Abstract
Etiology of adolescent idiopathic scoliosis (AIS), a complicated three-dimensional spinal deformity with early-onset, receives continuous attention but remains unclear. To gain an insight into AIS pathogenesis, this review searched PubMed database up to June 2019, using key words or medical subject headings terms including “adolescent idiopathic scoliosis,” “scoliosis,” “pathogenesis,” “etiology,” “genetics,” “mesenchymal stem cells,” and their combinations, summarized existing literatures and categorized the theories or hypothesis into nine aspects. These aspects include bone marrow mesenchymal stem cell studies, genetic studies, tissue analysis, spine biomechanics measurements, neurologic analysis, hormone studies, biochemical analysis, environmental factor analysis, and lifestyle explorations. These categories could be a guidance for further etiology or treatment researches to gain inspiration.
Keywords: Scoliosis, Pathogenesis, Etiology, Mesenchymal stem cells
Introduction
Adolescent idiopathic scoliosis (AIS) is a three-dimensional spine deformity that takes place at the early age around 11 to 18 years, which is the most common type of idiopathic scoliosis in children. The prevalence of AIS is 0.47% to 5.20% around the world,[1] A review by Qiu[2] showed the incidence of scoliosis in the Chinese population varied from 0.6% to 2.0%, while 90% of them were AIS. Zheng et al[3] from China showed the prevalence of AIS is 2.4%, which is higher in girls.[1]
Various theories are trying to explain the pathogenesis of AIS, which contains the initiation and the progression of AIS. The latest papers regarding AIS pathogenesis mostly focus on the genetic factors, while there are still numerous theories explaining the pathogenesis from other factors. We classified the theories into the following groups to make a better understanding of the multifactorial pathogenesis of AIS: genetics, mesenchymal stem cells, tissues, spine biomechanics, neurology, hormones, biochemistry, environment, and lifestyle. We also showed a previous theory which tried to integrate multiple former studies.
This review searched PubMed database up to June 2019, using keywords or medical subject headings terms including “adolescent idiopathic scoliosis,” “scoliosis,” “pathogenesis,” “etiology,” “genetics,” “mesenchymal stem cells,” and their combinations, summarized existing literatures and categorized the theories or hypothesis into nine aspects. Literatures relevant to AIS pathogenesis which mainly published in recent 10 years were included. Publications focusing on AIS diagnosis, bracing treatment or surgical techniques were excluded.
Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs)
BM-MSCs possess multipotency of differentiating into osteoblasts, chondrocytes, or adipocytes.[4] Decreased osteogenetic ability of MSCs and inclination of MSCs towards adipogenic differentiation have been revealed in AIS patients.[5,6] The resultant low bone mineral density status has also been reported in AIS patients,[7–9] implying that bone marrow-derived MSCs may regulate bone mass formation in AIS patients, thereby participating in AIS development.
At the expression level, in a proteomic analysis of MSCs from AIS patients, five bone growth-related proteins including pyruvate kinase M2 (PKM2), annexin A2, heat shock 27 k protein (HSP27), γ-actin, and β-actin were identified to be altered.[10] In this study by Zhuang et al,[10] up-regulated PKM2 was speculated as associated with increased cell proliferation, while down-regulated annexin A2, HSP27, γ-actin, and β-actin were speculated as associated with the diminished ossification process and low bone mass status. In a further microarray and pathway analysis study of Zhuang et al,[11] several differentially expressed genes were discovered, down-regulated mitogen-activated protein kinase kinase 1, heat shock 70 kDa protein 6, and up-regulated SMAD family member 3 were speculated to inhibit the osteogenic differentiation, while up-regulated homeobox C6/9 affected the global patterning of vertebrate axial skeleton, other dysregulated genes including general transcription factor IIi, CREB binding protein, phosphoinositide-3-kinase, regulatory sub-unit 2, and dual-specificity phosphatase 2 may also play roles in osteogenesis and bone formation. Chen et al[12] discovered the expression of melatonin receptors in AIS MSCs were down-regulated, which may lead to the reduction of response to melatonin treatment, since melatonin increases alkaline phosphatase activity and glycosaminoglycan (GAG) synthesis and other differentiation-related genes expression, lack of response to melatonin might alter this process and then influence membranous and endochondral ossification. Leptin receptors in AIS MSCs were also found to be down-regulated in another study, which might result in hyposensitivity of MSCs to circulating leptin.[5] Thyroid hormone-inducible nuclear protein (Spot14) and its messenger RNA (mRNA) were found to be more expressed in adipogenic MSCs from AIS patients than controls, and the higher expression was also found in the AIS patients’ adipose tissue, which also reflected the abnormal adipogenic differentiation.[13] Lower expression of mitogen-activated protein kinase 7 (MAPK7) was also identified in AIS MSCs, which might result in disturbance of MSCs osteogenic differentiation.[14] G protein-coupled receptor 126 (GPR126) gene was showed to have higher expression in the vertebral bodies from the convex side of scoliosis, knocking down of GPR126 would promote MSCs ossification.[15]
At the epigenetic level, long non-coding RNA (lncRNAs) and microRNAs (miRNAs) were analyzed in previous studies. LncRNAs are the transcripts that have the length longer than 200 nucleotides, which do not contain any functional open reading frame, lncRNAs can regulate gene expression by interfering the chromatin modification, transcriptional/post-transcriptional regulation. LncRNAs are expressed differently in different types of tissues and cells, a review has discussed the existence of relationships between lncRNAs of MSCs and various bone-related diseases such as osteoporosis, osteosarcoma and ankylosing spondylitis, it was speculated that lncRNAs participates in osteogenic differentiation of MSCs.[16] By applying microarray analysis of BM-MSCs in AIS patients and the control groups, Zhuang et al[17] have identified a novel lncRNA (ENST00000453347) that was prominently down-regulated, which was later named as lncAIS, in normal conditions, lncAIS was reported to maintain the stability of Homeobox D8 mRNA by interacting with nuclear factor 90 protein and further enhance Runt-related transcription factor 2 (RUNX2) transcription [Figure 1], and the down-regulation of lncAIS would inhibit RUNX2 expression, thus alter the osteogenic differentiation of MSCs, and finally result in osteopenia in AIS patients. RUNX2 as a transcription factor was also previously reported to participate in reduced bone mineral density (BMD) in AIS patients.[18] miRNAs are also non-coding RNA which are shorter than 20 nucleotides, miRNAs target specific mRNA and resulted in changes in gene expression. Through analysis of miRNA expression profile, gene ontology terms and Kyoto encyclopedia of genes and genomes, a novel study of Hui et al[19] has identified seven most significantly central up-regulated miRNA in AIS BM-MSCs including miR-17-5p, miR-106a-5p, miR-106b-5p, miR-16-5p, miR-93-5p, and miR-181b-5p, which could suppress osteogenic differentiation and bone formation, while miR-15a-5p could regulate cell apoptosis.
Figure 1.
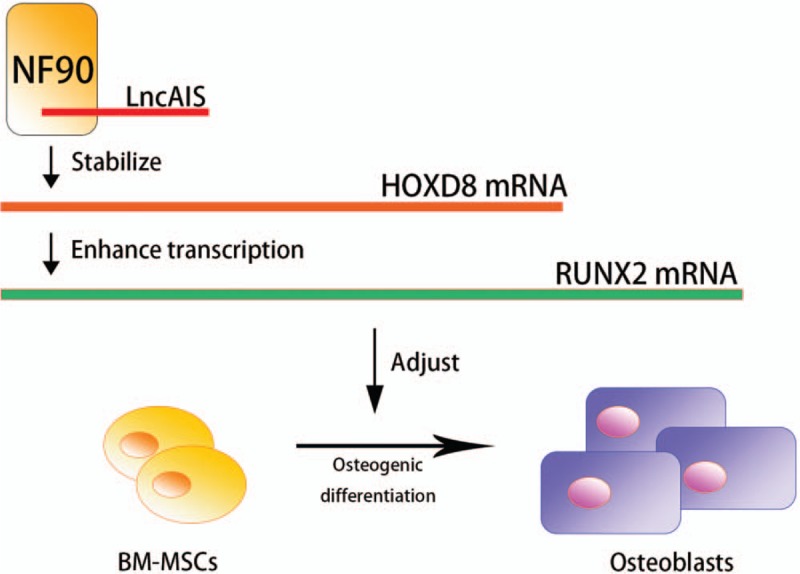
LncAIS interacts with NF90, stabilizing the HOXD8 mRNA and further enhances the transcription of RUNX2, which alters the osteogenic differentiation process of BM-MSCs. BM-MSCs: Bone marrow-derived mesenchymal stem cells; HOXD8: Homeobox D8; LncAIS: Long non-coding AIS (gene symbol: ENST00000453347); NF90: Nuclear factor 90; RUNX2: Runt-Related transcription factor 2.
