Highlights
-
•
First clinical outcomes of a new thin strut cobalt chromium biolimus-eluting stent.
-
•
The primary endpoint (9 month cardiac death, MI, ciTVR) occurred in 3.9%
-
•
Cardiac death occurred in 0.8%, MI in 1.1%, and ciTVR in 2.7%
-
•
Only 1 patient (0.25%) had a definite or probable stent thrombosis.
-
•
Pre-specified comparison with the LEADERS (BES arm) trial met non-inferiority.
Keywords: Drug eluting stent, Biodegradable polymer, Cobalt-chromium, Strut thickness, Myocardial infarction, Stent thrombosis
Abstract
Background
The biolimus-eluting stent (BES) was the first to elute anti-proliferative drug from a biodegradable polymer. In the randomized LEADERS trial, a stainless steel BES showed non-inferior efficacy compared to a sirolimus-eluting stent and a long-term safety advantage. We report the first clinical efficacy and safety outcomes of a new thin-strut cobalt chromium biolimus-eluting stent (CoCr-BES) from an international multi-centre registry.
Methods
We studied 400 all-comer patients with coronary disease receiving CoCr-BES at 12 centres, with follow-up at 9 months and 2 years. The primary endpoint was incidence of major adverse cardiac events (MACE) at 9 months comprising cardiac death, myocardial infarction (MI), and clinically indicated target vessel revascularization (ci-TVR). Key protocol elements were the same as the randomized LEADERS trial to enable a historical control for propensity-matched comparison.
Results
Mean patient age was 65 ± 11 years, 19% had diabetes, and 55% presented with unstable angina or MI. On discharge, 96% of patients were on dual antiplatelet therapy (DAPT) and 69% were on DAPT at 9 months. MACE at 9 months occurred in 3.9% of patients, cardiac death in 0.8%, MI in 1.1% and ci-TVR in 2.7%. One patient (0.25%) experienced definite or probable stent thrombosis (ST). A propensity-adjusted comparison showed similar clinical outcomes to the BES arm in the LEADERS trial for the primary endpoint MACE.
Conclusions
The new CoCr-BES showed low rates of MACE, MI, ci-TVR and ST at 9 months, similar to the BES arm in LEADERS.
1. Introduction
Drug-eluting stents (DES) constitute the current standard of care for acute and elective patients undergoing percutaneous coronary intervention [1], [2], [3]. While first-generation DES typically used durable polymers to store and modulate the release of the anti-proliferative drug, later generations have introduced biodegradable polymers with the intent to reduce untoward side effects occurring as an intolerance or hypersensitivity reaction of the vessel wall to the polymer. One of these 2nd generation DES, the Biolimus-eluting stent (BES) (Biomatrix Flex™, Biosensors International, Morges, Switzerland), released the drug Biolimus-A9 from a biodegradable polymer [4]. The BES was compared head-to-head with the first Sirolimus-eluting stent (SES) (Cypher™, Cordis, Miami Lakes, FL, USA) in the LEADERS trial. In this comparative study, the BES showed not only non-inferior efficacy at 9 months [5], but also a safety improvement with less very late stent thrombosis events from 3 to 5 years - an advantage that was attributed to the biodegradable polymer [6], [7].
Recently, an iteration of the BES was developed based on a CoCr thin-strut platform (83 um strut thickness), while all other design elements of the BES including the BA-9 drug, the drug dose, the PLA polymer and the drug release kinetics were kept the same. According to its similarity with the BES, the new CoCr-BES (Biomatrix Alpha™, Biosensors International, Morges, Switzerland) received CE-mark approval in 2015 through a regulatory pathway that does not require a new clinical trial.
The present study was designed as a post-market surveillance registry to evaluate the clinical safety and effectiveness data of the CoCr-BES stent in day-to-day clinical use. Key design elements of the registry protocol were kept the same as in the LEADERS trial so that it was possible to use the BES arm of the LEADERS study [5] as a historic reference in a propensity-matched comparison.
