Abstract
Carotenoids are important pigments in photosynthetic organisms where they play essential roles in photoreception and photoprotection. Chromochloris zofingiensis is a unicellular green alga that is able to accumulate high amounts of ketocarotenoids including astaxanthin, canthaxanthin and ketolutein when growing heterotrophically or mixotrophically with glucose as a carbon source. Here we elucidate the ketocarotenoid biosynthesis pathway in C. zofingiensis by analyzing five algal mutants. The mutants were shown to have a single nucleotide insertion or substitution in β-carotene ketolase (BKT) gene 1, which resulted in a lack of ketocarotenoid production in Cz-bkt1-1, and decreased ketocarotenoid content in the other four mutants. These mutants accumulated much higher amounts of non-ketocarotenoids (β-carotene, zeaxanthin and lutein). Interestingly, the Cz-bkt1-5 mutant synthesized 2-fold the ketolutein and only 1/30 of the canthaxanthin and astaxanthin as its parent strain, suggesting that the mutated BKT1 exhibits much higher activity in catalyzing lutein to ketolutein but lower activity in ketolating β-carotene and zeaxanthin. Mutant and WT BKT2 gene sequences did not differ. Taken together, we conclude that BKT1 is the key gene involved in ketocarotenoid biosynthesis in C. zofingiensis. Our study provides insight into the biosynthesis of ketocarotenoids in green algae. Furthermore, Cz-bkt1 mutants may serve as a natural source for the production of zeaxanthin, lutein, and β-carotene.
Keywords: Chromochloris zofingiensis, Astaxanthin, Ketocarotenoid, β-carotene ketolase
1. Introduction
Carotenoids are synthesized in all photosynthetic organisms, where they serve as essential constituents for the assembly of photosystems and light harvesting antenna complexes for photosynthesis and photoprotection (Rodriguez-Concepcion and Boronat, 2002). In addition to primary carotenoids, plants are able to accumulate much higher amounts of secondary carotenoids that are not involved in photosynthesis, from which human and animals obtain their essential carotenoids to maintain normal health, e.g., β-carotene from carrots for the synthesis of vitamin A, lutein and zeaxanthin from marigold flowers for macular pigments (Romer et al., 2000, Seddon et al., 1994, Ye et al., 2000). Although the common carotenogenesis pathways have been well studied in photosynthetic organisms (Cazzonelli, 2011, Cazzonelli and Pogson, 2010, Chen et al., 2010, Cuttriss et al., 2007, Park et al., 2002, Rodriguez-Concepcion, 2010, Welsch et al., 2000), additional algae-specific pathways remain less studied. For example, in contrast to higher plants that generally do not produce ketocarotenoids, some green algae, such as Haematococcus pluvialis and Chromochloris zofingiensis (formerly named as Chlorella zofingiensis), are able to accumulate high amounts of ketocarotenoids outside of plastids under stress conditions (Lemoine and Schoefs, 2010, Pelah et al., 2004, Solovchenko, 2015). β-Carotene ketolases (BKT/CrtW) are the key enzymes involved in astaxanthin biosynthesis (Huang et al., 2016, Roth et al., 2017). The Haematococcus BKTs catalyze β-carotene to canthaxanthin (Kajiwara et al., 1995), whereas C. zofingiensis BKT1 can convert β-carotene to canthaxanthin as well as zeaxanthin to astaxanthin (Wang and Chen, 2008). Whether BKT/CrtW can convert lutein to ketolutein remains largely unknown.
C. zofingiensis is a unicellular green alga regarded as a potential producer of high-value carotenoids and fatty acids (Mao et al., 2018). Due to its fast growth and easy cultivation, including three modes of growth, this alga has served as a model system for the study of carotenoid biosynthesis (Liu et al., 2014). C. zofingiensis was found to contain two BKT genes, of which the BKT1 might play an indispensable role in the synthesis of astaxanthin (Huang et al., 2006, Roth et al., 2017). In a recent study, we further confirmed the key role of BKT1 in the biosynthesis of astaxanthin in C. zofingiensis (Huang et al., 2018). However, the role of BKT2 and the biosynthesis of ketolutein remain to be elucidated.
In this study, we generated and analyzed five C. zofingiensis mutants that exhibited diverse ketocarotenoid profiles. The mutants were shown to result from a change in function of BKT1 caused by a single nucleotide insertion or substitution in its coding region. Depending on the mutation site, BKT1 mutations block or attenuate the formation of ketocarotenoids. Coupling the carotenoid profiles with the molecular basis of the mutants, we propose a putative ketocarotenoid biosynthetic pathway in C. zofinginesis.
