Abstract
Background
While the BRAF V600E mutation occurs in 5%–15% of metastatic colorectal cancer (mCRC), BRAF non-V600E mutations were recently reported to range from 1.6% to 5.1%. We have previously reported that BRAF non-V600E mutations could have a negative impact on efficacy outcomes as well as BRAF V600E mutation for antiepidermal growth factor receptor (EGFR) antibody treatment for pretreated patients with mCRC. Recently, simultaneous inhibitions of mitogen-activated protein kinase kinase (MEK), BRAF and EGFR exhibited relevant antitumour activities in patients with BRAF V600E mutant and also in BRAF non-V600E mutant but only in the preclinical model.
Trial design
The BIG BANG (study is a multicentre, phase II study to assess the efficacy, safety and proof of concept of the combinations of binimetinib+encorafenib+cetuximab in patients with BRAF non-V600E mutated mCRC, identified by either tumour tissue (tumour tissue group) or blood samples (liquid biopsy group). Key eligibility criteria include Eastern Cooperative Oncology Group Performance Status of ≤1, mCRC with BRAF non-V600E mutant and RAS wild type, refractory or intolerant to at least one fluoropyrimidine-based regimen and no prior history of regorafenib, and no prior history of anti-EGFR antibody treatment (primary analysis cohort and liquid biopsy cohort) or refractory to prior anti-EGFR antibody treatment in patients with class 3 BRAF mutations (anti-EGFR antibody refractory class three cohort). Enrolled patients receive binimetinib (45 mg, two times per day), encorafenib (300 mg, once a day) and cetuximab (initially 400 mg/m2 and subsequently 250 mg/m2, once per week). The primary endpoint is the confirmed objective response rate in the primary analysis cohort.
Trial registration numbers
UMIN000031857 and 000031860.
Keywords: BRAF non-V600E, metastatic colorectal cancer, binimetinib, encorafenib, cetuximab
Introduction
BRAF is a member of the RAF family of serine/threonine kinases that transduces signals in the mitogen-activated protein kinase (MAPK) pathway.1 The hotspot mutation of BRAF V600E is found in approximately 5%–15% of patients with metastatic colorectal cancer (mCRC).2–4 BRAF V600E has been established as a marker of poor prognosis and limited efficacy for antiepidermal growth factor receptor (EGFR) antibody therapy.3 5 6 Recently, combination therapy with binimetinib, encorafenib and cetuximab has demonstrated prolongation of overall survival (OS) in pretreated patients with BRAF V600E mutated mCRC.7
In contrast, several studies have reported BRAF mutations other than V600E (BRAF non-V600E) ranging from 1.6% to 5.1% in patients with mCRC,8–12 and these mutants are increasingly identified in clinical practice with next-generation sequencing. A recent large cohort retrospective study has indicated that clinicopathological features of patients with BRAF non-V600E mutated mCRC were different from those with BRAF V600E mutated mCRC.11 BRAF mutations can be classified into three groups based on their biochemical and signalling mechanisms.13 14 Class 1 is composed of mutations occurring in codon 600, including the V600E mutation, which exhibit high kinase activity and are RAS-independent because they can signal as monomers. Mutations outside of the codon 600 in BRAF are divided into class 2 and class 3. Class 2 mutants are activating and RAS-independent; they dimerise and signal without RAS activation. Class 3 mutants exhibit low kinase activity or are kinase-dead but activate the MAPK pathway through enhanced RAS binding and subsequent RAS-dependent CRAF activation. These mechanisms might lead to differences between the clinical characteristics of patients with BRAF non-V600E mutated mCRC and those with BRAF V600E mutation.
