Abstract
Background.
A U-tube drainage catheter (UTDC) is a novel intervention for necrotizing pancreatitis, with multiple benefits: bidirectional flushing, greater interface with large fluid collections, less risk of dislodgement, and creation of a large-diameter fistula tract for potential fistulojejunostomy. We report the first clinical experience with UTDC for necrotizing pancreatitis.
Methods.
From 2011 to 2014, all patients undergoing UTDC for necrotizing pancreatitis at our institution were identified. Clinical variables including patient, disease, and intervention-specific characteristics as well as long-term outcomes populated our dataset.
Results.
Twenty-two patients underwent UTDC for necrotizing pancreatitis; the median follow-up was 10.2 months. Necrotizing pancreatitis was most commonly owing to gallstones (n = 9; 41%), idiopathic disease (n = 5; 23%), and alcohol abuse (n = 4; 18%). During the course of UTDC and definitive operative therapy (when required), patients had median hospital stays of 31 days, 6 interventional radiology procedures, and 6 CT scans. Operative intervention was not necessary in 9 patients (41%). Among the other 13 patients, 4 patients underwent distal pancreatectomy/splenectomy, 8 had a fistulojejunostomy performed, and 1 underwent both procedures.
Conclusion.
UTDC for necrotizing pancreatitis patients is associated with effective drainage and low morbidity/hospital resource utilization. With skilled interventional radiologists and multidisciplinary coordination, this technique is a valuable means of minimizing morbidity for patients with necrotizing pancreatitis.
Acute pancreatitiss affects >200,000 people per year in the United States, and approximately 15–20% of these cases progress to necrotizing pancreatitis.1,2 A majority of patients with necrotizing pancreatitis eventually develop organized pancreatic necrosis, and intervention is required frequently to remove necrotic tissue, especially with any signs or symptoms of infected necrosis. Such invasive treatment is met with significant morbidity and mortality, and routinely poor outcomes have mandated innovative strategies to optimize patient outcomes.
Although early surgical debridement was historically the intervention of choice for necrotizing pancreatitis, there recently has been a shift toward less invasive and/or delayed interventions.2-4 Currently, there are endoscopic, surgical, and radiologically guided interventions available for treatment of necrotizing pancreatitis and its sequelae, and a multidisciplinary “step-up” approach to treatment involving initial drainage (percutaneous or transluminal) followed by operative or endoscopic debridement (if needed) is emerging as a treatment plan of choice.2-4 This intentional delay in operative intervention allows for liquefaction of necrotic tissue and improved demarcation of viable pancreas.2,5 Furthermore, resolution of the acute phases of pancreatitis allows for less morbid operative intervention.2,5
The optimal bridge to definitive operative therapy, however, is not well-defined. Drainage with single percutaneous catheters has been used with variable success, although this approach is plagued by obstruction and subsequent flushing/exchanging; each interventional procedure is associated with low (but not zero) risk, time, effort and cost.6-9 Endoscopic therapies continue to evolve but require technical skill and experience, carry their own risks, make future surgical therapy much more difficult (eg, fusion of stomach and retroperitoneum) and are not yet widely or routinely used for necrotizing pancreatitis. Minimally invasive operative interventions such as laparoscopic or retroperitoneoscopic debridement have also been advocated, although universal adoption of these techniques are limited by an inability to reproduce the clinical results achieved at the most experienced centers.10
A novel approach that has not yet been described for necrotizing pancreatitis is placement of a U-tube catheter for both drainage and fistula tract formation. A U-tube consists of a single large-bore, straight tube with multiple side holes and 2 percutaneous openings.11 Theoretical benefits of the U-tube compared with other forms of percutaneous drainage include more effective flushing (owing to the larger bore catheter, multiple side holes, and bidirectional flushing for clogged side holes for evacuation of necrotic debris), greater interface with large fluid collections (allowing for more rapid resolution of necrosis), less risk of dislodgement/fewer catheter exchanges, avoidance of early open necrosectomy, and creation of a large-diameter tract for future fistulojejunostomy, if required. U-tubes can be used either as an intermediate in the step up approach or as the initial drainage mechanism once intervention is required (our institutional approach) for necrotizing pancreatitis. We report the first clinical experience with UTDC for necrotizing pancreatitis.
METHODS
Patient selection and outcomes.
After obtaining Institutional Review Board approval at the University of Cincinnati, patients with necrotizing pancreatitis treated with a U-tube from 2011 to 2014 were identified from an institutional database. Patients were included if they had a U-tube placed initially or after other failed percutaneous intervention at an outside institution and did not undergo any surgical or endoscopic intervention prior to U-tube placement. Exclusion criteria were age <18 and patients who currently have U-tubes in place and have yet to reach their ultimate outcome.
