Abstract
Background:
Acute myeloid leukemia (AML) is a common type of hematological malignancy in elderly people. Geriatricians have developed comprehensive geriatric assessment (CGA) methods for elderly patients; however, the tools used for CGA in AML are not uniform. Thus, we aimed to validate the instrumental activities of daily living (IADL) scales, age, comorbidities (Charlson Comorbidity Index), and albumin (IACA) index, which is a new tool for CGA, in elderly patients with AML.
Methods:
Patients aged ≥60 years who had been diagnosed with AML were screened for eligibility. Among the IACA low-, intermediate-, and high-risk groups, continuous variables were compared using the Mann-Whitney U test, and categorical variables were compared using χ2 and Fisher exact tests. In addition, probabilities of overall survival (OS) were estimated using the Kaplan-Meier method.
Results:
A total of 21, 34, and 6 patients were categorized into IACA low-risk (0 point), intermediate-risk (1–2 points), and high-risk (≥3 points) groups, respectively. The rates of relapse/progression-related mortality were 23.8%, 58.8%, and 100.0% in the IACA low-, intermediate-, and high-risk groups, respectively (χ2 = 12.81, P < 0.001). The 2-year probabilities of OS were 47.7% (95% confidence interval [CI] 22.8%–72.6%) and 20.2% (95% CI 5.9%–34.5%) in the IACA low- and intermediate-risk groups, respectively (χ2 = 5.99, P = 0.014), which were significantly higher than those in the high-risk group (low-risk [47.7% (95% CI 22.8%–72.6%)] vs. high-risk [0], χ2 = 20.80, P < 0.001; intermediate-risk [20.2% (95% CI 5.9%–34.5%)] vs. high-risk [0], χ2 = 7.56, P = 0.006; respectively). In the IACA low-risk group, the 2-year probability of OS in patients receiving induction chemotherapy (50.8% [95% CI 24.1%–77.5%]) was significantly higher than that in those receiving best supportive care (0, χ2 = 25.74, P < 0.001).
Conclusion:
We suggest that the IACA index might be a simple and effective tool for comprehensive geriatric assessment in elderly AML patients.
Keywords: Acute myeloid leukemia, Albumin, Comorbidity, Instrumental activities of daily living, Comprehensive geriatric assessment
Introduction
Acute myeloid leukemia (AML) is a common type of hematological malignancy in elderly people, with the median age of 68 to 72 years at diagnosis and approximately one-third of newly diagnosed patients aged ≥75 years.[1,2] Survival is age-dependent with lower rates in elderly patients. Thus, elderly patients with AML should be carefully managed.
Achieving complete remission (CR) of AML is a requisite end-point for prolonged survival, and data from large population registries have validated the use of intensive induction approaches over less-intensive therapies in patients aged ≥80 years.[3] Thus, intensive remission induction chemotherapy (IC) should be the first choice for elderly patients with AML. However, the clinical outcomes of ICs for elderly patients with AML are often compromised by comorbid conditions and enhanced susceptibility to toxicities from therapy.[4] Selecting patients who are suitable to receive intensive IC is critical to improve clinical outcomes of elderly patients with AML.
Over the last few decades, geriatricians have developed comprehensive geriatric assessment (CGA) methods for elderly patients, which are useful for the evaluation of cancer patients.[5,6] Several studies have shown that CGA can predict the clinical outcomes of elderly patients with AML and may identify patients who would benefit from aggressive therapy[7–9]; however, the tools used for CGA were complicated and not uniform.[10]
Recently, Miura et al[11] proposed the age, comorbidities, and albumin (ACA) index, which is simple to use in elderly patients with diffuse large B-cell lymphoma (DLBCL) treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. Combining the use of instrumental activities of daily living (IADL) and the ACA index, we propose a new and more effective CGA system (ie, IACA index), which can effectively predict the outcomes of the elderly DLBCL patients.[12] Because the IACA index is simple to use, we suggested that it can also predict the clinical outcomes and guide the therapies in elderly patients with AML.
