Abstract
Objectives:
To test the feasibility, safety, and efficacy of intratracheal delivery of nitric oxide (NO) generated from air by pulsed electrical discharge via a Scoop catheter.
Study design:
We studied healthy 3- to 4-month-old lambs weighing 34±4 kg (mean±SD, n=6). A transtracheal Scoop catheter was inserted through a cuffed tracheostomy tube. U46619 was infused to increase mean pulmonary arterial pressure (mPAP) from 16±1 to 32±3 mmHg (mean±SD). Electrically generated NO was delivered via the Scoop catheter to awake lambs. A sampling line, to monitor NO and nitrogen dioxide (NO2) levels, was placed in the distal trachea of the lambs. The effect of varying doses of electrically generated NO, produced continuously, on pulmonary hypertension was assessed.
Results:
In awake lambs with acute pulmonary hypertension, NO was continuously delivered via the Scoop catheter at 400 ml/min. NO induced pulmonary vasodilation. NO2 levels, measured in the trachea, were below 0.5 ppm at intratracheal NO doses of 10–80 ppm. No changes were detected in the levels of methemoglobin in blood samples before and after 5 min of NO breathing.
Conclusions:
Continuously delivering electrically generated NO through a Scoop catheter produces vasodilation of the pulmonary vasculature of awake lambs with pulmonary hypertension. Transtracheal NO delivery may provide a long-term treatment for patients with chronic pulmonary hypertension as an outpatient without requiring a mask or tracheal intubation.
List of key words: inhaled nitric oxide, transtracheal Scoop catheter, pulsed electrical discharge, pulmonary hypertension, vasodilation
Introduction
Nitric oxide (NO) is an important mediator in the nervous, immune, and vascular systems of the human body. In December 1999, the FDA approved the inhalation of NO gas as a treatment for hypoxic newborns with persistent pulmonary hypertension (PPHN). Since then, NO has been used widely in major medical centers of the developed world to treat pulmonary hypertension in newborns, children, and adults [1; 2; 3; 4]. By 2018, more than a half million Americans with various causes of pulmonary hypertension had received in-hospital NO inhalation therapy. However, the delivery of NO therapy requires gas cylinders and a cylinder distribution network, a complex delivery device to regulate NO, nitrogen dioxide (NO2), and O2 concentrations, and a trained respiratory therapy staff. The cost of providing NO therapy in the USA for 5 days, to an average newborn patient with PPHN, is about $14,000 [5]. To overcome the barriers to treatment with NO, we developed a simple, lightweight, and economical NO generation device, which produces therapeutic levels of NO by pulsed electric discharges in air [5; 6; 7].
The transtracheal Scoop catheter is a small, flexible, plastic tube that can pass through the lower neck into the trachea, to deliver oxygen directly into the lungs. The Scoop catheter was first used to deliver O2 for long-term therapy in 1982 [8]. Since the first use tens thousands of patients in the US have had Scoop catheters inserted into the trachea for gas delivery, including patients with chronic obstructive pulmonary disease (COPD), interstitial lung disease (ILD), congestive heart failure, and patients awaiting heart-lung transplantation [9; 10]. The techniques for safely inserting a Scoop catheter have improved dramatically over the past thirty-seven years. The modified Seldinger technique (MST) [11] and “fast tract” procedure [8; 12] are the most commonly employed methods with few complications. There are several advantages to using the Scoop catheter to deliver O2: (1) the ability to deliver high gas flow rates (up to 12 L/min) of a humidified and warmed O2/air mixture; (2) the opportunity to “buy time” needed to stabilize a patient and thereby avoid tracheal intubation and mechanical ventilation; and (3) the ability to reduce a patient’s “work of breathing”[13], which may be especially beneficial for patients with COPD. Transtracheal oxygen therapy avoids a mask or tracheal intubation which many patients do not want. Because of previous studies demonstrating the benefits of delivering O2 via the Scoop catheter [8; 9; 10], we sought to determine the feasibility, safety, and efficacy of continuously delivering electrically generated NO in air via a Scoop catheter to awake, spontaneously breathing lambs. First, we sought to determine whether continuous intratracheal delivery of electrically generated NO in air would vasodilate the pulmonary vasculature of awake lambs with drug-induced pulmonary hypertension. Second, we measured NO2 and NO levels inside the lamb’s trachea during NO delivery.
