Abstract
Blood serum is enriched in lipids and has provided a platform to understand the pathogenesis of a number of human diseases with improved diagnosis and development of biomarkers. Understanding lipid changes in neurodegenerative diseases is particularly important because of the fact that lipids make up >50% of brain tissues. Frontotemporal dementia (FTD) is a common cause of early onset dementia, characterized by brain atrophy in the frontal and temporal regions, concomitant loss of lipids and dyslipidemia. However, little is known about the link between dyslipidemia and FTD pathophysiology. Here, we utilized an innovative approach – lipidomics based on mass spectrometry – to investigate three key aspects of FTD pathophysiology – mitochondrial dysfunction, inflammation, and oxidative stress. We analyzed the lipids that are intrinsically linked to neurodegeneration in serum collected from FTD patients and controls. We found that cardiolipin, acylcarnitine, lysophosphatidylcholine, platelet-activating factor, o-acyl-ω-hydroxy fatty acid and acrolein were specifically altered in FTD with strong correlation between the lipids, signifying pathophysiological changes in FTD. The lipid changes were verified by measurement of the common disease markers (e.g. ATP, cytokine, calcium) using conventional assays. When put together, these results support the use of lipidomics technology to detect pathophysiological changes in FTD.
Subject terms: Lipidomics, Development of the nervous system
Introduction
Lipidomics is a systems-level analysis and characterization of lipids1,2. Like other “omics” analyses, such as transcriptomics and proteomics, lipidomics is a global profiling of lipid species present in cells, tissues or body fluids3. Lipidomics allows detection and quantification of thousands of different lipid species of a broad range of lipid classes4. It has facilitated a greater understanding of the pathophysiology of a range of human diseases, particularly cardiovascular disease and diabetes. It has also provided invaluable data to identify risk factors and develop biomarkers for disease identification and classification. Recent advances in lipidomics technology based on mass spectrometry have significantly improved the detection of a vast array of lipids present in blood serum. Importantly, it has allowed detection of small, yet significant, differences in lipid levels that are intrinsically linked to disease processes. Application of lipidomics in the study of neurodegenerative diseases is particularly imperative because of the fact that lipids make up 39.6% of the brain grey matter and 64.6% of the white matter5.
Frontotemporal dementia (FTD) is the second most common form of younger-onset dementia after Alzheimer’s disease (AD), of which the main clinical syndrome is the behavioral variant (bvFTD)6. Clinically, bvFTD is characterized by progressive changes in behavior and personality, loss of empathy, apathy, as well as variable cognitive deficits that include executive function, language and, in a subset episodic memory. Pathologically, FTD is characterized by brain atrophy in the frontal and temporal regions, and concomitant loss of lipids7–10. Although some of the clinical features of FTD overlap with those found in AD, the underlying brain cellular pathologies are distinctive in the two diseases. In FTD, aggregates of microtubule associated protein tau (MAPT), tar DNA-binding protein-43 (TDP43) or fused in sarcoma (FUS) are present in the brain; whereas in AD, aggregates of amyloid-β peptides or neurofibrillary tangles (composed of tau) are present in the brain.
Blood fluids of FTD patients have been analyzed using conventional lipid assays based on enzymes, and these have shown that triglyceride (TG) levels are increased in FTD compared to controls11. Global lipid analysis has also shown that TG levels are increased in FTD compared to controls, along with changes in other lipids12. Furthermore, correlation studies have shown that TG levels are positively correlated with body mass index (BMI), whereas HDL cholesterol levels are negatively correlated with BMI11. Both TG and HDL cholesterol levels are also correlated to eating behavior (fat intake) and measures of cognition and disease duration13. Although these findings suggest manifestation of lipid dysregulation, the exact relationship between lipid dysregulation and FTD pathophysiology is largely unknown.
In the current study, we undertook lipidomics analysis of FTD serum to investigate three key aspects of FTD pathophysiology relevant to neurodegeneration: mitochondrial dysfunction, inflammation, and oxidative stress. We focused on specific lipids that are intrinsically involved in each of these three pathological processes. In the mitochondrial dysfunction study, we analyzed cardiolipin and acylcarnitine, both of which are involved in mitochondrial energy production14,15. In the inflammation study, we analyzed two pro-inflammatory lipids lysophosphatidylcholine and platelet-activating factor; both play important roles in innate immunity in the host defense cells16,17. Finally, in the oxidative stress study, we examined the three major lipid aldehydes, acrolein, malondialdehyde and 4-hydroxynonenal, all of which are known to cause oxidative damage to cells. The first aim of our study was to detect pathophysiological changes in FTD using serum lipids. The second aim was to uncover lipid species and lipid synthesis pathways that could be exploited to develop biomarkers for FTD.
Materials and Methods
Chemicals and materials
Lipids were extracted using chloroform, methanol and isopropanol (Sigma Aldrich, St. Louis, MO, USA) and ultrapure water (Millipore). All solvents used were HPLC grade or higher. Glass pipettes and tubes were used wherever possible and the use of plasticware was minimized during lipid extraction to avoid contamination of samples. Glass tubes and glass transfer pipettes were purchased from Sigma and vWR. Lipid internal standards (ISTDs) were purchased from Avanti Polar Lipids Inc. (Alabaster, AL, USA). These include phosphatidylcholine (19:0), sphingomyelin (12:0), phosphatidylethanolamine (17:0), phosphatidylglycerol (17:0), phosphatidylserine (17:0), phosphatidic acid (17:0), ceramide (d18:1, 12:0), diglyceride (1,3 18:0 d5), cholesteryl ester (19:0), monoglyceride (17:0), triglyceride mix d5 (Avanti Code LM-6000), diglyceride mix d5 (Avanti Code LM-6001), phosphatidylinositol (17:0 14:1), C12 GluCer, C12 sulfatide, C17 ceramide, C17 sphingosine, C17 S1P, C12 C1P, D3 C20 fatty acid, and C12 LacCer. Lipid internal standards were prepared as a mixture at 10 pmol/µl in methyl-tert butyl ether and methanol (MTBE:methanol, 1:1 v/v).
Patient blood serum
Individuals diagnosed with bvFTD (N = 40) and healthy controls (N = 22) were recruited at Neuroscience Research Australia in Sydney from FRONTIER, the frontotemporal dementia clinical research group, and from a panel of healthy study volunteers11 with no neurological (i.e. no evidence of cognitive impairment) or psychiatric disorders. The study was approved by the University of New South Wales human ethics committee (approval number: HC12573). All methods were carried out in accordance with the relevant guidelines and regulations. Blood samples were obtained following written informed consent from the participant and/or primary carer. All patients underwent a neurological examination, a comprehensive cognitive assessment and structural brain MRI, and met current consensus diagnostic criteria for bvFTD18, as previously described11. The mean age at assessment was 65 ± 8 years for bvFTD and 71 ± 5 years for controls. Blood samples (9 mL) were collected in tubes (BD Vacutainer SST II Advance Tube #367958), and serum prepared by centrifugation at 3,500 rpm for 10 min at 4 °C, which was then aliquoted and stored at −80 °C until use.
Human brain tissues
Frozen post-mortem brain tissue samples were obtained from Sydney Brain Bank and NSW Brain Tissue Resource Centre following appropriate ethical approvals (University of New South Wales Human Research Ethics approval number: HC15789). Frozen samples from the superior frontal cortex from 10 FTD cases, 10 AD cases and 11 controls without neurological, psychiatric or neuropathological diagnoses19,20 were used in this study. The mean age of the three groups were 72.9 ± 13.0, 73.7 ± 7.5 and 79.5 ± 12.1 years, respectively.
