Abstract
Purpose:
To investigate the efficacy of a single subconjunctival injection of dendrimer- dexamethasone to treat dry eye in a rabbit model of induced autoimmune dacryoadenitis (AID).
Method:
Dendrimer biodistribution after subconjuntival injection in AID animals was evaluated using Cy5-labelled dendrimer (D-Cy5) and confocal microscopy. Diseased animals were treated with free dexamethasone (Free-Dex), dendrimer-dexamethasone (DDex), or saline via a single subconjunctival injection. The efficacy was evaluated using various clinical evaluations, such as Schirmer’s test, tear breakup time (TBUT), and fluorescein and rose bengal staining. Histopathology was evaluated by H&E staining and immunostaining. Levels of inflammatory cytokines and aquaporin proteins in the LGs were determined by real-time PCR.
Result:
Subconjunctivally administered dendrimers selectively localized in the inflamed LGs, and were taken up by the infiltrated cells. At two weeks post-treatment, the D-Dex group showed improved clinical evaluations. No significant changes were observed in other groups. H&E staining demonstrated, less inflammatory cell infiltration and fewer atrophic acini in D-Dex group, compared to those treated with saline or Free-Dex. Immunohistochemistry demonstrated that the intensity of CD-18 (+) and RTLA (+) was weaker in LGs in the D-Dex group than in other treatment rabbits. Pro-inflammatory gene expression levels of MMP9, IL6, IL8, and TNFα were significantly decreased in the D-Dex group compared to the saline group.
Conclusion:
The dendrimer exhibits pathology-dependent biodistribution in the inflamed LGs. Subconjunctivally administered D-Dex suppressed LG inflammation, leading to partial recovery of LG function with clinical improvement in induced AID. Sjögren’s patients may benefit from this targeted nanomedicine approach.
Keywords: Sjögren’s syndrome, Dry eye, Autoimmune dacryoadenitis, Dendrimer, Dexamethasone, Subconjunctival injection
1. Introduction
Dry eye disease (DED) is a widely prevalent eye disorder affecting 46 million Americans, especially the elderly population [1] [3]. It is a multifactorial disease which causes various degrees of dryness of the eyes and an uncomfortable feeling, blurred vision, ocular surface damage, and even functional blindness [2]. One of the main causes of DED is Sjögren’s syndrome, an autoimmune disease characterized by focal lymphocytic infiltration and severe dysfunction of the exocrine organs, primarily the lacrimal gland (LG) and salivary gland [4–9]. Although the precise etiology of DED remains unclear, it is believed to be a chronic inflammatory disease especially in Sjögren’s syndrome induced DED. A wide range of immunologic, genetic, epigenetic, hormonal, viral, neural, and environmental variables are proposed as being involved in the pathogenesis of this disease, where lymphocytic infiltration of the LG is associated with exocrine insufficiency, leading to functional quiescence and eventual destruction of the secretory parenchyma [10, 11].
Although numerous murine models present with features resembling those of Sjögren’s syndrome in humans, none completely represents the pathophysiological characteristics of this condition [9]. An induced rabbit model of chronic autoimmune dacryoadenitis (AID) for studying the pathogenesis and pathophysiology of DED shares immune features of human Sjögren’s syndrome [12, 13]. Moreover, the rabbit LG exhibits greater similarity to the microanatomical and immunohistological features of the human LG [14]. As there is a precise onset of disease initiation in this inducible model, this animal model offers a good platform to identify and test candidate therapies, and to further explore underlying mechanisms [8, 12, 13, 15].
Current treatment for DEDs is mainly focused on reducing inflammation and restoring tears [11, 16]. Topical anti-inflammatory corticosteroid drops or immunosuppressive drugs such as cyclosporine (CsA) drops are most commonly treatments[11, 17–19]. Although, the aforementioned treatments achieve clinical success in relieving the symptoms and signs of moderate or severe dry eye in Sjögren’s syndrome [11, 15, 19], this treatment requires frequent topical application that often results in corneal irritation, toxicity, and patient noncompliance [20, 21]. Furthermore, very little is known about the topical anti-inflammatory drug treatment’s effect on inflammatory cellular response in LGs. Because the permeability of these topical drugs is not that desirable, it is less likely that a therapeutic concentration of the drugs can be delivered and maintained to the target site in LG. Drug formulations that offer sustained release, targeting, and increased bioavailability are needed to improve efficacy and reduce side effects in DED treatment [22–24].
Hydroxyl poly(amidoamine) (PAMAM) dendrimers have a well-defined structure, small size (~3–10 nm), good safety profile, and high water solubility, which makes them excellent candidates for ocular drug and gene delivery [25]. Numerous studies have examined the role of their unique nanoscale architecture on the delivery of therapeutics (drugs and genes) and imaging agents [25–30]. Due to their “neutral” surface charge and nanoscale size, hydroxylterminated PAMAM dendrimers are noncytotoxic with reduced nonspecific tissue interactions [22, 31, 32]. Systemic and intravitreal hydroxyl-PAMAM dendrimers are readily cleared intact from off-target organs, but are selectively localized and retained in the areas of inflammation in the brain and retina [33–36]. Dendrimer targets activated macrophages in the injured cornea upon subconjunctival administration, suggesting enhanced delivery and availability of corticosteroids (e.g., dexamethasone) to the very cells responsible for corneal inflammation [22].
Based on the targeted anti-inflammatory activity of the subconjunctival dendrimerdexamethasone (D-Dex) conjugate in the corneal alkali burn model [22], we hypothesized that the dendrimer will enhance intracellular delivery to and efficacy of drugs on target cells in the LG, in the rabbit autoimmune dacryoadenitis model. We investigated the efficacy of a single dose of subconjunctivally administered D-Dex in a well-established rabbit model of induced AID, investigated the dendrimer distribution in the LG, and evaluated the efficacy of dendrimer–drug therapy. We expect the dendrimers may potentially enhance the antiinflammatory effects of drugs in treatment of DED.
