Abstract
This literature review examines infectious wildlife disease research in Austria. We analyzed 226 research papers, published between 1980 and 2017. We determined that wildlife disease papers increased significantly from 0.8 ± 0.8 publications per year in the first decade (1980–1989) when compared to 2008–2017 with an average of 12.9 ± 4.1 publications per year. We illustrate information about the most investigated diseases and highlight the lack of research into certain wildlife pathogens. A special emphasis was given to diseases with zoonotic potential. The review showed that research focused on a few select species like the red fox (Vulpes vulpes), red deer (Cervus elaphus), and wild boar (Sus scrofa), all game species. Moreover, diseases affecting livestock and human health were seen more often. The review also found that only a low number of publications actually stated disease prevalence and confidence interval data. The reported diseases identified were classified according to their notifiable status and the distribution at the wildlife–human and wildlife–livestock interface. Furthermore, we try to argue why research into some diseases is prioritized, and why other diseases are underrepresented in current Austrian research. While spatiotemporal indicators could not be assessed due to the variability in methodologies and objectives of various studies, the information provided by this review offers the first comprehensive evaluation of the status of infectious wildlife disease research in Austria. Therefore, this study could assist investigators to identify further areas of priorities for research and conservation efforts and for wildlife management professionals to inform policy and funding strategies. With this review, we want to encourage research in the field of wildlife diseases in Austria to enhance current knowledge in the prevention of further loss in biodiversity and to find new measures to promote “One Health” on a global scale.
Keywords: wildlife, infectious, diseases, zoonosis, Austria, game, one health, EID
Introduction
We determined the status of infectious wildlife disease research in Austria based on a 37-year literature review between 1980 and 2017. The literature review was modeled on a similar study from the Republic of Korea in 2017 (1). One reason for conducting this review was to determine if an increase in so-called emerging infectious diseases (EIDs) had been noted in Austrian wildlife. It has previously been determined that that ~70% of EIDs have their origin in wildlife, and an estimated 60–80% of all EIDs also have zoonotic potential (2, 3). Increases in EIDs are related to the human impacts on landscapes (3, 4). In our constantly changing world, ecosystems continuously transform and offer pathogens novel opportunities to evolve, resulting in a greater diversity of adaptable and rapidly spreading infectious agents (5). Pathogens that can infect several species of hosts, so-called multihost pathogens, can also represent cause for concern with regard to wildlife conservation (6). Wildlife potentially contributes to the spread and maintenance of infectious diseases, and knowing the status supports addressing and mitigating impacts.
Austria has a population of ~8.8 million people (2017) with an average population density of 105 people/km2. The gross domestic product per capita is 37,100 euros (2016), which makes Austria one of the wealthiest countries in the European union (EU). A member of the European Union (EU) since 1995, the federal state of Austria is located in Central Europe and divided into nine provinces. Austria is a landlocked country with a land area of 83,879 km2 and a diversity of landscapes. About two-thirds of the land mass are mountainous, encompassing the Alps, the Carpathians, as well as the gneiss and granite highlands of the Bohemian Massif. The remaining third consists of the Vienna Basin and the Pannonian border regions of the Hungarian lowlands (7–9). The Austrian Fauna consists of roughly 45,870 different species, of which 626 are vertebrates. One hundred and ten vertebrates belong to the mammalian class, 418 to the avian class, 16 to the class of reptiles, 21 are amphibian species, and 60 species are fish (10).
Both active and passive surveillance programs for wildlife diseases exist in Austria (11, 12). Control programs were successful in eradicating rabies using widespread oral immunization of red fox (Vulpes vulpes). The last known rabies case occurred in 2003, and in 2008, Austria was declared rabies free. As of 2013, indicator animals (e.g., fox, badger, racoon dog, and raccoon) opportunistically were found dead, and all animals suspected of being rabid are examined at the National Reference Laboratory. Additional epizootic monitoring programs for Aujesky disease, bovine spongiform encephalopathy (BSE), scrapie, bluetongue virus, Brucella melitensis, bovine brucellosis, enzootic bovine leucosis, infectious bovine rhinotracheitis and infectious pustular vulvovaginitis, classical swine fever, and avian influenza are implemented. Only one zoonotic disease in wildlife is routinely monitored, namely, trichinosis in wild boars (Sus scrofa). In addition, in livestock animals, campylobacteriosis, echinococcosis, salmonellosis, trichinosis, and verotoxin-producing Escherichia coli are routinely monitored (13).
The wildlife-human interface in Austria consists of a number of critical points, such as game meat consumption, the hunting industry (e.g., direct contact between hunters, hunting dogs, and game animals) and tourism (e.g., hikers and recreational athletes). Occupations that involve a constant exposure to animals are related to a higher risk of infection, and this risk may be intensified through contact with livestock sharing pastures with wildlife. Risk groups include farmers, veterinarians, hunters, forestry workers, veterinary technicians, animal keepers, taxidermists, zookeepers, and people who prepare or consume game meat (14–18).
Wildlife diseases are often not included in epidemiological reflections, as the main focus remains on diseases in livestock and transmission to humans. It is important to note that the wildlife-livestock interface is bidirectional and dynamic with frequent pathogen exchange through shared resources, such as habitats, water, and feed (19, 20). These factors increase the risk of transmitting infectious diseases from wildlife to domestic animals and subsequently to humans (19). Our review serves not only as an overview but also as a reminder that research on wildlife diseases is important and must be encouraged. This review is the first and only comprehensive review of infectious wildlife diseases in Austria.
Materials and Methods
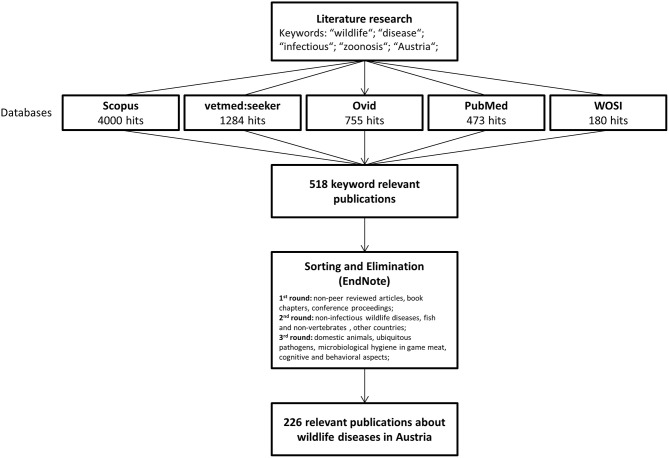
A detailed literature search was conducted between October 2018 and December 2018 using the following literature data bases: “vetmedseeker” (vetmedseeker, University of Veterinary Medicine Vienna, Austria), “Scopus” (Scopus, Elsevier, Netherlands), “Ovid” (“Ovid Technologies,” Wolters Kluwer, Netherlands), “Web of Science” (Web of Science Core Collection, Clarivate Analytics, United States), and “PubMed” (NCBI Pubmed, National Center for Biotechnology Information, United States). Search terms used included “wildlife,” “diseases,” “infectious,” and “zoonosis” adjusted by setting the time frame to 1980–2017 and the affiliation to Austria. This resulted in 2,696 hits on “Scopus,” 1,284 hits on the “vetmedseeker,” 755 on “Ovid,” 473 on “PubMed,” and 180 on “Web of Science.” Only articles published in peer-reviewed journals were retained. Papers that addressed wildlife-human or wildlife-domestic disease transmission were also included. Additional articles found manually from the respective citations were included as well. A schematic step-by-step of the literature search can be found in Figure 1.
Figure 1.
Overview of the work steps for drafting this review.
Following abstract review, 518 publications from “Scopus” appeared relevant. Papers were then compiled using a reference managing software (End Note Version X7.8, Thomson Reuters, United States). Duplicates, non-peer reviewed articles, book chapters, and conference proceedings were excluded in a first round. In a second round, we focused on infectious pathogens and diseases occurring in wild species. We excluded papers describing non-infectious, autoimmune, or idiopathic diseases. In addition, we restricted the search to vertebrates. Multicountry studies were only included in our review if “Austria” was mentioned in the materials and methods. In a third round, we excluded publications that addressed microbiological hygiene aspects in game meat. Subsequent to this process, it was clear that “Scopus” was the most efficient database for wildlife diseases, revealing 219 out of a total of 226 relevant publications. “PubMed,” in contrast, only showed 76 relevant articles. Finally, from all databases surveyed, 226 papers were retained and entered into a Microsoft Excel file for descriptive statistical analysis.
Publications were classified according to definitions by “Springer Nature” concerning article type (21) into original research articles, reviews, case reports, and short reports or letters. Original research articles were further subdivided into controlled studies and retrospective studies. Subsequently papers were categorized according to year of publication, type of pathogen, affected animal species, and group interface. The classification of group interfaces describes the primary group of interest (e.g., wildlife, humans, or livestock animals). Furthermore, we recorded whether the disease was notifiable according to the OIE List of notifiable diseases or the current Austrian legislation. If the paper covered various infectious agents, it was assigned to the categories “Notifiable in Austria” or “OIE-listed disease,” if at least one notifiable disease was included. In addition, it was noted if the prevalence and confidence intervals of the pathogen(s) were stated in the article.
Infectious agents were divided into parasitic, viral, bacterial, and fungal pathogens, and articles covering more than one pathogen or addressing more than one species were classified as “multiple infectious agents” or “multiple species affected.” Furthermore, agents were classified as zoonotic or having zoonotic potential. To determine the zoonotic potential of the diseases, the OIE list for infectious diseases, the “Merck Veterinary Manual” (22), as well as various textbooks (23–26) were consulted. Diseases that are not yet known to have zoonotic potential or are currently being researched have been labeled as “unknown.”
Results
Publication Frequency
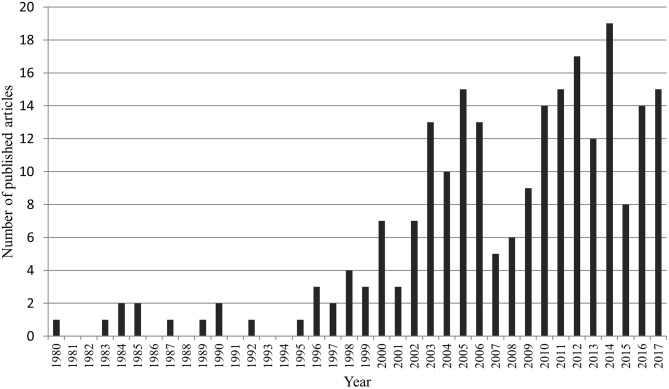
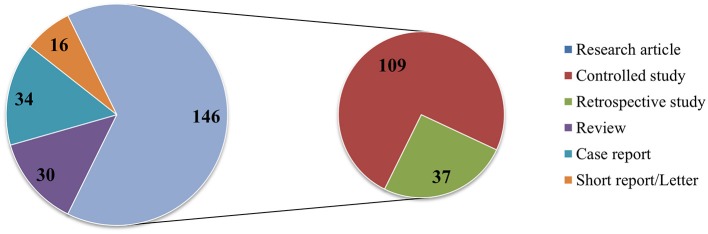
After review, we retained 226 publications, published between 1980 and 2017 (Figure 2). With the exception of the years 1981–1982, 1986, 1988, 1991, and 1993–1994, numerous wildlife disease papers were published. From 1995 onwards, there was a continuous increase in wildlife disease publications, with the most articles (n = 19) published in 2014. In 2007 and 2015, a drop in publications was noted, followed by an increase in the subsequent year. During the 37 years reviewed, 2000–2017 was most productive period with 89.4% of the retained articles published during this time period. Comparing the average yearly number of publications in the first decade (1980–1989 = 0.8 ± 0.8) to the last decade (2008–2017 = 12.9 ± 4.1) clearly shows a significant increase in interest in wildlife diseases (normality of data was tested by Shapiro-Wilk; two-tailed t-test: p = 0.01, t-statistics = −9.1178). Of the 226 papers, 146 publications were empirical studies, more specifically, 109 controlled studies, and 37 retrospective analyses. The rest was composed of 30 review articles, 34 case reports, and 16 letters or short reports (Figure 3).
Figure 2.
Frequency of publications addressing wildlife diseases in Austria between 1980 and 2017.
Figure 3.
Numerical amount of the different types of scientific publications according to the Springer classification.
Infectious Agents
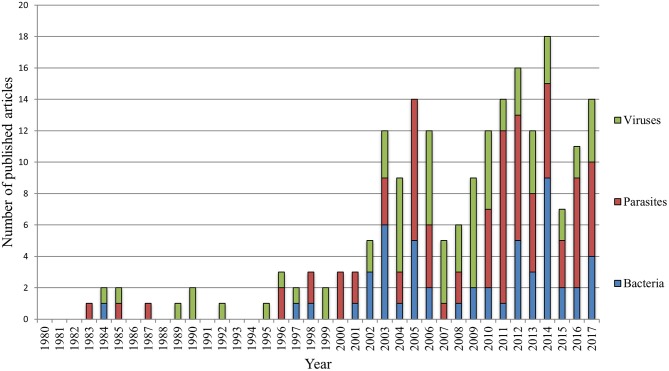
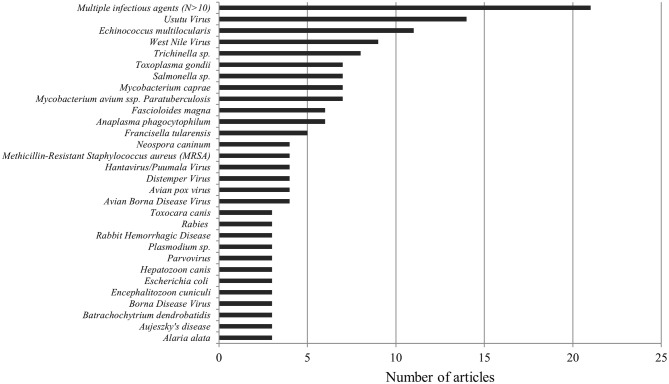
The largest proportion of publications, namely 84 (37%), addressed infectious diseases caused by parasites (endoparasites: protozoa and helminths, or ectoparasites: arachnids and insects). A total of 67 (30%) of publications discussed viral diseases and 53 (23%) bacterial diseases, 9 (4%) dealt with diseases of fungal origin, and 13 (6%) papers mentioned multiple infectious agents (27–41). We found no studies concerning diseases caused by prions (see Figure 4). In summary, we found 136 pathogens discussed in the 226 publications. For the complete list of all diseases and pathogens found in this study, see Table 1. Of these 136 infectious agents, 84 (62%) were only featured once, 23 (17%) were mentioned twice, and 12 (9%) were discussed three times. The remaining pathogens, namely 17 (12%), were featured more than three times. No clear trend was determined as to changes in which pathogenic agents were reported over the years (see Figure 5). Beyond the publications that discussed multiple pathogens (N > 10), Usutu virus was the most studied agent in Austria, with 14 published papers. It was followed by Echinococcus multilocularis (n = 11), West Nile Virus (n = 9), Trichinella sp. (n = 8), Toxoplasma gondii, Salmonella sp., Mycobacterium caprae, and Mycobacterium avium subsp. paratuberculosis with seven publications each. Also noteworthy are Fascioloides magna and Anaplasma phagocytophilum with six publications each, as well as Francisella tularensis, which was mentioned in five papers (see Figure 6).
Figure 4.
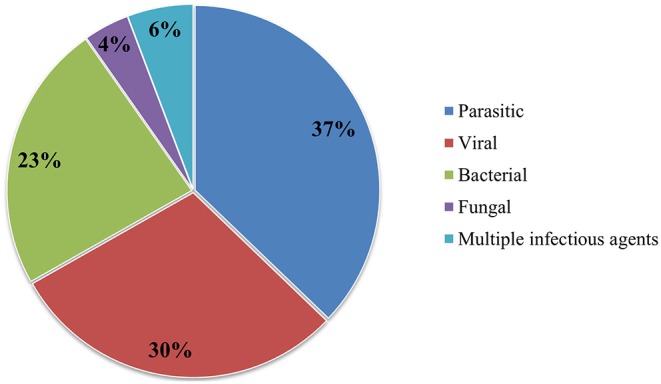
Percentage of the infectious agents mentioned in all publications.
Table 1.
