Abstract
Leishmaniases are neglected tropical diseases and Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis are the most important causative agents of leishmaniases in the New World. These two parasite species may co-circulate in a given endemic area but their interactions in the vector have not been studied yet. We conducted experimental infections using both single infections and co-infections to compare the development of L. (L.) infantum (OGVL/mCherry) and L. (V.) braziliensis (XB29/GFP) in Lutzomyia longipalpis and Lutzomyia migonei. Parasite labelling by different fluorescein proteins enabled studying interspecific competition and localization of different parasite species during co-infections. Both Leishmania species completed their life cycle, producing infective forms in both sand fly species studied. The same happens in the co infections, demonstrating that the two parasites conclude their development and do not compete with each other. However, infections produced by L. (L.) infantum reached higher rates and grew more vigorously, as compared to L. (V.) braziliensis. In late-stage infections, L. (L.) infantum was present in all midgut regions, showing typical suprapylarian type of development, whereas L. (V.) braziliensis was concentrated in the hindgut and the abdominal midgut (peripylarian development). We concluded that both Lu. migonei and Lu. longipalpis are equally susceptible vectors for L. (L.) infantum, in laboratory colonies. In relation to L. (V.) braziliensis, Lu. migonei appears to be more susceptible to this parasite than Lu. longipalpis.
Subject terms: Parasite development, Entomology
Introduction
Leishmaniases are important parasitic diseases, causing serious medical problems in many countries, as they rank in the top-three list of neglected tropical diseases caused by protists1. The causative agents, flagellates of the genus Leishmania (Kinetoplastida: Trypanosomatidae), subgenera Leishmania and Viannia, are transmitted by phlebotomine sand flies (Diptera: Psychodidae)2.
In Brazil, Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis are the most important causative agents of leishmaniases in humans3. L. (V.) braziliensis causes a typical cutaneous leishmaniasis (CL), which may progress to mucosal disease, whereas L. (L.) infantum infections is responsible for a life-threatening form of the disease – visceral leishmaniasis (VL). These two parasite species also differ in their development in the sand fly vector; both colonize the sand fly midgut, but only L. (V.) braziliensis was documented to colonize the hindgut (peripylarian development)4.
As different parasite species may co-circulate in a given endemic area5, the significance of co-infections by different Leishmania in sand flies is poorly understood. So far, rather limited number of investigations has been conducted to study the simultaneous development of different Leishmania species in the same sand fly6,7. In particular, it would be interesting and important to study simultaneous development of a suprapylarian Leishmania and a peripylarian Viannia.
The present study was designed to fill this gap in knowledge and conducted experimental co-infections to compare the development of L. (L.) infantum and L. (V.) braziliensis in Lutzomyia longipalpis, a known permissive vector8,9, and Lutzomyia migonei, which is susceptible to the development of L. (V.) braziliensis10 and to different strains of L. (L.) infantum11. Parasite labelling by different fluorescein proteins enabled us to study interspecific competition during co-infections.
Results
Experimental infections of Lu. migonei
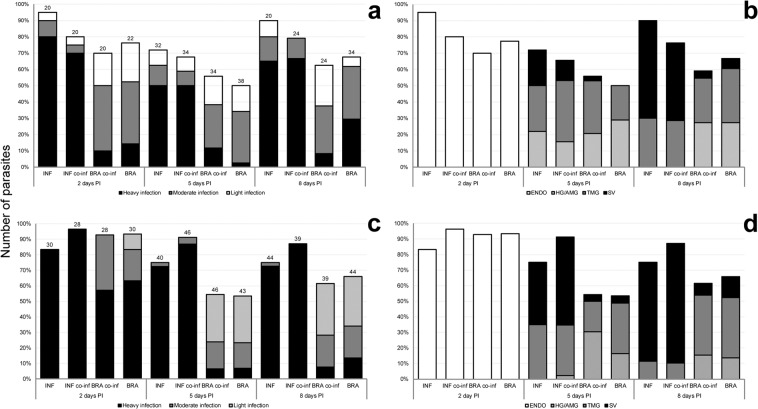
In total, 322 Lu. migonei females were dissected. On day 2 post infection (PI), L. (L.) infantum infection rate was 95% and parasites grew very vigorously, whereas L. (V.) braziliensis infection rate was 70% and parasites grew slower (Fig. 1a). Both parasite species were restricted to the endoperitrophic space (bloodmeal surrounded by peritrophic matrix) (Fig. 1b). There were no statistically significant differences in the infection rate (X2 = 4.29, df = 3, P = 0.23).
Figure 1.
