Corresponding Author

Key Words: cardiorenal benefits, mechanisms, SGLT2 inhibitors, sympathetic nervous system
Sodium glucose cotransporter 2 (SGLT2) inhibitors exert marked effects to prevent and treat both heart failure and renal disease in people with type 2 diabetes. Recent data from the DAPA-HF (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure) trial suggest that the improvement in heart failure outcomes is also observed in people who do not have diabetes. These remarkable benefits, which appear to manifest soon after treatment initiation, in addition to standard of care, and independent of glucose lowering, have culminated in several translational hypotheses and, in turn, driven multiple endeavors to identify the potential mechanistic underpinnings of the cardiorenal efficacy observed with SGLT2 inhibitors (1).
Some of the more commonly discussed mechanisms include natriuresis and diuresis, improved filling conditions (through reduction in preload and afterload), reduction in left ventricular mass (2), improved myocardial energetics (3), direct inhibitory effects on the cardiac sodium-hydrogen exchanger, reduction in cardiac inflammation, stimulation of cardiac autophagy and mitophagy, reduction in adipokines, increased provascular progenitor cell production, and stimulation of erythropoietin (EPO) production (4). However, despite the flurry of basic and translational research in this area, it remains unclear which mechanism(s) are primarily responsible for the observed cardiorenal benefits of the SGLT2 inhibitors.
A few important clues to the mechanisms responsible for the cardiorenal benefits can be gleaned from the recently completed DAPA-HF trial. In this trial, the observed reduction in the primary outcome (time to first event of either cardiovascular death or worsening heart failure) was reduced significantly by 26% in people with heart failure and reduced ejection fraction. Importantly, this benefit was consistent, in those individuals with and those without diabetes, and persisted when evaluated by baseline glycosylated hemoglobin (HbA1C) both categorically and continuously. Although the primary renal outcome in the DAPA-HF trial was numerically but not statistically significantly reduced, a closer look at the temporal estimated glomerular filtration rate (eGFR) suggests that participants with and without diabetes exhibited similar initial declines in eGFR. Furthermore, the broad renal composite outcome was numerically lower in those individuals with and those without diabetes. Finally, the heart failure benefits in the groups with and without diabetes were similar, even though the HbA1C in the latter was essentially unchanged during the trial. Therefore, it is reasonable to conclude that the cardiorenal mechanism of action of SGLT2 inhibitors is independent of baseline HbA1C and changes in HbA1C over time.
Having taken glycemic control off the table, could the benefits of SGLT2 inhibition be ascribed to diuresis and volume contraction? Again, translational insights from the DAPA-HF trial shed some light, albeit indirectly, on this matter. It has been widely held that the rise in hematocrit observed with SGLT2 inhibitors is secondary to diuresis and volume contraction. Although mediation analyses from the EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) trial indicated that hemoconcentration was statistically the most important mediator of the cardiovascular death benefit, and accordingly strengthened the belief that these classes of medications must be working through diuresis, the DAPA-HF trial data question this notion. In the DAPA-HF trial, a similar rise in hematocrit was observed in both individuals with and without diabetes. Given that people without diabetes would have presumably demonstrated less osmotic diuresis raised doubts that volume contraction drove this effect, particularly in the subcohort that did not have diabetes. Furthermore, the rise in hematocrit was observed to peak at approximately 4 months after treatment initiation. If this phenomenon was secondary to volume contraction, then it should have occurred simultaneously with the early drop in eGFR, as opposed to gradually rising and cresting at 4 months. Finally, although there was a reduction in N-terminal pro–B-type natriuretic peptide in the DAPA-HF trial, this was relatively modest and inconsistent with an agent that works primarily through diuresis.
Could the rise in hematocrit, which seems to be so closely associated with the cardiorenal efficacy, therefore reflect primary erythropoiesis (vs. diuresis)? Indeed, recent data (albeit from people with diabetes) suggest that within 1 month after initiation of empagliflozin, there is a significant increase in the plasma EPO levels (4). An increase in EPO may have several theoretical benefits on heart failure and systemic organ protection. However, whether EPO levels are increased by SGLT2 inhibition in people without diabetes remains unknown. Notably, pharmacological approaches aimed at raising EPO levels have been, to date, unsuccessful.
