Abstract
Antenatal register data from 62 clinics in 5 regions of Kenya were used to estimate women with human immunodeficiency virus (HIV) risk (partner HIV status, syphilis). With individual risk-guided preexposure prophylaxis (PrEP) offer in all regions, 39% of pregnant women would be offered PrEP nationally. Offering PrEP to all women in high-prevalence regions reached 26% of the pregnant women.
To reach elimination of mother-to-child transmission (EMTCT) of human immunodeficiency virus (HIV) targets, the World Health Organization (WHO) recommends offering preexposure prophylaxis (PrEP) to HIV-negative pregnant women in HIV high-burden settings.1,2 The WHO also recommends using risk-guided approaches where HIV risk assessment tools are used during routine antenatal care (ANC) visits to identify HIV-uninfected pregnant women who would benefit most PrEP.1 Alternatively, PrEP could be offered to all HIV-uninfected women in ANC in subnational regions with high HIV burden where HIV incidence is likely highest. Programmatic delivery of PrEP for pregnant women is being considered in sub-Saharan African settings,3 and there is a critical need for research on how best to operationalize PrEP delivery within ANC.4
Setting national and subnational PrEP targets could help decision makers and health planners in forecasting drug procurement and assessing the budgetary allocation for PrEP implementation for ANC clients. Targets also help motivate programs to reach pregnant and postpartum women who may benefit from PrEP services most. Countries planning to offer PrEP to pregnant women could use available epidemiologic data from surveys or ANC programs to make pragmatic initial targets which can be adjusted when demand is better understood after implementation.
Previous studies among pregnant women in Kenya have found HIV incidence rates between 3.4 to 6.8 per 100 person-years5,6 Kenya national guidelines recommend PrEP for individuals with high risk of HIV acquisition, including pregnant women,3 though large-scale implementation through ANC has not been rolled out. To inform scale-up of PrEP delivery for pregnant women in Kenya, we estimated the number and proportion of HIV-uninfected pregnant women in Kenya who could be offered PrEP under different pragmatic approaches, including offering PrEP based on regional HIV prevalence and/or individual-level HIV risk factors at the national level.
METHODS
Design
We conducted a secondary analysis using data from a large cross-sectional survey of standardized Ministry of Health ANC registers.7 The primary aim of the parent study was to evaluate programmatic PMTCT indicators, including maternal HIV testing in ANC. Facilities providing routine ANC services with 100 or greater annual HIV-infected ANC clients (as reported to the Kenya National AIDS and STI Control Program) were randomly selected for inclusion, using stratified random sampling (stratified by facility size). The number of facilities selected in each strata was proportional to the number of facilities within each strata; 62 facilities were selected from the 92 facilities in the overall sampling frame. Selected facilities were from the regions of Nyanza, Nairobi, Coast, Rift Valley, and Eastern. Data from the first 10% of all first ANC visits were included for each year from 2011 to 2013. Ethical approval was obtained from the ethical review committees at the Kenya Medical Research Institute, the University of Washington, and the US Centers for Disease Control and Prevention.
Data Abstraction
The ANC registers contained information on demographics, obstetric history, maternal and partner HIV status, and other routine maternal health indicators. Study staff scanned each ANC register page with identifying information blocked with an opaque cover. Data were digitized using Captricity, a proprietary cloud-based data transcription service that uses a combination of computer algorithms and manual checks to digitize handwritten data. Image capture and digitization were piloted before study implementation to ensure consistency between paper-based and digitized data. Data accuracy was verified daily during study implementation.
Definition of Variables
We defined high HIV risk based on individual-level characteristics as having syphilis (documentation rapid plasma reagin–positive results) and/or having a partner of unknown or positive HIV status. These characteristics were selected based on the availability of indicators in the registries, previous studies which identified these characteristics as risk factors in an empiric risk score for HIV acquisition among pregnant women in Kenya,8 and current Kenya National AIDS and STI Control Program guidelines3 which recommend PrEP for individuals who recently had an sexually transmitted infection (STI) and/or have sex partners of positive or unknown HIV status.
Statistical Analyses
We estimated the absolute number and proportion of HIV-uninfected pregnant women in Kenya who could be offered PrEP under three different public health approaches, including offering PrEP to: (1) all HIV-uninfected pregnant women nationally with high HIV risk based on our defined individual-level characteristics; (2) all HIV-uninfected pregnant women, regardless of individual-level risk, only in the region with highest HIV prevalence; and (3) all HIV-uninfected pregnant women, regardless of individual-level risk, only in the region with highest HIV prevalence and all HIV-uninfected pregnant women nationally with individual-level high HIV risk.
