Abstract
Background:
Current childhood asthma therapies have little effect on lung function trajectory.
Objective:
To determine if mouse allergen exposure reduction is associated with lung function growth in mouse-sensitized/exposed asthmatic children.
Methods:
350 mouse-sensitized/exposed asthmatic children (5–17y) were enrolled in a 1-year randomized trial of integrated pest management+education versus education alone. Pre-/post-bronchodilator (BD) spirometry was performed at baseline, 6 and 12 months, and bedroom floor mouse allergen was measured every 3 months. Mouse allergen reduction was defined as ≥75% decrease in mouse allergen from baseline. Treatment groups were combined for analyses, as there were no differences in outcomes between groups. Changes in lung function over time were modeled, adjusting for age, gender, race, atopy, group, BD reversibility and including an interaction term (allergen reduction*time).
Results:
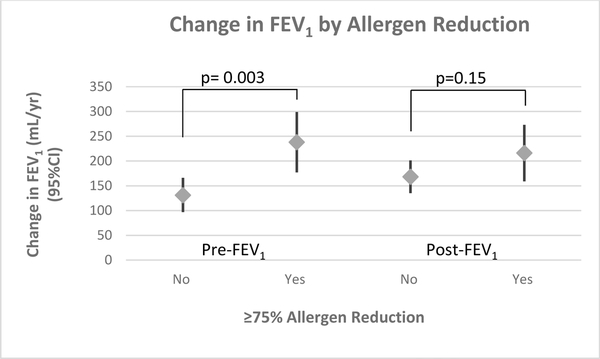
The study population was predominantly black (79.4%) and low-income (66.3% <$30,000). At baseline, median (IQR) mouse allergen level was 5.7ug/g (1.5–22.8); mean (SD) pre-BD FEV1/FVC% was 80.2%(9.0). 92(26.3%) participants had ≥75% mouse allergen reduction. For a 10-year-old black male, ≥75% allergen reduction was associated with an increase in pre-BD FEV1 of 238mL/yr (95% CI: (177–299mL/yr)); whereas <75% allergen reduction was associated with an increase in pre-BD FEV1 of 131mL/yr (97–166mL/yr). The estimated differences in pre- and post-BD FEV1 growth were: 107mL/yr (37–177mL/yr), pint=0.003 and 48mL/yr (−17–113mL/yr), pint=0.15, respectively. The estimated differences in pre- and post-BD FEF25–75 growth were: 182mL/yr (61–304mL/yr), pint=0.003 and 181mL/yr (48–314mL/yr), pint=0.008, respectively.
Conclusion:
Mouse allergen reduction is associated with greater increases in pre-BD FEV1 and pre-/post-BD FEF25–75 over 1 year among sensitized/exposed asthmatic children.
Keywords: Allergen exposure, allergen exposure reduction, lung function growth, lung function trajectory, mouse allergen exposure, allergen-sensitized and exposed asthmatic children, allergic asthma
Graphical Abstract
Capsule summary:
Mouse allergen exposure reduction was associated with greater increases in pre-BD FEV1 and pre-/post-BD FEF25–75 over 1 year in sensitized/exposed asthmatic children, supporting a causal relationship between allergen exposure and impaired lung function growth.
Introduction
Forced expiratory volume in 1 second (FEV1) increases in childhood, then plateaus during late adolescence and early adulthood before starting a gradual decline1. Children with asthma are at risk for abnormal lung function growth, including reduced growth and early decline in lung function2–4. The lung function effects that occur in childhood have long-term implications as children with abnormal lung function are more likely to have lower lung function as adults5, and low lung function in adulthood, including early adulthood, is a predictor of mortality6–8 and chronic obstructive pulmonary disease (COPD)9. Repeated studies have shown that inhaled corticosteroids (ICS), the mainstay of guidelines-based asthma therapy, do not affect lung function trajectory among children10–12. Therefore, there is a great need to identify modifiable risk factors for abnormal childhood lung function growth.
Allergen exposure is one such modifiable risk factor that may influence the long-term lung function trajectory among children with asthma. Cross-sectional studies have shown an association between indoor allergen exposure and lower lung function (FEV1, FEV1/Forced vital capacity (FVC)) among children with asthma who are sensitized to the allergen13,14. However, to our knowledge, there are no studies that examine the effect of allergen exposure reduction on lung function growth in sensitized asthmatic children. An association between allergen reduction and improved lung function growth over time would suggest that existing interventions to reduce indoor allergen exposure could alter the lung function trajectory of sensitized children with asthma. We therefore tested the hypothesis that a reduction in indoor allergen exposure is associated with greater lung function growth by examining relationships between mouse allergen reduction and one year lung function growth among a population of mouse allergen sensitized children with persistent asthma enrolled in the Mouse Allergen and Asthma Intervention Trial (MAAIT). The primary findings of MAAIT are reported elsewhere15. Here we perform an ancillary analysis of MAAIT using pre-specified allergen reduction cutoffs15 as our primary exposure variable.
Methods
Study Population and Recruitment Procedures
MAAIT is a multicenter, randomized clinical trial which randomized 361 children ages 5–17 years to intensive mouse allergen targeted integrated pest management (IPM) intervention plus education or to education alone. To be eligible for enrollment into the trial, children had to meet the following criteria: (1) be mouse sensitized (mouse-specific IgE ≥0.10 kU/L or a positive mouse skin test (wheal size ≥ 3mm)), (2) have uncontrolled asthma, which was defined as an exacerbation (an asthma-related emergency room or urgent care visit, overnight hospitalization, or oral steroid burst) in the last twelve months, (3) have persistent asthma, defined as use of a long-term controller medication or fulfillment of National Asthma Education and Prevention Program (NAEPP) guidelines for persistent disease, and (4) meet an exposure threshold for mouse allergen exposure, which was ≥0.4μg/g mouse allergen in bed dust or ≥0.5 μg/g mouse allergen in bedroom floor dust15.
