Reward-associated stimuli can both evoke conditioned responses and acquire reinforcing properties in their own right, becoming avidly pursued. Such conditioned stimuli (CS) can guide reward-seeking behavior in adaptive (e.g., locating food) and maladaptive (e.g., binge eating) ways. The basolateral amygdala (BLA) regulates conditioned responses evoked by appetitive CS, but less is known about how the BLA contributes to the instrumental pursuit of CS.
Keywords: basolateral amygdala, conditioned stimulus, optogenetics, reward
Abstract
Reward-associated stimuli can both evoke conditioned responses and acquire reinforcing properties in their own right, becoming avidly pursued. Such conditioned stimuli (CS) can guide reward-seeking behavior in adaptive (e.g., locating food) and maladaptive (e.g., binge eating) ways. The basolateral amygdala (BLA) regulates conditioned responses evoked by appetitive CS, but less is known about how the BLA contributes to the instrumental pursuit of CS. Here we studied the influence of BLA neuron activity on both behavioral effects. Water-restricted male rats learned to associate a light-tone cue (CS) with water delivery into a port. During these Pavlovian conditioning sessions, we paired CS presentations with photo-stimulation of channelrhodopsin-2 (ChR2)-expressing BLA neurons. BLA photo-stimulation potentiated CS-evoked port entries during conditioning, indicating enhanced conditioned approach and appetitive conditioning. Next, new rats received Pavlovian conditioning without photo-stimulation. These rats then received instrumental conditioning sessions where they could press an inactive lever or an active lever that produced CS presentation, without water delivery. Rats pressed more on the active versus inactive lever, and pairing CS presentation with BLA-ChR2 photo-stimulation intensified responding for the CS. This suggests that BLA-ChR2 photo-stimulation enhanced CS incentive value. In a separate experiment, rats did not reliably self-administer BLA-ChR2 stimulations, suggesting that BLA neurons do not carry a primary reward signal. Last, intra-BLA infusions of d-amphetamine also intensified lever-pressing for the CS. The findings suggest that BLA-mediated activity facilitates CS control over behavior by enhancing both appetitive Pavlovian conditioning and instrumental pursuit of CS.
SIGNIFICANCE STATEMENT Cues paired with rewards can guide animals to valuable resources such as food. Cues can also promote dysfunctional reward-seeking behavior, as in overeating. Reward-paired cues influence reward seeking through two major mechanisms. First, reward-paired cues evoke conditioned anticipatory behaviors to prepare for impending rewards. Second, reward-paired cues are powerful motivators and they can evoke pursuit in their own right. Here we show that increasing neural activity in the basolateral amygdala enhances both conditioned anticipatory behaviors and pursuit of reward-paired cues. The basolateral amygdala therefore facilitates cue-induced control over behavior by both increasing anticipation of impending rewards and making reward cues more attractive.
Introduction
Initially neutral cues (sights, sounds, or places) that predict rewards such as food and water exert powerful control over behavior. For instance, reward-paired cues [conditioned stimuli (CS)] can acquire incentive motivational value (Bolles, 1972; Bindra, 1978), thereby “goading an individual into action” (Flagel et al., 2009). In this regard, CS can (1) elicit approach and attention, allowing animals to prepare for impending rewards (Hearst and Jenkins, 1974), (2) energize ongoing reward-seeking behaviors (Rescorla and Solomon, 1967), (3) trigger reinstatement of extinguished reward-seeking behavior (de Wit and Stewart, 1981), and (4) reinforce learning of new instrumental behaviors (Mackintosh, 1974; Cardinal et al., 2002). Through these effects, CS guide behavior toward rewards necessary for survival. However, changes in the response to CS can contribute to excessive reward-seeking behaviors (as in addiction) or conversely, low levels of appetitive behavior (as in depression).
Prior studies have examined the role of the basolateral amygdala (BLA) in the capacity of CS to both evoke conditioned approach and influence instrumental behavior. BLA lesions (Burns et al., 1993) and optogenetic stimulation of BLA→nucleus accumbens shell neurons (Millan et al., 2017) both attenuate CS-evoked conditioned responses. Similar effects are seen with optogenetic inhibition of either BLA neurons expressing the Ppp1r1b gene (Kim et al., 2016) or BLA→nucleus accumbens core neurons (Stuber et al., 2011). The BLA is also thought to be necessary for the expression of CS-controlled instrumental behavior. Decreasing BLA function with lesions (Cador et al., 1989; Everitt et al., 1991; Brown and Fibiger, 1993; Burns et al., 1993; White and McDonald, 1993; McDonald and Hong, 2004; McDonald et al., 2010), pharmacological agents (Grimm and See, 2000; Kantak et al., 2002; McLaughlin and See, 2003; Rogers et al., 2008; Gabriele and See, 2010) or optogenetic methods (Stefanik and Kalivas, 2013) suppresses CS-controlled instrumental behavior. However, these studies used tasks that potentially confound the motivational effects of the CS and those of the unconditioned stimulus (UCS), and/or neuronal manipulation methods that do not allow control of neural activity coincident with CS occurrence (e.g., lesions/pharmacological agents).
In this context, key questions remain. First, how does increased BLA-mediated neuronal activity during CS presentation influence respectively, CS-evoked conditioned behaviors and the instrumental pursuit of CS? BLA neurons fire in response to CS presentations during appetitive conditioning (Tye and Janak, 2007; Ambroggi et al., 2008; Tye et al., 2008). The functional significance of this is not fully understood. Second, CS can motivate behavior through many dissociable psychological processes (Cardinal et al., 2002), what processes might BLA-dependent activity regulate? Increased BLA activity might mediate the specific incentive value attributed to the CS. If so, then increased BLA activity should alter CS motivational properties preferentially when it is explicitly paired with CS presentations. The BLA might also arouse a general motivational state, thereby “setting the occasion” to perform a CS-controlled goal-directed behavior (Lajoie and Bindra, 1976; Rescorla, 1988). If so, then increased BLA activity should alter CS incentive value, even when increased BLA activity is explicitly unpaired with CS presentations.
We addressed these questions using in vivo optogenetics combined with Pavlovian and instrumental conditioning procedures. First, we determined whether photo-stimulation of BLA neurons is intrinsically rewarding, as assessed by self-stimulation behavior. We compared self-stimulation of BLA neurons with self-stimulation of adjacent central amygdala (CeA) neurons, as rodents will self-stimulate into the CeA (Seo et al., 2016; Baumgartner et al., 2017; Kim et al., 2017). Second, we determined how photo-stimulation of BLA neurons influences appetitive conditioned responses, as assessed by CS-evoked approach behavior that indicates expectation of the primary reward (Tolman, 1932; Hearst and Jenkins, 1974). Finally, we assessed how photo-stimulation of BLA neurons influences CS-controlled instrumental behavior, by measuring the capacity of a CS to support the spontaneous learning of a new instrumental behavior (Mackintosh, 1974; Robbins, 1978; Cardinal et al., 2002).
Materials and Methods
Animals.
Male Sprague-Dawley rats (Charles River Laboratories; 200–275 g on arrival) were housed individually on a 12 h light/dark cycle (lights off at 8:30 A.M.). They were tested during the dark phase of the circadian cycle. Food and water were available ad libitum, except in Experiments 3–4, where water access was restricted to 2 h/d. This was to facilitate Pavlovian conditioning using water as the UCS (see 'Pavlovian conditioning' section). The Université de Montréal approved all procedures involving animals and procedures followed the guidelines of the Canadian Council on Animal Care.
Intracerebral surgery.
Rats weighing 325–375 g were anesthetized with isoflurane and placed on a stereotaxic apparatus. For photo-stimulation of amygdala neurons in Experiments 1–3, rats received bilateral infusions of AAV5-hSyn1-hChR2(H134R)-eYFP (provided by Dr. Karl Deisseroth; UNC Vector Core, NC) into either the BLA (mm relative to Bregma, AP: −2.8, ML: ±5.0; mm relative to skull surface, DV: −8.4) or CeA (mm relative to Bregma, AP: −2.6, ML: ±4.3; mm relative to skull surface, DV: −7.9). Control rats received an optically inactive AAV-eYFP virus (AAV5-hSyn1-eYFP, UNC Vector Core). The hSyn promoter is neuron-specific and allows gene expression in both excitatory and inhibitory neurons (Dittgen et al., 2004). Using a glass pipette (tip diameter, ∼50 μm) coupled to a Nanoject II (Drummond Scientific), we administered 27 microinjections of 36.8 nl each (23 nl/s, at 10 s intervals; total volume of ∼1 μl/hemisphere) into each brain region. After the infusions, the glass pipette was left in place for 10 more min. In Experiment 4, guide cannulae (26 GA, model C315G, HRS Scientific) were implanted 2 mm dorsal to the BLA (mm relative to Bregma, AP: −2.4, ML: ±5.5; mm relative to skull surface: DV −6.6) or dorsal to the amygdala, without targeting the BLA specifically, as a neuroanatomical control (referred to as “Amygdala”; mm relative to Bregma, AP: −2.3, ML: ±5.1; mm relative to skull surface, DV: −6.2). In Experiment 1, the craniectomy was sealed with bone wax (Ethicon). In Experiments 2–3, an optic fiber implant (∼300 μm core diameter, numerical aperture = 0.39; Thorlabs; glued with epoxy to a ferrule, model F10061F340, Fiber Instrument Sales) was implanted in each hemisphere, 0.2 mm dorsal to the virus injection site. Four to 6 stainless steel screws were then anchored to the skull, and optic fiber implants or cannulae were fixed with dental cement. Optic fiber implants were protected with a sleeve and a dummy. Guide cannulae were sealed with obturators (model C315CD, HRS Scientific). Optogenetic manipulations started at least 4 weeks following virus injection, to allow sufficient viral expression (Zhang et al., 2010).
In vivo electrophysiology.
