Abstract
Background:
Nipple discharge cytology is a simple non-invasive method that may provide valuable information for detecting underlying malignancy. Several studies have investigated the diagnostic value of cytology in breast cancer patients with pathological nipple discharge, but the results have been highly variable. Herein we presented a systematic review and meta-analysis of published studies pertaining to the diagnostic capacity of nipple discharge cytology in patients with breast cancer.
Methods:
A systematic literature search was performed (Medline/PubMed, Embase, Cochrane Library databases, and Google Scholar) to identify studies that investigated the diagnostic capacity of cytology with regard to breast cancer in patients with pathologic nipple discharge. Two independent researchers identified articles that assessed the sensitivity and specificity of cytological evaluation for breast cancer detection in patients with pathologic nipple discharge published between January 2000 and October 2018. Articles were only included in the meta-analysis if they met predetermined criteria. The characteristics of each study and the data they yielded were summarized. Quality assessment of all articles included was performed using the Methodological Index for Non-randomized Studies Criteria (MINORS) and the Quality Assessment of Diagnostic Accuracy Study 2 (QUADAS-2). Heterogeneity was tested via Cochran Q test and the I2 statistic using Stata 12.0 and Meta-DiSc 1.4 software, and meta-analysis was performed.
Results:
A total of 286 articles were identified, of which 12 articles including a total of 1476 patients were deemed eligible for inclusion in the meta-analysis. A random-effects model assessing the capacity of nipple discharge cytology to predict breast cancer yielded pooled sensitivity 63% (95% confidence interval [CI]: 53%–72%), specificity 95% (95% CI: 87%–98%), positive likelihood ratio 12.35 (95% CI: 4.87–31.34), and negative likelihood ratio 0.39 (95% CI: 0.30–0.50). The diagnostic odds ratio was 31.88 (95% CI: 11.30–89.98). The area under the summary receiver operating characteristic curve was 0.79 (95% CI: 0.75–0.82).
Conclusion:
The current meta-analysis suggests that nipple discharge cytology is a useful diagnostic modality for detection of breast cancer in patients with pathological nipple discharge, with moderate sensitivity and high specificity.
Keywords: Breast carcinoma, Cytology, Diagnosis, Meta-analysis, Nipple discharge
Introduction
Nipple discharge refers to the passage of liquid material through the nipple of the breast.[1] It is a common chief complaint in patients with breast disease and is indicative of a possible intraductal lesion, thus it requires further evaluation. It is most commonly caused by intraductal papilloma or benign duct ectasia, but is also associated with underlying malignancies such as ductal carcinoma in situ and invasive ductal carcinoma.[2]
Diagnostic imaging methods that can be used in patients with pathological nipple discharge include mammography and high-frequency ultrasound, which may not depict any pathological indications.[3] Consistent with previous findings, in a retrospective analysis[4] mammography and sonography used either alone or in combination were less sensitive with regard to diagnosing non-palpable breast cancer associated with nipple discharge. Magnetic resonance imaging (MRI) may assist in the detection of intraductal lesions, but a previous study[5] suggests that it is of limited value in patients with unilateral bloody nipple discharge but no accessible masses and negative mammography and ultrasound results. It has also been asserted that MRI should not replace duct excision as the gold standard for the exclusion of malignant tumors.[6] Additional examination options include galactography and fiberoptic ductoscopy, both of which are invasive. Although galactography has been used as an alternative to mammography for pre-operative evaluation of intraductal lesions causing nipple discharge, reported accuracy rates with regard to detecting breast cancer range from only 39% up to 70%.[7] Moreover, galactography is a poor predictor of underlying pathology and cannot exclude malignancy. Fiberoptic catheteroscopy (FDS) is an imaging technique that enables the direct observation of space-occupying lesions in the breast ducts, which may increase the sensitivity and specificity of breast cancer diagnosis. However, even with this technique, some lesions may be missed due to the narrowing of the distal catheter.[8]
Nipple discharge cytology is a potentially useful modality in this context. It is a simple, uninvasive, and inexpensive means of histological sampling-based detection of alterations in breast tissue. The aim of the present study was to perform a systematic review and meta-analysis of relevant literature published between January 2000 and October 2018 to estimate the value of nipple discharge cytology in the diagnosis of breast cancer in women with pathological nipple discharge.