Recruitment occurred between December 2010 and August 2014, utilizing pediatric emergency rooms, primary care clinics, and specialty clinics. Participants were followed for 1 year at clinical sites in Baltimore and Boston. The study was approved by the Johns Hopkins Medicine and Boston Children’s Hospital Institutional Review Boards and written, informed consent was obtained from parents or guardians of participants.
Clinic Visit Procedures
Questionnaires were used to capture socioeconomic, clinical, and environmental data and medication use was collected by questionnaire and by medications brought to the clinic visit by study participants. Participants underwent skin prick testing using the MultiTest II device (Lincoln Diagnostics, Decatur, Illinois) to 14 common aeroallergens and venipuncture was performed for mouse-specific IgE at baseline15.
Pre- and post-bronchodilator (BD) spirometry was obtained at baseline, 6, and 12 months using a Koko spirometer (Longmont, Colorado). Post-bronchodilator spirometry was obtained approximately 15–20 minutes after administration of albuterol by nebulizer (2.5mg/3mLs). Bronchodilator reversibility was defined as an increase in FEV1 by ≥12% following albuterol. Percent predicted values for participants 8 years and older were generated using Hankinson equations16. Percent predicted values for participants <8 years of age were not calculated. Spirometry data were reviewed for acceptability by the investigators in accordance with American Thoracic Society (ATS) guidelines17.
Home Assessment Visit
Dust samples were collected from the bedroom floor at baseline, 3, 6, 9, and 12 months using a handheld vacuum cleaner using standard methods18–20. The samples were analyzed for Mus m 1 by enzyme-linked immunosorbent assay (ELISA)21.
Study Design and Statistical Analysis
We performed a secondary analysis of 350 participants in MAAIT to determine if reduction in mouse allergen exposure is associated with improved lung function growth over time in mouse sensitized and exposed children with persistent asthma. Mouse allergen reduction was defined as at least a 75% decrease from baseline in the mean mouse allergen levels across all follow-up time points. The primary outcome was change in pre- and post-BD FEV1 and FVC over 1 year.
MAAIT had high retention, as more than 88% of participants were followed to 12 months. Both treatment groups of the parent trial achieved significant reduction in mouse allergen levels which were not different from one another and there were also no differences in clinical outcomes between groups15; therefore we combined groups for our analyses.
All analyses were performed using Stata SE 13.1 (College Station, TX). As we set out to determine whether the average mouse allergen reduction over the follow-up period of the study affected the change in FEV1 over 1 year, we evaluated the FEV1 trajectory over 1 year between participants who achieved ≥75% decrease from baseline in the mean mouse allergen level and those who did not achieve ≥75% decrease from baseline in the mean mouse allergen level. To accomplish this, linear regression models with generalized estimating equations (to account for repeated within person measures of the outcome) using an exchangeable correlation were used to model change in lung function over time. Independent generalized estimating equation models were fit with varying allergen reduction metrics (mean reduction ≥75&, ≥50%, and ≥95%) to examine the effect of different allergen reduction cutoffs. To determine if mouse allergen exposure reduction modified the relationship between time and lung function, we included an interaction term of allergen reduction*time. As lung function growth varies with age, we also included an interaction term of age*time. Final models were adjusted for age, gender, race, group assignment, BD reversibility, age*time and atopy (defined as number of +SPT). A two-tailed p<0.05 was considered statistically significant.
Results
Study Population
350 participants were included in the analysis of the effect of mouse allergen reduction on lung function growth over time and their characteristics are depicted in Table 1. Participants ranged in age from 5 to 17 years, with a mean age of 9.8 years. 63.2% were male, 79.4% were black or African American, and 21.4% were of Hispanic ethnicity. The large majority of participants were on public insurance (87.8%) with 66.3% of families reporting an annual household income of less than $30,000. 68.3% of participants were from Baltimore and the remaining 31.7% were from Boston. At baseline, 66.9% of participants had had an acute visit for asthma in the previous 3 months and 54.6% of participants were on step 4 or 5 asthma controller medication (equivalent to medium dose ICS with long-acting β-agonist or high dose ICS with or without long acting β-agonist). Baseline spirometry revealed 30.8% of participants 8 years and older had an FEV1 of less than 80% predicted and 64.3% of participants had an FEV1/FVC of less than 85%. All participants were sensitized and exposed to mouse. More than 50% of participants were also SPT positive to cockroach and cat. Median bedroom floor mouse allergen level was 5.7μg/g.
Table 1.