Lipid extraction
Serum lipid extraction was based on the Bligh and Dyer method21. Briefly, serum samples were thawed on ice and 80 µl aliquots were transferred into glass tubes. Methanol (600 µl), chloroform (1,000 µl) and ultrapure water (500 µl) were sequentially added with vortexing between each addition. Samples were then centrifuged at 3,000 rpm for 10 min at room temperature. The lower solvent phase was collected and transferred to a new glass tube using a glass Pasteur pipette. Chloroform (600 µl) was added to the upper phase, vortexed and centrifuged at 3,000 rpm for 10 min. The lower phase was collected and transferred into the same glass tube and dried under nitrogen gas. Dried lipid samples were reconstituted in 100 µl of isopropanol/methanol (1:1) and stored at −80 °C in glass LC-MS vials.
Lipidomics mass spectrometry
Lipid extracts (10 μl) were analyzed using a Q-Exactive Plus Mass Spectrometer coupled to a U3000 UPLC system (ThermoFisher Scientific). Chromatography was performed at 60 °C on a Waters CSH C18 UHPLC column 2.1 ×100 mm, 1.8 μM with VanGuard guard column. Solvent A was 6:4 acetonitrile:water and Solvent B was 1:9 acetonitrile:isopropanol, both with 10 mM ammonium formate and 0.1% formic acid. Lipids were chromatographed according to the method of Castro-Perez et al.22. Briefly, a 30 min gradient running from 30 to 100% of solvent B was performed, eluting lipids in order of hydrophobicity. Column eluate was directed into the electrospray ionization source of the mass spectrometer where a HESI probe was employed. Source parameters were broadly optimized on a range of lipid standards prior to the analysis. The mass spectrometer was run in data dependent acquisition mode. A survey scan over the mass range 200–1,200 at resolution 70 K was followed by 10 data dependent MS/MS scans on the most intense ions in the survey at 15 K resolution. Dynamic exclusion was used to improve the number of ions targeted. Cycle time was approximately 1 sec. Samples were run in both positive and negative polarities. The samples were run in a random order (generated using Microsoft Excel). This is important to avoid batch effects/changing instrument performance effects. Data were analyzed in LipidSearch software 4.1.16. Data were searched against the standard Lipidsearch database with all common mammalian lipid classes included. The search results were then grouped according to sample type and aligned for differential analysis. Aligned data (containing lipid identity, retention time, peak area etc.) were exported to Excel software. Relative abundance of lipids was obtained from peak areas normalized to internal standards.
Thin layer chromatography
Firstly, serum lipid concentrations were determined using the Sulfo-Phospho-Vanillin (SPV) method as previously described23. Briefly, 10 µl of lipid extracts were loaded into a microplate and the solvent removed by evaporation at 90 °C. Concentrated sulphuric acid (100 µl) was added and incubated for 20 min at 90 °C. The plate was rapidly cooled to room temperature and background absorbance measured at 540 nm. 50 µl of vanillin-phosphoric reagent (20% (w/v) vanillin in 17% (v/v) phosphoric acid) was added, the color developed for 10 min and absorbance measured at 540 nm. Total lipid was calculated based on a standard curve generated using fish oil (Blackmores). The serum lipids were then separated using thin layer chromatography (TLC) as previously described24. Briefly, volumes of extracted lipids equivalent to 20 µg were spotted onto TLC plates (Silca gel 60, Merck). The plates were developed firstly in chloroform/methanol/water (40:10:1) to 2 cm, then in chloroform/methanol/water (40:10:1) to 5 cm, followed by chloroform/methanol/acetic acid (47:2:0.5) to 8.5 cm and lastly n-hexane/diethyl ether/acetic acid (65:35:1) to the top of the plates. Lipids were visualized by misting the plate with 5% (w/v) CuSO4 in 15% (w/v) H3PO4 and heating for 10 min at 180 °C. Band intensities were determined using BioRad Image Lab.
ATP assay
Fluorometric ATP assay was carried out following the manufacturer’s protocol (Abcam, cat. # ab83355). Briefly, 50 µl of samples and standards were added to 96-well plates containing the ATP reaction mix and incubated at room temperature in the dark for 30 min. The plates were read using CLARIOstar microplate reader (BMG Labtech) at Ex/Em = 535/587 nm.
Inflammatory marker assays
IL-6 was measured using the magnetic human cytokine ELISA assay (Bio-Rad) following the manufacturer’s instructions. Plates containing the serum samples and standards were analyzed using Magpix plate reader (Luminex), and the concentration of IL-6 was determined using the Xponent software package (Luminex). C3 was measured by western blotting (see below). Calcium assay was carried out following the manufacturer’s protocol (Abcam, ab102505). Briefly, 50 µl of samples and standards were added to 96-well plates. Then, 90 µl of the chromogenic reagent was added to each well, followed by 60 µl of calcium assay buffer. The plates were incubated at room temperature for 10 min in the dark and read in a microplate reader at 575 nm.
Protein extraction
Tris-buffered saline (TBS)-soluble proteins were extracted from 100 mg of brain tissues as previously described25. Briefly, tissue samples were homogenized in ten volumes of TBS homogenization buffer (20 mM Tris, 150 mM NaCl, pH 7.4, 5 mM EDTA, 0.02% sodium azide) containing protease inhibitor cocktail (Roche) using Qiagen TissueLyser (3 × 30 sec, 30 Hz cycles), followed by centrifugation at 100,000 × g for 1 h at 4 °C. The supernatant was collected and transferred into new tubes. Protein concentration was measured using a bicinchoninic acid assay (Pierce BCA Protein Assay Kit) following the manufacturer’s instructions.
Western blotting
Serum (equal volumes) or protein lysates (10 µg) were heated with sample buffer (3.2% SDS, 32% glycerol, 0.16% bromophenol blue, 100 mM Tris-HCl, pH 6.8, 8% 2-mercaptoethanol). They were then electrophoresed on Criterion Stain-free 4–20% SDS-PAGE gels (Bio-Rad) and transferred onto nitrocellulose membranes at 100 volts for 30 min. The membranes were blocked with TBS containing 5% nonfat dry milk and probed with anti-C3 antibody (Santa Cruz, sc-28294, 1:1,000) or anti-acrolein antibody (NOVUS, NB200–556, 1:1,000) overnight at 4 °C. They were then washed three times in TBS containing 0.1% Tween 20 and incubated with horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature. Signals were detected using enhanced chemiluminescence and Gel Doc System (Bio-Rad). The blots were stripped and probed for housekeeper proteins transferrin (serum) or β-actin (tissue lysate). The signal intensity was quantified using Image Lab (Bio-Rad) and NIH ImageJ software (v1.45 s).
Malondialdehyde assay
Malondialdehyde (MDA) was measured using Lipid Peroxidation MDA Assay Kit (Abcam, cat. # ab118970) following the manufacturer’s protocol. Briefly, samples were prepared by addition of 500 µL of 42 mM H2SO4 and 125 µL of phosphotungstic acid solution to 50 µL of serum samples. After incubation and centrifugation, the pellet was dissolved in 200 µL of ddH2O by sonication. TBA solution was then added into each vial containing samples or standards and incubated at 95 °C for 1 h. Fluorescence at excitation 532 nm/emission 553 nm was read using CLARIOstar plate reader (BMG Labtech).