Methods
Search strategy
The international databases searched were Medline/PubMed, Embase, Cochrane Library databases, and Google Scholar. The publication period was from January 2000 to October 2018. The search terms used in literature searches were “breast cancer” OR “breast carcinoma” OR “mammary cancer” OR “breast tumor” AND “nipple discharge” OR “nipple aspiration” OR “nipple secretion” AND “cytology.” In an effort to identify additional articles of relevance, existing systematic reviews, meta-analyses, bibliographies of reports, and conference summaries were also perused. Where necessary, authors were contacted for additional information.
Inclusion and exclusion criteria
The studies included in the current meta-analysis all met the following criteria: (1) Histopathological confirmation of breast cancer diagnoses; (2) inclusion of the number of patients presenting with nipple discharge, and provision of a 2 × 2 contingency calculation based on sensitivity and specificity (or provision of sufficient raw data with which to perform these calculations); (3) assessment of the diagnostic performance of cytology in patients with pathological nipple discharge; (4) inclusion of patients with benign disease or healthy people who served as a control group; and (5) examinations were restricted to patients with breast cancer and a control group. Publications were excluded if they were case reports, editorial letters, review articles, articles on tissue or other cytology, or repeated publications, or if they contained unqualified or insufficient data.
Data extraction and quality assessment
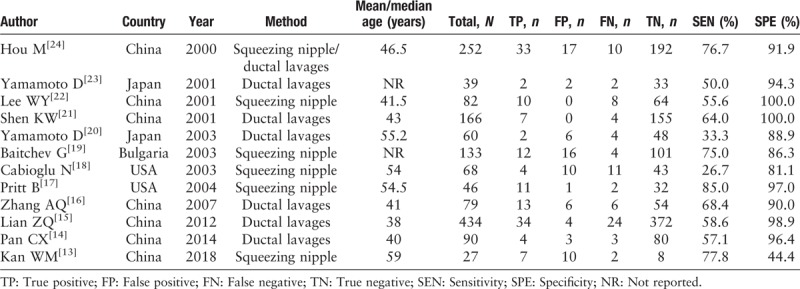
Two independent reviewers retrieved the following information from each eligible study: First author, year of publication, country of publication, mean age and median age, methods of nipple discharge collection, number of patients, and data for sensitivity and specificity [Table 1]. If a study included two different methods used to obtain nipple discharge, the study was split into two independent studies.[9] Any disagreements with regard to any of the information retrieved were resolved via discussion. Any disagreement over the controversial study was resolved through full discussion to reach a consensus.
Table 1.
Characteristics of eligible studies about the cytology of nipple discharge included in the meta-analysis.
Quality assessment was performed using the quality assessment of diagnostic accuracy studies-2 (QUADAS-2) tool, which consists of four parts and has proven to be an efficient tool in previous diagnostic accuracy studies.
Statistical analysis
All statistical analyses were performed using STATA SE 12.0 (Stata Corporation, College Station, TX, USA) and Meta-DiSc 1.4 software.[10] A 2 × 2 contingency table was constructed for each study based on sensitivity and specificity. Sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio were summarized using a diagnostic meta-analysis model, and a summary receiver operating characteristic curve was generated.[11] A P < 0.05 was deemed to indicate statistical significance, and I2 ≥ 50% was deemed to indicate significant heterogeneity.[12] Where applicable, confidence intervals (CIs) were calculated. Publication bias with regard to inclusion in the study was assessed using QUADAS-2 and a deeks funnel regression method. Spearman correlational coefficient was used to assess relationships between sensitivity and specificity.
