Abstract
Spontaneous electrical activity is a common feature of sensory systems during early development. This sensory-independent neuronal activity has been implicated in promoting their survival and maturation, as well as growth and refinement of their projections to yield circuits that can rapidly extract information about the external world. Periodic bursts of action potentials occur in auditory neurons of mammals before hearing onset. This activity is induced by inner hair cells (IHCs) within the developing cochlea, which establish functional connections with spiral ganglion neurons (SGNs) several weeks before they are capable of detecting external sounds. During this pre-hearing period, IHCs fire periodic bursts of Ca2+ action potentials that excite SGNs, triggering brief but intense periods of activity that pass through auditory centers of the brain. Although spontaneous activity requires input from IHCs, there is ongoing debate about whether IHCs are intrinsically active and their firing periodically interrupted by external inhibitory input (IHC-inhibition model), or are intrinsically silent and their firing periodically promoted by an external excitatory stimulus (IHC-excitation model). There is accumulating evidence that inner supporting cells in Kölliker’s organ spontaneously release ATP during this time, which can induce bursts of Ca2+ spikes in IHCs that recapitulate many features of auditory neuron activity observed in vivo. Nevertheless, the role of supporting cells in this process remains to be established in vivo. A greater understanding of the molecular mechanisms responsible for generating IHC activity in the developing cochlea will help reveal how these events contribute to the maturation of nascent auditory circuits.
Keywords: Cochlea, ATP, Glia, Spiral ganglion neuron, Inner hair cell
Introduction
A major goal for developmental neuroscience is to elucidate how neurons assemble into a network to carry out its specific functions. While substantial progress has been made in understanding the genetic programs and guidance molecules required for proper connections, much less is known about another fundamental feature of developing neural circuits — the role of intrinsically generated or “spontaneous” activity. Action potentials that are not initiated by input from the external environment have been observed in the developing nervous system of many species and in many distinct regions of the nervous system (Blankenship and Feller 2010), including the retina (Galli and Maffei 1988; Meister et al. 1991), spinal cord (Landmesser and O′Donovan 1984), hippocampus (Ben-Ari et al. 1989; Garaschuk et al. 1998), cerebellum (Watt et al. 2009), and cochlea (Kros et al. 1998; Tritsch et al. 2007). The pervasiveness of this spontaneous activity suggests that it plays an important role in the maturation of neural circuits. Indeed, it has been implicated in regulating the proliferation, differentiation, and migration of neurons (Wong and Ghosh 2002; Moody and Bosma 2005; Spitzer 2006), and is thought to influence their structural maturation (Cohen-Cory 2002), axonal arborization, and ultimately their integration into neuronal circuits (Katz and Shatz 1996; Friauf and Lohmann 1999; Zhang and Poo 2001; Moody and Bosma 2005; Huberman et al. 2008; Blankenship and Feller 2010; Kirkby et al. 2013).
In the auditory system, spontaneous electrical activity that is present before the onset of hearing (defined as the age when auditory neurons reliably respond to airborne acoustic stimulation) has also been implicated in shaping the organization of nascent circuits. Most studies have been performed in either chicken (in ovo) or neonatal altricial animals, such as rodents and cats, which are born deaf and remain unresponsive to airborne sound until approximately the end of the 2nd postnatal week. In rodents, the inability to sense ambient sound is not due to the inability of hair cells to transform mechanical stimulation into electrical signals (Lelli et al. 2009) or the lack of functional connections between central cochlear neurons (Hoffpauir et al. 2009). Rather, deafness results from the combination of physical occlusion of the external ear canal, poor ossicular transduction in the middle ear, underdeveloped mechanics in the organ of Corti, immature ionic composition in the endolymph, and absence of the endocochlear potential (Bosher and Warren 1971; Anniko and Wroblewski 1986; Woolf and Ryan 1988; Rybak et al. 1992; Geal-Dor et al. 1993; Abe et al. 2007). The time of hearing onset for some common experimental animals is embryonic day 16 (E16) in chicken (Jones et al. 2006), postnatal day (P) 10–12 in mouse (Mikaelian and Ruben 1965; Ehret 1983), P11–13 in rat (Uziel et al. 1981; Ehret 1983; Geal-Dor et al. 1993), P10 in cat (Walsh and McGee 1987), and P12 in gerbil (Woolf and Ryan 1984), with the day of birth defined as P0.
The properties of sound-independent activity present during auditory system development are distinct from those which occur after hearing onset. Previous studies have demonstrated that the majority of auditory nerve fibers (ANFs) in hearing cats and rodents are continuously active in the absence of acoustic stimulation. The spontaneous discharge rate of individual ANFs can vary from only a few Hz to more than 100 Hz (Kiang 1965; Liberman 1978; Walsh and McGee 1987; Taberner and Liberman 2005); the pattern of spiking over time is relatively continuous for a given fiber, which displays regular discharges without discernible periods of silence (Walsh and McGee 1987; Jones et al. 2007). Similar patterns of activity have also been recorded from brainstem auditory nuclei (Kopp-Scheinpflug et al. 2008; Sonntag et al. 2009; Crins et al. 2011), and in other species (guinea pig: Manley and Robertson 1976; Manley et al. 1991; chicken: Jones and Jones 2000). This regular firing behavior is distinct from the patterns of activity present in the developing auditory system. Before hearing onset, a larger proportion of ANFs and brainstem auditory neurons are silent and do not exhibit spontaneous discharges, a phenomenon that decreases with age (Romand and Marty 1975; Shnerson and Willott 1979; Romand 1984; Walsh and McGee 1987). Although the average discharge rate of active cells is only several Hz (Shnerson and Willott 1979; Brugge and O′Connor 1984; Romand 1984; Walsh and McGee 1987; Kotak and Sanes 1995; Jones et al. 2007; Sonntag et al. 2009; Crins et al. 2011), auditory neuron activity is concentrated into brief bursts that are followed by long periods of silence (Romand and Marty 1975; Shnerson and Willott 1979; Romand 1984; Walsh and McGee 1987; Kotak and Sanes 1995; Sonntag et al. 2009; Tritsch et al. 2010a; Crins et al. 2011). Similar firing behavior has been observed in the cochlear ganglion and in higher-order neurons in the embryonic chicken (Lippe 1994; Jones et al. 2001), in the auditory midbrain of pre-hearing horseshoe bats (Rubsamen and Schafer 1990), and in the cochlear nucleus from pouch young wallaby (Gummer and Mark 1994), suggesting that the mechanisms responsible for initiating this activity may be highly conserved.
In this review, we attempt to outline current knowledge about the generation, developmental changes, and function of spontaneous activity that occurs in the auditory system before hearing onset. For simplicity, we will focus on rodents and restrict our discussion of functional consequences to the auditory brainstem, where most experimental results have been obtained.
Spontaneous activity in the auditory system originates within the cochlea
Although spontaneous activity has been recorded within different central auditory nuclei, it is believed that this activity is initiated from the developing cochlea. Bursting activity in auditory brainstem neurons of embryonic chick was eliminated following removal of the cochlea or by application of tetrodotoxin (TTX) to the oval window to block firing of ANFs (Lippe 1994), and similar bursts of action potentials have been recorded directly from spiral ganglion neurons (SGNs) in vivo (Jones et al. 2001; 2007). Furthermore, it has been shown that the spontaneous action potentials recorded in vitro from SGNs exhibit a stereotyped pattern consisting of repeating mini-bursts, which is also seen in the spontaneous discharge of neurons in the medial nucleus of the trapezoid body (MNTB) and the central nucleus of inferior colliculus (IC) in vivo (Tritsch et al. 2010a). Although it is possible that this activity is intrinsically generated by SGNs, the spontaneous action potentials exhibited by SGNs are dependent on Ca2+-mediated transmitter release from inner hair cells (IHCs) (Robertson and Paki 2002; Tritsch and Bergles 2010), suggesting that this distinct firing behavior reflects events happening within the developing organ of Corti before the onset of hearing.