2. Methods
2.1. New CoCr-Biolimus-eluting stent design
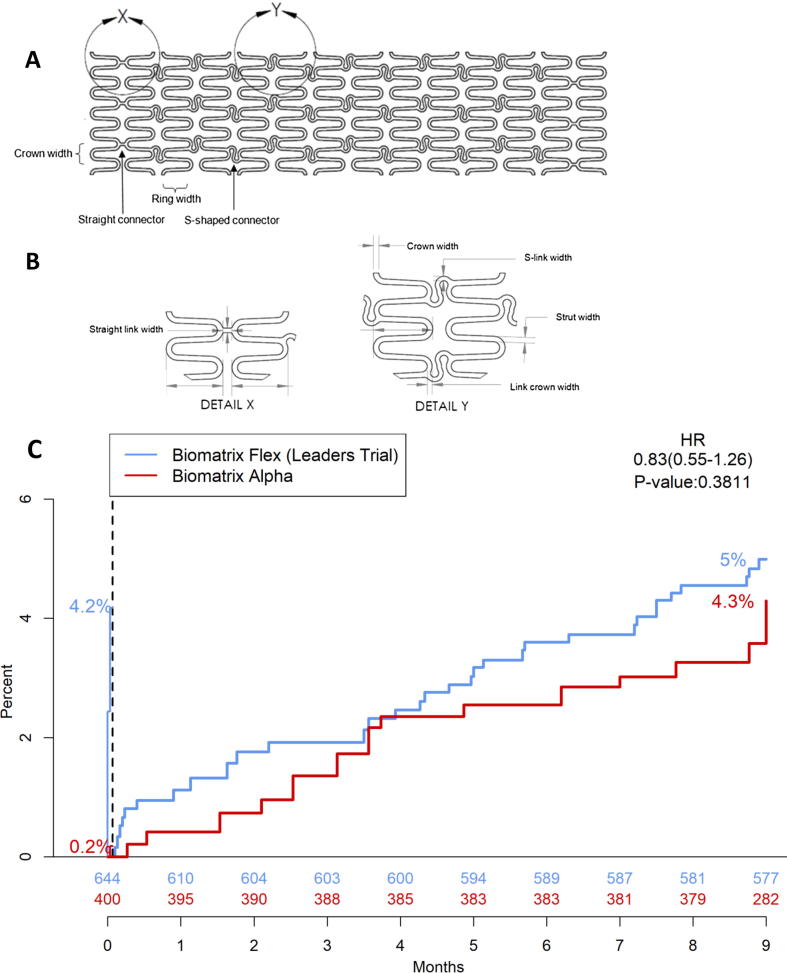
The CoCr-BES evaluated in this registry is abluminally coated with a mixture of the Biolimus-A9 drug and a PLA polymer matrix (50:50 by weight) in a dose of 15.6 µg/mm stent length. Biolimus-A9 is a Sirolimus derivative that has the same ring structure as Sirolimus, but a ligand modification that results in a 10-fold increased lipophilicity. Biolimus-A9 is an m-TOR inhibitor with a cytostatic mechanism of action that has close similarity to Sirolimus. The polymer is a biodegradable poly-lactic acid (PLA) which is absorbed within 6–9 months. While drug and polymer are identical to the BES in formulation and dose, the new CoCr-BES uses a cobalt-chromium (MP35N) rather than stainless steel (316L) stent platform enabling a reduction of stent strut thickness from 120 µm to 83 µm while maintaining a similar radial strength. All other stent design elements have remained unchanged including the stent platforms hybrid design of mid-section S-connectors for improved flexibility combined with straight connectors for higher longitudinal strength in the proximal and distal end sections of the stent (Fig. 1).
Fig. 1.
A: Flattened view of the cobalt chromium stent platform (small vessel model) B: Details of the straight and curved link connectors C: Comparison with LEADERS (historical control), with propensity matching and landmark analysis at day 3 for the primary endpoint of major adverse cardiac events at 9 months.