Genetics
Previous twin study of Simony et al,[20] aiming to found out the heritability of AIS, showed a higher concordance rate in monozygotic pairs (0.13) than dizygotic pairs (0.00). The higher prevalence in female inspired studies investigating the possibility of X-linked inheritance, a study by Justice et al[21] gave evidence of a region on the X chromosome that might be linked to AIS, another study by Ward et al[22] indicated the polygenic inheritance of AIS, the study also showed male to male transmission examples that negated the former X-linked heritance hypothesis. Despite the controversies of inheritance pattern, current researches showed that chromosome abnormality, variations of gene loci caused primary expressional alterations, and the epigenetic changes resulted from environmental factors further regulated the gene expression; these elements worked together inducing a dysfunction of cell activities, which further led to AIS development.
Chromosome abnormality and gene variations
Linkage and association studies are primary techniques to analyze the genotype-phenotype relationship.[23] linkage analysis identified mutants in gene loci such as MAPK7[24] and allele marker DS1034 on chromosome 19p13.3,[25] both of which were relevant to AIS etiology.
Genome-wide association study (GWAS) is another powerful tool to analyze the relationship between single nucleotide polymorphism (SNPs) in gene loci and phenotypes, it was widely used in genetic studies of polygenic diseases, a study by Ogura et al[26] showed ladybird homeobox 1 (LBX1), were related to AIS pathogenesis. GWAS was also used to analyze the copy number variants (CNV) associated with AIS, it has been revealed that CNV in chromosome 1q21.1, 2q13, 15q11.2, 16p11.2 harbor in AIS.[27] It is worth mentioning that Liu et al[28] found SNPs and CNV (such as deletion in TBX6) could also locate at one locus thus distort the calculations of the significance in associating SNPs to AIS.
Asides from the above gene loci where SNPs harbor, other gene loci including cell adhesion molecule L1 like, fibrillin (FBN), GPR126, insulin-like growth factor 1, LBX1, matrilin-1, matrix metalloproteinases-3, and interleukin-6 (IL-6), paired box 1, proteome of centrioles 5, transforming growth factor beta 1 (TGFB1), VANGL planar cell polarity protein 1 were shown to be possibly related with the pathogenesis of AIS.[29–38] Recently, Zhang et al[39] found a mutation of uts2ra could cause spinal curvature in zebrafish, the mutation could down-regulate the urotensin neuropeptides receptors of slow-twitch muscle fibers of the somite and; therefore, change the straightening of vertebrae axis process. Further researches can investigate the expressional alterations and regulation of these genes in AIS patients.
Epigenetics
The definition of epigenetics is the change in DNA or the paired proteins, with DNA sequence variation excluded, epigenetics plays roles in gene expression and cell division.[40] A study by Fendri et al[41] using microarray analysis and quantitative Reverse-transcription PCR found 145 genes differently expressed in AIS osteoblasts, which might be related with epigenetic regulation.
At the DNA methylation level, in the study by Mao et al,[42] the positive methylation on the cartilage oligomeric matrix protein (COMP) promoter resulted in the low expression of the COMP gene, which influenced the bone formation, correlating with young chronological age and high cobb angle of main curve. Meng et al[43] found the hypo-methylation of site cg01374129, which located near HAS2 gene, was reported to have a negative correlation with AIS curve severity. The positive methylation in the promoter of pituitary homeobox 1 gene was also related to larger Cob angles of main curve.
At the non-coding RNAs level, miRNAs and lncRNAs were also reported to be related with the pathogenesis,[17,19,44–46] the epigenetic alterations were located in both peripheral blood and MSCs.
Tissues
Bone
Histological methods, computed tomography scan with higher resolution[47] and reconstruction were used, identified the bone formation abnormalities at morphologic level which is in accordance with low BMD status. In the study of Tanabe et al,[48] from the histological sections of the spinous process taken from AIS patients, 67% showed sub-normal bone volume and 76% showed a high bone turnover rate. It has also been revealed from a study by Wang et al[49] that AIS patients with low or normal BMD both have abnormal bone quality and it might be resulting from altered endocortical apposition.
Muscle
Studies of paravertebral muscles have implied the alteration and asymmetry of paravertebral muscles caused the inharmony of the posture and movement control of the spine, thus resulted in AIS progression. A study by Acaroglu et al[50] showed a higher concentration of calmodulin in muscle tissue of the convex side, which might affect the contractility of muscles. In the study by Wang and Pessin,[51] through the biopsy from AIS patients, muscles of convex side have an increased portion of type I fibers than type II while a decreased portion was found in the concave side, Type I fiber has higher fatigue resistant but slower contractile speed, and may occur under long duration,[52] Stetkarova et al[53] considered that this change might be a secondary adaption to the higher load demand on the convex side, and are related with curve progression. Moreover, the alterations of paravertebral muscle have been verified at the genetic level, Buchan et al[38] identified rare variants in FBN1 and FBN2, which were correlated with curve progression, FBN genes were observed to up-regulate the TGF-β signaling pathway in paravertebral muscles. In the study of Nowak et al,[54] muscles of the concave side showed a higher transcript abundance of TGF-β2, TGF-3, and transforming growth factor beta receptor 2 (TGFBR2), these genetic expressions mostly affect the extracellular region of the paravertebral muscles.
Spine Biomechanics
Relative anterior spinal overgrowth (RASO) and the contraction from the anterior part of the torso led to asymmetry of the spinal growth. Back to 1996, Murray et al[55] built a simple model of idiopathic scoliosis to analyze the possible biomechanics behind the deformity, it was shown that the overgrowth of the anterior column relative to the posterior one caused the model to form a shape of idiopathic scoliosis. In the study by Shi et al,[56] finite elements model has then been simulated, verified the accelerated growth pattern affecting scoliotic progression, while it has also been speculated that RASO was a secondary change in the development of AIS since the model with a pre-set small kyphosis in the study did not develop scoliosis. Nevertheless, Crijns et al[57] built a hypokyphosis model with an anterior band simulating the contraction from the anterior muscles and ligaments, demonstrating that even without a pre-set left-right asymmetry of the spine, the restraining force from the anterior of the body can cause a lateral curve, which indicated the unmatched growth speed of the spine with that of the anterior banding components might induce the scoliotic deformity after the decrease of kyphosis or increase of lordosis resulting from RASO. A study by Guo et al[58] has revealed that the main ossification type of vertebral bodies of AIS patients was endochondral ossification, which was also faster than the membranous ossification of pedicles as parts of the posterior structure, which explained the principle of RASO.
The intervertebral discs might be another source of spinal asymmetry. In the study of Will et al,[59] it was shown that rather than vertebrae body, the discs wedging was found to contributed the most to scoliosis progression at the beginning of the growth spurt of AIS patients but gradually reversed along with the spurt. Another study by Brink et al[60] revealed the increase of the height of discs also accounted for the development of RASO, but the study considered RASO as the secondary phenomenon since RASO was also observed in other types of scoliosis as well.
The biomechanics between spine and other parts of the torso might also contribute to AIS. Zhu et al[61] found out the rib length asymmetry was most likely a secondary change to the scoliosis deformity. Another hypothesis proposed by Yang et al[62] was that the left-right handedness and the location and gravity of heart and aorta might play roles in the curve patterns of AIS, it was hypothesized that right handedness induced a stronger right extrinsic back muscles which cannot be counteracted by the intrinsic muscle, which caused the convexity of the right side. While comments have also been made to question the handedness part.[63] Another study of Chen et al[64] found that imbalance of growth between sternum and thoracic vertebrae might lead to scoliosis.
Neurology
Brain
The research of association between brain abnormalities and spinal deformity focused mainly on the neuroanatomical and neurofunctional alterations which were observed in the cerebrum, brain stem, and cerebellum.
Of the studies regarding cerebrum and brain stem, magnetic resonance imaging (MRI) images with a morphometric study by Shi et al[65] showed white matter attenuation in the corpus callosum and left internal capsule of the AIS patients with left thoracic curves. In another study by Wang et al[66] also found cerebrum abnormality that the cortical thickness of AIS patients was different from the normal control group, the differences were mostly observed in the region involving in motor and vestibular function. Opposite opinion was also given, a study by Lee et al[67] showed no significant glucose metabolic difference was found between AIS groups and normal groups, giving the contrary evidence of cerebrum abnormalities taking part in the pathogenesis of AIS. Another study by Geissele et al[68] regarding the brain stem discovered the ventral pons or medulla asymmetry in the area of the corticospinal tracts from 7 AIS patients. To sum up, despite abnormalities were observed in AIS patients, there is currently no solid evidence proves the alterations in the cerebrum and brain stem are primary changes in AIS development, nor it has been proved that the alterations in the cerebrum found in AIS patients affect neurological function yet.