2. Material and methods
2.1. Microalga and growth condition
C. zofingiensis (ATCC 30412) was obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). The cells were kept and cultivated in Kuhl medium as described by Huang et al. (2016). Cells were cultivated in 10 mL liquid Kuhl medium for 4 days (25 °C, 30 μmol photons m−2 s−1, 150 rpm) and then inoculated at 10% (v/v) into 50 mL fresh Kuhl medium for another 4 days to be used as seeds or induced with 30 g/L glucose for 9 h for RNA-Seq and 12 days for pigments and fatty acids.
2.2. Mutagenesis and mutant selection
Seed cells were inoculated at 10% (v/v) into 50 mL fresh Kuhl medium for 4 days. Cells were collected by centrifugation (2410 g for 10 min) and then washed with phosphate-buffered saline (PBS, pH 7.0) three times. Cells were treated with N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) in dark for 1 h and mutagenesis was terminated by adding 10% (v/v) Na2SO3. The treated cells were washed with PBS three times and recovered in fresh Kuhl medium for 24 h in the dark. Following recovery, the cells were harvested and spread on selective medium (Kuhl medium containing 30 g/L glucose and 1.5 g/L agar) to develop colonies. Colonies with different colors from the WT strain, e.g., yellow rather than orange, were selected and cultivated in Kuhl medium with glucose (30 g/L) for 12 days for analysis of carotenoid profiles.
2.3. Pigment analysis
Algal cells were centrifuged at 3800 g for 5 min and washed with distilled water three times. Acetone was used to extract pigments until cells were colorless. Pigments in extracts were filtered through a 0.22 μm Millipore organic membrane and then analyzed with an Agilent Ultra-Performance liquid chromatography (UPLC) 1290 Infinity. An agilent Eclipse plus C18 RRHD 1.8 μm column (2.1 × 50 mm) was used for the mobile phase, including elution A (20% water, 60% acetonitrile, 5% isopropanol and 15% methanol) and elution B (80% acetonitrile, 5% isopropanol and 15% methanol). The flow rate was 0.5 mL/min as the process blew: 100% A for 1 min followed by 100% A to 100% B with liner gradient in 1 min, and then 100% B for 6 min. The concentrations of zeaxanthin and lutein were measured according to the method reported by Huang et al. (2018). Individual carotenoids were identified and quantitated through comparison to standard carotenoid retention times, absorption spectra, standard curves and peak areas.
2.4. Dry cell weight measurement
Cell dry weight was measured as described by Liu et al. (2012). Briefly, cells were centrifuged at 3800 g for 5 min and washed with distilled water three times, and then filtered with a Whatman GF/C filter paper (1.2 μm pore size) which was pre-dried and weighted. Filter paper with alga cells were dried at 80 °C in a vacuum oven until the weight did not change; the weight of the algal cells were calculated by subtracting the weight of the pre-dried paper from the final weight.
2.5. Molecular characterization of mutants
The BKT1 and BKT2 genes were amplified using a PCR approach with the following primers:
-
(1)
BKT1F (ACAACTCAAAGCATACCACCCCA) + BKT1R (TCCTGGGCCGTGCTGTATTG);
-
(2)
BKT2F (CCCAATGCACCAACGAGCC) + BKT2R (TTGGTCCCAGACTTCAGCTTATTGT). The PCR thermal cycling conditions were 98 °C for 2 min, 98 °C for 20 s, 56 °C for 10 s and 72 °C for 2 min. PCR products were purified and sequenced. Bio Edit software was used for sequences alignment.
3. Results
3.1. Isolation of ketocarotenoid-deficient C. zofingiensis mutants
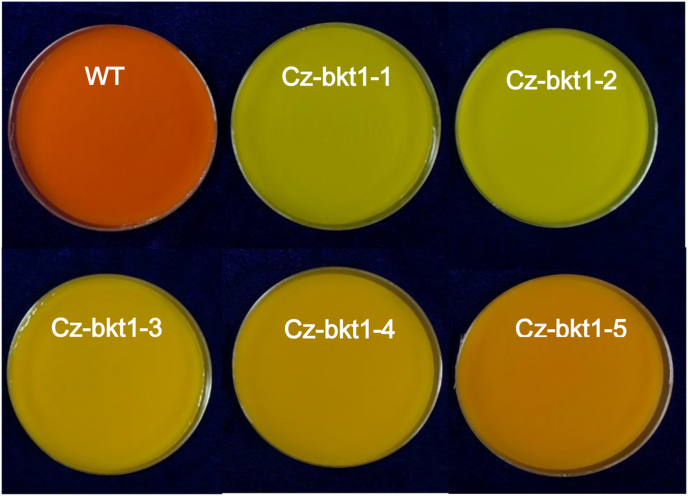
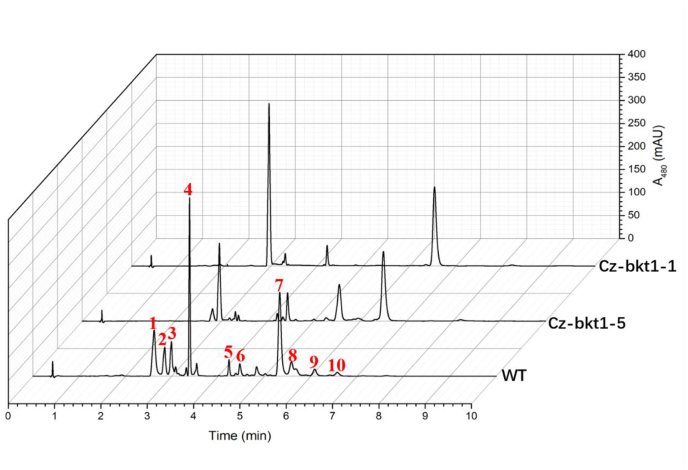
Based on our previous study (Huang et al., 2018), we used MNNG to mutate C. zofingiensis cells for strains with changed ketocarotenoid profiles and identified related mutants by phenotype, e.g. color changes. Treated algal cells were grown on 1/2 Kuhl medium containing 15 g/L glucose in which WT colonies exhibited orange due to the accumulation of red ketocarotenoids including astaxanthin, canthaxanthin and ketolutein. Colonies exhibiting different colors from WT were considered potential mutants. These colonies were isolated and sub-cultivated in Kuhl medium containing 30 g/L glucose to induce the biosynthesis of secondary carotenoids for 7 days. Of more than 500 colonies with phenotypes different from WT, five colonies (CZ-bkt1-(1–5)) demonstrated yellow to light orange rather than red of WT (Fig. 1). UPLC analysis showed that unlike WT, which accumulated mainly the keto-carotenoids astaxanthin, canthaxanthin, and ketolutein, the mutants mostly produced the non-ketocarotenoids β-carotene, zeaxanthin and lutein (Fig. 2). The carotenoid profiles coordinated well with the phenotypes between the mutants and WT strain since the non-ketocarotenoids are yellow while ketocarotenoids are red.
Fig. 1.
Phenotypes of WT and CZ-bkt1 mutants of C. zofingiensis cultivated in Kuhl medium containing 3% glucose.
Fig. 2.
UPLC chromatograms of pigments extracted from WT, CZ-bkt1-1 and Cz-bkt1-5 of C. zofingiensis cultivated in Kuhl medium containing 3% glucose. Peaks were identified as follows:1- astaxanthin; 2-ketolutein; 3-zeaxanthin + lutein; 4-canthaxanthin; 5-chlorophyll b; 6- chlorophyll a; 7-astaxanthin monoester; 8-ketolutein monoester; 9-adonixanthin; 10-β-carotene.
3.2. The growth and carotenoid profiles of Cz-bkt1-(1–5) mutants
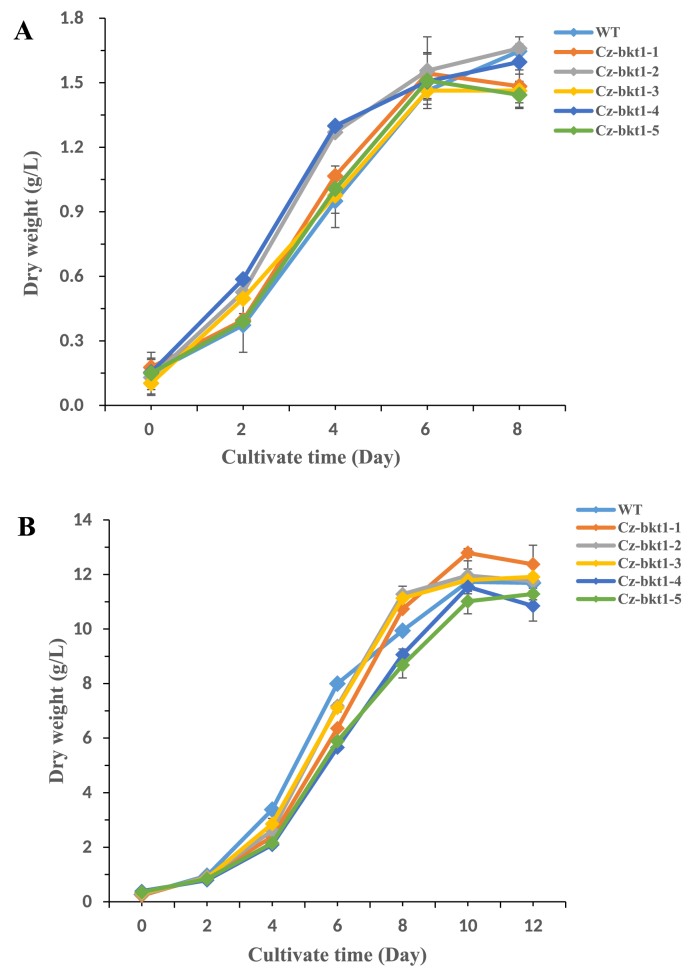
To elucidate the ketocarotenoid biosynthetic pathway in C. zofingiensis, we examined the carotenoid composition and content of five the five Cz-bkt1 mutants. The mutant strains were cultivated in Kuhl medium with or without glucose. As indicated by their growth curves, CZ-bkt1-(1–5) shared similar growth rates with WT regardless of growth mode (Fig. 2A and B). The maximum dry weight of CZ-bkt1-1 was higher than that of other mutants and WT. When cultivated in medium without glucose, all strains had similar carotenoid profiles, with lutein, chlorophylls and β-carotene as the predominant pigments (data not shown). However, when cultivated mixotrophically, all five mutants demonstrated different carotenoid profiles from WT (Fig. 3). WT accumulated