Abstract
Background
An average adult American consumes sulfur amino acids (SAA) at levels far above the Estimated Average Requirement (EAR) and recent preclinical data suggest that higher levels of SAA intake may be associated with a variety of aging-related chronic diseases. However, there are little data regarding the relationship between SAA intake and chronic disease risk in humans. The aim of this study was to examine the associations between consumption of SAA and risk factors for cardiometabolic diseases.
Methods
The sample included 11,576 adult participants of the Third National Examination and Nutritional Health Survey (NHANES III) Study (1988–1994). The primary outcome was cardiometabolic disease risk score (composite risk factor based on blood cholesterol, triglycerides, HDL, C-reactive protein (CRP), uric acid, glucose, blood urea nitrogen (BUN), glycated hemoglobin, insulin, and eGFR). Group differences in risk score by quintiles of energy-adjusted total SAA, methionine (Met), and cysteine (Cys) intake were determined by multiple linear regression after adjusting for age, sex, BMI, smoking, alcohol intake, and dietary factors. We further examined for associations between SAA intake and individual risk factors.
Findings
Mean SAA consumption was > 2.5-fold higher than the EAR. After multivariable adjustment, higher intake of SAA, Met, and Cys were associated with significant increases in composite cardiometabolic disease risk scores, independent of protein intake, and with several individual risk factors including serum cholesterol, glucose, uric acid, BUN, and insulin and glycated hemoglobin (p < 0.01).
Interpretation
Overall, our findings suggest that diets lower in SAA (close to the EAR) are associated with reduced risk for cardiometabolic diseases. Low SAA dietary patterns rely on plant-derived protein sources over meat derived foods. Given the high intake of SAA among most adults, our findings may have important public health implications for chronic disease prevention.
Funding
This study does not have any funding.
Keywords: Dietary sulfur amino acids, Methionine, Cysteine, Cardiometabolic diseases, Diabetes, Sulfur amino acids restriction
Abbreviations: SAA, sulfur amino acids; RDA, recommended dietary allowance; Met, methionine; Cys, cysteine; SAAR, sulfur amino acid restriction; IR, insulin resistance; EAR, estimated average requirement; NHANES III, Third National Examination and Nutritional Health Survey; CRP, C-reactive protein; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; MEC, mobile examination center
Research in Context.
Evidence before this study
Although sulfur amino acids play critical roles in metabolism and overall health maintenance, accumulating evidence from animal studies have suggested that diets restricted in sulfur amino acids are associated with many health benefits including increased longevity and reductions in aging-related diseases and disorders. Since the initial study by Orentreich et al. in 1993 demonstrating that lifelong feeding of an amino acid-defined diet low in methionine as the sole sulfur amino acid source increased maximum life span in rats, similar methionine restriction interventions have been shown to delay aging in a number of animal and cell-based models. Further, low sulfur amino acid diets have been associated with reductions in body weight, adiposity, and oxidative stress, improved glucose metabolism, and beneficial changes in the levels of a variety of blood biomarkers, including insulin, glucose, leptin, adiponectin, insulin-like grown factor-1, and fibroblast growth factor-21. To date, there is little data regarding the potential long-term health benefits of low sulfur amino acid diets in humans. Thus, our goal was to investigate whether diets low in sulfur amino acids were associated with reductions in risk factors for cardiometabolic diseases in a nationally representative study population.
Added value of this study
This is the first epidemiologic study to explore the association between sulfur amino acid intake and cardiometablic disease risk in adults. The study population consisted of a large nationally representative cohort from diverse socioeconomic backgrounds. Composite risk scores based on a series of relevant risk factors, calculated either categorically or continuously, served as primary outcomes and resulted in similar associations with sulfur amino acid intake. Further, different methods were used to control potential confounding by protein intake. The findings can serve as novel scientific evidence for the potential establishment of new sulfur amino acid intake recommendations for optimal long-term health in adults.
Implications of all the available evidence
Our findings of a positive relationship between sulfur amino acid intake and cardiometabolic disease risk are of significant public health importance given the high rates of these diseases and high intake of sulfur amino acids in many developed countries. These results, together with previous preclinical data provide strong evidence for a novel dietary approach for chronic disease prevention based on the reduction of sulfur amino acid intake. The finding that low sulfur amino acid diets are typically more heavily reliant on plant-derived proteins suggests that sulfur amino acid reduction may, in part, be responsible for health benefits associated with a plant-based diet and offer a feasible approach for reducing sulfur amino acid intake.
Alt-text: Unlabelled box
1. Introduction
Extensive investigations in animal models have highlighted the role of sulfur amino acids (SAA) restricted diets in delaying the aging process and inhibiting the onset of aging-related diseases and disorders [1,2]. Beneficial effects of dietary SAA restriction (SAAR) include life span extension [3], reductions in body weight and adiposity [4], reduced insulin resistance (IR), and positive changes in blood biomarkers including insulin, glucose, leptin, and adiponectin [2,5].
As an essential dietary component, established nutritional requirement levels for total SAA include the Estimated Average Requirement (EAR) of 15 mg/kg/day (required for meeting the needs of half of the population of healthy adults) and Recommended Daily Allowance (RDA) of 19 mg/kg/day (12.2 mg/kg/day for methionine (Met) and 6.6 mg/kg/day for cysteine (Cys)) (required for meeting the needs of 97%–98% of the population of healthy adults) [6,7]. However, nationally representative studies in the US indicate that the majority of adults consume diets that are well in excess of dietary SAA requirements [7,8]. While most amino acids are thought to be relatively safe when consumed at typical dietary levels, there is accumulating evidence of negative health consequences linked with high intakes of both Met and Cys [9,10]. In addition to laboratory animal studies showing health benefits of SAAR diets, toxicity associated with high levels of SAA intake include growth inhibition in laboratory animals [9] and elevated risk of cardiometabolic diseases, including type 2 diabetes and cardiovascular disease (CVD), which are associated with elevated homocysteine levels in animal models and humans [11,12]. Overall, these and other studies have led Met and Cys to be considered among the most toxic amino acids [13,14] with potential implications for enhanced risk for chronic diseases [12,15]. However, to date, human data regarding SAA intake and disease risk are sparse.
To further investigate the hypothesis that lower consumption of SAAs is protective against the development of cardiometabolic diseases, we conducted a cross-sectional analysis among NHANES III participants of SAA intake in relation to relevant risk-related biomarkers in heart disease-free adults. In particular, we examined dietary intake of SAA in relation to serum levels of total cholesterol, HDL cholesterol, triglycerides, insulin, glucose, glycated hemoglobin, C-reactive protein (CRP), uric acid, and blood urea nitrogen (BUN) as well as blood pressure, and estimated glomerular filtration rate (eGFR).
2. Methods
2.1. Ethics
Institutional review board approval is not required for secondary analysis using the NHANES data. The data collection process of the NHANES III has its own institutional review board and written and oral informed consent procedures [16].
