Abstract
Background
Vitamin and mineral deficiencies, particularly those of iron, vitamin A, and zinc, affect more than two billion people worldwide. Young children are highly vulnerable because of rapid growth and inadequate dietary practices. Multiple micronutrient powders (MNPs) are single‐dose packets containing multiple vitamins and minerals in powder form, which are mixed into any semi‐solid food for children six months of age or older. The use of MNPs for home or point‐of‐use fortification of complementary foods has been proposed as an intervention for improving micronutrient intake in children under two years of age. In 2014, MNP interventions were implemented in 43 countries and reached over three million children.
This review updates a previous Cochrane Review, which has become out‐of‐date.
Objectives
To assess the effects and safety of home (point‐of‐use) fortification of foods with MNPs on nutrition, health, and developmental outcomes in children under two years of age.
For the purposes of this review, home fortification with MNP refers to the addition of powders containing vitamins and minerals to semi‐solid foods immediately before consumption. This can be done at home or at any other place that meals are consumed (e.g. schools, refugee camps). For this reason, MNPs are also referred to as point‐of‐use fortification.
Search methods
We searched the following databases up to July 2019: CENTRAL, MEDLINE, Embase, and eight other databases. We also searched four trials registers, contacted relevant organisations and authors of included studies to identify any ongoing or unpublished studies, and searched the reference lists of included studies.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs with individual randomisation or cluster‐randomisation. Participants were infants and young children aged 6 to 23 months at the time of intervention, with no identified specific health problems. The intervention consisted of consumption of food fortified at the point of use with MNP formulated with at least iron, zinc, and vitamin A, compared with placebo, no intervention, or use of iron‐containing supplements, which is standard practice.
Data collection and analysis
Two review authors independently assessed the eligibility of studies against the inclusion criteria, extracted data from included studies, and assessed the risk of bias of included studies. We reported categorical outcomes as risk ratios (RRs) or odds ratios (ORs), with 95% confidence intervals (CIs), and continuous outcomes as mean differences (MDs) and 95% CIs. We used the GRADE approach to assess the certainty of evidence.
Main results
We included 29 studies (33,147 children) conducted in low‐ and middle‐income countries in Asia, Africa, Latin America, and the Caribbean, where anaemia is a public health problem. Twenty‐six studies with 27,051 children contributed data. The interventions lasted between 2 and 44 months, and the powder formulations contained between 5 and 22 nutrients. Among the 26 studies contributing data, 24 studies (26,486 children) compared the use of MNP versus no intervention or placebo; the two remaining studies compared the use of MNP versus an iron‐only supplement (iron drops) given daily. The main outcomes of interest were related to anaemia and iron status. We assessed most of the included studies at low risk of selection and attrition bias. We considered some studies to be at high risk of performance and detection bias due to lack of blinding. Most studies were funded by government programmes or foundations; only two were funded by industry.
Home fortification with MNP, compared with no intervention or placebo, reduced the risk of anaemia in infants and young children by 18% (RR 0.82, 95% CI 0.76 to 0.90; 16 studies; 9927 children; moderate‐certainty evidence) and iron deficiency by 53% (RR 0.47, 95% CI 0.39 to 0.56; 7 studies; 1634 children; high‐certainty evidence). Children receiving MNP had higher haemoglobin concentrations (MD 2.74 g/L, 95% CI 1.95 to 3.53; 20 studies; 10,509 children; low‐certainty evidence) and higher iron status (MD 12.93 μg/L, 95% CI 7.41 to 18.45; 7 studies; 2612 children; moderate‐certainty evidence) at follow‐up compared with children receiving the control intervention. We did not find an effect on weight‐for‐age (MD 0.02, 95% CI −0.03 to 0.07; 10 studies; 9287 children; moderate‐certainty evidence). Few studies reported morbidity outcomes (three to five studies each outcome) and definitions varied, but MNP did not increase diarrhoea, upper respiratory infection, malaria, or all‐cause morbidity.
In comparison with daily iron supplementation, the use of MNP produced similar results for anaemia (RR 0.89, 95% CI 0.58 to 1.39; 1 study; 145 children; low‐certainty evidence) and haemoglobin concentrations (MD −2.81 g/L, 95% CI −10.84 to 5.22; 2 studies; 278 children; very low‐certainty evidence) but less diarrhoea (RR 0.52, 95% CI 0.38 to 0.72; 1 study; 262 children; low‐certainty of evidence). However, given the limited quantity of data, these results should be interpreted cautiously.
Reporting of death was infrequent, although no trials reported deaths attributable to the intervention. Information on side effects and morbidity, including malaria and diarrhoea, was scarce.
It appears that use of MNP is efficacious among infants and young children aged 6 to 23 months who are living in settings with different prevalences of anaemia and malaria endemicity, regardless of intervention duration.
MNP intake adherence was variable and in some cases comparable to that achieved in infants and young children receiving standard iron supplements as drops or syrups.
Authors' conclusions
Home fortification of foods with MNP is an effective intervention for reducing anaemia and iron deficiency in children younger than two years of age. Providing MNP is better than providing no intervention or placebo and may be comparable to using daily iron supplementation. The benefits of this intervention as a child survival strategy or for developmental outcomes are unclear. Further investigation of morbidity outcomes, including malaria and diarrhoea, is needed. MNP intake adherence was variable and in some cases comparable to that achieved in infants and young children receiving standard iron supplements as drops or syrups.
Keywords: Child, Preschool; Humans; Infant; Food, Fortified; Avitaminosis; Avitaminosis/prevention & control; Deficiency Diseases; Dietary Supplements; Infant Nutritional Physiological Phenomena; Infant Nutritional Physiological Phenomena/physiology; Micronutrients; Micronutrients/administration & dosage; Micronutrients/deficiency; ; Randomized Controlled Trials as Topic; Trace Elements; Trace Elements/administration & dosage; Vitamins; Vitamins/administration & dosage
Plain language summary
Using a vitamin and mineral powder, mixed into complementary foods, to improve health and nutrition in children under two years of age
Review question
Does using a vitamin and mineral powder, mixed into complementary foods, improve health and nutrition in children under two years of age?
Background
Deficiencies of vitamins and minerals, particularly of iron, vitamin A, and zinc, affect more than two billion people worldwide. Young children are highly vulnerable because of rapid growth and inadequate dietary practices. Exclusive breastfeeding until six months of age, followed by complementary feeding combined with continued breastfeeding for at least two years, is recommended to maintain children's adequate health and nutrition. After six months of age, infants start to receive semi‐solid foods, but the quantities of vitamins and minerals can be insufficient to fulfil all of their requirements for growth and development. Multiple micronutrient powders (MNPs) are single‐dose packets of powder containing iron, vitamin A, zinc, and other vitamins and minerals that can be mixed into any semi‐solid food at home or at any other point of use, to increase the content of essential vitamins and minerals in the diet of infants and young children during this period. This is done without making any other changes to their usual diet.
Study characteristics
We searched up to July 2019 for all studies that assessed the use of MNP for improving the health and nutrition of children under two years of age. We included 29 studies that involved 33,147 infants and young children from low‐ and middle‐income countries in Asia, Africa, Latin America, and the Caribbean. Twenty‐six studies with 27,051 children contributed data. Of these 26 studies, 24 compared the use of MNP versus no intervention or placebo, and 2 compared the use of MNP versus an iron‐only supplement (iron drops) given daily. We found that a variety of MNP formulations containing between 5 and 22 vitamins and minerals were given for 2 to 44 months to infants and young children aged 6 to 23 months. Most studies were funded by government programmes or foundations; only 2 were funded by industry.
Key results
The use of MNP containing at least iron, zinc, and vitamin A for home fortification of foods was associated with reduced risk of anaemia of 18% and iron deficiency of 53% in children aged six months to two years compared with no intervention. Also, haemoglobin concentration and iron status improved. Studies did not find any effects on growth. There was no additional benefit in reducing risk of anaemia and improving haemoglobin concentration compared to usually recommended iron drops or syrups; however, only two studies compared these different interventions. No trials reported death attributable to the intervention. Information on deaths, side effects, and morbidity, including malaria and diarrhoea, was scarce. The use of MNP was beneficial for young children 6 to 23 months of age, independent of whether they lived in settings with different anaemia and malaria backgrounds and regardless of the length of the intervention.
MNP is better than no intervention or placebo and may be comparable to daily iron supplementation.The benefits of this intervention as a child survival strategy or for developmental outcomes are still unclear, and further investigation is required.
MNP intake adherence was variable and in some cases comparable to that achieved in infants and young children receiving standard iron supplements as drops or syrups.
Certainty of the evidence
For the comparison of MNP versus no intervention or placebo, we judged the certainty of evidence to be moderate for anaemia and high for iron deficiency. The certainty of evidence for all other outcomes in this comparison was either low or moderate.
Two trials that compared the use of MNP versus iron supplement showed similar effects on anaemia and haemoglobin but less diarrhoea; however, we judged the certainty of evidence as low for anaemia and very low for haemoglobin concentration due to the small number of study participants.
Summary of findings
Summary of findings for the main comparison. Home (point‐of‐use) fortification of foods with multiple micronutrient powders versus no intervention or placebo in children under two years of age.
| Population: children 6 to 23 months of age Settings: community settings Intervention: home fortification with multiple micronutrient powders (duration range: 2 to 36 months) Comparison: no intervention or placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) |
Relative effect (95% CI) |
№ of participants (studies) |
Certainty of the evidence (GRADE) | Comments | |
| Risk with no intervention or placebo | Risk with multiple micronutrient powders | |||||
| Anaemia (haemoglobin values lower than 110 g/L) | Study population |
RR 0.82 (0.76 to 0.90) |
9927 (16 RCTs) |
⊕⊕⊕⊝ Moderatea | ‐ | |
| 606 per 1000 | 497 per 1000 (461 to 545) | |||||
| Iron deficiency (as defined by trialists) | Study population | RR 0.47 (0.39 to 0.56) | 1634 (7 RCTs) |
⊕⊕⊕⊕ High | ‐ | |
| 335 per 1000 | 158 per 1000 (131 to 188) | |||||
| Haemoglobin concentration (g/L) | Mean haemoglobin concentration in control groups ranged from 91.2 g/L to 128 g/L | Mean haemoglobin concentration in intervention groups was, on average, 2.74 g/L higher (1.95 higher to 3.53 higher) | ‐ | 10,509 (20 RCTs) |
⊕⊕⊝⊝ Lowa,b | ‐ |
| Iron status (ferritin concentrations in μg/L) | Mean ferritin concentration in control groups ranged from 7.9 μg/L to 69.4 μg/L | Mean ferritin concentration in intervention groups was, on average, 12.93 μg/L higher (7.41 higher to 18.45 higher) | ‐ | 2612 (7 RCTs) |
⊕⊕⊕⊝ Moderatea | ‐ |
| Weight‐for‐age (z scores) | Mean weight‐for‐age z score in control groups ranged from −1.87 to 0.52 | Mean weight‐for‐age z score in intervention groups was, on average,0.02 lower (0.03 lower to 0.07 higher) | ‐ | 9287 (10 RCTs) |
⊕⊕⊕⊝ Moderateb | ‐ |
| All‐cause mortality | Not measured | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level because heterogeneity was high. bDowngraded one level because some studies are at high or unclear risk of selection and attrition bias.
Summary of findings 2. Home (point‐of‐use) fortification of foods with multiple micronutrient powders versus an iron‐only supplement in children under two years of age.
| Population: children 6 to 23 months of age Settings: community settings Intervention: home fortification with multiple micronutrient powders (duration 2 months) Comparison: iron‐only supplement | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) |
Relative effect (95% CI) |
№ of participants (studies) |
Certainty of the evidence (GRADE) | Comments | |
| Risk with iron supplements | Risk with multiple micronutrient powders | |||||
| Anaemia (haemoglobin values lower than 110 g/L) | Study population | RR 0.89 (0.58 to 1.39) | 145 (1 RCT) |
⊕⊕⊝⊝ Lowa | ‐ | |
| 467 per 1000 | 415 per 1000 (271 to 649) | |||||
| Iron deficiency (as defined by trialists) | Not measured | |||||
| Haemoglobin concentration (g/L) | Mean haemoglobin concentration ranged across control groups from 101 g/L to 110 g/L | Mean haemoglobin concentration in intervention groups was, on average, 2.81 g/L lower (10.84 lower to 5.22 higher) | ‐ | 278 (2 RCTs) |
⊕⊝⊝⊝ Very lowa,b | ‐ |
| Iron status (as defined by trialists) | Not measured | |||||
| Weight‐for‐age (z scores) | Not measured | |||||
| All‐cause mortality | Not measured | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels due to small sample size and wide 95% CI. bDowngraded one level due to considerable statistical heterogeneity and inconsistency in the results between trials.
Background
Description of the condition
Vitamin and mineral deficiencies affect more than two billion people worldwide (Micronutrient Initiative 2009), and there is a disproportionate burden of vitamin and mineral deficiencies in low‐ and middle‐income countries. Iron deficiency, which affects over half the world’s population, is the most common preventable nutritional deficiency. Together with vitamin A and zinc deficiencies, iron deficiency has the largest documented disease burden among micronutrients (Black 2008; WHO 2009; WHO 2016a). Infants and children are the most vulnerable groups to suffer from micronutrient malnutrition given the high vitamin and mineral intake they need for rapid growth relative to the amount of food they consume (Dewey 2003). The diets of infants and young children aged 6 to 23 months generally provide insufficient quantities of key micronutrients (particularly iron, vitamin A, zinc, and calcium) to meet their nutritional needs, and inclusion of animal‐source foods to fill the nutrient gap may not be practical or feasible for all populations in low‐ and middle‐income countries (PAHO 2001; WHO 2005). No global estimates of vitamin and mineral deficiencies are available specifically for children under two years of age; however, it is calculated that 190 million preschool children are affected by vitamin A deficiency (WHO 2009), and 273 million are affected by anaemia (Stevens 2013).
Vitamin A deficiency is the leading cause of childhood blindness (WHO 2009). Iron is essential for red blood cells and is involved in several metabolic reactions; compelling evidence shows that infants aged 6 to 23 months with iron deficiency anaemia are at risk for poor cognitive, motor, social‐emotional, and neurophysiological development (Lozoff 2007). Zinc is important during periods of accelerated growth and for tissues with rapid cellular differentiation and turnover such as the immune system and the gastrointestinal tract. Critical functions that are affected by zinc nutrition include physical growth, susceptibility to infection, and neurobehavioural development (Brown 2001).
Frequently, multiple vitamin and mineral deficiencies occur simultaneously, and their joint effects during the critical period from conception to two years of age can be associated with irreversible physical and cognitive consequences, increased perinatal mortality, and reduced physical work capacity and productivity (Lozoff 2007; Sanghvi 2007; WHO 2016a), leading to lifelong detrimental consequences for health, productivity, and economic growth. In fact, it has been estimated that nutritional risk factors, including underweight status, suboptimal breastfeeding, and vitamin and mineral deficiencies, particularly vitamin A, iron, and zinc, are responsible for 3.9 million deaths (35% of total deaths) and 144 million disability‐adjusted life years (DALYs) (33% of total DALYs) in children under five years of age worldwide (WHO 2009).
Description of the intervention
Interventions to prevent micronutrient malnutrition typically include exclusive breastfeeding during the first six months of life and continued breastfeeding until at least two years of age, dietary diversification to include foods with highly absorbable vitamins and minerals, fortification of staple and complementary foods, and provision of vitamin and mineral supplements (Bhutta 2013).
Vitamin A supplementation for children between six months and five years of age significantly reduces total mortality by about 23% to 30% (Beaton 1993; Fawzi 1993; Glasizou 1993; Imdad 2017), as well as childhood blindness by 70%. The reduction in mortality is believed to be mediated through improved vitamin A status, which may affect susceptibility to infection through an effect on the immune system (Stephensen 2001). Zinc supplementation leads to a 9% reduction in child mortality and a 23% reduction in the incidence of childhood diarrhoea (Brown 2009; WHO 2006). Because adequate iron status early in life is critical for motor and cognitive development, the World Health Organization (WHO) has recommended blanket iron supplementation for all infants and children aged 6 to 23 months in areas where the prevalence of anaemia is 40% or higher (INACG 1998; WHO 2016a). Micronutrient interventions, particularly vitamin A and zinc supplementation for children and fortification of foods with iron and iodine, have been shown to be among the most cost‐effective global development efforts (Horton 2008).
Despite the well‐recognised benefits of supplementation with one, two, or multiple micronutrients, implementation has been hindered by inadequate supply, low coverage, inadequate healthcare provider communication and support, poor intake adherence to dosing regimens, lack of perceived benefit, concerns about perceived negative effects, potential dose‐related side effects, and safety concerns (Galloway 1994; Galloway 2002; Sazawal 2006; Stoltzfus 2011; UNICEF 2011). In response to these operational constraints, 'home' or ‘point‐of‐use’ food fortification with multiple micronutrient powders (MNPs) was developed as an alternative to daily iron supplementation for delivering iron and other micronutrients with foods. MNPs for children aged 6 to 23 months are typically single‐dose packets of dry powder containing vitamins and minerals that parents or caregivers mix into any semi‐solid food right before eating; in institutional settings, multi‐serve sachets of MNPs are available that can be mixed into larger quantities of food before serving to groups of individuals. MNPs are not a substitute for food or breast milk and do not mix well with liquids. When MNPs are mixed into semi‐solid food and used correctly, there should be no change in colour, flavour, or taste of the food. Use of MNP does not require changes to typical complementary feeding practices, assuming local complementary foods are semi‐soft and not too liquid. For children aged 6 to 23 months, MNPs should reinforce and support appropriate infant and young child feeding practices, including exclusive breastfeeding for the first six months of life, not using MNP until a child is six months of age, continued breastfeeding for two years and beyond, and timely introduction of complementary foods at six months.
How the intervention might work
Although initial efficacy trials focused on formulations to reduce anaemia and iron deficiency, public health programmes recognised the opportunity to deliver additional micronutrients, and in programme settings, the most common formulation includes 5 or 15 vitamins and minerals, including iron, vitamin A, and zinc (UNICEF‐CDC 2013). The cost of increasing the number of micronutrients in the powder is minimal (the primary cost of the product is in the packaging) (De Pee 2008). In 2013, the MNP product cost of 180 sachets for one child for one year was estimated at USD4.50 (De Pee 2013). An additional benefit of MNPs over liquid iron supplements is that the potential for overdose is low with MNP (Zlotkin 2004). Further, MNP sachets are lightweight and relatively simple to store, transport, and distribute.
Ongoing research is examining the potential for morbidities with MNPs that contain iron. Providing iron in malaria‐endemic areas is a potential concern in that the malaria parasite requires iron for growth (Oppenheimer 2001). However, a recent Cochrane Review found that providing iron supplementation to children does not increase the risk of clinical malaria (risk ratio (RR) 0.93, 95% confidence interval (CI) 0.87 to 1.0; high‐quality evidence) (Neuberger 2016), and in areas where prevention and management services for malaria are available, there was a small reduction in the risk of clinical malaria with iron supplementation. The findings from this review, combined with other evidence, led to a recent update of WHO guidelines on iron supplementation in children, which state that in malaria‐endemic areas, providing iron supplementation for children should be done in conjunction with public health measures to prevent, diagnose, and treat malaria (WHO 2016a). Recent studies of iron MNP suggest that morbidity from iron may occur secondary to changes in the gut microbiome rather than to malaria (Paganini 2016). In a setting with high malaria burden where malaria treatment was available, a trial providing insecticide‐treated bed nets found a decreased incidence of malaria among young children consuming MNPs with iron daily compared to those given MNPs without iron (Zlotkin 2013); however, more children in the iron group were admitted to the hospital for diarrhoea during the intervention compared to the non‐iron group. In a large study of children in Pakistan aged 6 to 18 months, iron‐containing MNPs with or without zinc were associated with small but statistically significant increases in diarrhoea and chest in‐drawing (Soofi 2013 (C)). Finally, Jaeggi 2015 examined effects of daily MNPs with and without iron on the gut microbiome in six‐month‐old Kenyan infants, and found that infants consuming MNPs with iron had gut microbiome changes that favoured the growth of potentially harmful pathogens, involved limited beneficial strains, and were associated with increased intestinal inflammation. However, in a study that distributed iron‐containing MNPs through integrated market‐based community sales in a malaria‐endemic setting in Kenya, hospitalisations for diarrhoea and fever in the intervention versus control villages were decreased (Suchdev 2016). Public health MNP programmes have also reported maternal/caregiver perceptions of side effects in children after initiation of MNP intake, especially dark stools and loose stools (De Pee 2013). Overall, in both research and programmatic public health settings, most morbidity data are based on maternal or caregiver recall rather than on rigorous morbidity monitoring (Suchdev 2013). Programmes typically inform caregivers that these effects might occur as part of the behaviour change intervention strategy, which helps prepare caregivers and mitigates potential negative effects on MNP acceptability (De Pee 2013).
The demand for MNP public health programmes is increasing; the number of MNP programmes and the number of countries implementing them almost doubled between 2011 and 2013. In 2011, there were 34 MNP programmes in 22 countries, which increased to 61 MNP programmes in 43 countries in 2013. Most were integrated with infant and young child feeding programmes and used multiple channels to distribute MNP (UNICEF‐CDC 2013; UNICEF 2014). A majority of programmes have been designed and implemented with limited written guidance, and this is reflected in the considerable heterogeneity identified in programme design and implementation across countries (Jefferds 2013; Timmer 2013).
Why it is important to do this review
The WHO recommends exclusive breastfeeding until six months of age and continued breastfeeding for at least two years (PAHO 2001; WHO 2005). Intake of several vitamins and minerals after six months, including iron, zinc, calcium, selected B vitamins, and (in some settings) vitamin A, remains problematic because commonly available, frequently consumed low‐cost foods contain inadequate quantities of these nutrients.
Various Cochrane Reviews have evaluated the effects of supplementation with different vitamins and minerals among children. Neuberger 2016 examined the effects of iron supplementation with tablets or elixirs, alone or in combination with folic acid or other micronutrients, in children under 18 years of age living in malaria‐endemic areas. Published Cochrane Reviews have also assessed the effects of (1) iron supplementation for improving clinical, immunological, and virological outcomes in children infected with HIV (Adetifa 2009); (2) micronutrient supplementation among children and adults with HIV infection (Visser 2017); (3) oral or intramuscular iron therapy for improving psychomotor development and cognitive function in children under the age of three years with iron deficiency anaemia (Wang 2013); (4) iodine supplementation for preventing iodine deficiency disorders in children (Angermayr 2004); and (5) vitamin A supplementation for preventing mortality and morbidity among children aged six months to five years (Imdad 2017). A 2013 systematic review examined the effectiveness and safety of MNP with at least two nutrients in children of all ages and in women (Salam 2013).
A 2011 global assessment of home fortification interventions reported that 50% of MNP pilots or demonstration projects were planning to scale up the intervention for national distribution (UNICEF‐CDC 2013), and a 2013 global assessment reported that 50% of programmes currently implemented worldwide were designed for children aged 6 to 23 months (UNICEF 2014). An update of this systematic review (which was first published in 2011) (De‐Regil 2011), is needed to guide programmes on the effectiveness and safety of MNP, as well as to inform on appropriate dose, frequency, and duration of this intervention. This review is focused on nutrition, health, and developmental outcomes in infants and young children whose food is fortified with MNP, particularly iron, zinc, and vitamin A, before consumption. We also included effects of this intervention on morbidity and mortality outcomes.
Objectives
To assess the effects and safety of home (point‐of‐use) fortification of foods with MNPs on nutrition, health, and developmental outcomes in children under two years of age.
For the purposes of this review, home fortification with MNP refers to the addition of powders containing vitamins and minerals to semi‐solid foods immediately before consumption. This can be done at home or at any other place at which meals are consumed (e.g. schools, refugee camps). For this reason, MNPs are also referred to as point‐of‐use fortification.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs with individual randomisation or cluster randomisation.
Types of participants
Infants and young children younger than two years of age at the start of the intervention. We did not include infants under six months of age, as exclusive breastfeeding is recommended from birth to six months. We included apparently healthy children (as reported in the reviews) from the general population, although some may be at risk of having highly prevalent diseases such as malaria, HIV, diarrhoea, or even undernutrition. We included only studies from which we could obtain information on children younger than two years of age and those that included 51% or more children within our specified age range.
Types of interventions
MNPs including at least the three micronutrients iron, zinc, and vitamin A. We included MNPs given at the point of use at any dose, frequency, and duration.
Comparators were no intervention, placebo, or usual supplementation.
We included the following comparisons.
Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo.
Home (point‐of‐use) fortification of foods with MNP versus an iron‐only supplement.
Home (point‐of‐use) fortification of foods with MNP versus iron and folic acid supplements.
Home (point‐of‐use) fortification of foods with MNP versus the same multiple micronutrients as in supplements.
We included interventions that combined home provision of MNP for home (point‐of‐use) fortification with co‐interventions, such as education or other approaches, only if the other co‐interventions were the same in both intervention and comparison groups. We excluded studies examining supplementary food‐based interventions (e.g. lipid‐based supplements), micronutrient crushable tablets, fortified complementary foods, and other fortified foods.
Types of outcome measures
Primary outcomes
Anaemia (defined as haemoglobin values lower than 110 g/L)
Iron deficiency (as defined by trialists)
Haemoglobin concentration (g/L)
Iron status (as defined by trialists)
Weight for age (z scores)
All‐cause mortality
Secondary outcomes
Adherence (as defined by trialists)
Severe anaemia (as defined by trialists)
Length for age (z scores)
Weight for height (z scores)
All‐cause morbidity
Side effects (such as staining of teeth, vomiting, stool discolouration, constipation, coughing)
Diarrhoea
Upper respiratory tract infections
Ear infections
Iron overload
Serum retinol concentration (µmol/L)
Retinol binding protein (as defined by trialists)
Serum zinc concentration (g/dL)
Mental development and motor skill development (as defined by trialists, e.g. might include Bayley Mental Development Index, Bayley Psychomotor Development Index, Stanford‐Binet Test, DENVER II Developmental Screening Test)
For populations in malaria‐endemic areas, we planned to report on malaria incidence and malaria severity (De‐Regil 2011). Because this was not reported by trial authors, we included any measure that would describe effects of the intervention in malaria settings, such as malaria infectivity. When malaria prevalence was not reported, we defined an area as malaria endemic based on the WHO classification (WHO 2018).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases and trial registers up to July 2019.
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 7), in the Cochrane Library (searched 16 July 2019).
MEDLINE Ovid (1946 to July Week 1 2019).
MEDLINE In‐Process & Other Non‐Indexed Citations Ovid (searched 16 July 2019).
MEDLINE E‐Pub Ahead of Print Ovid (searched 16 July 2019).
Embase Ovid (1980 to Week 29 2019).
Cumulative Index to Nursing and Allied Health Literature (CINAHL EBSCOhost; 1937 to 16 July 2019).
Science Citation Index Web of Science (1970 to 16 July 2019).
Conference Proceedings Citation Index ‐ Science Web of Science (1990 to 16 July 2019).
African Index Medicus (indexmedicus.afro.who.int; searched 16 July 2019).
Latin American and Caribbean Health Science Information database (LILACS; lilacs.bvsalud.org/en; searched 16 July 2019).
Population Information Online (POPLINE) (www.popline.org; searched 16 July 2019).
ClinicalTrials.gov (clinicaltrials.gov; searched 16 July 2019).
International Standard Randomized Controlled Trials Number (ISRCTN) Registry (www.isrctn.com; searched 16 July 2019).
metaRegister of Clinical Trials (www.isrctn.com/page/mrct; last searched 28 February 2014, before service became unavailable).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch; searched 16 July 2019).
The search strategies for each source are provided in Appendix 1. We did not apply any date or language restrictions, and we found that no translation of relevant data was necessary.
Searching other resources
We searched the bibliographies of included studies and asked authors of included studies for lists of other studies that should be considered for inclusion. In January 2018, we contacted the following organisations for assistance in identifying ongoing or unpublished studies: Sprinkles Global Health Initiative; Home Fortification Technical Advisory Group; Nutrition Branch of the United Nations Children's Fund (i.e. UNICEF); World Food Programme; Nutrition International; Global Alliance for Improved Nutrition; Helen Keller International; Sight and Life Foundation; Departments of Nutrition for Health and Development at WHO; and US Centers for Disease Control and Prevention.
Data collection and analysis
In successive sections, we report only the methods that we used in this review. Please see our protocol ‐ De‐Regil 2011 ‐ and Table 3 for additionally planned but unused methods.
1. Unused methods.
| Section in protocol (De‐Regil 2011) | Pre‐planned method proposed in protocol (De‐Regil 2011) | Reason for non‐use |
| Types of outcome measures | We will group outcome time points as follows: immediately post intervention, 1 to 5 months post intervention, and 6 to 12 months post intervention | We limited our analyses to the end of the intervention, as only 1 study reported on continued follow‐up post intervention, and only for the intervention arm. We have described this in the Characteristics of included studies tables |
| Measures of treatment effect | We will use the SMD to combine trials that measure the same outcome but use different units of measurement | Outcomes were reported in same units |
| Subgroup analysis and investigation of heterogeneity | For comparisons related to malaria‐endemic areas, we will conduct a subgroup analysis by treatment and prevention of malaria | No information was available for this review |
SMD: standardised mean difference.
Selection of studies
EO and KSL screened titles and abstracts of all records yielded by the searches. All review authors then obtained the full‐text reports of those records deemed potentially relevant, or for which more information was needed to determine relevance, and assessed them for eligibility against the selection criteria (see Criteria for considering studies for this review).
If studies were published only as abstracts, or if study reports contained little information on methods, we attempted to contact the study authors to obtain further details on study design, populations, and interventions, to properly assess their eligibility for inclusion.
The selection process is record in the PRISMA flow diagram (Figure 1).
1.

Study flow diagram.
Data extraction and management
For eligible trials, two review authors independently extracted the information using a form designed and piloted for this review. This data collection form was adapted from a similar review. Two review authors extracted data from the trial reports and entered them into Review Manager 5 (Review Manager 2014). Two different review authors reviewed the data extracted and entered for accuracy. We resolved any discrepancies through discussion with the entire team and documented the process.
We completed the data collection form electronically and recorded information on the following.
Trial methods
Study design
Unit and method of allocation
Method of sequence generation
Masking of participants, personnel, and outcome assessors
Participants
Location of the study
Sample size
Age
Sex
Socioeconomic status (as defined by trialists and when such information was available)
Baseline prevalence of anaemia
Baseline prevalence of soil helminths
Baseline malaria prevalence
Inclusion and exclusion criteria
Interventions
Dose
Type of iron compound
Provision of MNP regimen
Duration of the intervention
Co‐intervention
Comparison group
No intervention
Placebo
Provision of iron supplements
Outcomes
Primary and secondary outcomes, as outlined under Types of outcome measures
Exclusion of participants after randomisation and proportion of losses at follow‐up
We recorded both pre‐specified and non‐pre‐specified outcomes, although we did not use the latter to underpin the conclusions of the review.
When information regarding any of the included trials was unclear, we attempted to contact authors of the original reports to obtain further details. If information was insufficient for us to be able to assess risk of bias, we categorised the trial as awaiting assessment, until further information is published or made available to us.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). Using the criteria set out in Appendix 2, both review authors assigned each study a rating of low, high, or unclear risk of bias for the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential sources of bias; we also assessed the overall risk of bias. We resolved any disagreement by discussion or by involving a third review author (JP‐R).
We assessed the overall risk of bias between trials by following the GRADE approach (Balshem 2010), as described in the 'Summary of findings' section below.
Measures of treatment effect
Dichotomous data
We presented results as risk ratios (RRs) or odds ratios (ORs) with 95% confidence intervals (CIs).
Continuous data
We used mean differences (MDs) with 95% CIs because studies measured outcomes in the same way.
Unit of analysis issues
Cluster‐randomised studies
We combined results from cluster‐randomised and individually randomised studies. All cluster‐randomised studies reported that sample size calculations reflected effects of clustering in the data. We conducted sensitivity analyses to examine the potential effects of clustering on CIs of the summary estimates (Sensitivity analysis). However, as the CI did not change significantly (P > 5%), we have not reported results of the sensitivity analyses. We labelled all cluster‐randomised studies with '(C)'.
Studies with more than two treatment groups
For studies with more than two intervention groups (multi‐arm studies), we reported all arms in the Characteristics of included studies tables and included the directly relevant arm only, including each group in the analysis only once (Higgins 2019). If we came across a study that compared home (point‐of‐use) fortification of foods with MNPs versus two of our comparison possibilities, we combined groups to create a single pair‐wise comparison (Christofides 2006 (C); Hirve 2007 (C)), as suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Dealing with missing data
We noted levels of attrition in all included studies in the Characteristics of included studies tables. Five included studies had high levels of attrition (all > 20%) (Adrianopoli 2014 (C); Esamai 2014; Luo 2017 (C); Sazawal 2014 (C); Somassè 2018 (C)), so we conducted sensitivity analyses to explore the impact of including these studies in the overall assessment of treatment effect (Sensitivity analysis).
We carried out analyses for all outcomes, as far as possible, on an intention‐to‐treat basis. We conducted analyses using available cases, and we conducted sensitivity tests (for dichotomous outcomes, assuming worst‐case scenario and assuming best‐case scenario) for primary outcomes, when data were available. For continuous outcomes, we used available data (i.e. no imputations) when data were missing.
Assessment of heterogeneity
We examined forest plots from the meta‐analyses to look for heterogeneity among studies. We assessed methodological heterogeneity by examining risk of bias, and clinical heterogeneity by examining similarities and differences between studies regarding types of participants, interventions, and outcomes. We considered the size and direction of effect and used tau², I², and Chi² statistics (P < 0.10) to quantify the level of statistical heterogeneity among the studies in each analysis. If we identified substantial heterogeneity (I² between 30% and 100%), we explored this by conducting pre‐specified subgroup analyses (Subgroup analysis and investigation of heterogeneity). We advise caution in interpretation of results with high levels of unexplained heterogeneity.
Assessment of reporting biases
When we suspected reporting bias (see "Selective reporting bias" under Assessment of risk of bias in included studies above), we contacted study authors to ask them to provide missing outcome data, or to clarify study design or other study issues. We advise caution in interpretation of results for which we suspected outcome reporting bias.
We used funnel plots to assess reporting bias (such as publication bias) and to investigate the relationship between effect size and standard error when 10 or more studies were included in a meta‐analysis.
Data synthesis
We carried out statistical analysis using RevMan 5 (Review Manager 2014). When we expected differences between studies in both the population and the intervention, or when we detected substantial statistical heterogeneity, we combined the data using a random‐effects model when it was clinically meaningful to do so, to provide an average treatment effect across studies. We used Mantel‐Haenszel weighting for dichotomous outcomes and inverse variance for continuous outcomes.
'Summary of findings'
We present the main findings of the review in our 'Summary of findings' tables, which we prepared using GRADE profiler software (GRADEpro GDT). Primary outcomes (anaemia, iron deficiency, haemoglobin concentration, iron status, weight‐for‐age, and all‐cause mortality) for the comparison of home (point‐of‐use) fortification of foods with multiple micronutrient powders versus no intervention or placebo and the comparison of home (point‐of‐use) fortification of foods with multiple micronutrient powders versus an iron‐only supplement have been listed, with estimates of relative effects along with numbers of participants and studies contributing data for those outcomes.
Two review authors assessed the certainty of evidence for each individual outcome included in the table, using the GRADE approach (Balshem 2010); differences were resolved through discussion or with involvement of another review author. The GRADE approach involves consideration of within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias. The results are expressed as having one of four levels of quality (high, moderate, low, or very low). We limited this assessment to the trials included in this review.
Subgroup analysis and investigation of heterogeneity
We conducted several subgroup analyses irrespective of heterogeneity. We interpreted all subgroup analyses cautiously. The planned subgroups arose from current clinical dilemmas and uncertainties (see Background). We explored subgroup analyses on the primary outcomes based on the following criteria.
By anaemic (defined as haemoglobin values < 110 g/L) status of participants at start of intervention (anaemic, non‐anaemic, or unknown anaemic status).
By iron status of participants at start of intervention as defined by trialists (iron deficient, not iron deficient, or unknown).
By age of participants at start of intervention (6 to 11 months, 12 to 17 months, 18 to 23 months).
By refugee status (yes, no, or unknown).
By malaria endemicity of the area at the time of the trial as reported by trialists (yes, no, or unknown).
By frequency (daily versus weekly versus flexible).
By duration of intervention (< 6 months versus ≥ 6 months).
By elemental iron content of product (< 12.5 mg versus ≥ 12.5 mg).
By zinc content of product (< 5 mg versus ≥ 5 mg).
For comparisons related to malaria‐endemic areas, we planned to conduct a subgroup analysis by treatment and prevention of malaria, but we found that limited information was available (Table 3).
Sensitivity analysis
We carried out sensitivity analyses to examine the effects of removing studies at high risk of bias (studies with poor or unclear allocation concealment and either lack of blinding or loss to follow‐up > 20% in each arm) from the analysis, and of including studies with children aged 6 to 59 months from which it was not possible to extract information for children aged 6 to 23 months only. Sensitivity analyses performed by removing high‐risk studies are post hoc analyses (see Differences between protocol and review) that were not originally planned in the protocol (De‐Regil 2011).
We also conducted sensitivity analyses to examine the potential effects of clustering on the CIs of summary estimates using a range of intracluster correlation coefficients (Unit of analysis issues). However, as the CI did not change significantly, we have not reported results of the sensitivity analyses in successive sections.
Results
Description of studies
Results of the search
Through the search strategy, we identified 26,442 references for possible inclusion, from which we removed 8582 duplicate references and excluded 4452 records because they were published prior to the first MNP trial in 2001. The screening process for the remaining 13,408 records is shown in Figure 1. We included 29 studies (Included studies) and excluded 34 (Excluded studies). Five studies are awaiting assessment (see Characteristics of studies awaiting classification tables).
Included studies
Below, we summarise the key characteristics of included studies. For a detailed description, please see the Characteristics of included studies tables.
We received additional information from the authors of two included studies (Hirve 2007 (C); Inayati 2012 (C)).
Study design
We included in this review 29 studies (from 43 reports) involving 33,147 children (Aboud 2011 (C); Adrianopoli 2014 (C); Adu‐Afarwuah 2007; Attanasio 2014 (C); Barffour 2019; Baum 2017 (C); Christofides 2006 (C); Clarke 2018 (C); Esamai 2014; Giovannini 2006; Hirve 2007 (C); Inayati 2012 (C); Jack 2012 (C); Kounnavong 2011; Lanou 2019 (C); Lundeen 2010 (C); Luo 2017 (C); Macharia‐Mutie 2012; Matias 2018 (C); Menon 2007 (C); Mridha 2016 (C); Olney 2018 (C); Osei 2015 (C); Sazawal 2014 (C); Sharieff 2006a; Somassè 2018 (C); Soofi 2013 (C); Larson 2018 (C); Young 2018 (C)). Of these, 26 studies (27,051 children) contributed data, and 3 studies did not contribute data (Luo 2017 (C); Olney 2018 (C); Young 2018 (C)). A total of 22 studies are new to this update (Aboud 2011 (C); Adrianopoli 2014 (C); Attanasio 2014 (C); Barffour 2019; Baum 2017 (C); Clarke 2018 (C); Esamai 2014; Inayati 2012 (C); Jack 2012 (C); Kounnavong 2011; Lanou 2019 (C); Luo 2017 (C); Macharia‐Mutie 2012; Matias 2018 (C); Mridha 2016 (C); Olney 2018 (C); Osei 2015 (C); Sazawal 2014 (C); Somassè 2018 (C); Soofi 2013 (C); Larson 2018 (C); Young 2018 (C).
All included studies are RCTs ‐ seven randomised at the individual level (Adu‐Afarwuah 2007; Barffour 2019; Esamai 2014; Giovannini 2006; Kounnavong 2011; Macharia‐Mutie 2012; Sharieff 2006a) and 22 cluster‐RCTs (Aboud 2011 (C); Adrianopoli 2014 (C); Attanasio 2014 (C); Baum 2017 (C); Christofides 2006 (C); Clarke 2018 (C); Hirve 2007 (C); Inayati 2012 (C); Jack 2012 (C); Lanou 2019 (C); Lundeen 2010 (C); Luo 2017 (C); Matias 2018 (C); Menon 2007 (C); Mridha 2016 (C); Olney 2018 (C); Osei 2015 (C); Sazawal 2014 (C); Somassè 2018 (C); Soofi 2013 (C); Larson 2018 (C); Young 2018 (C)). Although eligible for inclusion, no quasi‐RCTs were included.
Setting
The studies included in the review were carried out over the last 10 years in low‐ and middle‐income countries in Asia, Africa, Latin America, and the Caribbean, where anaemia is a public health problem (i.e. > 40% of the population is affected): Bangladesh (Aboud 2011 (C); Matias 2018 (C); Mridha 2016 (C)); Burkina Faso (Lanou 2019 (C)); Cambodia (Giovannini 2006; Jack 2012 (C)); China (Luo 2017 (C)); Colombia (Attanasio 2014 (C)); Guatemala (Olney 2018 (C)); Ghana (Adu‐Afarwuah 2007; Christofides 2006 (C)); Haiti (Baum 2017 (C); Menon 2007 (C)); India (Hirve 2007 (C); Larson 2018 (C); Sazawal 2014 (C); Young 2018 (C)); Indonesia (Inayati 2012 (C)); Kenya (Esamai 2014; Macharia‐Mutie 2012); Kyrzgyz Republic (Lundeen 2010 (C)); Lao People’s Democratic Republic (Barffour 2019; Kounnavong 2011); Mali (Clarke 2018 (C); Somassè 2018 (C)); Nepal (Osei 2015 (C)); Pakistan (Sharieff 2006a; Soofi 2013 (C)); and Tajikistan (Adrianopoli 2014 (C)).
Twenty studies reported anaemia prevalence at baseline (Adrianopoli 2014 (C); Adu‐Afarwuah 2007; Attanasio 2014 (C); Barffour 2019; Christofides 2006 (C); Esamai 2014; Giovannini 2006; Hirve 2007 (C); Inayati 2012 (C); Jack 2012 (C); Kounnavong 2011; Lanou 2019 (C); Lundeen 2010 (C); Luo 2017 (C); Macharia‐Mutie 2012; Menon 2007 (C); Osei 2015 (C); Somassè 2018 (C); Soofi 2013 (C); Larson 2018 (C)). In Adrianopoli 2014 (C), Giovannini 2006, and Osei 2015 (C), all infants were anaemic at the start of the intervention. In Esamai 2014, the baseline prevalence of anaemia was 0% and in the remaining studies ranged from 23% in Soofi 2013 (C) to 84% in Jack 2012 (C). Seven studies did not report the prevalence of anaemia (Aboud 2011 (C); Baum 2017 (C); Matias 2018 (C); Mridha 2016 (C); Sazawal 2014 (C); Sharieff 2006a; Young 2018 (C)).
According to the WHO classification (WHO 2018), 27 studies were performed in malaria‐endemic areas (Aboud 2011 (C); Adrianopoli 2014 (C); Adu‐Afarwuah 2007; Attanasio 2014 (C); Barffour 2019; Baum 2017 (C); Christofides 2006 (C); Clarke 2018 (C); Esamai 2014; Giovannini 2006; Hirve 2007 (C); Inayati 2012 (C); Jack 2012 (C); Kounnavong 2011; Lanou 2019 (C); Macharia‐Mutie 2012; Matias 2018 (C); Menon 2007 (C); Mridha 2016 (C); Olney 2018 (C); Osei 2015 (C); Sazawal 2014 (C); Sharieff 2006a; Somassè 2018 (C); Soofi 2013 (C); Larson 2018 (C); Young 2018 (C)). Only Kyrzgyz Republic and China, respectively, were classified as non‐malaria‐endemic (Lundeen 2010 (C); Luo 2017 (C)).
Inayati 2012 (C), Kounnavong 2011, and Lanou 2019 (C) reported that a malaria control programme was implemented in the study area. In the remaining reports, it is unclear whether malaria prevention and control programmes were in place at the study sites, or whether concomitant malaria interventions were made available for study participants.
None of the studies took place in refugee settings, so it was not possible to conduct a subgroup analysis for refugee status.
Participants
Participant age ranged from 6 to 60 months. When possible, we included data for children under 24 months of age only. All studies included children of both sexes. Sample sizes ranged from 45 in Esamai 2014 to 4292 in Larson 2018 (C). However, the analyses include only the estimated effective sample size, after data were adjusted to account for the clustering effect.
Interventions
Twenty‐six studies evaluated the effects of providing MNP versus no intervention or placebo (comparison 1) (Aboud 2011 (C); Adrianopoli 2014 (C); Adu‐Afarwuah 2007; Attanasio 2014 (C); Barffour 2019; Baum 2017 (C); Clarke 2018 (C); Esamai 2014; Giovannini 2006; Inayati 2012 (C); Jack 2012 (C); Kounnavong 2011; Lanou 2019 (C); Lundeen 2010 (C); Luo 2017 (C); Macharia‐Mutie 2012; Matias 2018 (C); Menon 2007; Mridha 2016 (C); Olney 2018 (C); Osei 2015 (C); Sazawal 2014 (C); Sharieff 2006a; Somassè 2018 (C); Soofi 2013 (C); Larson 2018 (C)). Of these studies, Luo 2017 (C) and Olney 2018 (C) did not contribute data to the analyses. Two studies compared effects of providing MNP versus an iron‐only supplement of iron drops or syrup (comparison 2) (Christofides 2006 (C); Hirve 2007 (C)). One study compared providing MNP versus iron and folic acid supplements (comparison 3) (Young 2018 (C)); however, this study did not contribute data to the analyses and so is not reported on further. No studies compared MNP versus multiple vitamin and mineral supplements (comparison 4).
Interventions lasted between two months in Christofides 2006 (C),Hirve 2007 (C),Lundeen 2010 (C),Menon 2007 (C), and Sharieff 2006a, and 44 months in Mridha 2016 (C). Study duration was variable in one study (Inayati 2012 (C)).
With the exception of Lanou 2019 (C), all studies used the same regimen of daily intake. Lanou 2019 (C) provided 15 MNP sachets to mothers every month (alternate‐day dosing).
