Abstract
Background
Patients with a history of prior cancer are frequently excluded from cancer trials. Previous studies indicated that prior cancer does not adversely impact clinical outcomes for patients with lung cancer older than 65 years. However, it remains unknown whether these results are applicable to patients with lung cancer aged younger than 65 years old. The study aimed to investigate the impact of prior cancer history on younger patients with lung cancer.
Methods
We identified younger patients with lung cancer (<65 years) diagnosed between 2004 and 2009 in the Surveillance, Epidemiology, and End Results database. Propensity score matching was performed to balance differences in baseline characteristics between groups. Kaplan-Meier method and the Cox proportional hazards model were used to evaluate the impact of prior cancer on overall survival (OS).
Results
Among 103 370 eligible patients with lung cancer, 15.18% had a history of prior cancer. Lung and bronchus (25.83%), breast (14.13%), prostate (8.85%) and cervix uteri (4.74%) were the most common prior cancer types. Of prior cancers, 61.56% are localised and regional stages. More than 67.98% of prior cancers were diagnosed within 5 years of the index lung cancer diagnosis. The median times of diagnosis for prior cancers were 38 months. Patients with prior cancer had the same/non-inferior OS as that of patients without a prior cancer diagnosis (propensity score-adjusted HR=1.01, 95% CI=0.99 to 1.04, p=0.324). Subgroup analyses stratified by timing of prior cancer displayed almost the same tendency (p>0.05). Interestingly, early-stage patients with a history of prior cancer had adverse survival curves (p<0.05). Advanced-stage patients with prior cancer had non-inferior survival (p>0.05).
Conclusions
A prior cancer diagnosis has a heterogeneous effect on the survival of patients with lung cancer aged <65 years across different stages, but further prospective studies are still warranted.
Keywords: lung cancer, younger patients, prior cancer, SEER
Key questions.
What is already known about this subject?
Previous studies indicated that a prior cancer history does not impact the overall survival of lung cancer patients older than 65 years.
What does this study add?
Our study further investigated whether prior cancer could impact the clinical outcomes of patients with lung cancer younger than 65 years.
How might this impact on clinical practice?
Our finding indicated that a prior cancer history had a heterogeneous effect on the survival of lung cancer patients aged <65 years across different stages, which could help clinicians to decide whether a prior cancer history should be adopted as a exclusion criteria for younger lung cancer patients.
Introduction
Lung cancer is one of the most common causes of cancer-related mortality, accounting for 25% of all cancer deaths. Compared to other cancer types, advanced improvement of survival for lung cancer is very slow, for which the 5‐year survival rate is 18%.1 To improve the diagnosis and treatment of lung cancer, numerous clinical trials are warranted to test whether new medical approaches work and how well they work. However, the enrolment rate and the completion rate of cancer clinical trials are unable to keep pace.2 3 In the USA, fewer than 5% of patients with cancer are included in clinical trials.4 5 The low accrual rate has been attributed to many factors, among which increasingly complex eligibility criteria are a major barrier.6 7 What’s worse, some eligibility criteria are applied in clinical trials just because similar protocols have been applied in a similar population, without scientific validation.7
A prior cancer diagnosis has been adopted as an exclusion criterion due to the belief that a prior cancer diagnosis could adversely affect the outcomes of clinical trials. Up to 18% of potential participants were excluded from lung cancer clinical trials for this reason alone.8 Little evidence has been found to support this assumption. Instead, previous studies based on Surveillance, Epidemiology, and End Results (SEER)–Medicare database indicated that prior cancer does not adversely impact clinical outcomes for patients with lung cancer older than 65 years across different stages.9 10 Due to the restrictions of SEER–Medicare data, the findings of these studies were limited to elderly patients. However, patients with lung cancer aged <65 years are also a significant proportion of the whole population, which accounts for 30.3%.11 The clinical characteristics of younger patients, such as treatment tolerance and effectiveness, are different from those of older patients.12 13 It remains unknown whether a prior cancer diagnosis impacts the survival of younger patients with lung cancer. To address these assumptions, we determined the characteristics and prognostic impact of prior cancer among patients with lung cancer using the SEER database.