2.2. Study population
NHANES III study participants were recruited from 1988 to 1994 as a nationally representative sample of the civilian, noninstitutionalized US population [17]. NHANES III was selected as subsequent NHANES datasets do not include SAA intake data. Participants gave informed consent and the study was conducted in accordance with principles of the Declaration of Helsinki. In NHANES III, each survey participant completed a household interview and underwent a physical examination [18]. We included individuals aged 18 years or older with complete data on diet, laboratory tests, and relevant covariates (N = 14,294). Because we were interested in risk factors contributing to CVD, we excluded 933 participants who had reported having either congestive heart failure, a heart attack, or who had reported they changed their diets due to a heart disease diagnosis. We also excluded 874 individuals who reported extreme daily energy intake (< 800 or > 4200 for males and < 500 or > 3500 for females); and 911 individuals who reported dietary intake of SAA below the EAR (15 mg/kg/day) [8] at baseline. After these exclusions, 11,576 NHANES III participants were included in the present analyses.
2.3. Dietary sulfur amino acid assessment
At the mobile examination center (MEC), anthropometric measurements were taken and MEC Questionnaire and 24-h Dietary Recall data were collected along with a variety of other tests and procedures as described previously [17]. Dietary information was obtained from in-person 24-h dietary recalls with use of a personal computer-based, automated, interactive data collection and coding system [17]. All MEC participants provided a single 24-h dietary recall including nutrients from foods and beverages, and a subsample of about 8% of participants provided a second 24-h dietary recall. The US Department of Agriculture Survey Nutrient Database (http://www.cdc.gov/nchs/nhanes/nh3data.htm) was used to calculate the total nutrient intakes based on the University of Minnesota Nutrition Coordinating Center nutrient database data. The nutritional assessment methodology for NHANES III was validated by the Nutrition Methodology Working Group, which consisted of National Center for Health Statistics staff with nutritional assessment expertise [18]. In the subset of subjects with two 24-h recalls, SAA intake was highly correlated between the two assessment days (r = 0.68). Because absolute Met and Cys intakes tend to be strongly correlated with overall energy and protein intake and with each other, exposures were assessed as absolute total SAA intake (mg/day) adjusted for total energy intake using the residual method [19]. In order to control for potential residual confounding from variation in protein intake, we further conducted a secondary analyses of SAA intake expressed as protein density (SAA intake as the percentage of total protein intake).
2.4. Biomarkers of cardiometabolic disease risk
Cardiometabolic diseases are represented by a cluster of diseases including CVD, diabetes, and metabolic syndrome [20]. The primary outcome in this study was a composite cardiometabolic disease risk score, based on blood pressure, eGFR, and blood levels of total cholesterol, HDL cholesterol, triglycerides, insulin, glucose, glycated hemoglobin, CRP, uric acid, and BUN. Inclusion of each of these components is supported by the results of factor analyses which indicated a high degree of inter-correlation in the underlying patterns or structure of these variables [21,22]. Blood biomarkers were analyzed as previously reported [18]. The eGFR was calculated by using the Chronic Kidney Disease Epidemiology equation [23]. Blood pressure was measured three times by trained personnel and recorded to the nearest even number according to a standardized protocol. The average of the three measurements was used in data analysis. Details about all laboratory procedures have been published in the NHANES III reference documents [18]. Biomarkers available in the NHANES III database, but excluded from the present analyses, include LDL cholesterol and lipids since only a smaller and less representative sample was available for these endpoints.
A cardiometabolic disease risk score was calculated based on the twelve risk factors described above. Risk scores were derived by standardizing the individual risk variables by regressing them onto age, sex, and race to account for any age/sex/race-related differences. For each of these variables, a Z score was computed as the number of standard deviation (SD) units from the sample mean after normalization of the variables, i.e., Z=[value-mean]/SD. The Z score was multiplied by −1 for HDL and eGFR since they are inversely related to cardiometabolic disease risk. The standardized residuals (Z score) for all twelve variables were summed to create a composite risk score. These variables were chosen based on their usage by the International Diabetes Federation and Adult Treatment Panel III as adult clinical criteria for metabolic syndrome [24,25]. A higher risk score indicates a less favorable cardiometabolic profile.
For each of the variables, a dichotomous indicator was also created as a secondary outcome, reflecting those with “high-risk” values (assigned a score of “1”) and “lower risk” values (assigned a score of “0”). Risk category cut-off values were based upon clinically accepted “high-risk” criteria: Serum level of cholesterol >199 mg/dL [26], HDL cholesterol <40 mg/dL [26], triglycerides >149 mg/dL [27], insulin >25 μU/mL [28], plasma glucose >100 mg/dL [29], glycated hemoglobin >5.7% [30], CRP <1 mg/dL [18], BUN >20 mg/dL [31], uric acid >6.0 mg/dL [32], eGFR <90 mL/min/1.73 m2 [33], systolic blood pressure >130 mm Hg [34] and diastolic blood pressure >80 mm Hg [34]. These indicator variables were then summed to create a composite cardiometabolic disease risk score with range from 0 to 12 (cumulative score).
2.5. Other variables
Age, sex, race/ethnic group (White, Black, and other), educational attainment (years), smoking status (ever smoked 100 cigarettes), poverty income ratio (components of family income and poverty threshold), physical activity (times/month) were self-reported. Height and weight were measured by using standardized methods, and body mass index (BMI) was calculated as weight divided by height squared. History of diabetes and hypertension were assessed by self-reporting, taking pills for such diseases, or changed diets because of such diseases. We included other dietary intake values as continuous covariates in our analyses including: alcohol, total fat, calcium, magnesium, sodium, potassium, vitamin A, vitamin C, vitamin B6, and folic acid. In addition, the Healthy Eating Index (HEI), a diet quality index that measures conformance to federal dietary guidelines [35], was calculated based on diet recall data to assess overall diet quality by SAA intake quintile. All data, including specimen collection for assay of cardiometabolic measures, measurement of body size and self-reported demographic information, were collected at the same visit. NHANES III was approved by the National Center Health Statistics Institutional Review Board. Written informed consent was obtained from all participants.
