Highlights
-
•
Aspirates allow to study cell death and proliferation in relation to thrombus age.
-
•
Apoptosis, etosis and proliferation are present in all stages of thrombus evolution.
-
•
Etosis is the only marker that associates significantly with older age of thrombus.
-
•
Etosis could serve potentially as tissue marker for thrombus aging and progression.
Keywords: Acute myocardial infarction, Coronary thrombosis, Cell death, Apoptosis, Etosis, Cell proliferation
Abstract
Background
Coronary thrombosis is a process with unpredictable clinical outcome. Changes of thrombus composition overtime influence tissue repair and stabilization. We investigated rates of cell deaths and cell proliferation at different time points after initiation of thrombosis.
Methods
Thrombectomy aspirates of 55 myocardial infarction patients were selected and histomorphologically classified as fresh (25), lytic (25), partially fibrocellular (10), completely fibrocellular (10). Paraffin sections were immunostained with anti-(cleaved) caspase-3/Casp3 (apoptosis), Citrullinated histone/CitH 3 (etosis), C-reactive protein/CRP and Ki67 (proliferation) in combination with either Feulgen counterstaining (DNA) or cell markers for granulocytes, macrophages, SMCs, platelets and endothelium. Rates of apoptosis, etosis and proliferation were measured as a percentage of total number of immunopositive pixels versus total number of DNA positive pixels, while co-localization with cell markers was assessed by digital image analysis.
Results
Positive staining of CitH3 was observed more frequently (93%) than Casp3 (70%), Ki67 (79%) or CRP (59%) (p < 0.05). Moreover, rate of etosis, found in granulocytes and macrophages, differed significantly among thrombi of different age, being higher in lytic (12.82) than in fresh (8.52) and late-organized (2.75) (p < 0.05). Such differences were not observed for the rates of apoptosis or cell proliferation related to thrombus age. CRP staining was present in fresh, lytic and organized thrombi, but did not reliably identify necrotic areas.
Conclusions
Different patterns of cell death and cell proliferation are noticed during progression of coronary thrombus overtime, but with significant differences for only etosis. Etosis could potentially serve as a biomarker for thrombus instability with clinical significance.
1. Introduction
Coronary thrombosis is usually initiated by atherosclerotic plaque disruption [1]. Further progression and eventual outgrowth of the thrombus mass is an unpredictable process, and is associated with a variety of clinical outcomes, ranging from no symptoms at all (clinically silent) to myocardial infarction (MI) or even sudden death [2]. Histopathological studies on thrombectomy materials from patients with acute myocardial infarction (AMI) have shown that plaque injury and subsequent thrombus formation do not always coincide directly with the onset of clinical symptoms [3]. Apparently, a coronary thrombus mass may grow over a period of time towards occlusion [2], [3]. On the other hand, also clinically unnoticed “healed” ruptured plaques have been identified at autopsy, implying that thrombus organization and stabilization had occurred in these instances without causing a critical stenosis [2], [4]. Finally, the thrombus structure can be of importance for the risk of complication such as distal embolization, which occurs particularly in the first two weeks after MI when thrombus structure is very fragile/lytic [5], [6].
Thrombus composition can be influenced by various locally active biological factors such as inflammation, cell death, oxidative stress, proteolytic activity, angiogenesis and fibrosis over time [7], [8]. From a pathological point of view, the progression of thrombus is a process of wound healing and tissue repair in which various forms of cell death take part. Cell death may induce tissue instability, which has been demonstrated not only in atherosclerotic plaques [9] but also in overlying thrombus mass of disrupted plaques [10]. On the other hand, cell death could also influence ongoing inflammatory activity and reparative mechanisms that eventually may stabilize a thrombus. Apoptosis, etosis and necrosis are important mechanisms of cell death [11] and have indeed previously been reported in coronary atherosclerosis and thrombosis [10], [12], and in experimental and clinical settings of venous thrombosis [13]. However, there is no previous study that simultaneously investigated these different types of cell death and cell proliferation in the coronary thrombus overtime. In the present study, we investigated the relative extent of apoptosis, etosis, necrosis and proliferation in relation to histologically defined stages of injury and repair in evolving coronary thrombus mass following myocardial infarction. For this purpose, we used thrombus aspirates of which the age of the thrombus was graded histologically according to previously defined criteria. In addition, the specific cell types involved in cell death and proliferation were also identified with the use of immunohistochemical (double) stains.
2. Materials and methods
2.1. Specimens
Coronary thrombus aspirates obtained from patients with acute ST elevation type of myocardial (STEMI) were used for this study. Thrombosuction is a part of the therapeutic intervention of many STEMI patients in our institution, and aspirates are sent to the pathology lab as left-over materials. All thrombectomies are immediately formalin-fixed, paraffin-embedded, and stored as tissue-blocks in the archive of the Pathology Department, Academic Medical Centre (AMC), Amsterdam, the Netherlands [3], [14]. The age of the thrombus specimens was graded using haematoxylin and eosin (HE)-stained sections as either fresh (composed of intact and viable-appearing red blood cells), lytic (featured of colliquation necrosis, cellular swelling or karyorrhexis) or organized (with ingrowth of micro vessels, smooth muscle cells/SMCs, or depositions of collagen fibres), as described previously [14], [15]. For the purpose of this study, we randomly retrieved 55 thrombus tissue blocks from the archive, which contained at least 2 mm total tissue area in the section. A series of 5 µm thin sections were cut, of which one was stained with HE to confirm the age of thrombus and presence of plaque material (lipid debris, foam cells, cholesterol clefts and/or calcification. From the specimens that contained fragments of more than one type of thrombus age, we selected another 15 fragments, which resulted in a total 70 different fragments for the purpose of this study. Adjacent sections were further used for immunohistochemistry.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. All materials were ‘left over materials’ (of no further diagnostic relevance) and were included in the study anonymously.