Materials and Methods
Measurements of inspired NO, NO2 and O2 levels
To monitor NO and NO2 levels, an NO analyzer (Sievers 280i Nitric Oxide Analyzer, GE Analytical Instruments, Boulder, CO), and a Cavity Attenuated Phase Shift (CAPS) NO2 monitor (Aerodyne Research Inc., Billerica, MA) were used, respectively. An oxygen analyzer (MiniOX I, Ohio Medical Corporation, Gurnee, IL) measured levels of O2.
Schematic of NO generator
The NO generator consists of a mini gas pump, an NO generation chamber controlled via a Bluetooth controller, and an intratracheal Scoop catheter which delivers continuously generated NO in air into the trachea of awake lambs (Fig. 1). The gas pump injects air into the NO generation chamber at a flow rate of 400 ml/min. The NO generation chamber includes an iridium spark plug, an NO2 scavenger consisting of 6.5 g Ca(OH)2, and a 0.22 μm high-efficiency particulate air (HEPA) filter. Energy is stored and released by an autotransformer and is delivered to the spark gap (2 mm) to create a plasma. The discharge of the electrodes is regulated by a microcontroller circuit. The level of NO production was controlled by four pulse pattern variables as previously described [5]: the number of spark groups per second (B); the number of spark discharges per group (N); the pulse duration in μs (P); and the period in μs between sparks (H). The settings of the four pulse variables in this study were B=1, N=210–1340, P=500, and H=250.
Fig. 1.
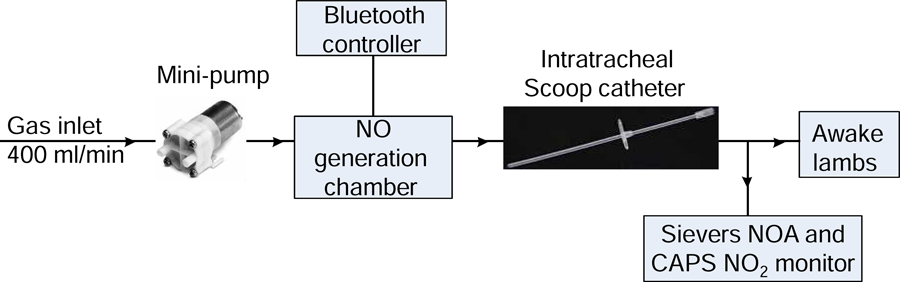
Schematic of NO injection device.
Animal studies
Sheep studies were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee (Boston, MA). We studied 6 healthy 3- to 4-month-old, 34±4 kg (mean±SD), Polypay lambs from a cesarean-derived, specific pathogen-free sheep flock (New England Ovis). One day before each experiment, a complete blood count was performed to confirm the healthy state of each animal. No abnormality in whole blood analysis was measured in any of the lambs. General anesthesia was induced with 5% inhaled isoflurane (1-chloro-2,2,2-trifluoroethyldifluromethyl ether, Baxter) in O2 delivered via a mask and then maintained with 1–4% isoflurane in 50% O2 during surgery. No paralyzing agents were used. During general anesthesia, all lambs were ventilated with a mechanical ventilator (model 7200, Puritan Bennett) at a tidal volume of 400 ml and respiratory rate of 10 to 14 breaths/min. After tracheal intubation, catheters were placed in the femoral artery and the pulmonary artery (via the external jugular vein), and a percutaneous tracheostomy was performed (femoral arterial catheter, Arterial Catheterization Set, Arrow International Inc., Cleveland, OH; pulmonary arterial catheter, 7.5F Swan-Ganz, Edwards Lifesciences, Irvine, CA; tracheostomy kit, Ciaglia Blue Rhino, Cook Medical LLC, Bloomington, IN; cuffed tracheostomy tube, 8.0 mm, Smiths Medical, Dublin, OH). Analgesia was provided intravenously using fentanyl (50 μg) before induction of general anesthesia and buprenorphine (3 mg) was administered during surgery. After tracheal intubation and vascular cannulation, isoflurane was discontinued and the animals were allowed to emerge from anesthesia and fully recover for 2–3 hrs. Awake, spontaneously breathing lambs were then studied. Lactated Ringer solution was administered IV at 10 ml/kg/hr. To induce pulmonary hypertension, the potent pulmonary vasoconstrictor U46619 (Cayman Chemical, Ann Arbor, MI), an analog of the endoperoxide prostaglandin H2, was infused intravenously at a rate of 0.8–0.9 μg/kg/min to increase the mean pulmonary arterial pressure (mPAP). All hemodynamic measurements and blood samples were obtained at baseline, during and at the end of each NO treatment. The mean arterial pressure and mPAP were continuously monitored using a Gould 6600 amplifier system (Gould Electronics, Inc., Eastlake, OH). Pulmonary capillary wedge pressure (PCWP), heart rate, and cardiac output were measured at baseline, during U46619 infusion, and before, during and after inhalation of NO. Cardiac output was assessed by thermal dilution as the average of three measurements after an intravenous bolus injection of 10 ml of ice-cold saline solution. Pulmonary vascular resistance (PVR) was calculated using a standard formula of [(mPAP-PCWP)/CO]*80.