Results
Included studies
The initial search identified 286 articles, 225 of which were excluded after screening their titles and abstracts. Of the remaining 61 articles, 49 were excluded because they did not fulfill the inclusion criteria, lost raw data, or were unable to be searched, because they were in a foreign language. Ultimately, 12 studies incorporating a combined total of 1476 patients were included in the current meta-analysis [Figure 1].[13–24]
Figure 1.

PRISMA flow chart of the literature search about the cytology of nipple discharge. PRISMA: Preferred reporting items for systematic reviews and meta-analyses.
Study characteristics and quality assessment
Of the collective total of 1476 patients in all the studies included in the current meta-analysis, 219 were reference-positive subjects and 1257 were reference-negative subjects. All of the studies used surgical excision or biopsy as the reference standard. Patients who did not undergo surgery or biopsy were excluded. All of the 12 studies were from one of four countries; China (n = 7), USA (n = 2), Japan (n = 2), and Bulgaria (n = 1). The sample sizes of studies ranged from 27 to 434 patients. Six studies obtained nipple discharge by squeezing the nipple. The other six used ductal lavages, including the ThinPrep cytology test (TCT) (Cytyc Corp, Marlborough, Massachusetts, USA).
With regard to the four parts of the QUADAS-2 evaluation tool, the readers agreed on 100% of them. The percentages of studies with low, high, or unclear risks of bias and the percentages of studies with low, high, or unclear concerns regarding applicability with respect to each domain of the QUADAS-2 are shown in Figure 2. Two studies were rated as having unclear risks of overall bias, and one additional study was rated as having unclear risks of bias in the flow domain and the timing domain. With those exceptions, all domains of all other studies were rated as having low risks of bias
Figure 2.

Proportion of studies with low, intermediate, high or unclear risk of bias and concerns regarding applicability determined with the quality assessment of diagnostic accuracy studies 2 tool.
Data analysis
Heterogeneity in sensitivity and specificity was evident between the twelve studies (I2 = 46.23%, 91.77%). Pooled results yielded a sensitivity of cytology of nipple discharge for the diagnosis of breast cancer of 63% (95% CI: 53%–72%) and a specificity of 95% (95% CI: 87%–98%) [Figures 3 and 4]. The combined positive likelihood ratio was 12.35 (95% CI: 4.87–31.34), indicating that patients with breast cancer had a nearly 12-fold greater chance of yielding a positive cytology result than patients without breast cancer. The combined negative likelihood ratio was 0.39 (95% CI: 0.30–0.50). The summary receiver operating characteristic curve for the studies included is shown in Figure 5. The area under the curve was 0.79, indicating good diagnostic accuracy. The diagnostic odds ratio was 31.88 (95% CI: 11.30–89.98) [Figure 6].
Figure 3.

Forest plot of pooled sensitivity for the diagnosis of breast cancer by nipple discharge cytology.
Figure 4.

Forest plot of pooled specificity for the diagnosis of breast cancer by nipple discharge cytology.
Figure 5.

Summary receiver operating characteristics curve for the diagnosis of breast cancer through nipple discharge cytology.
Figure 6.

Diagnostic odds ratio with Cochran Q value.
Publication bias
Deeks’ funnel regression method was used to further assess publication bias [Figure 7]. And the P value of the test was 0.169, indicating no publication bias.
Figure 7.

Regression analysis to evaluate publication bias in nipple discharge cytology assays.
Sensitivity analysis
In sensitivity analysis each of the 12 studies was sequentially eliminated one by one, and at each step meta-analysis of the remaining studies was conducted and the results were compared with those before the previous elimination. If the change in the combined effect was not large, the stability of the studies included was good and the result was credible. If the change was not small, the stability of the included studies was poor. The results suggested that the stability was good [Table 2].
Table 2.
The influence of each trial about the cytology of nipple discharge for the outcome of the meta-analysis.