Rates and intensities (a,c) and localization of Leishmania spp. (b,d) in Lu. migonei (a,b) and Lu. longipalpis (c,d) evaluated by fluorescence microscopy. Intensities of infections were classified into three categories: light (<100 parasites/gut), moderate (100–1000 parasites/gut), or heavy (>1000 parasites/gut). PI = post infections, INF = L. infantum, BRA = L. braziliensis. Columns represent intensity of the Leishmania species either in single infections (INF single, BRA single) of in coinfection (INF co-inf, BRA co-inf). Numbers of dissected sand fly females are shown above the bars.
On day 5 PI, L. (L.) infantum infection rate was still high (Fig. 1a), parasites colonized both parts of the midgut (abdominal and thoracic midgut) and, in more than 20% of female sand flies, also the stomodeal valve (Fig. 1b). On the other hand, L. (V.) braziliensis infection rate was lower (50%) and parasites colonized only the midgut and the hindgut. Differences in infection rates were non-significant (X2 = 4.52, df = 3, P = 0.21), whereas differences in the location of the parasites were significant (X2 = 6.94, df = 1, P < 0.01). Similar differences between L. (L.) infantum and L. (V.) braziliensis were also observed in parasite loads and their localization during coinfections (Fig. 1b).
On day 8 PI, in late stage infections, L. (L.) infantum developed very successfully (infection rate 90%), whereas L. (V.) braziliensis grew less successfully (infection rate 68%), but there was no statistical difference (X2 = 3.44, df = 1, P = 0.06). L (L.) infantum colonized the cardia and the stomodeal valve significantly more frequently (X2 = 14.43, df = 1, P < 0.01), whereas L. (V.) braziliensis did not colonize the stomodeal valve at all (Fig. 1b).
Co-infection did not alter the dynamics of infection of the individual parasite species and did not favour or harm any of the parasite species. In co-infections L. (L.) infantum produced high infection rates (Fig. 1a) and colonized the stomodeal valve in high numbers (Fig. 2D–F). In contrast, L. (V.) braziliensis infections were limited to the midgut and hindgut and some co-infected females became negative for L. (V.) braziliensis (Fig. 1b).
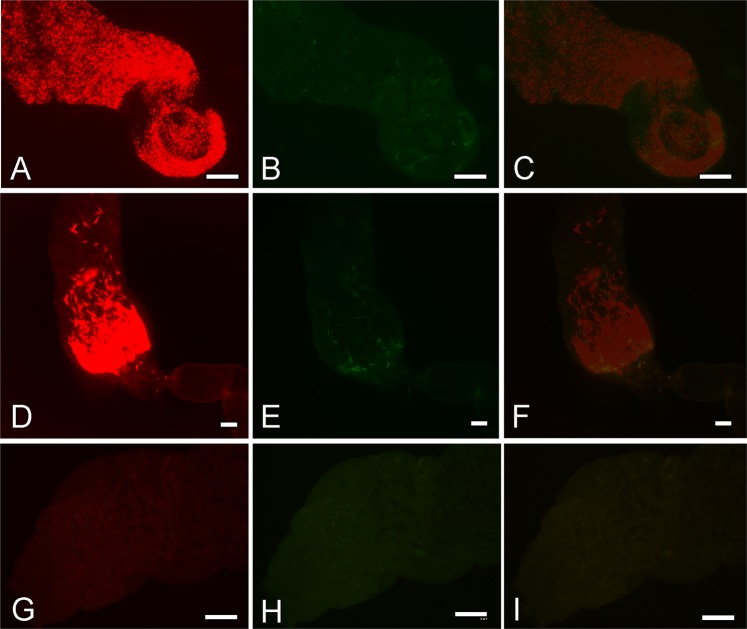
Figure 2.
Fluorescence micrographs of thoracic midguts with cardia section and stomodeal valve of Lutzomyia spp. females on days 8 postinfection. Cardia of Lu. longipalpis (A–C) and Lu. migonei (D–F) coinfected by Leishmania (Viannia) braziliensis (green) and Leishmania (Leishmania) infantum (red). Control uninfected gut of Lu. longipalpis (G–I). (A,D,G) images from red fluorescence, (B,E,H) images from green fluorescence and (C,F,I) merged images, scale bar: 5 mm.
Experimental infections of Lu. longipalpis
In total, 457 Lu. longipalpis females were dissected. On day 2 PI, infection rates were high in all parasite-vector combination (83%) (Fig. 1c), with parasites present in the endoperitrophic space (Fig. 1d).
On day 5 PI, L. (L.) infantum developed more successfully than L. (V.) braziliensis (infection rates of 75% and 53%, respectively (X2 = 4.16, df = 1, P = 0.04; X2 = 15.9, df = 1, P < 0.01).
