Abstract
Although Staphylococcus aureus is a common cause of bacteremia, treatment options are limited. The need for new therapies is particularly urgent for methicillin-resistant S. aureus bacteremia (SAB). Ceftobiprole is an advanced-generation, broad-spectrum cephalosporin with activity against both methicillin-susceptible and -resistant S. aureus. This is a Phase III, randomized, double-blind, active-controlled, parallel-group, multicenter, two-part study to establish the efficacy and safety of ceftobiprole compared with daptomycin in the treatment of SAB, including infective endocarditis. Anticipated enrollment is 390 hospitalized adult patients, aged ≥18 years, with confirmed or suspected complicated SAB. The primary end point is overall success rate. Target completion of the study is in the second half of 2021.
Clinicaltrials.gov identifier: NCT03138733
Keywords: : bacteremia, ceftobiprole, daptomycin, double-blind, infective endocarditis, noninferiority, Phase III, protocol, randomized, Staphylococcus aureus
Staphylococcus aureus is a common cause of bacteremia and the leading cause of infective endocarditis (IE) in the industrialized world [1,2]. Recent reviews have suggested an incidence of S. aureus bacteremia (SAB) and IE of 16–41 and 3.5–16.6, respectively, per 100,000 person-years [1,2]. S. aureus accounts for 15–40% of IE cases [1,2], and this proportion is increasing [1]. In adults, 30-day mortality from SAB is estimated at 15–25% [2], and in-hospital mortality for all causes of IE attributable to S. aureus is 22–66% [1]. Moreover, methicillin-resistant S. aureus (MRSA) bacteremia is associated with a significantly higher mortality rate than methicillin-susceptible S. aureus (MSSA) bacteremia [3–5], with a recent large, observational cohort study reporting mortality rates of 26 versus 13% (MRSA vs MSSA) in 2013–2015 [4].
Despite the considerable health burden associated with SAB, there are limited antibiotic treatment options available, particularly for patients with MRSA bacteremia. Furthermore, there remains a lack of robust, high-quality evidence supporting selection of antimicrobial agents, with few randomized, controlled studies in this condition [6,7]. Reports of an increasing prevalence of MRSA isolates with reduced susceptibility and poorer responses to agents such as vancomycin and daptomycin [8–11] further highlight the need for new antibacterial options for SAB.
Ceftobiprole, the active moiety of the prodrug ceftobiprole medocaril, is an advanced-generation, broad-spectrum cephalosporin β-lactam agent that has a rapid bactericidal effect against both MSSA and MRSA infections [12,13], and activity covering isolates of streptococci (including penicillin-resistant pneumococci), Enterococcus faecalis, Haemophilus influenzae, Moraxella catarrhalis, nonextended-spectrum β-lactamase-producing Enterobacteriaceae, and susceptible Pseudomonas aeruginosa [14,15]. It is currently approved in many European and non-European countries for the treatment of community- and hospital-acquired pneumonia (excluding ventilator-associated pneumonia) [16].
The purpose of the current trial is to evaluate the efficacy and safety of ceftobiprole for the treatment of SAB, including IE, in a randomized, double-blind trial.
Introduction to the trial
This Phase III study entitled ‘A randomized, double-blind, multicenter study to establish the efficacy and safety of ceftobiprole medocaril compared to daptomycin in the treatment of Staphylococcus aureus bacteremia, including infective endocarditis’ (clinicaltrials.gov identifier NCT03138733; EudraCT number 2017-001699-43; sponsored by Basilea Pharmaceutica International Ltd) is designed to demonstrate the noninferiority of ceftobiprole to daptomycin in the treatment of adult patients with SAB, including IE [17]. Given that a previous trial of daptomycin featured an open-label design [8], this represents the first double-blind, randomized, controlled, Phase III, registrational study conducted in this setting [7]. The first patient was enrolled on 29 August 2018, and recruitment is ongoing [17].
Background & rationale
Rationale for investigating ceftobiprole in SAB, including IE
Standard-of-care antibacterial treatments for SAB include vancomycin, daptomycin and several β-lactam antimicrobials [7,18,19]. For MRSA, only vancomycin and daptomycin are approved for the treatment of SAB and IE (right-sided IE for daptomycin) [20]. Ceftaroline fosamil is currently the only β-lactam antibiotic used to treat MRSA bacteremia, and this is in an off-label capacity with limited clinical data to guide optimal dosing [21,22]. To date, only a single registrational study has been completed in SAB [8]. In this trial, daptomycin was shown to be noninferior to standard-of-care and was effective against both MSSA and MRSA bacteremia.
One of the key limitations of established β-lactams in the treatment of SAB is their lack of activity against MRSA. Conversely, vancomycin is not as effective as β-lactams against MSSA [7]. With bactericidal activity against both MSSA and MRSA [12,13], ceftobiprole has the potential to address this shortcoming. Several studies in animal models have demonstrated the potency of ceftobiprole against IE caused by S. aureus and suggested the potential for superior outcomes compared with daptomycin (cardiac vegetations), and vancomycin and linezolid (cardiac vegetations, and spleen and kidney foci) in this setting [23–26].
Rationale for choosing daptomycin as a comparator
Daptomycin represents a logical choice of comparator for a study that includes both MSSA and MRSA bacteremia. It has a novel mechanism of action distinct from β-lactams and vancomycin, and provides similar overall clinical success rates against both MSSA and MRSA [7,8,27–30]. Daptomycin is approved for the treatment of SAB and right-sided IE in the USA and many countries worldwide, thereby allowing a global study of the treatment of SAB to be conducted. The pivotal trial for daptomycin in this setting used an open-label design, which was essential given that the standard-of-care treatment at that time varied according to the susceptibility of the causative isolate (antistaphylococcal penicillin for MSSA; vancomycin for MRSA) [1,7,8,18,19]. Selecting daptomycin as the sole standard-of-care comparator for our study enables a double-blind design, a first-in-kind advance for Phase III trials in SAB.