We used in vivo electrophysiology to confirm laser-induced action potentials in channelrhodopsin-2 (ChR2)-expressing neurons in the BLA and CeA. Anesthetized rats (urethane, 1.2 g/kg, i.p.) were placed inside a Faraday cage on a stereotaxic frame equipped with a body temperature controller. Optrodes were implanted above the BLA and CeA. Optrodes were constructed using an extracellular Parylene-coated tungsten electrode (1 MΩ, ∼125 μm outer diameter; FHC) glued with epoxy to an optical fiber (∼300 μm core diameter, numerical aperture = 0.39) with a ∼0.5 mm offset to ensure illumination of recorded neurons. A reference electrode (insulated silver wire, 0.25 mm diameter) was lowered into the back of the brain close to the cerebellum. The optrode and reference electrodes were fixed with stainless steel screws anchored to the skull and bee wax. The optrode was lowered by hydraulic microdrive into the BLA or the CeA to record single action potentials elicited by laser stimulation (465 nm blue diode laser). Optrodes were linked to the laser via patch-cords built as described by Trujillo-Pisanty et al. (2015).
The signal recorded from each optrode was fed into a high impedance headstage connected to a microelectrode amplifier (Model 1800, A-M Systems). During photo-stimulation, the low- and high-pass filters were set at 300 Hz and 5 kHz, respectively. To reduce the possibility of photoelectric artifacts, we grounded the laser head and patch-cord. Action potentials were displayed on an oscilloscope (Tektronix, Model TDS 1002). The signal was digitalized and stored using DataWave recording (USB 16 channels) and DataWave SciWorks Experimenter Package (DataWave Technologies).
Pavlovian conditioning.
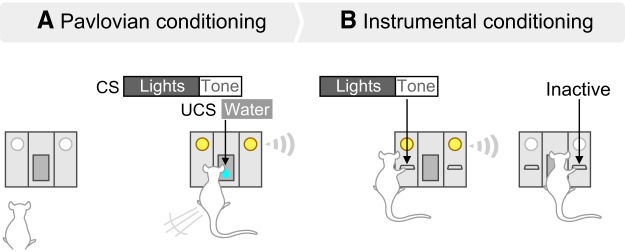
Training and testing took place in standard operant chambers (Med Associates) where a fan and a house-light were on. Rats had restricted water access for at least 3 d (2 h/d). Starting on the next day, they were trained to associate a light-tone cue (Fig. 1A; CS) with water delivery (UCS; 100 μl) into a recessed receptacle, using Pavlovian conditioning procedures. The light-tone cue consisted of illumination of two discrete lights for 5 s, combined with the extinction of the house-light. This was immediately followed by an 1800 Hz, 85-dB tone. The tone lasted 0.18 s and was coincident with water delivery. The CS-UCSs were presented on a variable interval of 60 s, 20 or 30 times/session. To determine the extent to which rats learned the CS-UCS contingency, we measured CS-evoked conditioned approach behavior. To this end, we quantified the number of nose-pokes into the recessed water receptacle during each 5 s light cue presentation [conditioned stimulus response (CSR)] versus during the 5 s period preceding each CS presentation [preconditioned stimulus response (PCSR)]. We computed a CSR/PCSR ratio for each animal, on each conditioning session.
Figure 1.
Pavlovian and Instrumental conditioning procedures. A, During Pavlovian conditioning, rats with limited access to water (2 h/d) learned that a cue (lights + tone, CS) predicts water (100 μl) delivery into a recessed dish. We assessed the acquisition of CS-evoked conditioned approach behavior by analyzing the ratio of the number of nose-pokes into the dish made during each 5 s cue presentation (CSR) over that made during the 5 s before each CS presentation (PCSR). B, After Pavlovian conditioning, rats were given instrumental conditioning sessions during which they were presented with two levers for the first time. Pressing the active lever produced the CS, whereas pressing the inactive lever had no programmed outcome. No water was delivered during instrumental conditioning sessions.
Instrumental conditioning.
To assess the capacity of the CS to control instrumental behavior, we determined whether after Pavlovian CS-UCS conditioning, rats would spontaneously learn a new instrumental response (lever-pressing) to earn CS presentations, without the UCS. This procedure dissociates incentive motivation for the CS versus that for the UCS, because the instrumental response is new and not previously reinforced by the UCS (Mackintosh, 1974; Robbins, 1978; Cardinal et al., 2002). First, rats were placed in the operant chambers for a lever habituation session, during which they could sample two test levers for the first time. As shown in Figure 1B, pressing the active lever produced the CS, without water delivery, according to a random-ratio 2 (RR2) schedule. Pressing on the active lever during CS presentation or on the inactive lever had no programmed consequences but was recorded. The lever habituation session ended after 10 active lever presses or 40 min. To measure the incentive motivational value of the CS, rats received additional instrumental test sessions. During these sessions, conditions were the same as during the lever habituation session, except that lever presses were not limited. Sessions ended after 20 or 40 min. We refer to these sessions as “operant responding for the CS”.
Experiment 1: effects of photo-stimulation on action potentials in ChR2-expressing BLA and CeA neurons in vivo.
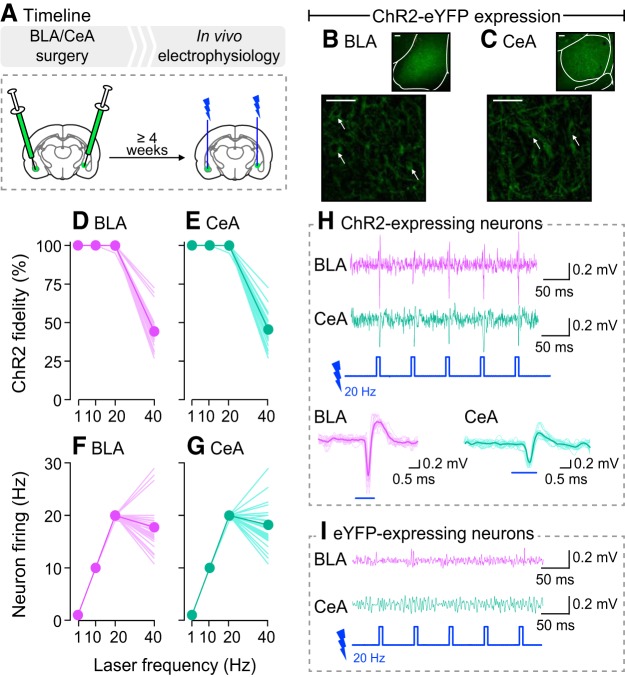
As shown in Figure 2A, rats received either the ChR2-eYFP (n = 4) or eYFP (n = 1) virus into the BLA of one hemisphere and into the CeA of the contralateral hemisphere. At least 4 weeks later, rats were anesthetized and in vivo neuronal firing was measured following photo-stimulation [squared light pulses of 5 ms delivered at 1, 10, 20, or 40 Hz at 10 mW; based on studies by Huff et al. (2013) and Robinson et al. (2014)]. These are the photo-stimulation parameters used in the behavioral studies below, with frequencies ≤20 Hz, at which we observed excellent ChR2 fidelity. Importantly, BLA neurons also fire in vivo at frequencies ≤20 Hz in behavioral tasks involving reward cues (Tye and Janak, 2007; Ambroggi et al., 2008; Tye et al., 2008).
Figure 2.
Photo-stimulation reliably induces action potentials only in BLA and CeA neurons expressing ChR2. A, In Experiment 1, rats received AAV5-hSyn1-hChR2(H134R)-eYFP (ChR2-eYFP) for transduction and activation of BLA or CeA neurons. Control rats received an optically inactive virus lacking ChR2 (AAV5-hSyn1-eYFP) in the BLA or CeA. At least 4 weeks later, we measured action potentials evoked by photo-stimulation using in vivo electrophysiology. B, C, ChR2-eYFP expression is shown in the BLA and CeA, respectively. Scale bars, 50 μm. Arrows indicate cell bodies. When laser-light is delivered, ChR2 reliably induced action potentials in (D) BLA and (E) CeA neurons, with stimulation frequencies ranging between 1 and 20 Hz. ChR2 fidelity was reduced at 40 Hz. Accordingly, firing frequency of (F) BLA and (G) CeA neurons matched laser stimulation frequency only between 1 and 20 Hz. Recordings in 4 rats/region; 10 observations/rat. Data are means, with each line representing individual observations. Examples of in vivo recordings show that laser-light induced action potentials in (H) ChR2-expressing BLA and CeA neurons but not in (I) eYFP-expressing BLA and CeA neurons.
Experiment 2: effects of photo-stimulating ChR2-expressing BLA or CeA neurons on lever-pressing behavior.
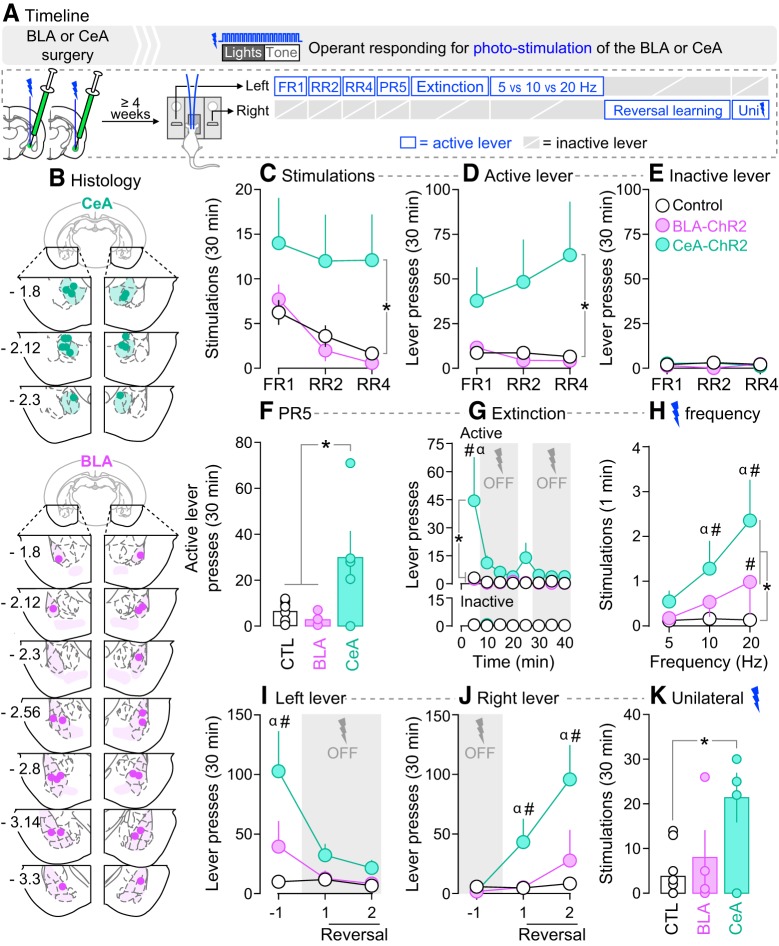
If photo-stimulation of BLA neurons is intrinsically rewarding, it could reinforce lever pressing behavior and this would confound interpretation of subsequent results. Thus, here we determined whether otherwise naive rats would reliably lever press for photo-stimulation of BLA. We also evaluated self-stimulation of CeA neurons, because photo-stimulation of CeA ChR2 has been reported to sustain self-stimulation (Seo et al., 2016; Baumgartner et al., 2017; Kim et al., 2017). As shown in Figure 3A, rats received bilateral injections of the ChR2-eYFP or eYFP virus into the BLA or CeA. Experimental rats were ChR2-eYFP rats (n = 5/subregion) allowed to lever press for photo-stimulation. Control rats included (1) rats expressing ChR2-eYFP in the BLA (n = 3) or CeA (n = 2) that could lever press but this did not produce photo-stimulation, and (2) rats expressing eYFP in the BLA (n = 3) or CeA (n = 2) and allowed to lever press for photo-stimulations. Throughout the study, lever-pressing behavior was similar across control groups. Thus, they were pooled together for final analysis (n = 10). Photo-stimulation was bilateral except where noted otherwise.