In the BRAF non-V600E mutated cell line, anti-EGFR antibody monotherapy showed modest antitumour activity due to downstream RAF homodimerisation or heterodimerisation. Although a MEK inhibitor temporarily reduced p-ERK activity, reactivation of p-ERK soon occurred and was controlled by EGFR. Indeed, simultaneous inhibition of EGFR and MEK exhibited powerful inhibition of MAPK signalling in both class 2 and class 3 BRAF non-V600E mutated cell lines. Furthermore, a combination of anti-EGFR antibody, BRAF inhibitor and MEK inhibitor exhibited more powerful antitumour activity in a BRAF non-V600E mutated cell line and in a xenograft model.15
In terms of the clinical effect of anti-EGFR antibody therapy, BRAF non-V600E mutations could have been negative predictive factors for anti-EGFR antibody treatment in patients with RAS wild-type mCRC in the Biomarker Research for Anti-EGFR Monoclonal Antibodies by ComprehensiveCancer Genomics (BREAC) study as well as in the MD Anderson Cancer Centre cohort.12 16 In addition, according to the previous phase I/II study on patients with BRAF V600E mutated mCRC,17 the combination of only anti-EGFR antibody and MEK inhibitor showed lower efficacy and greater grade 3 dermatological toxicities compared with the triple combination of anti-EGFR antibody, BRAF inhibitor and MEK inhibitor, suggesting that combining BRAF inhibitors has not only additional efficacies but also a toxicity-mitigating effect. Based on these facts, we planned a multicentre phase II study with a combination of a MEK inhibitor, binimetinib, a BRAF inhibitor, encorafenib and an anti-EGFR antibody, cetuximab, using the same regimen as the BEACON-CRC study for BRAF V600E mutated mCRC, in patients with anti-EGFR antibody naïve BRAF non-V600E mutated mCRC.
Contradicting reports to the BREAC study and the MD Anderson Cancer Centre cohort have been reported recently. In an Italian study, three of four patients treated with upfront chemotherapy plus cetuximab provided partial response (PR).18 Furthermore, our large cohort study including 118 patients with BRAF non-V600E mutated mCRC in Japan and the USA revealed that patients with class 2 BRAF mutated mCRC did not respond to anti-EGFR antibody treatment, while some of those with class 3 mutations did respond.19 Based on these results, we also added an exploratory anti-EGFR antibody refractory class 3 cohort to investigate the efficacy of triple combination therapy in those who are refractory to prior anti-EGFR antibody therapy.
Methods
Study design and treatment
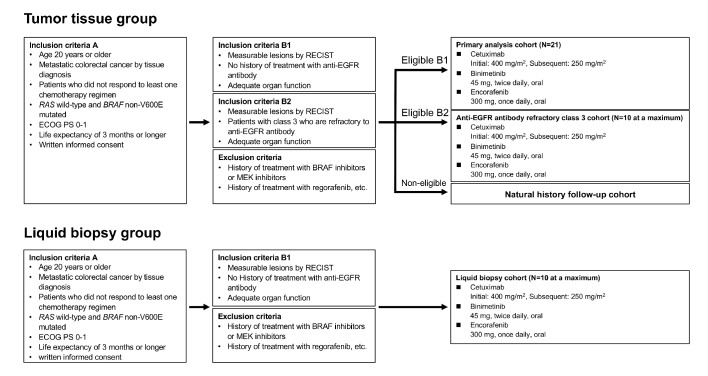
Our multicentre, proof-of-concept, phase II study aimed to evaluate the efficacy and safety of combination therapy of binimetinib, encorafenib and cetuximab in patients with BRAF non-V600E mutated mCRC. This study consists of two groups: patients with mCRC with the BRAF non-V600E mutation as determined by tumour tissue analysis are enrolled in the tumour tissue group, and those in with the BRAF non-V600E mutation as determined by blood samples analysis are enrolled in the liquid biopsy group. Patients who satisfy the eligibility of both groups are enrolled in the tumour tissue group. Tumour tissue analysis is mandatory for enrolment in the liquid biopsy group. Furthermore, the tumour tissue group includes the primary analysis cohort and anti-EGFR antibody refractory class 3 cohort (figure 1). Patients receive encorafenib 300 mg every day plus binimetinib 45 mg two times per day plus cetuximab 400 mg/m2, followed by 250 mg/m2 intravenously per week in 28-day cycles. The study treatment continues until disease progression, unacceptable toxicity, patient withdrawal, investigator’s decision, pregnancy or death.
