Abstract
Triturus cristatus and Triturus marmoratus are two protected and declining newts occurring in the administrative department of Vienne, in France. They have limited dispersal abilities and rely on the connectivity between habitats and their suitability. In a warming climate, the locations of suitable habitats are expected to change, as is the connectivity. Here, we wondered how climate change might affect shifts in habitat suitability and connectivity of habitat patches, as connectivity is a key element enabling species to realize a potential range shift. We used ecological niche modelling (ENM), combining large-scale climate suitability with local scale, high-resolution habitat features, to identify suitable areas for the two species, under low and high warming scenarios (RCP 2.6 and RCP 8.5). We associated it with connectivity assessment through graph theory. The variable ‘small ponds’ contributed most to land cover-only ENMs for both species. Projections with climate change scenarios revealed a potential impact of warming on suitable habitat patches for newts, especially for T. cristatus. We observed a decrease in connectivity following a decrease in patch suitability. Our results highlight the important areas for newt habitat connectivity within the study area, and define those potentially threatened by climate warming. We provide information for prioritizing sites for acquisition, protection or restoration, and to advise landscape policies. Our framework is a useful and easily reproducible way to combine global climate requirements of the species with detailed information on species habitats and occurrence when available.
Subject terms: Conservation biology, Biogeography
Introduction
Amphibians are declining globally and have become a high-priority group in conservation1. The multiple threats on amphibians include loss and fragmentation of habitats, invasive species, diseases, human-induced pollution and climate change2,3. Climate change, through its effects on temperature and water availability, is likely to affect amphibian survival, phenology and distribution4–6. Ecological niche modelling (ENM) has facilitated the modelling of potential range shifts in amphibians at several scales, forecasting range expansion or reduction depending on species7–9. ENM can provide useful information with which to plan conservation actions in the face of climate and/or land use change10,11.
Whereas climate change is a driver of range shift at global12 and regional scales13, amphibian conservation at a local scale is closely related to the landscape mosaic, connectivity and degree of fragmentation14,15. Landscape connectivity is defined as “the degree to which the landscape facilitates or impedes movement among resource patches”16 and is a key parameter that should be taken into account in conservation planning17. Urban and Keitt18 advocated the study of landscape connectivity through graph theory, considering habitat patches as “nodes” connected by “edges”, which refer to ecological corridors. Landscape graphs can be used to represent ecological networks and to analyse connectivity between habitats, with, for instance, the purpose of prioritizing sites for protection, improving connectivity, or assessing potential effect of an urban development project19,20. In this approach, the landscape between habitat patches is represented by a “resistance” or “cost” which estimates the relationships between environmental variables and movement of the considered species21. Resistance surfaces can be categorical, with a cost associated with land cover types22; continuous, for example from an output of ENM, where resistance increases when habitat suitability decreases23,24; or binary, assuming that the influence of the matrix on species movement is homogenous between patches25. Ziółkowska et al.26 have compared the efficiency of such resistance surface representation on landscape connectivity assessment and they recommend using continuous resistance surface whenever possible. Using a habitat suitability map from ENM helps to overcome a lack of sufficient data. The use of ENM output is also a way to explore predictions of changes in high habitat suitability sites and the associated connectivity of species populations in the face of climate change27. In addition, combining connectivity analyses for a group of species allows accounting for the variability of ecological requirements and movements through the landscape matrix28.
Newts, like most amphibians, have both aquatic and terrestrial life stages. The maintenance of their populations depends on the presence of suitable habitats for wintering and breeding seasons, as well as on movements allowing migration to breeding sites and the dispersal of juveniles. Here, we expected climate warming to reduce the distribution of suitable habitats for two newt species, Triturus marmoratus and Triturus cristatus, within Vienne, an administrative department of the region Nouvelle-Aquitaine, in France. We also expected a decrease in the related connectivity of habitat patches, as it is a key element enabling species to track their climatic niche, especially for low mobility species such as newts. We benefited from high-resolution data on the presence of these two species, to produce detailed identification of suitable habitats for newts and assessment of the related connectivity. This study has the advantage of accounting for relevant landscape data that are often lacking at larger scales, such as small ponds13, and provides a valuable tool for environmental planning at the scale of an administrative department. We used ecological niche modelling, combining large-scale climate suitability with local scale, high-resolution habitat features to identify suitable habitat patches within a detailed map of the study area. We associated it with connectivity assessment through graph theory to identify key areas for maintaining connectivity for newts. Such a tool can help to define conservation action strategy.
Materials and Methods
Study area and studied species
The study area corresponded to the administrative department of Vienne (7,308 km²), in Nouvelle-Aquitaine, France. We divided the study area into 50 × 50 m cells to allow the inclusion of high resolution land cover data in local scale ENMs.
The great crested newt Triturus cristatus is a boreal species ranging from Western Europe (France, Great Britain) to eastern Russia29. The marbled newt Triturus marmoratus has a smaller range, covering the north of Spain and Portugal and western France30. Both species and their habitat are protected in France and Europe31,32, and classified as near threatened in national and regional red lists33 and as least concern at the global scale29,30. The two newt species co-occur in Vienne, T. cristatus being at the southern limit of its range, with some sites sheltering the hybrid Triturus blasii (T. cristatus × T. marmoratus).
Modelling approach and connectivity analysis
Step 1: Global climate-only ENMs
Our method consisted of five steps (ESM 1). Steps 1, 2, 3 and 4 were repeated for each species. First, we ran climate-only ENMs across the full range of each species to account for their global climatic niche. For this first step, we used georeferenced data, gathered at a 10 × 10 km grid scale, across the full range of each species, from the Faune-France database (www.faune-france.org) and from the GBIF database34,35, compiling 8,599 points for T. cristatus and 3,708 points for T. marmoratus from 1970 to 2018. We used the Biomod2 platform for ensemble modelling36 under R software version 3.3.2, including bioclimatic variables from Worldclim, within a 10 × 10 km grid37. We applied a selection procedure proposed by Leroy et al.38 to select a set of uncorrelated variables based on Pearson correlation and variable importance for each species (see ESM 2 for variable description and correlation). Using this process we selected seven variables for T. cristatus: maximum temperature of warmest month, temperature annual range, mean temperature of wettest quarter, mean temperature of coldest quarter, annual precipitation, precipitation seasonality, and precipitation of driest quarter. We selected eight variables for T. marmoratus: annual mean temperature, mean diurnal range, temperature seasonality, mean temperature of wettest quarter, mean temperature of driest quarter, precipitation seasonality, precipitation of warmest quarter, and precipitation of coldest quarter. We ran the models with eight algorithms: grouping generalized additive model (GAM), generalized linear model (GLM), multivariate adaptative regression splines (MARS), artificial neural networks (ANN), flexible discriminant analysis (FDA), classification tree analysis (CTA), generalized boosting models (GBM) and random-forest (RF). We ran the models with five sets of 10,000 randomly selected pseudo-absences (PA), five runs per model. We adjusted weights of points to give equal weight for presence and PA. This process resulted in a total of 200 models. Model parameters are available in ESM 3. We split the observation dataset into 70% for training and 30% for evaluation of the area under the ROC curve (AUC-ROC39) and calculation of a True skill statistic (TSS40). We used ensemble modelling, including all runs with TSS over 0.7, to display central tendency across the modelling algorithms41. We assessed uncertainty in our ensemble models by calculating the coefficient of variation among single models used for ensemble modelling.