Methods
Data source and study population
All the data used in our study were extracted from the SEER database through SEER*Stat software V.8.3.5, which covers approximately 34.6% of the population in the USA (https://seer.cancer.gov/, accession numbers 13693-Nov2015 and lh8N79l2).14 15 Patients younger than 65 years old who were diagnosed with lung cancer from January 2004 to December 2009 were included in the study. Exclusion criteria were listed as follows: (1) <18 years of age at diagnosis, (2) patients with unknown follow-up information and survival data, (3) and patients with only death certificates or autopsy records. We extracted demographic and clinical characteristics from the SEER database, including age, sex, race, marital status, TNM stage (American Joint Committee on Cancer [AJCC] sixth edition), pathology grade, surgery records and radiotherapy records. The survival data were recorded in months. To include those who died within 1 month after the cancer diagnosis, we transformed 0-month survival into 0.5-month survival.16
Measures
As described in our previous study, we determined a prior cancer diagnosis according to SEER sequence numbers, which reflects the order of diagnosis time of all primary reportable neoplasms.17 We calculated the timing of prior cancer by subtracting the diagnosis date of the most recent prior cancer from the diagnosis date of the index lung cancer. The primary endpoint of the study was overall survival (OS). The cut-off date was set as 31 December 2014 to ensure at least 5 years of follow-up time for all included patients.
Statistical analysis
Patients included in our study were categorised into two groups: with a prior cancer diagnosis and without a prior cancer diagnosis. We assessed differences in the characteristics of these two group of patients, with t-test for continuous variables and Pearson χ2 analysis for categorical variables. Propensity score matching (PSM) method was employed to reduce the bias due to observed confounding variables in baseline characteristics.18 Propensity scores were calculated based on age, sex, race, marital status, TNM stage (AJCC sixth edition), pathology grade, surgery records and radiotherapy records. We performed a one-to-one PSM with a calliper of 0.2 and used these PSM pairs in subsequent analyses. With the Kaplan-Meier method, we estimated the OS of the patients and compared the differences between the two groups using log-rank tests. A multivariate Cox proportional hazards model was also built to identify whether prior cancer impacts the prognosis independently. The common demographic and clinical characteristics, including age, sex, race, marital status, TNM stage (AJCC sixth edition), pathology grade, surgery records and radiotherapy records, were entered as covariates. We set the significance level at 0.05 (two-sided). All the analyses were performed using R V.3.4.2 (Institute for Statistics and Mathematics, Vienna, Austria; www.r-project.org).
Results
In all, 103 370 eligible patients with lung cancer were identified, of whom 15 696 (15.18%) had a history of prior cancer. A prior cancer diagnosis was more common among women (53.9% vs 43.7%, p<0.001), the elderly (58.55 years vs 56.86 years, p<0.001), Caucasians (83.1% vs 78.6%, p<0.001) and married individuals (55.6% vs 53.0%, p<0.001). Other baseline patient characteristics are shown in table 1.
Table 1.
Baseline characteristics of patients with lung cancer in the original/matched data sets (N=15 696).
