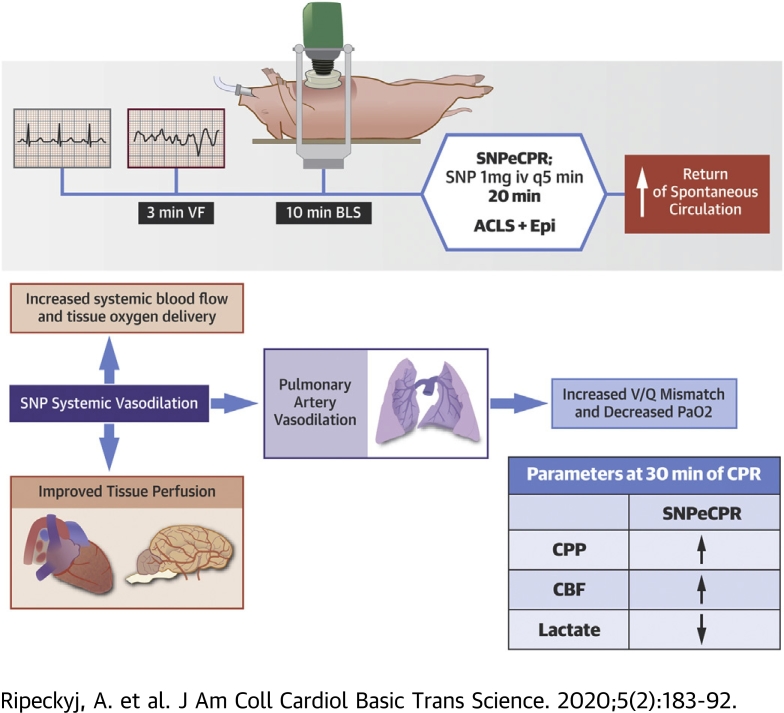
Visual Abstract
Key Words: cardiopulmonary resuscitation, coronary perfusion pressure, lactic acid, pulmonary vasodilation, sodium nitroprusside
Abbreviations and Acronyms: A-a, alveolar-arterial; ACLS, advanced cardiac life support; BLS, basic life support; CBF, carotid blood flow; CPP, coronary perfusion pressure; CPR, cardiopulmonary resuscitation; FiO2, fraction of inspired oxygen; ITD, impedance threshold device; ROSC, return of spontaneous circulation; SNP, sodium nitroprusside; SNPeCPR, sodium nitroprusside–enhanced cardiopulmonary resuscitation; VF, ventricular fibrillation
Highlights
-
•
SNPeCPR improves coronary perfusion pressure, tissue perfusion, and carotid blood flow compared to epinephrine-based standard advanced cardiac life support.
-
•
In a porcine model of prolonged resuscitation, SNPeCPR was associated with decreased arterial oxygen saturation but improved tissue oxygen delivery due to improvement in blood flow.
-
•
Oxygen supplementation led to alleviation of hypoxemia and maintenance of the SNPeCPR hemodynamic benefits.
-
•
Arterial oxygen saturation must be a safety endpoint that will be prospectively assessed in the first SNPeCPR clinical trial in humans.
Summary
Sodium nitroprusside–enhanced cardiopulmonary resuscitation has shown superior resuscitation rates and neurologic outcomes in large animal models supporting the need for a randomized human clinical trial. This study is the first to show nonselective pulmonary vasodilation as a potential mechanism for the hemodynamic benefits. The pulmonary shunting that is created requires increased oxygen treatment, but the overall improvement in blood flow increases minute oxygen delivery to tissues. In this context, hypoxemia is an important safety endpoint and a 100% oxygen ventilation strategy may be necessary for the first human clinical trial.
Each year, approximately 400,000 out-of-hospital cardiac arrests occur in the United States (1,2). Of these, approximately only 5% to 10% achieve neurologically intact survival (3). Approximately one-third of patients experiencing out-of-hospital cardiac arrests present with ventricular tachycardia/ventricular fibrillation (VF) (4). Epinephrine is a commonly used vasoconstrictor for standard cardiopulmonary resuscitation (CPR) in and out of hospital protocols in congruence with current American Heart Association guidelines; however, no study to date has shown improvement in long-term outcomes (5).
A new method of CPR has been proposed that significantly increases forward blood flow and overall patient outcomes (6, 7, 8, 9, 10, 11). Sodium nitroprusside–enhanced CPR (SNPeCPR) is the combination of 3 basic components: 1) SNP, a potent vasodilator, to decrease peripheral vascular resistance in tandem with abdominal binding (7,8) to decrease descending aorta runoff and redirect blood flow to the vital organs; 2) an impedance threshold device; and 3) active compression/decompression CPR to actively increase venous return (11). Together, these components act synergistically to increase coronary and cerebral perfusion during CPR (12).
Previous studies of SNPeCPR have indicated an improved carotid blood flow (CBF), end-tidal CO2, and return of spontaneous circulation (ROSC) rates and short-term 24- to 48-h survival rates with favorable neurologic function compared to those of standard CPR (8,12, 13, 14, 15, 16, 17, 18). There appears to be a vital timepoint after which hemodynamic decompensation during extended standard CPR is inevitable and irreversible (19). The use of SNPeCPR shifts the survival curve, increasing a valuable window for which to resuscitate the patient (8,12, 13, 14, 15, 16, 17, 18). This shift of the metabolic wall represents an invaluable clinical application of patient selection for furthered resuscitation efforts. As more patients are treated following prolonged periods of CPR, and with increasing access to enhanced CPR (eCPR) and veno-arterial extracorporeal membrane oxygenation therapies, providing a superior advanced cardiac life support (ACLS) and CPR method will become critical (20). Before the first clinical trial for SNPeCPR can be performed, safety must be further evaluated.
We sought to investigate the blood flow effects of SNPeCPR during prolonged CPR and to understand the predominant mechanism of its action. We hypothesized that the predominant effect of SNPeCPR is indiscriminate profound pulmonary vasodilation. As such, the ability to maintain adequate oxygenation with SNPeCPR was the main focus of this study.