Study Population Characteristics (n=350)
| n (%) | |
|---|---|
| Age (y), mean (SD) | 9.8 (3.2) |
| Male gender | 218 (63.2) |
| Race | |
| Black/African American | 278 (79.4) |
| White | 36 (10.3) |
| Other or Unknown | 36 (10.3) |
| Hispanic ethnicity | 75 (21.4) |
| Socioeconomic Measures | |
| Public insurance | 303 (87.8) |
| Annual income < $30K | 232 (66.3) |
| Study Site | |
| Baltimore | 239 (68.3) |
| Boston | 111 (31.7) |
| Asthma-related Acute Visits and Hospitalizations, last 3 months | |
| Acute (ED, urgent care, unscheduled PCP) visit | 234 (66.9) |
| Hospitalization | 44 (12.6) |
| Asthma Controller Medication (n=326) | |
| Step 1: Short acting β-agonist as needed | 38 (11.7) |
| Step 2: Low-dose inhaled corticosteroids (ICS) or leukotriene modifier | 61 (18.7) |
| Step 3: Low-dose ICS plus long acting β-agonist or medium-dose ICS | 49 (15.0) |
| Step 4: Medium-dose ICS plus long acting β-agonist | 16 (4.9) |
| Step 5: High dose ICS with or without long acting β-agonist | 162 (49.7) |
| Lung Physiology (Pre-BD) | |
| FEV1 % predicted, mean (SD)* | 87.1 (17.3) |
| FEV1 < 80% predicted* | 65 (30.8) |
| FVC % predicted, mean (SD)* | 96.8 (16.2) |
| FEV1/FVC%^ | 80.2 (9.0) |
| FEV1/FVC% < 85%^ | 211 (64.3) |
| Skin Prick Test (SPT) Sensitization (n=297) | |
| ≥1 +SPT | 291 (98.0) |
| No. +SPTs, mean (SD) | 6.1 (3.2) |
| Cockroach | 162 (54.6) |
| Cat | 159 (53.5) |
| Dust mite | 132 (44.4) |
| Dog | 67 (22.6) |
| Allergen bedroom floor level | |
| Mouse (μg/g), median (25th-75th%ile) | 5.7 (1.5–22.8) |
| ≥75% mean reduction from baseline | 92 (26.3) |
restricted to ≥8y, n=211; ^n=328
Associations between mouse allergen reduction and FEV1 growth
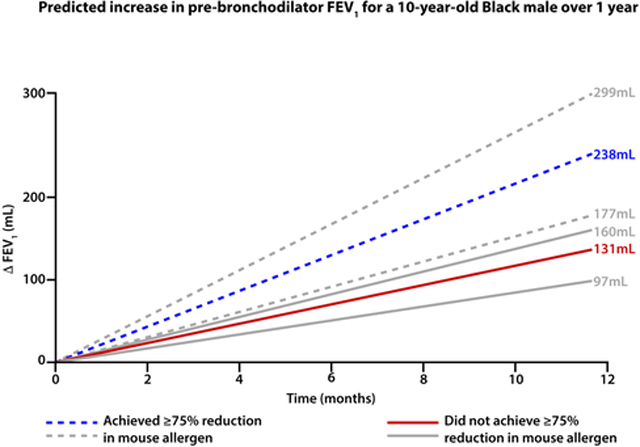
Linear regression models with generalized estimating equations were used to model the relationship between FEV1 and time and subsequently predict the estimated change in FEV1 over time for the average participant, a 10-year-old black male. A 10-year-old black male who achieved ≥75% mean mouse allergen reduction is estimated to have an increase in pre-BD FEV1 of 238mL (95% CI: (177 – 299mL)) over one year; a 10-year-old black male who did not achieve allergen reduction is estimated to have an increase in pre-BD FEV1 of 131mL (97 – 166mL) over one year, with a difference in estimated FEV1 growth of 107mL (37 – 177mL) (Table 2, Figures 1&2). The difference in predicted FEV1 growth between those who did and did not achieve allergen reduction was statistically significant (p=0.003 for interaction term mouse allergen reduction*time) after adjusting for age, sex, race, group assignment, BD reversibility, and atopy (number of positive SPT). For post-BD FEV1 growth, a 10-year-old black male who achieved allergen reduction is estimated to have an increase in post-BD FEV1 of 216mL (95% CI: (159–273mL))over one year compared to 168mL (135 – 201mL) increase in a 10-year-old black male who did not achieve reduction. This estimated difference in post-BD FEV1 growth of 48mL (−17 – 113mL) was not statistically significant (p=0.15). Sensitivity analyses that additionally adjusted for site, oral steroid use in the previous 3 months, controller medication treatment step, body mass index, and cockroach exposure did not substantively change the results (Supplemental Table S1). In addition, adjusting for baseline urine cotinine does not affect the results (data not shown). When we control for bronchodilator reversibility as a continuous variable, instead of a dichotomous variable cut at ≥12%, the estimated difference in pre-BD FEV1 growth is attenuated. The estimated difference in pre-BD FEV1 growth is predicted to be 58mL ((95% CI: −9 – 127), p=0.09) greater for a 10-year-old black male who achieved ≥75% mean reduction in mouse allergen exposure as compared a 10-year-old black male who did not achieve ≥75% mean reduction in mouse allergen exposure. Estimated difference for post-BD FEV1 is 49mL (95% CI: (−21 −119), p=0.17).