IHCs in the pre-hearing cochlea are capable of firing Ca2+based action potentials (termed “Ca2+ spikes”) (Kros et al. 1998), which have much slower kinetics than conventional Na+-based action potentials. These spikes are mediated primarily by L-type Ca2+ channels containing the Cav1.3 subunit (Platzer et al. 2000; Brandt et al. 2003), but the kinetics of these events are also modified by other ion channels (Marcotti et al. 2003a, 2003b, 2004; Kennedy 2012). Each Ca2+ spike allows a large bolus of Ca2+ to enter the cell, which is sufficient to trigger Ca2+-mediated glutamate release from immature ribbon synapses (Beutner and Moser 2001; Glowatzki and Fuchs 2002; Johnson et al. 2005). This synaptic activity induces SGNs to fire action potentials that are carried to the auditory brainstem via the eighth nerve. IHCs exhibit small Ca2+ currents and exocytotic membrane capacitance changes in response to current injection as early as E16.5 (Marcotti et al. 2003a; Johnson et al. 2005), and by E17.5 depolarization elicits broad Ca2+spikesinIHCs (Marcotti et al. 2003a).Thus, despite the immaturity of ribbon synapses and the lower Ca2+ efficiency of exocytosis (Sobkowicz et al. 1982; Beutner and Moser 2001; Johnson et al. 2005), IHCs are capable of releasing neurotransmitter several weeks before hearing onset.
Although the precise timing of synapse formation between SGNs and IHCs has not been clearly defined, the peripheral neurites of developing SGNs extend into the sensory epithelium even before hair-cell differentiation, with nascent contacts between these cells formed as early as E18 in both rat (Pujol et al. 1998) and mouse (Huang et al. 2007). Glutamate receptors are expressed by SGNs before birth (Luo et al. 1995; Puyal et al. 2002), and morphologically-defined synapses are present at P0, in which intracellular ribbon-like structures in IHCs are apposed to electron-dense regions of SGN dendritic membranes (Sobkowicz et al. 1982). Consistent with the presence of these synaptic structures, depolarization-evoked glutamate exocytosis from IHCs can trigger action potentials in SGNs at P0 (Tritsch and Bergles 2010). This activity is unlikely to be restricted to the cochlea, as the central projections of SGNs grow into the hindbrain quite early (Appler and Goodrich 2011) and reach the cochlear nucleus by E15.5 (Koundakjian et al. 2007). Within the brainstem, functional synapses between auditory relay neurons are also present at birth (Friauf and Kandler 1990; Kandler and Friauf 1995b; Hoffpauir et al. 2006, 2009; Rodriguez-Contreras et al. 2008). Together, these anatomical and functional studies indicate that the transduction pathway from IHCs to developing circuits of the brain is established well before hearing onset, providing a substrate that spontaneous activity can act upon to influence its developmental trajectory.
Despite the capacity of IHCs to generate Ca2+ spikes and release glutamate before hearing onset, it is still under debate whether the spontaneous activity exhibited by IHCs arises from cell intrinsic processes or is induced (or modified) by an external stimulus. Previous studies indicate that IHCs in the pre-hearing cochlea of rodents continuously fire Ca2+ spikes in vitro without apparent external stimulation (Kros et al. 1998; Marcotti et al. 2003a, 2003b, 2004; Brandt et al. 2007). To reconcile this pattern of activity with the discontinuous, bursting pattern recorded from auditory neurons in vivo, it has been proposed that phasic inhibitory cholinergic input from medial olivocochlear (MOC) efferents periodically interrupts the tonic firing of IHCs (Kros 2007) (Fig. 1). Indeed, cholinergic efferent discharge can hyperpolarize IHCs and disrupt the initiation of Ca2+ spikes (Glowatzki and Fuchs 2000); if these efferents fire prolonged, high-frequency bursts in vivo, they could suppress the generation of Ca2+ spikes (Goutman et al. 2005). In support of this model, inhibition of acetylcholine receptors in acutely isolated cochleae changed the firing pattern of IHCs from bursting to sustained activity (Johnson et al. 2011), suggesting that cholinergic efferents can sustain high rates of release even when removed from their cell bodies in the brainstem. The involvement of these cholinergic efferents could explain the discrepancy between in vitro and in vivo activity patterns, if the extent of release from these axons varies after cochlea isolation. This model satisfies the cochlear origin of spontaneous activity, and provides an explanation for the abrupt decline in burst firing at hearing onset, which coincides with the cessation of both MOC efferent innervation of IHCs (Katz et al. 2004; Roux et al. 2011) and Ca2+ spike generation by IHCs (Kros et al. 1998). Nevertheless, several observations suggest that cholinergic input is not essential for generating bursts of activity in IHCs and auditory neurons during this period.
Fig. 1.
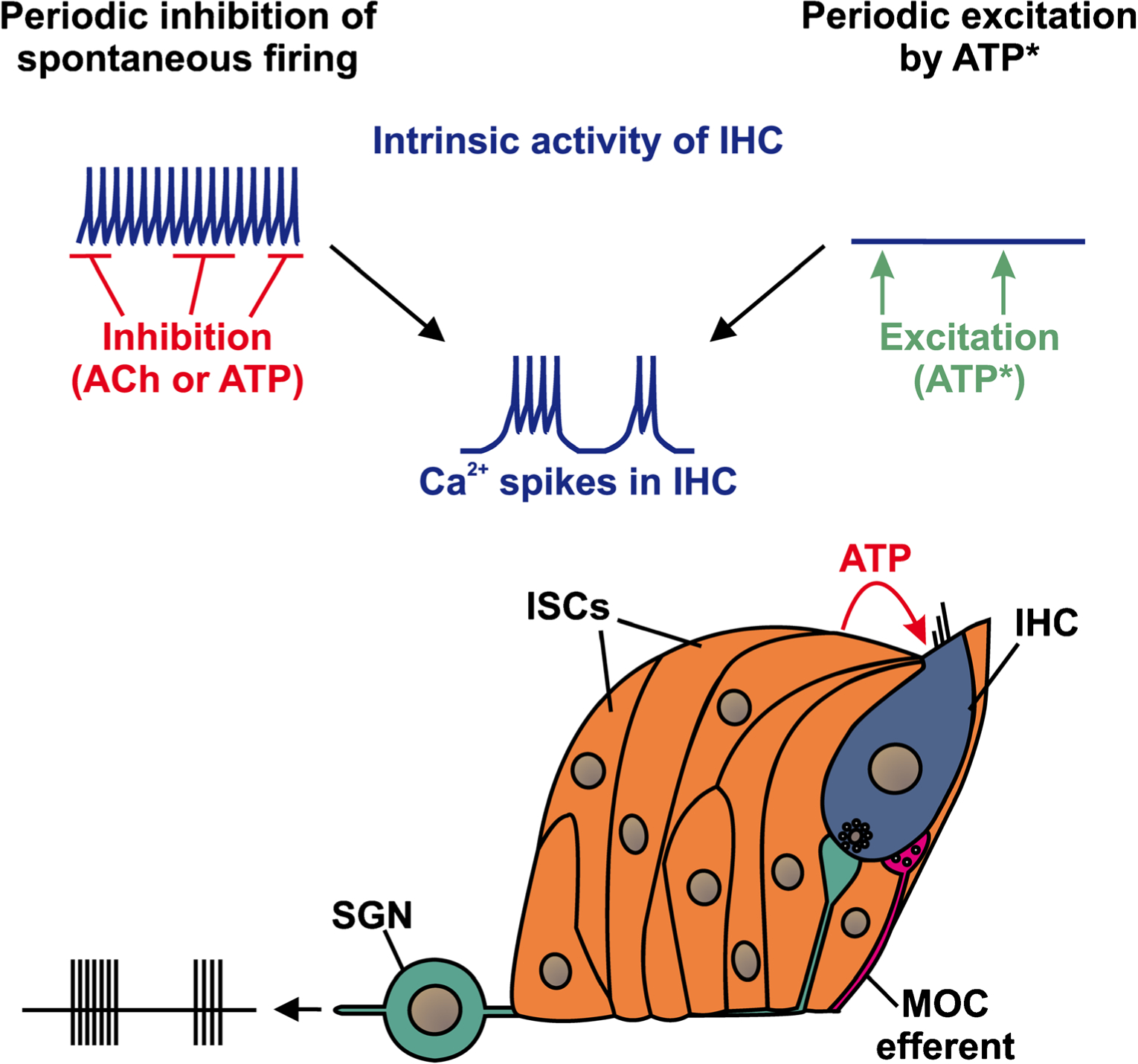
Two models to explain how spontaneous bursts of activity could be induced in IHCs before hearing onset. The “IHC-inhibition” model (at left) proposes that IHCs are depolarized and therefore tend to fire Ca2+ spikes continuously. Periodic inhibition of IHCs by an external modulator such as acetylcholine (ACh) or adenosine triphosphate (ATP) would interrupt this firing, transforming continuous activity into burst firing. The “IHC-excitation” model (at right) proposes that IHCs are hyperpolarized and thus predominantly silent in the absence of an external stimulation. When ATP (* or another excitatory modulator) is spontaneously released from ISCs, IHCs are slowly depolarized, triggering a brief train of Ca2+ spikes. In both scenarios, the bursts of IHC Ca2+ spikes induce glutamate release and bursts of action potentials in SGNs that are subsequently carried to the auditory brainstem via the eighth nerve
At present, there is a lack of consensus about whether IHCs fire continuously in vitro at this age. While some studies have shown that IHCs sustain tonic firing, as noted above (Kros et al. 1998; Marcotti et al. 2003a, 2003b, 2004; Brandt et al. 2007), others reported that IHCs fire bursts of Ca2+ spikes in the apex (Tritsch and Bergles 2010; Johnson et al. 2011), or all along the length of the cochlea (Sendin et al. 2014). Moreover, it has been shown that IHCs and SGNs exhibit burst activity in cochlear explants that have been maintained in vitro for several days (Tritsch et al. 2010a), despite the absence of cholinergic axons. Furthermore, to accommodate the long periods of silence between bursts observed in vivo, the cholinergic model requires that MOC neurons fire sustained discharges for up to several seconds; unfortunately, there have been no in vivo recordings from these neurons to determine if they exhibit such activity during this developmental stage. Finally, this model predicts that removal of MOC cholinergic input would result in a conversion of auditory neuron activity from bursting to continuous firing. However, a recent study showed that MNTB neurons in vivo continue to fire in discrete bursts in mice that lack the α9 acetylcholine receptor subunit, which is required for MOC-mediated inhibition of IHCs (Vetter et al. 1999; Clause et al. 2014). Together, these results indicate that inhibitory input from MOC efferents is not essential to induce burst firing in auditory neurons, and suggest that there may be other extrinsic mechanisms to trigger periodic excitation of IHCs.