Threshold effect and heterogeneity
The threshold effect is attributed to differences in sensitivity and specificity, and Spearman correlational coefficient is a reliable measure by which to evaluate it. In the current meta-analysis Spearman correlational coefficient was 0.014 with a P value of 0.966, suggesting that there was no significant heterogeneity arising from the threshold effect.
Whether heterogeneity had originated from the region of the study population, the method of obtaining nipple discharge, and/or the total number of specimens in the studies was investigated via meta-regression analysis. As shown in Table 3, none of the aforementioned variables contributed to the heterogeneity between the studies.
Table 3.
Sub-group analysis of meta-analysis data on the cytology of nipple discharge.

In a sub-group meta-regression analysis, the aforementioned variables in various sub-groups were analyzed based on P values. Due to small numbers of European and American subjects in the meta-analysis, the comparative heterogeneity of that sub-group may have been relatively large. In addition, the method of obtaining nipple discharge was evidently also a major cause of heterogeneity [Table 3].
Discussion
Nipple discharge in the non-lactating breast is considered the pathological discharge which is often associated with a variety of pathologic lesions. A portion of these lesions are underlying malignancies. Many approaches are employed to evaluate patients with pathological nipple discharge. There is no agreement about methods the diagnostic tests to confirm or exclude breast cancer in patients with nipple discharge. Nipple discharge cytology is a simple, non-invasive method that complements other methods and could provide valuable information in detecting an underlying malignancy. For galactography, the sensitivity was 56.3% to 83.0% and specificity was 26.7% to 62.4%.[25] It is reported that the sensitivity of FDS for detection of malignant lesions was 55.2% to 94.2%, the specificity was 61.5% to 100%.[26] But the diagnostic role of nipple discharge cytology in breast cancer is still uncertain. So we presented this systematic review and meta-analysis of the diagnostic value of pathological nipple discharge cytology for breast cancer.
In the present meta-analysis 12 articles were identified and used to derive an informed estimation of the diagnostic capacity of pathological nipple discharge cytology with regard to the detection of breast cancer. Most of the 12 studies were rated as being of high quality via the QUADAS-2 tool.
The diagnostic odds ratio combines sensitivity and specificity data into a single indicator of the accuracy of the diagnostic test.[27] The value of 31.88 obtained in the current meta-analysis suggests that cytology may be a useful method for the diagnosis of breast cancer in patients with nipple discharge. This contention is supported by the good diagnostic accuracy indicated by the area under the curve of 0.79.
Spontaneous or expressible nipple discharge may occur in palpable and non-palpable breast lesions. Lee[22] reported that the sensitivity and specificity of non-palpable and palpable breast lesions in patients with nipple discharge cytology were similar. Nipple discharge cytology is useful for detecting an underlying breast lesion even if the patient has no palpable mass in the breast. Thus, the present meta-analysis evaluated the diagnostic accuracy of nipple discharge cytology in palpable and non-palpable breast lesions.
Nipple discharge is usually the first symptom in patients with intraductal mass. There is no established consensus on whether or not such discharge should be regarded as generally indicative of an underlying malignancy. The current meta-analysis investigated the value of cytology analysis of nipple discharges of various types including milky, yellowish, and bloody discharges, and this heterogeneity of discharge types may have contributed to the low sensitivity result obtained.
There are diverse potential sources of heterogeneity in numerous fields of medical investigation, and in test accuracy studies one of the most important is the threshold effect. Spearman correlational coefficient was 0.014 (P = 0.966) in the present analysis; however, indicating that there was no significant heterogeneity arising from the threshold effect. In addition, the regions of the study populations, methods of obtaining cells, and sample sizes in the different studies could also have caused heterogeneity. To identify sources of heterogeneity meta-regression analysis was used to assess the contribution of the above variables, and that analysis suggested that none were a source of heterogeneity. A sub-group analysis including the aforementioned variables was then performed. Due to small numbers of European and American people in the total subject pool, relative heterogeneity within that sub-group may have been comparatively large. Notably, variation in the method of obtaining nipple discharge was also evidently a major cause of heterogeneity.
