Abstract
Long noncoding RNAs (lncRNAs) have recently been discovered and are increasingly recognized as vital components of modern molecular biology. Accumulating evidence shows that lncRNAs have emerged as important mediators in diverse biological processes such as cell differentiation, pluripotency, and tumorigenesis, while the function of lncRNAs in the field of normal and malignant hematopoiesis remains to be further elucidated. Here, we widely reviewed recent advances and summarize the characteristics and basic mechanisms of lncRNAs and keep abreast of developments of lncRNAs within the field of normal and malignant hematopoiesis. Based on gene regulatory networks at different levels of lncRNAs participation, lncRNAs have been shown to regulate gene expression from epigenetics, transcription and post transcription. The expression of lncRNAs is highly cell-specific and critical for the development and activation of hematopoiesis. Moreover, we also summarized the role of lncRNAs involved in hematological malignancies in recent years. LncRNAs have been found to play an emerging role in normal and malignant hematopoiesis, which may provide novel ideas for the diagnosis and therapeutic targets of hematological diseases in the foreseeable future.
Keywords: Long non-coding RNA, Hematopoiesis, Hematological malignancies
Introduction
With the development of the genome-wide transcriptome studies, pervasive researches are currently focusing on non-protein-coding regulatory RNAs (ncRNAs), which were regarded as junk or noises of transcripts previously.[1] The concept that non-coding RNAs do play crucial roles in regulating gene expression at the levels of transcription, RNA processing, and translation has been recognized for several years.[2–4] According to the transcript size, ncRNAs are generally classified into two major groups: short ncRNAs (<200 nucleotides) or long ncRNAs (>200 nucleotides). MicroRNAs (miRNAs), a typical representative of short ncRNAs, have been best studied and known to induce mRNA degradation or block mRNA translation via the RNA interference pathway[1,4] and recognized as powerful regulators of numerous genes and pathways in the pathogenesis of hematological diseases.[5–8] In contrast, abundant long non-coding RNAs (lncRNAs) have recently been discovered and are increasingly recognized as vital components of modern molecular biology. Accumulating evidence shows that lncRNAs can modulate diverse biological processes such as cell proliferation, differentiation, pluripotency, apoptosis, and tumorigenesis.[9–13] The exploration of the characteristics and mechanisms of them is at a relatively initial stage yet, and especially the function of lncRNAs in the field of hematopoiesis remains to be further elucidated. In this review, we will succinctly summarize the characteristics and mechanisms of lncRNAs and focus on the latest progress of lncRNAs in normal and malignant hematopoiesis.
Characteristics and Functions of Long Non-coding RNAs
Long non-coding RNAs are defined as a heterogeneous class of ncRNAs longer than 200 nucleotides which feature distinguishe them from small regulatory RNAs such as miRNAs, small nucleolar RNAs (snoRNAs), piwi-interacting RNAs (piRNAs), short interfering RNAs (siRNAs), and other short RNAs. LncRNAs could be localized to the nucleus or cytoplasm and are most abundant in the nucleus, which is different from mRNAs that are mostly transported to the cytoplasm.[14,15] According to the NONCODE database (current version v5.0, http://www.noncode.org), which is an integrated knowledge database dedicated to non-coding RNAs and currently recruits the lncRNA information of 17 species, there are 172,216 and 131,697 lncRNA transcripts of human and mouse at present, respectively.
Due to the high heterogeneity of the sequence, structure and biological function of lncRNAs, there are various classification methods so far. Generally, in light of their proximity to protein-coding mRNAs, lncRNAs could be classified into the following categories[16]: (1) the long intergenic ncRNA (lincRNA), which comes from the region between two genes; (2) intronic lncRNA, which originates from the intron region of secondary transcript (sometimes mRNA precursor sequence); (3) sense lncRNA, which overlaps with one or more exons of another protein-coding gene of the synonymous chain; (4) antisense lncRNA, which overlaps with one or more exons of another protein-coding gene in the opposite strand; and (5) bidirectional lncRNAs, whose transcription start site is very close to the protein gene encoding on the antisense strand, but the direction of transcription is the opposite. Based on the features and special functions of lncRNAs, it also includes lncRNA-activating (lncRNA-a), transcribed pseudogene lncRNAs, telomere-associated ncRNAs (TERRAs), transcribed ultraconserved regions (T-UCRs), enhancer RNAs (eRNAs), circular RNAs, etc.[17–19]
According to recent advances, the characteristics of lncRNAs can be summarized as follows: Firstly, no different than mRNA, most lncRNAs whose promoter can also bind transcription factors are also polyadenylated, spliced, and modified with 5′-cap and poly-A tail, and also transcribed by RNA polymerase II (RNAPII). They have dynamic expression and different splicing modes during differentiation.[20,21] Secondly, lncRNAs are relatively conservative in function although they contain fewer longer exons and lower evolutionary sequence conservation compared with mRNAs.[22,23] Thirdly, most lncRNAs are expressed at relatively lower levels but have more obvious temporal and spatial expression specificity compared with mRNAs in the process of tissue differentiation and development.[24–26]
Up to now, the precise mechanism of lncRNA is not entirely clear. Nevertheless, based on gene regulatory networks at different levels of lncRNA participation, lncRNAs have been shown to regulate gene expression in three ways: epigenetics, transcription, and post-transcription[27–29] [Figure 1]. First, lncRNAs can catalyze the synthesis of chromatin remodeling complexes into specific genomic loci. For example, the recruitment of polycomb complex to the HOXD gene cluster by lncRNA HOTAIR and Xist/RepA results in three methylations (me3K27) of histone H3 27th lysine in the X chromosome and induces heterochromatin formation, which finally inhibits gene expression in this region.[30] Second, lncRNAs may participate in transcriptional regulation of target genes by regulating the transcription of neighboring protein-coding genes, interacting with transcription factors, or forming three-helix complexes with DNA. For instance, lncRNA pncRNA-D (promoter-associated ncRNA-D) can bind to the gene cyclinD1 and recruit RNA binding protein TLS (Translocated in LipoSarcoma) to regulate histone acetyltransferase activity of protein CBP and P300, and then inhibiting the transcription of cyclin D1.[31] Third, lncRNAs may affect any step in post-transcriptional gene expression including regulating alternative splicing of mRNA precursor, being spliced into non-coding small RNA, regulating mRNA stability and abundance, or the role of competitive endogenous RNA (ceRNA).[32–34]
Figure 1.
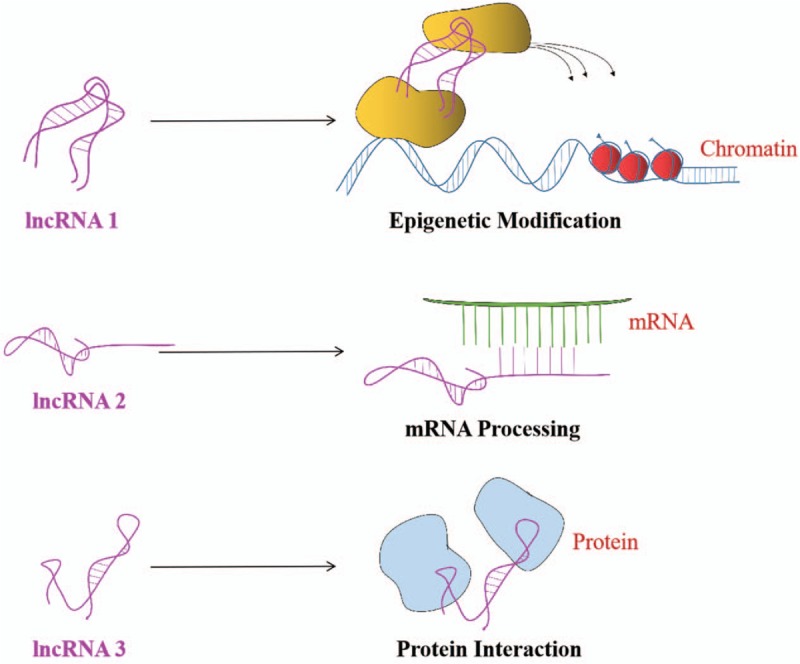
Schematic diagram of the basic regulatory mechanism of lncRNAs. The purple ones are lncRNAs. lncRNAs: Long non-coding RNAs.
