Abstract
Objective:
Assess the impact of alcohol use on HIV care cascade outcomes
Design:
Cross-sectional analyses
Methods
We evaluated HIV care cascade outcomes and alcohol use in adults (≥15 years) during baseline (2013–14) population-based HIV testing in 28 Kenyan and Ugandan communities. “Alcohol use” included any current use and was stratified by Alcohol Use Disorders Identification Test-Concise (AUDIT-C) scores: non-hazardous/low (1–3 men/1–2 women), hazardous/medium (4–5 men/3–5 women), hazardous/high (6–7), hazardous/very-high (8–12). We estimated cascade outcomes and relative risks associated with each drinking level using targeted maximum likelihood estimation, adjusting for confounding and missing measures.
Results
Among 118,923 adults, 10,268 (9%) tested HIV-positive. Of those, 10,067 (98%) completed alcohol screening: 1,626 (16%) reported drinking, representing 7% of women (467/6,499) and 33% of men (1,159/3,568). Drinking levels were: low (48%), medium (34%), high (11%), very-high (7%). Drinkers were less likely to be previously HIV diagnosed (58% [95%CI: 55–61%]) than non-drinkers (66% [95%CI: 65–67%]; RR: 0.87 [95%CI: 0.83–0.92]). If previously diagnosed, drinkers were less likely to be on ART (77%, [95%CI: 73–80%]) than non-drinkers (83% [95%CI 82–84%]; RR: 0.93[95%CI: 0.89–0.97]). If on ART, there was no association between alcohol use and viral suppression; however, very-high level users were less likely to be suppressed (RR: 0.80 [95%CI: 0.68–0.94]) versus non-drinkers. On a population level, viral suppression was 38% (95%CI: 36–41%) among drinkers and 44% (95%CI: 43–45%) among non-drinkers (RR: 0.87 [95%CI 0.82–0.94]), an association seen at all drinking levels.
Conclusions
Alcohol use was associated with lower viral suppression; this may be due to decreased HIV diagnosis and ART use.
Keywords: HIV infections, care continuum, Alcohol drinking, Substance-related disorders, Antiretroviral therapy, Viral load, Africa
INTRODUCTION
Guidelines recommend antiretroviral therapy (ART) for all persons living with HIV (PLHIV), yet, only an estimated 47% of the 25.7 million PLHIV in sub-Saharan Africa (SSA) were virally suppressed in 2018.[1] The HIV care cascade measures the continuum of care through which PLHIV must pass to achieve viral suppression. It can be simplified to three key steps in the era of universal treatment: knowing one’s HIV status, accessing ART, and achieving viral suppression.[1] Attrition at any of these steps results in gaps in the care cascade, with subsequent risk of morbidity and mortality for PLHIV and increased risk of onward HIV transmission. An urgent need exists to understand gaps along the HIV care cascade to enable improvement of care and treatment programs for PLHIV.
Alcohol use is a well-established risk factor for poor HIV care outcomes[2–4] and in SSA, heavy alcohol use is a growing problem.[5] It is the most common substance of disordered use[6] and heavy episodic drinking rates in SSA are among the highest in the world, affecting 17% of the general population and more than 50% of drinkers.[3] For PLHIV, the estimated lifetime incidence of alcohol use disorders is 29–60%, which is two to four times higher than in persons without HIV,[7] though estimates specific to SSA are less well described. Alcohol use is also a risk factor for HIV acquisition[8, 9] as well as onward transmission[3, 8], thereby contributing to persistently elevated HIV incidence rates in multiple settings.
Studies of HIV care cascade outcomes among alcohol users have largely found that drinking negatively impacts outcomes. These studies report that drinkers have decreased HIV testing uptake[10–12], engagement and retention in care[2, 13–15], ART use[15–17], adherence[2, 14, 18, 19], and viral suppression.[2, 14, 20] Although these studies offer compelling evidence of the negative impact of alcohol on HIV care, no study to date has assessed the effect of alcohol use across the entire cascade, from diagnosis to viral suppression. Understanding the relative impact of alcohol at each step along a single care cascade could be helpful to prioritize the use of resources to improve viral suppression. Data on HIV diagnosis—the critical first step in the care cascade—among alcohol users are particularly limited. A growing body of evidence in high-risk populations (men who have sex with men [MSM] and female sex workers) has shown that drinkers are at higher risk than non-drinkers of being HIV-positive but unaware of their diagnosis,[10, 11] but it is unknown if this holds true in more general populations. Lastly, while there are robust data on the negative impact of heavy and hazardous alcohol use on HIV care outcomes, associations between lower levels of alcohol use (i.e. levels that do not meet cutoffs for hazardous use or alcohol use disorder) and poor HIV outcomes have only recently been described among U.S. veterans[15] and are not well understood in SSA.
The Sustainable East Africa Research in Community Health (SEARCH) study is an HIV universal testing and treatment trial in Uganda and Kenya that achieved near-universal coverage of HIV testing and assessed alcohol use among almost all adults in 28 of the study communities. Leveraging data from this study population at trial baseline, we performed a cross-sectional evaluation of the associations between levels of alcohol use and HIV care cascade outcomes including HIV diagnosis, ART uptake, and viral suppression in a population-based cohort of PLHIV.