An alternative model proposes that IHCs are excited by the periodic release of adenosine triphosphate (ATP) from neighboring supporting cells. Previous studies have shown that a group of pseudostratified inner supporting cells (ISCs) that together form Kölliker’s organ (greater epithelial ridge), which lies medial to IHCs, exhibit spontaneous inward currents in the pre-hearing cochlea that are mediated by ATP. Similar events can be elicited by exogenous ATP or UTP, an agonist of P2Y receptors, and inhibited by purinergic receptor antagonists (suramin, PPADS). Strikingly, these cells shrink or crenate when stimulated with ATP or UTP, an effect that is triggered by a rise in intracellular Ca2+ (Tritsch et al. 2010b). Although the significance of these crenations is not known, it provides a mean to pinpoint the source of ATP — since all of the cells crenate when exposed to ATP, the site of crenation indicates where ATP was released. These studies indicate that the supporting cells themselves release ATP, which induces crenation by activation of P2 autoreceptors. Recordings from IHCs revealed that they exhibited similar slow inward currents, many of which were of sufficient magnitude and duration to induce bursts of Ca2+ spikes, and simultaneous recordings from IHCs and ISCs, while crenations were imaged, revealed that these three phenomena were coincident and required activation of purinergic receptors (Tritsch et al. 2007). Moreover, recordings from SGNs in isolated cochleae revealed that this IHC activity was sufficient to induce bursts of action potentials, providing a plausible mechanism to explain the periodic activity of auditory neurons observed in vivo. Indeed, subsequent studies revealed that SGNs exhibit a stereotyped firing pattern during this prehearing period, in which each burst is comprised of a series of mini-bursts, a reflection of the ability of each IHC Ca2+ spike to induce repetitive firing of SGNs (Tritsch et al. 2010a). This stereotyped firing pattern within bursts is also exhibited by neurons in the cochlear nucleus and the MNTB (Tritsch et al. 2010a; Clause et al. 2014), providing further support for the cochlear origin of this activity. Together, these experiments indicate that ATP is spontaneously released from ISCs in the prehearing cochlea, which activates purinergic receptors on IHCs (and ISCs), ultimately leading to bursts of action potentials in SGNs and auditory neurons of the brain (Fig. 1). Random variations in the location and amount of ATP released from ISCs provide an explanation for observed variations in the duration and frequency of action potential bursts in auditory neurons. This model also provides an explanation for the decline in phasic spontaneous activity around hearing onset, as Kölliker’s organ progressively regresses after birth, ultimately forming the inner sulcus around the time of hearing onset (Hinojosa 1977; Kelley 2007); ISCs and IHCs remain responsive to ATP after hearing onset (Tritsch and Bergles 2010), suggesting that a key event in the cessation of spontaneous activity is the decline in ATP release from ISCs. In this model, burst firing does not require sustained firing of MOC efferents, consistent with the preservation of bursting activity in MNTB neurons in α9 knockout animals (Clause et al. 2014), although this cholinergic input could provide feedback inhibition to influence burst firing (e.g., the duration and magnitude of bursts, as well as the number of IHCs that are activated). ATP appears to be released focally along the length of the cochlea, and is degraded by ectonucleotidases, which are expressed in the pre-hearing cochlea (O′Keeffe et al. 2010). As a result, each ATP release event is accompanied by the activation of discrete groups of IHCs proximal to the site of ATP release; such synchronous activity among IHCs, and thus SGNs, could help establish tonotopic wiring in the auditory pathway through Hebbian-like plasticity mechanisms (Tritsch et al. 2007).
Despite this evidence that supporting cell dependent excitation of IHCs is a primary driver for spontaneous activity in the developing auditory system, there are a number of unresolved issues, as well as experimental observations that appear to contradict this model. For example, it has been shown that exogenous ATP can have excitatory (Tritsch et al. 2007), inhibitory (Sendin et al. 2014), or dual effects (Johnson et al. 2011) on IHC firing, depending on the concentration and method of application used. In addition, one study reported that purinergic receptor inhibition increases the firing rate of IHCs (Johnson et al. 2011). Moreover, the activity of IHCs recorded in whole mount preparations from the early postnatal cochlea varies from infrequent Ca2+ spikes and bursting behavior (Brandt et al. 2007; Tritsch and Bergles 2010) to steady firing (Marcotti et al. 2003a; Sendin et al. 2014).
Although the reasons for these different observations have not yet been determined, it is possible that they reflect differences in experimental conditions between different laboratories. The organ of Corti is contained in a specialized environment in vivo, with one side exposed to endolymph containing a high K+ concentration (157 mM) and the other side surrounded by perilymph (6.0 mM K+ in perilymph of scala vestibule and 4.2 mM K+ in scala tympani) (Wangemann and Schacht 1996). Unfortunately, it is not possible to mimic such a polarized environment in vitro. Moreover, the distinct ionic compositions of endolymph and perilymph are not fully established until the end of first postnatal week (Bosher and Warren 1971; Anniko and Wroblewski 1986), a time when there are many physiological changes occurring in the organ of Corti. Although many laboratories perform experiments in 6 mM extracellular K+; it is unclear whether this K+ concentration mimics the in vivo ionic environment. In addition, the kinetics of Ca2+ spikes are affected by temperature and the degree of intracellular Ca2+ buffering (Johnson et al. 2011). While these conditions can be reasonably replicated by different laboratories, it is more difficult to control for differences in the state of the tissue after isolation. IHCs exhibit high resting Ca2+ levels following tissue isolation (Wang and Bergles, unpublished results) and reduced responsiveness to ATP. Although resting IHC Ca2+ levels are lowered in preparations cultured for as little as one day (Wang and Bergles, unpublished results), exposure to culture media and isolation from the CNS may induce other changes that cause it to diverge from the in vivo state. Finally, MOC efferent axons are isolated from their cell bodies upon cochlear isolation, which prevents an analysis of their physiological activity patterns and extent of feedback from central auditory neurons. Thus, although acutely isolated cochleae and cochlear slices preserve complex intercellular relationships and offer greater experimental access, they have significant limitations that prevent ready extrapolation to in vivo conditions, necessitating confirmation of key findings in the intact nervous system.
Developmental changes in spontaneous activity
As described above, anatomical and physiological studies indicate that the transduction pathway from IHCs to the brain is established in early postnatal life in rodents; however, there have been only a few in vivo studies to assess the developmental time course of spontaneous activity in the cochlea or auditory centers of the brain. Juxtacellular recordings from rat MNTB neurons revealed that they fire isolated spikes at P0, but the proportion of burst-firing neurons rapidly increases with age, and constitutes over 91 % of all units by P4 (Tritsch et al. 2010a). The principal neurons of MNTB are readily excited by glutamatergic inputs at P0–P1 as a result of their high input resistance (Rusu and Borst 2011), suggesting that the lack of burst-firing at P0 is not because principal neurons cannot respond to synaptic inputs, but because there is little peripheral input at this age. Another in vivo study in rat also showed that MNTB neurons exhibit bursting activity at P4, which transitions to continuous firing after the middle of the 2nd postnatal week, reaching the adult pattern after hearing onset (Crins et al. 2011). Similar observations have been reported in mice, where MNTB neurons shift rapidly from bursting activity at P8–P10 to a regular, non-bursting discharge pattern at P11–12, just before the onset of hearing (Sonntag et al. 2009). These results indicate that spontaneous activity in pre-hearing rodents emerges postnatally, with a distinct bursting pattern emerging before P4 that is maintained until hearing onset.