Vitamin and mineral composition
MNPs were formulated with 22 micronutrients in one study (Olney 2018 (C)), 19 micronutrients in another (Sazawal 2014 (C)), and 18 micronutrients in a third (Macharia‐Mutie 2012). In seven studies, MNPs contained 15 micronutrients (Barffour 2019; Baum 2017 (C); Clarke 2018 (C); Lanou 2019 (C); Matias 2018 (C); Mridha 2016 (C); Somassè 2018 (C)); in four studies, 14 micronutrients (Inayati 2012 (C); Jack 2012 (C); Kounnavong 2011; Osei 2015 (C)); in one study, 12 micronutrients (Luo 2017 (C)); in two studies, seven micronutrients (Larson 2018 (C); Young 2018 (C)); in four studies, six micronutrients (Adu‐Afarwuah 2007; Christofides 2006 (C); Giovannini 2006; Sharieff 2006a); and in eight studies, five micronutrients (Aboud 2011 (C); Adrianopoli 2014 (C); Attanasio 2014 (C); Hirve 2007 (C); Esamai 2014; Lundeen 2010 (C); Menon 2007; Soofi 2013 (C)).
Fourteen studies provided 12.5 mg of elemental iron as ferrous fumarate; eight studies used 10 mg of elemental iron (Clarke 2018 (C); Inayati 2012 (C); Kounnavong 2011; Lanou 2019 (C); Matias 2018 (C); Mridha 2016 (C); Osei 2015 (C); Somassè 2018 (C)); one study provided 9 mg of ferrous orthophosphate (Olney 2018 (C)); one study provided 6 mg of ferrous lactate (Luo 2017 (C)); and one study provided 6 mg of elemental iron (Barffour 2019). Macharia‐Mutie 2012 used 2.5 mg of iron as sodium‐iron‐ethylenediaminetetraacetic acid (NaFeEDTA), and Sharieff 2006a used 30 mg of elemental iron. Two studies also tested micronised ferrous pyrophosphate as the iron compound and three dosages of elemental iron (as ferrous fumarate) ‐ 12.5 mg, 20 mg, and 30 mg (Christofides 2006 (C); Hirve 2007 (C)).
Five mg of elemental zinc (as gluconate) was used in 15 studies. Of the 14 remaining studies, four used 10 mg of zinc (Barffour 2019; Jack 2012 (C); Sazawal 2014 (C); Soofi 2013 (C)); one used 8.0 mg of zinc (Olney 2018 (C)); one used 6.0 mg of zinc (Luo 2017 (C)); seven used 4.1 mg of zinc (Clarke 2018 (C); Kounnavong 2011; Lanou 2019 (C); Matias 2018 (C); Mridha 2016 (C); Osei 2015 (C); Somassè 2018 (C)); and one used 2.5 mg of zinc (Macharia‐Mutie 2012).
The content of vitamin A in the MNP was as follows: 300 μg vitamin A in 13 studies (Aboud 2011 (C); Adrianopoli 2014 (C); Adu‐Afarwuah 2007; Attanasio 2014 (C); Christofides 2006 (C); Esamai 2014; Giovannini 2006; Hirve 2007 (C); Jack 2012 (C); Lundeen 2010 (C); Sharieff 2006a; Soofi 2013 (C); Young 2018 (C)); 400 μg in 10 studies (Barffour 2019; Clarke 2018 (C); Kounnavong 2011; Lanou 2019 (C); Matias 2018 (C); Menon 2007; Mridha 2016 (C); Olney 2018 (C); Osei 2015 (C); Somassè 2018 (C)); 375 μg in one study (Inayati 2012 (C)); and 200 μg in one study (Luo 2017 (C)). In Macharia‐Mutie 2012, MNPs contained only 100 μg of vitamin A, and in Sazawal 2014 (C), MNP contained 978.26 μg of vitamin A.
Baum 2017 (C) used a 15‐nutrient formulation that was not described in detail. All studies also provided folic acid as part of the MNP formulations.
Outcomes
Primary outcomes
Sixteen studies measured anaemia, 7 measured iron deficiency, 20 measured haemoglobin concentration, 7 measured iron status, and 10 measured weight‐for‐age. No studies measured all‐cause mortality.
Secondary outcomes
Eleven studies measured length‐for‐age, 10 measured weight‐for‐height, 3 measured all‐cause morbidity, 5 measured diarrhoea, 3 measured upper respiratory tract infections, 1 measured serum retinol concentrations, 2 measured retinol binding protein, 5 measured serum zinc concentration, and 1 measured mental and motor skill development. Three studies measured malarial infection. No studies measured adherence, severe anaemia, ear infections, side effects, or iron overload.
Funding sources
Funding sources of included studies are described in the notes of each Characteristics of included studies table. A total of 14 studies were funded by government programmes, 17 by foundations, and three by UNICEF or WHO ((Jack 2012 (C);Kounnavong 2011; Sazawal 2014 (C)). Only two studies reported industry funding (Inayati 2012 (C); Macharia‐Mutie 2012).
Excluded studies
We excluded 34 studies (from 51 reports). We excluded one study because the study design was not an RCT nor a quasi‐RCT; only two clusters were assigned to experimental or control groups (Jyoti 2014). We excluded another study because the children were not randomised (Cardoso 2016). In four studies, participants were from other age groups, such as two to six years of age (Chen 2008), three to six years of age (Sharieff 2006b), or 5 to 11 (school‐age) years of age (Troesch 2011), or they were young women (Troesch 2009), and a further four studies recruited high‐risk populations (not apparently healthy children) into the study (Lemaire 2011; Singla 2014; Van der Kam 2016a; Van der Kam 2016b). We excluded four studies that assessed interventions with MNP but provided only one or two of the relevant micronutrients (Yousafzai 2014; Zlotkin 2001; Zlotkin 2003a; Zlotkin 2003b); two studies that evaluated food‐like tablets (Smuts 2005; Wijaya‐Erhardt 2007); one study that assessed fortification of rice for use in childcare centre meals (Bagni 2009); and one study that compared MNP versus drops (multi‐vitamin supplements) but the drops did not include all three micronutrients that we used as inclusion criteria: vitamin A, iron, and zinc (Geltman 2009). We also excluded a study that evaluated a combined package of MNP and albendazole (Wang 2017). Finally, we excluded 12 studies because they did not include any of the comparisons of interest (e.g. they did not compare MNP versus placebo or supplements) (Avula 2011; Barth‐Jaeggi 2015; Bilenko 2014; Ip 2009; Jaeggi 2015; Khan 2014; Munayco 2013; Neufeld 2008; Samadpour 2011; Shafique 2014; Teshome 2017; Zlotkin 2013), one study because it had no comparison arm (Goyena 2019), and two studies because they focused on marketing of MNPs (Rawat 2015; Suchdev 2012). Suchdev 2012 was included in the previous version of this review, but we excluded this study because it focused on marketing of MNPs, and because the control group may have received MNPs, as vendors were not prevented from selling Sprinkles MNP in control villages.
See the Characteristics of excluded studies tables for a detailed description of excluded studies along with reasons for their exclusion.
Studies awaiting assessment
We listed five studies (from seven reports) as awaiting classification (Fernandez‐Rao 2014; Hasan 2017; Islam 2018b; ISRCTN39244429; ISRCTN57594793). Three studies are RCTs that have been completed but have not yet published results (Fernandez‐Rao 2014; ISRCTN39244429; ISRCTN57594793). We contacted the study authors but received no reply. Two studies are protocols for individual RCTs evaluating effects of MNPs on cognitive development (Hasan 2017), and on diarrhoea morbidity and growth (Islam 2018b). These studies have not been completed, and no results are available.
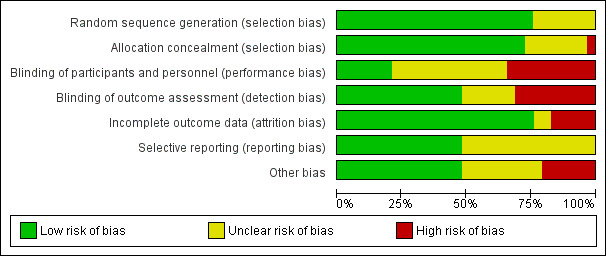
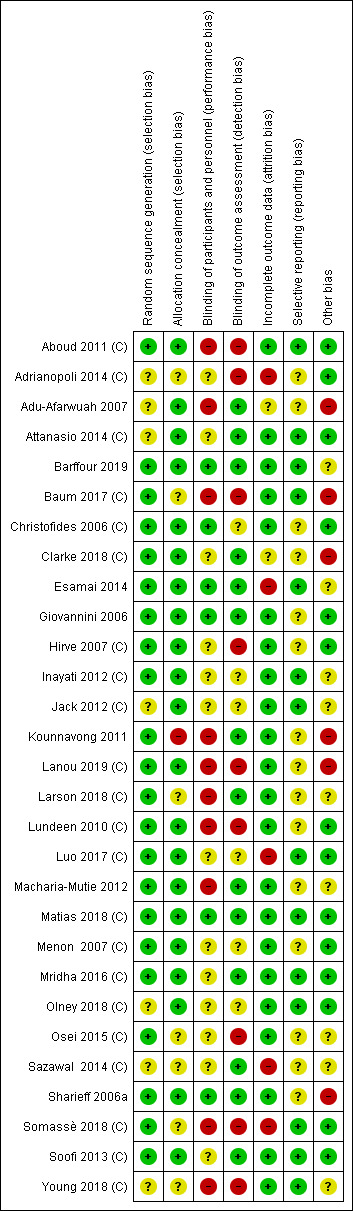
Risk of bias in included studies
See the 'Risk of bias' tables beneath the Characteristics of included studies tables for an assessment of the risk of bias in each included study, and see Figure 2 and Figure 3 for an overall summary of the risk of bias of all included studies. We considered 10 studies to be of high quality overall, according to our pre‐established criteria (Aboud 2011 (C); Barffour 2019; Giovannini 2006; Hirve 2007 (C); Inayati 2012 (C); Luo 2017 (C); Matias 2018 (C); Mridha 2016 (C); Christofides 2006 (C); Soofi 2013 (C)). We considered studies to be of high quality if they were assessed as having low risk of bias for both random sequence generation and allocation concealment (selection bias) and low risk of bias for either blinding (performance or detection bias) or incomplete outcome data (attrition bias). We considered the following 14 studies to be of low quality overall due to unclear or high risk of selection or attrition bias, or both: Adu‐Afarwuah 2007; Adrianopoli 2014 (C); Attanasio 2014 (C); Baum 2017 (C); Esamai 2014; Jack 2012 (C); Kounnavong 2011; Luo 2017 (C); Olney 2018 (C); Osei 2015 (C); Sazawal 2014 (C); Somassè 2018 (C); Larson 2018 (C); Young 2018 (C).
2.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
We assessed 22 studies as reporting adequate methods for generating the randomisation sequence (Aboud 2011 (C); Barffour 2019; Baum 2017 (C); Christofides 2006 (C); Clarke 2018 (C); Esamai 2014; Giovannini 2006; Hirve 2007 (C); Inayati 2012 (C); Kounnavong 2011; Lanou 2019 (C); Larson 2018 (C); Lundeen 2010 (C); Luo 2017 (C); Macharia‐Mutie 2012; Matias 2018 (C); Menon 2007 (C); Mridha 2016 (C); Osei 2015 (C); Sharieff 2006a; Somassè 2018 (C); Soofi 2013 (C)). Risk of selection bias was unclear in the seven remaining studies. One study allocated groups receiving the interventions randomly but not the group receiving no intervention, although the latter was randomly selected from the original population (Adu‐Afarwuah 2007). In Attanasio 2014 (C), individuals were not selected by simple random sampling from each cluster but instead among beneficiary households of elected mother leaders. The method of random sequence generation was not fully described in the other five studies (Adrianopoli 2014 (C); Jack 2012 (C); Olney 2018 (C); Sazawal 2014 (C); Young 2018 (C)).
Allocation concealment
We assessed 21 studies at low risk of bias for allocation concealment (Aboud 2011 (C); Adu‐Afarwuah 2007; Attanasio 2014 (C); Barffour 2019; Christofides 2006 (C); Clarke 2018 (C); Esamai 2014; Giovannini 2006; Hirve 2007 (C); Inayati 2012 (C); Jack 2012 (C); Lanou 2019 (C); Lundeen 2010 (C); Luo 2017 (C); Macharia‐Mutie 2012; Matias 2018 (C); Menon 2007 (C); Mridha 2016 (C); Olney 2018 (C); Sharieff 2006a; Soofi 2013 (C)). Seven studies did not describe the method of allocation concealment, and we judged these at unclear risk of bias (Adrianopoli 2014 (C); Baum 2017 (C); Larson 2018 (C); Mridha 2016 (C); Osei 2015 (C); Sazawal 2014 (C); Young 2018 (C)). Kounnavong 2011 had no method in place to conceal the allocation; consequently, we rated it at high risk of bias.
Blinding
Blinding of participants and personnel
We considered six studies to be at low risk of performance bias (Barffour 2019; Christofides 2006 (C); Esamai 2014; Giovannini 2006; Matias 2018 (C); Sharieff 2006a). We rated 13 studies at unclear risk of performance bias ‐ 6 because they were unable to blind allocation to treatment groups (Attanasio 2014 (C); Clarke 2018 (C); Hirve 2007 (C); Mridha 2016 (C); Osei 2015 (C); Sazawal 2014 (C)), and 7 because they provided insufficient or no methodological details (Adrianopoli 2014 (C); Inayati 2012 (C); Jack 2012 (C); Luo 2017 (C); Menon 2007 (C); Olney 2018 (C); Soofi 2013 (C)). We judged ten studies at high risk of performance bias ‐ eight studies did not attempt blinding (Aboud 2011 (C); Adu‐Afarwuah 2007; Baum 2017 (C); Lanou 2019 (C); Lundeen 2010 (C); Somassè 2018 (C); Larson 2018 (C); Young 2018 (C)), one study did not use placebo (Kounnavong 2011), and one study used only partial blinding (Macharia‐Mutie 2012).
Blinding of outcome assessment
Fourteen studies stated blinding of outcome assessors (Adu‐Afarwuah 2007; Attanasio 2014 (C); Barffour 2019; Clarke 2018 (C); Esamai 2014; Giovannini 2006; Kounnavong 2011; Macharia‐Mutie 2012; Matias 2018 (C); Mridha 2016 (C); Sazawal 2014 (C); Sharieff 2006a; Soofi 2013 (C); Larson 2018 (C)). We judged six studies at unclear risk of detection bias due to insufficient details provided (Christofides 2006 (C); Inayati 2012 (C); Jack 2012 (C); Luo 2017 (C); Menon 2007 (C); Olney 2018 (C)). The nine remaining studies did not attempt blinding of outcome assessment, and we rated these at high risk of bias (Aboud 2011 (C); Adrianopoli 2014 (C); Baum 2017 (C); Hirve 2007 (C); Lanou 2019 (C); Lundeen 2010 (C); Osei 2015 (C); Somassè 2018 (C); Young 2018 (C)).
Incomplete outcome data
We considered studies with more than 20% loss to follow‐up, or with imbalanced loss to follow‐up in different arms of the study, as inadequate in terms of completeness of outcome data. We rated 22 studies at low risk of attrition bias (Aboud 2011 (C); Attanasio 2014 (C); Barffour 2019; Baum 2017 (C); Christofides 2006 (C); Giovannini 2006; Hirve 2007 (C); Inayati 2012 (C); Jack 2012 (C); Kounnavong 2011; Lanou 2019 (C); Larson 2018 (C); Lundeen 2010 (C); Macharia‐Mutie 2012; Matias 2018 (C); Menon 2007 (C); Mridha 2016 (C); Olney 2018 (C); Osei 2015 (C); Sharieff 2006a; Soofi 2013 (C); Young 2018 (C)). We rated two studies at unclear risk of bias (Adu‐Afarwuah 2007; Clarke 2018 (C)). Due to high loss to follow‐up, we considered four studies at high risk of attrition bias (Adrianopoli 2014 (C); Luo 2017 (C); Sazawal 2014 (C); Somassè 2018 (C)). We also judged Esamai 2014 at high risk of attrition bias because 40% (18/45) of participants could not complete the isotope study.
Selective reporting
We rated 14 studies at low risk of reporting bias (Aboud 2011 (C); Attanasio 2014 (C); Barffour 2019; Baum 2017 (C); Esamai 2014; Inayati 2012 (C); Jack 2012 (C); Luo 2017 (C); Matias 2018 (C); Mridha 2016 (C); Olney 2018 (C); Somassè 2018 (C); Soofi 2013 (C); Young 2018 (C)), and we rated 15 studies at unclear risk of bias because they provided no or insufficient details about pre‐specified outcomes, or because a study protocol was not available (Adrianopoli 2014 (C); Adu‐Afarwuah 2007; Christofides 2006 (C); Clarke 2018 (C); Giovannini 2006; Hirve 2007 (C); Kounnavong 2011; Lanou 2019 (C); Lundeen 2010 (C); Macharia‐Mutie 2012; Menon 2007 (C); Osei 2015 (C); Sazawal 2014 (C); Sharieff 2006a; Larson 2018 (C)).
Other potential sources of bias
Fourteen studies appeared to be free of other sources of bias (Aboud 2011 (C); Adrianopoli 2014 (C); Attanasio 2014 (C); Christofides 2006 (C); Giovannini 2006; Hirve 2007 (C); Lundeen 2010 (C); Luo 2017 (C); Matias 2018 (C); Menon 2007 (C); Mridha 2016 (C); Olney 2018 (C); Somassè 2018 (C); Soofi 2013 (C)). We judged nine studies at unclear risk of other bias. It is unclear whether the cutoff for retinol binding protein to define vitamin A deficiency in Barffour 2019 was appropriate. Inayati 2012 (C) did not adjust data by length of the intervention, and it is unclear if Jack 2012 (C) conducted the analysis on an intention‐to‐treat basis. Macharia‐Mutie 2012 used multiple imputation to estimate missing data values. In Sazawal 2014 (C), milk was used as the vehicle for MNP, which may have led to high rejection rates by children because MNPs do not mix well with liquids. In Esamai 2014, Osei 2015 (C), Larson 2018 (C), and Young 2018 (C), there were imbalances in baseline numbers of boys versus girls between control and intervention groups. We rated six studies at high risk of other bias. In Baum 2017 (C), intervention and control groups differed in several baseline characteristics. In Adu‐Afarwuah 2007, there were no baseline values in the control group for any outcomes. In Kounnavong 2011, the haemoglobin concentration was significantly different at baseline between control and intervention groups. Sharieff 2006a did not measure haemoglobin and ferritin at baseline, making it difficult to judge comparability between groups. In Clarke 2018 (C), there was a wide range of intervention coverage between villages, ranging from 38% to 97%.
Effects of interventions
This review includes 29 studies with 33,147 children, of which 26 studies with 27,051 children contributed data; however, for trials with more than two treatment arms, we may not have included all arms in our analyses. We have organised the results by the different comparisons and by primary and secondary outcomes. Most of the included studies focused on anaemia and haematological indices; few reported on any of the other outcomes pre‐specified in the protocol (De‐Regil 2011). Because some results showed significant heterogeneity that could not be explained by standard sensitivity analyses, including quality assessment, we used a random‐effects model to analyse the results.
See the Data and analyses section for detailed results on primary and secondary outcomes. The main findings of the review are set out in Table 1 and Table 2, in which we also present the overall certainty of evidence for each primary outcome, by comparison.
1. Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo
Twenty‐six studies with 32,582 children under two years of age examined this comparison (Aboud 2011 (C); Adrianopoli 2014 (C); Adu‐Afarwuah 2007; Attanasio 2014 (C); Barffour 2019; Baum 2017 (C); Clarke 2018 (C); Esamai 2014; Giovannini 2006; Inayati 2012 (C); Jack 2012 (C); Kounnavong 2011; Lanou 2019 (C); Lundeen 2010 (C); Luo 2017 (C); Macharia‐Mutie 2012; Matias 2018 (C); Menon 2007 (C); Mridha 2016 (C); Olney 2018 (C); Osei 2015 (C); Sazawal 2014 (C); Sharieff 2006a; Somassè 2018 (C); Soofi 2013 (C); Larson 2018 (C)). Two studies did not contribute data to the analyses because of the ways in which they reported the primary outcomes (Luo 2017 (C); Olney 2018 (C)).
Primary outcomes
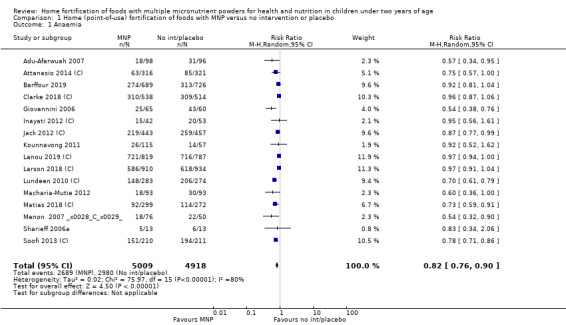
Anaemia (defined as haemoglobin values < 110 g/L)
Sixteen studies (9927 children) evaluated this outcome (Adu‐Afarwuah 2007; Attanasio 2014 (C); Barffour 2019; Clarke 2018 (C); Giovannini 2006; Inayati 2012 (C); Jack 2012 (C); Kounnavong 2011; Lanou 2019 (C); Lundeen 2010 (C); Macharia‐Mutie 2012; Matias 2018 (C); Menon 2007 (C); Sharieff 2006a; Soofi 2013 (C); Larson 2018 (C)). Children receiving MNPs were significantly less likely to have anaemia at follow‐up than children receiving no treatment or placebo (risk ratio (RR) 0.82, 95% confidence interval (CI) 0.76 to 0.90; P < 0.001; moderate‐certainty evidence; Analysis 1.1). The risk remained almost the same after four low‐quality trials were removed from the analysis (RR 0.79, 95% CI 0.70 to 0.89; 12 trials; 6817 children; analysis not shown) (Adu‐Afarwuah 2007; Jack 2012 (C); Kounnavong 2011; Larson 2018 (C)). As 16 trials were included in the meta‐analysis, we investigated the relationship between effect size and standard error by drawing a funnel plot (Figure 4), and we found no evidence of reporting bias.
1.1. Analysis.
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 1 Anaemia.
4.
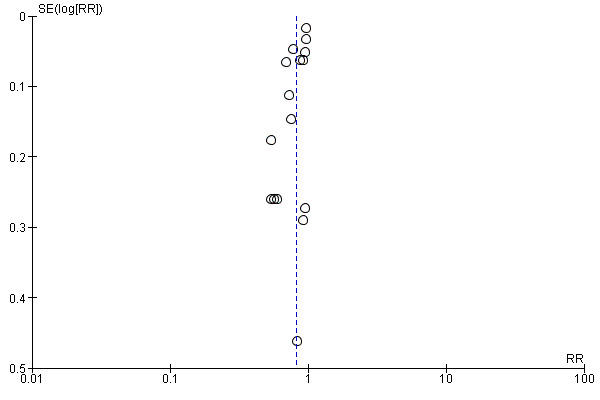
Funnel plot of comparison: 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, outcome: 1.1 Anaemia.
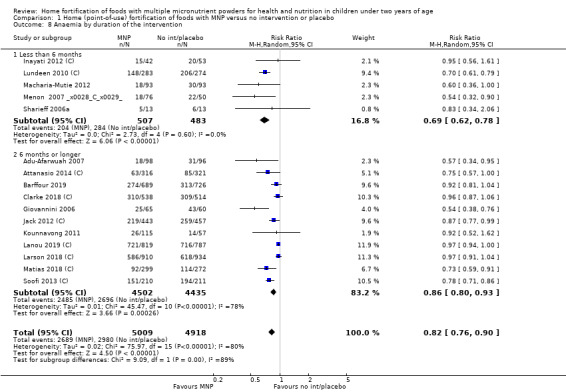
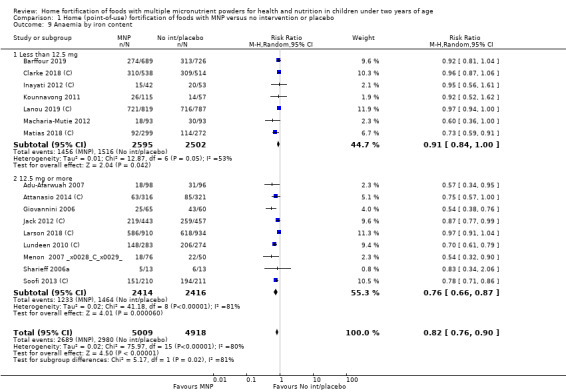
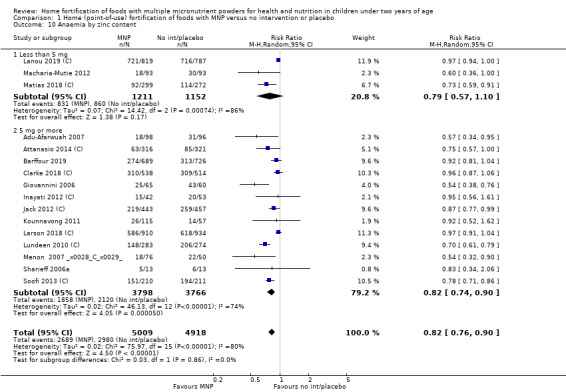
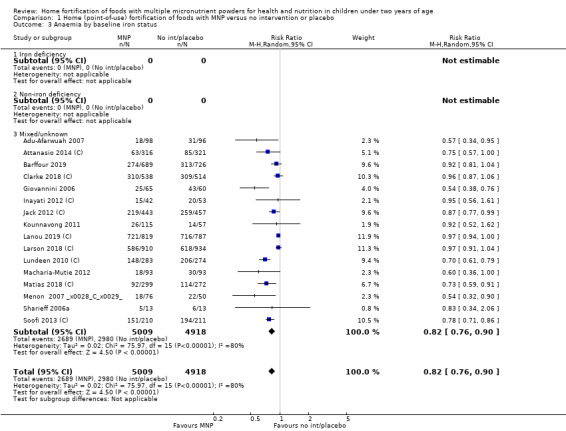
Visual examination of the subgroup analyses reveals that the intervention appeared equally effective among all infants aged 6 to 23 months (Analysis 1.4). The intervention was more effective in populations anaemic at baseline (RR 0.54, 95% CI 0.38 to 0.76) compared to populations with mixed/unknown anaemia status (RR 0.84, 95% CI 0.77 to 0.91; test for subgroup differences P = 0.01; Analysis 1.2) in non‐malarial versus malaria‐endemic settings (Analysis 1.6), with daily versus flexible intake (Analysis 1.7), and when the intervention lasted less than six months versus six months or longer (Analysis 1.8). When anaemia status was examined by iron content, MNPs with low iron content (< 12.5 mg) were noted to be less effective (RR 0.91, 95% CI 0.84 to 1.00) than MNPs with 12.5 mg of iron (RR 0.76, 95% CI 0.66 to 0.87; test for subgroup differences P = 0.02; Analysis 1.9). There were no differences by MNP zinc content (Analysis 1.10). No studies reported baseline iron status, and no studies were conducted among refugees (Analysis 1.3; Analysis 1.5).
1.4. Analysis.
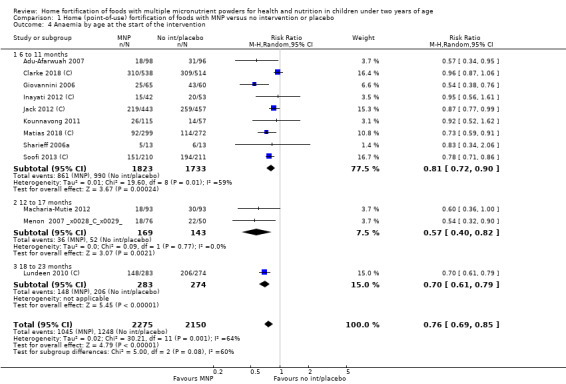
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 4 Anaemia by age at the start of the intervention.
1.2. Analysis.
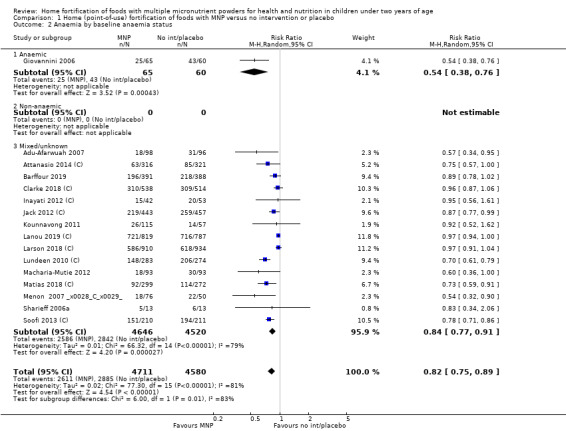
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 2 Anaemia by baseline anaemia status.
1.6. Analysis.
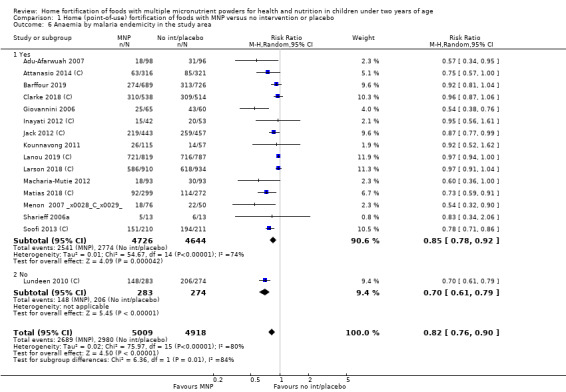
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 6 Anaemia by malaria endemicity in the study area.
1.7. Analysis.
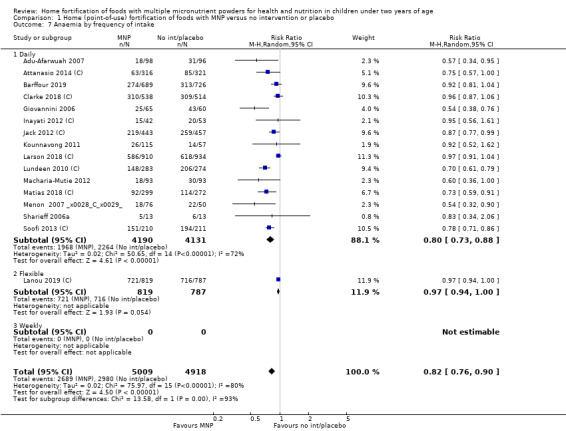
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 7 Anaemia by frequency of intake.
1.8. Analysis.
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 8 Anaemia by duration of the intervention.
1.9. Analysis.
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 9 Anaemia by iron content.
1.10. Analysis.
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 10 Anaemia by zinc content.
1.3. Analysis.
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 3 Anaemia by baseline iron status.
1.5. Analysis.
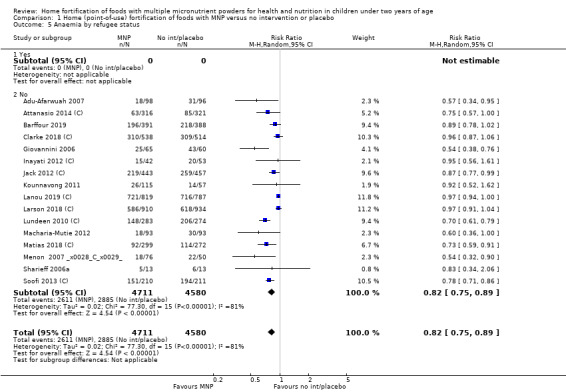
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 5 Anaemia by refugee status.
Iron deficiency (as defined by trialists)
Seven studies with 1634 children assessed iron deficiency at follow‐up (Adu‐Afarwuah 2007; Barffour 2019; Giovannini 2006; Macharia‐Mutie 2012; Matias 2018 (C); Sharieff 2006a; Soofi 2013 (C)). Five studies defined iron deficiency as ferritin concentration less than 12 μg/L (Adu‐Afarwuah 2007; Barffour 2019; Matias 2018 (C); Sharieff 2006a; Soofi 2013 (C)); one study as ferritin concentration < 12 μg/L or serum transferrin receptor > 8.3 mg/L, corrected for inflammation if C‐reactive protein > 5 mg/L (Macharia‐Mutie 2012); and one study as ferritin concentration < 15 μg/L (Sharieff 2006a). These studies found that children receiving MNP were significantly less likely to have iron deficiency at follow‐up than children receiving no treatment or placebo (RR 0.47, 95% CI 0.39 to 0.56; P < 0.001; high‐certainty evidence; Analysis 1.11). After one low‐quality study was removed from the analysis (Adu‐Afarwuah 2007), the RR was 0.44 (95% CI 0.35 to 0.56; 6 studies; 1475 children; analysis not shown).
1.11. Analysis.
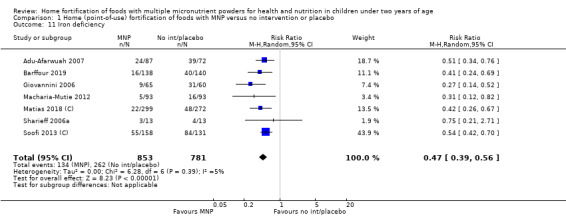
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 11 Iron deficiency.
There were no apparent differences among subgroups (Analysis 1.12; Analysis 1.14; Analysis 1.18; Analysis 1.19; Analysis 1.20). It was not possible to conduct subgroup analyses for iron status at baseline as all were of mixed status (Analysis 1.13), nor for refugee status (Analysis 1.15), malaria endemicity (Analysis 1.16), or frequency of intake (Analysis 1.17).
1.12. Analysis.
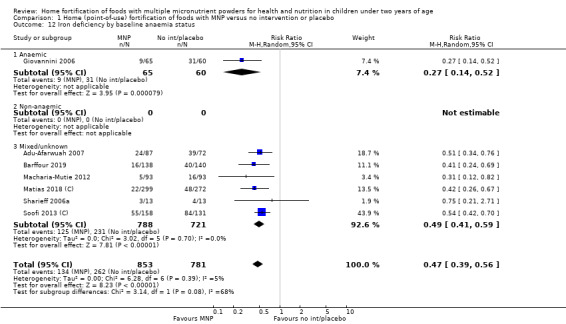
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 12 Iron deficiency by baseline anaemia status.
1.14. Analysis.
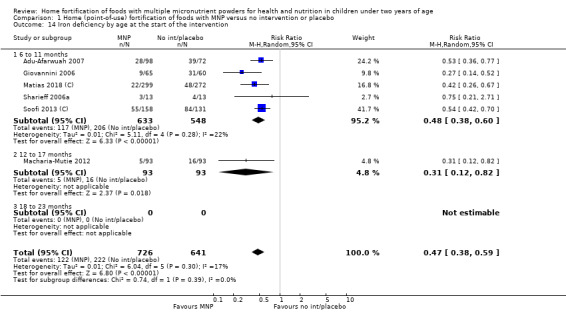
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 14 Iron deficiency by age at the start of the intervention.
1.18. Analysis.
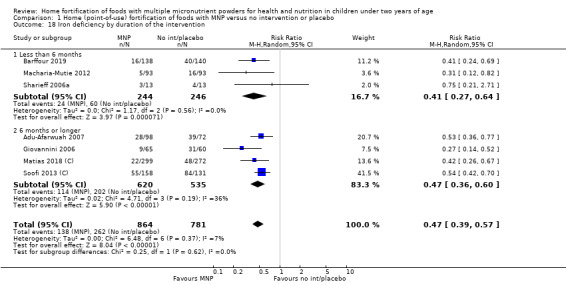
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 18 Iron deficiency by duration of the intervention.
1.19. Analysis.
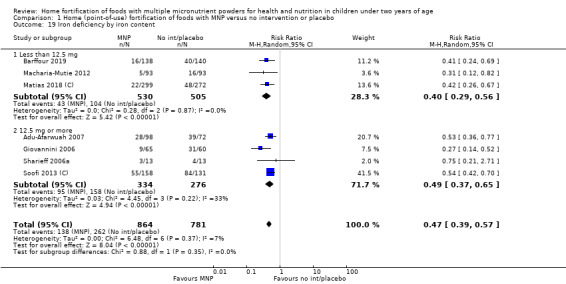
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 19 Iron deficiency by iron content.
1.20. Analysis.
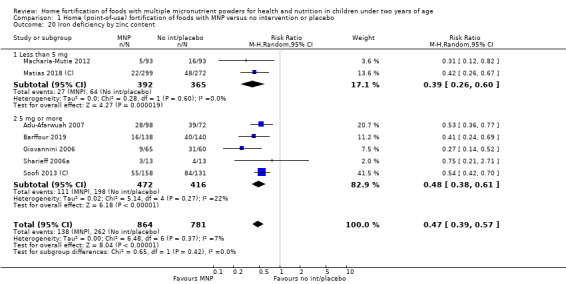
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 20 Iron deficiency by zinc content.
1.13. Analysis.
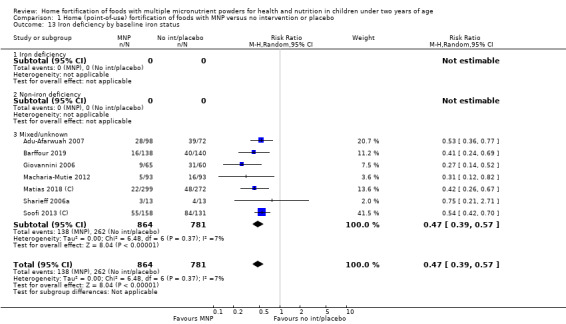
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 13 Iron deficiency by baseline iron status.
1.15. Analysis.
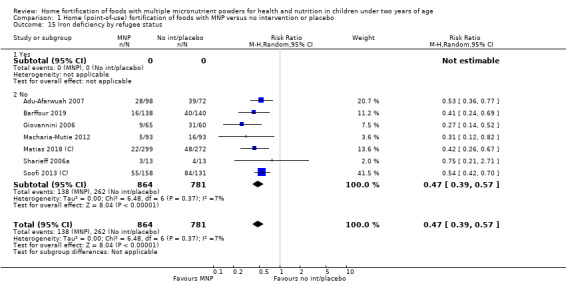
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 15 Iron deficiency by refugee status.
1.16. Analysis.
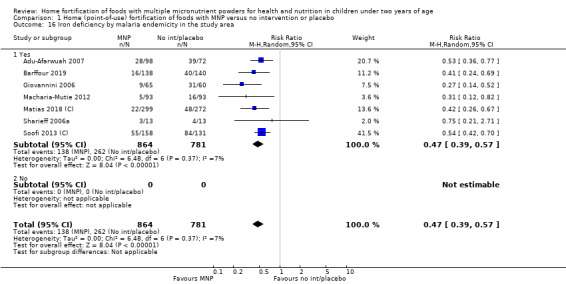
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 16 Iron deficiency by malaria endemicity in the study area.
1.17. Analysis.
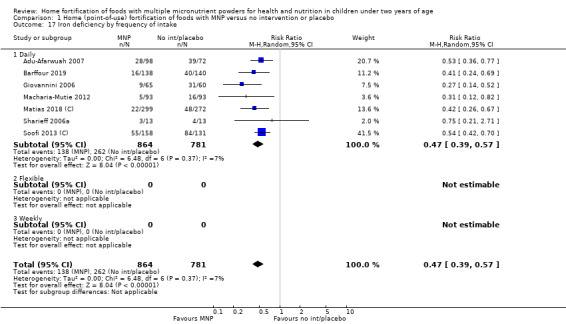
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 17 Iron deficiency by frequency of intake.
Haemoglobin concentration (g/L)
Twenty studies (10,509 children) evaluated this outcome (Adu‐Afarwuah 2007; Adrianopoli 2014 (C); Attanasio 2014 (C); Barffour 2019; Baum 2017 (C); Clarke 2018 (C); Esamai 2014; Giovannini 2006; Inayati 2012 (C); Jack 2012 (C); Kounnavong 2011; Lanou 2019 (C); Lundeen 2010 (C); Macharia‐Mutie 2012; Matias 2018 (C); Menon 2007 (C); Osei 2015 (C); Sazawal 2014 (C); Sharieff 2006a; Soofi 2013 (C)). Compared to children receiving no treatment or placebo, children receiving MNP had a 3.04 g/L higher haemoglobin concentration at follow‐up (MD 2.74 g/L, 95% CI 1.95 to 3.53; P < 0.001; low‐certainty evidence; Analysis 1.21). After nine low‐quality trials were removed from the analysis (Adu‐Afarwuah 2007; Adrianopoli 2014 (C); Attanasio 2014 (C); Baum 2017 (C); Esamai 2014; Jack 2012 (C); Kounnavong 2011; Osei 2015 (C); Sazawal 2014 (C)), the MD was 3.39 g/L (95% CI 2.39 to 4.39; 11 trials; 7690 children; analysis not shown). Visual inspection of the funnel plot suggests no evidence of reporting bias for this outcome (Figure 5).
1.21. Analysis.
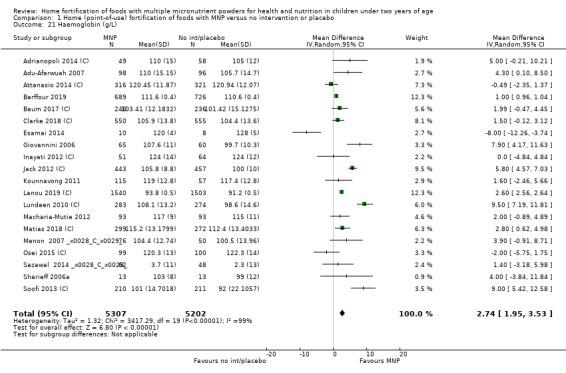
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 21 Haemoglobin (g/L).
5.
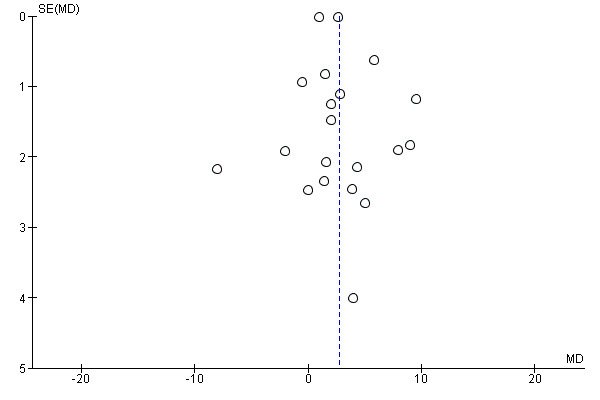
Funnel plot of comparison: 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, outcome: 1.21 Haemoglobin (g/L).
The intervention was not effective in children who were non‐iron deficient or anaemic at baseline (Analysis 1.22; Analysis 1.23), nor in children for whom the duration of the intervention was less than six months (Analysis 1.28). The intervention was effective in children aged 6 to 11 months old at the start of the intervention, but not in children aged 12 to 17 months or 18 to 23 months (Analysis 1.24). The intervention appeared to be more effective in non‐malaria‐endemic areas compared to malaria‐endemic areas (mean difference (MD) 9.50, 95% CI 7.19 to 11.81 compared to MD 2.30, 95% CI 1.49 to 3.11; test for subgroup differences P < 0.001; Analysis 1.26). MNP with higher iron content (12.5 mg or higher) was more effective than MNP with iron content less than 12.5 mg (MD 3.92, 95% CI 1.06 to 6.77 compared to MD 1.61, 95% CI 0.58 to 2.64; test for subgroup differences P = 0.14; Analysis 1.29). There was no difference by frequency of intake (Analysis 1.27) nor by zinc content (Analysis 1.30). It was not possible to perform subgroup analysis for refugee status (Analysis 1.25).
1.22. Analysis.
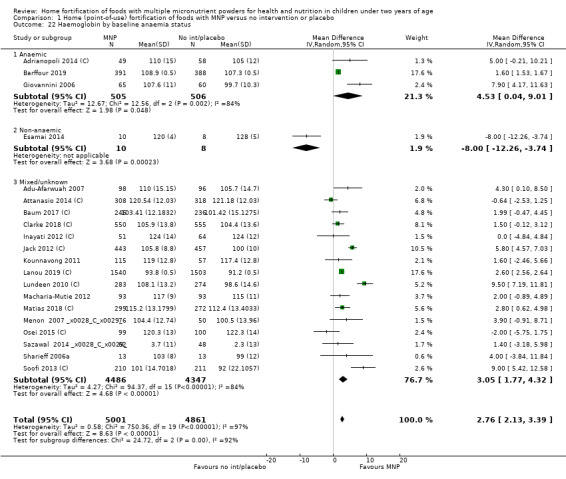
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 22 Haemoglobin by baseline anaemia status.
1.23. Analysis.
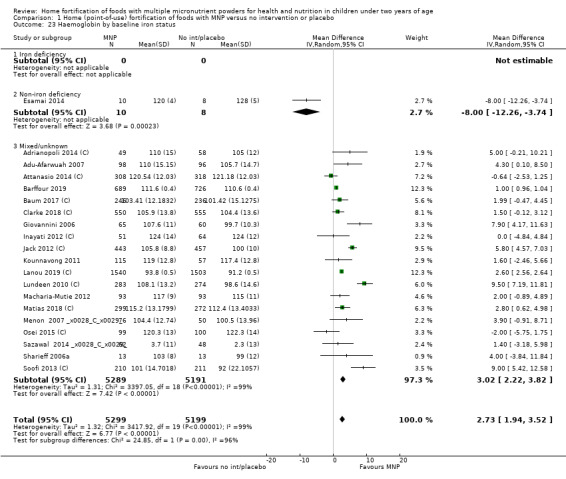
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 23 Haemoglobin by baseline iron status.
1.28. Analysis.
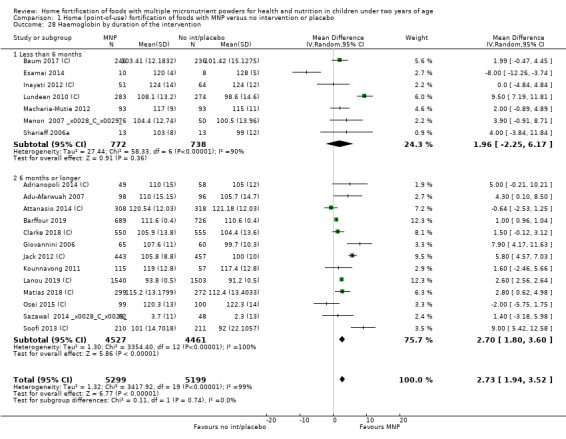
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 28 Haemoglobin by duration of the intervention.
