Abstract
Background
Recent studies have suggested an association between sleep apnea (SA) and atrial fibrillation (AF). We aimed to study the prevalence, characteristics, risk factors and type of sleep apnea (SA) in ablation candidates with paroxysmal AF.
Methods/Results
We prospectively studied 579 patients with paroxysmal AF, including 157 women (27.1%) and 422 men (72.9%). Mean age was 59.9 ± 9.6 years and mean body mass index (BMI) 28.5 ± 4.5 kg/m2. SA was diagnosed using polygraphy for two nights at home. The Epworth Sleepiness Scale (ESS), STOP-Bang Questionnaire, and Berlin Questionnaire (BQ) assessed the degree of SA symptoms. A total of 479 (82.7%) patients had an apnea-hypopnea index (AHI) ≥ 5, whereas moderate-severe SA (AHI ≥ 15) was diagnosed in 244 patients (42.1%). The type of SA was predominantly obstructive, with a median AHI of 12.1 (6.7–20.6) (range 0.4–85.8). The median central apnea index was 0.3 (0.1–0.7). AHI increased with age, BMI, waist and neck circumference, body and visceral fat. Using the Atrial Fibrillation Severity Scale and the SF-36, patients with more severe SA had a higher AF burden, severity and symptom score and a lower Physical-Component Summary score. Age, male gender, BMI, duration of AF, and habitual snoring were independent risk factors in multivariate analysis (AHI ≥ 15). We found no association between ESS and AHI (R2 = 0.003, p = 0.367).
Conclusions
In our AF population, SA was highly prevalent and predominantly obstructive. The high prevalence of SA detected in this study may indicate that SA is under-recognized in patients with AF. None of the screening questionnaires predicted SA reliably.
Keywords: Atrial fibrillation, CPAP, Prevalence, Sleep apnea
Abbreviations: AASM, American Academy of Sleep Medicine; ACE-I, Aangiotensin converting enzyme inhibitor; AF, Atrial fibrillation; AFSS, Atrial Fibrillation Severity Scale; AHI, Apnea-hypopnea index; ARB, Angiotensin receptor blocker; AUC, Area under the curve; BMI, Body mass index; BQ, Berlin Questionnaire; cAHI, Central apnea-hypopnea index; CI, Confidence interval; COPD, Chronic obstructive pulmonary disease; CPAP, Continuous positive airway pressure; CSA, Central sleep apnea; DC, Direct current; ESS, Epworth Sleepiness Scale; FEV1, Forced expiratory volume in 1 s; GERD, Gastroesophageal reflux disease; IQR, Interquartile range; NOAC, Novel oral anticoagulant; ODI, Oxygen desaturation index; OR, Odds ratio; OSA, Obstructive sleep apnea; PAF, Paroxysmal atrial fibrillation; PVI, Pulmonary vein isolation; SA, Sleep apnea; SD, Standard deviation; SF-36, Short form-36; TIA, Transient ischaemic attack
1. Introduction
Atrial fibrillation (AF) has an estimated prevalence of 2–3%, which increases with advancing age and is projected to increase 3-fold from the year 2000 to 2050 [1]. Five million new cases are diagnosed annually, making AF one of the cardiovascular epidemics of the 21st century [2]. With one-third of cases comprising silent AF, the total AF burden could be even higher [3], [4]. AF is associated with increased mortality, heart failure, dementia, and a 5-fold increased risk of stroke [5], [6], [7], [8], with tremendous clinical and socioeconomic implications [9]. To reduce the increasing AF burden worldwide, a multidisciplinary approach to AF prevention and treatment needs to be embraced [10]. Anti-arrhythmic drugs are widely used but have limited efficacy and a recognized potential for adverse events. Catheter ablation with pulmonary vein isolation continues to gain acceptance, but procedural complications are still an issue, and the recurrence rate is high with the need for continuous medication. Therefore, cardiometabolic factors, such as obesity, inactivity, and sleep apnea (SA), have gained interest. As early as 1983, Guilleminault et al. described a relationship between obstructive sleep apnea (OSA) and AF [11]. SA, which causes intermittent hypoxemia and hemodynamic changes, could have an unfavorable impact on cardiovascular physiology and is associated with AF, independent of age, gender, hypertension, heart failure, and body mass index (BMI). However, the pathway between SA and AF is complex and may be bidirectional [12], [13], [14]. The prevalence of SA in the general population has been reported to be 6–38% [15], [16], though it is much higher in older age groups [17] and significantly higher in patients with AF (21–62%) [18], [19], [20], [21], with great variation depending on age, sex, body habitus, and the definition of SA. Furthermore, the type of SA (OSA versus central sleep apnea (CSA)) is rarely reported. Thus, we aimed to classify both apneas and hypopneas into central or obstructive respiratory events and to prospectively evaluate the prevalence, characteristics, and risk factors for SA in patients with paroxysmal atrial fibrillation (PAF).
2. Methods
2.1. Study design and participants
This was a prospective cohort study of 579 consecutive patients with PAF aged 18–75 years conducted at two cardiology centers in Norway. A total of 472 patients were recruited from the catheter ablation waiting list, whereas 107 were from outpatient clinics. Patients who were previously diagnosed with SA, receiving OSA treatment, had persistent AF or no AF for the last three months were excluded.
Ethical approval was obtained from the local ethics committees, South-East Regional Ethics Committee (REK,ID: 2015/436) and the data inspector of Oslo University Hospital. The trial was conducted according to the 1975 Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent to participate in the study. The trial is registered on clinicaltrials.gov (NCT02727192).
2.2. Ambulatory sleep testing
Sleep recordings took place when the patients were admitted for evaluation for catheter ablation. Participants were equipped with a type 3 polygraphic recorder (T3, NOX Medical, Reykjavik, Iceland) for two consecutive nights of home sleep recording. Patients were provided with instructions on how to correctly attach the NOX monitor with sensors at a Clinical Trial Unit. Patients were given the opportunity to return the recording device in a prepaid envelope. The recorded signals included a nasal pressure transducer, respiratory inductance plethysmography, body position, oxygen saturation, and pulse rate. All sleep studies were analyzed as recommended by the American Academy of Sleep Medicine (AASM) [22]. The same experienced sleep specialist scored the recordings manually using Noxturnal software (version 5.1.0, Reykjavik, Iceland), unaware of the patient‘s identification. Apnea was defined as ≥90% decrease in airflow lasting 10 s or longer. Hypopnea was defined as ≥30% decrease in airflow lasting at least 10 s, followed by a ≥3% decrease in oxygen saturation.
Apneas were classified as obstructive if they were associated with continued or increased respiratory effort, central if there was an absence of respiratory effort, or mixed if there was an absence of respiratory effort in the initial portion, followed by resumption of the respiratory effort in the second portion. Hypopneas were classified as obstructive if snoring, increased inspiratory flattening of the nasal pressure or paradoxical thoracoabdominal movements were observed.
The apnea-hypopnea index (AHI) was defined as the number of apneas and hypopneas per hour of recording time. The AHI scores from the two nights of recording were averaged to give the final AHI severity classification.