To increase the resolution of our outputs, we used variables at higher resolution to project our climate-only ENMs throughout Vienne42–44. According to Guisan et al.45, increasing resolution when projecting species distribution should be done cautiously, because the span of potential values across the range decreases when the resolution of the data decreases. Therefore, prior to projections, we verified that the range of values found for each selected variable within the calibration dataset actually contained the range of values of the corresponding variable within the projection dataset (see ESM 4 for values). In this way, we projected the ensemble models to current climate conditions at a 1 km² grid, using a dataset of maximum temperature, minimum temperature and precipitation, initially calculated for France, and different from Worldclim data (ESM 2). We also projected our ensemble models with data depicting future conditions calculated for France (see ESM 2 for description of datasets calculated for France for current and future conditions13,46). This allowed us to increase the spatial resolution of our results. We calculated the bioclimatic variables from these sets of data using the dismo package47. We verified a correlation between Worldclim bioclimatic variables and our set of variables under current conditions, allowing us to use the latter to project our models (see ESM 5 for correlation values between bioclimatic variables). We then forecasted the climate suitability for the species under future conditions accounting for two scenarios of climate change. The Representative Concentration Pathway (RCP) 2.6 scenario forecasts a peak in radiative forcing followed by a decline, to remain under 500 ppm by 2100, allowing to maintain the increase of global mean temperature below 2 °C48. The RCP 8.5 scenario forecasts a rise of radiative forcing up to more than 1,300 ppm by 2100, with no specific climate mitigation48. We assessed the transferability of our ensemble models to these datasets by calculating the dissimilarity between variables used for calibrating the models and variables used to project the models through multivariate environmental similarity surface (MESS49), for both current and future conditions, using the dismo package47. The MESS allows comparison of the values of the variables across the study area to the distribution of values at reference points (i.e. occurrence of the species), and thus identifies where extrapolation can be an issue. The MESS is similar to a BIOCLIM analysis, but it allows negative values. Negative values indicate locations where at least one variable has a value outside the range of environments displayed by the reference set49. The more the values are negative, the more the location displays a novel environment, and extrapolation of the model is needed49.
Step 2: Local land cover-only ENMs
For step 2, we ran local scale ENMs across the study area, with the same eight algorithms of Biomod2. Night surveys are organised every year in Vienne during the amphibian breeding season (February to May), by the naturalist association Vienne Nature (www.vienne-nature.fr/), as part of monitoring, inventorying and improving knowledge on amphibian distribution. In order to homogenize the prospecting effort, the department of Vienne was divided into a 10 × 10 km grid. For this step 2, we used georeferenced observations for the two newt species within the study area, collected by the volunteer and professional naturalists of Vienne Nature between 2000 and 2018, during these field surveys. The database compiled 187 points for the great crested newt Triturus cristatus and 435 points for the marbled newt Triturus marmoratus. We spatially reduced presence points of the species from a distance of 50 m around each point to minimize sampling bias and point clustering50, leaving 183 points for T. cristatus and 398 points for T. marmoratus.
We used a set of 15 land cover variables (see ESM 2 for variable description, source and original resolution) to account for land cover suitability for the species within the study area. Vienne Nature inventoried data on small ponds, large ponds and springs across Vienne through remote sensing, by combining mapped information and orthophotos (ESM 2). The use of this dataset for the study area did not allow investigation of land-use change scenarios in this study. We computed different proxies to define land cover variables. For some variables, namely coniferous forests, broadleaved forests, woody moorlands, hedges, crops, large ponds, beaches, dunes and sand, natural grasslands, pastures, orchards, and vineyards, we computed an index of surface-compactness (SC) in each cell of the 50 × 50 m grid, using ArcGIS 10.3. We chose this index because it accounts for both the area and the shape of the variable, and it is commonly used to characterize the shape of habitat patches in landscape connectivity assessments, particularly in urban planning. Compactness is a measure of shape, thus the broader the heart of a patch, the more it has potential to be a core habitat. The compactness = (4*Pi*surface of the patch)/(perimeter of the patch²)51, thus the measure of compactness increases with the width of the heart of the patch. The SC index is the product of the compactness and the area of a patch. For some other variables, namely water courses, small ponds, and springs, we computed the distance to the closest element in each cell. However, we kept the original definition of the variables ‘elevation’ and ‘urban areas’ (see ESM 2). We ran all models with the 15 variables and selected the most important ones for each species according to the method of Leroy et al.38 (see ESM 2 for variable correlation). In this way we selected five variables important for T. cristatus: small ponds, springs, elevation, broadleaved forests and water courses. We also selected five variables for T. marmoratus: small ponds, elevation, springs, broadleaved forests and crops.
We ran the models with ten sets of 1,000 pseudo-absences (PA) selected at a minimal distance of 50 m from our occurrences (disk method), as this method has shown to perform well with few presence data52. We used five runs per model. We adjusted weights of points to give equal weight for presence and PA. This resulted in a total of 400 models. Model parameters are available in ESM 3. We used ensemble models to forecast the land cover suitability of the species under current conditions and displayed the related coefficient of variation between single models. We assessed the extent of extrapolation of the ensemble model through MESS49. We used 70% of the data for training and 30% for the evaluation of models by AUC-ROC and TSS.
Step 3: Habitat suitability index
At step 3, we calculated a map of habitat suitability index (HSI) accounting for both climate-only ENMs and land cover-only ENMs outputs from steps 1 and 2. The outputs of climate-only ENMs were continuous probabilities within cells of 1 × 1 km and the outputs of land cover-only ENMs were continuous probabilities within cells of 50 × 50 m. To combine the outputs of both types of ENMs, we disaggregated the outputs of climate-only ENMs at 50 × 50 m, keeping the same values as in larger original cells. We multiplied the outputs of climate-only and land cover-only ENMs for current conditions to obtain a HSI map varying from 0 to 1 for current conditions53,54. We multiplied the outputs of climate-only ENMs for future conditions and land cover-only ENMs for current conditions to obtain HSI maps for future conditions. This framework implies two underlying assumptions, which may lead to uncertainty: multiplying probabilities implies that climate and land cover have independent effects on habitat suitability, and multiplying current land cover-only ENM outputs with outputs of climate-only ENMs for future conditions implies no change of land cover in the future. We computed a global Moran’s I index on the residuals of current HSI maps55 to assess the spatial autocorrelation at species locations. This index ranges from −1 to 1, respectively standing for strong negative and strong positive spatial autocorrelation. A value of 0 indicates a random pattern.
Step 4: Landscape graphs and connectivity analysis
At step 4, we transformed the HSI map into a binary map using a threshold defined by the 10th percentile of training presence points56,57. This threshold allows removal of locations where HSI is lower than the suitability values of the bottom 10% of species occurrence, which could be affected by errors in the data or represent sink populations56. Locations with HSI lower than the threshold are considered as unsuitable whereas locations with HSI equal or higher than the threshold are considered as suitable. We used Graphab 2.2.6 to compute the landscape graphs and the connectivity analysis58. The nodes of the graphs corresponded to the patches of suitable habitat identified in the binary map of habitat suitability. We averaged the value of HSI across the cells composing habitat patches to obtain a value of capacity for each habitat patch. The capacity is considered as an indicator of the demographic potential of a patch. The value of capacity attributed to each habitat patch was equal to the mean of the HSI within the cells of each habitat patch, thus a value between 0 and 1. We calculated a resistance surface from the continuous HSI resulting from step 3, as: resistance = 1 when HSI was ≥ threshold; and resistance = e(ln(0.001)/threshold × HSI) × 10³ when HSI was <threshold23,59. The resulting resistance surface ranges from 1 to 1000. The edges were calculated in cost distance from the resistance surfaces. The graph allows one to network the suitable habitat patches (i.e. nodes), connected by the least costly edge between patches.
