Abstract
A 62-year-old woman with chronic kidney disease stage 4, sleep apnoea on continuous positive airway pressure and recent admission for acute-on-chronic diastolic heart failure presented to emergency room with weakness. She was hypotensive and had symptomatic bradycardia in the 30 s secondary to hyperkalaemia and beta-blockers, raising concern for BRASH syndrome. Antihypertensives were immediately held. Potassium-lowering agents (with calcium gluconate for cardiac stability) were begun, as were fluids and dopamine for vasopressor support. The patient was admitted to intensive care unit and electrophysiology was consulted. Over the next 2 days, the patient clinically improved: she remained off dopamine for over 24 hours; potassium levels and renal function improved; and heart rate stabilised in 60 s. The patient was eventually discharged and advised to avoid metolazone, bumetanide and carvedilol, with primary care provider and cardiology follow-up.
Keywords: adult intensive care, fluid electrolyte and acid-base disturbances, pacing and electrophysiology
Background
BRASH syndrome stands for Bradycardia, Renal failure, Atrioventricular (AV) node blocker, Shock and Hyperkalaemia. It is frequently under-recognised and is often confused with simple hyperkalaemia. Although hyperkalaemia is an entity that is aggressively managed within this syndrome, it is important to understand that it is the synergistic combination of both an AV blocker and hyperkalaemia that characterises a clinical presentation of BRASH syndrome.1
Case presentation
Our patient is a 62-year-old woman with a medical history of diastolic congestive heart failure (New York Heart Association (NYHA) Class 3), chronic kidney disease stage VI, sleep apnoea with continuous positive airway pressure, hypertension, hyperlipidaemia, type 2 diabetes mellitus and depression who presented to the emergency room shortly after a cardiology appointment. She was recently admitted for acute-on-chronic respiratory failure secondary to acute-on-chronic diastolic congestive heart failure; at that time, the patient was started on metolazone (2.5 mg weekly) in addition to the bumetanide (2.5 mg three times per day) she was already on. Potassium repletion and isosorbide dinitrate were also given with the intention to see outpatient cardiology for echo and stress test. At the said follow-up, the patient was bradycardic and she was told to stop her carvedilol (6.25 mg two times per day) with plan for Holter monitor the following week. The patient was asymptomatic at this appointment and the blood pressure (BP) was not documented. The patient had been on this combination of bumetanide, metolazone and carvedilol for approximately 15 days before being seen at this follow-up appointment. After the patient drove home, she was too weak to exit her car; she called emergency medical services and when they arrived, her heart rate (HR) was in the 30 s.
In the emergency department (ED), HR was 31 and BP was 63/32 with a mean arterial pressure of 42. On physical examination, the patient had a systolic ejection murmur in the left second intercostal space, crackles throughout lung bases bilaterally, appeared short of breath and 1+bilateral lower extremity pitting oedema. Her potassium was 8.0 meg/L on i-STAT, creatinine 4.06 mg/dL, blood urea nitrogen 76 mg/dL, brain natriuretic peptide 1795 pg/mL and lactate 3.0 mmol/L (table 1). Chest X-ray revealed no acute process. ECG demonstrated bradycardia with junctional rhythm. Concern was raised for BRASH syndrome. Dopamine drip was initiated to provide ionotropic (cardiac) and vasopressor support, as bradycardia was not responsive to atropine.2 Although the patient had oxygen saturation at 94% on room air, the patient still felt short of breath and so was put on 2 L oxygen through nasal cannula which improved saturation to >96%. Additionally, insulin, D50 and calcium gluconate were administered. EP was consulted. The patient was admitted to the intensive care unit (ICU) for further management.
Table 1.