The tracheostomy was performed to allow the insertion of a gas sampling line (ID=1 mm) into the distal trachea (just above carina) of a spontaneously breathing lamb. Thus, we could monitor the levels of NO and NO2 directly inside the trachea. A Scoop catheter (ID=2 mm, OD=3 mm, length=11 cm, Scoop, Transtracheal Technologies, Englewood, CO) was inserted via the cuffed tracheostomy tube (Fig. 2). A distance of 2.5–3 cm between the tips of the sampling line and the Scoop catheter was sufficient to avoid directly sampling the NO gas delivered from the Scoop catheter. To ease the irritation that the sampling line caused inside trachea of awake lambs, 2% lidocaine (1–2 ml) was injected into the trachea every 2 hours. During NO delivery, the settings of the NO generator were adjusted to produce the desired intratracheal NO concentrations (10, 20, 40, 60, and 80 ppm). Three lambs received increasing doses of NO (from 10–80 ppm) and the other 3 lambs received decreasing doses of NO (from 80–10). With the NM3 Respironics airway flowmeter (Philips Healthcare, Thornton, CO), we measured tidal volume, the I:E ratio, and respiratory rate in awake, spontaneously breathing lambs.
Fig. 2.
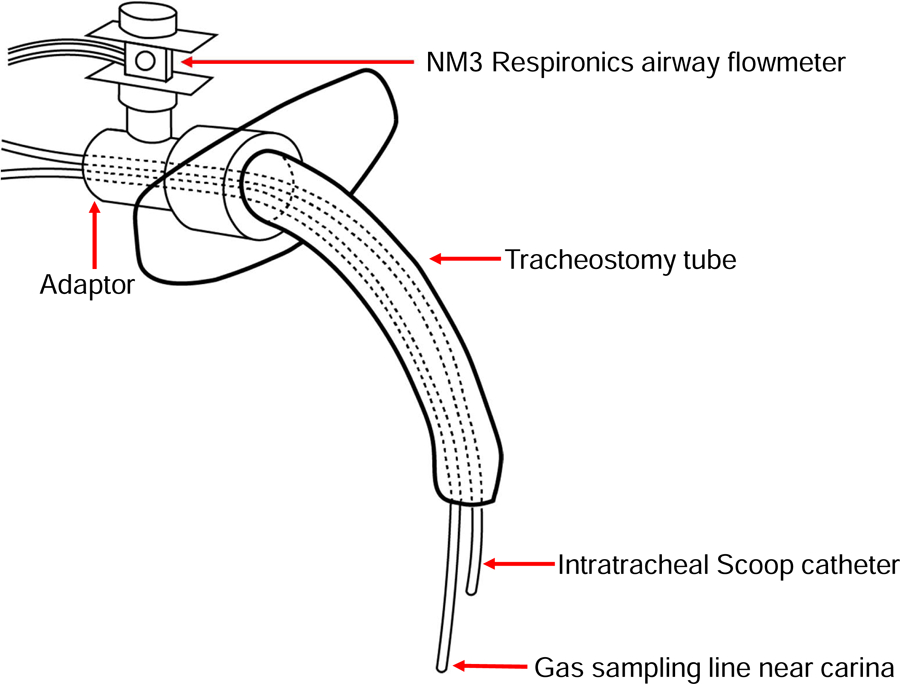
Diagram of the intratracheal Scoop catheter
The mPAP response to each level of NO delivery was continuously measured for 5 min. At the end of each trial, the mPAP was measured for an additional 3 min after NO production and delivery was discontinued. A 5 to 10 min interval, with the animal breathing air, was allowed before the next concentration of NO was tested. During continuous NO production, NO was measured in the distal trachea by chemiluminescence and NO2 levels was measured using CAPS. Sample gas was continuously withdrawn from the trachea at 300 ml/min and the peak values of sampled NO and NO2 were reported. Plasma levels of nitrate and nitrite were measured before and after breathing 80 ppm of NO for 5 min using chemiluminescence method, as previously described [14].
