Abstract
Introduction
Non-alcoholic fatty liver disease (NAFLD) has a wide spectrum, eventually leading to cirrhosis and hepatic carcinogenesis. We previously reported that a series of microRNAs (miRNAs) mapped in the 14q32.2 maternally imprinted gene region (Dlk1-Dio3 mat) are related to NAFLD development and progression in a mouse model. We examined the suitability of miR-379, a circulating Dlk1-Dio3 mat miRNA, as a human NAFLD biomarker.
Methods
Eighty NAFLD patients were recruited for this study. miR-379 was selected from the putative Dlk1-Dio3 mat miRNA cluster because it exhibited the greatest expression difference between NAFLD and non-alcoholic steatohepatitis in our preliminary study. Real-time PCR was used to examine the expression levels of miR-379 and miR-16 as an internal control. One patient was excluded due to low RT-PCR signal.
Results
Compared to normal controls, serum miR-379 expression was significantly up-regulated in NAFLD patients. Receiver operating characteristic curve analysis suggested that miR-379 is a suitable marker for discriminating NAFLD patients from controls, with an area under the curve value of 0.72. Serum miR-379 exhibited positive correlations with alkaline phosphatase, total cholesterol, low-density-lipoprotein cholesterol and non-high-density-lipoprotein cholesterol levels in patients with early stage NAFLD (Brunt fibrosis stage 0 to 1). The correlation between serum miR-379 and cholesterol levels was lost in early stage NAFLD patients treated with statins. Software-based predictions indicated that various energy metabolism–related genes, including insulin-like growth factor-1 (IGF-1) and IGF-1 receptor, are potential targets of miR-379.
Conclusions
Serum miR-379 exhibits high potential as a biomarker for NAFLD. miR-379 appears to increase cholesterol lipotoxicity, leading to the development and progression of NAFLD, via interference with the expression of target genes, including those related to the IGF-1 signaling pathway. Our results could facilitate future research into the pathogenesis, diagnosis, and treatment of NAFLD.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is an important cause of chronic liver injury, with an increasing incidence worldwide [1]. NAFLD, regarded as a hepatic manifestation of metabolic syndrome, is defined by significant lipid deposition in hepatocytes (excessive numbers of fat-laden hepatocytes are observed by light microscopy), unrelated to excessive alcohol consumption [2]. The prevalence of NAFLD is almost 25% worldwide and expected to increase with increasing incidence of obesity and metabolic diseases such as type 2 diabetes mellitus (T2DM) and hyperlipidemia [3].
The mechanism underlying the development of NAFLD has not been fully elucidated. Currently, the multiple parallel hit theory is the most widely accepted mechanism for the progression of NAFLD [4]. This theory suggests that the disease process begins with de novo lipogenesis (DNL) by increase fructose consumption by western style diet and the development of insulin resistance resulting from excessive energy intake [5, 6]. Fructose is a potent lipogenic carbohydrate contributing to hepatic steatosis. Fructose is taken into hepatocyte via glucose transporter 2 and converted into fructose-1-phosphate (F1P) by fructokinase. These physiological sequences are not controlled by insulin and induced by fructose [6]. Fructose-bisphosphate aldolase B (known as hepatic aldolase) converts F1P into glycogen, glucose, lactate, and acetyl-CoA. Fructose also upregulate key transcription factors for fatty acid synthesis such as sterol response element binding protein 1c and carbohydrate responsive element binding Protein [7]. Both acetyl-CoA oversupply and induce of lipogenic enzymes increase DNL in hepatocyte strongly. Insulin resistance in turn leads to hyperinsulinemia, resulting in upregulated hepatic DNL and adipose tissue lipolysis. These “primary hits” increase the susceptibility of hepatocytes to multiple pathogenetic factors, such as upregulated expression of pro-inflammatory cytokines and eicosanoids, Fas ligand, and Toll-like receptor ligands; increased reactive oxygen species (ROS) generation; and altered production of adipokines [8]. Whole-body organs such as adipose tissue, the gut, and gut microbiota are also involved in the pathologic process [9, 10]. Collectively, these factors promote hepatocyte apoptosis through mitochondrial dysfunction [11] and an endoplasmic reticulum stress reaction [12]. Such continuous liver tissue injury ultimately leads to fibrosis [13].
The clinical status of NAFLD patients is generally classified broadly into one of just two categories: non-alcoholic fatty liver (NAFL) or non-alcoholic steatohepatitis (NASH) [14]. NAFL encompasses most of the NAFLD spectrum and is a benign condition. NASH, on the other hand, is defined as the combination of steatosis with lobular inflammation and hepatocyte ballooning; it can progress to liver fibrosis and result in cirrhosis and cancerous malignancies [14]. In contrast to NAFL, NASH is a life-threatening disease. Indeed, a cohort study showed that 35% of NASH patients die during the 7.6-year average follow-up period, whereas no NAFL patients followed in that study died during the same period [15].
Considering the wide disease spectrum of NAFLD, which can result in significant differences in prognosis, it is likely that mechanisms that regulate one or more of these multiple-hit factors exist. Some risk factors for the development of liver fibrosis in NAFLD include age over 50 years, severe obesity, complications associated with T2DM, increased ferritin levels, and patatin-like phospholipase domain–containing 3 gene polymorphisms [16, 17]. However, more-sensitive and -reliable biomarkers are urgently needed to predict outcome in NAFLD patients and enable treatment to begin in the early stage.
MicroRNAs (miRNAs) are a class of endogenous, noncoding, small RNAs that regulate gene expression [18]. Mature miRNAs are introduced into RNA-induced silencing complexes (RISCs) [19]. A RISC bearing a miRNA binds to a partially complementary mRNA sequence and represses the translation of that mRNA. Because miRNAs cause incomplete base-pair matching with mRNAs, a single miRNA can inhibit the translation of hundreds to thousands of target genes [20]. As such, miRNAs play an important role in many cellular processes, including metabolism, inflammation, and fibrosis [21]. Accumulating evidence from both animal model and human patients indicates that miRNAs contribute to the pathogenesis and progression of NAFLD. For example, the expression levels of miR-29c, miR-34a, miR-155, and miR-200b in mouse model liver and miR-122 and miR-34a in human liver are thought to be involved in the development of NASH [22–24]. Our previous study showed that a series of miRNAs mapped in the 14q32.2 maternally imprinted gene cluster region delineated by the delta-like homolog 1 and type III iodothyronine deiodinase genes (Dlk1-Dio3 mat) are related to NAFLD development and progression in a NAFL/NASH mouse model (fatty liver Shionogi [FLS] and mutated leptin gene transferred FLS ob/ob) [25]. Seven miRNAs in the Dlk1-Dio3 mat (miR-127, -136, -376c, -379, -409-3p, -411, and -495) are strongly upregulated in both FLS and FLS ob/ob liver tissues. In contrast to previously reported NAFLD-related miRNAs, the expression of these seven miRNAs was higher in NAFL model mice than NASH model mice.
Recent studies have clearly indicated that miRNAs are secreted into circulating body fluids from various tissues [26]. A considerable amount of secreted miRNAs are protected from enzymatic and physical degradation by binding to proteins or lipoproteins that are then stored in exosomes [27]. These observations suggest that serum miRNAs are potential biomarkers for NAFLD, as they could reflect various pathologic changes in miRNA expression in the liver. Indeed, our preliminary study in human NAFLD patients indicated that serum levels of the respective human homologs of the candidate Dlk1-Dio3 mat miRNAs are related to NAFLD progression [25]. The aim of the present study was to examine the suitability of circulating 14q32.2 mat miRNA as a human NAFLD biomarker.
Materials and methods
Ethics statement
This study was approved by the committee for ethics in medical experiments on human subjects of the medical faculty of Tottori University (protocol no. 2374) and all collaborative medical institutes: Hiroshima University Hospital, JA Hiroshima General Hospital, Kawasaki University Hospital, and Shimane University Hospital. The study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from each patient before blood was collected.
Sample size calculation
The effective sample size has been calculated from the outcomes of our previous clinical study about serum Dlk1-Dio3 mat miRNA expression in NAFLD patients [25]. According to the criterion of our previous study, we set up the relevant difference of miR-379 expression level is 2log2. The associated standard deviation was estimated to be 1.6log2 based on the serum miR-379 expression of normal control in our previous study [25]. Given a statistical power of 80% and 0.05 level of significance, a sample size of 11 in each group will be sufficient to detect a clinically relevant difference between groups. To allow for dropout of patients (up to 20%) and further subgroup analysis, we aim to recruit 80 participants.
Patient recruitment and collection of blood and liver samples
Recruiting and obtaining liver tissue, blood sample and clinical data were performed at Tottori University Hospital and collaborative medical institutes: Hiroshima University Hospital, JA Hiroshima General Hospital, Kawasaki University Hospital, and Shimane University Hospital from July 2014 to March 2016. All participants were Japanese who live in the western part of Japanese main-island. NAFLD outpatients who underwent continuous clinical follow-up at Tottori University Hospital or collaborative medical institutes were recruited by interview with our study members. In interview, we gave clear exposition of the purpose, procedures, duration and potential adverse events of our study using the printed description which have been obtained the approval of the committee for ethics in medical experiments on human subjects of the medical faculty of Tottori University. Participants were given sufficient time to read the consent and have all questions answered before signing the consent form voluntary. Exclusion criteria included chronic hepatitis B or C virus infection (positive hepatitis B surface antigen or hepatitis C antibody), habitual alcohol consumption over 20 g/day, administration of liver steatotic drugs (such as glucocorticoids, tamoxifen, amiodarone, methotrexate, or valproate), primary biliary cholangitis (positive anti-mitochondrial antibody), or autoimmune liver disease (positive anti-nuclear antibody or anti-smooth muscle antibody). All NAFLD patients underwent liver biopsy to confirm the diagnoses of NAFLD, and the histologic grade and NAFLD stage was determined according to the Brunt system [28]. NAFL and NASH were defined by >5% fat-laden hepatocytes in biopsy samples and at least 6 months of continuous blood test results in which alanine aminotransferase (ALT) and aspartate aminotransferase (AST) remained at <2-fold of the normal range or in excess, respectively. Blood sample collection for serum miRNA isolation and clinical blood tests were performed at the same time and within 1 month of liver biopsy. Blood samples were collected in the fasted state. For each sample, blood serum was isolated by refrigerated centrifugation at 4°C and 1500 × g for 10 min and then stored at −80°C until use. Eighty NAFLD patients were enrolled in this study. One NAFLD patient was excluded from this study due to low RT-PCR signal, even after 60 PCR cycles. The NAFLD patients were divided into two subgroups as follows: 9 NAFL patients, and 70 NASH patients. In another analysis, NAFLD patients were also divided into early stage (n = 53) and advanced stage (n = 26) groups. Early stage was defined as Brunt fibrosis stage 0 or 1, and the advanced stage was defined as Brunt fibrosis stage 2 to 4. As normal control, 10 outpatients with asymptomatic gallbladder stones without liver function abnormalities and fatty liver changes by ultrasound imaging were recruited. The clinicopathologic features of each patient group are shown in Table 1. We assessed whether our NAFLD sample can be considered representative of a larger NAFLD population. Concerning age of the participants, the mean age of our NAFLD patients are 48.6 ± 15.4. The majority of NAFLD concerning studies indicate that the median age of NAFLD patients are in the range from 40 to 59 [1]. Meta-analytic assessment of prevalence and incidence of NAFLD showed that the prevalence of NAFLD increases with age, however, the prevalence rate are similar from age 40’s to 60’s (26.5% to 28.9%, respectively) [1]. Former large population studies support that the prevalence of NAFLD is higher in men than in women [29]. In our present study, male NAFLD patients are also more frequently observed than female patients (54 males and 25 females). Overweight is one of the strong risk factors for NAFLD [1]. Previous Japanese NAFLD patients study also showed a clear relationship between BMI and NAFLD development [30]. In our study, mean of body mass index of NAFLD patients excess 25 (29.3 ± 6.1), which indicate overweight. Development and resolution of NAFLD were both closely related to metabolic syndrome especially diabetes mellitus (DM) [30]. Hamaguchi et al. reported that 25–40% of Japanese NAFLD patients have established DM [30]. In our study, 24 cases (30%) of NAFLD patients had complication of DM. In subgroup, 1 of NAFL (11%) and 23 of NASH (33%), and 16 of NAFLD early stage (30%) and 8 of NAFLD advanced stage (31%) had DM. Because of these demographic features, we regarded that our NAFLD participants could be considered as the representative group of larger population of NAFLD.
