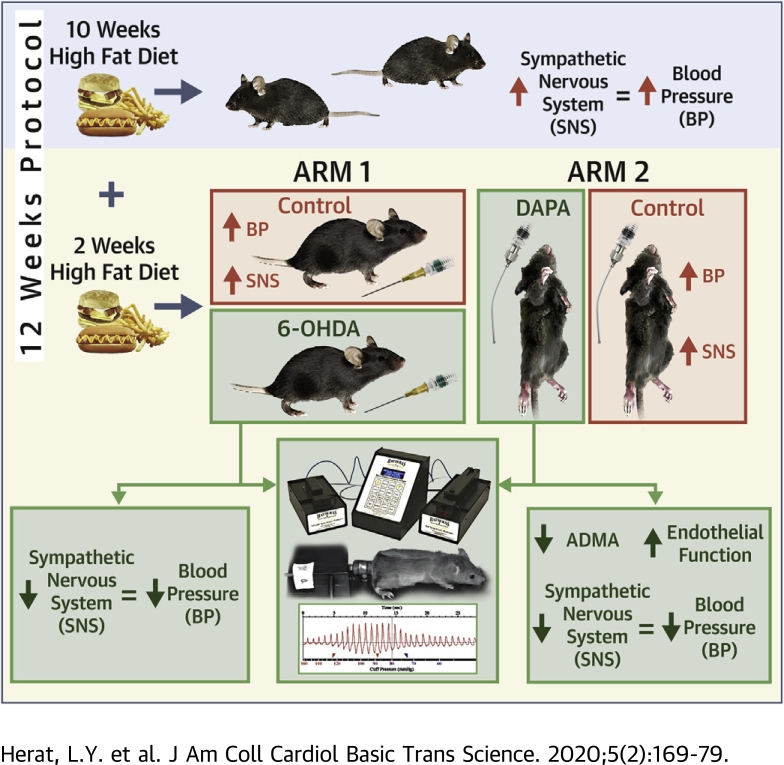
Visual Abstract
Key Words: denervation, heart, hypertension, kidney, SGLT2, sympathetic nervous system
Abbreviations and Acronyms: 6-OHDA, 6-hydroxydopamine; DBP, diastolic blood pressure; ELISA, enzyme-linked immunosorbent assay; HFD, high-fat diet; MAP, mean arterial pressure; SBP, systolic blood pressure; SCFA, short-chain fatty acid; SGLT2, sodium glucose cotransporter 2; SNS, sympathetic nervous system
Highlights
-
•
SGLT2 inhibitors improve cardiovascular outcomes.
-
•
SGLT2 inhibitor–induced sympathetic nervous system inhibition may be an underlying mechanism.
-
•
Chemical denervation in neurogenic hypertensive mice reduces renal SGLT2 expression.
-
•
SGLT2 inhibition lowered blood pressure and resulted in significantly reduced tyrosine hydroxylase and norepinephrine levels in the kidney tissue of neurogenic hypertensive mice.
-
•
Crosstalk between the sympathetic nervous system and SGLT2 regulation appears as a key mechanism of the cardiorenal protective effects demonstrated with SGLT2 inhibition.
Summary
Recent clinical trial data suggest a cardiorenal protective effect of sodium glucose cotransporter 2 (SGLT2) inhibition. We demonstrate that chemical denervation in neurogenic hypertensive Schlager (BPH/2J) mice reduced blood pressure, improved glucose homeostasis, and reduced renal SGLT2 protein expression. Inhibition of SGLT2 prevented weight gain, reduced blood pressure, significantly reduced elevations of tyrosine hydroxylase and norepinephrine, and protects against endothelial dysfunction. These findings provide evidence for significant crosstalk between activation of the sympathetic nervous system and SGLT2 regulation and possible ancillary effects on endothelial function, which may contribute to the observed cardiorenal protective effects of SGLT2 inhibition.
Inhibition of the renal sodium glucose cotransporter 2 (SGLT2) is now an established therapeutic approach to improve glucose control in patients with diabetes. Currently. SGLT2 is believed to be primarily expressed in the kidney (1). However, there is the possibility that other cellular sources of SGLT2 expression may exist. To date, the healthy or diseased heart has not been shown to be a source of SGLT2 expression (2). The EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) studies have recently demonstrated that the SGLT2 inhibitor empagliflozin improved glycemic control and is associated with a significant reduction in the progression of kidney disease (3) and substantial 38% relative risk reduction in death from cardiovascular causes (4). The cardiovascular and renal protective properties of SGLT2 inhibitors were confirmed in the CANVAS (Canagliflozin Cardiovascular Assessment Study) study (5). Interestingly, sudden cardiac death and hospitalization for heart failure were the major drivers of cardiovascular risk reduction, raising the possibility that SGLT2 inhibitors may have the capacity to antagonize increased sympathetic nervous system (SNS) activation commonly evident in these patients with diabetes.
Type 2 diabetes and hypertension frequently coexist (6) and hypertension is a risk factor for chronic kidney and cardiovascular disease. Thus, early treatment of hypertension, including intensive blood pressure control to reduce micro- and macrovascular complications and diabetes is critical in preventing cardiovascular disease and diabetic nephropathy. There is clear evidence to indicate that obesity, metabolic syndrome, and type 2 diabetes are associated with chronic activation of sympathetic nervous tone (7,8). Sympathoexcitation, as indicated by elevated urinary norepinephrine and metabolite levels, increased efferent muscle sympathetic nerve activity and elevated rates of spillover of norepinephrine to plasma has been observed in obese subjects (7). Furthermore, exaggerated responses to mental stress and elevated resting sympathetic activity could predispose to the development of insulin resistance, hypertension, renal disease, and cardiac perturbations such as diastolic dysfunction, left ventricular hypertrophy, and cardiac arrhythmias (7).