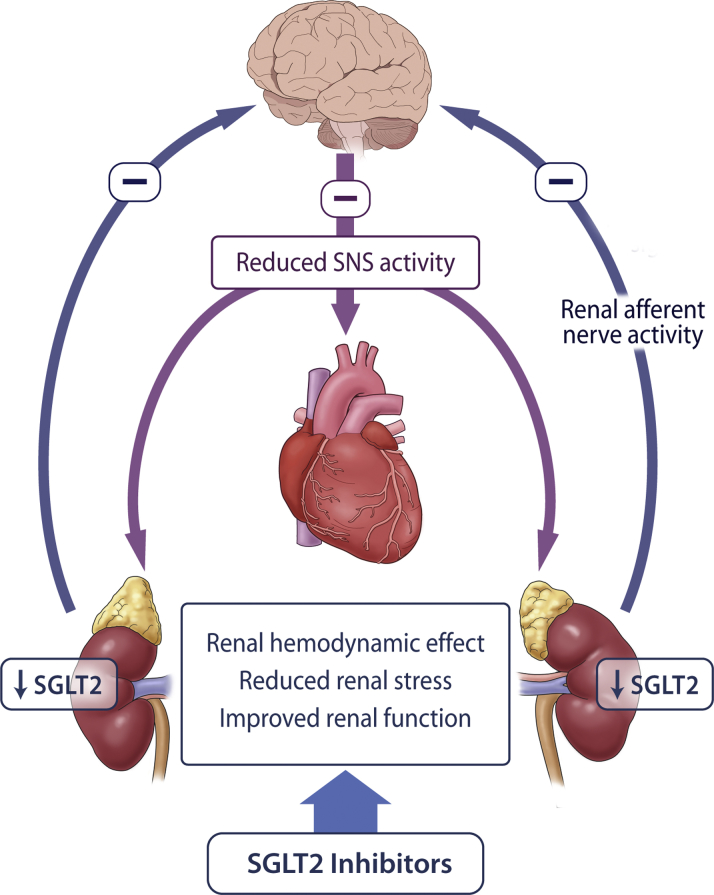
It has been postulated that SGLT2 inhibitors may exert a direct or indirect effect to inhibit the central sympathetic nervous system (SNS) (Figure 1). Aberrant SNS activation is believed to be an important pathophysiological determinant of heart failure and renal disease, and pharmacotherapies that attenuate SNS activity have been shown to reduce heart failure events in clinical trials (e.g., β-blockers). The fact that SGLT2 inhibitors lower blood pressure without a compensatory increase in heart rate suggests that they may inhibit the SNS. In addition, preliminary evidence indicates that SGLT2 inhibitors may attenuate sympathetic nerve activity in models of diabetes and obesity.
Figure 1.
SGLT2 Inhibition and SNS Regulation
Sodium glucose cotransporter 2 (SGLT2) inhibitors, secondary to reducing renal stress, may reduce afferent renal sympathetic nervous system (SNS) activation. A reduction in central SNS activation may serve as an important mechanism of heart failure protection and reduce renal SGLT2 expression.
In this issue of JACC: Basic to Translational Science, Herat et al. (5) provide evidence of an important relationship between SGLT2 inhibition and the SNS. The data suggest that an SNS-associated mechanism may be responsible for the cardiorenal benefits observed in clinical trials. In the first set of experiments, the authors demonstrated that chemical denervation of the SNS with 6-hydroxydopamine resulted in a reduced expression of renal SGLT2, with concomitant improvements in metabolic and hemodynamic parameters in hypertensive (nondiabetic) BPH/2J mice. In further experiments, the authors studied the effects of SGLT2 inhibition with dapagliflozin in BPH/2J mice fed a high-fat diet. The investigators observed that in addition to improvements in glycemia and blood pressure, dapagliflozin treatment was also associated with a reduction in cardiac and renal tyrosine hydroxylase staining (a measure of SNS activation), alongside a reduction in norepinephrine content. Coincident with a decrease in cardiorenal SNS activation, the authors found that measures of endothelial function were improved (through reductions in asymmetric dimethylarginine resulting in increased endogenous nitric oxide production), levels of proinflammatory cytokines were attenuated, and there was an increase in the anti-inflammatory cytokine interleukin-10 in the hearts and kidneys of the dapagliflozin-treated mice. Taken together, Herat et al. (5) concluded a bidirectional relationship between SGLT2 inhibition and SNS activity. They suggested that SGLT2 inhibitors promote a reduction in SNS activity within the heart and kidneys, which occurs in hypertensive, nondiabetic mice and that a reduction in SNS activation in turn also attenuates renal expression of SGLT2.