Rationale for study design
An added benefit of the double-blind design is that it allows for a postrandomization ascertainment (within a protocol-defined 7-day window) of underlying complications of the SAB without introducing potential bias. To maximize the generalizability of the study to clinical practice, patients with a broad range of complicated SAB are eligible for enrollment, including patients on chronic hemodialysis, patients with persistent SAB (i.e., documented failure of bloodstream clearance after prior appropriate antibiotic treatment), and patients with polymicrobial infection. In the pivotal study with daptomycin, none of these groups were included [8]. Patients with bacteremia due to either MSSA or MRSA are eligible for enrollment. The study does not use susceptibility as a stratification factor, as this information is not usually available at the baseline visit [7]; however, subgroup analyses based on the baseline pathogen (i.e., MSSA vs MRSA) are prespecified.
To reduce the potential heterogeneity of the study population, the study focuses on complicated forms of SAB (as defined by any of the inclusion criteria 6–9, Table 1). Complicated SAB is associated with the greatest risk of a poor outcome [5], and it represents the subset of SAB for which there is a paucity of good quality clinical trial data to guide treatment and a pressing need for new antibacterial options. Given the potential challenges in differentiating between complicated and uncomplicated SAB at study enrollment [7], the double-blind study design allows for a 7-day window to confirm complicated disease, and accommodates the unintentional enrollment of patients with uncomplicated SAB.
Table 1. . Inclusion and exclusion criteria for enrollment in Part 1.
| Inclusion criteria |
| All of the following: |
| 1. Male or female aged ≥18 years. |
| 2. Provision of informed consent. |
| 3. SAB, based on ≥1 positive blood culture: identified by culture laboratory report; or positive diagnostic test for Staphylococcus aureus obtained from a blood culture. |
| 4. At least one of the following signs or symptoms of bacteremia:† a. Fever >38°C/100.4°F measured orally‡; b. White blood cell count >10.0 × 109/l or <4.0 × 109/l, or >10% immature neutrophils; c. Tachycardia, defined as heart rate >90 bpm; or d. Hypotension, defined as systolic blood pressure <90 mmHg |
| 5. Required duration of study antibacterial treatment ≤28 days. |
| At least one of the following§: |
| 6. SAB in patients undergoing chronic intermittent hemodialysis or peritoneal dialysis. |
| 7. Persistent SAB with documented failure of bloodstream clearance, defined as a positive blood culture for S. aureus after prior appropriate antistaphylococcal treatment (except failure under daptomycin therapy) of ≥3 complete days. |
| 8. Other forms of complicated SAB, including:
a. Acute bacterial skin and skin structure infection; or b. Metastatic infection of native tissue requiring ≤28 days of study antibacterial treatment, including but not limited to: i. Septic arthritis or bacterial joint infection/empyema; ii. Septic or suppurative thrombophlebitis; iii. Visceral soft-tissue abscesses; or iv. Septic pulmonary emboli/infarction¶. |
| 9. Definitive native-valve right-sided IE by Modified Duke Criteria [31], if both of the following conditions are met: absence of clinical and/or radiological evidence of cerebral foci; and absence of other complications requiring ≥28 days of antibacterial treatment. |
| Exclusion criteria (any of the following at screening) |
| • Treatment with a potentially effective antistaphylococcal systemic antibacterial treatment for >48 h within 7 days before randomization#.
• Bloodstream or nonbloodstream concomitant infections with Gram-negative bacteria that are known at screening to be nonsusceptible to either ceftobiprole or aztreonam. • Confirmed uncomplicated SAB, in other, none of inclusion criteria 6–9. • Left-sided IE. • Prosthetic cardiac valves or valve support rings, cardiac pacemakers, automatic implantable cardioverter-defibrillator, or left-ventricular assist devices. • Complicated SAB in patients with intra- or extra-vascular foreign body material that cannot be removed within the 7 days after randomization, except patients with: a. Noninfected coronary stents; b. Noninfected prosthetic joints, plates, spinal hardware, or other extravascular material (if implanted ≥60 days before randomization); c. Noninfected intravascular prosthetic material or vena cava filters (if implanted ≥90 days before randomization). • Cardiac native-valve surgery planned within 3 days of randomization. • Community-or hospital-acquired pneumonia. • Osteomyelitis, including vertebral, sternal or long-bone osteomyelitis. • Epidural or cerebral abscess. • High probability of death within 7 days due to the underlying SAB or SAB-associated disease, or within 28 days from an unrelated underlying disease. • Clinically-relevant hypersensitivity to β-lactam antibacterials or daptomycin. • Known infection due to S. aureus that exhibits reduced susceptibility to daptomycin (MIC >1 mg/l) or ceftobiprole (MIC >2 mg/l). • Absolute neutrophil count <0.5 × 109/l. • History of opportunistic infections within 30 days prior to randomization, where underlying cause is still active (e.g., leukemia or transplant). • CD4 count <100 cells/mm3 in patients with AIDS, or treatment with cotrimoxazole as prophylaxis for pneumocystis pneumonia. • Expected requirement during the study for effective systemic antibacterial treatment unrelated to treatment of SAB (e.g., in the context of planned surgery) or other anticipated use of antibacterials (e.g., for treatment of acne vulgaris). • Requirement for continuous renal replacement therapy at randomization, or high likelihood of requirement for such therapy during the study. • ALT or AST levels ≥8 x ULN, or severe hepatic disease with Child-Pugh class C. • Women who are pregnant or nursing, or who are of childbearing potential and unwilling to use an acceptable method of birth control during the study. • Use of an investigational drug in a Phase I study within 30 days of the start of study treatment. |
Inclusion criteria 1–7 must be ascertained based on assessments within the 72 h screening window. For inclusion criteria 8 and 9, assessments performed up to 7 days before randomization may be used to confirm the diagnosis of complicated SAB.
