Abstract
Context.
Maternal obesity is a significant public health concern that contributes to unfavorable outcomes such as inflammation and insulin resistance. Women with obesity may have impaired metabolic flexibility (i.e. an inability to adjust substrate metabolism according to fuel availability). Impaired metabolic flexibility during pregnancy may mediate poor pregnancy outcomes in women with obesity.
Purpose.
The purposes of this study were to: 1) compare metabolic flexibility between overweight/obese and lean women; and 2) determine the relationships between metabolic flexibility, inflammation following a high-fat meal, and maternal metabolic health outcomes (i.e. gestational weight gain and insulin resistance).
Procedures.
This interventional physiology study assessed lipid oxidation rate via indirect calorimetry before and after consumption of a high-fat meal. The percent change in lipid metabolism was calculated to determine ‘metabolic flexibility’. Maternal inflammatory profiles (CRP, IL-6, IL-8, IL-10, IL-12, TNF-α) and insulin resistance (HOMA-IR) were determined via plasma analyses.
Main Findings.
64 women who were pregnant (lean=35, overweight/obese=29) between 32 and 38 weeks gestation participated. Lean women had significantly higher metabolic flexibility compared to overweight/obese women (lean 48.0±34.1% vs overweight/obese 29.3±34.3%, p=0.035). Even when controlling for pre-pregnancy BMI, there was a negative relationship between metabolic flexibility and percent change in CRP among the overweight/obese group (r=−0.526, p=0.017). Metabolic flexibility (per kg fat free mass) was negatively correlated with postprandial HOMA-IR (2 hr: r=−0.325, p=0.016; 4 hr: r=−0.319, p=0.019).
Conclusions.
Overweight and obese women who are pregnant are less ‘metabolically flexible’ than lean women, and this is related to postprandial inflammation and insulin resistance.
Keywords: pregnancy, obesity, high-fat meal, postprandial, lipid oxidation
1. Introduction
Maternal obesity is a significant public health concern in the United States as over 50% of women enter pregnancy overweight or obese(1). Maternal obesity is associated with a large number of unfavorable maternal outcomes including gestational weight gain, insulin resistance, and inflammation (2–10). Metabolic adaptations, specifically changes in lipid and carbohydrate metabolism, occur during normal physiologic pregnancy to provide an adequate nutrient supply to the developing fetus (11, 12). However, in women with obesity these metabolic adaptations may be augmented by additional maternal metabolic dysfunction during pregnancy (11, 13), which in turn may have important implications for maternal and neonatal health (14).
Metabolic flexibility is the ability to adjust substrate metabolism according to fuel availability and is a critical aspect of metabolic health(15). For example, in response to a high-fat meal, a metabolically flexible individual upregulates lipid oxidation while shifting away from carbohydrate oxidation. To the contrary, an inability to upregulate lipid oxidation in response to an increase in dietary fat, termed “metabolic inflexibility,” is associated with metabolic disease and may contribute to a positive energy balance and weight gain (16). In non-gravid adults, impaired fat oxidation and metabolic inflexibility has been linked with obesity and has significant implications for long-term health, as it contributes to increased triglycerides, impaired glucose metabolism, and insulin resistance (15, 17, 18). During pregnancy, the inability to upregulate fat oxidation in response to a high-fat meal (i.e. metabolic inflexibility) may result in a positive energy balance and excessive gestational weight gain, as well as exaggerated insulin resistance beyond what is considered to be a normal physiologic response to pregnancy; however, metabolic flexibility during pregnancy has not been studied.
Dampened metabolic flexibility has also been intricately linked to systemic inflammation in non-pregnant adults (18). Previous studies suggest obesity-associated over-nutrition may contribute to inflammation and this subsequent inflammation may lead to further metabolic dysfunction, including metabolic inflexibility (18), thus perpetuating a vicious cycle. Additionally, overweight/obese individuals tend to have high inflammatory responses to high-fat loads(19), which has been recognized as a risk factor for cardiovascular disease (20–22). During pregnancy, women with obesity have higher levels of inflammation during normal fasted conditions relative to lean women (23, 24) but the inflammatory responses following a high-fat meal are not well-established. Higher levels of inflammation following a high-fat meal among women who are pregnant and overweight or obese may be important as they could contribute to the risk for the development of common complications during pregnancy such as gestational diabetes and gestational hypertensive disorders (25, 26).
The relationships between maternal metabolic flexibility in response to a high-fat diet, inflammation, and metabolic outcomes during pregnancy among women with obesity are unknown. The primary purpose of this study was to compare metabolic flexibility between women who are lean and women who are overweight/obese during late-gestation, and to establish whether maternal metabolic flexibility is associated with maternal inflammation following a high-fat meal. This study also aimed to determine the relationships between maternal metabolic flexibility and inflammation following a high-fat meal and other maternal metabolic health outcomes (i.e. gestational weight gain and insulin resistance). We hypothesized that women who are pregnant and overweight/obese would be less metabolically flexible and have higher levels of inflammation compared to lean women who are pregnant in response to a high-fat meal. Additionally, we hypothesized that metabolic inflexibility and postprandial inflammation would be associated with gestational weight gain and insulin resistance.
2. Materials and Methods
2.1. Participants
Pregnant women (n=64) between 32 and 38 weeks of pregnancy were recruited from local obstetric clinics, university–wide emails, local pregnancy fairs sponsored through the regional Kentucky medical center affiliated with the study, and word of mouth. Late pregnancy was chosen because as pregnancy progresses, insulin resistance, inflammation, and blood lipids increase(27–29) (i.e. unfavorable metabolic adaptations to pregnancy are the most significant during late pregnancy). Therefore, late pregnancy is the most important time point during pregnancy to study metabolic dysregulation and how it could impact both mother and neonate. Inclusion criteria included: 1) age 18–44 years, 2) confirmed singleton viable pregnancy with no fetal abnormalities at routine 18–22 ultrasonography, 3) self-reported pre-pregnancy BMI between 18.5 and 45 kg/m2 (lean: 18.5–24.9 kg/m2, overweight: 25–29.9 kg/m2, obese: ≥30 kg/m2), 4) plans to deliver at the hospital affiliated with the study, and 5) obstetric provider release to participate in the study. Exclusion criteria included: multiple gestation pregnancy, inability to provide voluntary informed consent, current use of illegal drugs (cocaine, methamphetamine, opiates, etc.), current smoker who did not consent to cessation, current usage of daily medications by class: corticosteroids, anti-psychotics (known to alter insulin resistance and metabolic profiles), history of or current gestational diabetes, pre-pregnancy diabetes or prior macrosomic (>4500g) infant (each elevate the risk for gestational diabetes in the current pregnancy, or undiagnosed gestational diabetes), and dietary restrictions prohibiting them from consuming the standardized meal/high-fat load. All study procedures were approved by the affiliated university’s Institutional Review Board (IRB: 16–229) and all participants provided written informed consent.
2.2. Setting.
Western Kentucky University Health Sciences Building and the Medical Center in Bowling Green, Kentucky. Enrollment began in May 2016 and concluded in March 2018. All data collection for the present study occurred between June 2016 and June 2018.
2.3. Study Procedures
2.3.1. Maternal Metabolic Flexibility Study Visit
Following a 10-hour fast, participants reported to a fully equipped exercise laboratory located on the Western Kentucky University Medical Sciences Campus at ~8:00am. Participants were provided with written instructions for consuming a standardized meal the night before the study visit. The meal was comprised of approximately 50% carbohydrate, 30% fat, and 20% protein. The participant’s weight, height, and vital signs were taken upon arrival. Body composition was measured using skinfold anthropometry in order to determine maternal percent body fat. Body fat percentage was determined by pressing folds of the skin at seven sites with a caliper (Lange Skinfold Calipers, Beta Technology, Santa Cruz, CA), recording skin fold thickness, and entering the data into a standardized equation that accounts for age as previously described (30), a technique that has been used during pregnancy in prior studies (31, 32). Participants completed the National Institutes of Health’s validated Dietary History Questionnaire II (NIH DHQII) to determine potential differences in day-to-day diet (33).
After resting for at least 30 minutes, fasting metabolic measurements (resting metabolic rate, respiratory quotient, carbon dioxide production, oxygen consumption) were assessed for 15 minutes using the TrueOne Canopy Option and TrueOne Metabolic Cart (TrueOne 2400, Parvomedics, Sandy, UT). Lipid and carbohydrate oxidation rates were calculated by measurement of oxygen consumption and carbon dioxide production as previously described (34). During the 4-hour study period, participants were asked to remain reclined or seated and resting. After the baseline resting metabolism measurement, a baseline blood draw was obtained. After the baseline blood draw, participants consumed a standardized high-fat meal, similar in composition to previous studies (35, 36). The high-fat meal consisted of a standardized smoothie from Smoothie King© that was prepared specifically for the study. The smoothie was 1062 total kilocalories, of which 594 were from fat (55.93%), 312 from carbohydrates (29.38%), and 156 from protein (14.69%). The smoothie composition was representative of a typical meal in a western diet (35, 36). Participants were instructed to finish the shake within twenty minutes, and the timer was started as soon as they began drinking the smoothie. Additional blood samples were taken at 60 minutes (1 hour), 120 minutes (2 hours), and 240 minutes (4 hours). Blood samples were immediately centrifuged and plasma was separated and stored at −80 degrees Celsius until final analyses were performed. See Figure 1 for a more detailed description of which plasma analyses were conducted at the various time points.