Figure 1.
Study design.
In this study, Guardant360 is used to screen patients harbouring the non-V600E BRAF mutation and to monitor the emergence of mutations causing resistance to the therapy in the following translational research as a circulating tumour DNA (ctDNA) test in blood samples. Guardant360 is a panel that detects 74 cancer-associated genomic alterations of ctDNA extracted from blood samples, using a digital sequencing technology by detecting single-nucleotide variation with a sensitivity of 99.9% and a positive predictive value of 99.6%.
This study is registered in the University Hospital Medical Information Network.
Patients
Eligibility criteria are shown in box 1. Patients who meet all inclusion criteria A and all inclusion criteria in either B1 or B2 and do not meet any of the exclusion criteria are enrolled for study treatment. Patients who meet all inclusion criteria A, but meet none of the inclusion criteria in B1 or B2, or who do not meet any of the exclusion criteria are enrolled in the natural history follow-up cohort and are followed up for information on antitumour treatment, post-treatment and survival every 3 months as a historical control.
Box 1. Eligibility criteria.
Inclusion c riteria A ( a ll cohorts)
Age of 20 years or older on the day of signing informed consent.
Confirmed diagnosis of advanced (unresectable) or metastatic colorectal cancer by tissue diagnosis.
Patients who did not respond to or tolerate at least one chemotherapy regimen (including irinotecan or oxaliplatin) containing fluoropyrimidine drugs in the treatment of metastatic CRC and are thus eligible for second or later line treatment.
-
RAS wild-type and BRAF non-V600E mutated CRC. The diagnosis should be based on the results of associated genetic tests and the record should be available.
Patients with overlapping RAS mutation or BRAF V600E mutation in the same tumour sample are not considered eligible.
It is desirable that the tests be performed at a Clinical Laboratory Improvements Amendments-certified or quality-qualified central laboratory.
Patients with wild-type RAS and BRAF non-V600E mutation by tumour tissue analysis will be enrolled in the primary analysis cohort or anti-EGFR antibody refractory class 3 cohort. Patients with wild-type RAS and BRAF non-V600E mutation by blood sample analysis using liquid biopsy will be enrolled in the liquid biopsy cohort. If the patient satisfies the eligibility requirements of both the primary analysis cohort and the liquid biopsy cohort, the patient will be enrolled in the primary analysis cohort.
Eastern Cooperative Oncology Group Performance Status of 0 or 1.
Life expectancy of 3 months or longer.
Patients who signed a written informed consent.
Inclusion c riteria B1 (primary analysis cohort and liquid biopsy group)
Measurable lesions in accordance with the Response Evaluation Criteria in Solid Tumours (RECIST) V.1.1.
No history of treatment with epidermal growth factor receptor (EGFR) inhibitors, including anti-EGFR antibody drugs cetuximab or panitumumab.
-
Patients with the following organ functions:
-
Neutrophil count≥1500/mm3.
Platelet count≥100 000/mm3.
Haemoglobin≥90.0 g/L.
Serum creatinine≤1.5 mg/dL or calculated or measured values of creatinine clearance≥50 mL/min.
T-Bil<1.5 mg/dL, not applicable in the case of Gilbert’s syndrome.
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST)<100 or <200 IU/L with liver metastases.
-
-
Adequate cardiac function as characterised by the following at screening:
Left ventricular ejection fraction (LVEF) of ≥50% as determined by a multiple-gated acquisition technique (MUGA) scan or ECHO.
Mean triplicate QT interval corrected for heart rate using Fridericia's formula (QTcF) value of ≤480 ms.
Patients who can take oral medicine.
Women of childbearing potential who are negative for pregnancy in a urine pregnancy test.
Female patients and men must agree to take appropriate precautions to avoid pregnancy with screening until 90 days after the final administration of the investigational drugs (see section 6.5).