Figure 3.
Photo-stimulation of neurons in the CeA, but not BLA, is reinforcing. A, In Experiment 2, rats received AAV5-hSyn1-hChR2(H134R)-eYFP or an optically inactive control virus lacking ChR2 (AAV5-hSyn1-eYFP) in the BLA or CeA of both hemispheres. Optic fibers were also implanted bilaterally, above virus injection sites. B, Estimated optic fiber placements in the CeA and BLA (anteroposterior position is shown in mm relative to Bregma). At least 4 weeks after surgery, rats were allowed to press on two levers. Pressing the active lever produced photo-stimulation of BLA or CeA neurons, paired with presentation of a light-tone cue. Pressing the inactive lever had no programmed consequence. C–E, Lever pressing was measured under FR1, RR2, and RR4 schedules of laser reinforcement. Responding was also assessed under (F) a PR5 schedule of laser reinforcement, and during a (G) within-session extinction test. H, Effects of laser stimulation frequency on stimulations earned/min. I, J, Lever pressing under reversal learning conditions. K, Effects of unilateral stimulation, under a RR2 schedule of laser reinforcement. *p < 0.05. G, #p < 0.05 versus control rats and BLA-ChR2 rats; α p < 0.05, first 5 min block versus all other 5 min blocks in CeA-ChR2 rats. H, #p < 0.05 versus control rats at the same frequency; α p < 0.05 versus 5 Hz in CeA-ChR2 rats. I, #p < 0.05 versus control rats in Session −1; α p < 0.05 versus Sessions 1 and 2 in CeA-ChR2 rats. J, #p < 0.05 versus control rats in the same test session; α p < 0.05 versus Session −1 in CeA-ChR2 rats. n = 4–10/group. Values are mean ± SEM. Individual data are shown on histograms.
As shown in Figure 3A, the rats were allowed to press a lever to obtain a 5.18 s laser stimulation (20 Hz frequency, unless stated otherwise) paired with a 5.18 s presentation of the light-tone stimulus described above. Importantly, rats were previously naive to the light-tone stimulus, such that this stimulus had not previously been paired with water or any other outcome in these rats. During all sessions, active lever presses during photo-stimulation and inactive lever presses had no programmed consequences, but both were recorded. Daily sessions ended after self-administration of 30 stimulations or 30 min, unless stated otherwise. First, for at least two sessions (1 session/d), pressing the active lever produced photo-stimulation under a fixed-ratio of 1 (FR1) schedule of reinforcement. The rats were then tested under RR2 and RR4 schedules, with two sessions/schedule. Then, rats were given two sessions where photo-stimulation was available under a progressive ratio 5 schedule of reinforcement (PR5). During these sessions, the number of active lever presses required to earn each successive photo-stimulation increased by a factor of 5, and sessions ended after 30 stimulations or 30 min (Rossi et al., 2013). Extinction responding was then evaluated during two 40 min sessions, based on the study by Ilango et al. (2014). During minutes 0–5 and 20–25 of the extinction sessions, lever pressing was reinforced with photo-stimulation under RR2. For the remaining min of each session, lever pressing produced the light-tone stimulus, without photo-stimulation. At the 20 min mark, a single, noncontingent photo-stimulation combined with the tone-light cue indicated that photo-stimulation was available once again. Next, we assessed the influence of laser stimulation frequency on lever pressing behavior during three sessions (5, 10, and 20 Hz, 1 frequency/session/d, counterbalanced). We then assessed reversal learning for two sessions during which the active and inactive levers were switched. If photo-stimulation of BLA or CeA neurons is reinforcing, then ChR2-BLA rats and ChR2-CeA rats should stop responding on the newly non-reinforced lever, and increase responding on the newly reinforced lever. Last, the rats were given a final test session to determine whether unilateral photo-stimulations are sufficient to reinforce lever-pressing behavior. The stimulated hemisphere was counterbalanced within each group. After the extinction sessions, one rat in the BLA-ChR2 group was excluded from subsequent testing because of increasing aggressive behavior.
In this and subsequent experiments, the experimenter observed each rat during testing. Some rats experienced seizures with repeated photo-stimulation of ChR2-containing BLA neurons (rats in the other groups did not show seizure activity). This is consistent with the amygdala kindling model of epilepsy and neuronal plasticity (Goddard et al., 1969; McNamara et al., 1980; Fisher, 1989). Rats that experienced seizures were eliminated from final data analyses (Experiment 2, n = 0; Experiment 3a, n = 3; Experiment 3b, n = 1), except for one rat in Experiment 3a (see next section).
Experiment 3a: effects of photo-stimulating ChR2-expressing BLA neurons during Pavlovian CS-UCS conditioning on CS-evoked conditioned approach.
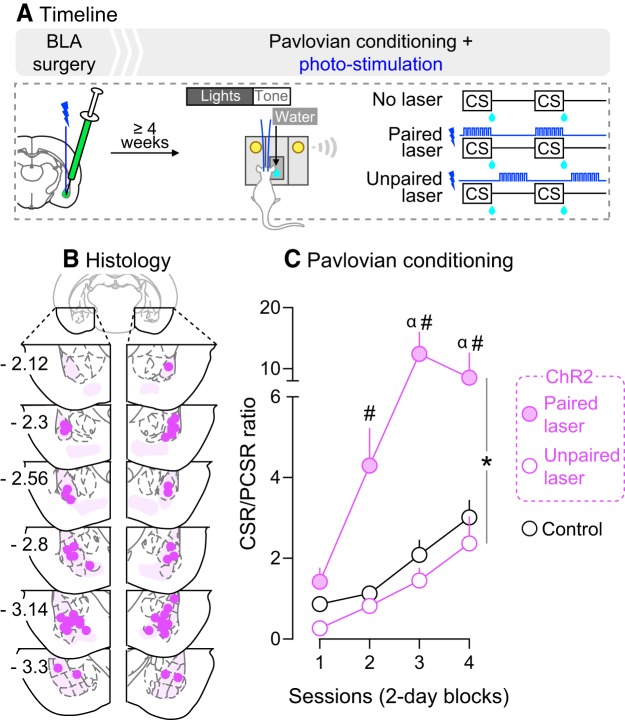
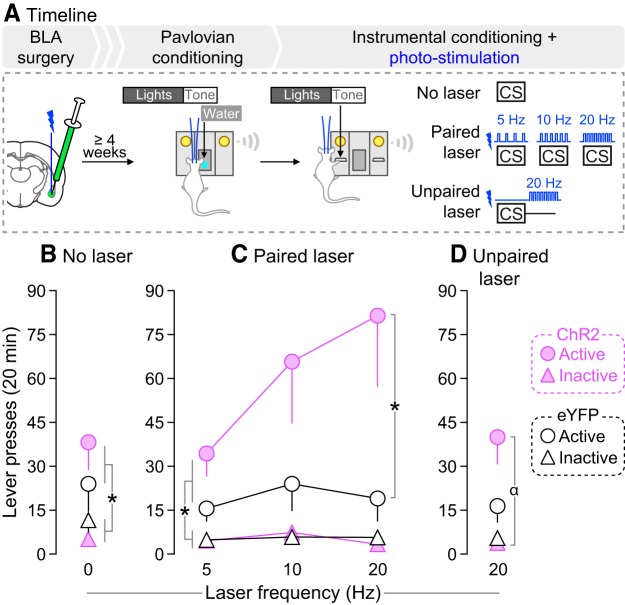
Experiment 2 showed that rats reliably lever pressed for photo-stimulation of CeA but not BLA neurons. Thus, we pursued the following experiments with BLA manipulations only, as the reinforcing effects of CeA photo-stimulation could confound data interpretation. We first determined whether photo-stimulation of BLA neurons during Pavlovian conditioning changes CS-evoked conditioned approach behavior, as measured by the CSR/PCSR ratio described above. As shown in Figure 4A, a new cohort of rats was prepared for optogenetic manipulations in the BLA as described. The rats then received Pavlovian conditioning under one of the following three conditions: (1) “No Laser”, where the CS was presented alone (ChR2, n = 11; eYFP, n = 5), (2) “Paired laser”, where photo-stimulation was paired with each CS presentation (ChR2, n = 3; eYFP, n = 3), and (3) “Unpaired laser”, where photo-stimulation and CS presentation were explicitly unpaired, by administering laser stimulation half-way between each CS-UCS presentation (ChR2, n = 3). The Unpaired laser group served to determine whether increased BLA neuronal activity had to coincide with CS presentation to influence CS-evoked conditioned approach. If so, then CSR/PCSR ratios in the Unpaired laser group should be similar to those in the ChR2-No Laser or eYFP rats. One Unpaired-ChR2 rat had a seizure on Session 9. Therefore, the effects of BLA photo-stimulation on CSR/PCSR ratios were analyzed on Sessions 1–8, with this rat included. There were no behavioral differences between ChR2-No laser, eYFP-Paired laser and eYFP-No laser rats under any test condition, and they were pooled into one group (controls, n = 19).