2. Methods
2.1. Animals and Materials
Female adult New Zealand white rabbits, each weighing between 3–4 kg, were obtained from Robinson Services Inc. (Mocksville, NC, USA). The animals were maintained and used in compliance with institutional guidelines and in accordance with the Association for Research in Vision and Ophthalmology Resolution on the Use of Animals in Ophthalmic Research and the U.K. Animals (Scientific Procedures) Act. The research was approved by the Johns Hopkins University animal review board committee. Clinical examinations were performed on the rabbits’ eyes prior to any experimental procedures to establish baseline data and to exclude any animals with ocular defects. Dexamethasone (dexamethasone 21phosphate disodium salt) was purchased from MP Biomedicals (Santa Ana, CA, USA). DDex and Dendrimer-cyanine 5 (Cy5, D-Cy5) were synthesized at our lab. Ful-Glo® fluorescein sodium ophthalmic strips and rose bengal strips were purchased from Akorn, Inc. (Lake Forest, IL, USA). Antibody specific for CD-18 was obtained from Spring Valley Laboratories, Inc. (Sykesville, MD, USA), and anti-rabbit T-lymphocyte antigen (RTLA) antibody was obtained from Cedarlane Laboratories (Burlington, Ontario, Canada). Aquaporin 5 (AQP5) antibody was obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Species-specific secondary antibodies were obtained from Thermo Fisher Scientific Inc. (Waltham, MA, USA). DAPI was obtained from Vector Laboratories Inc. (Burlingame, CA, USA).
2.2. Synthesis and characterization of D-Dex and fluorescently labelled dendrimers (D-Cy5):
D-Cy5 conjugates were used to evaluate the biodistribution of the subconjunctivally administered dendrimers in this model. D-Cy5 conjugates were synthesized using previously established synthetic protocols [32]. The detailed procedure for synthesis of D-Dex conjugates by 2 step synthesis protocol was reported previously [31]. Briefly, the D-Dex conjugates were synthesized using a two-step procedure. In the first step, dexamethasone21-glutarate (Dex-Linker) was synthesized by reacting the -OH group at the 21th position of Dex to the -COOH of the glutaric acid in the presence of triethylamine as a base. The DexLinker was purified using flash column chromatography. In the second step, the Dex-Linker was conjugated to the -OH groups on the dendrimer surface in the presence of coupling agent benzotriazol-1-yloxy tripyrrolidinophosphonium hexafluorophosphate ,diisopropylethylamine as a base and anhydrous DMF as solvent. The final product was purified using dialysis. The D-Dex conjugates were characterized using proton nuclear magnetic resonance (1H NMR) and high performance liquid chromatography (HPLC).
2.3. Autologous mixed cell reaction and induction of AID and subconjunctival treatment
Left inferior LGs were surgically removed under anesthesia for the isolation of purified epithelial cells and peripheral blood was collected from each rabbit for isolation of peripheral blood lymphocytes using methods described by Guo et al. [13]. AID was induced in the rabbits by injecting the activated lymphocytes back to right LGs. At 2 weeks after disease induction in this double-blinded study, the rabbits were randomly divided into 3 treatment groups: D-Dex, containing 2.5 mg of Dex in conjugated form (n=9); Free-Dex (2.5 mg) (n=6); or saline control (n=7). The treatment was administered only once through subconjunctival injection using a 30G insulin syringe under general anesthesia. Another 4 healthy rabbits were used as normal controls. Nine rabbits with IAD and three normal rabbits received subconjunctival injection of D-Cy5 for its LG biodistribution study.
2.4. Clinical Analysis
Ocular surface assessments and intraocular pressure (IOP) measurement were performed before model establishment and in diseased rabbits immediately preceding treatments and 2 weeks after treatments. IOP was measured with an Icare tonometer (Icare Finland Oy, Vantaa, Finland). Tear production was assessed using the Schirmer test without topical anesthesia. Tear film stability was evaluated by instilling fluorescein into the conjunctival sac and determining the tear breakup time (TBUT) under examination with a slitlamp biomicroscope. A standardized grading system scored the intensity of staining of the cornea and or conjunctiva in fluorescein and rose bengal staining, respectively. The cornea was divided into 3 segments: superior, mid- and inferior. The conjunctiva included 2 sectors: nasal bulbar and temporal bulbar conjunctiva [38]. Scoring used the following guidelines: no punctate staining on the cornea was assigned 0 points; punctate staining of 1–10 was assigned 1 point; punctate staining 11–30 was assigned 2 points; and either punctate staining >30 or clumped staining was assigned 3 points [39].
2.5. Tissue procurement and process
For the biodistribution study, the rabbits were sacrificed at 24 hrs, 72 hrs, and 2 weeks after D-Cy5 was given. Otherwise, rabbits were sacrificed 2 weeks following treatments, and the right LGs were removed and evaluated for appearance and size. The LGs were then bisected longitudinally. One part was fixed in 4% paraformaldehyde overnight and dehydrated with gradient sucrose followed by embedding in paraffin, cutting in 5 µm sections, and staining with hematoxylin and eosin (H&E), or in OCT for 8 µm cryosections and immunostaining. The other halves of the bisected LG tissues were minced into small pieces with fine scissors and snap frozen in liquid nitrogen. The frozen tissue was cryogenically ground into an evenly fine powder with a freezer mill (Mixer Mill MM400, Retsch, Hann, Germany) and stored at −80 °C until use.
2.6. Immunohistopathology
The paraffin-embedded sections were H&E stained. The cryosections were air-dried, rehydrated in phosphate-buffered saline, and blocked in 5% bovine serum albumin for 15 min. The sections were incubated at room temperature for 1 hr with the primary antibody at the following dilutions: mouse anti-rabbit CD-18 (1:1000), goat-anti-rabbit AQP5 (1:100) and goat anti-rabbit RTLA (1:200). Sections were then rinsed and incubated for 60 min with secondary antibodies conjugated with Alexa Fluor 488 or 568 (1:500) at RT for 1 hr. After rinsing, the sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). The stained specimens were observed and imaged with laser scanning confocal microscopy (LSM 710; Zeiss, Germany). A tile scan mode was used to scan a stained area of 2 mm x 2 mm at the same exposure settings. Three different regions were randomly chosen for each section, and 3 sections were used for each sample. Images were taken for each tile scan and analyzed with Image J software (NIH, Bethesda, MD, USA). The staining area for each antibody and the number of DAPI stained nuclei were counted in each image. Normalized staining ratio was defined and calculated as staining area divided by number of nuclei. For each sample, 9 staining ratios were obtained from 9 images for each antibody, and averaged to yield the mean value for comparisons between groups.