2.6. Statistical analysis
We used SAS software version 9.4 (SAS Institute) for all statistical analysis. In addition to Met and Cys intake, total SAA intake was defined as the sum of the two individual SAA, Met and Cys. We generated quintile categories of absolute intakes of total SAA, Met, and Cys, and generated quintile categories of protein density intakes of total SAA. Means and proportions of baseline characteristics, as well as dietary nutrient intake, were compared across absolute SAA intake quintiles by using chi-square for categorical variables and analysis of variance (ANOVA) for continuous variables. Full adjusted participant risk scores (both Z score and cumulative score) were compared across quintile categories of SAA intake (both absolute intake and protein density intake) by using the SAS procedure PROC GLM. We also performed analysis of covariance (PROC GLM) to examine the relation between dietary SAA intake and the means of individual risk factors continuously, with adjustment for their potential confounders, including age, sex, race/ethnic, BMI (categorical), smoking status (yes or no), alcohol intake (g/day), and intake of total fat (g/day), calcium (mg/day), magnesium (mg/day), sodium (g/day), potassium (g/day), vitamin A (IU/day), vitamin C (mg/day), vitamin B6 (mg/day), and folic acid (mcg/day), as well as history of diabetes and hypertension. Tests for trends for each risk factor outcome were conducted by assigning the median value to each quintile category and including these values in multiple linear regression models as a continuous variable. The results would indicate whether there is a significant trends between quintile groups. To further test for the robustness of the findings, we conducted several secondary analyses by including participants who consumed SAA less than 15 mg/kg/day and excluding participants who had reported changing their diets due to hypertension and/or diabetes (437 participants). In addition, we added animal protein and vegetable protein as additional covariates in a sensitivity analysis.
3. Results
3.1. Baseline characteristics and dietary nutrient intake of healthy NHANES participants by quintiles of absolute SAA intake
The 11,576 participants included in this study were representative of the over 99 million non-institutionalized US adults ≥18 years of age during the 1988–1994 survey period. Mean (± SD) SAA intake for the study sample was 2.83 ± 0.89 g/day with a median of 2.75 g/day. After accounting for body weight, the average intake of SAA (39.2 ± 18.1 mg/kg/day) was more than 2.5-fold higher than the EAR for adults (15 mg/kg/day) and, among participants in the highest SAA quintile, intake was over 4-times higher than the EAR. Selected baseline characteristics of participants according to quintiles of total dietary SAA (sum of energy-adjusted dietary Met and Cys) are provided in Table 1. Baseline characteristics for intake of total SAA as expressed by protein density were similar to the absolute SAA intake, and are provided in Supplemental Table 1. All characteristics were significantly different by quintile except for smoking status and physical activity. Participants with higher SAA intake were more likely to be men of a race other than white, and have higher BMI, be less educated, and have a history of diabetes. Table 2 presents intakes of selected dietary nutrients of participants from the cohort according to quintiles of total dietary SAA. Higher SAA intake was positively associated with vitamin A, vitamin B6, folic acids, calcium, magnesium, total protein, and animal protein. Higher SAA intake was positively associated with intake of almost every type of food except grains, vegetables, and fruit. It is noteworthy that HEI values were lower among participants with higher SAA intake compared to those with lower SAA intake.
Table 1.
Baseline characteristics by absolute total energy-adjusted SAA intake quintiles.
| Characteristics | Quintile of total SAAs intake |
||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |
| N | 2315 | 2315 | 2316 | 2315 | 2315 |
| Total SAA, g/day1 | |||||
| Median (range) | 1.50 (0.70 - 1.80) | 2.10 (1.80–2.30) | 2.60 (2.40–2.90) | 3.30 (2.90–3.70) | 4.50 (3.80–14.70) |
| Total SAA, mg/kg/day1 | |||||
| Median (range) | 20.1 (15.0–24.1) | 27.7 (24.1–31.6) | 35.5 (31.6–39.7) | 44.9 (39.7–51.7) | 62.7 (51.7–175.6) |
| Age, years | 43.6 | 47.7 | 49.1 | 48.6 | 45.7 |
| BMI, Kg/m2 | 26.1 | 26.4 | 26.9 | 27.3 | 27.5 |
| Gender, % | |||||
| Male | 49.0 | 37.8 | 39.7 | 44.7 | 57.7 |
| Female | 51.0 | 62.2 | 60.3 | 55.3 | 42.3 |
| Race, % | |||||
| White | 70.1 | 71.9 | 71.2 | 66.9 | 67.4 |
| Black | 27.8 | 25.0 | 25.1 | 27.6 | 28.1 |
| Other | 2.1 | 3.1 | 3.8 | 3.5 | 4.5 |
| Poverty Income Ratio [2] | 2.6 | 2.5 | 2.4 | 2.6 | 2.4 |
| Physical Activity, times/month | 11.9 | 7.8 | 7.5 | 7.4 | 8.1 |
| Education level, years | 11.8 | 11.3 | 11.1 | 11.0 | 11.0 |
| Smoked 100+ cigarettes, % | 53.2 | 46.6 | 47.5 | 48.8 | 52.4 |
| Alcohol intake, g/day | 15.7 | 7.0 | 4.9 | 5.2 | 6.0 |
| Diabetes, % | 3.5 | 4.8 | 7.0 | 10.4 | 11.0 |
| Hypertension, % | 30.9 | 34.7 | 36.2 | 37.9 | 35.0 |
Values for SAA intake are not adjusted for energy.
Poverty income ratio was computed as a ratio of two components: The numerator is the midpoint of the observed family income and denominator is the poverty threshold based on the age of the family reference person and the calendar year in which the family was interviewed.
Table 2.
Baseline nutrient intake in individuals by absolute total energy-adjusted SAA intake quintile.