| Characteristics | Original data set | Matched data set | ||||
| No prior cancer N=87 674 (%) |
With prior cancer N=15 696 (%) |
P value | No prior cancer N=15 696 (%) |
With prior cancer N=15 696 (%) |
P value | |
| Age (years), mean (SD) | 56.86 (6.64) | 58.55 (5.91) | <0.001 | 58.74 (5.64) | 58.55 (5.91) | 0.005 |
| Race (%) | <0.001 | 0.301 | ||||
| Black | 13 230 (15.1) | 2003 (12.8) | 1937 (12.3) | 2003 (12.8) | ||
| Unknown/others | 5552 (6.3) | 649 (4.1) | 615 (3.9) | 649 (4.1) | ||
| White | 68 892 (78.6) | 13 044 (83.1) | 13 144 (83.7) | 13 044 (83.1) | ||
| Gender | <0.001 | 0.892 | ||||
| Male | 49 396 (56.3) | 7242 (46.1) | 7255 (46.2) | 7242 (46.1) | ||
| Female | 38 278 (43.7) | 8454 (53.9) | 8441 (53.8) | 8454 (53.9) | ||
| Marital status | <0.001 | 0.699 | ||||
| Unmarried | 41 228 (47.0) | 6962 (44.4) | 6997 (44.6) | 6962 (44.4) | ||
| Married | 46 446 (53.0) | 8734 (55.6) | 8699 (55.4) | 8734 (55.6) | ||
| Primary site | <0.001 | 0.532 | ||||
| Main bronchus | 5534 (6.3) | 733 (4.7) | 734 (4.7) | 733 (4.7) | ||
| Upper lobe | 44 914 (51.2) | 8097 (51.6) | 8240 (52.5) | 8097 (51.6) | ||
| Middle lobe | 3535 (4.0) | 732 (4.7) | 686 (4.4) | 732 (4.7) | ||
| Lower lobe | 18 213 (20.8) | 3777 (24.1) | 3705 (23.6) | 3777 (24.1) | ||
| Overlapping lesion | 1274 (1.5) | 179 (1.1) | 164 (1.0) | 179 (1.1) | ||
| Lung, NOS | 14 204 (16.2) | 2178 (13.9) | 2167 (13.8) | 2178 (13.9) | ||
| AJCC group | <0.001 | 0.020 | ||||
| I | 12 420 (14.2) | 4290 (27.3) | 4152 (26.5) | 4290 (27.3) | ||
| II | 3728 (4.3) | 777 (5.0) | 805 (5.1) | 777 (5.0) | ||
| III | 21 389 (24.4) | 3596 (22.9) | 3714 (23.7) | 3596 (22.9) | ||
| IV | 44 175 (50.4) | 5649 (36.0) | 5765 (36.7) | 5649 (36.0) | ||
| Unknown | 5962 (6.8) | 1384 (8.8) | 1260 (8.0) | 1384 (8.8) | ||
| Surgery | <0.001 | 0.934 | ||||
| Yes | 19 232 (21.9) | 5457 (34.8) | 5465 (34.8) | 5457 (34.8) | ||
| No/unknown | 68 442 (78.1) | 10 239 (65.2) | 10 231 (65.2) | 10 239 (65.2) | ||
| Radiotherapy | <0.001 | 0.850 | ||||
| Yes | 39 779 (45.4) | 5525 (35.2) | 5508 (35.1) | 5525 (35.2) | ||
| No/unknown | 47 895 (54.6) | 10 171 (64.8) | 10 188 (64.9) | 10 171 (64.8) | ||
| Grade | <0.001 | 0.667 | ||||
| Well differentiated | 3069 (3.5) | 950 (6.1) | 897 (5.7) | 950 (6.1) | ||
| Moderately differentiated | 11 510 (13.1) | 2864 (18.2) | 2820 (18.0) | 2864 (18.2) | ||
| Poorly differentiated | 22 875 (26.1) | 4131 (26.3) | 4190 (26.7) | 4131 (26.3) | ||
| Undifferentiated | 5055 (5.8) | 680 (4.3) | 682 (4.3) | 680 (4.3) | ||
| Unknown | 45 165 (51.5) | 7071 (45.0) | 7107 (45.3) | 7071 (45.0) | ||
| Stage | <0.001 | 0.033 | ||||
| Distant | 53 977 (61.6) | 7078 (45.1) | 7162 (45.6) | 7078 (45.1) | ||
| Localised | 10 937 (12.5) | 3976 (25.3) | 3822 (24.4) | 3976 (25.3) | ||
| Regional | 19 995 (22.8) | 4011 (25.6) | 4142 (26.4) | 4011 (25.6) | ||
| Unknown/unstaged | 2765 (3.2) | 631 (4.0) | 570 (3.6) | 631 (4.0) | ||
AJCC, American Joint Committee on Cancer; NOS, not otherwise specified.