Figure 4.
Photo-stimulation of BLA neurons during CS presentation potentiates CS-evoked conditioned approach. A, In Experiment 3a, rats received AAV5-hSyn1-hChR2(H134R)-eYFP or an optically inactive control virus lacking ChR2 (AAV5-hSyn1-eYFP) in the BLA of both hemispheres. Optic fibers were also implanted bilaterally, above virus injection sites. B, Estimated optic fiber placements in the BLA (anteroposterior position is shown in mm relative to Bregma). At least 4 weeks after surgery, rats were water-restricted (2 h/d) and received Pavlovian conditioning sessions where a light-tone CS predicted water (100 μl) delivery (UCS) into a recessed dish. During conditioning sessions, photo-stimulation of BLA neurons was either explicitly paired or unpaired with CS presentation, in independent groups of rats. Control rats included ChR2 and eYFP rats that did not receive photo-stimulations and eYFP rats that received photo-stimulations. C, CS-paired but not CS-unpaired BLA photo-stimulation enhanced CSR/PCSR ratios (ratio of nose-pokes into the water dish during each 5 s CS presentation versus nose-pokes made during the 5 s period preceding each CS presentation). This indicates enhanced Pavlovian learning. n = 3–19/group. *p < 0.05 versus ChR2-Unpaired laser group and control group; #p < 0.05 versus control group on the same session; α p < 0.05 versus ChR2-Unpaired laser group on the same session. Values are mean ± SEM.
Experiment 3b: effects of photo-stimulating ChR2-expressing BLA neurons during operant responding for a CS.
Rats naive to laser stimulation (control rats from Experiment 3a, including 7 eYFP rats, and 8 ChR2 rats) received sessions where they could lever press for presentations of the CS, with or without CS-paired BLA photo-stimulation (0, 5, 10, or 20 Hz, one frequency/session, counterbalanced), as shown in Figure 5A. We then determined whether photo-stimulation of BLA neurons must be explicitly paired with CS presentations to alter operant responding for the CS. If so, then explicitly unpairing photo-stimulation and CS presentation during operant responding for the CS should have no or reduced effects on lever-pressing for that CS, compared with effects seen when photo-stimulation and CS presentation are paired. To address this, all rats were given an operant responding session during which photo-stimulation was explicitly unpaired with CS presentation (photo-stimulation applied 3 s after each CS presentation).
Figure 5.
Photo-stimulation of BLA neurons potentiates incentive motivation for a CS. A, In Experiment 3b, rats that had not received photo-stimulation of BLA neurons during previous Pavlovian CS-UCS conditioning (eYFP rats and ChR2-No laser control rats from Experiment 3a) were used to assess the effects of photo-stimulation of BLA neurons during instrumental responding for the CS. B, During a session without laser stimulation, both groups pressed more on the active versus inactive lever, and there were no group differences in lever-pressing behavior. C, During sessions where BLA photo-stimulation was paired with each earned CS presentation, ChR2 rats pressed more on the active lever than eYFP rats did. This indicates that photo-stimulation of BLA neurons during CS presentation enhances the incentive motivational value of the CS. D, During sessions where BLA photo-stimulation was explicitly unpaired with each earned CS presentation, ChR2 rats still pressed more on the active versus inactive lever, but lever-pressing behavior did not differ between ChR2 and eYFP rats. n = 7–8/group. *p < 0.05; α p < 0.05 active lever presses versus inactive lever presses in ChR2 rats. Values are mean ± SEM.
Experiment 4: effects of intra-amygdala d-amphetamine infusions on the incentive motivational effects of a CS.
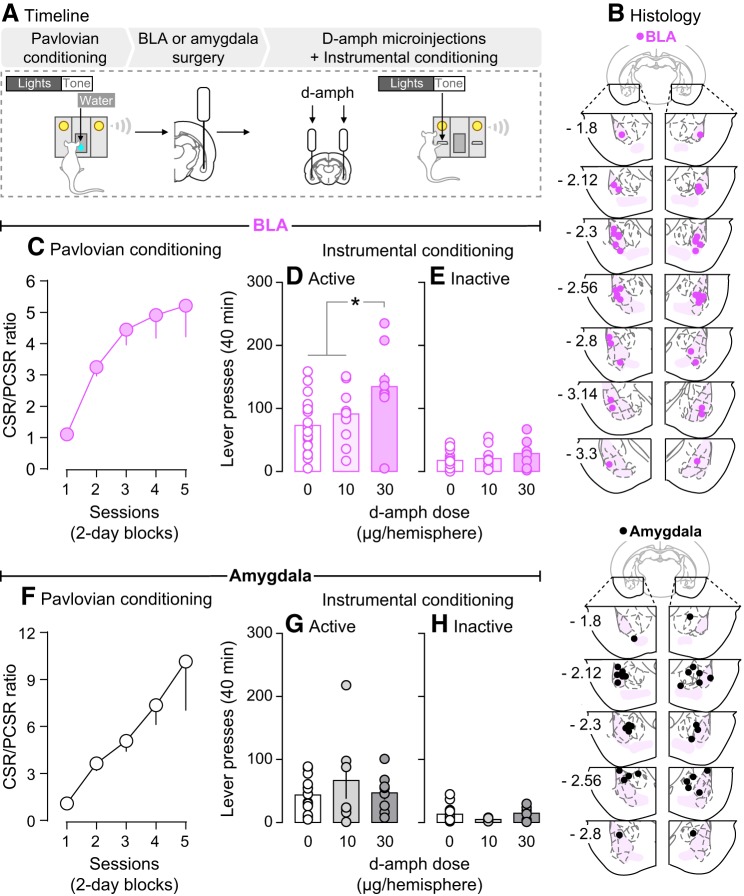
Experiment 3b showed that photo-stimulation of BLA neurons potentiates operant responding for a CS, suggesting that changes in BLA neuron activity influences the incentive motivational effects of the CS. Here we sought to extend these findings by using a pharmacological approach to influence BLA neuron activity. Thus, we determined whether injecting d-amphetamine into the BLA (n = 20) also changes operant responding for a CS. We also determined whether, within the amygdala, effects of d-amphetamine on CS incentive properties are specific to the BLA. To this end, we assessed the effects of infusing d-amphetamine into the amygdala, but without targeting the BLA specifically (n = 15). We predicted that d-amphetamine infused specifically into the BLA would enhance operant responding for a CS, based on work showing that intra-BLA infusions of d-amphetamine increase cue-induced reinstatement of extinguished cocaine seeking (Ledford et al., 2003). As shown in Figure 7A, following Pavlovian CS-UCS conditioning, intra-cerebral cannulae were implanted bilaterally. The rats were then given at least 2 weeks to recover. Rats then received a reminder Pavlovian conditioning session. Right after this session, rats received intracerebral saline infusions to habituate them to the infusion procedure. No behavior was recorded. On the next day, rats received a lever habituation session. Starting on the next day, rats received intracerebral saline or d-amphetamine (10 or 30 μg/hemisphere; Sigma-Aldrich; 1 injection/d, given every other day) and they were then allowed to lever press for the CS during a 40 min test session. This session length was chosen based on our previous work with intra-nucleus accumbens d-amphetamine injections (El Hage et al., 2015). Each rat received a maximum of three intracerebral injections to minimize tissue damage. This included (1) a saline microinjection for habituation, (2) a saline microinjection before testing, and (3) a d-amphetamine microinjection (10 or 30 μg/hemisphere) before testing (10 μg/hemisphere: n = 11 in BLA group, n = 7 in Amygdala group; 30 μg/hemisphere: n = 9 in BLA group, n = 8 in Amygdala group). Therefore, each rat received only one d-amphetamine microinjection. For intracerebral injections, injectors (33 GA, model C315I, HRS Scientific) were inserted to extend 2 mm beyond the cannulae. Microinjections were given in a volume of 0.5 μl/hemisphere and were infused over 1 min using a microsyringe pump (HARVARD PHD 200, HARVARD Apparatus). Injectors were left in place for an additional min after the infusion.
Figure 7.
Bilateral infusions of d-amphetamine into the BLA intensify the incentive value of a CS. A, In Experiment 4, rats received Pavlovian conditioning. Bilateral cannulae were then implanted specifically into the BLA (BLA group) or into the amygdala without targeting the BLA specifically (Amygdala group). B, Estimated injector tip placements in BLA rats and in Amygdala rats (anteroposterior position is shown in mm relative to Bregma). C, F, During Pavlovian conditioning, rats reliably learned the CS-unconditioned stimulus contingency, as indicated by increasing CSR/PCSR ratios over sessions (ratio of nose-pokes into the water receptacle made during each 5 s CS presentation versus during the 5 s period preceding each CS presentation). Next, we assessed the effects of intracerebral d-amphetamine infusions (0, 10, or 30 μg/hemisphere) on instrumental responding for the CS. Both (D, E) BLA and (G, H) Amygdala rats pressed more on the active versus inactive lever, indicating that the CS acquired incentive motivational value. D, d-Amphetamine influenced responding for the CS only when the drug was infused into the BLA. n = 7–20/group. *p < 0.05. Values are mean ± SEM. Individual data are shown on histograms.
Histology.
In Experiments 2–3, rats were anesthetized with urethane (1.2 g/kg, i.p.) and were transcardially perfused with PBS and 4% paraformaldehyde. Brains were then extracted and kept at room temperature for 1 week in a 30% sucrose/4% paraformaldehyde solution, and then stored at −80°C. In Experiment 4, rats were anesthetized with isoflurane (5%), brains were extracted and stored at −20°C. Forty μm-thick coronal slices were cut in a cryostat and optic fiber or injector placement was estimated using the Paxinos and Watson atlas (Paxinos and Watson, 1986).
Statistics.