| Nutrients Intakes | Quintile of total SAAs intake |
||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |
| N | 2315 | 2315 | 2316 | 2315 | 2315 |
| Energy intake, kcal/day | 2353 | 1905 | 1830 | 1882 | 2241 |
| Fiber intake, g/day | 19.1 | 16.9 | 16.5 | 16.6 | 18.1 |
| Total fat intake, g/day | 87.2 | 72.4 | 70.3 | 73.2 | 87.5 |
| Carbohydrate intake, g/day | 313 | 245 | 227 | 221 | 239 |
| Polyunsaturated:saturated fat ratio | 0.70 | 0.66 | 0.66 | 0.65 | 0.67 |
| Vitamin A intake, IU/day | 5943 | 5719 | 5863 | 6502 | 7736 |
| Vitamin C intake, mg/day | 107 | 92 | 86 | 88 | 93 |
| Vitamin B6 intake, mg/day | 1.7 | 1.5 | 1.6 | 1.8 | 2.3 |
| Folic acid intake, mcg/day | 282 | 261 | 265 | 274 | 312 |
| Calcium intake, mg/day | 721 | 693 | 719 | 754 | 863 |
| Magnesium intake, mg/day | 287 | 256 | 259 | 275 | 332 |
| Sodium intake, g/day | 3.3 | 3.1 | 3.0 | 3.2 | 4.0 |
| Potassium intake, g/day | 2.6 | 2.4 | 2.4 | 2.5 | 2.3 |
| Protein intake, g/day | 62 | 63 | 70 | 81 | 117 |
| Animal protein intake, g/day | 34.7 | 39.0 | 45.9 | 57.7 | 91.3 |
| Vegetable protein intake, g/day | 25.9 | 23.3 | 22.9 | 22.8 | 25.4 |
| Grain, serving/day | 7.2 | 6.4 | 6.2 | 6.1 | 6.8 |
| Fruit, serving/day | 1.7 | 1.6 | 1.4 | 1.4 | 1.4 |
| Meat, serving/day | 1.4 | 1.6 | 1.9 | 2.4 | 4.0 |
| Vegetables, serving/day | 3.1 | 2.8 | 2.9 | 3.0 | 3.4 |
| Dairy, serving/day | 1.7 | 1.6 | 1.7 | 1.8 | 2.1 |
| Legumes, serving/day | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Healthy Eating Index | 64.2 | 64.2 | 64.3 | 64.3 | 62.3 |
3.2. Cardiometabolic risk scores by quintiles of SAA intake
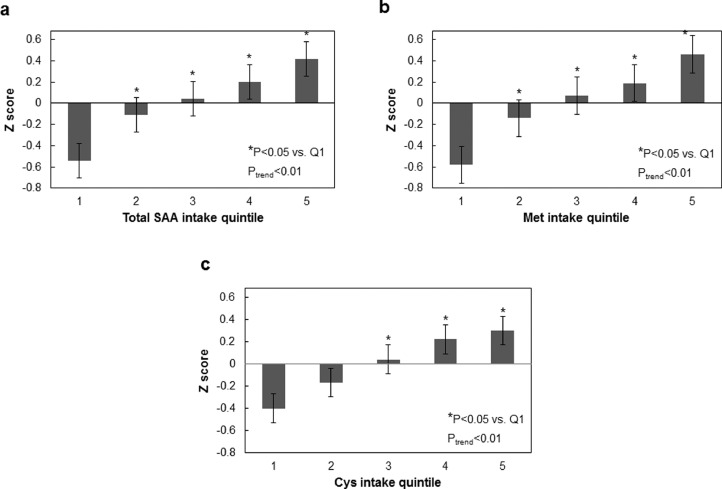
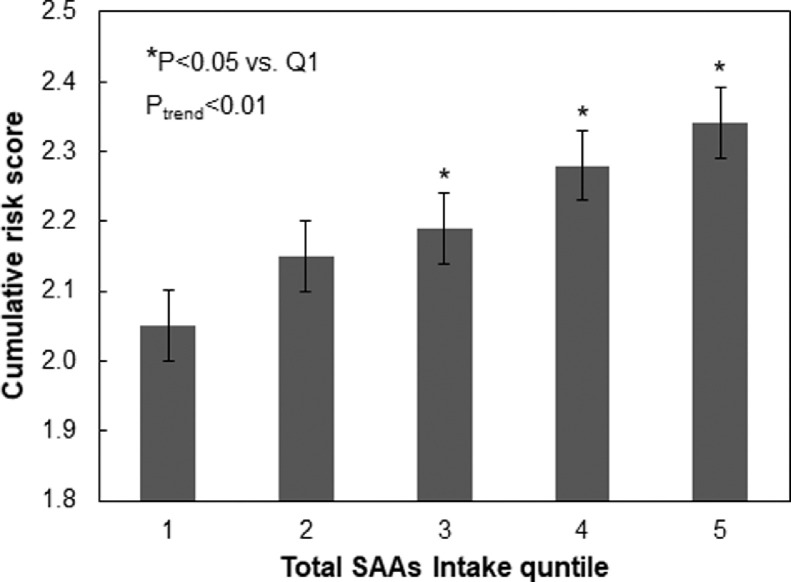
The associations of total SAA, Met, and Cys intake and cardiometabolic risk score are presented in Fig. 1. The continuous risk score was positively correlated with total SAA, Met, and Cys intake (Ptrend < 0.01). The risk scores for highest total SAA and Cys quintiles (3–5) were significantly higher than the lowest quintile (Ptrend < 0.01). The risk scores for highest Met quintiles (2–5) were significantly higher than the lowest quintile (Ptrend < 0.01). A positive relationship was additionally found for total SAA intake and cumulative cardiometabolic disease risk score (Ptrend < 0.01) (Fig. 2), with the risk scores for highest total SAA quintiles (3–5) being significantly higher compared to the lowest quintile (P < 0.01). In analyses that used protein density as the exposure, there was a marginally significant trend of increasing Z score with increasing total SAA intake with the exception of the highest quartile (Ptrend = 0.06) (Supplementary Fig. 1). In sensitivity analysis where participants who consumed SAA less than 15 mg/kg/day were included, and where participants reported having changed their dietary habits due to hypertension and/or diabetes were excluded, total SAA intake was also positively associated with increased Z score (Ptrend < 0.01) (Supplementary Figs. 2 and 3, respectively). In sensitivity analyses where we added animal and vegetable protein intake as covariates, total SAA intake was also positively associated with increased Z score (Ptrend = 0.05) (Supplementary Fig. 4).
Fig. 1.
Relationship between mean composite cardiometabolic disease risk factor Z-score and intake quintile of total SAA (a), Met (b) and Cys (c). Composite risk factor Z-scores were calculated based upon individual continuous risk factors as described in text. Error bars are standard error values.
Fig. 2.
Relationship between quintile of total SAA intake and mean cumulative categorical cardiometabolic disease risk score. Categorical risk scores were calculated as described in text. Error bars are standard error values.
3.3. Total SAA, Met, and Cys intake and specific cardiometabolic diseases risk factors
Associations of total SAA intake with specific cardiometabolic disease risk factors are presented in Table 3. Dietary intake of total SAA was positively associated with levels of glycated hemoglobin, eGFR, cholesterol, HDL cholesterol, glucose, uric acid, BUN, and insulin (Ptrend ≤ 0.05). No associations between SAA intake and blood pressure and serum levels of triglycerides, CRP were observed (Ptrend ≥ 0.10). All associations were similar for total SAA, Met and Cys except for eGFR which was not significant for Met (Ptrend = 0.32) (Supplemental Tables 1 and 2).
Table 3.