4-Hydroxynonenal assay
4-Hydroxynonenal (HNE) was measured using OxiSelectTM HNE Adduct Competitive ELISA Kit (Cell Biolabs, Inc., San Diego, CA) following the manufacturer’s protocol. Briefly, 50 µL of serum was added to HNE Conjugate pre-coated wells followed by addition of 50 µL of anti-HNE antibody. After 1-h incubation at room temperature and washing, 100 µL of secondary antibody-HRP conjugate was added to each well and incubated for 1 h at room temperature. The plate was washed three times. 100 µL of substrate solution followed by stop solution were added into each well. Absorbance at 450 nm was read on POLARstar Omega plate reader (BMG Labtech).
Statistical analysis
Statistical analyses were performed using SPSS Statistics software (IBM, Chicago, Illinois). For comparisons between FTD and control groups, either univariate analysis (general linear model) or Student’s t-test was used and statistical significance set at p < 0.05. When univariate analysis was performed, age and gender were included as covariates. Pearson’s correlations were used to determine if changes in lipid levels were associated with each other with statistical significance set at p < 0.05. Graphs were generated using GraphPad Prism 7.
Results
Validation of lipid analysis of FTD serum
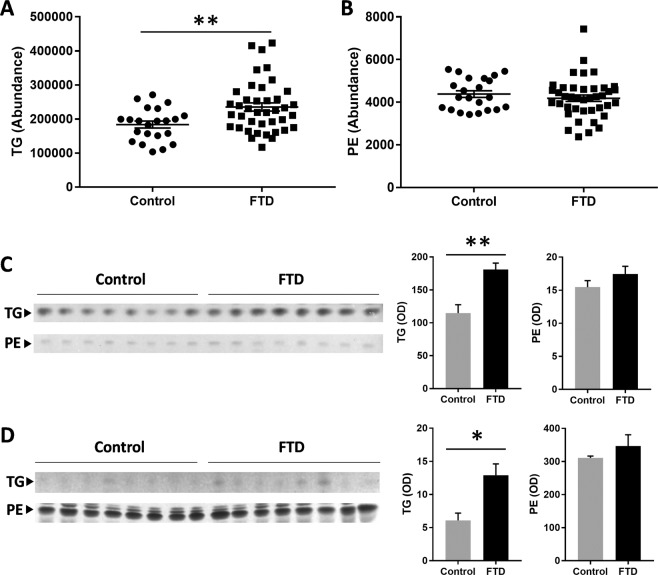
Previous studies based on untargeted lipidomics analysis of FTD blood have revealed global lipid changes in FTD12. Here, we used a focused approach to analyze specific serum lipids with the aim of detecting and understanding the pathophysiological changes in FTD. We were interested in three key aspects of FTD pathophysiology that are relevant to neurodegeneration – mitochondrial dysfunction, inflammation, and oxidative stress. We analyzed serum lipids from FTD patients and controls without dementia using sophisticated HPLC-MS and LipidSearch software. A summary of total abundance of all lipids analyzed is shown in Table 1. Firstly, for validation purpose, we compared our new data from the current study to those previously published. We analyzed the levels of two common lipids – triglyceride (TG) and phosphatidylethanolamine (PE) – that are increased and unaltered, respectively, in FTD11,12. As expected, TG was significantly increased in FTD serum compared to controls (Fig. 1A), and PE was unaltered (Fig. 1B). To further validate these data, we measured the two lipids in both serum and brain using an alternative method – thin layer chromatography. And once again, TG was significantly increased in both FTD serum and brain (Fig. 1C,D), and PE was unaltered in both FTD serum and brain (Fig. 1C,D).
Table 1.
A summary of total abundance of serum lipids analyzed.
| Lipid | Symbol | Unit | Control | FTD | Change(%) | P value |
|---|---|---|---|---|---|---|
| Lipids | ||||||
| Triglyceride | TG | Abundance | 183,600 ± 10,190 | 235,199 ± 11,880 | 28 | 0.0017 |
| Phosphatidylethanolamine | PE | Abundance | 4,382 ± 156 | 4,186 ± 151 | −4.5 | 0.3733 |
| Phosphatidylcholine | PC | Abundance | 387,876 ± 9,089 | 362,632 ± 5,998 | −6.5 | 0.0257 |
| Methylphosphatidylcholine | MPC | Abundance | 171,355 ± 3,774 | 151,426 ± 3,305 | −11.6 | 0.0002 |
| Mitochondrial lipids | ||||||
| Cardiolipin | CL | Abundance | 12.1 ± 0.5 | 9.8 ± 0.3 | −19 | 0.0006 |
| Acylcarnitine | AC | Abundance | 540 ± 35 | 381 ± 19 | −29 | 0.0004 |
| Inflammatory lipids | ||||||
| Lysophosphatidylcholine | LPC | Abundance | 88,056 ± 5,244 | 116,117 ± 4,477 | 32 | 0.0019 |
| Platelet-activating factor | PAF | Abundance | 470 ± 117 | 1,159 ± 154 | 147 | 0.0007 |
| O-acyl-ω-hydroxy fatty acid | OAHFA | Abundance | 23 ± 3.3 | 11 ± 1.4 | −52 | 0.0027 |
| Lipid aldehydes | ||||||
| Acrolein | AL | Abundance | 24 ± 2.8 | 40 ± 5.9 | 67 | 0.0279 |
| Malondialdehyde | MDA | nmol/mL | 5.1 ± 0.3 | 5.6 ± 0.4 | 9.8 | 0.3630 |
| 4-Hydroxynonenal | HNE | μg/mL | 0.62 ± 0.08 | 0.68 ± 0.13 | 9.7 | 0.7265 |
Figure 1.
Validation of lipid analysis of FTD serum and brain. (A) Triglyceride (TG) was significantly increased in FTD serum (N = 40) compared to controls (N = 22). (B) Phosphatidylethanolamine (PE) was unaltered in FTD serum compared to controls. (C) Thin layer chromatography (TLC) of serum TG and PE and optical density measurements of the bands. (D) TLC of TG and PE in FTD brain (N = 10) compared to control brain (N = 11) and optical density measurements of the bands. Data represent mean and SE as error bars, *P < 0.05, **P < 0.005.
Detection of mitochondrial dysfunction in FTD using serum lipids
Mitochondrial dysfunction is a common pathological feature in Alzheimer’s disease (AD) and Parkinson’s disease (PD)26. It is also increasingly evident in FTD27,28. Two lipids that play prominent roles in mitochondria are cardiolipin (CL) and acylcarnitine (AC). CL is almost exclusively located in the inner mitochondrial membrane, where it plays roles in numerous enzymatic functions that are involved in mitochondrial energy metabolism14. AC acts as a transporter of long-chain fatty acids into the mitochondria, where the fatty acids are oxidized to produce energy, i.e. ATP15. CL is a unique phospholipid synthesized from glycerol-3-phosphate (Fig. 2A), whereas AC is a simple lipid consisting of lysine derivative bound to fatty acids (Fig. 2B). The two lipids are structurally different and are synthesized under independent pathways. Changes in CL and AC levels would indicate changes in mitochondrial function/activity.
Figure 2.
Decreases in mitochondrial lipids and ATP in FTD serum. Biosynthetic pathways of mitochondrial lipids cardiolipin (CL) (A) and acylcarnitine (AC) (B). (C) Total CL levels were decreased in FTD compared to controls. (D) Eight of the twelve AC species were decreased in FTD. (E) Total AC levels were decreased in FTD. (F) A strong correlation between the two most significantly decreased AC species, 10:0 and 12:1 (Pearson’s correlation = 0.897; P = 6.4 × 10−23). (G) An extremely strong correlation between AC and CL levels (Pearson’s correlation = 0.910; P = 1.1 × 10−24). (H) ATP levels were decreased in FTD. FTD (N = 40), controls (N = 22), data represent mean and SE as error bars, *P < 0.05, **P < 0.005, ***P < 0.0005.