A more extensive in vitro assessment of developmental changes in the spontaneous firing of IHCs has been performed primarily using cochlear tissue isolated from rodents. Nevertheless, there remains a lack of consensus about the changes in IHC activity patterns before hearing onset. Some studies have reported that IHCs spontaneously fire Ca2+ spikes only during the early postnatal period in mouse (E17.5 to P6: Marcotti et al. 2003a; until P7: Brandt et al. 2007), but spontaneous IHC Ca2+ spikes have also been observed in P7–8 mice (Seal et al. 2008). In contrast, spontaneous generation of Ca2+ spikes has been documented in the rat cochlea from P3 until hearing onset (Brandt et al. 2007; Tritsch and Bergles 2010), while other studies showed that current injection is necessary to elicit Ca2+ spikes in IHCs of P7–P11 rats (Glowatzki and Fuchs 2000; Goutman et al. 2005). It has also been reported that IHCs in the apical and basal regions of cochlea exhibit different activities, with apical IHCs firing Ca2+ spikes in bursts and basal IHCs firing continuously (P2–P5) (Johnson et al. 2011). In contrast, a recent study found that both apical and basal IHCs exhibited burst-firing behavior (P1–P9) (Sendin et al. 2014).
The stereotyped firing behavior produced in SGNs by IHC Ca2+ spikes provides a template with which to evaluate in vivo activity patterns in auditory centers of the brain. These data suggest that from the end of the 1st postnatal week until hearing onset, IHCs fire brief trains of Ca2+ spikes that lead to bursts of action potentials in SGNs, which then propagate through neurons in various auditory centers of the brain. The patterns of activity that precede (developmentally) this burst firing has been more difficult to establish — in vivo recordings from the MNTB in anesthetized rats showed limited activity (Tritsch et al. 2010a), consistent with some in vitro studies (Brandt et al. 2007; Tritsch and Bergles 2010), but counter to other reports of robust spontaneous firing of IHCs at this early developmental stage (Marcotti et al. 2003a; Brandt et al. 2007; Johnson et al. 2011; Sendin et al. 2014). In-vivo recordings from central auditory neurons in prehearing rodents have consistently failed to detect high rates of continuous firing such as that reported by these latter studies, suggesting that such activity may be confined to the cochlea at this age or that cochlear isolation in some cases enhances the excitability of IHCs.
Functional roles of spontaneous activity in the auditory system
Spontaneous activity in the developing nervous system is thought to play an important role in the maturation of neural circuits (Feller 1999; O’Donovan 1999); however, the particular contributions of this activity to different aspects of circuit refinement, and the mechanisms through which it influences these changes, are not well-understood. The most intensively studied example of developmental spontaneous activity involves coordinated activity of retinal ganglion cells (retinal waves) that occurs before eye opening and its involvement in retinotopic refinement in the lateral geniculate nucleus (LGN) (Huberman et al. 2008). Cholinergic retinal waves are initially induced by the spontaneous release of acetylcholine from a subset of starburst amacrine cells (Feller et al. 1996). Mice lacking the β2-subunit of the neuronal nicotinic acetylcholine receptor (nAChR) do not exhibit cholinergic retinal waves (Bansal et al. 2000; Muir-Robinson et al. 2002), while retinal waves at other developmental stages are unaffected. Application of the high-affinity cholinergic agonist epibatidine also blocks cholinergic waves by desensitizing nAChRs (Penn et al. 1998), and elimination of spontaneous activity after intraocular injection of epibatidine has been demonstrated in vivo (Ackman et al. 2012). Using both transgenic mouse models and pharmacological manipulations, it has been shown that axons of retinal ganglion cells project to correct retinotopic positions in the superior colliculus and lateral geniculate nuclei when retinal waves have been blocked or reduced, but form abnormally diffuse arborizations (Grubb et al. 2003; McLaughlin et al. 2003; Pfeiffenberger et al. 2006). Thus, sensory-independent activity within the developing retina is necessary for establishing proper retinotopic maps (Kirkby et al. 2013).
Although some nascent synaptic connections between auditory nuclei are established embryonically (Hoffpauir et al. 2009), the auditory brainstem circuit experiences remarkable structural and functional modifications during the first two postnatal weeks, during which auditory neurons obtain their adult morphology (Sanes et al. 1992; Sanes and Takacs 1993) and electrical properties (Sanes 1993; Kandler and Friauf 1995a; Youssoufian et al. 2005; Lu et al. 2007). During this time, synapses undergo morphological changes (Hoffpauir et al. 2006; Youssoufian et al. 2008; Ford et al. 2009), they are strengthened or eliminated (Sanes 1993; Kotak and Sanes 1995; Taschenberger and von Gersdorff 2000; Brenowitz and Trussell 2001; Iwasaki and Takahashi 2001; Kim and Kandler 2003; Awatramani et al. 2005; Youssoufian et al. 2005; Hoffpauir et al. 2006; Lu et al. 2007), and undergo spatial refinement to achieve precise tonotopic connections (Sanes et al. 1992; Sanes and Takacs 1993; Gabriele et al. 2000a; Leake et al. 2002; Kim and Kandler 2003). Throughout this time of synaptic refinement, spontaneous bursts of activity propagate through auditory brainstem circuits, raising the possibility that correlated activity among inputs could help achieve tonotopic segregation (Friauf and Lohmann 1999; Rubel and Fritzsch 2002; Kandler et al. 2009). Unfortunately, little is known about the roles of this activity, in part because of our limited understanding of the cellular and molecular mechanisms that initiate this activity in the cochlea — knowledge that is required to perform targeted disruption of activity patterns in vivo, similarly to what has been achieved in the visual system. Previous studies addressed the role of spontaneous activity by neonatally deafening animals through cochlear ablation, by injecting ototoxic drugs, or by using transgenic or naturally-occurring animal models of deafness. Unfortunately, these approaches do more than simply block burst firing in the cochlea. For example, cochlear ablation and delivery of ototoxic drugs induce degeneration of afferent fibers, depriving neurons in cochlear nuclei of not only electrical input but also crucial trophic support. Also, the level of spontaneous activity during the pre-hearing period has not been examined in vivo in many deafness models, which may not be completely absent (Youssoufian et al. 2008). Furthermore, many studies have delayed their analysis of the consequences of deafferentation until after hearing onset, making it difficult to discriminate changes resulting from the lack of spontaneous activity from those that occur from the loss of sound-evoked activity. Moreover, the mutations that cause deafness in these models may also affect cells in the brainstem (Schug et al. 2006; Noh et al. 2010; Hirtz et al. 2011), and the consequences of deafness can vary between mutations and mouse strains (Kandler et al. 2009). Despite these limitations, these pioneering studies have revealed some possible functions of pre-hearing spontaneous activity.
The most consistent observation is that depriving SGNs of excitatory input, through either cochlear removal, IHC degeneration, or through use of genetic models that exhibit impaired Ca2+-dependent glutamate release from IHCs, leads to apoptotic degeneration of SGNs and neurons in the ventral cochlear nuclei (Hashisaki and Rubel 1989; Mostafapour et al. 2000; Glueckert et al. 2003; Harris and Rubel 2006; Seal et al. 2008; Hirtz et al. 2011). In the CNS, more severe neuronal loss was observed when cochlear input was eliminated during the pre-hearing period, while no degeneration was observed when the cochlea was ablated after the onset of hearing (Hashisaki and Rubel 1989; Tierney et al. 1997; Mostafapour et al. 2000), suggesting that there is a critical period of development, prior to sensory experience, when afferent activity from IHCs supports neuronal survival.