Methods
All studies were performed with approval from the Institutional Animal Care and Use Committee. Animal care was compliant with the National Research Council’s Guide of 1996 for the Care and Use of Laboratory Animals (protocol number 1802-35586A)(21).
Animal model
We used 25 Yorkshire pigs with an average weight of 51.5 ± 1.4 kg. Twelve animals were randomized to receive SNPeCPR and 13 to receive standard CPR. The surgical preparation, anesthesia, and data monitoring have been described thoroughly in previous studies (7,22). Intramuscular ketamine and xylazine was provided as sedation (5 ml of 100 mg/ml dose and 1 to 3 mg/kg, respectively). This was followed by inhaled isoflurane at a dose of 1% to 1.4%. Endotracheal intubation was performed with a 7.5-mm endotracheal tube. Animals were ventilated with a tidal volume of 10 ml/kg using room air volume control ventilation (Narkomed, Draeger Medical, Telford, Pennsylvania). The respiratory rate was adjusted to maintain partial pressure of carbon dioxide (PaCO2) of 40 mm Hg as measured by arterial blood (Gem 3500, Instrumentation Laboratory, Bedford, Massachusetts). Arterial blood gases were obtained at baseline and every 5 min until 30 min of CPR. Animal temperature was measured with an esophageal temperature probe and normothermia (37 ± 0.5 ºC) was maintained with convective warming unit (Covidien Warm Touch, Mansfield, Massachusetts). Vascular access was obtained in the femoral artery and the right external jugular vein percutaneously using ultrasound guidance with an 8-F and 6-F catheter, respectively. The central aortic blood pressure was measured with a Millar catheter (Millar Instruments, Houston, Texas) placed in the descending thoracic aorta. The right atrial pressure was also measured with a Millar catheter that was inserted via the right external jugular sheath. The measurements from these Millar catheters were used for calculation of coronary perfusion pressure as the difference between diastolic blood pressure and right atrial pressure in spontaneous circulation, and the difference between decompression phase arterial pressure and the right atrial pressure at maximal decompression during the arrest. Both sheaths were placed percutaneously with an ultrasound-guided Seldinger technique. The left common carotid artery was surgically exposed and a Doppler flow probe (Transonic, 400-Series Multi-channel, Transonic Systems Inc. Ithaca, New York) was placed around it to quantify the CBF. All animals received a 5,000-U intravenous bolus of heparin upon completion of surgical access. Hemodynamic data were continuously recorded (Labview 2015, National Instruments, Austin, Texas). Electrocardiograms were continuously recorded, as well as end-tidal CO2, tidal volume, minute ventilation, and blood oxygen saturation (Cardiocap/5, Datex-Ohmeda, Louisville, Colorado).
Experimental protocol
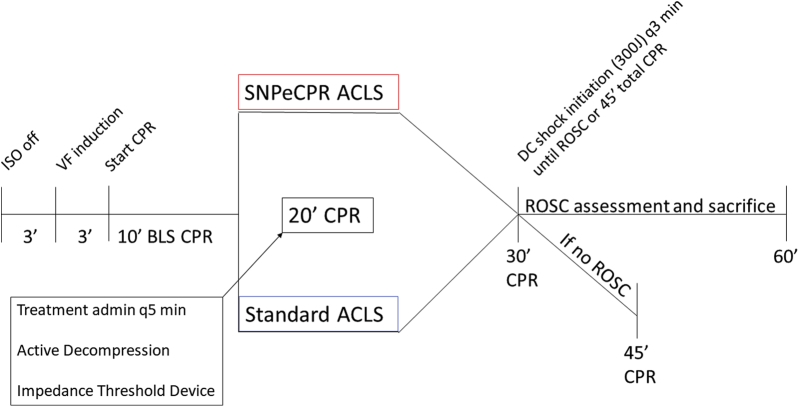
Following the aforementioned surgical preparation, baseline values were recorded. The timeline for the experimental protocol is outlined in Figure 1. VF was electrically induced in all animals with a pacing wire inserted through the right jugular vein sheath into the right ventricle. Upon inducing VF, mechanical ventilation was suspended. VF was left untreated for 3.5 min to mimic an arrival time for first responders. After untreated VF, basic life support (BLS) with active decompression (ACD) and an impedance threshold device (ITD) (ACD + ITD) and mechanical ventilations at a rate of 10 respirations per minute was initiated for all animals. The mechanical CPR parameters were held constant: compression/decompression duty cycle of 50%, rate of 100 compressions per minute, and target depth of 20% of the anteroposterior diameter. Mechanical ACD + ITD CPR was used for all animals to optimize perfusion during BLS (6). BLS was performed for 10 min to simulate the time required for arrival of ACLS providers.
Figure 1.
Study Protocol
ACLS = advanced cardiac life support; admin = administration (of); BLS = basic life support; CPR = cardiopulmonary resuscitation; DC = decompression; ISO = isoflurane; ROSC = return of spontaneous circulation; SNPeCPR = sodium nitroprusside–enhanced cardiopulmonary resuscitation; VF = ventricular fibrillation; q5 min = every 5 min.
Following 10 min of BLS, animals were randomized to either SNPeCPR or standard ACLS CPR groups.
SNPeCPR group
SNPeCPR added manual abdominal binding on the ongoing mechanical ACD + ITD CPR and SNP 1mg intravenous bolus every 5 min as previously described (7).
Standard ACLS group
The standard ACLS group continued to receive ACD + ITD CPR (similar to BLS) and received epinephrine 0.5 mg intravenous bolus every 5 min starting at minute 10 (6).
At 28 min of total CPR, all animals were administered 25 mg amiodarone and 50 mEq bicarbonate. At the 30-min CPR mark, animals were defibrillated with 200-J biphasic shocks. Shocks were performed every 2 to 3 min for an additional 15 min at which point efforts were terminated if no ROSC was achieved. Animals with ROSC were followed for a total of 60 min, at which point they were sacrificed.