1.24. Analysis.
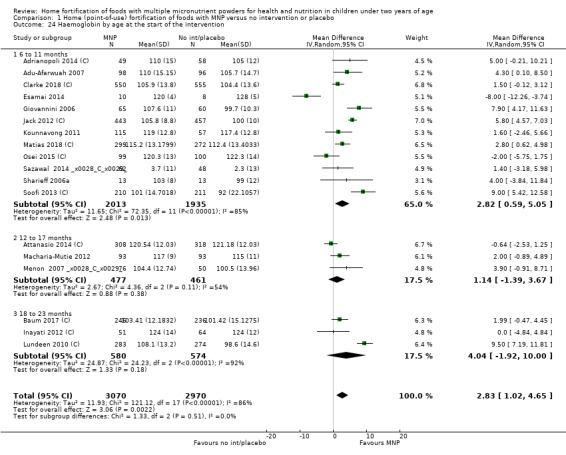
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 24 Haemoglobin by age at the start of the intervention.
1.26. Analysis.
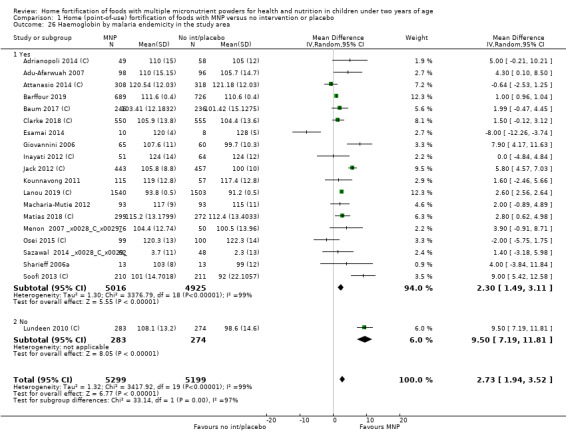
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 26 Haemoglobin by malaria endemicity in the study area.
1.29. Analysis.
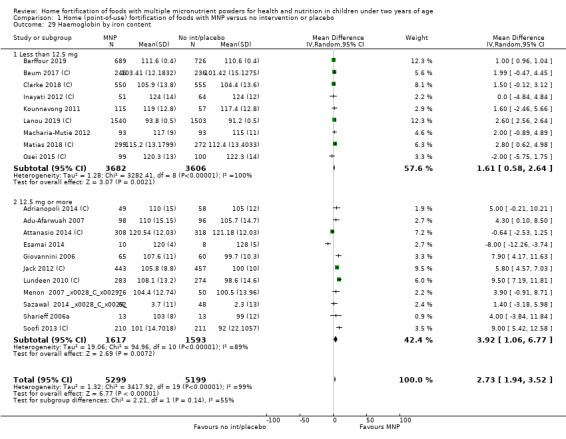
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 29 Haemoglobin by iron content.
1.27. Analysis.
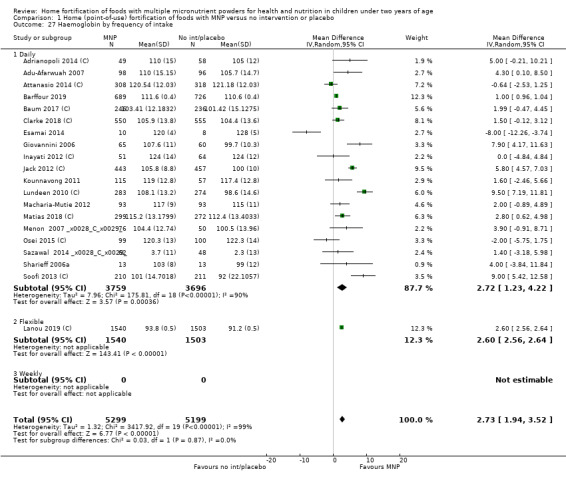
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 27 Haemoglobin by frequency of intake.
1.30. Analysis.
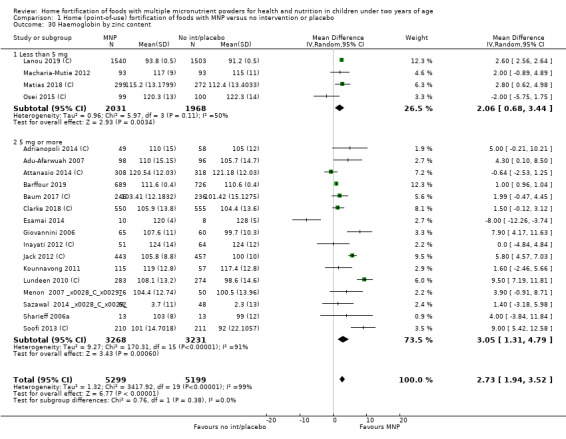
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 30 Haemoglobin by zinc content.
1.25. Analysis.
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 25 Haemoglobin by refugee status.
Iron status (as defined by trialists)
Seven studies (2612 children) provided information on ferritin concentrations (Adu‐Afarwuah 2007; Barffour 2019; Clarke 2018 (C); Esamai 2014; Giovannini 2006; Jack 2012 (C); Matias 2018 (C)). On average, children receiving MNP had 12.93 μg of ferritin more per litre at follow‐up than children receiving no treatment or placebo (MD 12.93 μg/L, 95% CI 7.41 to 18.45; P < 0.001; moderate‐certainty evidence; Analysis 1.31).
1.31. Analysis.

Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 31 Iron status (ferritin concentrations in μg/L).
It was not possible to perform subgroup analyses for age at the start of the intervention, refugee status, malaria endemicity, frequency of intake, nor zinc content (Analysis 1.34; Analysis 1.35; Analysis 1.36; Analysis 1.37; Analysis 1.40). MNP with low iron content (< 12.5 mg) was not effective (MD 6.76, 95% CI −2.25 to 15.77), whereas MNP with higher iron content (≥ 12.5 mg) was effective (MD 19.43, 95% CI 9.50 to 29.36), in improving iron status (test for subgroup differences P = 0.06; Analysis 1.39). All other subgroup analyses indicate that the intervention appeared equally effective in populations with different anaemia status at baseline (Analysis 1.32) and with different iron status at baseline (Analysis 1.33), and whether the intervention lasted less than six months or six months or longer (Analysis 1.38).
1.34. Analysis.
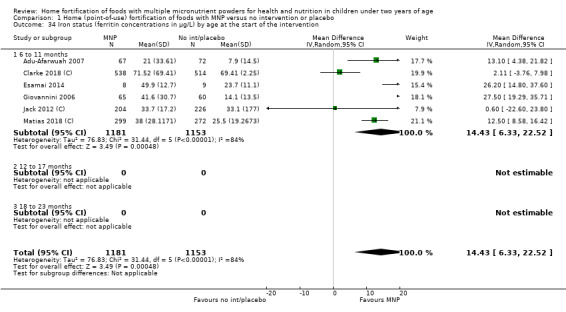
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 34 Iron status (ferritin concentrations in μg/L) by age at the start of the intervention.
1.35. Analysis.

Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 35 Iron status (ferritin concentrations in μg/L) by refugee status.
1.36. Analysis.
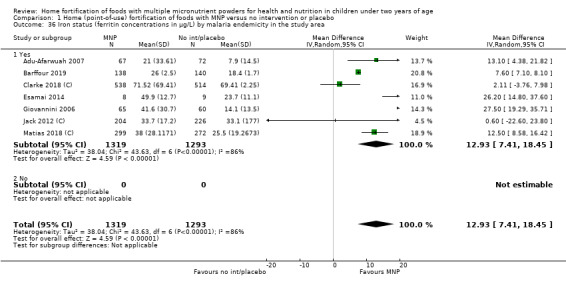
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 36 Iron status (ferritin concentrations in μg/L) by malaria endemicity in the study area.
1.37. Analysis.
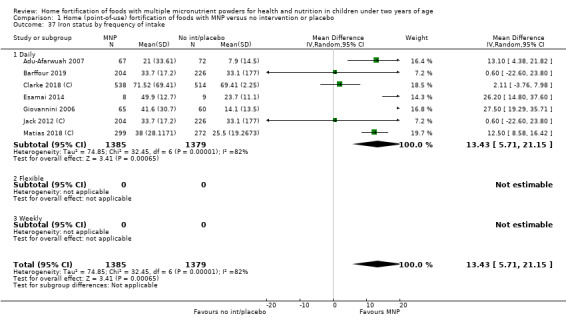
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 37 Iron status by frequency of intake.
1.40. Analysis.
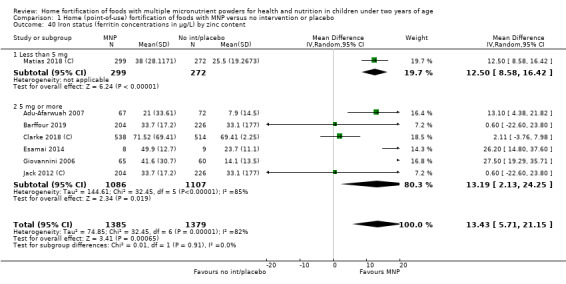
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 40 Iron status (ferritin concentrations in μg/L) by zinc content.
1.39. Analysis.
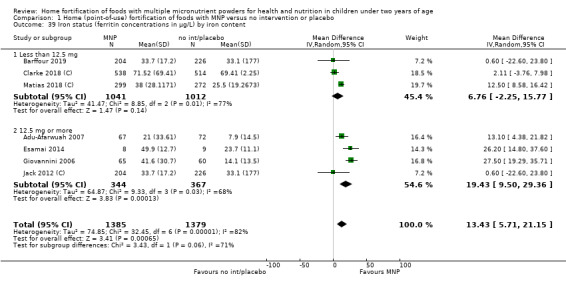
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 39 Iron status (ferritin concentrations in μg/L) by iron content.
1.32. Analysis.
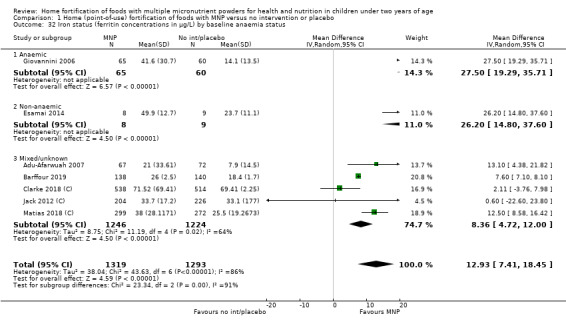
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 32 Iron status (ferritin concentrations in μg/L) by baseline anaemia status.
1.33. Analysis.
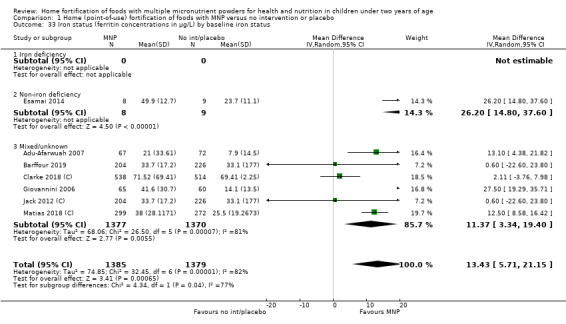
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 33 Iron status (ferritin concentrations in μg/L) by baseline iron status.
1.38. Analysis.
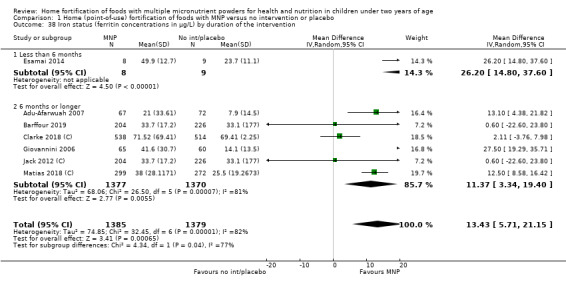
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 38 Iron status (ferritin concentrations in μg/L) by duration of the intervention.
Weight‐for‐age (z scores)
In 10 studies (9287 children) in which the intervention was given for 3 (Esamai 2014), 6 (Aboud 2011 (C); Adu‐Afarwuah 2007; Sazawal 2014 (C)), 9 (Barffour 2019), 11 (Osei 2015 (C)), 12 (Giovannini 2006; Larson 2018 (C)), or 18 months or longer (Clarke 2018 (C); Mridha 2016 (C)), researchers found no evidence of a difference in weight‐for‐age z scores between groups (MD 0.02, 95% CI −0.03 to 0.07; moderate‐certainty evidence; Analysis 1.41).
1.41. Analysis.
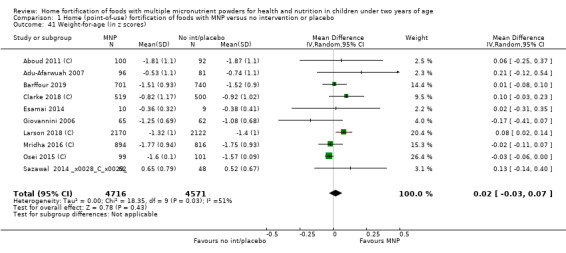
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 41 Weight‐for‐age (in z scores).
All‐cause mortality
Giovannini 2006 stated that no deaths occurred over the 12‐month intervention period. In Hirve 2007 (C), two deaths were reported after the intervention was finalised but were judged not to be related to the study. In Soofi 2013 (C), 37 deaths were reported, but no differences were reported by study group. In Matias 2018 (C), 15 postneonatal deaths occurred in the MNP group (15.6/1000 live births) and 23 postnatal deaths in the control group (25.6/1000 live births).
Secondary outcomes
Length‐for‐age (z scores)
In 11 studies (11,682 children) in which the intervention was given for 3 (Esamai 2014), 6 (Aboud 2011 (C); Adu‐Afarwuah 2007; Sazawal 2014 (C)), 9 (Barffour 2019), 11 (Osei 2015 (C)), 12 (Giovannini 2006; Lanou 2019 (C); Larson 2018 (C)), or 18 months or longer (Clarke 2018 (C); Mridha 2016 (C)), researchers found no evidence of a significant difference in length‐for‐age z scores between groups (MD −0.01, 95% CI −0.04 to 0.01; Analysis 1.42).
1.42. Analysis.
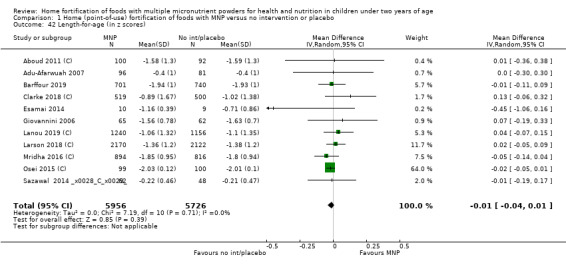
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 42 Length‐for‐age (in z scores).
Weight‐for‐height (z scores)
Ten studies (11,664 children) reported on weight‐for‐height z scores (Adrianopoli 2014 (C); Adu‐Afarwuah 2007; Barffour 2019; Clarke 2018 (C); Giovannini 2006;Lanou 2019 (C); Mridha 2016 (C); Osei 2015 (C); Sazawal 2014 (C); Larson 2018 (C)); researchers found no evidence of a difference in weight‐for‐height z scores between groups (MD 0.03, 95% CI −0.01 to 0.08; Analysis 1.43).
1.43. Analysis.
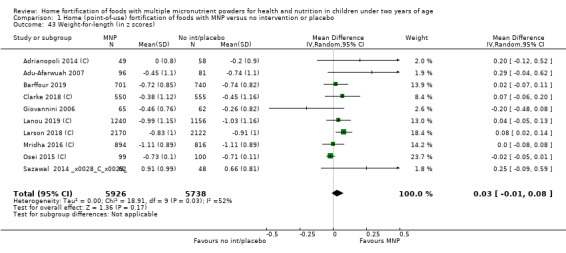
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 43 Weight‐for‐length (in z scores).
All‐cause morbidity
This outcome was reported by three trials with 2270 children (Aboud 2011 (C); Giovannini 2006; Lanou 2019 (C);). Results show no significant differences in all‐cause morbidity between intervention and control groups (RR 1.03, 95% CI 0.77 to 1.39; Analysis 1.44).
1.44. Analysis.
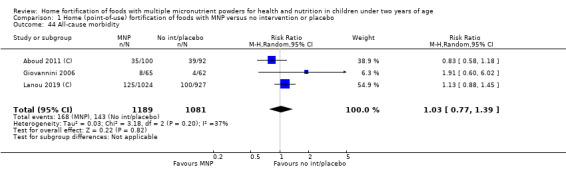
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 44 All‐cause morbidity.
Diarrhoea
In five trials with 5579 children (Barffour 2019; Larson 2018 (C); Mridha 2016 (C); Sharieff 2006a; Soofi 2013 (C)), children receiving MNP did not have more diarrhoea than those who received placebo (odds ratio (OR) 1.05, 95% CI 0.82 to 1.35; Analysis 1.45).
1.45. Analysis.
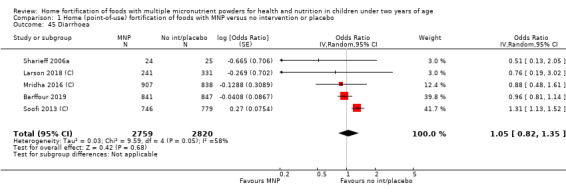
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 45 Diarrhoea.
Upper respiratory tract infections
Three studies with 3560 children found that upper respiratory infections were equally prevalent among children receiving MNP and those allocated to the placebo group (OR 0.89, 95% CI 0.76 to 1.06; Analysis 1.46) (Barffour 2019; Giovannini 2006; Mridha 2016 (C)).
1.46. Analysis.
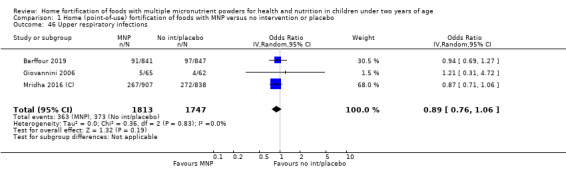
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 46 Upper respiratory infections.
Serum retinol concentration (μmol/L)
One trial with 386 children reported on serum retinol concentration in μmol/L to assess vitamin A status and found that children receiving MNP compared with children receiving no intervention or placebo had significantly lower retinol levels at follow‐up (MD −0.16, 95% CI −0.28 to −0.04; P = 0.009; Analysis 1.48) (Soofi 2013 (C)).
1.48. Analysis.
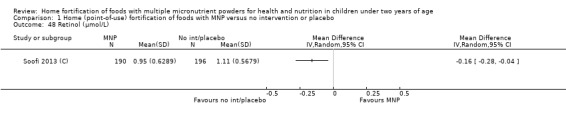
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 48 Retinol (μmol/L).
Retinol binding protein (μmol/L)
Two trials with 883 children reported on retinol binding protein to assess vitamin A status and found no differences between intervention and control groups (MD 0.00, 95% CI −0.04 to 0.04; Analysis 1.47) (Barffour 2019; Jack 2012 (C)).
1.47. Analysis.
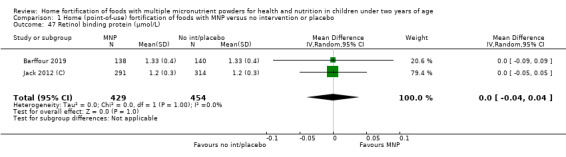
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 47 Retinol binding protein (μmol/L).
Serum zinc concentration (g/dL)
Five trials with 1426 children reported this outcome (Adu‐Afarwuah 2007; Barffour 2019; Esamai 2014; Jack 2012 (C); Soofi 2013 (C)); these researchers found no effect of daily provision of MNP plus zinc on children's serum zinc concentrations (MD 1.07, 95% CI −3.46 to 5.61; Analysis 1.49). The only study that provided 10 mg of zinc reported significant improvement in serum zinc concentrations (MD 6.20, 95% CI 5.67 to 6.73; P < 0.0001; analysis not shown) (Barffour 2019).
1.49. Analysis.
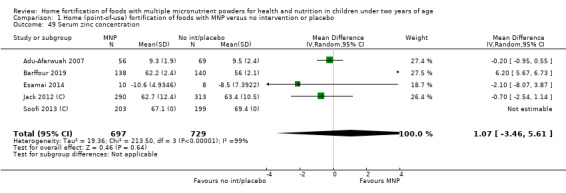
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 49 Serum zinc concentration.
Mental development and motor skill development (as defined by trialists)
One study with 179 children reported that children receiving MNP were more likely to walk independently at 12 months of age than those receiving no intervention (RR 1.58, 95% CI 1.02 to 2.46; Analysis 1.51) (Adu‐Afarwuah 2007).
1.51. Analysis.
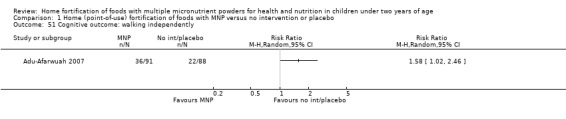
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 51 Cognitive outcome: walking independently.
One study with 192 children reported language and Home Observation for Measurement of the Environment (HOME) scores (Aboud 2011 (C)). Researchers found no evidence of an effect of MNP on mental development (MD −0.77, 95% CI −2.74 to 1.20; Analysis 1.52) nor on motor skill development (MD 0.31, 95% CI −1.18 to 1.80; Analysis 1.53).
1.52. Analysis.
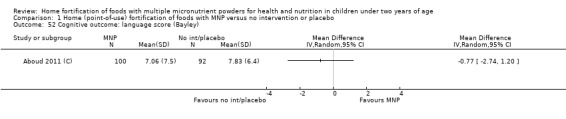
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 52 Cognitive outcome: language score (Bayley).
1.53. Analysis.
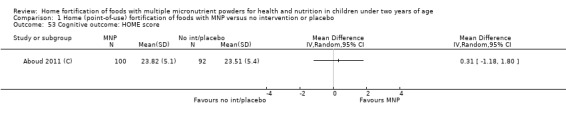
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 53 Cognitive outcome: HOME score.
One trial with 1710 children found evidence of a better repetitive language z score (MD 0.17, 95% CI 0.07 to 0.27, P < 0.001) and expressive language z score (MD 0.13, 95% CI 0.04 to 0.22, P = 0.006) at 24 months for children receiving MNP compared with those given the control intervention (Analysis 1.54) (Mridha 2016 (C)).
1.54. Analysis.
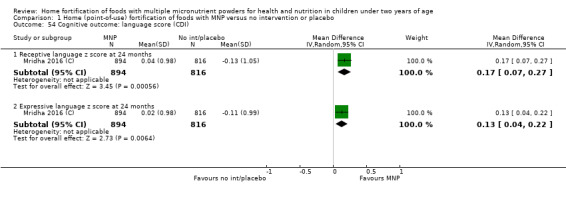
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 54 Cognitive outcome: language score (CDI).
Malaria outcomes
Although 27 studies were conducted in settings considered as malaria‐endemic, only three studies with 1737 children reported results on the presence of positive malaria smears (Adu‐Afarwuah 2007; Clarke 2018 (C); Lanou 2019 (C)); researchers found no differences between study groups (RR 0.88, 95% CI 0.62 to 1.24; Analysis 1.50).
1.50. Analysis.
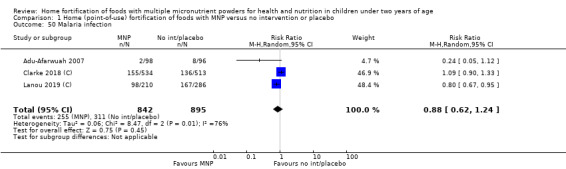
Comparison 1 Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo, Outcome 50 Malaria infection.
Other outcomes
No studies reported data on outcomes that we defined as all‐cause mortality, adherence, severe anaemia, non‐serious adverse effects, ear infections, or iron overload.
2. Home (point‐of‐use) fortification of foods with MNP versus an iron‐only supplement
Two studies with 565 children under two years of age examined this comparison (Christofides 2006 (C); Hirve 2007 (C)).
Primary outcomes
Anaemia (defined as haemoglobin values < 110 g/L)
One study with 145 children found no differences in anaemia between children who received MNP plus five nutrients and those who were supplemented with iron drops after two months of follow‐up (RR 0.89, 95% CI 0.58 to 1.39; P = 0.62; low‐certainty evidence; Analysis 2.1) (Hirve 2007 (C)).
2.1. Analysis.
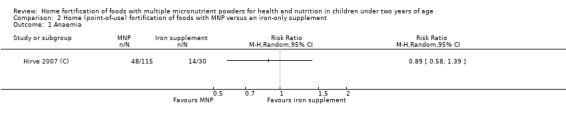
Comparison 2 Home (point‐of‐use) fortification of foods with MNP versus an iron‐only supplement, Outcome 1 Anaemia.
Haemoglobin concentration (g/L)
Data from both studies (278 children) show no evidence of a difference in haemoglobin concentrations between groups receiving MNP or iron supplements (MD −2.81, 95% CI −10.84 to 5.22; P = 0.49; very low‐certainty evidence; Analysis 2.2).
2.2. Analysis.
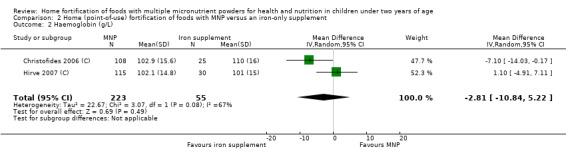
Comparison 2 Home (point‐of‐use) fortification of foods with MNP versus an iron‐only supplement, Outcome 2 Haemoglobin (g/L).
Secondary outcomes
Side effects
Data from both studies (395 children) show that children receiving MNP were less likely to have stained teeth than those receiving iron syrup daily (RR 0.37, 95% CI 0.16 to 0.82; P = 0.02; Analysis 2.3), and were less likely to have stool discolouration (RR 0.80, 95% CI 0.66 to 0.98; P = 0.04; Analysis 2.4). Only Hirve 2007 (C) reported on other side effects, finding an effect of MNP versus iron supplements on reducing the likelihood of children getting a cold (RR 0.84, 95% CI 0.73 to 0.97; P = 0.01; 262 children; Analysis 2.5) or fever (RR 0.59, 95% CI 0.42 to 0.82; P = 0.002; 262 children; Analysis 2.6) but not cough (RR 0.74, 95% CI 0.53 to 1.03; P = 0.08; 130 children; Analysis 2.7).
2.3. Analysis.
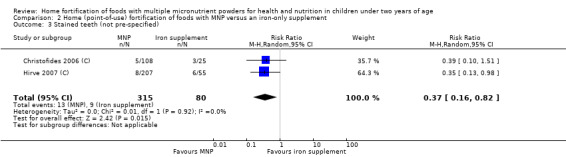
Comparison 2 Home (point‐of‐use) fortification of foods with MNP versus an iron‐only supplement, Outcome 3 Stained teeth (not pre‐specified).
2.4. Analysis.
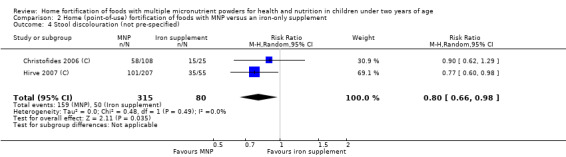
Comparison 2 Home (point‐of‐use) fortification of foods with MNP versus an iron‐only supplement, Outcome 4 Stool discolouration (not pre‐specified).
2.5. Analysis.
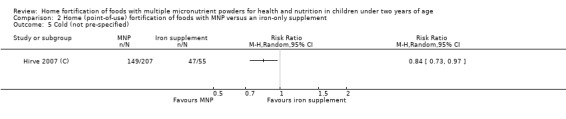
Comparison 2 Home (point‐of‐use) fortification of foods with MNP versus an iron‐only supplement, Outcome 5 Cold (not pre‐specified).
2.6. Analysis.
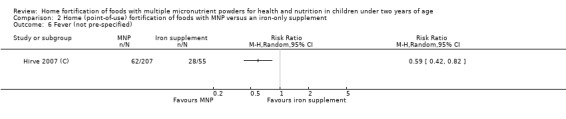
Comparison 2 Home (point‐of‐use) fortification of foods with MNP versus an iron‐only supplement, Outcome 6 Fever (not pre‐specified).
2.7. Analysis.
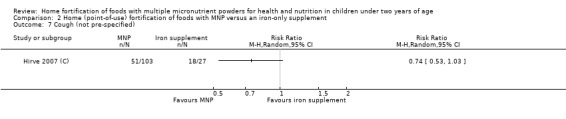
Comparison 2 Home (point‐of‐use) fortification of foods with MNP versus an iron‐only supplement, Outcome 7 Cough (not pre‐specified).
Hirve 2007 (C) (262 children) reported that children receiving MNP were less likely to have vomiting (RR 0.58, 95% CI 0.35 to 0.95; P = 0.03; Analysis 2.8), diarrhoea (RR 0.52, 95% CI 0.38 to 0.72; P < 0.001; Analysis 2.9), and recurrent diarrhoea (RR 0.27, 95% CI 0.10 to 0.73; P = 0.01; Analysis 2.10) than those receiving daily iron supplements.
2.8. Analysis.
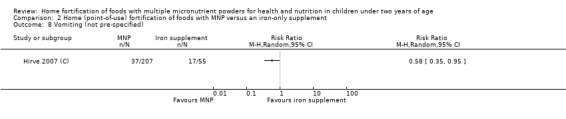
Comparison 2 Home (point‐of‐use) fortification of foods with MNP versus an iron‐only supplement, Outcome 8 Vomiting (not pre‐specified).
2.9. Analysis.
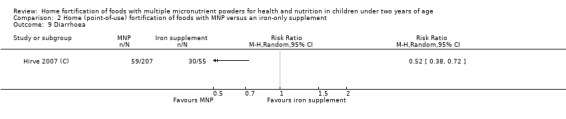
Comparison 2 Home (point‐of‐use) fortification of foods with MNP versus an iron‐only supplement, Outcome 9 Diarrhoea.
2.10. Analysis.
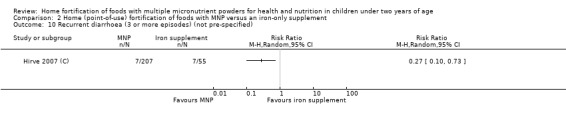
Comparison 2 Home (point‐of‐use) fortification of foods with MNP versus an iron‐only supplement, Outcome 10 Recurrent diarrhoea (3 or more episodes) (not pre‐specified).
Christofides 2006 (C) and Hirve 2007 (C) found no differences in the mean number of episodes of diarrhoea per child between interventions (RR 0.46, 95% CI 0.16 to 1.30; P = 0.14; 389 children; Analysis 2.11).
2.11. Analysis.
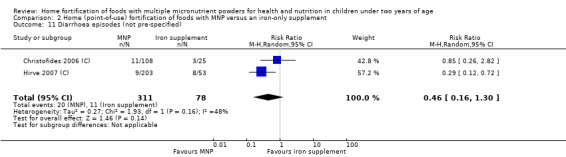
Comparison 2 Home (point‐of‐use) fortification of foods with MNP versus an iron‐only supplement, Outcome 11 Diarrhoea episodes (not pre‐specified).
Other outcomes
No studies reported data on the outcomes we defined as iron deficiency, iron status, weight‐for‐age, all‐cause mortality, adherence, severe anaemia, length‐for‐age, weight‐for‐height, all‐cause morbidity, upper respiratory tract infections, ear infections, iron overload, serum retinol concentration, retinol blinding protein, serum zinc concentration, mental development, and motor skill development, nor malaria incidence or malaria severity.
Discussion
Summary of main results
This review includes 29 studies, with 24 contributing data that compared groups of children receiving micronutrient powder (MNP) to groups receiving no treatment or placebo. Results show that providing MNP to infants and children aged six months to two years at the start of the intervention reduced anaemia by 18% and iron deficiency by 53%, and that those who received MNP had significantly higher haemoglobin concentrations and significantly higher ferritin concentrations compared to those who did not receive the intervention, or who received placebo. MNP with iron content of 12.5 mg or higher was more effective for reducing anaemia and for increasing haemoglobin and ferritin concentrations compared to MNP with low iron content (< 12.5 mg). The intervention led to no effects on zinc status, vitamin A status, or child growth. The haematological effects of MNP seemed comparable to those observed with daily oral iron supplementation with drops; however, given the small number of studies evaluating the equivalence between interventions, these results should be interpreted with caution.
Although the real effect of an intervention is context‐specific, providing MNP was effective in various settings with a high infectious disease burden, in both malaria‐ and non‐malaria‐endemic areas, among populations with a high prevalence of anaemia (25% to 100%), and when provided for two to six months or longer to infants and young children across the age span of 6 to 23 months.
Data on side effects and morbidity are scarce, and definitions for each of the outcomes are variable amongst studies, making it difficult to compare and assess the overall safety of this intervention (e.g. diarrhoea was reported as the average number of episodes of diarrhoea per child, longitudinal prevalence of any diarrhoea, or number of children with at least one episode of diarrhoea). Nonetheless, there were no increased effects of MNP on diarrheoa, upper respiratory infections, malarial infection, or all‐cause morbidity. Furthermore, none of the studies reported deaths attributable to the intervention, and the pattern of disease seemed similar to that in children receiving placebo or no intervention. It is clear that a standardised approach to reporting side effects and morbidity is needed, as are improved malaria surveillance and reporting in studies conducted in malaria settings. Data on neurocognitive development are scarce and difficult to pool due to the different tools used to measure mental and motor development.
Overall completeness and applicability of evidence
Use of MNP containing iron, zinc, and vitamin A for children younger than two years of age significantly reduces the prevalence of anaemia and iron deficiency in populations with high prevalence of anaemia, but information is insufficient to assess effects of MNP on other health and nutrition outcomes.
Public health programmes supporting home fortification with MNP for improving micronutrient intake in children younger than two years of age have rapidly expanded worldwide (UNICEF 2014). Although doses as high as 80 mg of elemental iron per day were initially used to test the efficacy of this intervention, as well as its equivalence to 40 mg of iron given as drops (Zlotkin 2001; Zlotkin 2003a), the widely used dose of 12.5 mg of elemental iron per sachet is based on the recommended daily dose to supplement children aged 6 to 23 months for iron deficiency anaemia prevention (INACG 1998; WHO 2016a). Its effectiveness was confirmed by a dose‐response trial in which 12.5 mg of elemental iron (as encapsulated ferrous fumarate) was as effective as 20 mg and 30 mg of iron in the same form and 20 mg of elemental iron (as ferrous sulphate drops) for improving haemoglobin and ferritin concentrations among anaemic children aged 6 to 18 months (Hirve 2007 (C)).
Most of the evidence included in this review examines a dose of 10 mg to 12.5 mg of iron given on a daily basis. However, another study suggested that providing MNP in a flexible regimen for two months, and hence at a lower overall monthly dose, produced the same haematological response as daily use of MNP (Hyder 2007). Other studies that have distributed MNP through flexible, market‐based distribution approaches have reported significant reductions in anaemia and iron deficiency (Suchdev 2012; Sun 2011). Intermittent provision of iron was proposed more than 25 years ago as a feasible public health strategy to supplement children's and women's diets and to reduce anaemia, as it is supposed to maximise absorption by providing iron in synchrony with the turnover of mucosal cells (Beaton 1999; Berger 1997; Viteri 1997).
The lasting effect of the benefits of using MNP on haematological outcomes is still unclear. However, evidence from two studies suggests that, independently of the dosing regimen, positive effects of MNP on anaemia prevalence may be maintained for a period of approximately six months after the end of the intervention (Ip 2009; Menon 2007 (C)).
MNPs can be prepared in various formulations, but for inclusion in this review, they had to contain zinc, vitamin A, and iron. In the included studies, these three nutrients were always accompanied by folic acid and vitamin C; approximately half (total of 14) of studies utilised MNP with 14 or more micronutrients (WHO/WFP/UNICEF 2007). When studies that provided a lower daily dose of elemental iron (< 12.5 mg) were compared with those that provided 12.5 mg or more of iron, MNP with higher iron content had a greater impact on haematological outcomes (haemoglobin and ferritin). Leaving iron aside, five studies evaluated the effects of MNP on zinc status (Adu‐Afarwuah 2007; Barffour 2019; Esamai 2014; Jack 2012 (C); Soofi 2013 (C)). Adding 5 mg of elemental zinc, which is a lower dose than that recommended to treat diarrhoea but is sufficient to avoid competition with iron for sites of absorption, did not improve serum zinc concentrations (WHO/UNICEF 2004), although providing 10 mg of elemental zinc did improve serum zinc concentrations in one study (Barffour 2019), but not in another (Jack 2012 (C)). An ongoing study in Bangladesh is comparing the effects of MNP at different doses and frequencies of zinc on diarrhoeal disease, zinc status, and growth (Islam 2018a). More evidence on the impact of MNP on other micronutrients (e.g. vitamin A, vitamin E, iodine, folic acid) is needed (Lobo 2019).
It is difficult to assess the safety of this intervention in malaria settings. Only three studies reported the incidence of malaria with no differences in malaria parasitaemia between MNP and control groups (Adu‐Afarwuah 2007; Clarke 2018 (C); Lanou 2019 (C)). Although a study in Ghana found a decreased incidence of malaria among young children who consumed daily MNP containing iron compared to MNP without iron (Zlotkin 2013), another study in Kenya found no differences in Plasmodium falciparum infection (Teshome 2017). We excluded these studies because the comparison group was given non‐iron‐containing MNP ‐ not placebo or no intervention.
Evidence shows that MNP was associated with increased risk of diarrhoea among children in one of five studies that reported on this outcome, although no effects were seen on prolonged diarrhoea or hospitalisation with diarrhoea (Soofi 2013 (C)). The mechanism for increased diarrhoea from MNP has been demonstrated and may reflect adaptation of gut microflora to increased iron intake (Paganini 2016). A recent MNP study in Kenyan infants demonstrated that addition of an exogenous prebiotic to the MNP, in the form of galacto‐oligosaccharides, mitigated the side effects of the iron MNP on the gut microbiome (Paganini 2016). In another Kenyan study, which distributed iron‐containing MNPs as part of a health product package that included insecticide‐treated bed nets, soap, and point‐of‐use water treatment, hospitalisations for diarrhoea were decreased as was fever in intervention versus control villages (Suchdev 2016). Additional research is needed to ascertain potential side effects of iron on the infant gut in different settings and potential strategies to mitigate these effects, including alternative formulations and dosing of iron, use of prebiotics or nutrients with antioxidant effects, and integrated and complementary interventions to address both nutritional deficiencies and infections.
Albeit they were not specific outcomes of this review, it is well known that intake adherence and acceptability of a product are essential for an intervention to be implemented successfully (De Barros 2016). Overall, MNP seems to be well accepted by children and families, and caregivers report perceiving multiple health benefits after use (De Pee 2013). Results of the randomised controlled trials (RCTs) included in this review, along with some RCTs that were excluded because of the limited number of nutrients, show that acceptance of the intervention is not always translated into better adherence. High intake adherence (defined by experts as consumption of four or more sachets per week) to daily provision of MNP has ranged from 50% to around 90% (De Barros 2016; Giovannini 2006), and high adherence has been observed in studies in which children received the product on an intermittent basis (Hyder 2007).
Evidence from the first version of this review was used to inform World Health Organization (WHO) guidelines on this intervention (WHO 2011; WHO 2016b). The results of this updated version confirm both findings of the previous review and the provision scheme proposed in the WHO guidelines (WHO 2016b).
Quality of the evidence
Although not all reports include detailed information on study methods employed, we made efforts to contact study authors to request more data. We classified nine studies at high risk of bias, and their exclusion from the analyses in a sensitivity analysis did not affect the significance of the results nor hence the review's conclusions. Blinding of mothers or caregivers and care providers was not attempted or was unclear in 80% of studies. Also, only two studies utilised a placebo control; thus we lumped these studies together with studies in which the control group received no intervention. In approximately 50% of studies, outcome assessors and technical staff carrying out laboratory investigations were reported to be unaware of group allocation. Although for some outcomes, lack of blinding is unlikely to have an impact on results (e.g. anaemia), for others (e.g. maternal reports on infant side effects), lack of blinding may represent a potentially serious source of bias. With the exception of five studies (Adrianopoli 2014 (C); Esamai 2014; Luo 2017 (C); Sazawal 2014 (C); Somassè 2018 (C)), we did not consider attrition to be a serious problem in the included studies.
We evaluated the certainty of available evidence using the GRADE method and presented our ratings in 'Summary of findings' tables for the primary outcomes of anaemia, iron deficiency, haemoglobin concentration, iron status, and weight‐for‐age z scores (Table 1; Table 2). We planned to conduct a GRADE assessment for all‐cause mortality also; however, no data were available for analysis for this outcome. In the four studies that did mention mortality, the outcome did not occur (Giovannini 2006), or the outcome was not related to the intervention (Hirve 2007 (C); Matias 2018 (C); Mridha 2016 (C)).
The certainty of evidence, as assessed via GRADE, was moderate for anaemia, high for iron deficiency, low for haemoglobin, moderate for iron status, and moderate for weight‐for‐age (z scores) for the comparison of 'fortification of foods with MNP versus no intervention or placebo in children under two years of age'. We downgraded the certainty ratings due to serious or unclear risk of bias and high heterogeneity. For the comparison of 'fortification of foods with MNP versus an iron‐only supplement in children under two years of age', we rated the certainty of evidence as low for anaemia and very low for haemoglobin due to considerable statistical heterogeneity, inconsistency in results between studies, and small sample sizes with wide 95% confidence intervals.
Potential biases in the review process
We were aware of the possibility of introducing bias at every stage of the review process. In this review, we tried to minimise bias in a number of ways: two review authors assessed study eligibility for inclusion, carried out data extraction, and assessed risk of bias; and each review author worked independently. Nevertheless, the process of assessing risk of bias, for example, is not an exact science and includes many personal judgements. Further, the process of reviewing research studies is known to be affected by prior beliefs and attitudes. It is difficult to control for this type of bias in the review process.
This intervention is very recent and is well known among implementing agencies; they were contacted as part of the search strategy. Thus, we consider there to be minimal risk of publication bias.
Agreements and disagreements with other studies or reviews
For this update, we have included 22 new studies and have excluded one previously included study (Suchdev 2012). Our findings are consistent with the previous version of this Cochrane Review, and our primary conclusions have not changed (De‐Regil 2011).
A systematic review on the efficacy and effectiveness of complementary feeding interventions carried out in developing countries evaluated various interventions that targeted children within the age range of 6 to 24 months (Dewey 2009). Interventions assessed included fortification of complementary foods with micronutrients (centrally processed fortified foods or home fortification products with or without additional energy). These review authors restricted the evaluation of MNP to anaemia prevention specifically and included two other types of micronutrient supplements that were added to home‐prepared complementary foods: crushable tablets and fat‐based products. They concluded that fortification of complementary foods (through either processed complementary foods or home fortification) was a feasible option for complementary feeding in most circumstances, given the cost of iron‐rich foods (such as liver or meat). Home fortification requires little change in dietary practices, thus allowing families to continue to use home‐prepared or purchased complementary foods as the basis for the child's diet.
Our review focused specifically on home fortification of foods with MNP, with an inclusion criterion that established three micronutrients of critical importance (iron, vitamin A, and zinc), and assessed a broader spectrum of outcomes. We excluded other types of home fortification with lipid‐based spreads or crushable tablets to isolate the effects of this single intervention. We also excluded 10 studies that did not compare MNP to placebo, no intervention, or usual supplementation. Future reviews assessing the independent effects of iron versus non‐iron‐containing MNP would be helpful.
Another systematic review, which included 17 studies, evaluated effects of MNP in women and children (aged six months to six years) on haematological, anthropometric, and morbidity outcomes (Salam 2013). In agreement with our review, Salam 2013 found that children receiving MNP were less likely to be anaemic at follow‐up and had significantly reduced iron deficiency anaemia and improved haemoglobin levels. This review also found a significant increase in diarrhoea among children receiving MNP but did not show an impact of MNP on growth.
An area of potential concern relates to iron interventions in areas of high malaria transmission. A technical working group convened by the US National Institutes of Health concluded that there was little evidence regarding the safety of iron‐containing home fortification mixtures in malaria‐endemic areas, and that there was no evidence that MNPs are not safe, but this group recognised that no published studies have been designed to examine safety in malaria‐endemic areas (Raiten 2011). Our systematic review concurs with the results of this report and acknowledges the limitations of studies designed specifically to examine the safety of home fortification with MNP in malaria‐endemic areas for malarial outcomes. The limited evidence does not seem to suggest that increased risk of mortality or morbidity is associated with malaria, but ongoing studies specifically addressing this issue will help us to better understand any potential risks associated with providing iron through home fortification with MNP.
Home fortification has most often targeted infants and young children. Although the results of this review are mainly applicable to this age group, a complementary Cochrane Review in preschool‐age and school‐age children shows similar findings (De‐Regil 2017).
Authors' conclusions
Implications for practice.
Use of MNP for home fortification of foods is an effective intervention to reduce anaemia and iron deficiency in infants and young children. This intervention can be integrated into strategies to prevent anaemia and reduce the risk of iron deficiency among infants and children aged 6 to 23 months, but its benefit in reducing the risk of other vitamin and mineral deficiencies has not yet been demonstrated. It can be hypothesised that improving dietary intake of vitamins and minerals in the daily diet through this mechanism is beneficial, but evidence is lacking, as most studies have focused on iron deficiency and anaemia outcomes.
In this context, the dose of 12.5 mg of elemental iron (as ferrous fumarate) along with 5 mg of zinc and 300 μg of vitamin A has proved effective, and the addition of other vitamins and minerals to address nutrient gaps could be considered within the recommended nutrient intake levels for this age group. Other, more bioavailable iron compounds or substances that can modify the effects of iron (e.g. prebiotics) may be used (Paganini 2017), but the evidence is limited. Use of sodium iron‐ethylenediaminetetraacetic acid (FeNaEDTA or iron‐EDTA) as a more bioavailable source of iron may be feasible, but consideration of safe levels of iron‐EDTA is important to avoid their excessive intake, particularly among infants.
Provision of the sachets seems to be well accepted by mothers and caregivers. Although the evidence is limited, in comparison to iron supplements (as drops or syrups), home fortification with MNP has similar benefits for haematological outcomes but is associated with less staining of teeth and discolouration of stools. If iron supplementation programmes are not in place, or are not successfully implemented, use of MNP for home fortification of foods can be considered for prevention of anaemia and iron deficiency in children 6 to 23 months of age.