Infectious pathogens in alphabetical order, listing their zoonotic potential, notifiable status, animals affected, and authors, as found through our study.
| Disease or pathogen | Disease agent | Notifiable in Austria | OIE notifiable disease | Zoonotic potential | Affected species | References |
|---|---|---|---|---|---|---|
| Agamid adenovirus 1 | Viral | No | No | No | Bearded dragon (Pogona vitticeps) | (42) |
| Alaria alata | Parasitic | No | No | Yes | Red fox (Vulpes vulpes), Wild boar (Sus scrofa) | (43–45) |
| Anaplasma phagocytophilum | Parasitic | No | No | Yes | Chamois (Rupicapra rupicapra), Ibex (Capra ibex), Mouflon (Ovis gmelini musimon), Red deer (Cervus elaphus), Roe deer (Capreolus capreolus), Timber wolf (Canis Lupus Occidentalis) | (46–51) |
| Ankylostoma spp. | Parasitic | No | No | Yes | Cheetah (Acinonyx jubatus) | (52) |
| Arboviruses | Viral | No | No | Yes | Multiple species* | (53) |
| Ascaris suum | Parasitic | No | No | Yes | Racoon (Procyon lotor), Red fox (Vulpes vulpes), Wild boar (Sus scrofa) | (54) |
| Ascaridia spp. | Parasitic | No | No | NA | Northern bald ibis (Geronticus eremita) | (55) |
| Aspergillus fumigates | Fungal | No | No | Yes | Falcon (Falco) | (56) |
| Aspergillus spp. | Fungal | No | No | Yes | Cheetah (Acinonyx jubatus) | (52) |
| Aujeszky's diseasea | Viral | Yes | Yes | No | Dog (Canis lupus familiaris), Wild boar (Sus scrofa) | (57–59) |
| Avian Borna disease virus | Viral | No | No | No | Canary bird (Serinus canaria) and multiple psittacine species* | (60–63) |
| Avian hepatitis E virus | Viral | No | No | Yes | Common buzzard (Buteo buteo), Feral pigeon (Columba livia domestica), Little owl (Athene noctua), Song thrush (Turdus philomelos) | (64) |
| Avian influenza virusa, b | Viral | Yes | Yes | Yes | Coot (Fulica atra), Duck (Anatidae), Egret (Ardeidae), Goose (Anatidae), Grebe (Podicipedidae), Swan (Cygnus), and other wild bird species | (65, 66) |
| Avian pox virus | Viral | No | No | No | Bald eagle (Haliaeetus leucocephalus), Great Bustard (Otis tarda), Great tit (Parus major), and multiple bird species* | (67–70) |
| Babesia canis | Parasitic | No | No | No | Eurasian golden jackal (Canis aureus) | (71) |
| Babesia capreoli | Parasitic | No | No | Unknown | Chamois (Rupicapra rupicapra), Ibex (Capra ibex), Mouflon (Ovis gmelini musimon), Red deer (Cervus elaphus), Roe deer (Capreolus capreolus) | (48) |
| Babesia divergens | Parasitic | No | Yes | Yes | Chamois (Rupicapra rupicapra), Ibex (Capra ibex), Mouflon (Ovis gmelini musimon), Red deer (Cervus elaphus), Roe deer (Capreolus capreolus) | (48, 51) |
| Babesia microti | Parasitic | No | No | Yes | Red fox (Vulpes vulpes) and other wild carnivores | (72, 73) |
| Babesia vesperuginis | Parasitic | No | No | Unknown | Bat (Chiroptera) | (74) |
| Babesia spp. | Parasitic | No | No | NA | Chamois (Rupicapra rupicapra), Fallow deer (Dama dama), Mouflon (Ovis gmelini musimon); Père David's deer (Elaphurus davidianus), Red deer (Cervus elaphus), Roe deer (Capreolus capreolus), Sika deer (Cervus nippon) | (75) |
| Bartonella spp. | Bacterial | No | No | Yes | Bank vole (Clethrionomys glareolus), Common vole (Microtus arvalis), Wood mouse (Apodemus sylvaticus), Yellow-necked field mouse (Apodemus flavicollis) | (76) |
| Batrachochytrium dendrobatidis | Fungal | No | Yes | Unknown | Alpine newt (Ichthyosaura alpestris), Alpine salamander (Salamandra atra), Crested newt (Triturus cristatus, T. carnifex, T. dobrogicus), Fire and yellow bellied toad (Bombina bombina, B. variegata), Poison dart frogs (Dendrobatidae), Smooth newt (Lissotriton vulgaris), Water frogs (Pelophylax) | (77–79) |
| Baylisascaris procyonis | Parasitic | No | No | Yes | Racoon (Procyon lotor), Red fox (Vulpes vulpes), Wild boar (Sus scrofa) | (54) |
| Baylisascaris spp. | Parasitic | No | No | Yes | Skunk (Mephitis mephitis) | (80) |
| Borna Disease Virusc | Viral | Yes | No | Yes | Bicolored shrew (Crocidura leucodon), Cheetah (Acinonyx jubatus), Domestic horse (Equus ferus caballus), and multiple other species* | (81–83) |
| Borrelia spp. | Bacterial | No | No | Yes | Bank vole (Clethrionomys glareolus), Common vole (Microtus arvalis), Wood mouse (Apodemus sylvaticus), Yellow-necked field mouse (Apodemus flavicollis) | (76) |
| Bot fly larvae “Cephenemyia auribarbis” | Parasitic | No | No | Unknown | Red deer (Cervus elaphus) | (84) |
| Bovine viral diarrhea virusd | Viral | Yes | Yes | No | Chamois (Rupicapra rupicapra), Fallow deer (Dama dama), Red deer (Cervus elaphus), Roe deer (Capreolus capreolus) | (85, 86) |
| Brucella microti | Bacterial | No | No | Unknown | Red fox (Vulpes vulpes) | (87, 88) |
| Brucella suisa | Bacterial | Yes | Yes | Yes | Red fox (Vulpes vulpes) | (89) |
| Brucella vulpis | Bacterial | No | No | Unknown | Red fox (Vulpes vulpes) | (90) |
| Brucella spp.b | Bacterial | Yes | No | NA | European brown hare (Lepus europaeus), Red fox (Vulpes vulpes) | (91, 92) |
| Campylobacter spp.b | Bacterial | Yes | No | Yes | Multiple wild bird species* | (93, 94) |
| Candidatus neoehrlichia spp. | Bacterial | No | No | Yes | Red fox (Vulpes vulpes) | (95) |
| Capillaria spp. | Parasitic | No | No | NA | Northern bald ibis (Geronticus eremita) | (55) |
| Capillaria boehmi | Parasitic | No | No | Unknown | Red fox (Vulpes vulpes) | (96) |
| Capillaria hepatica/Calodium hepaticum | Parasitic | No | No | Yes | Multiple species* | (97–99) |
| Chelonid Herpesvirus (ChHV) | Viral | No | No | No | Leopard tortoise (Stigmochelys pardalis) | (100) |
| Chlamydiaceae spp. | Bacterial | No | No | NA | Multiple wild rodent species* | (101) |
| Chrysosporium guarroi | Fungal | No | No | Unknown | Bearded dragon (Pogona vitticeps) | (102) |
| Clostridium botulinumb, Avian botulism | Bacterial | Yes | No | No | Garganey (Anas querquedula), Mallard (Anas platyrhynchos), Northern lapwing (Vanellus vanellus) and multiple other wild bird species* | (103, 104) |
| Clostridium perfringens | Bacterial | No | No | Yes | Blackbuck (Antilope cervicapra), Collared peccary (Pecari tajacu), Domestic goat (Capra aegagrus hircus), Domestic sheep (Ovis aries), Domestic swine (Sus scrofa domesticus), European reindeer (Rangifer tarandus), Japanese serow (Capricornis crispus), Lechwe (Kobus leche), Waterbuck (Kobus ellipsiprymnus) | (105) |
| Clostridium septicum | Bacterial | No | No | Yes | Chamois (Rupicapra rupicapra) | (106) |
| Coccidia | Parasitic | No | No | NA | Common hill myna (Gracula religiosa) | (107) |
| Collyriclum faba | Parasitic | No | No | Unknown | Starling (Sturnus vulgaris) | (108) |
| Coxiella burnetii | Bacterial | No | No | Yes | Bank vole (Clethrionomys glareolus), Common vole (Microtus arvalis), Wood mouse (Apodemus sylvaticus), Yellow-necked field mouse (Apodemus flavicollis) | (76) |
| Crane hepatitis herpesviruses | Viral | No | No | Unknown | Gray crowned crane (Balearica regulorum) | (109) |
| Cryptococcus spp. | Fungal | No | No | Yes | Feral pigeon (Columba livia domestica) | (110) |
| Cryptosporidium spp. | Parasitic | No | No | Yes | Corn snake (Pantherophis guttatus), Leopard gecko (Eublepharis macularius), and multiple reptile and snake species* | (111, 112) |
| Cryptosporidium suis | Parasitic | No | No | Yes | Wild boar (Sus scrofa) | (113) |
| Cryptosporidium scrofarum | Parasitic | No | No | Yes | Wild boar (Sus scrofa) | (113) |
| Demodex spp. | Parasitic | No | No | No | Cheetah (Acinonyx jubatus) | (52) |
| Dermatophilus congolensis | Bacterial | No | No | Yes | Orangutan (Pongo pygmaeus pygmaeus) | (114) |
| Dermatophytosis (Trichopphyton, Microsporum) | Fungal | No | No | Yes | Small mammals | (115) |
| Devriesea agamarum | Bacterial | No | No | Unknown | Bearded dragon (Pogona vitticeps) | (102) |
| Dirofilaria repens | Parasitic | No | No | Yes | Dog (Canis lupus familiaris), Red fox (Vulpes vulpes) | (116) |
| Dirofilaria immitis | Parasitic | No | No | Yes | Dog (Canis lupus familiaris), Red fox (Vulpes vulpes) | (116) |
| Distemper virus | Viral | No | No | No | Badger (Meles meles), Beech marten (Martes foina), Cheetah (Acinonyx jubatus), Dog (Canis lupus familiaris) | (82, 117–119) |
| Echinococcus multilocularisb | Parasitic | Yes | Yes | Yes | Beaver (Castor fiber), Dog (Canis lupus familiaris), Domestic cat (Felis silvestris catus), Red fox (Vulpes vulpes), and multiple mammalian or rodent species* | (54, 99, 120–128) |
| Eimeria leporis | Parasitic | No | No | No | European brown hare (Lepus europaeus) | (123) |
| Encephalitozoon cuniculi | Parasitic | No | No | Yes | Bearded dragon (Pogona vitticeps), Common vole (Microtus arvalis), European brown hare (Lepus europaeus), European water vole (Arvicola terrestris) | (129–131) |
| Encephalitozoon spp. | Parasitic | No | No | Yes | Multiple species* | (132) |
| Encephalomyocarditis virus | Viral | No | No | Yes | Domestic cat (Felis silvestris catus) and wild rodents | (133) |
| Entamoeba invadens | Parasitic | No | No | No | Boa constrictor (Boa constrictor) | (134) |
| Entamoeba spp. | Parasitic | No | No | NA | Multiple snake species* | (112) |
| ESBL/AmpC producing Enterobacteriaceae | Bacterial | No | No | Yes | Rook (Corvus frugilegus) | (135) |
| Escherichia coli | Bacterial | No | No | Yes | Mouflon (Ovis gmelini musimon) and multiple wild bird species* | (93, 94, 136) |
| European brown hare (Lepus europaeus) syndrome | Viral | No | No | No | European brown hare (Lepus europaeus), Rabbit (Oryctolagus cuniculus) | (137, 138) |
| Fascioloides magna | Parasitic | No | No | No | Fallow deer (Dama dama), Red deer (Cervus elaphus), Roe deer (Capreolus capreolus), and otherwild ungulates and ruminants | (139–144) |
| Feline Herpes Virus 1 | Viral | No | No | No | Cheetah (Acinonyx jubatus) | (82, 145) |
| Flavivirus, mosquito-borne | Viral | No | No | Yes | Multiple species* | (146) |
| Francisella tularensisb | Bacterial | Yes | Yes | Yes | Dog (Canis lupus familiaris), European brown hare (Lepus europaeus), Red fox (Vulpes vulpes) | (18, 89, 91, 147, 148) |
| Fur mites (Lynxacarus mustelae) | Parasitic | No | No | Unknown | Beech marten (Martes foina) | (149) |
| Geopetitia aspiculata | Parasitic | No | No | Unknown | Bearded barbet (Lybius dubius), Black-faced dacnis (Dacnis lineata), Brown-breasted barbet (Lybius melanopterus), Sociable weaver (Philetairus socius), Steere's liocichla (Liocichla steerii) | (150) |
| Hantavirus/Puumala Virusb | Viral | Yes | No | Yes | Bank vole (Clethrionomys glareolus), Common vole (Microtus arvalis), Domestic cat (Felis silvestris catus), House mouse (Mus musculus), Wood mouse (Apodemus sylvaticus), Yellow-necked field mouse (Apodemus flavicollis), and other wild rodents | (76, 133, 151, 152) |
| Hepatozoon canis | Parasitic | No | No | No | Eurasian golden jackal (Canis aureus), Red fox (Vulpes vulpes) | (71, 72, 153) |
| Herpesvirus | Viral | No | No | NA | Tortoises (Testudinidae) | (154) |
| Histoplasma capsulatum var. Capsulatum | Fungal | No | No | Yes | Badger (Meles meles) | (155) |
| Iridovirus (Genus Ranavirus) | Viral | No | No | Unknown | Leopard tortoise (Stigmochelys pardalis) | (100) |
| Klebsiella pneumoniae | Bacterial | No | No | Yes | Mouflon (Ovis gmelini musimon) | (136) |
| Koala retrovirus (KoRV) | Viral | No | No | Unknown | Koala (Phascolarctos cinereus) | (156) |
| Leishmania infantum | Parasitic | No | Yes | Yes | Eurasian golden jackal (Canis aureus) | (71) |
| Leptospira interrogansb | Bacterial | Yes | No | Yes | European brown hare (Lepus europaeus), Wild boar (Sus scrofa) | (91, 157) |
| Leptospira spp. | Bacterial | No | No | Yes | Bank vole (Clethrionomys glareolus), Common vole (Microtus arvalis), Wood mouse (Apodemus sylvaticus), Yellow-necked field mouse (Apodemus flavicollis) | (76) |
| Listeria monocytogenesb | Bacterial | Yes | No | Yes | Water fowl (Anatidae), Red deer (Cervus elaphus), Roe deer (Capreolus capreolus), Chamois (Rupicapra rupicapra), Wild boar (Sus scrofa), Mouflon (Ovis gmelini musimon), and other wild and domestic ruminants | (158, 159) |
| Listeria spp. | Bacterial | No | No | NA | Water fowl (Anatidae) and wild and domestic ruminants | (159) |
| Lymphocytic choriomeningitis virus | Viral | No | No | Yes | Bank vole (Clethrionomys glareolus), Common vole (Microtus arvalis), Wood mouse (Apodemus sylvaticus), Yellow-necked field mouse (Apodemus flavicollis) | (76) |
| Malignant catarrhal fever virus | Viral | No | No | No | Chamois (Rupicapra rupicapra), Fallow deer (Dama dama), Red deer (Cervus elaphus), Roe deer (Capreolus capreolus) | (160) |
| Methicillin-resistant Staphylococcus aureus (MRSA) | Bacterial | No | No | Yes | Eurasian Lynx (Lynx lynx), European brown hare (Lepus europaeus), Hedgehog (Erinaceus europaeus), Otter (Lutra lutra), Rook (Corvus frugilegus) | (93, 135, 161, 162) |
| Monocercomonas spp. | Parasitic | No | No | Unknown | Multiple snake species* | (112) |