In this pilot study, we aimed to validate the efficacy of the IACA index in the elderly patients with AML.
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of Beijing Hospital (No. 2018BJYYEC-154-02). Informed consent was obtained from all patients.
Patients
In this pilot study, patients ≥60 years of age who had been diagnosed with AML between May 5, 2011, and April 30, 2017, in the Department of Hematology, Beijing Hospital, China, were screened for eligibility. The final follow-up visits for survival analysis were conducted on May 31, 2018.
Therapeutic regimens
The decision to treat a patient and choice of type and intensity of treatment depends on the clinical judgment of the attending physician, who was blinded to the results of the IACA index in this study. Thirty-five patients received IC; the most common regimen was HA (harringtonine 2.0–2.5 mg·m−2·day−1 for 7 days and cytarabine 100–200 mg·m−2·day−1 for 7 days; n = 15, 42.9%), followed by CAG ± decitabine (granulocyte colony-stimulating factor 300 μg·m−2·day−1 for 15 days, aclacinomycin 20 mg·m−2·day−1 for 4 days, cytarabine 20 mg·m−2·day−1 for 14 days, and/or decitabine 20 mg·m−2·day−1 for 5 days; n = 14, 40.0%) and DA (daunorubicin 45–60 mg·m−2·day−1 for 3 days and cytarabine 100 mg·m−2·day−1 for 7 days; n = 3, 8.6%). One patient was diagnosed as AML-M3 according to morphology, but his PML-RARA was negative and the karyotype was normal; therefore, he received induction and consolidation chemotherapies which were the same as the therapies used to treat the non-acute promyelocytic leukemia AML patients. Four patients received decitabine as single drug treatment (20 mg·m−2·day−1 for 5 days; decitabine group). Twenty-two patients received the best supportive care (BSC group).
IACA index
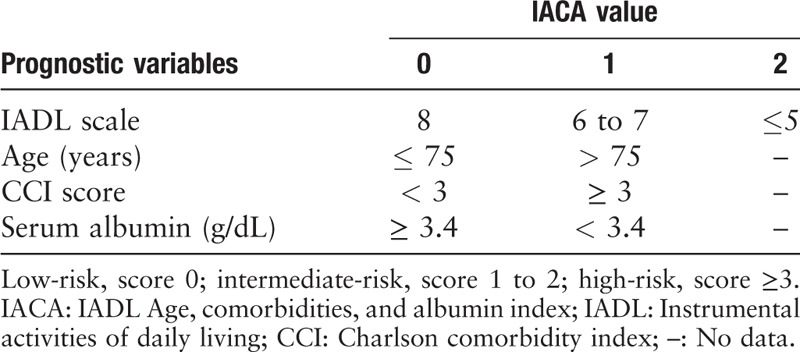
The IACA index was comprised of four risk factors: IADL scales (8 vs. 6–7 vs. 5), advanced age (>75 years), hypoalbuminemia (<3.4 g/dL, the lower limit of normal serum albumin levels in Beijing Hospital), and high burden of clinical comorbidities (Charlson Comorbidity Index [CCI] score of ≥3) [Table 1]. Based on the IACA index score, patients were categorized into low- (0 point), intermediate- (1–2 points), and high-risk (≥3 points) groups.[11]
Table 1.
Instrumental activities of daily living, age, comorbidities, and albumin index.