We defined SA using usual clinical thresholds [22]: no SA, AHI <5; mild SA, AHI ≥5 to <15; moderate SA, AHI ≥15 to <30; severe SA; AHI ≥30.
2.3. Demographic and clinical characteristics
Demographic and clinical characteristics were obtained the same day as the sleep recording was performed. We measured waist and hip circumference, and neck circumference was measured just below the laryngeal prominence. Participants self-reported their smoking habits, alcohol use, and activity level. In addition, spirometry with measurement of forced expiratory volume in 1 s (FEV1) was obtained with the highest of three recordings selected.
All questionnaires used in this study were obtained electronically using the online platform ViedocTM (PCG Solutions, Uppsala, Sweden).
2.3.1. Bioelectrical impedance analysis
Body composition data were collected using a bioelectric impedance weight (Tanita BC-545N; Tanita Corp., Arlington Heights, IL) [23]. The recorded variables included BMI, body fat, total body water, muscle mass, visceral fat, bone mass, metabolic age, and basal metabolic rate. A mean of three measurements were made for each patient.
2.3.2. Epworth sleepiness scale
The degree of subjective daytime sleepiness was assessed by the Epworth Sleepiness Scale (ESS), with scores ranging from 0 to 24 (most sleepy). The eight-item questionnaire is well-validated [24] and asks the subjects to rate the likelihood of falling asleep in different situations. A score ≥11 is considered to represent excessive daytime sleepiness.
2.3.3. Berlin questionnaire
Symptoms of SA were measured using the Berlin Questionnaire (BQ) [25]. The BQ consists of three categories: snoring and cessation of breathing (5 questions), daytime sleepiness (3 questions), and history of hypertension and BMI (1 question). Positive scores in two or more categories suggest a high risk of OSA.
2.3.4. STOP-Bang questionnaire
The STOP-Bang questionnaire was first developed in 2008 to screen for OSA in preoperative clinics [26]. It consists of two domains: A self-assessment questionnaire evaluates four subjective (STOP: Snoring, Tiredness, Observed apnea and high blood Pressure) and four demographic items (Bang: BMI, Age, Neck circumference, Gender). A score of ≥3 suggest risk for OSA. The questionnaire was translated into Norwegian by a forward and backward translation procedure.
2.3.5. Atrial fibrillation severity Scale
The disease burden of AF was quantified by the validated Atrial Fibrillation Severity Scale (AFSS) [27]. The questionnaire quantifies three domains of AF: event frequency (score 1–10), duration (score 1–10), and global episode severity (score 1–10). The total AF burden is calculated from the modified sum of the frequency, duration, and severity of AF (score 3–30). In addition, the AFSS assesses a symptom score based on seven items asking how bothered patients are by specified symptoms (score 0–35). Global well-being is a visual analogue scale ranging from 1 to 10 (indicating the best possible life). The questionnaire was translated into Norwegian by a forward and backward translation procedure.
2.3.6. SF-36
Quality of life was assessed by the widely used Medical Outcomes Study 36-item Short-Form Health Survey (SF-36), version 1.2. [28], [29]. Item scores were transformed into the Physical and Mental Component Summary scores (PCS and MCS). Scores range from 0 to 100, with higher scores indicating a better health state.
2.4. Statistical analysis
Statistical analyses were performed using SPSS statistical software, version 25 (IBM Corp. Released 2017. Armonk, NY). Characteristics of the study population are presented as mean with standard deviation or median with interquartile range for continuous variables, and by counts with percentages for categorical variables. To compare two groups, the Student‘s t test or Mann Whitney U test was used for continuous variables and Pearson‘s chi-squared test or Fisher‘s exact test for categorical variables. To compare the four groups of SA severity and analyze linear trends, the Pearson correlation or Jonckheere-Terpstra test were used for continuous variables as appropriate, and linear-by-linear association chi-squared test or Fisher‘s exact test where appropriate for categorical variables. A two-tailed p-value <0.05 was considered significant. A binary logistic regression analysis was performed to assess independent factors of the presence of SA. The diagnosis of moderate-severe SA (AHI ≥ 15) and severe SA (AHI ≥ 30) was used as the dichotomous dependent variable. For variable selection in the univariate model, a p-value of 0.157 was used to enter the multivariate model [30]. Because of multicollinearity, body fat percentage, muscle mass, visceral fat, and neck circumference were excluded from the model. No multicollinearity was observed among the variables age, male gender, BMI, duration of AF, habitual snoring, and hypertension (variance inflation factor < 1.2). Results were presented as odd ratios (ORs) with 95% confidence intervals (CIs).
3. Results
3.1. Sample size and population characteristics
Between August 2015 and December 2018, 1093 patients with PAF were invited to undergo sleep polygraphy. A total of 597 of 740 (80.7%) eligible AF patients consented to participate in the study. Eighteen patients were non-compliant, changed their mind, or had technical problems resulting in insufficient data, leaving 579 recordings in the analysis. The baseline characteristics of the cohort are presented in Table 1. Among the patients included in the analysis, 472 (82%) had been referred for catheter ablation (120 women (25%) and 352 (75%) men) and 107 (18%) were outpatients. Patients referred for catheter ablation were younger (mean 59 ± 9.3 years) than outpatients (62 ± 10.5 years), with higher AFSS burden, duration, and severity scores, used more antiarrhythmic medications, and more often had previous direct current (DC) cardioversion (39% vs. 22%, p = 0.001). Among patients referred for ablation, the female patients were older than the male patients (61 ± 8.3 years vs. 59 ± 9.5 years, p = 0.018). Mean BMI, body fat%, visceral fat, and the median AHI and other respiratory characteristics were similar between the groups (Table A.1 in the supplementary appendix).
Table 1.
Selected baseline characteristics by sleep apnea severity.