On day 8, most of the females of Lu. longipalpis infected with L. (L.) infantum had a heavy infection rate (75%), whereas in L. (V.) braziliensis parasites were less numerous and the infection rate (66%) was significantly lower (X2 = 38.09, df = 2, P < 0.01) (Fig. 2A–C). L. (L.) infantum parasites frequently colonized the stomodeal valve while L. (V.) braziliensis infections were mostly limited to in the midgut and pylorus region of the hindgut (Fig. 1d). The same differences in parasite localization were observed in coinfections.
Co-infection did not alter the dynamics of infection of the individual parasite species and did not favour or harm any of the parasite species and the same differences in parasite localization were observed in coinfections.
Morphometric analysis of promastigotes from midgut smears
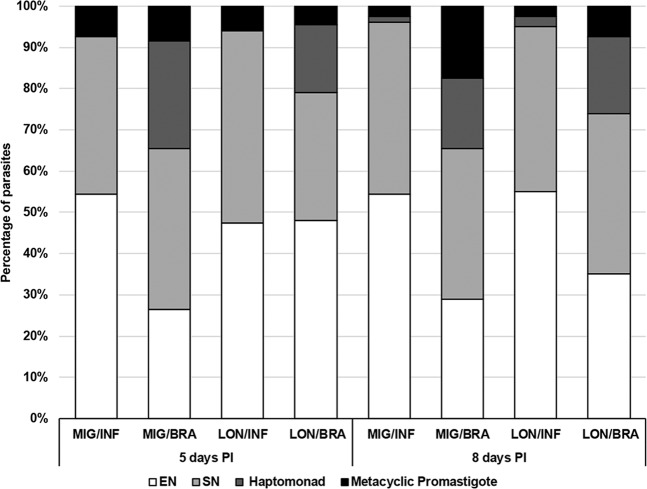
On day 5 PI, some differences in proportions of morphological forms in various parasite-vector combinations were documented. In L. (L.) infantum, elongated nectomonads were the prevailing forms in both vectors (55% Lu. migonei and 48% in Lu. longipalpis) (Fig. 3), and differences between various vectors were not significant (X2 = 3.00, df = 2, P = 0.22). On the other hand, significant differences were found between morphological forms of L. (V.) braziliensis (X2 = 20.95, df = 3, P < 0.01) in both vectors: elongated nectomonads (Fig. 4A) prevailed in Lu. longipalpis (48%) but short nectomonads (Fig. 4B) were the most numerous forms in Lu. migonei (39%). The metacyclic (Fig. 4C) forms were found in all parasite-vector combinations but in relatively low numbers, from 7.5 to 8.5% (Table 1).
Figure 3.
Morphological forms of Leishmania spp. during development in Lutzomyia spp. Morphological forms of Leishmania parasites in Lutzomyia migonei and Lutzomyia longipalpis were evaluated by light microscopy using oil-immersion objective on days 5 and 8 postinfection. MIG (Lu. migonei), BRA (L. (V.) braziliensis), LONG (Lu. longipalpis), INF (L. (L.) infantum), EN (elongated nectomonads), SN (short nectomonads).
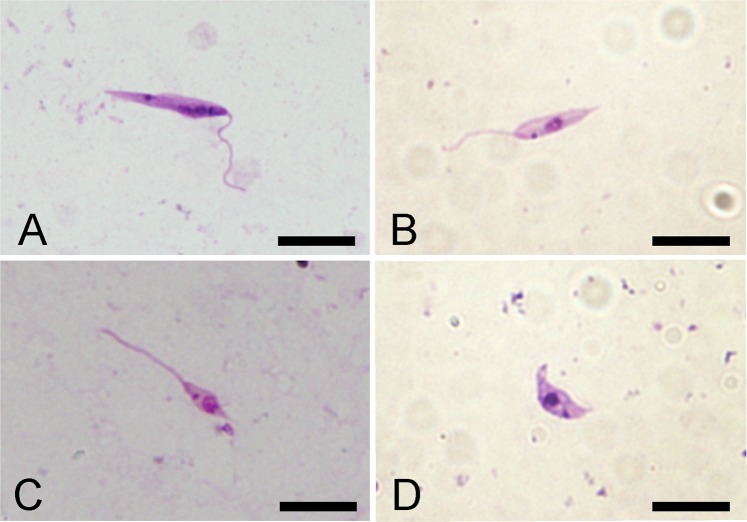
Figure 4.
Morphological forms of Leishmania occurring in sand fly midgut of Lu. migonei. Scale bar 10 μm. (A) Elongated nectomonad of L. (V.) braziliensis in Lu. migonei; (B) Short nectomonad of L. (L.) infantum in Lu. migonei (C) Metacyclic promastigote of L. (L.) infantum in Lu. migonei; (D) haptomonads of L. (L.) infantum in Lu. migonei.