A retrospective chart review populated the dataset with patient demographic information including age, sex, and comorbidities. A comprehensive inclusion of clinical resource utilization and outcomes was conducted to include type of operative intervention (if any), interventional radiology complications, perioperative complications and mortality, number of hospitalizations, hospital ICU and overall duration of stay (inclusive of surgical and nonsurgical hospital stays), number of CT scans, number of interventional radiology interventions, and long-term success (or failure) of the operative intervention (if required).
Percutaneous UTDC placement and management, and medical decision making.
Placement of UTDC requires a sufficiently large collection that it can be percutaneously accessed from 2 outer opposed aspects of a peripancreatic collection, and through the central collection. CT followed by fluoroscopy are the imaging modalities used for initial guidewire and catheter placement. A 21-G needle and guidewire are inserted into opposite aspects of the fluid collection, usually the anterior abdomen and left posterolateral flank, and each is converted to a 5-Fr catheter. Under fluoroscopic guidance, each percutaneous access to the collection is upsized to an 8-Fr sheath. A 5-Fr catheter with guidewire is inserted through one sheath and a 25-mm endovascular snare is inserted through the other sheath. The guidewire is grasped by the snare and pulled out leading to “through-and-through” guidewire access across the retroperitoneal collection. The track is dilated to 20-Fr. From 1 side of the patient, the 20-Fr Silastic catheter is inserted over the guidewire, through the collection, until the catheter tip exits the other side of the patient (the most commonly used UTDC is a 100-cm, 20-Fr, silicone elastomer [Silastic] catheter [Bentec Medical, Woodland, CA], with 8 cm of manufacturer-placed side holes in the central portion of the catheter and allowing for additional side hole creation to increase the internal drainage length for the catheter). Each end of the Silastic UTDC tubing is fitted and secured with a Molnar-type disk, which stabilizes the position of the UTDC across the peripancreatic collection. The anterior end of the UTDC is capped and the left posterolateral end is placed to a drainage bag. Irrigation of the UTDC can be performed by injecting saline from 1 or both ends.
If the UTDC becomes obstructed with debris, the Molnar-type disk can be removed and the catheter pulled out partially from 1 or both ends to allow manual extraction of debris. The UTDC is then reinserted and secured. If operative intervention is required, the UTDC can be either exchanged for an anterior tube to maintain patency of the fistulous tract for a potential fistulojejunostomy or left in situ. The latter approach is valuable in that, if a fistulojejunostomy is performed, the UTDC can be divided and the posterior limb can be left in place to stent the anastomosis between the small bowel and the fistula tract.
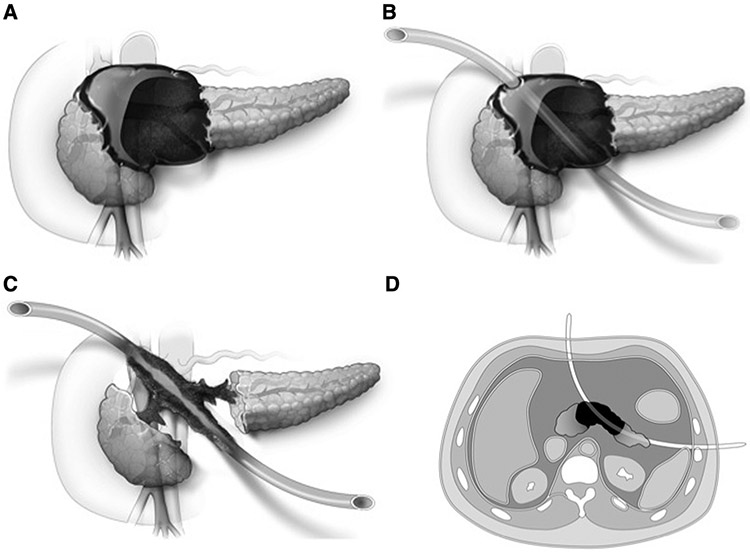
U-tube placement is shown in Fig 1, A-D, and a radiographic example in Fig 2, A, B. If a continued pancreatic duct leak is demonstrated arising from the head/genu despite pancreatic duct stent placement and watchful waiting, a fistulojejunostomy is planned; the risks of pancreaticoduodenectomy in this setting are too great owing to inflammation and fibrotic distortion of anatomy. If a persistent ductal leak is originating from residual tail parenchyma, the preferred and definitive operative approach is distal pancreatectomy/splenectomy; fistulojejunostomy is reserved as a second option should a distal pancreatectomy be technically impossible. As described, an anterior entry is mandatory to ensure that a fistulojejunostomy is possible as an alternative surgical option.
Fig 1.
U-tube placement. A, Common distribution of pancreatic necrosis and eventual liquefied/organized pancreatic necrosis. B, Placement of U-tube through affected portion of pancreas; note that one anterior limb is critical for future operative intervention. C, Appearance of retroperitoneum following complete drainage of liquefied necrosis; note a disconnected duct is well-drained, and a fistulous tract develops around U-tube within 3–4 months of placement (critical if fistulojejunostomy is ultimately required). D, Axial view of U-tube placement.