2.7. Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using an RNeasy Mini Kit (Qiagen N.V., Hilden, Germany), with on-column DNA digestion by RNase-free DNase (Qiagen). cDNA was synthesized using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA). After the first strand cDNA synthesis, 1 µl of 1:10 diluted cDNA was used for PCR in a 20 µl reaction volume consisting of 10 µl SYBR Green Master Mix (Thermo Fisher) and 0.4 µl primers mix by using a StepOne RT-PCR System (Thermo Fisher). PCR reactions were run in triplicate for each sample for the following genes: β-actin, MMP9, IL6, IL8, IL10, TNFα, AQP5, and AQP4. The specific primer sequences are available upon request. PCR conditions included an initial denaturing step for 3 min at 95 °C, followed by 40 cycles (95 °C for 15 s, 60 °C for 20 s). The validity of each individual PCR reaction was confirmed by melting curves, obtained by heating samples from 58 °C to 95 °C. Agarose gel analysis was performed to further confirm the amplicons. The fold change in relative expression of each gene was calculated using the ∆∆CT method with β-actin as the internal control.
2.8. Statistical analysis
All data are expressed as mean ± standard deviation (SD). Data were subjected to paired t-test or signed rank sum test using SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA) and analysis of variance (ANOVA). Multiple comparisons to the normal group were adjusted using Dunnett’s correction. A probability (p) value of 0.05 was considered statistically significant.
In addition, for better evaluation of clinical validity, we used a dichotomy approach [40] to categorize the examination of tear production and TBUT in all 3 treatment groups. To minimize the possibility of false positive results and maximize the intergroup statistical difference, we set a high cutoff value to guarantee the improvement of the dry eye condition after treatment. A 40% increase of tear production or 50% increase of TBUT was defined as valid/ otherwise invalid, and the effective rate was calculated for each group. Under such cutoff value, the control group showed no improvement by examination.
3. Results
3.1. D-Dex and D-Cy5 conjugates
We used near-IR dye Cy5 for labelling the dendrimer to avoid tissue autofluorescence. 1H NMR and HPLC characterization demonstrated that ~2 molecules of Cy5 were conjugated to the dendrimer surface and the purity of D-Cy5 was >95% [22]. We previously showed that DCy5 is stable in vivo in several animal models [32, 41]. The detailed synthesis of the D-Dex conjugate has been described previously [22]. Briefly, a glutaric acid linker was used to conjugate Dex to the dendrimer surface to avoid steric hindrance to conjugation, and to enable drug release through hydrolysis. 1H NMR characterization confirmed that ~8 molecules of dexamethasone were conjugated to each dendrimer. HPLC characterization of D-Dex conjugates demonstrated purity of >98%. The conjugates were soluble in aqueous solutions up to 100 mg/mL. D-Dex conjugates demonstrated improved anti-inflammatory activity at 10-fold lower concentration compared to Free Dex in lipopolysaccharide-activated murine macrophages [22].
3.2. Biodistribution of dendrimer in LG in induced AID
We used CD-18 and RTLA as markers for lymphocytes in LG. In a normal LG, a few CD 18+/RTLA+ cells were found scattered in the gland. Following disease onset, a significant increase in lymphocyte infiltration was noted in the inflamed LG (Fig. 1). To demonstrate both targeting and retention of dendrimers after subconjunctival injection, we used fluorescent-labelled dendrimer (D-Cy5). Upon subconjunctival administration, D-Cy5 was found localized and retained in the inflamed region in LG, wheras minimal D-Cy5 signal was seen in the healthy LG. In the inflamed LG, D-Cy5 was present in infiltrated cells, surrounding the acinar cells and the extracellular matrix, both 24 hrs and 72 hrs after administration (Fig. 1A, B, D, E).The imaging studies demonstrated pathology-dependent biodistribution of dendrimers: co-localization of D-Cy5 (red) and inflammatory cell markers (green) as CD-18 and RTLA staining was found in the LG of the AID model. D-Cy5 could still be found in the injured LG 2 weeks post-administration (Fig. 1G, H). However, the subconjunctival injection in healthy control animals demonstrated very minimal signal from D-Cy5 (Fig. 1C, F, I). It suggests that dendrimer biodistribution is restricted to inflamed and pathologic tissues/cells.
Figure 1.
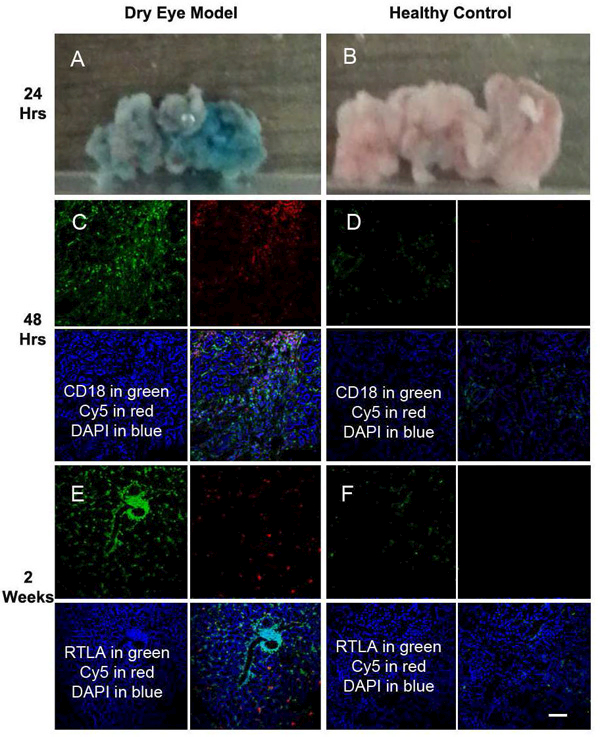
Biodistribution of subconjunctivally injected D-Cy5 in LG. Co-localization of the Cy5 signal (red) with inflammatory cell marker staining (green) as CD 18 (A, D, G) and RTLA (B, E, H) were found in LG with established disease. The upper panel (A-C), the middle panel (D-F) and the lower panel (G-I) represent samples from 24 hours, 72 hrs, and 2 weeks post-subconjunctival injection, respectively. Minimal uptake was found in LG of normal control group (C, F, I). Blue for DAPI staining. Scale Bar :100 µm.