Table 1.
Promastigotes from gut smears were measured under light microscopy with an oil-immersion objective. MIG (Lutzomyia migonei). BRA (Leishmania (Viannia) braziliensis). LONG (Lutzomyia longipalpis). INF (Leishmania (Leishmania) infantum). PI (postinfection). EN (elongated nectomonads). SN (short nectomonads). HAP (haptomonads). MET (metacyclic promastigotes).
| Day PI | Parasite-vector | Morphological form | n | Body length | Body width | Flagellar length | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean (µm) | Range (µm) | Mean (µm) | Range (µm) | Mean (µm) | Range (µm) | ||||
| 5 | MIG/INF | EN | 109 | 17.3 | 25.9-14 | 3 | 5.2-1.5 | 23.8 | 33.8-17.1 |
| SN | 76 | 11.9 | 13.9-7.3 | 3.1 | 5.9-1.6 | 13.11 | 25.3-3.2 | ||
| HAP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| MET | 15 | 9.4 | 13.5-6.7 | 3.7 | 5.8-2.0 | 23.8 | 33.8-17.1 | ||
| MIG/BRA | EN | 53 | 15.1 | 21.1-13.0 | 2.9 | 4.5-1.8 | 16.6 | 30.4-2.8 | |
| SN | 78 | 10.2 | 12.9-5.7 | 3.1 | 5.7-1.2 | 13.8 | 23.0-6.0 | ||
| HAP | 52 | 9.7 | 12.8-3.5 | 3.4 | 5.9-1.9 | 5.8 | 12.1-1.1 | ||
| MET | 17 | 9.1 | 11.8-6.2 | 3.5 | 4.5-2.0 | 20.8 | 25.5-14.8 | ||
| LON/INF | EN | 95 | 17.4 | 26.4-14.0 | 2.3 | 3.7-1.3 | 19.1 | 29.2-5.8 | |
| SN | 93 | 11.3 | 13.9-6.0 | 2.2 | 4.3-1.2 | 13.2 | 26.6-2.0 | ||
| HAP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| MET | 12 | 8.7 | 11.1-6.4 | 2.5 | 3.6-1.6 | 22.4 | 28.2-18.0 | ||
| LON/BRA | EN | 96 | 13.2 | 28.3-16.3 | 2.9 | 12.2-1.2 | 18.4 | 31.8-3.5 | |
| SN | 62 | 10.7 | 12.8-5.7 | 2.9 | 6.9-1.3 | 15.4 | 23.4-7.5 | ||
| HAP | 33 | 9.8 | 12.7-4.9 | 3 | 5.9-1.6 | 7.11 | 11.9-2.1 | ||
| MET | 9 | 8.4 | 12.2-2.8 | 3.1 | 5.6-1.5 | 21.3 | 27.9-12.4 | ||
| 8 | MIG/INF | EN | 109 | 16.6 | 22.5-14 | 2.8 | 4.9-1.3 | 15.2 | 24.7-2.9 |
| SN | 83 | 11.8 | 13.9-6.8 | 2.7 | 4.3-1.5 | 12.3 | 26.4-3.4 | ||
| HAP | 3 | 12.9 | 13.9-11.5 | 3 | 3.1-2.9 | 2.7 | 2.9-2.4 | ||
| MET | 5 | 8.6 | 9.5-7.6 | 2.8 | 3.3-2.0 | 19.5 | 24.7-16.9 | ||
| MIG/BRA | EN | 58 | 15 | 18.9-13.1 | 2.9 | 17.2-1.5 | 20.9 | 30.6-2.9 | |
| SN | 73 | 10.8 | 12.9-5.8 | 2.4 | 3.7-1.5 | 17 | 24.6-10.2 | ||
| HAP | 34 | 9.4 | 12.5-6.0 | 2.6 | 3.8-1.7 | 5.9 | 11.9-1.5 | ||
| MET | 35 | 9 | 12.7-4.9 | 2.6 | 2.3-1.5 | 22.7 | 30.9-14.5 | ||
| LON/INF | EN | 110 | 16.7 | 24.0-14.0 | 2.7 | 5.0-1.3 | 15.2 | 26.9-2.3 | |
| SN | 80 | 11.8 | 13.9-6.1 | 2.6 | 5.7-1.6 | 1.9 | 2.3-1.2 | ||
| HAP | 5 | 11.3 | 12.2-9.8 | 2.3 | 2.8-1.9 | 11.4 | 24.6-3.0 | ||
| MET | 5 | 9.6 | 13.2-6.9 | 3.6 | 5.1-2.2 | 23.4 | 31.1-23.4 | ||
| LON/BRA | EN | 70 | 14.6 | 21.2-9.4 | 2.3 | 5.0-1.6 | 16.3 | 30.6-3.6 | |
| SN | 78 | 10.6 | 12.9-5.5 | 2.3 | 5.0-1.3 | 15.2 | 23.7-6.2 | ||
| HAP | 37 | 10.6 | 12.7-6.5 | 2.2 | 3.5-1.2 | 6.4 | 23.7-1.8 | ||
| MET | 15 | 10.2 | 12.9-4.5 | 2 | 3.1-1.1 | 19.9 | 24.5-15.2 | ||
On day 8 PI, differences between Leishmania species were more pronounced: in Lu. migonei and Lu. longipalpis infected by L. (L.) infantum the majority of parasites were present as elongated nectomonads, whereas in Lu. migonei and Lu. longipalpis infected by L. (V.) braziliensis the prevailing forms were short nectomonads (Fig. 3). Metacyclic forms were found in all parasite-vector combinations, the highest proportion (17.5%) were found in Lu. migonei infected by L. (V.) braziliensis (Table 1).