Statistical analysis
All variables were found to be normally distributed by the Shapiro-Wilk test and are expressed as mean±SD. A two-way analysis of variance with repeated measures was performed to determine the effect of breathing electrically generated NO delivered via a Scoop catheter on the mPAP and PVR of lambs with pulmonary hypertension (GraphPad Prism v.6; GraphPad Software Inc.). Two-tailed analyses were performed, and a P-value < 0.05 was considered significant.
Results
Electrically-generated NO delivered through a Scoop catheter reduces mPAP and PVR in awake lambs with acute pulmonary hypertension
In this study, we investigated whether electrically generated NO could be used to deliver intratracheally to awake, ambulatory sheep with drug-induced pulmonary hypertension. Awake, spontaneously breathing lambs received a continuous IV infusion of the thromboxane analog U46619, which increased mPAP from 16±2 to 32±3 mmHg. The lambs spontaneously breathed with an average tidal volume of 154±27 ml, and the I:E ratio varied from 1:1.4 to 1:2.0, at an average respiratory rate of 41±10 breaths/min. The mPAP was subsequently measured while lambs breathed air and 10, 20, 40, 60, or 80 ppm NO delivered through a Scoop catheter. The amount of NO produced by the NO generator was adjusted based on the measured level of NO in the trachea. Breathing electrically generated NO at 20, 40, 60, or 80 ppm reduced the mPAP beginning 1 min after initiating production of NO. Breathing 20–80 ppm NO decreased mPAP by 20% at 20 ppm, 23% at 40 ppm, 25% at 60 ppm, and 35% at 80 ppm, respectively (P<0.05, mPAP before vs after NO breathing, Fig. 3A). After the NO generator was turned off, mPAP returned to 31±4 mmHg within 1 min. Thus, intratracheal delivery of electrically generated NO (20–80 ppm) decreased PVR in lambs with acute pulmonary hypertension (Fig. 3B).
Fig. 3.
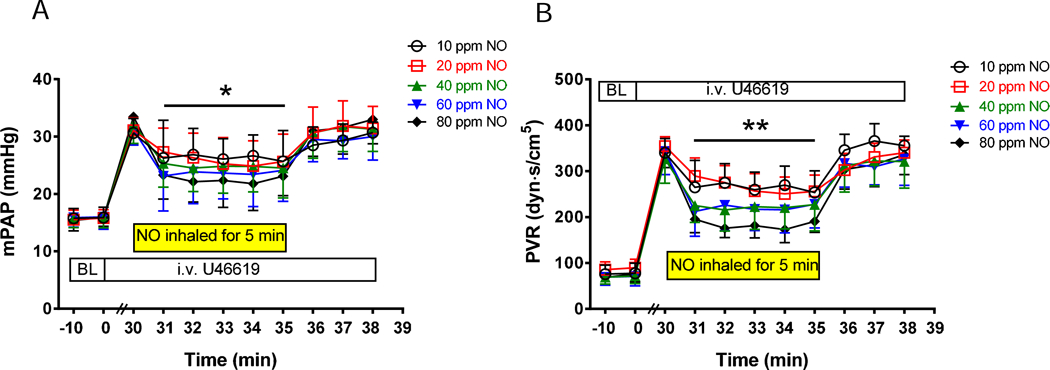
Breathing NO, generated continuously and delivered through an intratracheal catheter, reduces mPAP and PVR in awake lambs with acute pulmonary hypertension (n=6). Pulmonary hypertension was induced by IV administering U46619 to lambs for 30 min before treatment with NO (baseline (BL)). Electrically generated NO was administered at 30 min for 5 min and mPAP (A) and PVR (B) were measured each min before, during, and after breathing NO, at concentrations ranging from 10–80 ppm. *P<0.05 for time 31 to 35 min compared to 30 min (before NO breathing) at NO of 20, 40, and 60 ppm; P<0.001 for time 31 to 35 min versus 30 min at NO of 80 ppm. **P<0.05 for time 31 to 35 min versus 30 min (before NO breathing) at NO of 20, 40, and 60 ppm; P<0.01 for time 31 to 35 min versus 30 min at NO of 80 ppm.