Fig 2.
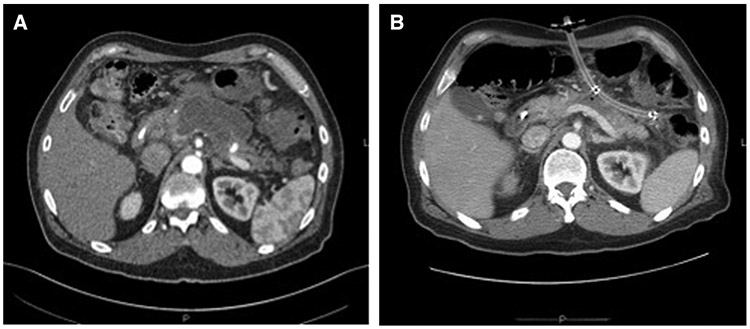
Radiographic representation of U-tube drainage. A, Initial necrosis prior to drain placement. B, Placement of U-tube with resolution of necrosis and large-bore fistulous tract visible anteriorly and laterally.
RESULTS
Baseline characteristics of patients treated for necrotizing pancreatitis at our institution from 2011 to 2014 are shown in Table I. Twenty-two patients underwent UTDC placement as primary intervention for necrotizing pancreatitis (Fig 3), with a median follow-up of 10.2 months. The cohort was primarily male (82%) with a median age of 52 years at time of U-tube placement. The leading etiologies of pancreatitis were gallstone disease (41%), idiopathic (23%) and alcohol (18%).
Table I.
Baseline characteristics and outcomes of the U-tube cohort
| Variable | Value |
|---|---|
| Gender | |
| Male | 18 (81.8%) |
| Female | 4 (18.2%) |
| Median age (y) | 52 (IQR: 45–65) |
| Median ASA score | 3 (IQR: 2–3) |
| NP etiology | |
| Alcohol | 4 (18.2%) |
| Gallstones | 9 (40.9%) |
| Idiopathic | 5 (22.7%) |
| HTG | 1 (4.6%) |
| Other | 3 (13.6%) |
| Surgery | |
| Yes | 13 (59.1%) |
| No | 9 (40.9%) |
| Reoperation rate | 2/13 (15.4%) |
| Outcome | |
| Definitive surgery | 12 (54.6%) |
| Definitive UTD | 7 (31.8%) |
| Death | 2 (9.1%) |
| Other | 1 (4.6%) |
| Positive drain fluid culture | 12 (54.5%) |
| Median duration of stay | 31 (IQR 17–41) |
| Median CT scans | 6 (IQR 4–10) |
| Median IR procedures | 6 (IQR 5–12) |
| Median collection size (cm) | 11 (IQR 8.8–15.3) |
| Prior PCD | 9 (40.9%) |
| Median time to intervention (d) | 36.5 (IQR 17–61.5) |
| Median follow-up (mo) | 10.2 (IQR 6.7–16.0) |
| Median duration of UTDC (mo) | 4.3 (IQR 2.6–7.4) |
ASA, American Society of Anesthesiologists; HTG, hypertriglyceridemia; IQR, interquartile range; IR, interventional radiology; NP, necrotizing pancreatitis; PCD, percutaneous drainage; UTDC, U-tube drainage catheter.
Fig 3.
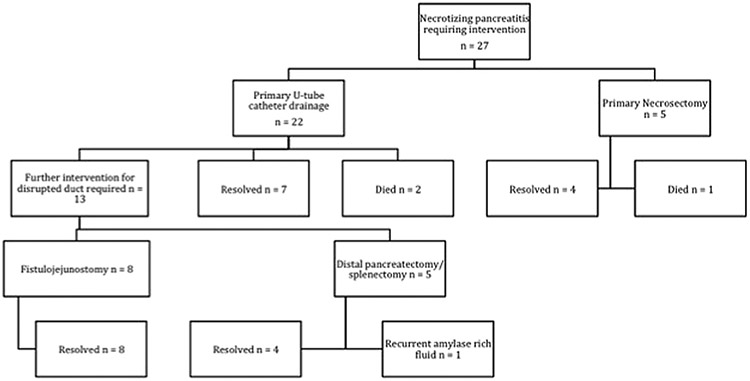
Flowchart of interventions performed.
Single U-drains were placed in 21 patients and 2 U-drains were placed in 1 patient. The drains ranged from 20 to 26 Fr in diameter. There were 19 exchanges of the UTDC, either for upsizing or cleaning, in 13 patients. In 9 patients, no drain exchange was performed. We placed 23 additional drainage catheters in 7 patients; the remaining 15 patients only had the single U-tube. Three of these additional drains were placed for assistance in evacuating the walled-off pancreatic necrosis. The other 20 drains were placed for management of abscesses and fluid collections distant from the central necrosis managed by the UTDC. The median time from radiographically confirmed necrosis to U-tube placement was 36.5 days. The median duration of the indwelling UTDC was 4.3 months. The median collection size, determined by the longest axis of the largest fluid collection, was 11 cm. Twelve patients (55%) had evidence of infected necrosis, determined by positive fluid culture.