Table 1. Clinicopathologic features of NAFLD patients and controls.
| Control (CON) (n = 10) | NAFL (n = 9) | NASH (n = 70) | p value | NAFLD early stage (n = 53) | NAFLD advanced stage (n = 26) | p value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NAFL and CON | NASH and CON | NAFL and NASH | Early stage and CON | Advanced stage and CON | Early stage and advanced stage | ||||||
| Age | 59.3 ± 16.6 | 44 ± 10 | 50 ± 16 | 0.080 | 0.162 | 0.533 | 45.4 ± 14.7 | 55.2 ± 14.9 | 0.023* | 0.742 | 0.021* |
| Gender M/F | 4 / 6 | 7 / 2 | 47 / 23 | 0.170 | 0.161 | 0.710 | 38 / 15 | 16 / 10 | 0.071 | 0.460 | 0.261 |
| BMI | 21.9 ± 5.2 | 26.4 ± 2.2 | 29.8 ± 6.3 | 0.270 | 0.002* | 0.259 | 29.8 ± 5.5 | 28.4 ± 7.2 | 0.003* | 0.024* | 0.628 |
| Brunt Stage | - | 0.89 ± 0.33 | 1.58 ± 0.87 | - | - | 0.041* | 1.0 ± 0.2 | 2.6 ± 0.6 | - | - | < 0.001* |
| Brunt Grade | - | 1.0 ± 0 | 1.58 ± 0.67 | - | - | 0.021* | 1.3 ± 0.6 | 1.9 ± 0.6 | - | - | 0.001* |
| T-Bil. | 0.8 ± 0.3 | 0.9 ± 0.3 | 1.0 ± 0.4 | 0.927 | 0.479 | 0.805 | 0.9 ± 0.4 | 1.2 ± 0.3 | 0.908 | 0.071 | 0.014 |
| Alb | 4.3 ± 0.4 | 4.6 ± 0.4 | 4.4 ± 0.4 | 0.123 | 0.175 | 0.701 | 4.5 ± 0.4 | 4.4 ± 0.4 | 0.378 | 0.880 | 0.470 |
| PT (%) | 96.7 ± 9.5 | 107.9 ± 12.5 | 99.2 ± 13.2 | 0.415 | 0.187 | 0.938 | 104.0 ± 12.6 | 92.4 ± 11.7 | 0.578 | 0.837 | 0.001* |
| AST (U/L) | 27.8 ± 18.8 | 40 ± 19 | 49 ± 19 | 0.360 | 0.005* | 0.404 | 45.5 ± 16.4 | 53.3 ± 23.1 | 0.021* | 0.002* | 0.198 |
| ALT (U/L) | 25.5 ± 15.1 | 72 ± 41 | 77 ± 40 | 0.028* | 0.001* | 0.923 | 78.3 ± 39.1 | 74.5 ± 41.6 | 0.001* | 0.002* | 0.910 |
| ALP (U/L) | 276.5 ± 91.7 | 259 ± 67 | 237 ± 84 | 0.886 | 0.350 | 0.752 | 240.5 ± 73.4 | 238.6 ± 100.2 | 0.434 | 0.451 | 0.995 |
| GGT (U/L) | 47.3 ± 45.6 | 65 ± 45 | 62 ± 45 | 0.667 | 0.598 | 0.980 | 63.7 ± 46.1 | 61.4 ± 41.6 | 0.542 | 0.676 | 0.976 |
| LDH (U/L) | 158.3 ± 45.6 | 215 ± 84 | 209 ± 47 | 0.244 | 0.226 | 0.958 | 216.3 ± 58.4 | 199.5 ± 32.6 | 0.144 | 0.391 | 0.362 |
| Ch-E (U/L) | 348.3 ± 66.2 | 351 ± 85 | 379 ± 82 | 0.997 | 0.511 | 0.634 | 388.9 ± 79.7 | 352.8 ± 84.8 | 0.310 | 0.988 | 0.150 |
| BUN (mg/dL) | 11.0 ± 2.4 | 13.8 ± 2.5 | 13.1 ± 2.4 | 0.216 | 0.301 | 0.766 | 13.1 ± 2.5 | 13.3 ± 1.9 | 0.296 | 0.276 | 0.955 |
| Cr (mg/dL) | 0.56 ± 0.17 | 0.79 ± 0.13 | 0.75 ± 0.15 | 0.054 | 0.092 | 0.638 | 0.76 ± 0.14 | 0.74 ± 0.16 | 0.068 | 0.109 | 0.920 |
| UA (mg/dL) | 5.7 ± 1.2 | 6.0 ± 1.1 | 6.3 ± 1.4 | 0.973 | 0.792 | 0.883 | 6.3 ± 1.4 | 6.2 ± 1.4 | 0.805 | 0.867 | 0.985 |
| Ferritin | 42.4 ± 33.0 | 142.1 ± 74.0 | 210.6 ± 174.5 | 0.723 | 0.338 | 0.477 | 190.6 ± 158.6 | 229.1 ± 186.6 | 0.439 | 0.287 | 0.614 |
| FBS (mg/dL) | 93.7 ± 9.7 | 104.0 ± 11.5 | 117.6 ± 45.6 | 0.849 | 0.204 | 0.621 | 117.7 ± 47.8 | 113.9 ± 33.8 | 0.220 | 0.394 | 0.923 |
| HgbA1c (%) | 6.3 ± 1.0 | 5.9 ± 0.6 | 6.3 ± 1.5 | 0.911 | 0.996 | 0.658 | 6.3 ± 1.5 | 6.2 ± 1.4 | 0.995 | 0.999 | 0.938 |
| IRI (μU/mL) | 17.1 ± 19.6 | 18.3 ± 13.5 | - | - | 0.820 | 18.8 ± 15.0 | 17.2 ± 12.8 | - | - | 0,897 | |
| HOMA-IR | 4.6 ± 5.7 | 5.3 ± 6.7 | - | - | 0.768 | 5.5 ± 7.4 | 4.9 ± 4.5 | - | - | 0.921 | |
| T-Chol (mg/dL) | 202 ± 44 | 199 ± 47 | 204 ± 35 | 0.978 | 0.988 | 0.913 | 206.6 ± 36.7 | 197.5 ± 36.3 | 0.936 | 0.940 | 0.936 |
| LDL-C (mg/dL) | 134.1 ± 37.4 | 130.3 ± 43.9 | 131.3 ± 33.2 | 0.974 | 0.978 | 0.996 | 135.1 ± 33.9 | 122.5 ± 34.9 | 0.997 | 0.709 | 0.288 |
| HDL-C (mg/dL) | 67.2 ± 34.3 | 50.9 ± 6.9 | 49.4 ± 9.0 | 0.033* | 0.004* | 0.930 | 49.1 ± 7.9 | 50.6 ± 10.6 | 0.003* | 0.012* | 0.853 |
| Non-HDL -C (mg/dL) | 150.2 ± 17.0 | 147.8 ± 47.0 | 154.1 ± 36.4 | 0.908 | 0.830 | 0.643 | 157.6 ± 5.2 | 144.5 ± 7.6 | 0.683 | 0.758 | 0.161 |
| TG (mg/dL) | 104.3 ± 64.8 | 112.1 ± 50.9 | 149.9 ± 69.0 | 0.967 | 0.139 | 0.255 | 154.5 ± 70.6 | 128.4 ± 60.7 | 0.104 | 0.629 | 0.255 |
| Compl. DM | 0 | 1 (11%) | 23 (33%) | - | - | 0.182 | 16 (30%) | 8 (31%) | - | - | 0.958 |
Value data are expressed as the mean ± standard deviation.
*: p < 0.05 in analysis of variance (ANOVA). NAFLD early stage was defined as Brunt fibrosis stage 0 or 1, and advanced stage was defined as Brunt fibrosis stage 2 to 4. T-Bil: total bilirubin, Alb: albumin, AST: alanine aminotransferase, ALT: aspartate aminotransferase, ALP: alkaline phosphatase, GGT: gamma-glutamyl transferase, LDH: lactate dehydrogenase, Ch-E: choline esterase, BUN: blood urea nitrogen, Cr: creatinine, UA: uric acid, FBS: fasting blood sugar, HgbA1c: hemoglobin A1c which was measured by national glycohemoglobin standardization program certified method, IRI: immunoreactive insulin, HOMA IR: homeostasis model assessment of insulin resistance, T-Chol: total cholesterol, LDL-C: low-density-lipoprotein cholesterol, HDL-C: high-density-lipoprotein cholesterol, Non-HDL-C: calculated by subtracting HDL-C from T-Chol levels, TG: triacylglycerol, Compl. DM: complication of diabetes mellitus, DM can be diagnosed if the patient has HgbA1c level of 6.3% or greater on two separate blood tests.
miRNA expression analysis with human serum
miRNA extract from the serum, Quantitative real-time polymerase chain reaction (RT-PCR) and data analysis were carried out at Tottori University. miR-379 was selected from the putative Dlk1-Dio3 mat miRNA cluster because it exhibited the greatest difference in serum expression between NAFL and NASH patients in our preliminary study [25]. Comparing to the normal controls, serum miR-379 overexpressed in simple steatosis (we named NAFL to simple steatosis in our previous report) group (3.3 ± 3.1 log2) and down regulated in NASH patients group (-7.4 ± 5.9 log2) in our previous study [25]. A miRNeasy serum/plasma kit (Qiagen Venlo, Nederland) was used to extract miRNAs from each 200-μL serum sample according to the manufacturer’s instructions. The miScript II reverse transcription kit (Qiagen) was used for reverse transcription of serum miRNA according to the manufacturer’s instructions. RT-PCR was used to examine the expression levels of miRNA, and data were analyzed using the ΔΔCT method of relative quantification. Applied Biosystems TaqMan® MicroRNA Assays (Applied Biosystems, Waltham, MA, USA) and an ABI7900HT system (Applied Biosystems) were used for quantitative RT-PCR amplification of serum miRNAs. The primer sequences of hsa-miR-379 was UGGUAGACUAUGGAACGUA. We selected miR-16 as an endogenous control. miR-16 is one of the most commonly used reference miRNAs in serum miRNA expression analyses [31, 32]. To the best of our knowledge, no previous reports have indicated a relationship between liver disease and miR-16. We also examined preliminary study about RT-PCR measurement in human serum between endogenous miR-16 and non-mammal spike in control miRNA. Serum samples were obtained from normal controls (n = 10). 1.6 x 108 copies of C. elegans (Ce)-miR-39-1 (Qiagen) and 1 μg bacteriophage MS2 RNA (Roche, Penzberg, Germany) as carrier RNA were added for each 200 μL serum sample. PT-PCR was performed using the same protocol of the present study. The primer sequence of hsa-miR-16 was UAGCAGCACGUAAAUAUUGGCG. The manufacturer does not disclose the primer sequence of Ce-miR-39-1. Both of miR-16 and Ce-miR-39-1 were stably expressed between samples and their standard deviations of threshold cycles were within ± 1 cycle (34.0 ± 0.8 and 23.9 ± 0.1, respectively). We concluded that endogenous miR-16 could be applied as internal control of RT-PCR in serum miRNA.