mainly ketocarotenoids: free astaxanthin and its mono-ester (peak 1, 7), ketolutein and its mono-ester (peak 2 and 8) canthaxanthin (peak 4), and adonixanthin monoester (peak 9). In contrast, CZ-bkt1-(1–5) mostly accumulated the yellow carotenoids, including lutein + zeaxanthin (peak 3), and β-carotene (peak 10), but no or only trace amounts of canthaxanthin and astaxanthin (Fig. 3). Glucose was able to induce C. zofingiensis to synthesize red ketocarotenoids. Hence, we further investigated the ketocarotenoid biosynthesis in CZ-bkt1-(1–5) that were cultivated in Kuhl medium containing 30 g/L glucose for 12 days. The carotenoid compositions and contents of these strains are summarized in Table 1.
Fig. 3.
The growth curves of WT and its mutants grown photoautotrophically (A) or mixautotrophically (B).
Table 1.
The contents of main pigments in WT and CZ-bkt1-(1–5) cultivated in Kuhl medium containing 30 g/L glucose in 12 days.
| Content (mg/g) | WT | Cz-bkt1-1 | Cz-bkt1-2 | Cz-bkt1-3 | Cz-bkt1-4 | Cz-bkt1-5 |
|---|---|---|---|---|---|---|
| Astaxanthin | 0.6 ± 0.0592 | TA | TA | TA | TA | TA |
| ketolutein | 0.1 ± 0.0219 | ND | ND | ND | 0.1 ± 0.0169 | 0.3 ± 0.0331 |
| Lutein | 0.5 ± 0.0593 | 1.3 ± 0.0401 | 1.0 ± 0.0817 | 0.7 ± 0.0037 | 0.9 ± 0.0100 | 0.6 ± 0.0263 |
| Zeaxanthin | 0.11 ± 0.0094 | 1.1 ± 0.0159 | 0.8 ± 0.0859 | 0.6 ± 0.0158 | 0.8 ± 0.0089 | 0.7 ± 0.0307 |
| Canthaxanthin | 0.5 ± 0.0620 | ND | ND | ND | ND | 0.1 ± 0.0027 |
| β-carotene | 0.0 ± 0.0007 | 0.7 ± 0.1946 | 0.7 ± 0.0024 | 0.8 ± 0.0286 | 1.40 ± 0.1164 | 0.8 ± 0.0129 |
| Total keto-carotenoids | 1.3 ± 0.1431 | TA | TA | TA | 0.1 ± 0.0174 | 0.3 ± 0.0362 |
| Total carotenoids | 2.1 ± 0.2125 | 3.1 ± 0.2506 | 2.6 ± 0.9058 | 2.3 ± 0.1001 | 3.3 ± 0.1572 | 2.6 ± 0.1088 |
“ND” means not detected, “TA” means trace amount. Total keto-carotenoids include astaxanthin, ketolutein and canthaxanthin.
The contents of astaxanthin and canthaxanthin in Cz-bkt1 (1–5) were 0–1/30 and 0–1/10 of that in WT, respectively. In contrast, the zeaxanthin contents in the five mutants were 7–11 times that in WT. The decreases of astaxanthin and canthaxanthin, and increases of zeaxanthin, suggest that steps converting β-carotene and zeaxanthin to canthaxanthin and astaxanthin might be interrupted, possibly resulting from the change in function of the algal BKT genes. Interestingly, Cz-bkt1-1 produced no ketolutein but 2-fold the lutein of WT. Similarly, Cz-bkt1-(2–4) also accumulated much higher amounts of non-ketocarotenoids and much lower amounts of ketocarotenoids than did WT. In contrast, Cz-bkt1-5 accumulated small amounts of canthaxanthin and astaxanthin, together with 2-fold the ketolutein of WT. Taken together, these results indicate that the biosynthesis of ketocarotenoids is blocked in Cz-bkt1-1 and interrupted in the other four mutants. To elucidate the underlying mechanisms of ketocarotenoid biosynthesis in the mutants, we investigated the genes that code for enzymes (i.e., the BKT genes) involved in the catalytic steps of ketocarotenoids (see Table 2).