We compared the levels of CL and AC in FTD and control sera. We identified a single CL species, which was significantly decreased in FTD compared to controls (Fig. 2C). We identified 12 AC species, of which 8 were significantly decreased in FTD (Fig. 2D); the total AC levels were also decreased in FTD (Fig. 2E). The decreases in AC species strongly correlated with one another, for example, the Pearson’s correlation for the two most significantly decreased AC species, 10:0 and 12:1, was 0.897 (P = 6.4 × 10−23) (Fig. 2F). Importantly, CL levels were strongly correlated to AC levels (Pearson’s correlation = 0.910; P = 1.1 × 10−24) (Fig. 2G) despite the fact that the two lipids are produced independently. These results strongly suggest mitochondrial dysfunction in FTD. To verify these findings, we then measured ATP level in the same sera. ATP level is a cardinal indicator of mitochondrial function, and it has already been shown that ATP synthesis is decreased in FTD brain and in the brain of mouse models of FTD27,29. We found that the ATP levels were significantly decreased in FTD compared to controls (Fig. 2H), supporting our lipid data. When put together, these data support the use of lipid measurements for detecting mitochondrial dysfunction in FTD serum.
Detection of inflammation in FTD using serum lipids
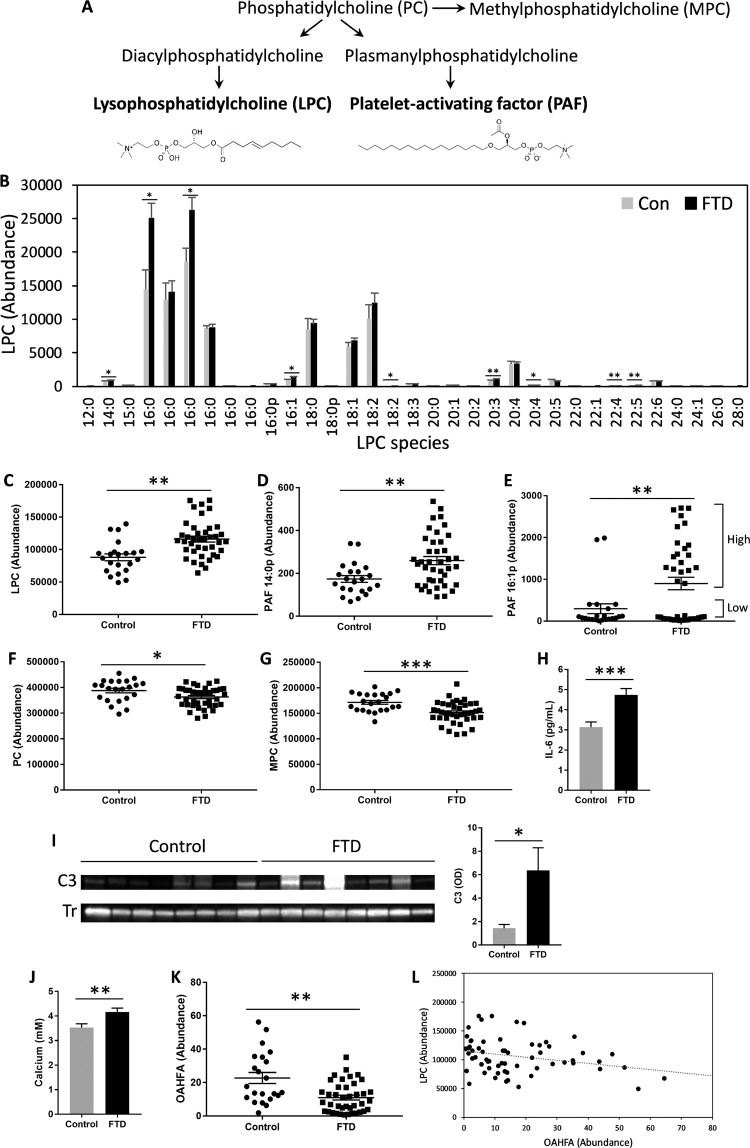
Inflammation is a prominent pathophysiological characteristic in neurodegenerative diseases, including FTD30,31. We were interested in whether inflammation in FTD could be detected by analysis of serum lipids. Two serum lipid classes that are known to participate in inflammatory response are lysophosphatidylcholine (LPC) and platelet-activating factor (PAF)16,17. LPC and PAF are pro-inflammatory lipids that are also known as second messengers or immediate-response molecules. LPC acts on a number of immune target cells, for example, it upregulates cytokine production and chemotaxis in macrophages and T-lymphocytes32–35. It also activates caspase-1 in microglia in the brain neuroinflammatory process36. PAF is also a potent activator of inflammatory cells involved in the innate immune system, including neutrophils, macrophages and platelets. It is also a potent neuromodulator37. Both LPC and PAF are glycerophospholipids that are derived from the parent lipid phosphatidylcholine (PC) (Fig. 3A). PC and another derivative of PC, methylphosphatidylcholine (MPC) (Fig. 3A), are non-inflammatory lipids.
Figure 3.
Changes in inflammatory lipids and cytokine in FTD serum. (A) Biosynthetic pathways of pro-inflammatory lipids lysophosphatidylcholine (LPC) and platelet-activating factor (PAF), and non-inflammatory lipids, phosphatidylcholine (PC) and methylphosphatidylcholine (MPC). (B) The abundance of LPC species, of which 9 were significantly increased in FTD compared to controls. (C) The total LPC levels were increased in FTD. (D) PAF 14:0p levels were increased in FTD. (E) PAF 16:1p levels were increased in FTD. (F) The total PC levels were decreased in FTD. (G) The total MPC levels were decreased in FTD. (H) The pro-inflammatory cytokine IL-6 levels were increased in FTD as measured by ELISA. (I) The pro-inflammatory marker C3 levels were increased in FTD as measured by western blotting; normalized by the housekeeper transferrin (Tr) (J) Calcium levels were increased in FTD. (K) Anti-inflammatory lipid o-acyl-ω-hydroxy fatty acids (OAHFA) levels were decreased in FTD. (L) An inverse correlation between LPC and OAHFA (Pearson’s correlation = −0.274; P < 0.05). FTD (N = 40), controls (N = 22), data represent mean and SE as error bars, *P < 0.05, **P < 0.005, ***P < 0.0005.
We compared the levels of LPC and PAF, as well as PC, in FTD and control sera. We identified 33 LPC species, of which 9 were significantly increased in FTD compared to controls (Fig. 3B). The total LPC was also significantly increased in FTD (Fig. 3C). We identified two PAF species (14:0p and 16:1p), both of which were significantly increased in FTD (Fig. 3D,E). Interestingly, there were two distribution clusters for the 16:1p species, i.e. “low” and “high” (Fig. 3E). Most of the control samples (i.e. 91%) were distributed to the low cluster, whereas 50% of the FTD samples were distributed to the low cluster and 50% to the high cluster (Fig. 3E). Within the FTD cohort, the mean of the high cluster was 28.6-fold higher (P = 1.1 × 10−10) than that of the low cluster with no difference in age (mean age = 65 y for both groups) or gender (male N = 10, female N = 10 for both groups). In terms of correlation, LPC levels were positively correlated to PAF levels (Pearson’s correlation = 0.278; P < 0.05). The increase in the lipid levels was specific to only the pro-inflammatory lipids as both non-inflammatory lipids, PC and MPC, were slightly decreased in FTD compared to controls (Fig. 3F,G).