Predicting miRNA targets
The physiological roles of miR-379 in liver are still hardly identified. Few former studies revealed the relationship between liver function and miR-379. A comprehensive investigation for miR-379 function should be carried out. We adopted the computational miRNA target genes prediction. Software prediction of miRNA target genes is a popular and reliable method to estimate miRNA physiological functions [33]. We used web-based software DIANA microT-CDS 5.0 (http://diana.cslab.ece.ntua.gr/) for miR-379 putative target gene analysis. The threshold for the target prediction score in DIANA microT-CDS was set to 0.7. One miRNA can interference hundreds to thousands genes [20]. We selected gene ontology (GO) annotation to select NAFLD related genes. Database for Annotation, Visualization, and Integrated Discovery (DAVID) 6.8 (http://david.abcc.ncifcrf.gov/) was used for GO annotation, and the Kyoto Encyclopedia of Genes and Genomes (KEGG) was used for pathway enrichment analysis.
Statistical analysis
Statistical analysis was performed using JMP 11.2.1 software (SAS Institute Inc., Cary, NC, USA). Value data are expressed as the mean ± standard deviation. The statistical significance of differences between groups was determined using the Student's t test or ANOVA, followed by Dunnett's test for multiple comparisons. Receiver operating characteristic (ROC) curve analysis was performed to assess NAFLD, NAFL, and NASH diagnostic accuracy. Linear regression analysis was used to examine correlations between miRNA levels and clinicopathologic parameters. Fisher’s exact test and the chi-square test were selected depending on the sample size and used to determine distribution differences of categorical variable. Differences were considered statistically significant at a p value < 0.05.
Results
Serum miR-379 expression was up-regulated in NAFLD patients
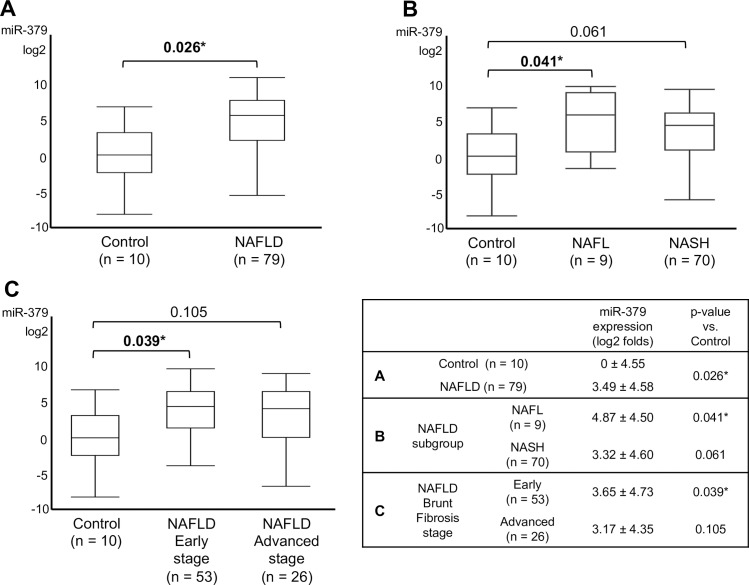
Compared to controls, serum miR-379 expression was significantly up-regulated in NAFLD patients (Fig 1A). In a subgroup analysis of NAFL and NASH patients, serum miR-379 expression was significantly higher in NAFL patients than normal controls (Fig 1B). We also compared early stage NAFLD (Brunt fibrosis stage 0 to 1) and advanced-stage NAFLD (Brunt fibrosis stage 2 to 4) patients with controls. Patients with early stage NAFLD exhibited significantly higher miR-379 expression than controls (Fig 1C). Expression of miR-379 in NASH patients was also higher than in controls, but the difference was not significant (p = 0.061) (Fig 1C). There was no significant difference in miR-379 expression between NAFL and NASH patients or between those with early or advanced-stage NAFLD.
Fig 1. Relative expression of serum miR-379 in NAFLD patients.
A: serum miR-379 expression level between NAFLD and normal control. B: serum miR-379 expression level between NAFL, NASH. C: serum miR-379 expression level between NAFLD early stage, advanced stage and normal control. NAFLD early stage was defined as Brunt fibrosis stage 0 or 1, and advanced stage was defined as Brunt fibrosis stage 2 to 4. Value data are expressed as the mean ± standard deviation. Quantitative real-time PCR (qRT-PCR) was used to examine miRNA levels. All qRT-PCR data were normalized to that for serum miR-16, and fold-change was calculated relative to data from normal controls. *p < 0.05.
Serum miR-379 is a potential NAFLD diagnostic marker
ROC curve analysis revealed that miR-379 is a potential marker for discriminating NAFLD patients from controls (area under the ROC curve [AUROC]: 0.72) (Fig 2). AUROC values for discriminating NAFL, NASH, and early and advanced-stage NAFLD patients from controls were 0.76, 0.72, 0.74, and 0.67, respectively (Fig 2).
Fig 2. Receiver operating characteristic (ROC) curve analysis.
ROC curves for serum miR-379 expression level for the diagnosis of NAFLD (A) and NAFLD subgroups (B: NAFL, C: NASH, D: NAFLD early stage and E: NAFLD advanced stage). Serum miR-379 expressions were normalized to that for serum miR-16. NAFLD early stage was defined as Brunt fibrosis stage 0 or 1, and advanced stage was defined as Brunt fibrosis stage 2 to 4. AUROC means area under the ROC curve.
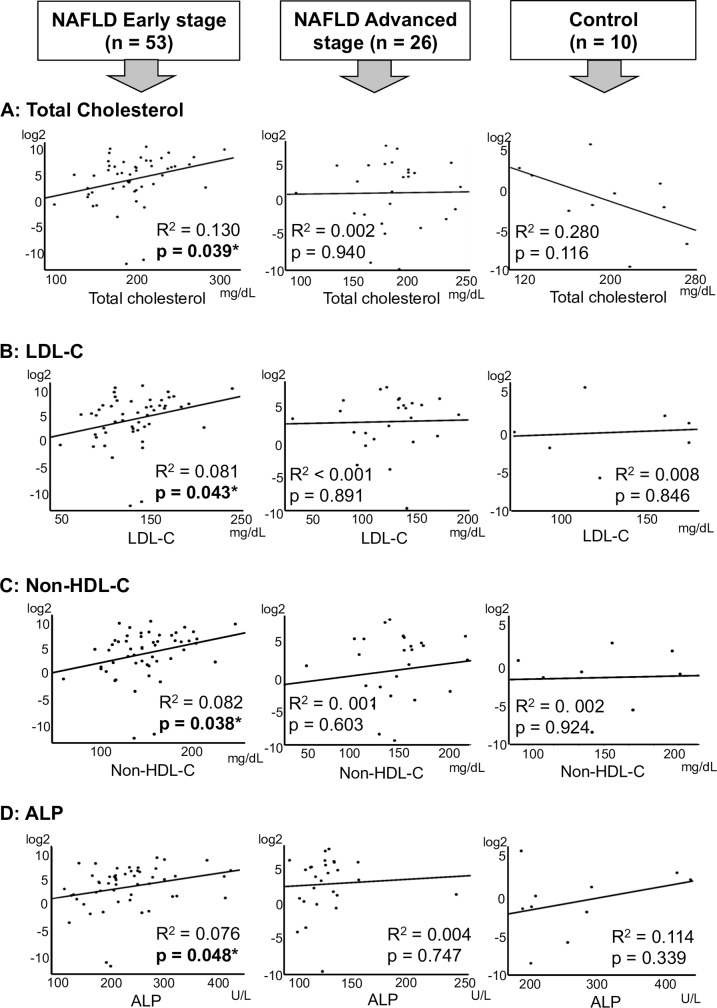
Positive correlations were observed between serum miR-379 and alkaline phosphatase (ALP) or cholesterol levels in patients with NAFL or early stage NAFLD
We analyzed the correlations between clinicopathologic parameters and serum miR-379 levels in NAFLD patients. No significant correlation was identified between serum miR-379 expression in NAFLD patients and any of the parameters examined (S1 Fig). However, positive correlations were observed between serum miR-379 expression and ALP, total cholesterol, low-density-lipoprotein cholesterol (LDL-C) and non-HDL-C Cholesterol (non-HDL-C) levels in patients with early stage NAFLD (Fig 3). In contrast, there was no correlation between these parameters and serum miR-379 levels in controls or patients with advanced-stage NAFLD (Fig 3, S3 Fig).
Fig 3. Correlation between miR-379 and T-Chol, LDL-C, non-HDL-C and ALP levels.
Linear regression analysis was used to examine correlations between serum miR-379 levels and clinicopathologic parameters. Left, middle, and right columns present the results for the NAFLD early stage, NAFLD advanced stage groups and normal control group, respectively. Rows A, B, C and D show miR-379 correlation between T-Chol, LDL-C, non-HDL-C and ALP levels, respectively. R2: coefficient of determination. *: p < 0.05. Non-HDL-C: calculated by subtracting HDL-C from T-Chol levels.
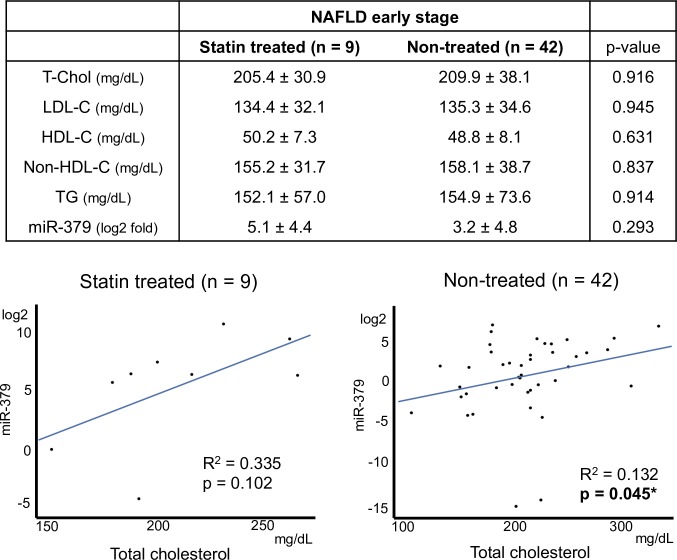
Statin treatment weakened the correlation between miR-379 and cholesterol level
Nine of 51 patients with early stage NAFLD were undergoing treatment for hypercholesterolemia with hydroxymethyl glutaryl coenzyme A reductase (HMG CoA-reductase) inhibitors; commonly called statins. Among statin-treated and non-treated patients with early stage NAFLD, serum levels of total cholesterol, LDL-C, and triglycerides were similar (Fig 4). miR-379 expression levels were higher in the statin-treated group than the non-treated group, but the difference was not significant (5.1 ± 4.4 and 3.2 ± 4.8 log2 folds, respectively. p = 0.29). Linear regression analysis showed the non-treated group exhibited a significant positive correlation between total cholesterol and serum miR-379 expression. This trend was also observed in the statin-treated group, but the correlation was not significant (p = 0.10) (Fig 4).