May be based on measurements obtained before or after informed consent, but within 72 h prior to randomization.
Or >38.5°C/101.3°F measured tympanically, >37.5°C/99.5°F measured by axillary method, or >39°C/102.2°F measured rectally.
Patients randomized with suspected complicated SAB who turn out not to meet one of these four criteria will be considered to have uncomplicated SAB, and will continue in the study.
Diagnosis must be based on radiological signs assessed using contrast-enhanced (preferred) or noncontrast-enhanced CT, or x-ray for those unable to undergo a CT scan.
An exception to this criterion will be made for patients with documented failure of bloodstream clearance, defined as a positive blood culture for S. aureus within the 72 h prior to randomization after prior appropriate antistaphylococcal treatment (except failure under daptomycin therapy) administered for at least 3 complete days.
CD4: Cluster of differentiation 4; CT: Computerized tomography; IE: Infective endocarditis; MIC: Minimum inhibitory concentration; SAB: S. aureus bacteremia; ULN: Upper limit of normal.
The primary end point, overall success at the post-treatment evaluation (PTE) visit (day 70 ± 5 days postrandomization), is similar to that used in the pivotal Phase III study for daptomycin [8], and consists of all of the following requirements: survival; absence of development of new SAB-related metastatic foci or complications; resolution of SAB-related symptoms; and microbiological eradication. Further details on the primary end point are provided in the outcome measures/end points section.
Given the often life-threatening nature of complicated SAB, a placebo-controlled design would clearly be unethical. Daptomycin was chosen as the comparator agent for the reasons provided in the previous subsection. In order to reflect contemporary clinical practice and treatment guidelines [28,29,32,33], study sites retained the option to administer daptomycin at doses exceeding the US FDA-approved daily dose of 6 mg/kg, as per local standard of care.
Design
Study design
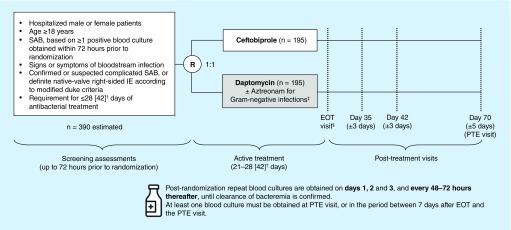
This is a randomized, double-blind, double-dummy, active-controlled, parallel-group, multicenter, two-part study in approximately 390 patients (Figure 1). In Part 1, treatment is administered for 21–28 days in the first 80 patients enrolled, to confirm that an extended duration of ceftobiprole does not increase the risk of convulsions. After an interim safety analysis and appropriate protocol amendment, the maximum duration of therapy may then be extended for up to 42 days (Part 2). The study is being conducted in accordance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice guidelines, the principles of the Declaration of Helsinki, and any applicable local laws and regulations. The protocol has been approved by the relevant Independent Ethics Committees and Institutional Review Boards, prior to the start of the study, as well as the FDA under a Special Protocol Assessment.
Figure 1. . Study design.
†Patients in Part 1 (N = 80) will receive active treatment for 21–28 days. For Part 2, maximum duration of therapy may be extended to 42 days, pending the outcome of the interim safety analysis and implementation of a protocol amendment.
‡Patients in the ceftobiprole group with Gram-negative infections receive placebo to maintain blinding.
§EOT visit to be conducted within 72 h of last study-drug administration.
EOT: End of treatment; IE: Infective endocarditis; PTE: Post-treatment evaluation; R: Randomization; SAB: Staphylococcus aureus bacteremia.
Eligibility criteria
Hospitalized male or female patients aged ≥18 years who have SAB, based on ≥1 positive blood culture obtained within 72 h prior to randomization, with signs or symptoms of bloodstream infection, and a requirement for ≤28 days (Part 1) of antibacterial treatment are eligible for the study. Full inclusion and exclusion criteria are detailed in Table 1.
Study populations
The following populations will be considered for the study:
Intent-to-treat (ITT) population: all randomized patients, analyzed according to the study medication assigned at randomization;
Modified ITT (mITT) population: a subset of the ITT population, including patients who received any dose of study medication and who have a blood culture positive for S. aureus at baseline, based on central microbiology laboratory assessment (or who have documented evidence of a positive culture at a local laboratory);
Clinically evaluable population: a subset of the mITT population, including patients who have no major protocol deviations and a completed primary outcome assessment;
Safety population: all randomized patients who received any dose of study medication, analyzed based on the first drug actually received;
Pharmacokinetic (PK) population: all patients who received ≥1 dose of ceftobiprole and have ≥1 plasma concentration measurement.
Planned sample size
The sample size has been estimated based on: a point estimate for overall success of 40% in each treatment group in the mITT population; a one-sided alpha level of 0.025; power of >80%; and a noninferiority margin of 15% for the between-group difference in the primary end point. The assumption of an overall success rate of 40% in each treatment group is based on the success rates in the Phase III study of daptomycin compared with standard-of-care treatment in patients with SAB including IE [8]. With these assumptions, enrollment of 350 patients (175 per group) is required for the mITT population. Based on the previously mentioned daptomycin Phase III study [8], it is assumed that approximately 90% of patients in the ITT population will have confirmed SAB and will be included in the mITT population, and therefore a total of 390 patients (195 per group) will need to be randomized and receive treatment. This sample size will provide ≥80% power to reject the null hypothesis (H0) against the alternative hypothesis (HA) at the one-sided alpha level of 0.025 as follows, using a two-group large-sample normal approximation test of proportions:
Planned study period
The total duration of patient participation in the study, including follow-up, is approximately 10 weeks after randomization. The study consists of three phases: screening of potentially eligible patients, conducted up to 72 h prior to randomization; randomization and subsequent active treatment (up to 28 days in Part 1) with intravenous study drug; and post-treatment, comprising an end-of-treatment (EOT) visit within 72 h of the last study drug administration and visits on day 35 (±3 days), day 42 (±3 days) and day 70 (±5 days; PTE visit).