Long Non-coding RNAs in Normal Hematopoiesis
Hematopoietic stem cells (HSCs) are characterized by the ability to execute a cascade of cell fates, including self-renewal, controlled expansion of progenitor cells, and timely differentiation into terminally mature blood cells.[35] The process of differentiation of hematopoietic lineages is critically driven by the interaction of external stimuli and intracellular regulatory programs. Lineage-specific lncRNA molecules are also emerging as important regulators of gene expression during hematopoiesis [Table 1 and Figure 2].
Table 1.
Long non-coding RNAs involved in the normal hematopoiesis.

Figure 2.
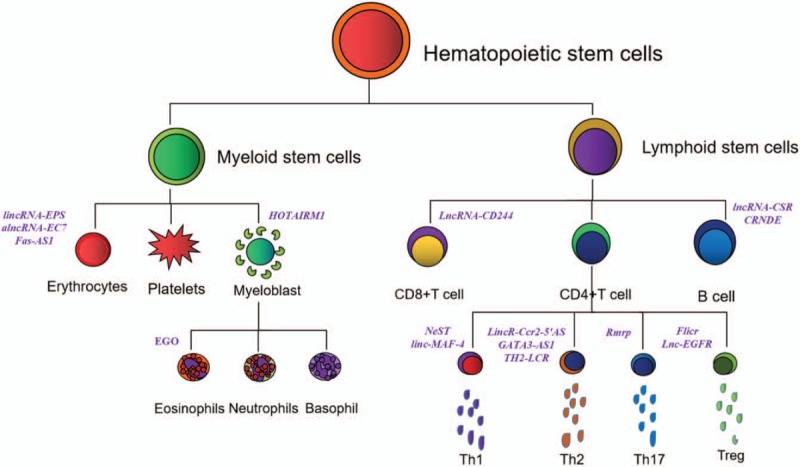
Schematic diagram of lncRNA regulating normal hematopoietic differentiation. The purple ones are the name of lncRNAs. lncRNAs: Long non-coding RNAs; Th1: Type 1 helper T cells; Th2: Type 2 helper T cells; Th17: Type 17 helper T cells; Treg: Regulatory T cells; lincRNA-EPS: lincRNA erythroid prosurvival; Fas-AS1 (or Saf): Fas-antisense 1; HOTAIRM1: HOX antisense intergenic RNA myeloid 1; EGO: Eosinophil granule ontogeny; NeST (Tmevpg1 or IFNG-AS1): Nettoie Salmonella pas Theiler's; cleanup Salmonella not Theiler's; GATA3-AS1: GATA3-antisense 1; TH2-LCR: TH2-locus control region; Flicr: Foxp3 long intergenic non-coding RNA; Inc-EGFR: Lnc-epidermal growth factor receptor; lncRNA-CSR: LncRNA-class switch DNA recombination; CRNDE: Colorectal neoplasia differentially expressed.
LncRNAs in Erythropoiesis
Erythropoiesis is a developmental process that is critically controlled by multiple regulators to ensure the proper generation of mature red blood cells and the transportation of oxygen to tissues.[36] Inspired by the “chromatin-state maps” approach pioneered by Guttman et al[59] less than a decade ago, the Biomedical Research group of Cambridge identified the first erythroid-specific lncRNA lincRNA erythroid prosurvival (lincRNA-EPS), which could facilitate erythropoiesis by repressing the expression of Pycard, a proapoptotic gene, without altering erythroid differentiation.[36,37] Subsequent studies also discovered multiple lncRNAs that are dynamically expressed during erythropoiesis and are targeted by key erythroid transcription factors such as GATA1, TAL1, or KLF1.[60]AlncRNA-EC7 was one of the identified lncRNAs. Reduction of alncRNA-EC7 expression in erythroblasts induced BAND3 (a major anion exchange protein present on erythrocyte membranes[61]) gene expression via chromatin interactions between the alncRNA-EC7 locus and the neighboring region of the BAND3 promoter leading to impaired erythrocyte maturation.[36,38] Recently, a research group also released their findings that lncRNA Fas-antisense 1 (Fas-AS1 or Saf) was induced during differentiation through the activity of essential erythroid transcription factors GATA-1 and KLF1. They further discovered that Saf was also negatively regulated by NF-κB and that over-expression of Saf in erythroblasts derived from CD34+ hematopoietic stem/progenitor cells of healthy donors could reduce surface levels of Fas and consequently conferred protection against Fas-mediated cell death signals.[39,40] Although current studies of lncRNAs in erythropoiesis are largely done in the murine models, these advances expand the repertoire of lncRNA functions and provide a novel genetic pathway that can be exploited with effective targets for the treatment of various anemia-related diseases in the future.[36]
LncRNAs in Myeloid Hematopoiesis
Almost a decade ago, Zhang et al[62] unveiled the first myeloid lineage-specific lncRNA HOTAIRM1 (HOX antisense intergenic RNA myeloid 1) which is transcribed between the human HOXA1 and HOXA2 genes. In-depth research revealed that HOTAIRM1 contributed to three-dimensional conformational changes of chromosomes which were required for the temporal collinear activation of HOXA genes.[41] Functional studies showed that knockdown of HOTAIRM1 could quantitatively impaire all-trans retinoic acid (ATRA)-induced myeloid differentiation and selectively attenuated differentiation-related transcripts such as CD11b, CD18, HOXA1, and HOXA4.[62] Subsequent studies showed that the master transcription factor PU.1 during myeloid differentiation could directly activate the expression of HOTAIRM1 through binding to the regulatory region of HOTAIRM1.[41] A recent study revealed that the high expression of HOTAIRM1 could enhance ATRA-induced PML-RARA degradation by affecting autophagic flux.[42] Conclusively, these advances indicate that HOTAIRM1 may be a novel potential therapeutic target for acute promyelocytic leukemia (APL).
The lncRNA EGO (eosinophil granule ontogeny) is a novel, nested, non-coding RNA, expressed during eosinophil development from CD34+ human HSCs and mature eosinophils. RNA silencing assays investigated that EGO regulated granule protein MBP (major basic protein) and EDN (eosinophil derived neurotoxin) transcript expression in developing CD34 hematopoietic progenitors.[43]
Lymphoid Differentiation-related lncRNAs
CD4+ T cells play central roles in mediating adaptive immunity against various pathogens. With T-cell receptor activation by specific cytokines, naive CD4+ T cells may differentiate into one of several lineages of T helper (Th) cells, including Th1, Th2, Th17, and inducible regulatory T cell (iTreg), as defined by their pattern of cytokine production and function.[63] The diversity of CD4+ T-cell subsets enables the adaptive immune system to adapt to many challenges during the expression of genes encoding cytokines and transcription factors.[64]NeST (Nettoie Salmonella pas Theiler's; cleanup Salmonella not Theiler's), formally known as Tmevpg1 or IFNG-AS1, is a long non-coding RNA specifically expressed in Th1 cells by a T-bet (a Th1-specific key transcription factor) dependent mechanism. NeST RNA was found to bind WDR5, a component of the histone H3 lysine 4 methyltransferase complex and to alter histone 3 methylation at the interferon-gamma locus, ultimately leading to the regulation of the expression of IFN-γ.[44,45] Another Th1-specific lncRNA is linc-MAF-4 whose expression is negatively correlated with MAF, a Th2-associated transcription factor. Studies suggest that linc-MAF-4 could regulate MAF transcription by recruiting chromatin modifiers and that down-regulation of linc-MAF-4 could skew T-cell differentiation toward the Th2 phenotype.[46]
Hu et al[47,48] identified 1524 lincRNA clusters from early T-cell progenitors to terminally differentiated T-helper subsets, among which lincR-Ccr2-5′AS, regulated by GATA-3 (a zinc-finger transcription factor, highly expressed in Th2 cells and critical to Th2 differentiation by regulating Th2 gene expression), was considered to be an essential part of the regulation in Th2-specific gene expression and important for Th2 cell migration. In the same year, another research group also reported a GATA-3-associated lncRNA GATA3-AS1 which was specifically expressed in primary Th2 cells. Their results indicate that the expression of GATA3-AS1 and GATA3 might be co-regulated by the same regulatory elements and might have shared functions in Th2 cell responses.[49] By whole-genome sequencing (RNA-seq), Spurlock et al[50] identified a cluster of antisense lncRNAs TH2-LCR, which was transcribed from the RAD50 locus that is co-expressed with IL4, IL5, and IL13 genes under Th2 polarizing conditions. Their analyses demonstrated that TH2-LCR is significantly required for the expression of genes encoding Th2 cytokines.