2.2. Study design and patients
The Biomatrix Alpha™ Registry was a prospective, single-arm, multi-centre post-market registry designed to enroll 400 patients with stable coronary artery disease or acute coronary syndromes, similar to the patients recruited into the LEADERS study. The registry was conducted in 12 centres in 4 countries in Europe and Asia. Patients were enrolled between October 2016 and October 2017. The registry was managed by the Cardiovascular European Research Center (CERC) in Massy, France.
Although not formally consecutive, an “all-comer” patient population was sought. Thus, patients were eligible for inclusion into the registry if they had undergone PCI in one or more coronary arteries or coronary bypass grafts with one or more CoCr-BES. There were no limitations as to the number of treated vessels, or the number, type and length of treated lesions. Patients were excluded if any additional stent(s) different from the study stent were implanted during the index procedure. There were no exclusion criteria related to clinical presentation. Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor was recommended as per clinical practice guidelines.
The study complied with the declaration of Helsinki and was approved by institutional ethics committees where applicable. All patients provided written informed consent for participation in the registry and were followed at 30 days and 9 months. The 2 years follow-up data collection is still ongoing at this point in time.
The primary endpoint was the incidence of major adverse cardiac events (MACE) at 9 months - a composite of cardiac death, myocardial infarction (MI) and clinically-indicated target vessel revascularization (ci-TVR). Pre-defined secondary endpoints included, among others, ARC definite/probable stent thrombosis (ST), the individual components of the primary endpoint, target lesion failure (TLF) – a composite of cardiac death, target-vessel MI or clinically-indicated target lesion revascularization, and the patient oriented composite endpoint (POCE) - a composite of all-cause mortality, MI, or any ciTVR.
2.3. Data and definitions
The study was conducted in accordance with GCP guidelines and the 1975 Declaration of Helsinki. Informed consent from each patient was obtained before data collection. Baseline data included demographic information, medical history, cardiovascular risk factors, lesion and procedure details and antithrombotic medications. Data were collected electronically at each participating centre and stored in a central database (BePATIENT, Paris, France). All data were checked for consistency. Electronic queries were issued as required. All reported MACE and ST events were monitored, checked against source documents and adjudicated by an independent Clinical Event Committee (CEC). Through a risk-based approach, the rate of overall source document verification was 10%.
Cardiac death was defined as any death due to immediate cardiac cause (e.g. MI, low-output failure, fatal arrhythmia), unwitnessed death and death of unknown cause. Myocardial infarction was defined through the Third Universal Definition of MI [8]. Ci-TVR was defined as a repeat PCI or bypass surgery of the target vessel associated with either a ≥ 70% vessel diameter reduction or a ≥ 50% diameter reduction in combination with angina and/or documented ischemia. ST was categorized as definite and/or probable according to the Academic Research Consortium (ARC) definitions [9], [10], and all the relevant angiograms were reviewed by the CEC.
2.4. Statistical analysis
2.4.1. Variables
For continuous variables, mean and standard deviation are reported. For categorical variables, counts and percentages are shown. The denominator for the calculation of percentages is based upon the number of non-missing values available, unless otherwise specified. Clinical events are reported as Kaplan-Meier estimates with corresponding confidence intervals based on the log-log transformation and hazard ratio derived from the Cox proportional hazard model. All data were analysed using SAS V.9.4 (SAS Institute, Cary, North Carolina, USA).
The registry was powered to compare the observed MACE rate to the historical MACE rate of 9.2% as observed in the BES arm of the LEADERS study at 9 months. Assuming a 9.2% event rate for the BES, a one-sided type I error (α) of 0.05, and a 4% non-inferiority margin, a sample size of 400 patients would have more than 80% power to conclude non-inferiority of the new CoCr-BES compared with the BES historical control. Non-inferiority would thus be met if the upper limit of the 90% Wald confidence interval for the 9 months MACE rate for the CoCr-BES was less than 13.2% (9.2% + 4%).