Adjusted geometric means of risk factors across quintiles of total SAA intake.
| Quintile of total SAA intake |
P-Value |
|||||||
|---|---|---|---|---|---|---|---|---|
| Risk factors | Q1 | Q2 | Q3 | Q4 | Q5 | Q5 vs Q1 | Trend | |
| Z Score | −0.60 | −0.27 | −0.07 | 0.29 | 0.64 | <0.01 | <0.01 | |
| Serum cholesterol (mg/dL) | ||||||||
| Unadjusted | 203 (201–205) | 204 (203–206) | 204(203–206) | 207 (205–209) | 206 (205–208) | 0.07 | <0.01 | |
| Adjusted* | 203 (201–205) | 204 (203–206) | 204 (203–206) | 207 (205–209) | 207 (205–208) | 0.04 | <0.01 | |
| Serum HDL cholesterol (mg/dL) | ||||||||
| Unadjusted | 52.0 (51.4–52.6) | 52.1 (51.4–52.7) | 51.7 (51.0–52.3) | 51.4 (50.8–52.0) | 50.5 (49.8–51.1) | <0.01 | <0.01 | |
| Adjusted* | 51.2 (50.5–51.8) | 51.0 (50.4–51.6) | 51.3 (50.7–51.9) | 51.9 (51.3–52.4) | 52.3 (51.7–52.9) | 0.09 | <0.01 | |
| Systolic pressure (mmHg) | ||||||||
| Unadjusted | 126 (124–127) | 125 (124–127) | 126 (125–127) | 126 (125–126) | 126 (125–128) | 0.95 | 0.30 | |
| Adjusted* | 125 (124–126) | 126 (125–127) | 127 (126–128) | 126 (125–128) | 125 (124–127) | 0.99 | 0.77 | |
| Diastolic pressure (mmHg) | ||||||||
| Unadjusted | 75.0 (73.6–76.3) | 75.1 (73.8–76.4) | 75.8 (74.5–77.1) | 76.1 (74.8–77.4) | 75.4 (74.1–76.7) | 0.99 | 0.49 | |
| Adjusted* | 74.6 (73.2–6.0) | 75.6 (74.3–76.9) | 76.3 (75.0–77.7) | 76.1 (74.8–77.4) | 74.7 (73.3–76.1) | 1.00 | 1.00 | |
| Serum glucose (mg/dL) | ||||||||
| Unadjusted | 95.9 (94.5–97.2) | 96.8 (95.4–98.2) | 98.1 (96.7–99.5) | 101 (99.9–103) | 103 (102- 105) | <0.01 | <0.01 | |
| Adjusted* | 97.6 (96.3–98.8) | 99.1 (97.9–100) | 99.0 (97.8–100) | 99.5 (98.3- 101) | 100 (99.1- 102) | 0.05 | <0.01 | |
| Serum triglycerides (mg/dL) | ||||||||
| Unadjusted | 137(133–141) | 137 (132–141) | 136 (132–141) | 142(138–147) | 150(145–154) | <0.01 | <0.01 | |
| Adjusted* | 139 (135–144) | 141 (137–146) | 139 (135–143) | 140 (136–145) | 142 (137–146) | 0.95 | 0.28 | |
| Glycated hemoglobin (%) | ||||||||
| Unadjusted | 5.39 (5.34–5.43) | 5.41 (5.36–5.45) | 5.50 (5.46–5.54) | 5.57 (5.53–5.61) | 5.67 (5.62–5.71) | <0.01 | <0.01 | |
| Adjusted* | 5.45 (5.41–5.49) | 5.47 (5.43–5.51) | 5.53 (5.49–5.56) | 5.51 (5.48–5.55) | 5.57 (5.54–5.61) | <0.01 | <0.01 | |
| Serum C-reactive protein (mg/dL) | ||||||||
| Unadjusted | 0.42 (0.39–0.45) | 0.45 (0.43–0.48) | 0.48 (0.45–0.51) | 0.45 (0.42–0.48) | 0.46 (0.43–0.49) | 0.43 | 0.16 | |
| Adjusted* | 0.44 (0.40–0.47) | 0.45 (0.42–0.48) | 0.47 (0.44–0.50) | 0.44 (0.41–0.47) | 0.48 (0.45–0.51) | 0.37 | 0.11 | |
| Serum uric acid (mg/dL) | ||||||||
| Unadjusted | 5.27 (5.21–5.33) | 5.15 (5.10–5.21) | 5.14 (5.08–5.20) | 5.32 (5.26–5.38) | 5.51 (5.45–5.56) | <0.01 | <0.01 | |
| Adjusted* | 5.17 (5.11–5.22) | 5.27 (5.23–5.32) | 5.24 (5.19–5.29) | 5.34 (5.29–5.39) | 5.37 (5.32–5.42) | <0.01 | <0.01 | |
| BUN (mg/dL) | ||||||||
| Unadjusted | 13.1 (12.9–13.3) | 13.6 (13.4–13.8) | 14.1 (14.0–14.4) | 14.7 (14.5–14.9) | 15.5 (15.3–15.7) | <0.01 | <0.01 | |
| Adjusted* | 13.1 (12.9–13.3) | 13.7 (13.6–13.9) | 14.3 (14.1–14.5) | 14.7 (14.5–14.9) | 15.3 (15.1–15.5) | <0.01 | <0.01 | |
| Serum insulin (μU/mL) | ||||||||
| Unadjusted | 10.5 (9.4–11.6) | 11.9 (10.8–13.0) | 11.6 (10.5–12.7) | 13.1 (12.0–14.2) | 14.3 (13.2–15.4) | <0.01 | <0.01 | |
| Adjusted* | 11.2 (10.1–12.4) | 12.5 (11.4–13.6) | 11.7 (11.6–12.8) | 12.4 (11.3–13.5) | 13.7 (12.5–14.8) | 0.04 | <0.01 | |
| eGFR | ||||||||
| Unadjusted | 69.2 (68.7–69.7) | 69.8 (69.3–70.4) | 69.9 (69.4–70.4) | 70.0 (69.5–70.5) | 69.8 (69.2–70.3) | 0.57 | 0.18 | |
| Adjusted* | 69.2 (68.6–69.7) | 69.7 (69.2–70.3) | 69.8 (69.3–70.3) | 70.0 (69.4–70.5) | 70.0 (69.4–70.5) | 0.26 | 0.05 | |
Note: adjusted for age (continuous), gender, total energy intake (kcal/day), smoking status (yes/no), alcohol intake (g/day) and BMI (categorical), total fat (g/day), calcium (mg/day), magnesium (mg/day), sodium (g/day), potassium (g/day), vitamin A (IU/day), vitamin C (mg/day), vitamin B6 (mg/day) folic acid (mcg/day) intake, diabetes, and hypertension history.
4. Discussion
In a large cross-sectional study of US adults, we observed consistent associations between consumption of SAA, including Met and Cys together or individually, with higher prevalence of cardiometabolic disease risk factors. These associations were independent of traditional CVD risk factors, including BMI, diabetes, and hypertension. These outcomes are consistent with a plethora of data in laboratory animals showing beneficial effects of reduced SAA diets on cardiometabolic disease-related pathways [2,4,36,37]. These new findings, together with previous preclinical data, highlight the importance of dietary SAA in the development of major chronic diseases and suggest that optimal intake levels of SAA for maintenance of long-term health are close to the minimum requirement values (RDA and EAR) and well-below those currently consumed by most adults.