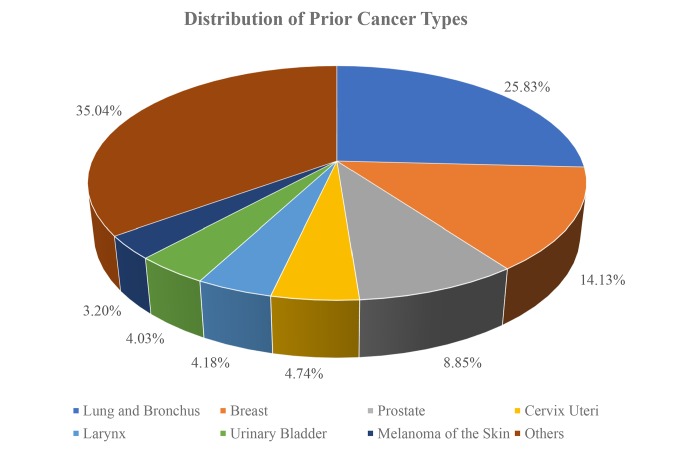
Lung and bronchus (25.83%), breast (14.13%), prostate (8.85%) and cervix uteri (4.74%) were the most common prior cancer types (figure 1). Among patients with prior cancer, distant stages were the most frequently observed, accounting for 45.1%. More than 67.98% of prior cancers were diagnosed within 5 years of the index lung cancer diagnosis. The median times between the index lung cancer date and the most recent diagnosis for prior cancers were 38 months. After propensity matching, the covariables were mostly balanced.
Figure 1.
Distributions of prior cancer types.
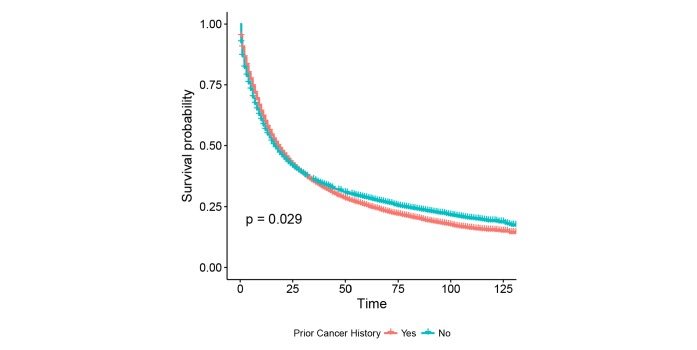
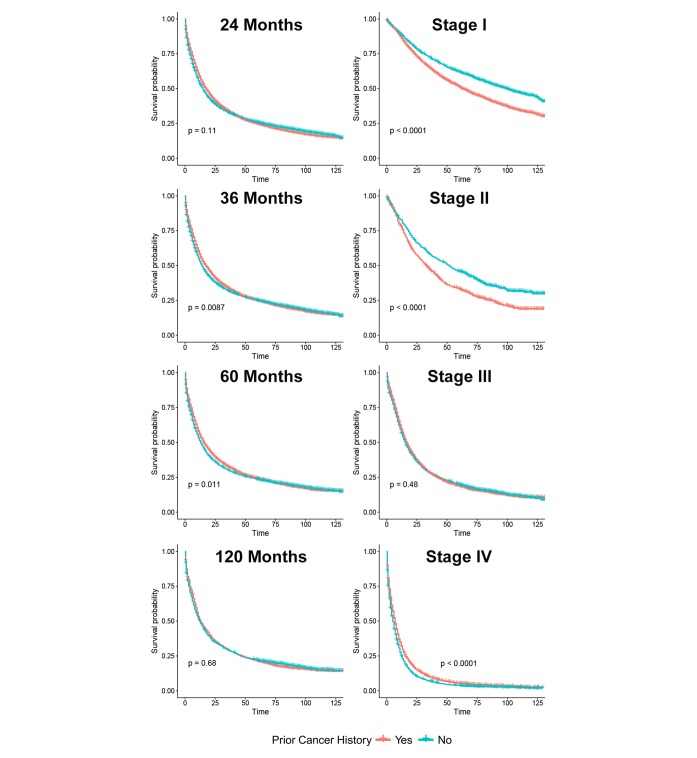
The unadjusted Kaplan-Meier curves in figure 2 showed an adverse effect of prior cancer on OS, compared with patients without a prior cancer diagnosis (log-rank tests, p=0.029). However, in Cox regression analysis, patients with prior cancer had the same/non-inferior OS as that of patients without a prior cancer diagnosis (HR=1.01, 95% CI=0.99 to 1.04, p=0.324). Subgroup analyses stratified by timing of prior cancer displayed almost the same tendency (p>0.05). Interestingly, early-stage patients with a prior cancer diagnosis had adverse survival than those without a history of prior cancer (p<0.05), while advanced-stage patients with and without a prior cancer had similar OS (p>0.05) (figure 3).