3.3. Dexamethasone treatment clinically alleviated AID
3.3.1. Tear production
As shown in Fig. 2A, tear production in the normal (healthy) group did not show appreciable change throughout the duration of the study. At two weeks following induction of AID, immediately before treatment, average decreases in tear production of 39%, 40%, and 38% were mesured in the D-Dex, Free-Dex, and saline groups, respectively, suggesting minimal variation between groups before the start of treatment. Two weeks after treatment, tear production increased by an average of 31% in the D-Dex group (statically significant [p<0.05], while only 12% and 5.5% increases were seen in Free-Dex and saline groups, respectively (not statistically significant). When setting a 40% increase of tear production as the criterion for treatment effectiveness in each rabbit based on our clinical experience, we found that the treatment was effective in 5 out of 9 rabbits (56%) in the D-Dex group, 0 out of 6 rabbits in the Free-Dex group, and 0 out of 7 rabbits in the saline group (Fig. 2D).
Figure 2.
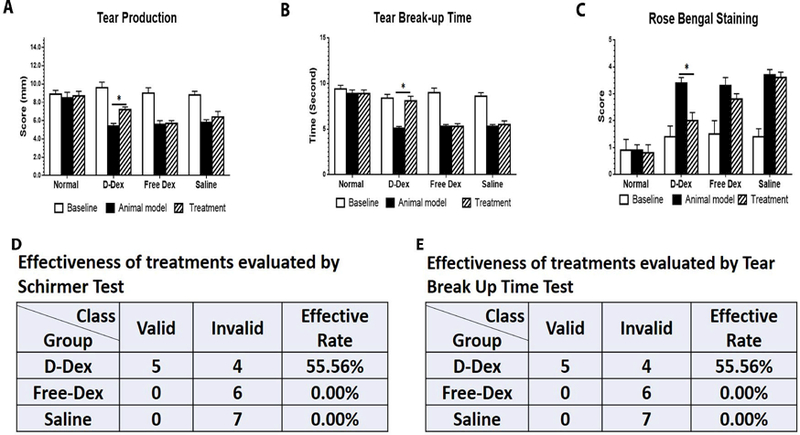
Clinical evaluation of ocular surface at baseline, 2 weeks post-disease model and 2 weeks post-treatment in 4 groups: (A) Tear production, Schirmer test was performed. (B) Tear break-up time demonstrates tear instability. Slit-lamp examination was performed. (C) Scores of rose bengal of the ocular surface was evaluated. (D) Effectiveness of treatments evaluated by Schirmer test: a 40% increase of tear production is used as the evaluation criterion. (E) Effectiveness of treatments evaluated by Tear break up time, a 50% increase is used as the evaluation criterion (* p<0.05).
3.3.2. Tear breakup time (TBUT)
TBUT values in normal rabbits remained similar throughout the study. In these 3 treatment groups, before treatment, a significant decrease was noticed at 2 weeks following induction of the animal model (mean 42%, 47% and 45% of decrease in D-Dex, Free-Dex and Saline groups respectively). Again, before the start of treatment, there was minimal difference between groups. After 2 weeks of treatment, D-Dex and Free-Dex groups have seen an average increase of 56% and 21% respectively while no increase was observed in Saline group (Fig. 2B). When setting a 50% increase as criterion for treatment effectiveness in each single rabbit, we found that the treatment was effective in 5 out of 9 rabbits (~56%) in D-Dex group, 0 out of 6 rabbits in Free-Dex group, and 0 out of 7 rabbits in saline group (Fig. 2E).
3.3.3. Rose bengal staining
Rose Bengal staining was assessed, to determine ocular surface defects due to deficiency in preocular tear film protection. The scores for rose bengal staining were low in the normal control (mean value=1.1) and for baseline examination in other treatment groups before treatment (the mean values are 0.93, 1.75, and 1.5 in D-Dex, Free-Dex, and saline groups, respectively). Staining score increased significantly by 2 weeks post-disease establishment in all 3 treatment groups (mean values: 3.2, 3.5, and 3.2 in D-Dex, Free-Dex, and saline groups, respectively). The staining became attenuated in the D-Dex group 2 weeks after the treatment to an average of 1.73 with statistical significance. In the other two treatment groups, minimal changes in rose bengal staining were observed (Fig. 2C).
3.3.4. Fluorescein staining
No remarkable changes were observed for fluorescein staining among groups, or between baseline and post induced AID/treatment (data not shown).
3.4. D-Dex treatment alleviated AID histologically
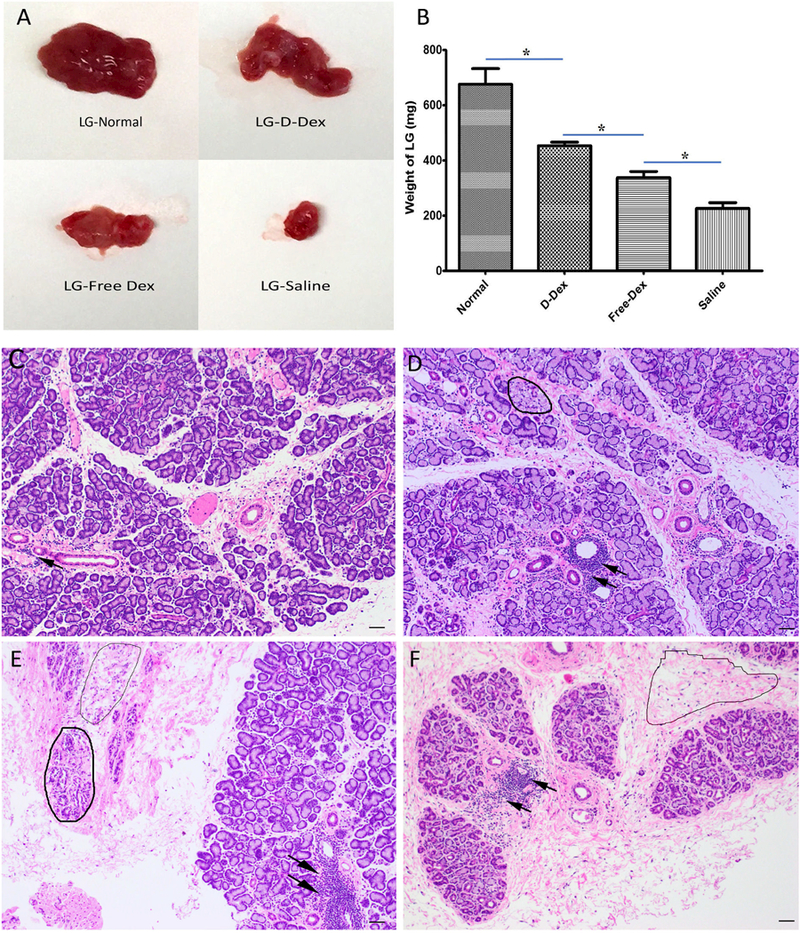
As shown in Fig. 3A, the LGs appeared different among groups. Compared to the normal LG, the diseased LGs in the treatment groups became smaller and had less glandular tissue. Especially in the saline treated rabbits, the LGs shrank significantly, and lacked the glossy and pink look. LGs in the D-Dex group appeared plumper and larger than those in the FreeDex group. At the experimental endpoint, LGs in the D-Dex group weighed 435 ± 27 grams, greater than those in the Free-Dex (337.75 ± 31 grams), and saline groups (226 ± 29 grams); but they were lighter than normal LGs (676 ± 80 grams) (Fig. 3B).