Although Herat et al. (5) are to be congratulated for their elegant study design, and for presenting cogent data linking SGLT2 inhibition to cardiorenal benefit, a few questions remain unanswered. First, the paper would have been further strengthened with details on cardiac structure and function, particularly in response to pressure overload or coronary artery occlusion. Second, the SNS is also a regulator of myocardial energetics, which has been implicated as a mechanism of benefit for SGLT2 inhibitors. Data on ketone bodies and measures of myocardial energetics (PGC1α) would have been beneficial. Probably the most important issue pertains to ascribing cause and effect. The benefits to lower tissue levels of SNS activation with SGLT2 inhibitors may simply represent a “bystander response” secondary to improvements in hemodynamics and metabolic stress and not causally related to the cardiovascular benefits observed. In mice that have SNS inactivated by chemical denervation, is there a heart failure benefit of SGLT2 inhibitors? Last, Herat et al. (5) did not provide much information on how the effects of SGLT2 inhibitors on the kidney may affect central SNS activity. One tantalizing hypothesis is that the primary effect of SGLT2 inhibitors is a renal hemodynamic effect and that through reducing renal stress, there are secondary benefits of attenuating renal afferent SNS activity. As discussed previously, a rise in EPO may represent a marker of generalized improvements in renal function, and the reduction in SNS activity may also be simply be secondary to a renoprotective effect. Cardiac function studies of SGLT2 inhibitors in models of nephrectomy may help shed light on this, as would studies of patients on dialysis.
The remarkable translational story of SGLT2 inhibitors continues. Although we continue to search for answers to explain these formidable cardiorenal benefits, we must be cognizant that these mechanisms are likely multifactorial and interrelated. Translational data from large clinical trials and correlating biomarkers to clinical outcomes may help get us closer.
Footnotes
Dr. Verma holds a Tier 1 Canada Research Chair in Cardiovascular Surgery, University of Toronto; and has received research grants and/or speaking honoraria from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, EOCI Pharmacomm Ltd., Janssen, Merck, Novartis, Novo Nordisk, Sanofi, Sun Pharmaceuticals, and the Toronto Knowledge Translation Working Group; and is the President of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not-for-profit physician organization.
The author attests they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Verma S., McMurray J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108–2117. doi: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 2.Verma S., Mazer C.D., Yan A.T. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation. 2019;140:1693–1702. doi: 10.1161/CIRCULATIONAHA.119.042375. [DOI] [PubMed] [Google Scholar]
- 3.Verma S., Rawat S., Ho K.L. Empagliflozin increases cardiac energy production in diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors. J Am Coll Cardiol Basic Trans Sci. 2018;3:575–587. doi: 10.1016/j.jacbts.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazer C.D., Hare G.M.T., Connelly P.W. Effect of empagliflozin on erythropoietin levels, iron stores and red blood cell morphology in patients with type 2 diabetes and coronary artery disease. Circulation. 2019 Nov 11 doi: 10.1161/CIRCULATIONAHA.119.044235. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Herat L.Y., Magno A.L., Rudnicka C. SGLT2 inhibitor–induced sympathoinhibition: a novel mechanism for cardiorenal protection. J Am Coll Cardiol Basic Trans Science. 2020;5:169–179. doi: 10.1016/j.jacbts.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]