Abstract
Background
Routine HIV pre-exposure prophylaxis (PrEP) and HIV care appointments provide opportunities for screening men who have sex with men (MSM) for hepatitis C virus infection (HCV). However, levels of screening required for achieving the WHO elimination target of reducing HCV incidence by 90% by 2030 among all MSM are unknown.
Methods
An HCV/HIV transmission model was calibrated to UK prevalence of HIV among MSM (4·7%) and chronic HCV infection among HIV-positive MSM (9·9%) and HIV-negative MSM (1.2%). Assuming 12·5% coverage of PrEP among HIV-negative MSM, we evaluated the relative reduction in overall HCV incidence by 2030 (compared to 2018 levels) of HCV screening every 12/6-months (alongside completing direct acting antiviral treatment within 6-months of diagnosis) in PrEP users and/or HIV-diagnosed MSM. We estimated the additional screening required among HIV-negative non-PrEP users to reduce overall incidence by 90% by 2030. The effect of 50% reduction in condom use among PrEP users (risk compensation) was estimated.
Results
Screening and treating PrEP users for HCV every 12 or 6-months decreases HCV incidence by 67·3% (uncertainty range 52·7–79·2%) or 70·2% (57·1–80·8%), respectively, increasing to 75·4% (59·0–88·6%) or 78·8% (63·9–90·4%) if HIV-diagnosed MSM are also screened at same frequencies. Risk compensation reduces these latter projections by <10%. To reduce HCV incidence by 90% by 2030 without risk compensation, HIV-negative non-PrEP users require screening every 5·6 (3·8–9·2) years if MSM on PrEP and HIV-diagnosed MSM are screened every 6-months, shortening to 4·4 (3·1–6·6) years with risk compensation. For 25·0% PrEP coverage, the HCV elimination target can be reached without screening HIV-negative MSM not on PrEP, irrespective of risk compensation.
Interpretation
At low PrEP coverage, increased screening of all MSM is required to achieve the WHO HCV-elimination targets for MSM in the UK, whereas at higher PrEP coverage this is possible through just screening HIV-diagnosed MSM and PrEP users.
Keywords: Pre-exposure prophylaxis, Hepatitis C virus, HIV, Men who have sex with men, Antiviral treatment, Prevention, Risk compensation
Abbreviations: PrEP, Pre-exposure prophylaxis; HCV, Hepatitis C virus; HIV, Human immunodeficiency virus; MSM, Men who have sex with men; DAA, Direct acting antiviral; PLHIV, People living with HIV; ART, Anti-retroviral therapy; STIs, Sexually transmitted infections; WHO, World Health organisation; NHS, National Health Service; EMIS, The European Men-Who-Have-Sex-With-Men Internet Survey; UK CHIC, UK Collaborative HIV Cohort
Research in context.
Evidence before this study
Recent modelling studies based on HIV-positive MSM in England, Switzerland and Berlin indicate that rapid access and scale up of HCV DAAs could lead to significant reductions in HCV incidence; but that behavioural change would be required to reach the HCV elimination targets. Similar modelling based on Victoria, Australia projected that treatment within 6 months of HCV diagnosis lowered HCV prevalence by 80% in just over 2 years, with a similar study based in France finding that treating 70% of those currently diagnosed every year resulted in an 80% reduction in HCV prevalence over 10 years. However, another study highlighted the potential drawbacks of treatment as prevention for HCV in MSM due to high re-infection rates, and so raised concerns about attaining the 2030 elimination targets.
Added value of this study
Our study is an important addition to this literature because it is the first to examine HCV elimination targets for the entire population of MSM, not just HIV-positive MSM. We also consider how HCV screening and treatment needs to evolve with the scale-up of PrEP, and should incorporate the possible effect of risk compensation on HCV elimination targets. These additions are useful for policy-makers working towards the WHO 2030 HCV elimination targets of reducing HCV incidence by 90% among MSM. Importantly, at low PrEP coverage our modelling suggests it will be difficult to reach these elimination targets among MSM without also including screening and treatment interventions for HIV-negative MSM, with frequent screening of MSM on PrEP being an important addition to ongoing HCV management strategies. However, at higher PrEP coverage, the elimination targets may be possible through just improving HCV screening in HIV-diagnosed MSM and MSM on PrEP.
Implications of all the available evidence
Our work supports the current literature, showing that the 2030 HCV elimination targets for incidence are achievable among MSM. However, this target may require increased HCV screening of all MSM with faster linkage of diagnosed individuals to HCV treatment. Elimination efforts that are currently only focussing on HIV-diagnosed MSM need to expand their scope to include other MSM groups.
Alt-text: Unlabelled box
1. Introduction
Globally, men who have sex with men (MSM) experience a high burden of HIV [1], with elevated levels of hepatitis C (HCV) co-infection occurring among HIV-positive MSM in high-income countries [2], but much lower transmission occurring among HIV-negative MSM [2,3]. This recent MSM HCV epidemic has been associated with sexual and drug-related behaviours [4], with the polarised pattern of HCV in HIV-infected MSM likely due to heterogeneity in risk behaviours and HIV sero-adaptive behaviours [5]. Over 2012–2017, HIV incidence has halved in the UK [6] mainly due to the UK achieving very high levels of antiretroviral therapy (ART) coverage and HIV viral suppression [6].
HIV pre-exposure prophylaxis (PrEP) is a pre-emptive anti-retroviral medication, which has high efficacy for preventing HIV acquisition [7,8]. Many countries are expanding the availability of PrEP among MSM [11]. However, as occurred with the expansion of ART [9], there are concerns that PrEP use could result in increased sexual risk taking, thus increasing the transmission of sexually transmitted infections (STIs), including HCV [7]. Recent studies have confirmed this, giving a consistent picture that the incidence of STIs increases following initiation of PrEP [10,11].
Although PrEP is freely available in Wales and Scotland [12], it has limited availability in England, with PrEP being restricted to individuals enrolled in the IMPACT trial [13]. In all these settings, PrEP is only available to individuals meeting specific eligibility criteria. In England, this mainly involves reporting on-going condomless sex or other factors posing similar HIV-risk [13]. The full IMPACT trial eligibility criteria is included in the Supplementary material, with there being similar eligibility criteria in Scotland and Wales [12].
The World Health organisation (WHO) recently developed a Global Health Strategy to eliminate HCV, aiming to reduce HCV incidence by 90% by 2030 [14]. Elimination initiatives are attempting to achieve this goal among MSM, mainly targeting HIV-diagnosed MSM [15–[16], [17]]. In the UK, HIV-diagnosed MSM are advised to be screened for HCV each year [18], while HIV-negative MSM are rarely tested [19] and there are no HCV testing guidelines for MSM using PrEP.
Previous modelling has considered what is required to eliminate HCV among HIV-diagnosed MSM [20,21], but none have accounted for the HCV transmission dynamics among HIV-negative MSM. In this study, we use modelling to determine what HCV testing and treatment strategies are needed to reduce the overall incidence of HCV among MSM by 90% by 2030 in the UK. We assess how PrEP and any associated changes in condom use may affect the impact achieved, and determine the need for HCV screening among PrEP users and other HIV-negative MSM to help inform future policy and guidelines.