Laboratory values from admission and discharge with institution ranges provided
| Admission laboratory values | Discharge laboratory values | Institution ranges | |
| Creatinine (mg/dL) | 4.06 (Pt baseline 1.6–1.9) | 1.84 | 0.60–1.40 |
| BUN (mg/dL) | 76 | 22 | 6–23 |
| BNP (pg/mL) | 1795 (Pt baseline 200–550) | 1038 | 0–100 |
| Potassium (meg/L) | 8.0 | 3.8 | 3.5–4.9 |
| TSH (μ(IU)/mL) | 9.73 | 4.61 | 0.35–4.01L |
| T4 (ng/dL) | 1.46 | 1.17 | 061–1.37 |
| T3 (pg/mL) | 3.8 | Not repeated | 2.8–4.4 |
| WBC (10ˆ3/μL) | 14.0 | 5.9 | 4.0–12 |
| Hgb (g/dL) | 11.2 | 10.5 | 12–15.0 |
| Glucose (mg/dL) | 241 | 183 | 65–66 |
| CPK (U/L) | 170 | 58 | 0–155 |
| Sodium (meq/L) | 130 | 141 | 135–145 |
| Lactate (mmol/L) | 3.0 | 0.9 | 0.2–1.8 |
BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CPK, creatine phosphokinase; Hgb, haemoglobin; TSH, thyroid-stimulating hormone; WBC, white blood cell count.
Investigations
EKG: bradycardia with junctional rhythm (figure 1).
Figure 1.
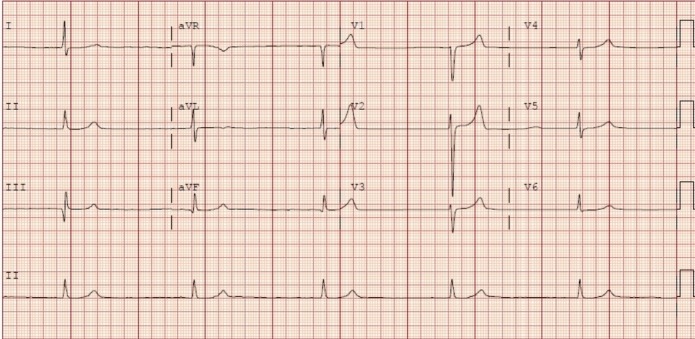
Admission EKG showing HR 31,junctional rhythm and peaked T-waves. HR, heart rate.
Bilateral renal ultrasound: left renal cyst, no hydronephrosis, norenal calculi, increased echogenicity consistent with medical renal disease.
Differential diagnosis
The list of possible pathology in a patient presenting with weakness is extensive, including infectious disease, congestive heart failure (cardiac), stroke (vascular), medication-induced, myasthenia gravis (autoimmune), hyponatraemia (nutritional/metabolic) and so on. The clinician must accordingly pay great attention to the history and physical in order to develop a differential diagnosis. Our patient presented with acute onset of weakness and history of recent admission for acute-on-chronic diastolic heart failure. Physical examination demonstrated a patient who was bradycardic, hypotensive, short of breath and had bilateral pitting oedema in the lower extremities. These findings along with the given history prompted initial testing and vasopressor support.
Infectious aetiology was quickly ruled out as the patient was afebrile, had an unremarkable chest X-ray and a normal white blood cell count. Hypothyroidism was also an unlikely cause for this patient’s decompensation as T3 levels were within the normal range. Initial EKG and troponin levels ruled out an acute ischaemic event. Further laboratory tests were consistent with acute-on-chronic renal failure, with creatinine 4.06 (baseline:~1.6) and potassium 8.0 with peaked T-waves seen on leads V2 and V5 in the patient’s EKG on admission (table 1, figure 1). Although these results guided us to hyperkalaemia as a possible aetiology of the patient’s weakness, substantial potassium elevation is needed for hyperkalaemia to cause bradycardia to this degree and our patient’s EKG did not demonstrate diffuse T wave changes or QT interval shortening. Additionally, the bradycardia, hypotension and recent carvedilol use also raised suspicion for beta-blocker toxicity; however, our patient reported medical compliance and denied the overuse of her beta-blocker medication. Thus, neither hyperkalaemia nor beta-blocker toxicity was likely to be the sole cause of this patient’s presentation.