Study procedures
Eligible patients are randomized 1:1 to treatment with ceftobiprole or daptomycin based on a computer-generated randomization schedule obtained via an interactive web response system. Randomization is stratified by study site, hemodialysis status and prior antibacterial treatment use (defined as use of any potentially effective systemic antibacterial treatment within 7 days of randomization). After randomization and during active treatment, patients receive intravenous infusions of either ceftobiprole or daptomycin, administered as described in Table 2. The target treatment duration in Part 1 is 21–28 days of study medication, with a maximum treatment duration of 28 days. To maintain blinding, patients in each treatment group also receive dummy infusions of physiological saline (0.9% NaCl) matching the active treatment allocated to the other treatment group.
Table 2. . Study drug administration.
| Study day | Duration of infusion | Normal renal function to mild renal impairment (CLCr ≥50 ml/min) | Renal impairment (nondialysis) | Intermittent hemodialysis or peritoneal dialysis |
|---|---|---|---|---|
| Ceftobiprole | ||||
| Days 1–8 | 2 h intravenous infusion | 500 mg q6h | CLCr 30 – <50 ml/min: 500 mg q8h
CLCr <30 ml/min: 250 mg q8h |
250 mg q24h |
| Day 9 onwards | 2 h intravenous infusion | 500 mg q8h | CLCr 30 – <50 ml/min: 500 mg q12h
CLCr <30 ml/min: 250 mg q12h |
250 mg q24h |
| Daptomycin† | ||||
| Day 1 onwards | 0.5 h intravenous infusion | 6 mg/kg q24h | 6 mg/kg q48h | 6 mg/kg q48h |
Blinded aztreonam (with daptomycin) or placebo (with ceftobiprole) may be used for coverage of Gram-negative infections.
In accordance with institutional standards, an increase in the dose of daptomycin (up to 10 mg/kg) may be implemented.
CLCr: Creatinine clearance (based on the Cockcroft–Gault formula); q6/8/12/24/48h: Every 6/8/12/24/48 h.
Concomitant use of nonstudy drug systemic antibacterials with activity against S. aureus is prohibited from randomization up to the PTE visit. Patients in either treatment group may receive concomitant nitrofurantoin, metronidazole or oral vancomycin, as required. Patients in the daptomycin group may receive aztreonam for Gram-negative infections (i.e., for polymicrobial bloodstream infections or Gram-negative nonbloodstream infections) using a standard-dose regimen at the study site, while patients in the ceftobiprole group who require coverage against such infections receive placebo to maintain blinding. In such cases, aztreonam or placebo is administered by an unblinded site pharmacist. All investigators and other study-site staff will remain blinded until the database has been locked for final analysis.
Blood cultures positive for S. aureus must be drawn within 72 h prior to randomization for patient eligibility. Two sets of blood cultures are obtained from each patient at baseline, and postrandomization repeat blood cultures are obtained on days 1, 2 and 3, and every 48–72 h thereafter, until clearance of bacteremia is confirmed. At least one blood culture must be obtained either at PTE, or in the period between 7 days after EOT and the PTE visit. Blood cultures are processed at the local laboratory. All unique organisms isolated from blood are later sent to a central microbiology laboratory for identification using matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry [34], and for susceptibility testing according to Clinical Laboratory Standards Institute methodology for both drugs [35]. Modified Duke Criteria [31] are used to define the presence of definite IE in all study patients, and are assessed in all patients at screening, EOT and PTE. Patients without definite IE are assessed at all visits from day 2 onwards. For patients with definite IE at screening, standard 2D or 3D transthoracic and/or transesophageal echocardiography performed within 10 days prior to randomization may be used to confirm eligibility. In these cases, echocardiography must be repeated within 72 h before, or 7 days after, randomization. For patients without definite IE at screening, echocardiography must be performed within 72 h prior to randomization.
Overall success is assessed by investigators at each visit from day 3 to the PTE visit, and by an independent Clinical Events Classification (CEC) committee (see following end points section for definition of overall success). Safety assessments, including adverse event monitoring, physical examination, vital signs, safety laboratory tests, and other assessments, will be performed regularly throughout the study. PK sampling is performed three times per day on days 3 and 12 for all patients, with an additional two samples taken on day 3 in a subset of 40 patients selected for richer PK sampling.
Outcome measures/end points
Primary & secondary end points
The primary objective of the study is to demonstrate noninferiority of ceftobiprole to daptomycin for overall success in patients with SAB, as assessed by the independent CEC committee at the PTE visit in the mITT population (primary end point). Detailed criteria for overall success and treatment failure are shown in Table 3. Secondary end points (Table 3) were selected based on clinical relevance, and include individual components of the primary end point such as all-cause mortality, microbiological eradication and development of new metastatic lesions, as well as an assessment of the primary end point in additional analysis populations.
Table 3. . Study end points.