2.4.2. Comparison with the LEADERS trial - propensity matched landmark analysis
We sought to compare the outcomes of the CoCr-BES in this registry with the patients in the BES arm in the LEADERS trial, and selected the 644 patients that that were not scheduled per protocol for follow-up angiography as a reference group in order to avoid an artificially elevated repeat revascularization rate [11]. In order to adjust for potential baseline patient condition discrepancies, we conducted a patient-level propensity score analysis between the datasets of the CoCr-BES in this registry and the BES arm of LEADERS. The propensity for each patient was modelled as the probability of being part of the new registry versus being part of the BES arm in LEADERS (propensity score), estimating this probability by logistic regression [12], [13], [14] using a pre-specified list of baseline covariates. The full list of baseline variables used in the propensity score calculation is provided in supplementary materials (Table S1). Two different propensity methods were used, including a 5-strata method as well as a propensity inverse probability weighting (IPW) [13] with KM estimate weighted by each patient’s inverse propensity score.
Although the CoCr-BES registry protocol was designed to emulate the LEADERS protocol, of note the updated Third Universal Definition of Myocardial Infarction [8] established in 2012 was used for the registry. Recognizing that the different definitions might introduce a potential discrepancy in MI reporting between the registry and the LEADERS trial, particularly for peri-procedural MI (within 48 h), we conducted for comparison a landmark analysis censoring clinical events which were part of the primary endpoint occurring before day 3 to make the comparison of the primary endpoint between the two trials clinically meaningful. An analysis without the day-3 landmark was also done as sensitivity analysis (Table S2 of the supplementary materials)
2.4.3. Comparison with other previous studies and registries of the stainless steel Biolimus-eluting stent
For additional comparison beyond LEADERS we used two previous randomized clinical studies and two clinical registries conducted with the BES to help put the outcomes of this new registry into appropriate perspective. The SORT-OUT VI study compared the BES with the Zotarolimus-eluting stent (Resolute Integrity™, Medtronic, Minneapolis, MN, USA) in a randomized fashion [15], and the SORT-OUT VIII trial compared the BES with the everolimus-eluting stent [16] (Synergy™, Boston Scientific, Malborough, MA, USA). The e-BioMatrix Registry included 5,472 patients in Europe and Middle East and was published in 2015 [17]. The e-Biomatrix French Registry recruited 2,365 patients and was published in 2017 [18].
3. Results
3.1. Baseline patient and lesion characteristics
Baseline patient and lesion characteristics of the CoCr-BES registry population are shown in Table 1. As considered typical for an all-comers population, the mean age was 64.7 years, 19% of patients had diabetes, 21% were smokers, 57% had dyslipidaemia and 57% had arterial hypertension. Over half of the patients presented with acute coronary syndromes (16.3% had ST segment elevation MI (STEMI), 24.8% had non-ST-elevation MI (NSTEMI), and 14% presented with unstable angina). Patients had an average of 1.40 lesions for intervention with a balanced distribution of lesion complexity; type B2 lesions in 25.6%, and type C in 26.7% of patients. The average lesion length was 21.7 mm, the average reference vessel diameter (RVD) was 3.05 mm, 26.2% of patients had small vessels treated with RVD < 2.75 mm, and the average total stented length was 25.5mmm. Following stent implantation, 95.5% of the patients were discharged on DAPT and 68.8% were still taking DAPT at 9 months.
Table 1.
Baseline characteristics of the study population.