Once the graph was computed, we calculated a measure of landscape connectivity on the graph. We used the local metric of interaction flux (IF) to quantify the potential connectivity at the level of habitat patches. IF is the sum of the products of one habitat patch capacity relative to those of all other habitat patches, weighted by their probability of interaction, and it ranges from 0 to the sum of capacities19,28. IF represents the local contribution of each habitat patch to the global connectivity within the study area28. The calculation of the IF involves specifying a distance relative to dispersal of the species. While dispersal in pond breeding amphibians is mostly achieved by juveniles60, most studies on amphibians are conducted on adult individuals, where radio tracking is easier, and movements attributed to newts are in the order of a few hundred meters28,61,62. Based on our field experience, we decided to use a maximum dispersal distance of 1 km for both newt species. We used the interpolation tool of Graphab to compute a spatial generalization of the IF in every pixel of the study area, using a decreasing weighting function from the edge of the patches28,63. By assuming that individuals can be found outside habitat patches64, interpolation allows assigning a value of the patch-based connectivity metric IF outside the patches considered as suitable to the species and thus the assessment of the potential connectivity of any pixel within the study area. This value decreases when distance to the patches increases28.
Step 5: Two-species combination and assessment of potential changes
Finally, at step 5, we combined the interpolations of the IF for each species in a two-species map by averaging the single maps of IF values computed for the two species at step 428. Because values of the IF depends on different graphs, we normalized the results by the mean and standard deviation, prior to combination, following the method of Sahraoui et al.28, to obtain comparable values for the two species, for current and future conditions. We evaluated the potential effect of climate change on overall connectivity for newts by computing the difference between two-species connectivity for current and future conditions.
Results
Global climate-only and local land cover-only ENMs
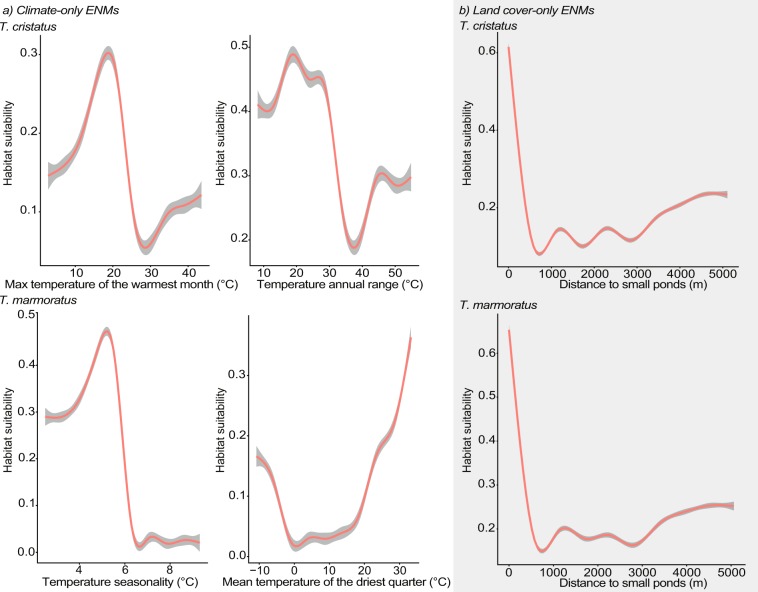
For global climate-only ENMs, we respectively obtained a value of TSS and AUC of 0.788 and 0.964 for the ensemble model of T. cristatus and 0.921 and 0.985 for the ensemble model of T. marmoratus. We found little variation (±0 to 0.1) between models combined in the final climate-only ensemble models (ESM 6). We did not find dissimilarity between values used to calibrate the models and values used to project the models in current conditions (0 to 50; see ESM 7 for MESS analysis in current conditions). For T. marmoratus, we did not find dissimilarity for projections for 2050 and 2100 under RCP 2.6 nor for 2050 under RCP 8.5, and we found little dissimilarity leading to little extrapolation in projection for 2100 under RCP 8.5 (−50 to 0; see ESM 7 for MESS analysis for T. marmoratus in future conditions). However, for T. cristatus, we found high dissimilarity leading to high extrapolation in all projections with future scenarios (−8000 to −4000; see ESM 7 for MESS analysis for T. cristatus in future conditions). Because of the high dissimilarity between current and future conditions, the results presented for T. cristatus in future conditions should be treated with caution. For T. cristatus, maximum temperature of the warmest month and temperature annual range showed the highest variable importance, with the highest habitat suitability around 20 °C for both variables (Fig. 1a). For T. marmoratus the most important variables were temperature seasonality, with highest habitat suitability around 5 °C, and mean temperature of the driest quarter, with highest habitat suitability around 30 °C (ESM 8, Fig. 1a).
Figure 1.
Response curves of the most important variables from (a) Climate-only ENMs and (b) Land cover-only ENMs for T. cristatus and T. marmoratus. Confidence interval is represented by grey background behind response curves.
For local land-cover-only ENMs, we obtained a value for TSS and AUC of 0.769 and 0.96 respectively for the ensemble model of T. cristatus, and 0.687 and 0.926 respectively for the ensemble model of T. marmoratus. We found very little variation between models (±0 to 0.2) in the suitable areas identified in final ensemble models for both species (ESM 6). We found dissimilarity leading to little extrapolation for the land cover-only ENMs, especially in some areas in the northern quarter of the study area for both species and in the southeast for T. cristatus (−163 to 0; ESM 7). Distance to small ponds showed high variable importance compared to other land cover variables for both species (ESM 8); habitat suitability dropped when distance to small pond increased (Fig. 1b).
Expected change in habitat suitability index
After combining climate-only and land cover-only ENMs, we found about 15% of the study area to be suitable for T. cristatus and about 24% to be suitable for T. marmoratus (Table 1, ESM 9). With climate change scenarios, we forecasted a decrease to less than 5% of the study area for T. cristatus under RCP 2.6 and we did not find any cells suitable for T. cristatus under RCP 8.5. The decrease in suitable areas was less important for T. marmoratus, for which suitable cells were mostly conserved under RCP 2.6. However, under RCP 8.5, the suitability of the study area decreased to about 20% in 2050, then was reduced to zero in 2100, as for T. cristatus. The suitable habitats of the two species overlapped in 10% of the study area in current climate conditions. The overlap was reduced to about 2% under RCP 2.6 and to zero under RCP 8.5. We did not find any significant spatial auto-correlation in models whatever the species considered (Moran’s I index = 0.0797 for T. cristatus (p-value = 0.118) and Moran’s I index = 0.008 for T. marmoratus (p-value = 0.078) (ESM 10)).
Table 1.
Area of total suitable habitat in km² (climate-only ENM output × land cover-only ENM output) for the two species within the study area, for current conditions and scenarios of future conditions.
| T. cristatus | T. marmoratus | |
|---|---|---|
| Current conditions | 1120.09 | 1730.99 |
| 2050 RCP 2.6 | 151.32 | 1713.14 |
| 2100 RCP 2.6 | 187.36 | 1729.74 |
| 2050 RCP 8.5 | 0 | 1443.53 |
| 2100 RCP 8.5 | 0 | 0 |
Landscape graphs and connectivity analysis
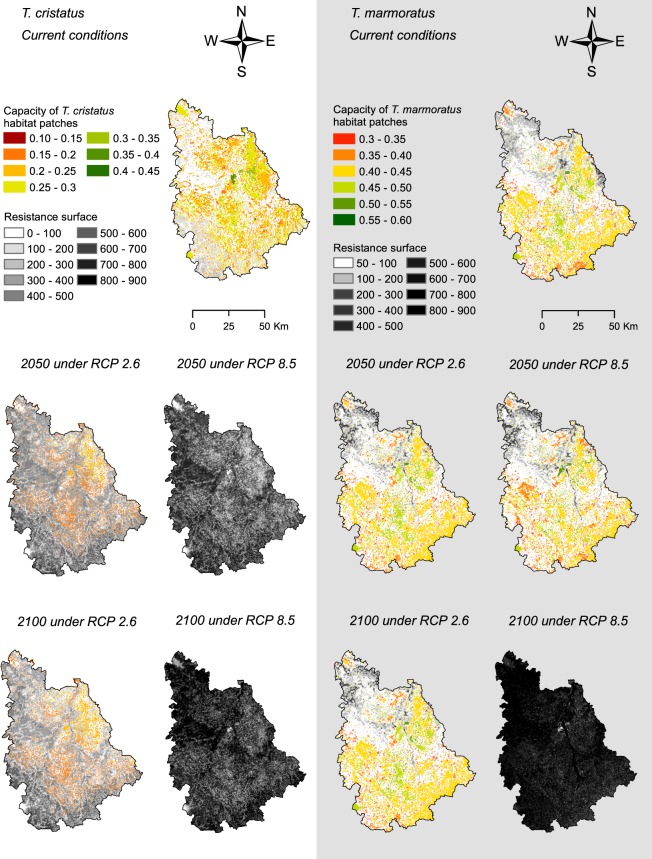
In current conditions, the northeast quarter of the study area seemed to hold some of the most suitable habitat patches (i.e. with highest capacity) for the two newt species, in addition to those of the centre-east of the study area for T. marmoratus (Fig. 2). The southwest quarter of the study area seemed less suitable for both species, but there were generally more habitat patches for T. marmoratus than for T. cristatus in the south of the study area. As the resistance of the landscape increased, the number and capacity of habitat patches decreased under RCP 2.6 for T. cristatus (Fig. 2) and we did not find any habitat patch suitable for T. cristatus under RCP 8.5. For T. marmoratus, although the resistance of the landscape increased under RCP 2.6 and in 2050 under RCP 8.5, the habitat patches seemed more sustainable for this species than for T. cristatus (Fig. 2). However, the high resistance of the landscape and low suitability did not allow any remaining habitat patches for T. marmoratus in 2100 under RCP 8.5. The high resistance of the landscape and the lack of suitable habitat patches for 2050 and 2100 for T. cristatus and for 2100 for T. marmoratus when we considered the scenario RCP 8.5 did not allow us to compute landscape graphs under such conditions.