To verify these lipid results, we used two non-lipid assays to measure the level of two independent pro-inflammatory markers in the same sera. Firstly, we used ELISA to measure interleukin 6 (IL-6), which is a major cytokine that regulates inflammatory responses. Polymorphisms of the IL-6 gene are associated with cognitive and behavioral performances of FTD patients38. Secondly, we used western blotting to measure complement component 3 (C3), which plays a central role in innate immunity and is commonly used as a blood test for the diagnosis of inflammatory conditions. C3 levels are associated with cognitive decline in FTD patients39. We found that both IL-6 and C3 levels were significantly increased in FTD compared to controls (Fig. 3H,I), supporting our lipid data. Furthermore, we analyzed calcium levels as a measure of LPC levels; increases in LPC levels induce increases in calcium levels via G-protein-coupled receptors40. Calcium dysregulation is also associated with FTD41,42. We found that calcium levels were significantly increased in FTD serum compared to controls (Fig. 3J), once again supporting our lipid data. These findings are consistent with earlier studies that showed pro-inflammatory cytokine levels are increased in FTD brain43.
Finally, we analyzed o-acyl-ω-hydroxy fatty acids (OAHFA), which are simple lipids composed of a long carbon-chain fatty acid esterified to an omega-hydroxy fatty acid. They are similar in structure to a group of lipids called fatty acid esters of hydroxy fatty acids (FAHFA). What is interesting is that these lipids exert anti-inflammatory effects44,45. We were therefore interested in whether OAHFA levels were altered in FTD. We found that the OAHFA levels were significantly decreased in FTD compared to controls (Fig. 3K), suggesting decreased anti-inflammatory activity. Furthermore, there was a significant inverse correlation between LPC and OAHFA levels (Pearson’s correlation = −0.274; P < 0.05) (Fig. 3L). When put together, these data support lipid assays as means to measure inflammation in FTD serum.
Detection of oxidative stress in FTD using serum lipids
In terms of chemistry, saturated fatty acids (i.e. contain no C=C double bonds) are very stable, whereas unsaturated fatty acids (i.e. contain one or more C=C double bonds) are prone to peroxidation (a process of oxidative degradation); the greater the number of C=C double bonds the greater the susceptibility to peroxidation (Fig. 4A). Lipid peroxidation results in the formation of lipid aldehydes – acrolein (AL), malondialdehyde (MDA) and 4-hydroxynonenal (HNE) (Fig. 4A). The three lipid aldehydes are highly reactive, and they conjugate (i.e. bind) to proteins, annulling their function. Increasing evidence indicates that lipid aldehydes contribute to the pathogenesis of neurodegenerative diseases, as well as in normal ageing46. It has already been demonstrated that the levels of HNE-conjugated proteins are increased in FTD brain43,47. Brain is particularly susceptible to lipid peroxidation due to a high lipid content and a high oxygen consumption.
Figure 4.
Increases in lipid peroxidation products in FTD serum and brain. (A) Unsaturated fatty acids (contain one or more C=C double bonds) are prone to lipid peroxidation, resulting in the formation of lipid aldehydes, acrolein (AL), malondialdehyde (MDA) and 4-hydroxynonenal (HNE). (B) The total abundance of unsaturated fatty acid was increased in FTD serum (N = 40) compared to controls (N = 22). (C) A comparison of abundance of unsaturated fatty acids with different number of C=C double bonds. (D) AL-, MDA- and HNE-conjugated protein levels in FTD serum and controls. (E) AL-conjugated protein levels were increased in the superior frontal cortex of FTD brain (N = 10) compared to control brain (N = 11) as detected by western blotting; normalized by the housekeeper β-actin (β-a) (F) AL-conjugated protein levels were increased in the superior frontal cortex of AD brain (N = 10) compared to control brain (N = 11). Data represent mean and SE as error bars, *P < 0.05, **P < 0.005, ***P < 0.0005.
Firstly, we analyzed the abundance of saturated and unsaturated fatty acids (i.e. in TG) present in the serum; fatty acids are usually derived from TG. The total level of unsaturated fatty acids was significantly increased in FTD compared to controls (Fig. 4B), indicating increased susceptibility to peroxidation (i.e. oxidative stress) in FTD. The significant increases occurred in fatty acids with 1–4 C=C double bonds (Fig. 4C). Secondly, we measured lipid aldehyde-conjugated proteins in the sera. There was a significant increase in the AL-conjugated protein levels in FTD compared to controls, whereas a non-significant trend for increase in the MDA- and HNE-conjugated protein levels (Fig. 4D). To verify these findings, we then measured AL-conjugated protein levels in the superior frontal cortex of FTD (with TDP43 pathology) and control brain, as well as in AD brain (positive control). The AL-conjugated protein levels were significantly increased in FTD and AD compared to controls (Fig. 4E,F), indicating increased oxidative stress in FTD and AD brain. These results support the use of lipid assays to detect oxidative stress in FTD.
Discussion
Utilization of lipid analysis has helped to understand the pathophysiology of a number of human diseases. With recent advances in mass spectrometry, lipidomics exploration of tissues and fluids has allowed identification of hundreds, if not thousands, of different lipid species, leading to improved diagnosis and development of peripheral biomarkers. In recent years there has been significant interest in the role of lipids in neurodegenerative processes, particularly in the aggregation and propagation of pathogenic proteins, e.g. amyloid-β and α-synuclein. Much of the work in this field has been focused on AD and to a lesser degree PD, with little on FTD. To address this, we asked a pertinent question – what are the changes in serum lipids that indicate FTD pathophysiology. We investigated three key aspects of FTD pathophysiology that are relevant to neurodegeneration, which are mitochondrial dysfunction, inflammation, and oxidative stress. There were two objectives to our investigation – (1) to detect pathophysiological changes in FTD using serum lipids, and (2) identify lipid species and pathways that could be exploited to develop biomarkers for FTD. The overall aim was to provide new insights into an under-recognized perturbed pathology in FTD.
The physiological status of mitochondria can be detected by the analysis of two mitochondrial lipid classes, CL and AC. We measured the levels of CL and AC in the sera, and both were significantly decreased in FTD compared to controls, suggesting mitochondrial dysfunction in FTD. We also verified our lipid data with measurement of ATP levels, which were significantly decreased in FTD serum compared to controls. Our data is consistent with earlier findings that both ATP level and ATP synthase expression/activity are decreased in FTD brain and FTD mouse models27,29. ATP synthesis is a major function of mitochondria, and therefore the level of ATP is a sensitive indicator of mitochondrial function/activity. CL and AC are structurally very different and are synthesized under independent pathways, yet their levels correlated with each other extremely strongly, providing further evidence for aberration in mitochondrial function. CL interacts with proteins in the mitochondria in the regulation of a number of mitochondrial processes, including the electron transport chain (i.e. ATP production), cell viability and apoptosis14,48,49. It is widely distributed throughout the brain in all cell types, suggesting its importance in the mitochondrial function in the brain. The multi-role of CL is thought to be due to its unique conical-shape structure that renders it to interact with a large array of proteins located within the mitochondrial membranes50. In one study, it was shown that CL mediates autophagy of damaged mitochondria in neurons51.
AC also plays a number of roles in the mitochondria, e.g. neuroprotection and antioxidant functions52–54. Supplementation of AC for the treatment of AD has been trialed. An oral treatment of AC for one year resulted in a slower rate of deterioration compared to placebo-controls, and significantly higher scores in a number of verbal and memory tests55. It is unknown whether AC supplementation is of any benefit in FTD. In in vitro studies, AC was shown to protect primary neurons and hippocampal cultures against neurotoxic substances56. Much work is needed to understand how AC is facilitating neuroprotection in mitochondria.