The differentiation of Th17 cells requires the nuclear hormone receptor RORγt which focuses on the activity of a cytokine-regulated transcriptional network including genes encoding the signature Th17 cytokines (IL-17A, IL-17F, IL-22).[65] Huang and colleagues identified the lncRNA Rmrp, RNA component of mitochondria RNA-processing endoribonuclease (RNase MRP), as a key DDX5 (DEAD-box protein 5, an RNA helicase possessing an important role in gene expression)-associated RNA, which could regulate the function of RORγt transcriptional complexes at a subset of critical genes implicated specifically in the Th17 effector program.[51,52]
Regulatory T cells (Tregs) characterized by the transcription factor FoxP3 are a fundamental component in maintaining immune homeostasis by negatively regulating several immunocyte lineages, especially during autoimmune, tumor, and lymphoproliferative pathologies.[66] A recent study identified a lncRNA Flicr whose expression profile and genomic localization displayed Treg specificity, partially overlapping Foxp3. Further assays revealed that Flicr dampens the Treg signature and may lower Treg stability, allowing stronger antiviral responses by destabilizing Foxp3.[53] Another novel Treg-related lncRNA is lnc-EGFR (lnc-epidermal growth factor receptor), whose up-regulation positively correlates with the expression of EGFR/Foxp3. Mechanism research shows that lnc-EGFR is a potential enhancer of EGFR and its downstream AP-1/NF-AT1 axis, and could augment immunosuppression by promoting Treg cell differentiation which may offer a potential therapeutic target for certain carcinomas.[54]
Wang et al[55,56] revealed that the expression of CD244, a T-cell-inhibitory molecule in CD8+ T-cell immune responses during tuberculosis (TB) infection, correlated with high levels of a lncRNA lncRNA-CD244. Functional assays demonstrated that lncRNA-CD244 could mediate IFN-γ and TNF-α expression and improve the protective immunity of CD8+ T cells by interacting with EZH2 (enhancer of zeste homolog 2, a chromatin-modification enzyme).[56]
B cells develop from the common lymphoid progenitor cells in the bone marrow and the initial antigen-independent phase is characterized by immunoglobulin gene rearrangements.[67] As the central drivers of immune humoral response, B cells development and function are influenced by a series of gene regulation.[68] Using RNA-seq and de novo transcript assembly, researchers have identified several lncRNAs involved in the development, activation, proliferation, and differentiation of B cells, such as lncRNA-CSR and CRNDE.[57,58,69]
Long Non-coding RNAs in Malignant Hematopoiesis
In addition to the normal hematopoietic process regulated by a variety of lncRNAs, abnormal interference of lncRNA regulation also inevitably leads to hematopoietic dysfunction, mainly in the occurrence of leukemia, lymphoma, and myeloma. At present, several lncRNAs related to hematological malignancies have been identified and summarized in the following sections [Table 2 and Figure 3].
Table 2.
Long non-coding RNAs involved in the malignant hematopoiesis.
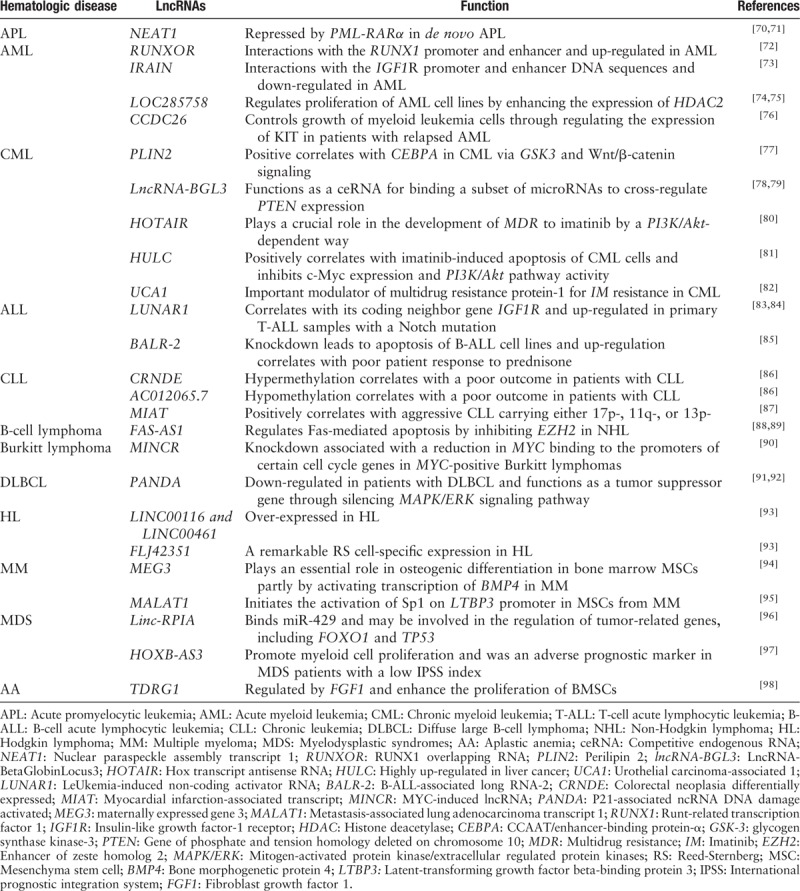
Figure 3.
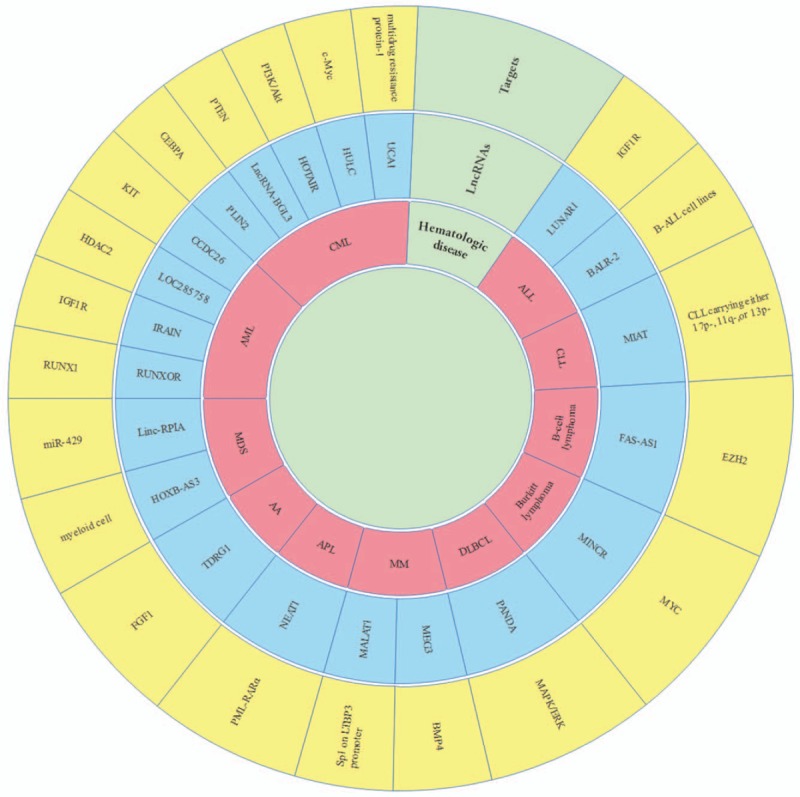
The relationship between lncRNA and its targets on the hematological diseases. The inner red part of the circle is the name of hematologic disease. The middle blue part of the circle is the name of lncRNAs. The outer yellow part of the circle is the target of hematologic disease regulated by lncRNAs. lncRNAs: Long non-coding RNAs; APL: Acute promyelocytic leukemia; AML: Acute myeloid leukemia; CML: Chronic myeloid leukemia; B-ALL: B-cell acute lymphocytic leukemia; CLL: Chronic leukemia; DLBCL: Diffuse large B-cell lymphoma; NHL: Non-Hodgkin lymphoma; HL: Hodgkin lymphoma; MM: Multiple myeloma; MDS: Myelodysplastic syndromes; AA: Aplastic anemia; NEAT1: Nuclear paraspeckle assembly transcript 1; RUNXOR: RUNX1 overlapping RNA; PLIN2: Perilipin 2; lncRNA-BGL3: LncRNA-BetaGlobinLocus 3; HOTAIR: Hox transcript antisense RNA; HULC: Highly up-regulated in liver cancer; UCA1: Urothelial carcinoma-associated 1; LUNAR1: LeUkemia-induced non-coding activator RNA; BALR-2: B-ALL-associated long RNA-2; CRNDE: Colorectal neoplasia differentially expressed; MIAT: Myocardial infarction-associated transcript; MINCR: MYC-induced lncRNA; PANDA: P21-associated ncRNA DNA damage activated; MEG3: Maternally expressed gene 3; MALAT1: Metastasis-associated lung adenocarcinoma transcript 1; RUNX1: Runt-related transcription factor 1; IGF1R: Insulin-like growth factor-1 receptor; HDAC: Histone deacetylase; CEBPA: CCAAT/enhancer-binding protein-α; GSK-3: Glycogen synthase kinase-3; PTEN: Gene of phosphate and tension homology deleted on chromosome 10; EZH2: Enhancer of zeste homolog 2; MAPK/ERK: Mitogen-activated protein kinase/extracellular regulated protein kinases; BMP4: Bone morphogenetic protein 4; LTBP3: Latent-transforming growth factor beta-binding protein 3; FGF1: Fibroblast growth factor 1.