Table 2.
The mutated sites of BKT1 in CZ-bkt1-(1–5).
| Mutant | Mutated site in coding region | Region | Substitution of amino acid |
|---|---|---|---|
| Cz-bkt1-1 | +G395 | highly conserved | ORF shift from 133 aa |
| Cz-bkt1-2 | A503G | highly conserved | H168 R |
| Cz-bkt1-3 | G152A | medium conserved | R51K |
| Cz-bkt1-4 | C851T | highly conserved | P284L |
| Cz-bkt1-5 | G752A | highly conserved | S251N |
3.3. Genetic characterization of the C. zofingiensis mutants
C. zofingiensis has two BKT genes (BKT1 and BKT2) that are possibly involved in converting zeaxanthin and β-carotene into astaxanthin and canthaxanthin (Huang et al., 2016, Huang et al., 2018, Roth et al., 2017). We hypothesized that at least one of the ketolase genes in mutants might have lost its function. To test this hypothesis, we cloned and sequenced both BKT1 and BKT2 from the five mutants.
Sequence alignment demonstrated that a single nucleotide insertion or substitution occurred in the BKT1 gene, resulting in a premature stop codon (Cz-bkt1-1) or amino acid substitutions (H168R in Cz-bkt1-2, R51K in Cz-bkt1-3, P284L in Cz-bkt1-4, or S251N in Cz-bkt1-5) in BKT1 (Table 1). The mutations with amino acid substitutions were localized near the conserved histidine motifs (His motifs) that have been proposed to bind iron and are required for enzymatic activity. Consistent with previous studies (Tao et al., 2006, Ye et al., 2006), we found that conserved residues outside the His motifs were important for full enzyme function. Interestingly, the BKT1 mutation S251N severely reduced canthaxanthin and astaxanthin production, but increased ketolutein production. No differences in BKT2 gene sequences were found between the mutants and WT.
4. Discussion
In this study, we elucidated the biosynthetic pathway of ketocarotenoids in the biotechnologically important green alga C. zofingiensis by investigating five mutants with deficient ketocarotenoids. The carotenoid profiles of the mutants were markedly different than that of WT. Whereas WT synthesized ketocarotenoids, the mutants predominantly synthesized non-ketocarotenoids. The carotenoid profiles suggested that the ketolating steps in the mutants were severely affected. Sequence analysis of the genes (BKT1 and BKT2) possibly involved in the ketolating steps showed that although BKT2 was normal in all five mutants, BKT1 had mutations. Thus, via reverse genetics, BKT1 was shown to be the key gene for the formation of ketocarotenoids in C. zofingiensis.
These results, together with those of a recent report (Huang et al., 2018), suggest that BKT1 is the key ketolase involved in the synthesis of astaxanthin as well as other ketocarotenoids in C. zofingiensis. BKT2 was previously proposed to be involved in the synthesis of ketolutein in C. zofingiensis (Huang et al., 2018). This proposal is not supported by the current study, because the BKT2 gene sequence in Cz-bkt1-1 is the same as in the WT; in addition, these mutants synthesize no ketolutein or other ketocarotenoids (canthaxanthin and astaxanthin). This result is similar in mutants Cz-bkt1-(2–4). Trace amounts of astaxanthin in the mutants might result from the weak activity of BKT2 toward zeaxanthin to form astaxanthin. Thus, BKT1 appears to be the key enzyme involved in the biosynthesis of all ketocarotenoids.
The BKT/CrtW enzymes are typical membrane-bound non-heme, iron-containing oxygenases that consist of conserved hydrophobic transmembrane domains and His motifs. Little is known regarding the structure and function of this group of enzymes due to the lack of data on their protein crystal structure. Previous research has proposed that CrtW proteins have at least two hydrophobic patches that may be involved in the interaction with hydrophobic carotenoid substrates---one located between the first two His clusters and the other near the C terminus (Ye et al., 2006). The mutated sites of BKT1 in the Cz-bkt1-4 and Cz-bkt1-5 mutants are located at the C terminus, leading to severe loss of activity in the conversion of β-carotene and zeaxanthin to canthaxanthin and astaxanthin. Interestingly, the severity of the effects of these mutations on ketolutein production varied between the mutants. For instance, Cz-bkt1-4 accumulated no ketolutein, whereas Cz-btk1-5 produced twice as much ketolutein as the parent strain. These findings support the hypothesis that the C terminus of BKT/CrtW is important to substrate specificity.