As providing MNP to children requires preparation of food by a caregiver, efforts should be made to ensure that basic sanitation is available, and that food hygiene and handling are done properly with safe water. All included studies were performed in low resource settings, where sanitation tends to be poor. Behavioural and communication campaigns, therefore, should promote appropriate use of MNP in addition to the hygienic preparation of complementary foods and hand washing (World Bank 2010). Furthermore, some evidence suggests that use of MNP may increase the prevalence of diarrhoea among children but provides limited information on severity; additional research is needed so we can better understand the potential risks and mitigating factors of MNP for diarrhoea.
The benefits of using MNP as a child survival strategy or for developmental outcomes are limited. Data on effects on malaria outcomes are also lacking, and further investigation in malaria settings is needed.
Implications for research.
This systematic review has highlighted some evidence gaps that merit further research, including the following.
Side effects (such as vomiting, constipation, diarrhoea, coughing), as well as iron overload associated with home fortification with MNP in settings where infection and malnutrition are common, need to be explored in greater depth, with emphasis on the harmonisation of outcome definitions that will better balance harms and benefits of this intervention in various contexts, particularly in areas with high transmission of malaria.
The prevalence of diarrhoea in association with MNP needs to be investigated in greater detail.
The use of alternative iron compounds, as well as dosing of iron and provision of prebiotics in MNP formulations, in areas with high infection burden needs to be examined.
The effective regimen for distribution and consumption of MNP in intermittent or flexible schemes as an alternative to daily provision of MNP needs to be identified.
The minimum effective duration of fortification with MNP and total iron intake must be ascertained.
The efficacy and effectiveness of home fortification with MNP for additional nutrient effects (e.g. iodine, vitamin D, vitamin A, calcium, phosphorus, magnesium, potassium, choline) and also for important functional outcomes, including growth and motor and cognitive development, need to be determined.
Feedback
Three queries concerning the "Home fortification with micronutrients" review, 14 October 2013
Summary
Comment
It was not clear to me why the numbers cited in the analysis seemed different from those given in the Hirve 2007 article [1]. For example, in analysis 2.1, N = 30 in the iron supplement control group was cited, yet the article flow chart described that n = 83 was assigned to the iron supplement (oral drop) group and 10 were lost to follow‐up. In analysis 2.1, 30 events of diarrhoea in 55 children were cited. In Table III of Hirve 2007, the mean episode per child was 1.05, which amounted to 77 events for all 73 children who completed iron supplement.
This review also cited unpublished data from the Suchdev 2011 study. Because it was unpublished, it was not available to me to evaluate. An article by Suchdev [2] published in 2012 seemed to report on this study as Suchdev 2011 cited in this review. The actual intervention was subtly different from this review. This trial did not give MNP to every family. The MNP was marketed and sold in the selected villages, so the family could buy if they wanted to. Although study authors said 93% of the intervention group received MNP at least once during the one‐year trial period, ˜ 40% of the control group also purchased MNP. These facts might help to explain the modest effect size of the trial.
The main results section of the Abstract said, "No deaths were reported in the trials ....", then qualified the statement as "In one of the trials, two deaths were reported after the intervention was finalized but were judged not to be related to the study (Hirve 2007 (C))." These statements were misleading. The second death occurred during the trial (in the seventh week of iron supplementation), not after the intervention was finalised. Deaths in clinical trials should be unambiguously and publicly reported but often were not [3]. In the Suchdev 2012 report, there were 12 deaths during the 12‐month follow‐up period of the trial. Regardless of the causes of death, discrepancy like this might cast doubt on the completeness of unpublished data.
References
Hirve S, Bhave S, Bavdekar A, Naik S, Pandit A, Schauer C, et al. Low dose 'Sprinkles' ‐ an innovative approach to treat iron deficiency anemia in infants and young children. Indian Pediatrics 2007;44(2):91–100.
Suchdev PS, Ruth LJ, Woodruff BA, Mbakay C, Mandava U, Flores‐Ayala R, et al. Selling Sprinkles micronutrient powder reduces anemia, iron deficiency, and vitamin A deficiency in young children in Western Kenya: a cluster‐randomized controlled trial. American Journal of Clinical Nutrition 2012;95:1223‐30.
Earley A, Lau J, Uhlig K. Haphazard reporting of deaths in clinical trials: a review of cases of ClinicalTrials.gov records and matched publications – a cross‐sectional study. BMJ Open 2013;3:e001963.
I agree with the conflict of interest statement below: I certify that I have no affiliations with or involvement in any organisation or entity with a financial interest in the subject matter of my feedback. Name: Jih‐I Yeh Email Address:jihiyeh@gms.tcu.edu.tw Affiliation: Department of Family Medicine, Buddhist Tzu‐Chi hospital & Tzu‐Chi University, Hualien, Taiwan
Reply
Response of review author team: this feedback is valid and helpful, and also timely. The review author team has recently been in discussions regarding an update to this review so that it reflects the current state of the art of this intervention. The last search was conducted almost three years ago, and since then a number of trials that potentially meet the inclusion criteria of this review have been published, including that of Dr Suchdev. Also, now more sophisticated statistical analyses are available for the subgroup analyses, and we (those authors who will contribute to the update) would also like this review be aligned with another Cochrane Review in development, "Point‐of‐use fortification of foods with micronutrient powders containing iron in children of preschool and school age" (De‐Regil 2017), to better inform policy‐making.
The Cochrane Developmental, Psychosocial and Learning Problems Editorial Board has accepted our plan to address the comments above, whilst updating the review as described, and we hope to complete this work in 2014.
Contributors
Dr Jih‐l Yeh (affiliation), Dr Luz de‐Regil (World Health Organization), Joanne Wilson (Managing Editor, CDPLPG), Jane Dennis (Feedback Editor, CDPLPG).
What's new
| Date | Event | Description |
|---|---|---|
| 8 October 2019 | New search has been performed | Updated following a new search in January 2018 and July 2019 |
| 8 October 2019 | New citation required but conclusions have not changed | Update includes 22 new studies and excludes 1 previously included study. Our conclusions have not changed |
History
Protocol first published: Issue 1, 2011 Review first published: Issue 9, 2011
| Date | Event | Description |
|---|---|---|
| 5 March 2014 | Amended | Minor edits made to references |
| 14 October 2013 | Feedback has been incorporated | 3 queries related to the "Home fortification of foods with multiple micronutrients" review and responses of the review author team |
Acknowledgements
We would like to thank the study authors and organisations that contributed additional data for this review. In addition, we would like to thank the staff of the Cochrane Developmental, Psychosocial and Learning Problems (CDPLP) Editorial Base for their support in the preparation of this review and, in particular, Geraldine Macdonald and Joanne Duffield, as well as Margaret Anderson, who helped with the search strategy.
As part of the pre‐publication editorial process, this review was commented on by three peers (a CDPLP editor and two referees (Professor Peter Emery, King's College London, UK; Professor Sue Horton, University of Waterloo, Canada) external to the CDPLP editorial team) and a statistician from CDPLP.
The findings and conclusions reported in this manuscript are those of the review authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library
# 1 MeSH descriptor Micronutrients, this term only # 2 MeSH descriptor Trace Elements, this term only # 3 MeSH descriptor Zinc, this term only # 4 MeSH descriptor Iron, this term only # 5 MeSH descriptor Vitamin A, this term only # 6 MeSH descriptor Iron, Dietary, this term only # 7 MeSH descriptor Ferric Compounds, this term only # 8 MeSH descriptor Ferrous Compounds, this term only # 9 micronutrient* or micro‐nutrient* or micro next nutrient* #10 multinutrient* or multi next nutrient* or multi* nutrient* #11 multimicronutrient* or multimicro next nutrient* #12 multivitamin* or multi* next vitamin* #13 multimineral* or multi* next mineral* #14 trace NEXT (element* or mineral* or nutrient*) #15 iron or ferric* or ferrous* or Fe or zinc or Zn or (vit* next A) or retinol* #16 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15) #17 MeSH descriptor Food, Fortified, this term only #18 MeSH descriptor Dietary Supplements, this term only #19 MeSH descriptor Foods, Specialized explode all trees #20 ((food* or meal* or drink* or beverage* or diet* or snack* or breakfast* or break‐fast* or lunch* or dinner*) near/5 (fortif* or enrich* or supplement*)) #21 "point of use" #22 home near/5 fortif* #23 (in NEXT home or at NEXT home) near/5 (fortif*) #24 mix* or powder* or supplement* or sachet* or packet* or powder* or MNP or MNPs #25 (#17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24) #26 Sprinkles Or Vita NEXT Shakti or Rahama or Anuka or Chispitas or BabyFer or Bebe NEXT Vanyan or Supplfer or Supplefem #27 (#16 AND #25) #28 (#26 OR #27) #29 (baby or babies or infant* or toddler* or preschool* or pre‐school* or child*) #30 MeSH descriptor Infant explode all trees #31 child near Mesh #32 (#29 OR #30 OR #31) #33 (#28 AND #32)
MEDLINE Ovid
1 micronutrients/ 2 iron/ or zinc/ or vitamin A/ 3 (micronutrient$ or micro‐nutrient$).tw. 4 (multinutrient$ or multi‐nutrient$ or multi$ nutrient$).tw. 5 (multimicro‐nutrient$ or multimicronutrient$).tw. 6 (multivitamin$ or multi‐vitamin$).tw. 7 (multimineral$ or multi‐mineral$).tw. 8 Trace elements/ 9 (trace adj (element$ or mineral$ or nutrient$)).tw. 10 iron,dietary/ 11 ferric compounds/ or ferrous compounds/ 12 (iron or Fe or ferric$ or ferrous$ or zinc or Zn or vit$ A or retinol$).mp. 13 or/1‐12 14 food, fortified/ 15 dietary supplements/ 16 food,specialized/ 17 ((food$ or meal$ or drink$ or beverage$ or diet$ or snack$ or breakfast$ or break‐fast$ or lunch$ or dinner$) adj5 (fortif$ or enrich$ or supplement$)).tw. 18 "point of use".tw. 19 (home adj5 fortif$).tw. 20 ((in‐home or at‐home) adj5 fortif$).tw. 21 (mix$ or powder$ or supplement$ or sachet$ or packet$ or powder$ or MNP or MNPs).tw. 22 or/14‐21 23 13 and 22 24 (Sprinkles or Vita Shakti or Rahama or Anuka or Chispitas or BabyFer or Bebe Vanyan or Supplefer or Supplefem).tw. 25 or/23‐24 26 (baby or babies or infant$ or toddler$ or preschool$ or pre‐school$ or child$).tw. 27 exp child/ or infant/ 28 26 or 27 29 25 and 28
MEDLINE In‐Process & Other Non‐Indexed Citations Ovid
1 (micronutrient$ or micro‐nutrient$).tw. 2 (multinutrient$ or multi‐nutrient$ or multi$ nutrient$).tw. 3 (multimicro‐nutrient$ or multimicronutrient$).tw. 4 (multivitamin$ or multi‐vitamin$).tw. 5 (multimineral$ or multi‐mineral$).tw. 6 (trace adj (element$ or mineral$ or nutrient$)).tw. 7 ((food$ or meal$ or drink$ or beverage$ or diet$ or snack$ or breakfast$ or break‐fast$ or lunch$ or dinner$) adj5 (fortif$ or enrich$ or supplement$)).tw. 8 "point of use".tw. 9 (home adj5 fortif$).tw. 10 ((in‐home or at‐home) adj5 fortif$).tw. 11 (mix$ or powder$ or supplement$ or sachet$ or packet$ or powder$ or MNP or MNPs).tw. 12 (Sprinkles or Vita Shakti or Rahama or Anuka or Chispitas or BabyFer or Bebe Vanyan or Supplefer or Supplefem).tw. 13 (baby or babies or infant$ or toddler$ or preschool$ or pre‐school$ or child$).tw. 14 or/7‐12 15 or/1‐6 16 or/7‐11 17 15 and 16 18 12 or 17 19 13 and 18
MEDLINE E‐Pub Ahead of Print Ovid
1 (micronutrient$ or micro‐nutrient$).tw. 2 (multinutrient$ or multi‐nutrient$ or multi$ nutrient$).tw. 3 (multimicro‐nutrient$ or multimicronutrient$).tw. 4 (multivitamin$ or multi‐vitamin$).tw. 5 (multimineral$ or multi‐mineral$).tw. 6 (trace adj (element$ or mineral$ or nutrient$)).tw. 7 ((food$ or meal$ or drink$ or beverage$ or diet$ or snack$ or breakfast$ or break‐fast$ or lunch$ or dinner$) adj5 (fortif$ or enrich$ or supplement$)).tw. 8 "point of use".tw. 9 (home adj5 fortif$).tw. 10 ((in‐home or at‐home) adj5 fortif$).tw. 11 (mix$ or powder$ or supplement$ or sachet$ or packet$ or powder$ or MNP or MNPs).tw. 12 (Sprinkles or Vita Shakti or Rahama or Anuka or Chispitas or BabyFer or Bebe Vanyan or Supplefer or Supplefem).tw. 13 (baby or babies or infant$ or toddler$ or preschool$ or pre‐school$ or child$).tw. 14 or/7‐12 15 or/1‐6 16 or/7‐11 17 15 and 16 18 12 or 17 19 13 and 18
Embase Ovid
1 exp trace element/ 2 vitamin mixture/ 3 (trace adj (element$ or mineral$ or nutrient$)).tw. 4 iron/ or ZINC/ or retinol/ 5 (iron or Fe or ferrous$ or ferric$ or zinc or Zn or vit$ A or retinol$).mp. 6 iron derivative/ 7 iron intake/ 8 ferric ion/ or ferrous ion/ 9 (micronutrient$ or micro‐nutrient$).tw. 10 (multinutrient or multi‐nutrient or multi$ nutrient$).tw. 11 (multimicronutrient$ or multi‐micronutrient$).tw. 12 (multivitamin$ or multi‐vitamin$).tw. 13 (multimineral$ or multi‐mineral$).tw. 14 or/1‐13 15 diet supplementation/ 16 ((food$ or meal$ or drink$ or beverage$ or diet$ or snack$ or breakfast$ or break‐fast$ or lunch$ or dinner$) adj5 (fortif$ or enrich$ or supplement$)).tw. 17 "point of use".tw. 18 (home$ adj5 fortif$).tw 19 ((in‐home or at‐home) adj5 fortif$).tw. 20 (mix$ or powder$ or packet$ or supplement$ or sachet$ or powder$ or MNP or MNPs).tw. 21 or/15‐20 22 14 and 21 23 (Sprinkles or Vita Shakti or Rahama or Anuka or Chispitas or BabyFer or Bebe Vanyan or Supplefer or Supplefem).tw. 24 22 or 23 25 exp child/ 26 infant/ 27 (baby or babies or infant$ or toddler$ or preschool or pre‐school$ or child$).tw. 28 25 or 26 or 27 29 24 and 28
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
S28 S24 and S27 S27 S25 or S26 S26 baby or babies or infant* or toddler* or pre‐school* or preschool* or child* S25 AG Infant, Newborn: birth‐1 month OR Infant: 1‐23 months or Child,Preschool: 2‐5 years S24 S22 or S23 S23 (Sprinkles or Vita Shakti or Rahama or Anuka or Chispitas or BabyFer or Bebe Vanyan or Supplefer or Supplefem) S22 S13 and S21 S21 S14 or S15 or S16 or S17 or S18 or S19 or S20 S20 (mix* or powder* or supplement* or sachet* or packet* or powder* or MNP or MNPs) S19 at‐home N5 fortif* S18 in‐home N5 fortif* S17 home N5 fortif* S16 "point of use" S15 (food* or meal* or drink* or beverage* or diet* or snack* or breakfast* or break‐fast* or lunch* or dinner*) AND (fortif* or enrich* or supplement*) S14 (MH "Food, Fortified") OR (MH "Dietary Supplements") S13 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 S12 iron or "Fe" or ferric* or ferrous* or zinc or "Zn" or "vit* A" or retinol* S11 trace element* or trace mineral* or trace nutrient* S10 (MH "Trace Elements") S9 (MH "Ferric Compounds") OR (MH "Ferrous Compounds") S8 multimineral* or multi‐mineral* S7 multivitamin* or multi‐vitamin* S6 (MH "Vitamin A") S5 multimicro‐nutrient* or multimicronutrient* S4 multinutrient* or multi‐nutrient* or multi* nutrient* S3 micronutrient* or micro‐nutrient* S2 (MH "Iron") OR (MH "Iron Compounds") OR (MH "Zinc") S1 (MH "Micronutrients")
Science Citation Index Web of Science
# 13 #12 AND #11 Indexes=SCI‐EXPANDED Timespan=All years # 12 TS=(baby or babies or infant* or toddler* or preschool* or pre‐school* or child* ) Indexes=SCI‐EXPANDED Timespan=All years # 11 #10 OR #1 Indexes=SCI‐EXPANDED Timespan=All years # 10 #9 AND #8 Indexes=SCI‐EXPANDED Timespan=All years # 9 #7 OR #6 OR #5 OR #4 Indexes=SCI‐EXPANDED Timespan=All years # 8 #2 OR #3 Indexes=SCI‐EXPANDED Timespan=All years # 7 TS=( supplement or supplements or sachet* or packet or packets or powder or powders or MNP or MNPs) Indexes=SCI‐EXPANDED Timespan=All years # 6 TS=( "home fortif*" or "at‐home fortif*" or "in‐home fortif*") Indexes=SCI‐EXPANDED Timespan=All years # 5 TS= ("point of use") Indexes=SCI‐EXPANDED Timespan=All years # 4 TS=((food* or meal* or drink* or beverage* or diet* or snack* or breakfast* or break‐fast* or lunch* or dinner*) NEAR/5 (fortif* or enrich* or supplement*)) Indexes=SCI‐EXPANDED Timespan=All years # 3 TS=(iron or Fe or ferric* or ferrous* or zinc or Zn or "vitamin A" or retinol*) Indexes=SCI‐EXPANDED Timespan=All years # 2 TS= (micronutrient* or micro‐nutrient* or multinutrient* or multi‐nutrient* or multi* nutrient* or multimicro‐nutrient* or multimicronutrient* or multivitamin* or multi‐vitamin* or MNP or MNPs) Indexes=SCI‐EXPANDED Timespan=All years # 1 TS= ("Sprinkles" or "Vita Shakti" or Rahama or Anuka or Chispitas or BabyFer or "Bebe Vanyan" or Supplefer or Supplefem) Indexes=SCI‐EXPANDED Timespan=All years
Conference Proceedings Citation Index ‐ Science Web of Science
# 13 #12 AND #11 Indexes=CPCI‐S Timespan=All years # 12 TS=(baby or babies or infant* or toddler* or preschool* or pre‐school* or child* ) Indexes=CPCI‐S Timespan=All years # 11 #10 OR #1 Indexes=CPCI‐S Timespan=All years # 10 #9 AND #8 Indexes=CPCI‐S Timespan=All years # 9 #7 OR #6 OR #5 OR #4 Indexes=CPCI‐S Timespan=All years # 8 #2 OR #3 Indexes=CPCI‐S Timespan=All years # 7 TS=( supplement or supplements or sachet* or packet or packets or powder or powders or MNP or MNPs) Indexes=CPCI‐S Timespan=All years # 6 TS=( "home fortif*" or "at‐home fortif*" or "in‐home fortif*") Indexes=CPCI‐S Timespan=All years # 5 TS= ("point of use") Indexes=CPCI‐S Timespan=All years # 4 TS=((food* or meal* or drink* or beverage* or diet* or snack* or breakfast* or break‐fast* or lunch* or dinner*) NEAR/5 (fortif* or enrich* or supplement*)) Indexes=CPCI‐S Timespan=All years # 3 TS=(iron or Fe or ferric* or ferrous* or zinc or Zn or "vitamin A" or retinol*) Indexes=CPCI‐S Timespan=All years # 2 TS= (micronutrient* or micro‐nutrient* or multinutrient* or multi‐nutrient* or multi* nutrient* or multimicro‐nutrient* or multimicronutrient* or multivitamin* or multi‐vitamin* or MNP or MNPs) Indexes=CPCI‐S Timespan=All years # 1 TS= ("Sprinkles" or "Vita Shakti" or Rahama or Anuka or Chispitas or BabyFer or "Bebe Vanyan" or Supplefer or Supplefem) Indexes=CPCI‐S Timespan=All years
African Index Medicus (indexmedicus.afro.who.int)
sprinkles OR micronutrients OR multimicronutrients OR mnp OR bebe vanyan or supplefer or vita shakti or babyfer or chispitas or anuka or rahama [Key Word]
LILACS (Latin American and Caribbean Health Science Information database; lilacs.bvsalud.org/en)
micronutrient$ or multinutrien$ or micro‐nutrient$ or multi‐nutrient$ [Words] and home$ or fortif$ or point‐of‐use [Words] or sprinkles or MNP or MNPs [Words]
POPLINE (www.popline.org)
((micronutrient* /micro‐nutrient*/multinutrient/ multi‐nutrient*/ multi* nutrient*) & (home fortif*/ point of use) ) /sprinkles
ClinicalTrials.gov (clinicaltrials.gov)
sprinkles OR "multinutrient powder" OR multimicronutrients OR mnp OR mnps | Child
ISRCTN Registry (www.isrctn.com)
First searched in 2016 to replace the metaRegister of Clinical Trials. Search terms entered in Advanced Search and limited to Categories "Nutritional Metabolic Endocrine" and "Children" include the following.
Sprinkles. Multi micronutrients. Micronutrient powder. Multi‐micronutrients. Home fortification. MNP. MNPs.
metaRegister of Clinical Trials (www.isrctn.com/page/mrct)
Last searched Februiary 2014, after which the service was unavailable.
sprinkles or multinutrients or multimicronutrients or MNP or MNPs
WHO ICTRP (apps.who.int/trialsearch)
Intervention: sprinkles or multinutrients or multimicronutrients or MNP or MNPs AND Clinical trials in children
Appendix 2. 'Risk of bias' assessment criteria
Random sequence generation (checking for selection bias)
We described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it produced comparable groups.
Low risk of bias: any truly random process; for example, random numbers table, computer random number generator.
High risk of bias: any non‐random process; for example, odd or even date of birth, hospital or clinic record number.
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias.
Allocation concealment (checking for possible selection bias)
We described the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment.
Low risk of bias: for example, telephone or central randomisation; consecutively numbered, sealed, opaque envelopes.
High risk of bias: open random allocation, unsealed or non‐opaque envelopes.
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias.
Blinding of participants and personnel (checking for possible performance bias)
We described all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received.
Low risk of bias: neither participants nor personnel giving the intervention were aware of the intervention.
High risk of bias: either participants or personnel were aware of the intervention.
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias.
Blinding of outcome assessment (checking for possible detection bias)
We described all measures used, if any, to blind outcome assessors from knowledge as to which intervention a participant received.
Low risk of bias: blinding of outcomes, which is unlikely to have been broken.
High risk of bias: for example, no blinding of outcome assessment where measurement is likely to be influenced by lack of blinding, or where blinding could have been broken.
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We assessed outcomes in each included study as follows.
Low risk of bias: either there were no missing outcome data or the missing outcome data were unlikely to bias the results based on the following considerations: study authors provided transparent documentation of participant flow throughout the study, the proportion of missing data was similar in the intervention and control groups, the reasons for missing data were provided and balanced across intervention and control groups, the reasons for missing data were not likely to bias the results (e.g. moving house).
High risk of bias: missing outcome data were likely to bias the results. Trials also received this rating if an 'as‐treated (per protocol)' analysis was performed with substantial differences between the intervention received and that assigned at randomisation, or if potentially inappropriate methods for imputation had been used.
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias.
Selective reporting (checking for possible reporting bias)
We stated how the possibility of selective outcome reporting was examined and what was found.
Low risk of bias: when it was clear that all the study's pre‐specified outcomes and all expected outcomes of interest to the review were reported.
High risk of bias: when not all of the study's pre‐specified outcomes were reported, one or more reported primary outcomes were not pre‐specified, outcomes of interest were reported incompletely and so could not be used, or the study failed to include results of a key outcome that would have been expected to have been reported.
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias.
Other bias (checking for other potential sources of bias not covered by the domains above)
We assessed if the study was free of other potential bias as follows.
Low risk of bias: when there was similarity between the outcome measures at baseline, similarity between potential confounding variables at baseline, or adequate protection of study arms against contamination.
High risk of bias: when there was no similarity between outcome measures at baseline, similarity between potential confounding variables at baseline, or adequate protection of study arms against contamination.
Unclear risk of bias: insufficient information to permit judgement of low or high risk of bias.
Overall risk of bias
We summarised the overall risk of bias at two levels: within trials (across domains) and across trials.
Overall risk of bias within trials
For the assessment within trials, we assessed the likely magnitude and direction of the bias in each of the 'Risk of bias' domains, and whether we considered they were likely to impact the findings. We considered trials at high risk of bias if they had poor or unclear allocation concealment and either inadequate blinding of both participants and personnel or high/imbalanced losses to follow‐up. We explored the impact of the level of bias through a Sensitivity analysis.
Data and analyses
Comparison 1. Home (point‐of‐use) fortification of foods with MNP versus no intervention or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anaemia | 16 | 9927 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.76, 0.90] |
| 2 Anaemia by baseline anaemia status | 16 | 9291 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.75, 0.89] |
| 2.1 Anaemic | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.38, 0.76] |
| 2.2 Non‐anaemic | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Mixed/unknown | 15 | 9166 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.77, 0.91] |
| 3 Anaemia by baseline iron status | 16 | 9927 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.76, 0.90] |
| 3.1 Iron deficiency | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Non‐iron deficiency | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Mixed/unknown | 16 | 9927 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.76, 0.90] |
| 4 Anaemia by age at the start of the intervention | 12 | 4425 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.69, 0.85] |
| 4.1 6 to 11 months | 9 | 3556 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.72, 0.90] |
| 4.2 12 to 17 months | 2 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.40, 0.82] |
| 4.3 18 to 23 months | 1 | 557 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.61, 0.79] |
| 5 Anaemia by refugee status | 16 | 9291 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.75, 0.89] |
| 5.1 Yes | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 No | 16 | 9291 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.75, 0.89] |
| 6 Anaemia by malaria endemicity in the study area | 16 | 9927 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.76, 0.90] |
| 6.1 Yes | 15 | 9370 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.78, 0.92] |
| 6.2 No | 1 | 557 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.61, 0.79] |
| 7 Anaemia by frequency of intake | 16 | 9927 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.76, 0.90] |
| 7.1 Daily | 15 | 8321 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.73, 0.88] |
| 7.2 Flexible | 1 | 1606 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 1.00] |
| 7.3 Weekly | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Anaemia by duration of the intervention | 16 | 9927 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.76, 0.90] |
| 8.1 Less than 6 months | 5 | 990 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.62, 0.78] |
| 8.2 6 months or longer | 11 | 8937 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.80, 0.93] |
| 9 Anaemia by iron content | 16 | 9927 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.76, 0.90] |
| 9.1 Less than 12.5 mg | 7 | 5097 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.84, 1.00] |
| 9.2 12.5 mg or more | 9 | 4830 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.66, 0.87] |
| 10 Anaemia by zinc content | 16 | 9927 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.76, 0.90] |
| 10.1 Less than 5 mg | 3 | 2363 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.57, 1.10] |
| 10.2 5 mg or more | 13 | 7564 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.74, 0.90] |
| 11 Iron deficiency | 7 | 1634 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.39, 0.56] |
| 12 Iron deficiency by baseline anaemia status | 7 | 1634 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.39, 0.56] |
| 12.1 Anaemic | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.14, 0.52] |
| 12.2 Non‐anaemic | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Mixed/unknown | 6 | 1509 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.41, 0.59] |
| 13 Iron deficiency by baseline iron status | 7 | 1645 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.39, 0.57] |
| 13.1 Iron deficiency | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Non‐iron deficiency | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Mixed/unknown | 7 | 1645 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.39, 0.57] |
| 14 Iron deficiency by age at the start of the intervention | 6 | 1367 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.38, 0.59] |
| 14.1 6 to 11 months | 5 | 1181 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.38, 0.60] |
| 14.2 12 to 17 months | 1 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.12, 0.82] |
| 14.3 18 to 23 months | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Iron deficiency by refugee status | 7 | 1645 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.39, 0.57] |
| 15.1 Yes | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 No | 7 | 1645 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.39, 0.57] |
| 16 Iron deficiency by malaria endemicity in the study area | 7 | 1645 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.39, 0.57] |
| 16.1 Yes | 7 | 1645 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.39, 0.57] |
| 16.2 No | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Iron deficiency by frequency of intake | 7 | 1645 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.39, 0.57] |
| 17.1 Daily | 7 | 1645 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.39, 0.57] |
| 17.2 Flexible | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Weekly | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Iron deficiency by duration of the intervention | 7 | 1645 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.39, 0.57] |
| 18.1 Less than 6 months | 3 | 490 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.27, 0.64] |
| 18.2 6 months or longer | 4 | 1155 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.36, 0.60] |
| 19 Iron deficiency by iron content | 7 | 1645 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.39, 0.57] |
| 19.1 Less than 12.5 mg | 3 | 1035 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.29, 0.56] |
| 19.2 12.5 mg or more | 4 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.37, 0.65] |
| 20 Iron deficiency by zinc content | 7 | 1645 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.39, 0.57] |
| 20.1 Less than 5 mg | 2 | 757 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.26, 0.60] |
| 20.2 5 mg or more | 5 | 888 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.38, 0.61] |
| 21 Haemoglobin (g/L) | 20 | 10509 | Mean Difference (IV, Random, 95% CI) | 2.74 [1.95, 3.53] |
| 22 Haemoglobin by baseline anaemia status | 20 | 9862 | Mean Difference (IV, Random, 95% CI) | 2.76 [2.13, 3.39] |
| 22.1 Anaemic | 3 | 1011 | Mean Difference (IV, Random, 95% CI) | 4.53 [0.04, 9.01] |
| 22.2 Non‐anaemic | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐8.0 [‐12.26, ‐3.74] |
| 22.3 Mixed/unknown | 16 | 8833 | Mean Difference (IV, Random, 95% CI) | 3.05 [1.77, 4.32] |
| 23 Haemoglobin by baseline iron status | 20 | 10498 | Mean Difference (IV, Random, 95% CI) | 2.73 [1.94, 3.52] |
| 23.1 Iron deficiency | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Non‐iron deficiency | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐8.0 [‐12.26, ‐3.74] |
| 23.3 Mixed/unknown | 19 | 10480 | Mean Difference (IV, Random, 95% CI) | 3.02 [2.22, 3.82] |
| 24 Haemoglobin by age at the start of the intervention | 18 | 6040 | Mean Difference (IV, Random, 95% CI) | 2.83 [1.02, 4.65] |
| 24.1 6 to 11 months | 12 | 3948 | Mean Difference (IV, Random, 95% CI) | 2.82 [0.59, 5.05] |
| 24.2 12 to 17 months | 3 | 938 | Mean Difference (IV, Random, 95% CI) | 1.14 [‐1.39, 3.67] |
| 24.3 18 to 23 months | 3 | 1154 | Mean Difference (IV, Random, 95% CI) | 4.04 [‐1.92, 10.00] |
| 25 Haemoglobin by refugee status | 20 | 10498 | Mean Difference (IV, Random, 95% CI) | 2.73 [1.94, 3.52] |
| 25.1 Yes | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 No | 20 | 10498 | Mean Difference (IV, Random, 95% CI) | 2.73 [1.94, 3.52] |
| 26 Haemoglobin by malaria endemicity in the study area | 20 | 10498 | Mean Difference (IV, Random, 95% CI) | 2.73 [1.94, 3.52] |
| 26.1 Yes | 19 | 9941 | Mean Difference (IV, Random, 95% CI) | 2.30 [1.49, 3.11] |
| 26.2 No | 1 | 557 | Mean Difference (IV, Random, 95% CI) | 9.5 [7.19, 11.81] |
| 27 Haemoglobin by frequency of intake | 20 | 10498 | Mean Difference (IV, Random, 95% CI) | 2.73 [1.94, 3.52] |
| 27.1 Daily | 19 | 7455 | Mean Difference (IV, Random, 95% CI) | 2.72 [1.23, 4.22] |
| 27.2 Flexible | 1 | 3043 | Mean Difference (IV, Random, 95% CI) | 2.60 [2.56, 2.64] |
| 27.3 Weekly | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Haemoglobin by duration of the intervention | 20 | 10498 | Mean Difference (IV, Random, 95% CI) | 2.73 [1.94, 3.52] |
| 28.1 Less than 6 months | 7 | 1510 | Mean Difference (IV, Random, 95% CI) | 1.96 [‐2.25, 6.17] |
| 28.2 6 months or longer | 13 | 8988 | Mean Difference (IV, Random, 95% CI) | 2.70 [1.80, 3.60] |
| 29 Haemoglobin by iron content | 20 | 10498 | Mean Difference (IV, Random, 95% CI) | 2.73 [1.94, 3.52] |
| 29.1 Less than 12.5 mg | 9 | 7288 | Mean Difference (IV, Random, 95% CI) | 1.61 [0.58, 2.64] |
| 29.2 12.5 mg or more | 11 | 3210 | Mean Difference (IV, Random, 95% CI) | 3.92 [1.06, 6.77] |
| 30 Haemoglobin by zinc content | 20 | 10498 | Mean Difference (IV, Random, 95% CI) | 2.73 [1.94, 3.52] |
| 30.1 Less than 5 mg | 4 | 3999 | Mean Difference (IV, Random, 95% CI) | 2.06 [0.68, 3.44] |
| 30.2 5 mg or more | 16 | 6499 | Mean Difference (IV, Random, 95% CI) | 3.05 [1.31, 4.79] |
| 31 Iron status (ferritin concentrations in μg/L) | 7 | 2612 | Mean Difference (IV, Random, 95% CI) | 12.93 [7.41, 18.45] |
| 32 Iron status (ferritin concentrations in μg/L) by baseline anaemia status | 7 | 2612 | Mean Difference (IV, Random, 95% CI) | 12.93 [7.41, 18.45] |
| 32.1 Anaemic | 1 | 125 | Mean Difference (IV, Random, 95% CI) | 27.5 [19.29, 35.71] |
| 32.2 Non‐anaemic | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 26.2 [14.80, 37.60] |
| 32.3 Mixed/unknown | 5 | 2470 | Mean Difference (IV, Random, 95% CI) | 8.36 [4.72, 12.00] |
| 33 Iron status (ferritin concentrations in μg/L) by baseline iron status | 7 | 2764 | Mean Difference (IV, Random, 95% CI) | 13.43 [5.71, 21.15] |
| 33.1 Iron deficiency | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 Non‐iron deficiency | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 26.2 [14.80, 37.60] |
| 33.3 Mixed/unknown | 6 | 2747 | Mean Difference (IV, Random, 95% CI) | 11.37 [3.34, 19.40] |
| 34 Iron status (ferritin concentrations in μg/L) by age at the start of the intervention | 6 | 2334 | Mean Difference (IV, Random, 95% CI) | 14.43 [6.33, 22.52] |
| 34.1 6 to 11 months | 6 | 2334 | Mean Difference (IV, Random, 95% CI) | 14.43 [6.33, 22.52] |
| 34.2 12 to 17 months | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.3 18 to 23 months | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35 Iron status (ferritin concentrations in μg/L) by refugee status | 7 | 2764 | Mean Difference (IV, Random, 95% CI) | 13.43 [5.71, 21.15] |
| 35.1 Yes | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 No | 7 | 2764 | Mean Difference (IV, Random, 95% CI) | 13.43 [5.71, 21.15] |
| 36 Iron status (ferritin concentrations in μg/L) by malaria endemicity in the study area | 7 | 2612 | Mean Difference (IV, Random, 95% CI) | 12.93 [7.41, 18.45] |
| 36.1 Yes | 7 | 2612 | Mean Difference (IV, Random, 95% CI) | 12.93 [7.41, 18.45] |
| 36.2 No | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37 Iron status by frequency of intake | 7 | 2764 | Mean Difference (IV, Random, 95% CI) | 13.43 [5.71, 21.15] |
| 37.1 Daily | 7 | 2764 | Mean Difference (IV, Random, 95% CI) | 13.43 [5.71, 21.15] |
| 37.2 Flexible | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.3 Weekly | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Iron status (ferritin concentrations in μg/L) by duration of the intervention | 7 | 2764 | Mean Difference (IV, Random, 95% CI) | 13.43 [5.71, 21.15] |
| 38.1 Less than 6 months | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 26.2 [14.80, 37.60] |
| 38.2 6 months or longer | 6 | 2747 | Mean Difference (IV, Random, 95% CI) | 11.37 [3.34, 19.40] |
| 39 Iron status (ferritin concentrations in μg/L) by iron content | 7 | 2764 | Mean Difference (IV, Random, 95% CI) | 13.43 [5.71, 21.15] |
| 39.1 Less than 12.5 mg | 3 | 2053 | Mean Difference (IV, Random, 95% CI) | 6.76 [‐2.25, 15.77] |
| 39.2 12.5 mg or more | 4 | 711 | Mean Difference (IV, Random, 95% CI) | 19.43 [9.50, 29.36] |
| 40 Iron status (ferritin concentrations in μg/L) by zinc content | 7 | 2764 | Mean Difference (IV, Random, 95% CI) | 13.43 [5.71, 21.15] |
| 40.1 Less than 5 mg | 1 | 571 | Mean Difference (IV, Random, 95% CI) | 12.5 [8.58, 16.42] |
| 40.2 5 mg or more | 6 | 2193 | Mean Difference (IV, Random, 95% CI) | 13.19 [2.13, 24.25] |
| 41 Weight‐for‐age (in z scores) | 10 | 9287 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.03, 0.07] |
| 42 Length‐for‐age (in z scores) | 11 | 11682 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.04, 0.01] |
| 43 Weight‐for‐length (in z scores) | 10 | 11664 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.01, 0.08] |
| 44 All‐cause morbidity | 3 | 2270 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.77, 1.39] |
| 45 Diarrhoea | 5 | 5579 | Odds Ratio (Random, 95% CI) | 1.05 [0.82, 1.35] |
| 46 Upper respiratory infections | 3 | 3560 | Odds Ratio (IV, Random, 95% CI) | 0.89 [0.76, 1.06] |
| 47 Retinol binding protein (μmol/L) | 2 | 883 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 48 Retinol (μmol/L) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 49 Serum zinc concentration | 5 | 1426 | Mean Difference (IV, Random, 95% CI) | 1.07 [‐3.46, 5.61] |
| 50 Malaria infection | 3 | 1737 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.62, 1.24] |
| 51 Cognitive outcome: walking independently | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 52 Cognitive outcome: language score (Bayley) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 53 Cognitive outcome: HOME score | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 54 Cognitive outcome: language score (CDI) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 54.1 Receptive language z score at 24 months | 1 | 1710 | Mean Difference (IV, Random, 95% CI) | 0.17 [0.07, 0.27] |
| 54.2 Expressive language z score at 24 months | 1 | 1710 | Mean Difference (IV, Random, 95% CI) | 0.13 [0.04, 0.22] |
Comparison 2. Home (point‐of‐use) fortification of foods with MNP versus an iron‐only supplement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anaemia | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Haemoglobin (g/L) | 2 | 278 | Mean Difference (IV, Random, 95% CI) | ‐2.81 [‐10.84, 5.22] |
| 3 Stained teeth (not pre‐specified) | 2 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.16, 0.82] |
| 4 Stool discolouration (not pre‐specified) | 2 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.66, 0.98] |
| 5 Cold (not pre‐specified) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Fever (not pre‐specified) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Cough (not pre‐specified) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Vomiting (not pre‐specified) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Diarrhoea | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10 Recurrent diarrhoea (3 or more episodes) (not pre‐specified) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11 Diarrhoea episodes (not pre‐specified) | 2 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.16, 1.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aboud 2011 (C).
| Methods |
Study design: cluster‐randomised field trial with 3 arms Unit and method of allocation: group (village level) Method of sequence generation: not reported Masking of participants, personnel, and outcome assessors: not reported |
|
| Participants |
Location of the study: Khansama subdistrict, Bangladesh Sample size: 302 children and their mothers Dropouts/withdrawals: 9 at post‐test, 17 at follow‐up Age (range): children aged 8 to 20 months Sex: both sexes (girls: control = 49%, RFS = 54%, RFS+ = 57%) Socioeconomic status: poor and very poor wealth categories Baseline prevalence of anaemia: not reported Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported Inclusion and exclusion criteria: mothers and their children aged 8 to 20 months were eligible for inclusion. Disabled children and those who had not started complementary feeding were excluded |
|
| Interventions | 45 clusters were randomly allocated to 1 of 3 groups Interventions
Comparison Group 3 (n = 16 villages, 110 children): control group received a health and nutrition child development programme, comprising 12 informational sessions Duration of intervention: 6 months |
|
| Outcomes |
Primary: nutritional and developmental outcomes (weight, weight‐for age z score, length, length‐for‐age z score), developmental outcomes (HOME inventory, mother‐child responsiveness, Bayley language scores) Secondary: self‐reported illness in the past week, observed feeding behaviours Timing of outcome assessment: baseline in April, post‐test in July (3 months later), follow‐up in December (8 months later) |
|
| Notes |
Study start date: May 2008 Study end date: December 2008 Conflict(s) of interest: not reported Funding source(s): Social Sciences and Humanities Research Council of Canada Malaria‐endemic area: yes Comment For the purposes of this review, we included only intervention groups 1 and 2. We analysed individual child outcomes on an intention‐to‐treat basis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: villages randomly pre‐assigned to treatment arm, then all mother‐child pairs of eligible age recruited for enrolment |
| Allocation concealment (selection bias) | Low risk | Comment: not described, but intervention was at village level, so low risk of selection bias at individual level |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not described, probably not performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not described, probably not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: low loss to follow‐up (5.6% overall) with minimal difference between groups; high adherence (80%) for MNP use |
| Selective reporting (reporting bias) | Low risk | Comment: appears to be no selective reporting, as outcomes pre‐specified in the trial registration were reported in the final publication |
| Other bias | Low risk | Comment: appears to be free of other sources of bias |
Adrianopoli 2014 (C).
| Methods |
Study design: cluster‐randomised pilot trial Unit and method of allocation: randomisation at village level Method of sequence generation: 2‐stage cluster‐sampling method was used. 20 clusters were selected and randomly assigned to 1 of 2 groups. 30 eligible households were selected from each cluster (n = 20). Method details were not described Masking of participants, personnel, and outcome assessors: not reported |
|
| Participants |
Location of the study: Tajikistan, Khatlon Province, and Gorno‐Badakhshan Autonomous Region Sample size: 209 Dropouts/withdrawals: 52 dropped out at first follow‐up (3 months), 1 dropped out at second follow‐up (6 months), 11 dropped out at third follow‐up (12 months) Age: 6 to 12 months Sex: both sexes. Male:female ratio = 45:55 in group A, 61:39 in group B Socioeconomic status: not reported Baseline prevalence of anaemia: 100% (moderate anaemia) Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported Inclusion and exclusion criteria: infants and young children (IYC) who were anaemic were the target population for the study. Blood haemoglobin levels and nutritional status were assessed during the screening phase. 209 IYC with a haemoglobin concentration > 7 g/dL and < 11 g/dL and aged 6 to 12 months were included. IYC presenting with severe clinical malnutrition (weight‐for‐height z score, or WHZ, < −3), haemoglobin level < 7 g/dL, severe chronic illness, and congenital abnormalities were excluded. Children receiving iron supplementation or enrolled in a therapeutic feeding programme were also excluded |
|
| Interventions | 209 IYC were randomly assigned to 1 of the 2 intervention groups Intervention: Group B (n = 105): received FBCFRs (food‐based complementary feeding recommendations) plus MNP (12.5 mg of iron, 5 mg of zinc, 300 μg of vitamin A, 160 μg of folic acid, and 30 mg of vitamin C in powder form, daily) Comparison: Group A (n = 104): received FBCFRs (age‐specific) Duration of intervention: 12 months |
|
| Outcomes |
Primary: haemoglobin concentrations, iron status, anthropometric measurements (z scores of the index weight‐for‐length), dietary assessment Secondary: morbidity, side effects (episodes of diarrhoea, respiratory illness, severe vomiting, and visits to physicians) Timing of outcome assessment: baseline, first follow‐up (3 months), second follow‐up (6 months), third follow‐up (12 months) |
|
| Notes |
Study start date: November 2007 Study end date: April 2009 Conflict(s) of interest: not reported Funding source(s): US Centers for Disease Control and Prevention (CDC) Malaria‐endemic area: yes Comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "randomly assigned" Comment: method details not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described, but blinding could not be possible due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not described and probably not attempted; however, non‐blinding of outcome assessment appears likely to have affected outcome measurements |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 56% (58/104) in group A and 47% (49/105) in group B completed all 3 follow‐up measurements |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol not available |
| Other bias | Low risk | Comment: appears to be free of other sources of bias |
Adu‐Afarwuah 2007.