In Experiment 2, mixed-model ANOVA was used to analyze group differences in self-administered photo-stimulations and lever pressing behavior (Group × Session: “Session” as a within-subjects variable; Group × Time: “Time” as a within-subjects variable; Group × Laser Frequency: “Frequency” as a within-subjects variable). One-way ANOVA was used to analyze group differences in both active lever presses during the PR5 session and the number of self-administered unilateral stimulations. In Experiment 3a, mixed-model ANOVA was used to analyze group differences in average CSR/PCSR ratios (Group × Session: Session as a within-subjects variable). In Experiment 3b, mixed-model ANOVA was used to analyze group differences in lever pressing for the CS (Group × Session or Lever Type: Session and “Lever Type” as within-subjects variables). In Experiment 4, one-way ANOVA was used to analyze CSR/PCSR ratios across sessions. The effects of d-amphetamine on lever pressing were analyzed using mixed-model ANOVA (Dose × Lever Type: Lever Type as a within-subjects variable). When an interaction and/or main effects were significant (p < 0.05), effects were analyzed further using Bonferroni's multiple-comparisons tests. Values in figures are mean ± SEM.
Results
Experiment 1: effects of photo-stimulation on action potentials in ChR2-expressing BLA and CeA neurons in vivo
Figure 2, B and C, shows ChR2-eYFP expression in the BLA and CeA. As seen in Figure 2, D and E, photo-stimulation of BLA or CeA neurons induced action potentials on average 100% of the time at 1, 10, and 20 Hz stimulation frequencies. However, at 40 Hz, spike fidelity decreased, and photo-stimulation produced action potentials only ∼45% of the time. In line with these observations, Figure 2, F and G, shows that the frequencies of neuron firing and photo-stimulation were closely matched at laser frequencies ≤20 Hz. However, at a stimulation frequency of 40 Hz, BLA and CeA neurons fired only at ∼18 Hz. This loss of fidelity is in accordance with the kinetic properties of ChR2(H134R), the ChR2 mutant used here. Indeed, when 5 ms pulses are given at a 40 Hz stimulation frequency, pulses are spaced by 20 ms, and this is shorter than the combined opening (∼3 ms) and closing (∼18 ms) rates of ChR2(H134R) (Lin et al., 2009). Importantly, laser application produced action potentials in ChR2-expressing BLA or CeA neurons (Fig. 2H), but not in eYFP-expressing BLA or CeA neurons (Fig. 2I). Thus, photo-stimulation reliably induced action potentials only in ChR2-expressing BLA or CeA neurons, and spike fidelity was excellent at laser frequencies ≤20 Hz. Thus, we used frequencies ≤20 Hz in the following studies.
Experiment 2: effects of photo-stimulation of BLA or CeA ChR2-expressing neurons on lever-pressing behavior
Here, we determined whether rats would reliably press on a lever for photo-stimulation of ChR2-expressing BLA or CeA neurons (Fig. 3A). Pressing on the active lever produced photo-stimulation paired with a light-tone cue, under FR1, RR2, and RR4 schedules of reinforcement (1 schedule/session). Pressing on the inactive lever had no programmed consequences.
Laser self-stimulation
Figure 3B shows estimated optic fiber placements in the CeA and BLA. Figure 3C shows that across different reinforcement schedules, CeA-ChR2 rats self-administered more laser stimulations than control rats (main effect of Group: F(2,17) = 5.5, p = 0.014; CeA-ChR2 versus control rats: F(1,13) = 7.5, p = 0.017). Accordingly, as seen in Figure 3D, CeA-ChR2 rats also pressed more on the active lever than control rats (main effect of Group: F(2,17) = 5.53, p = 0.014; CeA-ChR2 versus control rats: F(1,13) = 7.42, p = 0.017). In contrast, BLA-ChR2 and control rats earned a similar number of photo-stimulations and pressed a similar number of times on the active lever (Fig. 3C,D; all p values > 0.05). Presses on the inactive lever did not differ between groups (Fig. 3E; p > 0.05), suggesting that photo-stimulation of either BLA or CeA neurons did not produce nonspecific motor effects. Figure 3F shows the number of active lever presses for photo-stimulation under a PR5 schedule of reinforcement. Under this schedule, CeA-ChR2 rats pressed more on the active lever relative to BLA-ChR2 or control rats (main effect of Group: F(2,17) = 3.77, p = 0.007; CeA-ChR2 > controls, p = 0.014; CeA-ChR2 > BLA-ChR2, p = 0.013). BLA-ChR2 rats and controls were not different (p > 0.05). Thus, across a range of schedules of reinforcement, rats self-administered cued photo-stimulations of CeA neurons, but not BLA neurons. The findings suggest that photo-stimulation of CeA, but not BLA neurons is reinforcing.
Extinction responding
We assessed lever-pressing behavior under extinction conditions during a 40 min session where photo-stimulation was only available from minutes 0–5 and 20–25, under a RR2 schedule. As shown in Figure 3G (top), BLA-ChR2 rats did not differ from controls during this session (all p values > 0.05). Presses on the inactive lever also did not differ between groups (Fig. 3G, bottom; p > 0.05). However, Figure 3G (top) also shows that when photo-stimulation was available in the first 5 min of the session, CeA-ChR2 rats pressed more on the active lever relative to controls and BLA-ChR2 rats (Group × Time interaction: F(14,119) = 4.14, p < 0.0001; main effect of Group: F(2,17) = 7.69, p = 0.004; CeA-ChR2 vs control rats: F(1,13) = 10.3, p = 0.007; minutes 0–5, CeA-ChR2 > controls, p < 0.0001; CeA-ChR2 vs BLA-ChR2, F(1,8) = 5.11, p = 0.054; post hoc comparisons on minutes 0–5, CeA-ChR2 > BLA-ChR2, p = 0.0001. No other comparisons were significant). CeA-ChR2 rats also extinguished their lever-pressing behavior during the extinction session (Fig. 3G, top; main effect of Time: F(7,119) = 6.12, p < 0.0001; minutes 0–5 vs each subsequent 5 min block, all p values < 0.0001). Thus, only CeA-ChR2 rats lever-pressed for photo-stimulation when it was available, and decreased responding when it was not. In contrast, BLA-ChR2 rats and control rats lever-pressed very little, regardless of photo-stimulation availability.
Self-stimulation as a function of laser stimulation frequency
Figure 3H shows the influence of stimulation frequency (5, 10, and 20 Hz) on self-administration of photo-stimulations. Sessions stopped after 30 stimulations or 30 min. As a measure of the rate of responding, we analyzed the number of photo-stimulations earned per min. Relative to control rats, CeA-ChR2 rats earned more photo-stimulations/min at 10 and 20 Hz (Fig. 3H; Frequency × Group interaction: F(4,32) = 4.08; p = 0.009; main effect of Group: F(2,16) = 5.76, p = 0.013; CeA-ChR2 rats vs controls: F(1,13) = 10.67, p = 0.006; CeA-ChR2 > controls at 10 Hz, p = 0.046, at 20 Hz, p < 0.0001). CeA-ChR2 rats also earned more photo-stimulations/min as stimulation frequency was increased (Fig. 3H; main effect of Frequency: F(2,32) = 9.31, p = 0.0006; CeA-ChR2 rats, 10 > 5 Hz, p = 0.019, 20 > 5 Hz, p < 0.0001). BLA-ChR2 rats earned more photo-stimulations/min relative to controls only at the highest frequency tested (main effect of Group: F(1,12) = 6.18, p = 0.029; BLA-ChR2 > controls, at 20 Hz, p = 0.013). No other comparisons were statistically significant. Thus, compared with control rats, BLA-ChR2 rats earned more photo-stimulations/min at 20 Hz, whereas CeA-ChR2 rats earned more photo-stimulations/min at both 10 and 20 Hz. Furthermore, only CeA-ChR2 rats increased their self-stimulation behavior with increasing laser frequency.
Reversal learning
Here we determined whether photo-stimulation of CeA or BLA neurons supports reversal learning. Figure 3I shows pressing on a lever that produced laser stimulation on Session −1, but not on subsequent sessions. Figure 3J shows pressing on a lever that did not produce laser stimulation on Session −1, but did so on subsequent sessions. As seen in Figure 3I, CeA-ChR2 but not BLA-ChR2 rats pressed more on the reinforced lever relative to control rats (Group × Session interaction: F(4,32) = 5.46, p = 0.002; main effect of Group: F(2,16) = 10.05, p = 0.002; CeA-ChR2 vs controls: F(1,13) = 19.47, p = 0.0007; CeA-ChR2 > controls on Session −1, p < 0.0001). CeA-ChR2 rats also pressed significantly less on this lever after reversal versus before (main effect of Session: F(2,32) = 11.98, p = 0.0001; CeA-ChR2 rats, Session −1 > Session 1: p = 0.0002, Session −1 > Session 2: p < 0.0001). As seen in Figure 3J, after lever reversal, CeA-ChR2 but not BLA-ChR2 rats pressed more on the newly reinforced lever relative to controls (Group × Session interaction: F(4,32) = 5.94, p = 0.001; main effect of Group: F(2,16) = 8.59, p = 0.003; CeA-ChR2 rats > controls, Session 1: p = 0.033, Session 2: p < 0.0001). CeA-ChR2 rats also pressed more on this lever after reversal versus before (main effect of Session: F(2,32) = 11.84, p = 0.0001; CeA-ChR2 rats, Session −1 > Session 1: p = 0.035, Session −1 > Session 2: p < 0.0001). In summary, photo-stimulation of CeA neurons both reliably reinforced lever-pressing behavior and supported reversal learning, whereas photo-stimulation of BLA neurons supported neither response.
Unilateral laser stimulation
Last, we determined whether unilateral photo-stimulation of CeA or BLA neurons was reinforcing. Figure 3K shows that CeA-ChR2 but not BLA-ChR2 rats earned more unilateral laser stimulations relative to controls (main effect of Group: F(2,16) = 6.24, p = 0.01; CeA-ChR2 > controls, p = 0.009). Therefore, unilateral stimulation of CeA, but not BLA neurons sustains self-stimulation.