2.2. Immunohistochemistry
To visualize apoptosis, etosis, necrosis and cellular proliferation, the following antibodies were used: rabbit polyclonal anti (cleaved) Caspase-3 (Casp3, Cell signalling, Massachusetts, USA), identifies apoptotic cells [16]; rabbit polyclonal anti citrullinated histone H3 (CitH3, Abcam), identifies cells undergoing etosis [17], [18]; rabbit monoclonal anti C-reactive protein (CRP, Abcam, Cambridge, UK); and rabbit monoclonal anti Ki67 (ThermoFisher Scientific, Fremont, CA, USA), identifies proliferating cells [7], [19]. CRP has been forwarded as a marker for necrosis in pancreatic necrosis, myocardial infarction and acute coronary syndrome lesions [20], [21], [22]. Sections were dewaxed in xylene and rehydrated in graded-alcohols prior to antigen retrieval with heat-induced epitope retrieval (HIER) in a PT module (Thermo Fisher/Labvision, Fremont, CA, USA) using Tris-EDTA buffer (ThermoFisher Scientific). Incubation with primary antibody was followed by alkaline phosphatase (AP) conjugated anti rabbit polymer secondary antibody (Immunologic, Duiven, the Netherlands), and AP activity was detected with Perma Blue (Diagnostic BioSystems, California, USA). Sections were counterstained with Feulgen pink staining, by incubation in 1N HCl (Merck KGaA, Darmstadt, Germany) and in Schiff’s reagent (Merck) after washing in distilled water. Finally, sections were dried on a hotplate and coverslip sealed with Vectamount (Vector Laboratories, Burlingame, CA, USA). Positive controls for immunostaining consisted of human tonsil tissue and thrombosed coronary artery plaque. In each staining round, negative controls were applied by means of omission of primary antibodies. All stained sections were digitized with a Philips IntelliSite UFS scanner (Philips Digital Pathology Solutions, Best, the Netherlands).
To identify the types of cells involved, double immunostaining was performed in a sequential combination of either cell death or proliferation markers with cell specific antibodies: anti- CD15 (Dako, Glostrup, Denmark) for granulocytes, CD68 (clone PG-M, Dako) for macrophages, CD61 for platelets, α- smooth muscle actin/SMA (Dako) for smooth muscle cells/SMC and CD34 (Dako) for endothelial cells. Before starting the next staining round, the sections were scanned using the slide scanner and subsequently stripped to elute the dye and immune-complex, as previously described [23]. Using Image J digital image analysis software, the immunodouble-stained scanned sections were analysed by creating false colour composite images as previously described [12].
2.3. Image analysis
First, Casp3, CitH3, CRP and Ki67 stained sections were microscopically evaluated to establish and score the percentage of immunopositive samples per category. Quantitative measurement of Casp3, CitH3 and Ki67 stained areas was performed using digital image analysis (Image Pro Premier 9.3, MediaCybernetics, Rockville, USA). Areas of interest (±0.5–1 mm2, depending on the actual size of the fragments) were selected that exhibited the distinct histomorphologic characteristics of the respective age categories. In these areas, rates of apoptosis, etosis and proliferation were assessed by calculating the percentage of the total number of immunopositive pixels versus the total number of Feulgen (DNA) positive pixels.
2.4. Statistical analysis
Normality test was performed with Shapiro-Wilk. When the data were not normally distributed, non-parametric tests were used. In relation to histologically stages of thrombus age, numbers of samples containing positive staining of Casp3, CitH3, Ki67 and CRP were analysed with Chi-Square test. Rates of apoptosis, etosis or proliferation presented as individual dot plot were analysed with Kruskal Wallis test, followed by pairwise multiple comparisons with Bonferroni correction when the results were significant (p < 0.05). All statistical analysis were performed with SPSS 25.00 (IBM Corporation, Armonk, NY, USA).
3. Results
HE-stained sections confirmed the presence of fresh (n = 25), lytic (n = 25) and organized thrombus fragments (n = 20). Organized thrombi were further classified as early (partial ingrowth of SMC and endothelial cells in thrombus mass, n = 10) and late organizing (complete replacement of thrombus mass by fibromyxoid matrix, n = 10) thrombus fragments. In addition, 10 out of 70 thrombus specimens (14%) contained soft plaque components such as cholesterol clefts, lipid debris and/or foam cells.
3.1. CRP, Casp3, CitH3 and Ki67 immunostaining in relation to age of thrombus
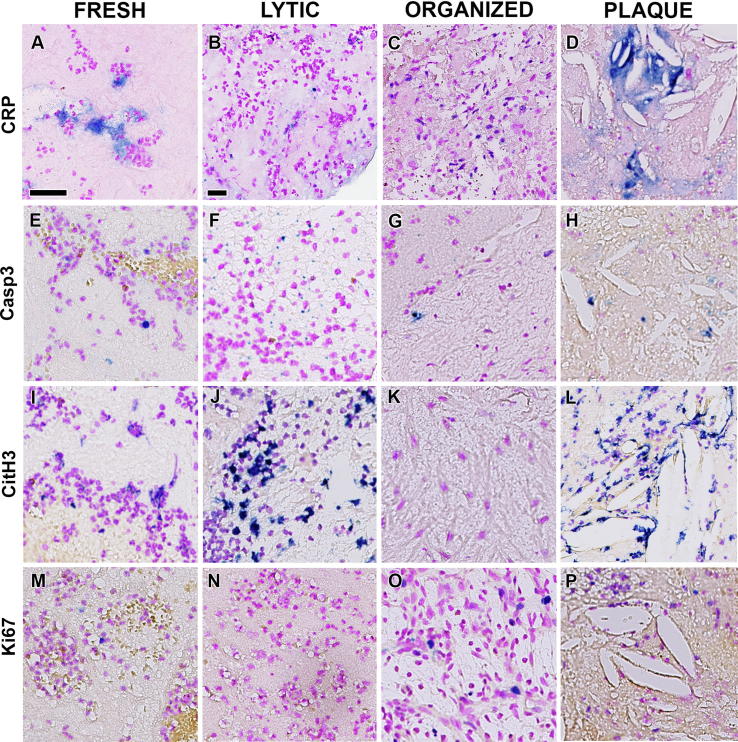
Substantial variation was noticed in the pattern of immunostaining in relation to thrombus age, and not all samples showed immunoreactivity with each of the antibodies applied. Representative examples of CRP, Casp3, CitH3 and Ki67 staining in relation to thrombus age and in plaque tissues are illustrated in Fig. 1. Details of the immunostaining for cell death and cell proliferation markers in the different thrombus age categories is presented in Table 1. CitH3 immunostaining showed that etosis was present in 93% of the lesions, which was significantly more than Casp3 (70%), Ki67 (79%) or CRP (59%) (p < 0.05, Table 1). Although diffuse CRP staining was observed in all areas containing lipid rich necrotic debris (Fig. 1D), CRP-positive areas did not always co localize with the necrotic areas identified in HE-stained sections. In contrast to the other stains, CRP showed staining of extracellular matrix (Fig. 1A) and was found more frequently in areas containing inflammatory cells (Fig. 1C), which appeared most prominent in the vital proliferative areas of organized thrombi.