METHODS
Study Design
We conducted a cross-sectional analysis comparing baseline HIV care cascade outcomes in drinkers versus non-drinkers among HIV-positive adults identified in 28 communities in the SEARCH trial. SEARCH () is a cluster-randomized HIV “test-and-treat” trial in 32 pair-matched, rural communities (approximately 10,000 persons each) in Kenya and Uganda, which has been previously described.[21, 22] This analysis includes both intervention and control communities at baseline, prior to study intervention.
Procedures
All communities underwent a door-to-door census to enumerate residents between April 2013-June 2014. Population-based HIV testing was performed using a combination of two-week multi-disease health campaigns, followed by home-based testing for enumerated residents who did not participate in the campaigns. All consenting adults were tested for HIV, irrespective of previous diagnosis, as previously described.[21] HIV-positive individuals underwent point-of-care CD4+ T-cell measurement (PIMA, Inverness Medical) and commercial HIV RNA measurements by plasma or fingerprick venous capillary blood testing at baseline screening.[23]
Measures
Demographic data were collected at baseline. Occupation was classified as formal sector (teacher, student, government worker, military worker, health worker, or factory worker), informal sector (fishmonger, fisherman, bar owner, bar worker, transport, tourism, farmer, shopkeeper, market vendor, hotel worker, homemaker, household worker, construction worker, or mining), other, and disabled/unemployed. Wealth quintiles were based on principal component analysis of household wealth surveys, as previously described.[21] Mobility was self-reported and dichotomous, defined as living away from the study community for ≥1 month in the past year.
Alcohol use was self-reported. Individuals reporting current alcohol use (in response to the question “Do you drink alcohol?”) were classified as “drinkers” and those with no alcohol use as “not current drinkers,” hereafter referred to as “non-drinkers.” Drinkers then were asked questions that allowed us to map onto the Alcohol Use Disorders Identification Test-Concise (AUDIT-C) questionnaire, a validated tool to identify alcohol misuse.[24] Drinking frequency was the number of days in the prior month and drinking quantity was the typical number of drinks per drinking day, captured as continuous variables. We mapped these numbers onto AUDIT-C response categories and scores. Binge-drinking was defined as ≥6 drinks on one occasion and assessed as never, less than monthly, monthly, weekly, or daily/almost daily. AUDIT-C scores ≥3 for women and ≥4 for men have optimal sensitivity and specificity for identifying unhealthy alcohol use.[24] We categorized the AUDIT-C scores further into non-drinker (score 0), non-hazardous/low (score 1–3 for men; 1–2 for women), hazardous/medium (score 4–5 for men; 3–5 for women), hazardous/high (score 6–7), and hazardous/very-high (score 8–12), consistent with prior literature showing increased severity of alcohol use with increasing AUDIT-C scores[25] and differences in clinical outcomes across similar categories.[15]
We assessed the HIV care cascade using three outcomes at trial baseline: previous HIV diagnosis, ART use if previously diagnosed, and viral suppression (<500 copies/mL) if on ART. We also assessed population-level viral suppression, defined as the proportion of HIV-positive adults with viral suppression in the population, regardless of prior diagnosis or ART use. Previous diagnosis was defined as having any of the following prior to the baseline HIV testing date: Ministry of Health record of HIV care or documented prior positive HIV test. ART use was assessed by review of Ministry of Health records for evidence of current ART prescription and engagement in care.[22] Additionally, HIV-positive individuals with suppressed viral loads at baseline were classified as on ART.
Statistical Analysis
This analysis included 28 of the 32 SEARCH study communities; 4 study communities in Kenya (2 matched pairs) were excluded due to an at-random procedural error in data collection in which alcohol questions were not included on the computer-based questionnaire. Adults aged ≥ 15 years at the conclusion of baseline testing and resident in one of the study communities were included.
We described baseline characteristics of drinkers and non-drinkers with documented HIV-positive status at the end of baseline testing. Median CD4+ T-cell counts are also described by alcohol and ART status. Targeted maximum likelihood estimation (TMLE)[26, 27] was used to 1) estimate HIV care cascade outcomes by drinking level, adjusting for missing measures of HIV status, alcohol use, and HIV RNA if HIV-positive among adult residents captured in the census; and, 2) compare relative risk of poor cascade outcomes between drinkers and non-drinkers, additionally adjusting for confounders (common predictors of alcohol use and care outcomes).[28] The primary reason for missing HIV RNA levels was assay failure at early campaigns, as previously described.[23] Our adjustment set included sex, age group, mobility, marital status, education level, occupation group, and wealth index. Analyses also adjusted for community as fixed effects, and the household was treated as the unit of independence. Super Learner, a machine-learning method, was used to reduce the risk of bias due to model misspecification. We also calculated unadjusted point estimates for HIV cascade outcomes by drinking level among those with known AUDIT-C, HIV status, and with viral load measured if HIV-positive. Analyses were conducted in R (R Foundation), version 3.5.1, using packages ltmle 1.1–0[26] and SuperLearner 2.0–24.[29]
Ethics
All participants provided verbal informed consent in their preferred language. HIV-positive adults were linked to HIV care, as previously described.[30, 31] The study was approved by the Makerere University School of Medicine Research and Ethics Committee (Kampala, Uganda), Uganda National Council for Science and Technology (Kampala, Uganda), Kenya Medical Research Institute Ethical Review Committee (Nairobi, Kenya), and the University of California, San Francisco Committee on Human Research (San Francisco, USA).