Inclusion c riteria B2 (anti-EGFR antibody refractory class 3 cohort)
Measurable lesions in accordance with the RECIST V.1.1.
Patients with class 3 BRAF non-V600E mutated metastatic CRC as determined from tumour tissue samples.
Patients who are refractory to an anti-EGFR antibody, including cetuximab or panitumumab.
-
Patients with the following organ functions:
Neutrophil count≥1500/mm3.
Platelet count≥1 00 000/mm3.
Haemoglobin≥9.0 g/L.
Serum creatinine of ≤1.5 mg/dL or calculated or measured values of creatinine clearance of ≥50 mL/min.
T-Bil<1.5 mg/dL, not applicable in the case of Gilbert’s syndrome.
ALT and AST<100 or <200 IU/L with liver metastases.
-
Adequate cardiac function as characterised by the following at screening:
LVEF of ≥50% as determined by a MUGA scan or ECHO.
Mean triplicate QTcF value ≤480 ms.
Patients who can take oral medicine.
Women of childbearing potential who are negative for pregnancy in a urine pregnancy test.
Female patients and men agree to take appropriate precautions to avoid pregnancy with screening until 90 days after the final administration of the investigational drugs if of childbearing potential (see section 6.5).
Exclusion c riteria ( a ll cohorts)
History of treatment with BRAF inhibitors or MEK inhibitors.
History of treatment with regorafenib.
Symptomatic brain metastases or meningeal dissemination.
Leptomeningeal disease.
Medical history, current condition or risk of retinal vein occlusion.
Inadequately controlled diabetes requiring insulin therapy.
Known acute or chronic pancreatitis.
Medical history of clinically significant cardiac diseases.
Gastrointestinal function or gastrointestinal diseases that significantly interfere with absorption, distribution, metabolism and excretion of the study drugs.
No history of other malignant tumours within the 3 years prior to the start of study treatment. In cases of lesions corresponding to carcinoma in situ and intramucosal carcinoma judged to be cured by local therapy, non-metastatic prostate cancer not requiring systemic therapy, and other solid cancers that do not require therapy or are not estimated to be adversely affected by the study treatment, patients will not be excluded from the study if the coordinating committee concludes after consultation that there is no effect on the patient’s prognosis.
Medical history of thromboembolism within 6 months.
Concurrent neuromuscular disorder that is associated with the potential of elevated creatine kinase (CK).
-
Previous treatment with any of the following:
Cyclical chemotherapy within a period of time shorter than the cycle length used for that treatment .
Bevacizumab, aflibercept or ramucirumab within 3 weeks.
Biological therapy (except bevacizumab, aflibercept or ramucirumab), immunotherapy, marketed small-molecular compounds or non-marketed investigational anticancer treatments within 4 weeks, or within a period ≤5-fold of the half-life (whichever is shorter).
Prior radiotherapy to ≥30% of bone marrow.
Patients who have not recovered from Common Terminology Criteria for Adverse Events grade2 or higher toxicity due to previous chemotherapy.
Major surgery within 2 weeks before the start of study treatment.
Women who are breastfeeding.
Known HIV infection.
Patients with active hepatitis B or C.
Other serious, acute or chronic, medically important abnormalities.
Patients taking herbal preparations/medications.
Use of known potent cytochrome P450 3A4 inhibitors.
History of Gilbert’s syndrome or patients with UGT1A1∗6/∗6, UGT1A1∗28/∗28 and UGT1A1∗6/∗28.
Endpoints and assessments
The primary endpoint is the confirmed objective response rate (ORR) by the investigators' assessment in the primary analysis cohort. The secondary endpoints are progression-free survival (PFS), duration of response (DoR), disease control rate (DCR) as determined by the investigators' assessment, confirmed ORR by central radiological assessment, OS and the incidence of adverse events (AEs). Efficacy will be evaluated according to Response Evaluation Criteria in Solid Tumours (RECIST) V.1.1 using CT every 4 weeks until the end of cycle 4, and thereafter every 8 weeks. ORR is defined as the proportion of patients who achieve a complete response (CR) or a PR. OS is defined as the period from enrolment to death from any cause, and it is censored on the last day the patient is alive. PFS is defined as the period from enrolment to progression or death from any cause, and is censored on the last day the patient is alive without progression. DoR is defined as the period from the confirmed response to progression or death from any cause and is censored on the last day the patient is alive without progression. DCR is calculated as the proportion with CR, PR or stable disease as evaluated by RECIST V.1.1. AEs are assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) V.4.0 before administration of the investigational drug on the administration day. In the liquid biopsy part, the same endpoints will be assessed.