Ketocarotenoids, especially astaxanthin and canthaxanthin, have been widely used as components of feeds by fisheries and poultry farms (Lorenz and Cysewski, 2000). Furthermore, astaxanthin has potential applications in treatment of a number of diseases (Hussein et al., 2006, Guerin et al., 2003, Yeum et al., 2005). Generally, higher plants lack BKT activity and therefore do not synthesize ketocarotenoids. However, as a production system for ketocarotenoids, the ability to express heterologous BKT genes makes plants attractive (Zhu et al., 2007). Overexpression of a heterologous BKT/CrtW gene from a number of microorganisms leads to the production of various amounts of astaxanthin (Breitenbach et al., 2014, Huang et al., 2013, Lin et al., 2017, Park et al., 2018, Zhu et al., 2007). Unexpectedly, many of the transgenic plants also generate 4-keto-α-carotene and/or ketolutein, indicating that BKT/CrtW ketolases are able to accept carotenoids with β- and α-ionone rings as substrates for the production of the ketocarotenoids (Breitenbach et al., 2014, Huang et al., 2013, Lin et al., 2017, Park et al., 2018, Zhu et al., 2007). These results are consistent with our finding that the BKT1 accepted both zeaxanthin and lutein as substrates for astaxanthin and ketolutein production. Lutein is the predominant carotenoids in plants (Kim and DellaPenna, 2006). However ketolutein production in green algae and transgenic plants is commonly much lower than astaxanthin, suggesting that BKT/CrtW may have a strong substrate preference for carotenoids with a β-ionone ring, e.g., β-carotene and zeaxanthin. Because Cz-bkt1-5 is able to predominantly accumulate ketolutein, this mutant provides a platform for elucidating the relationship between structure and function of BKT enzymes that lack structural information.
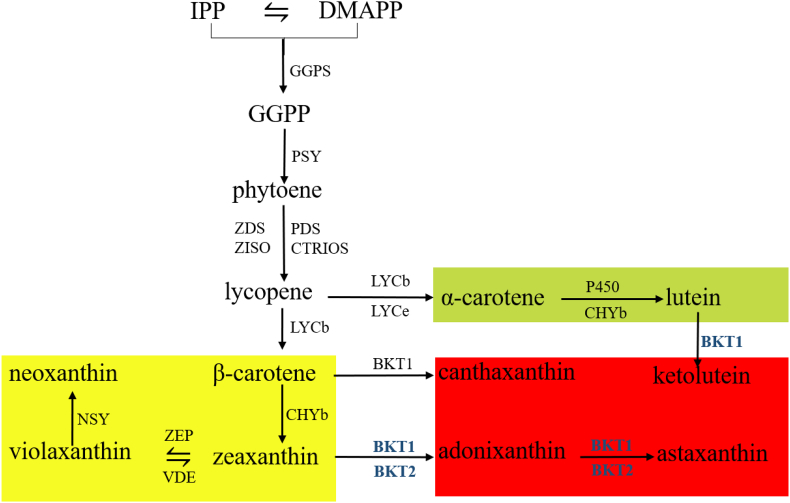
In summary, we have shown that BKT1 is the key gene for the formation of ketocarotenoids in C. zofingiensis. This finding allowed us to propose a biosynthetic pathway for carotenoids in C. zofingiensis (Fig. 4). C. zofingiensis shares the same biosynthetic pathway of primary carotenoids, including β-carotene, lutein, zeaxanthin, violaxanthin, and neoxanthin, as other green algae. The formation of astaxanthin from β-carotene requires the introduction of two hydroxyl groups at C3 and C3′ by Chy gene product and two keto groups at C4 and C4’ by the BKT gene product. C. zofingiensis Chy might fail to convert canthaxanthin to astaxanthin, and thus astaxanthin synthesis may start with the hydroxylation of β-carotene with zeaxanthin and adonixanthin as intermediates.
Fig. 4.
The biosynthetic pathway of carotenoids in C. zofingiensis. IPP, isoprenoid isopentenyl diphosphate; DMAPP, dimethylallyl diphosphate; GGPPS, Geranylgeranyl pyrophosphate synthase; GGPS, geranylgeranyl diphosphate synthase; GGPP, geranylgeranyl diphosphate; PSY, phytoene synthase; PDS, phytoene desaturase; LYCb, lycopene beta-cyclase; LYCe, lycopene ε-cyclase; CHYb, β-carotenoid hydroxylase; BKT1, β-carotene ketolase 1; P450, cytochrome ε-hydroxylase.
Declaration of Competing Interest
There is no conflict of interest.
Editor: Zhekun Zhou
Footnotes
Peer review under responsibility of Editorial Office of Plant Diversity.