Figure 3.
Morphology and histology of LG (A) Gross appearance of LG in normal rabbits and 3 treatment groups. (B) Comparison of LG weight among the 4 groups. (C-F) Representative micrographs (H&E staining) of LG sections in normal, D-Dex, Free-Dex, and saline treated rabbits. (C) Occasional scattered lymphocytic infiltration in the normal control group. (D) Lymphocytic foci (arrows) and slightly degenerating lobules (circle). (E) Severe lymphocytic infiltration and more degenerating lobules (circles) and connective tissue were present. (F) Most lobules lost normal structure and areas of fibrosis (circle) were common. Scale bar: 100µm.
Representative H&E-stained paraffin section from unmanipulated normal LGs is presented in Fig. 3C in which numerous lobules are well organized and separated by thin connective tissue, acini are packed densely in lobules with good polar morphology of acinar cells, and occasional lymphocytic infiltration is found around the ducts and venules (arrow). In the LG section of D-Dex treated rabbits, the lobules appeared a bit incompact, demonstrating some degenerating acinar units (as circled in Fig. 3D) and scattered large periductal and perivenular foci of lymphocytes (arrows in Fig. 3D). In the LG sections from Free-Dex rabbits, lymphocytic infiltration was serious (arrows in Fig. 3E), and some lobules appeared to be degenerating (e.g., the one in bold line circle in Fig. 3E) and some became atrophic (e.g., the one in light line circle in Fig. 3E). In addition, fibrosis was visible. LG sections from saline-treated rabbits showed an atrophic appearance with numerous degenerated lobules. Acinar units were sharply decreased and much glandular tissue was replaced by fibrotic connective tissue with lymphocytic infiltration (Fig. 3F).The histology revealed that D-Dex treated rabbits showed better and more integral structure of acini and lobules than the other two groups, though slight inflammatory infiltration was still observed in the D-Dex group.
3.5. D-Dex treatment inhibited infiltration of T-lymphocytes
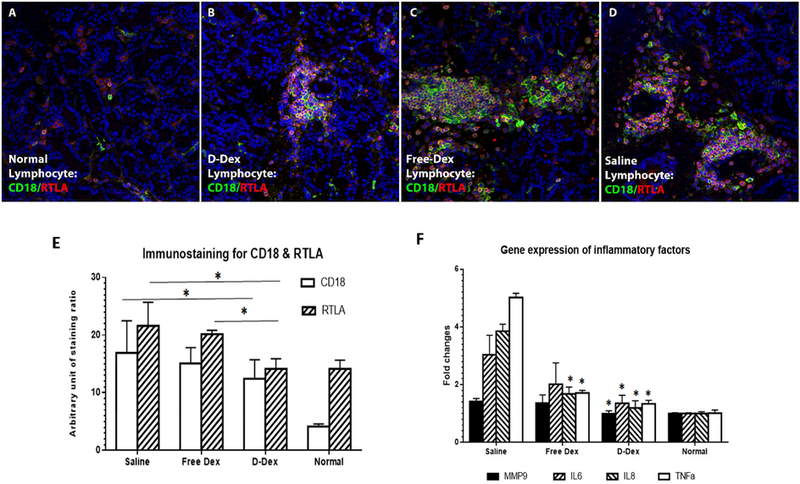
Representative images of cryosections of LG immunostained for RTLA and CD-18 for all groups and quantitative immunohistochemical analyses are presented in Figure 4. CD-18, as the beta chain of lymphocyte function-associated antigen 1, could be found on all T cells and also on B cells, macrophages, neutrophils, and NK cells [12], and is a well accepted marker for evaluating lymphocyte infiltration in rabbit autoimmune dacryoadenitis model [8]. Compared to the normal group, all 3 treated groups have more CD18 immunostaining. Among them, D-Dex group showed less staining for CD18 (Fig. 4 B, E). The other two group have similar immunostaining for CD18 (Fig. 4 C, D, E).
Figure 4.
Immunofluorescent (IF) staining of LG sections for CD-18 and RTLA. CD-18 and RTLA IF staining on normal LG sections showing a small amount of CD-18+ and RTLA+ cells with some co-staining for them (A, E). CD-18 and RTLA IF staining on D-Dex treated LG sections showing certain amount of positive staining cells (B, E). CD-18 and RTLA IF staining on Free-Dex treated LG sections showing more positive staining cells (C, E). IF staining for CD 18 and RTLA on Saline treated LG sections is even more than Free-Dex group (D, E). Quantitative analysis of immunostaining for CD18 and RTLA were performed respectively and comparisons among groups showed (E). Expression level of inflammatory cytokines were measured by qPCR. * p<0.05. Magnification times: 200X.
RTLA-positive staining, as a marker for T cells, was more commonly found as foci around the venules and often appeared as clusters. Based on the staining ratio, RTLA expression was less abundant in D-Dex (Fig. 4B, E) than in Free-Dex group (Fig. 4 C, E) or saline group (Fig. 4D, E), indicating attenuated infiltration of T lymphocytes in D-Dex treated rabbits.