| Characteristic | No of patients with data | Cohort = 579 | No SA (AHI < 5) N = 100 | Mild SA (AHI 5 to < 15) N = 235 | Moderate SA (AHI 15 to < 30) N = 174 | Severe SA (AHI ≥ 30) N = 70 | P-value |
|---|---|---|---|---|---|---|---|
| Men | 579 | 422 (72.9%) | 58 (58.0%) | 167 (71.1%) | 144 (82.8%) | 53 (75.7%) | <0.001 |
| Age, years | 579 | 59.9 (9.6) | 54.7 (11.9) | 59.5 (9.5) | 62.0 (7.7) | 63.7 (6.9) | <0.001 |
| BMI, kg/m2 | 579 | 28.5 (4.5) | 26.7 (3.9) | 28 (4.1) | 29.2 (4.4) | 31.3 (4.8) | <0.001 |
| Body fat % | 550 | 29.9 (8.3) | 28.8 (8.8) | 29.5 (8.2) | 29.8 (7.9) | 32.8 (8.3) | 0.005 |
| Muscle mass, kg | 550 | 60.1 (11.2) | 56.8 (12.1) | 59.5 (11.2) | 61.8 (10.6) | 62.3 (10.4) | <0.001 |
| Visceral fat score | 550 | 12.8 (4.0) | 10.1 (3.5) | 12.3 (3.7) | 14.1 (3.8) | 15.5 (3.8) | <0.001 |
| Neck circumference, cm | 569 | 38.9 (3.4) | 37.1 (3.2) | 38.6 (3.3) | 39.7 (3.3) | 40.5 (2.9) | <0.001 |
| Waist-to-hip ratio | 567 | 0.97 (0.08) | 0.93 (0.09) | 0.97 (0.08) | 0.99 (0.07) | 1.0 (0.07) | <0.001 |
| Higher education† | 540 | 295 (54.6%) | 57 (60.0%) | 112 (51.9%) | 86 (53.4%) | 40 (58.8%) | 0.899 |
| Married or cohabitant | 579 | 460 (79.4%) | 79 (79%) | 180 (76.6%) | 149 (85.6%) | 52 (74.3%) | 0.646 |
| In regular work | 572 | 337 (58.9%) | 64 (64.6%) | 141 (61.0%) | 99 (57.2%) | 33 (47.8%) | 0.762 |
| Alcohol units/week | 541 | 2.5 (1–5.5) | 2.5 (1–5) | 2.5 (0.5–5) | 2.5 (1–6) | 3 (1–6) | 0.348 |
| Current smoking | 560 | 37 (6.6%) | 7 (7.1%) | 15 (6.7%) | 14 (8.3%) | 1 (1.4%) | 0.246 |
| FEV1 % predicted | 517 | 99 (17.7) | 100.8 (16.5) | 99.2 (18.3) | 99.1 (17) | 95.7 (18.7) | 0.114 |
| Exercise 2–3 or more/week | 539 | 372 (69%) | 69 (72.6%) | 148 (68.5%) | 109 (68.1%) | 46 (67.6%) | 0.494 |
| Duration of AF, years | 543 | 4.8 (2–10) | 4.4 (1.5–10) | 4.8 (1.7–10) | 5 (2–10) | 5.3 (2.5–11) | 0.092 |
| Snoring | 565 | 408 (72.2%) | 55 (55.6%) | 151 (66.5%) | 146 (85.9%) | 56 (81.2%) | 0.056 |
| Observed apneas | 513 | 132 (25.7%) | 11 (12.1%) | 36 (17.6%) | 57 (37.3%) | 28 (43.8%) | <0.001 |
| Epworth Sleepiness Scale, score | 568 | 7.2 (4.0) | 6.9 (4.3) | 7.1 (4.0) | 7.5 (3.6) | 7.1 (4.2) | 0.367 |
| Berlin Questionnaire ≥ 2 | 565 | 240 (42.5%) | 25 (25.3%) | 83 (36.4%) | 92 (54.1%) | 40 (58.8%) | <0.001 |
| STOP-Bang Questionnaire ≥ 3 | 535 | 358 (66.9%) | 33 (35.9%) | 137 (62.8%) | 133 (83.1%) | 55 (84.6%) | <0.001 |
| Central apnea index | 579 | 0.8 (0.1–0.7) | 0.1 (0–0.3) | 0.2 (0.1–0.6) | 0.4 (0.1–0.9) | 0.8 (0.2–2.8) | <0.001 |
| Central AHI > obstructive AHI | 571 | 26 (4.6%) | 14 (14%) | 8 (3.5%) | 2 (1.2%) | 2 (2.9%) | <0.001 |
| SF-36§ | |||||||
| Physical Component Summary | 563 | 45.7 (9.4) | 47.8 (9.5) | 47.1 (8.6) | 44.1 (9.5) | 41.9 (9.5) | <0.001 |
| Mental Component Summary | 563 | 50.4 (9.1) | 51.2 (8.1) | 49.3 (10) | 51.4 (8.1) | 50.5 (9.7) | 0.594 |
| Medical history | |||||||
| Cardiovascular disease ‡ | 579 | 59 (10.2%) | 8 (8%) | 22 (9.4%) | 20 (11.5%) | 9 (12.9%) | 0.214 |
| Hypertension | 579 | 204 (35.2%) | 18 (18%) | 78 (33.2%) | 72 (41.4%) | 36 (51.4%) | <0.001 |
| Diabetes mellitus | 579 | 26 (4.5%) | 0 | 9 (3.8%) | 10 (5.7%) | 7 (10%) | 0.007 |
| Stroke/TIA | 579 | 25 (4.3%) | 1 (1%) | 14 (6%) | 8 (4.6%) | 2 (2.9%) | 0.205 |
| COPD/asthma | 579 | 60 (10.4%) | 12 (12%) | 25 (10.6%) | 15 (8.6%) | 8 (11.4%) | 0.633 |
| Depression | 578 | 41 (7.1%) | 6 (6%) | 19 (8.1%) | 13 (7.5%) | 3 (4.3%) | 0.772 |
| Anxiety | 578 | 38 (6.6%) | 2 (2%) | 21 (8.9%) | 10 (5.7%) | 5 (7.2%) | 0.103 |
| GERD | 577 | 135 (23.4%) | 21 (21%) | 43 (18.3%) | 49 (28.3%) | 22 (31.9%) | 0.014 |
| Family history of AF | 563 | 224 (40%) | 44 (50%) | 93 (41%) | 66 (40%) | 21 (30.4%) | 0.082 |
| Previous PVI | 579 | 63 (10.9%) | 10 (10%) | 29 (12.3%) | 16 (9.2%) | 8 (11.4%) | 0.850 |
| Previous DC cardioversion | 562 | 203 (36%) | 26 (26.3%) | 81 (35.5%) | 71 (42.3%) | 25 (37.3%) | 0.036 |
| Atrial Fibrillation Severity Scale | |||||||
| AF burden (3–30) | 557 | 15.7 (4) | 14.9 (4.3) | 15.5 (3.8) | 16 (4.1) | 16.5 (3.6) | 0.004 |
| Frequency (1–10) | 558 | 5.5 (2.4) | 5.4 (2.4) | 5.6 (2.3) | 5.3 (2.5) | 5.7 (2.5) | 0.978 |
| Duration (1–10) | 551 | 5.5 (2.4) | 5.4 (2.5) | 5.3 (2.4) | 5.6 (2.5) | 5.7 (2.3) | 0.287 |
| Severity (1–10) | 557 | 4.8 (2.3) | 4.2 (2.1) | 4.7 (2.3) | 5.2 (2.3) | 5.1 (2) | <0.001 |
| AF symptom score (0–35) | 564 | 10 (6) | 8.5 (5.8) | 10.2 (5.8) | 10 (6.1) | 11.6 (5.9) | 0.005 |
| Global well-being (0–10) | 560 | 6.9 (1.7) | 6.9 (1.7) | 7 (1.7) | 6.8 (1.6) | 6.9 (1.8) | 0.623 |
| Medication | |||||||
| Beta-blocker | 579 | 294 (50.8%) | 41 (41%) | 111 (47.2%) | 103 (59.2%) | 39 (55.7%) | 0.004 |
| ACE-I + ARB | 579 | 171 (29.5%) | 15 (15%) | 65 (27.7%) | 62 (35.6%) | 29 (41.4%) | <0.001 |
| Calcium channel blocker | 579 | 79 (13.6%) | 8 (8%) | 29 (12.3%) | 30 (17.2%) | 12 (17.1%) | 0.025 |
| Diuretic | 579 | 65 (11.2%) | 2 (2%) | 24 (10.2%) | 25 (14.4%) | 14 (20%) | <0.001 |
| Statin | 579 | 156 (26.9%) | 15 (15%) | 59 (25.1%) | 59 (33.9%) | 23 (32.9%) | 0.001 |
| NOACs | 579 | 349 (60.3%) | 43 (43%) | 138 (58.7%) | 122 (70.1%) | 46 (65.7%) | <0.001 |