The composite cardiometabolic disease Z score was used to assess disease risk clustering on a continuous scale based on it being statistically more sensitive and less error prone than dichotomous approaches [38]. We would expect that Z score would correlate well with the true level of cardiometabolic disease risk based on the nature of its subcomponent risk factors and the precise manner by which they are measured [39]. Results obtained from analysis of the composite score based on continuous risk factors were further confirmed when analyses were conducted using categorical risk factors. Because the primary exposure, dietary intake of SAA, is correlated with other nutrients including total energy and protein, we utilized an analysis approach which accounted for a variety of potential confounders. We also expressed dietary SAA intake not only as an absolute value, but also as protein density to control for total energy and protein intake. In this latter analysis, the relative intake of SAA independent of protein is addressed and the similarity of the results from both methods provides confidence in the positive associations observed between SAA intake and potential cardiometabolic risk.
When specific components of the composite risk factor were examined, we found that participants in the lowest SAA intake quintile had significantly lower levels of cholesterol, glucose, glycated hemoglobin, uric acid, BUN, insulin and eGFR. While there is limited previous data in this regard in humans, numerous animal studies have reported associations between SAAR and metabolic alterations associated with diabetes and metabolic syndrome [40], [41], [42]. The mechanism by which SAAR benefits glucose homeostasis and insulin sensitivity may be due to improvements in insulin secretion and its signaling pathway [43]. It has been suggested that by restricting SAA intake, reductions in Met metabolism and subsequent production of Cys and hydrogen sulfide (H2S) may act to combat the development of insulin resistance, an important factor in the pathogenesis of cardiometabolic diseases [44,45]. Overall, improvements in the balance of glucose and insulin sensitivity by SAAR have been consistently observed in animal models [40,46]. Our epidemiological findings are consistent with these dietary SAAR effects on glucose metabolism, demonstrating associations between lower SAA intake and reductions in serum glucose and glycated hemoglobin.
In the previously conducted animal studies, dietary SAAR was found to improve lipid metabolism and reduce body fat [42]. Rate-limiting lipogenic enzymes such as acetyl-CoA carboxylase 1, fatty acid synthase, and stearoly-CoA desaturase 1, were identified as transcriptional targets of SAAR leading to reductions in lipid accumulation [47]. Results from our current epidemiologic study showing associations between SAA intake and total cholesterol levels suggest that similar effects of low SAA diets on lipid metabolism may be occurring in humans. However, associations with HDL cholesterol are suggestive of an opposite effect regarding CVD risk. Also, no effects on serum triglycerides were observed, although, this finding could be impacted by inconsistentcies in fasting state prior to blood collection, resulting in higher variation and lower statistical power. Although participants were instructed to fast for 10–16 h prior to the examination, the instructions were not always followed uniformly.
In this study, no association was observed between SAA intake and blood pressure, an independent risk factor for CVD. This is consistent with findings from the Rotterdam Study [48] where no association was found between Cys intake and hypertension. In addition, CRP, the acute-phase protein reflecting inflammation and CVD risk, was not related with dietary SAA intake, and no data on high sensitivity CRP was available.
Our findings that dietary SAA intake was positively associated with serum uric acid, eGFR and BUN suggests that lower levels of SAA consumption is beneficial in regard to kidney function. This is consistent with previous studies in animal models where SAAR protected against the development of chronic kidney disease [46,49]. While the mechanisms responsible for these SAA effects are not known, potential effects on mitochondrial oxidative stress [50] and glutathione (GSH) levels resulting from SAAR may be involved [51]. Overall, SAAR will likely have several chronic effects which could work together to reduce the risk for cardiometabolic disease processes.
There are some important distinctions between dietary SAAR studies in animal models and our present results in adult humans. Many of the animal studies were performed by initiating SAAR diets in young and growing animals, a stage of the lifespan where SAA requirements are high based upon the added needs for growth (e.g., 9.8 g/kg diet for rats) [52]. Consequently, the rather severe (~80%) restriction in young animals led to intake levels far below their high requirements resulting in significant reductions in growth. However, when SAAR was initiated in adult animals where dietary SAA requirements are substantially lower (2.3 g/ kg diet), many if not most, of the same diet-induced beneficial effects were still apparent [53,54]. These later studies, together with our present findings, provide support for optimal dietary SAA levels being as close to, but not below, dietary requirements (EAR). Since we observed significantly lower risk values in the first quintile compared with the fourth and fifth quintiles as well as a significant overall trend, we make the assumption that the lowest quintile represents optimal SAA intake. It is important to note that the EAR represents an average for the population and that some individuals may have somewhat higher requirements for maintenance of nitrogen balance. For this reason, the RDA value, which is designed to meet the needs of 97%–98% of the population may be a more appropriate target for the population at large.
It is of interest to note that SAA is naturally higher in most meats than in vegetables based on both the overall content of protein and the Met and Cys content of those proteins. This is consistent with our present findings of increasing meat:vegetable protein ratios with increasing SAA consumption. This suggests that a likely effective approach to reduce dietary SAA content may include increasing the intake of plant-based foods. In particular, by moderating the intake of legumes products and diluting total protein intake by ingestion of ample amounts of fruits and other foods, it would appear possible to maintain the 15–29 mg/kg/day SAA intake value observed in the lowest quintile of the current study. These results also suggest that reduced SAA intake may be, in part, responsible for beneficial health effects attributed to plant-based diets [55].
While an important strength of our study is the use of a nationally representative sample of CVD-free adults, there are several limitations that are worthy of consideration. Since residual confounding is a common and unavoidable issue in observational studies, we sought to minimize the influence of potential confounders by controlling for variables including major lifestyle, dietary risk factors, and health status. In addition, in the 8% of subjects with two separate days of recall data, calculated SAA intake values were highly correlated (r = 0.68) between the two days. Also, two different methods to estimate dietary SAA intake were used and both resulted in consistent associations between lower dietary SAA intake and lower risks for cardiometabolic diseases. Therefore, our observations are unlikely to be explained by chance, bias, or confounding. A limitation was a lack of any time dimension in assessing factors associated with cardiometabolic risk due to the cross-sectional design of the study. Because both the outcome and risk factors were examined at one point in time, we do not know whether these factors preceded or followed the onset of cardiometabolic risk. However, while the direction of causality cannot be established, it is biologically most plausible that dietary SAAR lowers cardiometabolic disease risk. In addition, estimation of an individual's usual intake from a single 24-h food recall may not accurately reflect habitual intake can potentially lead to miss-classification as well as potential selection and recall bias which could limit our ability to detect significant results. However, despite this limitation, significant associations were observed which, we believe, strengthen our confidence in the results obtained. Further, previous studies report that dietary patterns are moderately stable over time [56], [57], [58], [59]. Finally, as is often the case in observational studies, there exists the possibility for residual confounding despite our efforts to control for this possibility.
Altogether, these new findings indicate that high levels of SAA intake, observed in a high proportion of adults, are associated with increased cardiometabolic disease risk. These results are consistent with animal studies showing beneficial effects of an SAAR diet on these same pathways. These findings suggest that optimal levels of SAA intake may be close to EAR levels, much lower than levels consumed in the typical American diet. More prospective studies, preferably in populations with heterogeneous eating habits, as well as randomized controlled trails are needed to further clarify the potential role of lower SAA intake in the prevention of cardiometabolic diseases.