Figure 2.
The unadjusted Kaplan-Meier survival curves of prior cancer impact on the OS in younger patients with lung cancer. The OS of younger patients with lung cancer with a prior cancer was marginally better than that of patients without a prior cancer (p<0.05). OS, overall survival.
Figure 3.
Subgroup analysis of prior cancer impact on the overall survival stratified by the timing of prior cancer and American Joint Committee on Cancer (AJCC) stage in younger patients with lung cancer. The younger patients with lung cancer with prior cancer showed a similar survival with patients without a prior cancer, regardless of the timing of a prior cancer diagnosis. Early-stage patients with a history of prior cancer had adverse survival curves (p<0.05). Advanced-stage patients with prior cancer had non-inferior survival (p>0.05).
Discussion
Our current study suggested that a prior cancer diagnosis was not rare among younger patients with lung cancer. On the whole, a prior cancer diagnosis did not convey a distinguishing affect on the survival of the patients, which was consistent with the research that focused on patients older than 65 years and our previous study.9 10 17 19 However, in patients aged ≥65 years, the impact of prior cancer did not vary across different stages of lung cancer, which was distinguished from the heterogeneous effect of tumour stage in our study. We found that a prior cancer diagnosis adversely affected the OS of the early-stage patients but had no significant influence on the survival of the advanced-stage patients. The unique impact of a prior cancer diagnosis on younger patients with lung cancer may result from their unique clinical characteristics. Younger patients with lung cancer tend to have better prognosis than older patients, especially in patients with early-stage non-small cell lung cancer (NSCLC). Nevertheless, the OS of younger patients with advanced-stage NSCLC is only marginally better than that of the older patients, although the younger patients have fewer comorbidities.20 Younger patients with early-stage lung cancer are more likely to be cured than those with advanced disease and older patients. Therefore, the risks of increasing treatment intolerance due to prior cancer treatment are more likely to worsen the survival of younger patients with early-stage lung cancer. While older patients and advanced-stage patients are more likely to die from the progression of lung cancer,21 the impact of prior cancer is relatively neglectable. Our study indicated that the heterogeneous survival impact of a prior cancer diagnosis might need to be taken into consideration when recruiting younger volunteers for lung cancer clinical trials.
In most lung cancer trials, the time interval must be at least 5 years if patients who have prior cancer diagnosis want to be enrolled in.9 In our study, more than 67.98% of prior cancer were diagnosed within the 5-year interval in relation to the index lung cancer diagnosis. Our findings suggested that the timing of a prior cancer diagnosis relative to the index lung cancer did not convey an appreciable adverse effect on patients’ OS, suggesting that the impact of prior cancer history on younger patients’ OS was independent of follow-up time. In addition, the median times of diagnosis for prior cancers were 38 months, which indicated that active surveillance was necessary for cancer survivors.