AML-related lncRNAs
The expression of nuclear paraspeckle assembly transcript 1 (NEAT1), a novel lncRNA localized specifically to nuclear paraspeckles, has a vital regulatory role in many human malignancies[70,99] and was indicated to be repressed by PML-RARα in de novo APL samples. Furthermore, significant NEAT1 up-regulation was observed during (ATRA)-induced NB4-cell differentiation.[71]
Another group performed transcriptome-wide lncRNA expression profiling of acute myeloid leukemia (AML) and identified that lncRNAs up-regulated in AML are associated with a lower degree of DNA methylation and a higher ratio of being bound by transcription factors such as SP1, STAT4, ATF-2, and ELK-1 compared with those down-regulated in AML. Moreover, they found that a novel lncRNA LOC285758 is associated with the poor prognosis in patients with AML and regulates the proliferation of AML cell lines by enhancing the expression of HDAC2 (histone deacetylase 2), a potential therapeutic target in AML blasts.[74,75]
Accumulating evidence has shown that the insulin-like growth factor type I receptor (IGF1R) is one of the most important regulators in the progression and therapeutic resistance of AML.[100] A novel intragenic lncRNA IRAIN was discovered to directly interact with the IGF1R promoter and enhancer chromatin DNA sequences. Moreover, IRAIN was down-regulated both in leukemia cell lines and high-risk patients with AML.[73]
Distinctive lncRNA profiles were found associated with common recurrent mutations in AML, such as FLT3-ITD (internal tandem duplications in the FLT3 gene) or NPM1, CEBPA, IDH2, and RUNX1 genes.[101] Utilizing a novel R3C (RNA-guided chromatin conformation capture) method,[102] Wang et al[72] described a RUNX1-intragenic lncRNA RUNXOR (RUNX1 overlapping RNA), which is transcribed by an upstream promoter and overlaps with RUNX1 (one of the most frequently mutated genes in AML[103]). RUNXOR was up-regulated in both AML samples and Ara-C-treated cell lines. The in-depth study showed that RUNXOR directly interacted with the RUNX1 promoter and enhancer chromatin DNA sequences through its 3′-terminal fragment.
Another lncRNA CCDC26, which is thought to transcriptionally regulate a set of genes and associated with pediatric AML,[104,105] was also proved to controls growth of myeloid leukemia cells through regulation the expression of KIT,[76] a receptor tyrosine kinase that have been considered to up-regulated in leukemia stem cells from patients with AML who relapsed after chemotherapy.[106]
CML-related lncRNAs
A comprehensive analysis of lncRNAs in human chronic myeloid leukemia (CML) cells was performed and a novel lncRNA lncRNA-BGL3 was observed to serve as a key regulator of Bcr-Abl-mediated cellular transformation. Functional assays suggested that lncRNA-BGL3 was highly induced in response to the disruption of Bcr-Abl expression or by inhibiting Bcr-Abl kinase activity in cell lines and patients with CML. Notably, lncRNA-BGL3 may function as a ceRNA that binds a subset of microRNAs to cross-regulate PTEN (phosphatase and tensin homolog, a critical tumor suppressor gene) expression.[78,79]
Hughes et al[77] identified more than 900 lncRNAs which are regulated by CEBPA (CCAAT/enhancer-binding protein-α), a critical regulator of myeloid differentiation, and many of them are induced during myeloid differentiation of AML cell lines including lncRNA PLIN2. Another group recently further investigated the potential roles of CEBPA-related lncRNA PLIN2 during CML. Results indicated that both CEBPA and PLIN2 were up-regulated in the process of CML and there was a positive correlation between CEBPA and PLIN2 in patients with CML. Moreover, they found that CEBPA-mediated up-regulation of PLIN2 expression promotes the development of CML via GSK3 and Wnt/β-catenin signaling.[107]
Recent studies have also reported some newly discovered lncRNAs that are associated with CML. For instance, lncRNA HOTAIR may play a crucial role in the development of multidrug resistance (MDR) to imatinib in CML via a PI3K/Akt-dependent mechanism.[80] Another lncRNA HULC was also revealed to be positively correlated with imatinib (IM)-induced apoptosis of CML cells and lead to the reduction of c-Myc expression and inhibition of PI3K/Akt pathway activity.[81]lncRNA UCA1 was identified as another important modulator of multidrug resistance protein-1 (MDR1) which is considered as the main reason for IM resistance in CML cells.[82]
T-lymphocytic leukemia-related lncRNAs
LUNAR1 (LeUkemia-induced non-coding activator RNA) is a pivotal lncRNA involved in T-cell leukemia (T-ALL), an aggressive hematological neoplasm derived from malignant T-lymphocyte progenitors with aberrant NOTCH1 signaling.[108] Evidence indicated that LUNAR1 is highly correlated with its coding neighbor gene IGF1R, which has been previously suggested to play a role in T-ALL.[83,84] Trimarchi et al[84] have revealed that the expression of LUNAR1 was up-regulated in primary T-ALL samples especially in ones with a Notch mutation, while down-regulated upon Notch inhibition.
B-lymphocytic leukemia-related lncRNAs
Unbiased microarray profiling was performed on human B-ALL samples and indicated that the expression of a subset of lncRNAs, termed BALRs (B-ALL associated long RNAs), corresponds with specific cytogenetic abnormalities in B-ALL. A functional assay suggested that knockdown of BALR-2 (a lncRNA among BALRs) led to apoptosis of B-ALL cell lines alone and up-regulated BALR-2 was correlated with poor patient response to prednisone and worse overall survival.[85]
By employing methyl-CpG-binding domain protein-enriched genome-wide sequencing (MBD-Seq), Subhash et al[86] identified 5800 hypermethylated and 12,570 hypomethylated chronic lymphocytic leukemia (CLL)-specific differentially methylated genes (cllDMGs), among which hypermethylated CRNDE and hypomethylated AC012065.7 were two novel lncRNAs validated in CLL samples. Notably, survival analysis revealed that hypermethylation of CRNDE and hypomethylation of AC012065.7 were correlated with poor outcome in patients with CLL. In addition, lncRNA MIAT is another lncRNA whose expression level was considered to be positively correlated with the aggressive CLL forms carrying either 17p-deletion, 11q-deletion, or trisomy 12 over indolent form carrying 13p-deletion.[87]
Lymphoma and multiple myeloma-related lncRNAs
Viré et al[88] firstly reported a lncRNA corresponding to an antisense transcript of Fas (FAS-AS1) which could effectively regulate Fas-mediated apoptosis in non-Hodgkin lymphoma (NHL). Results suggested that the FAS-AS1 expression was repressed because of its promotor being hyper-methylated by EZH2 (enhancer of zeste homologue 2), a histone-lysine N-methyltransferase enzyme that participates in histone methylation and leads to transcriptional repression, which is often mutated or over-expressed in lymphomas.[88] Functional assays indicated that treatment with Bruton's tyrosine kinase (BTK) inhibitor or EZH2 knockdown significantly could decrease the levels of EZH2 and then enhancing Fas-mediated apoptosis, which may provide a novel therapeutic target in lymphomas.[89]
Burkitt lymphoma is an aggressive hematological neoplasm with a poor prognosis and the deregulation of the oncogenic transcription factor MYC is considered to be the major driving force in lymphoma development.[109] Doose et al[90] identified 13 lncRNAs differentially expressed in IG-MYC-positive Burkitt lymphoma, among which a lncRNA named MYC-induced lncRNA (MINCR) showing a strong correlation with MYC expression in MYC-positive lymphomas. MINCR knockdown was associated with a reduction in MYC binding to the promoters of selected cell cycle genes. These findings suggested novel therapeutic opportunities for the fight against not only malignant lymphoma but possibly, all cancers that rely on MYC expression.