All lambs tolerated the Scoop catheter well and neither sedation nor restraints were required to prevent the animals from displacing the catheter. MAP, heart rates, and cardiac output were measured at baseline, and (with continuous i.v. infusion of U46619) before, during and after inhalation of NO (Table 1). Continuous infusion of U46619 increased MAP from 98±6 to 119±9 mmHg. NO breathing did not affect MAP, heart rate, or cardiac output. After breathing NO (80 ppm) for 5 min, the plasma nitrate level increased from 7.2±2.1 to 14.1±3.2 μM (n=5, P<0.05), and the plasma nitrite level increased from 0.4±0.1 to 0.7±0.2 μM (n=5, P<0.05). These results are consistent with previously published data [15; 16]. The NO2 levels sampled from the lambs’ trachea were below 0.5 ppm with tracheal delivery of NO between 10–80 ppm (Fig. 4). In addition, no significant changes were found in the levels of methemoglobin before and after breathing NO (Table 2). Taken together, the results show that electrically-generated NO can be delivered through a Scoop catheter to treat acute pulmonary hypertension in awake, ambulatory sheep.
Table 1.
Hemodynamic measurements before, during, and after NO inhalation in awake lambs (n=6)
| NO dose (ppm) | MAP (mmHg) | Heart rate (BMP) | Cardiac Output (L/min) | |
|---|---|---|---|---|
| Baseline (without NO) | 98±6 | 101±10 | 5.2±1.6 | |
| 80 | Pre-NO (with i.v. U46619) | 119±7 | 96±9 | 4.1±0.7 |
| During NO | 116±5 | 98±10 | 4.3±1.1 | |
| Post-NO | 121±4 | 92±11 | 4.6±0.8 | |
| 60 | Pre-NO (with i.v. U46619) | 116±8 | 95±8 | 5.0±1.3 |
| During NO | 118±4 | 90±10 | 4.5±0.4 | |
| Post-NO | 125±5 | 93±8 | 4.9±0.6 | |
| 40 | Pre-NO (with i.v. U46619) | 119±3 | 110±11 | 4.5±0.9 |
| During NO | 115±6 | 97±12 | 5.5±0.6 | |
| Post-NO | 116±5 | 95±9 | 5.2±0.7 | |
| 20 | Pre-NO (with i.v. U46619) | 122±6 | 92±9 | 5.4±1.2 |
| During NO | 120±5 | 90±8 | 5.2±1.7 | |
| Post-NO | 119±7 | 94±6 | 4.9±1.3 | |
| 10 | Pre-NO (with i.v. U46619) | 122±8 | 102±5 | 4.7±1.2 |
| During NO | 119±6 | 99±7 | 5.1±0.9 | |
| Post-NO | 123±5 | 110±6 | 5.7±1.6 | |
Fig. 4.
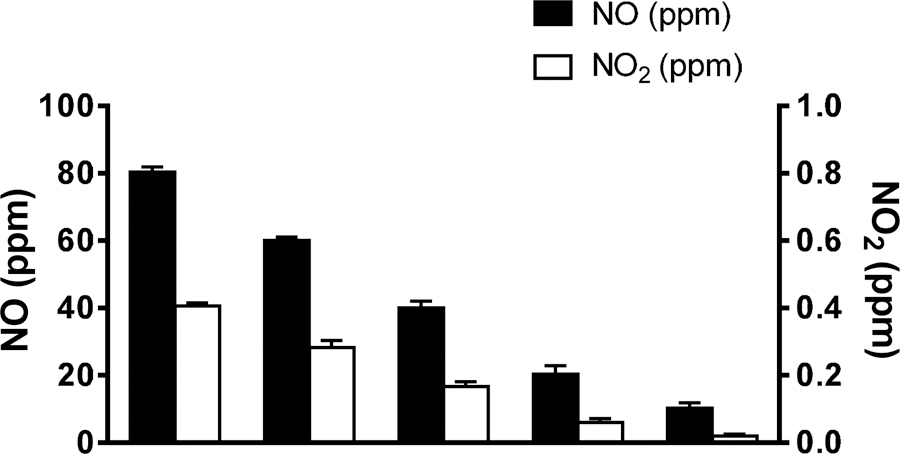
The level of NO2 was sampled inside the trachea near the carina, while NO was delivered at 400 ml/min in air via the Scoop catheter to awake lambs (n=6).
Table 2.