In addition to concerns about the survival impact of prior cancer diagnosis, there is another reason why patients with prior cancer are usually excluded. The treatment they received might lower the patient’s tolerance or decrease the efficacy of further treatment for the current lung cancer. Although treatment toxicities cannot be determined in our current analysis due to data restriction, we believe this concern can be allayed in other ways. For example, we can restrict the enrolment according to organ function so that we could exclude patients who are intolerant of treatment. Moreover, prior cancer treatment can be employed as an exclusion criterion, which is different from prior cancer diagnosis exclusion. This strategy has been adopted in many lung cancer trials.9
There are also several limitations in our study. First, detailed characteristics of a prior cancer cannot be achieved through the SEER database. We defined a prior cancer history according to the documented order, and we could only include the type and the timing of prior cancer in our study. Other information such as the efficacy and toxicity of treatment on prior cancer could not be considered due to lack of relevant data. Second, in our PSM analyses and regression models, comorbidities were not included because there were no relevant data in the SEER database. PSM analysis could not adjust for unobservable differences between groups. Therefore, hidden bias resulting from unobservable confounders remained after the PSM process. Finally, further study is warranted to confirm the generality of our findings since SEER data only cover approximately 34.6% of the total US population, thus making it necessary to confirm the generality of our findings.
Conclusions
In conclusion, a prior cancer diagnosis has a heterogeneous effect on the survival of patients with lung cancer aged <65 years across different stages. For younger patients with early-stage lung cancer, a prior cancer history might need to be cautiously considered when deciding the exclusion criteria, while for younger patients with advanced lung cancer, broader inclusion trial criteria could be adopted in terms of prior cancer diagnosis, which could increase the accrual rate and enable the outcomes of the clinical trials to benefit more patients.
Acknowledgments
The authors acknowledge the efforts of the Surveillance, Epidemiology, and End Results programme tumor registries in providing high-quality open resources for researchers.
Footnotes
JL, HZ and YZ contributed equally.
Presented at: This study was previously presented as an abstract at International Association for the Study of Lung Cancer Asia Conference on Lung Cancer 2018.
Contributors: JL, HZ, and YZ contributed equally to this work and should be regarded as cofirst authors. LZ, JL, HZ and YZ and were responsible for the conception and design of the study, the interpretation of data, drafting and writing of the article; WF, YY, SH, GC and SZ were responsible for acquisition, analysis and interpretation of data and drafting the text, and also participated in the drafting of the article; JS, WX, ZZ and XC were responsible for the interpretation of the data and drawing the figures. HZ and YH were responsible for the revision of the intellectual content. All authors participated in the final approval of the article and agreed to be accountable for all aspects of the work.
Funding: This work was supported by the National Key R&D Program of China (grant numbers 2016YFC0905500 and 2016YFC0905503), the Chinese National Natural Science Foundation project (grant numbers 81872499 and 81772476), the Science and Technology Program of Guangdong (grant number 2017B020227001) and the Science and Technology Program of Guangzhou (grant numbers 201607020031 and 201704020072).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open access repository.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2. Institute of M, Board on Health Care S, Committee on Cancer Clinical T, the NCICGP A national cancer clinical trials system for the 21st century: Reinvigorating the NCI cooperative group program. National Academies Press, 2010. [PubMed] [Google Scholar]