| Antidiabetic medication | 579 | 16 (2.8%) | 0 | 4 (1.7%) | 8 (4.6%) | 4 (5.7%) | 0.025 |
| Flecainide | 579 | 170 (29.4%) | 35 (35%) | 63 (26.8%) | 54 (31%) | 18 (25.7%) | 0.430 |
| Dronedarone | 579 | 81 (14%) | 13 (13%) | 30 (12.8%) | 31 (17.8%) | 7 (10%) | 0.785 |
| Amiodarone | 579 | 21 (3.6%) | 2 (2%) | 11 (4.7%) | 6 (3.4%) | 2 (2.9%) | 0.734 |
| Sleeping pills | 579 | 18 (3.1%) | 3 (3%) | 11 (4.7%) | 2 (1.1%) | 2 (2.9%) | 0.223 |
| No use of medication | 579 | 45 (7.8%) | 16 (16%) | 21 (8.9%) | 4 (2.3%) | 4 (5.7%) | <0.001 |
| Antidepressants | 579 | 14 (2.4%) | 2 (2%) | 2 (0.9%) | 9 (5.2%) | 1 (1.4%) | 0.041 |
Data are presented as mean (standard deviation), median (interquartile range), or n (%). Abbreviations: ACE-I = angiotensin converting enzyme inhibitor, AF = atrial fibrillation, AFSS = Atrial Fibrillation Severity Scale, AHI = apnea-hypopnea index ,ARB = angiotensin receptor blocker, BMI = body mass index, COPD = chronic obstructive pulmonary disease, DC = direct current, FEV1 = forced expiratory volume in 1 s, GERD = gastroesophageal reflux disease, NOAC = novel oral anticoagulant, PVI = pulmonary vein isolation, TIA = transient ischemic attack. ¶ Visceral fat score from 1 to 59, with healthy range from 1 to 12. † College or university degree. § SF-36 = Scores on the Medical Outcomes Study Short Form-36, score range from 0 to 100, with higher scores indicating better quality of life, ‡ Cardiovascular disease includes myocardial infarction, angina, coronary bypass surgery, angioplasty, and pacemaker insertion.
3.2. Prevalence and severity of SA
The median AHI was 12.1 (6.7–20.6) (range 0.4–85.8), which was the average of recording 1 and recording 2. Pearson‘s correlation coefficient between night 1 and night 2 was high, r = 0.875, p < 0.001. A total of 479 patients (82.7%) had an AHI ≥ 5, 244 (42.1%) had an AHI ≥ 15, and 70 (12.1%) had an AHI ≥ 30. The type of SA was predominantly obstructive. Fifty-one percent of the respiratory events were hypopneas, followed by obstructive apneas (39.2%), central apneas (5.2%), and mixed apneas (4.6%). Eighty-eight percent of all hypopneas were obstructive. The median central AHI (cAHI) was 1 (0.4–2.2) (range 0–33.2). None of the central apneas were characterized as cheyne-stokes respiration.
The clinical characteristics according to SA severity are shown in Table 1. Patients with moderate and severe SA were older, more likely male, and had higher BMI, waist-to-hip ratio, and neck circumference, higher body fat and muscle mass, and were more likely to snore. There was a significant difference (p < 0.001) in the median AHI between male and female patients (13.4 (7.4–22) vs. 9.4 (4.5–17)). However, in the group of severe SA, women had a higher median AHI than men, though the difference was not significant.
Only 4.6% of the participants had more central respiratory events than obstructive respiratory events, and they were more often in the group of no/mild SA (Table 1). We found no differences between gender and BMI, but mean age was lower (54.5 ± 11.2 years vs. 60.1 ± 9.5, p = 0.004) in the group of predominantly central respiratory. Mean duration of apnea and hypopnea was also significantly lower in the group of predominantly central respiratory events. A comparison between gender and respiratory characteristics is shown in Table 2.
Table 2.
Respiratory characteristics.
| Characteristic | Total n = 579 | Male N = 422 | Female N = 157 | P-value |
|---|---|---|---|---|
| Age, years | 59.9 (9.6) | 59 (9.9) | 62 (8.2) | 0.001 |
| BMI, kg/m2 | 28.5 (4.5) | 28.5 (4.1) | 28.7 (5.4) | 0.712 |
| Snoring, n = 565 | 408 (72.2) | 314 (76.4) | 94 (61) | <0.001 |
| Observed apneas during sleep, n = 513 | 132 (25.7) | 117 (31.5) | 15 (10.6) | <0.001 |
| AHI | ||||
| Mean (SD) | 15.3 (12.1) | 16.1 (11.6) | 13.3 (13.2) | |
| Median (IQR) | 12.1 (6.7–20.6) | 13.4 (7.4–22) | 9.4 (4.5–17) | <0.001 |
| AHI supine | ||||
| Mean (SD) | 26.1 (19.9) | 28.5 (20.3) | 19.5 (17.2) | |
| Median (IQR) | 21.6 (10.6–36.6) | 24.8 (12.4–39) | 15.8 (7.8–24) | <0.001 |
| ODI | ||||
| Mean (SD) | 15.9 (11.9) | 16.6 (11.3) | 14.1 (13.3) | |
| Median (IQR) | 12.7 (7.3–21) | 14.2 (8–23) | 10.3 (5.6–17) | <0.001 |
| Obstructive apnea hypopnea index | ||||
| Mean (SD) | 12.9 (10.7) | 13.2 (9.8) | 11.9 (12.6) | |
| Median (IQR) | 10.3 (5.1–17.5) | 11.4 (5.7–18.1) | 8.2 (3.8–14.9) | 0.003 |
| Central apnea hypopnea index | ||||
| Mean (SD) | 1.8 (2.7) | 2 (2.9) | 1.1 (1.7) | |
| Median (IQR) | 1 (0.4–2.2) | 1.2 (0.5–2.5) | 0.5 (0.2–1.2) | <0.001 |
| Mixed apnea index | ||||
| Mean (SD) | 0.7 (1.7) | 0.8 (1.9) | 0.3 (1) | |
| Median (IQR) | 0.1 (0–0.5) | 0.2 (0–0.6) | 0.1 (0–0.2) | <0.001 |
| Oxygen saturation % | ||||
| Mean (SD) | 93.1 (1.8) | 93.1 (1.7) | 93.3 (1.9) | 0.361 |
| Minimum (SD) | 84.2 (5.8) | 84.3 (7) | 84 (7) | 0.628 |
| SaO2 time < 90%, % of time | 1.4 (0.1–5.6) | 1.5 (0.1–5.8) | 1.3 (0.1–5.1) | 0.201 |
Data are presented as mean (standard deviation (SD) or median (interquartile range (IQR)) unless otherwise stated. Abbreviations: AHI = apnea-hypopnea index, BMI = body mass index, ODI = oxygen desaturation index, SaO2 = Oxygen saturation.
