Neuroinflammation is a complex immune response to brain trauma and disease (Filipov, 2019). Over the past 2 decades, researchers have determined that microglia are one of the key initiators of inflammatory reactions in neurodegenerative diseases, such as Alzheimer's disease (AD; Filipov, 2019). Findings from early animal studies first identified the presence of microglia-mediated inflammation in mouse models of AD (Benzing et al., 1999; Yoshiyama et al., 2007). These results motivated the development of in vivo positron emission tomography (PET) radiotracers, such as [11C] PK 11195, to quantify microglia-dependent neuroinflammatory reactions in humans with and without AD pathology (Cagnin et al., 2007). The [11C] PK 11195 PET tracer selectively binds to the mitochondrial translocator protein (TSPO), which is thought to be upregulated during microglial activation (Passamonti et al., 2018). Clinical research efforts have since shown that the binding of [11C] PK 11195 is significantly higher in distinct areas of the brain in people with AD than in age-matched neurotypical individuals, suggesting that there are region-specific pathological factors driving the microglial inflammatory response (Passamonti et al., 2018). Overall, while these findings propose that microglia-induced inflammation is closely tied to AD pathology, they do not establish a direct link between inflammation and changes in brain function.
In a recent article, Passamonti et al. (2019) performed a multimodal and multivariate neuroimaging study to determine (1) whether there is an association between widespread neuroinflammation and changes in functional connectivity across brain networks in people with AD compared with healthy individuals; and (2) whether the link between these two measures is related to the degree of cognitive impairment present in these groups. To evaluate these questions, the researchers quantified neuroinflammation using the [11C] PK 11195 tracer in a cross-sectional cohort that included patients with AD or mild cognitive impairment (MCI+), as well as in age-, sex-, and education-matched healthy control subjects. The same subjects also underwent resting-state functional magnetic resonance imaging to characterize baseline functional connectivity at the network level (Shirer et al., 2012). In addition, subjects were assessed on three cognitive tests to establish their relative cognitive index.
Using these measures, the researchers examined their first question: does microglia-mediated inflammation measured with [11C] PK 11195 relate to the resting-state functional connectivity of different brain networks? Consistent with their predictions, increased [11C] PK 11195 binding in the inferior and medial temporal areas was significantly related to increased internetwork functional connectivity among the default mode network (DMN), the hippocampus, and other subcortical areas, as well as decreased intranetwork functional connectivity within the main brain areas involved in the DMN (Passamonti et al., 2019).
Next, the authors tested whether the associations between neuroinflammation and network connectivity were linked to cognitive performance in each group. As predicted, the joint connection between neuroinflammation and network-level functional connectivity was negatively associated with performance on the cognitive tests in the patient group but not in control subjects. These results suggest that people in the AD/MCI+ sample that had a stronger association between increased neuroinflammation and differences in functional connectivity also had overall worse performance on the three measures of cognitive capacity (Passamonti et al., 2019).
The multivariate analytical strategy used by Passamonti et al. (2019) allowed them to investigate the relationship among neuroinflammation, functional connectivity, cognitive performance, and AD at the network level. Most research in humans to date has focused on either the molecular and structural basis of AD or its functional and cognitive components separately. However, AD is a complex multifactor disease affecting all of these variables. The results of the research by Passamonti et al. (2019) are powerful in their ability to explain the relationships among different characteristics of AD.
It is important to note that the rationale for investigating the influence of neuroinflammation on brain network function and cognition (Chen et al., 2016) arises from the need to better understand the role microglia play in the development and progression of AD (Bachiller et al., 2018). Passamonti et al. (2019) posited that because microglia activity underlies neuroinflammation, microglia must play a role in the abnormal network function and clinically described cognitive deficits seen in people with AD (Passamonti et al., 2019). However, several influential studies have shown that the functional role of microglia change dynamically with aging and AD progression according to the genes they express (Keren-Shaul et al., 2017; Liddelow et al., 2017; Clarke et al., 2018).