Definitions and assessments
Comorbidities at diagnosis were assessed based on CCI scores.[13] Patients’ ability to perform ADLs was assessed based on IADL scales.[14] Toxicities of therapy were rated according to the World Health Organization criteria.[15] High leukocyte counts at diagnosis were defined as ≥50 × 109 cells/L. The cytogenetic classification was based on the National Comprehensive Cancer Network criteria.[16] CR was defined as a morphologic leukemia-free state, including <5% blasts in the bone marrow, no blasts with Auer rods, no persistent extramedullary disease, and patients with incomplete platelet count recovery (<100,000) who were transfusion independent.[17] Relapse or disease progression was defined according to the criteria proposed by Cheson et al[17] Overall survival (OS) was defined as the time from the date of diagnosis to the date of death from any cause or last follow-up for censored patients. Patients without OS events were censored based on the last date with valid information for that end-point. Treatment-related mortality (TRM) was defined as death without evidence of disease relapse or progression. Relapse/progression-related mortality was defined as death with evidence of disease relapse or progression.
Statistical analyses
Among the IACA low-, intermediate-, and high-risk groups, continuous variables were compared using the Mann-Whitney U test; categorical variables were compared using χ2 and Fisher exact tests. In addition, the probabilities of OS were calculated using the Kaplan-Meier estimator.
Hazard ratios (HRs) for OS were estimated using univariate and multivariate Cox regression analyses. Factors included in the regression model were high leukocyte counts at diagnosis (yes vs. no), myelodysplastic syndrome-transformed AML (yes vs. no), cytogenetics (poor vs. intermediate vs. favorable), achieving CR (yes vs. no), and IACA index (high-risk vs. intermediate-risk vs. low-risk). All factors with P < 0.1 in the univariate analysis were included in multivariate regression, and P < 0.05 was considered statistically significant. All reported P-values were based on two-sided tests. Data analyses were primarily conducted using SPSS software (SPSS Inc., Chicago, IL, USA), while the R software package (version 2.6.1; http://www.r-project.org) was used for competing risk analysis.
Results
Patient characteristics
Table 2 summarizes the characteristics of 61 patients, and Table 3 shows the characteristics of comorbidities. Among them, 21, 34, and 6 patients were categorized into IACA low-, intermediate-, and high-risk groups, respectively. In the high-risk group, compared to patients showing low IADL scores, a higher number of patients showed multiple comorbidities (CCI score, ≥3) at diagnosis. Most patients in the low-risk group received IC regimens, while all patients in the high-risk group received BSC.
Table 2.
Patient characteristics according to IACA index.
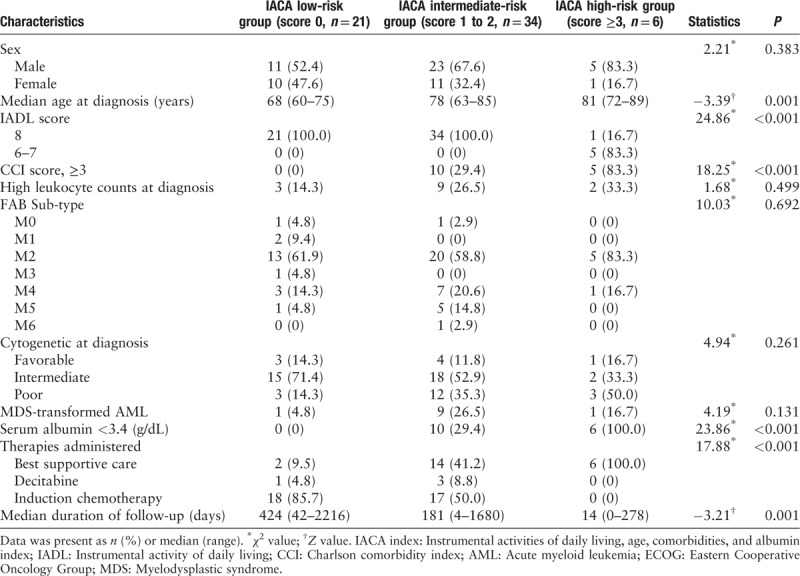
Table 3.
Characteristics for comorbidities at diagnosis.