In summary, across different schedules of reinforcement, operant testing conditions and photo-stimulation parameters, rats did not reliably self-administer photo-stimulation of BLA neurons. In contrast, rats reliably self-administered photo-stimulation of CeA neurons, indicating that it is reinforcing. These findings show that photo-stimulation of BLA versus CeA neurons has dissociable effects, and that CeA but not BLA neurons carry a primary reward signal.
Experiment 3a: effects of photo-stimulating ChR2-expressing BLA neurons during Pavlovian CS-UCS conditioning on CS-evoked conditioned approach
Figure 4B shows estimated optic fiber placements in the BLA. We first determined the effects of BLA photo-stimulation on CS-evoked conditioned approach behavior (Fig. 4A). This was assessed by analyzing the ratio of nose-pokes into the water receptacle during each 5 s light cue presentation (CSR), versus during the 5 s period preceding each CS presentation (PCSR). Figure 4C shows the effects of photo-stimulation of ChR2-expressing BLA neurons on the CSR/PCSR ratio over Pavlovian conditioning sessions. Average CSR/PCSR ratios progressively increased over sessions in all groups, indicating that rats learned the CS-UCS contingency (Fig. 4C; main effect of Session: F(3,66) = 20.12, p < 0.0001). Pairing photo-stimulation of BLA neurons with CS presentations (“ChR2-Paired laser” group) potentiated conditioned approach behavior relative to all other conditions (Fig. 4C; Group × Session interaction: F(6,66) = 8.31, p < 0.0001; main effect of Group: F(2,22) = 21.81, p < 0.0001; ChR2 Paired laser > Controls, Session 2: p = 0.025, Session 3: p < 0.0001, Session 4: p < 0.0001; ChR2 Paired laser > ChR2 Unpaired laser, Session 3: p < 0.0001, Session 4: p = 0.0004). No other comparisons were significant. Thus, photo-stimulation of BLA neurons potentiated CS-evoked conditioned approach behavior over time, but only if photo-stimulation was explicitly paired with CS presentation.
Experiment 3b: effects of photo-stimulating ChR2-expressing BLA neurons during operant responding for a CS
Here, we sought to determine whether BLA photo-stimulation would potentiate instrumental pursuit of the CS. To this end, we used rats from Experiment 3a that had undergone Pavlovian CS-UCS conditioning without laser stimulation. These are rats with ChR2-expressing BLA neurons that had not received laser photo-stimulation, and rats with eYFP-expressing BLA neurons. We determined in these rats whether BLA photo-stimulation during operant responding for the CS enhances responding for that CS (Fig. 5A). Figure 5B shows presses on an active lever that produced CS presentation and on an inactive lever, during a session where rats did not receive laser stimulation. Across groups, rats pressed more on the active versus inactive lever (main effect of Lever Type: F(1,13) = 13.86, p = 0.003). This indicates that the CS acquired incentive properties. There was neither a main effect of Group nor a Group × Lever Type interaction effect (all p values > 0.05). Thus, without laser stimulation, ChR2 and eYFP rats show similar incentive motivation for the CS.
Figure 5C shows presses on the active and inactive levers when CS presentations were paired with BLA photo-stimulation at different laser frequencies (5, 10, or 20 Hz). Figure 5C shows that both ChR2 and eYFP rats pressed more on the active versus inactive lever (main effect of Lever Type: F(1,26) = 18.3, p = 0.001; eYFP rats: F(1,12) = 6.31, p = 0.027; ChR2 rats: F(1,14) = 12.19, p = 0.004). Thus, both groups showed incentive motivation for the CS under these conditions. In addition, ChR2 rats pressed more on the active lever than did eYFP rats (Fig. 5C; main effect of Group: F(1,13) = 5.39, p = 0.04; no other comparisons were significant). This suggests that photo-stimulation of BLA neurons potentiates the expression of incentive motivation for the CS. Figure 5D shows lever-pressing behavior when pressing the active lever produced the CS and photo-stimulation 3 s later, such that the CS and photo-stimulation were unpaired. Only ChR2 rats pressed more on the active versus inactive lever (Fig. 5D; Group × Lever Type interaction: F(1,13) = 5.79, p = 0.032; main effect of Lever Type: F(1,13) = 20.13, p = 0.0006; ChR2 rats: active > inactive lever, p = 0.0004). However, ChR2 rats did not press more on the active lever than eYFP rats (p > 0.05). No other comparisons were significant. Last, BLA photo-stimulation, when it was either paired or unpaired with CS presentation, did not influence nose pokes into the water receptacle (data not shown, all p values > 0.05). This suggests that BLA photo-stimulation did not increase the urge to consume the associated water UCS. Together, these results show that photo-stimulation of BLA neurons during operant responding for the CS potentiated incentive motivation for that CS, and that this effect was strongest when photo-stimulation was explicitly paired with each CS presentation.
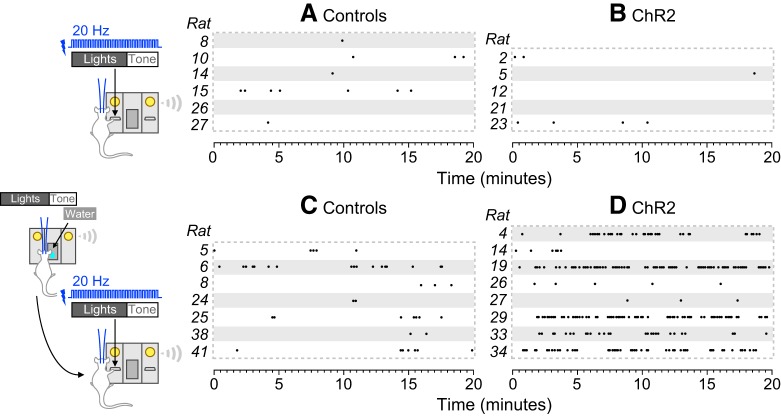
Together, the results of Experiments 2 and 3b indicate that BLA photo-stimulation increases instrumental pursuit of a discrete stimulus, if and only if that stimulus reliably predicts a primary reward (water). That is, BLA photo-stimulation selectively potentiates the pursuit of environmental stimuli that possess conditioned incentive properties. Figure 6 highlights this effect. It shows reinforcements earned by individual rats lever pressing for presentations of a light-tone stimulus not previously associated with a reward (Fig. 6A,B; rats from Experiment 2) or a light-tone stimulus previously associated with a water reward (Fig. 6C,D; rats from Experiment 3b). When the light-tone stimulus had no relationship with a primary reward, BLA photo-stimulation did not significantly change the number of stimulus presentations earned (Fig. 6A,B). After the light-tone stimulus had been paired with water, control rats pursued this CS more avidly (Fig. 6, compare A, C), and BLA photo-stimulation potentiated this effect (Fig. 6, compare C, D).
Figure 6.
Photo-stimulation of BLA neurons potentiates motivation for a discrete environmental stimulus if and only if this stimulus was previously associated with a primary reward. Each dot indicates reinforcements earned by individual rats that were lever pressing for presentations of a light-tone stimulus. Data are shown for individual control rats (A, C) and individual rats receiving photo-stimulation of ChR2-expressing BLA neurons paired with each stimulus presentation (B, D). A, B, When the light-tone stimulus had not previously been associated with a primary reward, BLA photo-stimulation did not change the number of stimulus presentations earned. C, D, When the light-tone stimulus had previously been associated with a water reward, BLA photo-stimulation enhanced responding.
Additionally, photo-stimulation of BLA neurons increased both CS-evoked conditioned approach (Fig. 4C; Experiment 3a) and operant responding for the CS (Fig. 5C; Experiment 3b). These two CS effects rely on common but also partially dissociable neurobiological and psychological processes (Flagel et al., 2011; Tabbara et al., 2016). In agreement, in the control rats represented in Figure 4C, there was no significant correlation between average CSR/PCSR ratios over the last 4 d of Pavlovian conditioning and active lever presses during a subsequent instrumental conditioning session (without laser; data not shown; r2 = 0.002, p = 0.85).
Experiment 4: effects of intra-amygdala d-amphetamine infusions on the incentive motivational effects of a CS
After CS-UCS Pavlovian conditioning, rats were given instrumental responding tests where they could lever-press for the CS (Fig. 7A). Immediately before these tests, rats received bilateral infusions of d-amphetamine (0, 10, or 30 μg/hemisphere) into the BLA or into the amygdala without targeting the BLA specifically. Figure 7B shows estimated location of injector tips when both cannulae were specifically in the BLA (top) or simply in the amygdala, but without targeting the BLA exclusively (bottom). The rats learned the CS-UCS contingency, as indicated by a progressive increase in CSR/PCSR ratio (Fig. 7C; main effect of Session: F(4,76) = 11.12, p < 0.0001; 7F; main effect of Session: F(4,56) = 5.04, p = 0.002). Figure 7D,E–G,H show that rats in both experimental groups pressed more on the active versus inactive lever (Fig. 7D,E; Dose × Lever Type interaction: F(2,37) = 5.31, p = 0.009; main effect of Lever Type: F(1,37) = 142.4; p < 0.0001; 7G,H; main effect of Lever Type: F(1,27) = 25.61, p < 0.0001). Thus, all rats spontaneously learned a new operant response to produce the CS, indicating that the CS acquired incentive value. d-Amphetamine influenced active lever pressing only when infused specifically into the BLA, such that active lever pressing was greatest at 30 μg/hemisphere d-amphetamine (Fig. 7D; main effect of Dose: F(2,37) = 4.5, p = 0.018; 30 vs 0 μg, p = 0.0002; 30 vs 10 μg, p = 0.027). In contrast, d-amphetamine did not alter lever-pressing behavior in rats that received infusions into the amygdala, without specifically targeting the BLA (Fig. 7G,H; all p values > 0.05). No other comparisons were statistically significant. Last, neither intra-BLA nor intra-amygdala d-amphetamine altered the number of nose pokes into the water receptacle (data not shown, all p values > 0.05). This suggests that d-amphetamine infusions into the amygdala did not increase the urge to consume the associated water UCS. Thus, the findings show that intra-BLA d-amphetamine intensified incentive motivation for the CS.