2. Methods
2.1. Model derivation
We adapt a previous model of HIV and HCV transmission among MSM [5] to include PrEP use (details in Supplementary material). The model (Supplementary Fig. S1) stratifies MSM by: HIV and PrEP status (susceptible on/off PrEP, acute HIV infection on/off PrEP, undiagnosed or diagnosed chronic HIV infection); HCV-status (susceptible, acute HCV infection, undiagnosed and diagnosed chronic HCV infection); and either low- or high-risk sexual behaviour, defined by the annual number of anal sex partners (high-risk defined as ≥ 15). Individuals are not assumed to change their risk.
Individuals enter the model susceptible to HIV and HCV, not using PrEP, and either low- or high-risk. HIV and HCV transmission occurs at rates related to an individuals’ sexual risk and prevalence of HIV and HCV among their sexual partners. HCV infectivity is elevated for HIV-HCV co-infected individuals based on evidence for vertical HCV transmission (details in Supplementary materials) [22]. MSM also mix preferentially, more commonly choosing partners of the same sexual risk and HIV-status (see Supplementary materials).
Depending on the PrEP scenario being modelled, HIV-negative individuals may initiate using PrEP, which confers protection to acquiring HIV [7,8,23]. PrEP was assumed to scale-up from 2018, with the coverage of PrEP reaching 12·5% of HIV-negative MSM by 2020 with the average duration on PrEP being 8·2 months [24]. PrEP users are screened quarterly for HIV and upon a positive diagnosis stop using PrEP. This frequent HIV-testing means PrEP users are diagnosed before reaching chronic HIV infection [25]. Non-PrEP users who acquire acute HIV infection, firstly transition to undiagnosed HIV infection and are then diagnosed at current UK HIV-testing rates (2·3 years between infection and diagnosis) [26]. Acute HIV is assumed to have elevated HIV transmission risk (26-fold) [27]. A proportion of HIV-diagnosed MSM are on ART, which increases survival 3·4-fold, with a proportion being virally supressed and having negligible HIV transmission risk (see Supplementary materials).
Newly HCV-infected individuals develop acute HCV infection, following which they either develop chronic HCV infection or spontaneously clear their infection and become susceptible again. The baseline model assumes HIV-negative MSM are diagnosed for HCV based upon symptomatic presentation after 5–15 years. Conversely, undiagnosed HIV-positive MSM receive testing for HCV following HIV diagnosis, with 88% of HIV-diagnosed MSM being screened annually in the UK [28]. At baseline, we assume 2.2 years from diagnosis to completing HCV treatment, consistent with UK data for pre-DAA treatments [28]. Before 2015, we assume different cure rates for HIV-positive (sustained viral response or SVR of 35–42%) [29] and HIV-negative MSM (SVR of 59–69%) [30] based on pre-DAA treatments, but then assume higher cure rates from 2015 for DAA therapies (SVR of 90–100%) [28]. From 2018, we then consider the impact of various scenarios of improved HCV screening with faster linkage-to-treatment following diagnosis (6 months to treatment completion); more consistent with current treatment rates [17]. This assumes a 3-month waiting time and an HCV treatment duration of 8–12 weeks. MSM failing treatment are retreated at the same rate as initial HCV treatment.
2.2. Parameterization of sexual risk behaviour
Sexual risk behaviours were parameterized using data from the UK component of the European MSM Internet Survey (EMIS-UK);[5], [31] EMIS was a pan-European internet survey on interventions, needs, behaviours and morbidities regarding HIV and STI transmission among MSM. Individuals could complete the survey online during Summer 2010. Over 180,000 men took part from 38 countries, including 18,000 in the UK [31]. From EMIS, we calculated the level of preferential mixing by HIV-status; proportion of MSM in the low and high-risk groups; prevalence of chem-sex; and levels of condom use stratified by the assumed HIV sero-concordancy. These model parameters are detailed in Supplementary Table S3, and summarised in Table 1. Briefly, EMIS data suggests 17·4% of MSM are high-risk, amongst whom the prevalence of chem-sex in last year is higher than among low-risk MSM (22.6% versus 11.5%). The baseline model assumed that MSM have sex more often with others of the same perceived HIV-status, with perceived HIV-positive concordant partnerships having lower condom use (13%) than other partnerships (68%) [5].
Table 1.
Key model parameters with ranges and details of estimation included. (For the full version see Supplementary Table S3.)
| Model parameters | Value and uncertainty range | References | Comment |
|---|---|---|---|
| HCV related parameters | |||
| Efficacy of HCV treatment with DAAs – after 2015 | 95% (90–100%) | [35,36] | Efficacy is the sustained viral response |
| Duration of HCV treatment in years | 0.19 (0.15–0.23) | [15] | Duration of DAA treatment generally 8 to 12 weeks |
| Rate of HCV screening in HIV-diagnosed MSM at baseline | 1.13 person-years | [28] | 88% of HIV-diagnosed MSM are HCV tested each year during routine HIV check-up appointments from UK-CHIC data |
| Rate of HCV screening in HIV-negative MSM at baseline | 0.10 (0.07–0.20) person-years | [44] | HIV-negative MSM not normally diagnosed with HCV until have symptoms unless they are high-risk, so assume 5–15 years |
| Average delay from HCV diagnosis to initiation of treatment. | 2.2 years | [28] | UK-CHIC data for HIV-diagnosed MSM and assume same for other MSM |
| Behavioural parameters | |||
| Proportion of MSM that are low-risk | 0.83 (0.79–0.86) | EMIS | Proportion of MSM with <15 anal sex partners in the last year. |
| Proportion of MSM that are high-risk | 0.17 (0.21–0.14) | EMIS | Proportion of MSM with ≥ 15 anal sex partners in the last year. |
| Annual number of anal sex partners in the last year in the low-risk group | 2.9 (2.3–3.5) | EMIS | Average number of anal sex partners among low-risk MSM with a +/− 20%. uncertainty range. |
| Annual number of anal sex partners in the last year in the high-risk group | 29.1 (23.3–34.9) | EMIS | Average number of anal sex partners among high-risk MSM with a +/− 20%. uncertainty range. |
| Relative risk of HIV acquisition among high-risk MSM compared to low-risk MSM | 1.3 (1.1–1.5) | EMIS and [45] | EMIS data on prevalence of chem-sex in last year for low and high risk MSM combined with estimated increased risk of HIV acquisition due to chem sex in MSM studies. Relative risk is difference between low and high risk MSM [47]. |
| Relative risk of HCV acquisition among high-risk MSM compared to low-risk MSM | 1.5 (1.1–1.9) | EMIS and [46] | EMIS data on prevalence of chem-sex in last year for low and high risk MSM combined with estimated increased risk of HCV acquisition due to chem sex in MSM studies. Relative risk is difference between low and high risk MSM [47]. |
| The proportion of MSM who mix like with like by HIV-status | 0.35 (0.28−0.42) | EMIS | EMIS data on proportion of partnerships chosen between people of the same HIV-status assuming no errors in judgement. |
| Probability of error in serosorting judgement (random mixing otherwise) | 24.9% (12.1–45.7%) | EMIS | EMIS data on proportion of individuals who make assumptions about partner's HIV-status based on unreliable reasons |
| Probability of condom usage between HIV diagnosed MSM and a partner assumed to be HIV-positive | 13.0% (10.4–15.6%) | EMIS | EMIS data on condom use with last casual partner when both sides of partnership are thought to be HIV-positive. Assume range +/− 20% either side. |
| Probability of condom usage in other MSM partnerships (not ones thought to be both HIV-positive) | 68.0% (54.4–81.6%) | EMIS | EMIS data on condom use with last casual partner when partnerships not thought to be sero-concordant. Assume range +/− 20% either side. |
| Efficacy of condoms per sex act | 0.91 (0.69–1.00) | [48] | Per act protection provided by condom use during anal sex |
| Proportion of MSM who mix like with like by risk status | 0.25 (0.00–0.50) | EMIS | EMIS data on whether individuals in each risk group meet their partners in the same places. Assume large uncertainty. |
| PrEP related parameters | |||
| Proportion of MSM taking up PrEP by 2020 | 12.5% or 25.0% | EMIS [25] | 12.5% coverage estimated from EMIS data using basic element of eligibility criteria from NHS England – recent condomless sex. Higher value assumed to encompass uncertainty. |
| Relative increase in coverage of PrEP in high-risk MSM versus low-risk MSM | 2.6 (2.4–2.8) | EMIS [25] | EMIS data applied to NHS England eligibility criteria for low and high-risk MSM |
| Efficacy of PrEP in reducing HIV incidence | 91.5% (86.0–97.0%) | [8,49] | Range in efficacy from UK PROUD study [8] and real-world study of PrEP use among MSM in France and Canada [49]. |
| Rate of HIV testing for PrEP users | Every 3 months | [13] | Standard of care for HIV testing in PrEP users in UK |
| Rate of HCV testing for PrEP users | Every 3–12 months; baseline same as HIV-negative MSM | Varied in different model scenarios | |
2.3. Baseline model calibration
Assuming historic levels of HCV screening and pre-DAA SVR rates with no PrEP, the model was firstly calibrated to give a stable HIV and HCV epidemic in 2012, in line with prevalence data from the UK Collaborative HIV Cohort (UK CHIC) study [28]. UK CHIC involved a collaboration of UK centres providing care for people living with HIV (PLHIV). The study gathered data relating to clinical care of PLHIV since 1996, including data on HCV incidence and prevalence among HIV-diagnosed MSM.