The long non-coding RNA PANDA (P21-associated ncRNA DNA damage activated), which is induced in a p53-dependent manner and interacts with the transcription factor NF-YA to limit expression of pro-apoptotic genes,[91] was recently reported to be down-regulated in patients with diffuse large B-cell lymphoma (DLBCL) and functioned as a tumor suppressor gene through silencing MAPK/ERK signaling pathway.[92]
A differential expression profiling between Hodgkin lymphoma (HL) and normal germinal center (GC)-B cells was performed and selected two lncRNAs (LINC00116 and LINC00461) which was over-expression in HL, DLBCL and lymphoblastoid cell lines, and another lncRNA (FLJ42351) which has a remarkable Reed-Sernberg (RS) cell-specific expression in HL and part of Burkitt lymphoma cell lines.[93]
Multiple myeloma (MM) is another large class of hematological malignancy characterized by the impaired osteogenic differentiation of mesenchymal stromal cells (MSCs). Zhuang et al[110] revealed that lncRNA maternally expressed gene 3 (MEG3, a tumor suppressor) played an essential role in osteogenic differentiation in bone marrow MSCs, partly by activating transcription of BMP4, a member of the transforming growth factor (TGF) family and participate in embryonic development, hematopoietic development, and mesenchymal development.[94,110] Functional assays indicated that MEG3 knockdown significantly reduced the expression of key osteogenic markers, including Runt-related transcription factor 2, osterix, and osteocalcin.[110]
Furthermore, another research group also reported an MM-related lncRNA MALAT1 (metastasis-associated lung adenocarcinoma transcript 1), which have been widely considered to play a role in the development of numerous cancers,[111,112] could initiate the activation of the key transcription factor Sp1 on Latent TGF-binding proteins (LTBP3, an important regulator for efficient secretion, folding, and activation of TGF-s and regulates the bioavailability of TGF- especially in the bone[113]) promoter in MSCs from MM.[95]
Myelodysplastic syndromes and aplastic anemia-related lncRNAs
Myelodysplastic syndromes (MDSs) are a group of myeloid clonal diseases with a high risk of transformation to AML.[114] Up to now, the molecular pathogenesis of MDS remains to be explored. Liu et al[96] have developed a network-based lncRNA co-module function annotation method, which integrated correlations between lncRNA, protein-coding genes and non-coding miRNAs, and generated lncRNA expression profiles from the HSCs from patients with MDS and healthy donors. Linc-RPIA was identified to potentially bind miR-429, which act as tumor-suppressor, and may be involved in the regulation of tumor-related genes, including FOXO1 and TP53.[96] A recent study revealed HOXB-AS3, a lncRNA located at the human HOXB cluster, maybe a potential risk factor in myeloid neoplasm especially MDS.[97] They demonstrated that high expression of HOXB-AS3 could promote myeloid cell proliferation which was consistent with previous researches.[101,115] Furthermore, clinical correlation analysis also showed that HOXB-AS3 was an adverse prognostic marker in patients with low IPSS index, compared with higher risk ones.[97]
Aplastic anemia (AA) is a group of hematopoietic failure syndrome caused by multiple factors.[116] At present, the underlying molecular mechanisms of AA are largely unknown. In a research of bone marrow mesenchymal stem cells (BMSCs) differentiation from AA patients, Jiang et al[98] found fibroblast growth factor 1 (FGF1) could regulate the expression of lncRNA TDRG1 through promoting acetylation in the TDRG1 promoter, thus enhancing the proliferation of BMSCs. This finding is a novel insight into the treatment of aplastic anemia patients. However, more research is needed to deepen the explanation of the relationship between lncRNA and AA.
Conclusion and Future Perspectives
To date, there is a large body of evidence to suggest that long non-coding RNAs are becoming fundamental regulators in diverse biological processes including cell proliferation, differentiation, pluripotency, apoptosis, and tumorigenesis and are increasingly recognized as vital components of modern molecular biology. With the publication of various studies in succession, the role of lncRNAs in hematopoiesis shows up prominently. Here we widely reviewed recent advances and summarize the characteristics and basic mechanisms of lncRNAs and keep abreast of developments of lncRNAs within the field of normal and malignant hematopoiesis. Based on gene regulatory networks at different levels of lncRNAs participation, lncRNAs have been shown to regulate gene expression from epigenetics, transcription, and post-transcription. The expression of lncRNAs is highly cell-specific and critical for the development and activation of hematopoiesis. Moreover, we also summarized the role of lncRNAs involved in hematological malignancies in recent years. With the advent of the post-genome era, there must be many additional hematopoiesis-related lncRNAs to be discovered and the underlying precise mechanisms also need to be further excavated. We believe that lncRNAs may provide novel ideas for the diagnosis and therapeutic targets of hematological diseases in the foreseeable future.
Funding
This study was supported by a grant from the Beijing Natural Science Foundation (No. 7162175).
Conflicts of interest
None.
Footnotes
How to cite this article: Wang FY, Gu ZY, Gao CJ. Emerging role of long non-coding RNAs in normal and malignant hematopoiesis. Chin Med J 2020;133:462–473. doi: 10.1097/CM9.0000000000000624
References
- 1.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, et al. Landscape of transcription in human cells. Nature 2012; 489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 2014; 157:77–94. doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Lafontaine DL. Noncoding RNAs in eukaryotic ribosome biogenesis and function. Nat Struct Mol Biol 2015; 22:11–19. doi: 10.1038/nsmb.2939. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohnken R, Wen J, Mundy-Bosse B, McConnell K, Keiter A, Grinshpun L, et al. Diminished microRNA-29b level is associated with BRD4-mediated activation of oncogenes in cutaneous T-cell lymphoma. Blood 2018; 131:771–781. doi: 10.1182/blood-2017-09-805663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morelli E, Biamonte L, Federico C, Amodio N, Di Martino MT, Gallo Cantafio ME, et al. Therapeutic vulnerability of multiple myeloma to MIR17PTi, a first-in-class inhibitor of pri-miR-17-92. Blood 2018; 132:1050–1063. doi: 10.1182/blood-2018-03-836601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace JA, O’Connell RM. MicroRNAs and acute myeloid leukemia: therapeutic implications and emerging concepts. Blood 2017; 130:1290–1301. doi: 10.1182/blood-2016-10-697698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang HY, Barbi J, Wu CY, Zheng Y, Vignali PD, Wu X, et al. MicroRNA-17 modulates regulatory T cell function by targeting co-regulators of the Foxp3 transcription factor. Immunity 2016; 45:83–93. doi: 10.1016/j.immuni.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Wang L, Feng G, Wang Y, Li Y, Li X, et al. Asymmetric expression of LincGET biases cell fate in two-cell mouse embryos. Cell 2018; 175:1887–1901. doi: 10.1016/j.cell.2018.11.039. [DOI] [PubMed] [Google Scholar]
- 10.Bach DH, Lee SK. Long noncoding RNAs in cancer cells. Cancer Lett 2018; 419:152–166. doi: 10.1016/j.canlet.2018.01.053. [DOI] [PubMed] [Google Scholar]
- 11.Zou Y, Xu S, Xiao Y, Qiu Q, Shi M, Wang J, et al. Long noncoding RNA LERFS negatively regulates rheumatoid synovial aggression and proliferation. J Clin Invest 2018; 128:4510–4524. doi: 10.1172/JCI97965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kretz M, Siprashvili Z, Chu C, Webster DE, Zehnder A, Qu K, et al. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature 2013; 493:231–235. doi: 10.1038/nature11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang P, Xue Y, Han Y, Lin L, Wu C, Xu S, et al. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science 2014; 344:310–313. doi: 10.1126/science.1251456. [DOI] [PubMed] [Google Scholar]