Figure 1. Study Visit Procedures.
All participants had fasting/baseline metabolic assessments and blood draws conducted at ~8am following an overnight fast. Metabolic assessments and blood draws were repeated at 1, 2 and 4 hours after consumption of a high-fat meal.
Indirect calorimetry was performed for 15 minutes from minute 120–135 (~2 hours post-meal) and minute 225 to 240 (~4 hours post-meal), respectively. Time points for assessment of metabolic responses to a high-fat load were chosen based on data from previous studies exploring metabolic inflexibility and inflammation in response to a high-fat meal in other populations (35, 37). Study visit procedures are outlined in Figure 1. Time points for assessment of metabolic responses to a high-fat load were chosen based on data from previous studies exploring metabolic inflexibility and inflammation in response to a high-fat meal in other populations(35, 37). Time points differ from some standardized mixed meal tolerance test protocols(38, 39); however, the meal in the present study was a much larger percentage of fats (~60% in present study vs. 20–30% in mixed meal tolerance test studies) which is likely to take longer to process and elicit metabolic changes. Therefore, the 4-hour endpoint was chosen to increase the stringency of the study design and capture additional metabolic responses beyond the first 2 hours.
2.3.2. Calculations
Metabolic flexibility was defined as the percent change in lipid oxidation rate over the 4-hour study period, in response to the high-fat meal (at 2 and 4 hours post-meal).
Adipose tissue lipolysis was calculated by the area under the curve (AUC) for free fatty acids (40, 41) assessed at baseline, 1 hour, 2 hours, and 4 hours post-meal.
Prior to leaving the study visit, participants were given an Actigraph GT9X Link Accelerometer (ActiGraph LLC, Pensacola, FL) to wear for a week in order to assess their physical activity levels as previously described (42). At delivery, maternal weight was recorded to allow for appropriate gestational weight gain determination.
2.3.3. Blood Sample Analyses
Blood draws were used to analyze maternal glucose, insulin, free fatty acids, lipids, and inflammatory markers. All blood samples were analyzed using standardized protocols. Blood samples for glucose were analyzed immediately with an automated glucose analyzer (OneTouch, Ultra 2, LifeScan, Inc). Insulin, lipid panels, free fatty acids, and CRP were analyzed, where assays were performed by trained experts and run in duplicate. Plasma insulin concentrations were assessed by electrochemiluminescence technology (Cobas c6000, Roche Diagnostics, Indianapolis, IN). Insulin and glucose levels assessed at baseline and 2 and 4 hours post-meal were used to calculate the homeostatic model assessment-insulin resistance (HOMA-IR), which is an index of insulin resistance (43, 44). High-sensitivity C-reactive protein (CRP) was measured by immunoturbidimetric assay (Roach Diagnostics, Indianapolis, IN). Plasma free fatty acid concentrations were determined by enzymatic colorimetric assay (Wako Pure Chemical Industries, Osaka, Japan). Total cholesterol, triglycerides, and direct HDL were run with enzymatic colorimetric assays completed using reagents manufactured by Roche Diagnostics and analyzed on the Cobas c6000 analyzer. LDL was calculated using the Friedwald equation (45). The inter-assay coefficients of variation in the Core Laboratory are ≤1.2% for glucose, ≤3.2% for CRP, ≤2.7% for free fatty acids, ≤2.6% for insulin, ≤1.7% for Triglycerides, ≤1.4% for cholesterol, and ≤ 1.6% for HDL cholesterol.
Inflammatory markers (interleukin-8 (IL-8), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), and tumor necrosis factor (TNF-α)) were analyzed in duplicate using The BD™ CBA Human Inflammatory Cytokines Kit Human Inflammatory Cytokine Kit (BD Sciences), according to the manufacturer’s protocol.
2.4. Statistical Procedures
2.4.1. Sample Size Calculation
Due to the lack of data regarding metabolic flexibility during pregnancy and the exploratory nature of the study, data from a previous study comparing metabolic flexibility between several non-gravid populations using the same methodology was used, where 29 subjects per group would provide 80% power to detect differences in metabolic flexibility (via indirect calorimetry) between groups of women who are pregnant (35).
2.4.2. Statistical Analyses
Normality of the distribution for each variable was tested using Kolmogorov-Smirnov tests. Minimal data was missing (at random) and these data points were removed from the data analysis. There were no missing data for the primary outcome of metabolic flexibility. Pearson product-moment correlation coefficients for normally distributed variables or Spearman’s rank-order correlation coefficient for non-normally distributed variables were used to assess the degree of the relationship between variables. Partial correlations were used to adjust for potential confounders. Student’s independent t-tests for normally distributed variables and Mann-Whitney U tests for non-normally distributed variables were used to compare outcomes between lean and obese women who are pregnant. For categorical data, chi-square tests were used to assess differences between groups. Comparisons between lean and overweight/obese women were performed with repeated-measures ANOVA with emphasis on “weight status” (lean, overweight/obese) X “time” (baseline, 2-hour post-meal, 4 hour post-meal) interaction, indicating that women who are lean and women who are overweight/obese responded differently to the high-fat meal. All data analyses were conducted using IBM SPSS Statistics, Version 22 (Armonk, New York) on raw or log-transformed data.
3. Results
Approximately 1200 women were potentially eligible for the study and given information regarding study participation via health care providers. Of those, ~150 women reached out to the study team regarding potential participation. Of those 150, ~100 were eligible based on inclusion/exclusion criteria, and 70 consented to participate. A total of 6 women were excluded from the study after consent for failure to complete or show up for the study visit (6). Demographic characteristics are presented in Table 1 (Total: N=64, lean women (LEAN): n=35; overweight/obese women (OW/OB): n=29). Chronologic and gestation ages at time of study visit, gestational weight gain, parity, ethnicity, and education level were all similar between groups. There was a significant difference in pre-pregnancy BMI (p<0.001) as well as body composition during late pregnancy (p<0.001) between LEAN and OW/OB groups. Systolic blood pressure (SBP) was the only other demographic measure assessed that was significantly different between groups. SBP was significantly higher among OW/OB compared to LEAN (p=0.01).
Table 1.
Participant Characteristics
| Participants, n = 64 | Lean = 35 | OW/OB = 29 | p-value |
|---|---|---|---|
| Demographic Characteristics | |||
| Age (y) | 29.8±4.6 | 30.0±3.4 | 0.87 |
| Pre-Pregnancy BMI (kg/m2)* | 22.2±1.5 | 32.2±5.7 | <0.001 |
| Body Fat Percentage (%)* | 20.9±3.9 | 28.3±4.7 | <0.001 |
| Gestation Age at Visit (weeks) | 34.4±1.8 | 34.5±1.5 | 0.81 |
| End of Pregnancy Gestational Weight Gain (kg) | 13.7±4.8 | 13.4±7.1 | 0.86 |
| Systolic Blood Pressure (mmHg)* | 116.8±13.4 | 127.8±12.5 | 0.01 |
| Diastolic Blood Pressure (mmHg) | 72.6±10.6 | 77.6±10.3 | 0.10 |
| Parity | |||
| Nulliparous | 16 (46%) | 15 (52%) | 0.63 |
| Multiparous | 19 (54%) | 14 (48%) | |
| Ethnicity | |||
| Caucasian | 34 (97.1%) | 28 (96.6%) | 0.36 |
| African American | 0 (0.0%) | 1 (3.4%) | |
| Hispanic | 1 (2.9%) | 0 (0.0%) | |
| Education | |||
| High School Graduate | 2 (5.7%) | 2 (6.9%) | 0.13 |
| Some College | 1 (2.8%) | 5 (17.2%) | |
| Trade School | 1 (2.8%) | 1 (3.4%) | |
| College Graduate | 13 (37.1%) | 14 (48.3%) | |
| Post-Graduate Degree | 18 (51.4%) | 7 (24.1%) | |
| Lifestyle Behaviors | |||
| Physical Activity | |||
| Sedentary Time (%) | 57.9±11.6 | 61.7±16.1 | 0.30 |
| Moderate Physical Activity (%) | 10.1± 4.3 | 10.0±5.3 | 0.91 |
| Diet | |||
| Total Kilocalories (kcal/day) | 1770.4±653 | 2118.1±1090 | 0.12 |
| Total Fat (g/day) | 71.6±36.6 | 81.2±45.1 | 0.35 |
| Total Carbohydrates(g/day) | 217.2±72.8 | 269.5±143.9 | 0.07 |
| Total Protein(g/day) | 73.8±33.8 | 86.8±47.6 | 0.21 |
Significant difference (p<0.05) between lean and overweight/obese
Lifestyle behaviors, including physical activity, sedentary time, and dietary intake, were compared between groups to ensure differences in outcomes were not simply due differences in these lifestyle factors, as both are closely linked to metabolism and inflammation (46) (Table 1). The objectively assessed percentage of time spent engaging in moderate physical activity and sedentary time were not different between groups. The overall estimates for total caloric and macronutrient intake were not different between groups.
Insulin, glucose, and blood lipids were assessed in the fasted state. These baseline metabolites are presented in Table 2 according to weight status group. High density lipoprotein, insulin, glucose, HOMA-IR, and free fatty acids were all significantly different between groups.