Figure 2.
Habitat patches and resistance surfaces resulting from habitat suitability maps of T. cristatus and T. marmoratus, for use in landscape graphs computation. Habitat patches correspond to the nodes and resistance surfaces are used to compute the edges in the graph. Capacity is the mean value of HSI within a habitat patch.
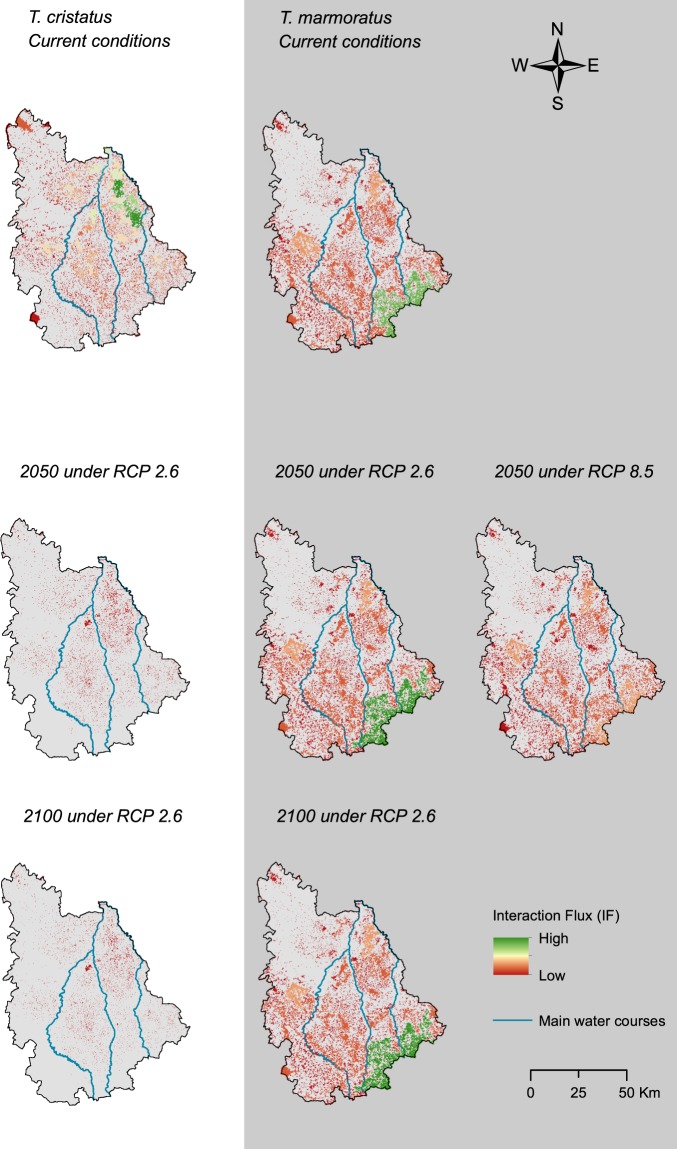
The calculation of the IF metric at the level of habitat patches showed that the most connected habitat patches were located in the northeast quarter of the study area for T. cristatus, whereas they were in the southeast part of the study area for T. marmoratus (Fig. 3). These areas were highly impacted by the decrease in patch number in T. cristatus forecasts, where most patches underwent a decline in interaction flux and thus of their potential of connectivity (Fig. 3). On the other hand, the best-connected areas persisted as such in 2050 and 2100 under the RCP 2.6 scenario for T. marmoratus (Fig. 3). The potential of connectivity of habitat patches generally decreased following the reduction in number of habitat patches. For T. marmoratus, the pattern of importance of patch connectivity in the southeast compared to the other patches within the study area was no longer so obvious in 2050 under the RCP 8.5 scenario, where some habitat patches reached the connectivity level of other patches in the northeast and west of the study area.
Figure 3.
Contribution of habitat patches to global connectivity through the study area identified through the interaction flux (IF, see materials and methods section for explanation) for T. cristatus and T. marmoratus.
Two-species combination and assessment of potential changes
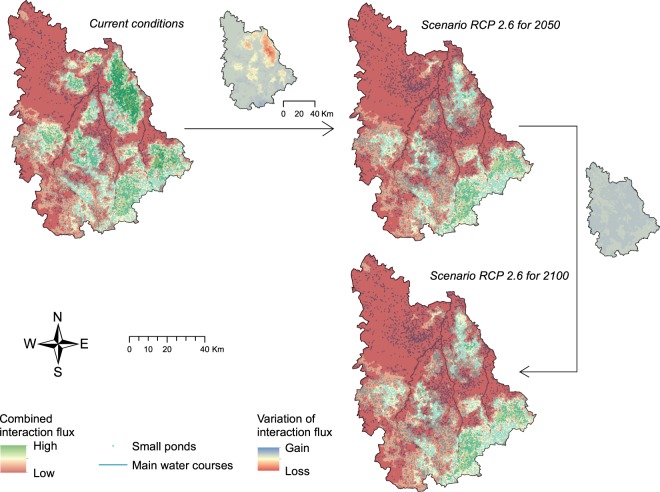
Through interpolation and combination of the IFs across the study area, we were able to sum up the potential connectivity for both newt species in a single map, for each condition where we found suitable habitat patches for both species (i.e. current conditions and future scenario RCP 2.6 for 2050 and 2100) (Fig. 4).
Figure 4.
Potential of landscape connectivity resulting from the combination of interaction flux for the two newt species within the study area. Small maps represent the variation of IF between large maps.
We found a high potential of connectivity for newts under current conditions in some patches located in the east, south-eastern and mid-western parts of the study area (Fig. 4). The results under future scenario RCP 2.6 showed an overall contraction of the most connected areas and a reduction in connectivity potential. Most of these changes happened in the north-eastern part of the study area between current conditions and conditions projected for 2050. According to the IFs, connectivity seemed quite stable between 2050 and 2100 in this scenario. Despite the loss of connectivity, we did not actually find any spatial shift of the areas considered best connected according to the IFs within the study area.