- 14.Seemann SE, Mirza AH, Hansen C, Bang-Berthelsen CH, Garde C, Christensen-Dalsgaard M, et al. The identification and functional annotation of RNA structures conserved in vertebrates. Genome Res 2017; 27:1371–1383. doi: 10.1101/gr.208652.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchese FP, Raimondi I, Huarte M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol 2017; 18:206.doi: 10.1186/s13059-017-1348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laurent GS, Wahlestedt C, Kapranov P. The Landscape of long noncoding RNA classification. Trends Genet 2015; 31:239–251. doi: 10.1016/j.tig.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ledford H. Circular RNAs throw genetics for a loop. Nature 2013; 494:415.doi: 10.1038/494415a. [DOI] [PubMed] [Google Scholar]
- 18.Smemo S, Campos LC, Moskowitz IP, Krieger JE, Pereira AC, Nobrega MA. Regulatory variation in a TBX5 enhancer leads to isolated congenital heart disease. Hum Mol Genet 2012; 21:3255–3263. doi: 10.1093/hmg/dds165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang XY, Pan HF, Leng RX, Ye DQ. Long noncoding RNAs: novel insights into gastric cancer. Cancer Lett 2015; 356:357–366. doi: 10.1016/j.canlet.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Jin JJ, Lv W, Xia P, Xu ZY, Zheng AD, Wang XJ, et al. Long noncoding RNA SYISL regulates myogenesis by interacting with polycomb repressive complex 2. Proc Natl Acad Sci U S A 2018; 115:E9802–E9811. doi: 10.1073/pnas.1801471115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engreitz JM, Haines JE, Perez EM, Munson G, Chen J, Kane M, et al. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 2016; 539:452–455. doi: 10.1038/nature20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Necsulea A, Soumillon M, Warnefors M, Liechti A, Daish T, Zeller U, et al. The evolution of lncRNA repertoires and expression patterns in tetrapods. Nature 2014; 505:635–640. doi: 10.1038/nature12943. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y. The novel regulatory role of lncRNA-miRNA-mRNA axis in cardiovascular diseases. J Cell Mol Med 2018; 22:5768–5775. doi: 10.1111/jcmm.13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulski JK. Long noncoding RNA HCP5, a hybrid HLA class I endogenous retroviral gene: structure, expression, and disease associations. Cells 2019; 8:pii:E480.doi: 10.3390/cells8050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosseini ES, Meryet-Figuiere M, Sabzalipoor H, Kashani HH, Nikzad H, Asemi Z. Dysregulated expression of long noncoding RNAs in gynecologic cancers. Mol Cancer 2017; 16:107.doi: 10.1186/s12943-017-0671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cabili MN, Trapnell C, Goff L, Koziol M, Tazon-Vega B, Regev A, et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev 2011; 25:1915–1927. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi X, Sun M, Liu H, Yao Y, Song Y. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett 2013; 339:159–166. doi: 10.1016/j.canlet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Ulitsky I, Bartel DP. LincRNAs: genomics, evolution, and mechanisms. Cell 2013; 154:26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet 2009; 10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 30.Meredith EK, Balas MM, Sindy K, Haislop K, Johnson AM. An RNA matchmaker protein regulates the activity of the long noncoding RNA HOTAIR. RNA 2016; 22:995–1010. doi: 10.1261/rna.055830.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoneda R, Suzuki S, Mashima T, Kondo K, Nagata T, Katahira M, et al. The binding specificity of translocated in LipoSarcoma/FUsed in sarcoma with lncRNA transcribed from the promoter region of cyclin D1. Cell Biosci 2016; 6:4.doi: 10.1186/s13578-016-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grelet S, Link LA, Howley B, Obellianne C, Palanisamy V, Gangaraju VK, et al. A regulated PNUTS mRNA to lncRNA splice switch mediates EMT and tumour progression. Nat Cell Biol 2017; 19:1105–1115. doi: 10.1038/ncb3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao L, Zhang P, Li J, Wu M. LAST, a c-Myc-inducible long noncoding RNA, cooperates with CNBP to promote CCND1 mRNA stability in human cells. Elife 2017; 6:pii:e30433.doi: 10.7554/eLife.30433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen L, Wang Q, Liu R, Chen Z, Zhang X, Zhou P, et al. LncRNA lnc-RI regulates homologous recombination repair of DNA double-strand breaks by stabilizing RAD51 mRNA as a competitive endogenous RNA. Nucleic Acids Res 2018; 46:717–729. doi: 10.1093/nar/gkx1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol 2006; 169:338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez-Dominguez JR, Hu W, Yuan B, Shi J, Park SS, Gromatzky AA, et al. Global discovery of erythroid long noncoding RNAs reveals novel regulators of red cell maturation. Blood 2014; 123:570–581. doi: 10.1182/blood-2013-10-530683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu W, Yuan B, Flygare J, Lodish HF. Long noncoding RNA-mediated anti-apoptotic activity in murine erythroid terminal differentiation. Genes Dev 2011; 25:2573–2578. doi: 10.1101/gad.178780.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paralkar VR, Mishra T, Luan J, Yao Y, Kossenkov AV, Anderson SM, et al. Lineage and species-specific long noncoding RNAs during erythro-megakaryocytic development. Blood 2014; 123:1927–1937. doi: 10.1182/blood-2013-12-544494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villamizar O, Chambers CB, Mo YY, Torry DS, Hofstrand R, Riberdy JM, et al. Fas-antisense long noncoding RNA is differentially expressed during maturation of human erythrocytes and confers resistance to Fas-mediated cell death. Blood Cells Mol Dis 2016; 58:57–66. doi: 10.1016/j.bcmd.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Villamizar O, Chambers CB, Mo YY, Torry DS, Hofstrand R, Riberdy JM, et al. Data in support of transcriptional regulation and function of Fas-antisense long noncoding RNA during human erythropoiesis. Data Brief 2016; 7:1288–1295. doi: 10.1016/j.dib.2016.03.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei S, Zhao M, Wang X, Li Y, Wang K. PU.1 controls the expression of long noncoding RNA HOTAIRM1 during granulocytic differentiation. J Hematol Oncol 2016; 9:44.doi: 10.1186/s13045-016-0274-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen ZH, Wang WT, Huang W, Fang K, Sun YM, Liu SR, et al. The lncRNA HOTAIRM1 regulates the degradation of PML-RARA oncoprotein and myeloid cell differentiation by enhancing the autophagy pathway. Cell Death Differ 2017; 24:212–224. doi: 10.1038/cdd.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner LA, Christensen CJ, Dunn DM, Spangrude GJ, Georgelas A, Kelley L, et al. EGO, a novel, noncoding RNA gene, regulates eosinophil granule protein transcript expression. Blood 2007; 109:5191–5198. doi: 10.1182/blood-2006-06-027987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomez JA, Wapinski OL, Yang YW, Bureau JF, Gopinath S, Monack DM, et al. NeST, a long noncoding RNA, controls microbial susceptibility and epigenetic activation of the Ifng locus. Cell 2013; 152:743–754. doi: 10.1016/j.cell.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collier SP, Collins PL, Williams CL, Boothby MR, Aune TM. Cutting edge: influence of Tmevpg1, a long intergenic noncoding RNA, on the expression of Ifng by Th1 cells. J Immunol 2012; 189:2084–2088. doi: 10.4049/jimmunol.1200774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranzani V, Rossetti G, Panzeri I, Arrigoni A, Bonnal RJ, Curti S, et al. LincRNA landscape in human lymphocytes highlights regulation of T cell differentiation by linc-MAF-4. Nat Immunol 2015; 16:318–325. doi: 10.1038/ni.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yagi R, Zhu J, Paul WE. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int Immunol 2011; 23:415–420. doi: 10.1093/intimm/dxr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu G, Tang Q, Sharma S, Yu F, Escobar TM, Muljo SA, et al. Expression and regulation of lincRNAs during T cell development and differentiation. Nat Immunol 2013; 14:1190–1198. doi: 10.1038/ni.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, Nestor CE, Zhao S, Lentini A, Bohle B, Benson M, et al. Profiling of human CD4+ T-cell subsets identifies the TH2-specific noncoding RNA GATA3-AS1. J Allergy Clin Immunol 2013; 132:1005–1008. doi: 10.1016/j.jaci.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 50.Spurlock CF, 3rd, Tossberg JT, Guo Y, Collier SP, Crooke PS, 3rd, Aune TM. Expression and functions of long noncoding RNAs during human T helper cell differentiation. Nat Commun 2015; 6:6932.doi: 10.1038/ncomms7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linder P, Jankowsky E. From unwinding to clamping-the DEAD box RNA helicase family. Nat Rev Mol Cell Biol 2011; 12:505–516. doi: 10.1038/nrm3154. [DOI] [PubMed] [Google Scholar]