To calibrate the model, we firstly randomly sampled parameter sets from their uncertainty ranges in Table S3. We then used non-linear least-squares fitting (see Supplementary) to estimate transmission parameters for HIV and HCV that result in each model run giving an overall HIV prevalence within the range 4·3–5·3% [32] and a chronic HCV prevalence within the range 9·6–10·2% for HIV-infected MSM at equilibrium [28]. Model runs were only accepted if they also projected a prevalence of HCV among HIV-negative MSM within the range of 0·6–2·1% [34]. In total, we performed 668 runs to obtain 500 fits (75% acceptance rate). For our results, we present the 2·5% to 97·5% percentile range from these 500 model fits, denoted as the 95% central range (95% CR).
For each model fit, we then assume that over 2012–2017 there is an increase in: (1) proportion of HIV-diagnosed MSM on ART from 85% [32] to 98% [6]; (2) proportion of those on ART that are virally suppressed from 72% to 97%; and (3) HIV testing frequency from every 3·2 years [33] to 2·3 years [26]. The resulting model projections (Supplementary Figs. S7–S12) were then validated against data suggesting a 55·5% (95% CI 34·4–72·7%) decrease in the annual rate of new HIV infections among MSM over 2012–2017 in the UK [6]. HCV prevalence and incidence also decrease over this period due to an increase to DAA SVR rates from 2015 [35,36]. Despite being not fit to this data, our model projections for HCV incidence among HIV-diagnosed MSM and HIV-negative MSM off PrEP for 2012 are comparable to UK data estimates from that period, as shown in Supplementary Figs. S7–S12 [37].
2.4. PrEP intervention scenarios
The calibrated model was used to estimate the impact of initiating a PrEP programme, with the coverage of PrEP scaling-up to 12·5% of HIV-negative MSM over the period 2018–2020 while assuming the average duration on PrEP was 8.2 months [24]. This coverage assumption was based on NHS-eligibility criteria, where only MSM recently participating in unprotected sex are eligible for PrEP. The relative coverage of PrEP among low- and high-risk MSM reflects this eligibility criteria (see Supplementary materials). The efficacy of PrEP for reducing the risk of HIV acquisition was assumed to be 91.5% (86–97%) [7,8,23].
PrEP driven risk compensation was also modelled. However, because of inconclusive data on how sexual behaviours may change [7,8,23,38], we only considered reductions in condom use among MSM using PrEP. In our modelling, we either assume no risk compensation (Scenario S0) or that all PrEP users halve their consistency of condom use from 68% to 34% with all partners (Scenario S1). This assumption is varied in our sensitivity analyses.
2.5. Model analyses
The main aim of the analysis is to determine what level of HCV screening and treatment is needed among different MSM sub-populations to eliminate HCV in all MSM, while incorporating the possible effects of PrEP scale-up. However, we firstly considered the possible impact of using DAAs as the new standard of care from 2015, and the effect that PrEP alone could have on the number of new HIV and HCV infections, as well as HCV prevalence and incidence in 2030 compared to 2018 levels.
We then evaluated the impact of different HCV screening and treatment scenarios initiated from 2020, to see what is needed to achieve the WHO elimination target. We firstly evaluated the impact of more frequent HCV screening for HIV-diagnosed MSM or PrEP users, with PrEP users otherwise having the same low level of HCV screening as HIV-negative MSM. The impact on HCV incidence among MSM using PrEP was estimated, with the relative change being compared to what the incidence was in those MSM in 2018. Similarly, the impact on HCV incidence in other groups was estimated. For these scenarios, we also assumed improved linkage-to-treatment for those MSM sub-groups with enhanced screening, with MSM completing treatment within 6 months of diagnosis. Lastly, we considered whether improved screening and linkage-to-treatment for HIV-negative MSM not using PrEP was needed to reach the elimination targets. For MSM using PrEP and HIV-diagnosed MSM, 3, 6 or 12-monthly screening were considered, while the screening frequency for HIV-negative MSM not using PrEP was fitted to give an overall 90% reduction in HCV incidence by 2030.
2.6. Uncertainty analysis
To ascertain which parameters are important for determining variability in the impact projections across the 500 baseline model fits, a linear regression analysis of covariance (ANCOVA) was performed on the projected decrease in overall HCV incidence (2018–2030) of undertaking 6-monthly screening among HIV-diagnosed MSM and MSM using PrEP (no risk compensation). The proportion of the sum of squares contributed by each parameter was calculated to determine each parameters’ importance to the variability in our projections.
We also performed a series of sensitivity analyses where we varied the following: (1) 4 versus 6 months between HCV diagnosis and treatment completion; (2) 25% versus 12·5% coverage of PrEP; (3) condom use among PrEP users decreases to 13% instead of 34% with risk compensation; (4) PrEP is distributed evenly between high and low-risk MSM, or to (5) just low-risk MSM or (6) just high-risk MSM; (7) no increased infectiousness of HCV with HIV co-infection; (8) 50% less mixing by HIV-status following PrEP introduction; (9) no increased risk associated with chem-sex; (10) HCV transmission rate decreased by 20% from 2012 to simulate a decreasing HCV epidemic before the introduction of DAA treatments, or conversely (11) increased by 20% to simulate an increasing HCV epidemic.