Fig. 1.
Cell death and cell proliferation in coronary thrombus. Positive staining of CRP (A-D), Casp3 (E-H), CitH3 (I-L) and Ki67 (M-P) visualized in Perma Blue and counterstained with Feulgen pink to identify necrosis (A-D), apoptosis (E-H), etosis (I-L) and proliferation (M-P), respectively, in different stages of thrombus progression: fresh (A, E, I and M), lytic (B, F, J and N), organized (C, G, K and O); and in plaque components within thrombus (D, H, L and P). CRP also stained non-necrotic areas in fresh (A) and organized (C) thrombus, therefore, is not a reliable marker for necrosis. Apoptosis and etosis mostly occurred in lytic (B and E, respectively), whereas cell proliferation in organized thrombus (O). Scale bar in A and B: 25 µm.
Table 1.
Number of specimens stained positive with markers for cell death and proliferation.
| Thrombus Age (total number) | Casp3 n (%) | CitH3 n (%) | CRP n (%) | Ki67 n (%) | p-value |
|---|---|---|---|---|---|
| Thrombus fragments | |||||
| Fresh (25) | 16 (64%) | 24 (96%) | 10 (40%) | 19 (76%) | 0.000 |
| Lytics (25) | 19 (76%) | 23 (92%) | 17 (68%) | 18 (72%) | 0.196 |
| Organized | |||||
| - Early (10) | 7 (70%) | 9 (90%) | 7 (70%) | 8 (80%) | 0.665 |
| - Late (10) | 7 (70%) | 9 (90%) | 7 (70%) | 10 (100%) | 0.197 |
| Total thrombus: 70 | 49 (70%) | 65 (93%) | 41 (59%) | 55 (79%) | 0.000 |
| Plaque remnants | |||||
| - Fresh (4) | 0 (0%) | 3 (30%) | 4 (40%) | 1 (10%) | |
| - Lytics (5) | 2 (20%) | 4 (40%) | 5 (50%) | 4 (40%) | |
| - Organized (1) | 0 (0%) | 1 (10%) | 1 (10%) | 1 (10%) | |
| Total plaque: 10 | 2 (20%) | 8 (80%) | 10 (100%) | 6 (60%) | 0.170 |
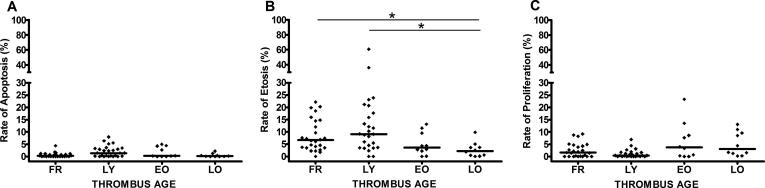
3.2. Extent of apoptosis, etosis and cell proliferation in relation to thrombus age
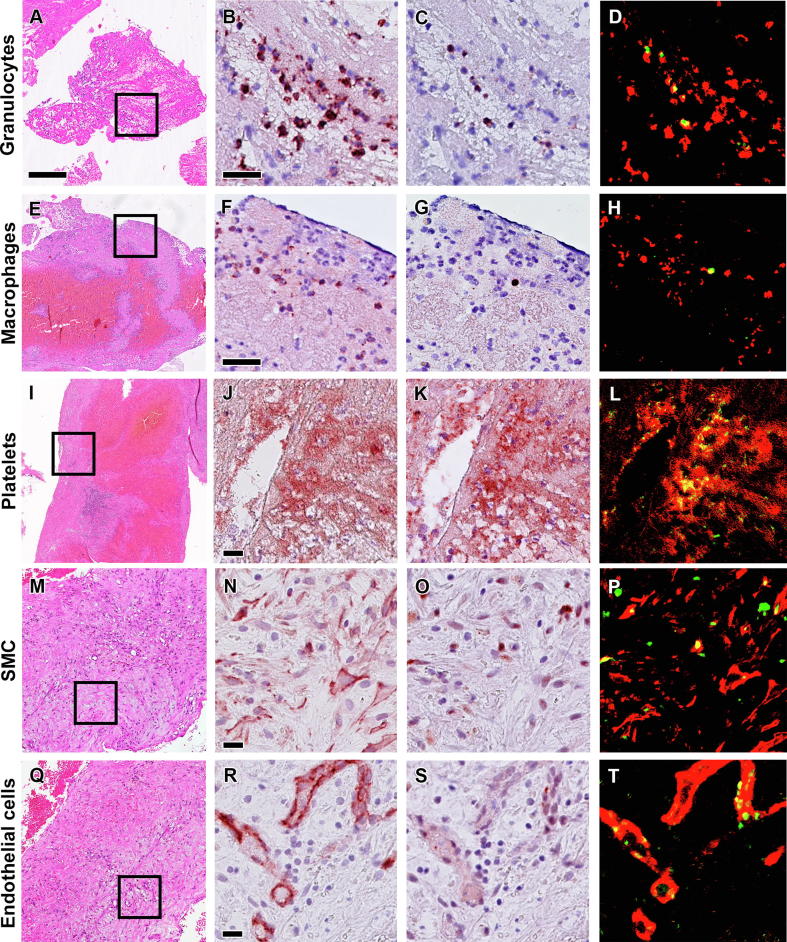
Rates of apoptosis, etosis and proliferation (calculated as a percentage of total number of immunopositive pixels versus total number of Feulgen positive pixels) in different thrombus age categories is presented in Fig. 2. Overall, the highest apoptotic rate was found in lytic- (2.09) and the lowest in late organized thrombus (0.45), but this finding was not significantly different (p = 0.079, Fig. 2A). Similarly, the apoptotic rate in plaque remnants (1.21, Fig. 1H) did not significantly differ from apoptosis in thrombus tissue. Using immuno doublestaining, we further identified the cell types undergoing apoptosis. We observed that all cell types known to be present in evolving thrombus have the potency to undergo apoptosis: granulocytes (CD15+/Casp3+), macrophages (CD68+/Casp3+), platelets (CD61+/Casp3+), endothelial cells (CD34+/Casp3+) and SMCs (αSMA+/Casp3+) (see Fig. 3).