The mechanisms linking obesity and sympathetic nervous system activation are likely to involve overeating, insulin resistance, adipokines, nonesterified free fatty acids, and alterations in beta-adrenergic responsiveness with sympathetic activation (8). Virtually all organs that play a key role in metabolic control are innervated by sympathetic nerves. A sustained increase in sympathetic outflow has adverse consequences on metabolic control and promotes the development of type 2 diabetes and its detrimental consequences, including lipolysis, impaired glucose uptake, and gluconeogenesis (9). The relevance of increased central sympathetic outflow to the kidney and its possible impact on regulation of the SGLT2 protein has not been explored in humans. Similarly, whether or not novel SGLT2 inhibitors have an impact on SNS activity in human subjects is unknown.
To this end, our group investigated the impact of SGLT2 inhibitors on SNS activity. We were the first to show through experiments in human proximal tubular cells and a mouse model of obesity that: 1) SNS activation through increased release of norepinephrine up-regulates SGLT2 expression and promotes translocation of SGLT2 to the cell membrane; 2) treatment of high-fat diet (HFD)–fed mice with the SGLT2 inhibitor dapagliflozin leads to marked glucosuria, reduced weight gain, and improved glucose control; and 3) dapagliflozin treatment reduced sympathetic innervation both in the kidney and the heart (10,11). These findings strongly suggest that SGLT2 expression is regulated by norepinephrine and that SGLT2 inhibition is associated with sympathoinhibition.
In our current study, we used a neurogenic hypertensive mouse model, known as the Schlager (BPH/2J) mouse (12,13), to further explore the direct interaction between sympathetic hyperactivity and SGLT2 regulation. We hypothesized that down-regulation of the SNS via either denervation or SGLT2 inhibition would reduce SGLT2 expression and hypertension in our neurogenic mouse model. Support for our hypothesis comes from a recent case report of a diabetic patient with heart failure who was treated with the SGLT2 inhibitor ipragliflozin (14). In this case report, cardiac sympathetic nerve function was assessed by 123I-MIBG scanning and showed a significant reduction in cardiac sympathetic nerve hyperactivity at 12-month follow-up compared with baseline (14). In our current study, hypertensive mice were treated with the chemical denervation agent 6-hydroxydopamine (6-OHDA) or the SGLT2 inhibitor for 2 weeks before being assessed for relevant cardiovascular and metabolic changes including blood pressure and markers of sympathetic activity.
Methods
Animals
Experiments were conducted in 12-week-old Schlager mice (BPH/2J strain) rederived and bred at the Animal Resource Centre (ARC, Perth, Australia). All animal experimentation was carried out at the Royal Perth Hospital animal holding facility in accordance with the guidelines of the Royal Perth Hospital Animal Ethics Committee (R537/17-20).
Mice were acclimatized for 1 week, and then all mice were fed a HFD (Specialty Feeds, Glen Forrest, Australia) for the 2 weeks of treatment. All mice were given unrestricted access to water and food. After acclimatization, mice were administered 5 intraperitoneal injections of 100-mg/kg 6-OHDA (Sigma, St. Louis, Missouri) in 0.9% saline (15) over a 2-week period. In a separate series of experiments, we explored the effect of SGLT2 inhibition on sympathetic activity in vivo using BPH/2J mice fed an HFD for 2 weeks and mice received either vehicle or 40-mg/kg dapagliflozin (4C Pharma Scientific Inc., Guelph, Canada) via oral gavage every 2 days for 2 weeks.
All animals undergoing blood pressure analysis were acclimatized for a period of 7 days. The blood pressure measurements were conducted on 13-, 14-, and 15-week-old BPH/2J mice using a noninvasive computer-automated multichannel blood pressure analysis system MC4000 (Hatters Instruments, Cary, North Carolina). Fifteen cycles were used in obtaining systolic blood pressure (SBP) (133 ± 9 mm Hg), diastolic blood pressure (DBP) (117 ± 16 mm Hg), and mean arterial pressure (MAP) (122 ± 14 mm Hg). Cycles with movement artifacts were excluded. All measurements were made in the morning (9:00 to 11:30 am). Data are presented as change from baseline readings.
Body weights were measured weekly for all mice. Fasting blood glucose was measured using Accu-Chek Performa blood glucose monitoring system (Roche Diagnostics, North Ryde, Australia). Urine glucose levels were measured for all mice using the Keta-Diabur-Test 5000 (Roche Diagnostics). At the conclusion of the experiment, all mice were anesthetized with methoxyflurane, underwent cardiac puncture to obtain blood and were euthanized by cervical dislocation. Kidney and heart tissue were collected and either fixed in paraformaldehyde and subsequently embedded in paraffin wax, or snap-frozen in liquid nitrogen and stored at –80°C. Tyrosine hydroxylase and SGLT2 staining and quantitation was conducted as reported by Matthews et al. (11). Aortic rings were isolated for vessel function assessment after perfusion of the animal with 0.9% saline in accordance to Jiang et al. (16). Cecal contents were collected from fasted mice.