We multiplied the total number of pregnant women nationally according to Kenya Demographic and Health Survey 2014 (1.53 million) by the proportion of women in Eastern, Coast, Rift Valley, Nairobi and Nyanza regions (74%) to estimate the number of pregnant women for all regions that contributed data to our study.9 The number of pregnant women with high HIV risk based on individual-level characteristics was estimated by multiplying the total number of pregnant women by the proportion with syphilis and/or a partner of unknown or positive HIV status. The number of pregnant women in Nyanza (the region with the highest HIV prevalence based on the Kenya AIDS Indicator Survey 2012) was estimated by multiplying the total number of pregnant women nationwide (1.53 million) by the proportion in Nyanza (26%). All analyses were weighted to account for the complex sampling design, including clustering at the clinic level.
RESULTS
Overall, 8634 ANC attendees (93% of all abstracted records) were HIV-uninfected and had data on syphilis or partner HIV status. The regional distribution of ANC attendees was as follows: Eastern, 1127 (13.1%); Rift Valley, 1284 (14.9%); Coast, 1396 (16.2%); Nairobi, 1619 (18.8%); and Nyanza, 3208 (37.2%). The median age of ANC attendees was 24 years, 18% were younger than 20 years, most were married (86%), and 37% were primigravidas.
Syphilis testing results were available for 69% of ANC attendees and among those with results, 1% were rapid plasma reagin–positive. Data on male partner HIV status were available for 85% of ANC attendees of which nearly half (54%) had documentation of unknown male partner HIV status. Only 3% had HIV tested as a couple and 1% reported knowing their male partner was HIV-infected. Among all regions, 39% of women had either documentation of syphilis infection and/or a partner of positive or unknown HIV status, which defined high HIV risk based on individual-level characteristics in our study; 34% of all women with high risk for HIV resided in Nyanza. Regions with 30% or greater of individuals having high HIV risk based on individual-level characteristics overlapped with higher HIV prevalence regions (Fig. 1A). Prevalence of high HIV risk was highest in Nyanza (51%), significantly higher than other regions (prevalence ratio, 1.5, 95% confidence interval, 1.1–2.2; P = 0.04). In all other regions, less than 40% of women had high HIV risk (Fig. 1A).
Figure 1.
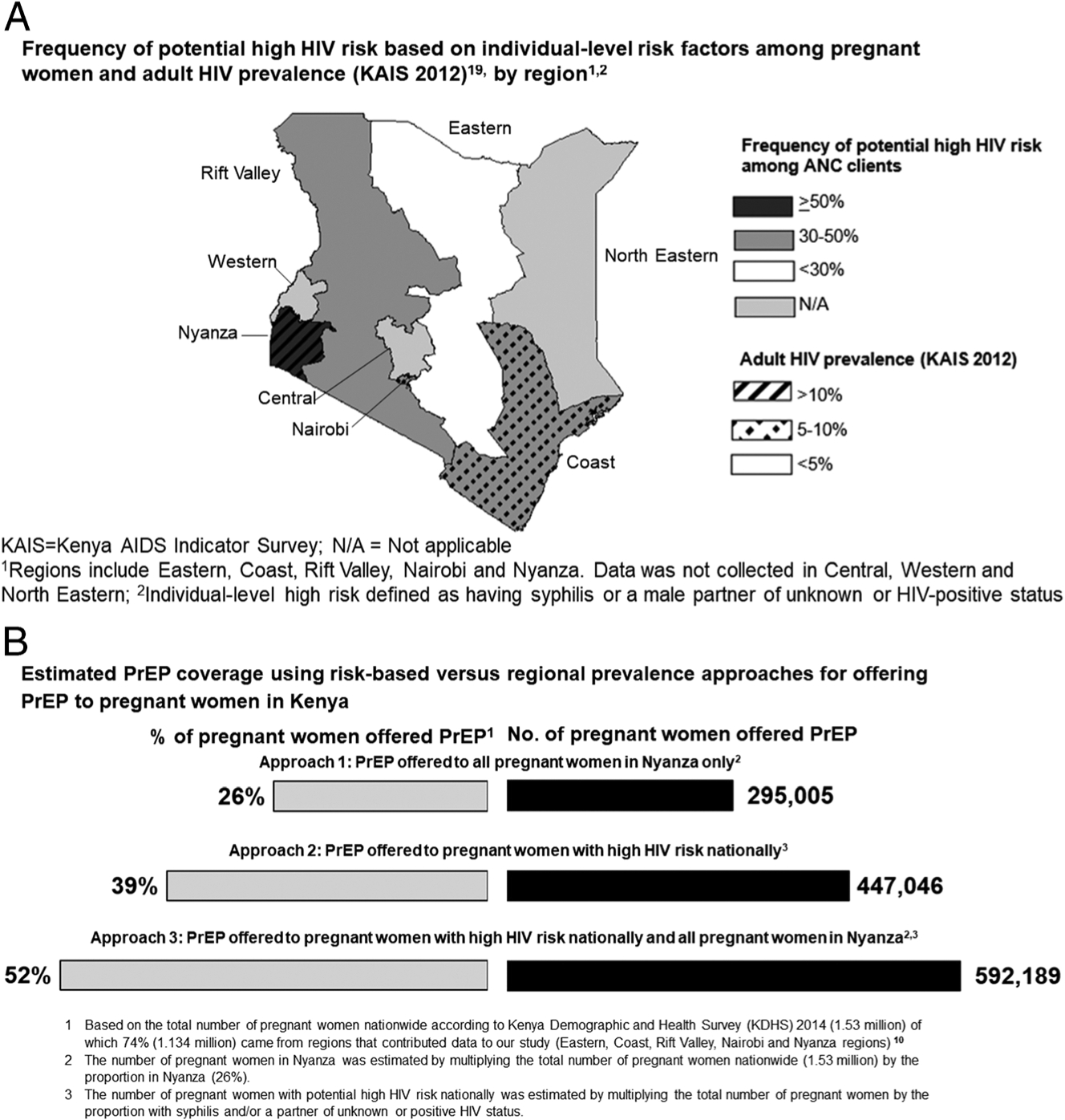
A, Frequency of potential high HIV risk based on individual-level risk factor among pregnant women and adult HIV prevalence (Kenya AIDS Indicator Survey 2012)19, by regoin.1,2 B, Estimated PrEP coverage using risk-based versus regional prevalence approaches for offering PrEP to pregnant women in Kenya.