The method used to obtain nipple discharge may have a substantial effect on the heterogeneity to diagnose breast cancer via nipple discharge cytology. In the current meta-analysis the method of obtaining discharge by squeezing the nipple was associated with greater heterogeneity than obtaining discharge via ductal lavage. Squeezing the nipple could facilitate cytologic diagnosis, and it became a routine part of the examination of every patient with nipple discharge. Notably, traditional exfoliative cell examination has relatively limited capacity for the early diagnosis and differential diagnosis of intraductal diseases. Contributory reasons may be that most malignant lesions are located in peripheral ducts, they tend to be small, and they may not be associated with substantial nipple discharge, making it less likely that malignant cells will be obtained by squeezing the nipple.[28]
Conversely there was no significant heterogeneity in the ductal lavage sub-group, indicating that it may be more reliable with respect to the diagnosis of breast cancer. Ductal lavage may increase the exfoliation of cells, which may in turn improve the sensitivity of cytology testing and reduce heterogeneity. One reason for this may be that ductal lavage cytology is always performed in conjunction with FDS, or galactography.[29] Owing to successful insertion into the lactiferous duct, the study could obtain an adequate amount of specimen from the catheter. Masukawa et al[30] reported that it was easier for cytopathologists to analyze two slides with many cells than several slides with fewer discharged cells, and that ductal lavage samples contained many more cells for examination and diagnosis. Another possible reason for the apparent superiority of cytology testing via ductal lavage is that it is often performed repeatedly until the specimens acquired are deemed sufficient. Therefore, the information derived from it is comparatively more reliable, and the associated heterogeneity is small. The ductal lavage sub-group in the present meta-analysis was derived from several studies that used the TCT[14–16] to investigate nipple discharge cytology. That test can reportedly significantly improve the positive detection rate, especially for malignant tumors, compared with the conventional exfoliated cell smear test.
Although the results of the current meta-analysis are promising, it had several limitations. Nine of the twelve studies it included were conducted in Asian countries, which may compromise the broader applicability of the results to other races. Another observation that potentially limits the conclusions that can be drawn from the analysis is that the heterogeneity of specificity pertaining to nipple discharge cytology for the detection of breast cancers was significant. Lastly, sensitivity and specificity values from different studies with different numbers of positive cells were pooled. A baseline reference for positive number of cells could be different. For example, different studies divide the severe atypical hyperplasia differently.
Given the high quality of the studies included in the current meta-analysis, the results it yielded suggest that cytology of pathological nipple discharge has potential diagnostic value with regard to breast cancer. Adequate sensitivity and specificity were both evident. Additional prospective multicenter studies with larger sample sizes are needed to verify the results of the current meta-analysis.
Funding
This study was supported by a grant from the Science and Technology Commission of Beijing Municipality (No. D16110000816002).
Conflicts of interest
None.
Footnotes
How to cite this article: Li XQ, Xu F, Lei CQ, Li J, Jiang HC. Accuracy for cytological evaluation in the detection of breast cancer among patients with pathologic nipple discharge: a PRISMA-compliant meta-analysis. Chin Med J 2020;133:435–443. doi: 10.1097/CM9.0000000000000643
Xiao-Qian Li and Feng Xu contributed equally to this work.