3.6. D-Dex treatment suppressed the expression of inflammatory mediators in dacryoadenitis rabbit LGs
The relative abundance of some inflammatory factors from whole LGs were quantified with qRT-PCR, including proinflammatory factors such as IL-6, IL-8, MMP-9 and TNF-α, and anti-inflammatory factor IL-10 (Fig. 4F). For MMP9, the gene expression level in normal control, D-Dex, Free-Dex and saline group is 1.00±0.03, 0.99±0.10, 1.36±0.28 and 1.42±0.10 respectively. For IL6, the gene expression level in normal control, D-Dex, FreeDex and saline group is 1.00±0.02, 1.35±0.28, 2.01±0.74 and 3.03±0.68 respectively. For IL8, the gene expression level in normal control, D-Dex, Free-Dex and saline group is 1.00±0.06, 1.19±0.25, 1.68±0.24 and 3.85±0.24 respectively. For TNFα, the gene expression level in normal control, D-Dex, Free-Dex and saline group is 1.01±0.11, 1.33±0.12, 1.71±0.09 and 5.02±0.14 respectively. Compared to saline treated rabbits with AID, the expression of all pro-inflammatory in LGs of D-Dex treated rabbits was significantly decreased (p<0.05). Free-Dex treated rabbits showed significantly lower IL8 and TNFα levels compared to saline controls (p<0.05). It is noted that expression of IL10 was detected only in some of the D-Dex treated rabbits (6 out of 9) and not in any rabbits in the other two treatment groups (data not shown).
3.7. Aquaporin changes in rabbit AID LGs
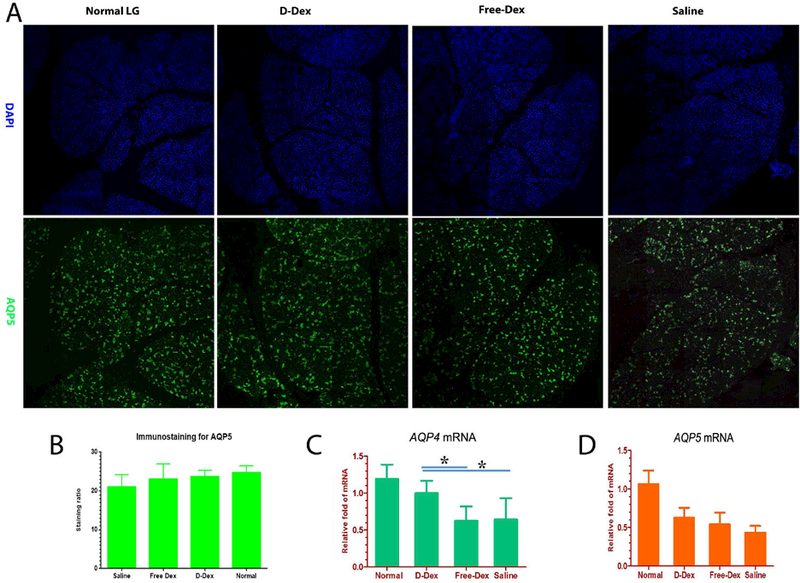
AQP5 immunoreactivity is found in the apical and basolateral membranes of acinar cells and is distributed among acini in a “mosaic” pattern. In our study, AQP5 was extensively expressed in the LG of all rabbits, but greater immunoreactivity was seen in normal rabbits compared to treated rabbits, with saline treated ones having the weakest immunoreactivity (Fig. 5A). However, no significantly difference in staining area among groups were detected.
Figure 5.
Immunofluorescent micrographs of LG sections from normal, D-Dex treatment, Free-Dex treatment or saline treatment, stained for nuclei (DAPI, blue) and AQP5 (green, A) as well as quantitative analysis and comparisons among groups (B). Expression level of AQP4 and AQP5 were measured by qPCR. * p<0.05. Magnification times: 200X.
We also used RT-qPCR to investigate expression levels of AQP5 and AQP4. Unlike the finding with immunofluorescent staining for AQP5, RT-qPCR revealed that the expression of AQP4 was downregulated in these treatment groups compared to normal controls, albeit the downregulation is significantly less in D-Dex than in the other two groups (Fig. 5B). AQP5 was significantly down-regulated in all 3 treatment groups although D-Dex and Free-Dex treated rabbits have a somewhat higher abundance than the saline treated ones (Fig. 5C).
4. Discussion
In this study, we demonstrated that a single subconjunctival injection of D-Dex conjugate has therapeutic effect for DED in an AID model. Current methods of drug introduction to treat DED are insufficient to successfully treat the disease. Rapid drug elimination from the ocular surface is a major obstacle for topical drug delivery, especially in the case of severe dry eye. Blinking, eyelashes, and reflexive tearing promote rapid removal of eye drops from the eye surface, typically within 15–30 seconds. The intraocular bioavailability of topically applied drugs are typically less than 5% [40]. As a result, many eye drops are prescribed or required to be dosed several times a day, decreasing patient compliance. Subconjunctival drug delivery can overcome such difficulty, especially in patients with severe dry eye. To better understand the bioavailability of dendrimer delivered drug in LG, we used Cy5-conjugated dendrimer to study its biodistribution in LGs upon subconjunctival administration. In patients with several dry eye, a monthly subconjunctioval treatment may be preferable to topical drops many times daily. The dendrimer exhibited pathology-dependent biodistribution, with significant preferential accumulation in the injured LG, compared to the healthy LG where it was readily cleared. In the injured LG, it was co-localized and retained in LG lymphocytes. Co-localization of dendrimer with lymphocytes suggests the possibility of enhanced delivery and availability of corticosteroids to the very cells responsible for LG inflammation. Therefore, dendrimers could enhance intracellular and targeted delivery in the injured LG, increasing drug efficacy, while reducing side effects, as illustrated in the rabbit AID model.
Inflammation and tear hyperosmolarity have recently been added as part of the features of DED [42]. Ocular surface and LG inflammation is identified in DED and plays a key role in the pathogenesis of DED. Dysfunction of the lacrimal functional unit from any cause alters the balance of tear film components, which destabilize the tear film that supports and protects the ocular surface. These changes in tear composition also promote inflammation on the ocular surface [43]. Topical steroids help to reduce ocular inflammation. They upregulate the expression of anti-inflammatory proteins and repress the expression of proinflammatory proteins. Moreover, they also elicit non-genomic effects, such as inhibition of vasodilation, vascular permeability and migration of leukocytes [42]. Some clinical studies have demonstrated the effectiveness of topical steroids for treatment of DED [44, 45]. Recovery of LG structure and function are essential to its treatment. In the inflamed LG, inhibition of inflammation is expected to play a key role in reversing its pathology and the changes in the ocular surface. Our data demonstrates that the downregulation of inflammatory cytokines in the D-Dex treatment groups,as well as less inflammatory cell infiltration in the D-Dex treatment group, which is considered to contribute to the pathological and clinical improvement with D-Dex treatment.