Ventilation, oxygen delivery strategy, and alveolar-arterial gradient calculation
All animals were ventilated as stated above at 10 breaths per minute with 10 ml/kg tidal volume. Room air was used during preparation and at the initiation of the VF. During CPR, oxygen was increased only if a saturation of <90% was observed (as indicated by arterial blood gas [ABG] values taken at 5-min intervals). Fraction of inspired oxygen (FiO2) was recorded and adjusted incrementally by ∼25%, only enough to increase the O2 saturation above 90%. After the FiO2 adjustment, the alveolar-arterial (A-a) gradient was calculated based on the recorded Fi02 and the arterial partial pressure of oxygen (PaO2) standard formula at the next ABG reading:
As such, the study sought to evaluate both the effect of SNPeCPR on pulmonary vasodilation but also the level of FiO2 support needed to maintain adequate tissue oxygenation over time.
Statistical analysis
All statistics were compiled using GraphPad Prism 6 software (GraphPad Software, La Jolla, California). All values are expressed as means ± SEM and categorical data as fractions. An unequal variance Student t test was utilized to analyze statistical differences in the hemodynamic and blood gas data. Two-way analysis of variance tests were used to evaluate treatment, time effects on lactic acid, and A-a gradient data; and Scheffe’s method was used for alpha-adjustment. A p value <0.05 was considered statistically significant. An additional random effect model analysis was completed in R studio (R development core team, 2018) to assess the effects of time to the value of lactate in the different treatment groups. The Fisher exact test was performed to assess the ROSC rates at the end of prolonged CPR. A Kaplan-Meier curve was constructed to assess the rate of decrease in coronary perfusion pressure over time between the 2 different groups. Log-rank test was used to assess the equality of the curves.
Results
Survival
All animals randomized received at least 30 min of CPR. The ROSC rate was significantly higher for SNPeCPR animals (9 of 12 [75%]) compared to standard ACLS animals (3 of 13 [23%]) (p = 0.017). ROSC efforts were continued for up to 15 min after completion of the 30-min CPR protocol. All animals that achieved ROSC survived for the full 1-h observation period.
Hemodynamics
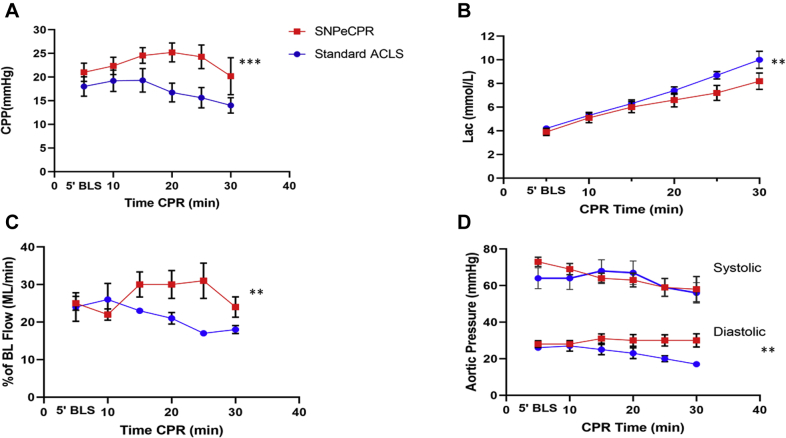
Use of SNPeCPR maintained higher systemic perfusion and minimized ischemia as observed through slower rise of lactic acid during prolonged CPR (Figure 2). Furthermore, use of SNPeCPR increased coronary perfusion pressure (p < 0.001) and CBF (p = 0.002) (Table 1). The hemodynamic effects of SNPeCPR were immediate and seen within the first 5 min after the first injection at minute 10 of CPR (Figure 1). Moreover, SNP animals had significantly higher diastolic blood pressure (p = 0.021), whereas no difference was observed between systolic blood pressure in the 2 groups (p = 0.394).
Figure 2.
Hemodynamic Comparison of SNP and Epinephrine During CPR
(A) Coronary perfusion pressure (B), lactate, (C) carotid flow, and (D) aortic pressure over the 30 min of cardiopulmonary resuscitation (CPR). All parameters except systolic blood pressure are statistically significant between treatment groups after the 20-min CPR time mark. The 10-min CPR directly precedes intervention of sodium nitroprusside (SNP) or epinephrine administration. Lactate p value for treatment is 0.003. p values for coronary perfusion pressure, carotid flow, systolic aortic pressure, and diastolic aortic pressure on relaxation were <0.001, 0.002, 0.394, and 0.021, respectively. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. BL = basic life; CPP = coronary perfusion pressure; other abbreviations as in Figure 1.
Table 1.
Hemodynamic Data at Baseline and at 5-Min Intervals During 30 Min of CPR
| Time | Treatment | RA Pressure, mm Hg | Compression RA Pressure, mm Hg | SBP, mm Hg | DBP, mm Hg | CPP, mm Hg | %CBF |
|---|---|---|---|---|---|---|---|
| BL | SNPeCPR | 5 ± 0.9 | Not applicable | 111 ± 17.0 | 76 ± 13.6 | 71 ± 12.7 | 100 ± 0 |
| Standard ACLS CPR | 4 ± 0.4 | Not applicable | 107 ± 15.5 | 72 ± 13.0 | 68 ± 8.9 | 100 ± 0 | |
| 5 min | SNPeCPR | 7 ± 1.1 | 72 ± 8.9 | 73 ± 9.3 | 28 ± 6.5 | 21 ± 5.8 | 25 ± 5.5 |
| Standard ACLS CPR | 8 ± 2.3 | 65 ± 18.8 | 64 ± 19.7 | 26 ± 5.2 | 18 ± 5.8 | 24 ± 10.8 | |
| 10 min∗ | SNPeCPR | 6 ± 1.7 | 68 ± 10.5 | 69 ± 11.1 | 28 ± 5.1 | 22 ± 5.6 | 22 ± 4.5 |
| Standard ACLS CPR | 8 ± 2.7 | 65 ± 22 | 64 ± 21.4 | 27 ± 10.0 | 19 ± 6.4 | 26 ± 12.1 | |
| 15 min | SNPeCPR | 7 ± 2.1 | 63 ± 10.8 | 64 ± 10.0 | 31 ± 9.2 | 24 ± 5.0† | 30 ± 10.0† |
| Standard ACLS CPR | 6 ± 1.6 | 69 ± 20.6 | 68 ± 21.4 | 25 ± 9.8 | 19 ± 7.1† | 23 ± 2.0† | |
| 20 min | SNPeCPR | 5 ± 2.9 | 61 ± 14.2 | 63 ± 13.7 | 30 ± 11.6 | 25 ± 6.0† | 30 ± 11.1† |
| Standard ACLS CPR | 6 ± 3.1 | 66 ± 20.9 | 67 ± 22.5 | 23 ± 10.2 | 17 ± 5.6† | 21 ± 4.4† | |
| 25 min | SNPeCPR | 6 ± 3.2 | 60 ± 18.2 | 59 ± 17.7 | 30 ± 11.1† | 24 ± 7.5† | 31 ± 14.1† |
| Standard ACLS CPR | 4 ± 1.8 | 58 ± 17.3 | 59 ± 17.6 | 20 ± 5.7† | 16 ± 6.1† | 17 ± 1.7† | |
| 30 min | SNPeCPR | 10 ± 6.5 | 59 ± 23.9 | 58 ± 25.2 | 30 ± 13.1† | 20 ± 11.7† | 24 ± 8.1† |
| Standard ACLS CPR | 3 ± 0.6 | 57 ± 17.8 | 56 ± 19.5 | 17 ± 5.2† | 14 ± 4.6† | 18 ± 3.1† |
Values are mean ± SEM.