3.3. Risk factors for OSA in patients with PAF
A binary logistic regression analysis was performed to assess independent factors for the presence of OSA. Univariate analysis of AHI ≥ 15 identified significant associations between SA and BMI, age, hypertension, habitual snoring, and male gender (Table A.2 in the supplementary appendix). In univariate analysis with AHI ≥ 30 as the dependent variable, male gender was no longer significant (Table A.3 in the supplementary appendix). In multivariate analysis, age, BMI, male gender, duration of AF, and habitual snoring remained significantly associated with SA for dichotomous AHI ≥ 15, with the largest OR for male gender (2.97, 95% CI 1.71–5.15, p < 0.001). For AHI ≥ 30, only age, BMI, and habitual snoring remained significant (Table 3).
Table 3.
Multivariable analysis of the association with moderate-severe and severe sleep apnea.
| AHI ≥ 15 as dependent variable | OR | Wald | 95% CI | P-value |
|---|---|---|---|---|
| BMI, kg/m2 | 1.14 | 19.37 | 1.07–1.20 | <0.001 |
| Male gender | 2.97 | 14.85 | 1.71–5.15 | <0.001 |
| Age, years | 1.06 | 16.38 | 1.03–1.09 | <0.001 |
| Duration of AF, years | 1.04 | 4.15 | 1.00–1.07 | 0.042 |
| Habitual snoring | 2.00 | 9.52 | 1.29–3.11 | 0.002 |
| Hypertension | 1.45 | 1.45 | 0.90–2.35 | 0.126 |
| AHI ≥ 30 as dependent variable | ||||
| BMI, kg/m2 | 1.15 | 12.86 | 1.06–1.24 | <0.001 |
| Male gender | 1.43 | 0.88 | 0.68–3.03 | 0.348 |
| Age, years | 1.05 | 6.26 | 1.01–1.10 | 0.012 |
| Duration of AF, years | 1.04 | 3.26 | 1.00–1.07 | 0.071 |
| Habitual snoring | 2.31 | 7.10 | 1.25–4.26 | 0.008 |
| Hypertension | 1.34 | 0.80 | 0.70–2.56 | 0.370 |
AHI ≥ 15 and AHI ≥ 30 were dichotomous dependent variables. AHI = apnea-hypopnea index, BMI = body mass index.
OR = adjusted odds ratio, CI = confidence interval, Habitual snoring = snoring every night or almost every night of the week.
3.4. Atrial fibrillation severity Scale
Patients with more severe SA had higher AF burden, severity, and symptom scores. The AFSS characteristics according to SA severity are presented in Table 1. AFSS symptom score had a weak, but significant correlation with ESS, Pearson‘s correlation coefficient r = 0.179, p < 0.001. The correlation was r = 0.212, p = 0.001 in patients with moderate-severe SA (AHI ≥ 15). The correlation was no longer significant in patients with severe SA. However, the correlation was higher when asking: “Do you feel tired, fatigued, or sleepy during daytime” from the STOP-Bang questionnaire (Pearson‘s correlation r = -0.361, p < 0.001). Women had a higher AFSS symptom score (11.5 ± 5.8) than men (9.5 ± 6, p < 0.001), and a higher AFSS episode duration score (5 ± 1.9 vs. 4.3 ± 1.9, p < 0.001). However, men had a higher AFSS frequency score (5.7 ± 2.4 vs. 5 ± 2.3, p = 0.003) and global well-being score (7 ± 1.7 vs. 6.7 ± 1.7, p = 0.041) than women. Previous cardioversion was more frequent in men (n = 164, 40%) than women (n = 39, 25%; p = 0.001).
3.5. Epworth sleepiness Scale
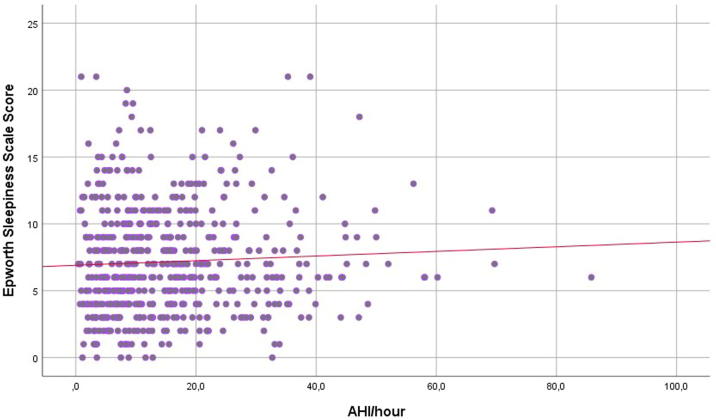
The mean ESS score was 7.2 ± 4. Excessive daytime sleepiness (ESS ≥ 11) was present in 19% of all participants and did not differ by SA severity (p = 0.989) or between males and females. 20.2%, 17.5%, 20.9% and 17.4% of patients diagnosed with no, mild, moderate and severe SA had ESS ≥ 11, respectively. Men and patients aged < 65 years had numerically higher scores than women and patients aged > 65 years, but the differences were not significant. We found no association between SA severity and daytime sleepiness (R2 = 0.003, p = 0.367; Fig. 1).
Fig. 1.
Scatterplot of the Epworth Sleepiness Scale Score and apnea-hypopnea index (AHI).
3.6. Berlin questionnaire
When using the BQ alone to diagnose SA, 38% of patients with AHI ≥ 5 were classified as “high risk” for OSA, compared to 23% in the group with AHI ≥ 15.
A BQ score ≥ 2 performed with a sensitivity of 46% and 56% and specificity of 75% and 70% for AHI ≥ 5 and ≥ 15, respectively. The area under the curve (AUC) was 0.61 for both cut-offs. The positive predictive value (PPV) was 90% and 55%, and negative predictive value (NPV) 23% and 67%, respectively.
3.7. STOP-Bang questionnaire
When using the STOP-Bang questionnaire to screen for OSA, 60.7% of patients with AHI ≥ 5 had a positive score ≥ 3, compared to 35.1% in the group with AHI ≥ 15. Mean score was 3.33 ± 1.5. A STOP-Bang score ≥ 3 performed with a sensitivity of 74% and 84% and specificity of 65% and 45%, respectively.The AUC was 0.71 and 0.68. The PPV was 91% and 53%, and the NPV was 34% and 79%, respectively.
3.8. SF-36
The mean PCS and MCS score was 45.7 (9.4) and 50.4 (9.1), respectively. With increasing SA severity, the PCS score decreased significantly (Table 1). Women had a lower mean PCS score (42.7 (9.5)) than men (46.8 (9)), p < 0.001. The MCS score was also lower among women than men (49 (9.6) vs 51 (8.9), p = 0.031.
4. Discussion
The main findings of this study were that (1) SA was highly prevalent in our patient cohort with AF; (2) the type of SA was mainly obstructive; (3) age, BMI, male gender, duration of AF, and habitual snoring are independent risk factors for moderate-severe SA based on the multivariate analysis; (4) female patients had more AF-related complaints according to the AFSS and lower quality of life according to the SF-36; (5) neither the ESS nor the BQ and STOP-Bang discriminated well between patients with or without SA.