Of the studies regarding cerebellum, it is known that cerebellum has crucial functions to adjust or coordinate the muscle movements and posture. In the study of Cheng et al,[69] tonsillar ectopia was found in a small part (7.3%) of AIS patients, this part of patients in the study had a higher prevalence of severe curve. Another study by Lee et al[70] showed a higher prevalence of 48% AIS patients, whose MRI images were taken under an upright rather than a supine position, had cerebellar tonsillar descent. The first study analyzing the regional cerebellum volume characteristics quantitatively revealed the enlargement of several cerebellar regions.[71] Another study by Chau et al[72] observed the prolonged latency of somatosensory-evoked potential (SEP) in AIS girls, among AIS patients with abnormal SEP, 58% were found to have cerebellar tonsillar ectopia. This study might show the functional impairments of morphological changes in the cerebellum of AIS patients. With these studies, we can speculate the possibility of cerebellum growth and functional changes having a relationship with AIS development.
Vestibular system
Deficits of the vestibular system may lead to asymmetrical body activities and senses thus contributes to AIS development. The vestibular system collects the postural information which is further integrated with other sensory information to maintain body balance. Byl et al[73] reported a postural imbalance in AIS patients and it was hypothesized that an asymmetric vestibular system which causes a rise in the asymmetric paraspinal muscle tone might play a role in the genesis of AIS.[74] Another study by Zeng et al[75] also showed a difference in the morphology of the vestibular system in the AIS patients. However, a systematic review by Catanzariti et al[76] in 2014 concluded that there has not been adequate evidence showing the unilateral contribution of vestibular dysfunction to the AIS pathogenesis. In the following study by Hitier et al,[77] lateral semicircular canal asymmetry was found in AIS patients, which was also associated with functional anomalies such as lower excitability and higher canal paresis, this asymmetry might even have developed before birth. Novel research by Antoniadou et al[78] also found vestibular deficits might cause verticality perception disorder, thus described the sensorimotor integration impairment in AIS.
Hormones
Melatonin and calmodulin
Whether the deficiency of melatonin accounts for the development of AIS remains controversial since studies have shown inter-opposite results of evaluating the serum melatonin level.[79,80] Experiments of pinealectomy in animals have shown a tendency to induce scoliosis, but Man et al[81] concerned that the surgery itself accounted for scoliosis and the differences existed between humans and chickens which were used in the experiments. Pinchuk et al[82] proposed that the disturbed biorhythm of secretion, rather than the deficiency of melatonin, might be the cause of scoliosis, which resulted from the imbalance of the suprachiasmatic nucleus/pineal gland activities. However, alterations in melatonin functioning may result in imbalance of cell proliferation and differentiation in different types of cells, thus disturb the regular bone mass formation and might lead to AIS development. Melatonin improves osteoblast cells proliferation and their secretion of osteoprotegerin (OPG), OPG further inhibits the binding of osteoclast differentiation factors to RANK, thus reduces the differentiation of osteoclast cells.[83] Melatonin receptor 1B (MT2) was expressed in osteoblasts cells, a study of Yim et al[84] discovered a quantitively lower expression of MT2 in AIS osteoblast cells than the normal group, which was statistically related to a longer arm span of AIS patients. Man et al[85] found AIS osteoblasts without MT2 expression showed a weakened proliferative ability, and gene polymorphisms of the MT2 gene such as rs4753426 were considered to be related with the risk of AIS.[86] In addition to the osteoblasts, growth plate chondrocytes (GPCs) were also found to had a decreased MT2 expression, melatonin was found to reduce GPCs proliferation and differentiation, lack of response to melatonin in AIS GPCs might result in an altered endochondral ossification.[87] As mentioned above, AIS MSCs had a lower MT2 expression which might be related to membranous and endochondral ossification as well.[12]
Calmodulin may be related to the muscle function and bone formation, which leads to AIS development. Calmodulin participates in different metabolism systems as a secondary messenger, it is widely expressed in different varieties of cells and takes part in the contractile system of cells and is also an inhibitor of melatonin.[83] According to the study of Lowe et al,[88] early studies showed an increased concentration of calmodulin in platelets of AIS patients, but whether it reflected the muscular changes or spine deformity remained controversial. Imbalance of the concentrations in the paraspinal muscles of AIS patients was discovered, but studies showed different results of which side has a higher concentration.[50,89] In the articular process of AIS patients, the expression of calmodulin was also found to be lower.[90]
Leptin
Leptin has central and peripheral functions to bone formation, the leptin-neurology functions are central functions, its direct interactions with bone-related tissues are peripheral functions, alterations in these functions can cause AIS development. Leptin is the hormone that takes part in the regulation of bone formation, and is regulated by hypothalamic nuclei and the sympathetic nerve system.[91] In the study of Qiu et al,[92] decreased circulating leptin level was found in AIS patients, and was believed to be related with lower body mass index (BMI) and BMD. Another study by Liang et al[5] proposed that the circulating leptin might be a secondary alteration caused by the low adipogenesis ability of AIS patients which resulted from their lower fat accumulation, the study also regarded the peripheral functions, leptin receptors were found to be down-regulated in induced AIS MSCs which explained their hyposensitivity to leptin and their weakened adipogenesis ability, the study also considered Janus tyrosine kinase 2/signal transducers and activators of transcription defects might exist in AIS patients. In the study of Wang et al,[93] the lower expression of membrane leptin receptors was found in the chondrocyte cells of AIS patients’ facet joints, it might be resulted from an imbalance between endocytosis and insertion of new receptors to the membrane, this alteration may cause decreased leptin sensitivity.
Estrogen
As one of the sex hormones resides in human body, estrogen has numerous functions, lack of estrogen leads to deficits of bone maturation which can further participate in AIS development. Reviews by Zhou et al and Leboeuf et al[94,95] have discussed that the response of cells to estrogen of AIS patients was altered and thus might result in the delay of menarche and osteopenia, which disturbed the maturation of bone. It was also speculated that estrogen receptors might influence the response of growth plates to strain which affected bone formation. The interactions between estrogen and other hormones were also discussed, melatonin and estrogen had opposite influences in the regulation of cAMP level in cells, calmodulin might also influence the estrogen functioning in signal pathways. It is clear that estrogen influences the growth pattern of spine, further research may reveal the specific mechanism and whether other environmental factors have effects on estrogen secretion or functions that can induce the development of AIS.
Growth hormone
The growth hormone (GH) has been considered to regulate the overall growth of the body, alterations in GH secretion and functions may affect the growth of the skeleton, which is related to AIS etiology. Studies have shown AIS patients were relatively taller than the normal groups,[96,97] In the study of Willner et al,[98] the basal hormone level was also found to be higher in AIS patients. Yang et al and Zhuang et al[99,100] identified gene polymorphisms of GH receptors in AIS patients, which were associated with the higher GH concentration in the blood and the low BMD condition. However, the GH gene promoter was considered not related to AIS etiology.[101] Through these studies we can conclude that GHR alterations may play as a more primary role than GH itself, despite GH still may participate in the etiology of AIS, further studies are needed to reveal the specific role of GH and the interactions between GH and other hormones.
Biochemistry
Bone mineral metabolism
Various mechanisms were considered to account for the low BMD status in AIS, low BMD might also result in stress increase and curve progression. In the study of Suh et al,[102] imbalance of the disturbed interaction between receptor activator of nuclear factor-κB ligand (RANKL) and OPG has been found to be related with low spinal BMD (LSBMD) and femoral neck BMD (FNBMD), the serum RANLK to OPG ratio was increased. Not only in the serum, but the increased RANKL expression was also found in osteoblasts by Zhou et al.[103] The RANK system plays an important role in bone metabolism, which induces the osteoclastic cells to generate bone resorption. In the study of Eun et al,[104] OPG gene and IL-6 gene, which was a candidate gene of osteopenia, were both confirmed to have polymorphisms in AIS patients and were considered to be associated with AIS pathogenesis. RUNX2 as an important transcription factor regulating osteoblast differentiation and skeletal formation, Wang et al[18] discovered that the expression of Runx2 decreased in osteoblasts cells of AIS patients and might be related to the LSBMD and FNBMD. Using the finite element model, Song et al[105] confirmed that low BMD in the concave side of thoracic scoliosis could enlarge the stress to the cortical bone, discs, and facets, altered their regular growth, thus caused curve progression.
Vitamin D
Vitamin D is correlated with calcium metabolism, besides, Ng et al[106] considered that it might also have influences on fibrosis regulation, postural control, which might result in AIS development. Vitamin D receptor (VDR) is another research point of vitamin D metabolism. It is still controversial whether the gene polymorphism of VDR Bsml contributes to the development of AIS.[107–109] Further researches should be taken to define which signaling pathway Vitamin D takes part in and whether it acts as a causal factor of AIS.