ACLS = advanced cardiac life support; BL = baseline values; CBF = carotid blood flow; %CBF = proportion of CBF as a percent of the initial baseline value; CPP = coronary perfusion pressure; CPR = cardiopulmonary resuscitation; DBP = diastolic blood pressure; RA = right atrial; SBP = systolic blood pressure; SNPeCPR = sodium nitroprusside–enhanced cardiopulmonary resuscitation.
The dividing line at 10 min indicates the initiation of randomization and drug administration.
p < 0.05.
ABGs
Arterial and venous blood gas results over the entire 30 min of prolonged CPR are shown in Table 2. SNPeCPR showed a significant decrease in PaO2 levels after SNP delivery which coincided with an increased A-a gradient. Increasing the FiO2 led to adequate PaO2 and overall tissue oxygen delivery while the increase in circulating blood flow induced by SNP infusion was maintained. The higher normal mixed venous oxygen tension (PvO2) in the SNPeCPR animals suggests increased tissue perfusion and oxygen delivery. The lower arteriovenous proportion of SO2 difference further suggests adequate oxygen delivery in agreement with the higher blood flow markers and perfusion pressures (Figure 3, Table 1).
Table 2.
Arterial and Venous Blood Gas Results at Baseline and at 5-Min Intervals During 30 Min of CPR
| Time | Treatment | pH | PaCO2 | PaO2 | PvO2 | Lac | FiO2 |
|---|---|---|---|---|---|---|---|
| BL | SNPeCPR | 7.48 ± 0.06 | 38 ± 7.6 | 113 ± 17 | 46 ± 3 | 1.0 ± 0.4 | 0.25 ± 0.017 |
| Standard ACLS CPR | 7.5 ± 0.04 | 41 ± 3.8 | 106 ± 20 | 44 ± 7.3 | 0.8 ± 0.2 | 0.26 ± 0.024 | |
| 5 min | SNPeCPR | 7.44 ± 0.08 | 30 ± 5.0 | 96 ± 17.5 | 29 ± 5.6 | 3.9 ± 1.1 | 0.25 ± 0.017 |
| Standard ACLS CPR | 7.43 ± 0.07 | 32 ± 5.7 | 103 ± 15.0 | 28 ± 7.5 | 4.2 ± 0.8 | 0.26 ± 0.016 | |
| 10 min∗ | SNPeCPR | 7.39 ± 0.04 | 29 ± 3.0 | 98 ± 18.4 | 26 ± 3.3 | 5.1 ± 1.5 | 0.26 ± 0.016 |
| Standard ACLS CPR | 7.39 ± 0.05 | 31 ± 4.9 | 102 ± 14.4 | 26 ± 8 | 5.3 ± 0.9 | 0.26 ± 0.016 | |
| 15 min | SNPeCPR | 7.36 ± 0.06 | 32 ± 3.6 | 67 ± 13.9† | 28 ± 5.5 | 6.0 ± 1.7 | 0.26 ± 0.025 |
| Standard ACLS CPR | 7.37 ± 0.05 | 29 ± 5.0 | 94 ± 17.7 | 23 ± 5.3 | 6.3 ± 1.1 | 0.26 ± 0.015 | |
| 20 min | SNPeCPR | 7.33 ± 0.08 | 34 ± 8.1† | 68 ± 18.1† | 29 ± 7.2† | 6.6 ± 2.1 | 0.43 ± 0.23† |
| Standard ACLS CPR | 7.35 ± 0.06 | 26 ± 5.4 | 95 ± 17.4 | 21 ± 8.5† | 7.4 ± 1.1 | 0.26 ± 0.016 | |
| 25 min | SNPeCPR | 7.33 ± 0.15 | 37 ± 11.2† | 64 ± 11.5† | 26 ± 7.4 | 7.2 ± 2.3† | 0.56 ± 0.31† |
| Standard ACLS CPR | 7.32 ± 0.06 | 26 ± 5.9 | 93 ± 16.7 | 20 ± 11.4 | 8.7 ± 1.1† | 0.26 ± 0.016 | |
| 30 min | SNPeCPR | 7.39 ± 0.13 | 51 ± 21.8 | 53 ± 14† | 26.4 ± 6.5 | 8.2 ± 2.5† | 0.58 ± 0.31† |
| Standard ACLS CPR | 7.37 ± 0.21 | 39 ± 20 | 77 ± 24.4† | 21 ± 6.0 | 10.0 ± 1.5† | 0.27 ± 0.037 |
Values are mean ± SEM. All partial pressures are shown in mm Hg.