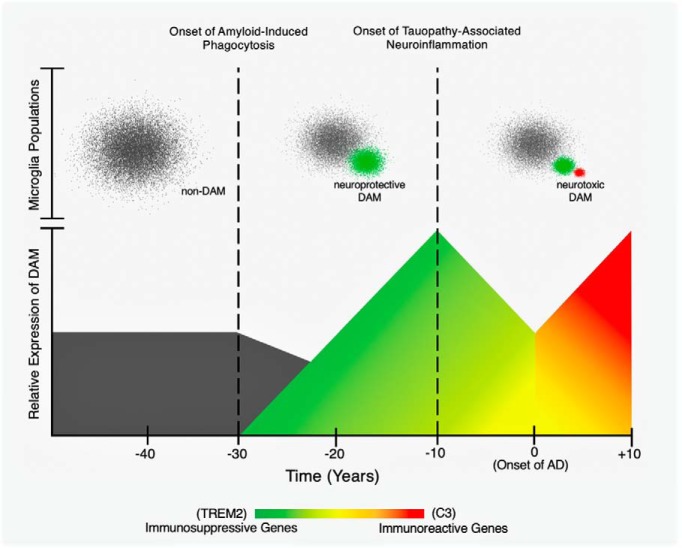
We can understand this idea by considering what one may call the “two faces” of disease-associated microglia. The first face is immunosuppressive and involves the expression of triggering receptor expressed on myeloid cells 2 (TREM2), a transmembrane receptor protein that controls microglial activity and survival (Keren-Shaul et al., 2017). While some genetic variants of TREM2 increase the chances of developing late-onset AD by twofold to fourfold, the primary function of TREM2 before or during the preliminary period of AD is to phagocytose amyloid peptides, which it does without triggering evident inflammation (Keren-Shaul et al., 2017; Gratuze et al., 2018). Thus, increased levels of TREM2 during this phase of brain aging is seen as a defensive or neuroprotective factor linked to amyloid clearance (Keren-Shaul et al., 2017; Parhizkar et al., 2019). Yet as AD progresses, it appears that the role of TREM2 evolves as well. In more advanced stages of the disease, TREM2-expressing microglia interact with accumulating tau tangles, and this interaction has been shown to cause extensive inflammation and neurodegeneration (Bemiller et al., 2017; Leyns et al., 2017). Moreover, the later phases of AD are associated with heightened microglial activation as a result of the innate immune defense system of the brain. Complement C3 is among the most highly upregulated genes involved in this immune reaction (Clarke et al., 2018). C3-expressing microglia are responsible for inducing excessive release of proinflammatory molecules and the proliferation of neurotoxic A1 reactive astrocytes (Liddelow et al., 2017). Thus, the C3 “face” of microglia is understood to be more harmful than helpful. This has been confirmed by studies using C3-knock-out mice that no longer seem to exhibit the same inflammatory or degenerative outcomes that occur in mouse models of amyloid and tau when C3 is present (Shi et al., 2017; Wu et al., 2019).
Overall, it is apparent that microglia can either assist the clearance of age-related amyloid accumulation or can promote extensive inflammation in reaction to amyloid or tau, eventually causing widespread neurodegeneration. Importantly, this difference might be driven by the temporal stage of the disease. In earlier periods of AD, microglia can be neuroprotective; but as either amyloid or tau pathology evolves, they can become neurotoxic (Fig. 1). Passamonti et al. (2019) most likely examined microglia in the latter state, given that their cohort consisted of MCI and AD patients. Therefore, the microglia in these individuals may have already lost their protective function and shifted toward their proinflammatory state. Because of this, these findings should be complemented by longitudinal studies using a similar multimodal approach in cognitively normal older adults at genetic risk for AD. This would allow researchers to track the possible “changing face” of microglia within the same individuals from preclinical to prodromal disease stages. With a more refined understanding of what mechanisms determine whether microglial activity has a neuroprotective or neurotoxic role in brain aging, researchers can begin to fully describe the dual role of microglia in the progression of AD.
Figure 1.
A schematic showing a hypothetical dual role for disease-associated microglia (DAM) in the progression of Alzheimer's disease (AD). The x-axis corresponds to time in years. Time 0 indicates the onset of symptomatic AD (i.e., mild cognitive impairment). The bottom panel depicts changes in the relative expression of genes upregulated and downregulated by DAM. These changes are associated with the transition from immunosuppressive phagocytosis to immunoreactive inflammation (Saito and Saido, 2018). The bottom y-axis represents the relative expression of immunosuppressive and immunoreactive genes regulated by DAM (e.g., TREM2 and C3). The top panel portrays non-DAM and DAM that are clustered according to their distinct transcriptional profiles (Keren-Shaul et al., 2017). The top y-axis outlines the functionally different populations of microglia (e.g., non-DAM, neuroprotective DAM, and neurotoxic DAM).
In conclusion, while the study by Passamonti et al. (2019) supports the development of therapeutic interventions targeted at regulating microglia activity, the efficacy of such approaches might be limited by what stage of the disease the patient is in. To prevent abnormal network activity and, as a result, to alleviate cognitive deficits, reducing neuroinflammation by decreasing microglia activation could be a potential mechanism. However, the differential expression of neuroprotective or neurotoxic signals by microglia in the aging brain poses a major problem to this proposal: it would be extremely detrimental to inhibit neuroprotective microglia activity in preclinical AD patients. Doing so could potentially set the stage for uncontrolled amyloid and tau pathology due to inhibited phagocytosis and possible disruption of other important but as of yet unknown functions of the immune system of the brain. Therefore, techniques that not only discriminate between the different types of microglia but also identify the factors controlling such varied expression may be a more reasonable therapeutic approach.
Footnotes
Editor's Note: These short reviews of recent JNeurosci articles, written exclusively by students or postdoctoral fellows, summarize the important findings of the paper and provide additional insight and commentary. If the authors of the highlighted article have written a response to the Journal Club, the response can be found by viewing the Journal Club at www.jneurosci.org. For more information on the format, review process, and purpose of Journal Club articles, please see https://www.jneurosci.org/content/jneurosci-journal-club.
