Abstract
Adult stem cells are essential for tissue homeostasis (1). In skeletal muscle, muscle stem cells (MuSCs) reside in a quiescent state (2, 3), but little is known about the mechanisms controlling homeostatic turnover. Here we show that the variation in MuSC activation rate among different muscles is determined by the levels of the transcription factor Pax3. We further show that Pax3 levels are controlled by alternative polyadenylation of its transcript, regulated by the small nucleolar RNA (snRNA) U1. Isoforms of the Pax3 mRNA that differ in their 3’ untranslated regions are differentially susceptible to regulation by miRNA-206, resulting in varying levels of Pax3 protein. These findings highlight a previously unrecognized mechanism of the homeostatic regulation of stem cell fate by multiple RNA species.
MuSCs are absolutely required for regeneration (4–6). Recent studies revealed that MuSCs in sedentary mice contribute to adult myofibers with high levels of contribution in diaphragm muscles and low levels in lower hindlimb muscles (7, 8). To understand the mechanisms that determine the extent of MuSC contribution to adult myofibers, we measured the extent to which MuSCs activate and enter the cell cycle in different uninjured muscles. When we pulsed mice in vivo with the nucleotide analog EdU to label cells undergoing DNA replication, we observed a wide range in EdU incorporation, with diaphragm, gracilis, and triceps muscles exhibiting the highest, and hindlimb muscles showing the lowest number of MuSCs that spontaneously break quiescence and enter the cell cycle under homeostatic conditions (Fig. S1A, S1B).
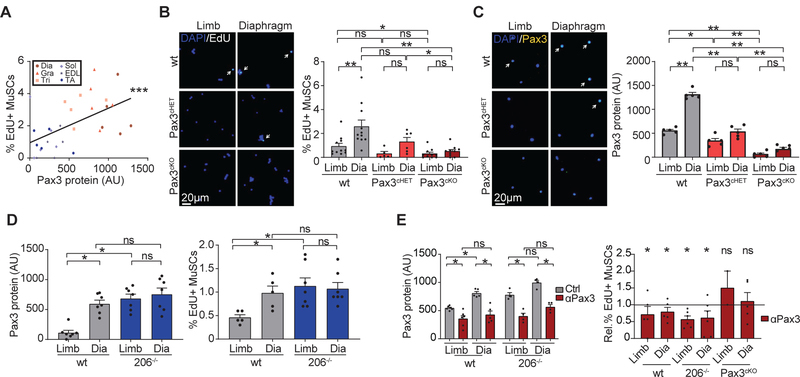
MuSCs in the diaphragm, gracilis, and triceps express high levels of the transcription factor Pax3, whereas MuSCs in most limb muscles do not (9). Given the established role of Pax3 in driving cell proliferation during embryogenesis (10) and in response to stress (11, 12), we asked whether Pax3 regulates the process of MuSC activation during homeostasis. Indeed, we observed that Pax3 staining positively correlated with EdU incorporation in MuSCs from different muscles (Fig. 1A). To test directly whether Pax3 plays a role in the balance between MuSC quiescence and activation, we conditionally deleted Pax3 from adult MuSCs using a Pax7-CreERT2 driver (Pax3cKO mice) and obtained a median reduction in Pax3 mRNA of 96% and 88% in diaphragm and hindlimb MuSCs, respectively. Deletion of Pax3 led to an increase in quiescence markers, a decrease in MyoD, and a decrease in EdU incorporation in the diaphragm, gracilis, and triceps, both in vivo and in vitro (Fig. 1B–C, S1C–E).
Figure 1: Pax3 controls MuSC activation under homeostatic conditions.