Table 2:
Associations between mouse allergen reduction and FEV1 growth*
| Lung function index | Achieved reduction ↑in mL(95%CI) | Did not achieve reduction ↑in mL(95%CI) | Difference in estimated FEV1 growth (95%CI)** | p-value of interaction term*** (reduction*time) |
|---|---|---|---|---|
| Reduction metric: ≥50% mean reduction from baseline | ||||
| Pre-BD FEV1 | 205mL (160 – 250) n=155 | 120mL (79 – 161) n=195 | 85mL (24 – 146) | p=0.006 |
| Post-BD FEV1 | 200mL (157 – 242) n=154 | 164mL (126 – 202) n=195 | 36mL (−21 – 93) | p=0.22 |
| Reduction metric: ≥75% mean reduction from baseline | ||||
| Pre-BD FEV1 | 238mL (177 – 299) n=92 | 131mL (97 – 166) n=258 | 107mL (37 – 177) | p=0.003 |
| Post-BD FEV1 | 216mL (159 – 273) n=92 | 168mL (135 – 201) n=257 | 48mL (−17 – 113) | p=0.15 |
| Reduction metric: ≥90% mean reduction from baseline | ||||
| Pre-BD FEV1 | 255mL (159 – 351) n=44 | 147mL (115 – 180) n=306 | 108mL (7 – 209) | p=0.04 |
| Post-BD FEV1 | 208mL (119 – 298) n=44 | 177mL (146 – 207) n=305 | 31mL (−63 – 126) | p=0.51 |
Average change over 1 year the mean participant (10yo black male)
Participants who achieved mean reduction minus participants who did not achieve mean reduction
Adjusted for age, sex, race, group assignment, BD reversibility, and atopy (number of positive SPT), n=296
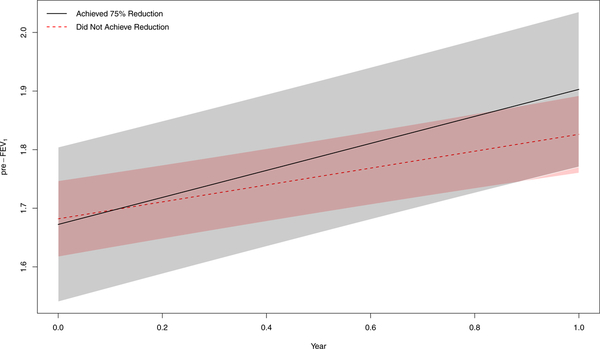
Figure 1:
Pre-BD FEV1 growth over 1 year for a 10-year-old black male who achieved ≥75% mouse allergen reduction (solid black line, 95% CI gray shading) versus a 10-year-old black male who did not achieve ≥75% mouse allergen reduction (dashed red line, 95% CI red shading).
Figure 2:
Stock plot of predicted change in FEV1 over 1 year for a 10-year-old black male who did not achieve ≥75% mouse allergen reduction versus a 10-year-old black male who did achieve ≥75% mouse allergen reduction. P-values derived from allergen reduction*time interaction term.
We also examined other allergen reduction cutoffs, including sustained mean reductions of ≥50% and ≥90% in bedroom floor mouse allergen level from baseline (Table 2). A 10-year-old black male who achieved sustained mean reduction of ≥50% is estimated to have an increase in pre-BD FEV1 of 205mL (95% CI: (160 – 250mL)) over one year compared to 120mL (79 – 161mL) increase in a 10-year-old black male who did not achieve reduction (estimated difference = 85mL (24 – 146mL), p=0.006). A 10-year-old black male who achieved sustained mean reduction of ≥90% is estimated to have an increase in pre-BD FEV1 of 255mL (159 – 351mL) over one year compared to 147mL (115 – 180mL) increase in a 10-year-old black male who did not achieve reduction (estimated difference = 108mL (7 – 209mL), p=0.04). Estimated changes in post-BD FEV1 growth were not statistically significant for either cutoff.
Associations between mouse allergen reduction and FVC growth
Linear regression models with generalized estimating equations stratified by allergen reduction were also used to predict the estimated change in FVC over time for the average participant, a 10-year-old black male. A 10-year-old black male who achieved ≥75% mean mouse allergen reduction is estimated to have an increase in pre-BD FVC of 270mL (95% CI:(191 – 348mL) over one year; a 10-year-old black male who did not achieve allergen reduction is estimated to have an increase in pre-BD FVC of 205mL (160 – 250mL) over one year, with a difference between these groups of people in estimated FVC growth of 65mL (−25 – 156mL) (Table 3). This difference in FVC growth did not reach statistical significance (p=0.16). For post-BD FVC growth, a 10-year-old black male who achieved allergen reduction is estimated to have an increase in post-BD FVC of 227mL (171 – 283mL) over one year compared to a 209mL (177 – 241mL) increase in a 10-year-old black male who did not achieve reduction, with an estimated difference in post-BD FVC growth of 18mL (−46 – 83mL) (p=0.58).
Table 3.
Associations between mouse allergen reduction and FVC growth*
| Lung function index | Achieved reduction ↑in mL(95%CI) | Did not achieve reduction ↑in mL(95%CI) | Difference in estimated FVC growth(95%CI)** | p-value of interaction term*** (reduction*time) |
|---|---|---|---|---|
| Reduction metric: ≥50% mean reduction from baseline | ||||
| Pre-BD FVC | 250mL (191 – 308) n=155 | 197mL (145 – 250) n=194 | 53mL (−26 – 132) | p=0.19 |
| Post-BD FVC | 219mL (177 – 260) n=152 | 209mL (171 – 246) n=192 | 10mL (−46 – 66) | p=0.73 |
| Reduction metric: ≥75% mean reduction from baseline | ||||
| Pre-BD FVC | 270mL (191 – 348) n=92 | 205mL (160 – 250) n=258 | 65mL (−25 – 156) | p=0.16 |
| Post-BD FVC | 227mL (171 – 283) n=90 | 209mL (177 – 241) n=254 | 18mL (−46 – 83) | p=0.58 |
| Reduction metric: ≥90% mean reduction from baseline | ||||
| Pre-BD FVC | 248mL (124 – 371) n=44 | 218mL (176 – 259) n=305 | 30mL (−100 – 161) | p=0.65 |
| Post-BD FVC | 196mL (108 – 285) n=42 | 215mL (185 – 245) n=302 | −19mL (−112 – 75) | p=0.69 |
Average change over 1 year the mean participant (10yo black male)
Participants who achieved mean reduction minus participants who did not achieve mean reduction
Adjusted for age, sex, race, group assignment, BD reversibility, and atopy (number of positive SPT), n=296
Additionally, we examined allergen reduction cutoffs of sustained mean reduction ≥ 50% and ≥ 90% reduction in bedroom floor mouse allergen level from baseline. There were no statistically significant differences in pre- or post-BD FVC growth for either allergen reduction metric (Table 3).