| Methods |
Study design: RCT with a non‐randomised control group Unit and method of allocation: individual level Method of sequence generation: random selection of 75% of participants who were randomly allocated to 1 of the intervention groups via opaque envelopes Masking of participants, personnel, and outcome assessors: not attempted for participants or personnel; outcome assessors blinded to the intervention |
|
| Participants |
Location of the study: Koforidua, Ghana Sample size: 409 infants Dropouts/withdrawals: 15 lost to follow‐up Age: 6 months Sex: both sexes Socioeconomic status: not reported Baseline prevalence of anaemia: 25% Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: baseline not reported. The percentage of children who had a positive malaria smear (at 12 months of age) ranged from 2% to 8.3% Inclusion and exclusion criteria: inclusion: 5 months of age, receiving any breast milk, not known to be asthmatic or allergic to peanuts, planning to stay at the study site during the next 7 months. Exclusion: not specified |
|
| Interventions | 313 infants were randomly allocated to 1 of the following groups Interventions
Comparison Control group* (n = 96): did not receive an intervention. It was not randomised but was randomly selected from the original population *For the purposes of this review, only group 1 and the control group were included Duration of intervention: 6 months |
|
| Outcomes |
Primary: growth (z scores for weight‐for‐age, length‐for‐age, and weight‐for‐length; head circumference) Secondary: morbidity, observed motor milestone acquisition, micronutrient status (haemoglobin, ferritin, transferrin receptor, plasma zinc), positive malaria smear Timing of outcome assessment: all outcomes were measured when infants were 12 months old (i.e. 6 months after start of the intervention). There were no baseline measurements in the control group |
|
| Notes |
Study start date: February 2004 Study end date: June 2005 Conflict(s) of interest: one of the study authors (AB) was a paid consultant for Nutriset, the company that manufactured NB until December 2003 Funding source(s): HJ Heinz Company has supported the technical development of micronutrient powders on a cost‐recovery basis Malaria‐endemic area: yes Comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: random selection of 75% of participants who were randomly allocated to 1 of the intervention groups using opaque envelopes. Control group (no intervention) was not randomised but belongs to the same sample population as the rest of the participants. Even though infants in the no‐intervention group were eligible for the trial at the same place in time, and comparability may be assumed, it is not clear what criteria were used by study authors to select the children because the paper states that "40 were not randomly selected" (quote) |
| Allocation concealment (selection bias) | Low risk | Comment: see above; however, as assignment to MNP was allocated via opaque envelopes and the control group was not part of the original study, it may be possible that the concealment was adequate |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not attempted for participants or caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: outcome assessors blinded to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: few dropouts in randomised groups (4.7%) with no differences among groups. It is impossible to assess dropouts in the control group because there was no follow‐up. Overall, only 96 out of 170 of the eligible population were included in the final analysis (56.4%) |
| Selective reporting (reporting bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Other bias | High risk | Comment: control group does not have baseline values for any of the outcomes, making it difficult to assess the comparability of groups, particularly for indicators such as ferritin, which show large, albeit non‐significant baseline variation among intervention groups |
Attanasio 2014 (C).
| Methods |
Study design: cluster‐randomised trial with 4 arms Unit and method of allocation: randomisation at the municipality level Method of sequence generation: computer‐generated codes to randomly assign 8 clusters each Masking of participants, personnel, and outcome assessors: not possible to blind allocation to treatment group; interviewers blinded to treatment status of participants |
|
| Participants |
Location of the study: 96 towns in Colombia, including Oriental region, coffee zone region, and central region Sample size: 1420 children Dropouts/withdrawals: 166 participants lost to follow‐up Age: children aged 12 to 24 months (mean = 18.27 (± 4.02) months in the control group, 17.96 (± 3.6) months in the intervention group) Sex: both sexes (50% male in the control group, 54% male in the intervention group) Socioeconomic status: mean wealth index = −0.08 (± 0.92) in the control group, 0.07 (± 1.06) in the intervention group Baseline prevalence of anaemia: 46% Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported Inclusion and exclusion criteria: children aged 12 to 24 months and their caregivers living in 8 of 32 departments in Colombia were eligible for inclusion. No exclusion criteria were reported |
|
| Interventions | 32 municipalities (clusters) were selected from 3 geographic regions. Then, households with children aged 12 to 24 months (mean age = 18 months) were randomly selected from each cluster to be enrolled in the study Interventions
Comparison Group 4* (n = 318 children): control, no intervention *For the purposes of this review, only groups 2 and 4 were compared Duration of intervention: 18 months |
|
| Outcomes |
Primary: Bayley Scales of Development (language, fine and gross motor scores), weight, length, haemoglobin level Secondary: Center for Epidemiologic Studies short depression scale (CES‐D 10), children's consumption of iron‐rich food based on maternal reports Timing of outcome assessment: baseline and after intervention |
|
| Notes |
Study start date: February to May 2010 Study end date: September to December 2011 Conflict(s) of interest: not reported Funding source(s): Economic and Social Research Council; Inter‐American Development Bank; World Bank; International Growth Center Malaria‐endemic area: yes Comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: municipalities (clusters) not completely random as they were in locations where the Familias en Acción programme was in operation. Individuals from each cluster were not selected by simple random sampling, but instead among beneficiary households of elected mother leaders, and it is unclear whether this could raise selection bias |
| Allocation concealment (selection bias) | Low risk | Comment: clusters assigned to treatment group via computer‐generated codes; intervention at municipality level, thus low risk of selection bias at individual level |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not possible to blind allocation to treatment group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: interviewers blinded to treatment status of participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: overall loss to follow‐up 10.7% for Bayley outcome with no significant differences between treatment arms |
| Selective reporting (reporting bias) | Low risk | Comment: appears to be no selective reporting, as outcomes pre‐specified in the trial registration were reported in the final publication |
| Other bias | Low risk | Comment: appears to be free of other sources of bias |
Barffour 2019.
| Methods |
Design: double‐blind, placebo‐controlled trial Unit and method of allocation: individual randomisation Method of sequence generation: statistician randomly assigned study ID numbers to the 4 study arms using a block randomisation scheme with block lengths of 4 or 8 Masking of participants, personnel, and outcome assessors: supplements were pre‐labelled by the manufacturer with 4 different numerical codes. In the field, the 4 numerical codes were assigned specific colours (one colour per intervention group) to ensure correct delivery to children in respective study groups. Each colour represented 1 type of preventive intervention to be taken daily and the corresponding therapeutic tablets for diarrhoea management. Micronutrient powder and control preventive interventions were formulated in powder form, whereas preventive zinc and therapeutic zinc were formulated in tablet form, potentially undermining the blinding procedures. Because there were 2 groups of powders and tablets, it was impossible to identify the exact intervention allocation for any particular group. The statistical analysis plan was published before analyses and was strictly followed to minimise bias. Group identities were revealed only after data analyses were completed and study investigators had reached consensus on interpretation of results |
|
| Participants |
Location of the study: rural communities in Khammouane Province, central Lao People's Democratic Republic; 300 villages from 5 districts (Nongbok, Xebangfai, Mahaxay, Xaibuathong, and Yommalat) Selection of participants: all villages in the 5 districts invited for enrolment, except in Nongbok, where only those with a mean stunting prevalence ≥ 25% were invited Sample size: 3407 randomised. Available data at end‐line by arm: group 1 = 739 children; group 2 = 701 children; group 3 = 763 children; and group 4 = 740 children (Figure 1). For biomarkers of nutritional status and health, data were collected from a subsample of 760 children. Available data at end‐line by arm: group 1 = 145 children; group 2 = 138 children; group 3 = 145 children; and group 4 = 140 children (Figure 1). Only group 2 and group 4 included in this analysis Age: 6 to 23 months at enrolment (mean age = 14.3 months at baseline) Sex: female and male (51.1% male) Socioeconomic status (as defined by trialists): not reported. Household Food Insecurity Access Scale 3.2 (±) 5.0 at baseline Baseline prevalence of anaemia: 54.8% Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported; Laos was classified as malaria‐endemic area, but study area was selected due to low malaria transmission (personal communication in Barffour 2019) Inclusion and exclusion criteria: inclusion: aged 6 to 23 months at enrolment; families intended to stay in study area for duration of study; willing to accept home visits; and caregiver provided written informed consent. When multiple siblings were within the eligible age range, only the youngest was enrolled. For twins, both were assigned to the same group and received all study‐related interventions and follow up, but only 1 was randomly selected for inclusion in data analysis. Exclusion: severe anaemia (haemoglobin < 70 g/L); severe wasting (weight‐for‐height z score < −3); bipedal oedema; severe illness warranting hospitalisation referral; congenital abnormalities that may interfere with growth; chronic medical conditions requiring frequent medical attention; known HIV infection of child or mother; ongoing use of micronutrient supplements; current participation in another research study |
|
| Interventions | Children were individually randomised into 1 of 4 groups
For all groups, caregivers were instructed to give diarrhoea treatment with therapeutic zinc (or placebo) tablets whenever the child had 3 or more liquid stools per day and to continue treatment for 10 days, until the blister pack was empty. Micronutrient powder and control arms were included in this analysis Duration of the intervention: 36 weeks |
|
| Outcomes | Study authors did not distinguish between primary and secondary outcomes Outcomes and timing of outcome assessment: mean length; weight; mid‐upper arm circumference (MUAC); length‐for‐age z score; weight‐for age z score; weight‐for‐length z score; stunting; wasting; underweight; mean haemoglobin; anaemia; mean micronutrient values and deficiency (zinc, ferritin, soluble transferrin receptor, retinol binding protein); diarrhoea (incidence, prevalence, duration); acute upper and lower respiratory tract infection (incidence, prevalence, duration) Timing of outcome assessment: anthropometry assessed at baseline, after 18 weeks, and at end‐line (32 to 40 weeks); haemoglobin, zinc, and iron status assessed at baseline and at end‐line; morbidity assessed weekly |
|
| Notes |
Study start date: September 2015 Study end date: April 2017 Conflict(s) of interest: 2 study authors work for the Bill and Melinda Gates Foundation (funder). No other conflicts of interest reported Funding source(s): Mathile Institute for the Advancement of Human Nutrition; Nutrition International; Bill and Melinda Gates Foundation Malaria‐endemic area: yes Comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: statistician randomly assigned study ID numbers to the 4 study arms using a block randomisation scheme with block length of 4 or 8 |
| Allocation concealment (selection bias) | Low risk | Comment: supplements were pre‐labeled by the manufacturer with 4 different numerical codes. In the field, the 4 numerical codes were assigned specific colours (1 colour per intervention group) to ensure correct delivery to children in the respective study groups. Each colour represented 1 type of preventive intervention to be taken daily and the corresponding therapeutic tablets for diarrhoea management. Micronutrient powder and control preventive interventions were formulated in powder forms, whereas preventive zinc and therapeutic zinc were formulated in tablet forms, potentially undermining blinding procedures. Because there were 2 groups of powders and tablets, it was impossible to identify the exact intervention allocation for any particular group |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: see above, under allocation concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: statistical analysis plan was published before analyses and was strictly followed to minimise bias. Group identities were revealed only after data analyses were completed and study investigators had reached consensus on interpretation of results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition was lower than expected but varied significantly by arm with micronutrient powder arm having highest attrition at 17% |
| Selective reporting (reporting bias) | Low risk | Comment: statistical analysis plan was published before analyses and was strictly followed to minimise bias |
| Other bias | Unclear risk | Comment: unknown if cutoff for retinol binding protein to define vitamin A deficiency is appropriate |
Baum 2017 (C).
| Methods |
Study design: cluster‐randomised, community‐based intervention trial Unit and method of allocation: 1 credit centre represented 1 cluster Method of sequence generation: computer‐generated list of random numbers Masking of participants, personnel, and outcome assessors: no blinding |
|
| Participants |
Location of the study: 34 Fonkoze credit centres in rural Haiti Sample size: 521 Dropouts/withdrawals: 31 participants lost to follow‐up Age: children aged 6 to 59 months (29.7 months in intervention group, 27.6 months in control group) Sex: both sexes (58% male in intervention group, 47% male in control group) Socioeconomic status: not reported Baseline prevalence of anaemia: haemoglobin concentration of 9.7 g/dL in intervention group and 9.8 g/dL in control group Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported Inclusion and exclusion criteria: participation was limited to children aged 6 to 59 months who lived in a household whose head was a member of 1 of the credit centres. Children who were anaemic (having a blood haemoglobin concentration < 7 g/dL) or severely malnourished (having a mid‐upper arm circumference < 110 mm) at baseline were excluded |
|
| Interventions | 34 clusters were randomly allocated to 1 of 2 groups Intervention (17 clusters, n = 262): MNP (vitamins A, B, B2, B6, B12, C, D, and E; folic acid; niacin; copper; iodine; iron; zinc; and selenium). Iron dose consisted of 2.5 mg ethylenediaminetetraacetic acid (EDTA) plus 2.5 mg ferrous lactate Comparison (17 clusters, n = 259): no intervention. Control group received MNP after 3‐month follow‐up at the conclusion of the study Duration of intervention: 3 months |
|
| Outcomes |
Primary: haemoglobin concentration Secondary: percentage of children with anaemia (those with haemoglobin < 11 g/dL) Timing of outcome assessment: baseline and during follow‐up 3 months later |
|
| Notes |
Study start date: February 2012 Study end date: March 2012 Conflict(s) of interest: not reported Funding source(s): Vitamin Angels. The first study author was supported by a sub‐award from the US Agency for International Development, for which Fonkoze is the prime awardee. Two study authors were employed by Fonkoze Malaria‐endemic area: yes Comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated list of random numbers was used |
| Allocation concealment (selection bias) | Unclear risk | Comment: not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Comment: no blinding Quote: "... participants and field staff members were not blinded to their credit centers’ group assignment..." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Comment: no blinding Quote: "... participants and field staff members were not blinded to their credit centers’ group assignment..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 31 participants lost to follow‐up; no evidence of a difference in attrition between intervention (246/262; 6.1%) and control (236/259; 8.9%) groups |
| Selective reporting (reporting bias) | Low risk | Comment: appears to be no selective reporting, as outcomes pre‐specified in the trial registration were reported in the final publication |
| Other bias | High risk | Comment: intervention and control groups differed in several variables at baseline |
Christofides 2006 (C).
| Methods |
Study design: 5‐arm, cluster‐randomised clinical trial Unit and method of allocation: at housing compound level Method of sequence generation: random digit generator used Masking of participants, personnel, and outcome assessors: participants blinded to multiple micronutrient composition groups; care providers and field staff blinded to form and dose of iron in the multiple MNPs; blinding of outcome assessors unclear |
|
| Participants |
Location of the study: Ghana Sample size: 133 Dropouts/withdrawals: 118 (89%) children completed the study Age: 6 to 18 months Sex: both sexes Socioeconomic status: not reported Baseline prevalence of anaemia: anaemic (100%) Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: 60% to 73.7% Inclusion and exclusion criteria: infants with body temperature ≤ 37.5 °C, no history of iron supplementation within 2 weeks before recruitment, and ingesting semi‐solid or solid weaning foods were eligible for inclusion. Infants with severe anaemia (haemoglobin < 70 g/L) were excluded |
|
| Interventions | 127 clusters of housing compounds (n = 133 children) were randomised to 5 groups Interventions*
Comparison Group 5* (n = 25, clusters 24): children received iron drops containing 15 mg of elemental iron per mL (as ferrous glycine sulphate drops) daily, between meals *For the purposes of this review, we have considered the 4 MNPs together and compared with group 5 Duration of intervention: 2 months |
|
| Outcomes |
Primary: haemoglobin Secondary: serum ferritin, serum transferrin receptors, iron deficiency anaemia, adherence, ease of use, diarrhoeal episodes per child, darkening of stools, staining of teeth Timing of outcome assessment: 3 and 8 weeks post intervention |
|
| Notes |
Study start date: April 2003 Study end date: September 2003 Conflict(s) of interest: S Zlotkin (last author) owns the intellectual property rights to micronutrient Sprinkles™. All profit net of expenses from the licensing of Sprinkles is donated to the Hospital for Sick Children Foundation. No other conflicts of interests Funding source(s): funded, in part, by grants from Canadian Institutes of Health Research and the HJ Heinz Company Foundation Malaria‐endemic area: yes Comments None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: cluster randomisation by housing compounds, using random digit generator |
| Allocation concealment (selection bias) | Low risk | Comment: not described; however, because the intervention was allocated at cluster (housing compounds) level, selection bias at the individual level is unlikely |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: participant blinded to the multiple micronutrient composition groups; care provider and field staff blinded to the form and dose of iron in the multiple MNP |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding of outcome assessment unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 7 out of 108 participants in the MNP groups (groups 1 to 4) lost to follow‐up and 4 out of 25 in the iron drop groups lost to follow‐up; no imbalance among groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Other bias | Low risk | Comment: appears to be free of other sources of bias |
Clarke 2018 (C).
| Methods |
Study design: 2‐arm cluster‐randomised effectiveness trial Unit and method of allocation: community/village with a functional centre for early cognitive development (ECD) Method of sequence generation: computer‐generated random list Masking of participants, personnel, and outcome assessors: evaluators and outcome assessors were blinded |
|
| Participants |
Location of the study: 60 communities in the circles of Sikasso and Yorosso, Mali Sample size: 40 children per village; 20 kids younger than 1 year (3 years at end of evaluation) and 20 who were 3 years of age or older (around 5 years at end of evaluation). This review includes results of the children who were 1 year old or younger at the start of the intervention. Total sample size was 583 in intervention group and 553 in control group Dropouts/withdrawals: 140 (∼ 9%) Age: 3 months to 5 years Sex: 52.8% male Socioeconomic status: not reported Baseline prevalence of anaemia: ∼ 85% Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: ∼ 62% Inclusion and exclusion criteria: all children previously randomly selected and enrolled in the surveys in 2013, who were still residents in the same village in 2016. New children were recruited in 2016, at random, from the same villages exposed to interventions to replace those lost to follow‐up |
|
| Interventions |
Intervention Group 1 (n = 600): children received home fortification with micronutrient powders, ECD and parenting education, seasonal malaria chemoprevention, and deworming plus vitamin A (by the government). Under this project, 120 MNP sachets containing 10 mg of iron were given to each child over a shorter, 4‐month period to avoid provision of iron‐containing supplements during the malaria transmission season (which ends in December and starts again in May‐June). The sachets contained 400 mcg of vitamin A; 5 mcg of vitamin D; 5 mg of vitamin E; 0.5 mg of vitamins B1, B2, and B6; 0.9 mcg of vitamin B12; 6 mg of niacinamide; 150 mcg of folate; 30 mg of vitamin C; 10 mg of iron; 4.1 mg of zinc; 0.56 mg of copper; 17 mcg of selenium; and 90 mcg of iodine. By May 2016, villages had received interventions from 2014 to 2016 Comparison Group 2 (n = 600): children received ECD and parenting education, seasonal malaria chemoprevention, and deworming plus vitamin A (by the government) There was an additional non‐randomised arm with no ECD, which, for the purposes of this review, was excluded from the analyses Duration of intervention: intervention lasted between 1 and 3 years, depending on age of enrolment to the study (i.e. 3 months to 3 years) |
|
| Outcomes |
Primary: anaemia, defined as haemoglobin < 110 g/L Secondary: haemoglobin concentration, serum ferritin, C‐reactive protein (CRP), acid glycoprotein (AGP), and soluble transferrin receptor (sTfR); height‐for‐age, weight‐for‐age, and weight‐for‐height z scores (HAZ, WAZ, and WHZ); malaria parasite density; malaria infectivity. Cognitive tests included expressive vocabulary (number of words); rapid automated naming time (seconds); digit span (maximum digit span); mixed instructions (number correct); heads, shoulders, knees, and toes (total score); listening comprehension (maximum 8 correct); letter recognition (maximum 20 correct); and number recognition (maximum 20 correct) Timing of outcome assessment: data on nutritional status and other health outcomes were collected in June and July 2016 at the end of the ECD school year. Children were assessed on cognitive and numerical tests between 3 and 5 years of age (on average) after the end of the intervention |
|
| Notes |
Study start date: June 2016 Study end date: July 2016 Conflict(s) of interest: none reported Funding source(s): World Bank, Save the Children, UBS Optimus Foundation Malaria‐endemic area: yes Comment Adherence was over 90% and did not differ between groups. Results reported by study authors were adjusted by the clustering effect. Amongst those that reported giving MNPs to their child, acceptability of the intervention was generally high (Table 7). Few parents had encountered problems in giving MNPs to their child, with 92% reporting no difficulty. Over 90% of parents reported that their child liked the food with the MNPs added, and 98% of parents would want to give their child MNPs again |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: study authors used a computer‐generated random list to allocate the intervention |
| Allocation concealment (selection bias) | Low risk | Comment: not described; however, because the intervention was allocated at cluster (housing compounds) level, selection bias at the individual level is unlikely |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "we did not use placebo tablets, but precautions were taken to blind evaluators to the intervention status of communities. Measurement of study outcomes [was] undertaken using standardized tests by independent field teams unaware of which communities have received the intervention. Slide microscopy was likewise performed by technicians blinded to the intervention status of communities, and data analysed in London by research staff blinded to intervention status of communities" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "we did not use placebo tablets, but precautions were taken to blind evaluators to the intervention status of communities. Measurement of study outcomes [was] undertaken using standardized tests by independent field teams unaware of which communities have received the intervention. Slide microscopy was likewise performed by technicians blinded to the intervention status of communities, and data analysed in London by research staff blinded to intervention status of communities" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "losses to follow‐up were approximately 20% amongst the two age groups of children recruited at baseline in 2013, and resampling was carried out to improve statistical power at endline. Under these circumstances, bias due to differential losses could be a potential concern. However, there was no circumstantial evidence to suggest that differential attrition had occurred between the two study arms: the interventions were reported to have been well received and popular with both parents and children; there were no documented refusals or withdrawals of consent; and losses to follow‐up were mainly due to outmigration, and thus can be assumed to be missing at random. Furthermore, the characteristics of the children examined at endline in 2016 were well balanced across the intervention and control arms" |
| Selective reporting (reporting bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Other bias | High risk |
Quote: "almost 80% of parents in the MNP intervention villages reported that they had given their child MNPs and 85% of those giving MNPs to their child had given them 4‐7 times in the preceding seven days. Although reported coverage of the MNP intervention exceeded 80% in more than half of the villages (17/30), there was some variation in the coverage between villages, which ranged from 38% to 97%" Comment Children in the control group received 1 round less of antimalaria treatment |
Esamai 2014.
| Methods |
Study design: double‐blind, randomised trial with 3 arms Unit and method of allocation: individual level Method of sequence generation: 45 pieces of paper with letters marked A, B, or C to represent the 3 study arms were rolled into balls and placed in a plastic container. The pieces were mixed thoroughly through shaking of the container. An independent person then picked the pieces one by one from the container Masking of participants, personnel, and outcome assessors: participants, personnel, and outcome assessors were masked |
|
| Participants |
Location of the study: rural western Kenya Sample size: 45 infants Dropouts/withdrawals: 18 dropouts due to withdrawals or technical difficulties with the metabolic study Age: 6 months Sex: both sexes Socioeconomic status: villages are characterised by extreme poverty, with 40% to 80% of the population living below the World Bank‐defined poverty level Baseline prevalence of anaemia: 0% Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: 0% Inclusion and exclusion criteria: included: born at term with birth weight > 2500 g, healthy appearance with no apparent congenital anomalies, current immunisation status, family social situation, agree to participate in the study, haemoglobin ≥ 10 g/dL, infant still breastfeeding at enrolment and intended to continue during the 3 months of the study period, and negative blood slide for malaria parasites. Excluded: existing acute malnutrition, current or planned use of infant formula or other nutrient‐fortified products, current or planned use of iron or zinc supplements, anaemia (defined as haemoglobin < 10 g/dL), previous hospitalisations for malaria within the last 4 weeks, and persistent diarrhoea (per WHO, > 3 loose stools/d lasting ≥ 14 days) |
|
| Interventions | 45 infants (6 months of age) were assigned to 1 of 3 MNP groups Interventions
Comparison Group 3 (n = 15): control with placebo sachets including no micronutrients (C) MNPs were provided at weekly intervals during the 3‐month intervention period from 6 to 9 months of age. At the end of each visit, 7 sachets were left with the mother plus 3 extra in case a delay in follow‐up visits occurred. Mothers were instructed to give 1 sachet daily and to add the entire contents of the sachet to 1 meal For the purposes of this review, groups 1 and 3 were compared Duration of intervention: 3 months |
|
| Outcomes | Study authors did not distinguish between primary and secondary outcomes Outcomes and timing of outcome assessment: for descriptive purposes, length and weight were obtained for infants at enrolment and at the end of the study period. Isotope studies were undertaken when participating infants were approximately 9 months of age (estimated amount of zinc in the meals). Baseline stool and urine specimens were taken before isotope dosing. Weighed duplicate diets were collected, and approximately 20‐mL urine samples were collected twice a day (am and pm) for days 4 to 7 after oral isotope administration. Blood was obtained at 6 and 9 months for serum zinc, biomarkers of iron status (ferritin and soluble transferrin receptor (sTfR)), and systemic inflammatory markers α1‐acid glycoprotein (AGP) and C‐reactive protein (CRP) |
|
| Notes |
Study start date: not reported Study end date: not reported Conflict(s) of interest: no conflict of interest Funding source(s): research was funded by International Atomic Energy Agency (IAEA); National Institutes of Health/National Center for Research Resources (NIH/NCRR); Colorado Clinical and Translational Sciences Institute (CCTSI) Malaria‐endemic area: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "forty‐five pieces of paper with letters marked A, B, or C to represent the three study arms were rolled into balls and placed in a plastic container. The pieces were mixed thoroughly through shaking of the container. An independent person then picked the pieces one by one from the container. The unique number‐letter became the subject and group assignment for each individual" |
| Allocation concealment (selection bias) | Low risk | Comment: see above; appears adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "all of the MNP sachets were marked with either A, B, or C by the manufacturer (Hexagon Nutrition Pvt. Ltd., Mumbai, India), and were otherwise indistinguishable. The code was known only to a senior investigator not involved in the field work (FE), thus assuring blinding of group assignment to the participating subjects and to the field research teams" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "thus, the isotope doses were the same for the 2 MNP groups containing zinc and lower for the placebo MNP preparation. The isotope preparations were indistinguishable except for labelling, to maintain the blinding of the MNP group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 18/45 (40%) could not complete isotope studies |
| Selective reporting (reporting bias) | Low risk | Comment: appears to be no selective reporting, as outcomes pre‐specified in the trial registration were reported in the publication |
| Other bias | Unclear risk | Comment: imbalances in baseline characteristics between control and intervention groups (there were more boys in the control group) |
Giovannini 2006.
| Methods |
Study design: double‐blind, placebo‐controlled trial Unit and method of allocation: individual level and sealed opaque envelopes Method of sequence generation: computer generated in blocks of 9 units and stratified on sex Masking of participants, personnel, and outcome assessors: participants, personnel, and outcome assessors were masked |
|
| Participants |
Location of the study: Tuk Phos district, Kompong Chhnang Province, Cambodia Sample size: 204 infants Dropouts/withdrawals: participation rate at the end of the study was 93.6% Age: 6 months Sex: both sexes (109 males, 95 females) Socioeconomic status: geographical area where people depend on farming and the main crop and complementary food is rice Baseline prevalence of anaemia: 100% (haemoglobin < 100 g/L). The prevalence of anaemia in Cambodian infants and young children is 63% Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported Inclusion and exclusion criteria: infants born between January and July 2003 and aged 6 months at recruitment were eligible for inclusion. Exclusion criterion was severe anaemia (haemoglobin < 70 g/L) |
|
| Interventions | Infants were randomly allocated to 1 of the following groups Interventions
Comparison Group 3* (n = 68): infants received daily placebo (potato maltodextrins in sprinkled powder form) Active Sprinkles and placebo, similar in powder form Administration of micronutrient powder started 7 (±) 2 days after baseline blood assessment. Content of each sachet was mixed with the infant’s meal after it was cooked *For the purposes of this review, only groups 1 and 3 were compared Duration of intervention: 12 months |
|
| Outcomes | Study authors did not distinguish between primary and secondary outcomes Outcomes and timing of outcome assessment: mortality and malaria parasitaemia; z scores for weight‐for‐age, length‐for‐age, and weight‐for‐length; haemoglobin concentration and serum ferritin at baseline and at the end of the intervention. Reported adverse events possibly linked to interventions: frequent or loose stool or diarrhoea within 14 days of administration; darkening of stool; mild constipation; mild vomiting |
|
| Notes |
Study start date: January 2003 Study end date: July 2003 Conflict(s) of interest: not reported Funding source(s): Canadian International Development Agency‐Health and Nutrition Initiatives Fund Malaria‐endemic area: yes Comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation to group designations based on allocation lists, computer generated in blocks of 9 units and stratified by sex |
| Allocation concealment (selection bias) | Low risk | Comment: randomisation to treatment was performed with sealed opaque envelopes containing group designations |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: parents and health workers unaware of group assignment until code was broken after completion of data analyses |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: research staff unaware of group assignment until code was broken after completion of data analyses |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 3/68 (4.4%) lost to follow‐up in MNP group and 6/68 (8.8%) in placebo group; analysis on intention‐to‐treat basis |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Other bias | Low risk | Comment: appears to be free of other sources of bias |
Hirve 2007 (C).
| Methods |
Design: double‐blinded, cluster‐randomised, community‐based trial Unit and method of allocation: village level. Random allocation. Study authors listed all villages on pieces of paper and blindly drew papers without replacement from an opaque bag to randomise the 22 villages to 5 groups Masking of participants, personnel, and outcome assessors: it is not possible to blind the difference between drops and MNP sachets. No blinding of outcome assessors |
|
| Participants |
Location of the study: Vadu Rural Health Program of KEM Hospital, which covers a rural population of 67,000 in 22 villages of Pune District, State of Maharashtra, India Selection of participants: surveillance database was used to identify and screen all children from these villages against eligibility criteria Sample size: 432 Age: 6 to 18 months Sex: females and males Socioeconomic status (as defined by trialists): not reported Baseline prevalence of anaemia: 58% Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: study area has only sporadic cases reported annually Inclusion and exclusion criteria: included: haemoglobin between 70 and 100 g/L; children should be taking semi‐solid or solid weaning foods; should not be taking hematinic; likely to remain within study area for 2 months; absence of any major illness. Excluded: severe anaemia (Hb < 70 g/L) |
|
| Interventions | Villages were randomised to 1 of 5 groups:
Four of the 5 groups were randomly assigned to receive identical looking Sprinkles sachets containing different dosages of elemental iron; the fifth group received iron drops. For the purposes of this review, we considered the 4 MNPs together and compared them with drops. For subgroups, we extracted relevant data if they were available Duration of the intervention: 2 months |
|
| Outcomes | Study authors did not distinguish between primary and secondary outcomes Outcomes: haemoglobin; serum ferritin; anaemia; side effects such as diarrhoea, vomiting and discolouration of stools, cough, cold, or fever in the past 7 days Timing of outcome assessment: side effects and compliance monitored through weekly visits; haemoglobin estimated at baseline and at 3 and 8 weeks; ferritin assessed at baseline and at 8 weeks |
|
| Notes |
Study start date: September 2004 Study end date: August 2005 Conflict(s) of interest: 1 co‐author owns the intellectual property rights to micronutrient Sprinkles™. The HJ Heinz Company is supporting the technical development of Sprinkles on a cost‐recovery basis. Any profit from administrative fees on the technology transfer of Sprinkles is currently donated to the Hospital for Sick Children Foundation. There are no other ‘competing interests’ Funding source(s): research grant from the Canadian Institutes for Health Research. Study authors acknowledge the support of Heinz India in facilitating the procurement of Sprinkles Malaria‐endemic area: yes Comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: all villages listed on pieces of paper. Random selection of pieces of paper from an opaque bag to allocated to study arms |
| Allocation concealment (selection bias) | Low risk | Comment: see above; appears adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not possible to blind differences between drops and MNP sachets |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not attempted |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 51/432 (11.8%) lost to follow‐up with no differences between groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: appears to be no selective reporting, as outcomes pre‐specified in the trial registration were reported in the publication; however, determination of haemoglobin levels at 8 months after planned first dosing was not reported |
| Other bias | Low risk | Comment: appears to be free of other sources of bias |
Inayati 2012 (C).
| Methods |
Design: randomised cluster trial with 4‐arm design Unit and method of allocation: villages (n = 29). Villages in the existing project areas were randomly allocated to groups 1 and 2 Method of sequence generation: manually, by use of random tables. Only groups 1 and 2 were randomised (information provided by the study author, Inayati 2012 (C)). For groups 3 and 4, the agency co‐ordinating the study opened new project sites that were distanced out of daily communication range with the first 2 groups' villages, to avoid spread of nutrition‐related knowledge. Groups 3 and 4 were not included in this analysis Masking of participants, personnel, and outcome assessors: participants and personnel were aware of the intervention, and no placebo was used. Outcome assessors were aware of the intervention (information provided by the study author, Inayati 2012 (C)) |
|
| Participants |
Location of the study: Archipelago of Nias in North Sumatra, Indonesia Selection of participants: eligible children were recruited in the project area from among all children who attended the monthly growth monitoring activities implemented by the Church World Service (CWS) or the Goverment of Indonesia, or both Sample size: 215 Age: ≥ 6 to < 60 months (mean age = ˜ 35 months) Sex: female and male (41% and 50% female in groups 1 and 2, respectively) Socioeconomic status (as defined by trialists): 90% of the children came from households with low socioeconomic status Baseline prevalence of anaemia: 64% and 62% for groups 1 and 2, respectively Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: malaria programme in areas; no baseline prevalence reported. Study authors report that when they conducted the study, the endemic malaria situation on Nias Island was stable and no index children suffered from malaria during the study period (information provided by the study author, Inayati 2012 (C)) Inclusion and exclusion criteria: children suffering from mild wasting (< −1.0 to ≥ −1.5 standard deviations) were eligible for inclusion. Children were individually discharged when they reached a weight‐for‐height z score (WHZ) of ≥ −1.0, or, when the intervention period ended, if they had not achieved WHZ ≥ −1.0 during the study period |
|
| Interventions | Villages were assigned to one of the following groups (only groups 1 and 2 were randomly allocated). Groups 3 and 4 were not randomly allocated (information provided by study author, Inayati 2012 (C))
For the purposes of this review, only groups 1 and 2 were compared Duration of the intervention: variable. Children were individually discharged when they reached weight‐for‐height z score (WHZ) ≥ −1.0 or failed to achieve WHZ ≥ −1.0 at the end of the intervention period. Length of stay of eligible children was 55 (± 34) days in group 1, 35 (± 14) days in group 2, 85 (± 19) days in group 3, and 83 (± 19) days in group 4. Final values were adjusted by length of stay in the programme |
|
| Outcomes | Study authors did not distinguish between primary and secondary outcomes Outcomes and timing of outcome assessment: weight, weight gain, height, length, weight‐for‐height z score (WHZ), height‐for‐age z score (HAZ), mid‐upper arm circumference (MUAC), haemoglobin, anaemia, reached discharge criterion, non‐reached discharge criterion, and adherence. Information on side effects (mortality, diarrhoea, acute respiratory infections, fever) was not reported in the publication but was provided by the study author (Inayati 2012 (C)) |
|
| Notes |
Study start date: October 2007 (with interruptions for Christmas and New Year) Study end date: September 2008 Conflict(s) of interest: study authors disclose no conflicts of interest Funding source(s): Neys‐van Hoogstraten Foundation; Eiselen Foundation; DSM Nutritional Products; Church World Service (CWS) Indonesia Malaria‐endemic area: yes Comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: generated manually by use of random tables; only groups 1 and 2 randomised. For groups 3 and 4, the agency co‐ordinating the study opened new project sites that were distanced out of daily communication range with the first 2 groups' villages, to avoid spread of nutrition‐related knowledge. These groups were not included in this analysis |
| Allocation concealment (selection bias) | Low risk | Comment: villages in existing project areas were randomly allocated to groups 1 and 2, and because the intervention was allocated at village level, selection bias at the individual level was unlikely |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: haemoglobin measured at discharge; 16.3% of participants have no data but balanced across groups |
| Selective reporting (reporting bias) | Low risk | Comment: data on side effects not reported in publication but provided by study author |
| Other bias | Unclear risk | Comment: data not adjusted by length of intervention (information confirmed by study author) |
Jack 2012 (C).
| Methods |
Design: cluster‐randomised trial Unit and method of allocation: health centres catchment area Method of sequence generation: clusters were randomly assigned to 1 of 2 interventions by a statistician not involved in study implementation. Unclear how 20 catchment areas were selected Masking of participants, personnel, and outcome assessors: allocation concealment not described, but intervention was at health centre catchment area level, so low risk of selection bias at individual level. Blinding of participants and personnel not described. Blinding of outcome assessors was not clearly described, but data entry was blinded |
|
| Participants |
Location of the study: Svay Rieng Operational Health District, Cambodia Selection of participants: 6‐ to 7‐month‐old infants residing in Svay Rieng Operational Health District, Cambodia, who were identified through listings of infants at health centre (HC) and village levels, were eligible to participate Sample size: 1350 subsample (675/arm) selected from original sample of 3112 infants Age: 6 to 7 months Sex: female and male (51% female in group 1, 50% female in group 2) Socioeconomic status (as defined by trialists): not reported but area described as rural; 15% in group 1 and 17% in group 2 had electricity, and 89% in both groups owned agricultural land Baseline prevalence of anaemia: approximately 84% in both groups Baseline prevalence of soil helminths: not assessed Baseline malaria prevalence: not assessed. Low malaria incidence rate (1 case/1000 population) Inclusion and exclusion criteria: all children aged 6 to 7 months living in the catchment areas of 20 health centres (clusters) in rural Cambodia were eligible for inclusion (n = 3112). A random subsample of 675 children per arm were randomly selected for the impact evaluation. No exclusion criteria were reported |
|
| Interventions | Health centres were randomly assigned to 1 of 2 groups
Infant and young child feeding education was provided to caregivers of all children in both intervention and control groups in verbal, written, and pictorial form, together with cooking demonstrations that focused on frequency, amount, consistency, and increased consumption of animal‐source foods. Immunisations, biannual vitamin A capsules, and mebendazole tablets (for deworming) were provided to all children, according to Cambodia Ministry of Health guidelines Duration of the intervention: infants were followed up to 24 months of age and outcomes were measured at 6, 12, 18, and 24 months of age |
|
| Outcomes |
Primary outcomes: anaemia, mean haemoglobin levels Secondary outcomes: retinol binding protein, zinc, ferritin, growth, MNP adherence Timing of outcome assessment: baseline and at 6‐month intervals to age 24 months |
|
| Notes |
Study start date: enrolment started March to August 2008 Study end date: intervention lasted 6 months with a subsample followed up at 6‐month intervals for data collection at ages 6, 12, 18, and 24 months Conflict(s) of interest: not stated. No financial disclosures reported Funding source(s): A2Z Micronutrient Project; Academy for Educational Development; USAID; Cambodia Health Sector Support Projects I & II (World Bank, Department for International Development, Australian Agency for International Development, UNICEF, UN Population Fund, French Cooperation); WHO Cambodia; Global Alliance for Improved Nutrition Malaria‐endemic area: yes Comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: clusters randomly assigned to treatment arm by external statistician; unclear how 20 catchment areas were selected |
| Allocation concealment (selection bias) | Low risk | Comment: not described, but intervention was provided at health centre catchment area level, so low risk of selection bias at individual level |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not clearly described but data entry was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: overall loss to follow‐up was 88/675 in the intervention group and 99/675 in the control group. In addition, on average, ˜ 25% of children were absent or refused at each visit, with no apparent differences by treatment group. Analyses were conducted separately for the 12‐, 18‐, and 24‐month visits |
| Selective reporting (reporting bias) | Low risk | Comment: appears to be no selective reporting, as outcomes pre‐specified in the trial registration were reported in the final publication |
| Other bias | Unclear risk | Comment: unclear if analyses on an intent‐to‐treat basis |
Kounnavong 2011.
| Methods |
Design: randomised controlled trial with a 3‐arm design Unit and method of allocation: a computer‐generated random number was used to randomise households to 1 of the 3 arms Method of sequence generation: computer randomised by household to 3 arms Masking of participants, personnel, and outcome assessors: No method was in place to conceal the allocation (confirmed by study author, Kounnavong 2011). Anthropometrists and trained technicians who collected haemoglobin data were unaware of the participant arm allocation. Because of technical and financial constraints, the placebo could not be produced locally, so trialists followed the normal standard of care in Lao People's Democratic Republic (PDR) at the time of the study; the control group received 6‐monthly high‐dose vitamin A supplementation instead of placebo. Village health volunteers monitored adherence and were aware of arm allocation of participants. They visited households every week and recorded the number of sachets consumed by children in the 2 intervention groups, any side effects, and any illnesses that occurred during the study period |
|
| Participants |
Location of the study: 6 communities in the Lahanam zone, Songkhone District, Savannakheth Province, 600 km south of the capital city, Vientiane, Lao PDR Selection of participants: since 2004, the entire population in the study area is registered in the Health and Demographic Surveillance System (HDSS) of the National Institute of Public Health (NIOPH). From the HDSS database, 367 eligible pre‐school‐age children were identified and invited to participate if they met all inclusion criteria. All eligible children in each household were enrolled and followed the same intervention randomly assigned to the household Sample size: 336. Of the 367 children who originally met the criteria, 17 were absent at the time of enrolment and 14 were excluded because they had infection with fever on the day of enrolment. Therefore, a total of 336 children were enrolled in the study Age: 6 to 52 months (mean = 32 months) Sex: female and male (58% female) Socioeconomic status (as defined by trialists): each household was categorised into 1 of 2 socioeconomic status groups: high (with electricity, improved water source, and latrine) or low (lacking one or all of these) Baseline prevalence of anaemia: 48.9% Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported Inclusion and exclusion criteria: "inclusion criteria were (i) age 6 to 53 months at the time of recruitment; (ii) willingness to participate; (iii) receiving complementary food in addition to breast milk; and (iv) apparently healthy. Exclusion criteria were: (i) having a fever or any illness on the day of enrolment; (ii) baseline level of haemoglobin less than 70 g/L; and (iii) currently receiving iron supplementation" (quote) |
|
| Interventions | Households were randomised to 1 of 3 groups
For the purposes of this review, results from groups 1 and 2 were combined and were reported separately in the subgroup assessing the scheme All subjects received a single high dose of vitamin A every 6 months, and those aged 24 months or older received a single dose of mebendazole for deworming in the 2 months before the study. Children who had not received mebendazole received it during the baseline survey. Because of technical and financial constraints, placebo could not be produced locally, so trialists followed the normal standard of care in Lao People’s Democratic Republic (PDR) at the time of the study; the control group received 6‐monthly, high‐dose vitamin A supplementation instead of placebo Duration of the intervention: 24 weeks |
|
| Outcomes | Study authors did not distinguish between primary and secondary outcomes Outcomes and timing of outcome assessment: haemoglobin, anaemia (measured at baseline, at week 12, and at week 24), height‐for‐age z score, weight‐for‐age z score, weight‐for‐height z score (taken every 4 weeks) |
|
| Notes |
Study start date: March 2009 for 24 weeks Study end date: October 2009 Conflict(s) of interest: none reported Funding source(s): this study was conducted as a part of the Eco‐Health Project of the Research Institute for Humanity and Nature, Kyoto (Japan), in collaboration with the National Institute of Public Health, Ministry of Health (MOH) of Lao PDR. MNP supplements were provided by the United Nations Children's Fund (UNICEF), Lao PDR. SK was in receipt of an Asian Health & Education Fund, Tokyo (Japan), and was in partial receipt of the Institute of Tropical Medicine, Nagasaki University (NEKKEN) Fellowship Malaria‐endemic area: yes Comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated random number used to randomise households to 1 of the 3 arms |
| Allocation concealment (selection bias) | High risk | Comment: no method in place to conceal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: because of technical and financial constraints, placebo could not be produced locally, so followed the normal standard of care in Lao People's Democratic Republic at the time of the study. The control group received 6‐monthly, high‐dose vitamin A supplementation instead of placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: assessment of haemoglobin concentration by trained technicians, who were unaware of the allocation of micronutrient supplement; anthropometric measurement by 2 field workers who were unaware of which group a child belonged to |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 98.5% of participants completed the study without imbalance between groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Other bias | High risk | Comment: although this was unintended, the haemoglobin concentration was significantly different at baseline in the control group (n = 110) compared with the 2 intervention groups. Children in the control group had, on average, a higher mean haemoglobin concentration and thus a lower incidence of anaemia compared with children in the 2 supplementation groups. Baseline anaemia prevalence varied by arm: 53.6% (daily); 58.6% (twice weekly); and 34.5% (control). Village health volunteers monitored adherence and were aware of arm allocation of participants. They visited households every week and recorded the number of sachets consumed by children in the 2 intervention groups, any side effects, and any illnesses that occurred during the study period |
Lanou 2019 (C).
| Methods |
Design: cluster‐randomised trial Unit and method of allocation: villages within 4 municipalities Method of sequence generation: a total of 74 clusters (villages) were eligible; 12 were excluded, resulting in 62 clusters. Villages were randomly allocated to intervention (n = 31) and control (n = 31) groups Masking of participants, personnel, and outcome assessors: allocation of villages to study groups using letter blindly selected from a text, "i", and selecting village names with first letter closest to "i" in the intervention group. Blinding of participants and personnel not described. Blinding of outcome assessors not clearly described |
|
| Participants |
Location of the study: rural area of the health district of Tougan, in Northwest Burkina Faso Sample size: 4629 representing 2233 children at baseline (control = 1132, intervention = 1101) and 2396 at end‐line survey (control = 1156, intervention = 1240) Age: children aged 6 to 23 months Sex: 50.8% female in intervention group, 49.7% female in control group Socioeconomic status: low socioeconomic status Baseline prevalence of anaemia: 81.2% in control group, 75.3 in intervention group Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: holoendemic, but baseline prevalence not reported Inclusion and exclusion criteria: all children aged 6 to 23 months living in study villages before and after intervention. No exclusion criteria reported |
|
| Interventions | 62 clusters were randomly allocated Intervention (31 clusters): intervention package consisted of (a) monthly supply of MNP to be taken on alternate days (15 micronutrients = 10 mg of elemental iron, 4.1 mg of zinc, 400 μg of vitamin A, 30 mg of vitamin C, 5 μg of vitamin D, 5 mg of vitamin E, 0.5 mg of B1, 0.5 mg of B2, 6 mg of B3, 0.5 mg of B6, 0.9 μg of B12, 150 μg of folic acid, 0.56 mg of copper, 17 μg of selenium, 90 μg of iodine); (b) child growth monitoring; (c) child‐centred counseling; and (d) cooking demonstrations Comparison (31 clusters): child growth monitoring only Duration of intervention: 12 months |
|
| Outcomes |
Primary: stunting, minimum dietary diversity Secondary: anthropometry (weight, height, mid‐upper arm circumference), haemoglobin, malaria (fever and rapid diagnostic kit), morbidity (fever, diarrhoea, lower respiratory tract infection) Timing of outcome assessment: 12 months |
|
| Notes |
Study start date: November 2014 Study end date: October 2015 Conflict(s) of interest: none reported Funding source(s): micronutrient initiative Malaria‐endemic area: yes Comment Study not powered to detect effects on anaemia. Analysis repeated cross‐sectional surveys, rather than comparing longitudinal changes in the same individuals in each study group. Suboptimal coverage and duration of intervention in some villages. Cluster‐level analyses were carried out. Sample sizes for growth data not reported, so results not included |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: all eligible villages were listed, and villages within a municipality were paired according to population size and distance from the nearest health centre |
| Allocation concealment (selection bias) | Low risk | Comment: allocation of villages to study groups using letter blindly selected from a text, "i", and selecting village names with first letter closest to "i" in the intervention group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not attempted; both researchers and caretakers were aware of the intervention, and no placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not attempted; both researchers and caretakers were aware of the intervention, and no placebo was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: repeated cross‐sectional surveys with similar sample size in baseline and end‐line surveys. |
| Selective reporting (reporting bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Other bias | High risk | Comment: suboptimal coverage and duration of intervention in some villages. Coverage or intake of MNP not reported |
Larson 2018 (C).