(A) MuSCs were isolated from mice treated with EdU for three days, stained for EdU and Pax3, and Pax3 levels were correlated with EdU levels. Dots represent individual mice, with muscles color coded as noted (low Pax3 level - blue; high Pax3 levels - red). A linear regression was fitted through the data points (n=5). (B,C) MuSCs were isolated from wt, Pax3cHET, and Pax3cKO mice treated with EdU for three days, stained for EdU (B, n=11) or Pax3 (C, n=5), and counterstained with DAPI. Left panels: representative images; right panels: graph of quantification. (D) Different strains of mice (wt and miR206−/−) were treated with EdU for three days. MuSCs were isolated and stained for Pax3 protein (left panel, n=7) or EdU (right panel, n=5). (E) Different strains of mice (wt, miR206−/−, or Pax3cKO) were treated with control or Pax3 knockdown AMOs and with EdU for three days. MuSCs were isolated and stained for Pax3 protein (left panel) or EdU (right panel, control is represented by the horizontal black line, normalized to 1) (n=5). (mean + SEM; 2-tailed t-test, * p<0.05, ** p<0.01, *** p<0.001)
Prior work from our lab showed that the microRNA miR206 inhibits Pax3 expression in MuSCs isolated from hindlimb muscles (9). We analyzed miR206−/− mice and found that they have increased Pax3 protein and corresponding increases in EdU incorporation in limb MuSCs, comparable to levels seen in the diaphragm (Fig. 1D; S1F,G), which was rescued by conditional deletion of Pax3 (Fig. S1H–J). Interestingly, Pax3 mRNA levels were unchanged, suggesting that miR206 regulates Pax3 protein translationally (Fig. S1K). Finally, we injected wt and 206−/− mice with antisense vivo-morpholino oligonucleotides (AMOs) complimentary to the translation initiation site in Pax3 to block Pax3 translation. This led to reduced Pax3 protein levels and reduced EdU incorporation in diaphragm MuSCs in both strains (Fig. 1E, Fig. S1L), but not in Pax3cKO mice (Fig. 1E, Fig. S1L). Conversely, miR206-blocking AMOs led to increased Pax3 protein levels and EdU incorporation in wt limb MuSCs, but not in miR206−/− or Pax3cKO animals (Fig. S1M–O). We conclude that Pax3 increases the propensity of quiescent MuSCs to exit the quiescent state.
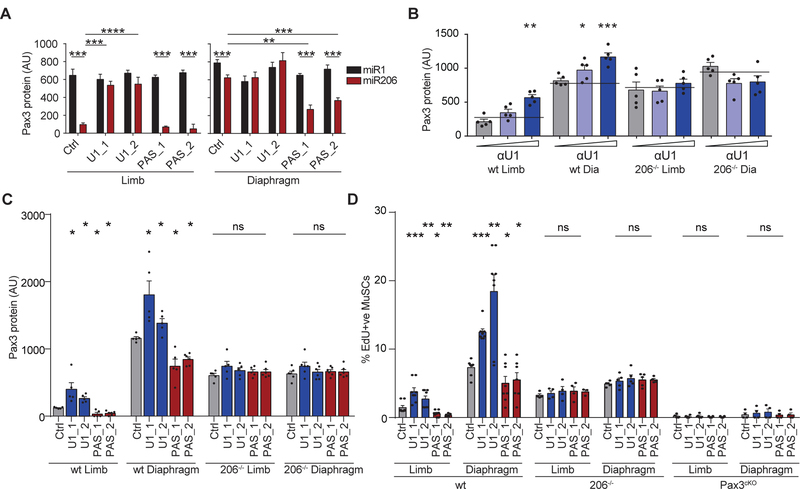
Previous results from our lab showed that MuSCs in the diaphragm express Pax3 transcript isoforms with shorter 3’UTRs that lack binding sites for miR206 (9). Using single molecule FISH (smFISH), we found that most diaphragm MuSC express mainly short isoforms, whereas most limb MuSCs express mainly long isoforms (Fig 2A,B Fig. S2A–C). We observed similar isoform distribution patterns in miR206−/− MuSCs, suggesting that miR206 does not affect Pax3 isoform expression patterns (Fig. S2D,E). This suggests a mechanism by which MuSCs tune Pax3 protein levels. In MuSC cDNA libraries, we observed increased levels of U1 snRNA in limb MuSCs compared to diaphragm MuSCs, but comparable levels of other known alternative polyadenylation factors (Fig. 2C, Fig. S2F). Moreover, using multiple sequence alignments of vertebrate Pax3 paralogs, we identified two conserved motifs upstream of the most proximal polyadenylation site (PAS) that match the consensus sequence for U1 snRNA (Fig. S2G), suggesting U1 snRNA could directly interact with Pax3 mRNA. U1 snRNA is the RNA scaffold of the small nucleolar riboprotein (snRNP) U1, the macromolecular complex that recognizes the 5’ splice site, acting outside of the spliceosome to protect native transcripts from premature transcription termination and polyadenylation (13). Knockdown of U1 snRNA resulted in the expression of shorter transcript isoforms (14), consistent with limb MuSCs expressing longer Pax3 isoforms and higher U1 snRNA levels.