Table 2.
Baseline/Fasted Metabolites
| Participants, n = 64 | Lean = 35 | OW/OB = 29 | p-value |
|---|---|---|---|
| Cholesterol (mg/dL) | 265.2±40.0 | 256.5±41.8 | 0.41 |
| LDL (mg/dL) | 150.1±41.5 | 145.9±37.0 | 0.68 |
| HDL (mg/dL)* | 72.1±21.6 | 62.4±16.7 | 0.05 |
| Insulin (uU/mL)* | 9.5±4.2 | 16.8±7.3 | <0.001 |
| Glucose (mg/dL)* | 80.6±6.9 | 88.22±8.6 | <0.001 |
| Triglycerides (mg/dL) | 209.8±77.9 | 245.7±85.8 | 0.09 |
| Free Fatty Acids (meq/L)* | 0.42±0.13 | 0.50±0.16 | 0.02 |
| HOMA-IR* | 1.9±0.9 | 4.3±3.2 | <0.001 |
Metabolic assessment data are presented in Table 3. As expected, resting metabolic rate (RMR) measures in the fasted and postprandial states were significantly higher (p≤0.001) among OW/OB relative to LEAN.
Table 3.
Metabolic Rate and Substrate Metabolism
| Participants, n = 64 | Lean = 35 | OW/OB = 29 | p-value |
|---|---|---|---|
| Baseline/Fasted | |||
| Resting Metabolic Rate (kcal/day)* | 1731±335 | 2124±356 | <0.001 |
| Respiratory Quotient | 0.82±0.07 | 0.82±0.06 | 0.885 |
| Lipid Oxidation (g/min) | 0.08±0.04 | 0.09±0.04 | 0.070 |
| Carbohydrate Oxidation (g/min) | 0.14±0.05 | 0.16±0.06 | 0.114 |
| Lipid Oxidation per Kg Fat Free Mass (g/min) | 0.0013 ±0.0007 | 0.0013±0.0005 | 0.725 |
| Carbohydrate Oxidation per Kg Fat Free Mass (g/min) | 0.0023±0.0010 | 0.0023±0.0010 | 0.948 |
| 2-Hour | |||
| Resting Metabolic Rate (kcal/day)* | 2047±334 | 2388±426 | 0.001 |
| Respiratory Quotient | 0.80±0.06 | 0.81±0.05 | 0.561 |
| Lipid Oxidation (g/min) | 0.10±0.05 | 0.11±0.04 | 0.397 |
| Carbohydrate Oxidation (g/min) | 0.13±0.07 | 0.17±0.08 | 0.054 |
| Lipid Oxidation per Kg Fat Free Mass (g/min) | 0.0017±0.0008 | 0.0015±0.0005 | 0.168 |
| Carbohydrate Oxidation per Kg Fat Free Mass (g/min) | 0.0023±0.00112 | 0.0025±0.0011 | 0.505 |
| 4-Hour | |||
| Resting Metabolic Rate (kcal/day)* | 1983±354 | 2426±395 | <0.001 |
| Respiratory Quotient | 0.79±0.07 | 0.81±0.05 | 0.160 |
| Lipid Oxidation (g/min) | 0.10±0.05 | 0.11±0.04 | 0.449 |
| Carbohydrate Oxidation (g/min)* | 0.12±0.07 | 0.16±0.07 | 0.021 |
| Lipid Oxidation per Kg FFM (g/min) | 0.0018±0.0008 | 0.0015±0.0005 | 0.109 |
| Carbohydrate Oxidation per Kg Fat Free Mass (g/min) | 0.0020±0.0012 | 0.0024±0.0010 | 0.181 |
| Assessment/s over all time points | |||
| Lipolytic Rate (meq·min/L) | 59.4±15.4 | 62.0±18.4 | 0.580 |
Significant difference (p<0.05) between lean and overweight/obese.
Metabolic Flexibility
There was a significant time effect for lipid oxidation (p<0.001) in response to the high-fat meal, and there was also a significant “weight status” X “time” interaction effect in response to the high-fat meal for lipid oxidation (p=0.012). Lipid oxidation rates and the percent changes in lipid oxidation in response to the high-fat meal (i.e. metabolic flexibility) are shown in Figure 2. Lipid oxidation measured 2 hours post-meal increased by 36.4±25.5% among women who were lean compared to 22.9±35.1% among the women who were overweight/obese (p=0.081) (Figure 2B). In order to ensure changes in metabolic responses to the meal were not simply due to differing amounts of fat free mass, lipid oxidation values and changes in lipid oxidation rates were both normalized to fat free mass. At 2 hours, percent change in lipid oxidation rate per kilogram fat free mass were similar to non-adjusted percentages (lean: 35.3±24.6% vs. overweight/obese: 22.8±36.3%), and the difference between groups was not statistically significant (p=0.081). At 4 hours post-meal, the lean group had significantly increased percent change lipid oxidation relative to women in the overweight/obese group (LEAN: 48.0±34.1% vs OW/OB: 29.3±34.3%, p=0.035) (Figure 2B), and these values were similar when adjusting for kilograms of fat free mass (lean: 47.1±34.2% vs. overweight/obese: 29.2±35.4%, p=0.05).
Figure 2. Maternal Lipid Oxidation and Metabolic Flexibility.
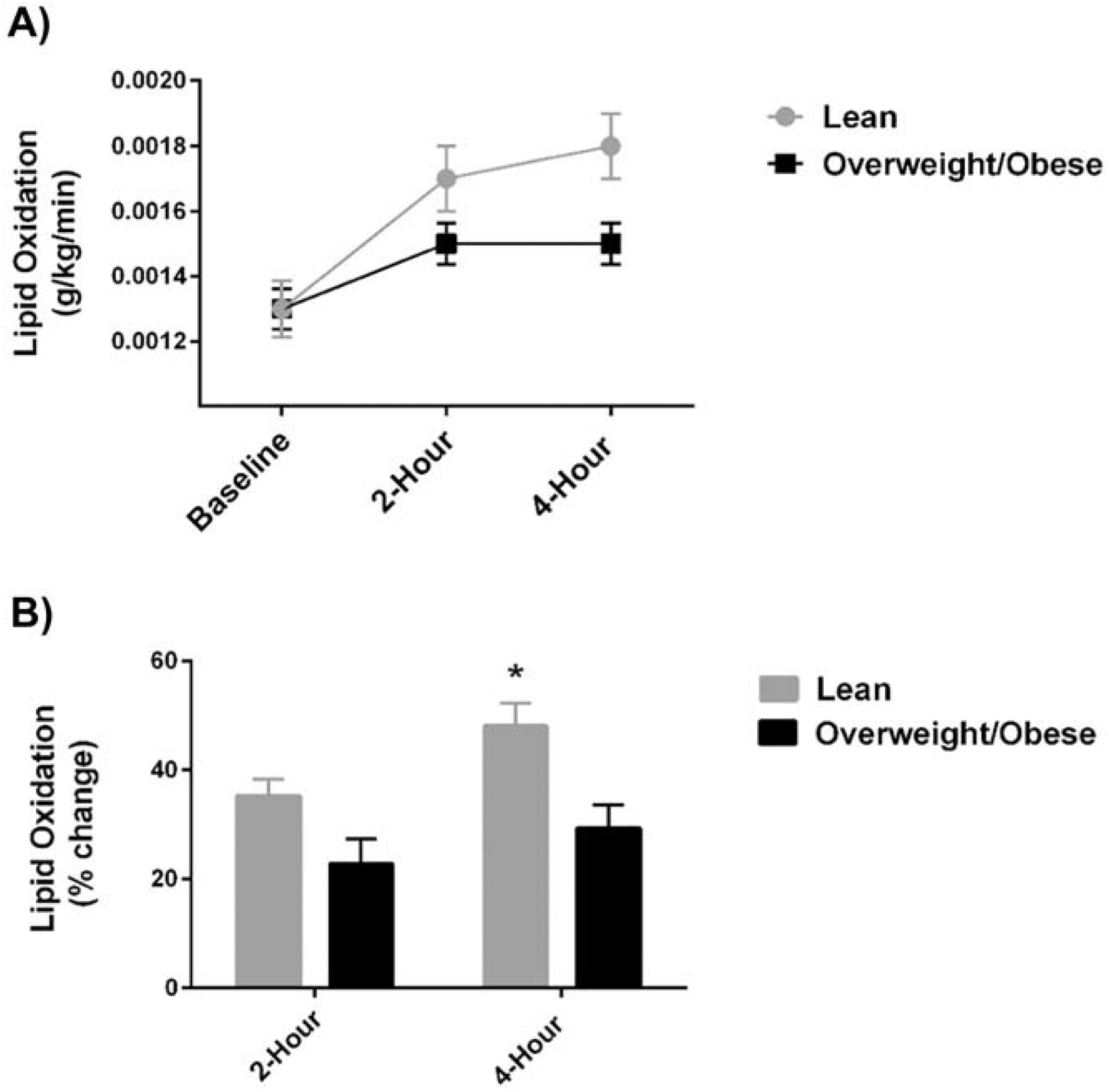
Maternal lipid oxidation rate is expressed per kilogram body mass at baseline and post high-fat meal (2-hour and 4-hour) (A) and metabolic flexibility is displayed as the percent change in maternal lipid oxidation at 2 hours and 4 hours post-meal (B). Women in the LEAN group had significantly increased metabolic flexibility (% change in lipid oxidation) compared to in the OW/OB group at 4 hours post-meal. These data suggest lean women were more metabolically flexible than women who are overweight/obese during pregnancy.