- 3. Meropol NJ, Kris MG, Winer EP. The American Society of clinical oncology's blueprint for transforming clinical and translational cancer research. J Clin Oncol 2012;30:690–1. 10.1200/JCO.2011.40.1125 [DOI] [PubMed] [Google Scholar]
- 4. Howerton MW, Gibbons MC, Baffi CR, et al. . Provider roles in the recruitment of underrepresented populations to cancer clinical trials. Cancer 2007;109:465–76. 10.1002/cncr.22436 [DOI] [PubMed] [Google Scholar]
- 5. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 2004;291:2720–6. 10.1001/jama.291.22.2720 [DOI] [PubMed] [Google Scholar]
- 6. Tournoux C, Katsahian S, Chevret S, et al. . Factors influencing inclusion of patients with malignancies in clinical trials. Cancer 2006;106:258–70. 10.1002/cncr.21613 [DOI] [PubMed] [Google Scholar]
- 7. Kim ES, Bernstein D, Hilsenbeck SG, et al. . Modernizing eligibility criteria for molecularly driven trials. J Clin Oncol 2015;33:2815–20. 10.1200/JCO.2015.62.1854 [DOI] [PubMed] [Google Scholar]
- 8. Gerber DE, Laccetti AL, Xuan L, et al. . Impact of prior cancer on eligibility for lung cancer clinical trials. J Natl Cancer Inst 2014;106:dju302 10.1093/jnci/dju302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laccetti AL, Pruitt SL, Xuan L, et al. . Effect of prior cancer on outcomes in advanced lung cancer: implications for clinical trial eligibility and accrual. J Natl Cancer Inst 2015;107:djv002 10.1093/jnci/djv002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laccetti AL, Pruitt SL, Xuan L, et al. . Prior cancer does not adversely affect survival in locally advanced lung cancer: a national SEER-medicare analysis. Lung Cancer 2016;98:106–13. 10.1016/j.lungcan.2016.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Noone AM HN, Krapcho M, Miller D, et al., eds SEER Cancer statistics review, 1975-2015, 2018. [Google Scholar]
- 12. Thomas DM, Albritton KH, Ferrari A. Adolescent and young adult oncology: an emerging field. J Clin Oncol 2010;28:4781–2. 10.1200/JCO.2010.30.5128 [DOI] [PubMed] [Google Scholar]
- 13. Rich AL, Khakwani A, Free CM, et al. . Non-small cell lung cancer in young adults: presentation and survival in the English National lung cancer audit. QJM 2015;108:891–7. 10.1093/qjmed/hcv052 [DOI] [PubMed] [Google Scholar]
- 14. Etemadi A, Abnet CC, Graubard BI, et al. . Anatomical subsite can modify the association between meat and meat compounds and risk of colorectal adenocarcinoma: findings from three large US cohorts. Int J Cancer 2018;143:2261–70. 10.1002/ijc.31612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lehtinen M, Luostarinen T, Vänskä S, et al. . Gender-Neutral vaccination provides improved control of human papillomavirus types 18/31/33/35 through herd immunity: results of a community randomized trial (III). Int J Cancer 2018;143:2299–310. 10.1002/ijc.31618 [DOI] [PubMed] [Google Scholar]
- 16. Shaikh WR, Weinstock MA, Halpern AC, et al. . The characterization and potential impact of melanoma cases with unknown thickness in the United States' surveillance, epidemiology, and end results program, 1989-2008. Cancer Epidemiol 2013;37:64–70. 10.1016/j.canep.2012.08.010 [DOI] [PubMed] [Google Scholar]
- 17. Zhou H, Huang Y, Qiu Z, et al. . Impact of prior cancer history on the overall survival of patients newly diagnosed with cancer: a pan-cancer analysis of the SEER database. Int J Cancer 2018;143:1569–77. 10.1002/ijc.31543 [DOI] [PubMed] [Google Scholar]
- 18. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pruitt SL, Laccetti AL, Xuan L, et al. . Revisiting a longstanding clinical trial exclusion criterion: impact of prior cancer in early-stage lung cancer. Br J Cancer 2017;116:717–25. 10.1038/bjc.2017.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arnold BN, Thomas DC, Rosen JE, et al. . Lung cancer in the very young: treatment and survival in the National cancer data base. J Thorac Oncol 2016;11:1121–31. 10.1016/j.jtho.2016.03.023 [DOI] [PubMed] [Google Scholar]
- 21. Janssen-Heijnen MLG, van Erning FN, De Ruysscher DK, et al. . Variation in causes of death in patients with non-small cell lung cancer according to stage and time since diagnosis. Ann Oncol 2015;26:902–7. 10.1093/annonc/mdv061 [DOI] [PubMed] [Google Scholar]