Treatment
In the ED, the patient was given atropine, calcium gluconate, insulin with D50 fluid, sodium bicarbonate push, sodium chloride, as well as a dopamine infusion. Home BP medications (ie, carvedilol, bumetanide, isosorbide dinitrate, terazosin, hydralazine and amlodipine) were all held. Patient was put on D50 intravenous fluids at 250 cc over 4 hours for acute-on-chronic renal failure. The next day, she was successfully weaned off dopamine infusion. Bicarb drip was started to correct lactic acidosis and polystyrene sulfonate was initiated for further correction of hyperkalaemia. The patient continued to improve clinically and was stable for transfer to the hospitalist group on the third day of admission.
On the medical floors, the patient remained stable and continued to improve. As the hyperkalaemia and lactic acidosis were corrected, polystyrene sulfonate and the bicarb drip were discontinued accordingly. The patient’s renal function improved while on intravenous fluids and creatinine values returned to baseline.
Outcome and follow-up
The patient was stable for discharge with a resolution of admission laboratory findings after completion of treatment and had outpatient cardiology follow-up (table 1). Our patient’s follow-up EKG showed normal sinus rhythm and non-peaked T-waves (figure 2). The patient was advised to avoid metolazone, bumetanide, carvedilol and was advised to follow-up within a week with primary care and cardiology. The patient was instructed to continue the rest of her home medications as well as dulaglutide, degludec and aspart.
Figure 2.
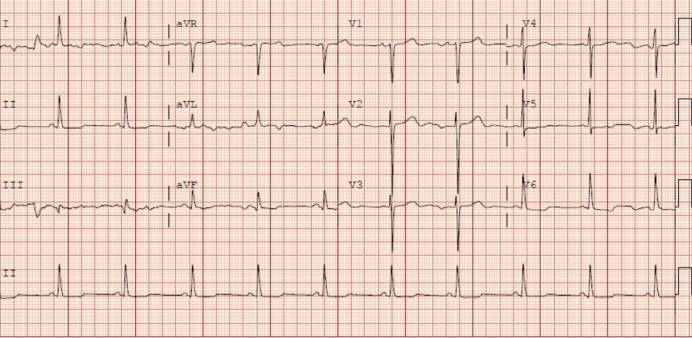
Follow-up EKG showing HR 61, normal sinus rhythm. HR, heart rate.
Discussion
BRASH syndrome is an acronym for Bradycardia, Renal failure, AV node blocker, Shock, and Hyperkalaemia. The syndrome is characterised by a pentad of these clinical findings and is often under-recognised or confused with simple electrolyte abnormalities such as hyperkalaemia.3 There are several triggers for BRASH syndrome4: antihypertensives, acute kidney injury and dehydration are all potential causes. However, the most common event that precipitates BRASH syndrome is hypovolaemia.5
Hypovolaemia (whether it be due decreased intake or increased loss) is one of the many possible causes for decreased perfusion that can trigger an acute kidney injury (serum creatinine by ≥0.3 mg/dL from baseline). Subsequently, the acute kidney injury leads to decreased excretion of potassium resulting in a hyperkalaemic state (K+level ≥5.5 mg/dL). The hyperkalaemic state is thought to synergistically enhance the effect of AV blocking agents within the body to exacerbate bradycardia (HR <60) and further decrease perfusion, thus increasing kidney injury.1 1 4 6 7 6
The treatment of BRASH syndrome is focused on managing haemodynamic status via vasopressors and fluids; hyperkalaemia via insulin, kayexalate or other potassium-lowering agents; and kidney injury via fluids and, in severe cases, dialysis. Our patient’s haemodynamic status and renal function were stabilised using dopamine and intravenous fluids. Additionally, her hyperkalaemia was managed using insulin and polystyrene sulfonate, with the addition of calcium gluconate for cardiac protection.