3. Results
3.1. Impact of PrEP on HIV
A 12·5% coverage of PrEP among HIV-negative MSM translates to 27·6% of high-risk HIV-negative MSM and 10·3% of low-risk HIV-negative MSM receiving PrEP. Over 2018–2030, this PrEP coverage results in 44·7% (95%CR 30·4–66·7%) of new HIV infections being prevented if sexual behaviours remain unchanged (scenario S0) as shown in Table 2. This large impact on HIV is due to PrEP being targeted to high-risk MSM resulting in the basic reproductive rate decreasing close to one. Less HIV-impact is achieved if PrEP is not targeted to high-risk MSM (Fig. S5).
Table 2.
Model projections of the impact of PrEP on HCV prevalence, incidence and the relative change in new HIV and HCV infections for 12·5% and 25·0% PrEP coverage with and without risk compensation. The risk compensation scenario assumes condom use among PrEP users reduces from 68% to 34%. *PrEP users in ‘No PrEP’ scenario are assumed to be representative of MSM who are eligible for PrEP in 2018.
| PrEP scenario | Relative change (%) in new infections from 2018 to 2030 compared to no PrEP scenario |
HCV prevalence by 2030 |
HCV Incidence by 2030 (per 100 person-years) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HIV | HCV | All MSM | HIV positive | On PrEP* | HIV negative not on PrEP | All MSM | HIV positive | On PrEP* | HIV negative not on PrEP | |
| No PrEP | 0.0% | 0.0% | 1.2% | 2.6% | 2.8% | 1.2% | 0.10 | 0.64 | 0.29 | 0.10 |
| 12·5% coverage of PrEP | ||||||||||
| Scenario S0 – no risk compensation | −44.7% | −1.0% | 1.2% | 2.3% | 2.1% | 1.0% | 0.10 | 0.58 | 0.17 | 0.09 |
| Scenario S1 – condom use in PrEP users falls from 68% to 34% | −40.5% | 42.5% | 1.5% | 2.9% | 3.0% | 1.2% | 0.17 | 0.81 | 0.39 | 0.13 |
| 25·0% coverage of PrEP | ||||||||||
| Scenario S0 – no risk compensation | −67.3% | −1.6% | 1.2% | 2.2% | 2.1% | 0.8% | 0.10 | 0.56 | 0.17 | 0.07 |
| Scenario S1 – condom use in PrEP users falls from 68% to 34% | −63.1% | 80.7% | 1.8% | 3.3% | 3.3% | 1.2% | 0.23 | 0.99 | 0.47 | 0.15 |
3.2. Impact of PrEP on HCV
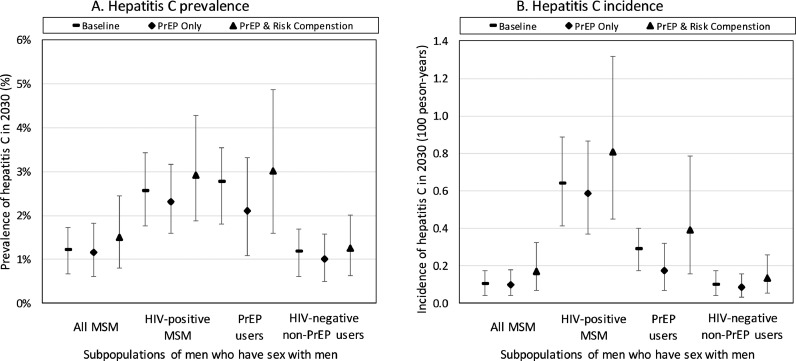
Due to switching to DAA therapies from 2015, HCV incidence will decrease by 42.1% (95%CR 20·8%−61·9%) over 2018–2030, even without the introduction of PrEP or enhanced HCV screening, and overall HCV prevalence will decrease by 32.6% (95%CR 20.5-44.4%) due mainly to a 62.2% (95%CR 50.8-71.2%) decrease in HCV prevalence in HIV-diagnosed MSM. With 12·5% coverage of PrEP, an additional small decrease in HCV incidence occurs (by 45·1% (95%CR 16·8–67·2%) – Fig. 1 and Table 2) if sexual behaviours remain unchanged (S0) and HCV screening remains the same. This decrease is due to PrEP reducing the number of HIV-infected MSM, among whom HCV is concentrated, thus translating to less HIV/HCV co-infections. Interestingly, the HCV prevalence and incidence among HIV-negative MSM using PrEP are both about 3-fold higher than other HIV-negative MSM (Fig. 1) due to many PrEP users being high-risk (Supplementary Fig. S3). However, although HIV-positive MSM and MSM on PrEP have the highest prevalence of HCV, HIV-negative MSM not using PrEP on average harbour 73·1% of all HCV infections (Supplementary Fig. S4).
Fig. 1.
Projections of HCV (A) prevalence and (B) incidence among different subgroups of MSM by 2030 for the baseline ‘no PrEP’ scenario and due to the introduction of PrEP both with and without risk compensation. The projections assume 12·5% coverage of PrEP in HIV-negative MSM and no additional HCV screening. The risk compensation scenario assumes condom use among PrEP users reduces from 68% to 34%. Point estimates are the median of our model projections with the whiskers representing the 2·5 to 97·5 percentiles from our 500 model fits. *PrEP users at baseline are assumed to be representative of MSM who are eligible for PrEP in 2018.
With a halving in condom use among PrEP users (S1), HCV incidence now only decreases by 5·9% (95%CR −59·4% to 49·5%) over 2018–2030.
3.3. Impact of increasing HCV screening in PrEP users
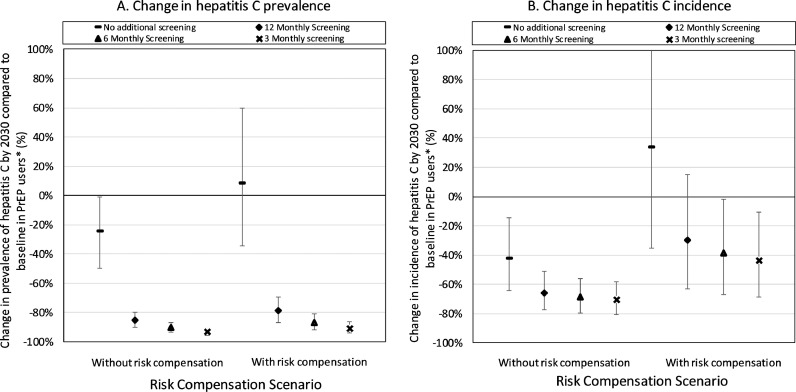
In the previous section, no additional HCV screening of PrEP users occurred. Assuming 12·5% coverage of PrEP and no risk compensation (S0), screening PrEP users every 12, 6 or 3-months (and reducing time to completing treatment to 6-months) reduces the HCV incidence among PrEP users by 65·7% (95%CR 51·1–77·5%), 68·8% (95%CR 55·9–79·7%) or 70·4% (95%CR 58·4–80·7%), respectively, all compared to 2018 levels (Fig. 2), and the HCV incidence among all MSM decreases by 67·3% (95%CR 52·7–79·2%), 70·2% (95%CR 57·1–80·8%) or 71·8% (95%CR 59·5–81·6%) (Fig. 3). This sizeable impact is due to HCV transmission being primarily driven by high-risk MSM, which predominate among PrEP users, with the large impact of HCV treatment in PrEP users having a large subsequent impact among other high-risk MSM (especially HIV-positive MSM) due to them preferentially mixing with each other (Supplementary materials). Risk compensation (S1) reduces the impact on HCV incidence in PrEP users of screening PrEP users by about half (Fig. 2).