Toxicities and TRM
A total of 39 patients received IC (n = 35) or decitabine (n = 4) after diagnosis. Table 4 summarizes the grade ≥3 toxicities. Hematologic toxicities were the most common (92.3%), followed by infectious (43.6%), cardiovascular (7.7%), central nervous system (5.1%), and gastrointestinal toxicities (5.1%).
Table 4.
Toxicities after therapies (Grade ≥ 3 toxicities).

Sixteen patients died because of TRM. The most common cause of TRM was infection (n = 11), followed by massive hemorrhage (n = 2), cerebrovascular accident (n = 2), and myocardial infarction (n = 1). For the 39 patients who received therapy after diagnosis, the rates of TRM were comparable between IACA low- and intermediate-risk groups (36.8% vs. 45.0%; χ2 = 0.27, P = 0.605), as well as between the decitabine and IC groups (25.0% vs. 42.9%; χ2 = 0.47, P = 0.631).
Response and relapse
Among the 39 patients receiving therapies after diagnosis, 19 (48.7%) achieved CR, and the rate of CR was significantly higher in the IACA low-risk group compared with that of the intermediate-risk group (68.4% vs. 30.0%, χ2 = 5.76, P = 0.016), and the rates were comparable between the decitabine and IC group (50.0% vs. 48.6%, χ2 = 0.003, P = 1.000).
All IACA high-risk patients received BSC and died from disease progression. The rate of relapse/progression-related mortality was 23.8%, 58.8%, and 100.0% in the IACA low-, intermediate-, and high-risk groups, respectively (χ2 = 12.81, P < 0.001). The rate of relapse/progression-related mortality was 95.5%, 50.0%, and 22.9% in the BSC, decitabine, and IC groups, respectively (χ2 = 28.49, P < 0.001).
Overall survival
The 2-year probability of OS was 27.5% (95% confidence interval [CI] 15.4%–39.6%) in the total population. The 2-year probabilities of OS were 47.7% (95% CI 22.8%–72.6%) and 20.2% (95% CI 5.9%–34.5%) in the IACA low- and intermediate-risk groups, respectively (χ2 = 5.99, P = 0.014), which were significantly higher than those in the high-risk group (low-risk [47.7% (95% CI 22.8%–72.6%)] vs. high-risk [0], χ2 = 20.80, P < 0.001; intermediate-risk [20.2% (95% CI 5.9%–34.5%)] vs. high-risk [0], χ2 = 7.56, P = 0.006; respectively) [Table 5, and Figure 1A]. The 2-year probability of OS in IC group was 42.6% (95% CI 24.6%–60.6%), which was higher than that in the decitabine (25.0% [95% CI 0–82.2%]; χ2 = 3.87, P = 0.049) and BSC groups (4.5% [95% CI 0%–15.8%]; χ2 = 30.12, P < 0.001) [Table 5 and Figure 1B].
Table 5.
Two-year overall survival of the patients.
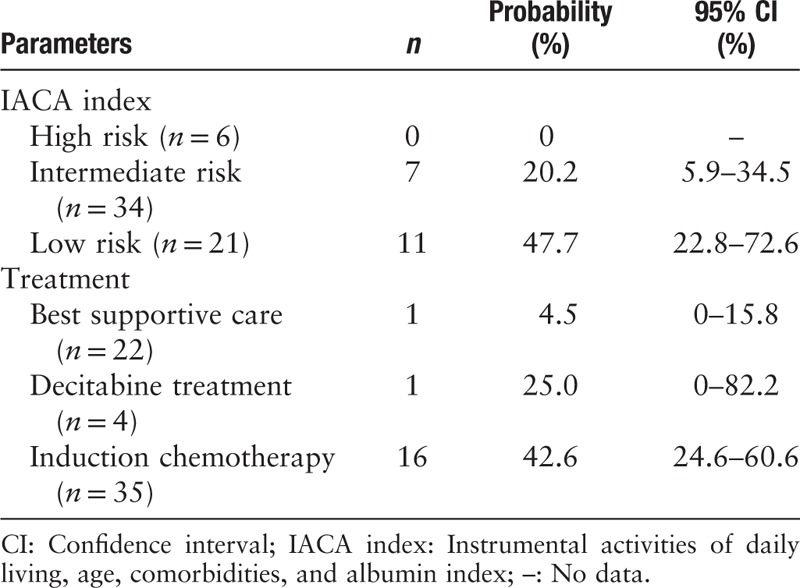
Figure 1.