FiO2 = fraction of inspired oxygen; Lac = lactate levels in mmol/l in arterial blood; PaCO2 = partial pressure of carbon dioxide in arterial blood; PaO2 = partial pressure of oxygen in arterial blood; PvO2 = partial pressure of oxygen in venous blood; other abbreviations as in Table 1.
The dividing line at 10 min indicates the initiation of randomization and drug administration.
p < 0.05.
Figure 3.
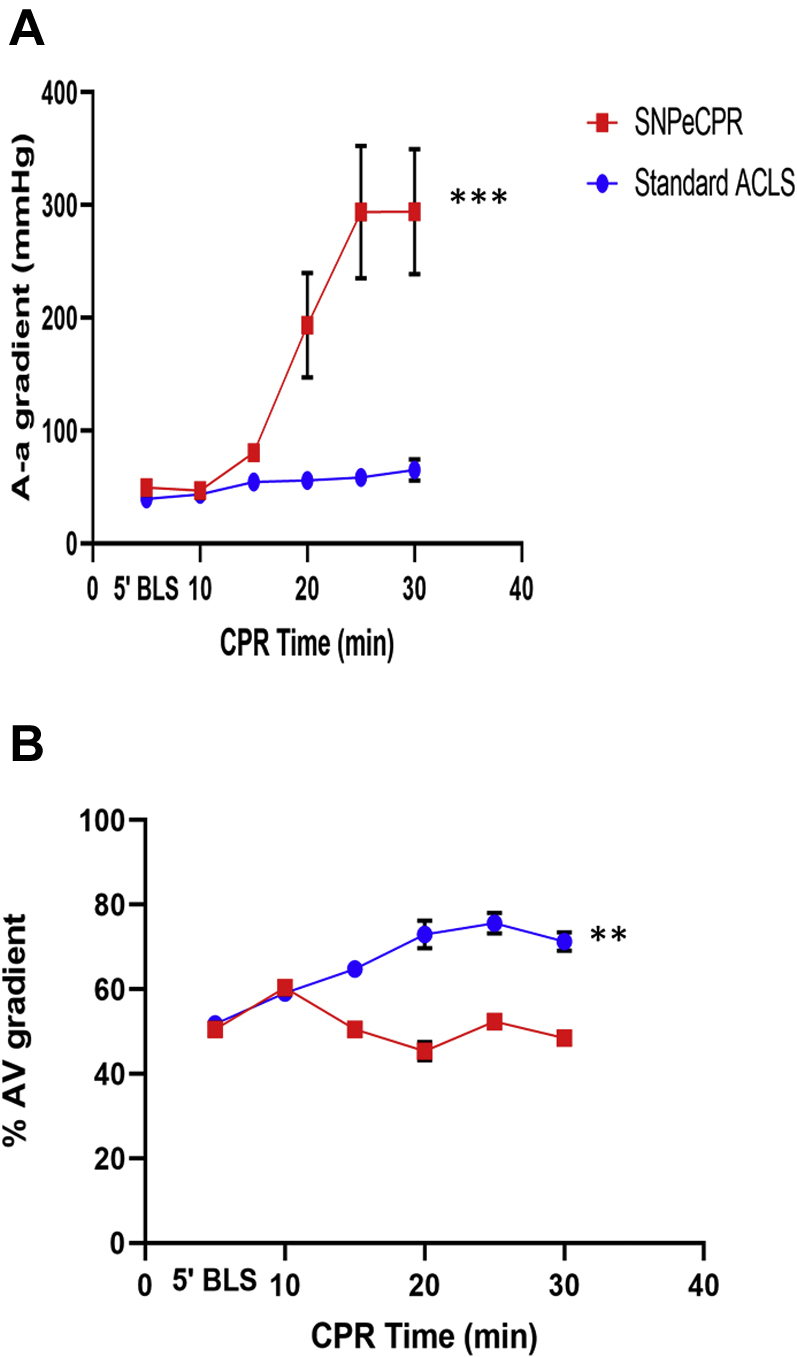
Blood Oxygenation Comparison Between SNP and Epinephrine During CPR
Alveolar-arterial (A-a) gradient (A) and arteriovenous oxygenation gradient (B) over the 30 min of CPR. All parameters are statistically significant between treatment groups after the 20-min CPR time mark. A-a gradient values are unitless. The 10-min CPR directly precedes intervention of SNP or epinephrine administration. The p values of treatment for A-a gradient and arteriovenous oxygenation gradient is <0.001 and 0.004, respectively. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Abbreviations as in Figures 1 and 2.
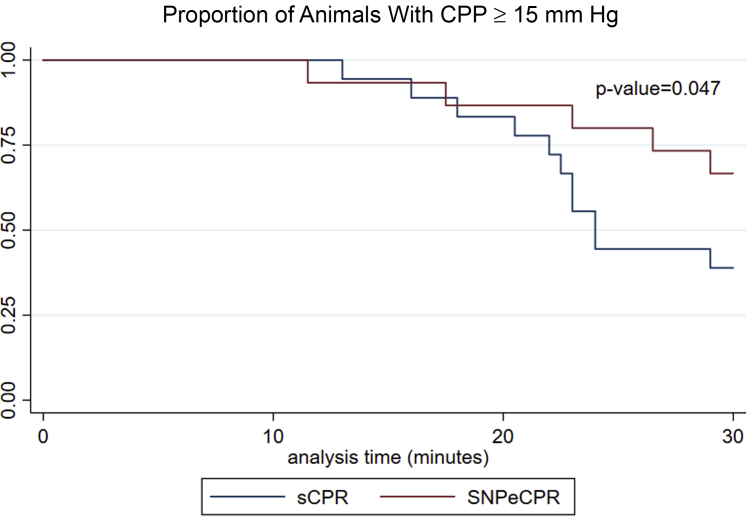
A coronary perfusion pressure of <15 mm Hg has been negatively associated with achieving ROSC and successful defibrillation (23,24). SNPeCPR animals fell below this threshold later than compared to control animals (Table 1, Figure 4). As it can be seen in the Kaplan-Meier plot (Figure 4), animals reaching of coronary perfusion pressure (CPP) <15 mm Hg are significantly delayed in the SNPeCPR cohort (p = 0.047).