| Patient characteristics | Biomatrix Alpha™ Registry Population N (%) |
|---|---|
| Age (years) | 64.7 ± 11 |
| Male | 314 (78.5%) |
| BMI (kg/m2) | 28.2 ± 5.0 |
| History of gastrointestinal bleeding | 9 (2.3%) |
| History of malignancy | 23 (5.8%) |
| Renal insufficiency | 46 (11.5%) |
| Prior MI | 75 (18.8%) |
| Prior CABG or PCI | 98 (24.6%) |
| Diabetes mellitus | 77 (19.3%) |
| Current smoker | 82 (21.0%) |
| Dyslipidaemia | 221 (56.7%) |
| Arterial hypertension | 225 (57.3%) |
| Family history of coronary artery disease | 135 (38.0%) |
| Stable angina pectoris | 134 (33.5%) |
| Silent ischemia | 27 (6.8%) |
| Unstable angina | 56 (14.0%) |
| ST-segment elevation MI (STEMI) | 65 (16.3%) |
| Non-ST-segment elevation MI (NSTEMI) | 99 (24.8%) |
| Ejection fraction (%) | 56.6 ± 12.4 |
| Lesions treated | 562 |
| Lesions per patient | 1.40 ± 0.64 |
| Lesion class | |
| Type A | 99 (17.6%) |
| Type B1 | 169 (30.1%) |
| Type B2 | 144 (25.6%) |
| Type C | 150 (26.7%) |
| Lesion length | 21.7 ± 12.8 |
| Lesion length >30 mm | 96 (17.1%) |
| Reference vessel diameter (RVD) | 3.05 ± 0.52 |
| Small vessel (RVD < 2.75 mm) | 147 (26.2%) |
| Total stent length | 25.5 ± 13.5 |
| De-novo lesions | 539 (95.9%) |
| In-stent restenotic lesions | 20 (3.6%) |
| Bifurcation | 145 (25.8%) |
| Stents implanted per lesion | 1.16 ± 0.47 |
| Overlapping lesion | 74 (13.4%) |
BMI = body mass index; MI = myocardial infarction; CABG = coronary artery bypass grafting; PCI = percutaneous coronary intervention.
3.2. Clinical outcomes at 9 months follow-up
Nine months clinical follow-up of the CoCr-BES registry population was obtained in 97.8% of patients. The primary endpoint MACE occurred in 15/400 patients (3.9%, 95% CI [2.39%-6.47%]). The incidence of all cause death was 6/400 patients (1.5%), and cardiac death occurred in 3/400 patients (0.8%). A total of 4/400 patients (1.1%) experienced myocardial infarction. ARC definite or probable stent thrombosis occurred in 1/400 of patients (0.25%), and 10/400 (2.6%) underwent clinically indicated target vessel revascularization (ciTVR). The patient oriented composite endpoint (POCE) occurred in 18/400 of patients (4.7%). Full details are shown in Table 2.
Table 2.
Clinical Event Rates at 9 months follow-up.
| Endpoint | Biomatrix Alpha™ Registry Population N (%) [95% CI] |
|---|---|
| Primary endpoint MACE (cardiac death, MI, ciTVR) | 15 (3.94%) [2.39–6.47] |
| All death | 6 (1.51%) [0.68–3.34] |
| Cardiac death | 3 (0.76%) [0.25–2.33] |
| Myocardial infarction (MI) | 4 (1.1%) [0.41%−2.95%] |
| Definite or probable stent thrombosis | 1 (0.25%) [0.04–1.77] |
| Target vessel revascularization (ciTVR) | 10 (2.6%) [1.41–4.79] |
| POCE (All death, MI, ciTVR) | 18 (4.68%) [2.97–7.34] |
MACE = major adverse cardiac event; MI = myocardial infarction; ciTVR = clinically indicated target vessel revascularization; POCE = patient orientated composite endpoint.
3.3. Comparison with the LEADERS trial
A number of differences in the patient baseline characteristics between the patients enrolled in the CoCr-BES registry versus the LEADERS trial were noted. More patients in the registry presented with renal failure (11.5% vs 5.3%), but fewer patients had hypertension (57.3% vs 72.5%), fewer patients had dyslipidaemia (56.7% vs 65,1%), fewer patients had previous MI (18.8% vs 33.2%) and fewer patients had a history of previous PCI or CABG (24.6% vs 44.7%). More details of patient characteristics are provided in Table S1.