| Methods |
Study design: cluster‐randomised effectiveness trial Unit and method of allocation: health subcentre communities randomly assigned to intervention and control Method of sequence generation: random number generator Masking of participants, personnel, and outcome assessors: only data collection and data entry were blinded |
|
| Participants |
Location of the study: West Champaran, Bihar, India Sample size: 4360 at baseline and 4292 at end‐line Age: 6 to 18 months Sex: 56% boys in micronutrient group, 28% boys in control group Socioeconomic status: similar at baseline between groups (report ethnicity, maternal education, family income, housing structure) Baseline prevalence of anaemia: 72% Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported Inclusion and exclusion criteria: age at enrolment = 6 to 18 months |
|
| Interventions | 70 health subcentres were randomised
Duration of intervention: 12 months |
|
| Outcomes |
Primary: anaemia, stunting, underweight and wasting, motor (gross and fine) and mental (language and personal‐social) development Secondary: adverse outcomes (diarrhoea, hospitalisations, fever) Timing of outcome assessment: assessed at baseline and end‐line (12 months) |
|
| Notes |
Study start date: January 2015 Study end date: December 2015 Conflict(s) of interest: none reported Funding source(s): Bill and Melinda Gates Foundation, Thrasher Research Fund Malaria‐endemic area: yes Comment 33% were stunted at baseline. Only 38% of children had consumed MNPs in the last month, and 24% were currently consuming MNP at end‐line. Design was nested, cross‐sectional, with pre‐test/post‐test control group design |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: cluster‐randomised effectiveness trial with random number generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: repeated cross‐sectional surveys with small number of refusals or missing data at each time point |
| Selective reporting (reporting bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Other bias | Unclear risk | Comment: information insufficient to permit judgement; delayed publication of primary study results |
Lundeen 2010 (C).
| Methods |
Study design: cluster‐randomised, community‐based effectiveness trial Unit and method of allocation: village or part of central district represented a cluster Method of sequence generation: random selection of small, medium, and large villages as well as parts of district centre. Excluded villages without functioning healthcare services Masking of participants, personnel, and outcome assessors: no blinding |
|
| Participants |
Location of the study: 2 rural areas of the Kyrgyz Republic and an urban quarter outside Bishkek, the nation's capital Sample size: 1869 (947 in intervention group and 922 in control group) Age: children aged 6 to 36 months (mean of 20.8 months in intervention group, 19.9 months in control group) Sex: 49.5% male in intervention group, 50.5% male in control group Socioeconomic status: low socioeconomic status Baseline prevalence of anaemia: 71.7% in intervention group, 72.2% in control group Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported Inclusion and exclusion criteria: participation was limited to children aged 6 to 36 months who were consuming semi‐solid food and were not currently taking any iron supplement. Severely anaemic children (haemoglobin < 7 g/dL) were excluded |
|
| Interventions | 24 clusters were randomly allocated to 1 of 2 groups Intervention (12 clusters, n = 1103): daily MNP (containing 12.5 mg of elemental iron as microencapsulated ferrous fumarate, 5 mg of zinc gluconate, 300 μg of vitamin A, 30 mg of vitamin C, and 0.16 mg of folic acid) Comparison (12 clusters, n = 1090): no intervention Duration of intervention: 2 months |
|
| Outcomes |
Primary: haemoglobin, anaemia Secondary: morbidity symptoms, side effects (diarrhoea, constipation, vomiting) Timing of outcome assessment: June 2007 (2 months after intervention) |
|
| Notes |
Study start date: March 2007 Study end date: June 2007 Conflict(s) of interest: none reported Funding source(s): Kyrgyz‐Swiss‐Swedish Health Project, Sprinkles Group at SickKids Malaria‐endemic area: no Comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: villages and parts of a central district randomly allocated to intervention and control groups using stratified randomisation to balance the size of the clusters. Sequence was generated by shuffling cards (in envelopes) |
| Allocation concealment (selection bias) | Low risk | Comment: not described; however because the intervention was allocated at village level, selection bias at the individual level is unlikely |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not attempted; both researchers and caretakers were aware of the intervention, and no placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not attempted; both researchers and caretakers were aware of the intervention, and no placebo was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 156/1103 (14%) of children lost to follow‐up in the MNP group, and 168/1090 (14.4%) in the control group mainly because they could not be located |
| Selective reporting (reporting bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Other bias | Low risk | Comment: appears to be free of other sources of bias |
Luo 2017 (C).
| Methods |
Study design: cluster‐randomised controlled trial Unit and method of allocation: randomisation at village level Method of sequence generation: randomly assigned 117 villages to control group and 234 villages to micronutrient group using computer code (in Stata version 12) Masking of participants, personnel, and outcome assessors: caregivers were not aware that they were in an RCT. Outcome assessor blinding was not clear |
|
| Participants |
Location of the study: Shaanxi Province, China Sample size: 1802 children Age: children aged 6 to 11 months Sex: 53.5% male in intervention group, 51.0% male in control group Socioeconomic status: relatively poor and rural Baseline prevalence of anaemia: 48% Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported Inclusion and exclusion criteria: all townships in each county were included except for 1 township per county that housed the county seat and townships that did not have any villages with at least 800 people |
|
| Interventions | Participants were randomly assigned to 1 of 3 groups and were fed at feeding centres Intervention (1192 children, 629 in cohort 1 and 563 in cohort 2): caregivers in the MNP arm were given a 6‐month supply of sachets every 6 months (each sachet containing 6.0 mg of iron as ferrous lactate, 4.8 mg of zinc, 200 μg of vitamin A, 50 mg of vitamin C, 5 μg of vitamin D, 1.55 mg of vitamin E, 0.3 mg of vitamin B1, 0.5 mg of vitamin B2, 0.3 mg of vitamin B6, 0.5 μg of vitamin B12, 66 μg of folic acid, and 3.0 mg of niacin). They were also given information about causes and consequences of anaemia along with oral and written instructions on how to use the powder, specifically to give 1 packet per day mixed with the child’s food. 'NurtureMate' is a Heinz‐produced MNP Comparison (610 children, 313 in cohort 1 and 297 in cohort 2): no intervention Duration of intervention: 18 months for both cohorts |
|
| Outcomes |
Primary: haemoglobin (measured in g/L), 2 sub‐indices of Bayley Scales of Infant Development Secondary: feeding behaviour Timing of outcome assessment: 6, 12, and 18 months after the start of the intervention |
|
| Notes |
Study start date: April 2013 for cohort 1 and October 2013 for cohort 2 Study end date: October 2014 for cohort 1 and April 2015 for cohort 2 Conflict(s) of interest: funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report Funding source(s): International Initiative for Impact Evaluation (3ie), National Natural Science Foundation of China (71473239, 71703083), UBS Optimus Foundation, China Medical Board, Bank of East Asia, Huaqiao Foundation, Bill & Melinda Gates Foundation‐HBGDki Initiative, Noblesse, HJ Heinz Company Foundation Malaria‐endemic area: not reported Comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomly assigned 117 villages to control group and 234 villages to micronutrient group using computer code (in Stata version 12) |
| Allocation concealment (selection bias) | Low risk | Comment: randomly assigned using computer code; concealment probably attempted |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: caregivers were not aware that they were in an RCT, but no placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: attrition rate was high in both control and treatment groups (approximately 28%), but there was no difference between arms |
| Selective reporting (reporting bias) | Low risk | Comment: appears to be no selective reporting, as outcomes were pre‐specified in the trial registration |
| Other bias | Low risk | Comment: intention‐to‐treat analysis performed |
Macharia‐Mutie 2012.
| Methods |
Study design: randomised, partially blinded, controlled trial Unit and method of allocation: individual Method of sequence generation: computer‐generated block randomisation by age and sex Masking of participants, personnel, and outcome assessors: blinded randomisation, but porridge prepared in amaranth and MNP groups notably different in colour and consistency |
|
| Participants |
Location of the study: Migwani and Nzauni administrative locations within the Migwani Division, Mwingi District, Kenya; semi‐arid area prone to food shortages Sample size: 276 (93 in MNP intervention group, 93 in control group, 93 in amaranth group ‐ last group not included) Age: children aged 12 to 59 months (35.8 months in intervention group, 37.3 months in control group) Sex: 51.6% male in intervention group, 49.5% male in control group Socioeconomic status: not reported, but study is located in an agro‐ecological zone in a semi‐arid area that experiences food shortages most parts of the year Baseline prevalence of anaemia: 40.9% in intervention group, 35.5% in control group Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: 0% Inclusion and exclusion criteria: participation was limited to children aged 12 to 59 months who were apparently healthy at the time of entry into the study, had lived in the village for at least 6 months before the intervention, and were continuing to live there for the next year |
|
| Interventions | Participants were randomly assigned to 1 of 3 groups and were fed at feeding centres Intervention (n = 93): daily unrefined maize porridge fortified at the time of consumption with MNP (2.5 mg of sodium‐iron‐ethylenediaminetetraacetic acid (NaFeEDTA), 100 µg of RE vitamin A as retinyl palmitate, 2.5 mg of zinc, 0.9 mg of folic acid, 60 mg of vitamin C, 5 µg of vitamin D3 cholecalciferol, 5 mg of TE vitamin E (1‐a tocopheryl acetate), 6 mg of niacin, 0.34 mg of copper, 30 µg of iodine, 0.5 mg of thiamine, 0.5 mg of riboflavin, 0.5 mg of vitamin B6, 0.9 µg of vitamin B12, 200 µg of calcium, 2 mg of pantothenic, 30 mg of vitamin K (phylloquinone), and 17 µg of selenium) Comparison (n = 93): refined plain maize porridge (control) Note: a third arm of n = 93 children fed daily unrefined maize porridge enriched with amaranth grain flour at the ratio of 30% maize flour and 70% amaranth was not included. All children who had not been de‐wormed within the last 3 months before the start of the study were de‐wormed with albendazole Duration of intervention: 16 weeks |
|
| Outcomes |
Primary: haemoglobin, anaemia, iron deficiency Secondary: stunting, underweight, wasting, malaria, C‐reactive protein (CRP) Timing of outcome assessment: baseline and post intervention (16 weeks) |
|
| Notes |
Study start date: not reported Study end date: not reported Conflict(s) of interest: none reported Funding source(s): International Nutrition Foundation/Ellison Medical Foundation, Nutricia Research Foundation, and Foundation Van Dam Nutrition Plan, Nestlé Foundation, DSM pharmaceuticals (provided the MNP) Malaria‐endemic area: yes Comment Target daily intake was 350 mL of porridge for all children, which was considered to be an amount that they could comfortably consume in 1 session. Elevated acute phase protein was defined as CRP > 5 mg/L, and a correction factor of 0.67 for those with elevated CRP was used to adjust plasma ferritin concentration |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: block randomisation by age and sex generated with Excel (Microsoft) by 1 investigator not involved in recruitment and data collection |
| Allocation concealment (selection bias) | Low risk | Comment: all serving bowls labelled with child's name and identification number; similar serving cups equivalent to 350 mL of porridge used to serve at all centres |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: partially blinded; maize‐only and maize with MNP porridge were identical in appearance and taste, but it was evident that amaranth porridge was different (amaranth was different in colour and thinner in consistency) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: laboratory personnel performing analysis not aware of treatment allocations |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: a total of 239 children completed the study, equivalent to 86% of children randomised at baseline. The endpoint measurement for biochemical indicators was not done for 19 children because their veins could not be detected (n = 5) or their caretakers declined (n = 14). 9 children were absent for the final measurement |
| Selective reporting (reporting bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Other bias | Unclear risk | Comment: missing data values for haemoglobin, ferritin, and TfR were imputed before primary analysis using multiple imputations. Data were imputed 5 times using the fully conditional specification method with the default PASW Statistics initialisation value. Treatment group, number of days attended, sex, age, and baseline and post‐intervention weight, height, haemoglobin, PF, and TfR concentrations were used as predictors in the imputation model. Pooled estimates from imputed data are reported |
Matias 2018 (C).
| Methods |
Study design: cluster‐randomised effectiveness trial with 4 arms in a ratio of 1:1:1:1 Unit and method of allocation: randomisation at union level Method of sequence generation: randomly assigned 64 clusters to the 4 arms equally by sorting on a randomly generated, uniformly distributed number (with use of SAS for Windows, release 9.2; SAS Institute) Masking of participants, personnel, and outcome assessors: personnel and outcome assessors were blinded to group assignment. However, participant blinding was not clear |
|
| Participants |
Location of the study: 11 rural unions of the Badarganj and Chirirbandar subdistricts of the northwest region of Bangladesh Sample size: 4011 pregnant women and 1346 unborn children Age: not reported Sex: both sexes (mentioned children) Socioeconomic status: not reported but study areas are among the poorest areas of Bangladesh Baseline prevalence of anaemia: not reported Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported Inclusion and exclusion criteria: all pregnant women residing in the area of work of any of the 64 community health workers were eligible if they met the following eligibility criteria at enrolment: (1) gestational age was ≤ 20 weeks and (2) had no plans to move out of study area for 3 years after enrolment |
|
| Interventions | Participants (2964 pregnant women) were randomly assigned to 1 of 3 groups and were fed at feeding centres: LNS‐LNS arm (1047 women with 327 livebirths), IFA‐LNS arm (930 women with 277 livebirths), and IFA‐MNP arm (1052 women with 324 livebirths). For the purposes of this review, arms 1 and 2 were not included Intervention (1052 women with 324 livebirths): IFA‐MNP arm; women received iron plus folic acid (IFA; 1 tablet containing 60 mg of Fe and 400 μg of folic acid) daily during pregnancy and every other day during the first 3 months postpartum, and their children received MNPs (1 sachet/d) containing 15 micronutrients (including 10 mg of iron, 400 μg of vitamin A, 4.1 mg of zinc, 0.5 mg of thiamin, 0.5 mg of riboflavin, 6 mg of niacin, 0.15 mg of folic acid, 0.5 mg of vitamin B6, 0.9 mg of vitamin B12, 30 mg of vitamin C, 5 mcg of vitamin D, 5 mg of vitamin E, 0.56 mg of copper, 90 mcg of iodine, 17 mcg of selenium) from 6 to 24 months of age Comparison (982 pregnant women with 298 livebirths): IFA‐control arm; women received IFA daily during pregnancy and every other day during the first 3 months postpartum, and their children received no supplements Duration of intervention: 18 months after postpartum |
|
| Outcomes |
Primary: anaemia (haemoglobin < 110 g/L), iron deficiency (ferritin < 12 µg/L or soluble transferrin receptor > 8.3 mg/L), iron deficiency anaemia (IDA) Secondary: not reported Timing of outcome assessment: 18 months postpartum |
|
| Notes |
Study start date: between 15 October 2011 and 31 August 2012 (pregnant women enrolment) Study end date: not reported (April 2013 and February 2014 as 18 months postpartum follow‐up) Conflict(s) of interest: none reported Funding source(s): US Agency for International Development Food and Nutrition Technical Assistance III Project (FANTA), managed by Family Health International 360 Malaria‐endemic area: not reported Comment: home fortification using lipid‐based nutrient supplements (LNS) or MNP reduced IDA in 18‐month‐old Bangladeshi children. Provision of LNS in both pregnancy and childhood also reduced child anaemia and iron deficiency. Ferritin concentrations were adjusted for inflammation using correction factors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomly assigned 64 clusters to the 4 arms equally by sorting, using a randomly generated, uniformly distributed number (with use of SAS for Windows, release 9.2; SAS Institute) |
| Allocation concealment (selection bias) | Low risk | Comment: random assignment performed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: team members were blinded to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: study evaluation teams were kept blind to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: attrition rate was 15% in treatment group and 17% in control group with no significant differences between arms |
| Selective reporting (reporting bias) | Low risk | Comment: appears to be no selective reporting, as outcomes were pre‐specified in the trial registration; protocol approved by institutional review board |
| Other bias | Low risk | Comment: intention‐to‐treat analysis performed |
Menon 2007 (C).
| Methods |
Study design: cluster‐randomised, pre‐post trial Unit and method of allocation: food distribution point represented a cluster Method of sequence generation: not reported Masking of participants, personnel, and outcome assessors: not reported |
|
| Participants |
Location of the study: Central Plateau region of Haiti Sample size: 415 (254 = MNP intervention group, 161 = control group) Age: children aged 6 to 21 months at enrolment to 18 to 33 months at follow‐up Sex: 54.3% male in intervention group, 43.5% male in control group Socioeconomic status: not reported but noted to not differ at baseline between 2 groups Baseline prevalence of anaemia: 52.3% in intervention group, 36.6% in control group Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported Inclusion and exclusion criteria: included enrolled children aged 6 to 21 months. Excluded those who were severely anaemic (haemoglobin < 7.0 g/dL), not receiving wheat‐soy‐blend, or not accompanied by their mother |
|
| Interventions | 10 FDPs (clusters) were randomly allocated to 1 of 2 groups
Both groups received 8 kg of wheat‐soy‐blend, 2.5 kg of oil (vitamin A fortified), and indirect ration of 10 kg of soy‐fortified bulgur and 2.5 kg of brown lentils Duration of intervention: 2 months |
|
| Outcomes |
Primary: haemoglobin concentration, prevalence of anaemia Secondary: diarrhoea Timing of outcome assessment: baseline, 2 and 9 months post intervention |
|
| Notes |
Study start date: March 2005 Study end date: March 2006 Conflict(s) of interest: none reported Funding source(s): Micronutrient Initiative, Food and Nutrition Technical Assistance (FANTA) Project, World Vision‐Haiti Malaria‐endemic area: yes Comment: individual level data were used and results were adjusted for child age, sex, and baseline haemoglobin (given differences in groups at baseline). The group that received MNP was followed up after 7 months post end of the intervention |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomised by computer‐generated random numbers (in SPSS) |
| Allocation concealment (selection bias) | Low risk | Comment: not mentioned; however, because the intervention was allocated at FDP level, selection bias at the individual level is unlikely |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described, but probably not attempted |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described, but probably not attempted |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 10/254 (3.9%) children lost to follow‐up in MNP group, and 7/154 (4.5%) in control group, mainly because children moved away from the intervention area |
| Selective reporting (reporting bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Other bias | Low risk | Comment: appears to be free of other sources of bias |
Mridha 2016 (C).
| Methods |
Study design: cluster‐randomised effectiveness trial Unit and method of allocation: cluster defined as supervision area of community health worker Method of sequence generation: computer‐generated randomisation, balanced across arms for mean cluster population, numbers of clinics and health workers, number of nutrition‐related non‐governmental organisations in the cluster, and source of funding Masking of participants, personnel, and outcome assessors: study team blinded, recipients not blinded to supplements due to differences in appearance and taste |
|
| Participants |
Location of the study: 11 rural unions of the Badarganj and Chirirbandar subdistricts in northwest Bangladesh Sample size: 4011 pregnant women followed through 24 months postpartum, 3379 children at 24 months Age: women = 22 years (mean), children = 6 to 24 months (range) Sex: not reported Socioeconomic status: similar between randomised groups Baseline prevalence of anaemia: not reported Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported Inclusion and exclusion criteria: enrolled pregnant women (≤ 20 gestational weeks) with no plans to move out of the study area for the following 3 years. All children eligible |
|
| Interventions | 64 clusters were randomly allocated to 1 of 4 arms Interventions
Comparison Group 4 (982 participants/16 clusters)*: control. Women received IFA (as described above) and children received no supplements (IFA‐control) *For the purposes of this review, only groups 3 and 4 were compared Duration of intervention: 44 months |
|
| Outcomes |
Primary outcomes and timing of outcome assessment: length‐for‐age z score,weight‐for‐length z score, weight‐for‐age z score, measured at 0, 6, 12, 18, and 24 months. Number of days of reported high fever, nasal discharge, cough, or diarrhoea in the 2 weeks before 6, 12, 18, and 24 months Secondary outcomes and timing of outcome assessment: language development at 12, 18, and 24 months, assessed using the MacArthur Communicative Development Inventories (CDI); acute upper respiratory infection and diarrhoea in the 2 weeks before 6, 12, 18, and 24 months |
|
| Notes |
Study start date: 15 January 2012 Study end date: May 2015 Conflict(s) of interest: none reported Funding source(s): USAID Food and Nutrition Technical Assistance III Project (FANTA) Malaria‐endemic area: yes Comment: reported adherence to MNP, based on consumption during previous 6 months, was high: 94% to 97% at 6 to 12 months and 97% to 99% at 12 to 24 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Comment: cluster‐randomised effectiveness trial; used multi‐stage cluster sampling procedure Quote: "... randomly generated, uniformly distributed number.." |
| Allocation concealment (selection bias) | Low risk | Comment: not mentioned; however, because the intervention was allocated at the cluster level, selection bias is unlikely |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Comment: blinding of participants not possible but blinding of personnel intended Quote: "...study evaluation teams were kept blind to group assignment, although this was difficult for home visit team members because they might have seen supplements in the home" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...researchers responsible for the collection of outcome data were kept blind to study assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: losses to follow‐up balanced between groups (15% to 17%) |
| Selective reporting (reporting bias) | Low risk | Comment: appears to be no selective reporting |
| Other bias | Low risk | Comment: appears to be free of other sources of bias |
Olney 2018 (C).
| Methods |
Study design: longitudinal, cluster‐randomised controlled trial Unit and method of allocation: health convergence centre randomised Method of sequence generation: unclear (120 (out of 215) eligible health convergence centres were stratified by size and randomly assigned to 1 of 6 study groups) Masking of participants, personnel, and outcome assessors: unclear |
|
| Participants |
Location of the study: Alta Verapaz in Guatemala Sample size: 4545 pregnant women and 4194 children Age: not reported Sex: both sexes (mentioned children) Socioeconomic status: highest percentage of households in the lowest wealth quintile (57%), among lowest levels of education (34% of women and 25% of men have had no education) and highest levels of illiteracy Baseline prevalence of anaemia: not reported Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported Inclusion and exclusion criteria: women living in PROCOMIDA (name of programme, not acronym) communities were eligible to enrol in the programme when they became pregnant and could participate in monthly food distributions, behaviour change communication, and other programme activities from time of enrolment until children were 24 months of age |
|
| Interventions | 120 clusters were randomly allocated to 1 of 6 study groups that varied the size of the family ration (full, reduced, or none) and the type of individual ration (corn‐soy blend (CSB), lipid‐based nutrient supplement, or MNP) Interventions
MNP contained 22 micronutrients containing 9 mg of iron (as ferrous orthophosphate), 8 mg of zinc, 400 µg of vitamin A, and vitamins C, D, E, K, B1, B2, niacin, B5, B6, folic acid, B12, copper, selenium, iodine, calcium, magnesium, manganese, phosphorus, and potassium Intervention beneficiaries received monthly family food rations for the duration of their participation in the programme. In addition, beneficiaries received a micronutrient‐fortified individual ration, which was intended to be consumed daily. Receipt of food rations was conditional on attending monthly BCC sessions and preventive health services. Monthly group BCC sessions were led by trained staff and were held at health convergence centres (primary healthcare facilities) Comparison Group 6 (753 women and 698 children): control group, which received no PROCOMIDA interventions *For the purposes of this review, only FFR + MNP and control arms were included Duration of intervention: 24 months after postpartum |
|
| Outcomes |
Primary: stunting, length‐for‐age z scores Secondary: not reported Timing of outcome assessment: 24 months after postpartum |
|
| Notes |
Study start date: August 2011 Study end date: May 2015 Conflict(s) of interest: not reported Funding source(s): US Agency for International Development (USAID), Food and Nutrition Technical Assistance III Project, CGIAR Research Program on Agriculture for Nutrition and Health led by the International Food Policy Research Institute Malaria‐endemic area: not reported Comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: 120 (out of 215) eligible health convergence centres were stratified by size and randomly assigned to 1 of 6 study groups |
| Allocation concealment (selection bias) | Low risk | Comment: random assignment performed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not described, but no inclusion of placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: at 24 months, between 84% and 87% of children had data at all time points |
| Selective reporting (reporting bias) | Low risk | Comment: appears to be no selective reporting, as outcomes were pre‐specified in the trial registration; protocol approved by institutional review board |
| Other bias | Low risk | Comment: intention‐to‐treat analysis performed |
Osei 2015 (C).
| Methods |
Study design: cluster‐randomised controlled substudy Unit and method of allocation: clusters defined as village development committees (VDCs) Method of sequence generation: simple random sampling to select 4 pairs of Ilakas matched on socioeconomic indicators Masking of participants, personnel, and outcome assessors: investigators, field workers, and participants not blinded |
|
| Participants |
Location of the study: Baitadi District, Nepal Sample size: 335 children Age: 6 to 9 months Sex: 47% female overall with no differences between groups Socioeconomic status: overall 32.6% of households in lower tertile with no difference between EHFP + MNP and EHFP‐only groups Baseline prevalence of anaemia: 67% Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported Inclusion and exclusion criteria: children who were not severely anaemic (haemoglobin < 70 g/L), underweight (weight‐for‐age z score (WAZ) < −3), stunted (length‐for‐age z score (HAZ) < −3), or wasted (weight‐for‐length z score (WHZ) < −3) at baseline who had mothers willing to remain in the study area for at least 1 year from the start of MNP supplementation were included in the trial |
|
| Interventions | For this MNP substudy, children were selected from the EHFP study (to assess the enhanced homestead food production (EHFP) programme, which offers home garden, animal husbandry, and nutrition education to beneficiary families). Children were randomised to 1 of 3 groups Intervention Group 1* (n = 115): EHFP + MNP (10 mg of iron (encapsulated ferrous fumarate), 4.1 mg of zinc (zinc gluconate), 90 μg of iodine (potassium iodide), 400 μg of vitamin A (vitamin A acetate), 150 μg of folic acid, 0.5 mg of vitamin B1 (thiamin mononitrate), 0.5 of mg vitamin B2 (riboflavin), 0.5 mg of vitamin B6 (pyridoxine), 0.9 μg of vitamin B12 (cyanocobalamin), 30 mg of vitamin C (ascorbic acid), 5 μg of vitamin D3 (cholecalciferol), 5 mg of vitamin E (vitamin E acetate), 6 mg of niacin (niacinamide), and 0.6 mg of copper (cupric gluconate)). Daily consumption of MNP Comparisons
*For the purposes of this review, only groups 1 and 2 were compared Duration of intervention: 11 months |
|
| Outcomes |
Primary: weight, recumbent length/height, haemoglobin Secondary: morbidity Timing of outcome assessment: primary outcomes assessed at enrolment and at end of MNP; secondary outcomes assessed 2 weeks before each interview at baseline and post intervention and during biweekly monitoring visits |
|
| Notes |
Study start date: September 2010 Study end date: February 2012 Conflict(s) of interest: none reported Funding source(s): Bill and Melinda Gates Foundation, Aline & Thrive Small Grants Program Malaria‐endemic area: yes Comment Estimated MNP distribution efficiency was 90.8%. Estimated compliance with MNP intake by children over the 11‐month period was 96.9% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: used multi‐stage cluster sampling procedure. Clusters were randomised to interventions or control using simple random sampling to select 4 pairs of Ilakas matched on socioeconomic indicators |
| Allocation concealment (selection bias) | Unclear risk | Comment: not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Comment: investigators and field workers not blinded to treatment assigned to children Quote: "the assignment of clusters rather than individuals to the study groups prevented participants in one group from knowing the treatment received by those in the other groups" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the non‐blinding of the data collectors may have also introduced some potential bias in our findings" (page 200) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 16/115 (14%) children lost to follow‐up in EHFP + MNP group (7 = younger than age criteria for inclusion at baseline, 8 = migrated; 1 = dropped out), 9/110 (8%) in EHFP group (8 = migrated; 1 = died), and 4/110 (4%) in control group (3 = migrated; 1 = died) |
| Selective reporting (reporting bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Other bias | Unclear risk | Quote: "stunting was significantly lower among children in the EHFP than those in the EHFP + MNP and control groups" |
Sazawal 2014 (C).
| Methods |
Study design: cluster‐randomised trial Unit and method of allocation: cluster (n = 12) Method of sequence generation: unclear Masking of participants, personnel, and outcome assessors: no blinding of field staff or participants; analysts blinded to group assignments |
|
| Participants |
Location of the study: Sangam Vihar, a low‐income, resettlement community in North Delhi, India Sample size: 292 Age: 6 to 24 months Sex: 58.8% boys in MNP group, 50.5% in fortified complementary food group, and 53.2% in control group Socioeconomic status: similar socioeconomic score between 3 groups Baseline prevalence of anaemia: not reported Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported Inclusion and exclusion criteria: eligible children selected from pre‐existing surveillance database were between 6 and 24 months old, consuming complementary food in addition to breastfeeding, not severely ill or with severe acute malnutrition requiring treatment, and willing to stay in study area for the next 6 months |
|
| Interventions | Participants were randomly allocated to the following groups Interventions
19 MNP formulation = 978.26 μg of vitamin A (IU), 0.98 mg of thiamin, 1.11 mg of riboflavin, 13.04 mg of niacin, 1.3 mg of pyridoxine, 3.91 μg of cyanocobalamin, 19.57 μg of biotin, 260.87 μg of folic acid, 39.13 mg of vitamin C, 6.52 μg of vitamin D, 9.78 μg of vitamin E, 494.02 mg of calcium, 69.13 mg of magnesium, 81.52 mg of phosphorus, 12.5 mg of iron, 10.0 mg of zinc, 1.3 mg of copper, 2.58 mg of manganese, 6.52 mg of pantothenic acid Intervention delivered to mother monthly for 6 months. Children assigned to MNP were to be fed 1 sachet daily. Children "less than 12 months of age were provided half the quantity of the sprinkle" ‐ it is unclear what half the quantity refers to Comparison Group 2* (n = 94): nutrition education alone as control. Education sessions occurred once a month *For the purposes of this review, only groups 1 and 2 were compared Duration of intervention: 6 months |
|
| Outcomes |
Primary: weight, height, length‐for‐age, weight‐for‐length, weight‐for‐age, haemoglobin, hematocrit, mean corpuscular volume, red cell distribution width, iron deficiency anaemia; anaemia defined as haemoglobin < 10 g/dL Secondary: intake compliance Timing of outcome assessment: weight and length measured at baseline and at end of study; complete haemogram estimation conducted at baseline and at end of study; information on compliance and anthropometry collected biweekly |
|
| Notes |
Study start date: not reported Study end date: not reported Conflict(s) of interest: not reported Funding source(s): Trasher Foundation, World Health Organization Malaria‐endemic area: yes Comment Does not state if results are adjusted for clustering. Used a lower haemoglobin cutoff than recommended by World Health Organization |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: clusters were matched but randomisation methods were not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: states cluster sampling was carried out to identify 12 clusters but no methods were described. Eligible children selected within clusters were randomly assigned to arms but methods were not described. Child characteristics were used to match clusters but unclear where matching was relevant |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not possible to blind field staff or mothers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: those responsible for laboratory and data analysis blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 5 lost to out‐migration in the MNP arm, and in control group, 2 lost to out‐migration and 1 died. Large proportion of refusals (those refusing to continue participating in the study because children did not like MNP or fortified complementary food) in the MNP arm (n = 30) |
| Selective reporting (reporting bias) | Unclear risk | Comment: in the MNP arm, 132 initially enrolled in the study, 30 (23%) withdrawn (children did not like the food with MNP), and 5 out‐migrated. In the control arm, 102 initially enrolled, 5 withdrew, 2 out‐migrated, and 1 died |
| Other bias | Unclear risk | Comment: results state that "usually, the sprinkle was added to milk, although it was acceptable with all other commonly‐consumed complementary foods" (quote). As a liquid drink, milk is not an appropriate vehicle to mix MNP. It is unclear if the milk was thick enough to stay on a spoon facing downward and thus had sufficient thickness for appropriate use. If mixed into liquids and the powder does not mix well, it is likely to be rejected by the child |
Sharieff 2006a.
| Methods |
Study design: randomised placebo‐controlled trial Unit and method of allocation: individual Method of sequence generation: computer‐generated random selection of children from each geographical zone Masking of participants, personnel, and outcome assessors: study participants, field staff, and data analyst were blinded |
|
| Participants |
Location of the study: Bilal Colony, an urban slum neighbourhood in Karachi, Pakistan Sample size: 75 infants Age: 6 to 12 months Sex: 56% boys in micronutrient group, 28% boys in control group Socioeconomic status: similar at baseline between groups (report ethnicity, maternal education, family income, housing structure) Baseline prevalence of anaemia: not reported Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported Inclusion and exclusion criteria: inclusion criteria included intent to reside in the area for 2 or more months, parental consent, and child able to ingest any type of semi‐solid weaning food, 1 or more episode of diarrhoea within previous 2 weeks |
|
| Interventions | Participants were randomly assigned to 1 of 3 groups Interventions
Comparison Group 3* (n = 25): children received daily placebo (containing ground purple rice with maltodextrin) Interventions were provided in small screw‐cap plastic containers with powders that were similar in appearance and taste; mothers and caregivers were instructed to open the containers and mix the entire contents with semi‐solid meal provided to the child once each day *For the purposes of this review, only groups 1 and 3 were compared Duration of intervention: 2 months |
|
| Outcomes |
Primary: longitudinal prevalence of diarrhoea (defined as 3 or more liquid or loose stools in the past 24 hours). Longitudinal prevalence was defined as percentage of days that the child had diarrhoea over the observation period Secondary: febrile days per child, compliance, haemoglobin concentrations, serum ferritin concentrations, anaemia, iron deficiency. Stool cultures at baseline (for Salmonella, Shigella, Campylobacter, Yersinia, enteropathic Escherichia coli and Vibrio cholerae, and Giardia lamblia) Timing of outcome assessment: number of diarrhoeal stools, days of diarrhoea, dysentery, vomiting, presence of fever (and intensity and duration), respiratory symptoms, and drug usage assessed twice a week; blood samples collected at end of 2 months |
|
| Notes |
Study start date: January 2003 Study end date: March 2003 Conflict(s) of interest: co‐author (S Zlotkin) owns intellectual property rights to micronutrient Sprinkles™ Funding source(s): Canadian Institutes of Health Research, HJ Heinz Foundation, Institut Rosell Lallemand Malaria‐endemic area: yes Comment Compliance was measured by counting the used supplements for each child whose supplements were not shared or lost. 91% of children consumed half of MNP (mean number 36) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: children assigned to treatment via a computer programme |
| Allocation concealment (selection bias) | Low risk | Comment: study authors provided identical, small screw‐cap plastic containers filled with micronutrients (with or without probiotic) or placebo in powder form, which were similar in appearance and taste |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: study authors provided identical, small screw‐cap plastic containers filled with micronutrients (with or without probiotic) or placebo in powder form, which were similar in appearance and taste. Study participants and field staff were blinded to random allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: data analysts blinded to random allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 1 child dropped out from the intervention group and 1 from the comparison group |
| Selective reporting (reporting bias) | Unclear risk | Comment: information insufficient to permit judgement |
| Other bias | High risk | Comment: haemoglobin and ferritin were not measured at baseline, making it difficult to judge comparability between groups. Only 61% of the original population agreed to provide a blood sample at the end of the study |
Somassè 2018 (C).
| Methods |
Study design: cluster‐randomised controlled community trial Unit and method of allocation: randomisation at the village level from 2 different municipalities Method of sequence generation: computer‐aided selection of 10 villages to intervention and 10 villages to control in each of 2 municipalities Masking of participants, personnel, and outcome assessors: not possible to blind allocation to treatment group; study staff also not blinded |
|
| Participants |
Location of the study: Circle of the Nioro, municipalities of Korera‐Kore and Sandare, Mali Sample size: 702 children (351 per arm) Age: 6 to 23 months Sex: 49% male in intervention group, 44% male in control group Socioeconomic status: approximately 80% of mothers with no school attendance; no differences between groups Baseline prevalence of anaemia: 89.9% Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported Inclusion and exclusion criteria: all children aged 6 to 23 months were eligible for inclusion. Those with severe acute malnutrition and those receiving iron supplementation at the time of the study were excluded |
|
| Interventions | Villages were randomised to 2 groups
Duration of intervention: 3 months |
|
| Outcomes |
Primary: weight, haemoglobin, anaemia Secondary: length, change in mid‐upper arm circumference (MUAC), reported illness (diarrhoea or fever) Timing of outcome assessment: primary outcomes, as well as length and MUAC, assessed at baseline and 3 months; illness assessed each month over 3 months of follow‐up |
|
| Notes |
Study start date: not reported Study end date: not reported Conflict(s) of interest: none reported Funding source(s): Red Cross of Belgium Malaria‐endemic area: yes Comment Analysis performed intent‐to‐treat using regression techniques, with standard errors taking account of the cluster design |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated assignment |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to blind allocation to treatment group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: field staff and investigators not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 24.9% loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: appears to be no selective reporting, as outcomes pre‐specified were reported in the final publication |
| Other bias | Low risk | Comment: appears to be free of other sources of bias |
Soofi 2013 (C).
| Methods |
Study design: cluster‐randomised trial Unit and method of allocation: cluster level within urban and rural strata Method of sequence generation: computer‐generated random numbers to 1 of 3 groups Masking of participants, personnel, and outcome assessors: investigators and field staff masked to composition of MNP preparations; participants not blinded |
|
| Participants |
Location of the study: urban and rural sites of Sindh, Pakistan. Urban areas were in Bilal colony, an urban squatter settlement in Karachi with population of 75,000. Rural areas in Matari district, about 200 km from Karachi, with population of 500,000. Both have functional health centres and research infrastructure and are broadly representative of urban and rural Pakistan Sample size: 2746 children in 256 clusters Age: 6 to 18 months Sex: not reported Socioeconomic status: similar between randomised groups (piped drinking water, underground sewage, monthly income) but lower underground sewage in rural vs urban clusters Baseline prevalence of anaemia: 23% Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported Inclusion and exclusion criteria: all children younger than 5 months in the 3 groups were eligible |
|
| Interventions | Participants were randomly assigned to 1 of 3 groups Interventions
MNP composition for groups B and C (all amounts represent elemental content) = 12.5 mg of iron (as microencapsulated ferrous fumarate), 50 mg of vitamin C (as ascorbic acid), 300 μg of vitamin A (as retinol acetate), 5 μg of vitamin D (as vitamin D3), and 150 μg of folate (as folic acid) 100 community health workers with at least 12 years of schooling were hired and trained for 1 week to support IYCN, use of oral rehydration solution and zinc for treatment of acute diarrhoea, and continued breastfeeding and complementary feeding during episodes. Also trained to distribute 2 weeks worth of MNP for daily use for children aged 6 to 18 months mixed with normal weaning food; told to continue MNP at the same dose during common illnesses; community health workers counted empty and remaining sachets every 2 weeks and replenished supplies Comparison Group A* (n = 947, 85 clusters): non‐supplemented control. Children received basic infant and young child feeding messages based on UNICEF/WHO recommendations; exclusive breastfeeding was promoted up to 6 months and continued breastfeeding with appropriate complementary feeding with locally available foods thereafter Duration of the intervention: 6 months. *For the purposes of this review, only groups A and C were compared |
|
| Outcomes |
Primary: growth, episodes of diarrhoea, acute lower respiratory tract infection, fever, incidence of admission to hospital Secondary: micronutrient status (haemoglobin, hematocrit, C‐reactive protein, ferritin, retinol, zinc) Timing of outcome assessment: information on presence or absence of diarrhoea, including presence of visible blood in the child's stools; respiratory signs; fever in the preceding week or 2 weeks obtained from families during regular household visits from recruitment to 24 months of age; monthly anthropometric assessments; blood samples collected at recruitment and at 6 and 18 months of age; routine stool samples collected at recruitment and at 6, 12, 18, and 24 months of age; diarrhoeal stool samples collected when mothers reported acute diarrhoea to visiting data collectors |
|
| Notes |
Study start date: 1 November 2008 Study end date: 31 December 2011 Conflict(s) of interest: none reported Funding source(s): Bill and Melinda Gates Foundation Malaria‐endemic area: yes Comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: within urban and rural strata, computer‐generated random numbers to 1 of 3 groups |
| Allocation concealment (selection bias) | Low risk | Comment: MNP sachets for groups B and C were identical except for colour (colour coding known only to the manager of Genera Pharmaceuticals (Islamabad), who produced the powders under license, and the Chair of the trial's DMC) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: field staff masked to composition of the MNP until after results of the trial were presented to the independent DMC. No placebo was used in the control group; therefore parents knew whether their child was receiving MNP but did not know whether the powder contained zinc |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: investigators and supervisory staff masked to composition of MNP until after results of the trial were presented to the independent DMC |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 108 children (11.4%) lost to follow‐up in group A and 87 children (9.8%) in group B at 24 months of age; no clusters lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: appears to be no selective reporting, as outcomes pre‐specified in trial registration were reported in the final publication |
| Other bias | Low risk | Comment: appears to be free of other sources of bias |
Young 2018 (C).