Spontaneous activity that occurs during this time may also influence the physiological properties of central auditory neurons. Cochlear removal before hearing onset impairs the developmental decrease of intracellular Cl− in auditory neurons, possibly through changes in the expression of K+Cl− co-transporter KCC2 (Shibata et al. 2004). This aberrant Cl− homeostasis prevents the shift of GABA/glycinergic responses from depolarizing to hyperpolarizing, and disrupts inhibition of neurons in lateral superior olive (LSO) and IC (Kotak and Sanes 1996; Vale and Sanes 2000, 2002; Vale et al. 2003). Moreover, principal neurons of the MNTB in congenitally deaf dn/dn mouse show enhanced excitability and non-synchronized firing in response to synaptic inputs (Leao et al. 2004a, 2005, 2006a), and lose their gradient in expression of ion channels along the tonotopic axis (Leao et al. 2006b). The firing properties and channel expression of LSO neurons also are altered in congenitally deaf mouse lines (Couchman et al. 2011; Hirtz et al. 2011), providing further indication that lack of peripheral excitatory drive disrupts the physiological maturation of central auditory neurons.
Spontaneous activity during development has also been shown to promote the functional elimination of synapses in brainstem auditory neurons, as well as their maturation. In particular, synaptic strength is enhanced at synapses between auditory nerves and bushy cells, the endbulbs of Held, in the congenitally deaf dn/dn mouse (Oleskevich and Walmsley 2002; Oleskevich et al. 2004; McKay and Oleskevich 2007). Moreover, the fenestration of calyceal synapses between bushy cells and MNTB principal cells (the calyx of Held) is disrupted in neonatal deafened gerbils and dn/dn mice (Youssoufian et al. 2008; Ford et al. 2009), but effects on synaptic strength have not been consistently observed (Oleskevich et al. 2004; Youssoufian et al. 2005; Erazo-Fischer et al. 2007). Abnormalities have also been documented in other synapses within the anteroventral cochlear nucleus, MNTB and LSO (Leao et al. 2004b; Lu et al. 2007; Cao et al. 2008; Clause et al. 2014), indicating that removal of afferent input results in widespread changes in the innervation of auditory neurons.
There is accumulating evidence that spontaneous activity during the pre-hearing period also helps to establish appropriate connections in the auditory pathway. Cochlear ablation in neonatal gerbils leads to ectopic projections from the cochlear nucleus to superior olive and IC (Moore and Kitzes 1985; Kitzes et al. 1995; Russell and Moore 1995). De-afferentation in older animals did not cause abnormal projections, indicating that this effect is similarly specific for pre-hearing spontaneous activity (Russell and Moore 1995). Moreover, the formation and maintenance of the tonotopically arranged afferent innervation patterns from the dorsal nucleus of lateral lemniscus to the IC is disrupted after deafening rats at an early postnatal age (Gabriele et al. 2000b; Franklin et al. 2006, 2008). In addition to these gross disruptions in neuronal projections, several studies have focused on the role of spontaneous activity in the formation and refinement of precise tonotopic maps in the auditory brainstem. Unexpectedly, the tonotopic organizations of brainstem auditory pathways in several congenitally deaf mouse lines appear normal (jerker: Cao et al. 2008; dn/dn: Youssoufian et al. 2008; Vglut3−/−: Noh et al. 2010). However, in neonatally deafened gerbil (Sanes and Takacs 1993) and cat (Leake et al. 2006), the axons of brainstem auditory neurons have more branches and broader arbors, resulting in less precise tonotopic organization. Afferent deprivation also causes degeneration of auditory neurons, so it is possible that these aberrant or broader projections might result from axonal sprouting secondary to neuronal loss, rather than the absence of spontaneous activity. Most recently, mice lacking cholinergic inhibition of IHCs exhibited axonal pruning and tonotopic refinement deficits (Clause et al. 2014); these animals exhibited different burst firing patterns, raising the possibility that the precise temporal structure of activity within bursts, or the timing and duration of bursts, influence the segregation of inputs in auditory nuclei according to their ultimate frequency response.
While most studies have focused on the central auditory pathway, spontaneous activity might also participate in the development of cochlea itself. In particular, it has been shown that the frequency and duration of Ca2+ spikes in IHCs is important for the maturation of Ca2+ efficiency of vesicle fusion at ribbon synapses (Johnson et al. 2013). And while this review has focused on spontaneous electrical activity, there may be many other forms of spontaneous activity, for example, driven by growth factor release, that affect the maturation of different cell types in the cochlea, which has downstream consequences for development of auditory circuits in the brain.
Conclusions
The auditory system establishes initial connections during early development, and is capable of conveying signals from the periphery to central neurons several weeks before normal hearing thresholds are established. During this postnatal, prehearing period in rodents, IHCs undergo periodic depolarizations, triggering bursts of Ca2+ spikes that reliably induce glutamate release onto SGNs. These events induce highly stereotyped bursts of action potentials in SGNs that are transmitted to auditory circuits of the brain, where it may promote neuronal survival, induce their physiological maturation, and refine their connections to establish tonotopically segregated pathways. However, in spite of extensive in vivo and in vitro studies over the past several decades, the roles of spontaneous activity in auditory development remain largely speculative. This lack of understanding is mainly due to our limited knowledge about the molecular mechanisms that initiate this activity in the cochlea and the lack of genetic tools to specifically manipulate this activity in vivo. The cochlear explant preparation has proven to be a powerful in vitro system to reveal the potential molecular targets involved in generating spontaneous activity, but a consensus about the precise sequence of events responsible for IHC activation during the pre-hearing period has not yet emerged. Two models have been proposed to explain the periodic excitation of IHCs necessary to trigger burst activity in SGNs — one that requires periodic inhibition of spontaneously active IHCs, and another which requires periodic excitation of otherwise silent IHCs. Genetic manipulation of distinct pathways accompanied by in vivo assessment of auditory neuron activity patterns will be required to establish which model is correct. Previous studies have revealed that removing activity entirely during this period leads to degeneration of afferent fibers, loss of trophic support, and apoptotic death of peripheral and central auditory neurons. Thus, an ideal manipulation to assess the role of spontaneous activity in the development of brain auditory circuits should selectively disrupt spontaneous activity, but preserve sound-evoked activity after hearing onset. A greater understanding of the mechanisms responsible for generating this activity and its effects on nervous system development may have broad implications for the development of other sensory systems, provide new strategies to restore auditory function after acoustic trauma, and facilitate integration of cochlear implants in hearing impaired patients.