Target sample size and statistical analysis
According to the three reports of anti-EGFR antibody treatment in patients with BRAF V600E mutated mCRC, the response rate was 0%–11%.5 20 21 Considering the sample size in each report, the pooled response rates were approximately 6% at best when several meta-analytic approaches were employed. The required sample size of the primary analysis cohort was calculated as 21 with an ORR of 30% deemed promising (one-sided α, 0.025; β, 0.2). Considering the feasibility issues, the planned sample size of anti-EGFR antibody refractory class 3 cohort and liquid biopsy cohort was set at a maximum of 10 patients, respectively.α
In the primary analysis cohort, a statistical significance will be declared when there are ≥5 responders in accordance with the RECIST V.1.1, which corresponds to a criterion of ORR of ≥23.8%. The Kaplan-Meier method will be used to perform survival analyses. The incidence of AEs in the safety population will also be reported.
Biomarker analyses and translational research
Serial blood samples will be collected at three time points: before the start of the protocol treatment, before cycle 2 and after the discontinuation of the protocol treatment. In patients who are able to have a tumour tissue biopsy, biopsy samples are also collected at the same three time points. We will investigate biomarkers for the efficacy of or resistance to the study treatment in patients with BRAF non-V600E mutated mCRC. The BRAF non-V600E test is performed at Clinical Laboratory Improvement Amendments or College of American Pathologists-certified laboratories. Blood samples will be analysed using Guardant360 to monitor the emergence of mutations causing resistance to the therapy. Additionally, we plan to establish new cancer cell lines with BRAF non-V600E mutated CRC using the tumour biopsy samples.
Conclusion
The BIG BANG study is the first phase II study to evaluate the efficacy, safety and proof-of-concept (POC) of combination therapy with binimetinib, encorafenib and cetuximab in patients with BRAF non-V600E mutated mCRC.
In this era of precision medicine, the findings will shed light on the potential value of triple targeted combination therapy for patients with BRAF non-V600E mutated mCRC.
Footnotes
Contributors: DK, HB and TY designed and prepared the protocol. SN is the chief statistician. All authors contributed to the collection and analysis of data in this study. All authors approved the final version of the manuscript for submission and agreed to be accountable for the contents.
Funding: The investigational drugs were provided by ONO Pharmaceutical Co. Ltd. and Merck Biopharma Co. Ltd. The Japan Agency for Medical Research and Development funded this study (18lk0201065h0002).