Comparison of growth curves in vitro
To investigate the in vitro growth of Leishmania spp., the cultures were maintained at a temperature 26 °C. For both parasite species, the initial doses were 104 promastigotes/ml and 105 promastigotes/ml and the cells were cultivated for seven days. The growth of the parasites in culture was similar to that occurring in the phlebotomine sand flies: L. (L.) infantum and L. (V.) braziliensis grew similarly until the third day of cultivation (Fig. 5a,b), there were no statistical differences (the initial dose of 104: X2 = 2.46, df = 3, P = 0.48, the initial dose of 105: X2 = 6.77, df = 3, P = 0.08). From day 4 PI, L. (V.) braziliensis grew slower compared to L. (L.) infantum. The differences were statistically significant (the initial dose 104: X2 = 256.2, df = 6, P < 0.01, the initial dose 105: X2 = 95.1, df = 6, P < 0.01).
Figure 5.
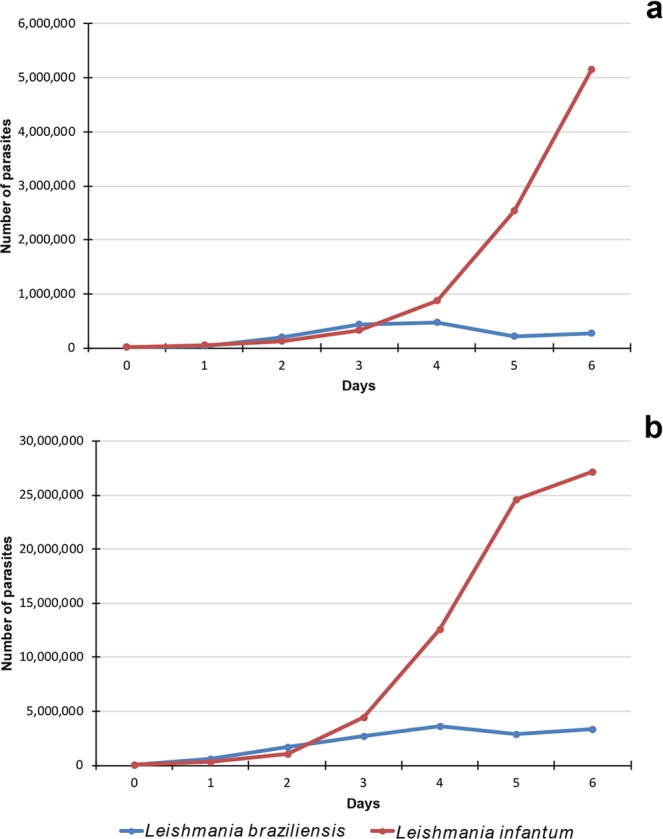
The curve growth of Leishmania spp. cultures at days 0, 1, 2, 3, 4, 5 and 6. Two initial concentrations were used for each Leishmania species. (a) Initial dose (at day 0) of 104 promastigotes/ml; (b) Initial dose (at day 0) of 105 promastigotes/ml.
Discussion
We evaluated the developmental patterns of two Leishmania spp. in two New World phlebotomine sand fly species, using both single infections and co-infections. Differences found between L. (L.) infantum and L. (V.) braziliensis were more pronounced than the differences between Lutzomyia species. In particular, L. (L.) infantum produced higher infection rates and grew more vigorously, as compared to L. (V.) braziliensis, in both Lu. longipalpis and Lu. migonei. Both sand fly species tested were similarly susceptible to infection by L. (L.) infantum, which confirms previous findings11.