- 52.Huang W, Thomas B, Flynn RA, Gavzy SJ, Wu L, Kim SV, et al. DDX5 and its associated lncRNA Rmrp modulate Th17 cell effector functions. Nature 2015; 528:517–522. doi: 10.1038/nature16193. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Zemmour D, Pratama A, Loughhead SM, Mathis D, Benoist C. Flicr, a long noncoding RNA, modulates Foxp3 expression and autoimmunity. Proc Natl Acad Sci U S A 2017; 114:E3472–E3480. doi: 10.1073/pnas.1700946114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang R, Tang J, Chen Y, Deng L, Ji J, Xie Y, et al. The long noncoding RNA lnc-EGFR stimulates T-regulatory cells differentiation thus promoting hepatocellular carcinoma immune evasion. Nat Commun 2017; 8:15129.doi: 10.1038/ncomms15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schlaphoff V, Lunemann S, Suneetha PV, Jaroszewicz J, Grabowski J, Dietz J, et al. Dual function of the NK cell receptor 2B4 (CD244) in the regulation of HCV-specific CD8+ T cells. PLoS Pathog 2011; 7:e1002045.doi: 10.1371/journal.ppat.1002045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Zhong H, Xie X, Chen CY, Huang D, Shen L, et al. Long noncoding RNA derived from CD244 signaling epigenetically controls CD8+ T-cell immune responses in tuberculosis infection. Proc Natl Acad Sci U S A 2015; 112:E3883–E3892. doi: 10.1073/pnas.1501662112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zan H, Casali P. Epigenetics of peripheral B-cell differentiation and the antibody response. Front Immunol 2015; 6:631.doi: 10.3389/fimmu.2015.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winkle M, Kluiver JL, Diepstra A, van den Berg A. Emerging roles for long noncoding RNAs in B-cell development and malignancy. Crit Rev Oncol Hematol 2017; 120:77–85. doi: 10.1016/j.critrevonc.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 59.Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 2009; 458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paralkar VR, Weiss MJ. A new ‘Linc’ between noncoding RNAs and blood development. Genes Dev 2011; 25:2555–2558. doi: 10.1101/gad.183020.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iwamoto S, Kamesaki T, Oyamada T, Okuda H, Kumada M, Omi T, et al. Reactivity of autoantibodies of autoimmune hemolytic anemia with recombinant rhesus blood group antigens or anion transporter band3. Am J Hematol 2001; 68:106–114. doi: 10.1002/ajh.1161. [DOI] [PubMed] [Google Scholar]
- 62.Zhang X, Lian Z, Padden C, Gerstein MB, Rozowsky J, Snyder M, et al. Amyelopoiesis-associated regulatory intergenic noncoding RNA transcript within the human HOXA cluster. Blood 2009; 113:2526–2534. doi: 10.1182/blood-2008-06-162164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol 2010; 28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 2010; 327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F, et al. A validated regulatory network for Th17 cell specification. Cell 2012; 151:289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smigiel KS, Srivastava S, Stolley JM, Campbell DJ. Regulatory T-cell homeostasis: steady-state maintenance and modulation during inflammation. Immunol Rev 2014; 259:40–59. doi: 10.1111/imr.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schatz DG, Ji Y. Recombination centres and the orchestration of V(D)J recombination. Nat Rev Immunol 2011; 11:251–263. doi: 10.1038/nri2941. [DOI] [PubMed] [Google Scholar]
- 68.Boothby M, Rickert RC. Metabolic regulation of the immune humoral response. Immunity 2017; 46:743–755. doi: 10.1016/j.immuni.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brazão TF, Johnson JS, Müller J, Heger A, Ponting CP, Tybulewicz VL. Long noncoding RNAs in B-cell development and activation. Blood 2016; 128:e10–e19. doi: 10.1182/blood-2015-11-680843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clemson CM, Hutchinson JN, Sara SA, Ensminger AW, Fox AH, Chess A, et al. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol Cell 2009; 33:717–726. doi: 10.1016/j.molcel.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeng C, Xu Y, Xu L, Yu X, Cheng J, Yang L, et al. Inhibition of long non-coding RNA NEAT1 impairs myeloid differentiation in acute promyelocytic leukemia cells. BMC Cancer 2014; 14:693.doi: 10.1186/1471-2407-14-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang H, Li W, Guo R, Sun J, Cui J, Wang G, et al. An intragenic long noncoding RNA interacts epigenetically with the RUNX1 promoter and enhancer chromatin DNA in hematopoietic malignancies. Int J Cancer 2014; 135:2783–2794. doi: 10.1002/ijc.28922. [DOI] [PubMed] [Google Scholar]
- 73.Sun J, Li W, Sun Y, Yu D, Wen X, Wang H, et al. A novel antisense long noncoding RNA within the IGF1R gene locus is imprinted in hematopoietic malignancies. Nucleic Acids Res 2014; 42:9588–9601. doi: 10.1093/nar/gku549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bug G, Ritter M, Wassmann B, Schoch C, Heinzel T, Schwarz K, et al. Clinical trial of valproic acid and all-trans retinoic acid in patients with poor-risk acute myeloid leukemia. Cancer 2005; 104:2717–2725. doi: 10.1002/cncr.21589. [DOI] [PubMed] [Google Scholar]
- 75.Lei L, Xia S, Liu D, Li X, Feng J, Zhu Y, et al. Genome-wide characterization of lncRNAs in acute myeloid leukemia. Brief Bioinform 2018; 19:627–635. doi: 10.1093/bib/bbx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hirano T, Yoshikawa R, Harada H, Harada Y, Ishida A, Yamazaki T. Long noncoding RNA, CCDC26, controls myeloid leukemia cell growth through regulation of KIT expression. Mol Cancer 2015; 14:90.doi: 10.1186/s12943-015-0364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hughes JM, Salvatori B, Giorgi FM, Bozzoni I, Fatica A. CEBPA-regulated lncRNAs, new players in the study of acute myeloid leukemia. J Hematol Oncol 2014; 7:69.doi: 10.1186/s13045-014-0069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martelli AM, Evangelisti C, Chappell W, Abrams SL, Bäsecke J, Stivala F, et al. Targeting the translational apparatus to improve leukemia therapy: roles of the PI3K/PTEN/Akt/mTOR pathway. Leukemia 2011; 25:1064–1079. doi: 10.1038/leu.2011.46. [DOI] [PubMed] [Google Scholar]
- 79.Guo G, Kang Q, Zhu X, Chen Q, Wang X, Chen Y, et al. A long noncoding RNA critically regulates Bcr-Abl-mediated cellular transformation by acting as a competitive endogenous RNA. Oncogene 2015; 34:1768–1779. doi: 10.1038/onc.2014.131. [DOI] [PubMed] [Google Scholar]
- 80.Wang H, Li Q, Tang S, Li M, Feng A, Qin L, et al. The role of long noncoding RNA HOTAIR in the acquired multidrug resistance to imatinib in chronic myeloid leukemia cells. Hematology 2017; 22:208–216. doi: 10.1080/10245332.2016.1258152. [DOI] [PubMed] [Google Scholar]
- 81.Lu Y, Li Y, Chai X, Kang Q, Zhao P, Xiong J, et al. Long noncoding RNA HULC promotes cell proliferation by regulating PI3K/AKT signaling pathway in chronic myeloid leukemia. Long noncoding RNA HULC promotes cell proliferation by regulating PI3K/AKT signaling pathway in chronic myeloid leukemia. Gene 2017; 607:41–46. doi: 10.1016/j.gene.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 82.Xiao Y, Jiao C, Lin Y, Chen M, Zhang J1, Wang J, et al. lncRNA UCA1 contributes to imatinib resistance by acting as a ceRNA against miR-16 in chronic myeloid leukemia cells. DNA Cell Biol 2017; 36:18–25. doi: 10.1089/dna.2016.3533. [DOI] [PubMed] [Google Scholar]
