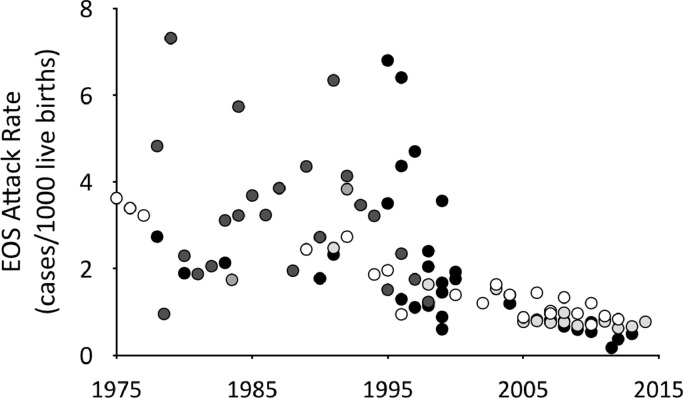
In recent decades, well-founded respect for rapidly progressive bacterial sepsis in newborn infants led to implementation of guidelines for ascertainment and management of early-onset neonatal sepsis (EOS). Limited ability to predict sepsis and frequent absence of early clinical signs resulted in empiric treatment of large numbers of infants – typically 30 to 40 – for each infant with confirmed EOS. Effective obstetrical measures to prevent neonatal sepsis, such as limitation of vaginal examinations, active management of labor to avoid prolonged duration of ruptured membranes, and prophylaxis for group B Streptococcus, led to a substantial reduction in rates of EOS by the middle of the last decade (Fig. 1). Because recommendations for empiric treatment remained categorical, however, the number of neonates treated per confirmed EOS case ballooned to more than 100 [1,2] (> 200 in some settings [3,4]). In concert with increasing recognition of adverse effects of antibiotic exposures, this has led to an emerging consensus that too many infants are receiving unnecessary and potentially harmful antibiotic treatment.
Fig. 1.
Declining rates of early-onset neonatal sepsis in high-income countries, 1975–2015. Data collected from 32 publications. A complete list of data sources is available from the authors upon request.  EOS at ≤ 2 days of age,
EOS at ≤ 2 days of age,  ≤3 days of age,
≤3 days of age,  < 5 days of age,
< 5 days of age,  < 6 days of age,
< 6 days of age,  < 7 days of age.
< 7 days of age.
Recognizing this trend, investigators from hospital systems in the United States sought a more efficient paradigm to identify infants at risk for EOS. A large proportion of infants at risk were not identified by risk stratification using a multivariate regression model based on maternal risk factors alone [5]. Addition of physical examination of the infants substantially improved recognition of EOS cases [6], but 40% of the EOS cases in the development data [6] or a subsequent birth cohort [3] still were not categorized as high-risk at birth. An online EOS Calculator incorporating the multivariate model and clinical findings [7] addressed this gap with additional recommendations: do more frequent vital signs and/or a blood culture for infants with ambiguous or intermediate risk estimates and “strongly consider antibiotics” for all with clinical signs of illness. The developers “recognized that a septic infant might appear well at birth and then develop symptoms later”; [7] a number of infants with EOS in their cohort must have been identified when ongoing examinations revealed postnatal development of clinical signs [3]. Validation of this tool is still needed, but this work makes important contributions to our understanding of EOS ascertainment: (1) traditional maternal risk factors alone are not sufficiently predictive, (2) standardized physical examination adds significant information, and (3) ongoing clinical monitoring is essential to ascertain infants with EOS otherwise not recognized.
In this issue, Pettinger et al. draw upon a collection of published instances of EOS for which available data suffice for comparison of case ascertainment by the calculator-based strategy to ascertainment using NICE guidelines [8]. The primary endpoint was “missed cases”, defined as instances in which the calculator did not initially recommend treatment but the NICE guidelines did. As noted by the calculator's developers [9], these cases may not have been truly “missed”, as they were (or almost certainly would have been) identified by ongoing clinical surveillance. The implication is not that the calculator-based strategy is inadequate or less safe (i.e., “missing” more cases), but rather just that it relies to a greater extent on ongoing evaluations of the babies. In practice, all approaches will initially fail to identify some neonates who will go on to have EOS. The analysis of Pettinger et al. supports Puopolo and Escobar's observation that “high-quality clinical examinations and protocols that ensure they happen consistently are the keys to timely identification of infected newborns” [9]. This vigilance must apply to all newborn infants, and not solely to those with intermediate estimates of risk, as more than 80% (29 of 36) of the infants with EOS who were not clinically ill at birth in the largest series reported to date had risk estimates below the threshold for incremental vigilance [3]. Provision of such oversight may be especially challenging in settings where birth at home or early hospital discharge are the prevalent practice. If serial examinations are not feasible for any reason, risk stratification by maternal risk factors may be useful.