| Methods |
Study design: longitudinal cross‐over trial Unit and method of allocation: health subcentre communities randomly assigned to intervention and control Method of sequence generation: not reported Masking of participants, personnel, and outcome assessors: none (open label) |
|
| Participants |
Location of the study: West Champaran, Bihar, India Sample size: 100 households Age: 6 to 23 months Sex: 50% female in health centre 1 and 60% female in health centre 2 Socioeconomic status: not reported Baseline prevalence of anaemia: not reported Baseline prevalence of soil helminths: not reported Baseline malaria prevalence: not reported Inclusion and exclusion criteria: children aged between 6 and 23 months and not currently taking iron supplements were eligible for inclusion. Exclusion criteria included wasting (mid‐upper arm circumference < 11.5 cm); suspected severe anaemia (through assessment of palmar pallor); history of haemoglobinopathy or repeated blood transfusions; and currently ill with pneumonia, fever, or acute diarrhoea |
|
| Interventions | Two health centres selected, and within each, 50 households randomly selected
Duration of intervention: 2.5 months |
|
| Outcomes |
Primary: adherence, acceptability Secondary: maternal preferences for MNP vs iron folic acid supplements Timing of outcome assessment: adherence and acceptability assessed by survey at baseline, 1 month after first intervention, and 1 month after second intervention; maternal preferences assessed by semi‐structured intervention at end of month 1 and month 2 |
|
| Notes |
Study start date: December 2015 Study end date: April 2016 Conflict(s) of interest: none reported Funding source(s): Bill and Melinda Gates Foundation Malaria‐endemic area: yes Comment No primary or secondary outcomes reported, so no data included |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised, but random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: < 5% loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: no apparent selective reporting |
| Other bias | Unclear risk | Comment: information insufficient to permit judgement |
CRP: C‐reactive protein. DMC: Data Monitoring Committee. ECD: early cognitive development. EDTA: ethylenediaminetetraacetic acid. EHFP: enhanced homestead food production. FBCFR: food‐based complementary feeding recommendation. FDP: food distribution point. Fe: iron. HAZ: height‐for‐age z score. Hb: haemoglobin. HDSS: Health and Demographic Surveillance System. HOME: Home Observation for Measurement of the Environment. IDA: iron deficiency anaemia. IFA: iron and folic acid. IYCF: Infant young child feeding LNS: lipid‐based nutrition supplement. MNP: micronutrient powder. MUAC: mid‐upper arm circumference. PASW: Predictive Analytics Software. PF: phenol formaldehyde resin. RBP: retinol binding protein. SPSS: Statistical Package for the Social Sciences. sTfR: soluble transferrin receptor. TfR: transferrin receptor. VDCs: village development committees. WAZ: weight‐for‐age z score. WHZ: weight‐for‐height z score.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Avula 2011 | Quasi‐experimental longitudinal design with randomisation at centre level. 1128 children aged 6 to 30 months living in Rajasthan, India, were selected from 30 Anganwadi centres (AWCs) and received the enhanced programme or the usual programme for 6 months. Enhancements to the Supplemental Nutrition Program (SNP) were provided through a network of AWCs delivered by Anganwadi workers (AWWs). AWCs were randomly selected and delivered the enhanced programme or the usual SNP programme
Outcomes were height‐for‐age, weight‐for‐age, and weight‐for‐height z scores. We excluded the study because there was no appropriate comparison arm (comparison vs placebo or other type of supplement) |
| Bagni 2009 | Randomised controlled trial with children between 12 and 60 months of age attending public childcare centres in Rio de Janeiro, Brazil. Participants were randomly assigned to receive a daily meal prepared with iron‐fortified rice or to receive non‐fortified placebo rice. The rice was fortified once a week. We excluded the study because the type of intervention provided (fortification) is outside the scope of this review |
| Barth‐Jaeggi 2015 | Randomised controlled, double‐blinded trial. 287 infants aged 6 months living in the catchment area of Kikoneni Health Center in rural coastal Kenya were selected if they had a haemoglobin level of at least 70 g/L and no acute or chronic illness. Infants were enrolled and randomised to 2 groups
Haemoglobin, serum ferritin, sTfR, body iron stores, C‐reactive protein, weight, height, and illness recall were assessed at baseline, 6 months, and 12 months. We excluded the study because both intervention and control groups received MNP and they differed only in iron supplementation. There was no appropriate comparison arm (comparison vs placebo or other type of supplement) |
| Bilenko 2014 | Open‐label, cluster‐randomised, controlled clinical trial with a 2‐arm design. Randomisation at cluster level (mother and child health (MCH) clinics). Stratification by ethnic population group (Bedouin and Jewish). 621 infants (328 Bedouin and 293 Jewish), aged 6 months old, living in Negev, Israel, who belonged to families attending MCH clinics during 2005 to 2007. Excluded infants with Hb < 10 g/dL; chronic haematological, immunological, metabolic, or malabsorption disorders; or major congenital malformations; or participating in another study Infants from 12 clusters were randomised to 2 groups
Length of intervention was 2 months. Outcomes included haemoglobin, hematocrit, mean cell volume, red blood cell distribution, serum ferritin, transferrin saturation, and nutritional evaluation at 6 and 12 months. We excluded the study because there was no appropriate comparison arm (comparison vs placebo or other type of supplement) |
| Cardoso 2016 | Multi‐centre, pragmatic, controlled trial among young Brazliian children, carried out in primary health centres, with data collected at baseline and at end‐line. Control group (CG) included children aged 10 to 14 months (n = 521) who were assessed at baseline only. CG participants were recruited during routine healthcare visits, where measurements of anaemia, anthropometry, and micronutrient status were collected. Intervention group (IG) children were 6 to 8 months of age at baseline (n = 462) and were given 60 micronutrient powder sachets to consume ‐ 1 daily over 60 days. Data were collected for IG children when they reached the age of CG children. Data were collected during different seasons and with no blinding. We excluded the study because children were not randomised |
| Chen 2008 | Randomised trial with 226 apparently healthy preschool children (2 to 6 years old) from 15 nurseries or kindergartens in the Banan District of Chongqing, China, who were randomly assigned to 1 of 3 groups for 6 months
Powders were to be sprinkled over porridge, soy milk, soup, or noodles after cooking, and were indistinguishable in taste, colour, and packaging. Food prepared with the powders was delivered to each child at lunchtime or afternoon snack time, 5 days a week, during the study period. We excluded the study because participants are outside of the age range for inclusion in this review |
| Geltman 2009 | Randomised clinical trial with 150 healthy 6‐month‐old infants. Each infant received a daily packet of MNPs, or multiple micronutrient drops. Follow‐up included alternating telephone and home visits biweekly for 3 months. Adherence was the primary outcome, whereas side effects and caregiver attitude about supplements were secondary outcomes. We excluded the study because the micronutrient powder formulation contained iron and vitamins A, C, and D, but not zinc, which was 1 of 3 main nutrients evaluated in this review |
| Goyena 2019 | Randomised community trial of children 6 to 23 months of age in the Phillippines receiving 2 different formulations of MNP. Primary outcomes were compliance and acceptability. We excluded the study because there was no comparison arm. Also, no data were presented on any of our primary or secondary outcomes |
| Ip 2009 | Cluster‐randomised trial with 362 Bangladeshi children (haemoglobin ≥ 70 g/L) aged 6 to 24 months who received 60 sachets of MNP daily over 2 months; flexibly over 3 months; or flexibly over 4 months. With a flexible regimen, mothers/caregivers decided how frequently to use MNP without exceeding 1 sachet per day. Post‐intervention outcomes included adherence, acceptability, and haematological status, which also was evaluated at 6 months post intervention. We excluded the study because it compared different schemes for providing MNP but did not compare them versus placebo or another type of supplement |
| Jaeggi 2015 | A double‐blind, randomised controlled trial in 6‐month‐old Kenyan infants (n = 115). Participants were randomly assigned to receive MNP with or without 2.5 mg of iron as sodium iron EDTA. Researchers dispensed 7 MNP sachets and 2 kg of maize flour directly to participating mothers each week for 4 months. We excluded the study because both intervention and control groups received MNP, differing only in iron supplementation |
| Jyoti 2014 | In India, from the records of registered children at respective Anganwadi centres, all children into the 6‐ to 36‐month age group were enrolled in the study through house‐to‐house visits. The experimental group (n = 790) received 75 g of the new supplementary baby mix (6 times a week) with micronutrient sprinkles (5 sachets per week). Micronutrient sprinkles were not administered to the control group (n = 540) The study was a quasi‐experimental longitudinal study. In the Tonk district, 2 blocks (namely, Tonk (rural) and Malpurawere) were selected randomly, and from the list of total Anganwadi centres in each block, a total of 15 Anganwadi centres were selected randomly. Tonk (rural) and Malpura were considered as experimental and control groups, respectively. We excluded the study because it was not an RCT |
| Khan 2014 | A double‐blind, randomised controlled (2‐month) trial in Bangladesh. Infants aged 6 to 11 months (n = 100) were randomised to 1 of 2 intervention groups given MNP (Sprinkles) containing Fe and other micronutrients, with or without Ca. We excluded the study because both intervention and control groups received MNP, differing only in Ca supplementation |
| Lemaire 2011 | A randomised, double‐blind, placebo‐controlled, non‐inferiority safety trial conducted in 268 Bangladeshi children used a 2‐month course of daily iron‐containing micronutrient powder (iron MNP) or placebo powder. This trial evaluated the effects of iron MNP on infectious morbidities when provided to children aged 12 to 24 months with moderate to severe malnutrition and anaemia. Primary outcomes included diarrhoea, dysentery, and lower respiratory tract infection episodes. Secondary outcomes included haemoglobin and anthropometric changes at 2 and 6 months. We excluded the study because MNP targeted to high‐risk populations is outside the scope of this review |
| Munayco 2013 | Randomised controlled trial with Peruvian infants (n = 400) aged 6 to 11 months. Participants were randomly assigned to 6 months daily, 6 months intermittent (every other day), 12 months daily, or 12 months intermittent MNP supplementation. MNPs were provided monthly, and field staff conducted home visits twice monthly. Unused MNP sachets were collected throughout, and at end‐line (12 months) all caregivers were asked about their experiences using MNP. The study was conducted between 2009 and 2011. We excluded the study because there was no comparison arm |
| Neufeld 2008 | A cluster‐randomised trial, implemented in the context of the Oportunidades programme, which is a conditional cash transfer programme implemented in rural areas in 1997 and urban areas in 2002 with authorisation from Oportunidades officials at federal, state, and local levels, at the National Institute of Public Health Ethics Commission, in Mexico. The study was designed to guide decisions within the programme to compare the effects of 3 nutritional supplements vs identical multiple micronutrient content (syrup, Nutrisano, MNP Sprinkles) on child growth and development and micronutrient status among Mexican children aged 6 to 12 months at baseline. 927 children were randomly assigned to:
We excluded the study because there was no comparison arm |
| Rawat 2015 | A cluster‐randomised, controlled trial was conducted with children aged 6 to 11 months in Bangladesh, to assess the impact of (1) sale of MNPs by BRAC (Bangladesh Rural Advancement Committee) front‐line workers (MNP intervention); or (2) enhanced nutrition interpersonal counselling (EIPC intervention), or both, on anaemia and iron deficiency. We excluded the study because it focused on the sale of MNP, which is outside the scope of this review |
| Samadpour 2011 | Randomised trial, conducted from May to September 2007 in an urban area of Iran, to compare the efficacy of daily MNP provided as foodlets (food‐like tablets), sprinkles, and drops on micronutrient status and growth in children aged 6 to 18 months. 362 children were allocated to receive a 4‐month daily dosage of:
Foodlets and sprinkles had the same 14 nutrients, whereas drops included only 9 of the 14 nutrients and did not contain zinc, vitamin B12, folic acid, copper, or iodine. Haemoglobin, serum ferritin, serum retinol, serum zinc, 25(OH)D concentration, and anthropometry were assessed at baseline and at 4 months. We excluded the study because it compared different schemes for providing MNP but did not compare them vs placebo or another type of supplement, or the same MNPs as supplements |
| Shafique 2014 | Community‐based, cluster‐randomised trial with 244 full‐term, low‐birthweight infants aged 6 to 12 months in rural Bangladesh (24 clusters). Participants were randomly assigned to 2 groups:
We excluded the study because this type of participant (low‐birthweight infants) is outside the scope of this review |
| Sharieff 2006b | 16 classes of 3‐ to 6‐year‐old children (n = 415) attending kindergarten in northern China were randomly assigned to 1 of 3 groups
The intervention lasted for 1 school term (13 weeks). Consumption of sachets was monitored for each child, and SF concentrations were measured at the end of the study. We excluded the study because this type of participant is outside the scope of this review |
| Singla 2014 | A cluster‐randomised trial to assess the effects of a 22‐element MNP on linear growth and mental development in full‐term, low‐birthweight children in rural Bangladesh. Children from 24 clusters were randomly allocated to receive:
Children at 16 to 22 months of age were assessed on subtests of the Bayley Scales of Infant and Toddler Development III, to measure cognitive and receptive and expressive language development. We excluded the study because this type of participant (full‐term, low‐birthweight children) is outside the scope of this review |
| Smuts 2005 | 290 term infants, aged 6 to 12 months, were recruited through the health posts of Valley of a Thousand Hills, Durban, KwaZulu‐Natal Province, South Africa; they were enrolled in the study and were randomly assigned to 1 of 4 groups:
The MNPs provided were large, chewable tablets or foodlets (food‐like tablets). We excluded the study because this type of intervention is outside the scope of this review |
| Suchdev 2012 | A cluster‐randomised trial with children aged 6 to 35 months in Western Kenya, from March 2007 to March 2009, to investigate (1) the effectiveness of the distribution of sprinkles MNP through an integrated health promotion and income‐generating programme; and (2) the impact of sales of sprinkles MNP on anaemia, iron deficiency, and vitamin A deficiency. Children from 60 villages were enrolled into the study and were randomly allocated to receive:
Sprinkles MNP was marketed and distributed in the intervention villages, but vendors were not prevented from selling sprinkles MNP in control villages. Outcomes included haemoglobin, ferritin, retinol binding protein, malaria, and anthropometric measurements at baseline and 12 months of follow‐up, along with sprinkles MNP sales and usage. We excluded the study because it was focused on marketing of MNP, which is outside the scope of this review |
| Teshome 2017 | Randomised, double‐blind, non‐inferiority trial with children aged 12 to 36 months in Western Kenya that included 3 arms: (1) experimental treatment (n = 112), given MNP containing 3 mg of iron as NaFeEDTA plus 13 other micronutrients taken daily for 30 days; (2) active control treatment (n = 114), given MNP containing 12.5 mg of iron as encapsulated ferrous fumarate plus 13 other micronutrients taken daily for 30 days; and (3) placebo (n = 112), given MNP containing no iron but 13 other micronutrients taken daily for 30 days. Outcomes included haemoglobin concentration, iron status, anaemia, and malaria (microscopy and dipstick test). We excluded the study because all 3 arms were given MNP with and without iron |
| Troesch 2009 | 101 apparently healthy, non‐pregnant, non‐lactating young women studying or working at the Institute of Food Science and Nutrition, Swiss Federal Institute of Technology, Zurich, and the University of Zurich, between January and April 2008, were randomly assigned to 1 of 6 groups receiving a maize porridge fortified with a micronutrient powder containing stable, isotope‐labelled elemental iron as ferrous sulphate or NaFeEDTA, along with different combinations of inhibitors and enhancers (ascorbic acid, calcium, phytase, l‐alpha‐glycerophosphocholine). Each woman consumed 2 meals in a cross‐over design for determination of iron absorption. We excluded this study because this type of participant and this type of intervention are not within the scope of this review |
| Troesch 2011 | 200 apparently healthy South African schoolchildren aged between 5 and 11 years from 2 primary schools in low socioeconomic areas of Kimberley, Northern Cape, South Africa, with low iron status, with haemoglobin higher than 90 g/L, and not taking nutritional supplements containing iron were randomly assigned to 1 of 2 groups:
Primary outcomes were iron and zinc status. Secondary outcome was somatic growth. We excluded the study because participants were school‐aged children, between 5 and 11 years of age, and thus they were outside the scope of this review |
| Van der Kam 2016a | 3‐Armed, partially blinded, randomised controlled trial; randomisation at individual level. 2213 children aged 6 to 59 months living in Goronyo, rural Nigeria, diagnosed with 1 or more of the 3 study diseases (malaria, diarrhoea, lower respiratory tract infection) were included in the study. Children were randomised in a 1:1:1 ratio to 1 of 3 intervention groups:
All groups (including the control group) received health education, including the message that following an illness, a child should eat 1 extra healthy meal per day for 2 weeks. Primary outcomes were negative nutritional outcome, defined as moderate malnutrition (MAM) or severe acute malnutrition (SAM), or both. For non‐malnourished children, weight‐for‐height z score < −2, MUAC < 115 mm, or nutritional oedema, whichever occurred first. For MAM, weight loss > 10% from baseline, weight‐for‐height z score < −2. For SAM, weight‐for‐height z score < −3, MUAC < 115 mm, nutritional oedema. Secondary outcomes included changes in anthropometric indicators, morbidity, and mortality. We excluded the study because high‐risk populations are outside the scope of this review |
| Van der Kam 2016b | 3‐Armed, partially blinded randomised controlled trial; randomisation at individual level. 2202 children aged 6 to 59 months living in Kaabong, Karamoja region, Uganda, diagnosed with 1 or more of the 3 study diseases (malaria, diarrhoea, lower respiratory tract infection) were included in the study. Children were randomised in a 1:1:1 ratio to 1 of 3 intervention groups:
All groups (including the control group) received health education, including the message that following an illness, a child should eat 1 extra healthy meal per day for 2 weeks. Primary outcomes were negative nutritional outcome, defined as weight‐for‐height z score < −2, MUAC < 115 mm, or nutritional oedema, whichever came first. Secondary outcomes included changes in anthropometric indicators, morbidity, and mortality. We excluded the study because high‐risk populations are outside the scope of this review |
| Wang 2017 | A double‐blind, randomised, placebo‐controlled clinical trial with rural, Malawian children aged 12 to 35 months. Intervention was the combined usage of a dose of albendazole, a course of zinc, and a daily MNP. Outcomes were compared to those of a placebo group after 12 and 24 weeks of intervention. We excluded this study because the type of intervention (a combined package of MNP and albendazole dose) is outside the scope of this review |
| Wijaya‐Erhardt 2007 | Randomised controlled trial conducted in children between 6 and 12 months of age in rural Indonesia, which assessed the efficacy and safety of 3 types of food‐like tablets (foodlets) given for 23 weeks, compared to placebo. The foodlets were given as daily iron (ferrous sulphate), daily multiple micronutrients (14 nutrients: vitamins A, D, E, K, and C, thiamin, riboflavin, vitamin B12, niacin, folate, iron, zinc, copper, iodine), and weekly multiple micronutrients (same 14 nutrients). Results showed an increase in iron stores in the daily iron and daily multiple micronutrients groups, but not in the weekly multiple micronutrients group. Observed side effects were vomiting and diarrhoea, with no significant differences between intervention groups. We excluded the study because foodlets are not an intervention within the scope of this review |
| Yousafzai 2014 | Community‐based, cluster‐randomised effectiveness trial in rural Sindh, Pakistan, from June 2009 to March 2012. The objective was to assess if responsive stimulation or enhanced care for nutrition intervention delivered alone, or a combined approach, would have an independent and additive or synergistic effect on child development, growth, and morbidity outcomes at 24 months. 1489 children younger than 24 months of age, from 80 clusters, were randomly allocated to receive:
MNP contained iron, folic acid, vitamin A, and vitamin C. Primary endpoints were child development at 12 and 24 months of age (assessed with Bayley Scales of Infant and Toddler Development) and growth at 24 months of age. We excluded the study because the MNP did not contain zinc and thus did not comply with the definition used in this review of MNP requiring 3 or more micronutrients with at least iron, vitamin A, and zinc |
| Zlotkin 2001 | Randomised controlled trial with 557 anaemic children between 6 and 18 months of age from rural Ghana. This trial assessed the efficacy of home fortification with MNP containing 80 mg of elemental iron (microencapsulated ferrous fumarate) + 50 mg of ascorbic acid added to weaning foods, compared to iron drops (40 mg of elemental iron given 3 times/d), given for 2 months. Outcomes included anaemia, ferritin, serum zinc, and growth concentration. We excluded the study because the MNP formulation tested in this study included only 2 micronutrients and thus did not comply with the definition used in this review of MNP requiring 3 or more micronutrients with at least iron, vitamin A, and zinc |
| Zlotkin 2003a | Randomised trial with 437 Ghanaian, non‐anaemic children aged 8 to 20 months who were ingesting a weaning food in addition to breast milk. Participants were randomised individually to 1 of 4 groups:
Primary outcome measures were change in haemoglobin and anaemic status at baseline and end of study. Prophylactic supplementation was provided to children for 6 months (October 1999 to March 2000). Children who maintained Hb concentration ≥ 100 g/L at the end of the intervention were reassessed at 12 months post intervention. Acceptability of the powders was better in comparison to the iron drops. No significant changes were seen in mean haemoglobin, ferritin, or serum retinol values from baseline to the end of the supplementation period among groups. The study area was considered a setting where intestinal parasites, malaria, and infectious diarrhoea are common. The supplementation period began at the end of the rainy season and was finished by the end of the dry season, when the burden of malaria was lower We excluded the study because the trial evaluated provision of MNP formulated with only 1 or 2 micronutrients and thus did not comply with the definition used in this review of MNP requiring 3 or more micronutrients with at least iron, vitamin A, and zinc |
| Zlotkin 2003b | Randomised controlled trial with 304 anaemic children between 6 and 18 months of age from rural Ghana. This trial compared the efficacy of home fortification with MNP including 80 mg of elemental iron (as microencapsulated ferrous fumarate) + 50 mg of ascorbic acid vs that of 80 mg of elemental iron (as microencapsulated ferrous fumarate) and 5 mg of zinc (as gluconate), over 2 months. Outcomes included anaemia, ferritin, serum zinc, and growth concentration. We excluded the study because interventions evaluated in this study included MNP with only 1 or 2 micronutrients and thus did not comply with the definition used in this review of MNP requiring 3 or more micronutrients with at least iron, vitamin A, and zinc. In addition, the study did not compare MNP vs placebo or any other supplement |
| Zlotkin 2013 | Double‐blind, cluster‐randomised trial with children 6 to 35 months of age, conducted over 6 months in a rural community setting in central Ghana ‐ an area with high malaria burden. This trial evaluated effects of providing MNP with or without iron on the incidence of malaria among children living in a high malaria‐endemic region. 1958 children living in 1552 clusters were randomly allocated to receive MNP with 12 mg/d iron (n = 967) or MNP without iron (n = 991). Outcomes included malaria (defined as parasitaemia of any density plus reported fever within 48 hours or axillary temperature higher than 37.5 °C), malaria with parasite density higher than 5000/μL, anaemia (haemoglobin level < 10 g/dL), iron deficiency (ferritin level < 30 μg/L), hospital admission, and clinical diagnoses of pneumonia, diarrhoea (> 3 loose or watery stools in 24 hours), cerebral malaria, or meningitis among children who were admitted to a health facility during the study period. We excluded this study because there was no appropriate comparison arm (comparison vs placebo or another type of supplement) |
Ca: calcium CG: control group. EDTA: ethylenediaminetetraacetic acid. Fe: iron. Hb: haemoglobin. MNP: micronutrient powder. MUAC: mid‐upper arm circumference. NaFeEDTA: mixture of ferric sodium ethylenediaminetetraacetate. RCT: randomised controlled trial. RUFT: ready‐to‐use therapeutic food. sTfR: soluble transferrin receptor.
Characteristics of studies awaiting assessment [ordered by study ID]
Fernandez‐Rao 2014.
| Methods |
Design: integrated, randomised, double‐masked, placebo‐controlled trial comprising 2 phases:
Unit of randomisation: individual level |
| Participants |
Setting: rural India Sample size: 480 Age range: (1) infant phase (enrolment age: 6 to 12 months); and (2) preschool phase (enrolment age: 36 to 48 months) Inclusion criteria: infants aged 6 to 14 months or preschoolers aged 30 to 48 months, inclusive, at time of recruitment; residing in the Nalgonda district of Telengana, India; preschoolers must attend an Anganwadi Center (preschool) in the Nalgonda district that is participating in Project Grow Smart; participating caregivers must be at least 18 years of age at the time of recruitment Exclusion criteria: developmental delays, congenital illnesses or disabilities, severe anaemia (haemoglobin < 7) |
| Interventions | Infants will be randomly allocated to 1 of 4 groups:
Administration: mixed into food served to the children Preschool children receive MNP (group 3) or placebo (group 1) |
| Outcomes |
Primary
Secondary
Timing of outcome assessment: 12 months |
| Notes |
Comments: awaiting assessment because report includes only background information and a description of the pilot phase of the trial. We contacted study authors but received no reply Funding source: University of Maryland Principal investigator: Maureen M Black, PhD; Madhavan K Nair, PhD Conflicts of interests: no conflicts of interest Trial register ID:NCT01660958 Study start date: August 2012 Study end date: December 2014 Current status: completed |
Hasan 2017.
| Methods |
Design: protocol for a 3‐arm, parallel, double‐blind, double‐dummy, placebo‐controlled superiority trial comparing 3 months of iron drops or iron‐containing micronutrient powders used for anaemia control in young children aged 8 months ± 14 days against placebo. Additional follow‐up 9 months post intervention. Researcher, caregiver, data collector, analysts, and participant‐blinded study design Unit of randomisation: individual. Participants randomly assigned to 1 of 3 arms with 1:1:1 allocation using computer‐generated schedule of randomly permuted blocks of fixed size stratification by sex and geographic union (each covered by different field team) to achieve balance between arms within each stratum. Randomisation list will be prepared by an independent statistician who will not reveal the block size. Allocation will occur by the field team according to the list, within the assigned union, once eligibility criteria are confirmed |
| Participants |
Setting: Rupganj, a rural subdistrict/Upazilla of Narayanganj district, Bangladesh (50 km from Dhaka, Bangaldesh). This setting is non‐malaria endemic and has a low groundwater iron level Sample size: 3300 total Age range: 8 months ± 14 days Inclusion criteria
Exclusion criteria
|
| Interventions | Participants will be randomised in a 1:1:1 ratio to 3 arms:
Administration: each participant will be asked to consume a syrup and sachet with powder every day for 3 months. After enrolment, assigned trained village health workers (VHWs) will visit child every week during 3 months of intervention and every month for the 9‐month post‐intervention period. VHWs will document and notify of any unscheduled hospital or clinic admissions by participants. VHSs will also record number of doses missed, collect empty bottles and sachets, and distribute new doses for the following week |
| Outcomes |
Primary
Secondary
Timing of outcome assessment: the primary outcome, cognitive development, will be assessed after 3 months of intervention. It will also be assessed as a secondary outcome at 9 months post intervention. Secondary outcomes 2 and 3 will be assessed at 3 and 9 months post intervention. Morbidity data will be collected weekly and monthly during the intervention and post intervention, respectively |
| Notes |
Comments: no mention of additional behaviour change strategies to support intake of syrups or powders, whether infant and young child feeding programmes are in place, or whether micronutrient powder content is mentioned or included in such programmes, as recommended by WHO Funding source: National Health and Medical Research Council (NHMRC) Principal investigator: Sant‐Rayn Pasricha Conflicts of interests: none declared Trial register ID:ACTRN12617000660381 Study start date: 1 June 2017 Study end date: 31March 2020 (anticipated) Current status: data collection |
Islam 2018b.
| Methods |
Design: randomised, double‐blind, community‐based efficacy trial of 5 different doses, forms, and frequencies of preventive zinc supplementation vs placebo among children aged 9 to 11 months in urban Dhaka, Bangladesh Unit of randomisation: individual. Children stratified by sex and then randomised to 1 of 6 groups using block randomisation, to ensure even distribution of groups across time |
| Participants |
Setting: Urban Dhaka, Bangladesh Sample size: 481 per group, 2886 total Age range: 9 to 11 months Inclusion criteria
Exclusion criteria
|
| Interventions | There are 6 study groups:
Administration: study workers will visit each study participant's household twice weekly to inquire about and record any morbidity that took place in previous 3 to 4 days. Study worker will ask about supplement intake during prior 3 or 4 days and will encourage continued future supplement intake. Unconsumed supplements and used supplement containers will be collected during these visits |
| Outcomes |
Primary
Secondary
Timing of outcome assessment: 24 weeks |
| Notes |
Comments: no results published Funding source: Bill and Melinda Gates Foundation Principal investigator: Robert Black Conflicts of interest: none reported Trial register ID:NCT03406793 Study start date: 20 February 2018 Study end date: 30 January 2020 (anticipated) Current status: data collection |
ISRCTN39244429.
| Methods |
Design: randomised controlled trial comparing a powder with multiple micronutrient vs iron‐only powder; follow‐up to an initial randomised, double‐blind controlled trial in 2010 Unit of randomisation: not reported |
| Participants |
Setting: Peru Sample size: 902 infants. In this study, 200 children of the 902 were randomly selected from the original study cohort for assessment of cognitive function (100 in each group) Age range: 6 to 17 months Inclusion criteria
Exclusion criteria
|
| Interventions | Participants will be randomly assigned to 1 of 2 groups:
Administration: each child will receive monthly 30 sachets of the corresponding supplement, to be consumed as 1 sachet per day mixed with a part of the main meal, for a period of 6 months |
| Outcomes |
Primary
Secondary
Timing of outcome assessment: 6 months |
| Notes |
Comment: aims to examine the long‐term cognitive and social‐emotional effects of MNP, which is outside the scope of this review. The original study is not published. We contacted the study authors but received no reply Funding source: United Nations Children's Fund (UNICEF) (USA) Principal investigator: Nelly Zavaleta Conflicts of interest: not provided Trial register ID:ISRCTN39244429 Study start date: 7 January 2010 Study end date: 31 January 2011 Current status: completed |
ISRCTN57594793.
| Methods |
Design: randomised controlled trial comparing multiple micronutrient powder containing 15 micronutrients vs a micronutrient supplement containing 3 micronutrients Unit of randomisation: individual randomisation |
| Participants |
Setting: Palestine Sample size: target sample size of 200; unclear how many enrolled Age range: 5 months + 2 weeks Inclusion criteria
Exclusion criteria
|
| Interventions | Individuals randomised to 2 groups:
Administration: both groups given 3 sachets/week for 12 months |
| Outcomes |
Primary
Secondary
Timing of outcome assessment: anthropometrics assessed at baseline and throughout the study period (every 3 to 6 months); haemoglobin concentration assessed secondary outcomes assessed at baseline, 6 months of intervention, and end of the intervention period; secondary outcomes assessed at baseline, end‐line, and 3 months after the end of the intervention |
| Notes |
Comments: no results published. We contacted study authors but received no reply Funding source: University Putra Malaysia Principal investigator: Ali Albelbeisi Conflicts of interest: none Trial register ID:ISRCTN57594793 Study start date: 4 September 2015 Study end date: 20 January 2017 Current status: unknown |
MNP: multiple micronutrient powder. MUAC: mid‐upper arm circumference. sTfR: soluble transferrin receptor. WLZ: weight‐for‐length z score.
Differences between protocol and review
Several differences (listed below) between the original protocol and the review are notable (De‐Regil 2011), as are differences between the original review ‐ De‐Regil 2013 ‐ and this update. We list these below.
Protocol and review
-
In our protocol, we stated that comparison groups would include no intervention, placebo, or usual supplementation. In the review, we listed the specific comparisons to make them explicit for the reader.
For clarity, we specified the types of interventions that are outside the scope of this review.
-
We added 'iron deficiency', as we had previously included iron status as a continuous variable but not a variable to diagnose the actual deficiency.
We moved 'all‐cause mortality' from the list of secondary outcomes to the list of primary outcomes, to be consistent with our objective, and so we could evaluate the safety of this intervention.
We replaced 'growth', which may refer to several indicators, with 'weight‐for‐age (z score)' (a variable used to evaluate growth).
-
We changed 'adverse effects (any)' to 'side effects' and complemented the definition with some examples such as staining of teeth, vomiting, stool discolouration, and coughing.
We included 'constipation' as a side effect; thus it is no longer specified as an independent secondary outcome.
We changed the order of the secondary outcomes proposed in the protocol, to improve the legibility of the text.
-
We added the International Clinical Trials Registry Platform (ICTRP) as a source of data and information about ongoing trials.
-
We added use of the odds ratio (OR) for reporting rare morbidity outcomes.
-
In the protocol, we said that we were not going to combine cluster‐randomised and individually randomised trial results. However, as the direction and magnitude of the effect were consistent among trials at either level of randomisation, we deemed it convenient to combine the results.
-
We added Tau² to quantify the level of statistical heterogeneity, and we specified that the P value for the Chi² test was set at < 0.10 for added clarity.
-
Subgroup analysis and investigation of heterogeneity
Given the small number of trials, we changed the definition of malaria setting from four categories to two categories.
Review and update
-
Authorship
The first author of the review was changed from De‐Regil to Suchdev. The latter also became corresponding author.
Juan Pablo Pena‐Rosas, Gunn Vist, and Silke Walleser withdrew themselves from the list of review authors and did not participate in any stage of preparation of the update.
Maria Elena Jefferds, Erika Ota, and Katharina da Silva Lopes are new review authors.
-
For this updated review, we searched two additional segments of MEDLINE: MEDLINE In‐Process and Other Non‐Indexed Citations and MEDLlNE EPub Ahead of Print.
The metaRegister of Clinical Trials service was not available, so we replaced it with the ISRCTN Registry.
-
Summary of findings
We included a new paragraph on Summary of findings, beneath Data synthesis in the Methods section, to summarise use of the GRADE approach to generate the 'Summary of findings' tables.
-
We included additional sensitivity analyses and examined the effects of removing from the analysis studies at high risk of bias (studies with poor or unclear allocation concealment and either lack of blinding or loss to follow‐up of more than 20% in each arm).
Contributions of authors
All review authors contributed to the development of this review. Parminder Suchdev is the guarantor for the review.
Sources of support
Internal sources
-
Emory University, USA.
Parminder Suchdev works as Professor of Pediatrics and Global Health for Emory University and is partially funded by the US Centers for Disease Control and Prevention (CDC), Atlanta (GA).
-
Nutrition International, Canada.
Luz Maria De‐Regil was a full‐time employee of Nutrition International between 2013 and 2018. Part of the update took place during this period.
External sources
-
Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, World Health Organization (WHO), Switzerland.
Erika Ota received financial support from the WHO to participate in this review.
Declarations of interest
Parminder Suchdev (PS)a receives partial salary support from the CDC for research activities in monitoring and evaluating nutrition interventions.
Luz Maria De‐Regil (LD‐R)b was a full‐time member of the staff of Nutrition International between December 2013 and December 2018. Some of the updates of this review took place during that period. Nutrition International is a non‐profit organization that receives and provides funds from or to different institutions to undertake research and implement nutrition programmes in which multiple micronutrient powders may be provided to different age groups. Some of those studies were eligible for inclusion in this review. LD‐R was not directly involved in any such studies and did not assess their eligibility for inclusion in this review. The time that LD‐R devoted to this review during her tenure at NI was partially covered with a grant from Global Affairs Canada to Nutrition International.
Maria Jefferds (MJ)a,b provides technical assistance to multiple countries for the design, implementation, monitoring, and evaluation of MNP programs. Multiple peer review and report publications have been disseminated or are under development based on that work. MJ has participated in UNICEF/CDC regional workshops on scaling up MNP interventions for young children aged 6 to 23 months. MJ is a co‐author for two publications included in a September 2013 Sight and Life supplement on MNP and was an Editor of that supplement. MJ was the co‐ordinator and writer of a monitoring manual for home fortification interventions, including micronutrient powders, for the Home Fortification Technical Advisory Group (HF‐TAG). With colleagues from CDC and UNICEF, MJ was an investigator on the first global assessment of home fortification interventions in 2011, which was published as an HF‐TAG report in 2013, and she wrote a summary journal article published in 2013. As part of UNICEF’s Nutridash System, MJ provided technical assistance in the development of an annual surveillance system of global MNP interventions. Collaborators with UNICEF and CDC also developed a home fortification toolkit and webinar series, which focused heavily on MNP. MJ has participated in executive committee meetings and strategic planning of the HF‐TAG historically and is a member of the Executive Committee.
Erika Ota ‐ none known.
Katharina da Silva Lopes ‐ none known.
aBoth PS and MJ were involved in Suchdev 2012 ‐ an study excluded from this update. They were not involved in assessing the eligibility of this study. LD‐R and EO assessed the study. bMJ and LD‐R are co‐authors on a Cochrane Review of MNP in children 2 to 12 years old (De‐Regil 2017).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aboud 2011 (C) {published data only}
- Aboud FE, Akhter S. A cluster‐randomized evaluation of a responsive stimulation and feeding intervention in Bangladesh. Pediatrics 2011;127(5):e1191‐7. [DOI: 10.1542/peds.2010-2160; PUBMED: 21502222] [DOI] [PubMed] [Google Scholar]
Adrianopoli 2014 (C) {published data only}
- Adrianopoli M, D’Acapito P, Ferrari M, Mistura L, Toti E, Maiani G, et al. Optimized feeding recommendations and in‐home fortification to improve iron status in infants and young children in the Republic of Tajikistan: a pilot project. In: Thompson B, Amoroso L editor(s). Improving Diets and Nutrition: Food Based Approaches. Rome: The Food and Agriculture Organization of the United Nations and CAB International, 2014:230‐245. [9781780642994 (CABI); 9789251073193 (FAO)] [Google Scholar]
Adu‐Afarwuah 2007 {published data only (unpublished sought but not used)}
- Adu‐Afarwuah S, Lartey A, Brown KH, Zlotkin S, Briend A, Dewey KG. Home fortification of complementary foods with micronutrient supplements is well accepted and has positive effects on infant iron status in Ghana. American Journal of Clinical Nutrition 2008;87:929‐38. [DOI: 10.1093/ajcn/87.4.929; PUBMED: 18400716] [DOI] [PubMed] [Google Scholar]
- Adu‐Afarwuah S, Lartey A, Brown KH, Zlotkin S, Briend A, Dewey KG. Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: effects on growth and motor development. American Journal of Clinical Nutrition 2007;86:412‐20. [CENTRAL: CN‐00703403; DOI: 10.1093/ajcn/86.2.412; PUBMED: 17684213] [DOI] [PubMed] [Google Scholar]
Attanasio 2014 (C) {published data only}
- Andrew A, Attanasio O, Fitzsimons E, Rubio‐Codina M. Why is multiple micronutrient powder ineffective at reducing anaemia among 12–24 month olds in Colombia? Evidence from a randomised controlled trial. SSM ‐ Population Health 2016;2:95‐104. [DOI: 10.1016/j.ssmph.2016.02.004; PMC5757801; PUBMED: 29349132] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attanasio OP, Fernández C, Fitzsimons EO, Grantham‐McGregor SM, Meghir C, Rubio‐Codina M. Using the infrastructure of a conditional cash transfer program to deliver a scalable integrated early child development program in Colombia: cluster randomized controlled trial. BMJ 2014;349:g5785. Erratum in: BMJ. 2014;349:g6126. [DOI: 10.1136/bmj.g5785; PMC4179481; PUBMED: 25266222] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barffour 2019 {published data only}
- Barffour MA, Hinnouho GM, Kounnavong S, Wessells KR, Ratsavong K, Bounheuang B, et al. Effects of daily zinc, daily multiple micronutrient powder, or therapeutic zinc for diarrhea prevention on physical growth, anemia, and micronutrient status in rural Laotian Children: a randomized controlled trial. Journal of Pediatrics 2019;207:80‐9. [DOI: 10.1016/j.jpeds.2018.11.022; PMC6448681; PUBMED: 30580974] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barffour MA, Hinnouho GM, Kounnavong S, Wessells KR, Ratsavong K, Bounheuang B, et al. Effects of therapeutic zinc supplementation for diarrhea and two daily preventive zinc interventions on incidence and duration of diarrhea and acute respiratory tract infections in rural Laotian children. BMJ (in press). [DOI] [PMC free article] [PubMed]
- Hess SY, Wessells KR, Hinnouho GM, Barffour MA, Sanchaisuriya K, Arnold CD, et al. Iron status and inherited haemoglobin disorders modify the effects of micronutrient powders on linear growth and morbidity among young Lao children in a double‐blind randomised trial. British Journal of Nutrition 2019;122(8):895‐909. [DOI: 10.1017/S0007114519001715; PUBMED: 31303184] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchdev P. Quick question about your Laos MNP trial [personal communication]. Email to: R Wessells, M Barffour, S Hess, C Arnold. 5 September 2019.
Baum 2017 (C) {published data only}
- Baum A, Elize W, Jean‐Louis F. Microfinance institutions' successful delivery of micronutrient powders: a randomized trial in rural Haiti. Health Affairs 2017;36(11):1938‐46. [DOI: 10.1377/hlthaff.2017.0281; PUBMED: 29137512] [DOI] [PubMed] [Google Scholar]
Christofides 2006 (C) {published data only}
- Christofides A, Asante KP, Schauer C, Sharieff W, Owusu‐Agyei S, Zlotkin S. Multi‐micronutrient Sprinkles including a low dose of iron provided as microencapsulated ferrous fumarate improves haematologic indices in anaemic children: a randomized clinical trial. Maternal & Child Nutrition 2006;2(3):169‐80. [DOI: 10.1111/j.1740-8709.2006.00060.x; PUBMED: 16881929] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 2018 (C) {unpublished data only}
- Clarke S, Toure D, Dicko A, Maiga B, Griffiths Y Kraemer K, et al. An integrated parenting, nutrition and malaria prevention package to improve nutrition and child development in infants and pre‐school children: impact evaluation of a randomized controlled trial in Southern Mali. Donor report. Save the Children, 2018.
Esamai 2014 {published data only}
- Esamai F, Liechty E, Ikemeri J, Westcott J, Kemp J, Culbertson D, et al. Zinc absorption from micronutrient powder is low but is not affected by iron in Kenyan infants. Nutrients 2014;6(12):5636‐51. [DOI: 10.3390/nu6125636; PMC4276989; PUBMED: 25493942] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Frank DN, Hendricks AE, Ir D, Esamai F, Liechty E, et al. Iron in micronutrient powder promotes an unfavorable gut microbiota in Kenyan infants. Nutrients 2017;9(7):776. [DOI: 10.3390/nu9070776; PMC5537890; PUBMED: 28753958] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giovannini 2006 {published data only}
- Agostoni C, Giovannini M, Sala D, Usuelli M, Livio L, Francescato C, et al. Double‐blind, placebo‐controlled trial comparing effects of supplementation of two micronutrient sprinkles on fatty acid status in Cambodian infants. Journal of Pediatric Gastroenterology and Nutrition 2007;44(1):136‐42. [DOI: 10.1097/01.mpg.0000243429.24463.2f; PUBMED: 17204967] [DOI] [PubMed] [Google Scholar]
- Giovannini M, Sala D, Usuelli M, Livio L, Francescato G, Braga M, et al. Double‐blind, placebo‐controlled trial comparing effects of supplementation with two different combinations of micronutrients delivered as sprinkles on growth, anemia, and iron deficiency in Cambodian infants. Journal of Pediatrics Gastroenterology and Nutrition 2006;42(3):306‐12. [DOI: 10.1097/01.mpg.0000189363.07040.4b00011; PUBMED: 16540800] [DOI] [PubMed] [Google Scholar]
Hirve 2007 (C) {published and unpublished data}
- Regil L. Request of information ‐ Cochrane Review on home fortification of food with multiple micronutrient powders in children under 2 years of age [personal communication]. Email to: S Bhave. 10 February 2011.
- Hirve S. KEM Pune sprinkles study ‐ Cochrane Review [personal communication]. Email to: L de Regil. 28 February 2011.
- Hirve S, Bhave S, Bavdekar A, Naik S, Pandit A, Schauer C, et al. Low dose 'Sprinkles' ‐ an innovative approach to treat iron deficiency anemia in infants and young children. Indian Pediatrics 2007;44(2):91‐100. [PUBMED: 17351300] [PubMed] [Google Scholar]
Inayati 2012 (C) {published data only}
- Regil L. Your paper on MNP [personal communication]. Email to: V Scherbaum. 17 July 2012; 2 August 2012; 29 January 2013; 5 February 2013.
- Inayati DA, Scherbaum V, Purwestri RC, Wirawan NN, Suryantan J, Hartono S, et al. Combined intensive nutrition education and micronutrient powder supplementation improved nutritional status of mildly wasted children on Nias Island, Indonesia. Asia Pacific Journal of Clinical Nutrition 2012;21(3):361‐73. [PUBMED: 22705425] [PubMed] [Google Scholar]
- Inayati DA, Scherbaum V, Purwestri RC, Wirawan NN, Suryantan J, Hartono S, et al. Improved nutrition knowledge and practice through intensive nutrition education: a study among caregivers of mildly wasted children on Nias Island, Indonesia. Food and Nutrition Bulletin 2012;33(2):117‐27. [DOI: 10.1177/156482651203300205; PUBMED: 22908693] [DOI] [PubMed] [Google Scholar]
- Purwestri RC, Scherbaum V, Inayati DA, Wirawan NN, Suryantan J, Bloem MA, et al. Supplementary feeding with locally‐produced Ready‐to‐Use Food (RUF) for mildly wasted children on Nias Island, Indonesia: comparison of daily and weekly program outcomes. Asia Pacific Journal of Clinical Nutrition 2012;21(3):374‐9. [PUBMED: 22705426] [PubMed] [Google Scholar]
- Scherbaum V. Your paper on MNP [personal communication]. Email to: L de Regil. 18 July 2012; 5 February 2013; 8 February 2013.
Jack 2012 (C) {published data only}
- Jack SJ, Ou K, Chea M, Chhin L, Devenish R, Dunbar M, et al. Effect of micronutrient sprinkles on reducing anemia: a cluster‐randomized effectiveness trial. Archives of Pediatrics & Adolescent Medicine 2012;166(9):842‐50. [DOI: 10.1001/archpediatrics.2012.1003; PUBMED: 22801933] [DOI] [PubMed] [Google Scholar]
Kounnavong 2011 {published data only}
- Kounnavong S, Sunahara T, Mascie‐Taylor CG, Hashizume M, Okumura J, Moji K, et al. Effect of daily versus weekly home fortification with multiple micronutrient powder on haemoglobin concentration of young children in a rural area, Lao People's Democratic Republic: a randomised trial. Nutrition Journal 2011;10:129. [DOI: 10.1186/1475-2891-10-129; PMC3266642 ; PUBMED: 22111770] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lanou 2019 (C) {published data only}
- Lanou HB, Osendarp SJM, Argaw A, Polnay K, Ouédraogo C, Kouanda S, et al. Micronutrient powder supplements combined with nutrition education marginally improve growth amongst children aged 6‐23 months in rural Burkina Faso: a cluster randomized controlled trial. Maternal & Child Nutrition 2019;15(4):12820. [DOI: 10.1111/mcn.12820; PUBMED: 30941887] [DOI] [PMC free article] [PubMed] [Google Scholar]
Larson 2018 (C) {published data only}
- Larson LM, Young MF, Bauer PJ, Mehta R, Webb Girard A, Ramakrishnan U, et al. Effectiveness of a home fortification programme with multiple micronutrients on infant and young child development: a cluster‐randomised trial in rural Bihar, India. British Journal of Nutrition 2018;120(2):176‐87. [DOI: 10.1017/S000711451800140X; PMC6088539; PUBMED: 29947323] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MF. Bihar MNP results [personal communication]. Email to: P Suchdev. 5 December 2019.