Functional changes in LGs were observed through the investigation of AQP proteins. AQP is a group of proteins that are responsible for rapid water transport across plasma membranes in many organisms. At least 2 subtypes of AQP have been found in LGs, including AQP-5 localized to the apical membranes and AQP-4 identified on the basolateral membranes of LG acinar and ductal cells. These AQP proteins play important roles in tear secretion. As Ding et al. reported, expression of AQPs is altered in rabbits with AID, and AQP-4 as a specific ductal segment plays an important role in lacrimal secretion [46]. In the injured LGs in our study, reduced expression of AQP-5 and down-regulation were observed. Both AQP-4 and 5 were upregulated in the D-Dex treatment group compared to the saline control, which had much lower than normal AQP expression. We speculate that tear secretion may be damaged by LG inflammation and, following the treatment, inhibition of inflammation may lead to partial recovery of tear secretion. In contrast, free-Dex yielded limited beneficial effects compared to D-Dex treatment suggesting the advantage of dendrimers as potential drug delivery vehicles.
While no corticosteroid-related complications were observed in short-term clinical trials for DED, toxicity associated with long-term usage is a major concern. This limits the use of more potent corticosteroids, such as dexamethasone, as a chronic therapy for DED. Our rabbit study showed no steroid-related side effects or complications, such as corneal ulceration, IOP increase and others during the 2-week observation period, however prolonged investigation is required to investigate this nanoparticle guided drug delivery with steroid. The introduction of cyclosporine-A (CsA) and Lifitegrast (Xiidra) ophthalmic solution offer good alternative anti-inflammatory treatments for DED. It was reported that long-term CsA topical administration reduced clinical signs of dry eye by reducing T-cell infiltration and inflammation in the LG of induced rabbit AID [15]. Yet, many patients discontinue topical CsA therapy because of ocular discomfort and delayed relief. In the long term, dendrimers could also deliver other anti-inflammatory or neuroprotective agents to address DED.
Some limitations of our study are noted. DED is a chronic condition. Extended observation longer than 2 weeks is needed for further evaluation of the treatment. Long-term effects of drugs to the lacrimal glands and reactive T cells and other immune cells were proposed to be evaluated in further study. Moreover, the mechanism and best time point to relieve ocular signs by dendrimer-guided steroid need to be further clarified. Repeated treatment effects, and long-term T cell resistance, development of drug resistance also need to be considered. However, compared to a previous study of long-term topical CsA treatment in the same rabbit AID model, the current study still shows a very promising result with better evaluation outcomes [15]. In addition, it would be valuable to compare the efficacy of dendrimer delivered CsA in the same animal model in a future study.
In summary, a single subconjunctival treatment with D-Dex conjugate, produced clinical improvement and reduced histopathology and inflammation in the LG in an experimentally induced AID model. The improved efficacy of D-Dex, compared to free Dex, in many functional measures, suggests that targeted, sustained delivery using dendrimers could be viable. With a single subconjunctival injection of the dendrimer conjugated anti-inflammatory drug, improved efficacy and patient compliance, decreased complaints of burning sensation, and reduced side effects could be achieved in future DED treatments, although long-term preclinical evaluation is warranted. In severe DED patients a single subconjuctival treatment every 1–2 months may be more viable than topical eye drops many times a day.
Supplementary Material
5. Acknowledgement
Supported in part by an unrestricted grant from Research to Prevent Blindness, New York, NY, to the Wilmer Eye Institute, and NIH/NEI R01 1R01EY025304–01 (RMK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest and financial or business interest related to this study.
Reference
- 1.Ding J, Sullivan DA. Aging and dry eye disease. Experimental gerontology 2012;47:483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ 2001;79:214–21. [PMC free article] [PubMed] [Google Scholar]
- 3.Paulsen AJ, Cruickshanks KJ, Fischer ME, Huang GH, Klein BE, Klein R, et al. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol 2014;157:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voulgarelis M, Tzioufas AG. Pathogenetic mechanisms in the initiation and perpetuation of Sjogren’s syndrome. Nature reviews Rheumatology 2010;6:529–37. [DOI] [PubMed] [Google Scholar]
- 5.Barone F, Colafrancesco S. Sjogren’s syndrome: from pathogenesis to novel therapeutic targets. Clinical and experimental rheumatology 2016;34:58–62. [PubMed] [Google Scholar]
- 6.Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J 1995;21:221–32. [PubMed] [Google Scholar]
- 7.Bayetto K, Logan RM. Sjogren’s syndrome: a review of aetiology, pathogenesis, diagnosis and management. Australian dental journal 2010;55 Suppl 1:39–47. [DOI] [PubMed] [Google Scholar]
- 8.Thomas PB, Zhu Z, Selvam S, Samant DM, Stevenson D, Mircheff AK, et al. Autoimmune dacryoadenitis and keratoconjunctivitis induced in rabbits by subcutaneous injection of autologous lymphocytes activated ex vivo against lacrimal antigens. Journal of autoimmunity 2008;31:116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen CQ, Peck AB. Unraveling the pathophysiology of Sjogren syndrome-associated dry eye disease. Ocul Surf 2009;7:11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Bultzingslowen I, Sollecito TP, Fox PC, Daniels T, Jonsson R, Lockhart PB, et al. Salivary dysfunction associated with systemic diseases: systematic review and clinical management recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;103 Suppl:S57 e1-15. [DOI] [PubMed] [Google Scholar]
- 11.Coursey TG, de Paiva CS. Managing Sjogren’s Syndrome and non-Sjogren Syndrome dry eye with anti-inflammatory therapy. Clinical ophthalmology 2014;8:1447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei RH, Thomas PB, Samant DM, Schechter JE, Mircheff AK, Trousdale MD. Autoimmune dacryoadenitis and sialadenitis induced in rabbits by intravenous injection of autologous lymphocytes activated ex vivo against lacrimal antigens. Cornea 2012;31:693–701. [DOI] [PubMed] [Google Scholar]