Because metabolism and metabolic dysfunction are likely to continue to change as the pregnancy progresses, these analyses were conducted a second time with adjustment for gestational age at time of study. The outcome did not change as a result of adjustment for gestational age. In order to account for other potential confounders, the analyses were performed a third time to examine the differences in metabolic flexibility at 4-hours between groups while adjusting for age, income, educational level, race, gestational weight gain, and physical activity levels. With all of these covariates included in the model, the difference between groups at 4-hours remained statistically significant (p=0.038).
3.1. Maternal Inflammatory Markers
Baseline and postprandial markers of inflammation were assessed in the fasted state and 4 hours post-meal (respectively). At baseline, inflammatory markers, specifically IL-6 and IL-8, were significantly higher among the OW/OB group when compared to the LEAN group (IL-6 p=0.003, IL-1β p=0.028) (Figure 3A). Following the high-fat meal, IL-8 tended to be higher in the OW/OB group vs. the LEAN group (p=0.078) (Figure 3B). CRP was significantly higher in the OW/OB group compared to the LEAN group at baseline and following the high-fat meal (baseline: CRP p=0.002, 4-Hour: CRP p=0.006) (Figure 3C). However, CRP was significantly lower following the high fat meal (p=0.006) in both groups.
Figure 3. Maternal Inflammatory Markers.
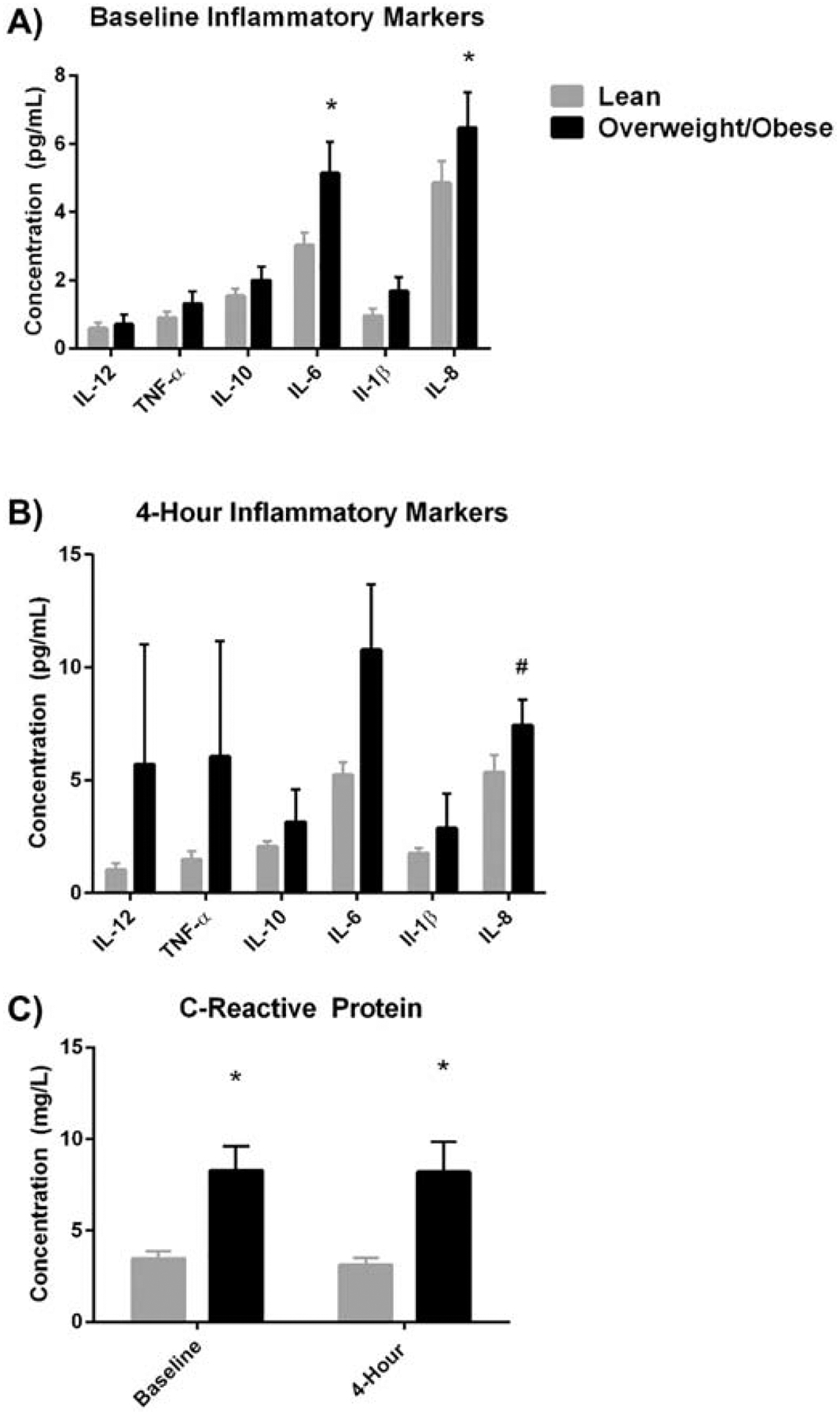
Women in the OW/OB group had higher levels of inflammation, particularly CRP, IL-6, and IL-8 at baseline and CRP at 4-hours post high-fat meal compared to women in the LEAN group.
3.2. Postprandial Glucose and Insulin Responses
Maternal insulin and glucose responses to the high-fat meal are shown in Figure 4. There was a significant time effect for maternal glucose and insulin (p<0.001) in response to the high-fat meal. There was a significant overall “weight status” effect (p<0.001) and a significant “weight status” X “time” interaction effect for insulin (p=0.004) (Figure 4A), but not for glucose (“weight status” effect p=0.106; “weight status” X “time” interaction effect p=0.110) (Figure 4B). Insulin levels were significantly higher in the OW/OB group at all 4 time points (p<0.05). Only baseline glucose was significantly higher in the OW/OB group (p<0.001); however, there was a trend for glucose to be elevated among the OW/OB group 1 hour post-meal when compared to the LEAN group (p=0.073).
Figure 4. Maternal Postprandial Glucose and Insulin Responses.
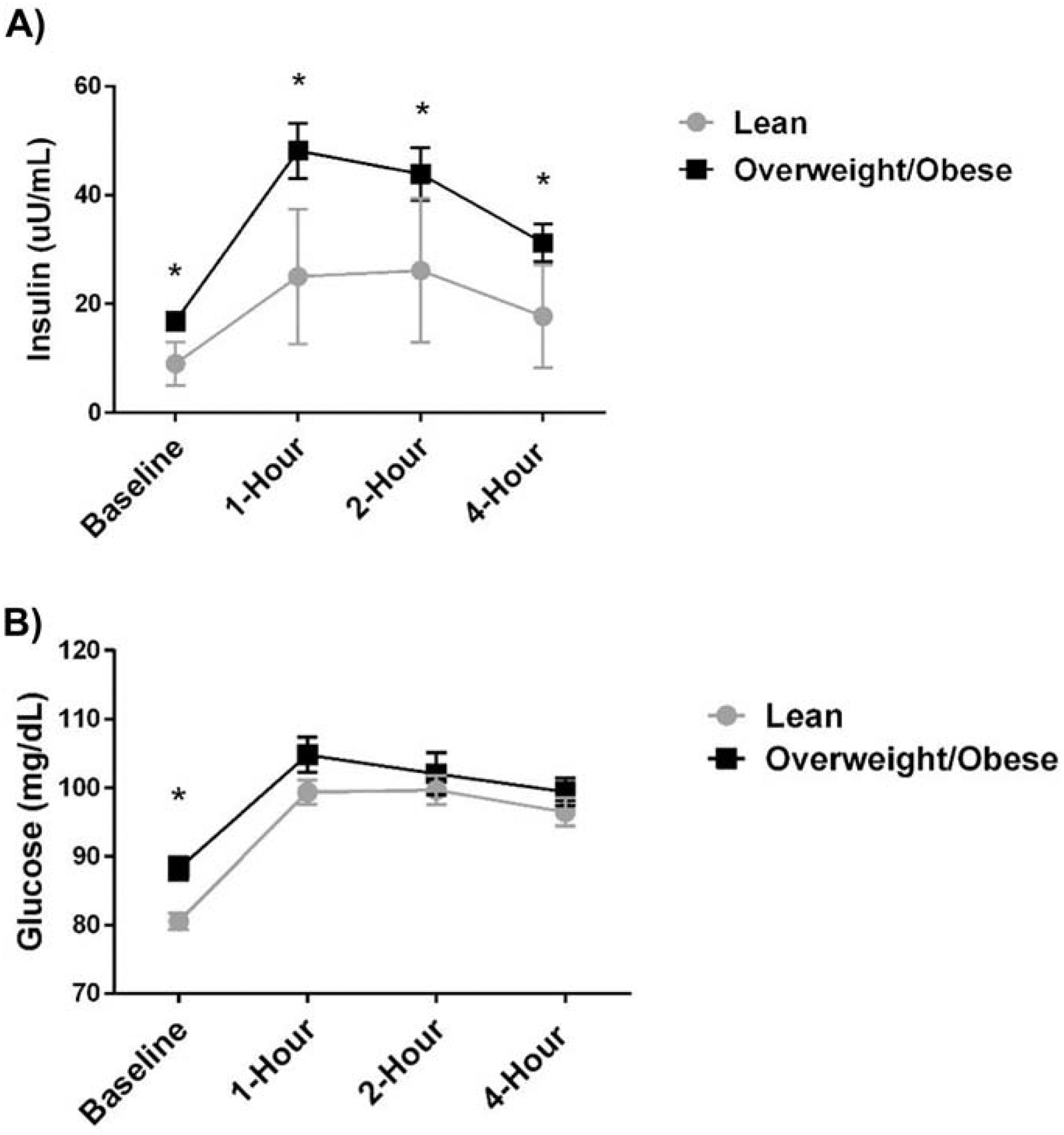
There was a significant time effect for maternal glucose and insulin (p<0.001) in response to the high-fat meal. There was a significant overall “weight status” effect (p<0.001) and a significant “weight status” X “time” interaction effect for insulin (p=0.004)(Figure 4A), but not for glucose (“weight status” effect p=0.106; “weight status” X “time” interaction effect p=0.110) (Figure 4B). Insulin levels were significantly higher among the OW/OB group at all 4 time points (p<0.05). Only baseline glucose was significantly higher among the OW/OB group (p<0.001),
Maternal Metabolic Flexibility, Postprandial Inflammation, and Postprandial Insulin Resistance
The change in lipid oxidation at 2 hours and 4 hours post-meal (indicators of metabolic flexibility and expressed per kg fat free mass) were negatively correlated with postprandial HOMA-IR (assessed at 2 hours post-meal) (2 hr: r=−0.325, p=0.016; 4 hr: r=−0.319, p=0.019). When adjusted for age, physical activity level, and ethnicity (all factors that have been connected to metabolic flexibility and/or insulin resistance in non-gravid populations(17, 47, 48)) the relationship between metabolic flexibility and postprandial HOMA-IR remained significant at the 4-hour time point. Metabolic flexibility was not associated with gestational weight gain.