| Mycobacterium avium ssp. Paratuberculosise | Bacterial | Yes | Yes | Yes | Capercaillie (Tetrao urogallus), Cattle (Bos taurus), Chamois (Rupicapra rupicapra), European brown hare (Lepus europaeus), Fallow deer (Dama dama), Ibex (Capra ibex), Mouflon (Ovis gmelini musimon), Mountain hare (Lepus timidus), Red deer (Cervus elaphus), Roe deer (Capreolus capreolus), Red fox (Vulpes vulpes), Yellow-necked field mouse (Apodemus flavicollis) | (163–169) |
| Mycobacterium avium subsp. silvaticum | Bacterial | No | Yes | Yes | Ural owl (Strix uralensis) | (170) |
| Mycobacterium bovisa, b | Bacterial | Yes | Yes | Yes | Badger (Meles meles), Red deer (Cervus elaphus), Wild boar (Sus scrofa) | (171) |
| Mycobacterium capraeb | Bacterial | Yes | Yes | Yes | Camel (Camelus), Cattle (Bos taurus), Domestic goat (Capra aegagrus hircus), Red deer (Cervus elaphus), Wild boar (Sus scrofa) | (16, 172–177) |
| Mycoplasma conjunctivae | Bacterial | No | No | Yes | Chamois (Rupicapra rupicapra), Domestic goat (Capra aegagrus hircus), Domestic sheep (Ovis aries), Domestic swine (Sus scrofa domesticus), Ibex (Capra ibex) | (178, 179) |
| Myxozoa | Parasitic | No | No | NA | Mole (Talpa europaea) | (180) |
| Neospora caninum | Parasitic | No | No | No | Common vole (Microtus arvalis), Dog (Canis lupus familiaris), European brown hare (Lepus europaeus), European water vole (Arvicola terrestris), Red fox (Vulpes vulpes), Parma wallaby (Macropus parma) | (129, 181–183) |
| Orthopoxvirus | Viral | No | No | Yes | Domestic cat (Felis silvestris catus) and wild rodents | (133) |
| Parapoxvirus | Viral | No | No | Yes | Domestic cat (Felis silvestris catus) and wild rodents | (133) |
| Parvovirus | Viral | No | No | No | Badger (Meles meles), Cheetah (Acinonyx jubatus) | (52, 82, 118) |
| Pasteurella spp. | Bacterial | No | No | NA | Chamois (Rupicapra rupicapra) | (184) |
| Pharyngomyia picta | Parasitic | No | No | Unknown | Red deer (Cervus elaphus) | (84) |
| Plasmodium spp.b | Parasitic | Yes | No | No | Humboldt penguins (Spheniscus humboldti), King penguins (Aptenodytes patagonicus), Puffins (Fratercula arctica), Rockhopper penguins (Eudyptes chrysocome), and multiple wild bird species* | (185–187) |
| Psittacine beak and feather disease virus | Viral | No | No | Unknown | Budgerigar (Melopsittacus undulatus) | (188) |
| Rabiesa, b | Viral | Yes | Yes | Yes | Red fox (Vulpes vulpes) | (189–191) |
| Rabbit hemorrhagic disease | Viral | No | Yes | No | European brown hare (Lepus europaeus), Rabbit (Oryctolagus cuniculus) | (137, 138, 192) |
| Rat hepatitis E virus | Viral | No | No | Yes | Black rat (Rattus rattus), Norway rat (Rattus norvegicus) | (193) |
| Rickettsia spp. | Bacterial | No | No | Yes | Bank vole (Clethrionomys glareolus), Common vole (Microtus arvalis), Wood mouse (Apodemus sylvaticus), Yellow-necked field mouse (Apodemus flavicollis) | (76) |
| Roe deer (Capreolus capreolus) papillomavirus | Viral | No | No | No | Red deer (Cervus elaphus) | (194) |
| Salmonella entericab | Bacterial | Yes | No | Yes | Red fox (Vulpes vulpes) and multiple reptile species* | (195, 196) |
| Salmonella spp.b | Bacterial | Yes | No | NA | Chamois (Rupicapra rupicapra), Lizards (Squamata), Mouflon (Ovis gmelini musimon), Red deer (Cervus elaphus), Roe deer (Capreolus capreolus), Snakes (Serpentes), Tortoises (Testudinidae), Turtles (Chelonii), Wild boar (Sus scrofa), and multiple wild bird, amphibian, reptile and other species* | (93, 94, 158, 197–200) |
| Scabies/Sarcoptic mangea | Parasitic | Yes | No | Yes | Chamois (Rupicapra rupicapra), Ibex (Capra ibex) | (201, 202) |
| Staphylococcus aureus | Bacterial | No | No | Yes | Multiple species* | (203) |
| Syngamus trachea | Parasitic | No | No | Yes | Northern bald ibis (Geronticus eremita) | (55) |
| Taenia crassiceps | Parasitic | No | No | Yes | Common vole (Microtus arvalis), European water vole (Arvicola terrestris) | (99, 204) |
| Taenia taeniaeformis | Parasitic | No | No | Yes | Common vole (Microtus arvalis), European water vole (Arvicola terrestris) | (99, 204) |
| Taenia spp. | Parasitic | No | No | Yes | Cheetah (Acinonyx jubatus) | (52) |
| Theileria spp. | Parasitic | No | Yes | No | Chamois (Rupicapra rupicapra), Fallow deer (Dama dama), Mouflon (Ovis gmelini musimon), Père David's deer (Elaphurus davidianus), Red deer (Cervus elaphus), Roe deer (Capreolus capreolus), Sika deer (Cervus nippon) | (75, 205) |
| Tickborne encephalitis virusb (TBEV) | Viral | Yes | No | Yes | Mouflon (Ovis gmelini musimon), Roe deer (Capreolus capreolus) | (206, 207) |
| Toxascaris leonina | Parasitic | No | No | No | Cheetah (Acinonyx jubatus) | (52) |
| Toxocara canis | No | No | Yes | Dog (Canis lupus familiaris), Domestic cat (Felis silvestris catus), Racoon (Procyon lotor), Red fox (Vulpes vulpes), Wild boar (Sus scrofa) |
(54, 208, 209) | |
| Toxocara cati | Parasitic | No | No | Yes | Dog (Canis lupus familiaris), Domestic cat (Felis silvestris catus), Red fox (Vulpes vulpes) | (208, 209) |
| Toxocara mystax | Parasitic | No | No | Yes | Cheetah (Acinonyx jubatus) | (52) |
| Toxoplasma gondii | Parasitic | No | No | Yes | Bank vole (Clethrionomys glareolus), Common vole (Microtus arvalis), European water vole (Arvicola terrestris), Pallas cat (Otocolobus manul), Racoon (Procyon lotor), Red fox (Vulpes vulpes), Wild boar (Sus scrofa), Wood mouse (Apodemus sylvaticus), Yellow-necked field mouse (Apodemus flavicollis) and multiple other species* |
(54, 76, 99, 181, 182, 210, 211) |
| Treponema spp. | Bacterial | No | No | Unknown | European brown hare (Lepus europaeus) | (212) |
| Trichinella britovi | Parasitic | No | Yes | Yes | Red fox (Vulpes vulpes) | (213) |
| Trichinella pseudospiralis | Parasitic | No | Yes | Yes | Wild boar (Sus scrofa) | (214) |
| Trichinella spp. | Parasitic | No | Yes | Yes | Domestic swine (Sus scrofa domesticus), Racoon (Procyon lotor), Red fox (Vulpes vulpes), Wild boar (Sus scrofa) |
(54, 215–221) |
| Trichomonas gallinae | Parasitic | No | No | No | Bearded vulture (Gypaetus barbatus), Budgerigar (Melopsittacus undulatus), Canary bird (Serinus canaria), Eurasian collared dove (Streptopelia decaocto), European greenfinch (Chloris chloris), Feral pigeon (Columba livia domestica), Hawfinch (Coccothraustes coccothraustes), Racing pigeons (Columba livia domestica), Yellowhammer (Emberiza citrinella) | (222, 223) |
| Trichophyton terrestre | Fungal | No | No | Unknown | Madagascar day gecko (Phelsuma m. madagascariensis) | (224) |
| Trichostrongylus tenius | Parasitic | No | No | Yes | Northern bald ibis (Geronticus eremita) | (55) |
| Tritrichomonas fetus | Parasitic | No | No | No | Quail (Coturnix coturnix) | (225) |
| Usutu Virus | Viral | No | No | Yes | Black bird (Turdus merula), Blue tit (Cyanistes caeruleus), Egyptian vulture (Neophron percnopterus), Eurasian eagle owl (Bubo bubo), Great gray owl (Strix nebulosa), House sparrow (Passer domesticus), Owls (Strigiformes), Snowy owl (Bubo scandiacus), Swallow (Hirundinidae), White stork (Ciconia ciconia), Ural owl (Strix uralensis), and multiple wild bird and other species* |
(226–239) |
| Verocytotoxin-producing Escherichia colib | Bacterial | Yes | No | Yes | Chamois (Rupicapra rupicapra), Cattle (Bos taurus) | (240) |
| West Nile Virusb, c | Viral | Yes | Yes | Yes | Bearded vulture (Gypaetus barbatus), Domestic horse (Equus ferus caballus), Goshawk (Accipiter gentilis), Gyrfalcon (Falco rusticolus), Kea (Nestor notabilis), Snowy owl (Bubo scandiacus), and multiple other bird species* | (237, 241–248) |
| Yersinia pseudotuberculosis | Bacterial | No | No | Yes | Cheetah (Acinonyx jubatus) | (52) |
Notifiable diseases according to the § 16. Federal law on epizootics in Austria.
Notifiable diseases according to the Tuberculosis Act, Epidemic Act 1950, and Sexually Transmitted Diseases Act in Austria.
Notifiable disease in Austria, as part of the Equine encephalitis disease complex.
Notifiable according to the BVD Regulation 2007, BGBl. II No. 178/2007.
Notifiable according to the Paratuberculosis Ordinance, BGBl. II No. 48/2006*.
Multiple species/other species, indicates more than 10 (N > 10) mentioned animal species.
Figure 5.
Distribution of publications addressing either bacterial, viral, or parasitic diseases between 1980 and 2017.
Figure 6.
Numerical depiction of diseases mentioned in all publications three times or more.
Zoonotic Potential
The review shows that 61% of papers discussed pathogens that have zoonotic potential, 21% of papers discussed diseases without zoonotic potential, and 5% discussed diseases with unclear zoonotic potential that would need further research (see Figures 7, 8).
Figure 7.
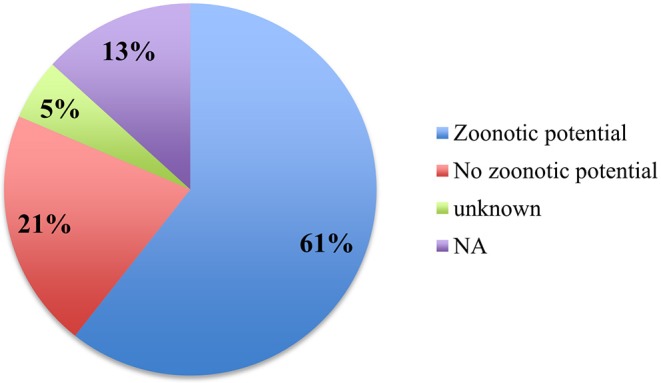
Percentage of publications discussing the zoonotic potential of diseases.
Figure 8.
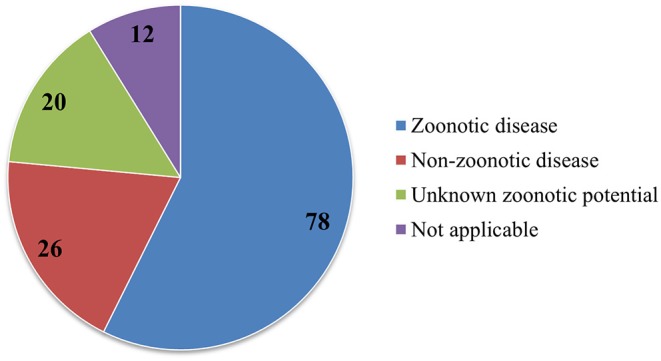
Numbers of diseases with or without zoonotic potential, as well as diseases with currently unknown zoonotic potential.
Notifiable Status
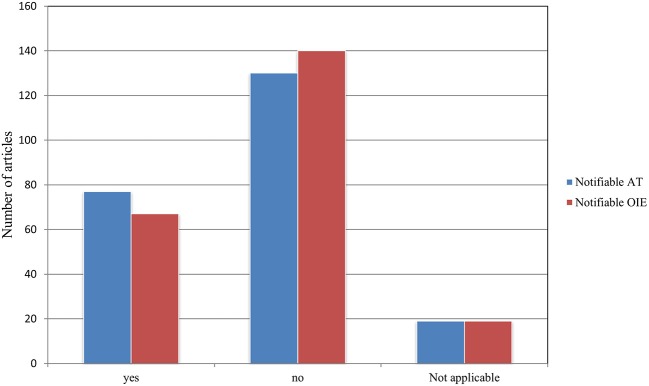
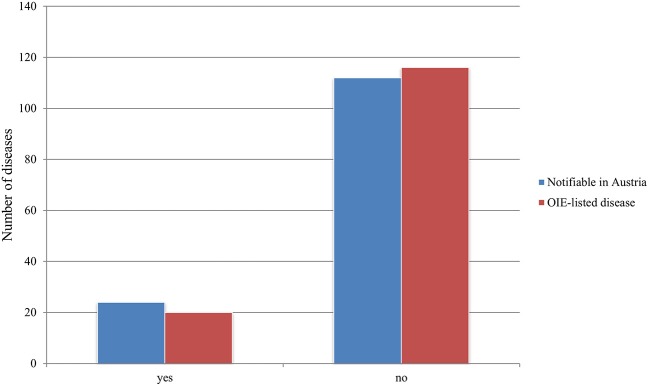
A total of 77 publications addressed diseases notifiable in Austria according to Austrian legislation including the § 16. Federal law on epizootics, the Tuberculosis Act (1968), the Epidemic Act (1950), the Sexually Transmitted Diseases Act (1945), the BVD Regulation (2007), and the Paratuberculosis Ordinance (2006), while 67 publications mentioned OIE notifiable diseases (See Supplementary Tables 1, 2). Of the 77 publications addressing notifiable diseases in Austria, 48 also addressed notifiable diseases according to the OIE list. For 19 publications, our criteria were not applicable, as they did not mention an exact pathogen but multiple pathogens (N > 10) (see Figure 9). Of the 136 diseases detected, 24 diseases are notifiable according to current Austrian law. Six of these 24 diseases are notifiable as per the Austrian § 16. Federal law on epizootics, the remaining diseases are notifiable according to the above-mentioned laws (See Supplementary Tables 1, 2). Moreover, 20 diseases were found to be OIE-listed diseases (see Figure 10). Lastly, of the 24 diseases notifiable in Austria, 11 are also notifiable according to the OIE list.
Figure 9.
Number of publications addressing diseases which are notifiable according to Austrian legislation and/or the OIE–World Organization for Animal Health compared to those addressing non-notifiable diseases.
Figure 10.
Number of notifiable diseases described in retained papers according to Austrian legislation and the OIE compared to non-notifiable diseases.
Prevalence and Confidence Interval
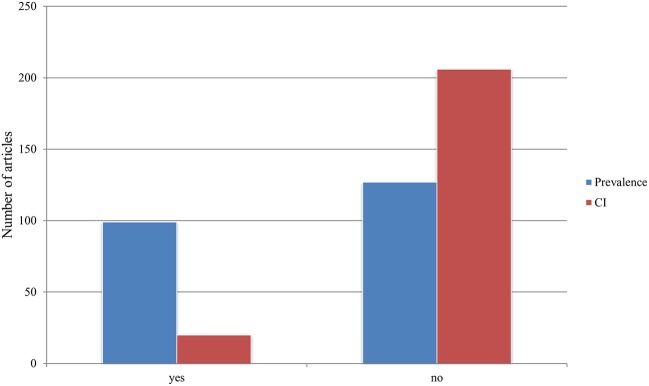
Prevalence was indicated in a total of 99 (44%) publications. The confidence intervals, on the other hand, were only mentioned in 20 (9%) publications (see Figure 11).
Figure 11.
Representation of the specification of prevalence and confidence interval (CI) in all the publications.
Animal Species
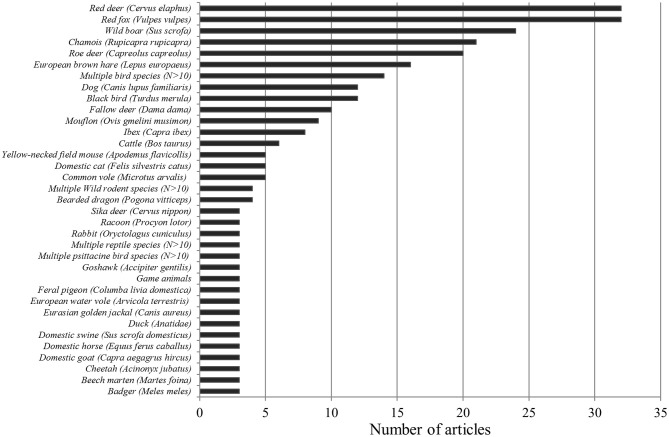
Both red deer (Cervus elaphus) and red foxes (V. vulpes) were each mentioned in 32 papers. These numbers were followed by wild boar (S. scrofa) (n = 25), chamois (Rupicapra rupicapra) (n = 21), roe deer (Capreolus capreolus) (n = 20), European brown hare (Lepus europeus) (n = 16), blackbird (Turdus merula) (n = 12), and fallow deer (Dama dama) (n = 10). Among the 10 most frequently described animal species, the domestic dog (Canis lupus familiaris) was the only domestic animal species. It occurred in a total of 12 papers. Furthermore, in 14 papers, multiple bird species were listed (see Figure 12).
Figure 12.
Numerical occurrence of the individual wild animal species or group in the investigated literature.
In summary, we found 131 animal species or groups (e.g., genera, families, and orders) in the 226 publications. Of these, 58 were birds, followed by 55 mammal species, and 2 marsupial species. Reptiles and amphibians appear underrepresented by nine species or groups and seven species, respectively. Thirteen more categories were formed to describe those papers addressing more than 10 species or not stating an exact species. These included multiple bird, rodent, reptile psittacine bird, snake, amphibian and mammalian species, and domestic ruminants, wild ruminants, wild carnivores, wild ungulates, and game animals. Ninety six species or categories were mentioned once, whereas 12 were mentioned twice.
Risk Group Interfaces
A total of 163 publications addressed diseases that occurred exclusively in wild animals, conducted research solely with wild animals or took samples only from wildlife. In contrast, 12 papers dealt with diseases at the wildlife-human interface, and all of these diseases were zoonotic. In these 12 studies, humans and animals were sampled or results reported in humans and animals were discussed. Moreover, 25 publications addressed transmission and prevalence of diseases at the wildlife-livestock interface. Last but not least, 26 publications pertained to all interfaces, i.e., between wild animals, domesticated animals, and humans (see Figure 13).
Figure 13.
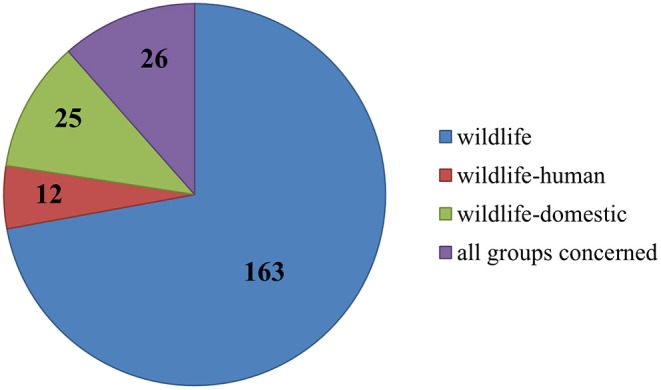
Numerical allocation of the observed interfaces addressed in all publications.