Fig 4. Statin treatment and serum miR-379 expression, and correlation with cholesterol levels.
Value data are expressed as the mean ± standard deviation. R2: coefficient of determination. *p < 0.05.
Software-based predictions of miR-379 target genes
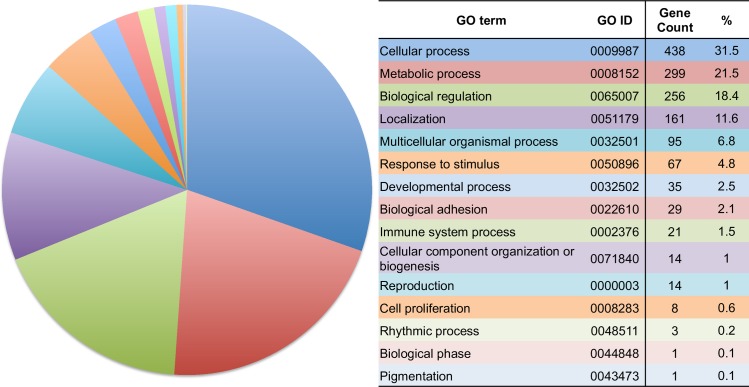
We predicted potential target genes of miR-379 using web-based software. Based on the selection criteria, 1423 human genes were identified as candidates. The candidate genes were classified according to GO annotation in Homo sapiens (Fig 5). Simple gene counting of GO terms showed that cellular process, metabolic process and biological regulation had a large proportion amount to over 70%.
Fig 5. Simple aggregation of Gene Ontology (GO) terms among putative miR-379 target genes.
The predicted miR-379 target gene dataset were fed into DAVID, version 6.8. Pie chart slices represent the number of genes associated with each GO term.
We also examined GO term enrichment analysis. The analysis can identify GO terms, which are significantly over-represented for DAVID pre-built human genome backgrounds [34]. miR-379 predicted target genes were richly represented in 12 GO terms compared to DAVID pre-built human genome backgrounds significantly (Table 2). Among 12 enriched GO, 10 terms related to cellular process regulations.
Table 2. GO-term enrichment analysis of predicted miR-379 target genes.
| Go Term | Gene Count | % | Fold enrichment | p value |
|---|---|---|---|---|
| Positive regulation of macromolecule biosynthetic process | 176 | 12.4 | 1.5 | > 0.001* |
| Positive regulation of RNA metabolic process | 156 | 11.0 | 1.5 | > 0.001* |
| Positive regulation of gene expression | 178 | 12.5 | 1.4 | 0.001* |
| Positive regulation of nucleobase-containing compound metabolic process | 175 | 12.3 | 1.4 | 0.001* |
| Positive regulation of cellular biosynthetic process | 181 | 12.7 | 1.4 | 0.002* |
| Positive regulation of transcription, DNA-templated | 148 | 10.4 | 1.5 | 0.002* |
| Regulation of cellular macromolecule biosynthetic process | 365 | 25.7 | 1.3 | 0.002* |
| Positive regulation of RNA biosynthetic process | 149 | 10.5 | 1.5 | 0.002* |
| Regulation of macromolecule biosynthetic process | 370 | 26.0 | 1.2 | 0.006* |
| Regulation of gene expression | 387 | 27.2 | 1.2 | 0.010* |
| Cellular protein modification process | 342 | 24.0 | 1.2 | 0.034* |
| Protein modification process | 342 | 24.0 | 1.2 | 0.034* |
Percentages indicate the number of predicted target genes associated with a GO term category compared to all predicted genes examined in the GO-term analysis. Fold-enrichment shows the abundance ratios of predicted miR-379 target genes and DAVID pre-built human genome backgrounds among GO terms. Only statistically significant results (p < 0.05) are displayed.
Each of the enriched GO terms still had hundreds genes (Table 2). It is one of the common weak points of this method that the output of genes can be large [34]. To select putative target gene for NAFLD pathology, we examined extending analysis using backend annotation database. Our GO term analysis showed that miR-379 seemed to relate biological regulations largely (Table 2). Therefore we selected KEGG pathway as the backend database. Ontology annotation via KEGG pathway mapping showed that biological functions have been identified for 32.8% of the candidate genes (467 of 1423 genes). Function-labeled miR-379 candidate target genes were primarily enriched in clusters associated with nutrition and energy regulation (FOXO and mTOR signaling pathways), cancer (melanoma, prostate cancer, p53 signaling, Hippo signaling, and transcriptional misregulation in cancer), and multi-functional cellular mechanisms or signaling pathways (cGMP-PKG signaling, focal adhesion, Hippo signaling pathway, pluripotency regulation in stem cells, TGF-beta signaling, and ubiquitin-mediated proteolysis) (Table 3).
Table 3. Enriched KEGG pathways among putative miR-379 target genes.
| KEGG pathway | Gene count | % | Fold enrichment | p value |
|---|---|---|---|---|
| FOXO signaling pathway | 21 | 1.5 | 2.3 | > 0.001* |
| TGF-beta signaling pathway | 15 | 1.1 | 2.6 | 0.001* |
| Ubiquitin mediated proteolysis | 20 | 1.4 | 2.2 | 0.002* |
| Hippo signaling pathway | 19 | 1.3 | 1.8 | 0.013* |
| Prostate cancer | 13 | 0.9 | 2.2 | 0.015* |
| Transcriptional misregulation in cancer | 20 | 1.4 | 1.9 | 0.018* |
| Signaling pathways regulating pluripotency of stem cells | 17 | 1.2 | 2.3 | 0.027* |
| p53 signaling pathway | 10 | 0.7 | 1.8 | 0.036* |
| cGMP-PKG signaling pathway | 18 | 1.3 | 1.6 | 0.038* |
| Focal adhesion | 21 | 1.5 | 2.1 | 0.038* |
| mTOR signaling pathway | 9 | 0.6 | 1.7 | 0.040* |
| Melanoma | 10 | 0.7 | 2.2 | 0.048* |
Percentages indicate the number of predicted miR-379 target genes associated with a KEGG pathway compared to all predicted genes explored in the KEGG pathway analysis. Fold-enrichment shows the abundance ratios of predicted miR-379 target genes and DAVID pre-built human genome backgrounds among KEGG terms. Only statistically significant results (p < 0.05) are displayed.
Finally, to identify probable miR-379 target genes related to the pathology of NAFLD, we conducted a keyword search of the U.S. National Library of Medicine database PubMed (https://www.ncbi.nlm.nih.gov/pubmed) using the terms “KEGG annotated putative target gene” and “NAFLD” or “NASH”. A total of 27 predicted genes were associated with NAFLD development or progression, including multi-functional cellular mechanisms or signaling pathways (HDAC2), fibrosis and inflammation (CAT, CTGF, IL10, PDGFA, PDGFRA, SMAD4, TGFBR1, and THBS1), cell survival and proliferation (Bcl2, CCNB1, HGF, PMAIP1, PTEN, and YAP1), and energy management, including gluconeogenesis and lipogenesis (CREB1, EIF4E, FOXO1, INSR, IGF1, IGF1R, ITPR2, PRKAA1 and 2, RICTOR, SOCS1, and TCF7L2) (Table 4) [35–60].
Table 4. Keyword search of the U.S. National Library of Medicine database PubMed to identify KEGG annotated miR-379 putative target genes associated with NAFLD or NASH.
| Gene Code | Protein name | Reference |
|---|---|---|
| Bcl2 | B-cell lymphoma 2 | Panasiuk et al. 2006 |
| CAT | Catalase | Kumar et al. 2013 |
| CCNB1 | Cyclin B1 | Gentric et al. 2015 |
| CREB1 | cAMP responsive element binding protein 1 | Oh et al. 2013 |
| CTGF | Connective tissue growth factor | Colak et al. 2012 |
| EIF4E | Eukaryotic translation initiation factor 4E | Wang et al. 2014 |
| FOXO1 | Forkhead box o1 | Pan et al. 2017 |
| HDAC2 | Histone deacetylase 2 | Kolodziejczyk et al. 2019 |
| HGF | Hepatocyte growth factor | Kosone et al. 2007 |
| INSR | Insulin receptor | Wu et al. 2017 |
| IGF1 | Insulin like growth factor 1 | Adamek et al. 2018 |
| IGF1R | Insulin like growth factor 1 receptor | Go et al. 2014 |
| IL10 | Interleukin 10 | Cintra et al. 2008 |
| ITPR2 | Inositol 1, 4, 5-trisphosphate receptor type 2 | Khamphaya et al. 2018 |
| PDGFA | Platelet derived growth factor subunit A | Hardy et al. 2017 |
| PDGFRA | Platelet derived growth factor receptor A | Abderrahmani et al. |
| PMAIP1 | Phorbol 12-myristate 13-acetate induced protein 1 | Kung et al. 2016 |
| PRKAA1 | 5’ AMP-activated protein kinase catalytic subunit alpha 1 | Garcia et al. 2019 |
| PRKAA2 | 5’ AMP-activated protein kinase catalytic subunit alpha 2 | Garcia et al. 2019 |
| PTEN | Phosphatase and tensin homolog | Matsuda et al. 2013 |
| RICTOR | Rapamycin-insensitive companion of mammalian target of rapamycin | Sydor et al. 2017 |
| SMAD4 | Small worm phenotype and mothers against decapentaplegic 4 | Qin et al. 2018 |
| SOCS1 | Suppressor of cytokine signaling 1 | Wang et al. 2017 |
| TCF7L2 | Transcription factor 7-like 2 | Musso et al. 2009 |
| TGFBR1 | Transforming growth factor beta receptor 1 | Matsubara et al. 2012 |
| THBS1 | Thrombospondin 1 | Li et al. 2017 |
| YAP1 | yes-associated protein 1 | Chen et al. 2018 |
Discussion
The present study revealed significantly higher serum levels of miR-379 in NAFLD patients compared to controls. Our previous study indicated that miR-379 expression in liver tissues of an NAFLD mouse model is strongly upregulated (>4 log2 compared to the normal control group) [25]. miR-379 secretion from liver tissue, probably via exosome particles rich in miR-379, appears to be related, at least in part, to the high circulating level observed in NAFLD patients.
Relatively little is known regarding the mechanism regulating miR-379 expression. miR-379 has been mapped to the miRNA cluster in the Dlk1-Dio3 mat region. Major regulators of Dlk1-Dio3 locus expression include methylated regulatory regions such as the germline-derived intergenic differentially methylated region and somatic MEG3-differentially methylated region [61, 62]. Moreover, CpG islands that are embedded in or near miRNA-coding regions also regulate the expression of Dlk1-Dio3 mat miRNA [63]. Dai et al. reported that miR-379 expression is directly regulated by DNA methylation [64]. In addition, histone acetylation functions synergistically with DNA methylation to regulate the Dlk1-Dio3 locus [63].
With respect to non–DNA methylation regulation, Guia and colleagues reported that the miRNA cluster miR-379/410 is a direct transcriptional target of the glucocorticoid receptor, which promotes insulin resistance and systemic dyslipidemia [65]. Guia et al. also showed that miR-379 is upregulated in liver tissue of obese subjects and that hepatic miR-379 expression in patients with obesity is correlated with both serum cortisol and triacylglycerol (TG) levels [65]. However, in our present study, TG levels in NAFLD patients did not differ significantly from those of controls (Table 1), and serum miR-379 expression was not correlated with TG level (p = 0.738, S1 Fig). This discrepancy may be related to differences between obese patients and NAFLD patients whose diagnosis was confirmed by liver biopsy. The mechanism of serum miRNA expression may also be related to this discrepancy. For example, sorting and selection occur during incorporation of cytosolic miRNAs into exosomes [66]. Because the level of circulating miRNAs is the sum total of miRNAs secreted from tissues/organs throughout the body, other metabolism-related organs may affect the level of circulating miRNA. Chartoumpekis et al. reported that miR-379 is overexpressed in white adipose tissue in an obese mouse model [67].