Fig. 2.
Apoptosis, etosis and proliferation rates in different stages of thrombus progression. Rates of apoptosis, etosis and proliferation were measured as a percentage of total number of immunopositive pixels of Casp3(A), CitH3(B) and Ki67(C), respectively, versus total number of Feulgen positive pixels in the selected region of interest in fresh (FR), lytic (LY), early (EO) and late organized (LO). The highest rates of etosis and apoptosis were found in lytic while the lowest in organized thrombi (*p < 0.05, significant to LO). In contrast, rate of proliferation was higher in organized than in fresh and lytic thrombi.
Fig. 3.
Cellular sources of apoptosis in coronary thrombus. Representative images of apoptotic cells in coronary thrombus (top to bottom): granulocytes (A-D), macrophages (E-H), platelets (I-L), smooth muscle cells/SMC (M-P) and endothelial cells (Q-T). Boxed areas in HE stains (A, E, I, M and Q) show the areas of interest for higher magnification of double immunostaining Casp3 (C, G, K, O and S) antibodies with cell specific antibodies: anti- CD15 (B), CD68 (F), CD61 (J), αSMA (N) and CD34 (R) as well as of false-colour images to show co-localization of the positive cells (in red) with Casp3+ (in green) as apoptotic cells (D, H, L, P and T in yellow). Scale bar in HE overview (A): 100 µm and in high power detail (B and F): 25 µm; (J, N and R): 10 µm.
Interestingly, significant differences in the rate of etosis between the different thrombus age categories were encountered. The etosis rate was significantly higher in lytic (12.82, Fig. 1J) and in fresh (8.52, Fig. 1I) than in late organized thrombi (2.75, Fig. 1K) (p < 0.05, Fig. 2B). High etosis rate was also found in the lipid rich plaque areas (9.8, Fig. 1L). Immunodouble stains with CitH3 antibody showed that only granulocytes and macrophages undergo etosis, both in plaque and in thrombus compartments of the samples.
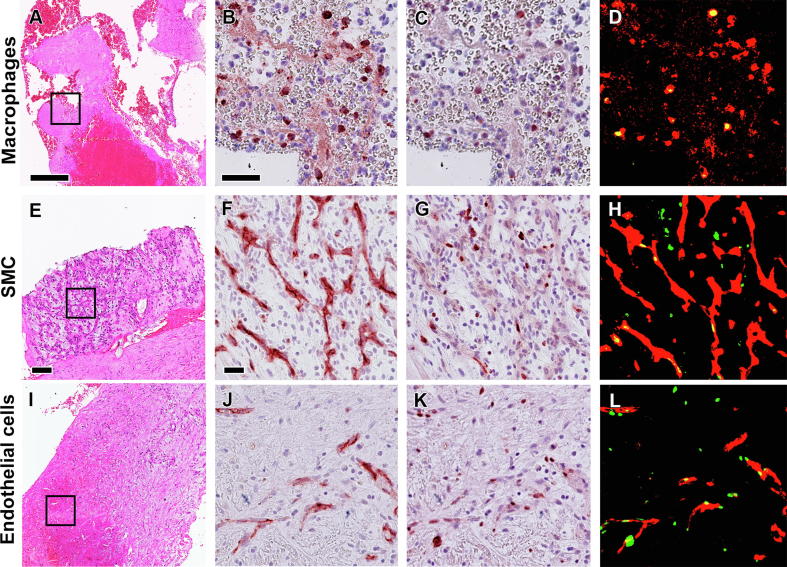
In contrast to apoptosis and etosis, the rate of proliferation was higher in organized thrombus (early: 6.70 and late: 5.79, Fig. 1O) than in the earlier stages of thrombus progression (fresh: 2.66; lytic: 1.12), or plaque tissue (2.40), although this finding was not significantly different (p = 0.076, Fig. 2C). Immuno double staining with Ki67 identified macrophages, endothelial cells and SMCs as proliferating cells (see Fig. 4).
Fig. 4.
Sources of proliferating cells in coronary thrombus. Representative images of cells undergoing proliferation in coronary thrombus (top to bottom): macrophages (A-D), smooth muscle cells/SMC (E-H), and endothelial cells (I-L). Boxed areas in HE stains (A, E, I) show the areas of interest for higher magnification of double immunostaining Ki67 (C, G, K) antibodies with cell specific antibodies: anti- CD68 (B), αSMA (F) and CD34 (J), as well as of false-colour images to show co-localization of the positive cells (in red) with Ki67+ (in green) as proliferating cells (D, H and L, in yellow). Scale bar in HE overviews (A and I): 100 µm and in high power detail (B and F): 25 µm.
4. Discussion
Histological composition of a thrombus or hematoma changes overtime in a chronological manner [14], [24]. Cell death and also cell proliferation are crucially involved in the processes of tissue remodelling and final repair (stabilization) that underlie the variety in histological composition. The thrombus aspirates of STEMI patients provide a valuable source of tissues to study these phenomena [14], [25], [26]. In the present study, we identified apoptosis, necrosis and etosis, simultaneously to be present in all stages of thrombus progression over time, from early onset (fresh) to the late organized stage of fibrocellular tissue only. Moreover, similar to cell death, also cell proliferation appears to be a feature of all different stages of thrombus age.
