitive testing and therefore are unable to assess if there were any differences in cognitive performance in this cohort of subject with T1D with IAH, T1D with NAH, and HC during euglycemia and hypoglycemia, as reported by some previous studies (36).
In conclusion, our data show that hippocampal neurochemical profile in subjects with longstanding T1D was not different compared to HC. Our data also show that subjects with T1D with sufficient exposure to recurrent hypoglycemia to create IAH did not have upregulation of Tmax/CMRglc compared with subjects with T1D-NAH or HC.
Acknowledgments
Financial Support: This study was supported by grants CTSI KL2TR000113 (A.M.) and National Institutes of Health R01 NS035192 (E.R.S., G.Ö.). C.M.R.R. was supported by grants from the National Institutes of Health P41 EB015894, P30 NS076408, and S10 OD017974. P.B. was partially supported by a NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation (grant no. 27238) and by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 846793.
Clinical Trial Information: CinicalTrials.gov no. NCT01852968.
Glossary
Abbreviations
- BMI
body mass index
- CMRglc
cerebral metabolic rate of glucose
- CRLB
Cramér-Rao lower bounds
- CSF
cerebrospinal fluid
- FLASH
fast low angle shot
- FWHM
full-width-at-half-maximum
- Glc
glucose
- GM
gray matter
- GMfr
mean fraction of gray matter
- HbA1c
hemoglobin A1C
- HC
health control
- IAH
impaired awareness of hypoglycemia
- IV
intravenous
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- MRS
magnetic resonaonce spectroscopy
- NAA
N-acetyl-aspartate
- NAH
normal awareness of hypoglycemia
- SNR
signal-to-noise ratio
- Tau
taurine
- TE
echo time
- Tmax
maximal transport rate
- TR
repetition time
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- Vd
physical distribution volume of glucose
- VOI
volume of interest
- WM
white matter
- WMfr
mean fraction of white matter
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Murray M, Stanley M, Lugar HM, Hershey T. Hippocampal volume in type 1 diabetes. Eur Endocrinol. 2014;10(1):14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McEwen BS, Magariños AM, Reagan LP. Studies of hormone action in the hippocampal formation: possible relevance to depression and diabetes. J Psychosom Res. 2002;53(4):883–890. [DOI] [PubMed] [Google Scholar]
- 3. McEwen BS, Reagan LP. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur J Pharmacol. 2004;490(1-3):13–24. [DOI] [PubMed] [Google Scholar]
- 4. Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3(3):169–178. [DOI] [PubMed] [Google Scholar]
- 5. den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, Hofman A, Breteler MM. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia. 2003;46(12):1604–1610. [DOI] [PubMed] [Google Scholar]
- 6. Bruehl H, Wolf OT, Sweat V, Tirsi A, Richardson S, Convit A. Modifiers of cognitive function and brain structure in middle-aged and elderly individuals with type 2 diabetes mellitus. Brain Res. 2009;1280:186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hershey T, Perantie DC, Warren SL, Zimmerman EC, Sadler M, White NH. Frequency and timing of severe hypoglycemia affects spatial memory in children with type 1 diabetes. Diabetes Care. 2005;28(10):2372–2377. [DOI] [PubMed] [Google Scholar]
- 8. Hershey T, Perantie DC, Wu J, Weaver PM, Black KJ, White NH. Hippocampal volumes in youth with type 1 diabetes. Diabetes. 2010;59(1):236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes. 2005;54(12):3592–3601. [DOI] [PubMed] [Google Scholar]
- 10. Fruehwald-Schultes B, Born J, Kern W, Peters A, Fehm HL. Adaptation of cognitive function to hypoglycemia in healthy men. Diabetes Care. 2000;23(8):1059–1066. [DOI] [PubMed] [Google Scholar]
- 11. Schultes B, Kern W, Oltmanns K, Peters A, Gais S, Fehm HL, Born J. Differential adaptation of neurocognitive brain functions to recurrent hypoglycemia in healthy men. Psychoneuroendocrinology. 2005;30(2):149–161. [DOI] [PubMed] [Google Scholar]