ROC curve analyses showed that miR-379 provides fair diagnostic accuracy for NAFLD. The AUROC of serum miR-379 for NAFLD diagnosis was >0.7 and similar to other single serologic markers for non-invasive detection of NAFLD, such as tumor necrosis factor–alpha, interleukin-6, and ferritin [68]. Most non-invasive NAFLD markers exhibit higher values and diagnostic accuracy in patients with liver fibrosis and cirrhosis [69]. In contrast to the majority of NAFLD diagnostic markers, the serum miR-379 level was significantly increased relative to NAFL, but there was no difference between NAFL and NASH. This distinctive feature of serum miR-379 may confer an advantage for detecting NAFLD in the early stage. For instance, serum miR-379 is a candidate factor for use in NAFLD diagnosis algorithms combining multiple biomarkers as a means of increasing sensitivity for early stage diagnosis [70].
Our present study showed that the serum miR-379 level is positively correlated with ALP in early stage NAFLD. Serum ALP is the traditional marker of cholestasis. However, the other cholestasis markers, such as bilirubin and gamma-glutamyl transferase, were not significantly correlated with miR-379 (S2 Fig). ALP is a plasma membrane–bound enzyme that catalyzes the hydrolysis of phosphate esters [71]. Though found in most body tissues, ALP is particularly abundant in the liver, bone, kidneys, and intestinal mucosa, with liver and bone serving as the predominate organs supplying ALP to circulating body fluids [71]. Chronic liver diseases, including NAFLD, increase serum ALP levels [72, 73]. Moreover, previous reports indicated that the serum ALP level is an independent marker of NAFLD development and progression. Pantsari et al. showed that a subset of NAFLD patients (elderly females) exhibit isolated elevation in ALP rather than aminotransferases [74]. Kocabay et al. reported that serum levels of ALP, but not gamma-glutamyl transferase, are increased in NAFLD patients with early fibrosis stage (stage 1 to 2) [75]. ALP is richly expressed in the canalicular membrane side of hepatocytes, and previous studies suggested that ALP relates the transport of bile acid, which plays a major role in cholesterol metabolism and excretion [76]. However, details regarding the physiologic functions of ALP are unclear. miR-379 may be related to NAFLD development and progression by directly or indirectly modulating ALP expression.
Our present study also showed that the serum miR-379 level is positively correlated with serum cholesterol in early stage NAFLD. The contribution of hypercholesterolemia to the development of NAFLD has not been fully elucidated; however, previous studies showed that hepatic cholesterol synthesis and circulating total cholesterol and LDL are increased in NAFLD [77]. Disruption of hepatic cholesterol homeostasis and free cholesterol (FC) accumulation in liver tissue is related to the pathogenesis of NAFLD [78, 79]. Some studies have shown that hepatic cholesterol synthesis is up-regulated in NAFL and NASH patients due to increased activity of a major regulator of cholesterol synthesis, sterol regulatory element–binding protein 2 and its downstream effector HMG CoA-reductase, which catalyzes a rate-limiting step in cholesterol synthesis [80–82]. Interestingly, Min et al. also reported that up-regulation of cholesterol synthesis was not observed in control obese subjects [80].
Regarding other cholesterol-related metabolic functions in the liver of NAFLD patients, cholesterol de-esterification is increased, and cholesterol catabolism to bile acid and cholesterol efflux via the bile duct are attenuated [80]. These NAFLD-specific changes in cholesterol metabolism are believed to increase FC levels in liver tissues. FC accumulation in hepatocytes induces mitochondrial dysfunction, which results in increased production of ROS and leads to the unfolded protein response in the endoplasmic reticulum, leading to localized stress and apoptosis [79]. Mari et al. also reported that FC loading (but not that of fatty acids or triglycerides) into hepatocyte mitochondria membranes sensitizes the hepatocyte to pro-inflammatory cytokines (e.g., tumor necrosis factor–alpha and Fas) in mouse models, resulting in steatohepatitis [83]. Moreover, FC accumulation in non-parenchymal cells in liver tissues such as Kupffer cells and stellate cells promotes activation of these cells [84, 85]. The activated Kupffer cells secrete pro-inflammatory cytokines such as interleukin-1β and tumor necrosis factor–alpha, and activated stellate cells differentiate into myofibroblasts, which exhibit a high ability to produce extracellular matrix and fibrogenic cytokines, such as transforming growth factor–β [84, 85]. It has been hypothesized that miR-379 promotes the development and progression of NAFLD as a result of continuous over-nutrition—manifested primarily as obesity—by increasing the lipotoxicity of cholesterol. Cirrhosis and hepatocellular carcinoma are the most common liver-related causes of morbidity associated with NAFLD [86]. However, cardiovascular disease is the most common cause of death in NAFLD patients without cirrhosis [15]. Therefore, some reviewers have recommended giving priority to the prevention of cardiovascular or renal diseases over liver-specific treatments in patients with non-aggressive NAFLD [87].
miR-379 has also been associated with the risk of cardiovascular disease in early stage NAFLD via up-regulation of the serum cholesterol level, which plays an important role in atherosclerosis development. In the present study, however, no significant correlation between serum miR-379 and cholesterol levels was observed in control subjects and NAFLD patients with advanced fibrosis (Brunt stage 2 to 4). This suggests that such a correlation is pertinent only under limited conditions, such as early stage NAFLD–specific pathophysiologic and nutritional states. The serum miR-379 level in controls was significantly lower than that in patients with early stage NAFLD. Normal levels of miR-379 may be insufficient to affect cholesterol metabolism. With respect to advanced-stage NAFLD, it is known that serum cholesterol levels decline with progression of liver fibrosis, independent of the etiology of chronic liver disease [88]. The effect of miR-379 on cholesterol metabolism may be attenuated by decreased hepatic parenchymal function.
The present study also demonstrated that the use of statins to treat hypercholesterolemia in NAFLD patients weakens the relationship between serum miR-379 and cholesterol levels. Statins target hepatocytes and inhibit HMG-CoA reductase, which catalyzes the rate-limiting step in the cholesterol biosynthesis pathway, known as the mevalonate pathway [89]. HMG-CoA reductase converts HMG-CoA into mevalonic acid, a cholesterol precursor. Stains have a higher binding affinity for HMG-CoA reductase than HMG-CoA and thus block access to the active site by the substrate [89]. Previous studies indicated that statins improve hepatic steatosis and reduce hepatic inflammation and fibrosis in NAFLD patients [90, 91]. Moreover, long-term observations of NAFLD patients indicated that continuous statin treatment reduces rates of liver-related death and liver transplantation [92]. Statins may attenuate the effect of miR-379 on cholesterol biosynthesis, resulting in reduced cholesterol lipotoxicity in NAFLD.
GO term annotation analyses showed enrichment of cellular biosynthesis and metabolism–related genes among predicted miR-379 targets. Aberrations in biosynthesis and metabolism play important roles in metabolic disorders such as NAFLD. miR-379 appears to affect the development and progression of NAFLD by interfering with these target genes.
KEGG pathway mapping of prospective miR-379 target genes extracted biological functions such as nutrition and energy regulation, the down-regulation of which leads to the development of NAFLD. Searches of PubMed combining keywords with the selected putative target genes identified in the KEGG pathway analysis and NAFLD identified a number of metabolism-, inflammation-, and fibrosis-related genes. Among the selected putative target genes, IGF1 and IGF1R were identified as targets of miR-379 interference in previous studies [93, 94]. IGF-1 is an insulin-like anabolic hormone primarily secreted by hepatocytes, and circulating IGF-1 levels reflect hepatic IGF-1 expression [95]. Previous studies reported that adults with growth hormone deficiency in which hepatic IGF-1 production is impaired exhibit an increased prevalence of NASH; IGF-1 substitution ameliorated NAFLD in a mouse model [96, 97]. In NAFLD patients without growth hormone deficiency, serum IGF-1 levels are also significantly reduced [95, 98]. The mechanism by which IGF-1 and its signaling pathways protect against NAFLD have been found to involve a variety of biological functions, such as improving insulin sensitivity, decreasing ROS production, and inducing senescence of hepatic stellate cells [99–101]. With respect to lipid metabolism, it has been reported that IGF-1 accelerates lipid oxidation and lipolysis [99]. Moreover, several previous studies revealed that serum IGF-1 is inversely correlated with serum levels of total cholesterol and LDL-C [102]. IGF1 appears to be one of the most significant miR-379 target genes with regard to promoting the development and progression of NAFLD via the enhancement of cholesterol lipotoxicity. Among other keyword-selected putative target genes, B-cell lymphoma 2 (BCL2), catalase (CAT), and cAMP responsive element binding protein 1 (CREB1) are reportedly down-regulated in the liver in NAFLD [36, 103, 104]. BCL2 and CAT are major anti-apoptosis genes that function by protecting against mitochondrial outer membrane permeabilization and detoxifying ROS, respectively [36, 103]. Down-regulation of BCL2 and CAT expression in liver tissue drives hepatocyte apoptosis, which is an important pathologic event in the development and progression of NAFLD. CREB1 is a transcription factor that regulates energy balance by suppressing hepatic fatty acid generation and accumulation via downregulation of hepatic-specific peroxisome proliferator activated receptor–𝛾 and fatty acid transporter CD36 expression [104]. miR-379 may affect the development and progression of NAFLD by interfering with the expression of these target genes, which is reportedly down-regulated in NAFLD.
A relationship with NAFLD has also been reported for other miR-379 target genes. For example, 5’-AMP–activated protein kinase catalytic subunit alpha 2 (PRKAA2) is the catalytic subunit alpha 2 of AMPK, a key sensor of energy status in mammalian cells. In the liver, AMPK phosphorylates and inactivates both acetyl-coenzyme A carboxylase and HMG-CoA reductase [105]. Acetyl-coenzyme A carboxylase regulates the biosynthesis of malonyl-CoA, which is the initial committed intermediate in fatty acid biosynthesis. Malonyl-CoA can inhibit carnitine palmitoyl transferase 1, which controls mitochondrial fatty acid oxidation [106]. Therefore, AMPK downregulation increases fatty acid and cholesterol biosynthesis and inhibits fatty acid oxidation, resulting in hepatic lipid accumulation. Although AMPK appears to be related to NAFLD development, details regarding levels of AMPK in hepatocytes are controversial [107].
Previous studies reported the relationship between miR-379 and various diseases. The majority of these studies suggest that miR-379 plays tumor suppressive role in many types of carcinomas, including nasopharyngeal carcinoma, cervical cancer, lung cancer, gastric cancer, hepatocellular carcinoma, bladder cancer, and osteosarcoma [108–113]. With regard to metabolic disorders as described above, de Guia et al. revealed a relationship between miR-379 and lipid homeostasis dysregulation [65]. Additionally, patients with a congenital disease known as maternal uniparental disomy for chromosome 14, which causes overexpression of miR-379 of the Dlk1-Dio3 mat miRNA cluster, exhibit characteristic weight gain in early childhood that results in truncal obesity [114].