Enzyme-linked immunosorbent assays
Frozen kidney or heart tissue were homogenized in cytosolic extraction buffer containing phosphatase and protease inhibitors and analyzed for norepinephrine, interleukin-6, and interleukin-10 content using the appropriate enzyme-linked immunosorbent assay (ELISA) kits according to manufacturer’s respective instructions (mouse norepinephrine NA ELISA kit [CSB-E07870m] [Cusabio, Wuhan, China], mouse interleukin-6 ELISA kit [ELISAkit.com, Melbourne, Australia], mouse interleukin-10 ELISA kit [ELISAkit.com]). Levels of renin (Duoset Mouse Renin ELISA kit [R&D Systems, Minneapolis, Minnesota]), asymmetric dimethylarginine (mouse asymmetric dimethylarginine ELISA kit [Cusabio]), and fibroblast growth factor 21 (Duoset mouse fibroblast growth factor-21 ELISA kit [R&D Systems]) were measured in serum using the appropriate ELISA kits according to manufacturer’s respective instructions.
Cecum short-chain fatty acids analysis
The concentration of short-chain fatty acids (SCFAs) acetate and butyric acid in cecum samples isolated from the gastrointestinal tract were assessed using gas chromatography-mass spectrometry as previously described (17,18). Data were calculated as nanomoles per milligram cecal content.
Statistical analysis
All quantitative data are presented as mean ± SEM. Significance was determined using Student’s t test. A p value <0.05 was considered statistically significant. Graphs were generated using GraphPad Prism 7 (GraphPad Software, San Diego, California) and the analyses were performed using Microsoft Excel (Microsoft Corporation, Redmond, Washington). Data were deemed approximately normally distributed as determined by Q-Q plots.
Results
Chemical denervation of the SNS promotes numerous metabolic benefits in hypertensive mice
Using the chemical denervation agent 6-OHDA, we were able to reduce SNS activity in BPH/2J mice, as evidenced by a reduction in tyrosine hydroxylase staining in heart and kidney tissue (Supplemental Figure 1). As tyrosine hydroxylase is a reliable marker of sympathetic innervation, we showed that sympathetic hyperactivity can be dampened in our genetically hypertensive mice with chemical denervation techniques.
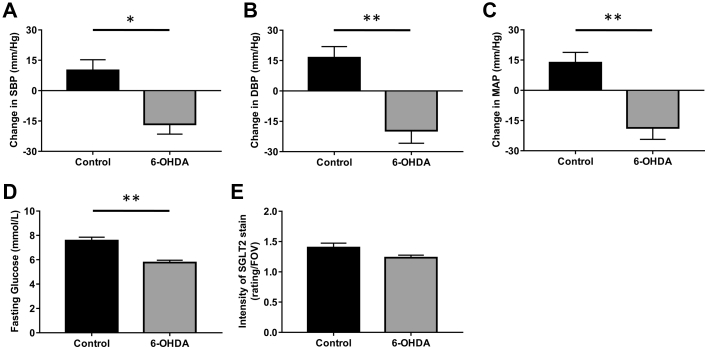
We next sought to establish the cardiovascular and metabolic benefits of using 6-OHDA denervation treatment in our genetically hypertensive mice. Intraperitoneal administration of 6-OHDA (100 mg/kg) prevented the increase in SBP, DBP, and MAP observed in BPH/2J mice (Figures 1A to 1C). 6-OHDA treatment significantly reduced the fasting glucose levels of BPH/2J mice (Figure 1D). Renal expression of the protein that promotes glucose reabsorption in the kidney, SGLT2, was also found to be reduced after treatment with 6-OHDA (Figure 1E, Supplemental Figure 2).
Figure 1.
Chemical Denervation With 6-OHDA Prevents the Increase in Blood Pressure and Lowers the Fasting Glucose and Renal SGLT2 Levels in Hypertensive BPH/2J Mice
Effects of 6-hydroxydopamine (6-OHDA) (100 mg/kg) on (A) systolic blood pressure (SBP), (B) diastolic blood pressure (DBP), and (C) mean arterial pressure (MAP) were measured using a tail-cuff apparatus. (D) Fasting glucose was measured after a week of treatment. (E) Intensity of sodium glucose cotransporter 2 (SGLT2) in proximal tubules in kidneys was rated on a scale 0–3 per field of view (FOV): 0 = no staining, 1 = low staining, 2 = intermediate staining, 3 = highest staining. n = 7–9 mice/group. *p < 0.05; **p < 0.001; all data presented as mean ± SEM.