| Primary end point (overall success) criteria | Secondary end points |
|---|---|
| Overall success is defined as all of the following: | • All-cause mortality at day 70 (±5 days) in the mITT population
• Microbiological eradication at day 70 (±5 days) in the mITT population • Overall success rate at day 70 (±5 days) in the CE population • Development of new metastatic foci or other complications of SAB after day 7 in the mITT population • Time to S. aureus bloodstream clearance in the mITT and CE populations • Safety and tolerability in the safety population, in terms of the incidence, type and severity of adverse events, and relationship to study medication, and changes in laboratory tests; and • Pharmacokinetics of ceftobiprole in the PK population |
| • Patient alive at day 70 (±5 days) postrandomization
• No new metastatic foci or complications of the SAB infection† • Resolution or improvement of SAB-related clinical signs and symptoms • Two negative blood cultures for Staphylococcus aureus, with no subsequent positive blood culture for S. aureus - ≥1 negative blood culture must be recorded while on active study treatment - Cultures must be confirmed by ≥1 subsequent negative blood culture for S. aureus at day 70 (±5 days) or between 7 days after the EOT visit and day 70 (±5 days) | |
| Treatment failure is defined as any of the following: | |
| • Premature discontinuation of study treatment due to lack of efficacy (as judged by the CEC committee), or for adverse events representing disease progression or relapse
• Development of new metastatic or other complications related to SAB between day 8 and PTE visit • SAB relapse or reinfection, based on evidence from a blood culture positive for S. aureus between EOT and PTE visits • Receipt of systemic nonstudy antibacterial treatments for SAB, other than those permitted under the protocol • Treatment of infections, other than SAB, with systemic nonstudy antibacterial treatment that is potentially effective against S. aureus and is considered by the CEC committee to have a relevant impact on the primary end point • Death for any reason • Indeterminate outcome (e.g., due to missing data, patients lost to follow-up or failure to meet the criteria for success or failure) • Requirement for longer systemic antibacterial treatment for SAB than is allowed by the protocol |
Foci identified from day 8 onwards are considered to be new foci.
CE: Clinically evaluable; CEC: Clinical events classification; EOT: End-of-treatment; mITT: Modified intent-to-treat; PK: Pharmacokinetic; PTE: Post-treatment evaluation.
SAB: S. aureus bacteremia.
Noninferiority margin
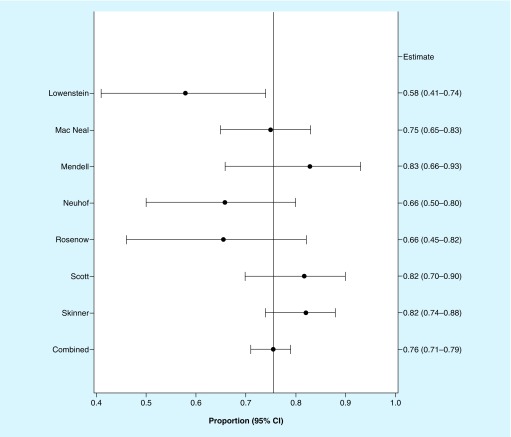
The primary end point will be tested for noninferiority of ceftobiprole versus daptomycin using a margin of 15%. To determine an appropriate noninferiority margin, it was first necessary to quantify the effect of the active control compared with placebo. Given the lack of placebo-controlled studies, literature from the pre-penicillin era was reviewed to determine the clinical outcome of patients with SAB who did not receive antibacterial treatments [36–42]. Because overall success was not reported in these studies, survival was considered as success. A random effects meta-analysis of the mortality data from these studies provided a conservative estimate of 71% mortality in untreated patients with SAB, based on the lower bound of the 95% CI (Figure 2).
Figure 2. . Random effects meta-analysis of clinical outcome (all-cause mortality) in untreated patients with Staphylococcus aureus bacteremia.
Seven original articles [36–42] were identified that described outcomes in case series of SAB in patients who did not receive antibacterial treatments. These studies were included in a random effects meta-analysis, which suggests an all-cause mortality of 76%. The lower bound of the 95% CI for this mortality estimate is 0.71, suggesting a conservative estimate of 71% mortality for patients with SAB who did not receive antibacterial treatment.
CIs were calculated using the Clopper Pearson formula.
SAB: Staphylococcus aureus bacteremia.
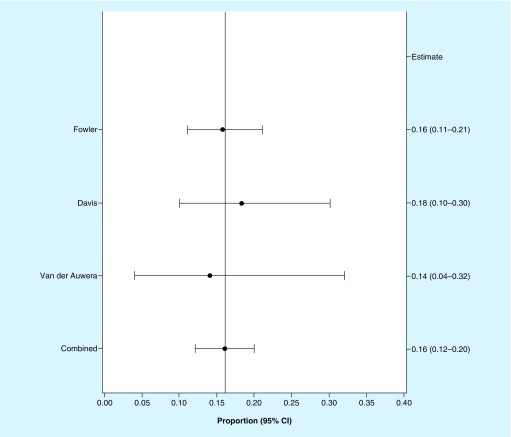
Mortality outcomes for SAB patients treated with an effective antibacterial treatment were estimated based on a separate random effects meta-analysis of three contemporary randomized studies with a variety of antibacterials, including the pivotal study with daptomycin [8,43,44]. This analysis indicated an all-cause mortality rate of 16% (95% CI: 0.12–0.20), with the upper bound of the CI suggesting an estimate of up to 20% mortality among patients with SAB who receive antibacterial treatment (Figure 3). Based on the all-cause mortality rates in untreated and treated patients, the effect of active control compared with placebo was estimated to be 51%. As it was not possible to estimate the effect size for an overall success end point, it was assumed that the effect size for an overall success end point would, like that of mortality, be very large (i.e., approximately 50%).
Figure 3. . Random effects meta-analysis of clinical outcome (all-cause mortality) in patients with Staphylococcus aureus bacteremia who receive antibacterial treatment.
Three original articles [8,43,44] were identified that described outcomes of SAB patients treated with antibacterial treatment in randomized controlled trials published since 1985. These studies were included in a random effects meta-analysis, which suggests an all-cause mortality of 16%. The upper bound of the 95% CI for this mortality estimate is 0.20, suggesting a conservative estimate of 20% mortality for patients with SAB who receive antibacterial treatment.
CIs were calculated using the Clopper Pearson formula.
SAB: S. aureus bacteremia.