References
- Abe T, Kakehata S, Kitani R, Maruya S, Navaratnam D, Santos-Sacchi J, Shinkawa H (2007) Developmental expression of the outer hair cell motor prestin in the mouse. J Membr Biol 215:49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackman JB, Burbridge TJ, Crair MC (2012) Retinal waves coordinate patterned activity throughout the developing visual system. Nature 490:219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anniko M, Wroblewski R (1986) Ionic environment of cochlear hair cells. Hear Res 22:279–293 [DOI] [PubMed] [Google Scholar]
- Appler JM, Goodrich LV (2011) Connecting the ear to the brain: Molecular mechanisms of auditory circuit assembly. Prog Neurobiol 93:488–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awatramani GB, Turecek R, Trussell LO (2005) Staggered development of GABAergic and glycinergic transmission in the MNTB. J Neurophysiol 93:819–828 [DOI] [PubMed] [Google Scholar]
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB (2000) Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci 20:7672–7681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL (1989) Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol 416:303–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner D, Moser T (2001) The presynaptic function of mouse cochlear inner hair cells during development of hearing. J Neurosci 21:4593–4599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Feller MB (2010) Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci 11:18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher SK, Warren RL (1971) A study of the electrochemistry and osmotic relationships of the cochlear fluids in the neonatal rat at the time of the development of the endocochlear potential. J Physiol 212:739–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A, Striessnig J, Moser T (2003) CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J Neurosci 23:10832–10840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt N, Kuhn S, Munkner S, Braig C, Winter H, Blin N, Vonthein R, Knipper M, Engel J (2007) Thyroid hormone deficiency affects postnatal spiking activity and expression of Ca2+ and K + channels in rodent inner hair cells. J Neurosci 27:3174–3186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz S, Trussell LO (2001) Maturation of synaptic transmission at endbulb synapses of the cochlear nucleus. J Neurosci 21:9487–9498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge JF O′Connor TA (1984) Postnatal functional development of the dorsal and posteroventral cochlear nuclei of the cat. J Acoust Soc Am 75:1548–1562 [DOI] [PubMed] [Google Scholar]
- Cao XJ, McGinley MJ, Oertel D (2008) Connections and synaptic function in the posteroventral cochlear nucleus of deaf jerker mice. J Comp Neurol 510:297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clause A, Kim G, Sonntag M, Weisz CJ, Vetter DE, Rubsamen R, Kandler K (2014) The precise temporal pattern of prehearing spontaneous activity is necessary for tonotopic map refinement. Neuron 82:822–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S (2002) The developing synapse: construction and modulation of synaptic structures and circuits. Science 298:770–776 [DOI] [PubMed] [Google Scholar]
- Couchman K, Garrett A, Deardorff AS, Rattay F, Resatz S, Fyffe R, Walmsley B, Leao RN (2011) Lateral superior olive function in congenital deafness. Hear Res 277:163–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crins TT, Rusu SI, Rodriguez-Contreras A, Borst JG (2011) Developmental changes in short-term plasticity at the rat calyx of held synapse. J Neurosci 31:11706–11717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G (1983) Development of hearing and response behavior to sound stimuli: behavioral studies In: Romand R (ed) Development of auditory and vestibular systems. Academic, New York, pp 211–237 [Google Scholar]
- Erazo-Fischer E, Striessnig J, Taschenberger H (2007) The role of physiological afferent nerve activity during in vivo maturation of the calyx of held synapse. J Neurosci 27:1725–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller MB (1999) Spontaneous correlated activity in developing neural circuits. Neuron 22:653–656 [DOI] [PubMed] [Google Scholar]
- Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ (1996) Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science 272:1182–1187 [DOI] [PubMed] [Google Scholar]
- Ford MC, Grothe B, Klug A (2009) Fenestration of the calyx of Held occurs sequentially along the tonotopic axis, is influenced by afferent activity, and facilitates glutamate clearance. J Comp Neurol 514:92–106 [DOI] [PubMed] [Google Scholar]
- Franklin SR, Brunso-Bechtold JK, Henkel CK (2006) Unilateral cochlear ablation before hearing onset disrupts the maintenance of dorsal nucleus of the lateral lemniscus projection patterns in the rat inferior colliculus. Neuroscience 143:105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin SR, Brunso-Bechtold JK, Henkel CK (2008) Bilateral cochlear ablation in postnatal rat disrupts development of banded pattern of projections from the dorsal nucleus of the lateral lemniscus to the inferior colliculus. Neuroscience 154:346–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friauf E, Kandler K (1990) Auditory projections to the inferior colliculus of the rat are present by birth. Neurosci Lett 120:58–61 [DOI] [PubMed] [Google Scholar]
- Friauf E, Lohmann C (1999) Development of auditory brainstem circuitry. Activity-dependent and activity-independent processes. Cell Tissue Res 297:187–195 [DOI] [PubMed] [Google Scholar]
- Gabriele ML, Brunso-Bechtold JK, Henkel CK (2000a) Development of afferent patterns in the inferior colliculus of the rat: projection from the dorsal nucleus of the lateral lemniscus. J Comp Neurol 416:368–382 [DOI] [PubMed] [Google Scholar]
- Gabriele ML, Brunso-Bechtold JK, Henkel CK (2000b) Plasticity in the development of afferent patterns in the inferior colliculus of the rat after unilateral cochlear ablation. J Neurosci 20:6939–6949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli L, Maffei L (1988) Spontaneous impulse activity of rat retinal ganglion cells in prenatal life. Science 242:90–91 [DOI] [PubMed] [Google Scholar]
- Garaschuk O, Hanse E, Konnerth A (1998) Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J Physiol 507(Pt 1):219–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geal-Dor M, Freeman S, Li G, Sohmer H (1993) Development of hearing in neonatal rats: air and bone conducted ABR thresholds. Hear Res 69:236–242 [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA (2000) Cholinergic synaptic inhibition of inner hair cells in the neonatal mammalian cochlea. Science 288:2366–2368 [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA (2002) Transmitter release at the hair cell ribbon synapse. Nat Neurosci 5:147–154 [DOI] [PubMed] [Google Scholar]
- Glueckert R, Wietzorrek G, Kammen-Jolly K, Scholtz A, Stephan K, Striessnig J, Schrott-Fischer A (2003) Role of class D L-type Ca2+ channels for cochlear morphology. Hear Res 178:95–105 [DOI] [PubMed] [Google Scholar]
- Goutman JD, Fuchs PA, Glowatzki E (2005) Facilitating efferent inhibition of inner hair cells in the cochlea of the neonatal rat. J Physiol 566:49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Rossi FM, Changeux JP, Thompson ID (2003) Abnormal functional organization in the dorsal lateral geniculate nucleus of mice lacking the beta 2 subunit of the nicotinic acetylcholine receptor. Neuron 40:1161–1172 [DOI] [PubMed] [Google Scholar]
- Gummer AW, Mark RF (1994) Patterned neural activity in brain stem auditory areas of a prehearing mammal, the tammar wallaby (Macropus eugenii). Neuroreport 5:685–688 [DOI] [PubMed] [Google Scholar]
- Harris JA, Rubel EW (2006) Afferent regulation of neuron number in the cochlear nucleus: cellular and molecular analyses of a critical period. Hear Res 216–217:127–137 [DOI] [PubMed] [Google Scholar]
- Hashisaki GT, Rubel EW (1989) Effects of unilateral cochlea removal on anteroventral cochlear nucleus neurons in developing gerbils. J Comp Neurol 283:5–73 [DOI] [PubMed] [Google Scholar]
- Hinojosa R (1977) A note on development of Corti′s organ. Acta Otolaryngol 84:238–251 [DOI] [PubMed] [Google Scholar]
- Hirtz JJ, Boesen M, Braun N, Deitmer JW, Kramer F, Lohr C, Muller B, Nothwang HG, Striessnig J, Lohrke S, Friauf E (2011) Cav1.3 calcium channels are required for normal development of the auditory brainstem. J Neurosci 31:8280–8294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffpauir BK, Grimes JL, Mathers PH, Spirou GA (2006) Synaptogenesis of the calyx of Held: rapid onset of function and one-to-one morphological innervation. J Neurosci 26:5511–5523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffpauir BK, Marrs GS, Mathers PH, Spirou GA (2009) Does the brain connect before the periphery can direct? A comparison of three sensory systems in mice. Brain Res 1277:115–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LC, Thorne PR, Housley GD, Montgomery JM (2007) Spatiotemporal definition of neurite outgrowth, refinement and retraction in the developing mouse cochlea. Development 134:2925–2933 [DOI] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B (2008) Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci 31:479–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Takahashi T (2001) Developmental regulation of transmitter release at the calyx of Held in rat auditory brainstem. J Physiol 534: 861–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Eckrich T, Kuhn S, Zampini V, Franz C, Ranatunga KM, Roberts TP, Masetto S, Knipper M, Kros CJ, Marcotti W (2011) Position-dependent patterning of spontaneous action potentials in immature cochlear inner hair cells. Nat Neurosci 14:711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Kuhn S, Franz C, Ingham N, Furness DN, Knipper M, Steel KP, Adelman JP, Holley MC, Marcotti W (2013) Presynaptic maturation in auditory hair cells requires a critical period of sensory-independent spiking activity. Proc Natl Acad Sci U S A 110:8720–8725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Marcotti W, Kros CJ (2005) Increase in efficiency and reduction in Ca2+ dependence of exocytosis during development of mouse inner hair cells. J Physiol 563:177–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Jones SM (2000) Spontaneous activity in the statoacoustic ganglion of the chicken embryo. J Neurophysiol 83:1452–1468 [DOI] [PubMed] [Google Scholar]