Distinct Study Features
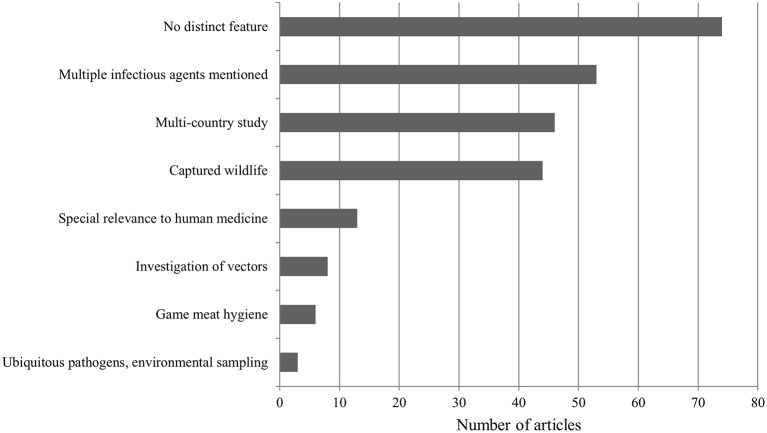
A total of 74 papers did not exhibit a specific trait according to our classification and were categorized under “No distinct feature.” In 53 publications, more than one specific pathogenic agent was mentioned. Furthermore, 46 papers referenced countries other than Austria. Diseases that emerged in captive wildlife were discussed in 44 publications. Thirteen studies addressed diseases emerging in wildlife but centered on human medicine (Figure 14). These included sampling of specific at-risk groups (e.g., hunters, veterinarians, animal keepers). Moreover, six papers dealt with game meat hygiene, and lastly, three publications were about environmental sampling for ubiquitous pathogens (e.g., Clostridium botulinum, avian botulism).
Figure 14.
Distribution of different distinct features exhibited by all publications.
Discussion
According to the WHO, 75% of new EIDs or emerging diseases originate from animal reservoirs and thus have zoonotic potential. In the last 30 years, these EIDs have appeared as a consequence of the intensifying codependence in multiuse landscapes at the interface between animals and humans (249). This review provides a first insight into the state of knowledge on wildlife diseases in Austria between 1980 and 2017.
This literature review identified 226 publications between 1980 and 2017. More than half of the publications (65%) involved actively conducted research. The review also revealed that 15% of all papers were case reports. A majority of these case reports were based on pathology reports from the NRL of the AGES, the Institute of Pathology and the Research Institute of Wildlife Ecology at the University of Veterinary Medicine in Vienna. The existence of several facilities addressing wildlife pathogens and disease appears to have had a positive impact on the generation of knowledge in this field.
In comparison to a similar study from the Republic of Korea, which focused on the time span between 1982 and 2014 and found an average rate of 6.1 publications per year, our review yielded a higher total number of publications as well as a higher average amount of publications per year. (1). However, it has to be mentioned that our study covered a longer period of time. In the Korean review, viral diseases were reported on more often than in Austria. The most frequently researched diseases in Korea were avian influenza virus and rabies. In Austria, Usutu virus and E. multilocularis clearly dominated. As far as interfaces are concerned, research in Korea focused primarily on human and livestock diseases, whereas in Austria the main focus was on diseases in wildlife. In Korea, 24 zoonotic diseases were reported, whereas in Austria 78 have been investigated since 1980. Fifteen diseases that are notifiable according to the OIE list were mentioned in the Korean review; in contrast, 20 were reported in Austria. Birds dominated in the Korean review, while mammals, namely the red fox (V. vulpes) and the red deer (C. elaphus), were most reported on in Austria (1).
Infectious Agents
Parasitic diseases accounted for 37% of the papers, 30% were viral, and 23% were bacterial. Fungal diseases were only mentioned in 4% of all studies. Furthermore, we did not find a single study on transmissible spongiform encephalopathies or other diseases caused by prions. This may be due to the fact that only eight cases of BSE were detected in Austria between 2001 and 2010 (250). Austria was also declared as a country with negligible BSE risk by the OIE in 2012. However, it is prudent not to ignore TSEs in wildlife in view of the sudden emergence of chronic wasting disease in reindeer (Rangifer tarandus) and moose (Alces alces) in Finland and Norway in April 2016 (251).
Viral diseases were addressed in 30% of publications. The most studied viral pathogens were Usutu Virus and West Nile Virus. Since its first detection in 2001, Usutu Virus has been responsible for massive die-off of blackbirds (T. merula) in Europe. The first two human cases of Usutu Virus were detected in 2003 in Austria, generating a greater interest in this vector-borne disease (252). To a much lesser extent, avian Borna disease virus, avian pox virus, distemper virus, and Hantavirus/Puumala virus were also investigated.
Parasitic diseases accounted for 37% of papers. E. multilocularis, Trichinella sp., T. gondii, F. magna, A. phagocytophilum, and Neospora caninum were most often reported on. With the exception of F. magna and N. caninum, all of these are zoonotic.
When it comes to the bacterial pathogens, the most reported bacteria were Salmonella sp., M. caprae and M. avium subsp. paratuberculosis, F. tularensis, and methicillin-resistant Staphylococcus aureus. These are all zoonotic, potentially food borne and thus of high relevance to human health. The risk of a spillover or spillback of these bacterial diseases from wildlife to livestock is potentially quite high, as livestock and wildlife share mountain pastures in Austria.
Batrachochytrium dendrobatidis, a fungus that infects amphibians and is responsible for the global decline and even extinction of amphibians, was the most reported fungi and a classic EIDs (253).
Zoonotic Potential
The recovered papers are biased toward zoonotic diseases (61% n = 78). This is most likely due to funding being more readily available for diseases representing a human health risk. However, wildlife diseases without zoonotic potential should not be ignored, as they can also pose a risk to society, for example by causing major direct economic losses (e.g., ASF) and the loss of ecosystem services through species loss.
Notifiable Status
Of the 35 notifiable animal diseases in the Austrian federal law on epizootics, 7 were reported in the examined literature. In addition, the BVD Regulation 2007, BGBl. II No. 178/2007, and the Paratuberculosis Ordinance, BGBl. II No. 48/2006*, were of relevance, as both BVD and paratuberculosis were reported in wildlife (See Supplementary Tables 1, 2). The OIE list includes 117 diseases that can possibly infect or infest animals (254). In this review, we noted 20 diseases that are notifiable according to the OIE. Of the 29 diseases discussed more than two times, seven are notifiable according to the OIE list and the Austrian legislation, four are only notifiable in Austria, and three are only notifiable according to the OIE list.
Prevalence and Confidence Interval
Reporting prevalence is essential and provides insight into the epidemiological situation of a pathogen or disease entity in the sampled region. The disease prevalence was stated in 44% of publications. Of concern is that CI were only mentioned in 9% of the papers, thereby seriously limiting the significance of most studies and respective results. We found that 17 publications that provided a confidence interval for their results were research articles (e.g., controlled studies or retrospective studies). Providing confidence intervals did not increase over the 37 years reviewed. The authors of this study are of the opinion that appropriate descriptive statistics are not sufficiently employed in Austrian wildlife disease research at the present time.
Animal Species
Of roughly 626 different vertebrate species (including fish) native to Austria, only 21% have been addressed in this review. This demonstrates a huge untapped research potential in wildlife health research.
Mammals
The most studied mammalian species were the red fox (V. vulpes), the red deer (C. elaphus), the wild boar (S. scrofa), the chamois (R. rupicapra), the roe deer (C. capreolus), and the European brown hare (L. europeus). It is worth mentioning that these species represent the main game species in Austria. Again, it becomes clear that the primary focus of research has lain on species that serve as food source and offer recreational opportunities for humans. These animals pose a higher risk of transmitting zoonoses, generate funding, and therefore are more interesting to research. The wild boar is a species that has been of great interest to research, as populations are rapidly expanding both in absolute numbers and spatial distribution. Similar to many European countries, wild boar population management is increasingly difficult in Austria. One reason for the rapid reproduction of wild boar is climate change, resulting in increased food availability from beech nuts and acorns coupled with an increased number of litters and offspring survival. Since infection pressure potentially increases with population density, the wild boar population poses an important potential risk for domesticated pigs and humans from ASF or Trichinella spp. (214, 255).
Birds
The most studied bird species in Austria was the blackbird (T. merula) with references in 12 publications. Interestingly, all studies about blackbirds focused on Usutu virus related to the outbreak in Europe in 2000–2001. The mosquito-borne zoonotic Usutu virus has caused widespread deaths in blackbirds. Before the emergence of the disease in Austria, no previous research on this virus existed. Besides blackbirds, goshawks (Accipiter gentilis), feral pigeons (Columba livia domestica), and ducks were also of special interest to research. The Usutu virus highlights the fact that a large proportion of research related to wildlife diseases is opportunistic and initially reactive.
Reptiles and Amphibians
The captive bearded dragon (Pogona vitticeps) was the most studied reptile in Austria between 1980 and 2017 and appeared in four studies. However, no disease was mentioned more than once. Moreover, we were not able to identify any individual amphibian species that appeared more than once in the investigated publications. The reason for this low number of studies could be the scarcity of amphibian and reptile species in Austria. There are only 21 known native amphibian species and 16 reptile species in Austria (10).
Risk Group Interfaces
The majority (72%) of publications either prospectively sampled wild animal species or evaluated research results in retrospective studies. Numerous publications address the possibility of transmission of pathogens between the different interfaces (e.g., wildlife-livestock, wildlife-human). The remaining studies (28%) dealt with diseases affecting the interfaces between wildlife, livestock, and humans. These papers actively collected samples or performed retrospective studies on existing data across different interfaces. Our study shows that most of the publications addressed the fact that wildlife diseases can emerge in and cross various interfaces.
Distinct Study Features
We were able to identify seven categories within which we could further classify a subset of the papers. Publications could also exhibit more than one distinct feature. Fifty-three publications addressed more than one pathogen of which 42 were research papers. Six publications represented reviews. Furthermore, in 46 studies, countries other than Austria were mentioned. Only 44 studies dealt with captive wildlife or exotic pets. Research at the wildlife-human interface was documented in 13 studies, and these sampled both animals and humans. As the interface between wildlife and humans expands across Austria, it appears prudent to increase research and facilitate human and veterinary medical collaborations. Disease research on trichinosis, tularemia, tuberculosis, and echinococcosis would especially benefit from such collaborative one-health approaches.
Conclusion
Wildlife diseases will challenge research and medicine alike for years to come, as major changes due to the growing human population and environmental impacts increase. These impacts contribute to increased occurrence of diseases originating in wild animal species spilling over to humans. This review shows that increasing numbers of studies are being conducted in Austria. However, there is still room for further research. With the exception of prions, all infectious agents (viruses, bacteria, parasites, and fungi) have been investigated. Although there have been numerous studies on zoonoses, we have to stress the importance for future research to focus more strongly on disease dynamics and transmission at the wildlife-human interface. We acknowledge that wildlife disease studies frequently lack prevalence data, or data are of poor quality, to perform robust prevalence calculations. However, it appears that data essential to prevalence statement are not always available, especially in single case reports or due to the bias produced by passive surveillance sampling. Therefore, researchers may often decide not to include prevalence data, since it could be misinterpreted or even speculative. We would suggest that future wildlife disease-related studies should include prevalence data if possible, or state other appropriate measures and methods for statistical and epidemiological characterization of a disease event. Similarly, it appears important to expand the scope of the species investigated in the future. Funding is most often only available during impactful events, such as outbreaks, but is notably lacking for baseline research on wildlife, ecosystems, and diseases. Our assumption that there would be an increase in the number of publications on wildlife diseases in Austria over the period examined was confirmed. Considering our hypothesis, spatiotemporal indicators would have been beneficial to our review. However, due to the quality of the available data, its variability in consistency regarding study design, objectives, and methodologies of the investigated studies (e.g., multiple sampling sites, sampling across longer periods of time, multiple diseases in the same study), stating spatiotemporal indicators would have exceeded the clearly defined scope of this initial study. We believe this question would be best addressed in a separate and future analysis. We hope that this review provides information about the current research status for wildlife ecologists, veterinarians, and officials alike and incentivizes further research that employs “One Health” approaches.
Author Contributions
NT did the literature research and wrote the manuscript. CW planned the review concept and working steps, and edited the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Dr. P. K. Walzer for improving and revising our manuscript in terms of grammar and style.
Glossary
Abbreviations
- ADNS
Animal Disease Notification System
- AGES
Austrian Agency for Health and Food Safety
- ASF
African swine fever
- AT
Austria
- AVR
Annual Veterinary Report
- BSE
Bovine spongiform encephalopathy
- BVD
Bovine viral diarrhea
- CI
Confidence interval
- CWD
Chronic wasting disease
- EFSA
European Food Safety Authority
- EID
Emerging infectious disease
- EU
European Union
- KVG
Kommunikationsplattform VerbraucherInnengesundheit
- MRSA
Methicillin-resistant Staphylococcus aureus
- NA
Not applicable
- NCBI
National Center for Biotechnology Information
- NRL
National Reference Laboratory of the AGES
- OIE
World Organization for Animal Health
- RZZA
Report of Zoonoses and Zoonotic Agents
- TSE
Transmissible spongiform encephalopathy
- TSG, Tierseuchengesetz
Federal law on epizootics
- WNV
West Nile Virus.
Footnotes
Funding. Funding was provided internally by the University of Veterinary Medicine of Vienna.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2020.00003/full#supplementary-material
References
- 1.Hwang J, Lee K, Kim YJ, Sleeman JM, Lee H. Retrospective analysis of the epidemiologic literature, 1990–2015, on wildlife-associated diseases from the republic of Korea. J Wildlife Dis. (2017) 53:5–18. 10.7589/2015-12-348 [DOI] [PubMed] [Google Scholar]
- 2.Hassell JM, Begon M, Ward MJ, Fèvre EM. Urbanization and disease emergence: dynamics at the wildlife–livestock–human interface. Trends Ecol Evol. (2017) 32:55–67. 10.1016/j.tree.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature. (2008) 451:990–3. 10.1038/nature06536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McFarlane R, Sleigh A, McMichael T. Synanthropy of wild mammals as a determinant of emerging infectious diseases in the Asian-Australasian region. EcoHealth. (2012) 9:24–35. 10.1007/s10393-012-0763-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coker R, Rushton J, Mounier-Jack S, Karimuribo E, Lutumba P, Kambarage D, et al. Towards a conceptual framework to support one-health research for policy on emerging zoonoses. Lancet Infect Dis. (2011) 11:326–31. 10.1016/S1473-3099(10)70312-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gortázar C, Ferroglio E, Höfle U, Frölich K, Vicente J. Diseases shared between wildlife and livestock: a European perspective. Eur J Wildlife Res. (2007) 53:241–56. 10.1007/s10344-007-0098-y [DOI] [Google Scholar]
- 7.StatistikAustria Bevölkerung. (2019). Available online at: https://www.statistik.at/web_de/statistiken/menschen_und_gesellschaft/bevoelkerung/index.html (accessed February 8, 2019).
- 8.Statisitk Austria. Austria. Data Figures Facts. (2018). Available online at: https://eu2018.statistik.at/fileadmin/euratspraesidentschaft/downloads/austria._data._figures._facts.pdf (accessed February 8, 2019).
- 9.Wikipedia Austria. (2019). Available online at: https://en.wikipedia.org/wiki/Austria#Geography (accessed February 08, 2019).
- 10.Geiser E. Wie viele tierarten leben in österreich? Erfassung, Hochrechnung und Abschätzung. Vcrii Zool Bot Ges Österreich. (1998) 135:81–93. [Google Scholar]
- 11.Herzog U, Damoser J, Höflechner-Pöltl A. Annual Veterinary Report 2017. Federal Ministry of Labour, Social Affairs, Health and Consumer Protection and Austrian and Agency for Health and Food Safety (AGES), Vienna (2017). p. 1–84. [Google Scholar]
- 12.Federal Ministry of Labour, Social Affairs, Health and Consumer Protection and Austrian Agency for Health and Food Safety. Zoonoses and Zoonotic agents in Austria. Report 2017. Vienna: (2018). p. 1–84. [Google Scholar]
- 13.Kommunikationsplattform VerbraucherInnengesundheit (KVG). Überwachung. (2019). Available online at: https://www.verbrauchergesundheit.gv.at/tiere/krankheiten/ueberwachung/ueberwachung.html (accessed May 8, 2019).
- 14.Deutz A, Fuchs K, Auer H, Schuller W, Nowotny N, Kerbl U, et al. Zoonoses, seroepidemiological examination of different persons for selected contact zoonoses: seroprevalences, risk factors and preventive measures. Fleischwirtschaft. (2002) 82:101–4. [Google Scholar]
- 15.Deutz A, Köfer J. Small game (fox, brown hare, pheasant and duck) as carriers of zoonoses. Berl Mun Tierarztl Wochenschr. (2000) 113:401–6. 10.7748/paed.12.3.11.s15 [DOI] [PubMed] [Google Scholar]
- 16.Fink M, Schleicher C, Gonano M, Prodinger WM, Pacciarini M, Glawischnig W, et al. Red deer as maintenance host for bovine tuberculosis, alpine region. Emerg Infect Dis. (2015) 21:464–7. 10.3201/eid2103.141119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kainz R, Paulsen P. Changes in EU hygiene legislation for meat from hunted game. Wien Tierarztl Monatsschr. (2005) 92:150–6. [Google Scholar]
- 18.Posautz A, Gyuranecz M, Denes B, Knauer F, Dier H, Walzer C. Seroprevalence of Francisella tularensis in Austrian hunting dogs. Vector Borne Zoon Dis. (2017) 18:117–9. 10.1089/vbz.2017.2193 [DOI] [PubMed] [Google Scholar]
- 19.Wiethoelter AK, Beltrán-Alcrudo D, Kock R, Mor SM. Global trends in infectious diseases at the wildlife-livestock interface. Proc Natl Acad Sci USA. (2015) 112:9662–7. 10.1073/pnas.1422741112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daszak P, Cunningham AA, Hyatt AD. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop. (2001) 78:103–16. 10.1016/S0001-706X(00)00179-0 [DOI] [PubMed] [Google Scholar]
- 21.Springer Types of Journal Articles. (2019). Avaialble online at: https://www.springer.com/gp/authors-editors/authorandreviewertutorials/writing-a-journal-manuscript/types-of-journal-articles/10285504 (accessed February 8, 2019).