Associations between mouse allergen reduction and FEF25–75 growth
Linear regression models with generalized estimating equations stratified by allergen reduction were again used to predict the estimated change in forced expiratory flow at 25–75% (FEF25–75) over time for the average participant, a 10-year-old black male. A 10-year-old black male who achieved ≥75% mean mouse allergen reduction is estimated to have a 245mL (95% CI:(140 – 63mL) increase in pre-BD FEF25–75 over one year, compared to a 63mL(2 – 123mL) increase in pre-BD FEF25–75 over one year for a 10-year-old black male who did not achieve ≥75% mean mouse allergen reduction (estimated difference 182mL (61–304mL), p=0.003, Table 4). For post-BD FEF25–75 growth, a 10-year-old black male who achieved allergen reduction is estimated to have an increase in post-BD FEF25–75 of 310mL (195 – 425mL) over one year compared to a 129mL (63 – 194mL) increase in a 10-year-old black male who did not achieve reduction, with an estimated difference in post-BD FVC growth of 181mL (48 – 314mL) (p=0.008).
Table 4:
Associations between mouse allergen reduction and FEF25–75 growth*
| Lung function index | Achieved reduction ↑in mL(95%CI) | Did not achieve reduction ↑in mL(95%CI) | Difference in estimated FEF25–75% growth (95%CI)** | p-value of interaction term*** (reduction*time) |
|---|---|---|---|---|
| Reduction metric: ≥50% mean reduction from baseline | ||||
| Pre-BD FEF25–75 | 174mL (95 – 253) n=153 | 55mL (−16 – 126) n=194 | 119mL (13 – 226) | p=0.03 |
| Post-BD FEF25–75 | 251mL (165 – 337) n=152 | 113mL (35 – 190) n=192 | 138mL (23 – 254) | p=0.02 |
| Reduction metric: ≥75% mean reduction from baseline | ||||
| Pre-BD FEF25–75 | 245mL (140 – 351) n=90 | 63mL (2 – 123) n=257 | 182mL (61 – 304) | p=0.003 |
| Post-BD FEF25–75 | 310mL (195 – 425) n=90 | 129mL (63 – 194) n=254 | 181mL (48 – 314) | p=0.008 |
| Reduction metric: ≥90% mean reduction from baseline | ||||
| Pre-BD FEF25–75 | 244mL (79 – 409) n=42 | 93mL (37 – 149) n=305 | 151mL (−23 – 325) | p=0.09 |
| Post-BD FEF25–75 | 275mL (96 – 456) n=42 | 163mL (102 – 224) n=302 | 112mL (−78 – 302) | p=0.25 |
Average change over 1 year the mean participant (10yo black male)
Participants who achieved mean reduction minus participants who did not achieve mean reduction
Adjusted for age, sex, race, group assignment, BD reversibility, and atopy (number of positive SPT), n=291
A similar trend for pre- and post-BD FEF25–75 growth was seen for a 10-year-old black male who achieved ≥50% mean mouse allergen reduction compared to a 10-year-old black male who did not achieve ≥50% mean mouse allergen reduction (estimated difference in pre-BD FEF25–75 growth 119mL (13 – 226mL), p=0.03; estimated difference in post-BD FEF25–75 growth 138mL (23 – 254mL), p=0.02, Table 4). The estimated differences in pre- and post-BD FEF25–75 growth were not statistically significant for a 10-year-old black male who achieved ≥90% mean mouse allergen reduction compared to a 10-year-old black male who did not achieve ≥90% mean mouse allergen reduction (Table 4).
Discussion
Our study’s objective was to determine whether mouse allergen exposure reduction was associated with a difference in lung function growth over time among low-income, urban-dwelling, mouse-sensitized and exposed asthmatic children and adolescents. In this study population, we found that mean reduction in mouse allergen exposure by ≥75% from baseline was associated with a greater increase in pre-BD FEV1 among participants who achieved this reduction metric compared with those who did not achieve this reduction metric. Those who achieved reduction had greater pre-BD FEV1 growth over 1 year, which was independent of age, sex, race, group assignment, BD reversibility, and atopy. Additionally, we found that ≥75% mean reduction in mouse allergen exposure was associated with a greater increase in pre- and post-BD FEF25–75. These findings suggest that mouse allergen exposure reduction may modify pre-BD FEV1 and pre- and post-BD FEF25–75 growth in sensitized and exposed asthmatic children.