Figure 2: U1 snRNA controls Pax3 length and sensitivity to miR206.
(A,B) MuSCs from wt and Pax3cKO were isolated from indicated muscles and stained with probe libraries complimentary to the open reading frame (ORF) or the 3’ untranslated region (3’UTR) of Pax3. (A) Representative smFISH images with assay schematic shown above; (B) graph of quantification (n=4). (C) MuSCs were analyzed for snRNA levels by RT-PCR. Values are relative to U2 snRNA (n=9). (D) MuSCs from mice treated with control AMOs, U1 site blocking AMOs, or PAS1 blocking AMOs were stained by smFISH and Pax3 isoforms per cell were quantified (n=4). (mean + SEM, 1 tailed student’s t-test, * p<0.05, *** p<0.001)
To evaluate the role of U1 snRNA in alternative polyadenylation in MuSCs, we created reporter genes that express the 3’UTR of Pax3 downstream of GFP (Fig. S2H). Knockdown or overexpression of U1 snRNA led to a reduction or increase of distal amplicons, respectively (Fig. S2I,J). To evaluate the role of U1 snRNA in MuSCs in vivo, we treated mice systemically with AMOs to knock down U1 snRNA and observed a decrease in long Pax3 isoforms in limb MuSCs, with no significant change in diaphragm MuSCs (Fig. S2K,L,M).
To test whether U1 snRNA regulates Pax3 isoform expression through the conserved binding motifs in the Pax3 3’UTR, we designed AMOs complementary to these motifs to compete with U1 snRNA at the Pax3 3’UTR. We observed a switch to shorter Pax3 isoforms in mice treated with these AMOs (Fig. 2D, S2N). We next designed AMOs complementary to two key conserved sequences in the most proximal PAS (PAS1) to compete with key polyadenylation factors and select for a more distal PAS (Fig. S2O). We observed an increase in longer isoforms in diaphragm MuSCs in mice treated with these AMOs (Fig. 2D, S2N). We conclude that U1 snRNA induces the expression of longer Pax3 transcript isoforms in MuSCs in vivo and that it works through conserved motifs within the Pax3 transcript.
We next asked whether changes in Pax3 isoform expression lead to differences in Pax3 protein levels. Since only the long Pax3 isoforms contain miR206 binding sites, only the long isoforms are expected to be sensitive to changes in miR206 levels. We tested this by treating miR206−/− mice with U1 site or PAS1 AMOs and transfecting the purified MuSCs with a miR206 mimic. After 24 hours in vitro, we measured Pax3 protein levels. In limb MuSCs (which express mainly long isoforms) from control AMO-treated mice, miR206 reduced Pax3 protein levels compared to control (miR1) (Fig. 3A). In contrast, limb MuSCs from miR206−/− mice treated with U1 site AMOs, did not show any reduction in Pax3 protein levels, whereas limb MuSCs from miR206−/− mice treated with PAS1 AMOs did show a strong reduction in Pax3 protein in response to miR206 (Fig. 3A). We observed comparable expression patterns in the diaphragm MuSCs, which at baseline express mainly short isoforms (Fig. 3A). Knockdown or overexpression of U1 snRNA led to increased or decreased Pax3 protein levels, respectively, in wt MuSCs but not in miR206−/− MuSCs (Fig. S3A,B).
Figure 3: U1 snRNA controls MuSC activation by controlling Pax3 levels.