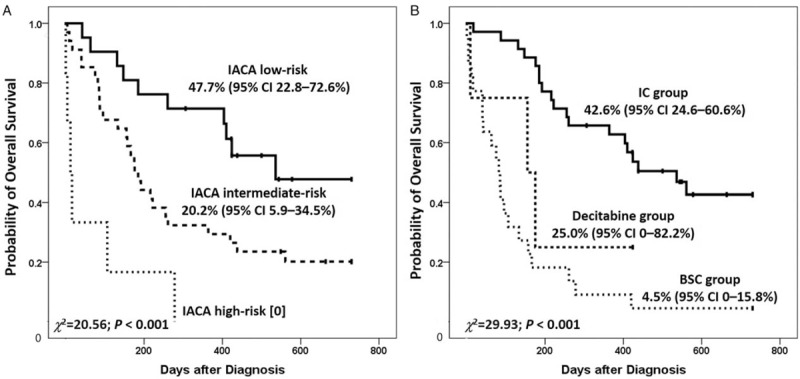
Overall survival according to instrumental activities of daily living, age, comorbidities, and albumin (IACA) index (A) and therapy regimens (B). IC: Induction chemotherapy; BSC: Best supportive care.
In the IACA low-risk group, the 2-year probability of OS in patients receiving IC (50.8%, 95% CI 24.1%–77.5%) was significantly higher than that in those receiving BSC (0, χ2 = 25.74, P < 0.001). In the IACA intermediate-risk group, the 2-year probability of OS in patients receiving IC was 34.3% (95% CI 9.8%–58.8%), which was higher than that in patients receiving decitabine (0, χ2 = 12.47, P < 0.001) and BSC (7.1% [95% CI 0–24.6%], χ2 = 8.74, P = 0.003).
In the multivariate analysis, high leukocyte counts at diagnosis, achieving CR, and IACA index were independent factors associated with OS [Table 6].
Table 6.
Univariate and multivariate analyses of variables for overall survival.

Discussion
In this pilot study, we observed that the IACA index could predict the clinical outcomes in elderly patients with AML. It is suggested that the IACA index is a concise and effective assessment of age, functional status (IADL scale), nutritional status (serum albumin), and comorbidity (CCI score) of elderly patients. Thus, we attempt to propose a simple and effective tool for CGA that may be suitable for elderly AML patients.
A complete geriatrician's assessment on the functional status should include the assessment of the patient's abilities to complete IADL skills, which are required to perform independent tasks in the community.[18] The evaluation of IADL skills can substantially add to the functional information provided by ECOG and Karnofsky performance status values, particularly in patients >80 years old.[5,18] The IADL scale is important for a geriatrician's assessment of patients with DLBCL,[19] multiple myeloma,[20] and chronic lymphoblastic leukemia.[21] Klepin et al[22] observed that 40.7% of elderly patients with AML reported impaired IADLs, and Wedding et al[23] observed that impaired IADL score (HR = 4.3, P = 0.001) significantly predicted median survival.