The second aspect of FTD pathophysiology we investigated using serum lipids was inflammation. For this analysis we targeted two known pro-inflammatory lipid classes, LPC and PAF, both of which are derived from the parent lipid PC. We found that the LPC and PAF levels were significantly increased in FTD compared to controls, whereas the non- inflammatory lipids, PC and MPC, levels were slightly decreased. Increases in LPC and PAF levels would indicate increased inflammatory activity. To verify our lipid data, we measured two key pro-inflammatory markers, IL-6 and C3, and calcium in the same sera and found that all were significantly increased in FTD compared to controls. These data are in accordance with the manifestation of inflammation in FTD30,31, in which the levels of IL-6, along with other cytokines, are increased in FTD serum/plasma57–59.
LPC regulates cytoskeleton and cellular Ca2+ homoeostasis, proliferation, survival, migration and adhesion40. PAF is a potent pro-inflammatory mediator implicated in neurodegenerative processes60. Initially, it was described as a factor that degranulates (i.e. aggregates) platelets, and thus its name61. Its physiological role is diverse and is implicated in a number of diseases62. It acts upon numerous inflammation pathways, including enhanced leukocyte adhesion, chemotaxis and leukocyte degranulation63. Its production is increased in response to specific stimuli in key host defense cells including macrophages and monocytes. Its level is regulated by PAF acetylhydrolase (PAF-AH), a phospholipase that hydrolyzes the acetyl residue, rendering the lipid inactive64. Virtually nothing is known about the role of LPC and PAF in FTD pathophysiology. In AD, increases in PAF levels correlate to the severity of cognitive impairment65,66. In one study, it was shown that PAF antagonists enhanced the intracellular degradation of amyloid-β42 in neurons via regulation of cholesterol ester hydrolases67. In another study, it was shown that the levels of PAF-AH were higher in AD patients compared to controls68.
The presence/absence of C=C double bonds, as well as the number of C=C double bonds, in fatty acids has significant impact on both biophysical and physiological properties of fatty acids. In terms of biophysical properties, unsaturated fatty acids (contain C=C double bonds) have lower melting points and are unstable, whereas saturated fatty acids have higher melting points and are stable. In terms of physiological properties, unsaturated fatty acids are susceptible to peroxidation and its lipid peroxidation products cause oxidative stress or damage to cells and tissues, whereas saturated fatty acids are largely not susceptible to peroxidation. The point of attack by free radicals in the process of lipid peroxidation is the C=C double bonds that contain the methylene -CH2- bridges; the greater the number of double bonds the greater the susceptibility to lipid peroxidation.
Toxic lipid peroxidation products are lipid aldehydes. The three major lipid aldehydes are AL, MDA and HNE; AL is the simplest form of lipid aldehydes (Fig. 4A). They are highly reactive and readily conjugate to proteins, altering or annulling the normal structure and function of proteins. Presence of aldehyde-conjugated proteins indicate cellular oxidative stress and can be used as bioactive markers to detect the extent of lipid peroxidation. In the past, much of the research has been focused on the detrimental effects of lipid peroxidation in the context of atherosclerosis. Accumulation of oxidized LDL in the arteries leads to foam cell formation and plaque development, causing atherosclerosis69. Lipid peroxidation is extremely important in the context of brain function since brain is highly enriched in lipids and highly oxygenated, and therefore particularly susceptible to oxidative damage. Growing evidence indicates that increases in lipid aldehydes in the brain are significant contributors to neurodegenerative processes associated with AD. The levels of AL-conjugated proteins are increased in AD brain, cerebral spinal fluid and serum/plasma70–72. Likewise, we found that the levels of AL-conjugated proteins were significantly increased in FTD serum and brain, indicating the prevalence of oxidative stress or damage in FTD. In terms of toxicity, AL is particularly toxic as it reacts with glutathione >100 times faster than HNE73. When compared to other reactive oxygen species, such as superoxide anion radical, hydrogen peroxide and hydroxyl radical (agents that induce oxidative stress), AL was found to be the most toxic74–76.
Lipidomics technology has been increasingly utilized in the analysis of peripheral fluids to understand lipid dysfunction in a number of diseases. Here, we applied lipidomics to detect pathophysiological changes in FTD. We investigated three key aspects of FTD pathophysiology – mitochondrial dysfunction, inflammation, and oxidative stress – that are important to neurodegeneration. Conclusively, all three pathophysiological changes in FTD were detected by the lipid analysis with evidence of similar changes in the brain. This represents the first focused lipidomics analysis of FTD serum that link lipid changes to neurodegeneration. Our study has not only provided useful data in understanding the pathogenesis of FTD, it has also opened avenues for biomarker development and for monitoring disease progression in FTD.
Acknowledgements
This work was supported by funding to ForeFront, a collaborative research group dedicated to the study of frontotemporal dementia and motor neuron disease, from the National Health and Medical Research Council of Australia (NHMRC) program grants (#1037746, #1132524) and the Australian Research Council Centre of Excellence in Cognition and its Disorders Memory Program (#CE110001021). G.M.H. is a NHMRC Senior Principal Research Fellow (#1079679) and O.P. is a NHMRC Senior Research Fellow (#1103258). We thank E. Jary, F. Atashrazm and S. Keshiya for technical assistance.
Author contributions
W.S.K. conceived and designed the study, analyzed the data, and wrote the manuscript. G.M.H. carried out the neuropathological diagnosis of the brain tissues, analyzed the data, and edited the manuscript. O.P. and J.R.H. recruited the patients and performed the neurological examinations. R.P. carried out the lipidomics mass spectrometry and processed the data using LipidSearch software. K.P., Y.H., S.B. and J.S.K. carried out the ELISA, western blotting and all other assays, and analyzed the data. All authors reviewed and approved the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Glenda M. Halliday, Email: glenda.halliday@sydney.edu.au
Woojin Scott Kim, Email: woojin.kim@sydney.edu.au.