Our study had some limitations associated with sample size and study design. We did not perform spike in control measurement in NAFLD patients. We cannot assess equalities in RNA extraction efficacies and RT-PCR accurately measurement between NAFLD serum samples especially the drop out case. We used software programs to predict target genes of the candidate miRNAs. Although this method is commonly used, it carries a risk of missing some real targets because the software is designed to assess the relative strength of partial sequence complementarity between mRNA and miRNA. Ontology selection was used to select putative targets that might be relevant to cellular functions. However, ontology selection can only identify proteins for which the function has been identified. Notably, our understanding of the detailed mechanisms that promote the development and progression of NAFLD to NASH is still developing, but new insights are being obtained regularly.
Moreover, we did not confirm whether miR-379 actually interfered with any of the predicted target genes in vivo (e. g. expression measurement in serum or liver tissue) or in vitro, such as direct binding experiments or miR-379 ectopic overexpression by gene transfection. Complex intracellular regulatory networks influence the tissue-specific function of miRNAs [115]. Therefore, further studies are needed to assess whether the predicted targets are actual targets of miR-379 in NAFLD.
Concerning the correlation between serum ALP and miR-379, we could not definitively conclude that the correlation reflects only liver tissue pathologic changes. Bone is another major ALP-secreting organ, and the serum level of the bone isozyme of ALP is elevated in children, adolescents, and elderly people due to bone tissue turnover [116, 117]. Regarding our study participants, all NAFLD patients and control subjects were adults (age ranging from 20 to 76 years), and there was no significant relationship between serum ALP level and age (R2 = 0.0286; p = 0.115). Additionally, no pregnant subjects were included. The number of patients in this study was small, at less than 100. Consequently, the statistical power of the human serum data was relatively limited.
In conclusion, the serum level of miR-379, a member of Dlk1-Dio3 mat miRNA cluster, exhibits high potential as a biomarker for NAFLD. miR-379 also appears to increase cholesterol lipotoxicity, which promotes the development and progression of NAFLD by interfering with the expression of target genes, including those of the IGF-1 signaling pathway. To confidently identify more associations between highly complex and interactive miRNAs with NAFLD, future longitudinal studies with greater sample sizes will be necessary.
Supporting information
Normalized relative to serum miR-16; miR-379 values represent fold-difference relative to the normal control.
(TIF)
Normalized relative to serum miR-16; miR-379 values represent fold-difference relative to the normal control.
(TIF)
Normalized relative to serum miR-16; miR-379 values represent fold-difference relative to the normal control.
(TIF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
Initials of the authors who received award: MK Grant number: 26461005 The full name of funder: A grant-in-aid for scientific research category C from the Japan Society for the Promotion of Science URL of funder: websitehttp://www.jsps.go.jp/english/e-grants/index.html Did the sponsors or funders play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript?: NO
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. Epub 2015/12/29. 10.1002/hep.28431 . [DOI] [PubMed] [Google Scholar]
- 2.Willner IR, Waters B, Patil SR, Reuben A, Morelli J, Riely CA. Ninety patients with nonalcoholic steatohepatitis: insulin resistance, familial tendency, and severity of disease. The American journal of gastroenterology. 2001;96(10):2957–61. Epub 2001/11/06. 10.1111/j.1572-0241.2001.04667.x . [DOI] [PubMed] [Google Scholar]
- 3.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nature reviews Gastroenterology & hepatology. 2018;15(1):11–20. Epub 2017/09/21. 10.1038/nrgastro.2017.109 . [DOI] [PubMed] [Google Scholar]
- 4.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52(5):1836–46. 10.1002/hep.24001 . [DOI] [PubMed] [Google Scholar]
- 5.Basaranoglu M, Basaranoglu G, Sabuncu T, Senturk H. Fructose as a key player in the development of fatty liver disease. World journal of gastroenterology: WJG. 2013;19(8):1166–72. 10.3748/wjg.v19.i8.1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, et al. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. Journal of hepatology. 2008;48(6):993–9. Epub 2008/04/09. 10.1016/j.jhep.2008.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jegatheesan P, De Bandt JP. Fructose and NAFLD: The Multifaceted Aspects of Fructose Metabolism. Nutrients. 2017;9(3). Epub 2017/03/10. 10.3390/nu9030230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. The Journal of clinical investigation. 2000;105(8):1067–75. 10.1172/JCI8814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stojsavljevic S, Gomercic Palcic M, Virovic Jukic L, Smircic Duvnjak L, Duvnjak M. Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease. World journal of gastroenterology: WJG. 2014;20(48):18070–91. 10.3748/wjg.v20.i48.18070 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miura K, Ohnishi H. Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease. World journal of gastroenterology: WJG. 2014;20(23):7381–91. 10.3748/wjg.v20.i23.7381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Begriche K, Massart J, Robin MA, Bonnet F, Fromenty B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology. 2013;58(4):1497–507. 10.1002/hep.26226 . [DOI] [PubMed] [Google Scholar]
- 12.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. American journal of physiology Endocrinology and metabolism. 2006;291(2):E275–81. 10.1152/ajpendo.00644.2005 . [DOI] [PubMed] [Google Scholar]
- 13.Hauff P, Gottwald U, Ocker M. Early to Phase II drugs currently under investigation for the treatment of liver fibrosis. Expert Opin Investig Drugs. 2015;24(3):309–27. 10.1517/13543784.2015.997874 . [DOI] [PubMed] [Google Scholar]
- 14.Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54(1):344–53. 10.1002/hep.24376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–21. Epub 2005/07/14. 10.1053/j.gastro.2005.04.014 . [DOI] [PubMed] [Google Scholar]
- 16.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–5. Epub 2008/09/30. 10.1038/ng.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nature reviews Gastroenterology & hepatology. 2013;10(6):330–44. Epub 2013/03/20. 10.1038/nrgastro.2013.41 . [DOI] [PubMed] [Google Scholar]
- 18.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294(5543):853–8. Epub 2001/10/27. 10.1126/science.1064921 . [DOI] [PubMed] [Google Scholar]
- 19.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. Epub 2004/01/28. 10.1016/s0092-8674(04)00045-5 . [DOI] [PubMed] [Google Scholar]
- 20.Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123(4):631–40. Epub 2005/11/08. 10.1016/j.cell.2005.10.022 . [DOI] [PubMed] [Google Scholar]
- 21.Szabo G, Bala S. MicroRNAs in liver disease. Nature reviews Gastroenterology & hepatology. 2013;10(9):542–52. Epub 2013/05/22. 10.1038/nrgastro.2013.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, et al. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology. 2008;48(6):1810–20. 10.1002/hep.22569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pogribny IP, Starlard-Davenport A, Tryndyak VP, Han T, Ross SA, Rusyn I, et al. Difference in expression of hepatic microRNAs miR-29c, miR-34a, miR-155, and miR-200b is associated with strain-specific susceptibility to dietary nonalcoholic steatohepatitis in mice. Lab Invest. 2010;90(10):1437–46. Epub 2010/06/16. 10.1038/labinvest.2010.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alisi A, Da Sacco L, Bruscalupi G, Piemonte F, Panera N, De Vito R, et al. Mirnome analysis reveals novel molecular determinants in the pathogenesis of diet-induced nonalcoholic fatty liver disease. Lab Invest. 2011;91(2):283–93. Epub 2010/10/20. 10.1038/labinvest.2010.166 . [DOI] [PubMed] [Google Scholar]
- 25.Okamoto K, Koda M, Okamoto T, Onoyama T, Miyoshi K, Kishina M, et al. A Series of microRNA in the Chromosome 14q32.2 Maternally Imprinted Region Related to Progression of Non-Alcoholic Fatty Liver Disease in a Mouse Model. PLoS One. 2016;11(5):e0154676 Epub 2016/05/03. 10.1371/journal.pone.0154676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. Epub 2008/09/04. 10.1038/cr.2008.282 . [DOI] [PubMed] [Google Scholar]
- 27.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–79. Epub 2002/08/03. 10.1038/nri855 . [DOI] [PubMed] [Google Scholar]
- 28.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. The American journal of gastroenterology. 1999;94(9):2467–74. 10.1111/j.1572-0241.1999.01377.x . [DOI] [PubMed] [Google Scholar]
- 29.Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a Sexual Dimorphic Disease: Role of Gender and Reproductive Status in the Development and Progression of Nonalcoholic Fatty Liver Disease and Inherent Cardiovascular Risk. Adv Ther. 2017;34(6):1291–326. Epub 2017/05/21. 10.1007/s12325-017-0556-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143(10):722–8. Epub 2005/11/17. 10.7326/0003-4819-143-10-200511150-00009 . [DOI] [PubMed] [Google Scholar]
- 31.Ng EK, Chong WW, Jin H, Lam EK, Shin VY, Yu J, et al. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58(10):1375–81. Epub 2009/02/10. 10.1136/gut.2008.167817 . [DOI] [PubMed] [Google Scholar]
- 32.Asaga S, Kuo C, Nguyen T, Terpenning M, Giuliano AE, Hoon DS. Direct serum assay for microRNA-21 concentrations in early and advanced breast cancer. Clin Chem. 2011;57(1):84–91. Epub 2010/11/03. 10.1373/clinchem.2010.151845 . [DOI] [PubMed] [Google Scholar]