Golchin et al reported an 84-year-old man with a medical history of hypertension who presented with weakness and polyuria. The interview found that the patient was on beta-blockers; his physical examination demonstrated hypotension and bradycardia in the 30s. Laboratory values revealed acute renal failure and hyperkalaemia of 7.1. The patient was given intravenous calcium, intravenous fluids, insulin with dextrose and put on dopamine drip. This patient required emergent dialysis and was discharged from the ICU 2 days later.1
Similarly, Aziz et al reported a 70-0year-old man who was admitted to the hospital for difficulty in breathing and generalised weakness. He presented with HR 38, creatinine 2.1, potassium 6.1 and BP 86/50. Home medications included valsartan, spironolactone and carvedilol for hypertension, and ECG revealed sinus arrest with high-grade heart block and junctional escape rhythm. He was treated with calcium chloride 1 gm intravenously, insulin 10 units intravenously and dextrose 50 mL of 50% solution. Within 24 hours, his potassium levels dropped to 4.2 and his ECG stabilised.3
In conclusion, BRASH syndrome is an under-recognised clinical diagnosis and its presentation is often confused with simple hyperkalaemia. However, this confusion is understandable as BRASH syndrome is quite variable in its presentation and further research is needed to establish consistent diagnostic criteria. The clinician should conceptualise this pathology as hypoperfusion that stems from a hyperkalaemic potentiation of AV blocker activity. This understanding can aid in faster recognition of this syndrome and can reduce the time until appropriate care is delivered thereby improving patient outcomes.
Learning points.
BRASH syndrome stands for Bradycardia, Renal failure, Atrioventricular node blocker, Shock and Hyperkalaemia
The syndrome is characterised by a pentad of these clinical findings, and it is often under-recognised and is variable in its presentation.
Hypoperfusion in BRASH syndrome results from a synergistic effect of hyperkalaemia increasing atrioventricular blocker activity.
This syndrome is treated by aggressive haemodynamic support, managing the patient’s hyperkalaemia and managing the patient’s renal function.
Footnotes
Contributors: SS: wrote the case summary and presentation, assisted in literature review, completed works cited and edited the final draft. TK: obtained consent; wrote the background/differential treatment/outcome, assisted in literature review and works cited, edited final draft. KH: wrote the discussion, assisted in the literature review and edited the final draft.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Golchin A, Zhou M, Khan A. Bradycardia, Renal Failure, AV-Nodal Blockers, Shock, and Hyperkalemia (BRASH) - A New Clinical Syndrome. ATS Journals 2018;B47. [Google Scholar]
- 2. Link MS, Berkow LC, Kudenchuk PJ, et al. Adult advanced cardiovascular life support. CPR and first aid, emergency cardiovascular care, 2015. Available: https://eccguidelines.heart.org/circulation/cpr-ecc-guidelines/part-7-adult-advanced-cardiovascular-life-support/ [Accessed 31 Jan 2020].
- 3. Aziz EF, Javed F, Korniyenko A, et al. Mild hyperkalemia and low eGFR a tedious recipe for cardiac disaster in the elderly: an unusual reversible cause of syncope and heart block. Heart Int 2011;6:e12 10.4081/hi.2011.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farkas J. PulmCrit- BRASH syndrome: bradycardia, renal failure, AV blocker, shock, hyperkalemia. Available: https://emcrit.org/pulmcrit/brash-syndrome-bradycardia-renal-failure-av-blocker-shock-hyperkalemia/ [Accessed 27 Nov 2019].
- 5. Gonuguntla K, Patil S, Manek G, et al. BRASH syndrome: lost in plain sight. Chest 2019;156:A2228 10.1016/j.chest.2019.08.2152 [DOI] [Google Scholar]
- 6. Spodick DH. Normal sinus heart rate: sinus tachycardia and sinus bradycardia redefined. Am Heart J 1992;124:1119–21. 10.1016/0002-8703(92)91012-P [DOI] [PubMed] [Google Scholar]
- 7. Bonvini RF, Hendiri T, Anwar A. Sinus arrest and moderate hyperkalemia. Ann Cardiol Angeiol 2006;55:161–3. 10.1016/j.ancard.2005.10.001 [DOI] [PubMed] [Google Scholar]