- Jones TA, Jones SM, Paggett KC (2001) Primordial rhythmic bursting in embryonic cochlear ganglion cells. J Neurosci 21:8129–8135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Jones SM, Paggett KC (2006) Emergence of hearing in the chicken embryo. J Neurophysiol 96:128–141 [DOI] [PubMed] [Google Scholar]
- Jones TA, Leake PA, Snyder RL, Stakhovskaya O, Bonham B (2007) Spontaneous discharge patterns in cochlear spiral ganglion cells before the onset of hearing in cats. J Neurophysiol 98:1898–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Clause A, Noh J (2009) Tonotopic reorganization of developing auditory brainstem circuits. Nat Neurosci 12:711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Friauf E (1995a) Development of electrical membrane properties and discharge characteristics of superior olivary complex neurons in fetal and postnatal rats. Eur J Neurosci 7:1773–1790 [DOI] [PubMed] [Google Scholar]
- Kandler K, Friauf E (1995b) Development of glycinergic and glutamatergic synaptic transmission in the auditory brainstem of perinatal rats. J Neurosci 15:6890–6904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E, Elgoyhen AB, Gomez-Casati ME, Knipper M, Vetter DE, Fuchs PA, Glowatzki E (2004) Developmental regulation of nicotinic synapses on cochlear inner hair cells. J Neurosci 24:7814–7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ (1996) Synaptic activity and the construction of cortical circuits. Science 274:1133–1138 [DOI] [PubMed] [Google Scholar]
- Kelley MW (2007) Cellular commitment and differentiation in the organ of Corti. Int J Dev Biol 51:571–583 [DOI] [PubMed] [Google Scholar]
- Kennedy HJ (2012) New developments in understanding the mechanisms and function of spontaneous electrical activity in the developing mammalian auditory system. J Assoc Res Otolaryngol 13:437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang N (1965) Discharge patterns of single fibers in the cat’s auditory nerve. M.I.T. Press, Cambridge, MA [Google Scholar]
- Kim G, Kandler K (2003) Elimination and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat Neurosci 6:282–290 [DOI] [PubMed] [Google Scholar]
- Kirkby LA, Sack GS, Firl A, Feller MB (2013) A role for correlated spontaneous activity in the assembly of neural circuits. Neuron 80: 1129–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzes LM, Kageyama GH, Semple MN, Kil J (1995) Development of ectopic projections from the ventral cochlear nucleus to the superior olivary complex induced by neonatal ablation of the contralateral cochlea. J Comp Neurol 353:341–363 [DOI] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Tolnai S, Malmierca MS, Rubsamen R (2008) The medial nucleus of the trapezoid body: comparative physiology. Neuroscience 154:160–170 [DOI] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH (1995) Synaptically evoked prolonged depolarizations in the developing auditory system. J Neurophysiol 74:1611–1620 [DOI] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH (1996) Developmental influence of glycinergic transmission: regulation of NMDA receptor-mediated EPSPs. J Neurosci 16:1836–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koundakjian EJ, Appler JL, Goodrich LV (2007) Auditory neurons make stereotyped wiring decisions before maturation of their targets. J Neurosci 27:14078–14088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kros CJ (2007) How to build an inner hair cell: challenges for regeneration. Hear Res 227:3–10 [DOI] [PubMed] [Google Scholar]
- Kros CJ, Ruppersberg JP, Rusch A (1998) Expression of a potassium current in inner hair cells during development of hearing in mice. Nature 394:281–284 [DOI] [PubMed] [Google Scholar]
- Landmesser LT O′Donovan MJ (1984) Activation patterns of embryonic chick hind limb muscles recorded in ovo and in an isolated spinal cord preparation. J Physiol 347:189–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Chair L, Snyder RL (2006) Neonatal deafness results in degraded topographic specificity of auditory nerve projections to the cochlear nucleus in cats. J Comp Neurol 497:13–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT (2002) Postnatal refinement of auditory nerve projections tothe cochlear nucleus in cats. J Comp Neurol 448:6–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao RN, Berntson A, Forsythe ID, Walmsley B (2004a) Reduced low-voltage activated K+ conductances and enhanced central excitability in a congenitally deaf (dn/dn) mouse. J Physiol 559:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao RN, Naves MM, Leao KE, Walmsley B (2006a) Altered sodium currents in auditory neurons of congenitally deaf mice. Eur J Neurosci 24:1137–1146 [DOI] [PubMed] [Google Scholar]
- Leao RN, Oleskevich S, Sun H, Bautista M, Fyffe RE, Walmsley B (2004b) Differences in glycinergic mIPSCs in the auditory brain stem of normal and congenitally deaf neonatal mice. J Neurophysiol 91:1006–1012 [DOI] [PubMed] [Google Scholar]
- Leao RN, Sun H, Svahn K, Berntson A, Youssoufian M, Paolini AG, Fyffe RE, Walmsley B (2006b) Topographic organization in the auditory brainstem of juvenile mice is disrupted in congenital deafness. J Physiol 571:563–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao RN, Svahn K, Berntson A, Walmsley B (2005) Hyperpolarization-activated (I) currents in auditory brainstem neurons of normal and congenitally deaf mice. Eur J Neurosci 22:147–157 [DOI] [PubMed] [Google Scholar]
- Lelli A, Asai Y, Forge A, Holt JR, Geleoc GS (2009) Tonotopic gradient in the developmental acquisition of sensory transduction in outer hair cells of the mouse cochlea. J Neurophysiol 101: 2961–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC (1978) Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am 63:442–455 [DOI] [PubMed] [Google Scholar]
- Lippe WR (1994) Rhythmic spontaneous activity in the developing avian auditory system. J Neurosci 14:1486–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Harris JA, Rubel EW (2007) Development of spontaneous miniature EPSCs in mouse AVCN neurons during a critical period of afferent-dependent neuron survival. J Neurophysiol 97:635–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Brumm D, Ryan AF (1995) Distribution of non-NMDA glutamate receptor mRNAs in the developing rat cochlea. J Comp Neurol 361: 372–382 [DOI] [PubMed] [Google Scholar]
- Manley GA, Kaiser A, Brix J, Gleich O (1991) Activity patterns of primary auditory-nerve fibres in chickens: development of fundamental properties. Hear Res 57:1–15 [DOI] [PubMed] [Google Scholar]
- Manley GA, Robertson D (1976) Analysis of spontaneous activity of auditory neurones in the spiral ganglion of the guinea-pig cochlea. J Physiol 258:323–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Holley MC, Kros CJ (2003a) Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J Physiol 548:383–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Kros CJ (2004) A transiently expressed SK current sustains and modulates action potential activity in immature mouse inner hair cells. J Physiol 560:691–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Rusch A, Kros CJ (2003b) Sodium and calcium currents shapeaction potentials in immature mouse inner hair cells. J Physiol 552:743–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay SM, Oleskevich S (2007) The role of spontaneous activity in development of the endbulb of Held synapse. Hear Res 230:53–63 [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O′Leary DD(2003) Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron 40:1147–1160 [DOI] [PubMed] [Google Scholar]
- Meister M, Wong RO, Baylor DA, Shatz CJ (1991) Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science 252:939–943 [DOI] [PubMed] [Google Scholar]
- Mikaelian D, Ruben RJ (1965) Development of hearing in the normal Cba-J mouse: correlation of physiological observations with behavioral responses and with cochlear anatomy. Acta Otolaryngol 59: 451–461 [DOI] [PubMed] [Google Scholar]
- Moody WJ, Bosma MM (2005) Ion channel development, spontaneous activity, and activity-dependent development in nerve and muscle cells. Physiol Rev 85:883–941 [DOI] [PubMed] [Google Scholar]
- Moore DR, Kitzes LM (1985) Projections from the cochlear nucleus to the inferior colliculus in normal and neonatally cochlea-ablated gerbils. J Comp Neurol 240:180–195 [DOI] [PubMed] [Google Scholar]
- Mostafapour SP, Cochran SL, Del Puerto NM, Rubel EW (2000) Patterns of cell death in mouse anteroventral cochlear nucleus neurons after unilateral cochlea removal. J Comp Neurol 426:561–571 [DOI] [PubMed] [Google Scholar]
- Muir-Robinson G, Hwang BJ, Feller MB (2002) Retinogeniculate axons undergo eye-specific segregation in the absence of eye-specific layers. J Neurosci 22:5259–5264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh J, Seal RP, Garver JA, Edwards RH, Kandler K (2010) Glutamate co-release at GABA/glycinergic synapses is crucial for the refinement of an inhibitory map. Nat Neurosci 13:232–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O′Donovan MJ(1999) The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr Opin Neurobiol 9: 94–104 [DOI] [PubMed] [Google Scholar]