Figure 4.
CPP-Guided Survival Function
Kaplan-Meier curve for the time until coronary perfusion pressure (CPP) < 15 mm Hg within the SNPeCPR and standard CPR cohorts. Our data suggest that after 20 min of CPR (within 10 min of ACLS initiation). CPP drops below 15 mm Hg much faster in the standard CPR cohort. CPp < 15 mm Hg has been adversely related to achieving ROSC (23). SNPeCPR delays the decay of CPP to below ROSC threshold. sCPR = standard cardiopulmonary resuscitation; other abbreviations as in Figures 1 and 2.
Discussion
SNPeCPR increased the cardiac output generated by chest compressions resulting in increased CPP and CBF and decreased lactic acid levels when compared to standard ACLS. SNP is the most potent vasodilator available with potent dilatory effects in both the arterial and venous circulation, including the pulmonary vasculature (25,26). This study has shown that pulmonary arterial vasodilation was an important mechanism of the positive hemodynamic effects of SNPeCPR. The 6-fold increase in A-a gradient suggested a substantial increase in transpulmonary flow resulting in increased left ventricular preload and increased cardiac output. Importantly, in the setting of prolonged CPR, the increased cardiac output and resulting increase in diastolic blood pressure provided by SNPeCPR prolonged the duration of ROSC-compatible hemodynamics by maintaining the CPP needed to achieve ROSC (27). This allowed for higher ROSC rates after 30 min of prolonged CPR and higher survival rates with ongoing observation (7,8,12, 13, 14, 15, 16, 17, 18).
The observed 6-fold increase in A-a gradient in the SNPeCPR group showed a substantial increase in pulmonary shunt due to increased transpulmonary blood flow. Profound pulmonary vasodilation induced by SNP increases perfusion throughout the pulmonary vasculature. This reversal of hypoxemic vasoconstriction and increased perfusion to nonventilated alveoli increased shunt physiology, thereby decreasing arterial oxygen content. In SNPeCPR animals, oxygen saturation of <90% was reversed by increasing FiO2 in 25% increments all the way up to 1.0, if needed, over the 20 min of ACLS. A moderate increase in FiO2 to 0.5 was adequate in the majority of the SNPeCPR treated animals to keep saturation above 90% to 92%. While arterial oxygen saturation was reduced, total oxygen delivery to tissues was maintained or even increased with SNPeCPR due to increased cardiac output. This was shown by the simultaneous increase in A-a gradient and decrease in PaO2 levels in association with lower lactic acid levels and similar mixed venous oxygen levels. Stable oxygen delivery despite decreased PaO2 levels suggested higher perfusion in agreement with the increased CPP and CBF.
The requirement for increased FiO2 did not compromise the outcomes in the SNPeCPR cohort as supported by the resuscitation rates between the 2 groups. CPR is a low-flow state, and the impact of high arterial oxygen levels on outcomes is poorly understood. In our study, although the SNPeCPR group had lower PaO2 and relative hypoxemia, tissue oxygen consumption was improved due to the increase in CPR-generated blood flow; therefore, there was no evidence of worsening tissue hypoxia compared to standard ACLS. The clinical effect of SNPeCPR, with the need for a higher FiO2, on neurological intact survival in humans can be only assessed with a clinical trial after safety has been documented in a Phase 1 trial.
SNPeCPR increased CBF immediately upon injection which was maintained throughout the 30 min of CPR performed in this study (12). Similar increases in CPP were also seen although coronary blood flow was not directly measured. It remains unknown if increases in CBF were due to SNP-induced vasodilation of cerebral vasculature or simply a manifestation of the increased cardiac output and arterial blood pressure caused by SNP combined with external carotid vasodilation. In addition, SNPeCPR animals appeared to have a higher PaCO2, most likely related to large ventilation perfusion mismatch. Although shunting immediately affects PaO2, PaCO2 does not begin to increase until shunt reaches or exceeds 50% (28). The combination of direct vasodilation and increased PaCO2 may contribute to an increase of cerebral blood flow. The consistently observed increase in CBF with SNPeCPR may be associated with the improved neurologic outcomes that have been reported in previous publications (8,12,14,15,17).
Prolonged CPR leads to death by progressive accumulation of tissue oxygen debt, ischemic injury, and cell death. As such, treatments meant to prolong patient viability during resuscitation should target this detrimental progression to increase the potential for ROSC and thereby limit brain ischemia and anoxic injury following prolonged periods of CPR. SNPeCPR extended ROSC potential and patient viability as shown by the slower accumulation of arterial lactic acid and preservation of CPP and CBF compared to that of control animals. Lactic acid is a reliable and reproducible indicator of tissue hypoxia that has been directly correlated with mortality (29) and poor neurologic outcomes (30). The slower rate of lactic acid accumulation in the SNPeCPR group indicates improved tissue oxygenation and, along with the preservation of CPP, supports an increase in the duration of patient viability and potential for ROSC (31). In recent studies, only 2.3% of patients receiving CPR longer than 30 min achieved ROSC (32, 33, 34, 35, 36). In contrast, this study has shown an ROSC rate of 75% with 30 min of CPR in pigs receiving SNPeCPR. In the setting of refractory VF, prolonged CPR may be particularly critical as patients are often transported to an eCPR-capable hospital. Transport may require additional time. Prolonged patient viability with SNPeCPR would be hypothesized to improve patient outcomes in this setting.
Study limitations
This study has multiple limitations. Although our study was designed to mimic the clinical reality of patients suffering refractory out-of-hospital VF cardiac arrest, unavoidable discrepancies persist. First, the animal model may have limited translatability to humans due to the relatively young age of the pigs and lack of coronary artery atherosclerosis and cardiovascular comorbidities such as diabetes. Second, during the ACLS phase, our study model provided no defibrillations to maintain the extended CPR model. Therefore, we cannot accurately assess the ability of SNPeCPR or control treatment to facilitate ROSC before 30 min of CPR. Furthermore, this leads to inclusion of all animals in the prolonged CPR group. This may also lead to inclusion of animals with more severe systemic injury that would have otherwise achieved ROSC early which would be expected to minimize differences between groups and minimize the observed relative effect of SNPeCPR. The use of anesthesia, as required for animal studies, may also limit the observed differences between groups as inhaled anesthetics such as isoflurane used in this study can provide cardioprotective effects. Moreover, the pulmonary vascular resistance was calculated indirectly and in the absence of direct assessment of cardiac output due to the inaccuracy of measurements in cardiac arrest due to significant motion artifact and the extreme low blood flow. Finally, we did not thoroughly examine the dose-dependent effects of SNP, but we have based the dosing on previously published studies where it was correlated with positive clinically relevant outcomes.