- 22.Aiello SE, Moses MA. The Merck Veterinary Manual. 11th ed Kenilworth, NJ: Merck & Co., Inc. (2016). [Google Scholar]
- 23.Boch J, Supperer R. Veterinärmedizinische Parasitologie. 3rd ed. Berlin: Parey; (2006). [Google Scholar]
- 24.Deplazes P, Eckert J, Samson-Himmelstjerna GV, Zahner H. Lehrbuch der Parasitologie für die Tiermedizin. 3rd ed. Stuttgart: Enke; (2013). [DOI] [PubMed] [Google Scholar]
- 25.Rolle M, Mayr A, Büttner M. Medizinische Mikrobiologie, Infektions- und Seuchenlehre. 8th ed. Stuttgart: Enke; (2007). [Google Scholar]
- 26.Selbit H-J, Truyen U, Valentin-Weigand P, Alber G. Tiermedizinische Mikrobiologie, Infektions- und Seuchenlehre. 9th ed. Stuttgart: Enke; (2011). [Google Scholar]
- 27.Rehbein S, Visser M. Die Endoparasiten des Sikawildes (Cervus nippon) in Österreich. Wien Klin Wochenschr. (2007) 119:96–101. 10.1007/s00508-007-0865-5 [DOI] [PubMed] [Google Scholar]
- 28.Winkelmayer R, Paulsen P. Parasites in free-living wild game: relevance for meat hygiene and possibilities of detection. Wien Tierarztl Monatsschr. (2011) 98:239–44. [Google Scholar]
- 29.Schwarz L, Frena M, Skalicky M, Prosl H. Endoparasite infestation of roe deer from a hunting ground in Lower Austria. Wien Tierarztl Monatsschr. (2011) 98:285–91. [Google Scholar]
- 30.Hoby S, Walzer C, Slotta-Bachmayr L, Segner H, Robert N. Pathological investigations in free-ranging ungulates in the National Park Hohe Tauern, Austria. Wien Tierarztl Monatsschr. (2006) 93:104–12. [Google Scholar]
- 31.Juncker-Voss M, Prosl H, Lussy H, Enzenberg U, Auer H, Lassnig H, et al. Screening for antibodies against zoonoses among employees of the Zoological Garden of Vienna, Schönbrunn, Austria. Berl Munch Tierarztl Wochenschr. (2004) 117:404–9. [PubMed] [Google Scholar]
- 32.Duscher G, Leschnik M, Führer HP, Joachim A. Wildlife reservoirs for vector-borne canine, feline and zoonotic infections in Austria. Int J Parasitol. (2015) 4:88–96. 10.1016/j.ijppaw.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deutz A, Fuchs K, Schuller W, Nowotny N, Auer H, Aspock H, et al. Seroepidemiological studies of zoonotic infections in hunters in southeastern Austria - prevalences, risk factors, and preventive methods. Berl Munch Tierarztl Wochenschr. (2003) 116:306–11. [PubMed] [Google Scholar]
- 34.Duscher T, HodŽić A, Glawischnig W, Duscher G. The raccoon dog (Nyctereutes procyonoides) and the raccoon (Procyon lotor) - their role and impact of maintaining and transmitting zoonotic diseases in Austria, Central Europe. Parasitol Res. (2017) 116:1411–6. 10.1007/s00436-017-5405-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deutz A, Hinterdorfer F. Diseases in brown hare: Post-mortem findings, range of pathogens and zoonotic aspects. Tierarztl Umschau. (2000) 55:628–35. [Google Scholar]
- 36.Khayal B, Hess M, Bagó Z. Pathomorphological investigations on wild birds during the winter season 2005/2006. Wien Tierarztl Monatsschr. (2010) 97:125–34. [Google Scholar]
- 37.Wascher CAF, Bauer AC, Holtmann AR, Kotrschal K. Environmental and social factors affecting the excretion of intestinal parasite eggs in graylag geese. Behav Ecol. (2012) 23:1276–83. 10.1093/beheco/ars113 [DOI] [Google Scholar]
- 38.Rehbein S, Visser M, Jekel I, Silaghi C. Endoparasites of the fallow deer (Dama dama) of the Antheringer Au in Salzburg, Austria. Wien Klin Wochenschr. (2014) 126 (Suppl.1):37–41. 10.1007/s00508-014-0506-8 [DOI] [PubMed] [Google Scholar]
- 39.Schmidt-Ukaj S, Hochleithner M, Richter B, Hochleithner C, Brandstetter D, Knotek Z. A survey of diseases in captive bearded dragons: a retrospective study of 529 patients. Vet Med. (2017) 62:508–15. 10.17221/162/2016-VETMED [DOI] [Google Scholar]
- 40.Hoby S, Schwarzenberger F, Doherr MG, Robert N, Walzer C. Steroid hormone related male biased parasitism in chamois, Rupicapra rupicapra. Vet Parasitol. (2006) 138:337–48. 10.1016/j.vetpar.2006.01.028 [DOI] [PubMed] [Google Scholar]
- 41.Deutz A, Köfer J. Pig and wild boar as carriers of zoonoses. Berl Munch Tierarztl Wochenschr. (1999) 112:305–10. [PubMed] [Google Scholar]
- 42.Kübber-Heiss A, Benetka V, Filip T, Benyr G, Schilcher F, Pallan C, et al. First detection of an adenovirus infection in a bearded dragon (Pogona vitticeps) in Austria. Wien Tierarztl Monatsschr. (2006) 93:68–72. [Google Scholar]
- 43.Duscher G. Duncker's muscle fluke—Alaria alata in red foxes from Austria in relation to the occurrence of wild boars. Wien Tierarztl Monatsschr. (2011) 98:251–4. [Google Scholar]
- 44.Paulsen P, Ehebruster J, Irschik I, Lücker E, Riehn K, Winkelmayer R, et al. Findings of Alaria alata mesocercariae in wild boars (Sus scrofa) in eastern Austria. Eur J Wildlife Res. (2012) 58:991–5. 10.1007/s10344-012-0642-2 [DOI] [Google Scholar]
- 45.Sailer A, Glawischnig W, Irschik I, Lücker E, Riehn K, Paulsen P. Findings of Alaria alata mesocercariae in wild boar in Austria: current knowledge, identification of risk factors and discussion of risk management options. Wien Tierarztl Monatsschr. (2012) 99:346–52. [Google Scholar]
- 46.Petrovec M, Sixl W, Schweiger R, Mikulašek S, Elke L, Wüst G, et al. Infections of wild animals with Anaplasma phagocytophila in Austria and the Czech republic. Ann NY Acad Sci. (2003) 990:103–6. 10.1111/j.1749-6632.2003.tb07345.x [DOI] [PubMed] [Google Scholar]
- 47.Polin H, Hufnagl P, Haunschmid R, Gruber F, Ladurner G. Molecular evidence of Anaplasma phagocytophilum in Ixodes ricinus ticks and wild animals in Austria. J Clin Microbiol. (2004) 42:2285–6. 10.1128/JCM.42.5.2285-2286.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silaghi C, Hamel D, Pfister K, Rehbein S. Babesia species and co-infection with Anaplasma phagocytophilum in free-ranging ungulates from tyrol (Austria). Wien Tierarztl Monatsschr. (2011) 98:268–74. [Google Scholar]
- 49.Silaghi C, Hamel D, Thiel C, Pfister K, Friche Passos LM, Rehbein S. Genetic variants of Anaplasma phagocytophilum in wild caprine and cervid ungulates from the alps in tyrol, Austria. Vector-Borne Zoonotic Dis. (2011) 11:355–62. 10.1089/vbz.2010.0051 [DOI] [PubMed] [Google Scholar]
- 50.Leschnik M, Kirtz G, Virányi Z, Wille-Piazzai W, Duscher G. Acute granulocytic anaplasmosis in a captive timber wolf (Canis lupus occidentalis). J Zoo Wildlife Med. (2012) 43:645–8. 10.1638/2011-0224R.1 [DOI] [PubMed] [Google Scholar]
- 51.Cezanne R, Mrowietz N, Eigner B, Duscher G, Glawischnig W, Führer HP. Molecular analysis of Anaplasma phagocytophilum and Babesia divergens in red deer (Cervus elaphus) in Western Austria. Mol Cell Probes. (2016) 31:55–8. 10.1016/j.mcp.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 52.Kotsch V, Kübber-Heiss A, Url A, Walzer C, Schmidt P. Diseases of captive cheetahs (Acinonyx jubatus) within the European endangered species program (EEP)—A 22-year retrospective histopathological study. Wien Tierarztl Monatsschr. (2002) 89:341–50. [Google Scholar]
- 53.Pfeffer M, Dobler G. Emergence of zoonotic arboviruses by animal trade and migration. Parasit Vect. (2010) 3:35. 10.1186/1756-3305-3-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Auer H. Parasites of huntable game dangerous for people in Austria. Wien Tierarztl Monatsschr. (2011) 98:245–50. [Google Scholar]
- 55.Frigerio D, Cibulski L, Ludwig SC, Campderrich I, Kotrschal K, Wascher CAF. Excretion patterns of coccidian oocysts and nematode eggs during the reproductive season in Northern Bald Ibis (Geronticus eremita). J Ornithol. (2016) 157:839–51. 10.1007/s10336-015-1317-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pardeike J, Weber S, Zarfl HP, Pagitz M, Zimmer A. Itraconazole-loaded nanostructured lipid carriers (NLC) for pulmonary treatment of aspergillosis in falcons. Eur J Pharm Biopharm. (2016) 108:269–76. 10.1016/j.ejpb.2016.07.018 [DOI] [PubMed] [Google Scholar]
- 57.Thaller D, Bilek A, Revilla-Fernández S, Bagó Z, Schildorfer H, Url A, et al. Diagnosis of aujeszky's disease in a dog in Austria. Wien Tierarztl Monatsschr. (2006) 93:62–7. [Google Scholar]
- 58.Leschnik M, Gruber A, Kübber-Heiss A, Bagó Z, Revilla-Fernández S, Wodak E, et al. Epidemiological aspects of aujeszky's disease in Austria by the means of six cases in dogs. Wien Tierarztl Monatsschr. (2012) 99:82–90. [Google Scholar]
- 59.Steinrigl A, Revilla-Fernández S, Kolodziejek J, Wodak E, Bagó Z, Nowotny N, et al. Detection and molecular characterization of Suid herpesvirus type 1 in Austrian wild boar and hunting dogs. Vet Microbiol. (2012) 157:276–84. 10.1016/j.vetmic.2011.12.033 [DOI] [PubMed] [Google Scholar]
- 60.Weissenböck H, Bakonyi T, Sekulin K, Ehrensperger F, Doneley RJT, Dürrwald R, et al. Avian bornaviruses in psittacine birds from Europe and Australia with proventricular dilatation disease. Emerg Infect Dis. (2009) 15:1453–9. 10.3201/eid1509.090353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weissenböck H, Sekulin K, Bakonyi T, Högler S, Nowotny N. Novel Avian bornavirus in a nonpsittacine species (canary; Serinus canaria) with enteric ganglioneuritis and encephalitis. J Virol. (2009) 83:11367–71. 10.1128/JVI.01343-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weissenböck H, Fragner K, Nedorost N, Mostegl MM, Sekulin K, Maderner A, et al. Localization of avian bornavirus RNA by in situ hybridization in tissues of psittacine birds with proventricular dilatation disease. Vet Microbiol. (2010) 145:9–16. 10.1016/j.vetmic.2010.02.030 [DOI] [PubMed] [Google Scholar]
- 63.Nedorost N, Maderner A, Kolodziejek J, Lussy H, Nowotny N, Weissenböck H. Identification of mixed infections with different genotypes of avian bornaviruses in psittacine birds with proventricular dilatation disease. Avian Dis. (2012) 56:414–7. 10.1637/10026-112911-ResNote.1 [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Bilic I, Troxler S, Hess M. Evidence of genotypes 1 and 3 of Avian hepatitis E virus in wild birds. Virus Res. (2017) 228:75–8. 10.1016/j.virusres.2016.11.028 [DOI] [PubMed] [Google Scholar]
- 65.Globig A, Baumer A, Revilla-Fernández S, Beer M, Wodak E, Fink M, et al. Ducks as sentinels for avian influenza in wild birds. Emerg Infect Dis. (2009) 15:1633–6. 10.3201/eid1510.090439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fink M, Fernández SR, Schobesberger H, Koefer J. Geographical spread of highly pathogenic avian influenza virus H5N1 during the 2006 outbreak in Austria. J Virol. (2010) 84:5815–23. 10.1128/JVI.01642-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loupal G, Schönbauer M, Jahn J. Pocken bei Zoo - und wildvögeln: licht - und elektronenmikroskopische untersuchungen. Z Vet Reihe B. (1985) 32:326–36. 10.1111/j.1439-0450.1985.tb01969.x [DOI] [PubMed] [Google Scholar]
- 68.Reiter AS, Loupal G. A case of pox in the great bustard (Otis tarda) of the hanság population in Austria. J Ornithol. (1995) 136:221–3. 10.1007/BF01651245 [DOI] [Google Scholar]
- 69.Gruber A, Grabensteiner E, Kolodziejek J, Nowotny N, Loupal G. Poxvirus infection in a great tit (Parus major). Avian Dis. (2007) 51:623–5. 10.1637/0005-2086(2007)51[623:PIIAGT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 70.Pichler R, Posautz A, Beer T. Avian pox virus in a female bald eagle (Haliaeetus leucocephalus) - a case report. Wien Tierarztl Monatsschr. (2016) 103:321–5. [Google Scholar]
- 71.Mitková B, Hrazdilová K, D'Amico G, Duscher G, Suchentrunk F, Forejtek P, et al. Eurasian golden jackal as host of canine vector-borne protists. Parasit Vect. (2017) 10:183. 10.1186/s13071-017-2110-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duscher G, Führer HP, Kubber-Heiss A. Fox on the run - molecular surveillance of fox blood and tissue for the occurrence of tick-borne pathogens in Austria. Parasit Vect. (2014) 7:521. 10.1186/PREACCEPT-1542240345144663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.HodŽić A, Zörer J, Duscher G. Dermacentor reticulatus, a putative vector of Babesia cf. microti (syn. theileria annae) piroplasm. Parasitol Res. (2017) 116:1075–7. 10.1007/s00436-017-5379-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corduneanu A, Hrazdilová K, Sándor AD, Matei IA, Ionicǎ AM, Barti L, et al. Babesia vesperuginis, a neglected piroplasmid: new host and geographical records, and phylogenetic relations. Parasit Vect. (2017) 10:598. 10.1186/s13071-017-2536-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hinaidy HK. Blutparasiten der wildlebenden wiederkäuer österreichs. J Veterin Med Ser B. (1987) 34:81–97. 10.1111/j.1439-0450.1987.tb00374.x [DOI] [PubMed] [Google Scholar]
- 76.Schmidt S, Essbauer SS, Mayer-Scholl A, Poppert S, Schmidt-Chanasit J, Klempa B, et al. Multiple infections of rodents with zoonotic pathogens in Austria. Vector Borne Zoo Dis. (2014) 14:467–75. 10.1089/vbz.2013.1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Richter B, Kübber-Heiss A. First detection of chytridiomycosis associated with fatalities in poison dart frogs (Dendrobates tinctorlus) in Austria. Wien Tierarztl Monatsschr. (2010) 97:157–60. [Google Scholar]
- 78.Sztatecsny M, Glaser F. From the eastern lowlands to the western mountains: first records of the chytrid fungus Batrachochytrium dendrobatidis in wild amphibian populations from Austria. Herpetol J. (2011) 21:87–90. [Google Scholar]
- 79.Lötters S, Kielgast J, Sztatecsny M, Wagner N, Schulte U, Werner P, et al. Absence of infection with the amphibian chytrid fungus in the terrestrial alpine salamander (Salamandra atra). Salamandra. (2012) 48:58–62. 10.5167/uzh-62111 [DOI] [Google Scholar]
- 80.d'Ovidio D, Pantchev N, Noviello E, Del Prete L, Maurelli MP, Cringoli G, et al. Survey of Baylisascaris spp. in captive striped skunks (mephitis mephitis) in some European areas. Parasitol Res. (2017) 116:483–6. 10.1007/s00436-016-5307-8 [DOI] [PubMed] [Google Scholar]
- 81.Dürrwald R, Kolodziejek J, Muluneh A, Herzog S, Nowotny N. Epidemiological pattern of classical Borna disease and regional genetic clustering of Borna disease viruses point towards the existence of to-date unknown endemic reservoir host populations. Microb Infect. (2006) 8:917–29. 10.1016/j.micinf.2005.08.013 [DOI] [PubMed] [Google Scholar]
- 82.Shibly S, Schmidt P, Robert N, Walzer C, Url A. Immunohistochemical screening for viral agents in cheetahs (Acinonyx jubatus) with myelopathy. Veterin Rec. (2006) 159:557–61. 10.1136/vr.159.17.557 [DOI] [PubMed] [Google Scholar]