Lipid metabolism
Disrupted lipid metabolism has been found in a serum metabolic analysis study by Sun et al,[110] the categories of lipids altered in the AIS patients were glycerophospholipids, glycerolipids, and fatty acid esters. Since the lipid metabolism are related with various kinds of hormones and regulative systems, it is necessary to find out the roles altered lipid metabolism played in the pathogenesis of AIS and to integrate the findings to other theories.
Biochemical characteristics in scoliotic disc
The biochemical alterations may also cause histological changes of discs, which may be accordance with some biomechanical effects, thus result in AIS curve progression. In the study of Ghosh et al,[111] the distribution of GAGs was reported to shift away from its original location in scoliotic vertebral discs, originally, the concentration should be highest in the nucleus pulposus. Another study by He et al[112] showed an increase of type I and type II collagen at the convex side of the discs relative to the concave side, which might result from the degeneration of the discs.
Environment and Lifestyle
High environmental selenium
After gathering the information of guppy fish developed an “S” curve deformity in the high-selenium environment, Yang et al[113] gave the hypothesis that high-selenium environment-induced uncoupled spinal neuro-osseous growth, and the overgrowth of the spine relative to the spinal cord resulted in the tethering of the spine, which might thus cause the curvature. Another cohort study by Ji et al[114] proved the hypothesis by giving evidence that the relative risk was 2.88.
Chlorine and the neurotoxic influences
A hypothesis by McMaster et al[115] postulated that the chloroform generated from heat swimming pools has a neurotoxic effect thus induces AIS development. It has been observed that the normal teenagers introduced to indoor heated swimming pools had a high prevalence (83%) of developing vertical spinous process asymmetry, this phenomenon was also observed in infants.[116]
Gut microbiome induced plasma proteome alterations in AIS patients
A novel hypothesis has been built on the findings of different structures of gut microbiome between AIS patients and healthy control groups.[117] It was found that the differences may result in alterations of the plasma proteins, and the abundance of fecal prevotella positively correlated with Cobb angles of the AIS patients. Although there is not sufficient evidence of direct participation of microbiome in the initiation or progression of the AIS.
Physical activities as controversial risk factors
Different physical activities with different training strategies may induce different outcomes, which may be associated with AIS etiology. Asides from the previously described indoor swimming pool exposure, several other physical activities have been evaluated. The better ability of toe touching was found to have a positive correlation with AIS occurrence, which might be resulted from connective tissues deficits.[118] In the same study, AIS children were shown to participated less frequently in dance, skating, gymnastics or karate, and football or hockey classes. Another study[119] discovered that different physical activities had different associations with AIS, the odds ratio of ballet training with AIS was reported to be 1.38.
Integrated theory
The double neuro-osseous theory, proposed by Burwell et al concluded the pathogenesis theories of AIS into a developmental disharmony between autonomic and somatic nervous systems of the spine and trunk, which was further exaggerated by hormones and thus induced a systemic skeletal overgrowth.[91] This theory postulated a leptin-hypothalamic-sympathetic nervous system involved in the pathogenesis of AIS, which showed the central functions of leptin, the genetic mutations of AIS patients induced an increased sensitivity of hypothalamus to leptin, exaggerated by somatotropic axis, then affected the skeletal growth. The somatic nervous system of AIS was described as a failure to control and compensate for the spinal deformity. As relatively low BMI was found in AIS patients in several studies,[96,120,121] it was also integrated into the double neuro-osseous theory, and were considered as a substitutional measure for body fat and circulating leptin levels.[122] The integrated theory was based on the former studies and required later confirmation.
Conclusions
The management of scoliosis includes surgery and conservative treatment[123–126]; however, the prevention of AIS are still under research, which partly because the etiology and pathogenesis of AIS is currently indefinite. Despite there have already been numerous theories or hypotheses investigating the pathogenesis of AIS, novel findings are still emerging constantly. In this review, we classified the known mechanisms of AIS pathogenesis and confirmed AIS as a multifactorial disease with intrinsic and extrinsic alterations. Limitations of our review exist because of the relatively simple inclusion and exclusion criteria, which may cause selective bias. According to the review, bone formation seems to be one of the key points of the etiology and pathogenesis of AIS, and was found to be related with changes in almost every field we classified from genetic to environmental factors. If further studies focus more on the bone formation differences of AIS patients, the connections between different fields may be well established. Moreover, the treatment of AIS may also find inspiration from the alteration in BM-MSCs and hormones. However, further studies are also expected to clarify the controversial parts and integrate the existing theories and findings.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (No. 81272054 and No. 81171673), the Beijing Talent Fund (No. 2015000021223ZK27), the Beijing Nova program Grant (No. 2014A019).
Conflicts of interest
None.
Footnotes
How to cite this article: Peng Y, Wang SR, Qiu GX, Zhang JG, Zhuang QY. Research progress on the etiology and pathogenesis of adolescent idiopathic scoliosis. Chin Med J 2020;133:483–493. doi: 10.1097/CM9.0000000000000652
References
- 1.Konieczny MR, Senyurt H, Krauspe R. Epidemiology of adolescent idiopathic scoliosis. J Child Orthop 2013; 7:3–9. doi: 10.1007/s11832-012-0457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qiu GX. Scoliosis in China: history and present status. Chin Med J 2017; 130:2521–2523. doi: 10.4103/0366-6999.217081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng Y, Dang Y, Wu X, Yang Y, Reinhardt JD, He C, et al. Epidemiological study of adolescent idiopathic scoliosis in Eastern China. J Rehabil Med 2017; 49:512–519. doi: 10.2340/16501977-2240. [DOI] [PubMed] [Google Scholar]
- 4.Andrzejewska A, Lukomska B, Janowski M. Concise review: mesenchymal stem cells: from roots to boost. Stem Cells 2019; 37:855–864. doi: 10.1002/stem.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang G, Gao W, Liang A, Ye W, Peng Y, Zhang L, et al. Normal leptin expression, lower adipogenic ability, decreased leptin receptor and hyposensitivity to leptin in adolescent idiopathic scoliosis. PLoS One 2012; 7:e36648.doi: 10.1371/journal.pone.0036648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park WW, Suh KT, Kim JI, Kim SJ, Lee JS. Decreased osteogenic differentiation of mesenchymal stem cells and reduced bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J 2009; 18:1920–1926. doi: 10.1007/s00586-009-1129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pourabbas Tahvildari B, Erfani MA, Nouraei H, Sadeghian M. Evaluation of bone mineral status in adolescent idiopathic scoliosis. Clin Orthop Surg 2014; 6:180–184. doi: 10.4055/cios.2014.6.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li XF, Li H, Liu ZD, Dai LY. Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J 2008; 17:1431–1440. doi: 10.1007/s00586-008-0757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng JC, Qin L, Cheung CS, Sher AH, Lee KM, Ng SW, et al. Generalized low areal and volumetric bone mineral density in adolescent idiopathic scoliosis. J Bone Miner Res 2000; 15:1587–1595. doi: 10.1359/jbmr.2000.15.8.1587. [DOI] [PubMed] [Google Scholar]