Elderly adults with AML commonly present with comorbidity at the time of diagnosis, which is another important factor affecting treatment decisions for AML in elderly patients. Comorbidity burden is typically measured using standardized indices, such as the CCI. Tawfik et al[24] reported that elderly patients with AML (≥60 years) had a higher comorbidity burden (CCI of ≥1, 58% vs. 26%, P < 0.001). In this study, 30 (49.2%) patients showed CCI >1, and 15 (24.6%) showed CCI ≥3. In addition, Etienne et al[25] reported that patients with CCI of >1 were less likely to achieve CR. Thus, some authors suggested that comorbidity scoring integrating patient- and disease-specific features may be the best way to define patients as fit and unfit for induction with intensive AML therapy.[26]
The 2-year OS rate was only 27.5% but can be approximately 50% in the low-risk group. This may be due to the fact that most patients in the low-risk group received IC. Juliusson[27] reported that intensive AML chemotherapy may improve elderly patients’ survival, even in those aged >70 years. Medeiros et al[28] reported that approximately 60% of elderly patients with AML remain untreated after the diagnosis; however, the use of anti-leukemic therapy was associated with a survival benefit in the elderly cohort. IC may lead to a substantially increased risk of early death in elderly patients with AML compared with younger patients,[29] and CGA may help identify patients who were fit for IC. Some studies showed that fit elderly patients with AML can tolerate IC.[30,31] We observed that the TRM rate was 42.9% in the IC group, and the 2-year OS rate was significantly higher in the IC group than that in the BSC group, particularly in the low-risk group. Therefore, IACA low-risk elderly patients with AML should receive IC after diagnosis.
Decitabine has also been investigated in elderly patients with AML who were not fit to receive IC.[32] In the study by Lübbert et al,[33] decitabine is well tolerated by elderly, medically non-fit patients with AML. The median OS was 5.5 months, and the 1-year survival rate was 28%. In addition, the remission rate was higher in decitabine compared with the BSC group,[34] and decitabine therapy may also be associated with similar survival rates as IC in elderly patients with AML.[35] In this study, the 2-year OS was 25% in the decitabine group, which was similar to that of the previous studies.[34] Only one patient receiving decitabine as the single drug treatment in low-risk group achieved long-term survival; however, the 2-year OS rate was poorer in the decitabine group compared to that of the IC group among the IACA intermediate-risk patients. Because only a small number of patients belong to the decitabine group, it is premature to conclude the superiority of IC over decitabine in elderly patients with AML in the present study.
This study has several limitations. First, this is a retrospective study with a relatively small number of elderly patients with AML, particularly for high-risk patients, which would have influenced the accuracy of our findings. In addition, patients in the IC group received different chemotherapy protocols, which may influence the interpretation of results. However, we observed that most of the patients received HA or CAG ± decitabine chemotherapy (29/35). Since the patient number was limited, the usefulness of the IACA index and IACA index directed therapy in the geriatric AML patients need to be further evidenced in an expanded cohort of patients in a prospective multicenter study.
In this pilot study, we observed that the IACA index can predict the clinical outcomes of elderly patients with AML, and IACA low-risk patients may benefit from IC. Thus, we suggested that the IACA index may be a simple and effective tool for CGA in elderly AML patients.
Acknowledgements
The authors thank Prof. Xiao-Dong Mo for his assistance in designing the IACA index.
Funding
This work was supported by grants from the Beijing Committee of Science and Technology (No. Z181100001718162, No. Z171100001017200, and No. Z171100001017084) and the Capital's Funds for Health Improvement and Research (No. 2018-4-4089).