Inhibition of SGLT2 activity in hypertensive mice imparts metabolic benefits on hypertensive mice
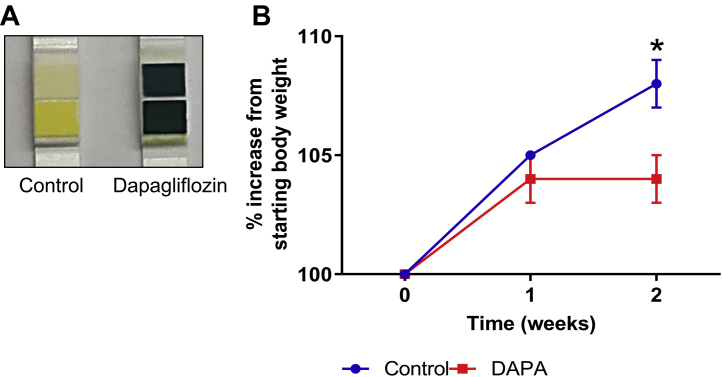
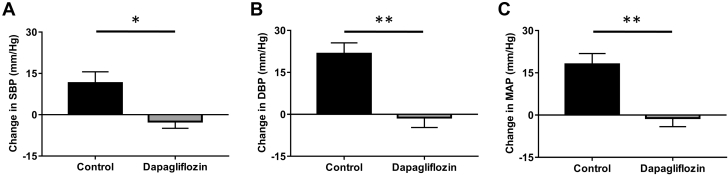
Taking into account the knowledge that chemical denervation decreases blood pressure, improves glucose homeostasis and reduces SGLT2 expression, we then aimed to determine whether therapeutic inhibition of SGLT2 activity with dapagliflozin may promote metabolic benefits in a mouse model of neurogenic hypertension. Dapagliflozin treatment was well tolerated by BPH/2J mice and had no significant impact on the renal expression of SGLT2 (Supplemental Figure 3). BPH/2J mice receiving dapagliflozin via oral gavage for 2 weeks exhibited glucosuria (Figure 2A) and gained significantly less weight (control group: 108 ± 1%; dapagliflozin group: 104 ± 1%; p = 0.034) (Figure 2B) in comparison with their control counterparts. Furthermore, we were able to demonstrate that SGLT2 inhibition with dapagliflozin prevents the elevation of SBP (control group: 11.5 ± 4.1 mm Hg; dapagliflozin group: –2.5 ± 2.4 mm Hg; p = 0.006), DBP (control group: 21.7 ± 3.2 mm Hg; dapagliflozin group: –1.2 ± 3.5 mm Hg; p < 0.001) and MAP (control group: 18.0 ± 3.7 mm Hg; dapagliflozin group: –1.1 ± 2.9 mm Hg; p < 0.001) in BPH/2J mice (Figure 3). Our data highlight that dapagliflozin was imparting metabolic benefits in our mouse model of neurogenic hypertension, similar to those observed in human studies (3,4).
Figure 2.
SGLT2 Inhibition Promotes Glucosuria and Decreases Weight Gain in Hypertensive BPH/2J Mice
(A) Representative image of glucose levels in the urine after 10 days of dapagliflozin (40 mg/kg) treatment (yellow = 0 mmol/l glucose, blue = 278 mmol/l glucose). (B) Increase in body weight of mice after 2 weeks of dapagliflozin (DAPA) treatment (starting at week 0), n = 6 to 8 mice/group. *p < 0.05; data presented as mean ± SEM. Abbreviation as in Figure 1.
Figure 3.
SGLT2 Inhibition With Dapagliflozin Treatment Prevents the Increase in Blood Pressure in Hypertensive BPH/2J Mice
Effects of dapagliflozin treatment on (A) SBP, (B) DBP, and (C) MAP were measured using a tail-cuff apparatus, n = 10–12 mice/group. *p < 0.01; **p < 0.001; all data presented as mean ± SEM. Abbreviations as in Figure 1.
SGLT2 inhibition with dapagliflozin reduces activation of the SNS in hypertensive mice fed an HFD
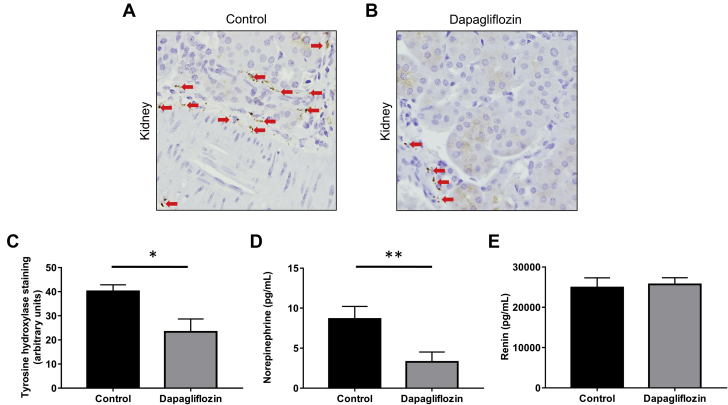
We next sought to assess whether SGLT2 inhibition may decrease markers of SNS innervation and activity. Kidneys from BPH/2J mice treated with dapagliflozin displayed significantly reduced tyrosine hydroxylase staining compared with control mice (control group: 40.1 ± 2.4 U dapagliflozin group: 23.4 ± 4.7 U p = 0.043) (Figures 4A to 4C). Dapagliflozin treatment also lowered the level of tyrosine hydroxylase staining observed in heart tissue from BPH/2J mice (Supplemental Figure 4). In addition, we examined the levels of norepinephrine, the major neurotransmitter of the SNS, in the kidney and found that dapagliflozin treatment significantly decreased norepinephrine content (control group: 8.6 ± 1.5 pg/ml; dapagliflozin group: 3.3 ± 1.2 pg/ml; p = 0.010) (Figure 4D). In contrast, measurements of renin serum levels showed no difference between BPH/2J mice with or without dapagliflozin treatment (Figure 4E). These observations strongly suggest that SGLT2 inhibition is associated with sympathoinhibition in this mouse model of hypertension, whereas renin does not appear to be affected.
Figure 4.
Inhibition of SGLT2 Reduces Sympathetic Innervation in the Kidney of Hypertensive BPH/2J Mice
Representative immunohistochemistry images of tyrosine hydroxylase expression in kidneys from (A) untreated BPH/2J mice or (B) BPH/2J mice treated with dapagliflozin. Tyrosine hydroxylase staining is indicated with arrows. Magnification 200×. (C) Quantitation of tyrosine hydroxylase expression in kidneys from BPH/2J mice, n = 4 to 6 mice/group. (D) Norepinephrine content in kidneys from BPH/2J mice with or without dapagliflozin treatment, n = 15 to 19 mice/group. (E) Renin concentration in serum from BPH/2J mice with or without dapagliflozin treatment, n = 14 to 18 mice/group. *p = 0.05; **p ≤ 0.01; all data presented as mean ± SEM. Abbreviation as in Figure 1.