- 10.Zhuang Q, Li J, Wu Z, Zhang J, Sun W, Li T, et al. Differential proteome analysis of bone marrow mesenchymal stem cells from adolescent idiopathic scoliosis patients. PLoS One 2011; 6:e18834.doi: 10.1371/journal.pone.0018834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhuang Q, Mao W, Xu P, Li H, Sun Z, Li S, et al. Identification of differential genes expression profiles and pathways of bone marrow mesenchymal stem cells of adolescent idiopathic scoliosis patients by microarray and integrated gene network analysis. Spine (Phila Pa 1976) 2016; 41:840–855. doi: 10.1097/brs.0000000000001394. [DOI] [PubMed] [Google Scholar]
- 12.Chen C, Xu C, Zhou T, Gao B, Zhou H, Chen C, et al. Abnormal osteogenic and chondrogenic differentiation of human mesenchymal stem cells from patients with adolescent idiopathic scoliosis in response to melatonin. Mol Med Rep 2016; 14:1201–1209. doi: 10.3892/mmr.2016.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Q, Yang J, Lin X, Huang Z, Xie C, Fan H. Spot14/Spot14R expression may be involved in MSC adipogenic differentiation in patients with adolescent idiopathic scoliosis. Mol Med Rep 2016; 13:4636–4642. doi: 10.3892/mmr.2016.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou T, Chen C, Xu C, Zhou H, Gao B, Su D, et al. Mutant MAPK7-induced idiopathic scoliosis is linked to impaired osteogenesis. Cell Physiol Biochem 2018; 48:880–890. doi: 10.1159/000491956. [DOI] [PubMed] [Google Scholar]
- 15.Xu EJ, Lin T, Jiang H, Ji Z, Shao W, Meng YC, et al. Asymmetric expression of GPR126 in the convex/concave side of the spine is associated with spinal skeletal malformation in adolescent idiopathic scoliosis population. Eur Spine J 2019; 28:1977–1986. doi: 10.1007/s00586-019-06001-5. [DOI] [PubMed] [Google Scholar]
- 16.Huo S, Zhou Y, He X, Wan M, Du W, Xu X, et al. Insight into the role of long non-coding RNAs during osteogenesis in mesenchymal stem cells. Curr Stem Cell Res Ther 2018; 13:52–59. doi: 10.2174/1574888x12666171115124112. [DOI] [PubMed] [Google Scholar]
- 17.Zhuang Q, Ye B, Hui S, Du Y, Zhao RC, Li J, et al. Long noncoding RNA lncAIS downregulation in mesenchymal stem cells is implicated in the pathogenesis of adolescent idiopathic scoliosis. Cell Death Differ 2019; 26:1700–1715. doi: 10.1038/s41418-018-0240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang WJ, Sun C, Liu Z, Sun X, Zhu F, Zhu ZZ, et al. Transcription factor Runx2 in the low bone mineral density of girls with adolescent idiopathic scoliosis. Orthop Surg 2014; 6:8–14. doi: 10.1111/os.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hui S, Yang Y, Li J, Li N, Xu P, Li H, et al. Differential miRNAs profile and bioinformatics analyses in bone marrow mesenchymal stem cells from adolescent idiopathic scoliosis patients. Spine J 2019; 19:1584–1596. doi: 10.1016/j.spinee.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Simony A, Carreon LY, Hjmark K, Kyvik KO, Andersen MO. Concordance rates of adolescent idiopathic scoliosis in a Danish twin population. Spine (Phila Pa 1976) 2016; 41:1503–1507. doi: 10.1097/brs.0000000000001681. [DOI] [PubMed] [Google Scholar]
- 21.Justice CM, Miller NH, Marosy B, Zhang J, Wilson AF. Familial idiopathic scoliosis: evidence of an X-linked susceptibility locus. Spine (Phila Pa 1976) 2003; 28:589–594. doi: 10.1097/01.Brs.0000049940.39801.E6. [DOI] [PubMed] [Google Scholar]
- 22.Ward K, Ogilvie J, Argyle V, Nelson L, Meade M, Braun J, et al. Polygenic inheritance of adolescent idiopathic scoliosis: a study of extended families in Utah. Am J Med Genet A 2010; 152a:1178–1188. doi: 10.1002/ajmg.a.33145. [DOI] [PubMed] [Google Scholar]
- 23.Borecki IB, Province MA. Linkage and association: basic concepts. Adv Genet 2008; 60:51–74. doi: 10.1016/s0065-2660(07)00403-8. [DOI] [PubMed] [Google Scholar]
- 24.Gao W, Chen C, Zhou T, Yang S, Gao B, Zhou H, et al. Rare coding variants in MAPK7 predispose to adolescent idiopathic scoliosis. Hum Mutat 2017; 38:1500–1510. doi: 10.1002/humu.23296. [DOI] [PubMed] [Google Scholar]
- 25.Sadat-Ali M, Al-Omran AS, Al-Othman AA. Genetic markers for idiopathic scoliosis on chromosome 19p 13.3 among Saudi Arabian girls: a pilot study. Indian J Hum Genet 2011; 17:13–16. doi: 10.4103/0971-6866.82187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogura Y, Kou I, Scoliosis J, Matsumoto M, Watanabe K, Ikegawa S. Genome-wide association study for adolescent idiopathic scoliosis. Clin Calcium 2016; 26:553–560. doi: CliCa1604553500. [PubMed] [Google Scholar]
- 27.Buchan JG, Alvarado DM, Haller G, Aferol H, Miller NH, Dobbs MB, et al. Are copy number variants associated with adolescent idiopathic scoliosis? Clin Orthop Relat Res 2014; 472:3216–3225. doi: 10.1007/s11999-014-3766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Zhou Y, Liu S, Song X, Yang XZ, Fan Y, et al. The coexistence of copy number variations (CNVs) and single nucleotide polymorphisms (SNPs) at a locus can result in distorted calculations of the significance in associating SNPs to disease. Hum Genet 2018; 137:553–567. doi: 10.1007/s00439-018-1910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu E, Shao W, Jiang H, Lin T, Gao R, Zhou X. A genetic variant in GPR126 causing a decreased inclusion of exon 6 is associated with cartilage development in adolescent idiopathic scoliosis population. Biomed Res Int 2019; 2019:4678969.doi: 10.1155/2019/4678969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu G, Liu S, Li X, Chen J, Chen W, Zuo Y, et al. Genetic polymorphisms of PAX1 are functionally associated with different PUMC types of adolescent idiopathic scoliosis in a northern Chinese Han population. Gene 2019; 688:215–220. doi: 10.1016/j.gene.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 31.Hassan A, Parent S, Mathieu H, Zaouter C, Molidperee S, Bagu ET, et al. Adolescent idiopathic scoliosis associated POC5 mutation impairs cell cycle, cilia length and centrosome protein interactions. PLoS One 2019; 14:e0213269.doi: 10.1371/journal.pone.0213269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu L, Sheng F, Xia C, Feng Z, Qiu Y, Zhu Z. VANGL1 is not associated with the susceptibility of adolescent idiopathic scoliosis in the Chinese population. Spine (Phila Pa 1976) 2018; 43:E580–E584. doi: 10.1097/brs.0000000000002497. [DOI] [PubMed] [Google Scholar]
- 33.Nada D, Julien C, Samuels ME, Moreau A. A replication study for association of LBX1 locus with adolescent idiopathic scoliosis in French-Canadian population. Spine (Phila Pa 1976) 2018; 43:172–178. doi: 10.1097/brs.0000000000002280. [DOI] [PubMed] [Google Scholar]
- 34.Sui W, Yang J, Huang Z, Wang Q, Fan H, Deng Y. Polymorphisms in promoter regions of MMP-3 and IL-6 genes are not associated to adolescent idiopathic scoliosis (AIS) gender bias. J Back Musculoskelet Rehabil 2017; 30:559–563. doi: 10.3233/bmr-150309. [DOI] [PubMed] [Google Scholar]
- 35.Guan M, Wang H, Fang H, Zhang C, Gao S, Zou Y. Association between IGF1 gene single nucleotide polymorphism (rs5742612) and adolescent idiopathic scoliosis: a meta-analysis. Eur Spine J 2017; 26:1624–1630. doi: 10.1007/s00586-016-4742-7. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Sun W, Qin X, Qiu Y, Zhu Z. The TGFB1 gene is associated with curve severity but not with the development of adolescent idiopathic scoliosis: a replication study in the Chinese population. BMC Musculoskelet Disord 2016; 17:15.doi: 10.1186/s12891-016-0863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu XS, Lv F, Zhu ZZ, Qian BP, Wang B, Yu Y, et al. Lack of association between the CHL1 gene and adolescent idiopathic scoliosis susceptibility in Han Chinese: a case-control study. BMC Musculoskelet Disord 2014; 15:38.doi: 10.1186/1471-2474-15-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buchan JG, Alvarado DM, Haller GE, Cruchaga C, Harms MB, Zhang T, et al. Rare variants in FBN1 and FBN2 are associated with severe adolescent idiopathic scoliosis. Hum Mol Genet 2014; 23:5271–5282. doi: 10.1093/hmg/ddu224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Jia S, Chen Z, Chong YL, Xie H, Feng D, et al. Cilia-driven cerebrospinal fluid flow directs expression of urotensin neuropeptides to straighten the vertebrate body axis. Nat Genet 2018; 50:1666–1673. doi: 10.1038/s41588-018-0260-3. [DOI] [PubMed] [Google Scholar]