- 13.Guo Z, Song D, Azzarolo AM, Schechter JE, Warren DW, Wood RL, et al. Autologous lacrimallymphoid mixed-cell reactions induce dacryoadenitis in rabbits. Exp Eye Res 2000;71:23–31. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Meagher CK, Moore CP, Phillips TE. M cells in the follicle-associated epithelium of the rabbit conjunctiva preferentially bind and translocate latex beads. Invest Ophthalmol Vis Sci 2005;46:4217–23. [DOI] [PubMed] [Google Scholar]
- 15.Thomas PB, Samant DM, Zhu Z, Selvam S, Stevenson D, Wang Y, et al. Long-term topical cyclosporine treatment improves tear production and reduces keratoconjunctivitis in rabbits with induced autoimmune dacryoadenitis. J Ocul Pharmacol Ther 2009;25:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5:108–52. [DOI] [PubMed] [Google Scholar]
- 17.Yang CQ, Sun W, Gu YS. A clinical study of the efficacy of topical corticosteroids on dry eye. Journal of Zhejiang University Science B 2006;7:675–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pflugfelder SC. Anti-inflammatory therapy of dry eye. Ocul Surf 2003;1:31–6. [DOI] [PubMed] [Google Scholar]
- 19.Colligris B, Alkozi HA, Pintor J. Recent developments on dry eye disease treatment compounds. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society 2014;28:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheppard JD, Scoper SV, Samudre S. Topical loteprednol pretreatment reduces cyclosporine stinging in chronic dry eye disease. J Ocul Pharmacol Ther 2011;27:23 7. [DOI] [PubMed] [Google Scholar]
- 21.Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology 2000;107:631–9. [DOI] [PubMed] [Google Scholar]
- 22.Soiberman U, Kambhampati SP, Wu T, Mishra MK, Oh Y, Sharma R, et al. Subconjunctival injectable dendrimer-dexamethasone gel for the treatment of corneal inflammation. Biomaterials 2017;125:38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J 2010;12:348–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandasana H, Prasad YD, Chhonker YS, Chaitanya TK, Mishra NN, Mitra K, et al. Corneal targeted nanoparticles for sustained natamycin delivery and their PK/PD indices: An approach to reduce dose and dosing frequency. International journal of pharmaceutics 2014;477:317–25. [DOI] [PubMed] [Google Scholar]
- 25.Iezzi R, Guru BR, Glybina IV, Mishra MK, Kennedy A, Kannan RM. Dendrimer-based targeted intravitreal therapy for sustained attenuation of neuroinflammation in retinal degeneration. Biomaterials. 2012;33:979–88. [DOI] [PubMed] [Google Scholar]
- 26.Menjoge AR, Kannan RM, Tomalia DA. Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug discovery today 2010;15:171–85. [DOI] [PubMed] [Google Scholar]
- 27.Florence AT, Hussain N. Transcytosis of nanoparticle and dendrimer delivery systems: evolving vistas. Advanced drug delivery reviews 2001;50 Suppl 1:S69-89. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi H, Brechbiel MW. Dendrimer-based macromolecular MRI contrast agents: characteristics and application. Molecular imaging 2003;2:1–10. [DOI] [PubMed] [Google Scholar]
- 29.Lee CC, MacKay JA, Frechet JM, Szoka FC. Designing dendrimers for biological applications. Nat Biotechnol 2005;23:1517–26. [DOI] [PubMed] [Google Scholar]
- 30.Hayder M, Poupot M, Baron M, Nigon D, Turrin CO, Caminade AM, et al. A phosphorus-based dendrimer targets inflammation and osteoclastogenesis in experimental arthritis. Science translational medicine 2011;3:81ra35. [DOI] [PubMed] [Google Scholar]
- 31.Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Advanced drug delivery reviews 2005;57:2215–37. [DOI] [PubMed] [Google Scholar]
- 32.Lesniak WG, Mishra MK, Jyoti A, Balakrishnan B, Zhang F, Nance E, et al. Biodistribution of fluorescently labeled PAMAM dendrimers in neonatal rabbits: effect of neuroinflammation. Molecular pharmaceutics 2013;10:4560–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandamme TF, Brobeck L. Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. Journal of controlled release : official journal of the Controlled Release Society 2005;102:23–38. [DOI] [PubMed] [Google Scholar]
- 34.Grinstaff MW. Designing hydrogel adhesives for corneal wound repair. Biomaterials 2007;28:5205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marano RJ, Toth I, Wimmer N, Brankov M, Rakoczy PE. Dendrimer delivery of an anti-VEGF oligonucleotide into the eye: a long-term study into inhibition of laser-induced CNV, distribution, uptake and toxicity. Gene therapy 2005;12:1544–50. [DOI] [PubMed] [Google Scholar]
- 36.Jaffe GJ, Ben-Nun J, Guo H, Dunn JP, Ashton P. Fluocinolone acetonide sustained drug delivery device to treat severe uveitis. Ophthalmology 2000;107:2024–33. [DOI] [PubMed] [Google Scholar]
- 37.Kambhampati SP, Kannan RM. Dendrimer nanoparticles for ocular drug delivery. J Ocul Pharmacol Ther 2013;29:151–65. [DOI] [PubMed] [Google Scholar]
- 38.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003;22:640–50. [DOI] [PubMed] [Google Scholar]
- 39.Li N, Deng XG, Gao Y, Zhang SH, He MF, Zhao DQ. Establishment of the mild, moderate and severe dry eye models using three methods in rabbits. Bmc Ophthalmol 2013;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoerauf H, Feltgen N, Weiss C, Paulus EM, Schmitz-Valckenberg S, Pielen A, et al. Clinical Efficacy and Safety of Ranibizumab Versus Dexamethasone for Central Retinal Vein Occlusion (COMRADE C): A European Label Study. Am J Ophthalmol 2016;169:258–67. [DOI] [PubMed] [Google Scholar]
- 41.Mishra MK, Beaty CA, Lesniak WG, Kambhampati SP, Zhang F, Wilson MA, et al. Dendrimer brain uptake and targeted therapy for brain injury in a large animal model of hypothermic circulatory arrest. Acs Nano 2014;8:2134–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hessen M, Akpek EK. Dry eye: an inflammatory ocular disease. Journal of ophthalmic & vision research 2014;9:240–50. [PMC free article] [PubMed] [Google Scholar]
- 43.Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol 2004;137:337–42. [DOI] [PubMed] [Google Scholar]
- 44.Marsh P, Pflugfelder SC. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjogren syndrome. Ophthalmology 1999;106:811–6. [DOI] [PubMed] [Google Scholar]
- 45.Pflugfelder SC, Maskin SL, Anderson B, Chodosh J, Holland EJ, De Paiva CS, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol 2004;138:444–57. [DOI] [PubMed] [Google Scholar]
- 46.Ding C, Nandoskar P, Lu M, Thomas P, Trousdale MD, Wang Y. Changes of aquaporins in the lacrimal glands of a rabbit model of Sjogren’s syndrome. Curr Eye Res 2011;36:571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