The outcomes of the conducted two-step comparison procedure including propensity score and a day-3 landmark analysis are provided in Fig. 1c showing Kaplan-Meier curves of the primary endpoint. The incidence of MACE was numerically lower for the CoCr-BES although this did not achieve statistical significance (4.3% vs 5.0%, HR 0.83; 95% CI [0.55–1.26], P = 0.38). Similarly, the incidence of each of the MACE components, as shown in Table 3, was numerically lower for the CoCr-BES. A trend towards a lower incidence of clinically driven target vessel revascularization in favour of the new CoCr Biomatrix Alpha™ stent was noted (ciTVR: 2.71% vs 4.24%, HR 0.63 95% CI [0.39 – 1.01], P = 0.056).
Table 3.
Propensity adjusted landmark analysis from day 3.
| Clinical event | Biomatrix Alpha™ Registry (N = 397) |
BES (LEADERS) Patients without protocol mandated angiographic follow up (N = 618) |
Hazard ratio [95% CI] |
P-value |
|---|---|---|---|---|
| Primary endpoint at 9 months (MACE) | 14 (4.29%) | 32 (4.99%) | 0.83 [0.55–1.26] | 0.38 |
| Cardiac death | 3 (0.72%) | 8 (1.23%) | 0.60 [0.24–1.49] | 0.27 |
| Myocardial infarction | 4 (1.28%) | 10 (1.57%) | 0.74 [0.34–1.59] | 0.44 |
| ciTVR | 9 (2.71%) | 29 (4.24%) | 0.63 [0.39–1.01] | 0.056 |
MACE = major adverse cardiac event, ciTVR = clinically indicated target vessel revascularization.
3.4. Comparison with previous studies and registries of the stainless steel biolimus eluting stent
To help place the results of the CoCr-BES registry in context, Table S3 in the supplementary material summarizes the key clinical outcomes this registry together with those of the stainless steel BES in the adjusted LEADERS arm, the SORT-OUT VI and SORT-OUT VIII randomized trials, the e-Biomatrix, and the e-Biomatrix France registries. While formal statistical analysis has not been undertaken as time-points at which event rates were reported were different, outcomes from this CoCr-BES registry compare favourably with those of the previous larger studies conducted with the stainless steel predecessor stent, particularly the low incidence of repeat revascularization and low incidence of stent thrombosis with the CoCr-BES.
4. Discussion
The key findings of the Biomatrix Alpha™ registry providing a first real-life clinical experience with the new CoCr-BES are low 9-months rates of MACE (3.9%), myocardial infarction (1.1%), target vessel revascularization (2.6%), and ARC definite or probable stent thrombosis (0.25%). Such low event rates are in line with the findings of contemporary studies of other latest generation DES [19]. In a recently published randomized trial comparing the Zotarolimus-eluting Onyx™ stent (Medtronic, Minneapolis, MN, USA) with the Sirolimus-eluting Orsiro™ stent (Biotronik, Bülach, Switzerland), the rates of target vessel failure were 4.5%, and 4.7%, respectively. Accordingly, our findings support the efficacy and safety profile of the new CoCr biolimus-eluting stent.
Given the absence of a control group, and with the aim of putting these CoCr-BES outcomes into a wider clinical perspective, we undertook a comparison with outcomes of the stainless steel BES arm of the LEADERS trial. This comparison is of relevance as the CoCr-BES registry was intentionally designed to be similar to the LEADERS trial keeping a number of key protocol elements the same including endpoints and follow-up duration. Further, a comprehensive statistical effort was made to make this comparison clinically meaningful including propensity analysis and a landmark analysis from day 3. While the incidence and time course of the primary endpoint MACE (Fig. 1c) was similar, there was a trend observed towards a lower incidence of repeat revascularization with CoCr-BES (Table 3). The low rate of ARC definite/probable stent thrombosis of 0.25% at 9 months in this all-comers cohort is also noteworthy.
It is reassuring that the CoCr-BES registry outcomes are similar to the two previous randomized trials (SORT-OUT VI and SORT-VIII) and two registries (e-Biomatrix Registry and the e-Biomatrix France registry) conducted with the stainless steel BES, with respect to cardiac death and MI, which is likely due the CoCr and stainless steel stents sharing a similar stent design, the same drug, the same drug dose, and the same biodegradable PLA polymer (Table S3).