Discussion
The study allowed both large-scale climate tolerances and fine-scale information on land cover to be accounted for, in identifying areas potentially suitable for T. cristatus and T. marmoratus. We found suitable patches across the whole study area for both species, except in the northwest quarter, where the combination of land-cover-only and climate-only ENMs revealed fewer proper conditions for the presence of newts. Closeness to small ponds took high importance in land cover-only ENMs for both species, and thus in the identification of habitat patches. This makes sense because small ponds are an important habitat for species survival and represent preferential spots for breeding. This emphasizes the relevance of integrating high-resolution data for modelling suitable habitats for species at this scale65. Especially for T. cristatus, for which climate change might have a high impact, our results emphasized that small ponds are key landscape elements in maintaining newt presence within the study area66.
Projections with climate change scenarios revealed a potential impact of warming on habitat patches suitable for newts. Indeed, under RCP 2.6, the scenario leading to the lowest greenhouse gas concentration levels through a “peak-and-decline” succession for 2050 and 2100, we still found a decline in habitat suitability in the study area for T. cristatus (from 24% to less than 5%), while habitat suitability for T. marmoratus was only slightly impacted. However, with the scenario RCP 8.5, leading to high greenhouse gas concentration levels through rising radiative forcing, habitat patches of T. cristatus disappeared from 2050 while those for T. marmoratus remained until 2050, and disappeared in 2100. The reduction in suitability for T. cristatus is probably related to the fact that it is at the southern limit of its range in our study area. Indeed, it has already been shown that climate change could lead to the displacement of species ranges at higher latitudes, particularly for T. cristatus13,67.
The calculation of the IF index provides an indication of the local contribution of each patch to overall connectivity within the study area, accounting for both the capacity (i.e. mean of HSI) of the patch and its contribution to overall interactions in the network. Despite a rather high number of suitable patches for both species under current conditions, graph analyses showed that most patches were poorly connected. However, IF calculations and interpolations showed high contribution to overall connectivity for areas in the northeast for T. cristatus and in the southeast for T. marmoratus, and for a few patches in the southwestern quarter of the study area that showed higher IF for both species. These areas seemed to hold relatively high intrinsic capacity, and to be well connected to other patches. Consequently, these areas could act as source populations within the study area68, and to enhance movement in newts, such as seasonal migrations or dispersal. These results could be further developed through genetic analyses to confirm the potential for connectivity among patches69.
With climate change scenarios, we observed a decrease in connectivity following the decrease of patch capacity. The variation in IF has made possible to highlight important areas of connectivity for newts and to sum up those potentially threatened by climate warming. In addition, we found that climate change might increase landscape resistance toward newt species. Climate warming is indeed assumed to have direct effects on amphibian ability to move through the landscape, for instance by increasing desiccation risk5. However, habitat loss and fragmentation remains a main threat to biodiversity and the related land cover is also subject to constant changes70, with impacts on landscape connectivity for amphibians28,71. In our forecasts, the increase of landscape resistance is only the result of climate change scenarios, because we did not account for any change in land cover. At this time, there is no coherent scenario of land cover change at the scale of our study area allowing the use of high-resolution data such as the ponds inventory. It would have been opportune to simulate changes in land cover and the evolution of preferential breeding areas for newts, such as ponds, whose maintenance in the landscape depends on both land use and water supply. Indeed, global warming is likely to affect the persistence of wetlands and ponds, and therefore their availability for amphibian reproduction72. Still, land use or land cover can possibly buffer impacts of climate warming on landscape connectivity, by ensuring functional connectivity between suitable habitat patches73. In fact, Quesnelle et al.74 found the amount of forest to be more important for amphibians than the amount of wetland, because it provides wintering and summer shelters, and because survival and dispersal is favoured in forested landscapes compared to open areas75–77.
The graphs and maps produced in this study are functional tools for environmental planning. The areas identified as suitable and well connected are valuable to the local actors of biodiversity conservation by informing a species conservation strategy; or by highlighting areas where to focus conservation actions, such as prioritizing sites for protection, land control and management, or restoring connectivity.
With the combination of connectivity assessments for both species, we have identified connectivity patches for newts, which it would be interesting to connect in the perspective of climate change. The area identified in the northwestern quarter of the study area was predicted to decrease in connectivity under RCP 2.6. Enhancing the sustaining functionality of the pond network and the connectivity with the surrounding patches might help to buffer this variation, especially by conserving and restoring small ponds and features such as hedgerows or deciduous patches61,78 favouring the species’ overwintering, protection against dehydration and seasonal breeding movements. In parallel, relevant actors such as NGOs or other associations in the field of biodiversity protection could systematically bring up the results when consulted on land planning and urban projects, and aware of any risk of habitat destruction or connectivity disruption, particularly in areas identified as sources for these species. Indeed, Matos et al.79 have found impermeable roads to act as barriers to movements between ponds during both migration and dispersal for T. cristatus, whereas smaller and permeable roads increased the size of core areas. Our results can argue for the proposal of measures such as small wildlife crossings to preserve the functionality of the pond network. The inclusion of our results in urban planning documents, for instance, schemes for territorial coherence (as defined by the French urban code) at the level of the administrative department, or local urban development plan (as defined by the French urban code) at the level of municipalities, which have the power to control the destruction of ponds in their respective territories, would improve newt protection. Moreover, maintaining functional connectivity for these low-mobile species would also favour connectivity for more mobile amphibians and other species that depend on small ponds.
Uncertainty in ENM can come from various sources, such as choice of variables, of algorithms, correlation among predictor variables, spatial autocorrelation at species occurrences and transferability of the models in space and time45. Here, both land-cover-only and climate-only ENMs obtained satisfying evaluations according to TSS and AUC, which tends to confirm the choice of predictor variables. However, it is noteworthy that the variable “hedges” was not retained during the variable selection procedure whereas it is considered as important for newts61. This could be explained by a lack of precision in the data available for the study area, or because this variable is not significant in our study area. We used poorly correlated variables in either climate-only and land-cover-only ENMs and reduced spatial autocorrelation and potential uneven sampling using a single occurrence in each grid-cell containing presence information. We did not find significant spatial autocorrelation on the residuals of our final habitat suitability outputs. In addition, since our models aim to help species conservation in the study area, we estimated areas where individual predictions of the different algorithms could vary, in order to inform practitioners about potential uncertainties. We also provided MESS maps to inform about uncertainty within our projections. We found little extrapolation for current conditions, which allows confidence in transferability of models. However, we found much more extrapolation in future projections for T. cristatus than for T. marmoratus. Indeed, T. cristatus is at the southern limit of its range in our study area and its climate-only ENM was calibrated on data from colder regions than that of T. marmoratus. Thus, extrapolation detected for T. cristatus under climate warming was the result of projection under non-existent conditions in its current range.
Another point of uncertainty comes from the dispersal distance used to assess the potential connectivity of the species. The choice of this distance for amphibians is not obvious because of little literature on this topic. Dispersal (i.e. an active or passive attempt to move from a natal ⁄ breeding site to another breeding site80), generally occurs during the juvenile stages in pond-breeding amphibians60, which is why it is difficult to quantify travelled distances. Here we used 1 km as a maximal dispersal distance for newts based on expert opinion, but it could be appropriate to test the effect of a range of dispersal distances23,81. The fact that we were not able to include land cover change scenarios at the scale of our study area leads to the unrealistic assumption of no land cover change between current and future conditions82. While land cover change is a threat to suitable habitats in the landscape through habitat loss and fragmentation, as explained above, the management of these habitats can also be a lever for species conservation. Moreover, the combination of climate only-ENM and land cover-only ENM outputs assumes that climate and land cover independently influence habitat suitability, which is a source of uncertainty in our framework, as we do not know properly the actual interactions between these two factors. Indeed, climate and land cover might interact through multiple direct and indirect ways, for instance by affecting species response to land cover types under different climatic conditions83,84. For all those reasons, our result should be taken with caution and better considered as scenarios than as definite predictions of current and future habitat suitability for the species.