RESULTS
Within the 28 communities included in the SEARCH trial, 131,552 adult (age ≥15 years) residents were identified, of which 118,963 (90%) underwent HIV-testing at study baseline. Among those tested, 10,268 (8.6%) HIV-positive individuals were identified. Alcohol screening was documented in 10,067 (98%) of those, who comprised the study sample for this analysis.
Baseline Demographics by Alcohol Use
Current alcohol use was reported by 1,626 (16%) HIV-positive adults; 8,441 (84%) reported no current alcohol use. By sex, current alcohol use was reported by 7% of women (467/6,499) and 33% of men (1,159/3,568). Characteristics of drinkers and non-drinkers are described in Table 1.
Table 1.
Baseline demographic and behavioral characteristics by reported alcohol use among adult residents known to be HIV-positive in East Africa (n=10,067)
| Drinkers | Non-drinkers | |||
|---|---|---|---|---|
| Characteristic | n | % | n | % |
| Total | 1,626 | 8,441 | ||
| Uganda | 1,160 | 71.3 | 3,232 | 38.3 |
| >44 | 460 | 28.3 | 2,020 | 23.9 |
| Female | 467 | 28.7 | 6,032 | 71.5 |
| Missing | 4 | 0.3 | 9 | 0.1 |
| Missing | 3 | 0.2 | 8 | 0.1 |
| Missing | 8 | 0.5 | 25 | 0.3 |
| Missing | 61 | 3.8 | 127 | 1.5 |
| MobileD | 277 | 17.0 | 826 | 9.8 |
| Very high (8–12) | 121 | 7.4 | ||
Abbreviations: HIV, human immunodeficiency virus; AUDIT-C, Alcohol Use Disorders Identification Test-Concise
Formal sector occupation defined as teacher, student, government worker, military worker, health worker, or factory worker
Informal sector occupation defined as fishmonger, fisherman, bar owner, bar worker, transport, tourism, farmer, shopkeeper, market vendor, hotel worker, homemaker, household worker, construction worker, or mining
Quintiles based on principal components analysis of household wealth survey
Mobile defined as living away from the study community for > 1 month in the past year
AUDIT-C scores range from 0–12 and are categorized as levels by score and gender as shown
Drinkers were more likely than non-drinkers to be Ugandan (71% vs. 38%), male (71% vs. 29%), and in the lowest household wealth quintile (26% vs 19%). Mobility was reported by 17% of drinkers compared to 10% of non-drinkers. Drinkers were more likely than non-drinkers to have completed primary school (34% vs 25%). Drinkers and non-drinkers were similar in age (mean 39 years vs. 37 years), and the majority were married (69% and 68%) and employed in the informal sector (83% and 85%). Of informal sector employees, the majority were farmers among both drinkers (56%) and non-drinkers (57%).
Baseline CD4+ Cell Count by Alcohol use
The median CD4+ count for those who underwent baseline CD4+ testing was 460 cells/μl (IQR 322–646) among drinkers and 519 cells/μl (IQR 357–710) among non-drinkers. Among individuals with a prior HIV diagnosis but not on ART, drinkers had a median CD4+ of 524 cells/μl (IQR 384–678) and non-drinkers 549 cells/μl (IQR 389–730).
Alcohol Use Levels
Among drinkers, AUDIT-C levels of drinking were low in 48%, medium in 34%, high in 11%, and very-high in 7%. Alcohol users reported a mean of 10.5 drinking days per month (IQR 3–15 days) and 2.2 drinks per session (IQR 1–3 drinks). Binge drinking (≥6 drinks in one session) at least monthly was reported by 20% of drinkers.
HIV CARE CASCADE OUTCOMES
Estimated proportions of HIV-positive adults who were diagnosed, on ART if diagnosed, suppressed if on ART, and suppressed if HIV-positive are given in Figure 1 by level of alcohol use and are adjusted for confounding as well as incomplete measurement of alcohol use, HIV serostatus, and viral load; unadjusted measures are provided for comparison in the associated table. Unadjusted counts and adjusted cascade proportions are presented in the accompanying table. Adjusted relative risks for each HIV care cascade outcome by alcohol use level are presented in Table 2.
Figure 1.