References
- 1.Chen L, Zhou WB, Zhao Y, Liu XA, Ding Q, Zha XM, et al. Bloody nipple discharge is a predictor of breast cancer risk: a meta-analysis. Breast Cancer Res Treat 2012; 132:9–14. doi: 10.1007/s10549-011-1787-5. [DOI] [PubMed] [Google Scholar]
- 2.Dolan RT, Butler JS, Kell MR, Gorey TF, Stokes MA. Nipple discharge and the efficacy of duct cytology in evaluating breast cancer risk. Surgeon 2010; 8:252–258. doi: 10.1016/j.surge.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Lippa N, Hurtevent-Labrot G, Ferron S, Boisserie-Lacroix M. Nipple discharge: the role of imaging. Diagn Interv Imaging 2015; 96:1017–1032. doi: 10.1016/j.diii.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Patel BK, Falcon S, Drukteinis J. Management of nipple discharge and the associated imaging findings. Am J Med 2015; 128:353–360. doi: 10.1016/j.amjmed.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 5.van Gelder L, Bisschops RH, Menke-Pluymers MB, Westenend PJ, Plaisier PW. Magnetic resonance imaging in patients with unilateral bloody nipple discharge; useful when conventional diagnostics are negative? World J Surg 2015; 39:184–186. doi: 10.1007/s00268-014-2701-1. [DOI] [PubMed] [Google Scholar]
- 6.Wang LJ, Wu P, Li XX, Luo R, Wang DB, Guan WB. Magnetic resonance imaging features for differentiating breast papilloma with high-risk or malignant lesions from benign papilloma: a retrospective study on 158 patients. World J Surg Oncol 2018; 16:234.doi: 10.1186/s12957-018-1537-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrogh M, Morris EA, Liberman L, Borgen PI, King TA. The predictive value of ductography and magnetic resonance imaging in the management of nipple discharge. Ann Surg Oncol 2007; 14:3369–3377. doi: 10.1245/s10434-007-9530-5. [DOI] [PubMed] [Google Scholar]
- 8.Feng XZ, Song YH, Zhang FX, Jiang CW, Mei H, Zhao B. Diagnostic accuracy of fiberoptic ductoscopy plus in vivo iodine staining for intraductal proliferative lesions. Chin Med J 2013; 126:3124–3129. doi: 10.3760/cma.j.issn.0366-6999.20130691. [PubMed] [Google Scholar]
- 9.Wang C, Luan S, Panayi AC, Xin MQ, Luan J. Methods used for evaluation of volume retention rate in autologous fat grafting for breast augmentation: a systematic review. Chin Med J 2019; 132:2223–2228. doi: 10.1097/CM9.0000000000000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006; 6:31.doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell AJ, Vaze A, Rao S. Clinical diagnosis of depression in primary care: a meta-analysis. Lancet 2009; 374:609–619. doi: 10.1016/s0140-6736(09)60879-5. [DOI] [PubMed] [Google Scholar]
- 12.Dinnes J, Deeks J, Kirby J, Roderick P. A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy. Health Technol Assess 2005; 9:1–113. doi: 10.3310/hta9120. [DOI] [PubMed] [Google Scholar]
- 13.Kan WM, Chen C, Kwong A. Implications of nipple discharge in Hong Kong Chinese women. Hong Kong Med J 2018; 24:18–24. doi: 10.12809/hkmj154764. [DOI] [PubMed] [Google Scholar]
- 14.Pan CX, Yang JH, Zou LM, Wu YY, Zhang XR, Mi XJ. The clinical value of nipple discharge diagnosis by fiberoptic ductoscopy system combined with thinprep cytology test (in Chinese). Guangdong Med J 2014; 35:2054–2056. doi: 10.3969/j.issn.1001-9448.2014.13.027. [Google Scholar]
- 15.Lian ZQ, Zhang JY, Wang Q, Zhang AQ, Zhu CX, Wu L. Evaluation of fiberoptic ductoscopy system and thinprep cytology test in diagnosis of pathological nipple discharge (in Chinese). Chin J Cancer Prev Treat 2012; 19:770–773. [Google Scholar]