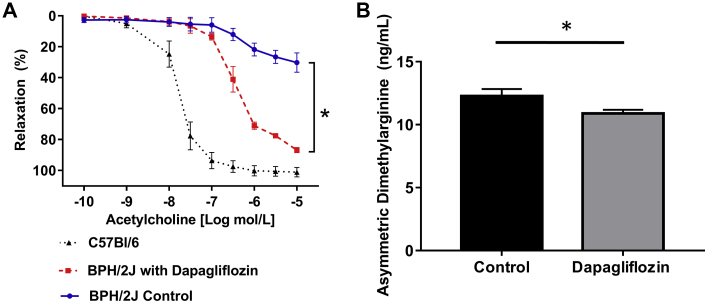
Effects of SGLT2 inhibition on endothelial dysfunction
Endothelial dysfunction is an important characteristic of hypertension. We showed that dapagliflozin treatment of BPH/2J mice stimulated acetylcholine-mediated relaxation of aortic rings compared with control C57BL6/J mice and BPH/2J mice (for acetylcholine log –5 mol/L, control group: 30.2 ± 5.3%; dapagliflozin group: 86.7 ± 2.6%; p = 0.017) (Figure 5A). Dapagliflozin treatment significantly decreased the level of the endogenous inhibitor of nitrous oxide synthase, asymmetric dimethylarginine, in the serum of BPH/2J mice (control group: 12.3 ± 0.5 ng/ml; dapagliflozin group: 10.9 ± 0.3 ng/ml; p = 0.037) (Figure 5B), suggesting that SGLT2 inhibition protects against endothelial dysfunction possibly mediated by reduced asymmetric dimethylarginine levels.
Figure 5.
Dapagliflozin Treatment Protects Against Endothelial Dysfunction in Hypertensive BPH/2J Mice
(A) Acetylcholine-mediated relaxation of mouse abdominal aortic rings in BPH/2J mice with and without dapagliflozin treatment and C57Bl/6 mice, n = 4 mice/group. (B) Measurement of the endogenous inhibitor of nitric oxide synthase, asymmetric dimethylarginine, in the serum of BPH/2J mice with and without dapagliflozin treatment, n = 9 to 10 mice/group. *p < 0.05; all data presented as mean ± SEM.
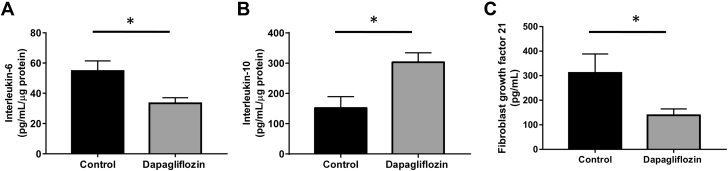
SGLT2 inhibition reduces expression of the hypertension-related cytokines in hypertensive mice
We also aimed to assess the levels of hypertension-related cytokines in our hypertensive mice treated with and without dapagliflozin. Dapagliflozin treatment mitigated cardiovascular inflammation as evidenced by a significant reduction in proinflammatory interleukin-6 protein levels in the heart of BPH/2J mice (control group: 54.8 ± 6.0 pg/ml/μg; dapagliflozin group: 33.4 ± 3.3 pg/ml/μg; p = 0.023) (Figure 6A). We also observed elevated expression of the anti-inflammatory cytokine interleukin-10 in renal (control group: 152.0 ± 33.5 pg/ml/μg; dapagliflozin group: 303.2 ± 28.1 pg/ml/μg; p = 0.014) (Figure 6B) and heart tissue from BPH/2J mice receiving dapagliflozin treatment (Supplemental Figure 5). Interestingly, dapagliflozin treatment resulted in a lower level of circulating fibroblast growth factor 21 in BPH/2J mice (control group: 311.5 ± 74.2 pg/ml; dapagliflozin group: 139.0 ± 24.4 pg/ml; p = 0.049) (Figure 6C).
Figure 6.
Assessment of Hypertension-Related Cytokines in Hypertensive BPH/2J Mice With and Without Dapagliflozin Treatment
(A) Interleukin-6 protein levels in hearts from BPH/2J mice with and without dapagliflozin treatment, n = 5 mice/group. (B) Renal interleukin-10 protein expression from BPH/2J mice with and without dapagliflozin treatment, n = 5 mice/group. (C) Fibroblast growth factor 21 protein levels in serum from BPH/2J mice with and without dapagliflozin treatment, n = 13 to 14 mice/group. *p < 0.05; all data presented as mean ± SEM.
Effects of SGLT2 inhibition in hypertensive mice on the production of SCFAs
The cecum contents of BPH/2J mice with or without dapagliflozin treatment were analyzed for levels of the SCFAs acetate and butyric acid. The concentrations of acetate and butyric acid were elevated in BPH/2J mice receiving dapagliflozin but not to a significant degree (Supplemental Figure 6).