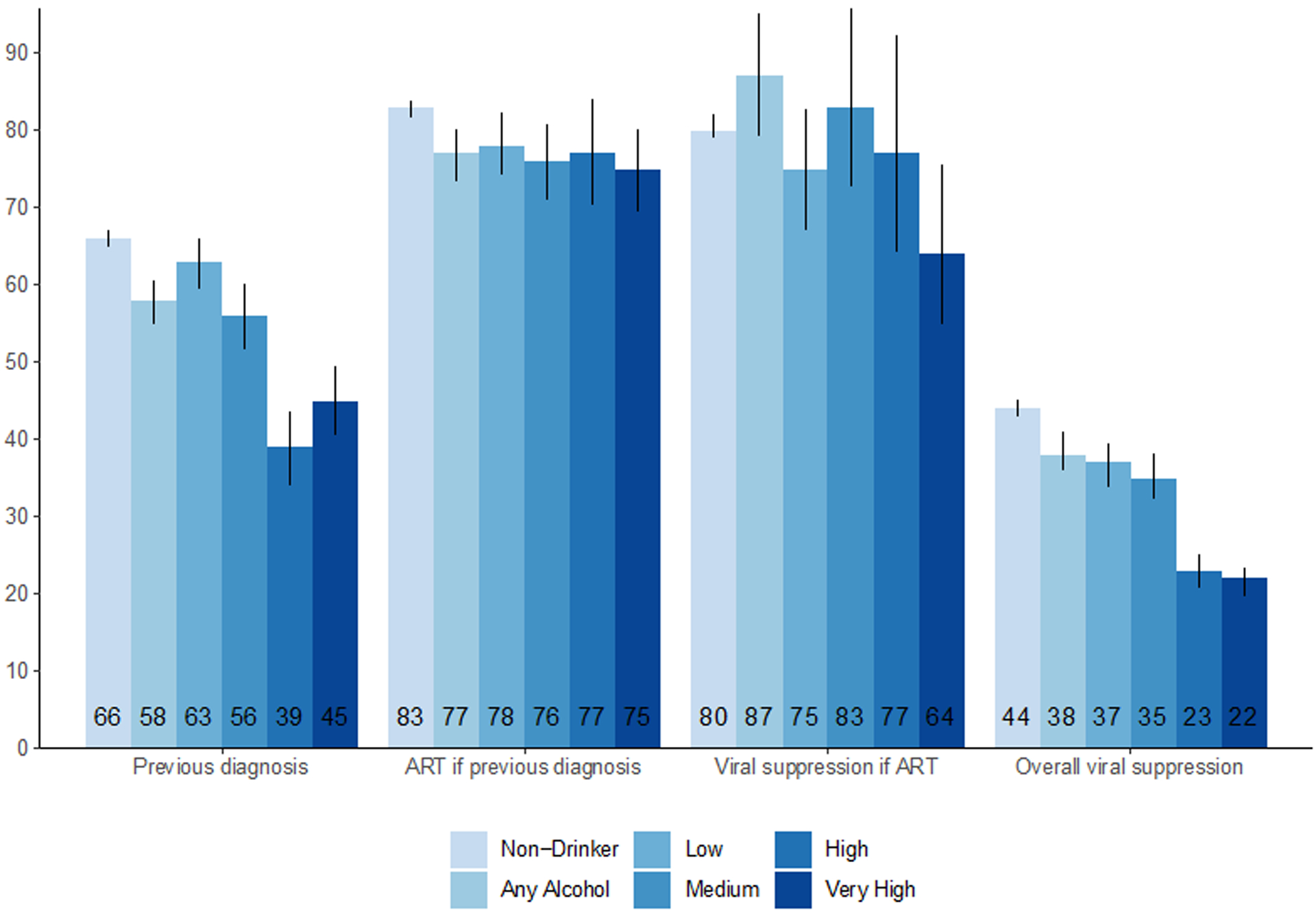
HIV care cascade outcomes by level of current alcohol use in 28 rural communities as assessed during baseline universal HIV testing: estimates adjusted* for confounding and incomplete measurement with TMLE shown in Figure (with black vertical lines for 95% confidence intervals) and unadjusted estimates† with absolute numbers shown in corresponding table.
| No. with previous diagnosis/ No. HIV+ (%) † | No. on ART/No. with previous diagnosis (%) † | No. virally suppressed/No. on ART with HIV RNA measured (%) † | No. virally suppressed/No. HIV+ with HIV RNA measured (%) † | |
|---|---|---|---|---|
| Non-drinker | 5761/8441 (68) | 4740/5761 (82) | 3209/3683 (87) | 3209/6211 (52) |
| Any Alcohol | 861/1626 (53) | 643/861 (75) | 430/491 (88) | 430/1192 (36) |
| Low | 438/774 (57) | 334/438 (76) | 231/257 (90) | 231/572 (40) |
| Medium | 294/561 (52) | 210/294 (71) | 142/159 (89) | 142/409 (35) |
| High | 77/170 (45) | 60/77 (78) | 37/49 (76) | 37/126 (29) |
| Very High | 52/121 (43) | 39/52 (75) | 20/26 (77) | 20/85 (24) |
Table 2.
Adjusted relative risks of HIV care cascade outcomes by level of current alcohol use in 28 rural Kenyan and Ugandan communities undergoing universal HIV testing
| Adjusted Relative Risk* | 95% Confidence Interval | p-value | |
|---|---|---|---|
| Prior diagnosis of HIV | |||
| Non-drinking | Reference | ||
| Any alcohol use | 0.87 | (0.83, 0.92) | <0.001 |
| Low-level | 0.95 | (0.90, 1.0) | 0.06 |
| Medium-level | 0.84 | (0.78, 0.91) | <0.001 |
| High-level | 0.58 | (0.52, 0.66) | <0.001 |
| Very high-level | 0.68 | (0.61, 0.75) | <0.001 |
| On ART if prior diagnosis | |||
| Non-drinking | Reference | ||
| Any alcohol use | 0.93 | (0.89, 0.97) | <0.001 |
| Low-level | 0.95 | (0.90, 1.0) | 0.04 |
| Medium-level | 0.91 | (0.86, 0.98) | 0.01 |
| High-level | 0.93 | (0.85, 1.02) | 0.11 |
| Very high-level | 0.90 | (0.84, 0.97) | 0.01 |
| Virally suppressed if on ART | |||
| Non-drinking | Reference | ||
| Any alcohol use | 1.08 | (0.98, 1.19) | 0.12 |
| Low-level | 0.93 | (0.83, 1.04) | 0.18 |
| Medium-level | 1.04 | (0.90, 1.20) | 0.62 |
| High-level | 0.96 | (0.80, 1.15) | 0.64 |
| Very high-level | 0.80 | (0.68, 0.94) | 0.01 |
| Virally suppressed overall | |||
| Non-drinking | Reference | ||
| Any alcohol use | 0.87 | (0.83, 0.94) | <0.001 |
| Low-level | 0.83 | (0.77, 0.90) | <0.001 |
| Medium-level | 0.80 | (0.73, 0.87) | <0.001 |
| High-level | 0.52 | (0.47, 0.57) | <0.001 |
| Very high-level | 0.49 | (0.45, 0.53) | <0.001 |
Adjusted for confounding and incomplete measures of alcohol use, HIV status and viral loads. The adjustment set included sex, age group, mobility, marital status, education, occupation, wealth index, and community.