Conclusion
Combining land cover-based and climate-based ENMs in a single output for graph analyses allowed us to determine patches with high capacity (i.e. mean of HSI) for each species. However, according to Unglaub et al.85, there is no obvious positive relationship between habitat suitability and performance of populations in T. cristatus. Thus, it would be interesting to couple our analyses with local scale population dynamic models or mechanistic models in order to refine our understanding of the relevance of the patches for newt populations. We also determined patches with high interaction flux, which were not necessarily the ones with highest capacity, but which stood apart through a relatively high capacity and high connectivity potential. We consider these as key patches to maintain connectivity and enable the movement of individuals during seasonal migrations or dispersal events through the study area. Thus, after field verifications, these patches could be the subject of protection measures or land control in order to maintain their high potential86.
Furthermore, improving patch connectivity of high capacity patches that seemed poorly connected should be considered to promote stepping-stone connectivity allowing a potential range shift, for instance for T. cristatus13,87. We believe that our framework is a useful and easily reproducible way to combine global climate tolerance of a species with detailed data on species habitats and occurrence when available. It provides information for prioritizing sites for conservation and advises on landscape policies. Our graphs can be reused to simulate potential risks associated with new urban developments, or to select areas in which to create new ponds to promote newt persistence and connectivity.
Supplementary information
Acknowledgements
We thank Vienne Nature and all associations of the Faune-France network. The study was supported by the Association Nationale de la Recherche et de la Technologie (CIFRE n°. 2016/0710), the Agence de l’eau Loire Bretagne, and the Communauté d’Agglomération de Grand Châtellerault. RB’s work was supported by the TULIP Laboratory of Excellence (ANR-10-LABX-41). We thank Marius Bredon for his help and we thank Julian Reynolds and Coralie Garron for assistance with the English.
Author contributions
C.P. designed the study, analysed the data and wrote the main manuscript. M.G. supervised species data acquisition in Vienne. Y.S. and M.G. provided expert opinion on amphibians. R.B. worked on climate datasets. F.G., F.I.N., Y.S., R.B. and M.G. revised and contributed to the final version of the manuscript. All authors approved the final manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author and under agreement with the naturalist associations that were partners of the study, on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-60479-4.
References
- 1.Nori J, et al. Amphibian conservation, land-use changes and protected areas: A global overview. Biol. Conserv. 2015;191:367–374. doi: 10.1016/j.biocon.2015.07.028. [DOI] [Google Scholar]
- 2.Blaustein, A. R. et al. In Year in Ecology and Conservation Biology Vol. 1223 Annals of the New York Academy of Sciences (eds Ostfeld, R. S. & Schlesinger, W. H.) 108–119 (Blackwell Science Publ, 2011). [DOI] [PubMed]
- 3.Hof C, Araujo MB, Jetz W, Rahbek C. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nat. 2011;480:516–519. doi: 10.1038/nature10650. [DOI] [PubMed] [Google Scholar]
- 4.Carey C, Alexander MA. Climate change and amphibian declines: is there a link? Diversity Distrib. 2003;9:111–121. doi: 10.1046/j.1472-4642.2003.00011.x. [DOI] [Google Scholar]
- 5.Li YM, Cohen JM, Rohr JR. Review and synthesis of the effects of climate change on amphibians. Integr. Zool. 2013;8:145–161. doi: 10.1111/1749-4877.12001. [DOI] [PubMed] [Google Scholar]
- 6.O’Regan SM, Palen WJ, Anderson SC. Climate warming mediates negative impacts of rapid pond drying for three amphibian species. Ecol. 2014;95:845–855. doi: 10.1890/13-0916.1. [DOI] [PubMed] [Google Scholar]
- 7.Araujo MB, Thuiller W, Pearson RG. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeography. 2006;33:1712–1728. doi: 10.1111/j.1365-2699.2006.01482.x. [DOI] [Google Scholar]
- 8.Dolgener N, Freudenberger L, Schneeweiss N, Ibisch PL, Tiedemann R. Projecting current and potential future distribution of the Fire-bellied toad Bombina bombina under climate change in north-eastern Germany. Reg. Env. Change. 2014;14:1063–1072. doi: 10.1007/s10113-013-0468-9. [DOI] [Google Scholar]
- 9.Girardello M, Griggio M, Whittingham MJ, Rushton SP. Models of climate associations and distributions of amphibians in Italy. Ecol. Res. 2010;25:103–111. doi: 10.1007/s11284-009-0636-z. [DOI] [Google Scholar]
- 10.Aguirre-Gutiérrez J, et al. Historical changes in the importance of climate and land use as determinants of Dutch pollinator distributions. J. Biogeography. 2017;44:696–707. doi: 10.1111/jbi.12937. [DOI] [Google Scholar]
- 11.Marshall L, et al. The interplay of climate and land use change affects the distribution of EU bumblebees. Glob. Chang. Biol. 2017;24:101–116. doi: 10.1111/gcb.13867. [DOI] [PubMed] [Google Scholar]
- 12.Thuiller W, Araujo MB, Lavorel S. Do we need land-cover data to model species distributions in Europe? J. Biogeography. 2004;31:353–361. doi: 10.1046/j.0305-0270.2003.00991.x. [DOI] [Google Scholar]
- 13.Préau C, Isselin-Nondedeu F, Sellier Y, Bertrand R, Grandjean F. Predicting suitable habitats of four range margin amphibians under climate and land-use changes in southwestern France. Reg. Env. Change. 2018;19:27–38. doi: 10.1007/s10113-018-1381-z. [DOI] [Google Scholar]
- 14.Arntzen JW, Abrahams C, Meilink WRM, Iosif R, Zuiderwijk A. Amphibian decline, pond loss and reduced population connectivity under agricultural intensification over a 38 year period. Biodivers. Conserv. 2017;26:1411–1430. doi: 10.1007/s10531-017-1307-y. [DOI] [Google Scholar]
- 15.Piha H, Luoto M, Merila J. Amphibian occurrence is influenced by current and historic landscape characteristics. Ecol. Appl. 2007;17:2298–2309. doi: 10.1890/06-1509.1. [DOI] [PubMed] [Google Scholar]
- 16.Taylor, P. D., Fahrig, L. & With, K. A. In Connectivity Conservation Conservation Biology (eds Kevin R. Crooks & M. Sanjayan) 29-43 (Cambridge University Press, 2006).