- 12. Zammitt NN, Warren RE, Deary IJ, Frier BM. Delayed recovery of cognitive function following hypoglycemia in adults with type 1 diabetes: effect of impaired awareness of hypoglycemia. Diabetes. 2008;57(3):732–736. [DOI] [PubMed] [Google Scholar]
- 13. McNay EC, Cotero VE. Mini-review: impact of recurrent hypoglycemia on cognitive and brain function. Physiol Behav. 2010;100(3):234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kumagai AK, Kang YS, Boado RJ, Pardridge WM. Upregulation of blood-brain barrier GLUT1 glucose transporter protein and mRNA in experimental chronic hypoglycemia. Diabetes. 1995;44(12):1399–1404. [DOI] [PubMed] [Google Scholar]
- 15. Uehara Y, Nipper V, McCall AL. Chronic insulin hypoglycemia induces GLUT-3 protein in rat brain neurons. Am J Physiol. 1997;272(4 Pt 1):E716–E719. [DOI] [PubMed] [Google Scholar]
- 16. Criego AB, Tkac I, Kumar A, Thomas W, Gruetter R, Seaquist ER. Brain glucose concentrations in patients with type 1 diabetes and hypoglycemia unawareness. J Neurosci Res. 2005;79(1-2):42–47. [DOI] [PubMed] [Google Scholar]
- 17. Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18(4):517–522. [DOI] [PubMed] [Google Scholar]
- 18. Gruetter R, Ugurbil K, Seaquist ER. Steady-state cerebral glucose concentrations and transport in the human brain. J Neurochem. 1998;70(1):397–408. [DOI] [PubMed] [Google Scholar]
- 19. Seaquist ER, Moheet A, Kumar A, Deelchand DK, Terpstra M, Kubisiak K, Eberly LE, Henry PG, Joers JM, Öz G. Hypothalamic glucose transport in humans during experimentally induced hypoglycemia-associated autonomic failure. J Clin Endocrinol Metab. 2017;102(9):3571–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bednařík P, Moheet A, Deelchand DK, Emir UE, Eberly LE, Bareš M, Seaquist ER, Öz G. Feasibility and reproducibility of neurochemical profile quantification in the human hippocampus at 3 T. NMR Biomed. 2015;28(6):685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Choi CG, Frahm J. Localized proton MRS of the human hippocampus: metabolite concentrations and relaxation times. Magn Reson Med. 1999;41(1):204–207. [DOI] [PubMed] [Google Scholar]
- 22. Oz G, Tkáč I. Short-echo, single-shot, full-intensity proton magnetic resonance spectroscopy for neurochemical profiling at 4 T: validation in the cerebellum and brainstem. Magn Reson Med. 2011;65(4):901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gruetter R, Tkác I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43(2):319–323. [DOI] [PubMed] [Google Scholar]
- 24. Tkác I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41(4):649–656. [DOI] [PubMed] [Google Scholar]
- 25. Deelchand DK, Adanyeguh IM, Emir UE, Nguyen TM, Valabregue R, Henry PG, Mochel F, Öz G. Two-site reproducibility of cerebellar and brainstem neurochemical profiles with short-echo, single-voxel MRS at 3T. Magn Reson Med. 2015;73(5):1718–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klose U. In vivo proton spectroscopy in presence of eddy currents. Magn Reson Med. 1990;14(1):26–30. [DOI] [PubMed] [Google Scholar]
- 27. Bednařík P, Henry PG, Khowaja AA, Rubin N, Kumar A, Deelchand DK, Eberly LE, Seaquist E, Oz G, Moheet A. Supplement to the paper: “Hippocampal neurochemical profile and glucose transport kinetics in patients with type 1 diabetes.” Researchgate.net; Uploaded 29 July 2019. 10.13140/RG.2.2.35741.15848/1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bartha R, Michaeli S, Merkle H, Adriany G, Andersen P, Chen W, Ugurbil K, Garwood M. In vivo 1H2O T2+ measurement in the human occipital lobe at 4T and 7T by Carr-Purcell MRI: detection of microscopic susceptibility contrast. Magn Reson Med. 2002;47(4):742–750. [DOI] [PubMed] [Google Scholar]
- 29. Joers JM, Deelchand DK, Lyu T, Emir UE, Hutter D, Gomez CM, Bushara KO, Eberly LE, Öz G. Neurochemical abnormalities in premanifest and early spinocerebellar ataxias. Ann Neurol. 2018;83(4):816–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kreis R. The trouble with quality filtering based on relative Cramér-Rao lower bounds. Magn Reson Med. 2016;75(1):15–18. [DOI] [PubMed] [Google Scholar]