Conflicts of interest
None
Footnotes
How to cite this article: Zhang CL, Feng R, Li JT, Wang T, Bai JF, Liu H. A new tool for comprehensive geriatric assessment in elderly patients with acute myeloid leukemia: a pilot study from China. Chin Med J 2020;133:381–387. doi: 10.1097/CM9.0000000000000645
References
- 1.Klepin HD, Rao AV, Pardee TS. Acute myeloid leukemia and myelodysplastic syndromes in older adults. J Clin Oncol 2014; 32:2541–2552. doi: 10.1200/JCO.2014.55.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juliusson G, Lazarevic V, Hörstedt AS, Hagberg O, Höglund M. Swedish Acute Leukemia Registry Group. Acute myeloid leukemia in the real world: why population-based registries are needed. Blood 2012; 119:3890–3899. doi: 10.1182/blood-2011-12-379008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juliusson G, Antunovic P, Derolf A, Lehmann S, Möllgård L, Stockelberg D, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 2009; 113:4179–4187. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- 4.Ossenkoppele G, Löwenberg B. How I treat the older patient with acute myeloid leukemia. Blood 2015; 125:767–774. doi: 10.1182/blood-2014-08-551499. [DOI] [PubMed] [Google Scholar]
- 5.Repetto L, Fratino L, Audisio RA, Venturino A, Gianni W, Vercelli M, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol 2002; 20:494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 6.Monfardini S, Ferrucci L, Fratino L, del Lungo I, Serraino D, Zagonel V. Validation of a multidimensional evaluation scale for use in elderly cancer patients. Cancer 1996; 77:395–401. doi: 10.1002/(SICI)1097-0142(19960115)77:2<395::AID-CNCR24>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 7.Sherman AE, Motyckova G, Fega KR, Deangelo DJ, Abel GA, Steensma D, et al. Geriatric assessment in older patients with acute myeloid leukemia: a retrospective study of associated treatment and outcomes. Leuk Res 2013; 37:998–1003. doi: 10.1016/j.leukres.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klepin HD, Geiger AM, Tooze JA, Kritchevsky SB, Williamson JD, Pardee TS, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood 2013; 121:4287–4294. doi: 10.1182/blood-2012-12-471680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deschler B, Ihorst G, Platzbecker U, Germing U, März E, de Figuerido M, et al. Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica 2013; 98:208–216. doi: 10.3324/haematol.2012.067892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao AV. Fitness in the elderly: how to make decisions regarding acute myeloid leukemia induction. Hematology Am Soc Hematol Educ Program 2016; 2016:339–347. doi: 10.1182/asheducation-2016.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miura K, Konishi J, Miyake T, Makita M, Hojo A, Masaki Y, et al. A host-dependent prognostic model for elderly patients with diffuse large B-cell lymphoma. Oncologist 2017; 22:554–560. doi: 10.1634/theoncologist.2016-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu H, Zhang CL, Feng R, Li JT, Tian Y, Wang T. Validation and refinement of the age, comorbidities, and albumin index in elderly patients with diffuse large B-cell lymphoma: an effective tool for comprehensive geriatric assessment. Oncologist 2018; 23:722–729. doi: 10.1634/theoncologist.2017-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9:179–186. [PubMed] [Google Scholar]
- 15.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 16. National Comprehensive Cancer Network. Version 1. NCCN clinical practice guidelines in oncology acute myloid leukemia; 2017. Available from: www.NCCN.org. Accessed February 24,2017. [Google Scholar]
- 17.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 2003; 21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 18.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol 2007; 25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 19.Tucci A, Ferrari S, Bottelli C, Borlenghi E, Drera M, Rossi G. A comprehensive geriatric assessment is more effective than clinical judgment to identify elderly diffuse large cell lymphoma patients who benefit from aggressive therapy. Cancer 2009; 115:4547–4553. doi: 10.1002/cncr.24490. [DOI] [PubMed] [Google Scholar]