Determining the largest clinically acceptable difference of a test drug compared with the active control for noninferiority is primarily based on clinical judgement. We postulated that a noninferiority margin of ≤20% could be considered to be clinically reasonable, given that this margin would preserve over half the previously described estimated active-control treatment effect over untreated patients, and also this was the margin used in the pivotal study that supported the regulatory approval of daptomycin for SAB [8]. To further explore the performance of noninferiority margins of ≤20%, the maximum allowable difference in the point estimate of overall response that would allow conclusion of noninferiority was calculated assuming: overall response rates of 40 or 50% in the active-control group [8]; noninferiority margins of 15, 17.5 or 20%; and mITT populations of 250–350 patients. Noninferiority margins of 15, 17.5 and 20% were calculated to allow a difference in the point estimate between ceftobiprole and daptomycin of <5, 5–7 and 8–10%, respectively. A noninferiority margin that allows a maximum difference in the point estimates for overall success with ceftobiprole versus daptomycin of <5% is, in our opinion, clinically justifiable, and therefore a margin of 15% was selected.
CEC committee
The CEC committee consists of a group of independent clinical experts, blinded to treatment allocation and not associated with study conduct. Data obtained for all patients who receive study medication will be reviewed independently by two CEC committee members. The CEC committee members will give their opinion on the diagnosis and classification of the SAB and the response assessment (overall success or failure) at the PTE visit. In addition, for deaths occurring during the study, a blinded assessment of the attribution of death to SAB or to another cause will be performed by the CEC committee members. Significant disagreements between the two reviewers will be adjudicated by the full committee.
Statistics
Primary end point analysis
The primary efficacy analysis will be based on the mITT population. The observed difference in the rates of overall success between the two treatment groups at day 70 ± 5 days (ceftobiprole group minus daptomycin group) will be determined and a two-sided 95% CI will be computed, with adjustment for geographical region, dialysis status and prior antibacterial treatment use. Cochran–Mantel–Haenszel weights will be used for the stratum weight in the calculation of the CI.
For assessing the noninferiority of ceftobiprole to daptomycin, a one-sided hypothesis test will be performed at the 2.5% level of significance. If the lower limit of the two-sided 95% CI for the difference in response rates in the mITT population is greater than −15%, noninferiority will be concluded. Analyses using risk ratios and odds ratios will also be performed. Subgroup analyses will be conducted for the primary efficacy outcome, including factors such as demographic characteristics, geographic region, baseline pathogen (i.e., MRSA vs MSSA), baseline SAB type, and antibacterial use prior to study drug.
Secondary end point analysis
Two-sided 95% CIs will be calculated for the observed difference between ceftobiprole and daptomycin for secondary end points, assessing all-cause mortality, microbiological eradication, overall success and development of new metastatic foci or other complications of SAB, with a similar approach to the primary end point. In addition, time-to-event analyses will be performed for all-cause mortality and time to S. aureus bloodstream clearance. Safety, tolerability and laboratory data will be summarized with descriptive statistics. Ceftobiprole plasma concentration data will be analyzed at each timepoint and also presented with descriptive statistics.
Interim analyses
At a minimum, interim safety analysis will be performed by an independent Data and Safety Monitoring Board after enrollment of 60 patients receiving ≥21 days of study treatment; 80 patients receiving ≥21 days of study treatment (completion of Part 1 of the study; dependent on the outcome of the interim safety analysis of 60 patients); and 200 and 300 patients. After completion of Part 1 of the study, the Data and Safety Monitoring Board will review the unblinded safety data and determine whether: the study can continue, with or without additional safety measures; the maximum study drug treatment duration can be extended from 28 to 42 days after an appropriate protocol amendment; and certain disease presentations requiring longer antibiotic treatment (e.g., osteomyelitis) that were excluded in Part 1 may be allowed in Part 2.
Conclusion
This double-blind study will establish whether ceftobiprole is noninferior to daptomycin in the treatment of complicated SAB, including IE. If noninferiority is established, ceftobiprole may become an important new treatment option in the antibiotic armamentarium, with activity against SAB caused by either MSSA or MRSA.
Executive summary.
There are limited antibiotic options available for the treatment of Staphylococcus aureus bacteremia (SAB), particularly for patients with methicillin-resistant S. aureus (MRSA) bacteremia.
There is also a lack of robust, high-quality evidence supporting the selection of antimicrobial agents, with few randomized, controlled studies in SAB.
Ceftobiprole is an advanced-generation, broad-spectrum cephalosporin β-lactam agent that has a rapid bactericidal effect against both methicillin-susceptible S. aureus (MSSA) and MRSA infections.
Ceftobiprole is currently approved in many European and non-European countries for the treatment of community- and hospital-acquired pneumonia (excluding ventilator-associated pneumonia).
Study design
This is the first double-blind, randomized, controlled, Phase III, registrational study in SAB.
The primary objective of the study is to determine whether ceftobiprole is noninferior to daptomycin for overall success in patients with SAB, including infective endocarditis.
Anticipated enrollment is 390 hospitalized adult patients who have SAB, based on ≥1 positive blood culture obtained within 72 h prior to randomization, with signs or symptoms of bloodstream infection and a requirement for ≤28 days of antibacterial treatment.
The primary end point will be overall success at the post-treatment evaluation visit (day 70 ± 5 days), defined as all of the following being met: survival; no new SAB-related metastatic foci or complications; resolution or improvement of SAB-related clinical signs and symptoms; and microbiological eradication.
Conclusion
Establishing ceftobiprole as noninferior to daptomycin would expand the range of treatment options against bacteremia caused by either MSSA or MRSA.
Acknowledgments
The authors would like to thank the patients, investigators and study site staff who are participating in this study.