- 40.Burwell RG, Dangerfield PH, Moulton A, Grivas TB. Adolescent idiopathic scoliosis (AIS), environment, exposome and epigenetics: a molecular perspective of postnatal normal spinal growth and the etiopathogenesis of AIS with consideration of a network approach and possible implications for medical therapy. Scoliosis 2011; 6:26.doi: 10.1186/1748-7161-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fendri K, Patten SA, Kaufman GN, Zaouter C, Parent S, Grimard G, et al. Microarray expression profiling identifies genes with altered expression in adolescent idiopathic scoliosis. Eur Spine J 2013; 22:1300–1311. doi: 10.1007/s00586-013-2728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mao SH, Qian BP, Shi B, Zhu ZZ, Qiu Y. Quantitative evaluation of the relationship between COMP promoter methylation and the susceptibility and curve progression of adolescent idiopathic scoliosis. Eur Spine J 2018; 27:272–277. doi: 10.1007/s00586-017-5309-y. [DOI] [PubMed] [Google Scholar]
- 43.Meng Y, Lin T, Liang S, Gao R, Jiang H, Shao W, et al. Value of DNA methylation in predicting curve progression in patients with adolescent idiopathic scoliosis. EBioMedicine 2018; 36:489–496. doi: 10.1016/j.ebiom.2018.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang JJ, Chen HX, Leung RKK, Choy KW, Lam TP, Ng BKW, et al. Aberrant miR-145-5p/beta-catenin signal impairs osteocyte function in adolescent idiopathic scoliosis. FASEB J 2018; 32:6537–6549. doi: 10.1096/fj.201800281. [DOI] [PubMed] [Google Scholar]
- 45.Ogura Y, Kou I, Takahashi Y, Takeda K, Minami S, Kawakami N, et al. A functional variant in MIR4300HG, the host gene of microRNA MIR4300 is associated with progression of adolescent idiopathic scoliosis. Hum Mol Genet 2017; 26:4086–4092. doi: 10.1093/hmg/ddx291. [DOI] [PubMed] [Google Scholar]
- 46.Liu XY, Wang L, Yu B, Zhuang QY, Wang YP. Expression signatures of long noncoding RNAs in adolescent idiopathic scoliosis. Biomed Res Int 2015; 2015:276049.doi: 10.1155/2015/276049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu F, Qiu Y, Yeung HY, Lee KM, Cheng CY. Trabecular bone micro-architecture and bone mineral density in adolescent idiopathic and congenital scoliosis. Orthop Surg 2009; 1:78–83. doi: 10.1111/j.1757-7861.2008.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanabe H, Aota Y, Nakamura N, Saito T. A histomorphometric study of the cancellous spinal process bone in adolescent idiopathic scoliosis. Eur Spine J 2017; 26:1600–1609. doi: 10.1007/s00586-017-4974-1. [DOI] [PubMed] [Google Scholar]
- 49.Wang ZW, Lee WY, Lam TP, Yip BH, Yu FW, Yu WS, et al. Defining the bone morphometry, micro-architecture and volumetric density profile in osteopenic vs non-osteopenic adolescent idiopathic scoliosis. Eur Spine J 2017; 26:1586–1594. doi: 10.1007/s00586-016-4422-7. [DOI] [PubMed] [Google Scholar]
- 50.Acaroglu E, Akel I, Alanay A, Yazici M, Marcucio R. Comparison of the melatonin and calmodulin in paravertebral muscle and platelets of patients with or without adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2009; 34:E659–E663. doi: 10.1097/BRS.0b013e3181a3c7a2. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Pessin JE. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr Opin Clin Nutr Metab Care 2013; 16:243–250. doi: 10.1097/MCO.0b013e328360272d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson JM, Loenneke JP, Jo E, Wilson GJ, Zourdos MC, Kim JS. The effects of endurance, strength, and power training on muscle fiber type shifting. J Strength Cond Res 2012; 26:1724–1729. doi: 10.1519/JSC.0b013e318234eb6f. [DOI] [PubMed] [Google Scholar]
- 53.Stetkarova I, Zamecnik J, Bocek V, Vasko P, Brabec K, Krbec M. Electrophysiological and histological changes of paraspinal muscles in adolescent idiopathic scoliosis. Eur Spine J 2016; 25:3146–3153. doi: 10.1007/s00586-016-4628-8. [DOI] [PubMed] [Google Scholar]
- 54.Nowak R, Kwiecien M, Tkacz M, Mazurek U. Transforming growth factor-beta (TGF-beta) signaling in paravertebral muscles in juvenile and adolescent idiopathic scoliosis. Biomed Res Int 2014; 2014:594287.doi: 10.1155/2014/594287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murray DW, Bulstrode CJ. The development of adolescent idiopathic scoliosis. Eur Spine J 1996; 5:251–257. doi: 10.1007/bf00301328. [DOI] [PubMed] [Google Scholar]
- 56.Shi L, Wang D, Driscoll M, Villemure I, Chu WC, Cheng JC, et al. Biomechanical analysis and modeling of different vertebral growth patterns in adolescent idiopathic scoliosis and healthy subjects. Scoliosis 2011; 6:11.doi: 10.1186/1748-7161-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crijns TJ, Stadhouder A, Smit TH. Restrained differential growth: the initiating event of adolescent idiopathic scoliosis? Spine (Phila Pa 1976) 2017; 42:E726–E732. doi: 10.1097/brs.0000000000001946. [DOI] [PubMed] [Google Scholar]
- 58.Guo X, Chau WW, Chan YL, Cheng JC, Burwell RG, Dangerfield PH. Relative anterior spinal overgrowth in adolescent idiopathic scoliosis--result of disproportionate endochondral-membranous bone growth? Summary of an electronic focus group debate of the IBSE. Eur Spine J 2005; 14:862–873. doi: 10.1007/s00586-005-1002-7. [DOI] [PubMed] [Google Scholar]
- 59.Will RE, Stokes IA, Qiu X, Walker MR, Sanders JO. Cobb angle progression in adolescent scoliosis begins at the intervertebral disc. Spine (Phila Pa 1976) 2009; 34:2782–2786. doi: 10.1097/BRS.0b013e3181c11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brink RC, Schlosser TPC, Colo D, Vavruch L, van Stralen M, Vincken KL, et al. Anterior spinal overgrowth is the result of the scoliotic mechanism and is located in the disc. Spine (Phila Pa 1976) 2017; 42:818–822. doi: 10.1097/brs.0000000000001919. [DOI] [PubMed] [Google Scholar]
- 61.Zhu F, Chu WC, Sun G, Zhu ZZ, Wang WJ, Cheng JC, et al. Rib length asymmetry in thoracic adolescent idiopathic scoliosis: is it primary or secondary? Eur Spine J 2011; 20:254–259. doi: 10.1007/s00586-010-1637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang ZD, Li M. There may be a same mechanism of the left-right handedness and left-right convex curve pattern of adolescent idiopathic scoliosis. Med Hypotheses 2011; 76:274–276. doi: 10.1016/j.mehy.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 63.Kouwenhoven JW, Janssen MM, Castelein RM. Letter to the editor concerning: Z.D. Yang et al., There may be a same mechanism of the left-right handedness and left-right convex curve pattern of adolescent idiopathic scoliosis, Med. Hypotheses (2010) Oct 29. Med Hypotheses 2011; 77:156.doi: 10.1016/j.mehy.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 64.Chen B, Tan Q, Chen H, Luo F, Xu M, Zhao J, et al. Imbalanced development of anterior and posterior thorax is a causative factor triggering scoliosis. J Orthop Translat 2019; 17:103–111. doi: 10.1016/j.jot.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi L, Wang D, Chu WC, Burwell RG, Freeman BJ, Heng PA, et al. Volume-based morphometry of brain MR images in adolescent idiopathic scoliosis and healthy control subjects. AJNR Am J Neuroradiol 2009; 30:1302–1307. doi: 10.3174/ajnr.A1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang D, Shi L, Chu WC, Burwell RG, Cheng JC, Ahuja AT. Abnormal cerebral cortical thinning pattern in adolescent girls with idiopathic scoliosis. Neuroimage 2012; 59:935–942. doi: 10.1016/j.neuroimage.2011.07.097. [DOI] [PubMed] [Google Scholar]
- 67.Lee JS, Kim SJ, Suh KT, Kim IJ, Kim YK. Adolescent idiopathic scoliosis may not be associated with brain abnormalities. Acta Radiol 2009; 50:941–946. doi: 10.1080/02841850903104161. [DOI] [PubMed] [Google Scholar]
- 68.Geissele AE, Kransdorf MJ, Geyer CA, Jelinek JS, Van Dam BE. Magnetic resonance imaging of the brain stem in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 1991; 16:761–763. doi: 10.1097/00007632-199107000-00013. [DOI] [PubMed] [Google Scholar]