Discussion
We evaluated the contributions of the BLA to appetitive Pavlovian conditioning and to the instrumental pursuit of a reward-predictive CS. First, photo-stimulation of BLA neurons was not intrinsically reinforcing, whereas photo-stimulation of neurons in the adjacent CeA was. Second, photo-stimulation of BLA neurons during Pavlovian CS-UCS conditioning enhanced CS-evoked conditioned approach, indicating potentiated anticipation of the primary reward. Third, photo-stimulation of BLA neurons potentiated operant responding for the CS, suggesting enhanced CS incentive value. Finally, intra-BLA infusions of d-amphetamine also augmented operant responding for the CS, suggesting that a local increase in monoamine neurotransmission is also involved in enhanced conditioned incentive motivation. Thus, increased neuronal activity within the BLA facilitates cue-controlled behavior by both increasing cue-induced anticipation of impending rewards and making reward cues more attractive.
Photo-stimulation of CeA, but not BLA neurons, is reinforcing
Rats reliably lever pressed for photo-stimulation of CeA, but not BLA neurons, suggesting that CeA neurons carry a primary reward signal. Our findings agree with earlier work showing that electrical stimulation of CeA cells is reinforcing (Prado-Alcalá and Wise, 1984; Kane et al., 1991). CeA neurons are mostly GABAergic, but they express different neuropeptides and have different anatomical connections. More recent studies show that stimulation of specific neuronal populations in the CeA can also be reinforcing. This includes CeA neurons expressing corticotropin-releasing hormone, somatostatin, neurotensin, and/or tachykinin 2 (Baumgartner et al., 2017; Kim et al., 2017), and CeA→medial prefrontal cortex neurons (Seo et al., 2016). In contrast, using photo-stimulation of CeA neurons without regards to cell subtype as done here, Berridge and colleagues report that CeA photo-stimulation is not reinforcing (Robinson et al., 2014; Warlow et al., 2017). This could involve the CeA subregion where photo-stimulation was applied. Robinson et al. (2014) and Warlow et al. (2017) implanted optic fibers in the posterior CeA, whereas we implanted in the anterior CeA. Our rats did not reliably self-administer photo-stimulation of BLA neurons. Rats will electrically self-stimulate some BLA subregions (Prado-Alcalá and Wise, 1984; Kane et al., 1991), and studies using optogenetic methods suggest that self-stimulation depends on the BLA circuit targeted. For instance, photo-stimulation of BLA→nucleus accumbens terminals is reinforcing (Stuber et al., 2011; Britt et al., 2012; Namburi et al., 2015), but photo-stimulation of BLA→medial CeA terminals produces avoidance (Namburi et al., 2015). The absence of BLA self-stimulation here could involve the hSyn promoter we used. It confers neuron-specific transgene expression, but it does not target neuron subtypes.
Via distinct cell types and connections, amygdala nuclei and subregions exert many functions, including both appetitive and defensive behaviors (Gallagher and Chiba, 1996). Future studies will be important to examine roles of specific CeA and BLA neuron subtypes and projections in appetitive behavior. As this research unfolds, our results support the idea that while the BLA and CeA are connected and can play similar roles in motivational processes (Wassum et al., 2011), they also have distinct appetitive functions (Corbit and Balleine, 2005; Robinson et al., 2014; Warlow et al., 2017).
Photo-stimulation of BLA neurons during CS-UCS conditioning enhances CS-evoked conditioned approach
During Pavlovian conditioning, we paired photo-stimulation of BLA neurons with CS presentation. This potentiated CS-evoked conditioned approach, as shown by more CS-triggered visits to the water dish. This suggests enhanced anticipation of the CS-associated water reward. Explicitly unpairing BLA stimulation and CS presentation did not influence CS-evoked conditioned approach. Thus, increasing BLA neuron activity when a CS is presented amplifies associative CS-UCS learning. Increased CS-triggered visits to the water dish could suggest that BLA photo-stimulation enhances the appetitive value of water. This is possible, but unlikely, because BLA lesions do not alter water consumption (Cador et al., 1989). Instead, enhanced CS-evoked conditioned approach likely involves changes in how BLA neurons represent the CS and/or how they encode the CS-UCS association. CS-triggered conditioned approach behaviors can reflect both the predictive and incentive effects of CS. BLA stimulation could increase CS-triggered visits to the water dish by enhancing either or both effects. For instance, rats might visit the water dish during CS presentation because the CS is evoking an incentive urge to drink the associated water (Weingarten, 1983). If so, then BLA photo-stimulation during the CS could increase visits to the water dish by enhancing this conditioned incentive urge (Holland et al., 2002). Similarly, the water dish is also a CS in our experiments, less predictive than the light-tone CS, but more proximal to the water UCS. As such, BLA photo-stimulation could have increased water dish visits by enhancing the incentive value of the dish. We do not believe this is the case, because BLA photo-stimulation increased the number of water dish visits only when this stimulation was explicitly paired with the light-tone CS.
Photo-stimulation of BLA neurons or d-amphetamine infusion into the BLA enhances CS incentive value
Once the CS had been imbued with incentive value through prior association with an appetitive UCS, BLA photo-stimulation amplified the expression of this incentive motivation (as measured by lever-pressing reinforced by the CS alone). Infusing d-amphetamine into the BLA had the same effect, suggesting that increases in monoamine-mediated neurotransmission in the BLA are involved (Ledford et al., 2003; Bernardi et al., 2009; Gremel and Cunningham, 2009; Lintas et al., 2011). This extends lesion studies showing that the BLA is necessary for operant responding reinforced by a CS (Cador et al., 1989; Burns et al., 1993). BLA photostimulation or d-amphetamine infusions into the BLA could have enhanced instrumental responding for the CS by potentiating the appetitive value of the associated water reward. This is unlikely, because neither manipulation influenced the number of water dish visits during instrumental tests. In addition, the increased lever pressing during tests of instrumental responding for the CS likely does not involve any intrinsically reinforcing effects of BLA photo-stimulation. Indeed, our BLA photo-stimulation parameters did not reliably support self-stimulation behavior. Instead, the BLA stores information about CS value, which is then used to guide behavior (Cardinal et al., 2002). As such, stimulation of BLA neurons could enhance operant responding for a CS by potentiating the incentive value of the CS itself or of the CS-associated reward representation (Mogenson, 1987; Everitt and Robbins, 1992).
Conclusions
Increased neuronal activity in BLA-dependent circuits amplifies control over behavior by an appetitive cue, and this involves two overlapping, but also dissociable psychological mechanisms. A first mechanism involves enhanced CS-UCS associative learning, such that the CS triggers increased conditioned approach, and increased anticipation of the primary reward. This prepares animals to engage with the forthcoming reward. The second mechanism involves amplified incentive motivation to pursue the CS, such that animals show enhanced instrumental responding for the CS. Thus, when reward cues are present in the environment, increased recruitment of BLA-dependent pathways could promote excessive pursuit of associated rewards both by augmenting anticipation for these rewards and making reward-paired cues more attractive in their own right.
Footnotes
This work was supported by Grants from the National Science and Engineering Research Council of Canada (Grant 355923) and the Canada Foundation for Innovation to A.-N.S. (Grant 24326). A.-N.S. holds a salary award from the Fonds de la Recherche du Québec-Santé (Grant 28988). We thank Nadia Chaudri, Franca Lacroix, Franz Villaruel, Ivan Trujillo-Pisanty, Jonathan Britt, Sean J. Reed, Mike J.F. Robinson, and Kent C. Berridge for generous advice on implementation of in vivo optogenetics procedures in our laboratory; and Ellie-Anna Minogianis for technical support.
Conflict of interest: A.-N.S. was a scientific consultant for H. Lundbeck A/S as this research was being carried out. This had no influence on the work. The remaining authors report no competing financial interests.