Postprandial lipid oxidation was associated with postprandial inflammation. Important relationships between postprandial lipid oxidation and inflammatory markers (IL-6, IL-8, IL-10) are shown in Figure 5. In response to the high-fat meal, when the percent change in CRP and metabolic flexibility at 4 hours was analyzed within the OW/OB and LEAN groups respectively (controlling for pre-pregnancy BMI), there was a negative relationship between the change in CRP and 4-hour metabolic flexibility among women in the OW/OB group (r=−0.526, p=0.017) but not among women in the LEAN group (r=−0.125, p=0.503) (Figure 6), Additional analyses were conducted and these relationships persisted when also controlling for age, gestational weight gain, and physical activity levels. When also adjusted for maternal HOMA-IR, the relationship between metabolic flexibility and change in inflammation was no longer significant. (r=−0.439, p=0.060).
Figure 5. Relationships between 4-Hour Postprandial Lipid Oxidation Rate and Inflammatory Markers (A: IL-6, B: IL-8, C: IL-10).
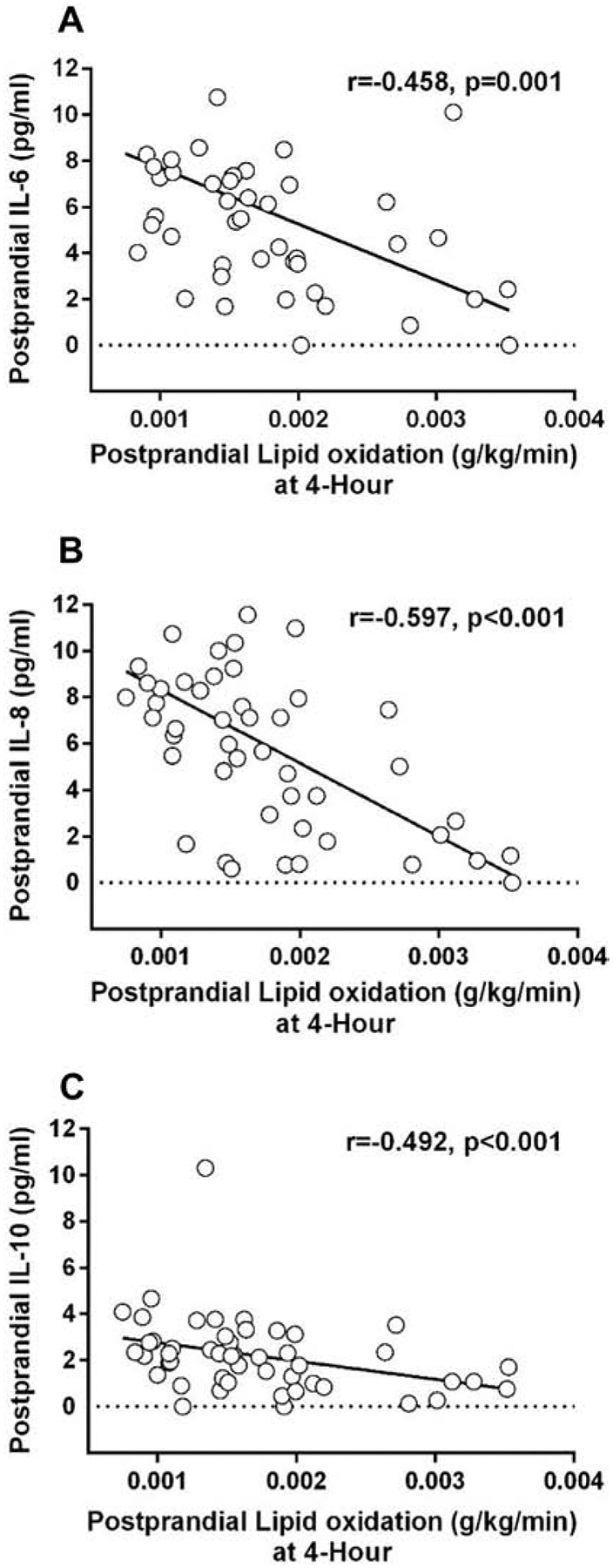
Postprandial lipid oxidation (g/kg/min) was significantly assoicated with postprandial IL-6, IL-8, and IL-10.
Figure 6. Metabolic Flexibility and Change in CRP between Baseline and 4 Hours Post High-Fat Meal.
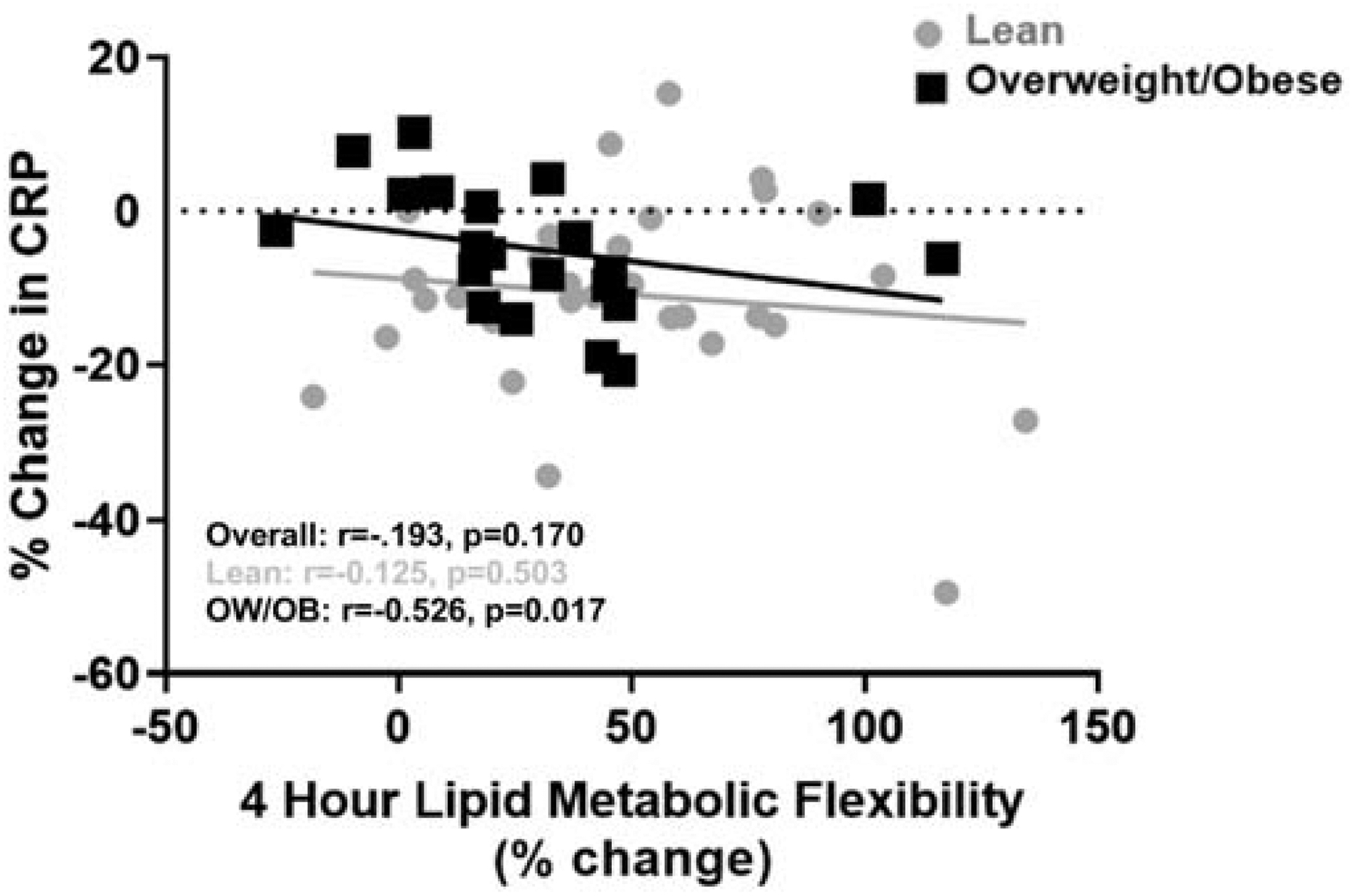
There was a negative relationship between the change in CRP and metabolic flexibility (i.e. % change in lipid oxidation) when controlling for pre-pregnancy BMI among the overweight/obese women (p=0.017) but not among the lean women (p=.503).