(A) MuSCs from miR206−/− mice treated with control, U1 site AMOs, or PAS1 AMOs were transfected with miR206 or control (miR1) and stained for Pax3 (n=6). (B) Different strains of mice (wt and miR206−/−) were treated with increasing amounts of control or U1 knockdown AMOs. MuSCs were isolated and stained for Pax3. Control treated mice are represented by the horizontal black line (n=5). (C) Different strains of mice (wt, miR206−/−, or Pax3cKO) were treated with control AMOs, U1 site AMOs, or PAS1 AMOs, and MuSCs were isolated and stained for Pax3 protein (n=5). (D) Different strains of mice (wt, miR206−/−, or Pax3cKO) were treated with control AMOs, U1 site AMOs, or PAS1 AMOs, and MuSCs were plated in the presence of EdU for 24 hours and stained for EdU (n=5). (mean + SEM, 1-tailed student’s t-test, *p<0.05, **p<0.01, ***p<0.005)
Having shown that longer isoforms are more susceptible to miR206 regulation, we next wanted to test whether such regulation occurs in vivo. Knockdown of U1 snRNA in wt, but not miR206−/−, mice led to a dose-dependent increase in Pax3 protein levels manner (Fig. 3B, S3C). We then treated wt mice with U1 site and PAS1 AMOs. Blocking the U1 snRNA binding sites led to increased Pax3 protein levels in wt, but not miR206−/−, MuSCs, whereas blocking PAS1 led to decreased Pax3 protein levels (Fig. 3C, S3D,E). We conclude that U1 snRNA induces a switch to longer Pax3 isoforms that leads to inhibition of Pax3 protein expression by miR206.
We next asked whether U1 snRNA, by regulating Pax3 protein levels, affects MuSC activation. We treated miR206−/− mice with U1 site or PAS1 AMOs, purified the MuSCs, and transfected them with miR206 in the presence of EdU. For mice treated with PAS1 AMOs, transfection of miR206 led to reduced EdU incorporation in both limb and diaphragm MuSCs. For the mice treated with U1 site AMOs no change in EdU incorporation was observed by miR206 transfection (Fig. S3F). Overexpression of U1 snRNA led to reduced EdU incorporation levels in wt but not miR206−/− or Pax3cKO MuSCs (Fig. S3G).
To provide further evidence for the role of U1 snRNA in the control of homeostatic MuSC activation, we treated wt mice with U1 site AMOs or PAS1 AMOs. Blocking the U1 binding sites led to increased EdU incorporation in wt limb and diaphragm MuSC, whereas blocking the proximal PAS led to decreased EdU incorporation (Fig. 3D). However, the AMOs had no effect in miR206−/− or Pax3cKO mice (Fig. 3D). These data suggest that U1 snRNA controls MuSC activation under homeostatic conditions via alternative polyadenylation of Pax3 (Fig. S3H).
To explore the functional relevance of Pax3 expression in MuSCs in vivo, we conditionally deleted Pax3 in MuSCs in three-month-old mice and saw no difference in MuSC numbers two weeks or nine months later (Fig. S4A–C). Using eYFP as a lineage tracer for MuSCs together with the Pax7Cre-ERT2 driver (Fig. S4D), we observed clear presence of eYFP in myofibers after nine months, with roughly 50% of fibers positive in the diaphragm and 10% positive in the TA of wild type mice (Fig. 4A,B), consistent with previous reports (7, 8). Strikingly, in mice in which Pax3 had been conditionally deleted, fewer diaphragm fibers exhibited evidence of fusion of MuSCs (Fig. 4A,B, S4E). In these mice, we observed a significant decrease in myofiber size in diaphragm and triceps, but not in TA muscles (Fig. 4C, S4F). Consistently, Pax3cKO mice displayed a decrease in grip strength (Fig. S4G) and reduced performance in a treadmill assay (Fig. 4D). We conclude that, in muscles in which MuSCs express high levels of Pax3, Pax3 expression in those cells is critical for structural and functional homeostasis of the tissue.
Figure 4: Pax3 determines MuSC contribution to mature myofibers.