References
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM (2008) Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron 59:648–661. 10.1016/j.neuron.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner HM, Olney JJ, Warlow SM, Schulkin J, Berridge KC (2017) Investigating corticotropin releasing factor in mediating appetitive behavior. Soc Neurosci Abstr 43:244.14. [Google Scholar]
- Bernardi RE, Ryabinin AE, Berger SP, Lattal KM (2009) Post-retrieval disruption of a cocaine conditioned place preference by systemic and intrabasolateral amygdala beta2- and alpha1-adrenergic antagonists. Learn Mem 16:777–789. 10.1101/lm.1648509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindra D. (1978) How adaptive behavior is produced: a perceptual-motivation alternative to response reinforcement. Behav Brain Sci 1:41–52. 10.1017/S0140525X00059380 [DOI] [Google Scholar]
- Bolles RC. (1972) Reinforcement, expectancy, and learning. Psychol Rev 79:394–409. 10.1037/h0033120 [DOI] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A (2012) Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76:790–803. 10.1016/j.neuron.2012.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EE, Fibiger HC (1993) Differential effects of excitotoxic lesions of the amygdala on cocaine-induced conditioned locomotion and conditioned place preference. Psychopharmacology 113:123–130. 10.1007/BF02244344 [DOI] [PubMed] [Google Scholar]
- Burns LH, Robbins TW, Everitt BJ (1993) Differential effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor activity potentiated by intra-accumbens infusions of d-amphetamine. Behav Brain Res 55:167–183. 10.1016/0166-4328(93)90113-5 [DOI] [PubMed] [Google Scholar]
- Cador M, Robbins TW, Everitt BJ (1989) Involvement of the amygdala in stimulus–reward associations: interaction with the ventral striatum. Neuroscience 30:77–86. 10.1016/0306-4522(89)90354-0 [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ (2002) Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 26:321–352. 10.1016/S0149-7634(02)00007-6 [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW (2005) Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. J Neurosci 25:962–970. 10.1523/JNEUROSCI.4507-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Stewart J (1981) Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology 75:134–143. 10.1007/BF00432175 [DOI] [PubMed] [Google Scholar]
- Dittgen T, Nimmerjahn A, Komai S, Licznerski P, Waters J, Margrie TW, Helmchen F, Denk W, Brecht M, Osten P (2004) Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc Natl Acad Sci U S A 101:18206–18211. 10.1073/pnas.0407976101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hage C, Bédard AM, Samaha AN (2015) Antipsychotic treatment leading to dopamine supersensitivity persistently alters nucleus accumbens function. Neuropharmacology 99:715–725. 10.1016/j.neuropharm.2015.03.012 [DOI] [PubMed] [Google Scholar]
- Everitt B, Robbins T (1992) Amygdala-ventral striatal interactions and reward-related processes. In: The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction, pp 401–409. New York: Wiley. [Google Scholar]
- Everitt BJ, Morris KA, O'Brien A, Robbins TW (1991) The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience 42:1–18. 10.1016/0306-4522(91)90145-E [DOI] [PubMed] [Google Scholar]
- Fisher RS. (1989) Animal models of the epilepsies. Brain Res Brain Res Rev 14:245–278. 10.1016/0165-0173(89)90003-9 [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE (2009) Individual differences in the attribution of incentive salience to reward-related cues: implications for addiction. Neuropharmacology 56:139–148. 10.1016/j.neuropharm.2008.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H (2011) A selective role for dopamine in stimulus–reward learning. Nature 469:53–57. 10.1038/nature09588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriele A, See RE (2010) Reversible inactivation of the basolateral amygdala, but not the dorsolateral caudate putamen, attenuates consolidation of cocaine-cue associative learning in a reinstatement model of drug-seeking. Eur J Neurosci 32:1024–1029. 10.1111/j.1460-9568.2010.07394.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Chiba AA (1996) The amygdala and emotion. Curr Opin Neurobiol 6:221–227. 10.1016/S0959-4388(96)80076-6 [DOI] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK (1969) A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol 25:295–330. 10.1016/0014-4886(69)90128-9 [DOI] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL (2009) Involvement of amygdala dopamine and nucleus accumbens NMDA receptors in ethanol-seeking behavior in mice. Neuropsychopharmacology 34:1443–1453. 10.1038/npp.2008.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, See RE (2000) Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology 22:473–479. 10.1016/S0893-133X(99)00157-8 [DOI] [PubMed] [Google Scholar]
- Hearst E, Jenkins HM (1974) Sign-tracking: the stimulus-reinforcer relation and directed action. Austin, TX: Psychonomic Society. [Google Scholar]
- Holland PC, Petrovich GD, Gallagher M (2002) The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol Behav 76:117–129. 10.1016/S0031-9384(02)00688-1 [DOI] [PubMed] [Google Scholar]
- Huff ML, Miller RL, Deisseroth K, Moorman DE, LaLumiere RT (2013) Posttraining optogenetic manipulations of basolateral amygdala activity modulate consolidation of inhibitory avoidance memory in rats. Proc Natl Acad Sci U S A 110:3597–3602. 10.1073/pnas.1219593110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilango A, Kesner AJ, Broker CJ, Wang DV, Ikemoto S (2014) Phasic excitation of ventral tegmental dopamine neurons potentiates the initiation of conditioned approach behavior: parametric and reinforcement-schedule analyses. Front Behav Neurosci 8:155. 10.3389/fnbeh.2014.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane F, Coulombe D, Miliaressis E (1991) Amygdaloid self-stimulation: a movable electrode mapping study. Behav Neurosci 105:926–932. 10.1037/0735-7044.105.6.926 [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB (2002) Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci 22:1126–1136. 10.1523/JNEUROSCI.22-03-01126.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Pignatelli M, Xu S, Itohara S, Tonegawa S (2016) Antagonistic negative and positive neurons of the basolateral amygdala. Nat Neurosci 19:1636–1646. 10.1038/nn.4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S (2017) Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron 93:1464–1479.e5. 10.1016/j.neuron.2017.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie J, Bindra D (1976) An interpretation of autoshaping and related phenomena in terms of stimulus-incentive contingencies alone. Can J Psychol 30:157–173. 10.1037/h0082057 [DOI] [Google Scholar]
- Ledford CC, Fuchs RA, See RE (2003) Potentiated reinstatement of cocaine-seeking behavior following d-amphetamine infusion into the basolateral amygdala. Neuropsychopharmacology 28:1721–1729. 10.1038/sj.npp.1300249 [DOI] [PubMed] [Google Scholar]
- Lin JY, Lin MZ, Steinbach P, Tsien RY (2009) Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys J 96:1803–1814. 10.1016/j.bpj.2008.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintas A, Chi N, Lauzon NM, Bishop SF, Gholizadeh S, Sun N, Tan H, Laviolette SR (2011) Identification of a dopamine receptor-mediated opiate reward memory switch in the basolateral amygdala-nucleus accumbens circuit. J Neurosci 31:11172–11183. 10.1523/JNEUROSCI.1781-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ. (1974) The psychology of animal learning. London; New York: Academic. [Google Scholar]
- McDonald RJ, Hong NS (2004) A dissociation of dorso-lateral striatum and amygdala function on the same stimulus-response habit task. Neuroscience 124:507–513. 10.1016/j.neuroscience.2003.11.041 [DOI] [PubMed] [Google Scholar]
- McDonald RJ, Yim TT, Lehmann H, Sparks FT, Zelinski EL, Sutherland RJ, Hong NS (2010) Expression of a conditioned place preference or spatial navigation task following muscimol-induced inactivations of the amygdala or dorsal hippocampus: a double dissociation in the retrograde direction. Brain Res Bull 83:29–37. 10.1016/j.brainresbull.2010.06.001 [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE (2003) Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology 168:57–65. 10.1007/s00213-002-1196-x [DOI] [PubMed] [Google Scholar]
- McNamara JO, Byrne MC, Dasheiff RM, Fitz JG (1980) The kindling model of epilepsy: a review. Prog Neurobiol 15:139–159. 10.1016/0301-0082(80)90006-4 [DOI] [PubMed] [Google Scholar]
- Millan EZ, Kim HA, Janak PH (2017) Optogenetic activation of amygdala projections to nucleus accumbens can arrest conditioned and unconditioned alcohol consummatory behavior. Neuroscience 360:106–117. 10.1016/j.neuroscience.2017.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ. (1987) Limbic-motor integration. Prog Psychobiol Physiol Psvchol 12:117–170. [Google Scholar]
- Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, Holden SS, Mertens KL, Anahtar M, Felix-Ortiz AC, Wickersham IR, Gray JM, Tye KM (2015) A circuit mechanism for differentiating positive and negative associations. Nature 520:675–678. 10.1038/nature14366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, Ed 2 Sydney; Orlando: Academic. [DOI] [PubMed] [Google Scholar]
- Prado-Alcalá R, Wise RA (1984) Brain stimulation reward and dopamine terminal fields: I. Caudate-putamen, nucleus accumbens and amygdala. Brain Res 297:265–273. 10.1016/0006-8993(84)90567-5 [DOI] [PubMed] [Google Scholar]
- Rescorla RA. (1988) Pavlovian conditioning: it's not what you think it is. Am Psychol 43:151–160. 10.1037/0003-066X.43.3.151 [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Solomon RL (1967) Two-process learning theory: relationships between Pavlovian conditioning and instrumental learning. Psychol Rev 74:151–182. 10.1037/h0024475 [DOI] [PubMed] [Google Scholar]
- Robbins TW. (1978) The acquisition of responding with conditioned reinforcement: effects of pipradrol, methylphenidate, d-amphetamine, and nomifensine. Psychopharmacology 58:79–87. 10.1007/BF00426794 [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Warlow SM, Berridge KC (2014) Optogenetic excitation of central amygdala amplifies and narrows incentive motivation to pursue one reward above another. J Neurosci 34:16567–16580. 10.1523/JNEUROSCI.2013-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE (2008) The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience 151:579–588. 10.1016/j.neuroscience.2007.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi MA, Sukharnikova T, Hayrapetyan VY, Yang L, Yin HH (2013) Operant self-stimulation of dopamine neurons in the substantia nigra. PLoS One 8:e65799. 10.1371/journal.pone.0065799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo DO, Funderburk SC, Bhatti DL, Motard LE, Newbold D, Girven KS, McCall JG, Krashes M, Sparta DR, Bruchas MR (2016) A GABAergic projection from the centromedial nuclei of the amygdala to ventromedial prefrontal cortex modulates reward behavior. J Neurosci 36:10831–10842. 10.1523/JNEUROSCI.1164-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kalivas PW (2013) Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Front Behav Neurosci 7:213. 10.3389/fnbeh.2013.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A (2011) Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475:377–380. 10.1038/nature10194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabbara RI, Maddux JM, Beharry PF, Iannuzzi J, Chaudhri N (2016) Effects of sucrose concentration and water deprivation on Pavlovian conditioning and responding for conditioned reinforcement. Behav Neurosci 130:231–242. 10.1037/bne0000138 [DOI] [PubMed] [Google Scholar]
- Tolman EC. (1932) Purposive behavior in animals and men. London: Century/Random House UK. [Google Scholar]
- Trujillo-Pisanty I, Sanio C, Chaudhri N, Shizgal P (2015) Robust optical fiber patch-cords for in vivo optogenetic experiments in rats. MethodsX 2:263–271. 10.1016/j.mex.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Janak PH (2007) Amygdala neurons differentially encode motivation and reinforcement. J Neurosci 27:3937–3945. 10.1523/JNEUROSCI.5281-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Stuber GD, de Ridder B, Bonci A, Janak PH (2008) Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature 453:1253–1257. 10.1038/nature06963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warlow SM, Robinson MJF, Berridge KC (2017) Optogenetic central amygdala stimulation intensifies and narrows motivation for cocaine. J Neurosci 37:8330–8348. 10.1523/JNEUROSCI.3141-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Cely IC, Balleine BW, Maidment NT (2011) Micro-opioid receptor activation in the basolateral amygdala mediates the learning of increases but not decreases in the incentive value of a food reward. J Neurosci 31:1591–1599. 10.1523/JNEUROSCI.3102-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten HP. (1983) Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science 220:431–433. 10.1126/science.6836286 [DOI] [PubMed] [Google Scholar]
- White NM, McDonald RJ (1993) Acquisition of a spatial conditioned place preference is impaired by amygdala lesions and improved by fornix lesions. Behav Brain Res 55:269–281. 10.1016/0166-4328(93)90122-7 [DOI] [PubMed] [Google Scholar]
- Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, Deisseroth K (2010) Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc 5:439–456. 10.1038/nprot.2009.226 [DOI] [PMC free article] [PubMed] [Google Scholar]