References
- 1.Wenk MR. The emerging field of lipidomics. Nat. Rev. Drug. Discov. 2005;4:594–610. doi: 10.1038/nrd1776. [DOI] [PubMed] [Google Scholar]
- 2.Watson AD. Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J. lipid Res. 2006;47:2101–2111. doi: 10.1194/jlr.R600022-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Dennis EA. Lipidomics joins the omics evolution. Proc. Natl Acad. Sci. USA. 2009;106:2089–2090. doi: 10.1073/pnas.0812636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quehenberger O, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. lipid Res. 2010;51:3299–3305. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Brien JS, Sampson EL. Lipid composition of the normal human brain: gray matter, white matter, and myelin. J. lipid Res. 1965;6:537–544. [PubMed] [Google Scholar]
- 6.Piguet O, Hornberger M, Mioshi E, Hodges JR. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol. 2011;10:162–172. doi: 10.1016/S1474-4422(10)70299-4. [DOI] [PubMed] [Google Scholar]
- 7.Broe M, et al. Staging disease severity in pathologically confirmed cases of frontotemporal dementia. Neurol. 2003;60:1005–1011. doi: 10.1212/01.WNL.0000052685.09194.39. [DOI] [PubMed] [Google Scholar]
- 8.Kril JJ, Halliday GM. Clinicopathological staging of frontotemporal dementia severity: correlation with regional atrophy. Dement. Geriatr. Cogn. Disord. 2004;17:311–315. doi: 10.1159/000077161. [DOI] [PubMed] [Google Scholar]
- 9.Kril JJ, Macdonald V, Patel S, Png F, Halliday GM. Distribution of brain atrophy in behavioral variant frontotemporal dementia. J. Neurol. Sci. 2005;232:83–90. doi: 10.1016/j.jns.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Gregory GC, Macdonald V, Schofield PR, Kril JJ, Halliday GM. Differences in regional brain atrophy in genetic forms of Alzheimer’s disease. Neurobiol. Aging. 2006;27:387–393. doi: 10.1016/j.neurobiolaging.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed RM, et al. Systemic metabolism in frontotemporal dementia. Neurol. 2014;83:1812–1818. doi: 10.1212/WNL.0000000000000993. [DOI] [PubMed] [Google Scholar]
- 12.Kim WS, et al. Lipidomics Analysis of Behavioral Variant Frontotemporal Dementia: A Scope for Biomarker Development. Front. Neurol. 2018;9:104. doi: 10.3389/fneur.2018.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed RM, et al. Lipid Metabolism and Survival Across the Frontotemporal Dementia-Amyotrophic Lateral Sclerosis Spectrum: Relationships to Eating Behavior and Cognition. J. Alzheimer’s disease: JAD. 2018;61:773–783. doi: 10.3233/JAD-170660. [DOI] [PubMed] [Google Scholar]
- 14.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am. J. Physiol. Cell Physiol. 2007;292:C33–44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 15.Vaz FM, Wanders RJ. Carnitine biosynthesis in mammals. Biochem. J. 2002;361:417–429. doi: 10.1042/0264-6021:3610417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu H, Valenzuela N, Fai S, Figeys D, Bennett SA. Targeted lipidomics - advances in profiling lysophosphocholine and platelet-activating factor second messengers. FEBS J. 2013;280:5652–5667. doi: 10.1111/febs.12423. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto T, Kobayashi T, Kamata K. Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr. Med. Chem. 2007;14:3209–3220. doi: 10.2174/092986707782793899. [DOI] [PubMed] [Google Scholar]
- 18.Rascovsky K, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montine TJ, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackenzie IR, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–113. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 22.Castro-Perez JM, et al. Comprehensive LC-MS E lipidomic analysis using a shotgun approach and its application to biomarker detection and identification in osteoarthritis patients. J. Proteome Res. 2010;9:2377–2389. doi: 10.1021/pr901094j. [DOI] [PubMed] [Google Scholar]
- 23.Cheng YS, Zheng Y, VanderGheynst JS. Rapid quantitative analysis of lipids using a colorimetric method in a microplate format. Lipids. 2011;46:95–103. doi: 10.1007/s11745-010-3494-0. [DOI] [PubMed] [Google Scholar]
- 24.Hirabayashi T, Murakami M, Kihara A. The role of PNPLA1 in omega-O-acylceramide synthesis and skin barrier function. Biochimica et biophysica acta. Mol. Cell Biol. lipids. 2019;1864:869–879. doi: 10.1016/j.bbalip.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia S, Kim WS, Shepherd CE, Halliday GM, Apolipoprotein D. Upregulation in Alzheimer’s Disease but Not Frontotemporal Dementia. J. Mol. Neurosci. 2019;67:125–132. doi: 10.1007/s12031-018-1217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowan K, Anichtchik O, Luo S. Mitochondrial integrity in neurodegeneration. CNS Neurosci. Ther. 2019;25:825–836. doi: 10.1111/cns.13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi SY, et al. C9ORF72-ALS/FTD-associated poly(GR) binds Atp5a1 and compromises mitochondrial function in vivo. Nat. Neurosci. 2019;22:851–862. doi: 10.1038/s41593-019-0397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Briston T, Hicks AR. Mitochondrial dysfunction and neurodegenerative proteinopathies: mechanisms and prospects for therapeutic intervention. Biochem. Soc. Trans. 2018;46:829–842. doi: 10.1042/BST20180025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.David DC, et al. Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L tau transgenic mice. J. Biol. Chem. 2005;280:23802–23814. doi: 10.1074/jbc.M500356200. [DOI] [PubMed] [Google Scholar]
- 30.McCauley ME, Baloh RH. Inflammation in ALS/FTD pathogenesis. Acta Neuropathol. 2019;137:715–730. doi: 10.1007/s00401-018-1933-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bright Fiona, Werry Eryn L., Dobson-Stone Carol, Piguet Olivier, Ittner Lars M., Halliday Glenda M., Hodges John R., Kiernan Matthew C., Loy Clement T., Kassiou Michael, Kril Jillian J. Neuroinflammation in frontotemporal dementia. Nature Reviews Neurology. 2019;15(9):540–555. doi: 10.1038/s41582-019-0231-z. [DOI] [PubMed] [Google Scholar]
- 32.Chang MY, Tsoi C, Wight TN, Chait A. Lysophosphatidylcholine regulates synthesis of biglycan and the proteoglycan form of macrophage colony stimulating factor. Arterioscler. Thromb. Vasc. Biol. 2003;23:809–815. doi: 10.1161/01.ATV.0000069208.20268.D0. [DOI] [PubMed] [Google Scholar]
- 33.Oestvang J, Anthonsen MW, Johansen B. Role of secretory and cytosolic phospholipase A(2) enzymes in lysophosphatidylcholine-stimulated monocyte arachidonic acid release. FEBS Lett. 2003;555:257–262. doi: 10.1016/s0014-5793(03)01242-0. [DOI] [PubMed] [Google Scholar]
- 34.Han KH, et al. Lysophosphatidylcholine up-regulates CXCR4 chemokine receptor expression in human CD4 T cells. J. Leukoc. Biol. 2004;76:195–202. doi: 10.1189/jlb.1103563. [DOI] [PubMed] [Google Scholar]
- 35.McMurray HF, Parthasarathy S, Steinberg D. Oxidatively modified low density lipoprotein is a chemoattractant for human T lymphocytes. J. Clin. investigation. 1993;92:1004–1008. doi: 10.1172/JCI116605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scholz H, Eder C. Lysophosphatidylcholine activates caspase-1 in microglia via a novel pathway involving two inflammasomes. J. Neuroimmunol. 2017;310:107–110. doi: 10.1016/j.jneuroim.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Chen C, Bazan NG. Lipid signaling: sleep, synaptic plasticity, and neuroprotection. Prostaglandins Other Lipid Mediat. 2005;77:65–76. doi: 10.1016/j.prostaglandins.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Rainero I, et al. Pro-inflammatory cytokine genes influence the clinical features of frontotemporal lobar degeneration. Dement. Geriatr. Cogn. Disord. 2009;27:543–547. doi: 10.1159/000225962. [DOI] [PubMed] [Google Scholar]