- 83.Weissenböck H, Bagó Z, Kolodziejek J, Hager B, Palmetzhofer G, Dürrwald R, et al. Infections of horses and shrews with Bornaviruses in upper Austria: a novel endemic area of borna disease. Emerg Microb Infect. (2017) 6:e52. 10.1038/emi.2017.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leitner N, Schwarzmann L, Zittra C, Palmieri N, Eigner B, Otranto D, et al. Morphological and molecular identification of nasopharyngeal bot fly larvae infesting red deer (Cervus elaphus) in Austria. Parasitol Res. (2016) 115:4417–22. 10.1007/s00436-016-5206-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krametter R, Nielsen SS, Loitsch A, Froetscher W, Benetka V, Moestl K, et al. Pestivirus exposure in free-living and captive deer in Austria. J Wildlife Dis. (2004) 40:791–5. 10.7589/0090-3558-40.4.791 [DOI] [PubMed] [Google Scholar]
- 86.Glawischnig W, Schoepf K, Matt M. Monitoring for Bovine viral diarrhea virus in Austrian red deer (Cervus elaphus elaphus) by using ear-notch samples. J Wildlife Dis. (2010) 46:1269–73. 10.7589/0090-3558-46.4.1269 [DOI] [PubMed] [Google Scholar]
- 87.Scholz HC, Hofer E, Vergnaud G, Fleche PL, Whatmore AM, Dahouk SA, et al. Isolation of Brucella microti from mandibular lymph nodes of red foxes, (Vulpes vulpes), in lower Austria. Vector Borne Zoo Dis. (2009) 9:153–5. 10.1089/vbz.2008.0036 [DOI] [PubMed] [Google Scholar]
- 88.Al Dahouk S, Hofer E, Tomaso H, Vergnaud G, Le Flèche P, Cloeckaert A, et al. Intraspecies diodiversity of the genetically homologous species Brucella microti. Appl Environ Microbiol. (2012) 78:1534–43. 10.1128/AEM.06351-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hofer E, Reisp K, Revilla-Fernández S, Plicka H, Romanek G, Bagó Z, et al. Isolation of Francisella tularensis and Brucella suis from red foxes (Vulpes vulpes). Tierarztli Umschau. (2010) 65:229–32. [Google Scholar]
- 90.Scholz HC, Revilla-Fernández S, Dahouk SA, Hammerl JA, Zygmunt MS, Cloeckaert A, et al. Brucella vulpis sp. nov, isolated from mandibular lymph nodes of red foxes (Vulpes vulpes). Intl J Syst Evol Microbiol. (2016) 66:2090–8. 10.1099/ijsem.0.000998 [DOI] [PubMed] [Google Scholar]
- 91.Winkelmayer R, Vodnansky M, Paulsen P, Gansterer A, Treml F. Explorative study on the seroprevalence of Brucella-, Francisella- and Leptospira antibodies in the European hare (Lepus europaeus) of the Austrian—Czech border region. Wien Tierarztl Monatsschr. (2005) 92:131–5. [Google Scholar]
- 92.Hofer E, Revilla-Fernández S, Al Dahouk S, Riehm JM, Nöckler K, Zygmunt MS, et al. A potential novel Brucella species isolated from mandibular lymph nodes of red foxes in Austria. Vet Microbiol. (2012) 155:93–9. 10.1016/j.vetmic.2011.08.009 [DOI] [PubMed] [Google Scholar]
- 93.Konicek C, VodráŽka P, Barták P, Knotek Z, Hess C, Račka K, et al. Detection of zoonotic pathogens in wild birds in the cross-border region Austria—Czech republic. J Wildlife Dis. (2016) 52:850–61. 10.7589/2016-02-038 [DOI] [PubMed] [Google Scholar]
- 94.Troxler S, Hess C, Konicek C, Knotek Z, Barták P, Hess M. Microdilution testing reveals considerable and diverse antimicrobial resistance of Escherichia coli, thermophilic Campylobacter spp. and Salmonella spp. isolated from wild birds present in urban areas. Eur J Wildlife Res. (2017) 63:68 10.1007/s10344-017-1125-2 [DOI] [Google Scholar]
- 95.HodŽić A, Cezanne R, Duscher G, Harl J, Glawischnig W, Führer HP. Candidatus Neoehrlichia sp. in an Austrian fox is distinct from candidatus neoehrlichia mikurensis, but closer related to candidatus neoehrlichia lotoris. Parasit Vectors. (2015) 8:1163. 10.1186/s13071-015-1163-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.HodŽić A, Bruckschwaiger P, Duscher G, Glawischnig W, Führer HP. High prevalence of Eucoleus boehmi (syn. Capillaria boehmi) in foxes from western Austria. Parasitol Res. (2016) 115:3275–8. 10.1007/s00436-016-5145-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Führer HP. An overview of the host spectrum and distribution of Calodium hepaticum (syn. Capillaria hepatica): part 1 - Muroidea. Parasitol Res. (2014) 113:619–40. 10.1007/s00436-013-3691-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Führer HP. An overview of the host spectrum and distribution of Calodium hepaticum (syn. Capillaria hepatica): part 2 - Mammalia (excluding Muroidea). Parasitol Res. (2014) 113:641–51. 10.1007/s00436-013-3692-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Führer HP, Schneider R, Walochnik J, Auer H. Extraintestinal helminths of the common vole (Microtus arvalis) and the water vole (Arvicola terrestris) in Western Austria (Vorarlberg). Parasitol Res. (2010) 106:1001–4. 10.1007/s00436-010-1753-x [DOI] [PubMed] [Google Scholar]
- 100.Benetka V, Grabensteiner E, Gumpenberger M, Neubauer C, Hirschmüller B, Möstl K. First report of an iridovirus (Genus Ranavirus) infection in a leopard tortoise (Geochelone pardalis pardalis). Wien Tierarztl Monatsschr. (2007) 94:243–8. [Google Scholar]
- 101.Stephan S, Guerra D, Pospischil A, Hilbe M, Weissenböck H, Novotný L, et al. Chlamydiaceae and chlamydia-like organisms in free-living small mammals in Europe and Afghanistan. J Wildlife Dis. (2014) 50:195–204. 10.7589/2013-08-194 [DOI] [PubMed] [Google Scholar]
- 102.Schmidt-Ukaj S, Loncaric I, Klang A, Spergser J, Häbich AC, Knotek Z. Infection with Devriesea agamarum and Chrysosporium guarroi in an inland bearded dragon (Pogona vitticeps). Vet Dermatol. (2014) 25:555–97. 10.1111/vde.12146 [DOI] [PubMed] [Google Scholar]
- 103.Kissling R, Krocza W. On the diagnosis of duck-sickness in an area near illmitz, Austria. Wien Tierarztl Monatsschr. (1984) 71:327–9. [Google Scholar]
- 104.Zechmeister TC, Kirschner AKT, Fuchsberger M, Gruber SG, Süß B, Rosengarten R, et al. Prevalence of botulinum neurotoxin C1 and its corresponding gene in environmental samples from low and high risk avian botulism areas. Altex. (2005) 22:185–95. [PubMed] [Google Scholar]
- 105.Sipos W, Fischer L, Schindler M, Schmoll F. Genotyping of Clostridium perfringens isolated from domestic and exotic ruminants and swine. J Veterin Med Ser B Infect Dis Veterin Pub Health. (2003) 50:360–2. 10.1046/j.1439-0450.2003.00690.x [DOI] [PubMed] [Google Scholar]
- 106.Burgstaller J, Burgstaller A, Mansfeld MD. Infection with Clostridium septicum in a chamois (Rupicapra rupicapra). Wien Tierarztl Monatsschr. (2014) 101:98–102. [Google Scholar]
- 107.Burtscher H. Große coccidien-gewebszysten im gehirn von Gracula religiosa (Aves: Sturnidae). Zentralblatt Veterinärmedizin Reihe B. (1983) 30:590–9. 10.1111/j.1439-0450.1983.tb01885.x [DOI] [PubMed] [Google Scholar]
- 108.Prosl H, Loupal G. Diagnosis of an infestation with Collyriclum faba (Bremser, 1831) in a starling (Sturnus vulgaris L.) in Vienna. Tierarztli Praxis. (1985) 13:177–179. [PubMed] [Google Scholar]
- 109.Foerster S, Chastel C, Kaleta EF. Crane hepatitis herpesviruses. J Veterin Med Ser B. (1989) 36:433–41. 10.1111/j.1439-0450.1989.tb00625.x [DOI] [PubMed] [Google Scholar]
- 110.Kielstein P, Hotzel H, Schmalreck A, Khaschabi D, Glawischnig W. Occurrence of Cryptococcus spp. in excreta of pigeons and pet birds Mycoses. (2000) 43:7–15. 10.1046/j.1439-0507.2000.00534.x [DOI] [PubMed] [Google Scholar]
- 111.Richter B, Nedorost N, Maderner A, Weissenböck H. Detection of Cryptosporidium species in feces or gastric contents from snakes and lizards as determined by polymerase chain reaction analysis and partial sequencing of the 18S ribosomal RNA gene. J Vet Diagn Invest. (2011) 23:430–5. 10.1177/1040638711403415 [DOI] [PubMed] [Google Scholar]
- 112.Richter B, Kübber-Heiss A, Weissenböck H, Schmidt P. Detection of Cryptosporidium spp., Entamoeba spp. and Monocercomonas spp. in the gastrointestinal tract of snakes by in-situ hybridization. J Compar Pathol. (2008) 138:63–71. 10.1016/j.jcpa.2007.11.001 [DOI] [PubMed] [Google Scholar]
- 113.Nemejc K, Sak B, Kvetonova D, Hanzal V, Janiszewski P, Forejtek P, et al. Cryptosporidium suis and Cryptosporidium scrofarum in Eurasian wild boars (Sus scrofa) in central Europe. Vet Parasitol. (2013) 197:504–8. 10.1016/j.vetpar.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brack M, Hochleithner C, Hochleithner M, Zenker W. Suspected dermatophilosis in an adult orangutan (Pongo pygmaeus pygmaeus). J Zoo Wildlife Med. (1997) 28:336–41. [PubMed] [Google Scholar]
- 115.Otcenásek M, Hubálek Z, Sixl W. Survey of dermatophytes in the hair of small mammals from Austria. Folia Parasitol. (1980) 27:83–7. [PubMed] [Google Scholar]
- 116.Führer HP, Auer H, Leschnik M, Silbermayr K, Duscher G, Joachim A. Dirofilaria in humans, dogs, and vectors in Austria (1978-2014)—from imported pathogens to the endemicity of Dirofilaria repens. PLoS Negl Trop Dis. (2016) 10:e0004547 10.1371/journal.pntd.0004547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kölbl S, Schnabel H, Mikula M. Distemper as the cause of death in badgers in Austria. Tierarztliche Praxis. (1990) 18:81–4. [PubMed] [Google Scholar]
- 118.Burtscher H, Url A. Evidence of canine distemper and suggestion of preceding parvovirus-myocarditis in a eurasian badger (Meles meles). J Zoo Wildlife Med. (2007) 38:139–42. 10.1638/05-016.1 [DOI] [PubMed] [Google Scholar]
- 119.Benetka V, Leschnik M, Affenzeller N, Mösti K. Phylogenetic analysis of Austrian canine distemper virus strains from clinical samples from dogs and wild carnivores. Vet Rec. (2011) 168:377. 10.1136/vr.c6404 [DOI] [PubMed] [Google Scholar]
- 120.Kreidl P, Allerberger F, Judmaier G, Auer H, Aspock H, Hall AJ. Domestic pets as risk factors for alveolar hydatid disease in Austria. Am J Epidemiol. (1998) 147:978–81. 10.1093/oxfordjournals.aje.a009388 [DOI] [PubMed] [Google Scholar]
- 121.Eckert J, Conraths FJ, Tackmann K. Echinococcosis: an emerging or re-emerging zoonosis? Int J Parasitol. (2000) 30:1283–94. 10.1016/S0020-7519(00)00130-2 [DOI] [PubMed] [Google Scholar]
- 122.Vuitton DA, Zhou H, Bresson-Hadni S, Wang Q, Piarroux M, Raoul F, et al. Epidemiology of alveolar echinococcosis with particular reference to China and Europe. Parasitology. (2003) 127(Suppl.):87–107. 10.1017/S0031182003004153 [DOI] [PubMed] [Google Scholar]
- 123.McCulloch CR, Prosl H, Schmidt P. A spontaneous and fatal jejunal intussusception in a European brown hare associated with Eimeria leporis. J Vet Med B Infect Dis Vet Public Health. (2004) 51:470–2. 10.1111/j.1439-0450.2004.00806.x [DOI] [PubMed] [Google Scholar]
- 124.Duscher G, Steineck T, Günter P, Prosl H, Joachim A. Echinococcus multilocularis in foxes in Vienna and surrounding territories. Wien Tierarztl Monatsschr. (2005) 92:16–20. [Google Scholar]
- 125.Duscher G, Pleydell D, Prosl H, Joachim A. Echinococcus multilocularis in Austrian foxes from 1991 until 2004. J Vet Med Series B. (2006) 53:138–44. 10.1111/j.1439-0450.2006.00930.x [DOI] [PubMed] [Google Scholar]
- 126.Schneider R, Aspöck H, Auer H. Unexpected increase of alveolar echincoccosis, Austria, 2011. Emerg Infect Dis. (2013) 19:475–7. 10.3201/eid1903.120595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gottstein B, Frey CF, Campbell-Palmer R, Pizzi R, Barlow A, Hentrich B, et al. Immunoblotting for the serodiagnosis of alveolar echinococcosis in alive and dead Eurasian beavers (Castor fiber). Vet Parasitol. (2014) 205:113–8. 10.1016/j.vetpar.2014.06.017 [DOI] [PubMed] [Google Scholar]
- 128.Posautz A, Parz-Gollner R, Hölzler G, Gottstein B, Schwaiger L, Beiglböck C, et al. First record of Echinococcus multilocularis in Austrian beavers (Castor fiber). Wien Tierarztl Monatsschr. (2015) 102:74–9. [Google Scholar]
- 129.Führer HP, Blöschl I, Siehs C, Hassl A. Detection of Toxoplasma gondii, Neospora caninum, and Encephalitozoon cuniculi in the brains of common voles (Microtus arvalis) and water voles (Arvicola terrestris) by gene amplification techniques in western Austria (Vorarlberg). Parasitol Res. (2010) 107:469–73. 10.1007/s00436-010-1905-z [DOI] [PubMed] [Google Scholar]
- 130.Richter B, Csokai J, Graner I, Eisenberg T, Pantchev N, Eskens HU, et al. Encephalitozoonosis in two inland bearded dragons (Pogona vitticeps). J Comp Pathol. (2013) 148:278–82. 10.1016/j.jcpa.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 131.Bartova E, Markova J, Sedlak K. Prevalence of antibodies to Encephalitozoon cuniculi in European hares (Lepus europaeus). Ann Agric Environ Med. (2015) 22:674–6. 10.5604/12321966.1185773 [DOI] [PubMed] [Google Scholar]
- 132.Hinney B, Sak B, Joachim A, Kváč M. More than a rabbit's tale – Encephalitozoon spp. in wild mammals and birds. Int J Parasitol. (2016) 5:76–87. 10.1016/j.ijppaw.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nowotny N. Serological examinations in domestic cats for virus infections of wild rodents with potential human pathogenicity. Zentralbl Hyg Umweltmed. (1996) 198:452–61. [PubMed] [Google Scholar]
- 134.Richter B, Kübber-Heiss A, Weissenböck H. Diphtheroid colitis in a Boa constrictor infected with amphibian Entamoeba sp. Vet Parasitol. (2008) 153:164–7. 10.1016/j.vetpar.2008.01.013 [DOI] [PubMed] [Google Scholar]
- 135.Loncaric I, Stalder GL, Mehinagic K, Rosengarten R, Hoelzl F, Knauer F, et al. Comparison of ESBL - and AmpC producing enterobacteriaceae and Methicillin-Resistant Staphylococcus aureus (MRSA) isolated from migratory and resident population of rooks (Corvus frugilegus) in Austria. PLoS ONE. (2013) 8:e84048. 10.1371/journal.pone.0084048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Loncaric I, Beiglböck C, Feßler AT, Posautz A, Rosengarten R, Walzer C, et al. Characterization of ESBL- and AmpC-producing and fluoroquinolone-resistant Enterobacteriaceae isolated from mouflons (Ovis orientalis musimon) in Austria and Germany. PLoS ONE. (2016) 11:e0155786. 10.1371/journal.pone.0155786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fuchs A, Weissenböck H. Comparative histopathological study of Rabbit haemorrhagic disease (RHD) and European brown hare syndrome (EBHS). J Comp Pathol. (1992) 107:103–13. 10.1016/0021-9975(92)90100-9 [DOI] [PubMed] [Google Scholar]
- 138.Nowotny N, Ros Bascuñana C, Ballagi-Pordány A, Gavier-Widén D, Uhlén M, Belák S. Phylogenetic analysis of rabbit haemorrhagic disease and European brown hare syndrome viruses by comparison of sequences from the capsid protein gene. Arch Virol. (1997) 142:657–73. 10.1007/s007050050109 [DOI] [PubMed] [Google Scholar]
- 139.Ursprung J, Joachim A, Prosl H. Epidemiology and control of the giant liver fluke, Fascioloides magna, in a population of wild ungulates in the Danubian wetlands east of Vienna. Berl Munch Tierarztl Wochenschr. (2006) 119:316–23. [PubMed] [Google Scholar]