Univariate outcomes are presented in Table I. Mortality was 9%. Of surviving patients, U-tube drainage/debridement was the only intervention required in 7 (32%); pancreatic resection or fistulojejunostomy was not necessary. Patients treated with UTDC had a median cumulative duration of stay of 31 days and underwent a median of 6 CT scans and 6 interventional radiology procedures (including placement of the U-tube). After UTDC placement, definitive operative intervention was required in 13 patients (59% of the cohort) for management of a persistent ductal disruption. Eight patients underwent fistulojejunostomy, 4 had a distal pancreatectomy/splenectomy, and 1 underwent fistulojejunostomy but required a follow-up distal pancreatectomy/splenectomy. Of the 13 patients who underwent distal pancreatectomy/splenectomy or fistulojejunostomy, the reoperation rate was 15% (n = 2).
We then compared our results with historical data (Table II).1,12-25 Our series reports an overall mortality rate of 9% and a median cumulative hospital duration of stay of 31 days. These data are favorable or similar to other large series, which have ranges of 5–39% and 19–93 days for perioperative mortality and median hospital duration of stay, respectively.
Table II.
Historical comparisons
| Study* | Intervention | No. of patients | Median hospital stay | Mortality (%) |
|---|---|---|---|---|
| Stahl 2015 | UTDC | 22 | 31 | 9.1 |
| Wormer 2014 | Pancreatic debridement | 1,783 | 46.2† | 29–15‡ |
| Madenci 2014 | OPN | 68 | 21.5 | 8.8 |
| van Brunschot 2014 | Endoscopic transluminal necrosectomy | 460 | NR | 6.1 |
| Pupelis 2013 | Focused open necrosectomy | 22 | 57 | 4.5 |
| Pupelis 2013 | OPN | 36 | 72 | 19.4 |
| van Santvoort 2011 | PCD (+additional necrosectomy if needed) | 130 | NR | 20.0 |
| van Santvoort 2011 | Primary necrosectomy | 78 | NR | 18.0 |
| van Santvoort 2010 | Step up | 43 | 50 | 19.0 |
| van Santvoort 2010 | Primary necrosectomy | 45 | 60 | 16.0 |
| Babu 2010 | OPN | 28 | 84 | 22.0 |
| Raraty 2010 | MARPN | 137 | 60 | 19.0 |
| Raraty 2010 | OPN | 52 | 36 | 38.0 |
| Rodriguez 2008 | OPN | 167 | 19 | 11.4 |
| Howard 2007 | OPN | 102 | NR | 11.8 |
| Reddy 2006 | OPN | 118 | NR | 38.1 |
| Olakowski 2006 | OPN | 126 | 61† | 20.6 |
| Rau 2005 | OPN | 285 | NR | 24.9 |
| Connor 2005 | Necrosectomy | 88 | 93 | 28.0 |
| Gotzinger 2002 | OPN | 340 | 54.5† | 39.1 |
DISCUSSION
We present the first series to date of U-tube drainage for necrotizing pancreatitis. We have shown that the potential benefits of UTDC seem to be realized: (1) relatively few drain complications, interventional radiology procedures and hospital days required over the course of the necrotizing pancreatitis process, (2) a substantial number of patients whose pancreatic necrosis is definitively drained with a U-tube, and (3) minimized morbidity and increased ease of distal pancreatectomy/splenectomy or fistulojejunostomy, if required.
A number of studies over the past 2 decades have reported the effectiveness of image-guided percutaneous drainage for pancreatic necrosis and subsequent fluid collections, although summarizing and comparing these studies is difficult (retrospective nature, selection bias, variations in the definetion of pancreatic complications [pancreatic necrosis, peripancreatic necrosis, organized pancreatic necrosis, fluid collections], variable infection rates and institutional approaches). In 2010 van Baal et al6 published a systematic review of 11 series reporting percutaneous catheter drainage in necrotizing pancreatitis from 1998 to 2010. The overall success rate of percutaneous drainage without surgical necrosectomy was 56%, mortality was 17%, and the complication rate 21%. When reported, drainage duration ranged from 16 to 98 days and the duration from drain placement until surgery ranged from 18 to 109 days. Most patients had ≥2 drains placed, with a maximum of 14. The number of individual interventional procedures performed per patient was not consistently reported.