Declaration of competing interest
We declare no competing interests.
Acknowledgment
There was no funding source for this study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2019.100248.
Appendix. Supplementary materials
References
- 1.Cavuoto P., Fenech M.F. A review of methionine dependency and the role of methionine restriction in cancer growth control and life-span extension. Cancer Treat Rev. 2012;38(6):726–736. doi: 10.1016/j.ctrv.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Miller R.A., Buehner G., Chang Y., Harper J.M., Sigler R., Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4(3):119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orentreich N., Matias J.R., DeFelice A., Zimmerman J.A. Low methionine ingestion by rats extends life span. J Nutr. 1993;123(2):269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- 4.Perrone C.E., Malloy V.L., Orentreich D.S., Orentreich N. Metabolic adaptations to methionine restriction that benefit health and lifespan in rodents. Exp Gerontol. 2013;48(7):654–660. doi: 10.1016/j.exger.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Malloy V.L., Krajcik R.A., Bailey S.J., Hristopoulos G., Plummer J.D., Orentreich N. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell. 2006;5(4):305–314. doi: 10.1111/j.1474-9726.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- 6.Rose W.C. Amino acid, requirements of man. Fed Proc. 1949;8(2):546–552. [PubMed] [Google Scholar]
- 7.Institute of Medicine . The National Academies Press; Washington, DC: 2005. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. [Google Scholar]
- 8.World Health Organization . Vol. 935. WHO; 2007. Protein and amino acid requirements in human nutrition. (Protein and amino acid requirements in human nutrition). Technical Report Series. [PubMed] [Google Scholar]
- 9.Benevenga N., Steele R. Adverse effects of excessive consumption of amino acids. Annu Rev Nutr. 1984;4(1):157–181. doi: 10.1146/annurev.nu.04.070184.001105. [DOI] [PubMed] [Google Scholar]
- 10.Dilger R.N., Baker D.H. Excess dietary L-cysteine causes lethal metabolic acidosis in chicks. J Nutr. 2008;138(9):1628–1633. doi: 10.1093/jn/138.9.1628. [DOI] [PubMed] [Google Scholar]
- 11.Baker DH, Dilger RN. Sulfur amino acid deficiency and toxicity: research with animal models. In: Masella R. and Mazza G, Eds. Glutathione and sulfur amino acids in human health and disease 2009: Wiley, 289–316.
- 12.Garlick P.J. Toxicity of methionine in humans. J Nutr. 2006;136(6):1722S–1725S. doi: 10.1093/jn/136.6.1722S. [DOI] [PubMed] [Google Scholar]
- 13.Sugiyama K., Kushima Y., Muramatsu K. Effect of dietary glycine on methionine metabolism in rats fed a high-methionine diet. J Nutr Sci Vitaminol. 1987;33(3):195–205. doi: 10.3177/jnsv.33.195. [DOI] [PubMed] [Google Scholar]
- 14.Garlick P.J. The nature of human hazards associated with excessive intake of amino acids. J Nutr. 2004;134(6):1633S–1639S. doi: 10.1093/jn/134.6.1633S. [DOI] [PubMed] [Google Scholar]
- 15.Yalçınkaya S., Ünlüçerçi Y., Olgaç V., Doğru-Abbasoğlu S., Uysal M. Oxidative and nitrosative stress and apoptosis in the liver of rats fed on high methionine diet: protective effect of taurine. Nutrition. 2009;25(4):436–444. doi: 10.1016/j.nut.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control, National Center for Health Statistics. Ethics review board (ERB) approval. Available from: https://www.cdc.gov/nchs/nhanes/irba98.htm. (Accessed Nov. 23, 2019).
- 17.National Center for Health Statistics Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital Health Stat. 1994;1:1–407. [PubMed] [Google Scholar]
- 18.United Department of Health . MD Centers for Disease Control and Prevention; 1996. Health services. Third national health and nutrition examination survey; pp. 1988–1994. NHANES III laboratory data file. Hyattsville. [Google Scholar]
- 19.Willett W., Stampfer M.J. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 20.Castro J.P., El-Atat F.A., McFarlane S.I., Aneja A., Sowers J.R. Cardiometabolic syndrome: pathophysiology and treatment. Curr Hypertens Rep. 2003;5(5):393–401. doi: 10.1007/s11906-003-0085-y. [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo R.A., Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 22.Meigs J.B., D'Agostino R.B., Wilson P.W., Cupples L.A., Nathan D.M., Singer D.E. Risk variable clustering in the insulin resistance syndrome: the Framingham Offspring Study. Diabetes. 1997;46(10):1594–1600. doi: 10.2337/diacare.46.10.1594. [DOI] [PubMed] [Google Scholar]
- 23.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., 3rd., Feldman H.I. A new equation to estimate glomerular filtration rate. Ann intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alberti K.G.M., Zimmet P., Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 25.National Cholesterol Education Program, National Heart, Lung, and Blood Institute . NIH Publication; Bethesda, MD: 2002. Detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) Final Report No. 02-5215. [Google Scholar]
- 26.Pencina M.J., Navar-Boggan A.M., D’Agostino Sr R.B., Williams K., Neely B., Sniderman A.D. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370(15):1422–1431. doi: 10.1056/NEJMoa1315665. [DOI] [PubMed] [Google Scholar]
- 27.McPherson R.A., Pincus M.R. Elsevier Health Sciences; 2017. Henry's clinical diagnosis and management by laboratory methods e-Book. [Google Scholar]
- 28.Melmed S., Polonsky K.S., Larsen P.R., Kronenberg H.M. Elsevier Health Sciences; 2015. Williams textbook of endocrinology. [Google Scholar]
- 29.Davies M.J., D'Alessio D.A., Fradkin J. Management of hyperglycaemia in type 2 diabetes, 2018. a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2018;61(12):2461–2498. doi: 10.1007/s00125-018-4729-5. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association Glycemic targets: standards of medical care in diabetes—2018. Diabetes Care. 2018;41:S55–S64. doi: 10.2337/dc18-S006. [DOI] [PubMed] [Google Scholar]
- 31.Kellerman R.D. Elsevier Health Sciences; 2017. Bope ET. Conn's current therapy 2018 e-Book. [Google Scholar]
- 32.Desideri G., Castaldo G., Lombardi A., Mussap M., Testa A., Pontremoli R. Is it time to revise the normal range of serum uric acid levels. Eur Rev Med Pharmacol Sci. 2014;18(9):1295–1306. [PubMed] [Google Scholar]
- 33.National Kidney Foundation. Estimated glomerular filtration rate (eGFR). Available from: https://www.kidney.org/atoz/content/gfr. (accessed Nov 26,2 2019).