4.1. Types of cell death and cell proliferation in relation to thrombus age
In the 70 thrombus specimens, positive staining of CitH3 antibody was observed more frequently than all the other markers (p < 0.05, see Table 1). Moreover, etosis rate appeared to be significantly different between the early (high) and the late stages of thrombus age (low) (p < 0.05). More apoptotic cells in lytic versus organized thrombus, and more proliferating (Ki67+) cells in organized thrombus than in the earlier stages on the average were also observed, but the findings were not statistically significant. Thus, etosis appears to be the most common form of cell death during thrombus progression, and a more distinctive marker to distinguish between different types of thrombus age than apoptosis or cell proliferation. Based on these results, we propose that etosis could serve potentially as a tissue biomarker for coronary thrombosis progression after the initiation. Furthermore, the occurrence of etosis as a form of cell death has also been reported in coronary [12] and cerebral thrombus [27], in venous thromboembolism (deep vein thrombosis and pulmonary embolism) [13], [28] and in dissecting aortic hematomas [24]. Previously, etosis has been reported to be pro-thrombogenic with the extrusion of extracellular traps provides scaffold for fibrin and platelets aggregation [29].
Formation of coronary thrombus after plaque disruption is a dynamic process with unpredictable clinical outcome and depends, at least in part, on the rate of progression towards critical stenosis or to total occlusion. Since nearly all aspirates with fragments of old thrombus, contained also fresh fragments, the growth of thrombus mass is indeed episodic. The clinical significance of old thrombus in coronary aspirates of STEMI patients was highlighted in several studies. Kramer et al. investigated aspirates of 1315 STEMI patients and found that patients with older thrombi had 16% higher mortality at 4 years follow-up time, longer ischemic time and more frequent incidence of distal embolization [30]. Li et al. showed that old age of a thrombus in STEMI patient represent an independent marker for mortality at 1 year follow up [31]. A recent study by Nishihara et al. in aspirates of 305 AMI patients, also demonstrated that older thrombus is not only a an independent predictor of major adverse cardiac and cerebrovascular events (MACCEs) such as death, stroke, or MI within 6 months of percutaneous coronary intervention (PCI), but also associated with impaired myocardial reperfusion [32]. It is speculated that cell and tissue death lead to destabilization and fragility of the thrombus structure which may increase the risk of embolization [6], [32]. In fact, both apoptosis and etosis may contribute to destabilizing thrombus tissue [10], [12].
Although necrotic areas can be recognized in HE stain on the basis of histomorphological characteristics [14], [26], it is not always possible to evaluate the extent of the necrotic areas. We attempted to immunostain necrotic areas with the use of CRP antibody. CRP has been widely used as a marker for cardiovascular risk resulting from myocardial necrosis [21] and a biochemical marker for pancreatic necrosis [20]. However, we found that not only all necrotic lesions recognized on HE stains as lytic areas were stained negative with CRP (Fig. 1B), but also fresh (Fig. 1A) and even the vital proliferative areas of organized thrombi (Fig. 1C) showed strong immunopositivity with this antibody. Apparently, immunohistochemistry for necrosis is hampered by the lack of a specific tissue marker and as such necrosis can still be better identified with the use of HE-staining. Furthermore, a previous study on post mortem atherosclerotic plaque tissues also reported that CRP not only stains necrotic core but also stains inflamed cellular regions with endothelium and SMCs [33]. Similarly, we also found strong CRP staining in the inflamed necrotic lipid areas of plaque fragments and in the inflamed areas with extracellular matrix in thrombus aspirates [34]. These could relate due to active local CRP release by macrophages and SMC [35], [33]. Clinically, CRP has been widely applied as a serum marker for inflammation in the setting of cardiovascular disease [36]. Therefore, we concluded that CRP is likely more as an immunohistochemical tissue marker for inflammation rather than for necrosis in human coronary thrombus specimens.
4.2. Cellular sources of cell death and proliferation in coronary thrombosis
Neutrophils and macrophages appeared to be the most prominent cell types in both apoptosis and etosis during all stages of thrombus progression. Neutrophils are short lived immune cell that usually undergo apoptosis after a day [10]. The presence of apoptotic neutrophils in particularly late stages of thrombosis could indicate a diminished functioning of macrophages since their function is to phagocytose apoptotic and “suicidal-etosis” cells (which are mainly neutrophils) and to clear away tissue debris. Not only granulocytes, but also macrophages undergo etosis in thrombus through formation of macrophage extracellular traps (MET) [12], [37]. Macrophage cell death, either by necrosis apoptosis or etosis is considered to contribute to the formation and expansion of plaque necrotic lipid core (‘graveyard for macrophages’), which contributes to the vulnerability of plaques [38]. However, functional repertoire of macrophages is highly heterogeneous, and macrophages also serve repair functions in atherosclerosis and thrombosis [39], [40].
Apart from white blood cells, we also observed Casp3+ immunostaining in thrombocytes (Fig. 3I-L). Our finding confirms earlier observations [10], and emphasizes the contribution of apoptotic platelets to the tissue decay in the earlier stages of thrombus progression. Apoptotic SMC (Fig. 3M and 3P) and endothelial cells (Fig. 3Q and T) were scarce and particularly noticed in late organized thrombus, which suggest a contributory role of apoptosis in tissue homeostasis.
Cell proliferation in this study was observed in macrophages, SMCs and endothelial cells (Fig. 4) which can be considered as the hallmark of tissue repair. Macrophages were previously detected as the predominant proliferating cell type in atherosclerotic plaques [41], [42] and in much smaller numbers in re-stenotic lesions after percutaneous vascular interventions [43]. Similar to macrophages, proliferating smooth muscle cells were found in all stages of thrombus age, early and late, which shows that repair processes can be identified already after the onset of thrombus formation.
4.3. Limitations of the study
In this study, the aspirated thrombi were used anonymously, and there were no available information regarding the clinical characteristics of the patients and the thrombectomy procedures performed by the cardiologist.
5. Conclusions
In conclusion, this study demonstrated that the occurrence of cell death and proliferation is a prominent feature in thrombus specimens of STEMI patients after the onset of myocardial infarction. Different patterns of cell death can be noted during progression of coronary thrombus overtime, but with significant differences for etosis only. Etosis is the dominant form of cell death during thrombus progression and could potentially serve as biomarker for thrombus instability. CRP likely represents a marker for lipid related inflammation in the plaques that witnessed a rupture event followed by thrombosis. Such biomarkers would be beneficial for assessing clinical risk and performing early detection, also for tailoring specific (anti thrombotic) therapy.