In the 4 largest individual series to date (n = 34–80 patients), the success rate for percutaneous drainage without subsequent surgery ranged from 35 to 49%, with surgical necrosectomy eventually required in 23–60% of patients.7-9,16 Delayed surgery for management of a pancreatic fistula was reported as 26% in 1 series7 and not reported in the others. Mortality rate ranged from 15 to 34%. Where reported, the mean number of drains placed per patient ranged from 2 to 3.3 and the mean number of drain exchanges ranged from 2 to 4 per patient. Reported duration of drainage ranged from 1 to 260 days, with means of 37 days and 85 days reported in 2 series.7,8 A summary of these series suggests that percutaneous drainage may be curative in up to one-half of appropriately selected patients, whereas overall mortality remains uniformly high. A common point emphasized by all authors is that percutaneous drainage in this challenging patient population is lengthy, laborious, and time intensive for the interventional radiology team.
In our series, the number of drains placed and the number of drain exchanges were significantly fewer than reported in any prior series using conventional drains. There were no instances of inadvertent drain dislodgement and nearly all patients were managed as outpatients after the initial UTDC placement; this attests to the effectiveness and ease of management of the U-tube. The duration of drainage was prolonged and in line with that reported in other series, reflecting the significant time required for necrotic collections to resolve even with adequate tube drainage. Perhaps most important, however, a UTDC anticipates definitive operative intervention, when required, for ductal disruption; most studies to date do not address this most difficult clinical end-game.
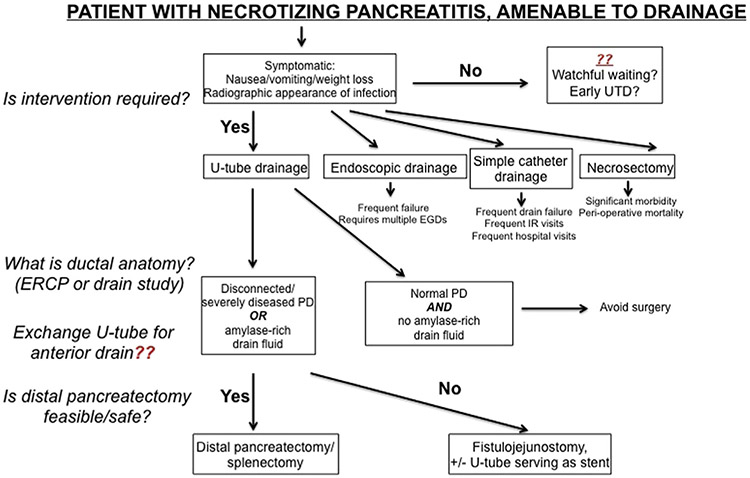
Ultimately, the purpose of our work is to make an evidenced-based argument for a novel, resource-wise, and clinically effective multidisciplinary approach to necrotizing pancreatitis—the current health care climate demands an evaluation of treatment strategies in terms of both clinical outcomes and resource utilization. Based on our recent clinical experience, a proposed treatment algorithm for necrotizing pancreatitis is found in Fig 4. If patients have sufficient liquefaction of necrotizing pancreatitis and are symptomatic (ie, have persistent symptoms or radiographic evidence of infection), intervention is mandatory. One question that we, as an institution, continue to debate is how best to deal with asymptomatic, sterile, organized pancreatic necrosis—it is unclear at what time point commitment to UTDC, or to any intervention-—is most appropriate. For patients with radiographic evidence of a disrupted pancreatic duct, we place U-tubes aggressively. With a low concern for a disrupted duct, however, the optimal treatment strategy continues to evolve.
Fig 4.
Proposed treatment algorithm for patients with necrotizing pancreatitis.
When intervention is required, U-tube drainage has become our preferred method of intervention (Fig 4, step 2). Our institutional paradigm shift away from necrosectomy as a first intervention when debridement/drainage of the pancreas is required is based on relatively poor outcomes in 5 patients treated with necrosectomy initially; the mortality was 20%, reoperation rate was 60%, and median duration of stay for these patients was nearly 2 months (Fig 3). Routine endoscopic, laparoscopic, or retroperitoneoscopic debridement of organized pancreatic necrosis is not performed routinely owing to both the success of our U-tube practice and failure of these approaches to have the same success as reported in the literature.
After a tube has been placed, patience is required to ensure complete drainage of any necrotizing process in the pancreatic bed. Once accomplished, endoscopic retrograde cholangiopancreatography, MR cholangiopancreatography, and/or drain study is required to delineate the pancreatic ductal anatomy (Figs 1 and 4 [step 3]). In the absence of a disconnected duct, significant ductal strictures or amylase rich fluid in the U-tube, it is likely that distal pancreatectomy/splenectomy or fistulojejunostomy can be avoided. If any 1 of those 3 are present, however, operative intervention becomes necessary, and a decision about exchanging the U-tube for a single anterior drain must be made. This is the second portion of our algorithm that continues to evolve. Exchanging the U-tube for an anterior drain ensures closure of the posterior tract, avoiding the potential for a persistent cutaneous fistula postoperatively, if fistulojejunostomy is performed. Conversely, leaving the U-tube in place allows for cutting the U-tube in half in the operating room, and using the posterior limb of the drain to serve as a stent if a fistulojejunostomy is required; this can be removed postoperatively once an anastomotic leak is ruled out.