Footnotes
Supplementary data
An infographic accompanies this paper at the end of the references section. To download the infographic that accompanies this paper, please visit the journal website at: www.future-science.com/doi/suppl/10.2217/fmb-2019-0332
Financial & competing interests disclosure
The study is funded in part with federal funds from the Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response and Biomedical Advanced Research and Development Authority (BARDA), under Contract No. HHSO100201600002C, and in part by Basilea Pharmaceutica International Ltd, Basel, Switzerland. K Hamed, M Engelhardt, ME Jones and M Saulay are employees of Basilea Pharmaceutica International Ltd. TL Holland is a scientific advisory board member for Motif Bio, and consultant for Basilea Pharmaceutica, Genentech, Motif Bio, Roivant and Theravance. H Seifert has received grants or research support from Accelerate, Cubist, Entasis, German Research Foundation (DFG), German Centre for Infection Research (DZIF), Novartis and Tetraphase; is a consultant for Basilea Pharmaceutica, Entasis, Genentech, MSD, Roche, Shionogi and Tetraphase; and has received payments for lectures from Gilead and Merck/MSD. VG Fowler Jr has received grants or research support from Advanced Liquid Logics, Affinergy, Basilea Pharmaceutica, Cerexa/Forest/Actavis/Allergan, ContraFect, Cubist/Merck, Genentech, Karius, Locus, Medical BioSurfaces, MedImmune, Novartis, Pfizer, Regeneron and Theravance; is a consultant for Achaogen, Affinergy, Affinium, Basilea Pharmaceutica, Bayer, Cerexa, ContraFect, Cubist, Debiopharm, Destiny, Durata, Galderma, Genentech, Janssen, Medicines Co., MedImmune, NovaDigm, Novartis, Pfizer, Regeneron, Tetraphase, Theravance, Trius and xBiotech; is Merck Co-Chair for V710 Vaccine; and has received educational fees from Cerexa, Cubist, Debiopharm, Durata, Green Cross and Theravance. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing support, under the direction of the authors, was provided by Stephanie Carter of Spirit Medical Communications Group Ltd, funded by Basilea Pharmaceutica International Ltd.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1. Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG., Jr Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management . Clin. Microbiol. Rev. 28(3), 603–661 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asgeirsson H, Thalme A, Weiland O. Staphylococcus aureus bacteraemia and endocarditis – epidemiology and outcome: a review . Infect. Dis. (Lond.) 50(3), 175–192 (2018). [DOI] [PubMed] [Google Scholar]; • A recent review of the epidemiology and burden of disease assocated with Staphylococcus aureus bacteremia (SAB).
- 3. Bassetti M, Peghin M, Trecarichi EM. et al. Characteristics of Staphylococcus aureus bacteraemia and predictors of early and late mortality. PLoS ONE 12(2), e0170236 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Austin ED, Sullivan SS, Nacesic N. et al. Reduced mortality of Staphylococcus aureus bacteremia in a retrospective cohort study of 2139 patients: 2007–2015. Clin. Infect. Dis. (2019) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. Predictors of mortality in Staphylococcus aureus bacteremia. Clin. Microbiol. Rev. 25(2), 362–386 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thwaites GE, Edgeworth JD, Gkrania-Klotsas E. et al. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect. Dis. 11(3), 208–222 (2011). [DOI] [PubMed] [Google Scholar]; • Discusses the evidence behind the key clinical decisions in the management of SAB, the priorities for immediate and long-term treatment and the direction of future research.
- 7. Holland TL, Chambers HF, Boucher HW. et al. Considerations for clinical trials of Staphylococcus aureus bloodstream infection in adults. Clin. Infect. Dis. 68(5), 865–872 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reviews the challenges in designing and executing interventional clinical trials for SAB, and provides recommendations for improvements in trial design.
- 8. Fowler VG, Jr, Boucher HW, Corey GR. et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus . N. Engl. J. Med. 355(7), 653–665 (2006). [DOI] [PubMed] [Google Scholar]; •• Documents the design and execution of the open-label trial establishing daptomycin as a treatment option for SAB.
- 9. Sharma M, Riederer K, Chase P, Khatib R. High rate of decreasing daptomycin susceptibility during the treatment of persistent Staphylococcus aureus bacteremia. Eur. J. Clin. Microbiol. Infect. Dis. 27(6), 433–437 (2008). [DOI] [PubMed] [Google Scholar]
- 10. Gasch O, Camoez M, Dominguez MA. et al. Emergence of resistance to daptomycin in a cohort of patients with methicillin-resistant Staphylococcus aureus persistent bacteraemia treated with daptomycin. J. Antimicrob. Chemother. 69(2), 568–571 (2014). [DOI] [PubMed] [Google Scholar]
- 11. Zhang S, Sun X, Chang W, Dai Y, Ma X. Systematic review and meta-analysis of the epidemiology of vancomycin-intermediate and heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. PLoS ONE 10(8), e0136082 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liapikou A, Cilloniz C, Torres A. Ceftobiprole for the treatment of pneumonia: a European perspective. Drug Des. Devel. Ther. 9, 4565–4572 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morosini MI, Diez-Aguilar M, Canton R. Mechanisms of action and antimicrobial activity of ceftobiprole. Rev. Esp. Quimioter. 32(Suppl. 3), 3–10 (2019). [PMC free article] [PubMed] [Google Scholar]
- 14. Giacobbe DR, De Rosa FG, Del Bono V. et al. Ceftobiprole: drug evaluation and place in therapy. Expert Rev. Anti. Infect. Ther. 17(9), 689–698 (2019). [DOI] [PubMed] [Google Scholar]
- 15. Pfaller MA, Flamm RK, Mendes RE. et al. Ceftobiprole activity against Gram-positive and -negative pathogens collected from the United States in 2006 and 2016. Antimicrob. Agents Chemother. 63(1), pii:e01566–e01518 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrates that cetfobiprole remains highly active in vitro against a large number of pathogens associated with serious infections compared with results from a decade ago.
- 16. Zevtera 500 mg powder for concentrate for solution for infusion (2019). https://www.medicines.org.uk/emc/product/9164
- 17. Ceftobiprole in the treatment of patients with Staphylococcus aureus bacteremia – NCT03138733 (2019). https://clinicaltrials.gov/ct2/show/NCT03138733
- 18. Hassoun A, Linden PK, Friedman B. Incidence, prevalence, and management of MRSA bacteremia across patient populations-a review of recent developments in MRSA management and treatment. Crit. Care 21(1), 211 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Habib G, Lancellotti P, Antunes MJ. et al. 2015 ESC Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 36(44), 3075–3128 (2015). [DOI] [PubMed] [Google Scholar]
- 20. Holland TL, Arnold C, Fowler VG., Jr Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 312(13), 1330–1341 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Summarizes the management strategies available for SAB, offers recommendations on the use of echocardiography and identifies the need for high-quality trials in SAB.