Discussion
We were able to demonstrate that 6-OHDA treatment of mice with neurogenic hypertension diminishes the expression of tyrosine hydroxylase protein, which is indicative of reduced sympathetic nerve innervation. In turn, this triggered a lowering of blood pressure, improvements in glucose metabolism and a reduction in SGLT2 expression. Furthermore, dapagliflozin treatment of hypertensive mice strikingly reduced renal sympathetic activity as evidenced by decreased innervation of tyrosine hydroxylase positive nerves and norepinephrine content, thereby promoting blood pressure reductions and preventing weight gain, protecting against endothelial dysfunction and stimulating beneficial changes in the gut microbiome. Our study provides compelling evidence for a crosstalk between the SNS and SGLT2 regulation, and that sympathoinhibition is a very likely mediator of SGLT2 inhibitor–induced cardiorenal protection. Our study supports the development of SNS modulators as potential therapeutics in obesity and type 2 diabetes with a particular focus on regulation of SGLT2 activity.
Sympathetic hyperactivity is characteristic of numerous metabolic diseases including obesity, type 2 diabetes and the metabolic syndrome (7,8). Data from the EMPA-REG OUTCOME (3,4) and CANVAS (5) studies indicate that SGLT2 inhibition promotes beneficial cardiovascular and renal outcomes; however, the mechanisms underlying the protective effect of SGLT2 inhibition have not been fully elucidated. We previously reported that the main sympathetic neurotransmitter, norepinephrine, up-regulates SGLT2 protein levels in vitro and we showed for the first time that SGLT2 inhibition exerts its protective renal and cardiac properties via negatively regulating the SNS in obese HFD-fed mice (11). In our current study, we utilized genetically hypertensive Schlager BPH/2J mice (12,13) to directly assess the interaction between the SNS and SGLT2 inhibition in the context of hypertension. The use of Schlager mice in our study is highly relevant because hypertension is often neurogenic in nature (19), and Schlager mice mimic human disease with increased sympathetic activity (13), elevated heart rate, and heightened blood pressure (20). Excitingly, our novel findings highlight that: 1) chemical denervation of the SNS promotes blood pressure lowering, improves glucose homeostasis, and decreases SGLT2 expression; and 2) hypertensive mice treated with the SGLT2 inhibitor dapagliflozin have reduced sympathetic innervation, which may underlie the improvements in blood pressure and endothelial function that we observed in these mice. Together, our novel data strongly suggest that sympathoinhibition may be an important mechanism underlying the cardioprotective effect of SGLT2 inhibition. Our data provide compelling evidence for the bidirectional nature of the interaction between the SNS and SGLT2 expression and activity.
We have previously shown that renal denervation lowers blood pressure and improves glucose control in humans (21). Based on these findings, we hypothesized that norepinephrine induced the up-regulation of SGLT2 and this perturbs glucose homeostasis. Our data suggest that denervation of the SNS promotes numerous metabolic benefits in the context of hypertension.
With the knowledge that chemical denervation is metabolically beneficial in our neurogenic mouse model of hypertension and that SGLT2 inhibition may promote sympathoinhibition in HFD-fed mice (11), we examined whether similar metabolic benefits could be induced in our hypertensive mice with SGLT2 inhibition. Indeed, we observed that dapagliflozin-treated mice exhibited significantly reduced renal expression of tyrosine hydroxylase and norepinephrine content compared with control mice. Sano (22) proposed that the kidney is at the core of sympathetic hyperactivation and that SGLT2 inhibition may reduce renal afferent nervous activity and suppress the central reflex mechanisms that promote sympathetic activation, thereby heightening the cardiovascular protective effect of SGLT2 inhibition. Given the adverse consequences of sustained sympathetic hyperactivity for both cardiac and renal outcomes (23,24), we propose that SGLT2 inhibitor–induced reductions in renal and cardiac sympathetic activity are also contributors to the drug’s blood pressure–lowering capacity.
We also investigated the levels of hypertension-related cytokines in our hypertensive mice treated with and without dapagliflozin. The proinflammatory cytokine interleukin-6 is positively correlated with heart failure (25), high blood pressure (26), and aortic stiffness (27) in humans, and was significantly reduced by dapagliflozin treatment in heart tissue from our neurogenic hypertensive mice. This finding is consistent with our current data highlighting that dapagliflozin treatment markedly reduces blood pressure, perhaps indicating that the beneficial effect of SGLT2 inhibition on blood pressure may be partially mediated by the lowering of interleukin-6 levels in the heart. Similar to our findings, other studies now highlight the anti-inflammatory nature of SGLT2 inhibition (28,29). The nod-like receptor family, pyrin domain-containing 3 (NLRP3) is a potent mediator of inflammation, and its activity has been shown to be attenuated by the SGLT2 inhibitor dapagliflozin (30). Interestingly, dapagliflozin was the SGLT2 inhibitor used in our current study, and it would be interesting to assess the activity of NLRP3 inflammasome in the future.
Chronic sympathetic hyperactivity is linked to endothelial dysfunction in obese hypertensive patients (31). We showed that dapagliflozin treatment in our hypertensive mice stimulated acetylcholine-mediated relaxation of abdominal aortic rings compared with control mice. These data demonstrate that SGLT2 inhibition in neurogenic hypertensive mice protects against endothelial dysfunction and improves vascular function possibly mediated by reduced levels of asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, the enzyme responsible for production of the vasodilator nitric oxide. Our findings indicate that SGLT2-induced alterations of the L-arginine/NO pathway may yet provide another mechanism through which SGLT2 inhibitors exert their cardioprotective and renoprotective effects.
Another mechanistic pathway by which hypertension may result is activation of the renin-angiotensin system. In our study SGLT inhibition with dapagliflozin did not alter renin levels. Interestingly, Canagliflozin has been shown to prevent intrarenal angiotensinogen augmentation (28,32). Different SGLT2 inhibitors may therefore vary in their capabilities to affect the renin-angiotensin system.