Author contributions
KRP, OJB and ACW designed the study. KRP, PG and ACW prepared and assessed the specimens. KRP, CM and PG performed experiments; KRP, OJB and CM analysed the data. KRP, OJB and ACW contributed to design the manuscript. KRP wrote the manuscript while all authors provided feedback to improve the manuscript. OJB and ACW supervised this study. All authors had final approval of the submitted versions.
Funding
This work was supported by the Indonesian Endowment Fund for Education, Ministry of Finance, Republic of Indonesia (LPDP).
Declaration of Competing Interest
Nothing to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2019.100439.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Badimon L., Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 2014;276:618–632. doi: 10.1111/joim.12296. [DOI] [PubMed] [Google Scholar]
- 2.Kramer M.C.A., Rittersma S.Z.H., de Winter R.J., Ladich E.R., Fowler D.R., Liang Y.H., Kutys R., Carter-Monroe N., Kolodgie F.D., van der Wal A.C., Virmani R. Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J. Am. Coll. Cardiol. 2010;55:122–132. doi: 10.1016/j.jacc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Rittersma S.Z.H., Van Der Wal A.C., Koch K.T., Piek J.J., Henriques J.P.S., Mulder K.J., Ploegmakers J.P.H.M., Meesterman M., De Winter R.J. Plaque instability frequently occurs days or weeks before occlusive coronary thrombosis: A pathological thrombectomy study in primary percutaneous coronary intervention. Circulation. 2005;111:1160–1165. doi: 10.1161/01.CIR.0000157141.00778.AC. [DOI] [PubMed] [Google Scholar]
- 4.J. Mann, M.J. Davies, Mechanisms of progression in native coronary artery disease : role of healed plaque disruption Mechanisms of progression in native coronary artery disease : role of healed plaque disruption, (1999) 265–268. [DOI] [PMC free article] [PubMed]
- 5.Yunoki K., Naruko T., Inoue T., Sugioka K., Inaba M., Iwasa Y., Komatsu R., Itoh A., Haze K., Yoshiyama M., Becker A.E., Ueda M. Relationship of thrombus characteristics to the incidence of angiographically visible distal embolization in patients with ST-segment elevation myocardial infarction treated with thrombus aspiration. JACC Cardiovasc. Interv. 2013;6:377–385. doi: 10.1016/j.jcin.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Grundeken M.J., Li X., Kurpershoek C.E., Kramer M.C., Vink A., Piek J.J., Tijssen J.G.P., Koch K.T., Wykrzykowska J.J., De Winter R.J., Van Der Wal A.C. Distal embolization of hydrophilic-coating material from Coronary guidewires after percutaneous coronary interventions. Circ. Cardiovasc. Interv. 2015;8:1–8. doi: 10.1161/CIRCINTERVENTIONS.114.001816. [DOI] [PubMed] [Google Scholar]
- 7.Li X., Kramer M.C., Van Der Loos C.M., Ploegmakers H.J.P., De Boer O.J., Koch K.T., Tijssen J.G.P., De Winter R.J., van Der Wal A.C. Early onset of endothelial cell proliferation in coronary thrombi of patients with an acute myocardial infarction: Implications for plaque healing. J. Thromb. Haemost. 2012;10:466–473. doi: 10.1111/j.1538-7836.2012.04620.x. [DOI] [PubMed] [Google Scholar]
- 8.Sadowski M., Zabczyk M., Undas A. Coronary thrombus composition: Links with inflammation, platelet and endothelial markers. Atherosclerosis. 2014;237:555–561. doi: 10.1016/j.atherosclerosis.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Kavurma M.M., Bhindi R., Lowe H.C., Chesterman C., Khachigian L.M. Vessel wall apoptosis and atherosclerotic plaque instability. J. Thromb. Haemost. 2005;3:465–472. doi: 10.1111/j.1538-7836.2005.01120.x. [DOI] [PubMed] [Google Scholar]
- 10.Maagdenberg C.G., de Boer O.J., Li X., Mackaay C., Niessen H.W., de Winter R.J., Van der Wal A.C. Time dependent apoptotic rates in the evolving coronary thrombus mass of myocardial infarction patients. Thromb. Res. 2016;145:12–17. doi: 10.1016/j.thromres.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W., Annicchiarico-Petruzzelli M., Antonov A.V., Arama E., Baehrecke E.H., Barlev N.A., Bazan N.G., Bernassola F., Bertrand M.J.M., Bianchi K., Blagosklonny M.V., Blomgren K., Borner C., Boya P., Brenner C., Campanella M., Candi E., Carmona-Gutierrez D., Cecconi F., Chan F.K.-M., Chandel N.S., Cheng E.H., Chipuk J.E., Cidlowski J.A., Ciechanover A., Cohen G.M., Conrad M., Cubillos-Ruiz J.R., Czabotar P.E., D’Angiolella V., Dawson T.M., Dawson V.L., De Laurenzi V., De Maria R., Debatin K.-M., DeBerardinis R.J., Deshmukh M., Di Daniele N., Di Virgilio F., Dixit V.M., Dixon S.J., Duckett C.S., Dynlacht B.D., El-Deiry W.S., Elrod J.W., Fimia G.M., Fulda S., García-Sáez A.J., Garg A.D., Garrido C., Gavathiotis E., Golstein P., Gottlieb E., Green D.R., Greene L.A., Gronemeyer H., Gross A., Hajnoczky G., Hardwick J.M., Harris I.S., Hengartner M.O., Hetz C., Ichijo H., Jäättelä M., Joseph B., Jost P.J., Juin P.P., Kaiser W.J., Karin M., Kaufmann T., Kepp O., Kimchi A., Kitsis R.N., Klionsky D.J., Knight R.A., Kumar S., Lee S.W., Lemasters J.J., Levine B., Linkermann A., Lipton S.A., Lockshin R.A., López-Otín C., Lowe S.W., Luedde T., Lugli E., MacFarlane M., Madeo F., Malewicz M., Malorni W., Manic G., Marine J.-C., Martin S.J., Martinou J.-C., Medema J.P., Mehlen P., Meier P., Melino S., Miao E.A., Molkentin J.D., Moll U.M., Muñoz-Pinedo C., Nagata S., Nuñez G., Oberst A., Oren M., Overholtzer M., Pagano M., Panaretakis T., Pasparakis M., Penninger J.M., Pereira D.M., Pervaiz S., Peter M.E., Piacentini M., Pinton P., Prehn J.H.M., Puthalakath H., Rabinovich G.A., Rehm M., Rizzuto R., Rodrigues C.M.P., Rubinsztein D.C., Rudel T., Ryan K.M., Sayan E., Scorrano L., Shao F., Shi Y., Silke J., Simon H.