- 69.Cheng JC, Guo X, Sher AH, Chan YL, Metreweli C. Correlation between curve severity, somatosensory evoked potentials, and magnetic resonance imaging in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 1999; 24:1679–1684. doi: 10.1097/00007632-199908150-00009. [DOI] [PubMed] [Google Scholar]
- 70.Lee RK, Griffith JF, Leung JH, Chu WC, Lam TP, Ng BK, et al. Effect of upright position on tonsillar level in adolescent idiopathic scoliosis. Eur Radiol 2015; 25:2397–2402. doi: 10.1007/s00330-015-3597-3. [DOI] [PubMed] [Google Scholar]
- 71.Shi L, Wang D, Hui SC, Tong MC, Cheng JC, Chu WC. Volumetric changes in cerebellar regions in adolescent idiopathic scoliosis compared with healthy controls. Spine J 2013; 13:1904–1911. doi: 10.1016/j.spinee.2013.06.045. [DOI] [PubMed] [Google Scholar]
- 72.Chau WW, Chu WC, Lam TP, Ng BK, Fu LL, Cheng JC. Anatomical origin of abnormal somatosensory-evoked potential (SEP) in adolescent idiopathic scoliosis with different curve severity and correlation with cerebellar tonsillar level determined by MRI. Spine (Phila Pa 1976) 2016; 41:E598–E604. doi: 10.1097/brs.0000000000001345. [DOI] [PubMed] [Google Scholar]
- 73.Byl NN, Holland S, Jurek A, Hu SS. Postural imbalance and vibratory sensitivity in patients with idiopathic scoliosis: implications for treatment. J Orthop Sports Phys Ther 1997; 26:60–68. doi: 10.2519/jospt.1997.26.2.60. [DOI] [PubMed] [Google Scholar]
- 74.Lambert FM, Malinvaud D, Glaunes J, Bergot C, Straka H, Vidal PP. Vestibular asymmetry as the cause of idiopathic scoliosis: a possible answer from Xenopus. J Neurosci 2009; 29:12477–12483. doi: 10.1523/jneurosci.2583-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeng W, Lui LM, Shi L, Wang D, Chu WC, Cheng JC, et al. Shape analysis of vestibular systems in adolescent idiopathic scoliosis using geodesic spectra. Med Image Comput Comput Assist Interv 2010; 13:538–546. doi: 10.1007/978-3-642-15711-0_67. [DOI] [PubMed] [Google Scholar]
- 76.Catanzariti JF, Agnani O, Guyot MA, Wlodyka-Demaille S, Khenioui H, Donze C. Does adolescent idiopathic scoliosis relate to vestibular disorders? A systematic review. Ann Phys Rehabil Med 2014; 57:465–479. doi: 10.1016/j.rehab.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 77.Hitier M, Hamon M, Denise P, Lacoudre J, Thenint MA, Mallet JF, et al. Lateral semicircular canal asymmetry in idiopathic scoliosis: an early link between biomechanical, hormonal and neurosensory theories? PLoS One 2015; 10:e0131120.doi: 10.1371/journal.pone.0131120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Antoniadou N, Hatzitaki V, Stavridis S, Samoladas E. Verticality perception reveals a vestibular deficit in adolescents with idiopathic scoliosis. Exp Brain Res 2018; 236:1725–1734. doi: 10.1007/s00221-018-5256-9. [DOI] [PubMed] [Google Scholar]
- 79.Sadat-Ali M, al-Habdan I, al-Othman A. Adolescent idiopathic scoliosis. Is low melatonin a cause? Joint Bone Spine 2000; 67:62–64. doi: 10.1016/S1169-8330(00)80050-2. [PubMed] [Google Scholar]
- 80.Hilibrand AS, Blakemore LC, Loder RT, Greenfield ML, Farley FA, Hensinger RN, et al. The role of melatonin in the pathogenesis of adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 1996; 21:1140–1146. doi: 10.1097/00007632-199605150-00004. [DOI] [PubMed] [Google Scholar]
- 81.Man GC, Wang WW, Yim AP, Wong JH, Ng TB, Lam TP, et al. A review of pinealectomy-induced melatonin-deficient animal models for the study of etiopathogenesis of adolescent idiopathic scoliosis. Int J Mol Sci 2014; 15:16484–16499. doi: 10.3390/ijms150916484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pinchuk DY, Bekshaev SS, Bumakova SA, Dudin MG, Pinchuk OD. Bioelectric activity in the suprachiasmatic nucleus-pineal gland system in children with adolescent idiopathic scoliosis. ISRN Orthop 2012; 2012:987095.doi: 10.5402/2012/987095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sanchez-Barcelo EJ, Mediavilla MD, Tan DX, Reiter RJ. Scientific basis for the potential use of melatonin in bone diseases: osteoporosis and adolescent idiopathic scoliosis. J Osteoporos 2010; 2010:830231.doi: 10.4061/2010/830231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yim AP, Yeung HY, Sun G, Lee KM, Ng TB, Lam TP, et al. Abnormal skeletal growth in adolescent idiopathic scoliosis is associated with abnormal quantitative expression of melatonin receptor, MT2. Int J Mol Sci 2013; 14:6345–6358. doi: 10.3390/ijms14036345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Man GC, Wong JH, Wang WW, Sun GQ, Yeung BH, Ng TB, et al. Abnormal melatonin receptor 1B expression in osteoblasts from girls with adolescent idiopathic scoliosis. J Pineal Res 2011; 50:395–402. doi: 10.1111/j.1600-079X.2011.00857.x. [DOI] [PubMed] [Google Scholar]
- 86.Yang P, Liu H, Lin J, Yang H. The association of rs4753426 polymorphism in the melatonin receptor 1B (MTNR1B) gene and susceptibility to adolescent idiopathic scoliosis: a systematic review and meta-analysis. Pain Physician 2015; 18:419–431. [PubMed] [Google Scholar]
- 87.Wang WW, Man GC, Wong JH, Ng TB, Lee KM, Ng BK, et al. Abnormal response of the proliferation and differentiation of growth plate chondrocytes to melatonin in adolescent idiopathic scoliosis. Int J Mol Sci 2014; 15:17100–17114. doi: 10.3390/ijms150917100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lowe TG, Burwell RG, Dangerfield PH. Platelet calmodulin levels in adolescent idiopathic scoliosis (AIS): can they predict curve progression and severity? Summary of an electronic focus group debate of the IBSE. Eur Spine J 2004; 13:257–265. doi: 10.1007/s00586-003-0655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao Y, Qiu GX. Expression of calmodulin and nNOS in the paraspinal muscles in idiopathic scoliosis (in Chinese). Natl Med J China 2004; 84:1358–1361. doi: 10.3760/j:issn:0376-2491.2004.16.016. [PubMed] [Google Scholar]
- 90.Qiu GX, Li J, Liu Y, Wu ZH, Zhao ZG. Expression of calmodulin in the articular process of vertebrae of adolescent idiopathic scoliosis patients (in Chinese). Natl Med J China 2006; 86:2017–2020. doi: 10.3760/j:issn:0376-2491.2006.29.001. [PubMed] [Google Scholar]
- 91.Burwell RG, Aujla RK, Grevitt MP, Dangerfield PH, Moulton A, Randell TL, et al. Pathogenesis of adolescent idiopathic scoliosis in girls - a double neuro-osseous theory involving disharmony between two nervous systems, somatic and autonomic expressed in the spine and trunk: possible dependency on sympathetic nervous system and hormones with implications for medical therapy. Scoliosis 2009; 4:24.doi: 10.1186/1748-7161-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Qiu Y, Sun X, Qiu X, Li W, Zhu Z, Zhu F, et al. Decreased circulating leptin level and its association with body and bone mass in girls with adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2007; 32:2703–2710. doi: 10.1097/BRS.0b013e31815a59e5. [DOI] [PubMed] [Google Scholar]
- 93.Wang YJ, Yu HG, Zhou ZH, Guo Q, Wang LJ, Zhang HQ. Leptin receptor metabolism disorder in primary chondrocytes from adolescent idiopathic scoliosis girls. Int J Mol Sci 2016; 17:E1160.doi: 10.3390/ijms17071160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou C, Wang H, Zou Y, Fang H. Research progress of role of estrogen and estrogen receptor on onset and progression of adolescent idiopathic scoliosis (in Chinese). Chin J Reparat Reconstru Surg 2015; 29:1441–1445. doi: 10.7507/1002-1892.20150308. [PubMed] [Google Scholar]
- 95.Leboeuf D, Letellier K, Alos N, Edery P, Moldovan F. Do estrogens impact adolescent idiopathic scoliosis? Trends Endocrinol Metab 2009; 20:147–152. doi: 10.1016/j.tem.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 96.Hershkovich O, Friedlander A, Gordon B, Arzi H, Derazne E, Tzur D, et al. Association between body mass index, body height, and the prevalence of spinal deformities. Spine J 2014; 14:1581–1587. doi: 10.1016/j.spinee.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 97.Yim AP, Yeung HY, Hung VW, Lee KM, Lam TP, Ng BK, et al. Abnormal skeletal growth patterns in adolescent idiopathic scoliosis--a longitudinal study until skeletal maturity. Spine (Phila Pa 1976) 2012; 37:E1148–E1154. doi: 10.1097/BRS.0b013e31825c036d. [DOI] [PubMed] [Google Scholar]