The reduction in strut thickness (CoCr-BES: 83um vs BES: 120um) is the most obvious change arising from the new CoCr-BES design. Conceptually, this may lead to improvements in stent deliverability with less associated vascular injury, and less intense foreign body reaction of the vessel wall to the implant, which may lead to more favourable healing. Almost two decades ago it was suggested through the ISAR STEREO Trial with bare-metal stents that a reduction in strut thickness may be associated with improved clinical outcomes, namely a lower incidence of angiographic restenosis as well as a lower repeat revascularization rate [20]. More recently, a meta-analysis of 69 randomized drug-eluting stent trials showed that patients receiving stents with ultra-thin struts have lower rates of myocardial infarction and stent thrombosis [21]. The numerically lower repeat revascularization rate of 2.6% (ciTVR), and the low rate of stent thrombosis (0.25%) in our registry could be related to reduction in strut thickness but they could also be related to advances in procedural technique and concomitant drug therapy over the past decade, not fully adjusted for in the propensity analysis. Overall, the favourable outcomes of our CoCr-BES registry compared with historical control will likely be interpreted as a manifestation of the benefits of thinner struts of the CoCr-BES. However, while the improvements are certainly within the expectations for a new CoCr thin strut stent design, and while it might appear intuitive to assume better clinical outcomes with thinner stent struts, we recommend interpreting the data with caution, as to prove this concept, a randomized clinical trial with the two stents only differing in the platform would be needed.
4.1. Study limitations
The major limitations of this registry are its relatively modest sample size and the lack of a randomized control arm. As these registry data represent the first clinical evidence with the new CoCr-BES, we sought to place the outcomes in clinically meaningful context by undertaking a careful detailed comparison with historical data derived from the stainless steel predecessor of the new stent, including a propensity adjustment with the patients in the BES arm that were not scheduled per protocol for angiographic follow-up, and a landmark analysis from day 3 to compensate for a discrepancy in the definition of peri-procedural MI. Despite these efforts, it is possible that some baseline differences remain as confounding factors.
In line with typical registry design elements, only 10% of the patients in this registry were fully monitored, thus there is a possibility of under-reporting of clinical events, although 100% adjudication of MACE events was undertaken.
4.2. Conclusion
In this contemporary all-comers registry, the thin strut CoCr-BES delivered safety and efficacy outcomes comparable to its predecessor, the stainless steel BES. The low MACE rate of 3.9% at 9 months and the low 0.25% definite/probable stent thrombosis rate are reassuring, in line with contemporary studies of other latest generation DES, and support the clinical benefit of the new biolimus-eluting stent technology incorporating a biodegradable polymer, the BA-9 drug and a thin-strut CoCr stent platform.
5. Grant support
Biosensors.
Declaration of Competing Interest
SC, SSS, and HPS are employed by Biosensors. IM has received conference sponsorship from Biosensors. No other authors have any conflicts to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100472.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Morice M.C., Serruys P.W., Sousa J.E. Randomized study with sirolimus-coated Bx velocity balloon-exandable stent in the treatment of patients with de novo native coronary artery lesions. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 2.Stone G.W., Ellis S.G., Cox D.A. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 3.Schauerte P., Sousa Uva M., Stefanini G.G. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur. Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 4.Costa R.A., Lansky A.J., Abizaid A. Angiographic results of the first human experience with the Biolimus A9 drug-eluting stent for de novo coronary lesions. Am. J. Cardiol. 2006;98:443–446. doi: 10.1016/j.amjcard.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 5.Windecker S., Serruys P.W., Wandel S. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet. 2008;372:1163–1173. doi: 10.1016/S0140-6736(08)61244-1. [DOI] [PubMed] [Google Scholar]