There is growing interest in the role the gut microbiota plays in the pathology of the metabolic syndrome and type 2 diabetes (33). SCFAs are metabolites of gut microbiota and include acetate and butyric acid, which may contribute to both immune and inflammatory responses of the host, as well as lipid and glucose homeostasis. Studies are emerging that suggest that various drug classes alter gut microbiota composition (18,33) and that a reduction in SCFA production is a characteristic of hypertension. We analyzed cecal contents from our dapagliflozin- and vehicle-treated hypertensive mice and present exciting evidence that SGLT2 inhibition promotes elevations in the levels of the SCFAs acetate and butyric acid in cecal contents from hypertensive Schlager mice. Our preliminary data suggest a potential beneficial impact of SGLT2 inhibitors on the microbiome. In addition, as our results were evident after only 2 weeks of oral gavage therapy, future studies should be conducted to allow a more thorough examination of the microbiome status and ascertain whether longer therapy duration promotes further benefits on the microbiome.
Study limitations
Our study has been conducted in a mouse model of neurogenic hypertension and the SGLT2 inhibitor treatment time was 2 weeks at a single dosage. These factors may be considered as limitations. Our findings should be translated in clinical cohorts and various time courses and dosages of the SGLT2 inhibitor should be examined.
Conclusions
We utilized the neurogenic hypertensive Schlager BPH/2J mouse model (34) to directly elucidate the crosstalk between the SNS and SGLT2 regulation. Our studies indicate that denervation of the SNS promotes blood pressure reductions, improvements in glucose homeostasis, and reduced SGLT2 expression. Furthermore, our findings provide novel insights into the mechanisms of dapagliflozin-induced reduction of sympathetic activity and blood pressure, protection against endothelial dysfunction, reduction of inflammation in the heart and kidney, and heightened synthesis of SCFAs. The apparent reduction in SNS activity with SGLT2 inhibition may at least in part account for the underlying renal and cardioprotective properties of SGLT2 inhibition via a range of diverse mechanisms. It is essential that future studies aim to assess whether SGLT2 inhibition will also result in sympathoinhibition in human hypertensive subjects.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Both the EMPA-REG OUTCOME and CANVAS studies indicate that SGLT2 inhibition promotes beneficial cardiovascular and renal outcomes; however, the mechanisms underlying the protective effect of SGLT2 inhibition have not been fully elucidated.
TRANSLATIONAL OUTLOOK: Our study highlights that dapagliflozin treatment in genetically hypertensive mice promotes significant improvements in hypertension and endothelial function and, diminishes sympathetic innervation and inflammation in the heart and kidney. Our studies will create the foundations for human clinical trials that aim to assess the mechanisms underlying cardiorenal protection.
Acknowledgment
The authors acknowledge Nicola P. Bondonno (School of Medicine, University of Western Australia, Royal Perth Hospital, Perth, Australia) for her assistance with the analysis of the endothelial dysfunction assays.
Footnotes
The authors kindly acknowledge funding from the Royal Perth Hospital Research Foundation and Diabetes Research Western Australia awarded to Drs. Schlaich and Matthews. Dr. Head is supported by a National Health and Medical Research Council Research Fellowship and has received research grant support from Boehringer Ingelheim. Prof. Schlaich is supported by a National Health and Medical Research Council Research Fellowship and has received research support from Medtronic, Abbott, Novartis, Servier, Pfizer, and Boehringer Ingelheim. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
For supplemental figures, please see the online version of this paper.
Appendix
References
- 1.Ghezzi C., Loo D.D.F., Wright E.M. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia. 2018;61:2087–2097. doi: 10.1007/s00125-018-4656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uthman L., Baartscheer A., Schumacher C.A. Direct cardiac actions of sodium glucose cotransporter 2 inhibitors target pathogenic mechanisms underlying heart failure in diabetic patients. Front Physiol. 2018;9:1575. doi: 10.3389/fphys.2018.01575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wanner C., Inzucchi S., Lachin J. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 4.Zinman B., Wanner C., Lachin J. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 5.Neal B., Perkovic V., Mahaffey K. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 6.Briasoulis A., Dhaybi O., Bakris G. SGLT2 inhibitors and mechanisms of hypertension. Curr Cardiol Rep. 2018;20:1. doi: 10.1007/s11886-018-0943-5. [DOI] [PubMed] [Google Scholar]
- 7.Schlaich M., Straznicky N., Lambert E., Lambert G. Metabolic syndrome: a sympathetic disease? Lancet Diabetes Endocrinol. 2015;3:148–157. doi: 10.1016/S2213-8587(14)70033-6. [DOI] [PubMed] [Google Scholar]
- 8.Thorp A., Schlaich M. Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J Diabetes Res. 2015;2015:341583. doi: 10.1155/2015/341583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000;43:533–549. doi: 10.1007/s001250051341. [DOI] [PubMed] [Google Scholar]
- 10.Elliot R., Matthews V., Rudnicka C., Schlaich M. Is it time to think about the sodium glucose co-transporter 2 sympathetically? Nephrology (Carlton) 2016;21:286–294. doi: 10.1111/nep.12620. [DOI] [PubMed] [Google Scholar]