Although the overall pattern of the associations between allergen reduction and lung function suggests that allergen reduction may be associated with improvements in lung function growth, the findings may instead be consistent with improvements in airway physiology. The uncertainty with respect to some of the post-BD lung function outcomes suggests that the overall findings may reflect improvements in airway obstruction or mid-flow volumes rather than lung growth. Studies designed specifically to examine the effects of allergen reduction on lung function growth will be needed to determine whether the effects seen here likely reflect improvements in physiology or lung growth. Additionally, while there is controversy about the significance of FEF25–75, particularly in pediatric populations, small airway disease has been associated with asthma, COPD,22 and asthma severity and morbidity in adulthood23, suggesting that the association between allergen reduction and greater pre- and post-BD FEF25–75 growth observed here is worth noting.
Lower lung function in adulthood, as early as age 217,8, has been repeatedly associated with mortality6–8. Recent literature has described lung function trajectories from childhood into adulthood4,24,25, which were predictable as early as preschool age in one study25. A number of risk factors for abnormal lung function trajectories have been identified. In children with mild-to-moderate asthma, male sex, baseline low FEV1, baseline airway hyper-responsiveness, and smaller BD response4 were associated with abnormal lung function growth. In population-based birth cohorts in the United Kingdom and Australia, childhood asthma, repeat wheezing, lung infections, allergic rhinitis, eczema, maternal smoking, and early allergic sensitization, including cat, dog, and dust mite, have been associated with abnormal childhood lung function trajectories24,25. Additionally, longitudinal studies in the United States, Europe, Mexico, and China have shown that air pollution and airborne particulate matter (PM) are associated with long-term abnormal lung function and lower lung function trajectories in children26–29. Each of these risk factors is important. While there have been no longitudinal studies in school-age children examining the long-term effects of allergen exposure on lung function growth, allergen sensitization and high exposure in the first 3 years of life has been associated with lower lung function at age 730. Our study is the first, to our knowledge, to describe an association between allergen exposure reduction and improved lung function increase. By demonstrating that allergen reduction is associated with a greater increase in lung function, this study extends prior cross-sectional observations. This finding strengthens the hypothesis that allergen exposure is causally related to impaired lung development in children. In conjunction with increased air pollution regulation and smoking bans and cessation, indoor allergen exposure reduction may represent another important step toward reducing exposure to modifiable risk factors for abnormal lung function growth in children.
Our study found estimated differences in pre-BD FEV1 growth from 85mL to 108mL over 1 year. These are large improvements in pre-BD FEV1, as adults are estimated to lose approximately 20–30mL in FEV1 per year31–33, making the improved FEV1 growth seen in our study equivalent to 3 to 4 years of expected FEV1 decline in adulthood. This change in FEV1 is also comparable to deficits and improvements seen with increased and decreased air pollution. Previous studies have shown an approximate 100mL difference in FEV1 in children living in Southern California communities with the highest air pollution versus those living in communities with the lowest air pollution34–36. Furthermore, regional improvements in air quality in Southern California have been associated with approximately 65mL to 90mL mean increase in FEV1 over 4 years per median community-specific decrease in PM (8.7μg/m3 PM10, 12.6μg/m3 PM2.5) and nitrogen dioxide (14.1 ppb), respectively37. Therefore, reduction of indoor allergen exposure could represent long-term lung function growth benefits in children similar to benefits seen from improvements in air quality and pollution.
There are notable strengths and limitations of our study. To our knowledge, our study is the first to suggest that there may be lung function growth benefits from reduction in indoor allergen exposure, and should this prove to be the case, indoor allergen exposure reduction could be a disease-modifying strategy for pediatric allergic asthma. In MAAIT, a significant proportion of participants achieved clinically meaningful allergen reduction, allowing us to examine the effect of indoor allergen exposure reduction on lung function growth in a population with high allergen exposure. MAAIT also had repeated, robust allergen measurement and pre-/post-BD spirometry over its 1 year duration. However, MAAIT’s participants were only followed for 1 year. Further follow up is needed to make inferences about the effect of allergen exposure reduction on lung function growth given the uncertainty of the some of the post-BD lung function measures. Additionally, MAAIT’s participants were primarily low-income, urban minorities who were all sensitized and exposed to mouse, potentially limiting the generalizability of our finding. However, this population has the highest asthma morbidity in the United States38,39 and is at risk for increased COPD morbidity and mortality in adulthood40–42 and would, therefore, greatly benefit from allergen exposure reduction interventions. While this study focused on mouse, mouse is a major contributor to asthma morbidity in low-income, minority populations19, which are at greater risk for adult pulmonary morbidity. Future studies are needed to assess the applicability of the findings of this study to other indoor allergens in sensitized and exposed populations. Lastly, as there were no differences in allergen reduction or clinical asthma outcomes by treatment group in the parent trial, we were unable to examine the effect of randomization to integrated pest management on lung function growth. An important next step would be to study the effect on randomization to integrated pest management on lung function growth.
While our results suggest that a reduction in allergen exposure may improve lung function growth, they are observational, from a single study, and there is greater uncertainty with respect to some of the post-BD lung function outcomes. Therefore these findings alone should not be considered proof of a causal relationship between mouse allergen reduction lung function growth. It is also possible that changes in other exposures that were not measured, including microbes and their constituents, such as endotoxin, could explain our findings. Unmeasured confounding therefore cannot be excluded as an explanation for the findings. Additionally, we are limited by our lack of other markers of inflammation (blood eosinophils, fraction of exhaled nitric oxide, total IgE), which if reduced, could suggest that change in mouse allergen exposure is associated with greater lung function growth over time through reduction of inflammation.