- 33.Liu W, Wang X. Prediction of functional microRNA targets by integrative modeling of microRNA binding and target expression data. Genome Biol. 2019;20(1):18 Epub 2019/01/24. 10.1186/s13059-019-1629-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1–13. Epub 2008/11/27. 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panasiuk A, Dzieciol J, Panasiuk B, Prokopowicz D. Expression of p53, Bax and Bcl-2 proteins in hepatocytes in non-alcoholic fatty liver disease. World journal of gastroenterology: WJG. 2006;12(38):6198–202. Epub 2006/10/13. 10.3748/wjg.v12.i38.6198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar A, Sharma A, Duseja A, Das A, Dhiman RK, Chawla YK, et al. Patients with Nonalcoholic Fatty Liver Disease (NAFLD) have Higher Oxidative Stress in Comparison to Chronic Viral Hepatitis. J Clin Exp Hepatol. 2013;3(1):12–8. Epub 2013/03/01. 10.1016/j.jceh.2012.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gentric G, Maillet V, Paradis V, Couton D, L'Hermitte A, Panasyuk G, et al. Oxidative stress promotes pathologic polyploidization in nonalcoholic fatty liver disease. The Journal of clinical investigation. 2015;125(3):981–92. Epub 2015/01/27. 10.1172/JCI73957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh KJ, Han HS, Kim MJ, Koo SH. CREB and FoxO1: two transcription factors for the regulation of hepatic gluconeogenesis. BMB Rep. 2013;46(12):567–74. Epub 2013/11/19. 10.5483/BMBRep.2013.46.12.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colak Y, Senates E, Coskunpinar E, Oltulu YM, Zemheri E, Ozturk O, et al. Concentrations of connective tissue growth factor in patients with nonalcoholic fatty liver disease: association with liver fibrosis. Dis Markers. 2012;33(2):77–83. Epub 2012/08/01. 10.3233/DMA-2012-0907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C, Hu L, Zhao L, Yang P, Moorhead JF, Varghese Z, et al. Inflammatory stress increases hepatic CD36 translational efficiency via activation of the mTOR signalling pathway. PloS one. 2014;9(7):e103071 Epub 2014/07/23. 10.1371/journal.pone.0103071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan X, Zhang Y, Kim HG, Liangpunsakul S, Dong XC. FOXO transcription factors protect against the diet-induced fatty liver disease. Sci Rep. 2017;7:44597 Epub 2017/03/17. 10.1038/srep44597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolodziejczyk AA, Zheng D, Shibolet O, Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol Med. 2019;11(2). Epub 2018/12/29. 10.15252/emmm.201809302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kosone T, Takagi H, Horiguchi N, Ariyama Y, Otsuka T, Sohara N, et al. HGF ameliorates a high-fat diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G204–10. Epub 2007/03/31. 10.1152/ajpgi.00021.2007 . [DOI] [PubMed] [Google Scholar]
- 44.Wu H, Zhang T, Pan F, Steer CJ, Li Z, Chen X, et al. MicroRNA-206 prevents hepatosteatosis and hyperglycemia by facilitating insulin signaling and impairing lipogenesis. Journal of hepatology. 2017;66(4):816–24. Epub 2016/12/28. 10.1016/j.jhep.2016.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adamek A, Kasprzak A. Insulin-Like Growth Factor (IGF) System in Liver Diseases. Int J Mol Sci. 2018;19(5). Epub 2018/04/28. 10.3390/ijms19051308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Go GW, Srivastava R, Hernandez-Ono A, Gang G, Smith SB, Booth CJ, et al. The combined hyperlipidemia caused by impaired Wnt-LRP6 signaling is reversed by Wnt3a rescue. Cell Metab. 2014;19(2):209–20. Epub 2014/02/11. 10.1016/j.cmet.2013.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cintra DE, Pauli JR, Araujo EP, Moraes JC, de Souza CT, Milanski M, et al. Interleukin-10 is a protective factor against diet-induced insulin resistance in liver. Journal of hepatology. 2008;48(4):628–37. Epub 2008/02/13. 10.1016/j.jhep.2007.12.017 . [DOI] [PubMed] [Google Scholar]
- 48.Khamphaya T, Chukijrungroat N, Saengsirisuwan V, Mitchell-Richards KA, Robert ME, Mennone A, et al. Nonalcoholic fatty liver disease impairs expression of the type II inositol 1,4,5-trisphosphate receptor. Hepatology. 2017. Epub 2017/10/13. 10.1002/hep.29588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardy T, Zeybel M, Day CP, Dipper C, Masson S, McPherson S, et al. Plasma DNA methylation: a potential biomarker for stratification of liver fibrosis in non-alcoholic fatty liver disease. Gut. 2017;66(7):1321–8. Epub 2016/03/24. 10.1136/gutjnl-2016-311526 PubMed Central PMCID: PMC5031527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abderrahmani A, Yengo L, Caiazzo R, Canouil M, Cauchi S, Raverdy V, et al. Increased Hepatic PDGF-AA Signaling Mediates Liver Insulin Resistance in Obesity-Associated Type 2 Diabetes. Diabetes. 2018;67(7):1310–21. Epub 2018/05/08. 10.2337/db17-1539 . [DOI] [PubMed] [Google Scholar]
- 51.Kung CP, Leu JI, Basu S, Khaku S, Anokye-Danso F, Liu Q, et al. The P72R Polymorphism of p53 Predisposes to Obesity and Metabolic Dysfunction. Cell Rep. 2016;14(10):2413–25. Epub 2016/03/08. 10.1016/j.celrep.2016.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia D, Hellberg K, Chaix A, Wallace M, Herzig S, Badur MG, et al. Genetic Liver-Specific AMPK Activation Protects against Diet-Induced Obesity and NAFLD. Cell Rep. 2019;26(1):192–208 e6. Epub 2019/01/04. 10.1016/j.celrep.2018.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuda S, Kobayashi M, Kitagishi Y. Roles for PI3K/AKT/PTEN Pathway in Cell Signaling of Nonalcoholic Fatty Liver Disease. ISRN Endocrinol. 2013;2013:472432 Epub 2013/02/23. 10.1155/2013/472432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sydor S, Sowa JP, Megger DA, Schlattjan M, Jafoui S, Wingerter L, et al. Acid sphingomyelinase deficiency in Western diet-fed mice protects against adipocyte hypertrophy and diet-induced liver steatosis. Mol Metab. 2017;6(5):416–27. Epub 2017/05/04. 10.1016/j.molmet.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qin G, Wang GZ, Guo DD, Bai RX, Wang M, Du SY. Deletion of Smad4 reduces hepatic inflammation and fibrogenesis during nonalcoholic steatohepatitis progression. J Dig Dis. 2018;19(5):301–13. Epub 2018/04/27. 10.1111/1751-2980.12599 . [DOI] [PubMed] [Google Scholar]
- 56.Wang H, Shao Y, Yuan F, Feng H, Li N, Zhang H, et al. Fish Oil Feeding Modulates the Expression of Hepatic MicroRNAs in a Western-Style Diet-Induced Nonalcoholic Fatty Liver Disease Rat Model. Biomed Res Int. 2017;2017:2503847 Epub 2017/07/12. 10.1155/2017/2503847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Musso G, Gambino R, Pacini G, De Michieli F, Cassader M. Prolonged saturated fat-induced, glucose-dependent insulinotropic polypeptide elevation is associated with adipokine imbalance and liver injury in nonalcoholic steatohepatitis: dysregulated enteroadipocyte axis as a novel feature of fatty liver. Am J Clin Nutr. 2009;89(2):558–67. Epub 2009/01/15. 10.3945/ajcn.2008.26720 . [DOI] [PubMed] [Google Scholar]
- 58.Matsubara T, Tanaka N, Sato M, Kang DW, Krausz KW, Flanders KC, et al. TGF-beta-SMAD3 signaling mediates hepatic bile acid and phospholipid metabolism following lithocholic acid-induced liver injury. J Lipid Res. 2012;53(12):2698–707. Epub 2012/10/05. 10.1194/jlr.M031773 PubMed Central PMCID: PMC3494264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Turpin CP, Wang S. Role of thrombospondin 1 in liver diseases. Hepatol Res. 2017;47(2):186–93. Epub 2016/08/06. 10.1111/hepr.12787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen P, Luo Q, Huang C, Gao Q, Li L, Chen J, et al. Pathogenesis of non-alcoholic fatty liver disease mediated by YAP. Hepatol Int. 2018;12(1):26–36. Epub 2018/01/14. 10.1007/s12072-017-9841-y . [DOI] [PubMed] [Google Scholar]
- 61.da Rocha ST, Edwards CA, Ito M, Ogata T, Ferguson-Smith AC. Genomic imprinting at the mammalian Dlk1-Dio3 domain. Trends Genet. 2008;24(6):306–16. Epub 2008/05/13. 10.1016/j.tig.2008.03.011 . [DOI] [PubMed] [Google Scholar]
- 62.Takada S, Paulsen M, Tevendale M, Tsai CE, Kelsey G, Cattanach BM, et al. Epigenetic analysis of the Dlk1-Gtl2 imprinted domain on mouse chromosome 12: implications for imprinting control from comparison with Igf2-H19. Hum Mol Genet. 2002;11(1):77–86. Epub 2002/01/05. 10.1093/hmg/11.1.77 . [DOI] [PubMed] [Google Scholar]
- 63.Saito Y, Liang G, Egger G, Friedman JM, Chuang JC, Coetzee GA, et al. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006;9(6):435–43. Epub 2006/06/13. 10.1016/j.ccr.2006.04.020 . [DOI] [PubMed] [Google Scholar]
- 64.Dai R, Lu R, Ahmed SA. The Upregulation of Genomic Imprinted DLK1-Dio3 miRNAs in Murine Lupus Is Associated with Global DNA Hypomethylation. PloS one. 2016;11(4):e0153509 Epub 2016/04/14. 10.1371/journal.pone.0153509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Guia RM, Rose AJ, Sommerfeld A, Seibert O, Strzoda D, Zota A, et al. microRNA-379 couples glucocorticoid hormones to dysfunctional lipid homeostasis. EMBO J. 2015;34(3):344–60. Epub 2014/12/17. 10.15252/embj.201490464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang J, Li S, Li L, Li M, Guo C, Yao J, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13(1):17–24. Epub 2015/03/01. 10.1016/j.gpb.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chartoumpekis DV, Zaravinos A, Ziros PG, Iskrenova RP, Psyrogiannis AI, Kyriazopoulou VE, et al. Differential expression of microRNAs in adipose tissue after long-term high-fat diet-induced obesity in mice. PloS one. 2012;7(4):e34872 Epub 2012/04/13. 10.1371/journal.pone.0034872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pearce SG, Thosani NC, Pan JJ. Noninvasive biomarkers for the diagnosis of steatohepatitis and advanced fibrosis in NAFLD. Biomark Res. 2013;1(1):7 Epub 2013/11/21. 10.1186/2050-7771-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846–54. Epub 2007/03/30. 10.1002/hep.21496 . [DOI] [PubMed] [Google Scholar]
- 70.Yang M, Xu D, Liu Y, Guo X, Li W, Guo C, et al. Combined Serum Biomarkers in Non-Invasive Diagnosis of Non-Alcoholic Steatohepatitis. PloS one. 2015;10(6):e0131664 Epub 2015/06/30. 10.1371/journal.pone.0131664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaplan MM. Alkaline phosphatase. Gastroenterology. 1972;62(3):452–68. Epub 1972/03/01. . [PubMed] [Google Scholar]
- 72.Poupon R. Liver alkaline phosphatase: a missing link between choleresis and biliary inflammation. Hepatology. 2015;61(6):2080–90. Epub 2015/01/22. 10.1002/hep.27715 . [DOI] [PubMed] [Google Scholar]
- 73.Tomizawa M, Kawanabe Y, Shinozaki F, Sato S, Motoyoshi Y, Sugiyama T, et al. Triglyceride is strongly associated with nonalcoholic fatty liver disease among markers of hyperlipidemia and diabetes. Biomed Rep. 2014;2(5):633–6. Epub 2014/07/24. 10.3892/br.2014.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pantsari MW, Harrison SA. Nonalcoholic fatty liver disease presenting with an isolated elevated alkaline phosphatase. Journal of clinical gastroenterology. 2006;40(7):633–5. Epub 2006/08/19. 10.1097/00004836-200608000-00015 . [DOI] [PubMed] [Google Scholar]
- 75.Kocabay G, Telci A, Tutuncu Y, Tiryaki B, Ozel S, Cevikbas U, et al. Alkaline phosphatase: can it be considered as an indicator of liver fibrosis in non-alcoholic steatohepatitis with type 2 diabetes? Bratisl Lek Listy. 2011;112(11):626–9. Epub 2011/12/21. . [PubMed] [Google Scholar]
- 76.Hatoff DE, Hardison WG. Bile acid-dependent secretion of alkaline phosphatase in rat bile. Hepatology. 1982;2(4):433–9. Epub 1982/07/01. 10.1002/hep.1840020407 . [DOI] [PubMed] [Google Scholar]
- 77.Barshop NJ, Sirlin CB, Schwimmer JB, Lavine JE. Review article: epidemiology, pathogenesis and potential treatments of paediatric non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;28(1):13–24. Epub 2008/04/10. 10.1111/j.1365-2036.2008.03703.x . [DOI] [PubMed] [Google Scholar]