- O′Keeffe MG, Thorne PR, Housley GD, Robson SC, Vlajkovic SM(2010) Developmentally regulated expression of ectonucleotidases NTPDase5 and NTPDase6 and UDP-responsive P2Y receptors in the rat cochlea. Histochem Cell Biol 133:425–436 [DOI] [PubMed] [Google Scholar]
- Oleskevich S, Walmsley B (2002) Synaptic transmission in the auditory brainstem of normal and congenitally deaf mice.J Physiol 540:447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleskevich S, Youssoufian M, Walmsley B (2004) Presynaptic plasticity at two giant auditory synapses in normal and deaf mice. J Physiol 560: 709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn AA, Riquelme PA, Feller MB, Shatz CJ (1998) Competition in retinogeniculate patterning driven by spontaneous activity. Science 279:2108–2112 [DOI] [PubMed] [Google Scholar]
- Pfeiffenberger C, Yamada J, Feldheim DA (2006) Ephrin-As and patterned retinal activity act together in the development of topographic maps in the primary visual system. J Neurosci 26:12873–12884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J (2000) Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 102:89–97 [DOI] [PubMed] [Google Scholar]
- Pujol R, Lavigne-Rebillard M, Lenoir M (1998) Development of sensory and neural structures in the mammalian cochlea In: Rubel EW, Popper AN, Fay RR (eds) Development of the auditory system. Springer handbook of auditory research. Springer, New York, pp 146–192 [Google Scholar]
- Puyal J, Sage C, Dememes D, Dechesne CJ (2002) Distribution of alpha-amino-3-hydroxy-5-methyl-4 isoazolepropionic acid and N-methyl-D-aspartate receptor subunits in the vestibular and spiral ganglia of the mouse during early development. Brain Res Dev Brain Res 139:51–57 [DOI] [PubMed] [Google Scholar]
- Robertson D, Paki B (2002) Role of L-type Ca2+ channels in transmitter release from mammalian inner hair cells. II. Single-neuron activity. J Neurophysiol 87:2734–2740 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Contreras A, van Hoeve JS, Habets RL, Locher H, Borst JG (2008) Dynamic development of the calyx of Held synapse. Proc Natl Acad Sci U S A 105:5603–5608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romand R (1984) Functional properties of auditory-nerve fibers during postnatal development in the kitten. Exp Brain Res 56:395–402 [DOI] [PubMed] [Google Scholar]
- Romand R, Marty R (1975) Postnatal maturation of the cochlear nuclei in the cat: a neurophysiological study. Brain Res 83:225–233 [DOI] [PubMed] [Google Scholar]
- Roux I, Wersinger E, McIntosh JM, Fuchs PA, Glowatzki E (2011) Onset of cholinergic efferent synaptic function in sensory hair cells of the rat cochlea. J Neurosci 31:15092–15101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B (2002) Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci 25:51–101 [DOI] [PubMed] [Google Scholar]
- Rubsamen R, Schafer M (1990) Ontogenesis of auditory fovea representation in the inferior colliculus of the Sri Lankan rufous horseshoe bat, Rhinolophus rouxi. J Comp Physiol A 167:757–769 [DOI] [PubMed] [Google Scholar]
- Russell FA, Moore DR (1995) Afferent reorganisation within the superior olivary complex of the gerbil: development and induction by neonatal, unilateral cochlear removal. J Comp Neurol 352:607–625 [DOI] [PubMed] [Google Scholar]
- Rusu SI, Borst JG (2011) Developmental changes in intrinsic excitability of principal neurons in the rat medial nucleus of the trapezoid body. Prospect Dev Neurobiol 71:284–295 [DOI] [PubMed] [Google Scholar]
- Rybak LP, Whitworth C, Scott V (1992) Development of endocochlear potential and compound action potential in the rat. Hear Res 59: 189–194 [DOI] [PubMed] [Google Scholar]
- Sanes DH (1993) The development of synaptic function and integration in the central auditory system. J Neurosci 13:2627–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH, Song J, Tyson J (1992) Refinement of dendritic arbors along the tonotopic axis of the gerbil lateral superior olive. Brain Res Dev Brain Res 67:47–55 [DOI] [PubMed] [Google Scholar]
- Sanes DH, Takacs C (1993) Activity-dependent refinement of inhibitory connections. Eur J Neurosci 5:570–574 [DOI] [PubMed] [Google Scholar]
- Schug N, Braig C, Zimmermann U, Engel J, Winter H, Ruth P, Blin N, Pfister M, Kalbacher H, Knipper M (2006) Differential expression of otoferlin in brain, vestibular system, immature and mature cochlea of the rat. Eur J Neurosci 24:3372–3380 [DOI] [PubMed] [Google Scholar]
- Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Clause A, Kandler K, Noebels JL, Glowatzki E, Lustig LR, Edwards RH (2008) Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron 57:263–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendin G, Bourien J, Rassendren F, Puel JL, Nouvian R (2014) Spatiotemporal pattern of action potential firing in developing inner hair cells of the mouse cochlea. Proc Natl Acad Sci U S A 111:1999–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Kakazu Y, Okabe A, Fukuda A, Nabekura J (2004) Experience-dependent changes in intracellular Cl- regulation in developing auditory neurons. Neurosci Res 48:211–220 [DOI] [PubMed] [Google Scholar]
- Shnerson A, Willott JF (1979) Development of inferior colliculus response properties in C57BL/6 J mouse pups. Exp Brain Res 37:373–385 [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Rose JE, Scott GE, Slapnick SM (1982) Ribbon synapses in the developing intact and cultured organ of Corti in the mouse. J Neurosci 2:942–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag M, Englitz B, Kopp-Scheinpflug C, Rubsamen R (2009) Early postnatal development of spontaneous and acoustically evoked discharge activity of principal cells of the medial nucleus of the trapezoid body: an in vivo study in mice. J Neurosci 29:9510–9520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC (2006) Electrical activity in early neuronal development. Nature 444:707–712 [DOI] [PubMed] [Google Scholar]
- Taberner AM, Liberman MC (2005) Response properties of single auditory nerve fibers in the mouse. J Neurophysiol 93:557–569 [DOI] [PubMed] [Google Scholar]
- Taschenberger H, von Gersdorff H (2000) Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci 20: 9162–9173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney TS, Russell FA, Moore DR (1997) Susceptibility of developing cochlear nucleus neurons to deafferentation-induced death abruptly ends just before the onset of hearing. J Comp Neurol 378:295–306 [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Bergles DE (2010) Developmental regulation of spontaneous activity in the Mammalian cochlea. J Neurosci 30:1539–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Rodriguez-Contreras A, Crins TT, Wang HC, Borst JG, Bergles DE (2010a) Calcium action potentials in hair cells pattern auditory neuron activity before hearing onset. Nat Neurosci 13: 1050–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE (2007) The origin of spontaneous activity in the developing auditory system. Nature 450: 50–55 [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Zhang YX, Ellis-Davies G, Bergles DE (2010b) ATP-induced morphological changes in supporting cells of the developing cochlea. Purinergic Signal 6:155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziel A, Romand R, Marot M (1981) Development of cochlear potentials in rats. Audiology 20:89–100 [DOI] [PubMed] [Google Scholar]
- Vale C, Sanes DH (2000) Afferent regulation of inhibitory synaptic transmission in the developing auditory midbrain. J Neurosci 20: 1912–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale C, Sanes DH (2002) The effect of bilateral deafness on excitatory and inhibitory synaptic strength in the inferior colliculus. Eur J Neurosci 16:2394–2404 [DOI] [PubMed] [Google Scholar]
- Vale C, Schoorlemmer J, Sanes DH (2003) Deafness disrupts chloride transporter function and inhibitory synaptic transmission. J Neurosci 23:7516–7524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter DE, Liberman MC, Mann J, Barhanin J, Boulter J, Brown MC, Saffiote-Kolman J, Heinemann SF, Elgoyhen AB (1999) Role of alpha9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron 23:93–103 [DOI] [PubMed] [Google Scholar]
- Walsh EJ, McGee J (1987) Postnatal development of auditory nerve and cochlear nucleus neuronal responses in kittens. Hear Res 28:97–116 [DOI] [PubMed] [Google Scholar]
- Wangemann P, Schacht J (1996) Homeostatic Mechanisms in the Cochlea In: Dallos P, Popper AN, Fay RR (eds) The cochlea. Springer handbook of auditory research. Springer, New York, pp 130–185 [Google Scholar]
- Watt AJ, Cuntz H, Mori M, Nusser Z, Sjostrom PJ, Hausser M (2009) Traveling waves in developing cerebellar cortex mediated by asymmetrical Purkinje cell connectivity. Nat Neurosci 12:463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RO, Ghosh A (2002) Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci 3:803–812 [DOI] [PubMed] [Google Scholar]
- Woolf NK, Ryan AF (1984) The development of auditory function in the cochlea of the Mongolian gerbil. Hear Res 13:277–283 [DOI] [PubMed] [Google Scholar]
- Woolf NK, Ryan AF (1988) Contributions of the middle ear to the development of function in the cochlea. Hear Res 35:131–142 [DOI] [PubMed] [Google Scholar]
- Youssoufian M, Couchman K, Shivdasani MN, Paolini AG, Walmsley B (2008) Maturation of auditory brainstem projections and calyces in the congenitally deaf (dn/dn) mouse. J Comp Neurol 506:442–451 [DOI] [PubMed] [Google Scholar]
- Youssoufian M, Oleskevich S, Walmsley B (2005) Development of a robust central auditory synapse in congenital deafness. J Neurophysiol 94:3168–3180 [DOI] [PubMed] [Google Scholar]
- Zhang LI, Poo MM (2001) Electrical activity and development of neural circuits. Nat Neurosci 4(Suppl):1207–1214 [DOI] [PubMed] [Google Scholar]