Offering PrEP to all HIV-uninfected pregnant women regardless of individual-level risk in Nyanza would result in an estimated 295,005 women offered PrEP (Fig. 1B). By offering PrEP nationwide to women with high HIV risk in all regions based on individual-level characteristics, 447,046 pregnant women would be offered PrEP; offering PrEP additionally to all women in Nyanza would result in 145,143 more pregnant women being offered PrEP (592,189 women overall). We repeated the analyses to estimate the proportion of pregnant women nationally covered in each of the approaches for offering PrEP (Fig. 1B). Offering PrEP to all women in Nyanza in addition to all women in other regions with high HIV risk based on individual-level characteristics would raise coverage of offering PrEP to 52% of all pregnant women in Kenya.
DISCUSSION
Our study is the first to estimate the number of HIV-uninfected pregnant women who could be offered PrEP in a high HIV prevalence country under pragmatic scenarios. In this large survey, frequency of high HIV risk was common based on routinely collected ANC indicators. We found that an approach for offering PrEP that incorporates individual-level HIV risk and regional HIV prevalence would result in PrEP offer to 52% of pregnant women in Kenya. Restricting PrEP offer to all women in high prevalence region would involve 26% of all pregnant women in Kenya, whereas offer to women with individual risk factors in all regions would result in offering 39% of women PrEP. Understanding regional differential HIV incidence during pregnancy will be important to refine programmatic decisions. Our findings could inform the development of conceptual models for PrEP delivery in pregnancy and parameterization of more comprehensive mathematical models of different PrEP delivery approaches.
Less than 5% of ANC attendees in our study tested for HIV with their partners, and over half did not know their partner’s HIV status. Accurate partner HIV status information is key to preventing new HIV infections in pregnant women and to targeting effective prevention strategies.10,11 Promoting male partner HIV testing, including with HIV self-test kits, may reduce the number of pregnant women who initiate PrEP.12,13 Syphilis is associated with increased incidence of HIV among pregnant women and routinely screened for within ANC.5,14 Nearly one third of ANC attendees were missing syphilis results in our study, highlighting the need for continued dual EMTCT of HIV and syphilis efforts. In our study, high HIV risk based on individual-level characteristics varied from 28% to 51% across regions. Future mathematical models that evaluate national or regional prioritization of PrEP for pregnant women are needed to understand the cost-effectiveness of PrEP in this population.