In this AF population, we found a significantly high correlation between the two nights of recording, and therefore no first-night effect. These findings are consistent with a previously Canadian study in 100 patients [31], but is at variance with another study that found a considerable night-to-night variability in SA severity assessed by a long term pacemaker-based SA monitoring (MicroPort) algorithm, suggesting that SA severity on a specific night relates to AF risk during the same day [32]. Whether one or multiple nights of sleep recording is sufficient in patients with AF, needs to be evaluated in further studies.
We found a higher prevalence of SA (82.7% with AHI ≥ 5 and 42.1% with AHI ≥ 15) than in the general population (6–38%) [15], [16], which in Norway is estimated to be 16% and 8% for AHI ≥ 5 and ≥ 15, respectively [33]. Previous studies have also reported a higher prevalence of SA in patients with AF, but the variation is high, from 21 to 62% [18], [19], [20], [21]. This is probably due to different study populations and changes in measurement techniques and definitions for classifying respiratory events, which have changed over time. The prevalence is also highly dependent on the device used (in-hospital polysomnography vs. portable polygraphy) and on technical factors, with nasal pressure sensors detecting more hypopneas and, therefore, a higher AHI than thermistors [34]. Furthermore, the 2012 AASM criteria [22] propose a more liberal definition of hypopneas compared to the definitions used earlier [17].
A recent study of 197 patients with AF in Japan reported that 53.8% and 15.7% had an AHI ≥ 15 and ≥ 30, respectively [20]. Another recent study from Canada using polysomnography, which included 100 AF patients [35], reported a prevalence of OSA of 85% for AHI ≥ 5, which is similar to our results. The average BMI was lower in the Japanese study (25 kg/m2). However, the higher prevalence in the Japanese study may be due to the higher proportion of men (95%) included and predictors other than BMI accounting for the presence of SA.
Nearly three-quarters of the study participants were male, with 3-times as many males than females referred for ablation, despite female patients having more AF-related complaints according to the AFSS and a lower SF-36 Physical and Mental Summary score. The prevalence of AF is greater among men than women and increases with age. As women have a longer life span than men, the absolute number of males and females with AF is about equal in the general population [36]. Previous studies have reported that women have been under-referred for catheter ablation [37], [38], but the reason why is not completely understood. In our study, the lower number of female referrals could be due to our limitation of patients up to 75 years old and the lower acceptance rate for catheter ablation in older people. A previous Norwegian study reported that 67% of patients with “lone AF” were men [39], which is quite similar to our male:female ratio in patients with PAF.
The type of SA may be important when choosing whether to treat SA or not [40], but has usually not been reported in previous studies dealing with the prevalence of SA in AF. Only 4.6% of all participants in our study had predominantly central respiratory events, despite reports of an increased incidence of AF in patients with CSA and normal left ventricular function [41], [42]. May et al. found that CSA was associated with incident AF in older men (age ≥ 76 years). We found that men had significantly higher cAHI scores than women, but we found no significant differences in cAHI between different age groups, not even in the older group with age ≥ 70 years. However, our study was limited to patients aged 18–75 years. Surprisingly, we found that patients with predominantly central respiratory events were significantly younger than those with predominantly obstructive respiratory events, and had significantly lower duration of apneas and hypopneas. Similar to the results of the SNOoze-AF study [43] patients with predominant central events were more often in the group of no or mild SA, p < 0.001. However, the low number of patients (n = 26) with predominant central respiratory events makes the results less certain, but is worthy of further study.
Similar to other studies, we found that age, BMI, and male gender were independent risk factors for SA with dichotomous AHI ≥ 15 [19], [35]. In addition, we found that habitual snoring and duration of AF were independent risk factors. This and previous studies suggest that the relationship between SA and AF may be bidirectional [12], [13], [14]. However, for dichotomous AHI ≥ 30, male gender and duration of AF were no longer risk factors. This could be explained by the smaller number of patients (n = 70) with severe SA or due to the possible heterogeneity of pathogenesis in patients with severe SA.
One of the symptoms of SA is increased sleepiness, but in the present study we found no correlation between sleepiness as assessed by the ESS and AHI. Even patients with the most severe SA, with hypopneas lasting up to 112 s, did not complain of daytime sleepiness. This and other data [43], [44], [45] suggest that excessive daytime sleepiness measured by the ESS cannot be relied on as a marker of SA in AF patients, and that we cannot exclude the possibility of SA in patients without excessive daytime sleepiness. The reason why remains speculative, but could be due to the increased sympathetic tone in patients with AF [46].
Excessive daytime sleepiness is an important symptom of SA that can impair quality of life. However, only 20.9% and 17.4% of patients diagnosed with moderate and severe SA had ESS ≥ 11. In patients with mild SA the presence of excessive daytime sleepiness can be a determinant factor starting SA treatment or not. 40 (17.5%) patients with mild SA had excessive daytime sleepiness. 244 patients had an AHI ≥ 15 and would likely be considered for SA treatment even without excessive daytime sleepiness. The clinical implication of this is that 284 of 579 (49.1%) patients in our cohort could have an indication for SA treatment. Neither the ESS, the STOP-Bang nor the BQ questionnaire discriminated well between patients with or without SA. The BQ alone predicted a lower prevalence of moderate-severe SA compared to polygraphy and performed with a sensitivity of 56% and specificity of 70%. The landmark study by Gami et al. showed better performance in a subgroup of patients with AF (86% sensitivity and 89% specificity) [21].
Still, current guidelines from the European Society of Cardiology recommend screening for symptoms of SA in patients with AF, and to consider SA therapy to improve AF [47]. However, the patient selection for SA screening is challenging without any questionnaires detecting SA more accurately [43], [48]. In the future we need to clarify at which AHI-threshold SA should be treated in AF patients. AHI might not reflect characteristics of SA like hypoxemic burden and long-term night-to-night SA variability [14], [32]. There is a need for more appropriate metrics to assess SA severity, and a better understanding of the potential bidirectional relationship between AF and SA. In the absence of large randomized controlled trials it still remains unclear, whether CPAP treatment of comorbid SA benefits patients with AF.
4.1. Study strengths and limitations
A major strength of this study was the relatively large sample size. In addition, all polygraphic recordings were done in the patient‘s home environment and two nights of recordings were averaged. We found a high correlation between the two nights and, therefore, no first night effect. In addition, the same sleep physiologist manually scored all recordings, and both apneas and hypopneas were classified into central or obstructive respiratory events. Finally, modern recording techniques with nasal pressure sensors and scoring criteria were used.
However, this study also has several limitations. First, the individuals included in this study were almost exclusively of white European origin (n = 576, 99.5%), which makes the generalization to people from other ethnicities limited. Only 27% of the participants in our study were female, probably due to the 3-times higher referral for ablation among men.
Most of the patients with AF were scheduled for catheter ablation and differed from the outpatients; they were slightly younger, more often had previous DC conversions, and had a higher subjective symptom burden. However, their AHI and other respiratory characteristics were similar.