-U., Sistigu A., Stockwell B.R., Strasser A., Szabadkai G., Tait S.W.G., Tang D., Tavernarakis N., Thorburn A., Tsujimoto Y., Turk B., Vanden Berghe T., Vandenabeele P., Vander Heiden M.G., Villunger A., Virgin H.W., Vousden K.H., Vucic D., Wagner E.F., Walczak H., Wallach D., Wang Y., Wells J.A., Wood W., Yuan J., Zakeri Z., Zhivotovsky B., Zitvogel L., Melino G., Kroemer G. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pertiwi K.R., de Boer O.J., Mackaaij C., Pabittei D.R., de Winter R.J., Li X., van der Wal A.C. Extracellular traps derived from macrophages, mast cells, eosinophils and neutrophils are generated in a time-dependent manner during atherothrombosis. J. Pathol. 2019;247:505–512. doi: 10.1002/path.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savchenko A.S., Martinod K., Seidman M.A., Wong S.L., Borissoff J.I., Piazza G., Libby P., Goldhaber S.Z., Mitchell R.N., Wagner D.D. Neutrophil extracellular traps form predominantly during the organizing stage of human venous thromboembolism development. J. Thromb. Haemost. 2014;12:860–870. doi: 10.1111/jth.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer M.C., van der Wal A.C., Koch K.T., Rittersma S.Z., Li X., Ploegmakers H.P., Henriques J.P., van der Schaaf R.J., Baan J., Vis M.M., Meesterman M.G., Piek J.J., Tijssen J.G., de Winter R.J. Histopathological features of aspirated thrombi after primary percutaneous coronary intervention in patients with ST-Elevation myocardial infarction. PLoS One. 2009;4:2–7. doi: 10.1371/journal.pone.0005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verouden N.J.W., Kramer M.C., Li X., Meuwissen M., Koch K.T., Henriques J.P.S., Baan J., Vis M.M., Piek J.J., Van Der Wal A.C., Tijssen J.G.P., De Winter R.J. Histopathology of aspirated thrombus and its association with ST-segment recovery in patients undergoing primary percutaneous coronary intervention with routine thrombus aspiration. Catheter. Cardiovasc. Interv. 2011;77:35–42. doi: 10.1002/ccd.22616. [DOI] [PubMed] [Google Scholar]
- 16.Gown A.M., Willingham M.C. Improved detection of apoptotic cells in archival paraffin sections: Immunohistochemistry using antibodies to cleaved caspase 3. J. Histochem. Cytochem. 2002;50:449–454. doi: 10.1177/002215540205000401. [DOI] [PubMed] [Google Scholar]
- 17.Brinkmann V., Abed U.A., Goosmann C., Zychlinsky A. Immunodetection of NETs in paraffin-embedded tissue. Front. Immunol. 2016;7 doi: 10.3389/fimmu.2016.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masuda S., Nakazawa D., Shida H., Miyoshi A., Kusunoki Y., Tomaru U., Ishizu A. NETosis markers: Quest for specific, objective, and quantitative markers. Clin. Chim. Acta. 2016;459:89–93. doi: 10.1016/j.cca.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 19.Sun X., Kaufman P.D. Ki-67: more than a proliferation marker. Chromosoma. 2018;127:175–186. doi: 10.1007/s00412-018-0659-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barauskas G., Svagzdys S., Maleckas A. C-reactive protein in early prediction of pancreatic necrosis. Medicina (Kaunas). 2004;40:135–140. [PubMed] [Google Scholar]
- 21.Goldberg A., Gruberg L., Roguin A., Petcherski S., Rimer D., Markiewicz W., Beyar R., Aronson D. Preprocedural C-reactive protein levels predict myocardial necrosis after successful coronary stenting in patients with stable angina. Am. Heart J. 2006;151:1265–1270. doi: 10.1016/j.ahj.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Andrié R.P., Bauriedel G., Braun P., Höpp H.W., Nickenig G., Skowasch D. Increased expression of C-reactive protein and tissue factor in acute coronary syndrome lesions. Correlation with serum C-reactive protein, angioscopic findings, and modification by statins. Atherosclerosis. 2009;202:135–143. doi: 10.1016/j.atherosclerosis.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 23.Pertiwi K.R., Van Der Wal A.C., Pabittei D.R., Mackaaij C., Van Leeuwen M.B., Li X., De Boer O.J. Neutrophil extracellular traps participate in all different types of thrombotic and haemorrhagic complications of coronary atherosclerosis. Thromb. Haemost. 2018 doi: 10.1055/s-0038-1641749. [DOI] [PubMed] [Google Scholar]
- 24.Visonà S.D., de Boer O.J., Mackaaij C., de Boer H.H., Pertiwi K.R., de Winter R.W., Osculati A., van der Wal A.C. Immunophenotypic analysis of the chronological events of tissue repair in aortic medial dissections. Cardiovasc. Pathol. 2018;34:9–14. doi: 10.1016/j.carpath.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Yunoki K., Naruko T., Sugioka K., Inaba M., Itoh A., Haze K., Yoshiyama M., Ueda M. Thrombus aspiration therapy and coronary thrombus components in patients with acute ST-elevation myocardial infarction. J. Atheroscler. Thromb. 2013;20:524–537. doi: 10.5551/jat.17608. [DOI] [PubMed] [Google Scholar]
- 26.Šteiner I., Špaček J., Matějková A., Vojáček J., Bis J., Dušek J. Histopathology of aspirated thrombi during primary percutaneous coronary intervention in patients with acute myocardial infarction. Cardiovasc. Pathol. 2014;23:267–271. doi: 10.1016/j.carpath.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Laridan E., Denorme F., Desender L., François O., Andersson T., Deckmyn H., Vanhoorelbeke K., De Meyer S.F. Neutrophil extracellular traps in ischemic stroke thrombi. Ann. Neurol. 2017;82:223–232. doi: 10.1002/ana.24993. [DOI] [PubMed] [Google Scholar]