- 140.Haider M, Hörweg C, Liesinger K, Sattmann H, Walochnik J. Recovery of Fascioloides magna (Digenea) population in spite of treatment programme? Screening of Galba truncatula (Gastropoda, Lymnaeidae) from Lower Austria. Vet Parasitol. (2012) 187:445–51. 10.1016/j.vetpar.2012.01.032 [DOI] [PubMed] [Google Scholar]
- 141.Hörweg C, Prosl H, Wille-Piazzai W, Joachim A, Sattmann H. Prevalence of Fascioloides magna in Galba truncatula in the Danube backwater area east of Vienna, Austria. Wien Tierarztl Monatsschr. (2011) 98:261–7. [Google Scholar]
- 142.Ursprung J, Prosl H. The American Giant Liver Fluke (Fascioloides magna) in the Danube floodplain forests east of Vienna, Austria. Occurrence and control 2000 - 2010. Wien Tierarztl Monatsschr. (2011) 98:275–84. [Google Scholar]
- 143.Sattmann H, Hörweg C, Gaub L, Feix AS, Haider M, Walochnik J, et al. Wherefrom and whereabouts of an alien: the American liver fluke Fascioloides magna in Austria: an overview. Wiener klinische Wochenschrift. (2014) 126 (Suppl.1):23–31. 10.1007/s00508-014-0499-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Husch C, Sattmann H, Hoerweg C, Ursprung J, Walochnik J. Genetic homogeneity of Fascioloides magna in Austria. Vet Parasitol. (2017) 243:75–8. 10.1016/j.vetpar.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 145.Walzer C, Url A, Robert N, Kübber-Heiss A, Nowotny N, Schmidt P. Idiopathic acute onset myelopathy in cheetah (Acinonyx jubatus) cubs. J Zoo Wildlife Med. (2003) 34:36–46. 10.1638/1042-7260(2003)34[0036:IAOMIC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 146.Weissenböck H, Hubálek Z, Bakonyi T, Nowotny N. Zoonotic mosquito-borne flaviviruses: Worldwide presence of agents with proven pathogenicity and potential candidates of future emerging diseases. Vet Microbiol. (2010) 140:271–80. 10.1016/j.vetmic.2009.08.025 [DOI] [PubMed] [Google Scholar]
- 147.Hubálek Z, Sixl W, Halouzka J. Francisella tularensis in Dermacentor reticulatus ticks from the Czech Republic and Austria. Wiener klinische Wochenschrift. (1998) 110:909–10. [PubMed] [Google Scholar]
- 148.Deutz A, Guggenberger T, Gasteiner J, Steineck T, Bagó Z, Hofer E, et al. Investigation of the prevalence of tularaemia under the aspect of climate change. Wien Tierarztl Monatsschr. (2009) 96:107–13. [Google Scholar]
- 149.Visser M, Messner C, Rehbein S. Massive infestation with fur mites (Lynxacarus mustelae) of a stone marten (Martes foina) from Tyrol. Wiener klinische Wochenschrift. (2011) 123 (Suppl. 1):36–42. 10.1007/s00508-011-0005-0 [DOI] [PubMed] [Google Scholar]
- 150.Juncker-Voss M, Kübber-Heiss A, Kutzer E, Kanzler P, Schratter D, Prosl H. Geopetitia aspiculata: Endemic in zoobirds in Austria. Wien Tierarztl Monatsschr. (2001) 88:106–13. [Google Scholar]
- 151.Aberle SW, Lehner P, Ecker M, Aberle JH, Arneitz K, Khanakah G, et al. Nephropathia epidemica and Puumala virus in Austria. Eur J Clin Microbiol Infect Dis. (1999) 18:467–72. 10.1007/s100960050325 [DOI] [PubMed] [Google Scholar]
- 152.Plyusnina A, Aberle S, Aberle J, Plyusnin A. Genetic analysis of Puumala hantavirus strains from Austria. Scand J Infect Dis. (2006) 38:512–9. 10.1080/00365540600585040 [DOI] [PubMed] [Google Scholar]
- 153.Duscher G, Kubber-Heiss A, Richter B, Suchentrunk F. A golden jackal (Canis aureus) from Austria bearing Hepatozoon canis - import due to immigration into a non-endemic area? Ticks Tick Borne Dis. (2013) 4:133–7. 10.1016/j.ttbdis.2012.10.040 [DOI] [PubMed] [Google Scholar]
- 154.Kübber-Heiss A, Schilcher F, Möstl K. Herpesvirus-infections in tortoises in Austria. Wien Tierarztl Monatsschr. (1999) 86:78–82. [Google Scholar]
- 155.Bauder B, Kübber-Heiss A, Steineck T, Kuttin ES, Kaufman L. Granulomatous skin lesions due to histoplasmosis in a badger (Meles meles) in Austria. Medical Mycology. (2000) 38:249–53. 10.1080/mmy.38.3.249.253 [DOI] [PubMed] [Google Scholar]
- 156.Tsangaras K, Siracusa MC, Nikolaidis N, Ishida Y, Cui P, Vielgrader H, et al. Hybridization capture reveals evolution and conservation across the entire koala retrovirus genome. PLoS ONE. (2014) 9:e95633. 10.1371/journal.pone.0095633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Deutz A, Fuchs K, Schuller W, Müller M, Kerbl U, Klement C. Studies on the seroprevalence of antibodies against Leptospira interrogans in hunters and wild boar from south-eastern Austria. Eur J Wildlife Res. (2002) 48:60–5. 10.1007/BF02285358 [DOI] [Google Scholar]
- 158.Paulsen P, Winkelmayer R. Seasonal variation in the microbial contamination of game carcasses in an Austrian hunting area. Eur J Wildlife Res. (2004) 50:157–9. 10.1007/s10344-004-0054-z [DOI] [Google Scholar]
- 159.Linke K, Rückerl I, Brugger K, Karpiskova R, Walland J, Muri-Klinger S, et al. Reservoirs of Listeria species in three environmental ecosystems. Appl Environ Microbiol. (2014) 80:5583–92. 10.1128/AEM.01018-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Benetka V, Krametter-Froetscher R, Baumgartner W, Moestl K. Investigation of the role of Austrian ruminant wildlife in the epidemiology of malignant catarrhal fever viruses. J Wildl Dis. (2009) 45:508–11. 10.7589/0090-3558-45.2.508 [DOI] [PubMed] [Google Scholar]
- 161.Loncaric I, Kübber-Heiss A, Posautz A, Stalder GL, Hoffmann D, Rosengarten R, et al. Characterization of methicillin-resistant Staphylococcus spp. carrying the mecC gene, isolated from wildlife. J Antimicrob Chemother. (2013) 68:2222–5. 10.1093/jac/dkt186 [DOI] [PubMed] [Google Scholar]
- 162.Loncaric I, Kübber-Heiss A, Posautz A, Stalder GL, Hoffmann D, Rosengarten R, et al. mecC- and mecA-positive meticillin-resistant Staphylococcus aureus (MRSA) isolated from livestock sharing habitat with wildlife previously tested positive for mecC-positive MRSA. Vet Dermatol. (2014) 25:147–8. 10.1111/vde.12116 [DOI] [PubMed] [Google Scholar]
- 163.Deutz A, Spergser J, Rosengarten R, Köfer J. First detection of intrauterine transmission of paratuberculosis in red deer and chamois. Z Jagdwiss. (2003) 49:314–9. 10.1007/BF02189639 [DOI] [Google Scholar]
- 164.Deutz A, Spergser J, Wagner P, Steineck T, Köfer J, Rosengarten R. Paratuberculosis in wild animals - An observed increase in the clinical incidence. Tierarztl Umsch. (2003) 58:482–9. [Google Scholar]
- 165.Deutz A, Spergser J, Wagner P, Rosengarten R, Köfer J. Mycobacterium avium subsp. paratuberculosis in wild animal species and cattle in Styria/Austria. Berl Munch Tierarztl Wochenschr. (2005) 118:314–20. [PubMed] [Google Scholar]
- 166.Glawischnig W, Steineck T, Spergser J. Infections caused by Mycobacterium avium subspecies avium, hominissuis, and paratuberculosis in free-ranging red deer (Cervus elaphus hippelaphus) in Austria, 2001-2004. J Wildlife Dis. (2006) 42:724–31. 10.7589/0090-3558-42.4.724 [DOI] [PubMed] [Google Scholar]
- 167.Spergser J, Fuchs K, Deutz A. Molecular characterisation of M. avium subsp paratuberculosis isolated from cattle and wild animal species from Styria. Wien Tierarztl Monatsschr. (2006) 93:47–52. [Google Scholar]
- 168.Gerritsmann H, Stalder GL, Spergser J, Hoelzl F, Deutz A, Kuebber-Heiss A, et al. Multiple strain infections and high genotypic diversity among Mycobacterium avium subsp. paratuberculosis field isolates from diseased wild and domestic ruminant species in the eastern Alpine region of Austria. Infect Genet Evol. (2014) 21:244–51. 10.1016/j.meegid.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 169.Glawischnig W, Khaschabi D. Paratuberculosis in a free living red deer (Cervus elaphus hippelaphus) from Vorarlberg. Wien Tierarztl Monatsschr. (2001) 88:66–9. [Google Scholar]
- 170.Scope A, Spergser J, Vobornik A, Gumpenberger M, Hooijberg EH, Reifinger M. Atypical mycobacteriosis in a ural owl (Strix uralensis, PALLAS 1771) from the Austrian reintroduction project. Wien Tierarztl Monatsschr. (2013) 100:85–92. [Google Scholar]
- 171.Rivière J, Carabin K, Le Strat Y, Hendrikx P, Dufour B. Bovine tuberculosis surveillance in cattle and free-ranging wildlife in EU Member States in 2013: A survey-based review. Vet Microbiol. (2014) 173:323–31. 10.1016/j.vetmic.2014.08.013 [DOI] [PubMed] [Google Scholar]
- 172.Prodinger WM, Eigentler A, Allerberger F, Schönbauer M, Glawischnig W. Infection of red deer, cattle, and humans with Mycobacterium bovis subsp. caprae in western Austria. J Clin Microbiol. (2002) 40:2270–2. 10.1128/JCM.40.6.2270-2272.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Glawischnig W, Allerberger F, Messner C, Schönbauer M, Prodinger WM. Tuberculosis in free-living red deer (Cervus elaphus hippelaphus) in the northern Alps. Wien Tierarztl Monatsschr. (2003) 90:38–44. [Google Scholar]
- 174.Prodinger WM, Brandstatter A, Naumann L, Pacciarini M, Kubica T, Boschiroli ML, et al. Characterization of Mycobacterium caprae isolates from Europe by mycobacterial interspersed repetitive unit genotyping. J Clin Microbiol. (2005) 43:4984–92. 10.1128/JCM.43.10.4984-4992.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Weikel J, Glawischnig W, Hofer E, Schoepf K. Tuberculosis in a deer (Capreolus capreolus) detected in Tyrol (Austria). Wien Tierarztl Monatsschr. (2010) 97:287–9. [Google Scholar]
- 176.Schoepf K, Prodinger WM, Glawischnig W, Hofer E, Revilla-Fernandez S, Hofrichter J, et al. A two-years' survey on the prevalence of tuberculosis caused by Mycobacterium caprae in red deer (Cervus elaphus) in the Tyrol, Austria. Vet Sci. (2012) 2012:245138. 10.5402/2012/245138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rettinger A, Broeckl S, Fink M, Prodinger WM, Blum H, Krebs S, et al. The region of difference four is a robust genetic marker for subtyping mycobacterium caprae isolates and is linked to spatial distribution of three subtypes. Transbound Emerg Dis. (2017) 64:782–92. 10.1111/tbed.12438 [DOI] [PubMed] [Google Scholar]
- 178.Belloy L, Janovsky M, Vilei EM, Pilo P, Giacometti M, Frey J. Molecular epidemiology of Mycoplasma conjunctivae in Caprinae: Transmission across species in natural outbreaks. Appl Environ Microbiol. (2003) 69:1913–9. 10.1128/AEM.69.4.1913-1919.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zimmermann L, Jambresic S, Giacometti M, Frey J. Specificity of Mycoplasma conjunctivae strains for alpine chamois Rupicapra r. rupicapra. Wildlife Biol. (2008) 14:118–24. 10.2981/0909-6396(2008)14[118:SOMCSF]2.0.CO;2 [DOI] [Google Scholar]
- 180.Friedrich C, Ingolic E, Freitag B, Kastberger G, Hohmann V, Skofitsch G, et al. A myxozoan-like parasite causing xenomas in the brain of the mole, Talpa europaea L., 1758 (Vertebrata, Mammalia). Parasitology. (2000) 121:483–92. 10.1017/S0031182099006769 [DOI] [PubMed] [Google Scholar]
- 181.Wanha K, Edelhofer R, Gabler-Eduardo C, Prosl H. Prevalence of antibodies against Neospora caninum and Toxoplasma gondii in dogs and foxes in Austria. Vet Parasitol. (2005) 128:189–93. 10.1016/j.vetpar.2004.11.027 [DOI] [PubMed] [Google Scholar]
- 182.Bartova E, Sedlak K, Treml F, Holko I, Literak I. Neospora caninum and Toxoplasma gondii antibodies in European brown hares in the Czech Republic, Slovakia and Austria. Vet Parasitol. (2010) 171:155–8. 10.1016/j.vetpar.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 183.Cronstedt-Fell A, Richter B, Voracek T, Kübber-Heiss A. Neosporosis in a Captive Parma Wallaby (Macropus parma). J Comp Pathol. (2012) 146:274–7. 10.1016/j.jcpa.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 184.Posautz A, Loncaric I, Kübber-Heiss A, Knoll A, Walzer C. Acute die-off of chamois (Rupicapra rupicapra) in the eastern Austrian alps due to bacterial bronchopneumonia with Pasteurellaceae. J Wildlife Dis. (2014) 50:616–20. 10.7589/2013-04-090 [DOI] [PubMed] [Google Scholar]
- 185.Loupal G, Kutzer E. Infections with Plasmodium spec, in puffins (Fractercula arctica). Kleintierpraxis. (1996) 41:901–6. [Google Scholar]
- 186.Dinhopl N, Mostegl MM, Richter B, Nedorost N, Maderner A, Fragner K, et al. Application of in-situ hybridization for the detection and identification of avian malaria parasites in paraffin wax-embedded tissues from captive penguins. Avian Pathol. (2011) 40:315–20. 10.1080/03079457.2011.569533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dinhopl N, Nedorost N, Mostegl MM, Weissenbacher-Lang C, Weissenbock H. In situ hybridization and sequence analysis reveal an association of Plasmodium spp. with mortalities in wild passerine birds in Austria. Parasitol Res. (2015) 114:1455–62. 10.1007/s00436-015-4328-z [DOI] [PubMed] [Google Scholar]
- 188.Hess M, Scope A, Heincz U. Comparitive sensitivity of polymerase chain reaction diagnosis of psittacine beak and feather disease on feather samples, cloacal swabs and blood from budgerigars (Melopsittacus undulates, Shaw 18005). Avian Pathol. (2004) 33:477–81. 10.1080/03079450400003619 [DOI] [PubMed] [Google Scholar]
- 189.Jahn J, Timischl W. Mathematical-analysis of the spread of wildlife rabies in Austria. Empirical and theoretical background. Wien Tierarztl Monatsschr. (1984) 71:149–55. [Google Scholar]
- 190.Kollaritsch H, Maurer W. Tollwut: Epidemiologie, prä- und postexpositionelle Immunisierung. Wien Klinis Wochenschr. (2006) 118:312–20. 10.1007/s00508-006-0606-1 [DOI] [PubMed] [Google Scholar]
- 191.Müller T, Bätza HJ, Beckert A, Bunzenthal C, Cox JH, Freuling CM, et al. Analysis of vaccine-virus-associated rabies cases in red foxes (Vulpes vulpes) after oral rabies vaccination campaigns in Germany and Austria. Arch Virol. (2009) 154:1081–91. 10.1007/s00705-009-0408-7 [DOI] [PubMed] [Google Scholar]
- 192.Kölbl S, Settele J, Schönbauer M. The first appearance of infectious hemorrhagic disease of rabbits in Austria. Berl Munch Tierarztl Wochenschr. (1990) 103:261–6. [PubMed] [Google Scholar]
- 193.Ryll R, Bernstein S, Heuser E, Schlegel M, Dremsek P, Zumpe M, et al. Detection of Rat hepatitis E virus in wild Norway rats (Rattus norvegicus) and Black rats (Rattus rattus) from 11 European countries. Vet Microbiol. (2017) 208:58–68. 10.1016/j.vetmic.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 194.Erdélyi K, Gál J, Sugár L, Ursu K, Forgách P, Szeredi L, et al. Papillomavirus-associated fibropapillomas of red deer (Cervus elaphus). Acta Vet Hungar. (2009) 57:337–44. 10.1556/AVet.57.2009.2.14 [DOI] [PubMed] [Google Scholar]
- 195.Geue L, Löschner U. Salmonella enterica in reptiles of German and Austrian origin. Vet Microbiol. (2002) 84:79–91. 10.1016/S0378-1135(01)00437-0 [DOI] [PubMed] [Google Scholar]