- 17.Rudnick D, et al. The role of landscape connectivity in planning and implementing conservation and restoration priorities. Issues Ecol. 2012;16:1–20. [Google Scholar]
- 18.Urban D, Keitt T. Landscape connectivity: A graph-theoretic perspective. Ecol. 2001;82:1205–1218. doi: 10.1890/0012-9658(2001)082[1205:LCAGTP]2.0.CO;2. [DOI] [Google Scholar]
- 19.Foltête J-C, Girardet X, Clauzel C. A methodological framework for the use of landscape graphs in land-use planning. Landsc. Urban. Plan. 2014;124:140–150. doi: 10.1016/j.landurbplan.2013.12.012. [DOI] [Google Scholar]
- 20.Tarabon S, Bergès L, Dutoit T, Isselin-Nondedeu F. Environmental impact assessment of development projects improved by merging species distribution and habitat connectivity modelling. J. Environ. Manag. 2019;241:439–449. doi: 10.1016/j.jenvman.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 21.Zeller KA, McGarigal K, Whiteley AR. Estimating landscape resistance to movement: a review. Landsc. Ecol. 2012;27:777–797. doi: 10.1007/s10980-012-9737-0. [DOI] [Google Scholar]
- 22.Clauzel C, Bannwarth C, Foltete J-C. Integrating regional-scale connectivity in habitat restoration: An application for amphibian conservation in eastern France. J. Nat. Conserv. 2015;23:98–107. doi: 10.1016/j.jnc.2014.07.001. [DOI] [Google Scholar]
- 23.Duflot R, Avon C, Roche P, Bergès L. Combining habitat suitability models and spatial graphs for more effective landscape conservation planning: An applied methodological framework and a species case study. J. Nat. Conserv. 2018;46:38–47. doi: 10.1016/j.jnc.2018.08.005. [DOI] [Google Scholar]
- 24.Le Roux M, et al. Conservation planning with spatially explicit models: a case for horseshoe bats in complex mountain landscapes. Landsc. Ecol. 2017;32:1005–1021. doi: 10.1007/s10980-017-0505-z. [DOI] [Google Scholar]
- 25.Baranyi G, Saura S, Podani J, Jordán F. Contribution of habitat patches to network connectivity: Redundancy and uniqueness of topological indices. Ecol. Indic. 2011;11:1301–1310. doi: 10.1016/j.ecolind.2011.02.003. [DOI] [Google Scholar]
- 26.Ziółkowska E, Ostapowicz K, Radeloff VC, Kuemmerle T. Effects of different matrix representations and connectivity measures on habitat network assessments. Landsc. Ecol. 2014;29:1551–1570. doi: 10.1007/s10980-014-0075-2. [DOI] [Google Scholar]
- 27.Goncalves J, Honrado JP, Vicente JR, Civantos E. A model-based framework for assessing the vulnerability of low dispersal vertebrates to landscape fragmentation under environmental change. Ecol. Complex. 2016;28:174–186. doi: 10.1016/j.ecocom.2016.05.003. [DOI] [Google Scholar]
- 28.Sahraoui Y, Foltete J-C, Clauzel C. A multi-species approach for assessing the impact of land-cover changes on landscape connectivity. Landsc. Ecol. 2017;32:1819–1835. doi: 10.1007/s10980-017-0551-6. [DOI] [Google Scholar]
- 29.Arntzen, J. W. et al. Triturus cristatus. The IUCN Red List of Threatened Species 2009: e.T22212A9365894, 10.2305/IUCN.UK.2009.RLTS.T22212A9365894.en (2009).
- 30.Arntzen, J. W. et al. Triturus marmoratus. The IUCN Red List of Threatened Species 2009: e.T59477A11949129, 10.2305/IUCN.UK.2009.RLTS.T59477A11949129.en (2009).
- 31.M d’Etat, Ministère de l’écologie du développement et de l’aménagement durables, pêche. Arrêté du 19 novembre 2007 fixant les listes des amphibiens et des reptiles protégés sur l’ensemble du territoire et les modalités de leur protection. Journal Officiel n°0293 du 18 décembre (2007).
- 32.Council of the European Union. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. pp. 0007 – 0050 (1992).
- 33.PCN. Liste rouge du Poitou-Charentes: chapitre Amphibiens et Reptiles. (Fontaine-le-Comte, 2016), http://www.poitou-charentes-nature.asso.fr/wp-content/uploads/2019/06/Liste_Rouge_AMPHIBIENS-REPTILES_PC_2016.pdf. Accessed 05 July (2019).
- 34.GBIF.org (16th March 2018) GBIF Occurrence Download, 10.15468/dl.mkv0mx.
- 35.GBIF.org (21st June 2018) GBIF Occurrence Download, 10.15468/dl.eyhf39.
- 36.Thuiller, W., Georges, D., Engler, R., Georges, M. D. & Thuiller, C. W. Package ‘biomod2’, https://cran.r-project.org/web/packages/biomod2/biomod2.pdf (2012).
- 37.Fick SE, Hijmans RJ. WorldClim 2: new 1‐km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017;37:4302–4315. doi: 10.1002/joc.5086. [DOI] [Google Scholar]
- 38.Leroy B, et al. Forecasted climate and land use changes, and protected areas: the contrasting case of spiders. Diversity Distrib. 2014;20:686–697. doi: 10.1111/ddi.12191. [DOI] [Google Scholar]
- 39.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 40.Allouche O, Tsoar A, Kadmon R. Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS) J. Appl. Ecol. 2006;43:1223–1232. doi: 10.1111/j.1365-2664.2006.01214.x. [DOI] [Google Scholar]
- 41.Araújo MB, New M. Ensemble forecasting of species distributions. Trends Ecol. Evolution. 2007;22:42–47. doi: 10.1016/j.tree.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Meller L, et al. Ensemble distribution models in conservation prioritization: from consensus predictions to consensus reserve networks. Diversity Distrib. 2014;20:309–321. doi: 10.1111/ddi.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takolander A, Hickler T, Meller L, Cabeza M. Comparing future shifts in tree species distributions across Europe projected by statistical and dynamic process-based models. Reg. Env. Change. 2019;19:251–266. doi: 10.1007/s10113-018-1403-x. [DOI] [Google Scholar]
- 44.Thuiller W, Lavorel S, Araújo MB, Sykes MT, Prentice IC. Climate change threats to plant diversity in Europe. Proc. Natl Acad. Sci. U S Am. 2005;102:8245. doi: 10.1073/pnas.0409902102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guisan, A., Zimmermann, N. E. & Thuiller, W. In Habitat Suitability and Distribution Models: With Applications in R Ecology, Biodiversity and Conservation (eds Antoine Guisan, Niklaus E. Zimmermann, & Wilfried Thuiller) (Cambridge University Press, 2017).
- 46.Bertrand R, et al. Ecological constraints increase the climatic debt in forests. Nat. Commun. 2016;7:12643. doi: 10.1038/ncomms12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hijmans, R. J. & Elith, J. package ‘dismo’, https://cran.r-project.org/web/packages/dismo/vignettes/sdm.pdf (2017).
- 48.IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change 1535 pp (Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2013).
- 49.Elith J, Kearney M, Phillips S. The art of modelling range‐shifting species. Methods Ecol. Evolution. 2010;1:330–342. doi: 10.1111/j.2041-210X.2010.00036.x. [DOI] [Google Scholar]
- 50.Boria RA, Olson LE, Goodman SM, Anderson RP. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014;275:73–77. doi: 10.1016/j.ecolmodel.2013.12.012. [DOI] [Google Scholar]
- 51.Miller, V. C. A Quantitative Geomorphic Study of Drainage Basin Characteristics in the Clinch Mountain Area, Virginia and Tennessee 389–402 (Department of Geology Columbia University, New York, 1953).