- 31. Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3): 968–980. [DOI] [PubMed] [Google Scholar]
- 32. Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23(2):724–738. [DOI] [PubMed] [Google Scholar]
- 33. Press GA, Barshop BA, Haas RH, Nyhan WL, Glass RF, Hesselink JR. Abnormalities of the brain in nonketotic hyperglycinemia: MR manifestations. AJNR Am J Neuroradiol. 1989;10(2):315–321. [PMC free article] [PubMed] [Google Scholar]
- 34. Henry PG, Criego AB, Kumar A, Seaquist ER. Measurement of cerebral oxidative glucose consumption in patients with type 1 diabetes mellitus and hypoglycemia unawareness using (13)C nuclear magnetic resonance spectroscopy. Metabolism. 2010;59(1):100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bednarik P, Moheet AA, Grohn H, Kumar AF, Eberly LE, Seaquist ER, Mangia S. Type 1 diabetes and impaired awareness of hypoglycemia are associated with reduced brain gray matter volumes. Front Neurosci. 2017;11:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moheet A, Mangia S, Seaquist ER. Impact of diabetes on cognitive function and brain structure. Ann N Y Acad Sci. 2015;1353:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brundel M, van den Heuvel M, de Bresser J, Kappelle LJ, Biessels GJ; Utrecht Diabetic Encephalopathy Study Group Cerebral cortical thickness in patients with type 2 diabetes. J Neurol Sci. 2010;299(1-2):126–130. [DOI] [PubMed] [Google Scholar]
- 38. Rupsingh R, Borrie M, Smith M, Wells JL, Bartha R. Reduced hippocampal glutamate in Alzheimer disease. Neurobiol Aging. 2011;32(5):802–810. [DOI] [PubMed] [Google Scholar]
- 39. Mangia S, Kumar AF, Moheet AA, Roberts RJ, Eberly LE, Seaquist ER, Tkáč I. Neurochemical profile of patients with type 1 diabetes measured by ¹H-MRS at 4 T. J Cereb Blood Flow Metab. 2013;33(5):754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wiegers EC, Rooijackers HM, van Asten JJA, Tack CJ, Heerschap A, de Galan BE, van der Graaf M. Elevated brain glutamate levels in type 1 diabetes: correlations with glycaemic control and age of disease onset but not with hypoglycaemia awareness status. Diabetologia. 2019;62(6):1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang WT, Lee P, Yeh HW, Smirnova IV, Choi IY. Effects of acute and chronic hyperglycemia on the neurochemical profiles in the rat brain with streptozotocin-induced diabetes detected using in vivo ¹H MR spectroscopy at 9.4 T. J Neurochem. 2012;121(3):407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Duarte JM, Carvalho RA, Cunha RA, Gruetter R. Caffeine consumption attenuates neurochemical modifications in the hippocampus of streptozotocin-induced diabetic rats. J Neurochem. 2009;111(2):368–379. [DOI] [PubMed] [Google Scholar]
- 43. Ozsoy E, Doganay S, Dogan M, Alkan A, Firat PG. Evaluation of metabolite changes in visual cortex in diabetic retinopathy by MR-spectroscopy. J Diabetes Complications. 2012;26(3):241–245. [DOI] [PubMed] [Google Scholar]
- 44. Sarac K, Akinci A, Alkan A, Aslan M, Baysal T, Ozcan C. Brain metabolite changes on proton magnetic resonance spectroscopy in children with poorly controlled type 1 diabetes mellitus. Neuroradiology. 2005;47(7):562–565. [DOI] [PubMed] [Google Scholar]
- 45. Northam EA, Rankins D, Lin A, Wellard RM, Pell GS, Finch SJ, Werther GA, Cameron FJ. Central nervous system function in youth with type 1 diabetes 12 years after disease onset. Diabetes Care. 2009;32(3):445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Heikkilä O, Lundbom N, Timonen M, Groop PH, Heikkinen S, Mäkimattila S. Hyperglycaemia is associated with changes in the regional concentrations of glucose and myo-inositol within the brain. Diabetologia. 2009;52(3):534–540. [DOI] [PubMed] [Google Scholar]
- 47. Bischof MG, Brehm A, Bernroider E, Krssák M, Mlynárik V, Krebs M, Roden M. Cerebral glutamate metabolism during hypoglycaemia in healthy and type 1 diabetic humans. Eur J Clin Invest. 2006;36(3):164–169. [DOI] [PubMed] [Google Scholar]