- 196.Glawischnig W, Lazar J, Wallner A, Kornschober C. Cattle-derived Salmonella enterica serovar dublin infections in red foxes (Vulpes vulpes) in Tyrol, Austria. J Wildlife Dis. (2017) 53:361–3. 10.7589/2016-04-087 [DOI] [PubMed] [Google Scholar]
- 197.Pfleger S, Benyr G, Sommer R, Hassl A. Pattern of Salmonella excretion in amphibians and reptiles in a vivarium. Int J Hyg Environ Health. (2003) 206:53–9. 10.1078/1438-4639-00184 [DOI] [PubMed] [Google Scholar]
- 198.Awad-Masalmeh M, Romanik G, Herkal C. The occurrence of Salmonellae in reptiles kept privateley or in zoos in Austria. Tierarztliche Umschau. (2005) 60:70–4. [Google Scholar]
- 199.Paulsen P, Smulders FJM, Hilbert F. Salmonella in meat from hunted game: a Central European perspective. Food Res Int. (2012) 45:609–16. 10.1016/j.foodres.2011.06.055 [DOI] [Google Scholar]
- 200.Hilbert F, Smulders FJM, Chopra-Dewasthaly R, Paulsen P. Salmonella in the wildlife-human interface. Food Res Int. (2012) 45:603–8. 10.1016/j.foodres.2011.08.015 [DOI] [Google Scholar]
- 201.Fuchs K, Deutz A, Gressmann G. Detection of space-time clusters and epidemiological examinations of scabies in chamois. Vet Parasitol. (2000) 92:63–73. 10.1016/S0304-4017(00)00269-7 [DOI] [PubMed] [Google Scholar]
- 202.Gressmann G, Deutz A. Considerations on reducing the risk of scabies infestation in chamois by targeted hunting of individual age classes. Z Jagdwiss. (2001) 47:34–42. 10.1007/BF02242412 [DOI] [Google Scholar]
- 203.Monecke S, Gavier-Widén D, Hotzel H, Peters M, Guenther S, Lazaris A, et al. Diversity of Staphylococcus aureus isolates in european wildlife. PLoS ONE. (2016) 11:e0168433. 10.1371/journal.pone.0168433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Führer HP, Siehs C, Schneider R, Auer H. Morphometrical analysis of Taenia taeniaeformis and Taenia crassiceps in the common vole (Microtus arvalis) and the water vole (Arvicola terrestris) in Vorarlberg, Austria. Helminthologia. (2012) 49:169–73. 10.2478/s11687-012-0034-x [DOI] [Google Scholar]
- 205.Führer HP, Biro N, Harl J, Worliczek HL, Beiglbock C, Farkas R, et al. Molecular detection of Theileria sp. ZS TO4 in red deer (Cervus elaphus) and questing Haemaphysalis concinna ticks in Eastern Austria. Vet Parasitol. (2013) 197:653–7. 10.1016/j.vetpar.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 206.Bagó Z, Bauder B, Kolodziejek J, Nowotny N, Weissenböck H. Tickborne encephalitis in a mouflon (Ovis ammon musimon). Vet Rec. (2002) 150:218–20. 10.1136/vr.150.7.218 [DOI] [PubMed] [Google Scholar]
- 207.Duscher G, Wetscher M, Baumgartner R, Walder G. Roe deer sera used for TBE surveillance in Austria. Ticks Tick Borne Dis. (2015) 6:489–93. 10.1016/j.ttbdis.2015.03.018 [DOI] [PubMed] [Google Scholar]
- 208.Deutz A, Fuchs K, Auer H, Kerbl U, Aspöck H, Köfer J. Toxocara-infestations in Austria: a study on the risk of infection of farmers, slaughterhouse staff, hunters and veterinarians. Parasitol Res. (2005) 97:390–4. 10.1007/s00436-005-1469-5 [DOI] [PubMed] [Google Scholar]
- 209.Auer H, Aspöck H. The diagnosis of Toxocara infestations and of human toxocarosis. Lab Med. (2006) 30:1–12. 10.1515/JLM.2006.001 [DOI] [Google Scholar]
- 210.Basso W, Edelhofer R, Zenker W, Möstl K, Kübber-Heiss A, Prosl H. Toxoplasmosis in Pallas' cats (Otocolobus manul) raised in captivity. Parasitology. (2005) 130:293–9. 10.1017/S0031182004006584 [DOI] [PubMed] [Google Scholar]
- 211.Edelhofer R, Prossinger H. Infection with Toxoplasma gondii during pregnancy: seroepidemiological studies in Austria. Zoonoses Public Health. (2010) 57:18–26. 10.1111/j.1863-2378.2009.01279.x [DOI] [PubMed] [Google Scholar]
- 212.Posautz A, Leidinger E, Knauer F, Hoffmann D, Suchentrunk F, Walzer C, et al. Seroprevalence of Treponema sp. in European brown hares (Lepus europaeus) in Austria and Germany. Wien Tierarztl Monatsschr. (2014) 101:281–5. [Google Scholar]
- 213.Krois E, Nöckler K, Duscher G, Joachim A, Kapel CMO, Prosl H. Trichinella britovi in Austrian red foxes (Vulpes vulpes). Wien Tierarztl Monatsschr. (2005) 92:308–14. [Google Scholar]
- 214.Glawischnig W, Wunsch A, Fötschl H, Bagó Z, Vanek E. First report of Trichinella pseudospiralis in wild boar in Austria. Wien Tierarztl Monatsschr. (2016) 103:183–187. [Google Scholar]
- 215.Edelhofer R, Prosl H, Kutzer E. Trichinellosis and toxoplasmosis in wild pigs of Eastern Austria. Wien Tierarztl Monatsschr. (1996) 83:225–9. [Google Scholar]
- 216.Pozio E. Trichinellosis in the European union: epidemiology, ecology and economic impact. Parasitol Today. (1998) 14:35–8. 10.1016/S0169-4758(97)01165-4 [DOI] [PubMed] [Google Scholar]
- 217.Paulsen P, Winkelmayer R, Gneist M, Riedl C, Gansterer A, Gabler C, et al. Establishing a Trichinella proficiency testing scheme in Austria. First communication: a preliminary study on Trichinella inspection of wild boar meat in Lower Austria by authorized trained hunters employing the compression method. Wien Tierarztl Monatsschr. (2003) 90:91–7. [Google Scholar]
- 218.Auer H. Human trichinellosis in Austria. Wien Tierarztl Monatsschr. (2005) 92:288–94. [Google Scholar]
- 219.Sattmann H, Prosl H. History of early research on trichinellae and trichinelloses. Wien Tierarztl Monatsschr. (2005) 92:283–7. [Google Scholar]
- 220.Winkelmayer R, Paulsen P. Trichinella examination of free range wild boar in Lower Austria. Wien Tierarztl Monatsschr. (2005) 92:322–4. [Google Scholar]
- 221.Duscher G, Winkelmayer R, Prosl H. Wild boar distribution in Austrian areas with Trichinella spp. findings in red foxes. Wien Tierarztl Monatsschr. (2005) 92:315–21. [Google Scholar]
- 222.Grabensteiner E, Bilic I, Kolbe T, Hess M. Molecular analysis of clonal trichomonad isolates indicate the existence of heterogenic species present in different birds and within the same host. Vet Parasitol. (2010) 172:53–64. 10.1016/j.vetpar.2010.04.015 [DOI] [PubMed] [Google Scholar]
- 223.Ganas P, Jaskulska B, Lawson B, Zadravec M, Hess M, Bilic I. Multi-locus sequence typing confirms the clonality of Trichomonas gallinae isolates circulating in European finches. Parasitology. (2014) 141:652–61. 10.1017/S0031182013002023 [DOI] [PubMed] [Google Scholar]
- 224.Schilcher F, Kübber-Heiss A, Breuer-Strosberg R, Barth S. Dermatomycosis due to Trichphyton terrestre in Madagascar Day Geckos (Phelsuma m. madagascariensis). Wien Tierarztl Monatsschr. (1998) 85:131–5. [Google Scholar]
- 225.Mostegl MM, Richter B, Nedorost N, Maderner A, Dinhopl N, Kübber-Heiss A, et al. Identification of a putatively novel trichomonad species in the intestine of a common quail (Coturnix coturnix). Vet Parasitol. (2012) 183:369–72. 10.1016/j.vetpar.2011.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Weissenböck H, Kolodziejek J, Url A, Lussy H, Rebel-Bauder B, Nowotny N. Emergence of Usutu virus, an African mosquito-borne Flavivirus of the Japanese encephalitis virus group, central Europe. Emerg Infect Dis. (2002) 8:652–6. 10.3201/eid0807.020094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Weissenböck H, Kolodziejek J, Fragner K, Kuhn R, Pfeffer M, Nowotny N. Usutu virus activity in Austria, 2001-2002. Microb Infect. (2003) 5:1132–6. 10.1016/S1286-4579(03)00204-1 [DOI] [PubMed] [Google Scholar]
- 228.Chvala S, Kolodziejek J, Nowotny N, Weissenböck H. Pathology and viral distribution in fatal Usutu virus infections of birds from the 2001 and 2002 outbreaks in Austria. J Comp Pathol. (2004) 131:176–85. 10.1016/j.jcpa.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 229.Bakonyi T, Gould EA, Kolodziejek J, Weissenböck H, Nowotny N. Complete genome analysis and molecular characterization of Usutu virus that emerged in Austria in 2001: comparison with the South African Strain SAAR-1776 and other flaviviruses. Virology. (2004) 328:301–10. 10.1016/S0042-6822(04)00525-2 [DOI] [PubMed] [Google Scholar]
- 230.Hassler D, Pfeffer M, Weissenbock H, Nowotny N. Usutu virus in Austria reminds one of the West Nile virus in the USA. Dtsch Med Wochenschr. (2004) 129:2339–40. [PubMed] [Google Scholar]
- 231.Weissenböck H, Bakonyi T, Chvala S, Nowotny N. Experimental Usutu virus infection of suckling mice causes neuronal and glial cell apoptosis and demyelination. Acta Neuropathol. (2004) 108:453–60. 10.1007/s00401-004-0916-1 [DOI] [PubMed] [Google Scholar]
- 232.Chvala S, Bakonyi T, Bukovsky C, Meister T, Brugger K, Rubel F, et al. Monitoring of Usutu virus activity and spread by using dead bird surveillance in Austria, 2003-2005. Vet Microbiol. (2007) 122:237–45. 10.1016/j.vetmic.2007.01.029 [DOI] [PubMed] [Google Scholar]
- 233.Meister T, Lussy H, Bakonyi T, Sikutova S, Rudolf I, Vogl W, et al. Serological evidence of continuing high Usutu virus (Flaviviridae) activity and establishment of herd immunity in wild birds in Austria. Vet Microbiol. (2008) 127:237–48. 10.1016/j.vetmic.2007.08.023 [DOI] [PubMed] [Google Scholar]
- 234.Rubel F, Brugger K, Hantel M, Chvala-Mannsberger S, Bakonyi T, Weissenböck H, et al. Explaining Usutu virus dynamics in Austria: Model development and calibration. Prev Vet Med. (2008) 85:166–86. 10.1016/j.prevetmed.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 235.Brugger K, Rubel F. Simulation of climate-change scenarios to explain Usutu-virus dynamics in Austria. Prev Vet Med. (2009) 88:24–31. 10.1016/j.prevetmed.2008.06.023 [DOI] [PubMed] [Google Scholar]
- 236.Buchebner N, Zenker W, Wenker C, Steinmetz HW, Sós E, Lussy H, et al. Low Usutu virus seroprevalence in four zoological gardens in central Europe. BMC Vet Res. (2013) 9:153. 10.1186/1746-6148-9-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Stiasny K, Aberle SW, Heinzl FX. Retrospective identification of human cases of West nile virus infection in Austria (2009 to 2010) by serological differentiation from Usutu and other flavivirus infections. Eurosurveillance. (2013) 18:20614. 10.2807/1560-7917.ES2013.18.43.20614 [DOI] [PubMed] [Google Scholar]
- 238.Bakonyi T, Busquets N, Nowotny N. Comparison of complete genome sequences of Usutu virus strains detected in Spain, Central Europe, and Africa. Vector Borne Zoon Dis. (2014) 14:324–9. 10.1089/vbz.2013.1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Bakonyi T, Erdélyi K, Brunthaler R, Dán Á, Weissenböck H, Nowotny N. Usutu virus, Austria and Hungary, 2010-2016. Emerg Microb Infect. (2017) 6:85. 10.1038/emi.2017.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Freidl G, Stalder G, Kostić T, Sessitsch A, Beiglböck C, Walzer C. Verocytotoxin-producing Escherichia coli in chamois (Rupicapra rupicapra) and cattle in Austria. J Wildlife Dis. (2011) 47:704–8. 10.7589/0090-3558-47.3.704 [DOI] [PubMed] [Google Scholar]
- 241.Weissenböck H, Hubálek Z, Halouzka J, Pichlmair A, Maderner A, Fragner K, et al. Screening for West Nile virus infections of susceptible animal species in Austria. Epidemiol Infect. (2003) 131:1023–7. 10.1017/S0950268803001031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Linke S, Muehlen M, Niedrig M, Ellerbrok H, Kaiser A, Fiedler W, et al. Assessing the exposure of German and Austrian bird ringers to West Nile virus (Flavivirus) and evaluating their potential risk of infection. J Ornithol. (2008) 149:271–5. 10.1007/s10336-007-0270-x [DOI] [Google Scholar]
- 243.Wodak E, Richter S, Bagó Z, Revilla-Fernández S, Weissenböck H, Nowotny N, et al. Detection and molecular analysis of West Nile virus infections in birds of prey in the eastern part of Austria in 2008 and 2009. Vet Microbiol. (2011) 149:358–66. 10.1016/j.vetmic.2010.12.012 [DOI] [PubMed] [Google Scholar]
- 244.Bakonyi T, Ferenczi E, Erdelyi K, Kutasi O, Csorgo T, Seidel B, et al. Explosive spread of a neuroinvasive lineage 2 West Nile virus in Central Europe, 2008/2009. Vet Microbiol. (2013) 165:61–70. 10.1016/j.vetmic.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 245.Beck C, Jimenez-Clavero MA, Leblond A, Durand B, Nowotny N, Leparc-Goffart I, et al. Flaviviruses in europe: complex circulation patterns and their consequences for the diagnosis and control of west nile disease. Int J Environ Res Public Health. (2013) 10:6049–83. 10.3390/ijerph10116049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hernández-Triana LM, Jeffries CL, Mansfield KL, Carnell G, Fooks AR, Johnson N. Emergence of West Nile virus lineage 2 in Europe: a review on the introduction and spread of a mosquito-borne disease. Front Public Health. (2014) 2:271. 10.3389/fpubh.2014.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Rizzoli A, Jiménez-Clavero MA, Barzon L, Cordioli P, Figuerola J, Koraka P, et al. The challenge of West nile virus in Europe: knowledge gaps and research priorities. Eurosurveillance. (2015) 20:21135. 10.2807/1560-7917.ES2015.20.20.21135 [DOI] [PubMed] [Google Scholar]
- 248.Bakonyi T, Gajdon GK, Schwing R, Vogl W, Häbich AC, Thaller D, et al. Chronic West Nile virus infection in kea (Nestor notabilis). Vet Microbiol. (2016) 183:135–9. 10.1016/j.vetmic.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 249.Bidaisee S, Macpherson CNL. Zoonoses and one health: a review of the literature. J Parasitol Res. (2014) 2014:874345. 10.1155/2014/874345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Austrian Agency for Health and Food Safety (AGES). Transmissible Spongiforme Enzephalopathie (TSE). (2019). Available online at: https://www.ages.at/themen/krankheitserreger/tse-bsescrapiecwd/tab/3/ (accessed May 10, 2019).
- 251.European Food Safety Authority (EFSA). Chronic Wasting Disease: Addressing Risks for the EU (CWD). (2017). Available online at: https://www.efsa.europa.eu/en/press/news/170118 (accessed May 6, 2019).
- 252.Austrian Agency for Health and Food Safety (AGES). Usutu Virus. (2019). Available online at: https://www.ages.at/themen/krankheitserreger/usutu-virus/tab/1/ (accessed May 9, 2019).
- 253.Dobson A, Foufopoulos J. Emerging infectious pathogens of wildlife. Philo Trans R Soc B Biol Sci. (2001) 356:1001–12. 10.1098/rstb.2001.0900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.World Organisation for Animal Health (OIE). OIE Listed Diseases. (2019). Available online at: http://www.oie.int/en/animal-health-in-the-world/oie-listed-diseases-2019/ (accessed May 7, 2019).
- 255.Vetter SG, Ruf T, Bieber C, Arnold W. What is a mild winter? Regional differences in within-species responses to climate change. PLoS ONE. (2015) 10:e0132178. 10.1371/journal.pone.0132178 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.