- 11.Matthews V., Elliot R., Rudnicka C., Hricova J., Herat L., Schlaich M. Role of the sympathetic nervous system in regulation of the sodium glucose co-transporter 2. J Hypertens. 2017;35:2059–2068. doi: 10.1097/HJH.0000000000001434. [DOI] [PubMed] [Google Scholar]
- 12.Schlager G., Sides J. Characterization of hypertensive and hypotensive inbred strains of mice. Lab Anim Sci. 1997;47:288–292. [PubMed] [Google Scholar]
- 13.Davern P., Nguyen-Huu T.-P., Greca L.L., Abdelkader A., Head G. Role of the sympathetic nervous system in Schlager genetically hypertensive mice. Hypertension. 2009;54:852–859. doi: 10.1161/HYPERTENSIONAHA.109.136069. [DOI] [PubMed] [Google Scholar]
- 14.Kiuchi S., Hisatake S., Kabuki T. Long-term use of ipragliflozin improved cardiac sympathetic nerve activity in a patient with heart failure: a case report. Drug Discov Ther. 2018;12:51–54. doi: 10.5582/ddt.2017.01069. [DOI] [PubMed] [Google Scholar]
- 15.Lin J.-C., Peng Y.-J., Wang S.-Y., Young T.-H., Salter D., Lee H.-S. Role of the sympathetic nervous system in carbon tetrachloride-induced hepatotoxicity and systemic inflammation. PLoS One. 2015;10 doi: 10.1371/journal.pone.0121365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang R., Hodgson J., Mas E., Kroft K., Ward N. Chlorogenic acid improves ex vivo vessel function and protects endothelial cells against HOCl-induced oxidative damage, via increased production of nitric oxide and induction of Hmox-1. J Nutr Biochem. 2016;27:53–60. doi: 10.1016/j.jnutbio.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Tan S., Caparros-Martin J.A., Matthews V.B. Isoquercetin and inulin synergistically modulate the gut microbiome to prevent development of the metabolic syndrome in mice fed a high fat diet. Sci Rep. 2018;8:10100. doi: 10.1038/s41598-018-28521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caparros-Martin J.A., Lareu R.R., Ramsay J.P. Statin therapy causes gut dysbiosis in mice through a PXR-dependent mechanism. Microbiome. 2017;5:95. doi: 10.1186/s40168-017-0312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grassi G., Arenare F., Pieruzzi F., Brambilla G., Mancia G. Sympathetic activation in cardiovascular and renal disease. J Nephrol. 2009;22:190–195. [PubMed] [Google Scholar]
- 20.Chiu C., Jackson K., Hearn N., Steiner N., Head G., Lind J. Identification of genes with altered expression in male and female Schlager hypertensive mice. BMC Med Genet. 2014;15:101–106. doi: 10.1186/s12881-014-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahfoud F., Schlaich M., Kindermann I. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension. Circulation. 2011;123:1940–1946. doi: 10.1161/CIRCULATIONAHA.110.991869. [DOI] [PubMed] [Google Scholar]
- 22.Sano M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J Cardiol. 2018;71:471–476. doi: 10.1016/j.jjcc.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Zoccali C., Mallamaci F., Parlongo S. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105:1354–1359. doi: 10.1161/hc1102.105261. [DOI] [PubMed] [Google Scholar]
- 24.Schlaich M., Kaye D., Lambert E., Sommerville M., Socratous F., Esler M. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation. 2003;108:560–565. doi: 10.1161/01.CIR.0000081775.72651.B6. [DOI] [PubMed] [Google Scholar]
- 25.Collier P., Watson C., Voon V. Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail. 2011;13:1087–1095. doi: 10.1093/eurjhf/hfr079. [DOI] [PubMed] [Google Scholar]
- 26.Bautista L., Vera L., Arenas I., Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-a) and essential hypertension. J Hum Hypertension. 2005;19:149–154. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 27.Desjardins M., Sidibe A., Fortier C. Association of interleukin-6 with aortic stiffness in end-stage renal disease. J Am Soc Hypertens. 2018;12:5–13. doi: 10.1016/j.jash.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Bakris G.L. Major advancements in slowing diabetic kidney disease progression: focus on SGLT2 inhibitors. Am J Kidney Dis. 2019;5:573–575. doi: 10.1053/j.ajkd.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Yaribeygi H., Katsiki N., Butler A.E., Sahebkar A. Effects of antidiabetic drugs on NLRP3 inflammasome activity, with a focus on diabetic kidneys. Drug Discov Today. 2019;24:256–262. doi: 10.1016/j.drudis.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Ye Y., Bajaj M., Yang H.C., Perez-Polo J.R., Birnbaum Y. SGLT-2 inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. Further augmentation of the effects with saxagliptin, a DPP4 inhibitor. Cardiovasc Drugs Ther. 2017;31:119–132. doi: 10.1007/s10557-017-6725-2. [DOI] [PubMed] [Google Scholar]
- 31.Gamboa A., Figueroa R., Paranjape S., Farley G., Diedrich A., Biaggioni I. Autonomic blockade reverses endothelial dysfunction in obesity-associated hypertension. Hypertension. 2016;68:1004–1010. doi: 10.1161/HYPERTENSIONAHA.116.07681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woods T.C., Satou R., Miyata K. Canagliflozin prevents intrarenal angiotensinogen augmentation and mitigates kidney injury and hypertension in mouse model of type 2 diabetes mellitus. Am J Nephrol. 2019;49:331–342. doi: 10.1159/000499597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montandon S., Jornayvaz F. Effects of antidiabetic drugs on gut microbiota composition. Genes (Basel) 2017;8:250. doi: 10.3390/genes8100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herat L., Magno A., Kiuchi M. The Schlager mouse as a model of altered retinal phenotype. Neural Regen Res. 2020;15:512–518. doi: 10.4103/1673-5374.266069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.