4. Discussion
The overall goal of this study was to compare metabolic flexibility between women with overweight/obesity and women who are lean during pregnancy and to examine the potential relationships between metabolic flexibility, postprandial inflammation, and maternal metabolic outcomes (insulin resistance and gestational weight gain). We found, for the first time, that women who are overweight/obese were less metabolically flexible compared to women who were lean in response to a high-fat meal during late pregnancy, and metabolic flexibility was negatively associated with maternal postprandial inflammation and insulin resistance. Contrary to our hypothesis, metabolic inflexibility was not associated with higher gestational weight gain (data not shown). Mounting evidence among non-gravid populations indicates that the ability to adjust substrate oxidation according to nutrient availability, termed “metabolic flexibility”, has significant implications for long-term health as it contributes to weight status and insulin resistance (17). Additionally, it is well established that obesity and other metabolic disorders are characterized by chronic low grade inflammation in the fasted state that increases to a greater extent following a high-fat meal (49). Our findings suggest that during pregnancy, overweight and obese women may also have lower metabolic flexibility, and that this lower ability to oxidize ingested lipids may contribute to other unfavorable maternal comorbidities such as maternal insulin resistance and elevated postprandial inflammation. This is clinically relevant for both maternal and fetal health, as evidence suggests that overweight/obese women with subclinical metabolic impairments, including insulin resistance and elevated inflammation, are at increased risk of adverse fetal programming (50–52).
In the present study among women in late pregnancy, metabolic flexibility was assessed by measuring the change in lipid oxidation in response to a high-fat meal challenge. We found that women who are pregnant and overweight/obese had a dampened ability to increase lipid oxidation at 4 hours post the high-fat meal relative to their lean counterparts (i.e. were less metabolically flexible). Others have suggested that in adults who are not pregnant, chronic over-nutrition leads to a state of metabolic confusion, inflexibility, and insensitivity that is associated with impaired nutrient sensing, dampened substrate switching, impaired energy homeostasis, and possibly insulin resistance (53). In the case of over-nutrition, mitochondrial “indecision” creates a cellular “metabolic gridlock” which contributes to incomplete substrate oxidation, increases in oxidative stress, overabundances of metabolic intermediates, and subsequent ectopic storage depots (53). It is this evidence that suggests in non-gravid individuals, the failure to appropriately upregulate fatty acid oxidation in response to a high-fat meal can cause intracellular lipid accumulation and insulin resistance over time (47). Our data support this as we found that women who overweight/obese had lower metabolic flexibility (Figure 2B) and significantly elevated insulin at baseline and in response to the high-fat meal (Figure 4A) during pregnancy. They also had significantly higher glucose and HOMA-IR at baseline. Additionally, we noted that metabolic flexibility and postprandial HOMA-IR were negatively correlated, suggesting that women who are pregnant and insulin resistant have a dampened ability to upregulate lipid oxidation in response to a high-fat meal compared to lean women.
Previous work exploring potential mechanisms underlying metabolic inflexibility has been conducted in non-gravid individuals. This study is the first, to our knowledge, to demonstrate metabolic inflexibility in response to a high-fat meal in overweight/obese women during pregnancy. Additionally, the majority of work describing metabolic dysfunction and impaired substrate shifting during pregnancy has focused on women with gestational diabetes mellitus (GDM). For example, women with GDM have impaired lipid oxidation and a dampened ability to alter substrate metabolism in response to physiological cues during late pregnancy compared to those with normal glucose tolerance characteristics (54, 55). However, little is known about cellular mechanisms potentially underlying impaired postprandial substrate shifting among women with obesity during pregnancy that have not been diagnosed with GDM. One previous study described cellular mechanisms in skeletal muscle that varied between lean and obese women during pregnancy, which could help explain metabolic differences between groups in the current study (56). Specifically, they found that women who are pregnant with obesity have decreased mitochondrial enzyme activity and antioxidant capacity, coupled with increased oxidative stress markers (56). They reported that the mitochondrial NAD-dependent deacetylase sirtuin-3 (SIRT3) was significantly lower in the skeletal muscle from women who are pregnant with obesity, and suspected that this protein may play an important role in higher oxidative stress and impaired mitochondrial enzyme capacity evident with obesity during pregnancy (56).
In the current study, the women who are pregnant and overweight/obese had elevated markers of systemic inflammation and were less metabolically flexible compared to their lean counterparts. There is sufficient evidence in non-gravid populations to suggest that individuals who are obese, with lower metabolic flexibility exhibit a more pro-inflammatory profile(57), which may lead to further metabolic inflexibility and dysfunction (18), thus likely perpetuating a vicious cycle. Our study demonstrates the same may be true during pregnancy. In this study, we found that baseline IL-6, IL-8, and CRP (markers of systemic inflammation) were significantly higher among the overweight/obese women relative to the lean (Figure 3A and 3C), which is consistent with findings from other studies (13, 58–61). Excessive inflammation during pregnancy is associated with an increased risk for the development of maternal metabolic complications during pregnancy, including insulin resistance and hypertension (25, 26), as well as increased risk for future development of maternal metabolic syndrome, insulin resistance, diabetes, hypertension, and cardiovascular disease (62). Our findings support these relationships as postprandial CRP was related to maternal systolic blood pressure and insulin resistance.
Another novel finding of the present study was the divergent inflammatory response to the high-fat meal between the overweight/obese and lean groups, which was associated with metabolic flexibility. Postprandial CRP was significantly higher among the overweight/obese group relative to the lean. In fact, the women in the OW/OB group saw a 2.67% increase in CRP values, while the LEAN group actually saw a 10.83% decrease in CRP following the high-fat meal. Of note, the change in plasma CRP values was associated with metabolic inflexibility in overweight/obese women. It is possible that metabolic cells faced with the constant metabolic stress of obesity-associated over-nutrition, leading to metabolic inflexibility, may initiate an inflammatory response mediated by the activation of multiple signaling pathways that may be nutrient-induced (63). In contrast, the relationship between a change in plasma CRP values and metabolic flexibility was not present in lean women during pregnancy. Perhaps the more metabolically flexible overweight/obese individuals may be less likely to exhibit postprandial spikes in inflammation relative to overweight/obese individuals that are less metabolically flexible.
It is also important to note that in the current study, postprandial HOMA-IR and postprandial CRP values were significantly correlated. Again, this suggests there is a link between metabolic flexibility, inflammation, and insulin resistance during pregnancy, a relationship that has been shown in non-gravid individuals (15, 17, 18). Interestingly, the relationship between metabolic flexibility and change in CRP was no longer significant when adjusting for maternal HOMA-IR; suggesting a potential mediating role of insulin resistance in the relationship between metabolic flexibility and inflammation. Inflammation may be involved in the pathogenesis of insulin resistance and via versa, potentially creating a vicious cycle(64). Our findings suggest that metabolic flexibility may lead to insulin resistance (as previously demonstrated in non-gravid adults and based on the relationship between metabolic flexibility and postprandial HOMA-IR) and that this insulin resistance may then contribute to subsequent inflammation. However, it is difficult to parse out the direction of these relationships as these variables have complicated and often interdependent pathways.
Taken together, findings from the present study suggest that metabolic flexibility could play an important role in maternal health during pregnancy. Our data suggest that physical activity in late pregnancy was not highly correlated with metabolic flexibility. These findings, which are in in contrast to findings among the non-gravid, are surprising. Previous research in men and women who are not pregnant has shown that endurance exercise training can improve metabolic flexibility in skeletal muscle regardless of body weight status (46). Therefore, exercise may be an intervention strategy that can improve metabolic flexibility and thus, improve outcomes. Clinically, any potentially modifiable characteristic (such as metabolic flexibility) that could positively alter inflammation, insulin resistance, gestational weight gain, and blood pressure (four of the most common and worrisome pregnancy-related ailments (65)) warrants further investigation. However, the fact that there was not a relationship between metabolic flexibility and physical activity levels may mean that during pregnancy, weight control may be of upmost importance to maintain metabolic health, which is in contrast to some literature in non-gravid populations that suggests improvements in fitness levels may improve metabolic health status regardless of body weight(66). In addition, it has been suggested that the metabolic, inflammatory, and endocrine stresses of pregnancy may “unmask” risk of future disease (56). Therefore, understanding the metabolic health of women during pregnancy, and the role of metabolic flexibility, is important far beyond the 9 months of pregnancy as it will have serious long-term implications for the mother.