- 83.Medyouf H, Gusscott S, Wang H, Tseng JC, Wai C, Nemirovsky O, et al. High-level IGF1R expression is required for leukemia-initiating cell activity in T-ALL and is supported by Notch signaling. J Exp Med 2011; 208:1809–1822. doi: 10.1084/jem.20110121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trimarchi T, Bilal E, Ntziachristos P, Fabbri G, Dalla-Favera R, Tsirigos A, et al. Genome-wide mapping and characterization of a Notchregulated long non-coding RNAs in acute leukemia. Cell 2014; 158:593–606. doi: 10.1084/jem.20110121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fernando TR, Rodriguez-Malave NI, Waters EV, Yan W, Casero D, Basso G, et al. LncRNA expression discriminates karyotype and predicts survival in B-lymphoblastic leukemia. Mol Cancer Res 2015; 13:839–851. doi: 10.1158/1541-7786.MCR-15-0006-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Subhash S, Andersson PO, Kosalai ST, Kanduri C, Kanduri M. Global DNA methylation profiling reveals new insights into epigenetically deregulated protein coding and long noncoding RNAs in CLL. Clin Epigenetics 2016; 8:106.doi: 10.1186/s13148-016-0274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sattari A, Siddiqui H, Moshiri F, Ngankeu A, Nakamura T, Kipps TJ, et al. Upregulation of long noncoding RNA MIAT in aggressive form of chronic lymphocytic leukemias. Oncotarget 2016; 7:54174–54182. doi: 10.18632/oncotarget.11099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Viré E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature 2006; 439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 89.Sehgal L, Mathur R, Braun FK, Wise JF, Berkova Z, Neelapu S, et al. FAS-antisense 1 lncRNA and production of soluble versus membrane Fas in B-cell lymphoma. Leukemia 2014; 28:2376–2387. doi: 10.1038/leu.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Doose G, Haake A, Bernhart SH, López C, Duggimpudi S, Wojciech F, et al. MINCR is a MYC-induced lncRNA able to modulate MYC's transcriptional network in Burkitt lymphoma cells. Proc Natl Acad Sci U S A 2015; 112:E5261–E5270. doi: 10.1073/pnas.1505753112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hung T, Wang Y, Lin MF, Koegel AK, Kotake Y, Grant GD, et al. Extensive and coordinated transcription of noncoding RNAs within cell cycle promoters. Nat Genet 2011; 43:621–629. doi: 10.1038/ng.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y, Zhang M, Xu H, Wang Y, Li Z, Chang Y, et al. Discovery and validation of the tumor-suppressive function of long noncoding RNA PANDA in human diffuse large B-cell lymphoma through the inactivation of MAPK/ERK signaling pathway. Oncotarget 2017; 8:72182–72196. doi: 10.18632/oncotarget.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tayari MM, Winkle M, Kortman G, Sietzema J, de Jong D, Terpstra M, et al. Long noncoding RNA expression profiling in normal B-cell subsets and Hodgkin lymphoma reveals Hodgkin and Reed-Sternberg cell-specific long noncoding RNAs. Am J Pathol 2016; 186:2462–2472. doi: 10.1016/j.ajpath.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 94.Khurana S, Buckley S, Schouteden S, Ekker S, Petryk A, Delforge M, et al. A novel role of BMP4 in adult hematopoietic stem and progenitor cell homing via Smad independent regulation of integrinalpha4 expression. Blood 2013; 121:781–790. doi: 10.1182/blood-2012-07-446443. [DOI] [PubMed] [Google Scholar]
- 95.Li B, Chen P, Qu J, Shi L, Zhuang W, Fu J, et al. Activation of LTBP3 gene by a long noncoding RNA (lncRNA) MALAT1 transcript in mesenchymal stem cells from multiple myeloma. J Biol Chem 2014; 289:29365–29375. doi: 10.1074/jbc.M114.572693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu K, Beck D, Thoms JAI, Liu L, Zhao W, Pimanda JE, et al. Annotating function to differentially expressed LincRNAs in myelodysplastic syndrome using a network-based method. Bioinformatics 2017; 33:2622–2630. doi: 10.1093/bioinformatics/btx280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang HH, Chen FY, Chou WC, Hou HA, Ko BS, Lin CT, et al. Long non-coding RNA HOXB-AS3 promotes myeloid cell proliferation and its higher expression is an adverse prognostic marker in patients with acute myeloid leukemia and myelodysplastic syndrome. BMC Cancer 2019; 19:617.doi: 10.1186/s12885-019-5822-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang S, Xia M, Yang J, Shao J, Liao X, Zhu J, et al. Novel insights into a treatment for aplastic anemia based on the advanced proliferation of bone marrow-derived mesenchymal stem cells induced by fibroblast growth factor 1. Mol Med Rep 2015; 12:7877–7882. doi: 10.3892/mmr.2015.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chakravarty D, Sboner A, Nair SS, Giannopoulou E, Li R, Hennig S, et al. The oestrogen receptor alpharegulated lncRNA NEAT1 is a critical modulator of prostate cancer. Nat Commun 2014; 5:5383.doi: 10.1038/ncomms6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Doepfner KT, Spertini O, Arcaro A. Autocrine insulin-like growth factor-I signaling promotes growth and survival of human acute myeloid leukemia cells via the phosphoinositide 3-kinase/Akt pathway. Leukemia 2007; 21:1921–1930. doi: 10.1038/sj.leu.2404813. [DOI] [PubMed] [Google Scholar]
- 101.Garzon R, Volinia S, Papaioannou D, Nicolet D, Kohlschmidt J, Yan PS, et al. Expression and prognostic impact of lncRNAs in acute myeloid leukemia. Proc Natl Acad Sci U S A 2014; 111:18679–18684. doi: 10.1073/pnas.1422050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang H, Jiao W, Sun L, Fan J, Chen M, Wang H, et al. Intrachromosomal looping is required for activation of endogenous pluripotency genes during reprogramming. Cell Stem Cell 2013; 13:30–35. doi: 10.1016/j.stem.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 103.Sood R, Kamikubo Y, Liu P. Role of RUNX1 in hematological malignancies. Blood 2017; 129:2070–2082. doi: 10.1182/blood-2016-10-687830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Radtke I, Mullighan CG, Ishii M, Su X, Cheng J, Ma J, et al. Genomic analysis reveals few genetic alterations in pediatric acute myeloid leukemia. Proc Natl Acad Sci U S A 2009; 106:12944–12949. doi: 10.1073/pnas.0903142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Di Ruscio A, Ebralidze AK, Benoukraf T, Amabile G, Goff LA, Terragni J, et al. DNMT1-interacting RNAs block gene-specific DNA methylation. Nature 2013; 503:371–376. doi: 10.1038/nature12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huntly BJP, Gilliland DG. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer 2005; 5:311–321. doi: 10.1038/nrc1592. [DOI] [PubMed] [Google Scholar]
- 107.Sun C, Luan S, Zhang G, Wang N, Shao H, Luan C. CEBPA-mediated upregulation of the lncRNA PLIN2 promotes the development of chronic myelogenous leukemia via the GSK3 and Wnt/β-catenin signaling pathways. Am J Cancer Res 2017; 7:1054–1067. [PMC free article] [PubMed] [Google Scholar]
- 108.Koch U, Radtke F. Notch in T-ALL: new players in a complex disease. Trends Immunol 2011; 32:434–442. doi: 10.1016/j.it.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 109.Molyneux EM, Rochford R, Griffin B, Newton R, Jackson G, Menon G, et al. Burkitt's lymphoma. Lancet 2012; 379:1234–1244. doi: 10.1016/S0140-6736(11)61177-X. [DOI] [PubMed] [Google Scholar]
- 110.Zhuang W, Ge X, Yang S, Huang M, Zhuang W, Chen P, et al. Upregulation of lncRNA MEG3 promotes osteogenic differentiation of mesenchymal stem cells from multiple myeloma patients by targeting BMP4 transcription. Stem Cells 2015; 33:1985–1997. doi: 10.1002/stem.1989. [DOI] [PubMed] [Google Scholar]
- 111.Schmidt LH, Spieker T, Koschmieder S, Schäffers S, Humberg J, Jungen D, et al. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J Thorac Oncol 2011; 6:1984–1992. doi: 10.1097/JTO.0b013e3182307eac. [DOI] [PubMed] [Google Scholar]
- 112.Lin R, Maeda S, Liu C, Karin M, Edgington TS. A large noncoding RNA is a marker for murine hepatocellular carcinomas and a spectrum of human carcinomas. Oncogene 2007; 26:851–858. doi: 10.1038/sj.onc.1209846. [DOI] [PubMed] [Google Scholar]
- 113.Dabovic B, Chen Y, Colarossi C, Obata H, Zambuto L, Perle MA, et al. Bone abnormalities in latent TGF- binding protein (Ltbp)-3-null mice indicate a role for Ltbp-3 in modulating TGF- bioavailability. J Cell Biol 2002; 156:227–232. doi: 10.1083/jcb.200111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 115.Papaioannou D, Petri A, Dovey OM, Terreri S, Wang E, Collins FA, et al. The long non-coding RNA HOXB-AS3 regulates ribosomal RNA transcription in NPM1-mutated acute myeloid leukemia. Nat Commun 2019; 10:5351.doi: 10.1038/s41467-019-13259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Young NS. Aplastic Anemia N Engl J Med 2018; 379:1643–1656. doi: 10.1056/NEJMra1413485. [DOI] [PMC free article] [PubMed] [Google Scholar]