Competing interests: DK reports receiving honoraria from Takeda, Chugai, Lilly and Merck Serono. HB reports receiving lecturer fee from Taiho and Lilly, and research expenses from Taiho, AstraZeneca and Sysmex. HT reports receiving honoraria from Takeda, Chugai, Merck Serono, Taiho, Bayer, Lilly, Yakult and Sanofi. TM reports receiving honoraria from Taiho, Merck Serono, Chugai, Yakult, Takeda, Lilly, Bayer and Sanofi, and research funding from Yakult, MSD, Daiichi Sankyo and Ono. YK reports receiving honoraria from Takeda, Chugai, Bristol-Myers Squibb, Ono, Merck Biopharma, Taiho, Bayer, Lilly, Yakult Honsha, Sanofi, Nipro, Moroo, Asahi Kasei, Mitsubishi Tanabe, Otsuka, Medical Review and Shiseido and research funding from MSD, Daiichi Sankyo, NanoCarrier, Eisai, Sysmex, Shionogi, IQVIA, Parexel International, Astellas, Mediscience, Sumitomo Dainippon, A2 Healthcare, Ono, Taiho, Bayer, Yakult Honsha and Sanofi. TN reports receiving honoraria from Taiho, Lilly, Chugai, Kyowa Hakko Kirin, Sawai, Takeda, BMS, Ono, Bayer, Dainippon Sumitomo, Merck Serono, MSD, Sanofi, Nippon Kayaku, Celltrion Healthcare Japan and Teijin, and research funding from Taiho, Ono, Chugai, Merck Serono, Takeda, MSD, Dainippon Sumitomo, Eisai, Sanofi, Daiichi Sankyo, A2 Healthcare, Lilly, Nippon Kayaku and Solasia Pharma. TS reports receiving honoraria from Chugai, Merck Serono, Bristol-Myers Squibb, Takeda, Yakult Honsha, Lilly, Bayer Yakuhin, Ono, Merck, Astellas Pharma, Taiho and Nihonkayaku; having a consulting or advisory role at Bayer, Lilly, Ono, Takara Bio, Merck Serono and Nihonkayaku; and receiving research funding from Yakult Honsha, Chugai, Ono, Sanofi, Lilly, Daiichi Sankyo, Merck Serono, Gilead Sciences and Dainippon Sumitomo. TE reports receiving honoraria from Lilly, Taiho, Bristol-Myers Squibb Japan, Eisai, Daiichi Sankyo, Merck Serono, Chugai, Ono, Takeda, Bayer and Sanofi, and research funding from Daiichi Sankyo, Merck Serono, MSD, Novartis, Dainippon Sumitomo, Ono, Astellas Pharma, Lilly, Bayer, Nihonkayaku, Pfizer and Bristol-Myers Squibb Japan. SN reports receiving honoraria from Taiho and Astrazeneca. AS reports receiving research funding from MSD, Eisai, Ono, Taiho, Takeda and Bayer. AO reports receiving honoraria from Ono, BMS, Chugai, Taiho, Eisai and Amgen; research funding from Bristol-Myers Squibb; and, being an immediate family member, has been employed by Celgene. TY reports receiving research funding from Novartus, MSD, Sumitomo Dainippon, Chugai, Sanofi, Daiichi-Sankyo, Parexel, Ono, GlaxoSmithKline and Boehringer Ingelheim.
Patient consent for publication: Not required.
Ethics approval: This study was conducted in accordance with the guidelines for Good Clinical Practice of the International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, as well as the ethical guidelines for medical and health research involving human subjects. All patients were required to sign written informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available.
References
- 1. Holderfield M, Deuker MM, McCormick F, et al. . Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat Rev Cancer 2014;14:455–67. 10.1038/nrc3760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davies H, Bignell GR, Cox C, et al. . Mutations of the BRAF gene in human cancer. Nature 2002;417:949–54. 10.1038/nature00766 [DOI] [PubMed] [Google Scholar]
- 3. Sanz-Garcia E, Argiles G, Elez E, et al. . Braf mutant colorectal cancer: prognosis, treatment, and new perspectives. Ann Oncol 2017;28:2648–57. 10.1093/annonc/mdx401 [DOI] [PubMed] [Google Scholar]
- 4. Yaeger R, Chatila WK, Lipsyc MD, et al. . Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell 2018;33:125–36. 10.1016/j.ccell.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seymour MT, Brown SR, Middleton G, et al. . Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (Piccolo): a prospectively stratified randomised trial. Lancet Oncol 2013;14:749–59. 10.1016/S1470-2045(13)70163-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Brummelen EMJ, de Boer A, Beijnen JH, et al. . BRAF Mutations as Predictive Biomarker for Response to Anti-EGFR Monoclonal Antibodies. Oncologist 2017;22:864–72. 10.1634/theoncologist.2017-0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kopetz S, Grothey A, Yaeger R, et al. . Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer. N Engl J Med 2019;381:1632–43. 10.1056/NEJMoa1908075 [DOI] [PubMed] [Google Scholar]