Bold p-value indicates statistical significance (p<0.05)
HIV Diagnosis
Among 8441 adult non-drinkers diagnosed with HIV at or before study baseline, 2,680 (32%) were newly diagnosed and 5,761 (68%) had a previous diagnosis. Among 1,626 drinkers, 765 (47%) were newly diagnosed and 861 (53%) had a previous diagnosis. Adjusting for missing measures of HIV status and alcohol use, a smaller proportion of HIV-positive drinkers (58% [95%CI: 55–61%]) had a previously known HIV diagnosis preceding study baseline testing compared to HIV-positive non-drinkers (66% [95%CI: 65–67%]). Drinkers were 13% less likely to have been previously diagnosed with HIV compared to non-drinkers (RR 0.87, [95%CI: 0.83–0.91]). At each hazardous level of alcohol use, drinkers were less likely to have been previously diagnosed when compared to non-drinkers: medium-level drinkers were 16% less likely (RR 0.84, [95%CI: 0.78–0.91]), high-level drinkers 42% less likely (RR 0.58, [95%CI: 0.52–0.66]), and very-high-level drinkers 32% less likely (RR 0.68, [95%CI: 0.61–0.75]). There was not a significant association between low-level alcohol use and being previously HIV diagnosed compared to non-drinkers (RR 0.95 [95%CI: 0.9–1.00]).
Antiretroviral Therapy Uptake Among Individuals with Previous HIV Diagnosis
HIV-positive drinkers who knew their diagnosis were less likely to be on ART (77%, [95%CI: 73–80%]) compared to non-drinkers (83% [95%CI 82–84%]), yielding a relative risk of 0.93 (95%CI: 0.89–0.97). There was a trend toward a decreased likelihood of ART use compared to non-drinkers at all levels of drinking, as shown in Table 2.
HIV Viral Suppression Among Individuals on ART
Lastly, viral suppression among those on ART was similar between drinkers and non-drinkers (87% [95%CI: 79–95%] vs. 80% [95%CI: 79–82%], respectively, with a relative risk of 1.08 (95%CI: 0.98–1.19). Similarly, there was no evidence of an association between low-, medium-, or high-level alcohol use and viral suppression among those on ART. However, very-high-level drinkers were less likely to achieve viral suppression (RR 0.80 [95%CI: 0.68–0.94]) compared to non-drinkers.
Population-Level Viral Suppression at Baseline
When assessing viral suppression regardless of known diagnosis or ART use, drinkers had a lower proportion suppressed (38% [95%CI: 36–41%]) than non-drinkers (44% [95%CI: 43–45%]). Any alcohol use was associated with being 13% less likely to be virally suppressed (RR 0.87, [95%CI 0.82–0.94]). There was a trend toward decreasing viral suppression with increasing severity of drinking, ranging from low-level use (RR 0.83, [95%CI 0.77–0.90]) to very-high-level use (RR 0.49, [95%CI 0.45–0.53]).
DISCUSSION
In one of the largest population-based evaluations of the HIV care cascade and current alcohol use in SSA, we found that, via the multiple steps of the cascade, HIV-positive drinkers had significantly worse viral suppression outcomes than non-drinkers. Underpinning this disparity were lower rates of HIV diagnosis and ART uptake among drinkers. Our findings highlight the impact of alcohol use on stepwise attrition along the HIV care cascade leading to cumulative, large gaps in viral suppression between drinkers and non-drinkers. As universal HIV testing and treatment expands across SSA to achieve and surpass UNAIDS 90-90-90 goals[1], addressing the negative impact of alcohol use on the care cascade will be critical.
This study leveraged a community-wide HIV testing approach that achieved near-universal HIV testing (90%) to assess drinkers’ HIV care cascade outcomes from diagnosis to viral suppression in SSA. Data on the impact of alcohol use on the complete HIV care cascade have been limited, particularly due to the challenge of estimating the number of HIV-positive drinkers in the population who are undiagnosed. Given the burden of HIV and expanding alcohol epidemic in SSA[5], understanding the effect of alcohol on HIV outcomes in this socioeconomic and cultural context is essential. Furthermore, the large sample size and use of AUDIT-C scores allowed us to examine alcohol use at several levels. The majority of available studies have assessed any drinking (dichotomous), hazardous drinking (AUDIT-C ≥3 for women, ≥4 for men), or binge drinking. Stratified levels of alcohol use, including low-level use, provide a more nuanced assessment of associations between alcohol and HIV outcomes.