- Young MF, Mehta R, Gosdin L, Kekre P, Verma P, Larson L, et al. Impact of home fortification of complementary foods program on child anemia and stunting in Bihar, India. FASEB Journal 2017;31(1 Suppl). [Abstract #165.7] [Google Scholar]
Lundeen 2010 (C) {published and unpublished data}
- Lundeen E, Schueth T, Toktobaev N, Zlotkin S, Hyder SM, Houser R. Daily use of Sprinkles micronutrient powder for 2 months reduces anemia among children 6 to 36 months of age in the Kyrgyz Republic: a cluster‐randomized trial. Food and Nutrition Bulletin 2010;31(3):446‐60. [DOI: 10.1177/156482651003100307; PUBMED: 20973465 ] [DOI] [PubMed] [Google Scholar]
Luo 2017 (C) {published data only}
- Luo R, Yue A, Zhou H, Shi Y, Zhang L, Martorell R, et al. The effect of a micronutrient powder home fortification program on anemia and cognitive outcomes among young children in rural China: a cluster randomized trial. BMC Public Health 2017;17(1):738. [DOI: 10.1186/s12889-017-4755-0; PMC5613507; PUBMED: 28946866] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macharia‐Mutie 2012 {published data only}
- Macharia‐Mutie CW, Moretti D, Briel N, Omusundi AM, Mwangi AM, Kok FJ, et al. Maize porridge enriched with a micronutrient powder containing low‐dose iron as NaFeEDTA but not amaranth grain flour reduces anemia and iron deficiency in Kenyan preschool children. Journal of Nutrition 2012;142(9):1756‐63. [DOI: 10.3945/jn.112.157578; PUBMED: 22810982] [DOI] [PubMed] [Google Scholar]
- Macharia‐Mutie CW, Omusundi AM, Mwai JM, Mwangi AM, Brouwer ID. Simulation of the effect of maize porridge fortified with grain amaranth or micronutrient powder containing NaFeEDTA on iron intake and status in Kenyan children. Public Health Nutrition 2013;16(9):1605‐13. [DOI: 10.1017/S1368980012005174; PUBMED: 23218415] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matias 2018 (C) {published data only}
- Matias SL, Mridha MK, Young RT, Khan MSA, Siddiqui Z, Ullah MB, et al. Prenatal and postnatal supplementation with lipid‐based nutrient supplements reduces anemia and iron deficiency in 18‐month‐old Bangladeshi children: a cluster‐randomized effectiveness trial. Journal of Nutrition 2018;148(7):1167‐76. [DOI: 10.1093/jn/nxy078; PUBMED: 29901736] [DOI] [PubMed] [Google Scholar]
Menon 2007 (C) {published and unpublished data}
- Loechl CU, Menon P, Arimond M, Ruel MT, Pelto G, Habicht JP, et al. Using programme theory to assess the feasibility of delivering micronutrient Sprinkles through a food‐assisted maternal and child health and nutrition programme in rural Haiti. Maternal & Child Nutrition 2009;5(1):33‐48. [DOI: 10.1111/j.1740-8709.2008.00154.x; PUBMED: 19161543] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon P, Ruel MT, Loechl CU, Arimond M, Habicht JP, Pelto G, et al. Micronutrient Sprinkles reduce anemia among 9‐ to 24‐mo‐old children when delivered through an integrated health and nutrition program in rural Haiti. Journal of Nutrition 2007;137(4):1023‐30. [DOI: 10.1093/jn/137.4.1023; PUBMED: 17374671] [DOI] [PubMed] [Google Scholar]
Mridha 2016 (C) {published data only}
- Dewey KG, Mridha MK, Matias SL, Arnold CD, Cummins JR, Khan MS, et al. Lipid‐based nutrient supplementation in the first 1000 d improves child growth in Bangladesh: a cluster‐randomized effectiveness trial. American Journal of Clinical Nutrition 2017;105(4):944‐57. [DOI: 10.3945/ajcn.116.147942; PUBMED: 28275125] [DOI] [PubMed] [Google Scholar]
- Matias SL, Mridha MK, Tofail F, Arnold CD, Khan MS, Siddiqui Z, et al. Home fortification during the first 1000 d improves child development in Bangladesh: a cluster‐randomized effectiveness trial. American Journal of Clinical Nutrition 2017;105(4):958‐69. [DOI: 10.3945/ajcn.116.150318; PUBMED: 28275128] [DOI] [PubMed] [Google Scholar]
- Mridha MK, Matias SL, Chaparro CM, Paul RR, Hussain S, Vosti SA, et al. Lipid‐based nutrient supplements for pregnant women reduce newborn stunting in a cluster‐randomized controlled effectiveness trial in Bangladesh. American Journal of Clinical Nutrition 2016;103(1):236‐49. [DOI: 10.3945/ajcn.115.111336; PMC6443293; PUBMED: 26607935] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah MB, Mridha MK, Arnold CD, Matias SL, Khan MSA, Siddiqui Z, et al. Provision of pre‐ and postnatal nutritional supplements generally did not increase or decrease common childhood illnesses in Bangladesh: a cluster‐randomized effectiveness trial. Journal of Nutrition 2019;149(7):1271‐81. [DOI: 10.1093/jn/nxz059; PUBMED: 31162588] [DOI] [PubMed] [Google Scholar]
Olney 2018 (C) {published data only}
- Olney DK, Leroy J, Bliznashka L, Ruel MT. PROCOMIDA, a food‐assisted maternal and child health and nutrition program, reduces child stunting in Guatemala: a cluster‐randomized controlled intervention trial. Journal of Nutrition 2018;148(9):1493‐505. [DOI: 10.1093/jn/nxy138; PMC6118165; PUBMED: 30184223] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osei 2015 (C) {published data only}
- Osei KA, Pandey P, Spiro D, Adhikari D, Haselow N, Morais C, et al. Adding multiple micronutrient powders to a homestead food production programme yields marginally significant benefit on anaemia reduction among young children in Nepal. Maternal & Child Nutrition 2015;11(Suppl 4):188‐202. [DOI: 10.1111/mcn.12173; PUBMED: 25682798] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sazawal 2014 (C) {published data only}
- Sazawal S, Dhingra P, Dhingra U, Gupta S, Iyengar V, Menon VP, et al. Compliance with home‐based fortification strategies for delivery of iron and zinc: its effect on haematological and growth markers among 6‐24 months old children in north India. Journal of Health, Population and Nutrition 2014;32(2):217‐26. [PMC4216958; PUBMED: 25076659] [PMC free article] [PubMed] [Google Scholar]
Sharieff 2006a {published data only}
- Sharieff W, Bhutta Z, Schauer C, Tomlinson G, Zlotkin S. Micronutrients (including zinc) reduce diarrhoea in children: the Pakistan Sprinkles Diarrhoea Study. Archives of Disease in Childhood 2006;91(7):573‐9. [PMC2082848; PUBMED: 16556612] [DOI] [PMC free article] [PubMed] [Google Scholar]
Somassè 2018 (C) {published data only}
- Somassè YE, Dramaix M, Traoré B, Ngabonziza I, Touré O, Konaté M, et al. The WHO recommendation of home fortification of foods with multiple‐micronutrient powders in children under 2 years of age and its effectiveness on anaemia and weight: a pragmatic cluster‐randomized controlled trial. Public Health Nutrition 2018;21(7):1350‐8. [DOI: 10.1017/S1368980017003858; PUBMED: 29352829] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soofi 2013 (C) {published data only}
- Soofi S, Cousens S, Iqbal SP, Akhund T, Khan J, Ahmed I, et al. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster‐randomised trial. Lancet 2013;382(9886):29‐40. [DOI: 10.1016/S0140-6736(13)60437-7; PUBMED: 23602230] [DOI] [PubMed] [Google Scholar]
Young 2018 (C) {published data only}
- Young MF, Girard AW, Mehta R, Srikantiah S, Gosdin L, Menon P, et al. Acceptability of multiple micronutrient powders and iron syrup in Bihar, India. Maternal & Child Nutrition 2018; Vol. 14, issue 2:e12572. [DOI: 10.1111/mcn.12572; PMC5900720; PUBMED: 29210507] [DOI] [PMC free article] [PubMed]
References to studies excluded from this review
Avula 2011 {published data only}
- Avula R, Frongillo EA, Arabi M, Sharma S, Schultink W. Enhancements to nutrition program in Indian integrated child development services increased growth and energy intake of children. Journal of Nutrition 2011;141(4):680‐4. [DOI: 10.3945/jn.109.116954; PUBMED: 21346106] [DOI] [PubMed] [Google Scholar]
Bagni 2009 {published data only}
- Bagni UV, Baiao MR, Souza Santos MMA, Luiz RR, Veiga GV. Effect of weekly rice fortification with iron on anaemia prevalence and haemoglobin concentration among children attending public daycare centres in Rio de Janeiro, Brazil [Efeito da fortificao semanel do arroz com ferro quelato sobre a frequencia de anemia e concentracao de hemoglobina em criancas de creches municipais de Rio de Janeiro, Brasil]. Cadernos de Saude Publica 2009;25(2):291‐302. [PUBMED: 19219236] [DOI] [PubMed] [Google Scholar]
Barth‐Jaeggi 2015 {published data only}
- Barth‐Jaeggi T, Moretti D, Kvalsvig J, Holding PA, Njenga J, Mwangi A, et al. In‐home fortification with 2.5 mg iron as NaFeEDTA does not reduce anaemia but increases weight gain: a randomised controlled trial in Kenyan infants. Maternal & Child Nutrition 2015;11(Suppl 4):151‐62. [DOI: 10.1111/mcn.12163; PUBMED: 25420455] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bilenko 2014 {published data only}
- Bilenko N, Belmaker I, Vardi H, Fraser D. Efficacy of multiple micronutrient supplementations on child health: study design and baseline characteristics. Israel Medical Association Journal 2010;12(6):342‐7. [PUBMED: 20928987] [PubMed] [Google Scholar]
- Bilenko N, Fraser D, Vardy H, Belmaker I. Impact of multiple micronutrient supplementation ("sprinkles") on iron deficiency anemia in Bedouin Arab and Jewish infants. Israel Medical Association Journal 2014;16(7):434‐8. [PUBMED: 25167690] [PubMed] [Google Scholar]
Cardoso 2016 {published data only}
- Cardoso MA, Augusto RA, Bortolini GA, Oliveira CS, Tietzman DC, Sequeira LA, et al. ENFAC Working Group. Effect of providing multiple micronutrients in powder through primary healthcare on anemia in young Brazilian children: a multicentre pragmatic controlled trial. PLoS One 2016;11(3):e0151097. [DOI: 10.1371/journal.pone.0151097; PMC4871551 ; PUBMED: 27192494] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva LLS, Augusto RA, Tietzmann DC, Sequeira LAS, Hadler MCCM, Muniz PT, et al. ENFAC Working Group. The impact of home fortification with multiple micronutrient powder on vitamin A status in young children: a multicenter pragmatic controlled trial in Brazil. Maternal & Child Nutrition 2017;13(4):e12403. [10.1111/mcn.12403; PUBMED: 27925426] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2008 {published data only}
- Chen K, Li TY, Chen L, Qu P, Liu YX. Effects of vitamin A, vitamin A plus iron and multiple micronutrient‐fortified seasoning powder on preschool children in a suburb of Chongqing, China. Journal of Nutritional Science and Vitaminology 2008;54(6):440‐7. [PUBMED: 19155581] [DOI] [PubMed] [Google Scholar]
- Chen K, Zhang X, Li TY, Chen L, Wei XP, Qu P, Liu YX. Effect of vitamin A, vitamin A plus iron and multiple micronutrient‐fortified seasoning powder on infectious morbidity of preschool children. Nutrition 2011;27(4):428‐34. [DOI: 10.1016/j.nut.2010.04.004; PUBMED: 20605698] [DOI] [PubMed] [Google Scholar]
Geltman 2009 {published data only}
- Geltman PL, Hironaka LK, Mehta SD, Padilla P, Rodrigues P, Meyers AF, et al. Iron supplementation of low‐income infants: a randomized clinical trial of adherence with ferrous fumarate sprinkles versus ferrous sulfate drops. Journal of Pediatrics 2009;154(5):738‐43. [DOI: 10.1016/j.jpeds.2008.11.003; PUBMED: 19111318 ] [DOI] [PubMed] [Google Scholar]
Goyena 2019 {published data only}
- Goyena EA, Barba CVC, Talavera MTM, Paunlagui MM, Rola AC, Tandang NA. Acceptance and compliance with micronutrient powder and complementary food blend use by Filipino mothers and their promotion by community workers. Food and Nutrition Bulletin 2019;40(2):202‐20. [DOI: 10.1177/0379572119833853; PUBMED: 30866669] [DOI] [PubMed] [Google Scholar]
Ip 2009 {published data only}
- Ip H, Hyder SM, Haseen F, Rahman M, Zlotkin SH. Improved adherence and anaemia cure rates with flexible administration of micronutrient Sprinkles: a new public health approach to anaemia control. European Journal of Clinical Nutrition 2009;63(2):165‐72. [DOI: 10.1038/sj.ejcn.1602917; PUBMED: 17895911 ] [DOI] [PubMed] [Google Scholar]
Jaeggi 2015 {published data only}
- Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015;64:731‐42. [DOI: 10.1136/gutjnl-2014-307720; NCT01111864.; PUBMED: 25143342] [DOI] [PubMed] [Google Scholar]
Jyoti 2014 {published data only}
- Jyoti V, Sharma S. Impact of micronutrients sprinkle on weight and height of children aged 6‐36 months in Tonk district of Rajasthan state. Indian Journal of Community Health 2014;26:294‐9. [Google Scholar]
Khan 2014 {published data only}
- Khan WU, Shafique S, Shikder H, Shakur YA, Sellen DW, Chowdhury JS, et al. Home fortification with calcium reduces Hb response to iron among anaemic Bangladeshi infants consuming a new multi‐micronutrient powder formulation. Public Health Nutrition 2014;17:1578. [DOI: 10.1017/S1368980013001742; PUBMED: 23816321] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lemaire 2011 {published data only}
- Lemaire M, Islam QS, Shen H, Khan MA, Parveen M, Abedin F, et al. Iron‐containing micronutrient powder provided to children with moderate‐to‐severe malnutrition increases hemoglobin concentrations but not the risk of infectious morbidity: a randomized, double‐blind, placebo‐controlled, noninferiority safety trial. American Journal of Clinical Nutrition 2011;94(2):585‐93. [DOI: 10.3945/ajcn.110.009316; PUBMED: 21715512] [DOI] [PubMed] [Google Scholar]
Munayco 2013 {published data only}
- Harding K, Zavaleta N, Roche M, Neufeld L. Caregiver perceptions, practices, and preferences relating to multiple micronutrient powders for children 6‐23 mo of age in the northern highlands of Peru. FASEB Journal 2014;28(1 Suppl). [Abstract #: 804.28] [Google Scholar]
- Munayco CV, Ulloa‐Rea ME, Medina‐Osis J, Lozano‐Revollar CR, Tejada V, Castro‐Salazar C, et al. Evaluation of the impact of multiple micronutrient powders on children anemia in three Andean regiones in Peru [Evaluación del impacto de los multimicronutrientes en polvo sobre la anemia infantil en tres regiones andinas del Perú]. Revista Peruana de Medicina Experimental y Salud Publica 2013;30(2):229‐34. [PUBMED: 23949507] [PubMed] [Google Scholar]
- Trelles S, Munayco CV. Impact and adherence of multi‐micronutrient supplementation in Peruvian children [Impacto y adherencia de la suplementación con multimicronutrientes en niños de Perú]. Revista Peruana de Medicina Experimental y Salud Publica 2019;36(1):147‐8. [DOI: 10.17843/rpmesp.2019.361.4051; PUBMED: 31116330] [DOI] [PubMed] [Google Scholar]
Neufeld 2008 {unpublished data only}
- Aburto NJ, Ramirez‐Zea M, Neufeld LM, Flores‐Ayala R. The effect of nutritional supplementation on physical activity and exploratory behavior of Mexican infants aged 8‐12 months. European Journal of Clinical Nutrition 2010;64(6):644‐51. [DOI: 10.1038/ejcn.2010.52; PUBMED: 20354559] [DOI] [PubMed] [Google Scholar]
- Colchero A, Neufeld LM. Cost estimations of different types of micronutrient supplements for children and pregnant women. FASEB Journal 2008; Vol. 22, issue 1 Suppl. [Abstract #: 678.21]
- García‐Guerra A, Neufeld LM, Domínguez CP, García‐Feregrino R, Hernández‐Cabrera A. Effect of three supplements with identical micronutrient content on anemia in Mexican children. FASEB Journal 2008; Vol. 22, issue 1 Suppl. [Abstract #: 677.5]
Rawat 2015 {published data only}
- Rawat R, Saha K, Kennedy A, Ruel M, Menon P. Sale of micronutrient powders (MNPs) by frontline workers (FLWs) enables high reach, but low uptake limits impact on anemia and iron status: a cluster randomized study in Bangladesh. FASEB Journal 2015;29(1 Suppl):391.6. [Google Scholar]
Samadpour 2011 {published data only}
- Samadpour K, Long KZ, Hayatbakhsh R, Marks GC. Randomised comparison of the effects of Sprinkles and Foodlets with the currently recommended supplement (Drops) on micronutrient status and growth in Iranian children. European Journal of Clinical Nutrition 2011;65(12):1287‐94. [DOI: 10.1038/ejcn.2011.124; PUBMED: 21750564] [DOI] [PubMed] [Google Scholar]
Shafique 2014 {published data only}
- Shafique S, Singla D, Aboud F, Jolly S, Zlotkin S. Multiple micronutrient powders reduce stunting and anemia and improve language development among full‐term low birth weight children in Bangladesh (389.3). FASEB Journal 2014;28(1 Suppl):389.3. [Google Scholar]
Sharieff 2006b {published data only}
- Sharieff W, Yin SA, Wu M, Yang Q, Schauer C, Tomlinson G, et al. Short‐term daily or weekly administration of micronutrient Sprinkles has high compliance and does not cause iron overload in Chinese schoolchildren: a cluster‐randomized trial. Public Health Nutrition 2006;9(3):336‐44. [PUBMED: 16684385] [DOI] [PubMed] [Google Scholar]
Singla 2014 {published data only}
- Shafique S, Sellen DW, Lou W, Jalal CS, Jolly SP, Zlotkin SH. Mineral‐ and vitamin‐enhanced micronutrient powder reduces stunting in full‐term low‐birth‐weight infants receiving nutrition, health, and hygiene education: a 2 × 2 factorial, cluster‐randomized trial in Bangladesh. American Journal of Clinical Nutrition 2016;103:1357‐69. [DOI: 10.3945/ajcn.115.117770; PUBMED: 27053383] [DOI] [PubMed] [Google Scholar]
- Singla DR, Shafique S, Zlotkin SH, Aboud FE. A 22‐element micronutrient powder benefits language but not cognition in Bangladeshi full‐term low‐birth‐weight children. Journal of Nutrition 2014;144(11):1803‐10. [DOI: 10.3945/jn.114.193094; PUBMED: 25143374] [DOI] [PubMed] [Google Scholar]
Smuts 2005 {published data only}
- Smuts CM, Dhansay MA, Faber M, Stuijvenberg ME, Swanevelder S, Gross R, et al. Efficacy of multiple micronutrient supplementation for improving anemia, micronutrient status, and growth in South African infants. Journal of Nutrition 2005;135(3):653S‐9S. [DOI: 10.1093/jn/135.3.653S; PUBMED: 15735110] [DOI] [PubMed] [Google Scholar]
Suchdev 2012 {published and unpublished data}
- Centers for Disease Control and Prevention. Baseline data from the Nyando Integrated Child Health and Education Project ‐ Kenya, 2007. Morbidity and Mortality Weekly Report: MMWR 2007;56(42):1109‐13. [PUBMED: 17962803] [PubMed] [Google Scholar]
- Suchdev PS, Addo OY, Martorell R, Grant FKE, Ruth LJ, Patel MK, et al. Effects of community‐based sales of micronutrient powders on morbidity episodes in preschool children in Western Kenya. American Journal of Clinical Nutrition 2016;103:934‐41. [DOI: 10.3945/ajcn.115.118000; NCT01088958; PUBMED: 26864367] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchdev PS, Addo Y, Martorell R, Grant FK, Ruth LJ, Patel M, et al. Effects of community‐based sales of micronutrient powders on morbidity episodes in preschool children in Western Kenya. American Journal of Clinical Nutrition 2016;103:934‐41. [DOI: 10.3945/ajcn.115.118000; PUBMED: 26864367 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchdev PS, Leeds IL, McFarland DA, Flores R. Is it time to change guidelines for iron supplementation in malarial areas?. Journal of Nutrition 2010;140(4):875‐6. [DOI: 10.3945/jn.109.118638; PUBMED: 20147465] [DOI] [PubMed] [Google Scholar]
- Suchdev PS, Ruth LJ, Woodruff BA, Mbakaya C, Mandava U, Flores‐Ayala R, et al. Selling Sprinkles micronutrient powder reduces anemia, iron deficiency, and vitamin A deficiency in young children in Western Kenya: a cluster‐randomized controlled trial. American Journal of Clinical Nutrition 2012;95(5):1223‐30. [DOI: 10.3945/ajcn.111.030072; NCT01088958; PUBMED: 22492366] [DOI] [PMC free article] [PubMed] [Google Scholar]
Teshome 2017 {published data only}
- Teshome EM, Andang'o PEA, Osoti V, Terwel SR, Otieno W, Demir AY, et al. Daily home fortification with iron as ferrous fumarate versus NaFeEDTA: a randomised, placebo‐controlled, non‐inferiority trial in Kenyan children. BMC Medicine 2017;15(1):89. [DOI: 10.1186/s12916-017-0839-z; PMC5408380; PUBMED: 28449690] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshome EM, Oriaro VS, Andango PEA, Prentice AM, Verhoef H. Adherence to home fortification with micronutrient powders in Kenyan pre‐school children: self‐reporting and sachet counts compared to an electronic monitoring device. BMC Public Health 2018;18(1):205. [DOI: 10.1186/s12889-018-5097-2; PMC5796300; PUBMED: 29391008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshome EM, Otieno W, Terwel SR, Osoti V, Demir AY, Andango PEA, et al. Comparison of home fortification with two iron formulations among Kenyan children: rationale and design of a placebo‐controlled non‐inferiority trial. Contemporary Clinical Trials Communications 2017;7:1‐10. [DOI: 10.1016/j.conctc.2017.04.007; PMC5898495; PUBMED: 29696163] [DOI] [PMC free article] [PubMed] [Google Scholar]
Troesch 2009 {published data only}
- Troesch B. Optimized micronutrient powder containing low levels of highly bioavailable iron and zinc together with EDTA, phytase and ascorbic acid improves the nutritional status of children. Sight and Life Magazine 2010, issue 3:9‐14.
- Troesch B, Egli I, Zeder C, Hurrell RF, Pee S, Zimmermann MB. Optimization of a phytase‐containing micronutrient powder with low amounts of highly bioavailable iron for in‐home fortification of complementary foods. American Journal of Clinical Nutrition 2009;89(2):539‐44. [DOI: 10.3945/ajcn.2008.27026; PUBMED: 19106242] [DOI] [PubMed] [Google Scholar]
Troesch 2011 {published data only}
- Troesch B, Stujivenberg ME, Smuts CM, Kruger HS, Biebinger R, Hurrell RF, et al. A micronutrient powder with low doses of highly absorbable iron and zinc reduces iron and zinc deficiency and improves weight‐for‐age Z‐scores in South African children. Journal of Nutrition 2011;141(2):237‐42. [DOI: 10.3945/jn.110.129247; PUBMED: 21178093] [DOI] [PubMed] [Google Scholar]
Van der Kam 2016a {published data only}
- Kam S, Salse‐Ubach N, Roll S, Swarthout T, Gayton‐Toyoshima S, Jiya NM, et al. Effect of short‐term supplementation with ready‐to‐use therapeutic food or micronutrients for children after illness for prevention of malnutrition: a randomised controlled trial in Nigeria. PLoS Medicine 2016;13(2):e1001952. [DOI: 10.1371/journal.pmed.1001952; PMC4747530; PUBMED: 26859559] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Kam 2016b {published data only}
- Kam S, Roll S, Swarthout T, Edyegu‐Otelu G, Matsumoto A, Kasujja FX, et al. Effect of short‐term supplementation with ready‐to‐use therapeutic food or micronutrients for children after illness for prevention of malnutrition: a randomised controlled trial in Uganda. PLoS Medicine 2016;13(2):e1001951. [DOI: 10.1371/journal.pmed.1001951; PMC4747529; PUBMED: 26859481] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2017 {published data only}
- Trehan I. A combined package of micronutrients and albendazole does not improve environmental enteric dysfunction or stunting in rural Malawian children. Journal of Pediatric Gastroenterology and Nutrition 2016;63(Suppl 2):S38. [Google Scholar]
- Wang AZ, Shulman RJ, Crocker AH, Thakwalakwa C, Maleta KM, Devaraj S, et al. A combined intervention of zinc, multiple micronutrients, and albendazole does not ameliorate environmental enteric dysfunction or stunting in rural Malawian children in a double‐blind randomized controlled trial. Journal of Nutrition 2017;147(1):97‐103. [DOI: 10.3945/jn.116.237735; NCT02253095; PUBMED: 27807040] [DOI] [PubMed] [Google Scholar]
Wijaya‐Erhardt 2007 {published data only}
- Wijaya‐Erhardt M, Erhardt JG, Untoro J, Karyadi E, Wibowo L, Gross R. Effects of daily or weekly multiple‐micronutrient and iron foodlike tablets on body iron stores of Indonesian infants aged 6‐12 mo: a double‐blind, randomized, placebo‐controlled trial. American Journal of Clinical Nutrition 2007;86(6):1680‐6. [DOI: 10.1093/ajcn/86.6.1680; PUBMED: 18065586] [DOI] [PubMed] [Google Scholar]
Yousafzai 2014 {published data only}
- Yousafzai AK, Obradović J, Rasheed MA, Rizvi A, Portilla XA, Tirado‐Strayer N, et al. Effects of responsive stimulation and nutrition interventions on children's development and growth at age 4 years in a disadvantaged population in Pakistan: a longitudinal follow‐up of a cluster‐randomised factorial effectiveness trial. Lancet Global Health 2016;4(8):e548‐58. [DOI: 10.1016/S2214-109X(16)30100-0; PUBMED: 27342433] [DOI] [PubMed] [Google Scholar]
- Yousafzai AK, Rasheed MA, Rizvi A, Armstrong R, Bhutta ZA. Effect of integrated responsive stimulation and nutrition interventions in the lady health worker programme in Pakistan on child development, growth, and health outcomes: a cluster‐randomised factorial effectiveness trial. Lancet 2014;384(9950):1282‐93. [DOI: 10.1016/S0140-6736(14)60455-4.; PUBMED: 24947106] [DOI] [PubMed] [Google Scholar]
Zlotkin 2001 {published data only}
- Zlotkin S, Arthur P, Antwi KY, Yeung G. Treatment of anemia with microencapsulated ferrous fumarate plus ascorbic acid supplied as sprinkles to complementary (weaning) foods. American Journal of Clinical Nutrition 2001;74(6):791‐5. [DOI: 10.1093/ajcn/74.6.791; PUBMED: 11722961] [DOI] [PubMed] [Google Scholar]
Zlotkin 2003a {published data only}
- Zlotkin S, Antwi KY, Schauer C, Yeung G. Use of microencapsulated iron (II) fumarate sprinkles to prevent recurrence of anaemia in infants and young children at high risk. Bulletin of the World Health Organization 2003;81(2):108‐15. [PUBMED: 12756979 ] [PMC free article] [PubMed] [Google Scholar]
Zlotkin 2003b {published data only}
- Zlotkin S, Arthus P, Schauer C, Antwi KY, Yeung G, Piekarz A. Home‐fortification with iron and zinc sprinkles or iron sprinkles alone successfully treats anemia in infants and young children. Journal of Nutrition 2003;133(4):1075‐80. [DOI: 10.1093/jn/133.4.1075; PUBMED: 12672922 ] [DOI] [PubMed] [Google Scholar]
Zlotkin 2013 {published data only}
- Zlotkin S, Newton S, Aimone AM, Azindow I, Amenga‐Etego S, Tchum K, et al. Effect of iron fortification on malaria incidence in infants and young children in Ghana: a randomized trial. JAMA 2013;310(9):938‐47. [DOI: 10.1001/jama.2013.277129; PUBMED: 24002280] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Fernandez‐Rao 2014 {published data only}
- Fernandeez‐Rao S, Hurley K, Nair M, Balakrishna N, Tilton N, Radhakrishna V, et al. Multiple micronutrients and early learning interventions promote Infant micronutrient status and development. FASEB Journal 2015;29(1 Suppl):28.2. [Google Scholar]
- Fernandez‐Rao S, Hurley KM, Nair KM, Balakrishna N, Radhakrishna KV, Ravinder P, et al. Integrating nutrition and early child‐development interventions among infants and preschoolers in rural India. Annals of the New York Academy of Sciences 2014;1308:218‐31. [DOI: 10.1111/nyas.12278; PUBMED: 24673168] [DOI] [PubMed] [Google Scholar]
Hasan 2017 {published and unpublished data}
- Hasan MI, Hossain SJ, Braat S, Dibley MJ, Fisher J, Grantham‐McGregor S, et al. Benefits and Risks of Iron interventionS in Children (BRISC): protocol for a three‐arm parallel‐group randomised controlled field trial in Bangladesh. BMJ Open 2017;7(11):e018325. [DOI: 10.1136/bmjopen-2017-018325; PMC5695407; PUBMED: 29146650] [DOI] [PMC free article] [PubMed] [Google Scholar]
Islam 2018b {published data only}
- Islam MM, McDonald CM, Krebs NF, Westcott J, Rahman AE, Arifeen S, et al. Study protocol for a randomized, double‐blind, community‐based efficacy trial of various doses of zinc in micronutrient powders or tablets in young Bangladeshi children. Nutrients 2018;10(2):132. [DOI: 10.3390/nu10020132; PMC5852708; PUBMED: 30720778] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN39244429 {published and unpublished data}
- ISRCTN39244429. Nutritional supplement sprinkles with zinc and micronutrients [Efficacy of the nutritional supplement sprinkles with zinc and micronutrients on anaemia and acute diarrhoea in Peruvian children]. www.isrctn.com/ISRCTN39244429 (first received 12 February 2010).
- Warthon‐Medina M, Qualter P, Zavaleta N, Dillon S, Lazarte F, Lowe NM. The long term impact of micronutrient supplementation during infancy on cognition and executive function performance in pre‐school children. Nutrients 2015;7(8):6606‐27. [DOI: 10.3390/nu7085302; PMC4555141 ; PUBMED: 26262642] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN57594793 {unpublished data only}
- ISRCTN57594793. Multiple micronutrient supplementation improves growth and hemoglobin of infants in Gaza Strip, Palestine [Multiple micronutrient supplementation improves growth and hemoglobin of infants in Gaza Strip, Palestine: a randomized community trial]. www.isrctn.com/ISRCTN57594793 (first received 2 January 2018).
Additional references
Adetifa 2009
- Adetifa I, Okomo U. Iron supplementation for reducing morbidity and mortality in children with HIV. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006736.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Angermayr 2004
- Angermayr L, Clar C. Iodine supplementation for preventing iodine deficiency disorders in children. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD003819.pub2] [DOI] [PubMed] [Google Scholar]
Balshem 2010
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI: 10.1016/j.jclinepi.2010.07.015; PUBMED: 21208779 ] [DOI] [PubMed] [Google Scholar]
Beaton 1993
- Beaton G, Martorell R, Aronson K, Edmonston B, McCabe G, Ross CA, et al. Effectiveness of vitamin A supplementation in the control of young child morbidity and mortality in developing countries. UN, ACC/SCN State‐of‐the‐Art Series. Nutrition Policy Discussion Paper No 13. 1993.
Beaton 1999
- Beaton GH, McCabe GP. Efficacy of intermittent iron supplementation in the control of iron deficiency anaemia in developing countries. An analysis of experience: final report to the Micronutrient Initiative. Ottowa, Micronutrient Initiative. [ISBN 1‐894217‐08‐X]
Berger 1997
- Berger J, Aguayo VM, Tellez W, Lujan C, Traissac P, San Miguel JL. Weekly iron supplementation is as effective as 5 day per week iron supplementation in Bolivian school children living at high altitude. European Journal of Clinical Nutrition 1997;51(6):381‐6. [PUBMED: 9192196] [DOI] [PubMed] [Google Scholar]
Bhutta 2013
- Bhutta Z, Rizvi A, Gaffey MF, Walker N, Horton S, Webb P, et al. Evidence‐based interventions for improvement of maternal and child nutrition: what can be done and at what cost?. Lancet 2013;382(9890):452‐77. [DOI: 10.1016/S0140-6736(13)60996-4; PUBMED: 23746776] [DOI] [PubMed] [Google Scholar]
Black 2008
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371(9608):243‐60. [DOI: 10.1016/S0140-6736(07)61690-0; PUBMED: 18207566 ] [DOI] [PubMed] [Google Scholar]
Brown 2001
- Brown KH, Wuehler SE, Peerson JM. The importance of zinc in human nutrition and estimation of the global prevalence of zinc deficiency. Food and Nutrition Bulletin 2001;22(2):113‐25. [Google Scholar]
Brown 2009
- Brown KH, Baker SK, IZiNCG Steering Committee (International Zinc Nutrition Consultative Group). Galvanizing action: conclusions and next steps for mainstreaming zinc interventions in public health programs. Food and Nutrition Bulletin 2009;30(1 Suppl 1):179‐84. [DOI] [PubMed] [Google Scholar]
De Barros 2016
- Barros SF, Cardoso MA. Adherence to and acceptability of home fortification with vitamins and minerals in children aged 6 to 23 months: a systematic review. BMC Public Health 2016;16:299. [PUBMED: 27056182] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Pee 2008
- Pee S, Kraemer K, Briel T, Boy E, Grasset C, Moench‐Pfanner R, et al. Quality criteria for micronutrient powder products: report of a meeting organized by the World Food Programme and Sprinkles Global Health Initiative. Food and Nutrition Bulletin 2008;29(3):232‐41. [DOI: 10.1177/156482650802900309; PUBMED: 18947036] [DOI] [PubMed] [Google Scholar]
De Pee 2013
- Pee S, Flores‐Ayala R, Jefferds ME, Irizarry L, Kraemer K, Monterrosa E, et al. editor(s). Home Fortification With Micronutrient Powders (MNP). Basel: Sight and Life on behalf of the Home Fortification Technical Advisory Group (HF‐TAG), 2013. [Google Scholar]
De‐Regil 2017
- De‐Regil LM, Jefferds MED, Peña‐Rosas JP. Point‐of‐use fortification of foods with micronutrient powders containing iron in children of preschool and school‐age. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD009666.pub2; PMC6486284; PUBMED: 29168569] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dewey 2003
- Dewey KG, Brown KH. Update on technical issues concerning complementary feeding of young children in developing countries and implications for intervention programs. Food and Nutrition Bulletin 2003;24(1):5‐28. [DOI: 10.1177/156482650302400102; PUBMED: 12664525] [DOI] [PubMed] [Google Scholar]
Dewey 2009
- Dewey KG, Adu‐Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Maternal and Child Nutrition 2008;4(Suppl 1):24‐85. [DOI: 10.1111/j.1740-8709.2007.00124.x; PUBMED: 18289157 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fawzi 1993
- Fawzi WW, Chalmers TC, Herrera MG, Mosteller F. Vitamin A supplementation and child mortality. A meta‐analysis. JAMA 1993;269(7):898‐903. [PUBMED: 8426449] [PubMed] [Google Scholar]
Galloway 1994
- Galloway R, McGuire J. Determinants of compliance with iron supplementation: supplies, side effects or psychology. Social Science and Medicine 1994;39:381‐90. [PUBMED: 7939855] [DOI] [PubMed] [Google Scholar]
Galloway 2002
- Galloway R, Dusch E, Elder L, Achadi E, Grajeda R, Hurtado E, et al. Women's perceptions of iron deficiency and anemia prevention and control in eight developing countries. Social Science and Medicine 2002;55(4):529‐44. [PUBMED: 12188461] [DOI] [PubMed] [Google Scholar]
Glasizou 1993
- Glasziou PP, Mackerras DE. Vitamin A supplementation in infectious diseases: a meta‐analysis. BMJ 1993;306(6874):366‐70. [PUBMED: 8461682 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 2 June 2019. Hamilton (ON): McMaster University (developed by Evidence Prime).
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC, (editors), on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2019
- Higgins JPT, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Horton 2008
- Horton S, Alderman H, Rivera JA. Copenhagen Consensus 2008. Challenge paper: hunger and malnutrition. www.copenhagenconsensus.com/Admin/Public/Download.aspx?file=Files%2fFiler%2fCC08%2fPapers%2fOfficial+papers%2fCopenhagen_Consensus_2008_‐_hunger_and_malnutrition.pdf (accessed prior to 6 September 2018).
Hyder 2007
- Hyder SMZ, Haseen F, Rahman M, Tondeur M, Zlotkin SH. Effect of daily versus once‐weekly home fortification with micronutrient Sprinkles on hemoglobin and iron status among young children in rural Bangladesh. Food Nutrition Bulletin 2007;28(2):156‐64. [DOI: 10.1177/156482650702800204; PUBMED: 24683674] [DOI] [PubMed] [Google Scholar]
Imdad 2017
- Imdad A, Mayo‐Wilson E, Herzer K, Bhutta ZA. Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD008524.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
INACG 1998
- International Nutritional Anemia Consultative Group (INACG). Guidelines for iron supplementation to prevent iron deficiency anemia. In: Stoltzfus RJ, Dreyfuss ML editor(s). Guidelines for the Use of Iron Supplements to Prevent and Treat Iron Deficiency Anemia. Washington DC: ILSI Press, 1998. [Google Scholar]
Islam 2018a
- Islam MM, McDonald CM, Krebs NF, Westcott J, Rahman AE, Arifeen S, et al. Study protocol for a randomized, double‐blind, community‐based efficacy trial of various doses of zinc in micronutrient powders or tablets in young Bangladeshi children. Nutrients 2018;10(2):132. [DOI: 10.3390/nu10020132; PMC5852708; PUBMED: 30720778] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jefferds 2013
- Jefferds ME, Irizarry L, Timmer A, Tripp K. UNICEF‐CDC 2011 Home Fortification Global Assessment: current status, new directions, and implications for policy and programmatic guidance. Food and Nutrition Bulletin 2013;34(4):434‐43. [DOI: 10.1177/156482651303400409; PUBMED: 24605694 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lobo 2019
- Lobo LMC, Schincaglia RM, Peixoto MDRG, Hadler MCCM. Multiple micronutrient powder reduces vitamin E deficiency in Brazilian children: a pragmatic, controlled clinical trial. Nutrients 2019;11(11):2730. [DOI: 10.3390/nu11112730; PMC6893530 ; PUBMED: 1717948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lozoff 2007
- Lozoff B. Iron deficiency and child development. Food and Nutrition Bulletin 2007;28(4 Suppl 4):S560‐71. [DOI] [PubMed] [Google Scholar]
Menon 2007
- Menon P, Ruel MT, Loechl CU, Arimond M, Habicht JP, Pelto G, et al. Micronutrient Sprinkles reduce anemia among 9‐ to 24‐mo‐old children when delivered through an integrated health and nutrition program in rural Haiti. Journal of Nutrition 2007;137(4):1023‐30. [DOI: 10.1093/jn/137.4.1023; PUBMED: 17374671] [DOI] [PubMed] [Google Scholar]
Micronutrient Initiative 2009
- Micronutrient Initiative, Flour Fortification Initiative, USAID, GAIN, WHO, The World Bank, UNICEF. Investing in the Future: A United Call to Action on Vitamin and Mineral Deficiencies: Global Report 2009. Ottawa, Canada: Micronutrient Initiative, 2009. [ISBN 978‐1‐894217‐31‐6] [Google Scholar]
Neuberger 2016
- Neuberger A, Okebe J, Yahav D, Paul M. Oral iron supplements for children in malaria‐endemic areas. Cochrane Database of Systematic Reviews 2016;2:CD006589. [PUBMED: 26921618] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oppenheimer 2001
- Oppenheimer SJ. Iron and its relation to immunity and infectious disease. Journal of Nutrition 2001;131(2 Suppl):616S‐635S. [DOI: 10.1093/jn/131.2.616S] [DOI] [PubMed] [Google Scholar]
Paganini 2016
- Paganini D, Uyoga MA, Zimmermann MB. Iron fortification of foods for infants and children in low‐income countries: effects on the gut microbiome, gut inflammation, and diarrhea. Nutrients 2016;8(8):E494. [DOI: 10.3390/nu8080494; PUBMED: 27529276] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paganini 2017
- Paganini D, Uyoga MA, Cercamondi CI, Moretti D, Mwasi E, Schwab C, et al. Consumption of galacto‐oligosaccharides increases iron absorption from a micronutrient powder containing ferrous fumarate and sodium iron EDTA: a stable‐isotope study in Kenyan infants. American Journal of Clinical Nutrition 2017;106(4):1020‐31. [DOI: 10.3945/ajcn.116.145060; NCT02666417; PUBMED: 28814396] [DOI] [PubMed] [Google Scholar]
PAHO 2001
- PAHO/WHO. Guiding Principles for Complementary Feeding of the Breastfed Child. Washington DC: Pan American Health Organization, 2001. [Google Scholar]
Raiten 2011
- Raiten DJ, Namasté S, Brabin B. Considerations for the safe and effective use of iron interventions in areas of malaria burden ‐ executive summary. International Journal for Vitamin and Nutrition Research 2011;81(1):57‐71. [DOI: 10.1024/0300-9831/a000051; PUBMED: 22002219] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salam 2013
- Salam R, MacPhail C, Das J, Bhutta Z. Effectiveness of micronutrient powders (MNP) in women and children. BMC Public Health 2013;13(Suppl 3):S22. [DOI: 10.1186/1471-2458-13-S3-S22] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanghvi 2007
- Sanghvi T, Ross J, Heymann H. Why is reducing vitamin and mineral deficiencies critical for development? The links between VMD and survival, health, education and productivity. Food and Nutrition Bulletin 2007;28(1 Suppl 2):S167‐S173. [PubMed] [Google Scholar]
Sazawal 2006
- Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community‐based, randomized, placebo‐controlled trial. Lancet 2006;367(9505):133‐43. [DOI: 10.1016/S0140-6736(06)67962-2; PUBMED: 16413877] [DOI] [PubMed] [Google Scholar]
Stephensen 2001
- Stephensen CB. Vitamin A, infection, and immune function. Annual Review of Nutrition 2001;21:167‐92. [DOI: 10.1146/annurev.nutr.21.1.167; PUBMED: 11375434 ] [DOI] [PubMed] [Google Scholar]
Stevens 2013
- Stevens GA, Finucane MM, De‐Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. Global, regional and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non‐pregnant women for 1995‐2011: a systematic analysis of population‐representative data. Lancet Global Health 2013;1:e16‐e25. [DOI: 10.1016/S2214-109X(13)70001-9; PUBMED: 25103581] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoltzfus 2011
- Stoltzfus RJ. Iron interventions for women and children in low‐income countries. Journal of Nutrition 2011;141(4):756S–762S. [DOI: 10.3945/jn.110.128793; PUBMED: 21367936] [DOI] [PubMed] [Google Scholar]
Suchdev 2013
- Suchdev PS, Neufeld LM. Micronutrient powders for young children. Lancet 2013;382(9899):1171. [DOI: 10.1016/S0140-6736(13)62051-6; PUBMED: 24095188] [DOI] [PubMed] [Google Scholar]
Suchdev 2016
- Suchdev PS, Addo OY, Martorell R, Grant FK, Ruth LJ, Patel MK, et al. Effects of community‐based sales of micronutrient powders on morbidity episodes in preschool children in Western Kenya. American Journal of Clinical Nutrition 2016;103(3):934‐41. [PUBMED: 26864367] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2011
- Sun J, Dai Y, Zhang S, Huang J, Yang Z, Huo J, et al. Implementation of a programme to market a complementary food supplement (Ying Yang Bao) and impacts on anaemia and feeding practices in Shanxi, China. Maternal & Child Nutrition 2011;7(Suppl 3):96‐111. [DOI: 10.1111/j.1740-8709.2011.00353.x; PUBMED: 21929638] [DOI] [PMC free article] [PubMed] [Google Scholar]
Timmer 2013
- Timmer A, Irizarry L, Tripp K, Flores‐Ayala R, Jefferds MED. UNICEF and CDC collaboration: leveraging regional workshops to support the scaling‐up of home fortification to improve the quality of complementary foods for young children 6‐23 months of age. Sight and Life 2013:42‐50.
UNICEF 2011
- UNICEF. The State of the World's Children 2011. Adolescence: An Age of Opportunity. New York: United Nations Children’s Fund, 2011. [ISBN: 978‐92‐806‐4555‐] [Google Scholar]
UNICEF 2014
- UNICEF. NutriDash: Global Report 2014. New York (NY): UNICEF, 2014. [www.ign.org/p142002958.html?from=0142002801] [Google Scholar]
UNICEF‐CDC 2013
- UNICEF‐CDC. Global Assessment of Home Fortification Interventions, 2011. Geneva: Home Fortification Technical Advisory Group, 2013. [DOI] [PMC free article] [PubMed]
Visser 2017
- Visser ME, Durao S, Sinclair D, Irlam JH, Siegfried N. Micronutrient supplementation in adults with HIV infection. Cochrane Database of Systematic Reviews 2017, Issue 5. [DOI: 10.1002/14651858.CD003650.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Viteri 1997
- Viteri FE. Iron supplementation for the control of iron deficiency in populations at risk. Nutrition Reviews 1997;55(6):195‐209. [PUBMED: 9279056] [DOI] [PubMed] [Google Scholar]
Wang 2013
- Wang B, Zhan S, Gong T, Lee L. Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD001444.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2005
- World Health Organization. Guiding Principles for Feeding Non‐Breastfed Children 6‐24 Months of Age. Geneva: World Health Organization, 2005. [ISBN: 9241593431] [Google Scholar]
WHO 2006
- World Health Organization. Workshop to review the results of studies evaluating the impact of zinc supplementation on childhood mortality and severe morbidity: conclusions and next steps; 2006 15‐16 Sept, Geneva, Switzerland. www.who.int/child_adolescent_health/documents/pdfs/zinc_mortality_workshop_2007.pdf (accessed prior to 6 September 2018).
WHO 2009
- World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva: World Health Organization, 2009. [ISBN 9789241563871] [Google Scholar]
WHO 2011
- World Health Organization. WHO Guideline: Use of Multiple Micronutrient Powders for Home Fortification of Foods Consumed by Infants and Children 6–23 Months of Age. Geneva: World Health Organization, 2011. [ISBN 9789241549943] [PubMed] [Google Scholar]
WHO 2016a
- World Health Organization. Daily Iron Supplementation in Infants and Children. Guideline. Geneva: World Health Organization, 2016. [ISBN: 9789241549523] [Google Scholar]
WHO 2016b
- WHO. WHO Guideline: Use of Multiple Micronutrient Powders for Point‐of‐Use Fortification of Foods Consumed by Infants and Young Children Aged 6–23 Months and Children Aged 2–12 Years. Geneva: WHO, 2016. [Licence: CC BY‐NC‐SA 3.0 IGO] [PubMed] [Google Scholar]
WHO 2018
- WHO. World Malaria Report 2018. Geneva: WHO, 2018. [Licence: CC BY‐NC‐SA 3.0 IGO] [Google Scholar]
WHO/UNICEF 2004
- World Health Organization/The United Nations Children's Fund. Clinical management of acute diarrhoea. apps.who.int/iris/bitstream/handle/10665/68627/WHO_FCH_CAH_04.7.pdf?sequence=1 (accessed prior to 07 September 2018). [UNICEF/PD/Diarrhoea/01; WHO/FCH/CAH/04.7 ]
WHO/WFP/UNICEF 2007
- World Health Organization, World Food Programme, The United Nations Children's Fund. Preventing and controlling micronutrient deficiencies in populations affected by an emergency. www.who.int/nutrition/publications/WHO_WFP_UNICEFstatement.pdf (accessed prior to 07 September 2018).
World Bank 2010
- World Bank, UNICEF, WHO, WFP. Scaling up nutrition: a framework for action.. Food and Nutrition Bulletin 2010;31(1):178‐86. [DOI] [PubMed] [Google Scholar]
Zlotkin 2004
- Zlotkin SH, Tondeur M. Specific strategies to address micronutrient deficiencies in the young child: supplementation and home fortification. In: Pettifor J, Zlotkin S editor(s). Micronutrient Deficiencies During the Weaning Period and the First Years of Life. Nestlé Nutrition Workshops Series Pediatric Program. Vol. 54, Basel: Nestec Ltd, 2004:233‐48. [Google Scholar]
References to other published versions of this review
De‐Regil 2011
- De‐Regil LM, Suchdev PS, Vist GE, Walleser S, Pena‐Rosas JP. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD008959] [DOI] [PubMed] [Google Scholar]
De‐Regil 2013
- De‐Regil LM, Suchdev PS, Vist GE, Walleser S, Pena‐Rosas JP. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age (Review). Evidence‐Based Child Health 2013;8(1):112‐201. [DOI: 10.1002/ebch.1895] [DOI] [PubMed] [Google Scholar]