Last, the decision to perform a fistulojejunostomy versus a distal pancreatectomy/splenectomy can only be made in the operating room. Although planning is required to ensure a fistulojejunostomy is possible if required, the most definitive intervention is resection of the distal pancreas. We have found that a right-sided pancreatectomy (ie, Whipple procedure) is essentially never performed for the reasons outlined. In virtually every case, persistent pancreatic leaks originate from that tail and, as such, if a distal pancreatectomy is deemed safe, this is preferentially performed. A fistulojejunostomy is performed as a “fall back” plan, as described previously by our institution.26
There are limitations to this study. We have a relatively small sample size of surgery-first controls at our institution, which limits the power of comparative statistical analysis. Second, there are institutional and provider biases that may skew our results. Third, U-tube placement and management requires significant technical skill from interventional radiologists, and our results may not be generalizable to other centers. Last, our comparison with historical controls is made recognizing that, in many other series, results are based on a heterogeneous patient population, with various stages of disease. Nevertheless, this study provides evidence for the feasibility and effectiveness of UTDC as the primary intervention for necrotizing pancreatitis, when procedural intervention is indeed required. Based on initial successes, our institution is placing ≥10-15 U-tube drains per year, and we look forward to updating our clinical experience with significantly more patients in years to come.
Although our institutional approach has evolved, and we strongly recommend the U-tube approach to minimize the morbidity of persistent drain clogging, catheter exchange, drain dislodgement and unnecessary hospital stays, there are also disadvantages. First, patient discomfort—primarily during the periprocedural time–can be significant. A strength of the catheter is its large diameter, this is also a limitation—especially because it must traverse the abdominal wall in 2 places. Frequently, oral narcotics—which the patients are frequently taking for their necrotizing pancreatitis—are required for initial pain management. Second, U-tubes are (intentionally) often in place for months, while the team intentionally waits for a fistulous tract to develop. The inconvenience and odor associated with this relatively long time frame are also frequent patient complaints. Our belief is that the risk/benefit analysis favors safer, less morbid operative intervention associated with U-tube placement, admittedly at some expense to patient quality of life.
In conclusion, U-tube drainage is a cornerstone of our institutional approach to necrotizing pancreatitis. Our preliminary data demonstrate favorable outcomes, both in terms of effectiveness and resource utilization, when compared with historical controls. Wider adoption of this treatment strategy could reduce morbidity, mortality, and cost associated with necrotizing pancreatitis.
Biography
Dr Michael Ujiki (Evanston, IL): The U-tube obviously seems to work very well. I was not sure if it was possible to always place. It seems like our radiologists sometimes have a hard time placing just a single tube. That tube in particular has to come anterior, not posterior. It looks like other organs could potentially get in the way. Were you able to place it in everybody? If not, what did you do?
Next, the morbidity and mortality rates certainly are better and they have improved. However, there are so many confounding factors that must be considered. How do you know that the U-tube itself is responsible for the better morbidity and mortality? How do you know it is not just our improvements in our ICU care, maybe getting away from using antibiotics and so forth? There might be other factors that are involved in your morbidity and mortality.
You actually described it well in the presentation, but as I looked up the papers that you had mentioned, I was impressed with their morbidity and mortality rates for an open necrosectomy. I guess you explained in your presentation that they might not have included everything that we would evaluate. Again, are there any other confounding factors?
Next, are there any down sides to the tube? Is there any reason why we should not use it? Is there anything bad about the tube that you did not like? It seems like, if it does get clogged, it is easier to flush than some other ones. As far as I am concerned, there is good and bad to everything, so there must be some down side to the U-tube.
If there is not much of a down side to it, are there other things we can use it for? Is it just pancreatic drainage or can we use it for intraabdominal abscesses, abscess after emergency abdominal surgery or any other kind of surgery where drains can get clogged up?
Dr Daniel Abbott (Cincinnati, OH): Number 1, the success rate. Certainly, the credit has to go to the interventional radiologists. We have a fantastic bunch. They have yet to be unable to place a U-tube in any patients. Of course, there are caveats. I can think of ≥1 patient who was not hypotensive or critically ill where they have actually asked us to wait to place it until the fluid collection got larger so they have a better target.
Regarding technique, they access it 1 way, put a little endovascular wire in, and then through the contralateral approach, grab it with an endoscopic snare and then serially dilate up the track. It is kind of 2 simple catheters, but then joined. Number 2 is, in some instances, place 1 drain first and actually wait until another day to complete the procedure after maybe the colon has moved out of the way or the stomach presents a better window.