Several cohort and cross-sectional studies have shown that alcohol use is a risk factor for HIV infection[32], and others have found that alcohol use is associated with a lack of HIV infection awareness among key populations.[10, 12, 33] A study in female sex workers in Malawi showed that harmful drinking and alcohol use disorders were 2.7 and 3 times more prevalent, respectively, among those unaware of their HIV-positive status compared to those aware of their HIV-positive status.[33] In a study of Peruvian MSM, individuals with alcohol use disorders were twice as likely to be unaware of their HIV-positive status.[11] We found that medium, high, and very-high-level drinkers (i.e. hazardous users) were much less likely to be aware of their status, ranging from 39–56% knowing their status, compared to 66% among non-drinkers. Potential explanations of these finding are that hazardous alcohol use increases risk of HIV acquisition[8, 9, 34], enriching the population of drinkers for undiagnosed HIV.[32] Alternatively, alcohol use may lead to poor health-seeking behavior or intentional avoidance of HIV testing due to self-assessed high risk or desire to avoid alcohol-related stigma.[12] Notably, our population of drinkers was primarily men—who typically have lower rates of HIV testing than women in SSA—however, even after adjusting for sex, the associations between alcohol use and HIV diagnosis remained. Our findings suggest that alcohol users should be a priority group for HIV testing and retesting to enhance early diagnosis, prevent negative health effects, and reduce transmission.
Our finding that alcohol use was associated with decreased ART use at study entry (i.e. prior to the implementation of the SEARCH treatment intervention) is consistent with earlier studies in general populations in South Africa, Uganda, and the United States.[4, 16, 17] Poor ART uptake among drinkers has several potential explanations, including poor engagement and retention in care[13] and healthcare provider decisions to defer or withhold treatment.[35] The data for this analysis were collected in 2013–2014, at which time Ugandan and Kenyan recommendations for ART were based on CD4+<350 cells/μl and expanded to CD4+<500 cells/μl by mid-2014. Future work should examine ART uptake among drinkers compared to non-drinkers in the present context of universal HIV treatment (i.e. ART eligibility regardless of CD4 cell count) as barriers to treatment access are removed and differentiated care models of ART delivery expand.
Interestingly, we found no difference in viral suppression at baseline between drinkers and non-drinkers prescribed ART. In a meta-analysis of alcohol-related HIV care continuum studies primarily from high-income, Western countries, 14 of 17 studies found an association with alcohol and lower rates of viral suppression; the remaining three found no significant association.[2] Subsequent studies in Botswana and Russia have also shown an association between alcohol use and worse viral suppression.[14, 20] Notably, we did find that very-high-level drinkers were less likely to be virally suppressed, consistent with studies which have focused on hazardous alcohol use and alcohol use disorders. The stratification of alcohol levels revealed that medium-level and high-level drinkers —who are often grouped with very-high-level drinkers as “hazardous” — did not have a significantly different likelihood of achieving viral suppression compared to non-drinkers. This may be due to a true absence of difference in achieving viral suppression among low to high-level drinkers once offered ART compared to non-drinkers; prior studies have shown a dose-response relationship between level of drinking and adherence.[19] Alternative explanations for this finding include the possibility that clinicians may offer ART to select drinkers based on individual assessments of their potential for treatment adherence, as well as the possibility that prior ART initiation among HIV-positive persons who had dropped out of care was ascertained less completely for drinkers than non-drinkers.
While this study offers a population-level view of the gaps in the HIV care cascade for drinkers versus non-drinkers, many questions remain regarding how to address these gaps. Interventions to improve outcomes among drinkers have typically focused on hazardous alcohol use; however, we found that even low- and medium-level alcohol use were associated with lower rates of diagnosis and ART uptake. These findings are similar to a U.S.-based study that examined alcohol use levels on the HIV care cascade and found a dose-response effect of alcohol use levels on engagement in care, ART use, and viral suppression in a large cohort of U.S. veterans.[15] It is yet to be determined if solutions to these gaps will be a one-size-fits-all for alcohol users, or require targeted interventions by level of alcohol use.
This study has several limitations. First, we relied on self-report to measure alcohol use, which may have resulted in underreporting as a result of social desirability and recall biases.[36] This phenomenon has been demonstrated by comparison of self-report with an alcohol biomarker (phosphatidylethanol) in a Ugandan cohort.[36] Non-standard drink sizes and concentrations may also contribute to misclassification of alcohol use level. However, we used AUDIT-C scores, a self-reporting metric commonly used across SSA, which allows for comparison across multiple settings. Underreporting of alcohol use, if independent of cascade outcomes, should bias results toward the null, underestimating the effect of alcohol use on HIV care cascade outcomes; however, overestimates are also possible if drinkers engaged in the care cascade were less likely to report alcohol use than those who were not. A second limitation is that a number of individuals were missing HIV testing and viral load data. However, only 10% of the population did not test for HIV, the majority of missing viral loads were due to logistic issues (assay failure),[23] and we adjusted for measured differences between those with known versus missing HIV serostatus and viral suppression. A third limitation is that the assessment of ART uptake may appear low due to the inclusion of previously diagnosed individuals who were ineligible for ART initiation per CD4-based guidelines at that time. Inclusion of these individuals, however, allows results to be compared to the current standard of care and to be interpreted in the context of UNAIDS’ “90-90-90” cascade data, which also do not restrict by CD4 eligibility.[37] Lastly, this cross-sectional analysis does not assess the longitudinal dynamics between levels of drinking and care cascade outcomes.