- 48. Gonder-Frederick LA, Zrebiec JF, Bauchowitz AU, Ritterband LM, Magee JC, Cox DJ, Clarke WL. Cognitive function is disrupted by both hypo- and hyperglycemia in school-aged children with type 1 diabetes: a field study. Diabetes Care. 2009;32(6):1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tesfaye N, Seaquist ER. Neuroendocrine responses to hypoglycemia. Ann N Y Acad Sci. 2010;1212:12–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Simpson IA, Appel NM, Hokari M, Oki J, Holman GD, Maher F, Koehler-Stec EM, Vannucci SJ, Smith QR. Blood-brain barrier glucose transporter: effects of hypo- and hyperglycemia revisited. J Neurochem. 1999;72(1):238–247. [DOI] [PubMed] [Google Scholar]
- 51. McNay EC, Sherwin RS. Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats. Diabetes. 2004;53(2):418–425. [DOI] [PubMed] [Google Scholar]
- 52. Boyle PJ, Kempers SF, O’Connor AM, Nagy RJ. Brain glucose uptake and unawareness of hypoglycemia in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1995;333(26):1726–1731. [DOI] [PubMed] [Google Scholar]
- 53. Segel SA, Fanelli CG, Dence CS, Markham J, Videen TO, Paramore DS, Powers WJ, Cryer PE. Blood-to-brain glucose transport, cerebral glucose metabolism, and cerebral blood flow are not increased after hypoglycemia. Diabetes. 2001;50(8):1911–1917. [DOI] [PubMed] [Google Scholar]
- 54. Bingham EM, Dunn JT, Smith D, Sutcliffe-Goulden J, Reed LJ, Marsden PK, Amiel SA. Differential changes in brain glucose metabolism during hypoglycaemia accompany loss of hypoglycaemia awareness in men with type 1 diabetes mellitus. An [11C]-3-O-methyl-D-glucose PET study. Diabetologia. 2005;48(10):2080–2089. [DOI] [PubMed] [Google Scholar]
- 55. McCrimmon RJ, Jacob RJ, Fan X, McNay EC, Sherwin RS. Effects of recurrent antecedent hypoglycaemia and chronic hyperglycaemia on brainstem extra-cellular glucose concentrations during acute hypoglycaemia in conscious diabetic BB rats. Diabetologia. 2003;46(12):1658–1661. [DOI] [PubMed] [Google Scholar]
- 56. Prasad S, Sajja RK, Naik P, Cucullo L. Diabetes mellitus and blood-brain barrier dysfunction: an overview. J Pharmacovigil. 2014;2(2):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hwang JJ, Jiang L, Sanchez Rangel E, Fan X, Ding Y, Lam W, Leventhal J, Dai F, Rothman DL, Mason GF, Sherwin RS. Glycemic variability and brain glucose levels in type 1 diabetes. Diabetes. 2019;68(1):163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bingham EM, Hopkins D, Smith D, Pernet A, Hallett W, Reed L, Marsden PK, Amiel SA. The role of insulin in human brain glucose metabolism: an 18fluoro-deoxyglucose positron emission tomography study. Diabetes. 2002;51(12):3384–3390. [DOI] [PubMed] [Google Scholar]
- 59. Seaquist ER, Damberg GS, Tkac I, Gruetter R. The effect of insulin on in vivo cerebral glucose concentrations and rates of glucose transport/metabolism in humans. Diabetes. 2001;50(10):2203–2209. [DOI] [PubMed] [Google Scholar]
- 60. Hasselbalch SG, Knudsen GM, Videbaek C, Pinborg LH, Schmidt JF, Holm S, Paulson OB. No effect of insulin on glucose blood-brain barrier transport and cerebral metabolism in humans. Diabetes. 1999;48(10):1915–1921. [DOI] [PubMed] [Google Scholar]
- 61. Terpstra M, Moheet A, Kumar A, Eberly LE, Seaquist E, Öz G. Changes in human brain glutamate concentration during hypoglycemia: insights into cerebral adaptations in hypoglycemia-associated autonomic failure in type 1 diabetes. J Cereb Blood Flow Metab. 2014;34(5):876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rubin N, Moheet A, Eberly L, Kumar A, Mangia S, Seaquist E. Does hypoglycemia awareness status on gold and clark questionnaires predict hormonal and symptomatic responses to hypoglycemia (HG) in type 1 diabetes (T1D)? Diabetes 2018;67:379. [Google Scholar]
- 63. van de Ven KC, van der Graaf M, Tack CJ, Heerschap A, de Galan BE. Steady-state brain glucose concentrations during hypoglycemia in healthy humans and patients with type 1 diabetes. Diabetes 2012;61:1974–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]