Sand fly vectors have been classified into two categories, specific vectors and permissive vectors, based on their ability to support late-stage development of different Leishmania species8,9. Specific vectors, like Phlebotomus papatasi support development of a single parasite species or two closely related parasite species6,12 In contrast, permissive vectors support experimental infections by a broad range of Leishmania spp. The most important example of a permissive vector is Lu. longipalpis, which was involved in the establishment of L. (L.) infantum in Latin America9 and is proven vector of this parasite in many countries, from Costa Rica to northern Argentina5,13. Here we confirmed that Lu. migonei should be also considered as the permissive vector, as females of this species support the development of all Leishmania species tested so far, namely Leishmania (Leishmania) amazonensis, L. (V.) braziliensis and L. (L.) infantum10,11.
Nevertheless, our detailed comparative study revealed that Lu. migonei was more susceptible to L. (V.) braziliensis than Lu. longipalpis. Previously, several sand fly species were described as vectors of L. (V.) braziliensis14, but the role of Lu. longipalpis and Lu. migonei in the circulation of this parasite was unknown5. Natural infections of Lu. migonei by L. (V.) braziliensis were repeatedly found in the Rio de Janeiro State10,15. Nieves and Pimenta10 conducted a laboratory study on susceptibility of Lu. migonei to L. (V.) braziliensis resulting in 77% positivity of the dissected females. Lu. longipalpis carrying DNA of L. (V.) braziliensis has been described in the South-Eastern region of Brazil16 and in laboratory conditions 70% of Lu. longipalpis females developed late stage infections of L. (V.) braziliensis17.
In single infections and co-infections, L. (L.) infantum developed more rapidly in both sand fly species than L. braziliensis. This correlates with growth curves we observed in vitro: during initial three days both species grew similarly, but then, from day 3 onwards, L. (L.) infantum grew significantly faster. These results suggest that development in the sand fly is affected not only by the susceptibility of the vectors, but also by the growth characteristics of the studied strains. In late-stage infections, L. (L.) infantum was present in all midgut regions, the cardia and stomodeal valve in both sand fly species from day 5 PI, showing typical suprapylarian type of development. In contrast, L. (V.) braziliensis was concentrated in the hindgut and the abdominal midgut (peripylarian development). The same happens in the co infections, demonstrating that the two parasites conclude their development and do not compete with each other. Previous study with Lu. migonei and two Leishmania species was done using single infections only, but observations by Nieves and Pimenta10 were similar to our results: suprapylarian L. (L.) amazonensis grew faster than peripylarian L. (V.) braziliensis.This interspecific difference appears to be strain-independent as almost identical patterns of development were described for the L. (V.) braziliensis strain m290310 and for two different L. (L.) infantum strains M4192 and CUK3 in Lu. migonei11.
From an epidemiological point of view, it is very important to carry out studies, assessing the development of two species of parasites that cause different forms of leishmaniasis, especially when they coexist in endemic regions18. Both Leishmania species completed the life cycle, producing infective forms in both sand fly species studied. We conclude that both Lu. migonei and Lu. longipalpis are equally susceptible vectors for L. (L.) infantum, in laboratory colonies. In relation to L. (V.) braziliensis, Lu. migonei appears to be more susceptible to this parasite than Lu. longipalpis.
Material and Methods
Sand fly colonies and Leishmania strains
Established laboratory colonies of Lu. longipalpis (from Jacobina, Brazil) and Lu. migonei (from Baturité, Brazil) were used and maintained under standard conditions, as previously described19. A fluorescent strains of L. (V.) braziliensis (XB29 marked with GFP) and L. (L.) infantum (OGVL marked with mCherry) by stable integration of mCherry into the 18 S rRNA locus as previously described20. We have used parasites within less than 10 passages in vitro and were maintained at 23 °C on Medium 199 (Sigma-Aldrich, USA) supplemented with 10% fetal calf serum (Thermo Fisher Scientific, USA), 1% BME vitamins (Sigma-Aldrich), 2% human urine and 250 μg/ml amikin (Bristol-Myers Squibb, USA).
For study of growth curves in-vitro, two initial doses were used: 104 promastigotes/ml and 105 promastigotes/ml. The concentration of parasites was analyzed daily for 7 days, using counting in Burker chamber. The experiments were repeated twice.
Experimental infections of sand flies
Female sand flies (3–6 days old) of both species were fed through a chick-skin membrane on heat-inactivated rabbit blood containing 106 promastigotes/ml for single infections and 5 × 105 of each species for co-infections (half a dose used in single infections). Three groups of females of Lu. migonei were studied, the first infected with L. (V.) braziliensis (XB29 marked with GFP), the second with L. (L.) infantum (OGVL marked with mCherry) and the third co-infected with both Leishmania species. The same procedure was performed with L. longipalpis. Engorged females were separated and maintained in the same conditions as the colony and dissected on days 2, 5 and 8 post-infection (PI). Individual guts were placed into a drop of saline and analyzed by fluorescence microscopy for the localization, infection intensity and morphology of Leishmania infections. Parasite loads were graded according to Myskova et al.21 as light (<100 parasites per gut), moderate (100 to 1000 parasites per gut) and heavy (>1000 parasites per gut). The experiments were repeated four times.