- 28.Laridan E., Martinod K., De Meyer S.F. Neutrophil extracellular traps in arterial and venous thrombosis. Semin. Thromb. Hemost. 2019 doi: 10.1055/s-0038-1677040. [DOI] [PubMed] [Google Scholar]
- 29.T.A. Fuchs, A. Brill, D. Duerschmied, D. Schatzberg, M. Monestier, Extracellular DNA traps promote thrombosis, 107 (2010) 15880–15885. 10.1073/pnas.1005743107/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed]
- 30.Kramer M.C.A., Van Der Wal A.C., Koch K.T., Ploegmakers J.P.H.M., Van Der Schaaf R.J., Henriques J.P.S., Baan J., Rittersma S.Z.H., Vis M.M., Piek J.J., Tijssen J.G.P., De Winter R.J. Presence of older thrombus is an independent predictor of long-term mortality in patients with ST-elevation myocardial infarction treated with thrombus aspiration during primary percutaneous coronary intervention. Circulation. 2008;118:1810–1816. doi: 10.1161/CIRCULATIONAHA.108.780734. [DOI] [PubMed] [Google Scholar]
- 31.Li X., Kramer M.C., Damman P., van der Wal A.C., Grundeken M.J., van Straalen J.P., Koch K.T., Henriques J.P., Baan J., Vis M.M., Piek J.J., Fischer J.C., Tijssen J.G.P., de Winter R.J. Older coronary thrombus is an independent predictor of 1-year mortality in acute myocardial infarction. Eur. J. Clin. Invest. 2016;46:501–510. doi: 10.1111/eci.12619. [DOI] [PubMed] [Google Scholar]
- 32.Nishihira K., Shibata Y., Yamashita A., Kuriyama N., Asada Y. Relationship between thrombus age in aspirated coronary material and mid-term major adverse cardiac and cerebrovascular events in patients with acute myocardial infarction. Atherosclerosis. 2018;268:138–144. doi: 10.1016/j.atherosclerosis.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Yasojima K., Schwab C., McGeer E.G., McGeer P.L. Generation of C-reactive protein and complement components in atherosclerotic plaques. Am. J. Pathol. 2001;158:1039–1051. doi: 10.1016/S0002-9440(10)64051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X.F., Kramer M.C., van der Loos C.M., Koch K.T., de Boer O.J., Henriques J.P.S., Baan J., Vis M.M., Piek J.J., Tijssen J.G.P., de Winter R.J., van der Wal A.C. A pattern of disperse plaque microcalcifications identifies a subset of plaques with high inflammatory burden in patients with acute myocardial infarction. Atherosclerosis. 2011;218:83–89. doi: 10.1016/j.atherosclerosis.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 35.Wu J., Stevenson M.J., Brown J.M., Grunz E.A., Strawn T.L., Fay W.P. C-Reactive protein enhances tissue factor expression by vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2008;28:698–704. doi: 10.1161/ATVBAHA.107.160903. [DOI] [PubMed] [Google Scholar]
- 36.Martínez V.B., González-Juanatey J.R. Markers of inflammation and cardiovascular disease. Am. J. Cardiovasc. Drugs. 2009;9:3–7. doi: 10.2165/1153161-S0-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Doster R.S., Rogers L.M., Gaddy J.A., Aronoff D.M. Macrophage extracellular traps: A scoping review. J. Innate Immun. 2018;10:3–13. doi: 10.1159/000480373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ball R.Y., Stowers E.C., Burton J.H., Cary N.R.B., Skepper J.N., Mitchinson M.J. Evidence that the death of macrophage foam cells contributes to the lipid core of atheroma. Atherosclerosis. 1995;114:45–54. doi: 10.1016/0021-9150(94)05463-s. [DOI] [PubMed] [Google Scholar]
- 39.L.H. Charney, R.B. Vascular, Macrophages in atherosclerosis : a dynamic balance, 13 (2015) 709–721. https://doi.org/10.1038/nri3520.Macrophages.
- 40.Spronk H., Padro T., Siland J., Prochaska J., Winters J., van der Wal A., Posthuma J., Lowe G., d’ Alessandro E., Wenzel P., Coenen D., Reitsma P., Ruf W., van Gorp R., Koenen R., Vajen T., Alshaikh N., Wolberg A., Macrae F., Asquith N., Heemskerk J., Heinzmann A., Moorlag M., Mackman N., van der Meijden P., Meijers J., Heestermans M., Renné T., Dólleman S., Chayouâ W., Ariëns R., Baaten C., Nagy M., Kuliopulos A., Posma J., Harrison P., Vries M., Crijns H., Dudink E., Buller H., Henskens Y., Själander A., Zwaveling S., Erküner O., Eikelboom J., Gulpen A., Peeters F., Douxfils J., Olie R., Baglin T., Leader A., Schotten U., Scaf B., van Beusekom H., Mosnier L., van der Vorm L., Declerck P., Visser M., Dippel D., Strijbis V.J., Pertiwi K., ten Cate-Hoek A., ten Cate H. Atherothrombosis and thromboembolism: position paper from the second maastricht consensus conference on thrombosis. Thromb. Haemost. 2018;118:229–250. doi: 10.1160/TH17-07-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rekhter M.D., Gordon D. Active proliferation of different cell types, including lymphocytes, in human atherosclerotic plaques. Am. J. Pathol. 1995;147:668–677. [PMC free article] [PubMed] [Google Scholar]
- 42.Andrés V., Pello O.M., Silvestre-Roig C. Macrophage proliferation and apoptosis in atherosclerosis. Curr. Opin. Lipidol. 2012;23:429–438. doi: 10.1097/MOL.0b013e328357a379. [DOI] [PubMed] [Google Scholar]
- 43.Kearney M., Pieczek A., Haley L., Losordo D.W., Andres V., Schainfeld R., Rosenfield K., Isner J.M. Histopathology of in-stent restenosis in patients with peripheral artery disease. Circulation. 1997;95:1998–2002. doi: 10.1161/01.cir.95.8.1998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.