- 20.Bila J, Jelicic J, Djurasinovic V, Vukovic V, Sretenovic A, Andjelic B, et al. Prognostic effect of comorbidity indices in elderly patients with multiple myeloma. Clin Lymphoma Myeloma Leuk 2015; 15:416–419. doi: 10.1016/j.clml.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Goede V, Bahlo J, Chataline V, Eichhorst B, Dürig J, Stilgenbauer S, et al. Evaluation of geriatric assessment in patients with chronic lymphocytic leukemia: results of the CLL9 trial of the German CLL study group. Leuk Lymphoma 2016; 57:789–796. doi: 10.3109/10428194.2015.1091933. [DOI] [PubMed] [Google Scholar]
- 22.Klepin HD, Tooze JA, Pardee TS, Ellis LR, Berenzon D, Mihalko SL, et al. Effect of intensive chemotherapy on physical, cognitive, and emotional health of older adults with acute myeloid leukemia. J Am Geriatr Soc 2016; 64:1988–1995. doi: 10.1111/jgs.14301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wedding U, Röhrig B, Klippstein A, Fricke HJ, Sayer HG, Höffken K. Impairment in functional status and survival in patients with acute myeloid leukaemia. J Cancer Res Clin Oncol 2006; 132:665–671. doi: 10.1007/s00432-006-0115-7. [DOI] [PubMed] [Google Scholar]
- 24.Tawfik B, Pardee TS, Isom S, Sliesoraitis S, Winter A, Lawrence J, et al. Comorbidity, age, and mortality among adults treated intensively for acute myeloid leukemia (AML). J Geriatr Oncol 2016; 7:24–31. doi: 10.1016/j.jgo.2015.10.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Etienne A, Esterni B, Charbonnier A, Mozziconacci MJ, Arnoulet C, Coso D, et al. Comorbidity is an independent predictor of complete remission in elderly patients receivinginduction chemotherapy for acute myeloid leukemia. Cancer 2007; 109:1376–1383. doi: 10.1002/cncr.22537. [DOI] [PubMed] [Google Scholar]
- 26.Swords R, Santini V. In elderly patients with AML, which patients should be considered fit or unfit for standardinduction therapy? Hematology Am Soc Hematol Educ Program 2012; 2012:74–75. doi: 10.1182/asheducation-2012.1.74. [DOI] [PubMed] [Google Scholar]
- 27.Juliusson G. Older patients with acute myeloid leukemia benefit from intensive chemotherapy: an update from the Swedish Acute Leukemia Registry. Clin Lymphoma Myeloma Leuk 2011; 11: Suppl 1: S54–S59. doi: 10.1016/j.clml.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Medeiros BC, Satram-Hoang S, Hurst D, Hoang KQ, Momin F, Reyes C. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol 2015; 94:1127–1138. doi: 10.1007/s00277-015-2351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krug U, Röllig C, Koschmieder A, Heinecke A, Sauerland MC, Schaich M, et al. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: a web-based application for prediction of outcomes. Lancet 2010; 376:2000–2008. doi: 10.1016/S0140-6736(10)62105-8. [DOI] [PubMed] [Google Scholar]
- 30.Thomas X. The management and treatment of acute leukemias in the elderly population. Expert Rev Hematol 2017; 10:975–985. doi: 10.1080/17474086.2017.1382345. [DOI] [PubMed] [Google Scholar]
- 31.Löwenberg B, Ossenkoppele GJ, van Putten W, Schouten HC, Graux C, Ferrant A, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med 2009; 361:1235–1248. doi: 10.1056/NEJMoa0901409. [DOI] [PubMed] [Google Scholar]
- 32.Malik P, Cashen AF. Decitabine in the treatment of acute myeloid leukemia in elderly patients. Cancer Manag Res 2014; 6:53–61. doi: 10.2147/CMAR.S40600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lübbert M, Rüter BH, Claus R, Schmoor C, Schmid M, Germing U, et al. A multicenter phase II trial of decitabine as first-line treatment for older patients with acute myeloid leukemia judged unfit for induction chemotherapy. Haematologica 2012; 97:393–401. doi: 10.3324/haematol.2011.048231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of olderpatients with newly diagnosed acute myeloid leukemia. J Clin Oncol 2012; 30:2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quintás-Cardama A, Ravandi F, Liu-Dumlao T, Brandt M, Faderl S, Pierce S, et al. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood 2012; 120:4840–4845. doi: 10.1182/blood-2012-06-436055. [DOI] [PMC free article] [PubMed] [Google Scholar]