- 98.Willner S, Nilsson KO, Kastrup K, Bergstrand CG. Growth hormone and somatomedin A in girls with adolescent idiopathic scoliosis. Acta Paediatr Scand 1976; 65:547–552. doi: 10.1111/j.1651-2227.1976.tb04930.x. [DOI] [PubMed] [Google Scholar]
- 99.Yang Y, Wu Z, Zhao T, Wang H, Zhao D, Zhang J, et al. Adolescent idiopathic scoliosis and the single-nucleotide polymorphism of the growth hormone receptor and IGF-1 genes. Orthopedics 2009; 32:411.doi: 10.3928/01477447-20090511-08. [DOI] [PubMed] [Google Scholar]
- 100.Zhuang QY, Wu ZH, Qiu GX. Is polymorphism of CALM1 gene or growth hormone receptor gene associated with susceptibility to adolescent idiopathic scoliosis (in Chinese)? Natl Med J China 2007; 87:2198–2202. doi: 10.3760/j.issn:0376-2491.2007.31.011. [PubMed] [Google Scholar]
- 101.Qiu XS, Deng LS, Yang XE, Zheng ZY, Qiu Y. Genetic polymorphism of growth hormone gene in adolescent idiopathic scoliosis (in Chinese). Chin J Surg 2008; 46:1741–1743. doi: 10.3321/j.issn:0529-5815.2008.22.019. [PubMed] [Google Scholar]
- 102.Suh KT, Lee SS, Hwang SH, Kim SJ, Lee JS. Elevated soluble receptor activator of nuclear factor-kappaB ligand and reduced bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J 2007; 16:1563–1569. doi: 10.1007/s00586-007-0390-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou S, Wang W, Zhu Z, Sun X, Zhu F, Yu Y, et al. Increased expression of receptor activator of nuclear factor-kappaB ligand in osteoblasts from adolescent idiopathic scoliosis patients with low bone mineral density. J Huazhong Univ Sci Technolog Med Sci 2012; 32:686–690. doi: 10.1007/s11596-012-1018-2. [DOI] [PubMed] [Google Scholar]
- 104.Eun IS, Park WW, Suh KT, Kim JI, Lee JS. Association between osteoprotegerin gene polymorphism and bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J 2009; 18:1936–1940. doi: 10.1007/s00586-009-1145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Song XX, Jin LY, Li XF, Qian L, Shen HX, Liu ZD, et al. Effects of low bone mineral status on biomechanical characteristics in idiopathic scoliotic spinal deformity. World Neurosurg 2018; 110:e321–e329. doi: 10.1016/j.wneu.2017.10.177. [DOI] [PubMed] [Google Scholar]
- 106.Ng SY, Bettany-Saltikov J, Cheung IYK, Chan KKY. The role of vitamin D in the pathogenesis of adolescent idiopathic scoliosis. Asian Spine J 2018; 12:1127–1145. doi: 10.31616/asj.2018.12.6.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yin X, Wang H, Guo J, Zhang L, Zhang Y, Li L, et al. Association of vitamin D receptor BsmI rs1544410 and ApaI rs7975232 polymorphisms with susceptibility to adolescent idiopathic scoliosis: a systematic review and meta-analysis. Medicine (Baltimore) 2018; 97:e9627.doi: 10.1097/md.0000000000009627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen WJ, Qiu Y, Zhu F, Zhu ZZ, Sun X, Liu Z, et al. Vitamin D receptor gene polymorphisms: no association with low bone mineral density in adolescent idiopathic scoliosis girls (in Chinese). Chin J Surg 2008; 46:1183–1186. doi: 10.3321/j.issn:0529-5815.2008.15.020. [PubMed] [Google Scholar]
- 109.Xia CW, Qiu Y, Sun X, Qiu XS, Wang SF, Zhu ZZ, et al. Vitamin D receptor gene polymorphisms in female adolescent idiopathic scoliosis patients (in Chinese). Natl Med J China 2007; 87:1465–1469. doi: 10.3760/j:issn:0376-2491.2007.21.007. [PubMed] [Google Scholar]
- 110.Sun ZJ, Jia HM, Qiu GX, Zhou C, Guo S, Zhang JG, et al. Identification of candidate diagnostic biomarkers for adolescent idiopathic scoliosis using UPLC/QTOF-MS analysis: a first report of lipid metabolism profiles. Sci Rep 2016; 6:22274.doi: 10.1038/srep22274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ghosh P, Bushell GR, Taylor TK, Pearce RH, Grimmer BJ. Distribution of glycosaminoglycans across the normal and the scoliotic disc. Spine (Phila Pa 1976) 1980; 5:310–317. doi: 10.1097/00007632-198007000-00004. [DOI] [PubMed] [Google Scholar]
- 112.He Y, Qiu Y, Zhu F, Zhu Z. Quantitative analysis of types I and II collagen in the disc annulus in adolescent idiopathic scoliosis. Stud Health Technol Inform 2006; 123:123–128. doi: 10.1061/9780784478530.009. [PubMed] [Google Scholar]
- 113.Yang Z, Xie Y, Chen J, Zhang D, Yang C, Li M. High selenium may be a risk factor of adolescent idiopathic scoliosis. Med Hypotheses 2010; 75:126–127. doi: 10.1016/j.mehy.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 114.Ji XR, Yang ZD, Yang XH, Liu DD, Ni HJ, Li M. Change of selenium in environment and risk of adolescent idiopathic scoliosis: a retrospective cohort study. Eur Rev Med Pharmacol Sci 2013; 17:2499–2503. doi: 10.1358/dof.2013.38.9.2040082. [PubMed] [Google Scholar]
- 115.McMaster ME. Heated indoor swimming pools, infants, and the pathogenesis of adolescent idiopathic scoliosis: a neurogenic hypothesis. Environ Health 2011; 10:86.doi: 10.1186/1476-069x-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McMaster ME, Lee AJ, Burwell RG. Indoor heated swimming pools: the vulnerability of some infants to develop spinal asymmetries years later. Stud Health Technol Inform 2006; 123:151–155. [PubMed] [Google Scholar]
- 117.Shen N, Chen N, Zhou X, Zhao B, Huang R, Liang J, et al. Alterations of the gut microbiome and plasma proteome in Chinese patients with adolescent idiopathic scoliosis. Bone 2019; 120:364–370. doi: 10.1016/j.bone.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 118.McMaster ME, Lee AJ, Burwell RG. Physical activities of patients with adolescent idiopathic scoliosis (AIS): preliminary longitudinal case-control study historical evaluation of possible risk factors. Scoliosis 2015; 10:6.doi: 10.1186/s13013-015-0029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Watanabe K, Michikawa T, Yonezawa I, Takaso M, Minami S, Soshi S, et al. Physical activities and lifestyle factors related to adolescent idiopathic scoliosis. J Bone Joint Surg Am 2017; 99:284–294. doi: 10.2106/jbjs.16.00459. [DOI] [PubMed] [Google Scholar]
- 120.Tarrant RC, Queally JM, Moore DP, Kiely PJ. Prevalence and impact of low body mass index on outcomes in patients with adolescent idiopathic scoliosis: a systematic review. Eur J Clin Nutr 2018; 72:1463–1484. doi: 10.1038/s41430-018-0095-0. [DOI] [PubMed] [Google Scholar]
- 121.Burwell RG, Aujla RK, Kirby AS, Dangerfield PH, Moulton A, Cole AA, et al. Body mass index of girls in health influences menarche and skeletal maturation: a leptin-sympathetic nervous system focus on the trunk with hypothalamic asymmetric dysfunction in the pathogenesis of adolescent idiopathic scoliosis? Stud Health Technol Inform 2008; 140:9–21. doi: 10.3233/978-1-58603-888-5-9. [PubMed] [Google Scholar]
- 122.Grivas TB, Burwell RG, Mihas C, Vasiliadis ES, Triantafyllopoulos G, Kaspiris A. Relatively lower body mass index is associated with an excess of severe truncal asymmetry in healthy adolescents: Do white adipose tissue, leptin, hypothalamus and sympathetic nervous system influence truncal growth asymmetry? Scoliosis 2009; 4:13.doi: 10.1186/1748-7161-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang HQ, Gao QL, Ge L, Wu JH, Liu JY, Guo CF, et al. Strong halo-femoral traction with wide posterior spinal release and three dimensional spinal correction for the treatment of severe adolescent idiopathic scoliosis. Chin Med J 2012; 125:1297–1302. doi: 10.3760/cma.j.issn.0366-6999.2012.07.020. [PubMed] [Google Scholar]
- 124.Yu B, Zhang JG, Qiu GX, Lu WC, Wang YP, Shen JX, et al. Selective anterior thoracolumbar/lumbar fusion and instrumentation in adolescent idiopathic scoliosis patients. Chin Med J 2010; 123:3003–3008. doi: 10.3760/cma.j.issn.0366-6999.2010.21.010. [PubMed] [Google Scholar]
- 125.Li QY, Zhang JG, Qiu GX, Wang YP, Shen JX, Zhao Y, et al. Primary effect of dual growing rod technique for the treatment of severe scoliosis in young children. Chin Med J 2010; 123:151–155. doi: 10.3760/cma.j.issn.0366-6999.2010.02.005. [PubMed] [Google Scholar]
- 126.Qiu Y, Wang B, Zhu F. Comparison of the curative effects of video assisted thoracoscopic anterior correction and small incision, thoracotomic anterior correction for idiopathic thoracic scoliosis. Chin Med J 2008; 121:1369–1373. doi: 10.1097/00029330-200808010-00007. [PubMed] [Google Scholar]