- 39.Lui H, et al. Progranulin Deficiency Promotes Circuit-Specific Synaptic Pruning by Microglia via Complement Activation. Cell. 2016;165:921–935. doi: 10.1016/j.cell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer zu Heringdorf D, Jakobs KH. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim. Biophys. Acta. 2007;1768:923–940. doi: 10.1016/j.bbamem.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 41.Imamura K, et al. Calcium dysregulation contributes to neurodegeneration in FTLD patient iPSC-derived neurons. Sci. Rep. 2016;6:34904. doi: 10.1038/srep34904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palluzzi F, et al. A novel network analysis approach reveals DNA damage, oxidative stress and calcium/cAMP homeostasis-associated biomarkers in frontotemporal dementia. PLoS one. 2017;12:e0185797. doi: 10.1371/journal.pone.0185797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liou CJ, Tong M, Vonsattel JP, de la Monte SM. Altered Brain Expression of Insulin and Insulin-Like Growth Factors in Frontotemporal Lobar Degeneration: Another Degenerative Disease Linked to Dysregulation of Insulin Metabolic Pathways. ASN Neuro. 2019;11:1759091419839515. doi: 10.1177/1759091419839515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oh DY, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yore MM, et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell. 2014;159:318–332. doi: 10.1016/j.cell.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reed TT. Lipid peroxidation and neurodegenerative disease. Free. Radic. Biol. Med. 2011;51:1302–1319. doi: 10.1016/j.freeradbiomed.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 47.Martinez A, et al. Type-dependent oxidative damage in frontotemporal lobar degeneration: cortical astrocytes are targets of oxidative damage. J. Neuropathol. Exp. Neurol. 2008;67:1122–1136. doi: 10.1097/NEN.0b013e31818e06f3. [DOI] [PubMed] [Google Scholar]
- 48.Peyta L, et al. Reduced cardiolipin content decreases respiratory chain capacities and increases ATP synthesis yield in the human HepaRG cells. Biochim. Biophys. Acta. 2016;1857:443–453. doi: 10.1016/j.bbabio.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Cheng H, et al. Shotgun lipidomics reveals the temporally dependent, highly diversified cardiolipin profile in the mammalian brain: temporally coordinated postnatal diversification of cardiolipin molecular species with neuronal remodeling. Biochem. 2008;47:5869–5880. doi: 10.1021/bi7023282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 2000;39:257–288. doi: 10.1016/S0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- 51.Chu CT, et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat. Cell Biol. 2013;15:1197–1205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazzio E, Yoon KJ, Soliman KF. Acetyl-L-carnitine cytoprotection against 1-methyl-4-phenylpyridinium toxicity in neuroblastoma cells. Biochem. Pharmacol. 2003;66:297–306. doi: 10.1016/S0006-2952(03)00261-2. [DOI] [PubMed] [Google Scholar]
- 53.Calabrese V, et al. Acetylcarnitine induces heme oxygenase in rat astrocytes and protects against oxidative stress: involvement of the transcription factor Nrf2. J. Neurosci. Res. 2005;79:509–521. doi: 10.1002/jnr.20386. [DOI] [PubMed] [Google Scholar]
- 54.Hagen TM, et al. Acetyl-L-carnitine fed to old rats partially restores mitochondrial function and ambulatory activity. Proc. Natl Acad. Sci. USA. 1998;95:9562–9566. doi: 10.1073/pnas.95.16.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spagnoli A, et al. Long-term acetyl-L-carnitine treatment in Alzheimer’s disease. Neurol. 1991;41:1726–1732. doi: 10.1212/wnl.41.11.1726. [DOI] [PubMed] [Google Scholar]
- 56.Forloni G, Angeretti N, Smiroldo S. Neuroprotective activity of acetyl-L-carnitine: studies in vitro. J. Neurosci. Res. 1994;37:92–96. doi: 10.1002/jnr.490370112. [DOI] [PubMed] [Google Scholar]
- 57.Bossu P, et al. Loss of function mutations in the progranulin gene are related to pro-inflammatory cytokine dysregulation in frontotemporal lobar degeneration patients. J. Neuroinflammation. 2011;8:65. doi: 10.1186/1742-2094-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gibbons L, et al. Plasma levels of progranulin and interleukin-6 in frontotemporal lobar degeneration. Neurobiol. Aging. 2015;36:1603 e1601–1604. doi: 10.1016/j.neurobiolaging.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller ZA, et al. TDP-43 frontotemporal lobar degeneration and autoimmune disease. J. neurology, neurosurgery, psychiatry. 2013;84:956–962. doi: 10.1136/jnnp-2012-304644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ryan SD, et al. Amyloid-beta42 signals tau hyperphosphorylation and compromises neuronal viability by disrupting alkylacylglycerophosphocholine metabolism. Proc. Natl Acad. Sci. USA. 2009;106:20936–20941. doi: 10.1073/pnas.0905654106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benveniste J, Henson PM, Cochrane CG. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J. Exp. Med. 1972;136:1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schlondorff D, Neuwirth R. Platelet-activating factor and the kidney. Am. J. Physiol. 1986;251:F1–11. doi: 10.1152/ajprenal.1986.251.1.F1. [DOI] [PubMed] [Google Scholar]
- 63.Sanchez-Crespo M, Alonso F, Egido J. Platelet-activating factor in anaphylaxis and phagocytosis. I. Release from human peripheral polymorphonuclears and monocytes during the stimulation by ionophore A23187 and phagocytosis but not from degranulating basophils. Immunology. 1980;40:645–655. [PMC free article] [PubMed] [Google Scholar]
- 64.Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. Platelet-activating factor and related lipid mediators. Annu. Rev. Biochem. 2000;69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 65.Farooqui AA, Ong WY, Farooqui T. Lipid mediators in the nucleus: Their potential contribution to Alzheimer’s disease. Biochim. Biophys. Acta. 2010;1801:906–916. doi: 10.1016/j.bbalip.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Hershkowitz M, Adunsky A. Binding of platelet-activating factor to platelets of Alzheimer’s disease and multiinfarct dementia patients. Neurobiol. Aging. 1996;17:865–868. doi: 10.1016/S0197-4580(96)00073-5. [DOI] [PubMed] [Google Scholar]
- 67.Simmons C, Ingham V, Williams A, Bate C. Platelet-activating factor antagonists enhance intracellular degradation of amyloid-beta42 in neurons via regulation of cholesterol ester hydrolases. Alzheimers Res. Ther. 2014;6:15. doi: 10.1186/alzrt245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bacchetti T, et al. Higher Levels of Oxidized Low Density Lipoproteins in Alzheimer’s Disease Patients: Roles for Platelet Activating Factor Acetyl Hydrolase and Paraoxonase-1. J. Alzheimer’s disease: JAD. 2015;46:179–186. doi: 10.3233/JAD-143096. [DOI] [PubMed] [Google Scholar]
- 69.Nenseter MS, Drevon CA. Dietary polyunsaturates and peroxidation of low density lipoprotein. Curr. Opin. Lipidol. 1996;7:8–13. doi: 10.1097/00041433-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Igarashi K, Yoshida M, Waragai M, Kashiwagi K. Evaluation of dementia by acrolein, amyloid-beta and creatinine. Clin. Chim. Acta. 2015;450:56–63. doi: 10.1016/j.cca.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 71.Calingasan NY, Uchida K, Gibson GE. Protein-bound acrolein: a novel marker of oxidative stress in Alzheimer’s disease. J. Neurochem. 1999;72:751–756. doi: 10.1046/j.1471-4159.1999.0720751.x. [DOI] [PubMed] [Google Scholar]
- 72.Tsou HH, et al. Alterations in Acrolein Metabolism Contribute to Alzheimer’s Disease. J. Alzheimer’s disease: JAD. 2018;61:571–580. doi: 10.3233/JAD-170736. [DOI] [PubMed] [Google Scholar]
- 73.Uchida K. Current status of acrolein as a lipid peroxidation product. Trends Cardiovasc. Med. 1999;9:109–113. doi: 10.1016/S1050-1738(99)00016-X. [DOI] [PubMed] [Google Scholar]
- 74.Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007;8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 75.Sharmin S, et al. Polyamine cytotoxicity in the presence of bovine serum amine oxidase. Biochem. Biophys. Res. Commun. 2001;282:228–235. doi: 10.1006/bbrc.2001.4569. [DOI] [PubMed] [Google Scholar]
- 76.Yoshida M, et al. Acrolein toxicity: Comparison with reactive oxygen species. Biochem. Biophys. Res. Commun. 2009;378:313–318. doi: 10.1016/j.bbrc.2008.11.054. [DOI] [PubMed] [Google Scholar]