Conclusions
In conclusion, HIV care cascade outcomes were significantly worse among drinkers than non-drinkers in Uganda and Kenya, across all levels of drinking at the time of baseline population HIV testing and prior to implementation of a test-and-treat intervention. Our findings suggest that for PLHIV who use alcohol, diagnosis and ART initiation remain the largest roadblocks to achieving viral suppression. With the advent of ‘treat all,’ it remains to be seen if removing systemic barriers to HIV testing and universal ART access may be sufficient to bridge these gaps or if interventions targeted specifically for drinkers, a traditionally hard-to-reach population, are needed to improve the downstream cascade to viral suppression.
Acknowledgments
The authors gratefully acknowledge the Ministries of Health of Uganda and Kenya, our research and administrative teams in San Francisco, Uganda, and Kenya, collaborators and advisory boards, and especially all communities and participants involved.
Sources of Funding
This work was supported by grants from the Division of AIDS, NIAID of the National Institutes of Health (NIH) (UM1AI068636 and U01AI099959; D.V.H.) and in part by the President’s Emergency Plan For AIDS Relief and Gilead Sciences. Additional funding by the National Institute on Alcohol Abuse and Alcoholism (K24 AA022586; J.A.H) and the National Institute of Mental Health (T32MH19105 supporting S.B.P) of the NIH.
Footnotes
Conflicts of Interest and Source of Funding:
DK, JAH, EDC, CRC, MRK, MLP, DVH, and GC have received grants from the National Institutes of Health (NIH). GC and CRC have received grants from the Bill & Melinda Gates Foundation. DVH has received non-financial support from Gilead Sciences. CRC has also received grants CIFF, personal fees from legal consulting about a malpractice case, and personal fees from Symbiomix. All other authors declare no competing interests.
REFERENCES
- 1.Joint United Nations Programme on HIV/AIDS. Miles to go: closing gaps, breaking barriers, righting injustices. Geneva: UNAIDS; 2018. [Google Scholar]
- 2.Vagenas P, Azar MM, Copenhaver MM, Springer SA, Molina PE, Altice FL. The Impact of Alcohol Use and Related Disorders on the HIV Continuum of Care: a Systematic Review : Alcohol and the HIV Continuum of Care. Curr HIV/AIDS Rep 2015; 12(4):421–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global Status Report on Alcohol and Health. In. Geneva; 2018. [Google Scholar]
- 4.Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend 2010; 112(3):178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jernigan DH, Babor TF. The concentration of the global alcohol industry and its penetration in the African region. Addiction 2015; 110(4):551–560. [DOI] [PubMed] [Google Scholar]
- 6.Lancaster KE, Hetrick A, Jaquet A, Adedimeji A, Atwoli L, Colby DJ, et al. Substance use and universal access to HIV testing and treatment in sub-Saharan Africa: implications and research priorities. J Virus Erad 2018; 4(Suppl 2):26–32. [PMC free article] [PubMed] [Google Scholar]
- 7.Petry NM. Alcohol use in HIV patients: what we don’t know may hurt us. Int J STD AIDS 1999; 10(9):561–570. [DOI] [PubMed] [Google Scholar]
- 8.Rehm J, Gmel GE Sr., Gmel G, Hasan OSM, Imtiaz S, Popova S, et al. The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction 2017; 112(6):968–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiwanuka N, Ssetaala A, Ssekandi I, Nalutaaya A, Kitandwe PK, Ssempiira J, et al. Population attributable fraction of incident HIV infections associated with alcohol consumption in fishing communities around Lake Victoria, Uganda. PLoS One 2017; 12(2):e0171200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bengtson AM, L’Engle K, Mwarogo P, King’ola N. Levels of alcohol use and history of HIV testing among female sex workers in Mombasa, Kenya. AIDS Care 2014; 26(12):1619–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vagenas P, Ludford KT, Gonzales P, Peinado J, Cabezas C, Gonzales F, et al. Being unaware of being HIV-infected is associated with alcohol use disorders and high-risk sexual behaviors among men who have sex with men in Peru. AIDS Behav 2014; 18(1):120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fatch R, Bellows B, Bagenda F, Mulogo E, Weiser S, Hahn JA. Alcohol consumption as a barrier to prior HIV testing in a population-based study in rural Uganda. AIDS Behav 2013; 17(5):1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monroe AK, Lau B, Mugavero MJ, Mathews WC, Mayer KH, Napravnik S, et al. Heavy Alcohol Use Is Associated With Worse Retention in HIV Care. J Acquir Immune Defic Syndr 2016; 73(4):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amirkhanian YA, Kelly JA, DiFranceisco WJ, Kuznetsova AV, Tarima SS, Yakovlev AA, et al. Predictors of HIV Care Engagement, Antiretroviral Medication Adherence, and Viral Suppression Among People Living with HIV Infection in St. Petersburg, Russia. AIDS Behav 2018; 22(3):791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams EC, McGinnis KA, Edelman EJ, Matson TE, Gordon AJ, Marshall BDL, et al. Level of Alcohol Use Associated with HIV Care Continuum Targets in a National U.S. Sample of Persons Living with HIV Receiving Healthcare. AIDS Behav 2019; 23(1):140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kader R, Seedat S, Govender R, Koch JR, Parry CD. Hazardous and harmful use of alcohol and/or other drugs and health status among South African patients attending HIV clinics. AIDS Behav 2014; 18(3):525–534. [DOI] [PubMed] [Google Scholar]