- 16.Zhang AQ, Wang Q, Zhang JY, et al. Intraductal aspiration TCT by Fiberoptic ductoscopy for patients with nipple discharge (in Chinese). Chin J Breast Dis (electronic version) 2007; 1:19–21. doi: 10.3969/j.issn.1674-0807.2007.02.006. [Google Scholar]
- 17.Pritt B, Pang Y, Kellogg M, St John T, Elhosseiny A. Diagnostic value of nipple cytology: study of 466 cases. Cancer 2004; 102:233–238. doi: 10.1002/cncr.20379. [DOI] [PubMed] [Google Scholar]
- 18.Cabioglu N, Hunt KK, Singletary SE, Stephens TW, Marcy S, Meric F, et al. Surgical decision making and factors determining a diagnosis of breast carcinoma in women presenting with nipple discharge. J Am Coll Surg 2003; 196:354–364. doi: 10.1016/s1072-7515(02)01606-x. [DOI] [PubMed] [Google Scholar]
- 19.Baitchev G, Gortchev G, Todorova A, Dikov D, Stancheva N, Daskalova I. Intraductal aspiration cytology and galactography for nipple discharge. Int Surg 2003; 88:83–86. doi: 10.1177/106689690301100217. [PubMed] [Google Scholar]
- 20.Yamamoto D, Senzaki H, Nakagawa H, Okugawa H, Gondo H, Tanaka K. Detection of chromosomal aneusomy by fluorescence in situ hybridization for patients with nipple discharge. Cancer 2003; 97:690–694. doi: 10.1002/cncr.11091. [DOI] [PubMed] [Google Scholar]
- 21.Shen KW, Wu J, Lu JS, Han QX, Shen ZZ, Nguyen M, et al. Fiberoptic ductoscopy for breast cancer patients with nipple discharge. Surg Endosc 2001; 15:1340–1345. doi: 10.1007/s004640080108. [DOI] [PubMed] [Google Scholar]
- 22.Lee WY. Cytology of abnormal nipple discharge: a cyto-histological correlation. Cytopathology 2003; 14:19–26. doi: 10.1046/j.1365-2303.2003.00419.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto D, Shoji T, Kawanishi H, Nakagawa H, Haijima H, Gondo H, et al. A utility of ductography and fiberoptic ductoscopy for patients with nipple discharge. Breast Cancer Res Treat 2001; 70:103–108. doi: 10.1023/a:1012990809466. [DOI] [PubMed] [Google Scholar]
- 24.Hou M, Tsai K, Lin H, Chai C, Liu C, Huang T. A simple intraductal aspiration method for cytodiagnosis in nipple discharge. Acta Cytol 2000; 44:1029–1034. doi: 10.1159/000328592. [DOI] [PubMed] [Google Scholar]
- 25.Istomin A, Masarwah A, Pitkanen M, Joukainen S, Sutela A, Vanninen R, et al. Galactography is not an obsolete investigation in the evaluation of pathological nipple discharge. PLoS One 2018; 13:e0204326.doi: 10.1371/journal.pone.0204326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu GY, Lu JS, Shen KW, Wu J, Chen CM, Hu Z, et al. Fiberoptic ductoscopy combined with cytology testing in the patients of spontaneous nipple discharge. Breast Cancer Res Treat 2008; 108:271–277. doi: 10.1007/s10549-007-9598-4. [DOI] [PubMed] [Google Scholar]
- 27.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 2003; 56:1129–1135. doi: 10.1016/S0895-4356(03)00177-X. [DOI] [PubMed] [Google Scholar]
- 28.Kamali S, Bender O, Kamali GH, Aydin MT, Karatepe O, Yuney E. Diagnostic and therapeutic value of ductoscopy in nipple discharge and intraductal proliferations compared with standard methods. Breast Cancer 2014; 21:154–161. doi: 10.1007/s12282-012-0377-7. [DOI] [PubMed] [Google Scholar]
- 29.Denewer A, El-Etribi K, Nada N, El-Metwally M. The role and limitations of mammary ductoscope in management of pathologic nipple discharge. Breast J 2008; 14:442–449. doi: 10.1111/j.1524-4741.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- 30.Masukawa T. Discovery of psammoma bodies and fungus organisms in the nipple secretion with improved breast cytology technique. Acta Cytol 1972; 16:408–415. [PubMed] [Google Scholar]