Number 2, the improvement in mortality is certainly multifactorial and no answer I will give you is going to be great. We are operating on people when they are generally well, instead of operating on people when they are generally very sick. Maybe 1 metric that we could use, for example, is when you are doing an open necrosectomy, not only might there be multisystem organ failure with respect to lungs and kidneys, but their nutrition is undoubtedly crummy.
When we do these operations 3–4 months after the U-tube is placed, with or without the assistance of tube feeds, we check their prealbumin. And if it is not 20, we wait. There is no emergency once this is well-drained. Their nutritional parameters are certainly much better.
As evidence to that approach, I will tell you, if you look at our mortalities, they are not in the 13 patients who we operated on; it is patients who died of multisystem organ failure after the U-tube was placed. That is not because of source control problems; they died of multiple organ failure.
Point 3 about the down sides of the U-tube. Certainly, it is not perfect. I would say that the things that the patients complain about the most are the large size of the catheters that come out in 2 places. These are huge hoses and you are piercing them in 2 places. They do not like that. Also, when the catheter is clogged, you get pancreatic enzymes leaking out on the skin. Instead of it leaking in 1 spot, it irritates the skin in 2 spots. They do not like that either.
Last, other uses of the U-tube. This was originally described for bilobar hepatic drainage, when people had central biliary obstructions. These are essentially bilobar PTCs meeting in the middle. For whatever reason, this approach has never gained traction. Our interventional radiologists do also use it for abscesses for other pathology, but we primarily use it for necrotizing pancreatitis.
Dr John Mellinger (Springfield, IL): I would like to know what percentage of your patients actually had infected necrosis as their indication. I think your mean hospitalization time of 31 days might suggest what you just said is true, that you are catching these people early enough that you are treating them at a better phase. I wonder if some of them, by our usual paradigms of maturation for noninfected disease, might still have fallen into a nonoperatively managed group or noninterventionally managed group if you had waited a little longer.
The other question I had was did you factor in the hospitalization for those who required surgery, which still ended up being a sizable percentage of your series into the 31-day mark you quoted? We have been doing a lot of endoscopic debridements at our institution, and one of the benefits, I think, is avoiding that ultimate fistula procedure that you described.
You might have more interventions endoscopically that you have to do for the debridement serially, but you create a fistula to do it so you do not end up having to do, in a vast majority of the cases, the secondary surgical procedure.
Dr Daniel Abbott: Number 1, the rate of infection is very high. From a historical perspective, in Dr Rodriquez’s study from Mass General, they had what they thought was an initial 50% infection rate. When they drained everybody, it turned out to be about a 70% infection rate.
Our numbers are even higher, >80%. You culture what is in there, and it is positive a majority of the time. Is that clinically relevant in someone who has a normal white count and is afebrile? I am not sure, but, certainly, by a microbiologic evaluation, a vast majority of patients have infected necrosis.
Number 2, yes, the hospitalization for those that required surgery was included in the duration of hospital stay. Really, when you talk about a distal pancreatectomy, again, that is a 5-day hospital stay. The health care resources that they use are front loaded, just as they are when you operate early or do other things; it is just that the severity of the intervention up front is a little bit less, because it is U-tubes instead of a big laparotomy.
REFERENCES
- 1.Madenci AL, Michailidou M, Chiou G, Thabet A, Fernandezdel Castillo C, Fagenholz PJ. A contemporary series of patients undergoing open debridement for necrotizing pancreatitis. Am J Surg 2014;208:324–31. [DOI] [PubMed] [Google Scholar]
- 2.da Costa DW, Boerma D, van Santvoort HC, Horvath KD, Werner J, Carter CR et al. Staged multidisciplinary step-up management for necrotizing pancreatitis. Br J Surg 2014;101:e65–79. [DOI] [PubMed] [Google Scholar]
- 3.Trikudanathan G, Arain M, Attam R, Freeman ML. Interventions for necrotizing pancreatitis: an overview of current approaches. Expert Rev Gastroenterol Hepatol 2013;7:463–75. [DOI] [PubMed] [Google Scholar]