- 17.Martinez P, Andia I, Emenyonu N, Hahn JA, Hauff E, Pepper L, et al. Alcohol use, depressive symptoms and the receipt of antiretroviral therapy in southwest Uganda. AIDS Behav 2008; 12(4):605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalichman S, Mathews C, Banas E, Kalichman M. Alcohol-related intentional nonadherence to antiretroviral therapy among people living with HIV, Cape Town, South Africa. AIDS Care 2019:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res 2005; 29(7):1190–1197. [DOI] [PubMed] [Google Scholar]
- 20.Gross R, Bellamy SL, Ratshaa B, Han X, Steenhoff AP, Mosepele M, et al. Effects of sex and alcohol use on antiretroviral therapy outcomes in Botswana: a cohort study. Addiction 2017; 112(1):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chamie G, Clark TD, Kabami J, Kadede K, Ssemmondo E, Steinfeld R, et al. A hybrid mobile approach for population-wide HIV testing in rural east Africa: an observational study. Lancet HIV 2016; 3(3):e111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen M, Balzer L, Kwarsiima D, Sang N, Chamie G, Ayieko J, et al. Association of Implementation of a Universal Testing and Treatment Intervention With HIV Diagnosis, Receipt of Antiretroviral Therapy, and Viral Suppression in East Africa. JAMA 2017; 317(21):2196–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain V, Petersen ML, Liegler T, Byonanebye DM, Kwarisiima D, Chamie G, et al. Population levels and geographical distribution of HIV RNA in rural Ugandan and Kenyan communities, including serodiscordant couples: a cross-sectional analysis. Lancet HIV 2017; 4(3):e122–e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res 2007; 31(7):1208–1217. [DOI] [PubMed] [Google Scholar]
- 25.Rubinsky AD, Kivlahan DR, Volk RJ, Maynard C, Bradley KA. Estimating risk of alcohol dependence using alcohol screening scores. Drug Alcohol Depend 2010; 108(1–2):29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwab JLS, Petersen M, Gruber S, van der Laan M. tmle: Longitudinal targeted maximum likelihood estimation. In. [Google Scholar]
- 27.Schuler MS, Rose S. Targeted Maximum Likelihood Estimation for Causal Inference in Observational Studies. Am J Epidemiol 2017; 185(1):65–73. [DOI] [PubMed] [Google Scholar]
- 28.Balzer L, Schwab J, van der Laan MJ, Petersen ML. Evaluation of Progress Towards the UNAIDS 90-90-90 HIV Care Cascade: A Description of Statistical Methods Used in an Interim Analysis of the Intervention Communities in the SEARCH Study. UC Berkeley Division of Biostatistics Working Paper Series 2017; (Working Paper 357). [Google Scholar]
- 29.Polley ELE, Kennedy C, Lendle S, van der Laan M. SuperLearner: Super Learner Prediction. In. [Google Scholar]
- 30.Havlir DV, Balzer LB, Charlebois ED, Clark TD, Kwarisiima D, Ayieko J, et al. HIV Testing and Treatment with the Use of a Community Health Approach in Rural Africa. N Engl J Med 2019; 381(3):219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwarisiima D, Kamya MR, Owaraganise A, Mwangwa F, Byonanebye DM, Ayieko J, et al. High rates of viral suppression in adults and children with high CD4+ counts using a streamlined ART delivery model in the SEARCH trial in rural Uganda and Kenya. J Int AIDS Soc 2017; 20(Suppl 4):21673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisher JC, Bang H, Kapiga SH. The Association Between HIV Infection and Alcohol Use: A Systematic Review and Meta-Analysis of African Studies. 2007; 34(11):856–863. [DOI] [PubMed] [Google Scholar]
- 33.Lancaster KE, Go VF, Lungu T, Mmodzi P, Hosseinipour MC, Chadwick K, et al. Substance use and HIV infection awareness among HIV-infected female sex workers in Lilongwe, Malawi. Int J Drug Policy 2016; 30:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalichman SC, Simbayi LC, Kaufman M, Cain D, Jooste S. Alcohol use and sexual risks for HIV/AIDS in sub-Saharan Africa: systematic review of empirical findings. Prev Sci 2007; 8(2):141–151. [DOI] [PubMed] [Google Scholar]
- 35.Ferro EG, Culbert GJ, Wickersham JA, Marcus R, Steffen AD, Pauls HA, et al. Physician Decisions to Defer Antiretroviral Therapy in Key Populations: Implications for Reducing Human Immunodeficiency Virus Incidence and Mortality in Malaysia. Open Forum Infect Dis 2017; 4(1):ofw219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bajunirwe F, Haberer JE, Boum Y 2nd, Hunt P, Mocello R, Martin JN, et al. Comparison of self-reported alcohol consumption to phosphatidylethanol measurement among HIV-infected patients initiating antiretroviral treatment in southwestern Uganda. PLoS One 2014; 9(12):e113152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.UNAIDS. The gap report. Geneva, Switzerland: 2014. [Google Scholar]