Morphometry of parasites
Smears from midguts of Lu. migonei and Lu. longipalpis infected with L. (L.) infantum and L. (V.) braziliensis, alone or in combination (i.e., co-infected) were prepared on days 5 and 8 PI, being fixed with methanol and stained with Giemsa. Stained smears were then examined under a light microscope with an oil-immersion objective and photographed with an Olympus D70 camera (Olympus, Hong-Kong, China). Body length, body width and flagellar length of 200 randomly selected promastigotes from four females/smears were measured for each sand fly species and time intervals using Image-J 1.x software. Promastigote forms were distinguished according to Walters et al.22 and Sadlova et al.23. L. (L.) infantum, developmental forms were identified as follows: (i) elongated nectomonads (body length ≥14 μm) (ii) short nectomonads (body length <14 μm and flagellar length ≤2 times body length); (iii) metacyclic promastigotes (body length <14 μm and flagellar length ≥2 times body length); and (iv) haptomonads (flagellum 0–3 µm, present during the late stage infections in cardia). L. (V.) braziliensis, developmental forms were identified as follows: (i) elongated nectomonads (body length ≥13 μm); (ii) short nectomonads (body length <13 μm and flagellar length ≤ 2 times body length); (iii) metacyclic promastigotes (body length <13 μm and flagellar length ≥2 times body length); and (iv) haptomonads (flagellar length ≤ body length, present in hind gut and cardia).
Ethical approval
Animals used for blood-feeding of sand fly colonies were maintained and handled in the animal facility of Charles University in Prague, in accordance with institutional guidelines and Czech legislation (Act of the Czech National Assembly on the Protection of Animals Against Cruelty No. 246/1992, latest amendment No. 359/2012), which complies with all relevant European Union guidelines for experimental animals. All experiments were approved by the Committee on the Ethics of Laboratory Experiments of the Charles University in Prague and were performed under the Certificate of Competency (Registration Number: CZ 03069).
Acknowledgements
This study was partially supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (grant number CNPq-PVE-CsF 400699/2014-1), Research Center UNCE 204072 to T.L. and M.J., Grant Agency of the Czech Republic (grant number17-10656S) and Russian Science Foundation (grant 19-15-00054, (establishing of fluorescent Leishmania strain) to V.Y., and by ERD funds MSMT CZ.02.1.01/0.0/0.0/16_019/0000759 to P.V., V.Y. and J.S. F.D.T. is the recipient of a research fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 313118/2018-3).
Author contributions
P.V. and J.S. designed the study. S.P.B.F. and P.V. were responsible for the financial support of the project. J.A., J.S., T.L., L.P., B.V., V.Y. and M.J. conducted the laboratory work, including establishing of the fluorescent strains of parasites, conducting of sand fly infections and dissections. J.A. and J.S. contributed to data analysis. J.A., P.V. and F.D.T. drafted the manuscript; all authors read, discussed and approved the final version of the paper
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fenwick A. The global burden of neglected tropical diseases. Public Health. 2012;126:233–6. doi: 10.1016/j.puhe.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Maroli M, et al. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med. Vet. Entomol. 2013;27:123–47. doi: 10.1111/j.1365-2915.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- 3.Alvar J, et al. Leishmaniasis worldwide and global estimates of its incidence. Plos One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lainson R, Ready PD, Shaw JJ. Leishmania in phlebotomid sandflies. VII. On the taxonomic status of Leishmania peruviana, causative agent of Peruvian ‘uta’, as indicated by its development in the sandfly, Lutzomyia longipalpis. Proc. R. Soc. Lond. 1979;206:307–18. doi: 10.1098/rspb.1979.0107. [DOI] [PubMed] [Google Scholar]
- 5.Dvorak, V, Shaw, J. J. & Volf, P. Parasite biology: The vectors. In The leishmaniases: Old neglected tropical diseases (ed. Bruschi, F. & Gradoni, L.) 31–78 (Springer International Publishing, 2018).