4.1. Limitations/Strengths
The present study has several notable limitations. First, our study design did not allow us to determine cause and effect. It is not clear if excess body weight contributes to metabolic inflexibility or if metabolic inflexibility lead to excess weight gain and other related metabolic dysfunction. Further research is needed to elucidate the relationships between and mechanisms linking inflammation, metabolic inflexibility, and obesity. Next, our sample consisted of predominately Caucasian women; therefore, our results cannot be generalized to all populations. Another limitation is that recent dietary intake can influence metabolic responsiveness to a meal (67, 68); thus, recent dietary patterns may have influenced results. However, we did have participants complete an extensive dietary history questionnaire for the month prior to the visit in order to account for differences in diet. In addition, we also instructed participants to consume a standardized meal the night prior to the metabolic visit in an effort to reduce this as a potential confounding variable.
Despite the limitations, the major strength of our study is that we demonstrated, for the first time, that pregnant women who are overweight/obese have impaired metabolic flexibility, and that it is connected to other unfavorable outcomes (i.e. inflammation and insulin resistance). While metabolic flexibility has been studied in non-pregnant populations, understanding the role that metabolic dysfunction could play is particularly important during pregnancy as there are both maternal and offspring implications. Our study is also novel in that we carefully examined postprandial metabolism among women who are pregnant, which is important as most pregnant women spend the majority of the day in a postprandial state. Further, we studied metabolic responsiveness to a high-fat meal, which is timely as shifts towards high-fat diets are contributing to the incidence of obesity, type II diabetes, heart disease, and other chronic diseases that ultimately lower life expectancy (69). In addition, our study was rigorously designed and implemented. We carefully assessed and/or accounted for as many variables as possible that could influence metabolic outcomes (e.g. gestational weight gain, gestation age, physical activity level, dietary intake). In addition, we measured metabolic flexibility at 2-hours and 4-hours post-high fat meal during a 5–6 hour study visit. Given that there were significant relationships and differences between groups in metabolic flexibility detected more often at 4 hours versus 2 hours post high-fat meal, the present study provides important information for future study replication; future studies exploring metabolic flexibility during pregnancy should last at least 4-hours post-meal, which is consistent with studies of metabolic flexibility in non-gravid populations(35, 37).
4.2. Conclusions
Overall, our data present compelling evidence linking metabolic inflexibility with overweight/obesity, insulin resistance, and inflammation among women in late pregnancy. The metabolic and inflammatory environment play an important role in maternal health outcomes, and thus, should be considered when designing future intervention strategies to combat obesity and its associated conditions during pregnancy. Additionally, health care providers should explore methods to improve metabolic responsiveness and function and decrease inflammation during pregnancy.
First study to look at metabolic response to high-fat meal during pregnancy
Lean pregnant women are better able to metabolize high-fat meals
The inability to metabolize dietary fats is linked to inflammation in pregnancy
Post-meal fat metabolism during pregnancy is related to insulin resistance
Acknowledgements:
We would like to acknowledge Alyssa Olenick, Bailey Pitts, Kolbi Edens, and Kristin Yoho for their assistance with data collection procedures.
Funding: This work was supported by the NIH National Institute of General Medical Science IDeA Grant 5P20GM103436 and Western Kentucky University’s Research and Creative Activity Program Grant 17-8011.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trail Registration Number:
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Financial Disclosure Statement: The authors have nothing to disclose.
References
- 1.Deputy NP, Dub B, Sharma AJ. Prevalence and Trends in Prepregnancy Normal Weight - 48 States, New York City, and District of Columbia, 2011–2015. MMWR Morb Mortal Wkly Rep. 2018;66(51–52):1402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chasan-Taber L, Schmidt MD, Pekow P, Sternfeld B, Solomon CG, Markenson G. Predictors of excessive and inadequate gestational weight gain in Hispanic women. Obesity (Silver Spring). 2008;16(7):1657–66. [DOI] [PubMed] [Google Scholar]
- 3.Althuizen E, van Poppel MN, Seidell JC, van Mechelen W. Correlates of absolute and excessive weight gain during pregnancy. J Womens Health (Larchmt). 2009;18(10):1559–66. [DOI] [PubMed] [Google Scholar]
- 4.Lain KY, Catalano PM. Factors that affect maternal insulin resistance and modify fetal growth and body composition. Metab Syndr Relat Disord. 2006;4(2):91–100. [DOI] [PubMed] [Google Scholar]
- 5.Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, Comstock CH, et al. Obesity, obstetric complications and cesarean delivery rate--a population-based screening study. American journal of obstetrics and gynecology. 2004;190(4):1091–7. [DOI] [PubMed] [Google Scholar]
- 6.Miller RS, Thompson ML, Williams MA. Trimester-specific blood pressure levels in relation to maternal pre-pregnancy body mass index. Paediatr Perinat Epidemiol. 2007;21(6):487–94. [DOI] [PubMed] [Google Scholar]
- 7.Walsh SW. Obesity: a risk factor for preeclampsia. Trends in endocrinology and metabolism: TEM. 2007;18(10):365–70. [DOI] [PubMed] [Google Scholar]
- 8.Yaniv-Salem S, Shoham-Vardi I, Kessous R, Pariente G, Sergienko R, Sheiner E. Obesity in pregnancy: what’s next? Long-term cardiovascular morbidity in a follow-up period of more than a decade. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2015:1–5. [DOI] [PubMed] [Google Scholar]
- 9.Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. The American journal of clinical nutrition. 2009;90(5):1303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Group HSCR, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. The New England journal of medicine. 2008;358(19):1991–2002. [DOI] [PubMed] [Google Scholar]
- 11.Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. The American journal of clinical nutrition. 2000;71(5 Suppl):1256S–61S. [DOI] [PubMed] [Google Scholar]
- 12.Wiznitzer A, Mayer A, Novack V, Sheiner E, Gilutz H, Malhotra A, et al. Association of lipid levels during gestation with preeclampsia and gestational diabetes mellitus: a population-based study. Am J Obstet Gynecol. 2009;201(5):482 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tinius RA, Cahill AG, Strand EA, Cade WT. Altered maternal lipid metabolism is associated with higher inflammation in obese women during late pregnancy. Integr Obes Diabetes. 2015;2(1):168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grieger JA, Bianco-Miotto T, Grzeskowiak LE, Leemaqz SY, Poston L, McCowan LM, et al. Metabolic syndrome in pregnancy and risk for adverse pregnancy outcomes: A prospective cohort of nulliparous women. PLoS Med. 2018;15(12):e1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodpaster BH, Sparks LM. Metabolic Flexibility in Health and Disease. Cell metabolism. 2017;25(5):1027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horton TJ, Drougas H, Brachey A, Reed GW, Peters JC, Hill JO. Fat and carbohydrate overfeeding in humans: different effects on energy storage. The American journal of clinical nutrition. 1995;62(1):19–29. [DOI] [PubMed] [Google Scholar]
- 17.Bergouignan A, Antoun E, Momken I, Schoeller DA, Gauquelin-Koch G, Simon C, et al. Effect of contrasted levels of habitual physical activity on metabolic flexibility. J Appl Physiol (1985). 2013;114(3):371–9. [DOI] [PubMed] [Google Scholar]
- 18.Calcada D, Vianello D, Giampieri E, Sala C, Castellani G, de Graaf A, et al. The role of low-grade inflammation and metabolic flexibility in aging and nutritional modulation thereof: a systems biology approach. Mech Ageing Dev. 2014;136–137:138–47. [DOI] [PubMed] [Google Scholar]
- 19.Blackburn P, Despres JP, Lamarche B, Tremblay A, Bergeron J, Lemieux I, et al. Postprandial variations of plasma inflammatory markers in abdominally obese men. Obesity (Silver Spring). 2006;14(10):1747–54. [DOI] [PubMed] [Google Scholar]
- 20.Miglio C, Peluso I, Raguzzini A, Villano DV, Cesqui E, Catasta G, et al. Antioxidant and inflammatory response following high-fat meal consumption in overweight subjects. Eur J Nutr. 2013;52(3):1107–14. [DOI] [PubMed] [Google Scholar]
- 21.Emerson SR, Kurti SP, Harms CA, Haub MD, Melgarejo T, Logan C, et al. Magnitude and Timing of the Postprandial Inflammatory Response to a High-Fat Meal in Healthy Adults: A Systematic Review. Adv Nutr. 2017;8(2):213–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klop B, Proctor SD, Mamo JC, Botham KM, Castro Cabezas M. Understanding postprandial inflammation and its relationship to lifestyle behaviour and metabolic diseases. Int J Vasc Med. 2012;2012:947417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madan JC, Davis JM, Craig WY, Collins M, Allan W, Quinn R, et al. Maternal obesity and markers of inflammation in pregnancy. Cytokine. 2009;47(1):61–4. [DOI] [PubMed] [Google Scholar]