(A-C) TA and diaphragm muscles of wtPax7Cre-ERT2;eYFP/eYFP and Pax3cKO;eYFP/eYFP mice were sectioned nine months after recombination and stained for eYFP (green) and with wheat germ agglutinin (WGA, red) and counterstained with DAPI. (A) Example images with YFP-positive fibers marked *; (B) graph of quantifications of eYFP-positive myofibers (n=6); (C)graph of mean fiber diameter (n=6). (D) Wt and Pax3cKO mice were tested on a treadmill with an endurance running protocol. Total distance run was plotted for individual mice (n=15. (mean + SEM, 2-tailed student’s t-test, ** p<0.01, *** p<0.001)
Previous work revealed different levels of fusion of MuSCs with mature myofibers among various muscles across the body (7, 8). We now provide evidence for a molecular basis of such heterogeneity by showing that Pax3 expression regulates the propensity of MuSCs to contribute to muscle homeostasis. Furthermore, we provide evidence of the molecular mechanisms by which Pax3 is differentially expressed in MuSCs in different muscles. We find that U1 snRNA interacts with Pax3 mRNA to determine transcript isoform expression patterns and, thus, sensitivity to inhibition by miR206. Together, U1 snRNA, miR206, and Pax3 mRNA create a rheostat of Pax3 protein expression that controls spontaneous activation and cell cycle entry of MuSCs under homeostatic conditions.
Supplementary Material
Acknowledgements
We thank Dr. S. Conway for providing Pax3fl/fl mice, Dr. E. Olsen for providing miR206−/− mice, and Dr. C. Keller for providing Pax7CreERT2/CreERT2 mice. We thank Dr. Corey Cain and Dr. Lusijah Rott and the Palo Alto VA Flow Cytometry Core for assistance with flow cytometry experiments.
Funding: This work was supported by funding from the Glenn Foundation for Medical Research, the Muscular Dystrophy Association (MDA313960 to AM), the FSH Society (to AM and TAR), the Lundbeck Foundation (R232-2016-2459 to JF), The Danish Council for Independent Research (5053-00195 to JF), the NIH (R01 AR062185, R37 AG023806, and P01 AG036695 to TAR), and the Departments of Veterans Affairs (BLR&D Merit Review to TAR).
Footnotes
Competing interests
The authors declare no competing interests.
Data and materials availability
All data are available in the manuscript or the supplementary material.
References
- 1.Simons BD, Clevers H, Strategies for homeostatic stem cell self-renewal in adult tissues. Cell (2011), , doi: 10.1016/j.cell.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 2.Cheung TH, Rando TA, Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol (2013), doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Velthoven CTJ, Rando TA, Stem Cell Quiescence: Dynamism, Restraint, and Cellular Idling. Cell Stem Cell (2019), , doi: 10.1016/j.stem.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sambasivan R. et al. , Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 138, 3647–3656 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Lepper C, Partridge TA, Fan C-M, An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 138, 3639–3646 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G, Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 138, 3625–3637 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keefe AC et al. , Muscle stem cells contribute to myofibres in sedentary adult mice. Nat. Commun 6, 1–11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pawlikowski B, Pulliam C, Betta ND, Kardon G, Olwin BB, Pervasive satellite cell contribution to uninjured adult muscle fibers. Skelet. Muscle 5, 1–13 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boutet SC et al. , Alternative Polyadenylation Mediates MicroRNA Regulation of Muscle Stem Cell Function. Cell Stem Cell. 10, 327–336 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Relaix F, Rocancourt D, Mansouri A, Buckingham M, Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes Dev. 18, 1088–1105 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Der Vartanian A. et al. , PAX3 Confers Functional Heterogeneity in Skeletal Muscle Stem Cell Responses to Environmental Stress. Cell Stem Cell (2019), doi: 10.1016/j.stem.2019.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scaramozza A. et al. , Lineage Tracing Reveals a Subset of Reserve Muscle Stem Cells Capable of Clonal Expansion under Stress. Cell Stem Cell (2019), doi: 10.1016/j.stem.2019.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaida D. et al. , U1 snRNP protects pre-mRNAs from premature cleavage and polyadenylation. Nature. 468, 664–8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg MG et al. , U1 snRNP determines mRNA length and regulates isoform expression. Cell. 150, 53–64 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.