References
- Breitenbach J., Bai C., Rivera S.M., Canela R., Capell T., Christou P., Zhu C.F., Sandmann G. A novel carotenoid, 4-keto-alpha-carotene, as an unexpected by-product during genetic engineering of carotenogenesis in rice callus. Phytochemistry. 2014;98:85–91. doi: 10.1016/j.phytochem.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Cazzonelli C.I. Carotenoids in nature: insights from plants and beyond. Funct. Plant Biol. 2011;38(11):833–847. doi: 10.1071/FP11192. [DOI] [PubMed] [Google Scholar]
- Cazzonelli C.I., Pogson B.J. Source to sink: regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010;15(5):266–274. doi: 10.1016/j.tplants.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Chen Y., Li F.Q., Wurtzel E.T. Isolation and characterization of the Z-ISO gene encoding a missing component of carotenoid biosynthesis in plants. Plant Physiol. 2010;153(1):66–79. doi: 10.1104/pp.110.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttriss A.J., Chubb A.C., Alawady A., Grimm B., Pogson B.J. Regulation of lutein biosynthesis and prolamellar body formation in Arabidopsis. Funct. Plant Biol. 2007;34(8):663–672. doi: 10.1071/FP07034. [DOI] [PubMed] [Google Scholar]
- Guerin M., Huntley M.E., Olaizola M. Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol. 2003;21(5):210–216. doi: 10.1016/S0167-7799(03)00078-7. [DOI] [PubMed] [Google Scholar]
- Huang J.C., Wang Y., Sandmann G., Chen F. Isolation and characterization of a carotenoid oxygenase gene from Chlorella zofingiensis (Chlorophyta) Appl. Microbiol. Biotechnol. 2006;71(4):473–479. doi: 10.1007/s00253-005-0166-8. [DOI] [PubMed] [Google Scholar]
- Huang J.C., Zhong Y.J., Liu J., Sandmann G., Chen F. Metabolic engineering of tomato for high-yield production of astaxanthin. Metab. Eng. 2013;17:59–67. doi: 10.1016/j.ymben.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Huang W.P., Lin Y., He M.X., Gong Y.H., Huang J.C. Induced high-yield production of zeaxanthin, lutein, and beta-carotene by a mutant of Chlorella zofingiensis. J. Agric. Food Chem. 2018;66(4):891–897. doi: 10.1021/acs.jafc.7b05400. [DOI] [PubMed] [Google Scholar]
- Huang W.P., Ye J.R., Zhang J.J., Lin Y., He M.X., Huang J.C. Transcriptome analysis of Chlorella zofingiensis to identify genes and their expressions involved in astaxanthin and triacylglycerol biosynthesis. Algal Res. 2016;17:236–243. [Google Scholar]
- Hussein G., Sankawa U., Goto H., Matsumoto K., Watanabe H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006;69(3):443–449. doi: 10.1021/np050354+. [DOI] [PubMed] [Google Scholar]
- Kajiwara S., Kakizono T., Saito T., Kondo K., Ohtani T., Nishio N., Nagai S., Misawa N. Isolation and functional identification of a novel Cdna for astaxanthin biosynthesis from Haematococcus-Pluvialis, and astaxanthin synthesis in escherichia-coli. Plant Mol. Biol. 1995;29(2):343–352. doi: 10.1007/BF00043657. [DOI] [PubMed] [Google Scholar]
- Kim J., DellaPenna D. Defining the primary route for lutein synthesis in plants: the role of Arabidopsis carotenoid beta-ring hydroxylase CYP97A3. Proc. Natl. Acad. Sci. U. S. A. 2006;103(9):3474–3479. doi: 10.1073/pnas.0511207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine Y., Schoefs B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: a multifunctional response to stress. Photosynth. Res. 2010;106(1–2):155–177. doi: 10.1007/s11120-010-9583-3. [DOI] [PubMed] [Google Scholar]
- Lin Y.J., Chang J.J., Lin H.Y., Thia C., Kao Y., Huang C.C., Li W.H. Metabolic engineering a yeast to produce astaxanthin. Bioresour. Technol. 2017;245:899–905. doi: 10.1016/j.biortech.2017.07.116. [DOI] [PubMed] [Google Scholar]
- Liu J., Huang J.C., Jiang Y., Chen F. Molasses-based growth and production of oil and astaxanthin by Chlorella zofingiensis. Bioresour. Technol. 2012;107:393–398. doi: 10.1016/j.biortech.2011.12.047. [DOI] [PubMed] [Google Scholar]
- Liu J., Sun Z., Gerken H., Liu Z., Jiang Y., Chen F. Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: biology and industrial potential. Mar. Drugs. 2014;12(6):3487–3515. doi: 10.3390/md12063487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz R.T., Cysewski G.R. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000;18(4):160–167. doi: 10.1016/s0167-7799(00)01433-5. [DOI] [PubMed] [Google Scholar]