- 6.Stefanini G.G., Kalesan B., Serruys P.W. Long-term clinical outcomes of biodegradable polymer biolimus-eluting stents versus durable polymer sirolimus-eluting stents in patients with coronary artery disease (LEADERS): 4 year follow-up of a randomised non inferiority trial. Lancet. 2011;378:1940–1948. doi: 10.1016/S0140-6736(11)61672-3. [DOI] [PubMed] [Google Scholar]
- 7.Serruys P.W., Farooq V., Kalesan B. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5-year report of the LEADERS (limus eluted from a durable versus ERodable stent coating) randomized, noninferiority trial. JACC Cardiovasc. Interv. 2013;6:777–789. doi: 10.1016/j.jcin.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Thygesen K., Alpert J.S., Jaffe A.S. Third Universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 9.Cutlip D.E., Windecker S., Mehran R. Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 10.Garcia H.M., McFadden E.M., Farb A. Standardized end point definitions for coronary intervention trials. Eur. Heart J. 2018;39:2192–2207. doi: 10.1093/eurheartj/ehy223. [DOI] [PubMed] [Google Scholar]
- 11.Misumida N., Kobayashi A., Kim S.M. Role of routine follow-up coronary angiography after percutaneous coronary intervention – systematic review and meta-analysis. Circ. J. 2017;82:203–210. doi: 10.1253/circj.CJ-17-0410. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbaum P.R., Rubin D.B. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am. Stat. 1985;39:33–38. [Google Scholar]
- 13.D’Agostino R.B. Tutorial in biostatistics: propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.D’Agostino R.B. Propensity scores in cardiovascular research. Circulation. 2007;115:2340–2343. doi: 10.1161/CIRCULATIONAHA.105.594952. [DOI] [PubMed] [Google Scholar]
- 15.Raungaard B., Okkels Jensen L., Tilsted H.H. Zotarolimus-eluting durable-polymer coated stent versus a biolimus-eluting biodegradable-polymer-coated stent in unselected patients undergoing percutaneous coronary intervention (SORT OUT VI): a randomized non-inferiority trial. Lancet. 2015;385:1527–1535. doi: 10.1016/S0140-6736(14)61794-3. [DOI] [PubMed] [Google Scholar]
- 16.Maeng M., Christiansen H.E., Raungaard B. Everolimus-eluting versus biolimus-eluting stents with biodegradable polymers in unselected patients undergoing percutaneous coronary intervention: a randomized noninferiority trial with 1 year follow-up (SORT OUT VIII Trial) J. Am. Coll. Cardiol. Intv. 2019;12:624–633. doi: 10.1016/j.jcin.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 17.Urban P., Valdes M., Menown I. Outcomes following implantation of the Biolimus-A9 Eluting biomatrix coronary stent: primary analysis of the e-BioMatrix Registry. Catheter. Cardiovasc. Intervent. 2015;86:1151–1160. doi: 10.1002/ccd.25892. [DOI] [PubMed] [Google Scholar]
- 18.Maupas E., Lipiecki J., Levy R. Safety and efficacy outcomes of 3rd Generation DES in an All-Comer Population of Patients Undergoing PCI: 12-Month and 24-Month Results of the e-Biomatrix French Registry. Catheter. Cardiovasc. Intervent. 2017;90:890–897. doi: 10.1002/ccd.27081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Von Birgelen C., Zocca P., Buiten R.A. Thin composite wire strut, durable polymer-coated (Resolute Onyx) versus ultrathin cobalt-chromium strut, bio-resorbable polymer-coated (Orsiro) drug-eluting stents in all-comers with coronary artery disease (BIONYX): an international, single-blind, randomized non-inferiority trial. Lancet. 2018;392:1235–1245. doi: 10.1016/S0140-6736(18)32001-4. [DOI] [PubMed] [Google Scholar]
- 20.Kastrati A., Mehili J., Dirschinger J. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR STEREO) Trial. Circulation. 2001;103:2816–2821. doi: 10.1161/01.cir.103.23.2816. [DOI] [PubMed] [Google Scholar]
- 21.Iantorno M., Lipinski M.J., Garcia-Garcia H.M. Meta-analysis of the impact of strut thickness on outcomes in patients with drug-eluting stents in a coronary artery. Am. J. Cardiol. 2018;122:1652–1660. doi: 10.1016/j.amjcard.2018.07.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.