- 6.Chajbullinova A, et al. The development of Leishmania turanica in sand flies and competition with L. major. Parasit. Vectors. 2012;5:219. doi: 10.1186/1756-3305-5-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inbar E, et al. The mating competence of geographically diverse Leishmania major strains in their natural and unnatural sand fly vectors. Plos Genet. 2013;9:e1003672. doi: 10.1371/journal.pgen.1003672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamhawi S. Phlebotomine sand flies and Leishmania parasites: friends or foes? Trends Parasitol. 2006;22:439–45. doi: 10.1016/j.pt.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Volf P, Myskova J. Sand flies and Leishmania: specific versus permissive vectors. Trends Parasitol. 2007;23:91–92. doi: 10.1016/j.pt.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieves E, Pimenta PF. Development of Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis in the sand fly Lutzomyia migonei (Diptera: Psychodidae) Ann. Entomol. Soc. Am. 2000;37:134–40. doi: 10.1603/0022-2585-37.1.134. [DOI] [PubMed] [Google Scholar]
- 11.Guimarães VCFV, et al. Lutzomyia migonei is a permissive vector competent for Leishmania infantum. Parasit. Vectors. 2016;9:159. doi: 10.1186/s13071-016-1444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pimenta PF, et al. Evidence that the vectorial competence of phlebotomine sand flies for different species of Leishmania is controlled by structural polymorphisms in the surface lipophosphoglycan. Proc. Natl. Acad. Sci. USA. 1994;91:9155–9. doi: 10.1073/pnas.91.19.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lainson R, Rangel EF. Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil: a review. Mem. Inst. Oswaldo Cruz. 2005;100:811–27. doi: 10.1590/S0074-02762005000800001. [DOI] [PubMed] [Google Scholar]
- 14.Brazil RP, et al. Sand fly vectors of Leishmania in the Americas-A mini review. Entomol. Ornithol. Herpetol. 2015;4:144. [Google Scholar]
- 15.Pita-Pereira D, et al. Identification of naturally infected Lutzomyia intermedia and Lutzomyia migonei with Leishmania (Viannia) braziliensis in Rio de Janeiro (Brazil) revealed by a PCR multiplex non-isotopic hybridisation assay. Trans. R. Soc. Trop. Med. Hyg. 2005;99:905–13. doi: 10.1016/j.trstmh.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Lana Rosana Silva, Michalsky Érika Monteiro, Fortes-Dias Consuelo Latorre, França-Silva João Carlos, Lara-Silva Fabiana de Oliveira, Rocha Lima Ana Cristina Vianna Mariano da, Moreira de Avelar Daniel, Martins Juliana Cristina Dias, Dias Edelberto Santos. Phlebotomine Sand Fly Fauna andLeishmaniaInfection in the Vicinity of the Serra do Cipó National Park, a Natural Brazilian Heritage Site. BioMed Research International. 2015;2015:1–9. doi: 10.1155/2015/385493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hlavacova J, Votypka J, Volf P. The effect of temperature on Leishmania (Kinetoplastida: Trypanosomatidae) development in sand flies. J. Med. Entomol. 2013;50:955–8. doi: 10.1603/ME13053. [DOI] [PubMed] [Google Scholar]
- 18.Dantas-Torres F, et al. Cutaneous and visceral leishmaniosis in dogs from a rural community in northeastern Brazil. Vet. Parasitol. 2010;170:313–317. doi: 10.1016/j.vetpar.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 19.Volf P, Volfova V. Establishment and maintenance of sand fly colonies. J. Vector Ecol. 2011;36(Suppl 1):S1–S9. doi: 10.1111/j.1948-7134.2011.00106.x. [DOI] [PubMed] [Google Scholar]
- 20.Kraeva N, et al. Leptomonas seymouri: adaptations to the dixenous life cycle analyzed by genome sequencing, transcriptome profiling and co-infection with Leishmania donovani. Plos Path. 2015;11(8):e1005127. doi: 10.1371/journal.ppat.1005127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myskova J, Votypka J, Volf P. Leishmania in sand flies: comparison of quantitative polymerase chain reaction with other techniques to determine the intensity of infection. J. Med. Entomol. 2008;45:133–13. doi: 10.1093/jmedent/45.1.133. [DOI] [PubMed] [Google Scholar]
- 22.Walters LL, et al. Ultrastructural biology of Leishmania (Viannia) panamensis (=Leishmania braziliensis panamensis) in Lutzomyia gomezi (Diptera: Psychodidae): a natural host-parasite association. Am. J. Trop. Med. Hyg. 1989;40:19–39. doi: 10.4269/ajtmh.1989.40.19. [DOI] [PubMed] [Google Scholar]
- 23.Sadlova J, et al. The stage-regulated HASPB and SHERP proteins are essential for differentiation of the protozoan parasite Leishmania major in its sand fly vector, Phlebotomus papatasi. Cell Microbiol. 2010;12:1765–79. doi: 10.1111/j.1462-5822.2010.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]