- 21. Casapao AM, Davis SL, Barr VO. et al. Large retrospective evaluation of the effectiveness and safety of ceftaroline fosamil therapy. Antimicrob. Agents Chemother. 58(5), 2541–2546 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pani A, Colombo F, Agnelli F. et al. Off-label use of ceftaroline fosamil: a systematic review. Int. J. Antimicrob. Agents 54(5), 562–571 (2019). [DOI] [PubMed] [Google Scholar]
- 23. Chambers HF. Evaluation of ceftobiprole in a rabbit model of aortic valve endocarditis due to methicillin-resistant and vancomycin-intermediate Staphylococcus aureus . Antimicrob. Agents Chemother. 49(3), 884–888 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Entenza JM, Veloso TR, Vouillamoz J, Giddey M, Majcherczyk P, Moreillon P. In vivo synergism of ceftobiprole and vancomycin against experimental endocarditis due to vancomycin-intermediate Staphylococcus aureus . Antimicrob. Agents Chemother. 55(9), 3977–3984 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fernandez J, Abbanat D, Shang W. et al. Synergistic activity of ceftobiprole and vancomycin in a rat model of infective endocarditis caused by methicillin-resistant and glycopeptide-intermediate Staphylococcus aureus . Antimicrob. Agents Chemother. 56(3), 1476–1484 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tattevin P, Basuino L, Bauer D, Diep BA, Chambers HF. Ceftobiprole is superior to vancomycin, daptomycin, and linezolid for treatment of experimental endocarditis in rabbits caused by methicillin-resistant Staphylococcus aureus . Antimicrob. Agents Chemother. 54(2), 610–613 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Documents the superiority of ceftobiprole versus standard therapies in treating experimental endocarditis caused by methicillin-resistant Staphylococcus aureus in rabbits.
- 27. Canepari P, Boaretti M, Lleo MM, Satta G. Lipoteichoic acid as a new target for activity of antibiotics: mode of action of daptomycin (LY146032). Antimicrob. Agents Chemother. 34(6), 1220–1226 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. CUBICIN Prescribing Information (2018). https://www.merck.com/product/usa/pi_circulars/c/cubicin/cubicin_pi.pdf
- 29. Cubicin Summary of Product Characteristics (2019). https://www.ema.europa.eu/en/documents/product-information/cubicin-epar-product-information_en.pdf
- 30. Steenbergen JN, Alder J, Thorne GM, Tally FP. Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive infections. J. Antimicrob. Chemother. 55(3), 283–288 (2005). [DOI] [PubMed] [Google Scholar]
- 31. Li JS, Sexton DJ, Mick N. et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 30(4), 633–638 (2000). [DOI] [PubMed] [Google Scholar]
- 32. Liu C, Bayer A, Cosgrove SE. et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin. Infect. Dis. 52(3), 285–292 (2011). [DOI] [PubMed] [Google Scholar]
- 33. Timbrook TT, Caffrey AR, Luther MK, Lopes V, Laplante KL. Association of higher daptomycin dose (7 mg/kg or greater) with improved survival in patients with methicillin-resistant Staphylococcus aureus bacteremia. Pharmacotherapy 38(2), 189–196 (2018). [DOI] [PubMed] [Google Scholar]
- 34. Angeletti S. Matrix assisted laser desorption time of flight mass spectrometry (MALDI-TOF MS) in clinical microbiology. J. Microbiol. Methods 138, 20–29 (2017). [DOI] [PubMed] [Google Scholar]
- 35. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 23rd Informational supplement M100-S23. Clinical and Laboratory Standards Institute, PA, USA: (2013). [Google Scholar]
- 36. Macneal W, Frisbee F. One hundred patients with Staphylococcus septicemia receiving bacteriophage service. Am. J. Med. Sci. 191, 179–195 (1936). [Google Scholar]
- 37. Lowenstein P. Staphylococcus septicemia . Am. J. M. Sc. 181, 196–203 (1936). [Google Scholar]
- 38. Mendell TH. Staphylococcic septicemia: a review of thirty-five cases, with six recoveries, twenty-nine deaths and sixteen autopsies . AMA. Arch. Intern. Med. (Chic.) 63(6), 1068–1083 (1939). [Google Scholar]
- 39. Neuhof H, Aufses A, Hirshfeld S. Pyogenic sepsis. Surg. Gynecol. Obstet. LVIII, 886–896 (1934). [Google Scholar]
- 40. Rosenow ECJ, Brown AE. Septicemia: a review of cases, 1934–1936 inclusive. Proc. Staff Meet. Mayo Clin. 13, 89–93 (1938). [Google Scholar]
- 41. Scott WJM. The principles of the treatment of septicemia. JAMA 105, 1246–1249 (1935). [Google Scholar]
- 42. Skinner D, Keefer C. Significance of bacteremia caused by Staphylococcus aureus . Arch. Intern. Med. (Chic.) 68, 851–875 (1941). [Google Scholar]
- 43. Davis JS, Sud A, O'sullivan MVN. et al. Combination of vancomycin and beta-lactam therapy for methicillin-resistant Staphylococcus aureus bacteremia: a pilot multicenter randomized controlled trial. Clin. Infect. Dis. 62(2), 173–180 (2016). [DOI] [PubMed] [Google Scholar]
- 44. Van Der Auwera P, Klastersky J, Thys JP, Meunier-Carpentier F, Legrand JC. Double-blind, placebo-controlled study of oxacillin combined with rifampin in the treatment of staphylococcal infections. Antimicrob. Agents Chemother. 28(4), 467–472 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]