The levels of metHb before and after NO breathing (n=6)
| Awake lambs | MetHb (%) |
|---|---|
| baseline | 0.6±0.1 |
| After iNO (80 ppm, 5 min) | 1.0±0.5 |
| After iNO (60 ppm, 5 min) | 1.1±0.4 |
| After iNO (40 ppm, 5 min) | 1.0±0.3 |
| After iNO (20 ppm, 5 min) | 0.8±0.2 |
| After iNO (10 ppm, 5 min) | 0.8±0.1 |
iNO: inhalation of NO
Discussion
In this study, we found that NO delivered through a Scoop catheter vasodilated the pulmonary vasculature in awake, spontaneously breathing, lambs with drug-induced pulmonary hypertension. The gas sampling line, placed inside the lamb’s distal trachea via the tracheostomy tube, measured the level of NO delivered to the animals. Electrically generated NO (20–80 ppm) decreased mPAP and PVR in awake ambulatory lambs with pulmonary hypertension. Breathing 20 ppm, but not 10 ppm, significantly reduced pulmonary hypertension, showing that 20 ppm was the minimally effective dose. All of the lambs tolerated the Scoop catheter without requiring sedation. The data demonstrate that it is feasible and effective to deliver electrically generated NO through a Scoop catheter to vasodilate the pulmonary vasculature.
NO reacts with O2 to form NO2. In the current study, a 6.5 gram Ca(OH)2 scavenger was sufficient to maintain the tracheal NO2 levels below 0.5 ppm for all NO doses. This level of NO2 is significantly below the 1 ppm limit set by the Occupational Safety and Health Administration for daily 8 hour exposure [17]. Delivering NO directly into the trachea is important, because it decreases dead space and thereby reduces the production of NO2 and reduces the rate of consumption of the NO generator’s NO2 scavenger.
Various electrical systems have been developed and used to produce NO for biomedical purposes, including pulsed arc [18; 19; 20; 21], gliding arc [22], dielectric barrier [23; 24], microwave [25], corona [26], radio frequency induced coupled discharge [27], and non-thermal atmospheric pressure high-frequency plasma discharge [28; 29]. These methods produce large amounts of NO2 and O3 as toxic byproducts requiring complex purification systems. We have developed a simple and safe method to generate NO from air by pulsed electrical discharge [5; 6; 7]. NO can be delivered through a ventilator, face mask or nasal cannula. However, several problems associated with current delivery systems prevent inhaled NO therapy in ambulatory patients outside of the hospital. These problems include the high flow rates that are required for face mask NO delivery and the side effects of chronic gas delivery through a nasal cannula including ear irritation, sinus infection, and recurrent epistaxis [12]. The delivery of oxygen through a transtracheal Scoop catheter has been used for nearly 40 years to provide long-term oxygen therapy to patients with chronic hypoxemia. The transtracheal Scoop catheter provides an alternative to a face mask or nasal cannula, and has been used to deliver oxygen for patients with COPD, ILD, pulmonary fibrosis, and other advanced lung diseases. Transtracheal oxygen therapy permits the use of gas flow rates up to 12 L/min, and decreases the patient’s work of breathing by reducing the anatomic dead space of the airway. Transtracheal oxygen therapy reduces the oxygen amount required for face mask or nasal cannula about 50% at rest and 30% with exercise, this allows use of smaller, lighter ambulatory units with extended functional capacity [10]. Because transtracheal therapy permits the use of decreased oxygen flow rates, patients are no longer confined to their homes and have an improved quality of life [30]. In a previous study, Snell and colleagues delivered 40 ppm of NO via a Scoop catheter to a patient with end-stage pulmonary hypertension for 68 days as a “bridge” to heart-lung transplantation [9]. The transtracheal route of NO administration proved acceptable to the patient and, compared to NO delivered by mask or nasal cannula, reduced episodes of sudden NO withdrawal. Our previous studies demonstrated that a single HEPA filter and a 12 g Ca(OH)2 scavenger effectively removed all metal particles and the potential toxic NO2 gas during NO generation [6]. We reported that mice, exposed to breathing electrically generated NO (50 ppm, 28 days), did not develop pulmonary inflammation or structural changes in the airway. NO generated electrically from the electric NO device makes NO breathing a feasible treatment for long-term therapy.
In conclusion, the recent development of an electric, lightweight NO generation device makes it practical to deliver NO therapy for prolonged periods to ambulatory patients. Transtracheal delivery of NO may provide an alterative for patients who require long-term NO therapy, but cannot tolerate a face mask or nasal cannula.