- 4.Freeman ML, Werner J, van Santvoort HC, Baron TH, Besselink MG, Windsor JA, et al. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas 2012;41:1176–94. [DOI] [PubMed] [Google Scholar]
- 5.Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013;13(4 Suppl 2): e1–15. [DOI] [PubMed] [Google Scholar]
- 6.van Baal MC, van Santvoort HC, Bollen TL, Bakker OJ, Besselink MG, Gooszen HG, et al. Systematic review of percutaneous catheter drainage as primary treatment for necrotizing pancreatitis. Br J Surg 2011;98:18–27. [DOI] [PubMed] [Google Scholar]
- 7.Freeny PC, Hauptmann E, Althaus SJ, Traverso LW, Sinanan M. Percutaneous CT-guided catheter drainage of infected acute necrotizing pancreatitis: techniques and results. Am J Roentgenol 1998;170:969–75. [DOI] [PubMed] [Google Scholar]
- 8.Bruennler T, Langgartner J, Lang S, Wrede CE, Klebl F, Zierhut S, et al. Outcome of patients with acute, necrotizing pancreatitis requiring drainage-does drainage size matter? World J Gastroenterol 2008;14:725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mortelé KJ, Girshman J, Szejnfeld D, Ashley SW, Erturk SM, Banks PA, et al. CT-guided percutaneous catheter drainage of acute necrotizing pancreatitis: clinical experience and observations in patients with sterile and infected necrosis. Am J Roentgenol 2009;192:110–6. [DOI] [PubMed] [Google Scholar]
- 10.Bucher P, Pugin F, Morel P. Minimally invasive necrosectomy for infected necrotizing pancreatitis. Pancreas 2008; 36:113–9. [DOI] [PubMed] [Google Scholar]
- 11.Thompson JS, Burnett DA, Hodgson PE. The many uses of the U tube. Am J Surg 1986;152:616–20. [DOI] [PubMed] [Google Scholar]
- 12.Wormer BA, Swan RZ, Williams KB, Bradley JF 3rd, Walters AL, Augenstein VA, et al. Outcomes of pancreatic debridement in acute pancreatitis: analysis of the nationwide inpatient sample from 1998 to 2010. Am J Surg 2014;208:350–62. [DOI] [PubMed] [Google Scholar]
- 13.van Brunschot S, Fockens P, Bakker OJ, et al. Endoscopic transluminal necrosectomy in necrotising pancreatitis: a systematic review. Surg Endosc 2014;28:1425–38. [DOI] [PubMed] [Google Scholar]
- 14.Pupelis G, Fokin V, Zeiza K, Plaudis H, Suhova A, Drozdova N, et al. Focused open necrosectomy in necrotizing pancreatitis. HPB (Oxford) 2013;15:535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmed Ali U, Schrijver AM, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology 2011;141:1254–63. [DOI] [PubMed] [Google Scholar]
- 16.van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010;362:1491–502. [DOI] [PubMed] [Google Scholar]
- 17.Babu BI, Sheen AJ, Lee SH, O’Shea S, Eddleston JM, Siriwardena AK. Open pancreatic necrosectomy in the multidisciplinary management of postinflammatory necrosis. Ann Surg 2010;251:783–6. [DOI] [PubMed] [Google Scholar]
- 18.Raraty MG, Halloran CM, Dodd S, Ghaneh P, Connor S, Evans J, et al. Minimal access retroperitoneal pancreatic necrosectomy: improvement in morbidity and mortality with a less invasive approach. Ann Surg 2010;251:787–93. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez JR, Razo AO, Targarona J, Thayer SP, Rattner DW, Warshaw AL, et al. Debridement and closed packing for sterile or infected necrotizing pancreatitis: insights into indications and outcomes in 167 patients. Ann Surg 2008;247:294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard TJ, Patel JB, Zyromski N, Sandrasegaran K, Yu J, Nakeeb A, et al. Declining morbidity and mortality rates in the surgical management of pancreatic necrosis. J Gastrointest Surg 2007;11:43–9. [DOI] [PubMed] [Google Scholar]
- 21.Reddy M, Jindal R, Gupta R, Yadav TD, Wig JD. Outcome after pancreatic necrosectomy: trends over 12 years at an Indian centre. ANZ J Surg 2006;76:704–9. [DOI] [PubMed] [Google Scholar]
- 22.Olakowski M, Dranka-Bojarowska D, Szlachta-Swiatkowska E, Lekstan A, Lampe P. Management of necrotizing pancreastitis: flexible approach depending on intra-operative assessment of necrosis. Acta Chir Belg 2006;106:172–6. [DOI] [PubMed] [Google Scholar]
- 23.Rau B, Bothe A, Beger HG. Surgical treatment of necrotizing pancreatitis by necrosectomy and closed lavage: changing patient characteristics and outcome in a 19-year, single center series. Surgery 2005;138:28–39. [DOI] [PubMed] [Google Scholar]
- 24.Connor S, Alexakis N, Raraty MG, Ghaneh P, Evans J, Hughes M, et al. Early and late complications after pancreatic necrosectomy. Surgery 2005;137:499–505. [DOI] [PubMed] [Google Scholar]
- 25.Gotzinger P, Sautner T, Kriwanek S, Beckerhinn P, Barlan M, Armbruster C, et al. Surgical treatment for severe acute pancreatitis: extent and surgical control of necrosis determine outcome. World J Surg 2002;26: 474–8. [DOI] [PubMed] [Google Scholar]
- 26.Nair RR, Lowy AM, McIntyre B, Sussman JJ, Matthews JB, Ahmad SA. Fistulojejunostomy for the management of refractory pancreatic fistula. Surgery 2007;142:636–42. [DOI] [PubMed] [Google Scholar]