Our study has limitations. We only selected facilities with 100 or more annual HIV-infected ANC clients and therefore our results may over represent areas with higher community-level HIV risk. We abstracted data from registries that were initially completed for nonresearch purposes. Thus, an appreciable proportion of entries had missing data. Important markers of HIV risk, such as intimate partner violence and nonsyphilis STIs are not captured in ANC registers. Frequency of true HIV serodiscordance is likely higher than what was self-reported as few women tested as a couple. Our analysis does not include mathematical modeling or cost-effectiveness analyses. Additionally, we focus on estimations for offering PrEP without incorporating data on uptake. Women with low HIV risk may not consider taking PrEP. Forecasting programmatic needs in the future should include uptake of PrEP among pregnant women.
In conclusion, data from ANC registers may be useful for estimating numbers and regional distribution of women at risk for HIV who would benefit from PrEP in ANC/PNC settings.
Sources of Funding:
President’s Emergency Plan for AIDS Relief (PEPFAR) and Centers for Disease Control and Prevention (COAG#U2GPS002047). JP was supported by a NIH training grant T32AI07140 and F32NR017125. GJS was supported by an NIH K24 grant (HD054314). The CHIME Team was supported by the University of Washington’s Global Center for Integrated Health of Women Adolescents and Children (Global WACh) and Center for AIDS Research (CFAR) (P30 AI027757).
Footnotes
Publisher's Disclaimer: CDC Disclaimer:
The findings and conclusions in this paper are those of the author(s) and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention, United States National Institutes of Health and Government of Kenya.
REFERENCES
- 1.World Health Organization. WHO Technical brief: Preventing HIV during pregnancy and breastfeeding in the context of pre-exposure prophylaxis (PrEP). Geneva: World Health Organization; 2017; Licence: CC BY-NC-SA 3.0 IGO. 2017. [Google Scholar]
- 2.Mofenson LM, Baggaley RC, Mameletzis I. Tenofovir disoproxil fumarate safety for women and their infants during pregnancy and breastfeeding: systematic review. AIDS 2017; 31:213–232. [DOI] [PubMed] [Google Scholar]
- 3.Ministry of Health NASCP. Guidelines on use of antiretroviral drugs for treating and preventing HIV infection in Kenya. 2016. Nairobi, Kenya; July 2016. [Google Scholar]
- 4.Joseph Davey DL, Bekker LG, Gorbach PM, et al. Delivering preexposure prophylaxis to pregnant and breastfeeding women in Sub-Saharan Africa: The implementation science frontier. AIDS 2017; 31:2193–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinuthia J, Kiarie JN, Farquhar C, et al. Cofactors for HIV-1 incidence during pregnancy and postpartum period. Curr HIV Res 2010; 8: 510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinuthia J, Drake AL, Matemo D, et al. HIV acquisition during pregnancy and postpartum is associated with genital infections and partnership characteristics. AIDS 2015; 29:2025–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng’ang’a L, McGrath CJ, Langat A, et al. Mother-to-child transmission of HIV in Kenya: a multi-year national evaluation (Abstract #761). Seattle, WA, USA: Conference on Retroviruses and Opportunistic Infections (CROI), 2017. [Google Scholar]
- 8.Pintye J, Drake AL, Kinuthia J, et al. A risk assessment tool for identifying pregnant and postpartum women who may benefit from pre-exposure prophylaxis (PrEP). Clin Infect Dis 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenya National Bureau of Statistics MoHK, National AIDS Control Council/Kenya, Kenya Medical Research Institute, and National Council for Population and Development/Kenya Kenya Demographic and Health Survey. 2014. Rockville, Maryland, USA, 2015. [Google Scholar]
- 10.Moodley D, Esterhuizen T, Reddy L, et al. Incident HIV infection in pregnant and lactating women and its effect on mother-to-child transmission in South Africa. J Infect Dis 2011; 203:1231–1234. [DOI] [PubMed] [Google Scholar]
- 11.Moodley D, Esterhuizen TM, Pather T, et al. High HIV incidence during pregnancy: compelling reason for repeat HIV testing. AIDS 2009; 23:1255–1259. [DOI] [PubMed] [Google Scholar]
- 12.Masters SH, Agot K, Obonyo B, et al. Promoting partner testing and couples testing through secondary distribution of HIV self-tests: A randomized clinical trial. PLoS Med 2016; 13:e1002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thirumurthy H, Masters SH, Mavedzenge SN, et al. Promoting male partner HIV testing and safer sexual decision making through secondary distribution of self-tests by HIV-negative female sex workers and women receiving antenatal and post-partum care in Kenya: A cohort study. Lancet HIV 2016; 3:e266–e274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osoti AO, John-Stewart G, Kiarie J, et al. Home visits during pregnancy enhance male partner HIV counselling and testing in Kenya: A randomized clinical trial. AIDS 2014; 28:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]