In conclusion, among low-income, urban, minority children with persistent asthma who are mouse-sensitized and exposed, mouse allergen exposure reduction was associated with a greater increase in pre-BD FEV1 and pre-and post-BD FEF25–75 over 1 year. These findings suggest that mouse allergen reduction may improve lung function growth in sensitized and exposed asthmatics, potentially altering the lung function growth trajectory from childhood into adulthood.
Supplementary Material
Clinical Implications:
Mouse allergen exposure reduction was associated with greater increases in pre-BD FEV1 and pre-/post-BD FEF25–75 over 1 year in sensitized/exposed children, suggesting allergen exposure reduction may improve lung function growth..
Acknowledgments
This study was supported by the following NIH grants: U01 Al 083238, K24 AI 106822, K24 AI114769 R01 ES023447, P50ES015903, and P01 ES018176.
Abbreviations:
- ATS
American Thoracic Society
- BD
Bronchodilator
- COPD
Chronic obstructive pulmonary disease
- ELISA
Enzyme-linked immunosorbent assay
- FEF25–75
Forced expiratory flow at 25–75%
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced vital capacity
- ICS
Inhaled corticosteroids
- IgE
Immunoglobulin E
- IPM
Integrated pest management
- MAAIT
Mouse Allergen and Asthma Intervention Trial
- NAEPP
National Asthma Education and Prevention Program
- SPT
Skin prick test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study is registered under at clinicaltrials.gov
COI Disclosure: All authors have nothing to disclose.
References
- 1.Lebowitz MD, Holberg CJ, Knudson RJ, Burrows B. Longitudinal study of pulmonary function development in childhood, adolescence, and early adulthood. Development of pulmonary function. Am Rev Respir Dis. 1987. July;136(1):69–75. [DOI] [PubMed] [Google Scholar]
- 2.Strunk RC, Weiss ST, Yates KP, Tonascia J, Zeiger RS, Szefler SJ. Mild to moderate asthma affects lung growth in children and adolescents. J Allergy Clin Immunol. 2006. November;118(5):1040–7. [DOI] [PubMed] [Google Scholar]
- 3.Sears M, Greene J, Willan A, Wiecek E, Taylor R, Flannery E, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med 2003;349:1414–22. [DOI] [PubMed] [Google Scholar]
- 4.McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, et al. Patterns of Growth and Decline in Lung Function in Persistent Childhood Asthma. N Engl J Med. 2016. May 12;374(19):1842–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limb SL, Brown KC, Wood RA, Wise RA, Eggleston PA, Tonascia J, et al. Irreversible lung function deficits in young adults with a history of childhood asthma. J Allergy Clin Immunol. 2005. December;116(6):1213–9. [DOI] [PubMed] [Google Scholar]
- 6.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest 2005;127:1952–1959. [DOI] [PubMed] [Google Scholar]
- 7.Agustí A, Noell G, Brugada J, Faner R. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med. 2017. December;5(12):935–945. [DOI] [PubMed] [Google Scholar]
- 8.Vasquez MM, Zhou M, Hu C, Martinez FD, Guerra S. Low Lung Function in Young Adult Life Is Associated with Early Mortality. Am J Respir Crit Care Med. 2017. May 15;195(10):1399–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R. et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N Engl J Med. 2015. July 9;373(2):111–22. [DOI] [PubMed] [Google Scholar]
- 10.Anselmo M. Pediatric Asthma Controller Therapy. Paediatr Drugs. 2011. February 1;13(1):11–7. [DOI] [PubMed] [Google Scholar]
- 11.Strunk RC, Sternberg AL, Szefler SJ, Zeiger RS, Bender B, Tonascia J. Long-term budesonide or nedocromil treatment, once discontinued, does not alter the course of mild to moderate asthma in children and adolescents. J Pediatr. 2009;154(5):682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006. May 11;354(19):1985–97. [DOI] [PubMed] [Google Scholar]
- 13.Langley SJ, Goldthorpe S, Craven M, Morris J, Woodcock A, Custovic A. Exposure and sensitization to indoor allergens: association with lung functions, bronchial reactivity, and exhaled nitric oxide measures in asthma. J Allergy Clin Immunol. 2003. August;112(2):362–8. [DOI] [PubMed] [Google Scholar]
- 14.Sheehan WJ, Permaul P, Petty CR, Coull BA, Baxi SN, Gaffin JM, et al. Association Between Allergen Exposure in Inner-City Schools and Asthma Morbidity Among Students. JAMA Pediatr. 2017. January 1;171(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsui EC, Perzanowski M, Peng RD, Wise RA, Balcer-Whaley S, Newman M, et al. Effect of an Integrated Pest Management Intervention on Asthma Symptoms Among Mouse-Sensitized Children and Adolescents With Asthma. A Randomized Clinical Trial. JAMA. 2017;317(10):1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the US general population. Am J Respir Crit Care Med. 1999. January;159(1):179–87. [DOI] [PubMed] [Google Scholar]
- 17.Standardization of Spirometry, 1994 Update. American Thoracic Society. AmJRespirCrit Care Med. 1995;152(3):1107–36. [DOI] [PubMed] [Google Scholar]
- 18.Wood RA, Eggleston PA, Lind P, Ingemann L, Schwartz B, Graveson S, et al. Antigenic analysis of household dust samples. AmRevRespirDis. 1988;137(2):358–63 [DOI] [PubMed] [Google Scholar]