Fig. 2.
Relative reduction in HCV (A) prevalence and (B) incidence over 2018 to 2030 compared to 2018 levels among PrEP users when they are screened for HCV every 3, 6 or 12 months and complete HCV treatment within 6 months of diagnosis. Projections are given with and without risk compensation, with the risk compensation scenario assuming condom use among PrEP users reduces from 68% to 34%. The projections with no additional screening are also shown for comparison. Point estimates are the median of our model projections with the whiskers representing the 2·5 to 97·5 percentiles from our 500 model fits. *This is compared to the prevalence of HCV in MSM who are eligible for PrEP in 2018.
Fig. 3.
Relative decrease in overall HCV incidence over 2018 to 2030 due to different HCV screening and treatment scenarios among MSM on PrEP and/or HIV-diagnosed MSM. MSM subgroups with enhanced screening also complete HCV treatment within 6 months of diagnosis. Projections are given (A) without and (B) with risk compensation, with the risk compensation scenario assuming condom use among PrEP users reduces from 68% to 34%. Dashed line in each figure gives change in HCV incidence by 2030 with no change in HCV screening. Point estimates are the median of our model projections with whiskers representing the 2·5 to 97·5 percentile range from our 500 model fits.
3.4. Screening requirements for reaching WHO HCV elimination targets
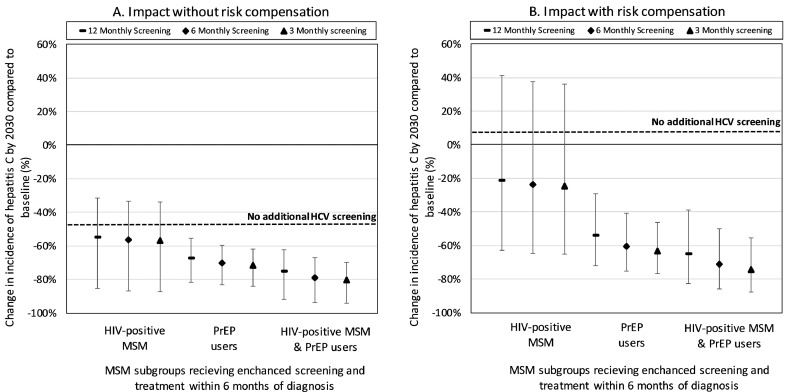
Fig. 3 shows that screening HIV-diagnosed MSM and/or PrEP users more often (3, 6 or 12-monthly) and reducing the time to completing treatment (6 months) can dramatically reduce the overall incidence of HCV by 2030. For instance, without risk compensation if we improve screening to 3-monthly in only HIV-diagnosed MSM then incidence will decrease by 57·1% (95%CR 27·0–80·1%) by 2030, whereas it will decrease by 80·4% (95%CR 66·6–91·2%) if we also undertake 3-monthly screening in PrEP users. With risk compensation, less impact is achieved, although this is small (10% reduction) when both PrEP users and HIV-diagnosed MSM are screened and treated.
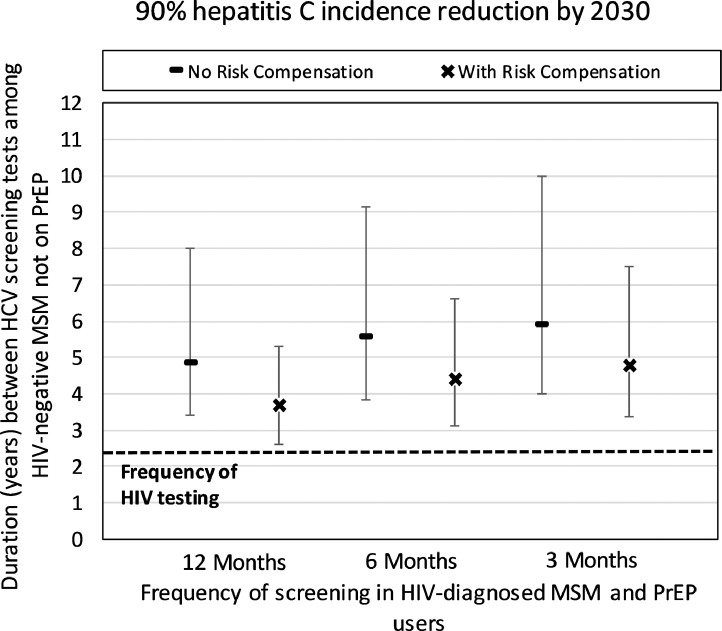
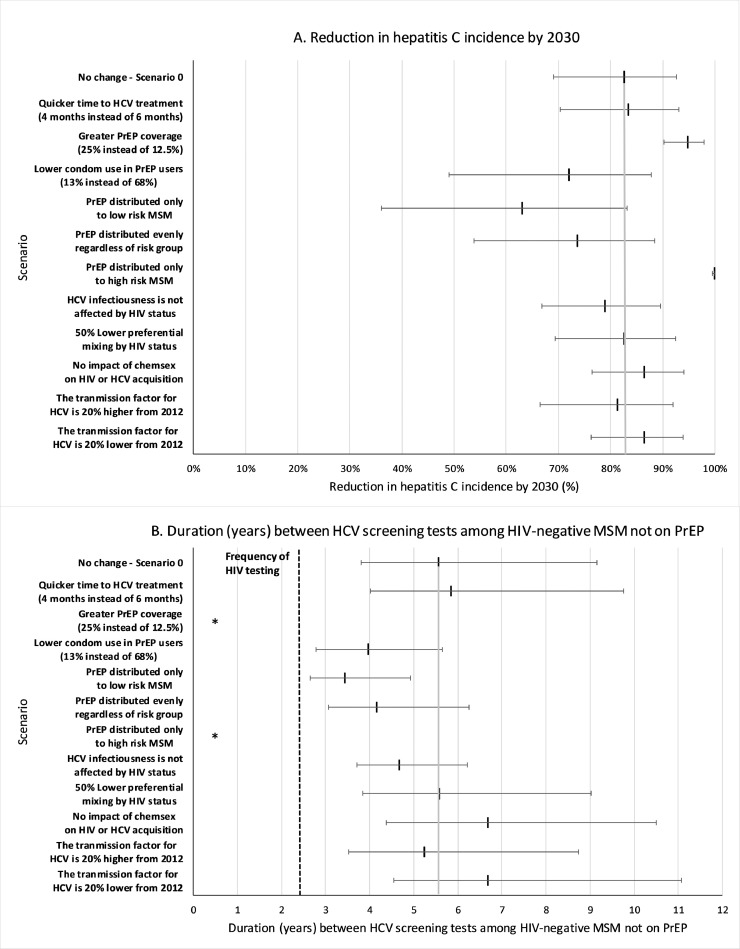
Although considerable impact can be achieved from screening HIV-diagnosed MSM and PrEP users, at 12.5% PrEP coverage it is unlikely to achieve a 90% decrease in overall incidence by 2030 unless we also enhance HCV screening and linkage-to-treatment among HIV-negative MSM not using PrEP. Without risk compensation, screening HIV-negative non-PrEP users every 4·8 (95%CR 3·4–8·0) years will result in a 90% reduction in incidence by 2030 if PrEP users and HIV-diagnosed MSM are screened yearly (Fig. 4). These screening frequencies reduce if PrEP users and HIV-diagnosed MSM are screened more frequently but increase with risk compensation.