Highlights:
Electrically generated NO was used to treat pulmonary hypertension
NO was delivered by transtracheal delivery via a Scoop catheter
Delivering electrically generated NO via a Scoop catheter permits long-term therapy
The minimal effective dose of NO was 20 ppm
Acknowledgments
Source of funding and conflict of interest statement:
This study was partially supported by grants from NHLBI B-BIC/NCAI (#U54HL119145) to W.M.Z., an NHLBI grant (#R21HL130956) to B.Y., NIH/NHLBI grant #1K23HL128882-01A1 to L.B., and funds from the German Research Foundation (DFG) #FI2429/1-1 to A.F. and #WI5162/2-1 to S.B.W. This study was also supported by laboratory funds of the Anesthesia Center for Critical Care Research of the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital (MGH). W.M.Z. and B.Y. received patents at MGH on the electric generation of nitric oxide (NO). W.M.Z. is on the scientific advisory board of Third Pole Inc., which has licensed patents on electric NO generation from MGH. Other authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Roberts JD, Polaner DM, Lang P, Zapol WM, Inhaled nitric oxide in persistent pulmonary hypertension of the newborn, Lancet 340 (1992) 818–9. [DOI] [PubMed] [Google Scholar]
- [2].Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, Roy BJ, Keszler M, Kinsella JP, Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group, N. Engl. J. Med 342 (2000) 469–74. [DOI] [PubMed] [Google Scholar]
- [3].Kinsella JP, Neish SR, Shaffer E, Abman SH, Low-dose inhalation nitric oxide in persistent pulmonary hypertension of the newborn, Lancet 340 (1992) 819–20. [DOI] [PubMed] [Google Scholar]
- [4].Abman SH, Inhaled nitric oxide for the treatment of pulmonary arterial hypertension, Handb. Exp. Pharmacol 218 (2013) 257–76. [DOI] [PubMed] [Google Scholar]
- [5].Yu B, Muenster S, Blaesi AH, Bloch DB, Zapol WM, Producing nitric oxide by pulsed electrical discharge in air for portable inhalation therapy, Sci Transl Med 7 (2015) 294ra107. [DOI] [PubMed] [Google Scholar]
- [6].Yu B, Blaesi AH, Casey N, Raykhtsaum G, Zazzeron L, Jones R, Morrese A, Dobrynin D, Malhotra R, Bloch DB, Goldstein LE, Zapol WM, Detection and removal of impurities in nitric oxide generated from air by pulsed electrical discharge, Nitric Oxide 60 (2016) 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yu B, Ferrari M, Schleifer G, Blaesi AH, Wepler M, Zapol WM, Bloch DB, Development of a portable mini-generator to safely produce nitric oxide for the treatment of infants with pulmonary hypertension, Nitric Oxide 75 (2018) 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Christopher KL, Schwartz MD, Transtracheal oxygen therapy, Chest 139 (2011) 435–440. [DOI] [PubMed] [Google Scholar]
- [9].Snell GI, Salamonsen RF, Bergin P, Esmore DS, Khan S, Williams TJ, Inhaled nitric oxide used as a bridge to heart-lung transplantation in a patient with end-stage pulmonary hypertension, Am J Respir Crit Care Med 151 (1995) 1263–6. [DOI] [PubMed] [Google Scholar]
- [10].O’Donohue WJ Jr., Transtracheal oxygen: a step beyond the nasal cannula for long-term oxygen therapy, Nebr Med J 77 (1992) 291–5. [PubMed] [Google Scholar]
- [11].Mehta AC, Stelmach K, Khalil HY, Insertion of a pre-SCOOP transtracheal oxygen therapy catheter with use of a Raulerson syringe, Chest 101 (1992) 887–8. [DOI] [PubMed] [Google Scholar]
- [12].Christopher KL, Transtracheal oxygen catheters, Clin Chest Med 24 (2003) 489–510. [DOI] [PubMed] [Google Scholar]
- [13].Nahum A, Tracheal gas insufflation, Crit Care 2 (1998) 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Moore DF, Scott LT, Gladwin MT, Altarescu G, Kaneski C, Suzuki K, Pease-Fye M, Ferri R, Brady RO, Herscovitch P, Schiffmann R, Regional cerebral hyperperfusion and nitric oxide pathway dysregulation in Fabry disease: reversal by enzyme replacement therapy, Circulation 104 (2001) 1506–12. [DOI] [PubMed] [Google Scholar]