- 34.Brook R.D., Rajagopalan S. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Soc Hypertens. 2017;12(3):238. doi: 10.1016/j.jash.2018.01.004. 2018. [DOI] [PubMed] [Google Scholar]
- 35.Guenther P.M., Casavale K.O., Reedy J., Kirkpatrick S.I., Hiza H.A., Kuczynski K.J. Update of the healthy eating index: HEI-2010. J Acad Nutr Diet. 2013;113(4):569–580. doi: 10.1016/j.jand.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pamplona R., Barja G. Mitochondrial oxidative stress, aging and caloric restriction: the protein and methionine connection. Biochim Biophys Acta. 2006;1757(5):496–508. doi: 10.1016/j.bbabio.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Richie J.P., Komninou D., Leutzinger Y., Kleinman W., Orentreich N., Malloy V. Tissue glutathione and cysteine levels in methionine-restricted rats. Nutrition. 2004;20(9):800–805. doi: 10.1016/j.nut.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Ragland D.R. Dichotomizing continuous outcome variables: dependence of the magnitude of association and statistical power on the cutpoint. Epidemiology. 1992;3(5):434–440. doi: 10.1097/00001648-199209000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Eisenmann J.C. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc Diabetol. 2008;7(1):17. doi: 10.1186/1475-2840-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ables G.P., Perrone C.E., Orentreich D., Orentreich N. Methionine-restricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PLoS One. 2012;7(12):e51357. doi: 10.1371/journal.pone.0051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stone K.P., Wanders D., Orgeron M., Cortez C.C., Gettys T.W. Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes. 2014;63(11):3721–3733. doi: 10.2337/db14-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou X., He L., Wan D., Yang H., Yao K., Wu G. Methionine restriction on lipid metabolism and its possible mechanisms. Amino Acids. 2016;48(7):1533–1540. doi: 10.1007/s00726-016-2247-7. [DOI] [PubMed] [Google Scholar]
- 43.Yin J., Ren W., Chen S., Li Y., Han H., Gao J. Metabolic regulation of methionine restriction in diabetes. Mol Nutr Food Res. 2018;62(10) doi: 10.1002/mnfr.201700951. [DOI] [PubMed] [Google Scholar]
- 44.Kaneko Y., Kimura Y., Kimura H., Niki I. L-cysteine inhibits insulin release from the pancreatic β-cell: possible involvement of metabolic production of hydrogen sulfide, a novel gasotransmitter. Diabetes. 2006;55(5):1391–1397. doi: 10.2337/db05-1082. [DOI] [PubMed] [Google Scholar]
- 45.Feng X., Chen Y., Zhao J., Tang C., Jiang Z., Geng B. Hydrogen sulfide from adipose tissue is a novel insulin resistance regulator. Biochem Biophys Res Commun. 2009;380(1):153–159. doi: 10.1016/j.bbrc.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 46.Grant L., Lees E.K., Forney L.A., Mody N., Gettys T., Brown P.A. Methionine restriction improves renal insulin signalling in aged kidneys. Mech Ageing Dev. 2016;157:35–43. doi: 10.1016/j.mad.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Orgeron M.L., Stone K.P., Wanders D., Cortez C.C., Van N.T., Gettys T.W. The impact of dietary methionine restriction on biomarkers of metabolic health. Prog Mol Biol Transl Sci. 2014;121:351. doi: 10.1016/B978-0-12-800101-1.00011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altorf-van der Kuil W., Engberink M.F., De Neve M., van Rooij F.J., Hofman A., van’t Veer P. Dietary amino acids and the risk of hypertension in a Dutch older population: the Rotterdam Study. Am J Clin Nutr. 2013;97(2):403–410. doi: 10.3945/ajcn.112.038737. [DOI] [PubMed] [Google Scholar]
- 49.Caro P., Gomez J., Sanchez I., Naudi A., Ayala V., Lopez-Torres M. Forty percent methionine restriction decreases mitochondrial oxygen radical production and leak at complex I during forward electron flow and lowers oxidative damage to proteins and mitochondrial DNA in rat kidney and brain mitochondria. Rejuvenation Res. 2009;12(6):421–434. doi: 10.1089/rej.2009.0902. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez-Roman I., Barja G. Regulation of longevity and oxidative stress by nutritional interventions: role of methionine restriction. Exp Gerontol. 2013;48(10):1030–1042. doi: 10.1016/j.exger.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 51.Wanders D., Stone K.P., Forney L.A., Cortez C.C., Dille K.N., Simon J. Role of GCN2-independent signaling through a noncanonical PERK/NRF2 pathway in the physiological responses to dietary methionine restriction. Diabetes. 2016;65(6):1499–1510. doi: 10.2337/db15-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.National Research Council . Nutrient requirements of laboratory animals. 4th ed. National Academies Press; Washington, DC: 1995. Nutrient requirement of the laboratory rat. [Google Scholar]
- 53.Lees E.K., Król E., Grant L., Shearer K., Wyse C., Moncur E. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell. 2014;13(5):817–827. doi: 10.1111/acel.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee B.C., Kaya A., Ma S., Kim G., Gerashchenko M.V., Yim S.H. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino acid status. Nat Commun. 2014;5:3592. doi: 10.1038/ncomms4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Craig W.J. Nutrition concerns and health effects of vegetarian diets. Nutr Clin Pract. 2010;25(6):613–620. doi: 10.1177/0884533610385707. [DOI] [PubMed] [Google Scholar]
- 56.Jensen O.M., Wahrendorf J., Rosenqvist A., Geser A. The reliability of questionnaire-derived historical dietary information and temporal stability of food habits in individuals. Am J Epidemiol. 1984;120(2):281–290. doi: 10.1093/oxfordjournals.aje.a113891. [DOI] [PubMed] [Google Scholar]
- 57.Jain M., Howe G., Harrison L., Miller A. A study of repeatability of dietary data over a seven-year period. Am J Epidemiol. 1989;129(2):422–429. doi: 10.1093/oxfordjournals.aje.a115146. [DOI] [PubMed] [Google Scholar]
- 58.Thompson F.E., Metzner H.L., Lamphiear D.E., Hawthorne V.M. Characteristics of individuals and long term reproducibility of dietary reports: the Tecumseh Diet Methodology Study. J Clin Epidemiol. 1990;43(11):1169–1178. doi: 10.1016/0895-4356(90)90018-k. [DOI] [PubMed] [Google Scholar]
- 59.Sijtsma F.P., Meyer K.A., Steffen L.M., Shikany J.M., Van Horn L., Harnack L. Longitudinal trends in diet and effects of sex, race, and education on dietary quality score change: the coronary artery risk development in young adults study. Am J Clin Nutr. 2012;95(3):580–586. doi: 10.3945/ajcn.111.020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.