- 78.Simonen P, Kotronen A, Hallikainen M, Sevastianova K, Makkonen J, Hakkarainen A, et al. Cholesterol synthesis is increased and absorption decreased in non-alcoholic fatty liver disease independent of obesity. Journal of hepatology. 2011;54(1):153–9. Epub 2010/10/16. 10.1016/j.jhep.2010.05.037 . [DOI] [PubMed] [Google Scholar]
- 79.Gan LT, Van Rooyen DM, Koina ME, McCuskey RS, Teoh NC, Farrell GC. Hepatocyte free cholesterol lipotoxicity results from JNK1-mediated mitochondrial injury and is HMGB1 and TLR4-dependent. Journal of hepatology. 2014;61(6):1376–84. Epub 2014/07/30. 10.1016/j.jhep.2014.07.024 . [DOI] [PubMed] [Google Scholar]
- 80.Min HK, Kapoor A, Fuchs M, Mirshahi F, Zhou H, Maher J, et al. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012;15(5):665–74. Epub 2012/05/09. 10.1016/j.cmet.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beg ZH, Stonik JA, Brewer HB Jr., Phosphorylation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase and modulation of its enzymic activity by calcium-activated and phospholipid-dependent protein kinase. J Biol Chem. 1985;260(3):1682–7. Epub 1985/02/10. . [PubMed] [Google Scholar]
- 82.Caballero F, Fernandez A, De Lacy AM, Fernandez-Checa JC, Caballeria J, Garcia-Ruiz C. Enhanced free cholesterol, SREBP-2 and StAR expression in human NASH. Journal of hepatology. 2009;50(4):789–96. Epub 2009/02/24. 10.1016/j.jhep.2008.12.016 . [DOI] [PubMed] [Google Scholar]
- 83.Mari M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, et al. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 2006;4(3):185–98. Epub 2006/09/05. 10.1016/j.cmet.2006.07.006 . [DOI] [PubMed] [Google Scholar]
- 84.Leroux A, Ferrere G, Godie V, Cailleux F, Renoud ML, Gaudin F, et al. Toxic lipids stored by Kupffer cells correlates with their pro-inflammatory phenotype at an early stage of steatohepatitis. Journal of hepatology. 2012;57(1):141–9. Epub 2012/03/20. 10.1016/j.jhep.2012.02.028 . [DOI] [PubMed] [Google Scholar]
- 85.Teratani T, Tomita K, Suzuki T, Oshikawa T, Yokoyama H, Shimamura K, et al. A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology. 2012;142(1):152–64 e10. Epub 2011/10/15. 10.1053/j.gastro.2011.09.049 . [DOI] [PubMed] [Google Scholar]
- 86.Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–54. Epub 2014/08/16. 10.1002/hep.27368 . [DOI] [PubMed] [Google Scholar]
- 87.Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. Journal of gastroenterology. 2018;53(3):362–76. Epub 2017/12/17. 10.1007/s00535-017-1415-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cicognani C, Malavolti M, Morselli-Labate AM, Zamboni L, Sama C, Barbara L. Serum lipid and lipoprotein patterns in patients with liver cirrhosis and chronic active hepatitis. Arch Intern Med. 1997;157(7):792–6. Epub 1997/04/14. . [PubMed] [Google Scholar]
- 89.Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med. 2001;5(4):378–87. Epub 2002/06/18. 10.1111/j.1582-4934.2001.tb00172.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eslami L, Merat S, Malekzadeh R, Nasseri-Moghaddam S, Aramin H. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2013;(12):CD008623 Epub 2014/01/01. 10.1002/14651858.CD008623.pub2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dongiovanni P, Petta S, Mannisto V, Mancina RM, Pipitone R, Karja V, et al. Statin use and non-alcoholic steatohepatitis in at risk individuals. Journal of hepatology. 2015;63(3):705–12. Epub 2015/05/20. 10.1016/j.jhep.2015.05.006 . [DOI] [PubMed] [Google Scholar]
- 92.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149(2):389–97 e10. Epub 2015/05/04. 10.1053/j.gastro.2015.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li K, Wang Y, Zhang A, Liu B, Jia L. miR-379 Inhibits Cell Proliferation, Invasion, and Migration of Vascular Smooth Muscle Cells by Targeting Insulin-Like Factor-1. Yonsei Med J. 2017;58(1):234–40. Epub 2016/11/23. 10.3349/ymj.2017.58.1.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang DJ, Huang JZ, Yang J, Li YH, Luo YC, He HY, et al. Bioinformatic identification of IGF1 as a hub gene in hepatocellular carcinoma (HCC) and in-vitro analysis of the chemosensitizing effect of miR-379 via suppressing the IGF1/IGF1R signaling pathway. Eur Rev Med Pharmacol Sci. 2016;20(24):5098–106. Epub 2017/01/05. . [PubMed] [Google Scholar]
- 95.Garcia-Galiano D, Sanchez-Garrido MA, Espejo I, Montero JL, Costan G, Marchal T, et al. IL-6 and IGF-1 are independent prognostic factors of liver steatosis and non-alcoholic steatohepatitis in morbidly obese patients. Obes Surg. 2007;17(4):493–503. Epub 2007/07/05. 10.1007/s11695-007-9087-1 . [DOI] [PubMed] [Google Scholar]
- 96.Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML, Endocrine S. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2011;96(6):1587–609. Epub 2011/05/24. 10.1210/jc.2011-0179 . [DOI] [PubMed] [Google Scholar]
- 97.Nishizawa H, Takahashi M, Fukuoka H, Iguchi G, Kitazawa R, Takahashi Y. GH-independent IGF-I action is essential to prevent the development of nonalcoholic steatohepatitis in a GH-deficient rat model. Biochem Biophys Res Commun. 2012;423(2):295–300. Epub 2012/06/05. 10.1016/j.bbrc.2012.05.115 . [DOI] [PubMed] [Google Scholar]
- 98.Arturi F, Succurro E, Procopio C, Pedace E, Mannino GC, Lugara M, et al. Nonalcoholic fatty liver disease is associated with low circulating levels of insulin-like growth factor-I. The Journal of clinical endocrinology and metabolism. 2011;96(10):E1640–4. Epub 2011/08/06. 10.1210/jc.2011-1227 . [DOI] [PubMed] [Google Scholar]
- 99.Ichikawa T, Nakao K, Hamasaki K, Furukawa R, Tsuruta S, Ueda Y, et al. Role of growth hormone, insulin-like growth factor 1 and insulin-like growth factor-binding protein 3 in development of non-alcoholic fatty liver disease. Hepatol Int. 2007;1(2):287–94. Epub 2007/06/01. 10.1007/s12072-007-9007-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, et al. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295(5):H1882–94. Epub 2008/09/02. 10.1152/ajpheart.412.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nishizawa H, Iguchi G, Fukuoka H, Takahashi M, Suda K, Bando H, et al. IGF-I induces senescence of hepatic stellate cells and limits fibrosis in a p53-dependent manner. Sci Rep. 2016;6:34605 Epub 2016/10/11. 10.1038/srep34605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lam CS, Chen MH, Lacey SM, Yang Q, Sullivan LM, Xanthakis V, et al. Circulating insulin-like growth factor-1 and its binding protein-3: metabolic and genetic correlates in the community. Arterioscler Thromb Vasc Biol. 2010;30(7):1479–84. Epub 2010/04/10. 10.1161/ATVBAHA.110.203943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zaki SM, Abdel Fattah S, Hassan DS. The differential effects of high-fat and high fructose diets on the liver of male albino rat and the proposed underlying mechanisms. Folia Morphol (Warsz). 2018. Epub 2018/07/17. 10.5603/FM.a2018.0063 . [DOI] [PubMed] [Google Scholar]
- 104.Liu Y, Cheng F, Luo Y, Zhan Z, Hu P, Ren H, et al. PEGylated Curcumin Derivative Attenuates Hepatic Steatosis via CREB/PPAR-gamma/CD36 Pathway. Biomed Res Int. 2017;2017:8234507 Epub 2017/08/05. 10.1155/2017/8234507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hardie DG. Regulation of fatty acid and cholesterol metabolism by the AMP-activated protein kinase. Biochim Biophys Acta. 1992;1123(3):231–8. Epub 1992/02/12. 10.1016/0005-2760(92)90001-c . [DOI] [PubMed] [Google Scholar]
- 106.Kim KH. Regulation of acetyl-CoA carboxylase. Curr Top Cell Regul. 1983;22:143–76. Epub 1983/01/01. 10.1016/b978-0-12-152822-5.50009-9 . [DOI] [PubMed] [Google Scholar]
- 107.Song Z, Deaciuc I, Zhou Z, Song M, Chen T, Hill D, et al. Involvement of AMP-activated protein kinase in beneficial effects of betaine on high-sucrose diet-induced hepatic steatosis. Am J Physiol Gastrointest Liver Physiol. 2007;293(4):G894–902. Epub 2007/08/19. 10.1152/ajpgi.00133.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao X, Chu J. MicroRNA-379 suppresses cell proliferation, migration and invasion in nasopharyngeal carcinoma by targeting tumor protein D52. Exp Ther Med. 2018;16(2):1232–40. Epub 2018/08/18. 10.3892/etm.2018.6302 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109.Shi X, Xiao X, Yuan N, Zhang S, Yuan F, Wang X. MicroRNA-379 Suppresses Cervical Cancer Cell Proliferation and Invasion by Directly Targeting V-crk Avian Sarcoma Virus CT10 Oncogene Homolog-Like (CRKL). Oncol Res. 2018;26(7):987–96. Epub 2018/01/04. 10.3727/096504017X15140534417184 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu M, Qin S, Cao F, Ding S, Li M. MicroRNA-379 inhibits metastasis and epithelial-mesenchymal transition via targeting FAK/AKT signaling in gastric cancer. Int J Oncol. 2017;51(3):867–76. Epub 2017/07/18. 10.3892/ijo.2017.4072 . [DOI] [PubMed] [Google Scholar]
- 111.Wu D, Niu X, Tao J, Li P, Lu Q, Xu A, et al. MicroRNA-379-5p plays a tumor-suppressive role in human bladder cancer growth and metastasis by directly targeting MDM2. Oncol Rep. 2017;37(6):3502–8. Epub 2017/05/13. 10.3892/or.2017.5607 . [DOI] [PubMed] [Google Scholar]
- 112.Xie X, Li YS, Xiao WF, Deng ZH, He HB, Liu Q, et al. MicroRNA-379 inhibits the proliferation, migration and invasion of human osteosarcoma cells by targetting EIF4G2. Biosci Rep. 2017;37(3). Epub 2017/04/07. 10.1042/BSR20160542 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 113.Chen JS, Li HS, Huang JQ, Dong SH, Huang ZJ, Yi W, et al. MicroRNA-379-5p inhibits tumor invasion and metastasis by targeting FAK/AKT signaling in hepatocellular carcinoma. Cancer Lett. 2016;375(1):73–83. Epub 2016/03/06. 10.1016/j.canlet.2016.02.043 . [DOI] [PubMed] [Google Scholar]
- 114.Mitter D, Buiting K, von Eggeling F, Kuechler A, Liehr T, Mau-Holzmann UA, et al. Is there a higher incidence of maternal uniparental disomy 14 [upd(14)mat]? Detection of 10 new patients by methylation-specific PCR. Am J Med Genet A. 2006;140(19):2039–49. Epub 2006/08/15. 10.1002/ajmg.a.31414 . [DOI] [PubMed] [Google Scholar]
- 115.Liang Y, Ridzon D, Wong L, Chen C. Characterization of microRNA expression profiles in normal human tissues. BMC genomics. 2007;8:166 10.1186/1471-2164-8-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Turan S, Topcu B, Gokce I, Guran T, Atay Z, Omar A, et al. Serum alkaline phosphatase levels in healthy children and evaluation of alkaline phosphatase z-scores in different types of rickets. J Clin Res Pediatr Endocrinol. 2011;3(1):7–11. Epub 2011/03/31. 10.4274/jcrpe.v3i1.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kuo TR, Chen CH. Bone biomarker for the clinical assessment of osteoporosis: recent developments and future perspectives. Biomark Res. 2017;5:18 Epub 2017/05/23. 10.1186/s40364-017-0097-4 [DOI] [PMC free article] [PubMed] [Google Scholar]