Since clinical vigilance is inescapable, considerations of which strategy to adopt should primarily be driven by the numbers of newborn infants who undergo diagnostic evaluation and/or receive empiric antibiotic treatment with each strategy. The largest study suggests that the NICE guidelines result in empiric treatment of 16% of all infants (at least 96 per case of EOS) – nearly four times as many as would have been the case using the EOS calculator [10]. Further surveillance of very large populations will be required to determine which approaches are most efficient at balancing risks. Comparisons such as that presented by Pettinger et al. will be necessary to determine which strategy might be most appropriate in each different environment. Policy choices will be facilitated by additional reports such as those used in this analysis, including data for each EOS case sufficient to assign management recommendations and to identify paradigm components that lead to case ascertainment, as well as population-based data to evaluate rates of false-positive recommendations leading to testing or treatment of infants who are not infected.
Declaration of Competing Interest
Dr. Benitz and Dr. Achten declare that they have nothing to disclose.
Acknowledgments
Author Contributions
Dr. Benitz collected the data for and prepared the Figure and drafted the initial manuscript. Dr. Achten revised the manuscript. Both authors reviewed and approved the final manuscript and take responsibility for the content of the commentary.
Funding
No external funding supported this work. No funding agency or entity had any role in writing or the decision to submit the manuscript.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2019.100255.
Appendix. Supplementary materials
References
- 1.Mukhopadhyay S., Eichenwald E.C., Puopolo K.M. Neonatal early-onset sepsis evaluations among well-appearing infants: projected impact of changes in CDC GBS guidelines. J Perinatol. 2013;33:198–205. doi: 10.1038/jp.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiser C., Nawab U., McKenna K., Aghai Z.H. Role of guidelines on length of therapy in chorioamnionitis and neonatal sepsis. Pediatrics. 2014;133:992–998. doi: 10.1542/peds.2013-2927. [DOI] [PubMed] [Google Scholar]
- 3.Kuzniewicz M.W., Puopolo K.M., Fischer A., Walsh E.M., Li S., Newman T.B. A quantitative, risk-based approach to the management of neonatal early-onset sepsis. JAMA Pediatr. 2017;171:365–371. doi: 10.1001/jamapediatrics.2016.4678. [DOI] [PubMed] [Google Scholar]
- 4.Schulman J., Benitz W.E., Profit J., Lee H.C., Duenas G., Bennett M.V. Newborn antibiotic exposures and association with proven infection. Pediatrics. 2019;144 doi: 10.1542/peds.2019-1105. [DOI] [PubMed] [Google Scholar]
- 5.Puopolo K.M., Draper D., Wi S., Newman T.B., Zupancic J., Lieberman E. Estimating the probability of neonatal early-onset infection on the basis of maternal risk factors. Pediatrics. 2011;128:e1155–e1163. doi: 10.1542/peds.2010-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escobar G.J., Puopolo K.M., Wi S., Turk B.J., Kuzniewicz M.W., Walsh E.M. Stratification of risk of early-onset sepsis in newborns ≥ 34 weeks' gestation. Pediatrics. 2014;133:30–36. doi: 10.1542/peds.2013-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuzniewicz M.W., Walsh E.M., Li S., Fischer A., Escobar G.J. Development and implementation of an early-onset sepsis calculator to guide antibiotic management in late preterm and term neonates. Jt Comm J Qual Patient Saf. 2016;42:232–239. doi: 10.1016/s1553-7250(16)42030-1. [DOI] [PubMed] [Google Scholar]
- 8.Pettinger K.J., Mayers K., McKechnie L., Philllips B. Sensitivity of the Kaiser Permanente early-onset sepsis calculator: a systematic review and meta-analysis. E Clinical Med. 2019 doi: 10.1016/j.eclinm.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puopolo K.M., Escobar G.J. Neonatal sepsis evaluation: facing the certainty of uncertainty. JAMA Pediatr. 2019;173:1015–1016. doi: 10.1001/jamapediatrics.2019.2832. [DOI] [PubMed] [Google Scholar]
- 10.Goel N., Shrestha S., Smith R., Mehta A., Ketty M., Muxworthy H. Screening for early onset neonatal sepsis: nice guidance-based practice versus projected application of the Kaiser Permanente sepsis risk calculator in the UK population. Arch Dis Child Fetal Neonatal Ed. 2019 doi: 10.1136/archdischild-2018-316777. Epub ahead of print July 13, 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.