- 24.Basu S, Haghiac M, Surace P, Challier JC, Guerre-Millo M, Singh K, et al. Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obesity (Silver Spring). 2011;19(3):476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozgu-Erdinc AS, Yilmaz S, Yeral MI, Seckin KD, Erkaya S, Danisman AN. Prediction of gestational diabetes mellitus in the first trimester: comparison of C-reactive protein, fasting plasma glucose, insulin and insulin sensitivity indices. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2014:1–6. [DOI] [PubMed] [Google Scholar]
- 26.Wolf M, Kettyle E, Sandler L, Ecker JL, Roberts J, Thadhani R. Obesity and preeclampsia: the potential role of inflammation. Obstet Gynecol. 2001;98(5 Pt 1):757–62. [DOI] [PubMed] [Google Scholar]
- 27.Watts DH, Krohn MA, Wener MH, Eschenbach DA. C-reactive protein in normal pregnancy. Obstet Gynecol. 1991;77(2):176–80. [DOI] [PubMed] [Google Scholar]
- 28.Pusukuru R, Shenoi AS, Kyada PK, Ghodke B, Mehta V, Bhuta K, et al. Evaluation of Lipid Profile in Second and Third Trimester of Pregnancy. J Clin Diagn Res. 2016;10(3):QC12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonagra AD, Biradar SM, K D, Murthy DSJ. Normal pregnancy- a state of insulin resistance. J Clin Diagn Res. 2014;8(11):CC01–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Medicine and science in sports and exercise. 1980;12(3):175–81. [PubMed] [Google Scholar]
- 31.Kannieappan LM, Deussen AR, Grivell RM, Yelland L, Dodd JM. Developing a tool for obtaining maternal skinfold thickness measurements and assessing inter-observer variability among pregnant women who are overweight and obese. BMC Pregnancy Childbirth. 2013;13:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taggart NR, Holliday RM, Billewicz WZ, Hytten FE, Thomson AM. Changes in skinfolds during pregnancy. Br J Nutr. 1967;21(2):439–51. [DOI] [PubMed] [Google Scholar]
- 33.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–99. [DOI] [PubMed] [Google Scholar]
- 34.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. Journal of applied physiology: respiratory, environmental and exercise physiology. 1983;55(2):628–34. [DOI] [PubMed] [Google Scholar]
- 35.Heilbronn LK, Gregersen S, Shirkhedkar D, Hu D, Campbell LV. Impaired fat oxidation after a single high-fat meal in insulin-sensitive nondiabetic individuals with a family history of type 2 diabetes. Diabetes. 2007;56(8):2046–53. [DOI] [PubMed] [Google Scholar]
- 36.Jakulj F, Zernicke K, Bacon SL, van Wielingen LE, Key BL, West SG, et al. A high-fat meal increases cardiovascular reactivity to psychological stress in healthy young adults. J Nutr. 2007;137(4):935–9. [DOI] [PubMed] [Google Scholar]
- 37.Brandauer J, Landers-Ramos RQ, Jenkins NT, Spangenburg EE, Hagberg JM, Prior SJ. Effects of prior acute exercise on circulating cytokine concentration responses to a high-fat meal. Physiol Rep. 2013;1(3):e00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Besser RE, Shields BM, Casas R, Hattersley AT, Ludvigsson J. Lessons from the mixed-meal tolerance test: use of 90-minute and fasting C-peptide in pediatric diabetes. Diabetes Care. 2013;36(2):195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujioka Y, Okura T, Sumi K, Matsumoto K, Shoji K, Nakamura R, et al. Normal meal tolerance test is preferable to the glucagon stimulation test in patients with type 2 diabetes that are not in a hyperglycemic state: Comparison with the change of C-peptide immunoreactivity. J Diabetes Investig. 2018;9(2):274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–31. [DOI] [PubMed] [Google Scholar]
- 41.Allirot X, Seyssel K, Saulais L, Roth H, Charrie A, Drai J, et al. Effects of a breakfast spread out over time on the food intake at lunch and the hormonal responses in obese men. Physiol Behav. 2014;127:37–44. [DOI] [PubMed] [Google Scholar]
- 42.van Hees VT, Renstrom F, Wright A, Gradmark A, Catt M, Chen KY, et al. Estimation of daily energy expenditure in pregnant and non-pregnant women using a wrist-worn tri-axial accelerometer. PLoS One. 2011;6(7):e22922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen O, Epstein GS, Weisz B, Homko CJ, Sivan E. Longitudinal assessment of insulin sensitivity in pregnancy. Validation of the homeostasis model assessment. Clinical endocrinology. 2006;64(6):640–4. [DOI] [PubMed] [Google Scholar]
- 44.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. [DOI] [PubMed] [Google Scholar]
- 45.Fukuyama N, Homma K, Wakana N, Kudo K, Suyama A, Ohazama H, et al. Validation of the Friedewald Equation for Evaluation of Plasma LDL-Cholesterol. J Clin Biochem Nutr. 2008;43(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Battaglia GM, Zheng D, Hickner RC, Houmard JA. Effect of exercise training on metabolic flexibility in response to a high-fat diet in obese individuals. Am J Physiol Endocrinol Metab. 2012;303(12):E1440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galgani JE, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab. 2008;295(5):E1009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karakelides H, Irving BA, Short KR, O’Brien P, Nair KS. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes. 2010;59(1):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106 Suppl 3:S5–78. [DOI] [PubMed] [Google Scholar]
- 50.Borgono CA, Hamilton JK, Ye C, Hanley AJ, Connelly PW, Sermer M, et al. Determinants of insulin resistance in infants at age 1 year: impact of gestational diabetes mellitus. Diabetes Care. 2012;35(8):1795–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamashita H, Yasuhi I, Fukuda M, Kugishima Y, Yamauchi Y, Kuzume A, et al. The association between maternal insulin resistance in mid-pregnancy and neonatal birthweight in uncomplicated pregnancies. Endocr J. 2014;61(10):1019–24. [DOI] [PubMed] [Google Scholar]
- 52.Rasmussen JM, Graham AM, Entringer S, Gilmore JH, Styner M, Fair DA, et al. Maternal Interleukin-6 concentration during pregnancy is associated with variation in frontolimbic white matter and cognitive development in early life. Neuroimage. 2019;185:825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muoio DM. Metabolic inflexibility: when mitochondrial indecision leads to metabolic gridlock. Cell. 2014;159(6):1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okereke NC, Huston-Presley L, Amini SB, Kalhan S, Catalano PM. Longitudinal changes in energy expenditure and body composition in obese women with normal and impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2004;287(3):E472–9. [DOI] [PubMed] [Google Scholar]
- 55.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180(4):903–16. [DOI] [PubMed] [Google Scholar]
- 56.Boyle KE, Newsom SA, Janssen RC, Lappas M, Friedman JE. Skeletal muscle MnSOD, mitochondrial complex II, and SIRT3 enzyme activities are decreased in maternal obesity during human pregnancy and gestational diabetes mellitus. J Clin Endocrinol Metab. 2013;98(10):E1601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282(22):2131–5. [DOI] [PubMed] [Google Scholar]
- 58.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab. 2002;87(9):4231–7. [DOI] [PubMed] [Google Scholar]
- 59.Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. C-reactive protein and gestational diabetes: the central role of maternal obesity. J Clin Endocrinol Metab. 2003;88(8):3507–12. [DOI] [PubMed] [Google Scholar]
- 60.Tinius RA, Cahill AG, Cade WT. Low-intensity Physical Activity is Associated with Lower Maternal Systemic Inflammation during Late Pregnancy. J Obes Weight Loss Ther. 2017;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tinius RA, Cahill AG, Strand EA, Cade WT. Maternal inflammation during late pregnancy is lower in physically active compared with inactive obese women. Appl Physiol Nutr Metab. 2016;41(2):191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kosus N, Kosus A, Turhan N. Relation between abdominal subcutaneous fat tissue thickness and inflammatory markers during pregnancy. Archives of medical science: AMS. 2014;10(4):739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. [DOI] [PubMed] [Google Scholar]
- 64.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Obesity and Pregnancy. The American College of Obstetricians and Gynecologists; April 2016. Accessed at https://www.acog.org/Patients/FAQs/Obesity-and-Pregnancy?IsMobileSet=false. [Google Scholar]
- 66.Lee S, Kuk JL, Katzmarzyk PT, Blair SN, Church TS, Ross R. Cardiorespiratory fitness attenuates metabolic risk independent of abdominal subcutaneous and visceral fat in men. Diabetes Care. 2005;28(4):895–901. [DOI] [PubMed] [Google Scholar]
- 67.Chavez-Jauregui RN, Mattes RD, Parks EJ. Dynamics of fat absorption and effect of sham feeding on postprandial lipema. Gastroenterology. 2010;139(5):1538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robertson MD, Henderson RA, Vist GE, Rumsey RD. Extended effects of evening meal carbohydrate-to-fat ratio on fasting and postprandial substrate metabolism. Am J Clin Nutr. 2002;75(3):505–10. [DOI] [PubMed] [Google Scholar]
- 69.Tilman D, Clark M. Global diets link environmental sustainability and human health. Nature. 2014;515(7528):518–22. [DOI] [PubMed] [Google Scholar]