Fig. 4.
Required duration between HCV screening tests among HIV-negative MSM not on PrEP (assuming 12·5% PrEP coverage) in order to reach a 90% HCV incidence reduction by 2030 compared to 2018 levels. HIV-diagnosed MSM and/or MSM on PrEP are screened every 3, 6 or 12 months, with all MSM subgroups completing HCV treatment within 6 months of diagnosis. Projections are given with and without risk compensation, with the risk compensation scenario assuming condom use among PrEP users reduces from 68% to 34%. Dashed line shows estimated current frequency of HIV testing in UK. Point estimates are the median of our model projections with whiskers representing the 2·5 to 97·5 percentile range from our 500 model fits.
3.5. Uncertainty analysis
Uncertainty in the increased infectiousness of HCV due to HIV co-infection and the ratio of the number of high-risk MSM on PrEP compared to low-risk MSM accounted for 71.8% of the overall variation in the projected impact on HCV incidence (2018–2030) of undertaking 6-monthly screening among HIV-diagnosed MSM and HIV-negative MSM on PrEP (Supplementary Tables S5 and S6). Otherwise, the efficacy of condoms and the probability of condom usage between pairings other than a HIV-diagnosed MSM and a partner perceived to be HIV-positive accounted for 9·0% and 7·7% of the variation, respectively.
Fig. 5 shows the effect of our sensitivity analyses, with higher coverage of PrEP among HIV-negative MSM (25·0% instead of 12·5%) resulting in the biggest increase in impact aside from giving PrEP to all high-risk MSM. Indeed, in these two scenarios just screening HIV-diagnosed MSM and MSM on PrEP can now result in HCV elimination. Otherwise, impact was reduced by 15–20% if either there was a higher level of risk compensation (to 13% condom use among PrEP users) or PrEP was equally used by low- and high-risk MSM. Other changes in the model assumptions had a small effect, including shorter time to HCV treatment completion after diagnosis (4 instead of 6 months) and a 50% decrease in preferential mixing by HIV-status after the introduction of PrEP. Importantly, none of the sensitivity analyses resulted in the HCV screening frequency of HIV-negative MSM being more frequent than the average screening frequency for HIV in this group (every 2·3 years; shown as dashed line in Fig. 5).
Fig. 5.
One-way sensitivity analysis on the (A) percentage reduction in HCV incidence over 2018 to 2030, and (B) the frequency of screening needed in HIV-negative non-PrEP users to reach an overall 90% reduction in HCV among MSM by 2030, given 6 monthly HCV screening in HIV-positive MSM and PrEP users. Scenario 0 shown for reference as point and whisker and grey vertical line. Point and whiskers represent the median values along with the 2·5 to 97·5 percentiles of the model projections across the 500 baseline model fits. *Note: the 25% PrEP coverage scenario and distribution of PrEP to only high-risk MSM is not shown in (B) because it does not require any screening in HIV-negative MSM not on PrEP to reach an overall 90% reduction in HCV among MSM by 2030.
4. Discussion
Our results highlight that PrEP users as well as HIV-diagnosed MSM are important and convenient groups for targeting HCV screening and treatment initiatives because of their higher risk and frequent health check-ups, which are normally every 3 and 6 months, respectively [25]. Indeed, with 12·5% coverage of PrEP among HIV-negative MSM, yearly HCV screening of MSM on PrEP with rapid linkage-to-treatment could reduce the overall HCV incidence among MSM by up to 67·3% (95%CR 52·7–79·2%) over 2018 to 2030, with this increasing to 79·6% (95% CR 64·6–91·0%) if HIV-diagnosed MSM also receive equivalent screening and treatment. However, although considerable impact is possible from just reaching these groups, at this lower PrEP coverage (12.5%) the WHO HCV elimination targets cannot be reached without also improving screening (and linkage-to-treatment) among HIV-negative MSM not on PrEP to every 3–8 years. This changes, though, at higher PrEP coverage (25·0%), where only HIV-diagnosed MSM and PrEP users will require improved screening to reach the elimination target. Importantly, the required HCV screening frequencies in all MSM groups for achieving elimination are less than their average frequency of HIV testing in the UK [25,26], suggesting that these increases in HCV testing should be feasible if done at the same time as existing HIV testing using the same blood samples.
Our projections also highlight the importance of maintaining condom use among PrEP users, possibly through including counselling on STI and HCV risk for PrEP users as part of routine healthcare visits. Otherwise, reductions in condom use among PrEP users will increase HCV and STI transmission, especially among PrEP users and HIV-diagnosed MSM. Importantly, though, enhanced HCV screening in PrEP users may offset these additional HCV risks with our modelling suggesting similar impact can be achieved from our screening activities irrespective of the level of risk compensation.
The strength of our analysis is in modelling the full epidemic of HIV and HCV among MSM in the UK, while accounting for heterogeneities in sexual risk, patterns of mixing by HIV-status, and using detailed UK data. Despite these strengths, there are limitations.
Firstly, because the model is UK-specific, it may have limited generalisability to other settings. Although most developed countries have a similar predominance of HCV in HIV-diagnosed MSM, some epidemics were increasing or decreasing before the introduction of DAAs [3], while it was relatively stable in the UK in 2012 [28]. Our sensitivity analyses suggest that it will be harder to control an increasing HCV epidemic, with a greater frequency of HCV screening being needed among HIV-negative MSM not on PrEP to reach the elimination targets than for a stable or decreasing HCV epidemic. Unfortunately, more precise parameterisation to specific settings is needed to form more detailed conclusions about the impact of our interventions’ in other settings. Additionally, our projections should not be generalised to lower and middle-income country settings which have different patterns of HIV and HCV prevalence among MSM [2].
Secondly, to simplify our modelling we did not attempt to recreate the entire historical HCV epidemic among MSM in the UK [28,33]; rather we assumed a stable HCV epidemic until 2012 (before the introduction of DAA treatments) as approximated by data from around that time. We also did not explicitly model the role of chem-sex; which we simplified by assuming a factor increase in transmission risk, reflective of what is found in the literature (see Supplementary materials). MSM were also assumed to have similar risk behaviour over their entire lifetime [39] due to insufficient data to parameterise transitions between risk groups over time.
Lastly, uncertainty exists in the data used by the model which propagates to uncertainty in our model projections. For instance, as with all large MSM datasets which include detailed sexual behavioural data, the EMIS dataset is likely to be biased towards more highly educated and higher risk MSM due to the need to perform convenience sampling to obtain a large number of respondents [40]. However, because EMIS was widely distributed and undertaken by 18,000 men in UK, these biases should be less than for much smaller surveys undertaken in gay venues [40]. Our uncertainty analyses show the model is most sensitive to the increased infectiousness of HCV among HIV co-infected MSM and the level of heterogeneity in risk in the two risk groups. Better data on these parameters would improve our model projections. Importantly, we also cannot be certain of the exact scale-up of PrEP, nor the level of risk compensation that may occur among PrEP users. Our model projections suggest that uncertainty in the coverage of PrEP could have a large effect on our findings, while encouragingly, uncertainty in levels of risk compensation has little effect on the impact of large increases in screening.