- 19.Ahluwalia SK, Peng RD, Breysse PN, Diette GB, Curtin-Brosnan J, Aloe C, et al. Mouse allergen is the major allergen of public health relevance in Baltimore city. J Allergy Clin Immunol. 2013. October;132(4):830–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breysse PN, Buckley TJ, Williams D, Beck CM, Jo SJ, Merriman B, et al. Indoor exposures to air pollutants and allergens in the homes of asthmatic children in inner-city Baltimore. EnvironRes. 2005;98(2):167–76. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton RG. Assessment of indoor allergen exposure. Curr Allergy Asthma Rep. 2005. September;5(5):394–401. [DOI] [PubMed] [Google Scholar]
- 22.Shaw RJ, Djukanovic R, Tashkin DP, Millar AB, du Bois RM, Orr PA. The role of small airways in lung disease. Respir Med. 2002. February;96(2):67–80. [DOI] [PubMed] [Google Scholar]
- 23.Riley CM, Wenzel SE, Castro M, Erzurum SC, Chung KF, Fitzpatrick AM, et al. Clinical Implications of Having Reduced Mid Forced Expiratory Flow Rates (FEF25–75), Independently of FEV1, in Adult Patients with Asthma. PLoS One. 2015. December 30;10(12):e0145476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bui DS, Lodge CJ, Burgess JA, Lowe AJ, Perret J, Bui MQ, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. 2018. July;6(7):535–544. [DOI] [PubMed] [Google Scholar]
- 25.Belgrave DCM, Granell R, Turner SW, Curtin JA, Buchan IE, Le Souëf PN, et al. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir Med. 2018. July;6(7):526–534. [DOI] [PubMed] [Google Scholar]
- 26.Götschi T, Heinrich J, Sunyer J, Künzli N. Long-term effects of ambient air pollution on lung function: a review. Epidemiology. 2008. September;19(5):690–701. [DOI] [PubMed] [Google Scholar]
- 27.Majewska R, Pac A, Mróz E, Spengler J, Camann D, Mrozek-Budzyn D, et al. Lung function growth trajectories in non-asthmatic children aged 4–9 in relation to prenatal exposure to airborne particulate matter and polycyclic aromatic hydrocarbons - Krakow birth cohort study. Environ Res.2018. October;166:150–157. [DOI] [PubMed] [Google Scholar]
- 28.He QQ, Wong TW, Du L, Jiang ZQ, Gao Y, Qiu H, et al. Effects of ambient air pollution on lung function growth in Chinese schoolchildren. Respir Med. 2010. October;104(10):1512–20. [DOI] [PubMed] [Google Scholar]
- 29.Roy A, Hu W, Wei F, Korn L, Chapman RS, Zhang JJ. Ambient particulate matter and lung function growth in Chinese children. Epidemiology. 2012. May;23(3):464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Illi S1, von Mutius E, Lau S, Niggemann B, Grüber C, Wahn U; Multicentre Allergy Study (MAS) group. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006. August 26;368(9537):763–70. [DOI] [PubMed] [Google Scholar]
- 31.Peat JK, Woolcock AJ, Cullen K. Rate of decline of lung function in subjects with asthma. Eur J Respir Dis 1987;70:171–9. [PubMed] [Google Scholar]
- 32.Panizza JA, James AL, Ryan G, de Klerk N, Finucane KE. Mortality and airflow obstruction in asthma: a 17-year follow-up study. Intern Med J 2006;36:773–80. [DOI] [PubMed] [Google Scholar]
- 33.Dijkstra A1, Vonk JM, Jongepier H, Koppelman GH, Schouten JP, ten Hacken NH, et al. Lung function decline in asthma: association with inhaled corticosteroids, smoking and sex. Thorax. 2006. February;61(2):105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gauderman WJ, McConnell R, Gilliland F, et al. Association between air pollution and lung function growth in southern California children. Am J Respir Crit Care Med. 2000;162(pt 1):1383–1390. [DOI] [PubMed] [Google Scholar]
- 35.Gauderman WJ, Gilliland GF, Vora H, et al. Association between air pollution and lung function growth in southern California children: results from a second cohort. Am J Respir Crit Care Med. 2002;166:76–84. [DOI] [PubMed] [Google Scholar]
- 36.Gauderman WJ, Avol E, Gilliland F, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351: 1057–1067. [DOI] [PubMed] [Google Scholar]
- 37.Gauderman WJ, Urman R, Avol E, Berhane K, McConnell R, Rappaport E, et al. Association of improved air quality with lung development in children. N Engl J Med. 2015. March 5;372(10):905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flores G, Snowden-Bridon C, Torres S, Perez R, Walter T, Brotanek J, et al. Urban minority children with asthma: substantial morbidity, compromised quality and access to specialists, and the importance of poverty and specialty care. J Asthma. 2009. May;46(4):392–8. [DOI] [PubMed] [Google Scholar]
- 39.Bryant-Stephens T. Asthma disparities in urban environments. J Allergy Clin Immunol. 2009. June;123(6):1199–206; quiz 1207–8. [DOI] [PubMed] [Google Scholar]
- 40.Foreman MG, Zhang L, Murphy J, Hansel NN, Make B, Hokanson JE, et al. Early-onset chronic obstructive pulmonary disease is associated with female sex, maternal factors, and African American race in the COPDGene Study. Am J Respir Crit Care Med. 2011. August 15;184(4):414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prescott E, Vestbo J. Socioeconomic status and chronic obstructive pulmonary disease. Thorax. 1999. Aug;54(8):737–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gershon AS, Hwee J, Victor JC, Wilton AS, To T. Trends in socioeconomic status-related differences in mortality among people with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014. Oct;11(8):1195–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.