Our study group was enrolled from among respondents to a letter, 20.5% of which were not answered. This raises the possibility that our group could be biased towards those with symptoms of SA. Furthermore, both the AFSS and the STOP-Bang questionnaire were rigorously translated into Norwegian for use in this study, but the validity and reliability of the Norwegian version has not been fully evaluated. However, both questionnaires had previously been translated into Swedish, which is quite similar to Norwegian. Finally, all sleep recordings in this study were done by polygraphy and did not include an EEG. Polysomnography remains the gold-standard for diagnosis and would have increased the reliability of the scoring. Nonetheless, previous studies have suggested that polygraphy also provides reasonably accurate results [49].
5. Conclusions
SA was highly prevalent in this AF population and our results indicate that SA may be under-recognized in these patients. The type of SA was predominantly OSA. In multivariate analysis, age, male gender, BMI, duration of AF, and habitual snoring were significantly associated with moderate-severe SA. None of the screeningtools used in this study discriminated well between patients with or without OSA. Whether treatment of OSA in patients with PAF reduces the AF burden is worth further study.
6. Role of the funding sources
Oslo University Hospital, University of Oslo and the Norwegian Health Association funded this study. ResMed Norway provided the home sleep devices and the ResMed Science Center an unrestricted grant. The funders of the study had no involvement in the study design, data collection, data analysis, data interpretation, or writing of this article. The corresponding author had full access to all data in the study. A Steering Committee was responsible for the clinical and scientific conduct of the study and publication of the results. Members of the steering committee are listed in Appendix B.1.
Declaration of Competing Interest
Gunn Marit Traaen has received speaker honoraria from ResMed, Norway. The authors declare no other potential conflicts of interest.
Acknowledgements
All authors of this manuscript have certified that they comply with the principles of ethical publishing in the International Journal of Cardiology, Heart & Vasculature [50]. The authors thank Nina Bredesen, Line Hansen, Karin Ausen, Merete Gulbrandsen Nordstad, Tobias Erik Herrscher, Per Anton Sirnes, Even Holt, Liv Sofie Vartdal and Hanne Berdal for patient recruitment. We thank Ragnhild Falk for statistical advice. We appreciate the permission to use the AFSS Questionnaire from Paul Dorian, University of Toronto, Ontario, Canada.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2019.100447.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Chugh S.S., Havmoeller R., Narayanan K., Singh D., Rienstra M., Benjamin E.J. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyasaka Y., Barnes M.E., Gersh B.J., Cha S.S., Bailey K.R., Abhayaratna W.P. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 3.Camm A.J., Corbucci G., Padeletti L. Usefulness of continuous electrocardiographic monitoring for atrial fibrillation. Am J Cardiol. 2012;110:270–276. doi: 10.1016/j.amjcard.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Healey J.S., Connolly S.J., Gold M.R., Israel C.W., Van Gelder I.C., Capucci A. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 5.Wolf P.A., Abbott R.D., Kannel W.B. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 6.Wang T.J., Larson M.G., Levy D., Vasan R.S., Leip E.P., Wolf P.A. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin E.J., Wolf P.A., D'Agostino R.B., Silbershatz H., Kannel W.B., Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 8.Miyasaka Y., Barnes M.E., Petersen R.C., Cha S.S., Bailey K.R., Gersh B.J. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a community-based cohort. Eur Heart J. 2007;28:1962–1967. doi: 10.1093/eurheartj/ehm012. [DOI] [PubMed] [Google Scholar]
- 9.Wattigney W.A., Mensah G.A., Croft J.B. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation. 2003;108:711–716. doi: 10.1161/01.CIR.0000083722.42033.0A. [DOI] [PubMed] [Google Scholar]
- 10.Du X., Dong J., Ma C. Is Atrial Fibrillation a Preventable Disease? J Am Coll Cardiol. 2017;69:1968–1982. doi: 10.1016/j.jacc.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Guilleminault C., Connolly S.J., Winkle R.A. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52:490–494. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 12.Gami A.S., Hodge D.O., Herges R.M., Olson E.J., Nykodym J., Kara T. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 13.Naruse Y., Tada H., Satoh M., Yanagihara M., Tsuneoka H., Hirata Y. Radiofrequency catheter ablation of persistent atrial fibrillation decreases a sleep-disordered breathing parameter during a short follow-up period. Circ J. 2012;76:2096–2103. doi: 10.1253/circj.cj-12-0014. [DOI] [PubMed] [Google Scholar]
- 14.Linz D., Baumert M., Catcheside P., Floras J., Sanders P., Levy P. Assessment and interpretation of sleep disordered breathing severity in cardiology: Clinical implications and perspectives. Int J Cardiol. 2018;271:281–288. doi: 10.1016/j.ijcard.2018.04.076. [DOI] [PubMed] [Google Scholar]
- 15.Senaratna C.V., Perret J.L., Lodge C.J., Lowe A.J., Campbell B.E., Matheson M.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Benjafield A.V., Ayas N.T., Eastwood P.R., Heinzer R., Ip M.S.M., Morrell M.J. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7:687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinzer R., Vat S., Marques-Vidal P., Marti-Soler H., Andries D., Tobback N. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel D., Mohanty P., Di Biase L., Shaheen M., Lewis W.R., Quan K. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol. 2010;3:445–451. doi: 10.1161/CIRCEP.109.858381. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson I.H., Teichtahl H., Cunnington D., Ciavarella S., Gordon I., Kalman J.M. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J. 2008;29:1662–1669. doi: 10.1093/eurheartj/ehn214. [DOI] [PubMed] [Google Scholar]
- 20.Kohno T., Kimura T., Fukunaga K., Yamasawa W., Fujisawa T., Fukuoka R. Prevalence and clinical characteristics of obstructive- and central-dominant sleep apnea in candidates of catheter ablation for atrial fibrillation in Japan. Int J Cardiol. 2018;260:99–102. doi: 10.1016/j.ijcard.2018.01.103. [DOI] [PubMed] [Google Scholar]
- 21.Gami A.S., Pressman G., Caples S.M., Kanagala R., Gard J.J., Davison D.E. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–367. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 22.Berry R., Brooks R., Gamaldo C., Harding S., Marcus C., Vaughn B. The AASM manual for the scoring of sleep and associated events: Rules, Terminology and Technical Specifications. Version 2.0. Darien, Illinois: Amer. Acad. Sleep Med. 2012 [Google Scholar]