Several modelling studies have projected the impact of HCV treatment among HIV-diagnosed MSM, but none have modelled the full HCV transmission dynamics and HCV treatment elimination requirements for all MSM [20,21,41,42]. Our analysis builds on these previous studies by evaluating the level of HCV screening and treatment needed in all MSM sub-groups. Our analysis is novel in considering the effect of PrEP on HCV elimination targets, and the additional screening opportunities this provides. Our work compliments recent modelling from the US showing the beneficial impact that PrEP scale-up could have on STI transmission through more frequent STI screening at routine PrEP check-ups [43].
In the era of PrEP scale-up, our modelling suggests that at low PrEP coverage, 12, 6 or 3-monthly HCV screening of HIV-diagnosed MSM and PrEP users, alongside less frequent screening (every 3–8 years) of HIV-negative non-PrEP users, could achieve the WHO elimination target for decreasing HCV incidence by 2030 in the UK. At higher PrEP coverage, this may be achievable with just increased screening in HIV-diagnosed MSM and PrEP users. Importantly, these elimination targets are not possible through only screening HIV-diagnosed MSM (as most elimination initiatives are doing), with the scale-up of PrEP providing a valuable opportunity for increasing HCV-screening among higher-risk MSM. Importantly, though, this added impact of screening PrEP users relies on the assumption that PrEP users are in regular contact with care, which may not be the case if MSM acquire PrEP through unofficial channels. This emphasises the importance of making PrEP freely available through formal channels to ensure that HCV and other STIs can be tested and treated effectively.
Lastly, our study has direct implications for the NHS-England commitment to eliminate HCV through giving specific screening targets for achieving this goal. For other countries with similar HCV epidemics in MSM [3], our findings highlight the need to look beyond just screening HIV-diagnosed MSM, to also screening PrEP users and HIV-negative non-PrEP users.
Declaration of Competing Interest
NKM has received unrestricted research grants from Gilead, and honoraria from Gilead. NKM has also received honorarium of Merck. MH personal fees from MSM Gilead and Jansen. JN has recieved research grants from Gilead Sciences Europe. PV has received unrestricted research grants off Gilead, and honoraria off Gilead and Abbvie. LM, MD, FH and PW have nothing to declare.
Acknowledgments
Partial presentation of results in meetings
N/A.
Financial support
Engineering and Physical Sciences Research Council (EPSRC) studentship for the Ph.D. studies of Louis MacGregor. This work was also partly funded by the Health Protection Research Unit in Evaluation of Interventions.
Acknowledgements
EMIS was funded by a grant of the European Commission under the EU Health Programme 2008-2013, as stipulated in Grant Agreement 2008 12 14 of the Executive Agency for Health and Consumers (EAHC). Further funding was received from CEEISCat (Centre d’Estudis Epidemiològics sobre les ITS/HIV/SIDA de Catalunya, Spain); Department of Health for England; Maastricht University (The Netherlands); Regione del Veneto (Italy); and Robert Koch Institute (Germany). Further funding was provided by: German Ministry of Health for the participation of men living in Ukraine and Moldova; Finnish Ministry of Health for Finland; Norwegian Institute of Public Health for Norway; Swedish Board of Health and Welfare for Sweden; and Bundeszentrale für gesundheitliche Aufklärung (BzGA) for German resident men.
MH and PV would also like to acknowledge support from the NIHR funded Health Protection Research Unit in Evaluation of Interventions. PV would also like to acknowledge the NIHR funded Health Protection Research Unit in STIs and BBVs. NM was supported by the National Institute for Drug Abuse [grant number R01 DA037773] and the University of California San Diego Center for AIDS Research (CFAR), a National Institute of Health (NIH) funded program [grant number P30 AI036214].
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2019.11.010.
Appendix. Supplementary materials
References
- 1.Beyrer C., Baral S.D., van Griensven F. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380(9839):367–377. doi: 10.1016/S0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Platt L., Easterbrook P., Gower E. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16(7):797–808. doi: 10.1016/S1473-3099(15)00485-5. [DOI] [PubMed] [Google Scholar]
- 3.Ghisla V., Scherrer A.U., Nicca D., Braun D.L., Fehr J.S. Incidence of hepatitis C in HIV positive and negative men who have sex with men 2000–2016: a systematic review and meta-analysis. Infection. 2017;45(3):309–321. doi: 10.1007/s15010-016-0975-y. [DOI] [PubMed] [Google Scholar]
- 4.Danta M., Brown D., Bhagani S. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS. 2007;21(8):983–991. doi: 10.1097/QAD.0b013e3281053a0c. [DOI] [PubMed] [Google Scholar]
- 5.MacGregor L, Martin NK, Mukandavire C. Behavioural, not biological, factors drive the HCV epidemic among HIV-positive MSM: HCV and HIV modelling analysis including HCV treatment-as-prevention impact. Int J Epidemiol. 2017;46(5):1582–1592. doi: 10.1093/ije/dyx075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nash S, Desai S, Croxford S. Progress towards ending the HIV epidemic in the United Kingdom 2018 report. Public Health England; 2018. [Google Scholar]
- 7.Molina J.M., Capitant C., Spire B. On-Demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373(23):2237–2246. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- 8.McCormack S., Dunn D.T., Desai M. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bezemer D., de Wolf F., Boerlijst M.C. A resurgent HIV-1 epidemic among men who have sex with men in the era of potent antiretroviral therapy. AIDS. 2008;22(9):1071–1077. doi: 10.1097/QAD.0b013e3282fd167c. [DOI] [PubMed] [Google Scholar]
- 10.Traeger M.W., Cornelisse V.J., Asselin J. Association of HIV preexposure prophylaxis with incidence of sexually transmitted infections among individuals at high risk of HIV infection. JAMA. 2019;321(14):1380–1390. doi: 10.1001/jama.2019.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kojima N., Davey D.J., Klausner J.D. Pre-exposure prophylaxis for HIV infection and new sexually transmitted infections among men who have sex with men. AIDS. 2016;30(14):2251–2252. doi: 10.1097/QAD.0000000000001185. [DOI] [PubMed] [Google Scholar]
- 12.Terrence Higgens Trust. I want Prep now. https://www.iwantprepnow.co.uk. Accessed 2019.
- 13.National Health Service (NHS) England. Prep impact trial. https://www.prepimpacttrial.org.uk. Accessed 2017.
- 14.World Health Organization (WHO). Combating hepatitis B and C to Reach Elimination by 2030. 2016.
- 15.National Health Service (NHS) England Eliminating Hepatitis C in England. All-Party Parliamentary Group on Liver Health Inquiry Report. 2018 [Google Scholar]
- 16.Kracht P.A.M., Arends J.E., van Erpecum K.J. Strategies for achieving viral hepatitis C micro-elimination in the Netherlands. Hepatol Med Policy. 2018;3:12. doi: 10.1186/s41124-018-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boerekamps A., van den Berk G.E., Lauw F.N. Declining hepatitis C virus (HCV) incidence in Dutch human immunodeficiency virus-positive men who have sex with men after unrestricted access to HCV therapy. Clin Infect Dis. 2018;66(9):1360–1365. doi: 10.1093/cid/cix1007. [DOI] [PubMed] [Google Scholar]
- 18.National AIDS Trust. Hepatitis C and HIV Co-Infection. https://www.nat.org.uk/sites/default/files/publications/Jan-2012-Hepatitis-C-and-HIV-co-infection.pdf. Accessed 2019.