Conclusions
SNPeCPR improves vital organ blood flow and tissue oxygenation during prolonged resuscitation. This is predominantly achieved by a significant decrease in pulmonary artery resistance that leads to a substantial increase in the A-a oxygen gradient over time. However, tissue oxygen delivery is not compromised when FiO2 can be increased to compensate. Our results support the implementation of the first clinical trial of SNPeCPR in humans. It further informs us of the necessary use of the 100% oxygen ventilation strategy and hypoxia as the primary safety endpoints.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: SNPeCPR is a novel CPR method that uses an advanced mechanical CPR platform with SNP—a potent vasodilator—to improve vital organ flow. SNPeCPR causes peripheral systemic vasodilation and, as our study shows, a significant and profound pulmonary circulation vasodilation. SNPeCPR has been shown to increase ROSC rates and short-term neurologically intact survival rates in multiple studies and is ready for a phase 1 clinical trial.
TRANSLATIONAL OUTLOOK: The current study identifies hypoxemia as a potential safety issue during ventilation with room air and suggests that SNPeCPR should be tested in humans with an FiO2 >0.5. Despite relative hypoxemia with lower FiO2 ventilation, SNPeCPR oxygen delivery to the tissues is increased due to higher blood flow generation.
Footnotes
This study was funded by an R01 research grant (R01HL108926) from the National Institutes of Health National Heart, Lung, and Blood Institute to Dr. Yannopoulos. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Shinozaki K., Nonogi H., Nagao K., Becker L.B. Strategies to improve cardiac arrest survival: a time to act. Acute Med Surg. 2016;3:61–64. doi: 10.1002/ams2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin E.J., Virani S.S., Callaway C.W. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 3.Kudenchuk P.J., Cobb L.A., Copass M.K. Amiodarone for resuscitation after out-of-hospital cardiac arrest due to ventricular fibrillation. N Engl J Med. 1999;341:871–878. doi: 10.1056/NEJM199909163411203. [DOI] [PubMed] [Google Scholar]
- 4.Daya M.R., Schmicker R.H., Zive D.M. Out-of-hospital cardiac arrest survival improving over time: results from the Resuscitation Outcomes Consortium (ROC) Resuscitation. 2015;91:108–115. doi: 10.1016/j.resuscitation.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olasveengen T.M., Sunde K., Brunborg C., Thowsen J., Steen P.A., Wik L. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. JAMA. 2009;302:2222–2229. doi: 10.1001/jama.2009.1729. [DOI] [PubMed] [Google Scholar]
- 6.Aufderheide T.P., Frascone R.J., Wayne M.A. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: a randomised trial. Lancet. 2011;377:301–311. doi: 10.1016/S0140-6736(10)62103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yannopoulos D., Bartos J.A., George S.A. Sodium nitroprusside enhanced cardiopulmonary resuscitation improves short term survival in a porcine model of ischemic refractory ventricular fibrillation. Resuscitation. 2017;110:6–11. doi: 10.1016/j.resuscitation.2016.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yannopoulos D., Matsuura T., Schultz J., Rudser K., Halperin H.R., Lurie K.G. Sodium nitroprusside enhanced cardiopulmonary resuscitation improves survival with good neurological function in a porcine model of prolonged cardiac arrest. Crit Care Med. 2011;39:1269–1274. doi: 10.1097/CCM.0b013e31820ed8a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yannopoulos D., McKnite S., Aufderheide T.P. Effects of incomplete chest wall decompression during cardiopulmonary resuscitation on coronary and cerebral perfusion pressures in a porcine model of cardiac arrest. Resuscitation. 2005;64:363–372. doi: 10.1016/j.resuscitation.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Yannopoulos D., Nadkarni V.M., McKnite S.H. Intrathoracic pressure regulator during continuous-chest-compression advanced cardiac resuscitation improves vital organ perfusion pressures in a porcine model of cardiac arrest. Circulation. 2005;112:803–811. doi: 10.1161/CIRCULATIONAHA.105.541508. [DOI] [PubMed] [Google Scholar]
- 11.Yannopoulos D., Aufderheide T.P., Abella B.S. Quality of CPR: an important effect modifier in cardiac arrest clinical outcomes and intervention effectiveness trials. Resuscitation. 2015;94:106–113. doi: 10.1016/j.resuscitation.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Schultz J., Segal N., Kolbeck J., McKnite S., Caldwell E., Yannopoulos D. Sodium nitroprusside enhanced cardiopulmonary resuscitation (SNPeCPR) improves vital organ perfusion pressures and carotid blood flow in a porcine model of cardiac arrest. Resuscitation. 2012;83:374–377. doi: 10.1016/j.resuscitation.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debaty G., Matsuura T.R., Bartos J.A. Sodium nitroprusside–enhanced cardiopulmonary resuscitation facilitates intra-arrest therapeutic hypothermia in a porcine model of prolonged ventricular fibrillation. Crit Care Med. 2015;43:849–855. doi: 10.1097/CCM.0000000000000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultz J., Segal N., Kolbeck J. Sodium nitroprusside enhanced cardiopulmonary resuscitation prevents post-resuscitation left ventricular dysfunction and improves 24-hour survival and neurological function in a porcine model of prolonged untreated ventricular fibrillation. Resuscitation. 