- 52.Barbet-Massin M, Jiguet F, Albert CH, Thuiller W. Selecting pseudo-absences for species distribution models: how, where and how many? Methods Ecol. Evolution. 2012;3:327–338. doi: 10.1111/j.2041-210X.2011.00172.x. [DOI] [Google Scholar]
- 53.Fournier A, Barbet-Massin M, Rome Q, Courchamp F. Predicting species distribution combining multi-scale drivers. Glob. Ecol. Conserv. 2017;12:215–226. doi: 10.1016/j.gecco.2017.11.002. [DOI] [Google Scholar]
- 54.Hattab T, et al. Towards a better understanding of potential impacts of climate change on marine species distribution: a multiscale modelling approach. Glob. Ecol. Biogeography. 2014;23:1417–1429. doi: 10.1111/geb.12217. [DOI] [Google Scholar]
- 55.Moran PAP. A Test for the Serial Independence of Residuals. Biometrika. 1950;37:178–181. doi: 10.1093/biomet/37.1-2.178. [DOI] [PubMed] [Google Scholar]
- 56.Capinha C, Larson ER, Tricarico E, Olden JD, Gherardi F. Effects of climate change, invasive species, and disease on the distribution of native European crayfishes. Conserv. Biol. 2013;27:731–740. doi: 10.1111/cobi.12043. [DOI] [PubMed] [Google Scholar]
- 57.Pearson RG, Raxworthy CJ, Nakamura M, Townsend Peterson A. ORIGINAL ARTICLE: Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J. Biogeography. 2007;34:102–117. doi: 10.1111/j.1365-2699.2006.01594.x. [DOI] [Google Scholar]
- 58.Foltête J-C, Clauzel C, Vuidel G. A software tool dedicated to the modelling of landscape networks. Environ. Model. Softw. 2012;38:316–327. doi: 10.1016/j.envsoft.2012.07.002. [DOI] [Google Scholar]
- 59.Keeley ATH, Beier P, Gagnon JW. Estimating landscape resistance from habitat suitability: effects of data source and nonlinearities. Landsc. Ecol. 2016;31:2151–2162. doi: 10.1007/s10980-016-0387-5. [DOI] [Google Scholar]
- 60.Pittman SE, Osbourn MS, Semlitsch RD. Movement ecology of amphibians: A missing component for understanding population declines. Biol. Conserv. 2014;169:44–53. doi: 10.1016/j.biocon.2013.10.020. [DOI] [Google Scholar]
- 61.Trochet A, et al. Postbreeding Movements in Marbled Newts (Caudata, Salamandridae): A Comparative Radiotracking Study in Two Habitat Types. Herpetologica. 2017;73:1–9. doi: 10.1655/Herpetologica-D-15-00072. [DOI] [Google Scholar]
- 62.Jehle R, Arntzen JW. Post-breeding migrations of newts (Triturus cristatus and T. marmoratus) with contrasting ecological requirements. J. Zool. 2000;251:297–306. doi: 10.1111/j.1469-7998.2000.tb01080.x. [DOI] [Google Scholar]
- 63.Foltete JC, Clauzel C, Vuidel G, Tournant P. Integrating graph-based connectivity metrics into species distribution models. Landsc. Ecol. 2012;27:557–569. doi: 10.1007/s10980-012-9709-4. [DOI] [Google Scholar]
- 64.Hirzel AH, Le Lay G. Habitat suitability modelling and niche theory. J. Appl. Ecol. 2008;45:1372–1381. doi: 10.1111/j.1365-2664.2008.01524.x. [DOI] [Google Scholar]
- 65.Trumbo DR, et al. Integrating local breeding pond, landcover, and climate factors in predicting amphibian distributions. Landsc. Ecol. 2012;27:1183–1196. doi: 10.1007/s10980-012-9770-z. [DOI] [Google Scholar]
- 66.Curado N, Hartel T, Arntzen JW. Amphibian pond loss as a function of landscape change – A case study over three decades in an agricultural area of northern France. Biol. Conserv. 2011;144:1610–1618. doi: 10.1016/j.biocon.2011.02.011. [DOI] [Google Scholar]
- 67.Walther G-R, et al. Ecological responses to recent climate change. Nat. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 68.Stevens VM, Baguette M. Importance of Habitat Quality and Landscape Connectivity for the Persistence of Endangered Natterjack Toads. Conserv. Biol. 2008;22:1194–1204. doi: 10.1111/j.1523-1739.2008.00990.x. [DOI] [PubMed] [Google Scholar]
- 69.Luque S, Saura S, Fortin M-J. Landscape connectivity analysis for conservation: insights from combining new methods with ecological and genetic data. Landsc. Ecol. 2012;27:153–157. doi: 10.1007/s10980-011-9700-5. [DOI] [Google Scholar]
- 70.Titeux N, et al. Biodiversity scenarios neglect future land-use changes. Glob. Change Biol. 2016;22:2505–2515. doi: 10.1111/gcb.13272. [DOI] [PubMed] [Google Scholar]
- 71.Decout S, Manel S, Miaud C, Luque S. Integrative approach for landscape-based graph connectivity analysis: a case study with the common frog (Rana temporaria) in human-dominated landscapes. Landsc. Ecol. 2012;27:267–279. doi: 10.1007/s10980-011-9694-z. [DOI] [Google Scholar]
- 72.McMenamin SK, Hadly EA, Wright CK. Climatic change and wetland desiccation cause amphibian decline in Yellowstone National Park. Proc. Natl Acad. Sci. U S Am. 2008;105:16988–16993. doi: 10.1073/pnas.0809090105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Albert CH, Rayfield B, Dumitru M, Gonzalez A. Applying network theory to prioritize multispecies habitat networks that are robust to climate and land-use change. Conserv. Biol. 2017;31:1383–1396. doi: 10.1111/cobi.12943. [DOI] [PubMed] [Google Scholar]
- 74.Quesnelle PE, Lindsay KE, Fahrig L. Relative effects of landscape-scale wetland amount and landscape matrix quality on wetland vertebrates: a meta-analysis. Ecol. Appl. 2015;25:812–825. doi: 10.1890/14-0362.1. [DOI] [PubMed] [Google Scholar]
- 75.Rothermel BB, Semlitsch RD. An experimental investigation of landscape resistance of forest versus old-field habitats to emigrating juvenile amphibians. Conserv. Biol. 2002;16:1324–1332. doi: 10.1046/j.1523-1739.2002.01085.x. [DOI] [Google Scholar]
- 76.Todd BD, Blomquist SM, Harper EB, Osbourn MS. Effects of timber harvesting on terrestrial survival of pond-breeding amphibians. For. Ecol. Manag. 2014;313:123–131. doi: 10.1016/j.foreco.2013.11.011. [DOI] [Google Scholar]
- 77.Todd BD, Luhring TM, Rothermel BB, Gibbons JW. Effects of forest removal on amphibian migrations: implications for habitat and landscape connectivity. J. Appl. Ecol. 2009;46:554–561. doi: 10.1111/j.1365-2664.2009.01645.x. [DOI] [Google Scholar]
- 78.Jehle R. The terrestrial summer habitat of radio-tracked great crested newts (Triturus cristatus) and marbled newts (T-marmoratus) Herpetological J. 2000;10:137–142. [Google Scholar]
- 79.Matos C, Petrovan SO, Wheeler PM, Ward AI. Landscape connectivity and spatial prioritization in an urbanising world: A network analysis approach for a threatened amphibian. Biol. Conserv. 2019;237:238–247. doi: 10.1016/j.biocon.2019.06.035. [DOI] [Google Scholar]
- 80.Clobert J, Le Galliard J-F, Cote J, Meylan S, Massot M. Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol. Lett. 2009;12:197–209. doi: 10.1111/j.1461-0248.2008.01267.x. [DOI] [PubMed] [Google Scholar]
- 81.Avon C, Bergès L. Prioritization of habitat patches for landscape connectivity conservation differs between least-cost and resistance distances. Landsc. Ecol. 2016;31:1551–1565. doi: 10.1007/s10980-015-0336-8. [DOI] [Google Scholar]
- 82.Stanton JC, Pearson RG, Horning N, Ersts P, Akcakaya HR. Combining static and dynamic variables in species distribution models under climate change. Methods Ecol. Evolution. 2012;3:349–357. doi: 10.1111/j.2041-210X.2011.00157.x. [DOI] [Google Scholar]
- 83.Newbold T. Future effects of climate and land-use change on terrestrial vertebrate community diversity under different scenarios. Proc. R. Soc. B-Biological Sci. 2018;285:20180792. doi: 10.1098/rspb.2018.0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oliver TH, Morecroft MD. Interactions between climate change and land use change on biodiversity: attribution problems, risks, and opportunities. Wiley Interdiscip. Reviews: Clim. Change. 2014;5:317–335. [Google Scholar]
- 85.Unglaub B, Steinfartz S, Kuhne D, Haas A, Schmidt BR. The relationships between habitat suitability, population size and body condition in a pond-breeding amphibian. Basic. Appl. Ecol. 2018;27:20–29. doi: 10.1016/j.baae.2018.01.002. [DOI] [Google Scholar]
- 86.Shoo LP, et al. Engineering a future for amphibians under climate change. J. Appl. Ecol. 2011;48:487–492. doi: 10.1111/j.1365-2664.2010.01942.x. [DOI] [Google Scholar]
- 87.Kujala H, Moilanen A, Araujo MB, Cabeza M. Conservation Planning with Uncertain Climate Change Projections. PLoS One. 2013;8:12. doi: 10.1371/journal.pone.0053315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author and under agreement with the naturalist associations that were partners of the study, on reasonable request.