- [15].Yu B, Volpato GP, Chang K, Bloch KD, Zapol WM, Prevention of the pulmonary vasoconstrictor effects of HBOC-201 in awake lambs by continuously breathing nitric oxide, Anesthesiology 110 (2009) 113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Baron DM, Yu B, Lei C, Bagchi A, Beloiartsev A, Stowell CP, Steinbicker AU, Malhotra R, Bloch KD, Zapol WM, Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide, Anesthesiology 116 (2012) 637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].C.f.D.C.a. Prevention, 1988 OSHA PEL project documentation, Atlanta, 1989. [Google Scholar]
- [18].Sakai S, Matsuda M, Wang D, Kiyan T, Namihira T, Akiyama H, Okamoto K, Toda K, A compact nitric oxide supply for medical application, IEEE 1 (2007) 752–755. [Google Scholar]
- [19].Hu H, Liang H, Li J, Zhao Q, He J, Study on production of inhaled nitric oxide for medical applications by pulsed discharge, IEEE Trans. Plasma Sci 35 (2007) 619–625. [Google Scholar]
- [20].Qin Y, Zajda J, Brisbois EJ, Ren H, Toomasian JM, Major TC, Rojas-Pena A, Carr B, Johnson T, Haft JW, Bartlett RH, Hunt AP, Lehnert N, Meyerhoff ME, Portable Nitric Oxide (NO) Generator Based on Electrochemical Reduction of Nitrite for Potential Applications in Inhaled NO Therapy and Cardiopulmonary Bypass Surgery, Mol Pharm 14 (2017) 3762–3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li SR, Huang YF, Liu Z, Sui MH, Liu JM, Yan KP, Production of medically useful nitric monoxide using AC arc discharge, Nitric Oxide 73 (2018) 89–95. [DOI] [PubMed] [Google Scholar]
- [22].Benstaali B, Boubert P, Cheron BG, Addou A, Brisset JL, Denstity and rotational temperature measurements of the OH and NO radicals produced by a gliding arc in humid air, Plasma Chem. Plasma Process 22 (2002) 553–571. [Google Scholar]
- [23].Stefanovic I, Bibinov NK, Deryugin AA, Vinogradov IP, Napartovich AP, Wiesemann K, Kinetics of ozone and nitric oxides in dielectric barrier discharges in O2/NOx and N2/O2/NOx mixtures, Plasma Sources Sci. Technol 10 (2001) 406–416. [Google Scholar]
- [24].Kornev K, Yavorovsky N, Preis S, Khaskelberg M, Isaev U, Chen BN, Generation of active oxidant species by pulsed dielectric barrier discharge in water-air mixtures, J. Int. Ozone Assoc 28 (2006) 207–215. [Google Scholar]
- [25].Wojtowicz MA, Miknis FP, Grimes RW, Smith WW, Serio MA, Control of nitric oxide, nitrous oxide, and ammonia emissions using microwave plasmas, J. Hazard Mater 74 (2000) 81–9. [DOI] [PubMed] [Google Scholar]
- [26].Hill RD, Rahmim I, Rinker RG, Experimental study of the production of nitric oxide, nitrous oxide, and ozone in a simulated atmospheric corona, Ind. Eng. Chem. Res 27 (1988) 1264–1269. [Google Scholar]
- [27].Stoffels E, Gonzalvo YA, Whitmore TD, Seymour DL, Rees JA, A plasma needle generates nitric oxide, Plasma Sources Sci. Technol 15 (2006) 501–506. [Google Scholar]
- [28].Kuhn S, Bibinov N, Gesche R, Awakowicz P, Non-thermal atmospheric pressure HF plasma source: generation of nitric oxide and ozone for bio-medical applications, Plasma Sources Sci. Technol 19 (2010) 1–8. [Google Scholar]
- [29].Matsuo K, Yoshida H, Choi J, Hamid R, Hosseini R, Namihira T, Katsuki S, Akiyama H, Nitric oxide generated by atmospheric pressure air microplasma, IEEE 8 (2009) 999–1003. [Google Scholar]
- [30].Siddiqui FM, Campbell S, Ie S, Biscardi F, Rubio E, Three decades of transtracheal oxygen therapy: A review of the associated complications with an illustrative case presentation, Lung India 34 (2017) 448–451. [DOI] [PMC free article] [PubMed] [Google Scholar]