- 8. Ciardiello F, Normanno N, Maiello E, et al. . Clinical activity of FOLFIRI plus cetuximab according to extended gene mutation status by next-generation sequencing: findings from the CAPRI-GOIM trial. Ann Oncol 2014;25:1756–61. 10.1093/annonc/mdu230 [DOI] [PubMed] [Google Scholar]
- 9. Haley L, Tseng L-H, Zheng G, et al. . Performance characteristics of next-generation sequencing in clinical mutation detection of colorectal cancers. Mod Pathol 2015;28:1390–9. 10.1038/modpathol.2015.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shen Y, Wang J, Han X, et al. . Effectors of epidermal growth factor receptor pathway: the genetic profiling of KRAS, BRAF, PIK3CA, NRAS mutations in colorectal cancer characteristics and personalized medicine. PLoS One 2013;8:e81628 10.1371/journal.pone.0081628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones JC, Renfro LA, Al-Shamsi HO, et al. . Non-V600BRAF Mutations Define a Clinically Distinct Molecular Subtype of Metastatic Colorectal Cancer. J Clin Oncol 2017;35:2624–30. 10.1200/JCO.2016.71.4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shinozaki E, Yoshino T, Yamazaki K, et al. . Clinical significance of BRAF non-V600E mutations on the therapeutic effects of anti-EGFR monoclonal antibody treatment in patients with pretreated metastatic colorectal cancer: the biomarker research for anti-EGFR monoclonal antibodies by comprehensive cancer genomics (BREAC) study. Br J Cancer 2017;117:1450–8. 10.1038/bjc.2017.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yao Z, Yaeger R, Rodrik-Outmezguine VS, et al. . Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature 2017;548:234–8. 10.1038/nature23291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schirripa M, Biason P, Lonardi S, et al. . Class 1, 2, and 3 BRAGF mutated metastatic colorectal cancer: a detailed clinical, pathological and molecular characterization. Clin Cancer Res 2019;13:3954–61. [DOI] [PubMed] [Google Scholar]
- 15. Kotani H, Adachi Y, Kitai H, et al. . Distinct dependencies on receptor tyrosine kinases in the regulation of MAPK signaling between BRAF V600E and non-V600E mutant lung cancers. Oncogene 2018;37:1775–87. 10.1038/s41388-017-0035-9 [DOI] [PubMed] [Google Scholar]
- 16. Johnson B, Loree JM, Jacome AA, et al. . Atypical, non-V600 BRAF mutations as a potential mechanism of resistance to EGFR inhibition in metastatic colorectal cancer. J Clin Oncol Precis Oncol 2019. [Epub ahead of print: 5 Aug 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corcoran RB, André T, Atreya CE, et al. . Combined BRAF, EGFR, and MEK Inhibition in Patients with BRAF V600E-Mutant Colorectal Cancer. Cancer Discov 2018;8:428–43. 10.1158/2159-8290.CD-17-1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cremolini C, Di Bartolomeo M, Amatu A, et al. . BRAF codons 594 and 596 mutations identify a new molecular subtype of metastatic colorectal cancer at favorable prognosis. Ann Oncol 2015;26:2092–7. 10.1093/annonc/mdv290 [DOI] [PubMed] [Google Scholar]
- 19. Yaeger R, Kotani D, Mondaca S, et al. . Response to anti-EGFR therapy in patients with BRAF non-V600–Mutant metastatic colorectal cancer. Clin Cancer Res 2019;25:7089–97. 10.1158/1078-0432.CCR-19-2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kopetz S, McDonough SL, Lenz H-J, et al. . Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406). J Clin Oncol 2017;35:3505 10.1200/JCO.2017.35.15_suppl.3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shitara K, Yonesaka K, Denda T, et al. . Randomized study of FOLFIRI plus either panitumumab or bevacizumab for wild-type KRAS colorectal cancer-WJOG 6210G. Cancer Sci 2016;107:1843–50. 10.1111/cas.13098 [DOI] [PMC free article] [PubMed] [Google Scholar]