- 23.Jaffrin M.Y. Body composition determination by bioimpedance: an update. Curr Opin Clin Nutr Metab Care. 2009;12:482–486. doi: 10.1097/MCO.0b013e32832da22c. [DOI] [PubMed] [Google Scholar]
- 24.Beiske K.K., Kjelsberg F.N., Ruud E.A., Stavem K. Reliability and validity of a Norwegian version of the Epworth sleepiness scale. Sleep Breath. 2009;13:65–72. doi: 10.1007/s11325-008-0202-x. [DOI] [PubMed] [Google Scholar]
- 25.Netzer N.C., Stoohs R.A., Netzer C.M., Clark K., Strohl K.P. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 26.Chung F., Yegneswaran B., Liao P., Chung S.A., Vairavanathan S., Islam S. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–821. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 27.Dorian P., Jung W., Newman D., Paquette M., Wood K., Ayers G.M. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J. Am. Coll. Cardiol. 2000;36:1303–1309. doi: 10.1016/s0735-1097(00)00886-x. [DOI] [PubMed] [Google Scholar]
- 28.Garratt A.M., Stavem K. Measurement properties and normative data for the Norwegian SF-36: results from a general population survey. Health Qual Life Outcomes. 2017;15:51. doi: 10.1186/s12955-017-0625-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ware J.E., Jr., Kosinski M., Gandek B., Aaronson N.K., Apolone G., Bech P. The factor structure of the SF-36 Health Survey in 10 countries: results from the IQOLA Project. International Quality of Life Assessment. J. Clin. Epidemiol. 1998;51:1159–1165. doi: 10.1016/s0895-4356(98)00107-3. [DOI] [PubMed] [Google Scholar]
- 30.Heinze G., Wallisch C., Dunkler D. Variable selection - A review and recommendations for the practicing statistician. Biom. J. 2018;60:431–449. doi: 10.1002/bimj.201700067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abumuamar A.M., Dorian P., Newman D., Shapiro C.M. A Comparison of two nights of ambulatory sleep testing in arrhythmia patients. Sleep Disord. 2018;2018:2394146. doi: 10.1155/2018/2394146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linz D., Brooks A.G., Elliott A.D., Nalliah C.J., Hendriks J.M.L., Middeldorp M.E. Variability of sleep apnea severity and risk of atrial fibrillation: The VARIOSA-AF study. JACC Clin. Electrophysiol. 2019;5:692–701. doi: 10.1016/j.jacep.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Hrubos-Strom H., Randby A., Namtvedt S.K., Kristiansen H.A., Einvik G., Benth J. A Norwegian population-based study on the risk and prevalence of obstructive sleep apnea. The Akershus Sleep Apnea Project (ASAP) J. Sleep Res. 2011;20:162–170. doi: 10.1111/j.1365-2869.2010.00861.x. [DOI] [PubMed] [Google Scholar]
- 34.Thurnheer R., Xie X., Bloch K.E. Accuracy of nasal cannula pressure recordings for assessment of ventilation during sleep. Am. J. Respir. Crit. Care Med. 2001;164:1914–1919. doi: 10.1164/ajrccm.164.10.2102104. [DOI] [PubMed] [Google Scholar]
- 35.Abumuamar A.M., Dorian P., Newman D., Shapiro C.M. The prevalence of obstructive sleep apnea in patients with atrial fibrillation. Clin Cardiol. 2018;41:601–607. doi: 10.1002/clc.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rienstra M., Van Veldhuisen D.J., Hagens V.E., Ranchor A.V., Veeger N.J., Crijns H.J. Gender-related differences in rhythm control treatment in persistent atrial fibrillation: data of the rate control versus electrical cardioversion (RACE) study. J. Am. Coll. Cardiol. 2005;46:1298–1306. doi: 10.1016/j.jacc.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 37.Dagres N., Clague J.R., Breithardt G., Borggrefe M. Significant gender-related differences in radiofrequency catheter ablation therapy. J Am. Coll. Cardiol. 2003;42:1103–1107. doi: 10.1016/s0735-1097(03)00925-2. [DOI] [PubMed] [Google Scholar]
- 38.Forleo G.B., Tondo C., De Luca L., Dello Russo A., Casella M., De Sanctis V. Gender-related differences in catheter ablation of atrial fibrillation. Europace. 2007;9:613–620. doi: 10.1093/europace/eum144. [DOI] [PubMed] [Google Scholar]
- 39.Nyrnes A., Mathiesen E.B., Njolstad I., Wilsgaard T., Lochen M.L. Palpitations are predictive of future atrial fibrillation. An 11-year follow-up of 22,815 men and women: the Tromso Study. Eur. J. Prev. Cardiol. 2013;20:729–736. doi: 10.1177/2047487312446562. [DOI] [PubMed] [Google Scholar]
- 40.Cowie M.R., Woehrle H., Wegscheider K., Angermann C., d'Ortho M.P., Erdmann E. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl. J. Med. 2015;373:1095–1105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung R.S., Huber M.A., Rogge T., Maimon N., Chiu K.L., Bradley T.D. Association between atrial fibrillation and central sleep apnea. Sleep. 2005;28:1543–1546. doi: 10.1093/sleep/28.12.1543. [DOI] [PubMed] [Google Scholar]
- 42.May A.M., Blackwell T., Stone P.H., Stone K.L., Cawthon P.M., Sauer W.H. Central sleep-disordered breathing predicts incident atrial fibrillation in older men. Am. J. Respir. Crit. Care Med. 2016;193:783–791. doi: 10.1164/rccm.201508-1523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kadhim K., Middeldorp M.E., Elliott A.D., Jones D., Hendriks J.M.L., Gallagher C. Self-reported daytime sleepiness and sleep-disordered breathing in patients with atrial fibrillation: SNOozE-AF. Can. J. Cardiol. 2019;35:1457–1464. doi: 10.1016/j.cjca.2019.07.627. [DOI] [PubMed] [Google Scholar]
- 44.Albuquerque F.N., Calvin A.D., Sert Kuniyoshi F.H., Konecny T., Lopez-Jimenez F., Pressman G.S. Sleep-disordered breathing and excessive daytime sleepiness in patients with atrial fibrillation. Chest. 2012;141:967–973. doi: 10.1378/chest.11-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altmann D.R., Ullmer E., Rickli H., Maeder M.T., Sticherling C., Schaer B.A. Clinical impact of screening for sleep related breathing disorders in atrial fibrillation. Int. J. Cardiol. 2012;154:256–258. doi: 10.1016/j.ijcard.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 46.Wasmund S.L., Li J.M., Page R.L., Joglar J.A., Kowal R.C., Smith M.L. Effect of atrial fibrillation and an irregular ventricular response on sympathetic nerve activity in human subjects. Circulation. 2003;107:2011–2015. doi: 10.1161/01.CIR.0000064900.76674.CC. [DOI] [PubMed] [Google Scholar]
- 47.Kirchhof P., Benussi S., Kotecha D., Ahlsson A., Atar D., Casadei B. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 48.Reuter H., Herkenrath S., Treml M., Halbach M., Steven D., Frank K. Sleep-disordered breathing in patients with cardiovascular diseases cannot be detected by ESS, STOP-BANG, and Berlin questionnaires. Clin. Res. Cardiol. 2018;107:1071–1078. doi: 10.1007/s00392-018-1282-7. [DOI] [PubMed] [Google Scholar]
- 49.Santos-Silva R., Sartori D.E., Truksinas V., Truksinas E., Alonso F.F., Tufik S. Validation of a portable monitoring system for the diagnosis of obstructive sleep apnea syndrome. Sleep. 2009;32:629–636. doi: 10.1093/sleep/32.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coats A.J. Ethical authorship and publishing. Int J Cardiol. 2009;131:149–150. doi: 10.1016/j.ijcard.2008.11.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.