2011;82(suppl 2):S35–S40. doi: 10.1016/S0300-9572(11)70149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yannopoulos D., Segal N., McKnite S., Aufderheide T.P., Lurie K.G. Controlled pauses at the initiation of sodium nitroprusside–enhanced cardiopulmonary resuscitation facilitate neurological and cardiac recovery after 15 mins of untreated ventricular fibrillation. Crit Care Med. 2012;40:1562–1569. doi: 10.1097/CCM.0b013e31823e9f78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schultz J.C., Segal N., Caldwell E. Sodium nitroprusside–enhanced cardiopulmonary resuscitation improves resuscitation rates after prolonged untreated cardiac arrest in 2 porcine models. Crit Care Med. 2011;39:2705–2710. doi: 10.1097/CCM.0b013e31822668ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yannopoulos D., Segal N., Matsuura T. Ischemic post-conditioning and vasodilator therapy during standard cardiopulmonary resuscitation to reduce cardiac and brain injury after prolonged untreated ventricular fibrillation. Resuscitation. 2013;84:1143–1149. doi: 10.1016/j.resuscitation.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore J.C., Bartos J.A., Matsuura T.R., Yannopoulos D. The future is now: neuroprotection during cardiopulmonary resuscitation. Curr Opin Crit Care. 2017;23:215–222. doi: 10.1097/MCC.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 19.Bartos J.A., Carlson K., Carlson C. Surviving refractory out-of-hospital ventricular fibrillation cardiac arrest: critical care and extracorporeal membrane oxygenation management. Resuscitation. 2018;132:47–55. doi: 10.1016/j.resuscitation.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 20.Yannopoulos D., Bartos J.A., Aufderheide T.P. The evolving role of the cardiac catheterization laboratory in the management of patients with out-of-hospital cardiac arrest: a scientific statement from the American Heart Association. Circulation. 2019;139:e530–e552. doi: 10.1161/CIR.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 21.National Research Council . Eighth Edition. The National Academies Press; Washington, DC: 1996. Guide for the Care and Use of Laboratory Animals.https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf Available at: [Google Scholar]
- 22.Segal N., Matsuura T., Caldwell E. Ischemic postconditioning at the initiation of cardiopulmonary resuscitation facilitates functional cardiac and cerebral recovery after prolonged untreated ventricular fibrillation. Resuscitation. 2012;83:1397–1403. doi: 10.1016/j.resuscitation.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halperin H.R., Lee K., Zviman M. Outcomes from low versus high-flow cardiopulmonary resuscitation in a swine model of cardiac arrest. Am J Emerg Med. 2010;28:195–202. doi: 10.1016/j.ajem.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Paradis N.A., Martin G.B., Rivers E.P. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1106–1113. [PubMed] [Google Scholar]
- 25.Ranadive S.M., Eugene A.R., Dillon G., Nicholson W.T., Joyner M.J. Comparison of the vasodilatory effects of sodium nitroprusside vs. nitroglycerin. J Appl Physiol. 2017;123:402–406. doi: 10.1152/japplphysiol.00167.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kieler-Jensen N., Milocco I., Ricksten S.E. Pulmonary vasodilation after heart transplantation. A comparison among prostacyclin, sodium nitroprusside, and nitroglycerin on right ventricular function and pulmonary selectivity. J Heart Lung Transplant. 1993;12:179–184. [PubMed] [Google Scholar]
- 27.Reynolds J.C., Salcido D.D., Menegazzi J.J. Coronary perfusion pressure and return of spontaneous circulation after prolonged cardiac arrest. Prehospital Emerg Care. 2010;14:78–84. doi: 10.3109/10903120903349796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Alonzo G.E., Dantzker D.R. Respiratory failure, mechanisms of abnormal gas exchange, and oxygen delivery. Med Clin North Am. 1983;67:557–571. doi: 10.1016/s0025-7125(16)31189-0. [DOI] [PubMed] [Google Scholar]
- 29.Neumar R.W., Brown C.G., Van Ligten P., Hoekstra J., Altschuld R.A., Baker P. Estimation of myocardial ischemic injury during ventricular fibrillation with total circulatory arrest using high-energy phosphates and lactate as metabolic markers. Ann Emerg Med. 1991;20:222–229. doi: 10.1016/s0196-0644(05)80927-8. [DOI] [PubMed] [Google Scholar]
- 30.Raina K.D., Callaway C., Rittenberger J.C., Holm M.B. Neurological and functional status following cardiac arrest: method and tool utility. Resuscitation. 2008;79:249–256. doi: 10.1016/j.resuscitation.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartos JA, Grunau, B, Carlson C, et al. Metabolic effects of prolonged resuscitation for refractory ventricular fibrillation cardiac arrest: the survival benefit associated with extracorporeal life support. Submitted to Circ January.
- 32.Grunau B., Puyat J., Wong H. Gains of continuing resuscitation in refractory out-of-hospital cardiac arrest: a model-based analysis to identify deaths due to intra-arrest prognostication. Prehosp Emerg Care. 2018;22:198–207. doi: 10.1080/10903127.2017.1356412. [DOI] [PubMed] [Google Scholar]
- 33.Goto Y., Funada A., Goto Y. Relationship between the duration of cardiopulmonary resuscitation and favorable neurological outcomes after out-of-hospital cardiac arrest: a prospective, nationwide, population-based cohort study. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reynolds J.C., Grunau B.E., Rittenberger J.C., Sawyer K.N., Kurz M.C., Callaway C.W. Association between duration of resuscitation and favorable outcome after out-of-hospital cardiac arrest: implications for prolonging or terminating resuscitation. Circulation. 2016;134:2084–2094. doi: 10.1161/CIRCULATIONAHA.116.023309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds J.C., Grunau B.E., Elmer J. Prevalence, natural history, and time-dependent outcomes of a multi-center North American cohort of out-of-hospital cardiac arrest extracorporeal CPR candidates. Resuscitation. 2017;117:24–31. doi: 10.1016/j.resuscitation.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 36.Sporer K., Jacobs M., Derevin L., Duval S., Pointer J. Continuous quality improvement efforts increase survival with favorable neurologic outcome after out-of-hospital cardiac arrest. Progn Prehospital Emerg Care. 2017:1–10. doi: 10.1080/10903127.2016.1218980. [DOI] [PubMed] [Google Scholar]