- 19.World Health Organization (WHO). World Health Organization: Hepatitis C Fact Sheet. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. Accessed 2019.
- 20.Martin N.K., Boerekamps A., Hill A.M., Rijnders B.J.A. Is hepatitis C virus elimination possible among people living with HIV and what will it take to achieve it. J Int AIDS Soc. 2018;21(Suppl 2) doi: 10.1002/jia2.25062. e25062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin N, Jansen K, van der Heiden M. Can HCV be eliminated among HIV-infected MSM in Berlin? Modeling a setting with increasing incidence and high treatment rates. J Hepatol. 2018;68(Suppl 1):S192–S193. [Google Scholar]
- 22.Benova L., Mohamoud Y.A., Calvert C., Abu-Raddad L.J. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59(6):765–773. doi: 10.1093/cid/ciu447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcus J.L., Glidden D.V., Mayer K.H. No evidence of sexual risk compensation in the iPrEx trial of daily oral HIV preexposure prophylaxis. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0081997. e81997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spinelli M.A., Scott H.M., Vittinghoff E. Missed visits associated with future preexposure prophylaxis (PrEP) discontinuation among prep users in a municipal primary care health network. Open Forum Infect Dis. 2019;6(4) doi: 10.1093/ofid/ofz101. ofz101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NHS England. Clinical Commissioning Policy Proposition: Pre-Exposure Prophylaxis (PrEP) to Prevent the Acquisition of HIV in Adults. 2016.
- 26.Ong K.J., Desai S., Field N. Economic evaluation of HIV pre-exposure prophylaxis among men-who-have-sex-with-men in England in 2016. Euro Surveill. 2017;22(42) doi: 10.2807/1560-7917.ES.2017.22.42.17-00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollingsworth T.D., Anderson R.M., Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198(5):687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 28.Martin NK, Thornton A, Hickman M. Can hepatitis C virus (HCV) direct-acting antiviral treatment as prevention reverse the HCV epidemic among men who have sex with men in the United Kingdom? Epidemiological and modeling insights. Clin Infect Dis. 2016;62(9):1072–1080. doi: 10.1093/cid/ciw075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies A., Singh K.P., Shubber Z. Treatment outcomes of treatment-naive hepatitis C patients co-infected with HIV: a systematic review and meta-analysis of observational cohorts. PLoS One. 2013;8(2):e55373. doi: 10.1371/journal.pone.0055373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borroni G., Andreoletti M., Casiraghi M.A. Effectiveness of pegylated interferon/ribavirin combination in 'real world' patients with chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2008;27(9):790–797. doi: 10.1111/j.1365-2036.2008.03657.x. [DOI] [PubMed] [Google Scholar]
- 31.Weatherburn P, Schmidt AJ, Hickson F. The European men-who-have-sex-with-men internet survey (EMIS): design and methods. Sexuality Research and Social Policy. 2013;10(4):243–257. doi: 10.1007/s13178-012-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aghaizu A, Brown AE, Nardone A. HIV in the United Kingdom 2013 Report. Public Health England; 2013. [Google Scholar]
- 33.Birrell P.J., Gill O.N., Delpech V.C. HIV incidence in men who have sex with men in England and Wales 2001–10: a nationwide population study. Lancet Infect Dis. 2013;13(4):313–318. doi: 10.1016/S1473-3099(12)70341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price H, Gilson R, Mercey D. Hepatitis C in men who have sex with men in London – a community survey. HIV Med. 2013;14(9):578–580. doi: 10.1111/hiv.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scotto R, Buonomo AR, Moriello NS. Real-world efficacy and safety of pangenotypic direct-acting antivirals against hepatitis C virus infection. Rev Recent Clin Trials. 2019;14(3):173–182. doi: 10.2174/1574887114666190306154650. [DOI] [PubMed] [Google Scholar]
- 36.Hezode C. Treatment of hepatitis C: results in real life. Liver Int. 2018;38(Suppl 1):21–27. doi: 10.1111/liv.13638. [DOI] [PubMed] [Google Scholar]
- 37.Jin F., Matthews G.V., Grulich A.E. Sexual transmission of hepatitis C virus among gay and bisexual men: a systematic review. Sex Health. 2017;14(1):28–41. doi: 10.1071/SH16141. [DOI] [PubMed] [Google Scholar]
- 38.Traeger MW, Schroeder SE, Wright EJ. Effects of pre-exposure prophylaxis for the prevention of HIV infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. 2018;67(5):676–686. doi: 10.1093/cid/ciy182. [DOI] [PubMed] [Google Scholar]
- 39.Basten M., Heijne J.C.M., Geskus R., Den Daas C., Kretzschmar M., Matser A. Sexual risk behaviour trajectories among MSM at risk for HIV in Amsterdam, the Netherlands. AIDS. 2018;32(9):1185–1192. doi: 10.1097/QAD.0000000000001803. [DOI] [PubMed] [Google Scholar]
- 40.Prah P, Hickson F, Bonell C. Men who have sex with men in Great Britain: comparing methods and estimates from probability and convenience sample surveys. Sex Transm Infect. 2016;92(6):455–463. doi: 10.1136/sextrans-2015-052389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott N., Stoove M., Wilson D.P. Eliminating hepatitis C virus as a public health threat among HIV-positive men who have sex with men: a multi-modelling approach to understand differences in sexual risk behaviour. J Int AIDS Soc. 2018;21(1) doi: 10.1002/jia2.25059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salazar-Vizcaya L., Wandeler G., Fehr J. Impact of direct-acting antivirals on the burden of HCV infection among persons who inject drugs and men who have sex with men in the Swiss HIV cohort study. Open Forum Infect Dis. 2018;5(7) doi: 10.1093/ofid/ofy154. ofy154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenness S.M., Weiss K.M., Goodreau S.M. Incidence of gonorrhea and chlamydia following human immunodeficiency virus preexposure prophylaxis among men who have sex with men: a modeling study. Clin Infect Dis. 2017;65(5):712–718. doi: 10.1093/cid/cix439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.British HIV Association Guidelines for the Management of Hepatitis Viruses in Adults Infected with HIV 2013. HIV Medicine. 2013;14(Suppl 4):1–71. doi: 10.1111/hiv.12106. [DOI] [PubMed] [Google Scholar]
- 45.Pakianathan M, Whittaker W, Lee MJ. Chemsex and new HIV diagnosis in gay, bisexual and other men who have sex with men attending sexual health clinics. HIV Med. 2018;19(7):485–490. doi: 10.1111/hiv.12629. [DOI] [PubMed] [Google Scholar]
- 46.Vaux S., Chevaliez S., Saboni L. Prevalence of hepatitis C infection, screening and associated factors among men who have sex with men attending gay venues: a cross-sectional survey (PREVAGAY), France, 2015. BMC Infect Dis. 2019;19(1):315. doi: 10.1186/s12879-019-3945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J., Yu K.F. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 48.Johnson W.D., O'Leary A., Flores S.A. Per-partner condom effectiveness against HIV for men who have sex with men. AIDS. 2018;32(11):1499–1505. doi: 10.1097/QAD.0000000000001832. [DOI] [PubMed] [Google Scholar]
- 49.Molina J.M., Charreau I., Spire B. Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV. 2017;4(9) doi: 10.1016/S2352-3018(17)30089-9. e402-e10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.