- Mao X.M., Wu T., Sun D.Z., Zhang Z., Chen F. Differential responses of the green microalga Chlorella zofingiensis to the starvation of various nutrients for oil and astaxanthin production. Bioresour. Technol. 2018;249:791–798. doi: 10.1016/j.biortech.2017.10.090. [DOI] [PubMed] [Google Scholar]
- Park H., Kreunen S.S., Cuttriss A.J., DellaPenna D., Pogson B.J. Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell. 2002;14(2):321–332. doi: 10.1105/tpc.010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., Binkley R.M., Kim W.J., Lee M.H., Lee S.Y. Metabolic engineering of Escherichia coli for high-level astaxanthin production with high productivity. Metab. Eng. 2018;49:105–115. doi: 10.1016/j.ymben.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Pelah D., Sintov A., Cohen E. The effect of salt stress on the production of canthaxanthin and astaxanthin by Chlorella zofingiensis grown under limited light intensity. World J. Microbiol. Biotechnol. 2004;20(5):483–486. [Google Scholar]
- Rodriguez-Concepcion M. Supply of precursors for carotenoid biosynthesis in plants. Arch. Biochem. Biophys. 2010;504(1):118–122. doi: 10.1016/j.abb.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Concepcion M., Boronat A. Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol. 2002;130(3):1079–1089. doi: 10.1104/pp.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer S., Fraser P.D., Kiano J.W., Shipton C.A., Misawa N., Schuch W., Bramley P.M. Elevation of the provitamin A content of transgenic tomato plants. Nat. Biotechnol. 2000;18(6):666–669. doi: 10.1038/76523. [DOI] [PubMed] [Google Scholar]
- Roth M.S., Cokus S.J., Gallaher S.D., Walter A., Lopez D., Erickson E., Endelman B., Westcott D., Larabell C.A., Merchant S.S., Pellegrini M., Niyogi K.K. Chromosome-level genome assembly and transcriptome of the green alga Chromochloris zofingiensis illuminates astaxanthin production. Proc. Natl. Acad. Sci. U. S. A. 2017;114(21):E4296–E4305. doi: 10.1073/pnas.1619928114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon J.M., Ajani U.A., Sperduto R.D., Hiller R., Blair N., Burton T.C., Farber M.D., Gragoudas E.S., Haller J., Miller D.T., Yannuzzi L.A., Willett W. Dietary carotenoids, vitamin-a, vitamin-C, and vitamin-E, and advanced age-related macular degeneration. JAMA – J. Am. Med. Assoc. 1994;272(18):1413–1420. [PubMed] [Google Scholar]
- Solovchenko A.E. Recent breakthroughs in the biology of astaxanthin accumulation by microalgal cell. Photosynth. Res. 2015;125(3):437–449. doi: 10.1007/s11120-015-0156-3. [DOI] [PubMed] [Google Scholar]
- Tao L., Mogilner A., Civelekogiu-Scholey G., Wollman R., Evans J., Stahlberg H., Scholey J.M. A homotetrameric kinesin-5, KLP61F, bundles microtubules and antagonizes Ncd in motility assays. Curr. Biol. 2006;16(23):2293–2302. doi: 10.1016/j.cub.2006.09.064. [DOI] [PubMed] [Google Scholar]
- Wang Y., Chen T. The biosynthetic pathway of carotenoids in the astaxanthin-producing green alga Chlorella zofingiensis. World J. Microbiol. Biotechnol. 2008;24(12):2927–2932. [Google Scholar]
- Welsch R., Beyer P., Hugueney P., Kleinig H., von Lintig J. Regulation and activation of phytoene synthase, a key enzyme in carotenoid biosynthesis, during photomorphogenesis. Planta. 2000;211(6):846–854. doi: 10.1007/s004250000352. [DOI] [PubMed] [Google Scholar]
- Ye S., LaBelle J., Weatherwax A.T. Further study of flickering auroral roar emission: 1. South Pole observations. J. Geophys. Res. Space. 2006;111(A7) [Google Scholar]
- Ye X.D., Al-Babili S., Kloti A., Zhang J., Lucca P., Beyer P., Potrykus I. Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science. 2000;287(5451):303–305. doi: 10.1126/science.287.5451.303. [DOI] [PubMed] [Google Scholar]
- Yeum K.J., Zhao X.F., Aldini G., Johnson E.J., Kraemer K., Krinsky N.I., Russell R.M., Mayer J. Effect of a physiologic dose of mixed carotenoids on DNA damage in elderly women. FASEB J. 2005;19(5) A1458-A1458. [Google Scholar]
- Zhu C., Gerjets T., Sandmann G. Nicotiana glauca engineered for the production of ketocarotenoids in flowers and leaves by expressing the cyanobacterial crtO ketolase gene. Transgenic Res. 2007;16(6):813–821. doi: 10.1007/s11248-007-9151-6. [DOI] [PubMed] [Google Scholar]