The author declares no competing financial interests.
References
- Bachiller S, Jiménez-Ferrer I, Paulus A, Yang Y, Swanberg M, Deierborg T, Boza-Serrano A (2018) Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front Cell Neurosci 12:488. 10.3389/fncel.2018.00488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemiller SM, McCray TJ, Allan K, Formica SV, Xu G, Wilson G, Kokiko-Cochran ON, Crish SD, Lasagna-Reeves CA, Ransohoff RM, Landreth GE, Lamb BT (2017) TREM2 deficiency exacerbates tau pathology through dysregulated kinase signaling in a mouse model of tauopathy. Mol Neurodegener 12:74. 10.1186/s13024-017-0216-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzing WC, Wujek JR, Ward EK, Shaffer D, Ashe KH, Younkin SG, Brunden KR (1999) Evidence for glial-mediated inflammation in aged APPSW transgenic mice. Neurobiol Aging 20:581–589. 10.1016/S0197-4580(99)00065-2 [DOI] [PubMed] [Google Scholar]
- Cagnin A, Kassiou M, Meikle SR, Banati RB (2007) Positron emission tomography imaging of neuroinflammation. Neurotherapeutics 4:443–452. 10.1016/j.nurt.2007.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WW, Zhang X, Huang WJ (2016) Role of neuroinflammation in neurodegenerative diseases (review). Mol Med Rep 13:3391–3396. 10.3892/mmr.2016.4948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, Liddelow SA, Chakraborty C, Münch AE, Heiman M, Barres BA (2018) Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A 115:E1896–E1905. 10.1073/pnas.1800165115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipov NM. (2019) Overview of peripheral and central inflammatory responses and their contribution to neurotoxicity. Adv Neurotoxicol 3:169–193. 10.1016/bs.ant.2018.10.001 [DOI] [Google Scholar]
- Gratuze M, Leyns CEG, Holtzman DM (2018) New insights into the role of TREM2 in Alzheimer's disease. Mol Neurodegener 13:66. 10.1186/s13024-018-0298-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I (2017) A unique microglia type associated with restricting development of Alzheimer's disease. Cell 169:1276–1290.e17. 10.1016/j.cell.2017.05.018 [DOI] [PubMed] [Google Scholar]
- Leyns CEG, Ulrich JD, Finn MB, Stewart FR, Koscal LJ, Remolina Serrano J, Robinson GO, Anderson E, Colonna M, Holtzman DM (2017) TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc Natl Acad Sci U S A 114:11524–11529. 10.1073/pnas.1710311114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parhizkar S, Arzberger T, Brendel M, Kleinberger G, Deussing M, Focke C, Nuscher B, Xiong M, Ghasemigharagoz A, Katzmarski N, Krasemann S, Lichtenthaler SF, Müller SA, Colombo A, Monasor LS, Tahirovic S, Herms J, Willem M, Pettkus N, Butovsky O, et al. (2019) Loss of TREM2 function increases amyloid seeding but reduces plaque associated ApoE. Nat Neurosci 22:191–204. 10.1038/s41593-018-0296-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Rodríguez PV, Hong YT, Allinson KSJ, Bevan-Jones WR, Williamson D, Jones PS, Arnold R, Borchert RJ, Surendranathan A, Mak E, Su L, Fryer TD, Aigbirhio FI, O'Brien JT, Rowe JB (2018) PK11195 binding in Alzheimer disease and progressive supranuclear palsy. Neurology 90:e1989–e1996. 10.1212/WNL.0000000000005610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Tsvetanov KA, Jones PS, Bevan-Jones WR, Arnold R, Borchert RJ, Mak E, Su L, O'Brien JT, Rowe JB (2019) Neuroinflammation and functional connectivity in Alzheimer's disease: interactive influences on cognitive performance. J Neurosci 39:7218–7226. 10.1523/JNEUROSCI.2574-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Saido TC (2018) Neuroinflammation in mouse models of Alzheimer's disease. Clin Exp Neuroimmunol 9:211–218. 10.1111/cen3.12475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD (2012) Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex 22:158–165. 10.1093/cercor/bhr099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Chowdhury S, Ma R, Le KX, Hong S, Caldarone BJ, Stevens B, Lemere CA (2017) Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci Transl Med 9:eaaf6295. 10.1126/scitranslmed.aaf6295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Dejanovic B, Gandham VD, Gogineni A, Edmonds R, Schauer S, Srinivasan K, Huntley MA, Wang Y, Wang TM, Hedehus M, Barck KH, Stark M, Ngu H, Foreman O, Meilandt WJ, Elstrott J, Chang MC, Hansen DV, Carano RAD, et al. (2019) Complement C3 is activated in human AD brain and is required for neurodegeneration in mouse models of amyloidosis and tauopathy. Cell Rep 28:2111–2123.e6. 10.1016/j.celrep.2019.07.060 [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM (2007) Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53:337–351. 10.1016/j.neuron.2007.01.010 [DOI] [PubMed] [Google Scholar]